Abstract
Although biologics have demonstrated to be effective in T2-high asthma patients, there is little experience with these drugs in asthma–COPD overlap (ACO). The aim of this study was to compare the effectiveness of biologics in these two conditions. We included 318 patients (24 ACO and 297 asthma) treated with monoclonal antibodies and followed for at least 12 months. Omalizumab was the most frequently employed biologic agent both in patients with ACO and asthma. Asthma control test (ACT) scores after at least 12 months of biologic therapy were not significantly different between groups. The percentage of patients with ≥1 exacerbation and ≥1 corticosteroid burst was significantly higher in ACO patients (70.8 vs 27.3 and 83.3% vs 37.5%, respectively), whereas the percentage of “controlled” patients (with no exacerbations, no need for corticosteroids and ACT ≥ 20) was significantly lower (16.7% vs 39.7%). In conclusion, this report suggests that patients with ACO treated with biologics reach worse outcomes than asthma patients.
Keywords: asthma, asthma–COPD overlap, COPD
Asthma–COPD overlap (ACO) is the coincidence of two complex conditions in a single individual. Or it can be considered as a part of the chronic airway disease continuum (Dutch hypothesis), being placed somewhere between the “pure” forms of asthma and COPD.1 Whichever the case, ACO is challenging for the clinician.2 To choose the best therapeutic option is particularly difficult, given the lack of specific studies and the fact that most clinical trials for asthma excluded patients with features of COPD, and vice versa. Although biologics have demonstrated to be effective in T2-high asthma patients,2 there is little experience with these drugs in ACO.
The aim of this study was to compare the effectiveness of biologics in asthma and ACO. Data were sourced from the GEMA-DATA register, a national (Spain), multicenter (40 Asthma Units), observational initiative with retrospective and prospective data collection. This study was conducted in accordance with the Declaration of Helsinki, ethical governance was provided by Santa Creu i Sant Pau Hospital (Barcelona) and subjects signed informed consent prior to study commencement. Data collection started in October 2017 and is currently ongoing. GEMA-DATA includes ≥18-year-old patients diagnosed with asthma according to Global Initiative for Asthma (GINA) criteria and who receive treatment according to GINA Step 5 or experience uncontrolled asthma at Step 4.3 Only patients who received a monoclonal antibody and have been followed up for a period longer than 12 months were included. Demographic and spirometric data, as well as T2 biomarker values, were collected from the first recorded visit. Data concerning clinical outcome were extracted from the last recorded visit.
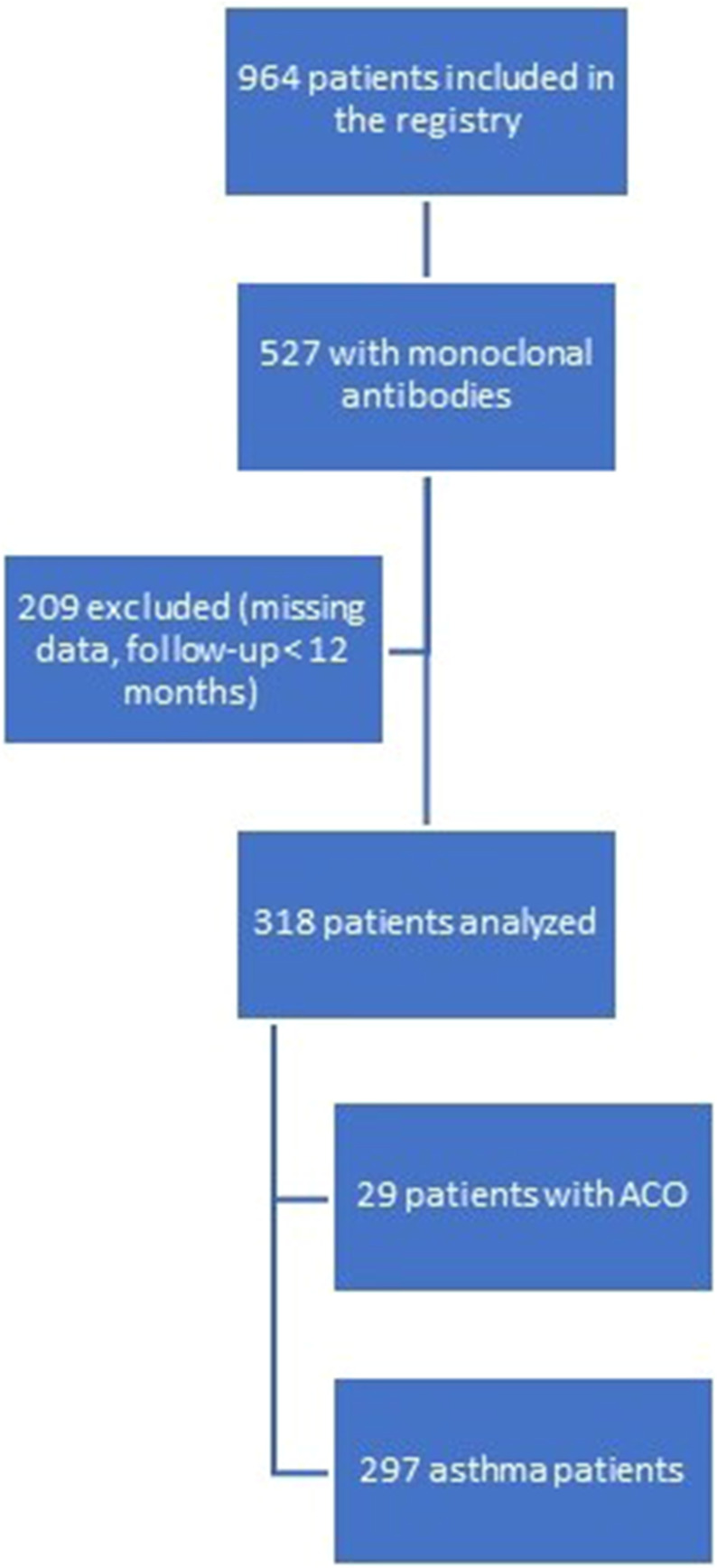
For the purpose of this study, only patients with ≥ 12 months follow-up and with no missing data on crucial variables (smoking history, spirometry, monoclonal antibodies use, ACT, systemic corticosteroids use, exacerbations) were analyzed. The flowchart (Figure 1) represents the consecutive stages of the patient inclusion.
Figure 1.
Stages of the patient inclusion.
Patients were classified into non-smokers or smokers (current or past smokers with a smoking history of ≥10 pack-years). ACO was diagnosed in those smokers who showed bronchial obstruction (FEV1/FVC <70%) despite maintenance treatment with bronchodilators or systemic corticosteroids. This definition fits in well with GINA´s proposal (history of smoking and/or other toxic exposures, any asthma features and persistent expiratory airflow limitation with or without bronchodilator reversibility).3 Asthma control test (ACT),4 severe exacerbations (admissions, emergency room visits, or the need for oral steroids for ≥3 days) in the preceding 12 months, unscheduled medical visits during this period, the need for regular systemic corticosteroids and the number of corticosteroids´ bursts were used to assess clinical outcome. “Clinical control” was defined as absence of severe exacerbations in the previous year, ACT ≥20 and non-use of systemic corticosteroids. Analyses were performed using Chi-squared or Fisher’s exact test for categorical data and Student’s t test or Wilcoxon rank sum test for continuous data as appropriate. A p-value <0.05 was considered significant. All analyses were conducted using SPSS software v22 (IBM Corporation, Armonk, NY, USA).
Twenty-four patients diagnosed with ACO and 297 with asthma were included. Demographic and clinical characteristics are summarized in Table 1. ACO was more frequently diagnosed in males, reflecting the fact that smoking is more prevalent in males and, remarkably, bronchiectasis were more frequent in this group, maybe limiting the scope for improvement. As it should be expected, FEV1 was significantly lower in ACO patients than in asthma. However, biomarkers of T2 inflammation showed similar values in both groups, in consonance with the finding that the proportion of patients with atopy, chronic rhinosinusitis and nasal polyps did not differ significantly between ACO and asthma patients.
Table 1.
Demographic and Clinical Characteristics of Biologic-Treated Severe Asthma and Asthma–COPD Overlap Patients. Parameters of Clinical Response
| Variable | Asthma n = 297 | ACO n = 24 | p value |
|---|---|---|---|
| Male, frequency (%) | 80 (26.9) | 14 (58.3) | 0.002 |
| Age, median (IQR) | 57.4 (45.5–65.7) | 59.9 (52.7–64.7) | 0.26 |
| Non-smokers, frequency (%) | 261 (87.9) | 0 | |
| Ex-smokers, frequency (%) | 36 (12) | 20 (83.3) | |
| Current smokers, frequency (%) | 0 | 4(16.6) | |
| Smoking history (pack-yrs), median (IQR) | 5.0 (4.0–9.0) | 20.0 (11.3–30.0) | <0.001 |
| Diagnosis < 12 yrs (%) | 22.7 | 4.3 | 0.036 |
| Atopy (%) | 70.5 | 77.3 | 0.627 |
| Comorbidities (%) | |||
| -Rhinosinusitis. | 65.0 | 66.7 | >0.99 |
| -Nasal Polyps. | 36.4 | 25.0 | 0.375 |
| -Bronchiectasis. | 18.2 | 37.5 | 0.031 |
| -Obesity. | 8.2 | 0.0 | <0.005 |
| -Gastroesophageal reflux. | 20.5 | 12.5 | 0.435 |
| -Anxiety/depression | 25.3 | 4.2 | 0.706 |
| Post-bronchodilator FEV1/FVC (%), median (IQR) | 71.0 (61.5–77.1) | 57.4 (43.6–66.1) | <0.001 |
| Post-bronchodilator FEV1 (% predicted), median (IQR) | 80.5 (63.2–95.5) | 66.3 (50.4–80.7) | <0.001 |
| Post-bronchodilator FVC (% predicted), median (IQR) | 94.9 (82.3–108.2) | 94.6 (72.2–111.5) | 0.287 |
| FENO (ppb), median (IQR) | 39.0 (20.0–66.2) | 33.7 (10.8–58.3) | 0.327 |
| IgE (IU/mL) | 236.5 (91.0–532.8) | 451.0 (193.0–630.0) | 0.140 |
| Blood eosinophils (cells/mm3) | |||
| -Last recorded value, median (IQR) | 110.0 (22.5–310.0) | 109.0 (37.6–307.5) | 0.902 |
| -Maximum historical value, median (IQR) | 670.0 (330.0–1100.0) | 500.0 (200.0–1000.0) | 0.08 |
| High-dose ICS/LABA (%) | 100 | 100 | |
| LAMA (%) | 54.2 | 87.5 | <0.05 |
| Biologic therapy | |||
| -Omalizumab, n (%). | 132 (44.4) | 15 (62.5) | |
| -Mepolizumab, n (%). | 108 (36.3) | 5 (20.8) | |
| -Reslizumab, n (%). | 28 (9.4) | 1 (4.2) | |
| -Benralizumab, n (%). | 28 (9.4) | 3 (12.5) | |
| -Dupilumab, n (%). | 1 (0.3) | 0 | |
| Parameters of clinical response | |||
| ACT, median (IQR) | 22.0 (17.0–24.0) | 18.5 (14.0–23.3) | 0.096 |
| ACT<20 (%) | 36.5 | 59.1 | 0.109 |
| ≥1 severe exacerbation (%) | 27.3 | 70.8 | <0.001 |
| ≥1 hospitalization (%) | 8.1 | 29.2 | 0.004 |
| ≥1 unscheduled visit | 19.2 | 54.2 | <0.001 |
| Corticosteroid bursts, median (IQR) | 0.0 (0.0–1.0) | 2.0 (1.0–4.0) | <0.001 |
| ≥ 1 burst (%) | 37.5 | 83.3 | <0.001 |
| Maintenance corticosteroids (%) | 22.2 | 12.5 | 0.437 |
| Corticosteroids´ dose (prednisone equivalent, mg), mean (SD) | 12.5 (17.8) | 10.0 (5.0) | 0.594 |
| Clinical control (%) | 39.7 | 16.7 | 0.028 |
Notes: Atopy: Positive skin prick test or serum specific IgE. Clinical control: absence of severe exacerbations in the prior 12 months, ACT ≥ 20 and no need for systemic corticosteroids.
Abbreviations: ACO, asthma–COPD overlap; ACT, asthma control test; FENO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 second; FVC, Forced vital capacity; ICS, inhaled corticosteroids; IgE, immunoglobulin E; IQR, interquartile range; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist.
Omalizumab was the most frequently employed biologic agent both in patients with ACO and asthma. ACT scores after at least 12 months of biologic therapy were not significantly different between groups. The number of severe exacerbations, hospitalizations and unscheduled visits were significantly higher in ACO patients whereas the percentage of “controlled” patients was significantly lower (Table 1 and Figures S1–S4).
This study offers a real-life snapshot of the effectiveness of biologic therapy in patients with ACO. Not surprisingly, the outcome variables showed better results for asthmatics, but it must be mentioned that 16% of the biologic-treated patients with ACO attained clinical control. Only 39.7% of the asthmatics were controlled with monoclonal antibodies, but this figure is in line with those reported in other real-life studies, which reflected that the majority of the patients achieve “partial response”.4
There is little experience with the use of biologic agents in ACO patients. Omalizumab improved asthma control and quality of life, without significantly improving lung function, in 11 current or ex-smokers with severe allergic asthma and ACO (FEV1 <80%) enrolled in the Australian Xolair Registry.5 However, it should be noted that mean ACQ score persisted above 2 after 6 months of treatment. Besides, a post-hoc analysis of the PROSPERO6 study found that 50 patients with ACO (defined as medical history of asthma, a postbronchodilator FEV1/FVC <0.7 and smoking history ≥10 pack-years), treated with omalizumab had similar clinical outcomes to patients with asthma and with ACO in terms of exacerbation frequency and ACT scores. Nonetheless, FEV1 did not significantly increase and mean ACT clearly remained below 20.7 These studies assessed how much the patients improved after therapy compared to their values before therapy, whereas our study focused on outcome variables that reflect the level of control achieved after biologic treatment. Another difference is that prior reports were restricted to the use of omalizumab while in this study different monoclonal antibodies were employed. All these drugs have demonstrated effectiveness in patients with asthma2 but, in COPD patients, only mepolizumab and benralizumab were tested to date.8 Mepolizumab 100 mg reduces the rate of moderate or severe exacerbations by 19% in COPD patients with an eosinophil count of at least 150/μL and benralizumab 100 mg reduces the rate of severe exacerbations requiring hospitalization in patients with ≥220/μL blood eosinophils (benralizumab 10 mg probably has the same effect on this specific subset).9 Given that biologic therapy is not part of standard of care for ACO, the discussion about which biologic to preferentially use is merely speculative, but it seems reasonable to choose a drug with proved efficacy in asthma and probable benefit in eosinophilic COPD patients.
The study has obvious limitations due to its retrospective design. Since many patients entered the register already receiving treatment with a biologic medication it is not possible to assess how much they improved with respect to baseline. Besides, a relatively small number of ACO patients were included, as a consequence of the strict definition adopted in this paper and reflecting the fact that this therapy is not officially indicated for this condition. Moreover, only nine patients were treated with anti-IL-5 biologics, and it cannot be excluded that results would have differed with a higher prevalence of this agents.
In conclusion, this report suggests that patients with ACO treated with biologics reach worse outcomes than asthma patients in terms of spirometric parameters, exacerbations, symptoms and OCS use. Nevertheless, it should be interpreted as hypothesis generating and more studies are needed in order to clarify the role of these drugs in this clinical setting.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Dr. Pérez de Llano reports grants, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from GSK, grants and personal fees from TEVA, personal fees and non-financial support from Novartis, personal fees and non-financial support from Chiesi, personal fees from Sanofi, personal fees from Menarini, personal fees from Leo-Pharma, personal fees from GEBRO, personal fees from GILEAD, grants and personal fees from Esteve, personal fees from ROVI, personal fees from MSD, personal fees from TECHDOW PHARMA, non-financial support from FAES, outside the submitted work. Dr. Dacal Rivas reports personal fees and non-financial support from Esteve, personal fees and non-financial support from Boehringer-Ingelheim, non-financial support from GSK, non-financial support from Novartis, non-financial support from TEVA, non-financial support from Chiesi, non-financial support from Ferrer, outside the submitted work. Dr. Marina Malanda reports payment for lectures including Service on speakers bureaus, payment for development of educational presentations and travel/accommodations/meeting expenses, outside the submitted work. Dr. Plaza Moral reports grants and personal fees from AstraZeneca, personal fees from Boehringer-Ingelheim, personal fees from GSK, personal fees from Merck, grants and personal fees from Chiesi, personal fees from Novartis, grants from Menarini, personal fees from Sanofi, outside the submitted work. Dr. Gullón has nothing to disclose. Dr. Muñoz Esquerre reports grants, personal fees and non-financial support from AstraZeneca, grants, personal fees and non-financial support from GSK, personal fees and non-financial support from TEVA, personal fees and non-financial support from Novartis, personal fees and non-financial support from Chiesi, personal fees and non-financial support from Sanofi, personal fees and non-financial support from Menarini, personal fees from Ferrer, personal fees and non-financial support from FAES PHARMA, personal fees and non-financial support from MUNDIPHARMA, personal fees and non-financial support from ORIONPHARMA, personal fees and non-financial support from ALK ABELLÓ, outside the submitted work. Dr. García-Moguel reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, Sanofi, GSK, Chiesi, Orionpharma, Leti, Stallergenes, Allergy therapeutics; payment for expert testimony from AstraZeneca, Sanofi and GSK, support for attending meetings and/or travel from Novartis, Allergy therapeutics, Stallergenes Greer and TEVA; participation on a data safety monitoring board or advisory board from GSK, AstraZeneca and Sanofi; leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid from AstraZeneca; receipt of equipment, materials, drugs, medical writing, gifts or other services from GSK. Dr. Díaz Campos reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from GSK, Pfizer and Novartis; payment for expert testimony from GSK, AstraZeneca; support for attending meetings and/or travel from Chiesi, Menarini; Participation on a Data Safety Monitoring Board or Advisory Board from TEVA, GSK. Dr. Martinez-Moragón, declares that in the last three years received honoraria for speaking at sponsored meetings from AstraZeneca, Boehringer-Ingelheim, Chiesi, GlaxoSmithKline, Sanofi, Novartis, TEVA and ALK; and as a consultant for AstraZeneca, Boehringer-Ingelheim and GlaxoSmithKline. Dr. Harbenau Mena reports payment for expert testimony from GSK, AstraZeneca, Sanofi and Novartis. Dr. Cosio reports personal fees from AstraZeneca, grants and personal fees from Boehringer-Ingelheim, grants and personal fees from Novartis, grants and personal fees from Chiesi, personal fees from Sanofi, grants from Menarini, personal fees from Esteve, grants and personal fees from GSK, outside the submitted work. Dr. Padilla-Galo reports grants, personal fees and non-financial support from Astra-Zeneca, personal fees and non-financial support from GSK, TEVA, Chiesi and Novartis, and personal fees from ALK, Sanofi, Mundipharma and Bial, outside the submitted work. Dr. Cisneros reports grants, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from GSK, grants and personal fees from TEVA, personal fees and non-financial support from Novartis, personal fees and non-financial support from Chiesi, Pfizer, Mundipharma, personal fees from Sanofi, personal fees from Menarini, grants and personal fees from Esteve, personal fees from ROVI, personal fees from MSD, personal fees from TECHDOW PHARMA, non-financial support from FAES, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Pérez de Llano L, Miravitlles M, Golpe R, et al. A proposed approach to Chronic Airway Disease (CAD) using therapeutic goals and treatable traits: a look to the future. Int J Chron Obstruct Pulmon Dis. 2020;15:2091–2100. doi: 10.2147/COPD.S263430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashimoto S, Sorimachi R, Jinnai T, Ichinose M. Asthma and chronic obstructive pulmonary disease overlap according to the Japanese Respiratory Society Diagnostic Criteria: the Prospective, Observational ACO Japan Cohort Study. Adv Ther. 2021;38(2):1168–1184. doi: 10.1007/s12325-020-01573-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agache I, Beltran J, Akdis C, et al. Efficacy and safety of treatment with biologicals (benralizumab, dupilumab, mepolizumab, omalizumab and reslizumab) for severe eosinophilic asthma. A systematic review for the EAACI Guidelines - recommendations on the use of biologicals in severe asthma. Allergy. 2020;75(5):1023–1042. doi: 10.1111/all.14221 [DOI] [PubMed] [Google Scholar]
- 4.Global initiative for asthma. Available from: https://ginasthma.org/wp-content/uploads/2020/04/GINA-2020-full-report_-final-_wms.pdf. Accessed March 13, 2021.
- 5.Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi: 10.1016/j.jaci.2003.09.008 [DOI] [PubMed] [Google Scholar]
- 6.Hanania NA, Chipps BE, Griffin NM, Yoo B, Iqbal A, Casale TB. Omalizumab effectiveness in asthma-COPD overlap: post hoc analysis of PROSPERO. J Allergy Clin Immunol. 2019;143(4):1629–1633.e2. doi: 10.1016/j.jaci.2018.11.032 [DOI] [PubMed] [Google Scholar]
- 7.Eger K, Kroes JA, Ten Brinke A, Bel EH. Long-term therapy response to anti-IL-5 biologics in severe asthma-a real-life evaluation. J Allergy Clin Immunol Pract. 2021;9(3):1194–1200. doi: 10.1016/j.jaip.2020.10.010 [DOI] [PubMed] [Google Scholar]
- 8.Donovan T, Milan SJ, Wang R, Banchoff E, Bradley P, Crossingham I. Anti-IL-5 therapies for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2020;12:CD013432. doi: 10.1002/14651858.CD013432.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maltby S, Gibson PG, Powell H, McDonald VM. Omalizumab treatment response in a population with severe allergic asthma and overlapping COPD. Chest. 2017;151(1):78–89. doi: 10.1016/j.chest.2016.09.035 [DOI] [PubMed] [Google Scholar]