Abstract
New, stable, highly water-soluble, nontoxic polysaccharide conjugates of amphotericin B (AmB) are described. AmB was conjugated by a Schiff-base reaction with oxidized arabinogalactan (AG). AG is a highly branched natural polysaccharide with unusual water solubility (70% in water). A high yield of active AmB was obtained with the conjugates which were similarly highly water soluble and which could be appropriately formulated for injection. They showed comparable MICs for Candida albicans and Cryptococcus neoformans (MICs, 0.1 to 0.2 μg/ml). The reduced AmB conjugate, which was synthesized at pH 11 for 48 h at 37°C, was nonhemolytic and was much safer than conventional micellar AmB-deoxycholate. It was the least toxic AmB-AG conjugate among those tested with mice (maximal tolerated dose, 50 mg/kg of body weight), and histopathology indicated no damage to the liver or kidneys. This conjugate, similarly to the liposomal formulation (AmBisome), was more effective than AmB-deoxycholate in prolonging survival. It was more effective than both the liposomal and the deoxycholate formulations in eradicating yeast cells from target organs. The overall results suggest that after further development of the AmB-AG conjugate, it may be a potent agent in the treatment of fungal infections.
Amphotericin B (AmB)-deoxycholate (AmB-DOC) is the drug of choice for the treatment of mycotic infections caused by a wide range of fungi (11). Clinical use of this drug is continuously growing as a result of the increasing incidence of life-threatening fungal infections, particularly in immunocompromised hosts such as cancer patients (3), patients who have undergone organ transplantation (13), and patients with AIDS (8). However, AmB therapy is frequently associated with nephrotoxicity, central nervous system and liver damage, and side effects, such as nausea, fever, and chills, all of which are dose related. Therefore, the daily dose is limited to 1.5 mg/kg of body weight, and at times it may be necessary to reduce the dose or even discontinue therapy early (6, 20, 25).
AmB is a hydrophobic molecule with negligible solubility in aqueous solutions and poor solubility in most organic solvents. The marketed formulation, Fungizone, is based on a micellar dispersion that is obtained following the addition of water to the lyophilized sodium deoxycholate-AmB mixture. Fungizone exhibits three major clinical limitations: (i) its toxicity is not selective enough, and therefore, its therapeutic index is narrow (7, 20); (ii) the new opportunistic fungal infections, such as fusariosis, that are appearing in immunocompromised patients are resistant to this marketed drug (22); and (iii) the treatment is less effective against new clinical manifestations such as chronic disseminated candidosis, mainly due to poor penetration of this drug into lesions (24).
To improve the therapeutic efficacy and to reduce the toxicity of AmB even at high doses, several strategies including the use of combination therapy, modification of the AmB molecule, and modification of the physical state of AmB or changes in the drug delivery system have been used (4). New drug delivery systems, such as liposomal formulations, lipid complexes, lipid emulsions, and colloidal dispersions, have been introduced, and most of these lipid products are used in clinical practice (14, 15, 18, 26).
However, due to the currently inadequate treatments for severe systemic fungal infections, the development of new, effective, parenteral antifungal drug delivery systems has assumed great importance. One of the approaches for improving drug performance and reducing toxicity is conjugation to a polymeric carrier (21). Conjugation of an insoluble drug such as AmB to a biodegradable water-soluble polymer may increase the water solubility of the drug, increase drug circulation time, and increase the level of accumulation in the diseased tissue, resulting in an improved therapeutic effect and reduced toxicity.
Arabinogalactan (AG) is a highly branched natural polysaccharide with an unusual water solubility (70% in water). It is extracted from the Larix tree and is available in a 99.9% pure form with reproducible molecular weight (MW) and physicochemical properties (1). The high water solubility, biocompatibility, biodegradability, and ease of drug conjugation in an aqueous medium make AG attractive as a potential drug carrier.
In this paper we describe a new class of AmB derivatives which overcome the limitations (insolubility, instability, and toxicity) of AmB-DOC for systemic administration. These derivatives were synthesized on the basis of AmB conjugation with the oxidized form of the natural, inert, water-soluble polysaccharide AG. The interaction of drug and polymer yields an amine or imine conjugates, increased solubility and stability of AmB in aqueous solutions, and significantly reduced toxicity. In addition, the imine derivative was effective in the treatment of murine candidosis and cryptococcosis.
MATERIALS AND METHODS
Synthesis and formulation.
AmB was conjugated to AG to produce AmB-AG conjugate in two steps: preparation of the dialdehyde AG (DAAG) and conjugation of DAAG with AmB through an imine bond with the amino group of AmB (see details below and Fig. 1).
FIG. 1.
Scheme of binding of free AmB to polysaccharide conjugated via imine or amine bonds of the AmB amino side group, AmB(−NH2).
Preparation of DAAG.
In a typical synthesis, AG (99% pure; Larex; St. Paul, Minn.) with an average MW of 20,000 (1 g; 0.0599 mol of saccharide units) was dissolved in 20.0 ml of deionized water. Potassium periodate (1.275 g, 0.0554 mol) was added to the mixture, which was stirred at room temperature until it was completely dissolved (∼2 h). The DAAG that was formed was purified from excess periodate and reaction by-products by applying it through a column (6 by 80 mm; volume = 2.0 ml) filled with Dowex-1 in the acetate form (Sigma, St. Louis, Mo.). Dowex-1–acetate was obtained by pretreatment of the commercial anion exchanger with aqueous 1 M acetic acid solution. The purified DAAG at a concentration of 50.0 mg/ml exhibited a degree of oxidation in the range of 50%, as determined by the iodometric method (19).
Conjugation of DAAG and AmB.
In a typical experiment, to a 0.2 M borate buffer solution (pH 11 ± 0.1) containing purified DAAG (40 ml, 12.5 mg/ml), 250 mg of AmB (950 U/mg; Dumex, Copenhagen, Denmark) was added (final AmB concentration, 6.25 mg/ml). After 48 h at 37°C the clear solution was purified by dialysis through a 12,000-MW-cutoff dialysis tube against deionized water for 48 h at 4°C, followed by centrifugation at 2,000 × g for 10 min and lyophilization. The lyophilized yellow product (90% yield) contained 15 to 20% AmB as evaluated by UV absorption at 385 and 416 nm with a Kontron Instruments Uvikon model 930 instrument.
The amine derivative (reduced AmB-AG) was obtained by reduction of the imine conjugate before purification by adding 1.2 M excess sodium borohydride to the conjugation reaction solution at 4°C for 60 min with stirring. The amine conjugate was purified by dialysis as described above for the imine derivative and was stored in dry form following lyophilization.
Sterile conjugates were obtained by filtration through a 0.2-μm-pore-size cellulose acetate sterile filter (Schleicher & Schuell, Dassel, Germany), followed by lyophilization under sterile conditions. The sterility of the solutions was assessed with the BACTEC 46 apparatus (Johnson Laboratories, Towson, Md.) by measuring radioactive carbon dioxide quantitatively in BACTEC culture vials inoculated with samples to be tested.
Stability study.
The chemical stability of AmB-DAAG conjugates was determined with gel permeation chromatography (GPC) systems. The system consists of a Spectra Physics (Darmstadt, Germany) P1000 pump with UV detection (Applied Bioscience 759A Absorbency UV detector) at 254 nm, a Rheodyne (Coatati, Calif.) injection valve with a 20-μl loop, a Spectra Physics Data Jet integrator, and a WINner/286 computer analyzer. Samples were eluted with double-distilled water in 0.05 M NaNO3 through a Shodex (KB-804) column at a flow rate of 1 ml/min. The MWs of the eluted DAAG derivatives were estimated with pollulan standards in the MW range of 5,000 to 110,000. When the AmB-AG conjugate was injected into the GPC system and the absorbance at 405 nm was read by the UV detector, it exhibited a typical peak at 7 to 8 min, which corresponds to the high-MW fraction (MW = 30,000) of the conjugated AmB, and another peak at 16 to 17 min, which corresponds to the low-MW fraction (MW = 1,000) of free AmB that is detached from the polymer. The peak ratio is the ratio between the absorbance at 16 to 17 min/absorbance at 7 to 8 min. Samples of lyophilized conjugates (100 mg) were packed in tightly closed brown glass containers, and the containers were stored at room temperature for 1 year. Samples (0.5 mg/ml in water solution) were taken at time zero, 1 week, 1 month, 3 months, 6 months, and 1 year, and the peak ratio was measured. The average MW was determined by GPC, the UV absorption at 405 nm was determined in water solution, and the antifungal activity was determined by comparing the MICs and hemolytic values as described below.
Susceptibility testing by broth dilution method.
Two yeast strains were used for susceptibility testing: Candida albicans CBS 562 (a reference type strain) and Cryptococcus neoformans B-3501 (serotype D; ATCC 34873). MICs were determined by the broth microdilution method according to the recommendations of the National Committee for Clinical Laboratory Standards (NCCLS) (23). This method involves the use of small volumes of broth dispensed in sterile plastic 96-well microtitration trays with wells that are round or conical on the bottom. Each well contains 0.1 ml of broth with the serially diluted drug. Briefly, twofold serial dilutions of drugs from stock solutions were prepared in RPMI 1640 broth medium (Sigma) buffered to a final pH of 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS; Sigma) and 1 M NaOH and were sterilized by filtration. A stock solution of 10 mg/ml was prepared in dimethyl sulfoxide (DMSO) for free AmB or in water for AmB conjugates. The final drug concentrations in the test ranged from 128 to 0.015 μg/ml in a final volume of 0.1 ml. Yeast inocula were prepared from 24-h (Candida sp.) or 48-h (C. neoformans) cultures on Sabouraud dextrose agar (SDA) plates (Difco, Detroit, Mich.) in RPMI 1640 broth medium to yield a final inoculum concentration of 104 yeast cells per ml, as measured by counting with a hemacytometer. The microdilution wells, which contained 0.1 ml of the serially diluted drug, were inoculated with 0.1 ml of the resulting suspension. The final inoculum concentration after dilution with the drug suspension was 103 yeast cells per ml. Two wells containing drug-free medium and inoculum were used as controls. The inoculated plates were incubated at 35°C for 24 h (Candida sp.) or 48 h (C. neoformans). The growth in each well was then estimated visually. The MIC was defined as the lowest drug concentration that resulted in complete inhibition of visible growth.
The minimum fungicidal concentration (MFC) was determined by assaying the number of CFU on SDA plates. Three 50-μl samples from wells that showed no growth, obtained just after determination of the MIC, were subcultured (0.05 ml) on SDA plates that were then incubated at 35°C for 24 h (Candida sp.) or 48 h (C. neoformans). The MFC was established as the lowest concentration of drug with which subcultures were negative.
In vitro toxicity study (hemolysis).
Sheep erythrocytes (SRBCs) were suspended in phosphate-buffered saline (PBS; 5% [vol/vol]) and were washed twice in the same buffer by centrifugation (3,000 × g for 10 min). The hemolysis reaction was conducted in glass tubes containing 0.1 ml of the serially diluted drug and 0.9 ml of SRBCs. The results were recorded visually after 1 h of incubation at 37°C in a water bath.
Toxicity in animal model.
Male albino BALB/c mice weighing ∼20 g were injected through the tail vein with various doses of each AmB formulation. Each dosage form was administered intravenously as single bolus injections of 0.1 ml of the same dose every 10 min to a group of 10 mice until death was observed. AmB formulations were prepared in 5% dextrose and were filter sterilized prior to injection through a sterile 0.2-μm-pore-size cellulose acetate filter (Schleicher & Schuell, Dassel, Germany). The survival of mice that received the maximal tolerated dose (MTD) was monitored for 8 days.
Multiple dose toxicity.
The safety of a 0.2-ml intravenous injection of five consecutive daily therapeutic doses of the AmB formulations was also examined with a group of 10 male albino BALB/c mice weighing ∼20 g. The study of multiple-dose treatment for murine candidosis and cryptococcosis was needed as a control for the efficacy study. Survival was monitored for up to 30 days.
Histopathology.
Specific-pathogen-free BALB/c male albino mice were injected intravenously with various doses of the AmB formulations following administration of five consecutive daily doses of the drug. The mice (a group of two) were killed on the sixth day; the livers and kidneys were removed, fixed with formalin, and prepared for hematoxylin and eosin histopathologic staining. The preparations were randomly evaluated by a pathologist.
Therapeutic efficacy study.
For the efficacy studies, C. albicans CBS 562 and C. neoformans B-3501 (serotype D; ATCC 34873) were used to induce systemic murine candidosis and cryptococcosis, respectively. Yeast inocula were injected into the tail veins of male albino BALB/c mice (weight, 20 ± 3 g) by administration of a single bolus of a 0.2-ml suspension in PBS. The inocula ranged from 104 to 106 yeast cells per mouse from a 24-h culture for C. albicans and from a 48-h culture for C. neoformans on SDA at 30°C. With these inocula systemic infections are regularly produced in mice, and they cause total killing within 10 to 20 days. The appropriate inoculum for each experiment was experimentally determined. The yeast concentration was determined by counting with a hemacytometer. The viable count was measured as the number of CFU on SDA plates after 24 to 48 h of incubation at 30°C.
Mice infected as described above were treated with AmB-DOC (Fungizone), liposomal AmB (AmBisome), and AmB-AG conjugates at various doses. Ten mice were used for each treatment and were maintained in separate cages. Treatment started 24 h after the initiation of the infection by intravenous injection of a daily single bolus (0.2 ml) of the drug for 5 consecutive days. Each dose comprised an AmB equivalent in a dosage range of 1 to 8 mg/kg/day, depending on the specific treatment (for the exact dosage, see Results). A control group of 10 infected mice treated with PBS instead of the conjugate was also included. The number of surviving animals in each group was recorded daily over a period of 30 days.
The efficacy of treatment with the various AmB formulations was also determined with the surviving mice by monitoring the yeast density in target organs: kidneys for murine candidosis and brains for murine cryptococcosis. The surviving mice were killed by carbon dioxide asphyxiation; the target organs were removed aseptically, weighed, and homogenized in saline; and appropriate dilutions were plated in duplicate on SDA plates. The viable count was estimated as the number of CFU after 24 to 48 h of incubation at 30°C.
Statistical analysis.
The survival data results were analyzed by the Kolmogorov-Smirnov goodness-of-fit procedure (28), which was originally developed for use with continuous data to detect a difference of a given magnitude between observed and hypothesized cumulative frequency distributions. In the present case, the statistical comparison was carried out first between each conjugate-treated group and the control group and then between conjugate-treated groups of animals in an attempt to identify a significant difference between the various survival curves. The results of the effects of various therapeutic treatments on the multiplication of the yeasts in the target organs were analyzed by the t-test (28).
RESULTS
Synthesis and characterization of AmB-AG conjugate.
The synthesis of AmB-AG conjugates involved four steps (Fig. 1) as follows: (i) step 1, formation of active polysaccharide by oxidation of AG with periodate to DAAG; (Fig. 1A and B); (ii) step 2, purification of the oxidized AG from the interfering oxidative anions by anion-exchange chromatography; (iii) step 3, conjugation of AmB to the oxidized AG through the imine bond (Fig. 1B and C); and (iv) step 4, further reduction of the resulting AmB-AG imine bond to a more stable amine bond with sodium borohydride (Fig. 1C and D).
The formation of pure oxidized AG (the first step) was conducted by dissolving AG in a solution of the oxidizing agent periodate and allowing the oxidation to occur for a few hours at room temperature. Under these mild conditions, a minimal change in the MW of the AG was observed. The oxidized AG, with about 50% of its saccharide units oxidized to dialdehydes, was purified by ion-exchange chromatography with a strongly basic anion exchanger (Dowex-1) in the acetate form. The ion-exchange chromatography removed excess periodate, iodate, and formate ions from the reaction solution, which was then lyophilized to a powder which was stable under refrigeration for at least 1 year. This second step was fast and efficient, and the anion exchanger could easily be regenerated afterward.
The conjugation conditions were optimized by investigating the proper pH, type, and concentration of the buffer solution and the reaction temperature. Carbonate, phosphate, and borate buffers with pHs in the range of 10 to 12 and at 0.1 M concentrations were used for the conjugation reaction. From these solutions the best results were obtained with borate buffer at pH 11, at which the drug has some solubility. The oxidized AG was soluble in these buffers, while AmB was added as a powder. As the reaction proceeded, the AmB powder was gradually dissolved in the solution, which was used as an indication of conjugation progress. The optimal reaction time was 24 h at 37°C.
The conjugation reaction yields two products: an unreduced AmB-AG conjugate (Fig. 1, structure C) with an imine bond, and a reduced AmB-AG conjugate (Fig. 1, structure D), which is formed via reduction of the imine bond to a more stable amine bond. In addition, unreacted aldehyde groups on the oxidized AG were also reduced to the corresponding alcohols.
Both amine and imine AmB-AG conjugates were stable at room temperature for at least 1 year as dry powders, as determined by GPC with UV detection at 405 nm, which allows the detection of changes in MW and the formation of low-MW components (i.e., free drug). Figure 2 illustrates the chemical stability of reduced conjugate 11R. Similar results were obtained with unreduced conjugate 11UR (data not shown). It can be seen (Fig. 2) that after storage for 1 year at 25°C there was no change in the ratio between the large peak at 7 to 8 min (representing the high-MW fraction of AmB conjugated to AG) and the small peak at 16 to 17 min (representing the low-MW peak of free AmB or possibly AmB conjugated to small fractions of the polymer).
FIG. 2.
Chemical stability of AmB-AG conjugate 11R. The chemical stability was detected with the GPC system by using a UV detector at 405 nm. The absorbance of lyophilized powder kept at 25°C was measured at the beginning of the experiment (a) and after 1 year (b), with peak ratios of 12.0 and 10.0, respectively (the peak ratio is the ratio between the absorbance at 16 to 17 min/absorbance at 7 to 8 min).
The most stable, reproducible, and least toxic conjugate was 11R, which was synthesized by reaction of AmB with oxidized AG in a 20:80 weight ratio in borate buffer (pH 11.0) for 48 h at 37°C. This AmB-AG conjugate contained 20% AmB (wt/wt), as measured by determining the UV absorption at 405 nm with a calibration curve of conjugates containing 5 to 30% AmB (assuming a 95% conjugation yield of the AmB reaction entry), and was highly water soluble (>1,000 mg/ml), contrary to free AmB, which is almost insoluble in water (0.1 mg/ml, as measured at pH ≤ 2 and pH ≥ 11). Stability data collected after 1 year of storage of this conjugate at 25°C as a lyophilized powder showed no change in its chemistry or effectiveness. These data included the MW determined by GPC (an MW of 22,700 at time zero versus an MW of 23,200 after 1 year of storage; Fig. 2) and in vitro biological activity, such as the MIC (0.1 μg/ml) and the hemolytic index (>1 mg/ml), which were the same at time zero and after storage for 1 year.
Antifungal activity of the AmB-AG conjugate against pathogenic yeasts.
The antifungal activities of the AmB-AG conjugates against C. albicans and C. neoformans were determined. The MICs and MFCs (Table 1) of both reduced and unreduced AmB-AG conjugates, as measured according to the recommendations of NCCLS (23), were similar to those of free AmB and AmB-DOC. This indicates that covalent binding of AmB to AG under different reaction conditions did not reduce the antifungal activity of AmB.
TABLE 1.
MICs and MFCs of AmB-AG conjugatea
| AmB formulationb |
C. albicans
|
C. neoformans
|
||
|---|---|---|---|---|
| MIC (μg/ml) | MFC (μg/ml) | MIC (μg/ml) | MFC (μg/ml) | |
| AmB | 0.12–0.25 | 0.25–0.50 | 0.12–0.25 | 0.25–0.50 |
| AmB-DOC | 0.12–0.25 | 0.25–0.50 | 0.12–0.25 | 0.25–0.50 |
| 11UR (pH 11 for 48 h, unreduced) | 0.12–0.25 | 0.25–1.00 | 0.12–0.25 | 0.25–0.50 |
| 11R (pH 11 for 48 h, reduced) | 0.12–0.25 | 0.50–1.00 | 0.12–0.25 | 0.25–1.00 |
| 12URc (pH 12 for 5 min, unreduced) | 0.12–0.25 | 0.50–2.00 | 0.12–0.25 | 0.25–1.00 |
| 12R (pH 12 for 5 min, reduced) | 0.12–0.50 | 0.50–1.00 | 0.12–0.50 | 0.25–0.50 |
The MICs and MFCs were measured by the NCCLS broth microdilution method (23) in RPMI broth.
Synthesis conditions are given in parentheses.
At pH 12 the AmB-DAAG solution became clear within a few minutes.
In vitro toxicity of AmB conjugates to SRBCs.
In vitro toxicity was determined by the hemolysis of SRBCs by the various AmB formulations. All AmB-AG conjugates were nonhemolytic at concentrations up to 1 mg/ml, the highest concentration studied, whereas free AmB and AmB-DOC were hemolytic at a much lower concentration of 8 μg/ml.
Toxicity study in vivo.
The results presented in Fig. 3 indicate that the 11R conjugate exhibited the highest MTD of all conjugates tested (50 mg/kg). The pH of the conjugation reaction had a dramatic effect on the acute toxicity, as the conjugate prepared in a pH 12 solution was very toxic (10 mg/kg) (it should be noted that the conjugate prepared in a pH 12 solution was nonhemolytic and exhibited in vitro antifungal activity similar to that of the conjugate which was prepared at pH 11). A decrease in the pH to 11 significantly reduced the toxicity. A slight difference in MTDs was detected between the reduced and the unreduced AmB-AG conjugates (MTDs, 50 and 40 mg/kg, respectively). Since the 11R conjugate was the least toxic AmB-AG conjugate among those tested in vivo, it was chosen for use in further therapeutic efficacy studies.
FIG. 3.
Acute toxicity of AmB formulations. MTD, maximal tolerated dose (equivalent of AmB) that did not kill the mice 8 days after drug injection; 12UR and 12R, unreduced and reduced AmB-AG conjugated at pH 12 for 5 min, respectively; 11UR and 11R, unreduced and reduced AmB-AG conjugated at pH 11 for 48 h, respectively.
Therapeutic efficacy of AmB-AG conjugate 11R in systemic murine candidosis.
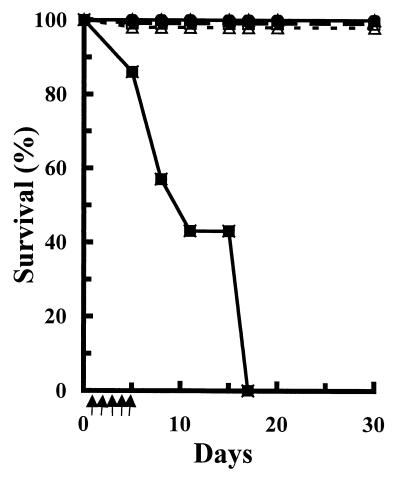
The therapeutic efficacy of AmB-AG conjugate 11R was studied with mice infected with C. albicans (the experiment was conducted three times; Fig. 4 exhibits the results of a representative study). The results indicate that 100% of the mice treated with any AmB formulation even at a low dosage of 1 mg/kg/day survived the whole period of the experiment (30 days), while the control mice, which were treated only with 5% dextrose, died within 17 days. The difference in the survival curves was clearly significant (P < 0.01) according to the Kolmogorov-Smirnov goodness-of-fit procedure (28). AmB-AG conjugate 11R was also highly effective in eradicating the yeast cells from the mouse kidney (the target organ in murine candidosis), as determined with the kidneys of the surviving mice (Fig. 5). At the low dosage (1 mg/kg/day), the three formulations (AmB-DOC, AmBisome, and AmB-AG conjugate) were equally effective in eradicating yeast cells from the mouse kidneys, while at the higher dosage of 4 mg/kg/day, the AmB-AG conjugate was significantly more effective than the liposomal formulation (AmBisome) (P < 0.01). Total yeast eradication was found in the kidneys of mice treated with the high dosage of the AmB-AG conjugate (8 mg/kg/day) (data not shown).
FIG. 4.
Efficacy of treatment of murine candidosis with various AmB formulations. Survival of mice infected with C. albicans (5 × 104 yeast cells per mouse) was determined for control mice (■), mice treated with AmB-DOC (Fungizone) at 1 mg/kg/day (□) and liposomal AmB (AmBisome) at 1 mg/kg/day (▵) and 4 mg/kg/day (▴), and mice treated with reduced AmB-AG conjugate 11R at 1 mg/kg/day (○) and 4 mg/kg/day (●). The mice were infected intravenously by administration of a single bolus of 0.2 ml of a C. albicans suspension in PBS. The treatment (↑) was started at 24 h postinfection by intravenous injection of a daily single bolus (0.2 ml) of the drug or 5% dextrose for the controls and continued for 5 consecutive days.
FIG. 5.
Yeast density in the kidneys of C. albicans-infected mice. The kidneys of the surviving mice (see Fig. 4) were homogenized and plated on SDA plates. Counts were determined after 48 h of incubation at 30°C.
Therapeutic efficacy of AmB-AG conjugate 11R in systemic murine cryptococcosis.
AmB-AG conjugate 11R was also effective in the treatment of murine cryptococcosis. A clear significant difference (P < 0.01) in the survival curve was noted for all groups treated with the various AmB formulations compared to that for the control group (the experiment was conducted three times; Fig. 6 exhibits the results of a representative study). At the high dosage (4 mg/kg/day) the 11R AmB-AG conjugate and AmBisome were equally effective, and most of the mice treated with these formulations survived for 30 days (86%), while all the mice in the control group died within 17 days. At the lower dosage (1 mg/kg/day) the conjugate and AmB-DOC were similarly effective (33% survival for 30 days) but were less effective than an equal dosage of AmBisome (86% survival). Furthermore, treatment with the AmB-AG conjugate was highly effective in eradicating the yeast cells from the mouse brain (the target organ in murine cryptococcosis), as determined with the brains of the surviving mice (Fig. 7). It was nearly as effective as the liposomal formulation at the dosage of 4 mg/kg/day (average of 8 × 106 versus 7 × 106 CFU/brain). However, 57% of the mice treated with the AmB-AG conjugate at this dosage had less than 1 × 104 CFU/brain, while all the mice treated with the same dosage of AmBisome had more than 5 × 105 CFU/brain.
FIG. 6.
Efficacy of treatment of murine cryptococcosis with various AmB formulations. Survival of mice infected with C. neoformans (5 × 105 yeasts per mouse) was determined for control mice (■), mice treated with AmB-DOC (Fungizone) at 1 mg/kg/day (□) and liposomal AmB (AmBisome) at 1 mg/kg/day (▵) and 4 mg/kg/day (▴), and mice treated with reduced AmB-AG conjugate 11R at 1 mg/kg/day (○) and 4 mg/kg/day (●). The mice were infected intravenously by administration of a single bolus of 0.2 ml of C. neoformans suspension in PBS. The treatment (↑) started at 24 h postinfection by intravenous injection of a daily single bolus (0.2 ml) of the drug or 5% dextrose for the controls and continued for 5 consecutive days.
FIG. 7.
Yeast density in the brains of C. neoformans-infected mice. The brains of surviving mice (see Fig. 6) were homogenized and plated on SDA plates. The counts were determined after 48 h of incubation at 30°C.
Histopathology.
Hematoxylin and eosin-stained slides of specimens that have been taken from the kidneys and livers of mice treated with various AmB formulations were randomly evaluated by a pathologist. Treatment with the AmB-AG conjugate, contrary to treatment with the marketed AmB-DOC, did not result in any pathological changes in either the kidney or the liver. In AmB-DOC-treated mice, all the kidneys exhibited areas with tubular necrosis, and one liver had single-cell necrosis of hepatocytes with neutrophil aggregation.
DISCUSSION
Our aim was to develop a new formulation of AmB which is highly water soluble and safer than the conventional AmB-DOC formulation without decreasing its therapeutic effectiveness. This was achieved by conjugating AmB to the polysaccharide AG via an amine or imine bond. Formation of pure oxidized AG was the first step, followed by ion-exchange chromatography to remove excess oxidizing agents. This purification step was essential to avoid oxidative degradation of AmB, a highly oxidation-sensitive drug (2), during the conjugation step. Omission of this step led to measurable decreases in the antifungal activity and the UV absorption at 405 nm.
The subsequent conjugation step was conducted at 37°C in borate buffer solution at pH 11, at which the drug has some solubility. At pH 10 or below, the yield was low, with most of the AmB powder remaining intact even after 48 h at 37°C. At pH 12, the AmB powder was solubilized within 5 min at room temperature, and the conjugation reaction was complete in 10 min. Although the resulting conjugate was as effective in vitro as AmB in antifungal testing and was not hemolytic, it was toxic to mice.
The use of reaction solutions in which AmB is soluble, such as DMSO and mixtures of DMSO with water or borate buffer solutions, did not improve the reaction yield or decrease the toxicity. It should be noted that previous reports described natural solutions or those with a pH of up to 9 for drug conjugation (17). In an earlier study (9), nystatin, a polyene antibiotic with a structure similar to that of AmB, was conjugated to dextran in borate buffer at pH 9 with a high yield, and it had a high degree of efficacy. The reason for the success at this low pH was probably the degradation of dextran at a pH higher than 9. In our study, no change in the MW of AG was observed, which is related to the fact that the main chain of AG does not contain vicinal hydroxyl groups and is thus less susceptible to oxidation.
The reduction step converts the imine and aldehyde bonds in the polymer conjugate to amine and alcohol groups, respectively. Reduction of the excess aldehydes eliminates further binding of the AmB-AG conjugate to proteins and body components in vivo. This reduction step did not affect the polymer’s MW or AmB content.
The antifungal activity of AmB is explained by its preferential binding to ergosterol in fungal cell membranes over cholesterol in mammalian cell membranes. Our studies indicate that covalent binding of AmB to AG does not reduce the antifungal activity of AmB in vitro (with no dependence on the reaction conditions used; Table 1). However, the affinity of AmB to cholesterol is thought to be a major cause of its toxicity (4, 6), so that the hemolytic index of SRBCs is one of the parameters used to detect the in vitro toxicity of AmB (10). The hemolytic indices of different conjugates synthesized under various reaction conditions were at least 2 orders of magnitude higher than those of free AmB and AmB-DOC (>1 and 0.008 mg/ml, respectively). There were no significant differences in the hemolytic indices between the various conjugates, suggesting that the conjugation itself eliminates the affinity between the SRBC cholesterol and the conjugated AmB.
In contrast to the in vitro toxicity, the pH of the coupling reaction is a major factor that affects the acute toxicity (Fig. 3). An increase in the pH of the conjugation reaction from 11 to 12 produced a significant decrease in MTD (from 50 to 10 mg/kg). The chemical reduction of the conjugate also had a considerable impact on the in vivo toxicity. Reduced AmB-AG conjugate 11R was safer than unreduced conjugate 11UR (Fig. 3). We hypothesize that the difference in the MTD lies in the strength of the bond between AmB and AG. Under physiological conditions free AmB is possibly released from the conjugate, which was synthesized at a pH of > 11. This in agreement with the high degree of stability of the amine bond, in contrast to the far lower degree of stability of the imine bond. The increased toxicity of the conjugate prepared at pH 12 can be explained either by formation of a physical complex of free toxic AmB molecules with the already conjugated drug molecules or by degradation of AmB into toxic products at high pH. Extraction of the conjugate prepared at pH 12 with DMSO resulted in the isolation of unbound AmB.
AmB-AG conjugate 11R was significantly less toxic than the marketed AmB-DOC formulation (MTDs, 50 and 4 mg/kg, respectively; Fig. 3) and, like several lipid-based formulations, was better tolerated in the murine model even at high dosages (more than 1.0 mg/kg/day) (4, 18). In the mice treated with AmB-DOC, hepatocellular and tubular necroses were observed, whereas no damage was detected in these organs of mice treated with conjugate 11R, even at a much higher dosage of AmB equivalent (8 versus 1 mg/kg/day). This low level of toxicity of AmB-AG, as evinced by the low MTD and the histopathological evaluation, could be partially explained by the reduced hemolytic activities of the conjugates. In addition, there is a possibility that the polymeric matrix prevented the AmB from penetrating the liver and kidney membranes. AmB-AG conjugate 11R was chosen for further therapeutic efficacy studies due to its low level of toxicity and high degree of stability.
Conjugate 11R was as effective as AmB-DOC or AmBisome in mice treated with 1 mg/kg/day in prolonging survival and eradicating yeasts from the kidneys in the murine candidosis model (Fig. 4). Moreover, it was significantly (P < 0.01) more effective than AmBisome (4 mg/kg) in reducing the counts of C. albicans in the kidneys (Fig. 5). AmB-DOC was toxic at this dose (MTD, 4 mg/kg; Fig. 3) and therefore was not tested. Furthermore, due to the higher doses of AmB that could be safely given with the 11R conjugate (4 to 8 mg/kg/day), it was possible to obtain total eradication of C. albicans from the kidneys.
In the murine model of cryptococcosis, the 11R conjugate was as effective as AmB-DOC at the low dosage of 1 mg/kg/day but was less effective than an equal dose of AmBisome in both prolonging the survival of the infected mice (Fig. 6) and eradicating yeasts from the target organ (Fig. 7). A high dosage of the 11R conjugate (4 mg/kg/day) could be safely given with a high degree of therapeutic effectiveness, which was similar to that of AmBisome. This was probably due to a good penetration through the blood-brain barrier of the conjugate at this high dosage.
The main difference between the conjugated drug and the lipid-based formulations is that the lipid-based formulations are particulate systems, which may present physical and chemical stability problems. In comparison, conjugated AmB is water soluble, is easily sterilized by filtration, and is physically and chemically stable as a lyophilized powder and as a reconstituted solution. The reagents, conjugation reaction, and method of preparation for the conjugate are simple and inexpensive compared to those for the lipid formulations.
The therapeutic activity of AmB-AG is comparable to those described for lipid formulations used in similar murine models of candidosis (5, 27) and cryptococcosis (5, 12, 16). However, in our study the AmB-AG conjugate was significantly more effective than the liposomal formulation at equal dosages of 4 mg/kg/day in eradicating the yeast cells from the target organ in murine candidosis (Fig. 5) and is as effective in eradicating the yeast cells from the target organ in murine cryptococcosis. Yet, despite the availability of the various AmB lipid formulations, a water-soluble, nontoxic dosage form of AmB remains a highly desirable alternative to the conventional treatment with AmB-DOC. We hypothesize that since AmB-AG is water soluble and differs chemically from the various lipid formulations, it is likely that its pharmaceutical properties (including tissue distribution, organ toxicity, and pharmacokinetics) will be favorably different, making it a useful alternative for the treatment of fungal infections.
The present paper provides evidence that the toxic effects of AmB can be mitigated by conjugating it to AG. In a series of in vitro and in vivo studies with mice, reduced AmB-AG conjugate 11R was found to be highly water soluble, significantly less toxic, and more effective in eradicating C. albicans and C. neoformans than AmB-DOC. This implies that it may have potential therapeutic applications. Pharmacokinetic and organ distribution studies are planned as part of the further investigation of the safety and efficacy of this formulation.
ACKNOWLEDGMENTS
We thank Liliana Waldmann for technical assistance in the in vivo experiments.
A. J. Domb is affiliated with the David Bloom Center for Pharmacy and the Alex Grass Center for Drug Design.
REFERENCES
- 1.Adams M F, Douglas C. Arabinogalactan—a review of the literature. TAPPI J. 1963;46:544–558. [Google Scholar]
- 2.Andrews F A, Beggs W H, Sarosi G. Influence of antioxidants on the bioactivity of amphotericin B. Antimicrob Agents Chemother. 1977;11:615–618. doi: 10.1128/aac.11.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodey G P. Infectious complications in the cancer patient. Curr Probl Cancer. 1977;1:1–63. doi: 10.1016/s0147-0272(77)80007-x. [DOI] [PubMed] [Google Scholar]
- 4.Brajtburg J, Bolard J. Carrier effects on biological activity of amphotericin B. Clin Microbiol Rev. 1996;9:512–531. doi: 10.1128/cmr.9.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brajtburg J, Elberg S, Travis S J, Kobayashi G S. Treatment of murine candidiasis and cryptococcosis with amphotericin B incorporated into egg lecithin-bile salt mixed micelles. Antimicrob Agents Chemother. 1994;38:294–299. doi: 10.1128/aac.38.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buler W T. Pharmacology, toxicity, and therapeutic usefulness of amphotericin B. JAMA. 1966;195:127–131. [PubMed] [Google Scholar]
- 7.Chabot G G, Pazdur R, Valeriote F A, Baker L H. Pharmacokinetics and toxicity of continuous infusion of amphotericin B in cancer patients. J Pharm Sci. 1989;78:307–310. doi: 10.1002/jps.2600780409. [DOI] [PubMed] [Google Scholar]
- 8.Denning D W. Epidemiology and pathogenesis of systemic fungal infections in the immunocompromised host. J Antimicrob Chemother. 1991;28(Suppl. B):1–16. doi: 10.1093/jac/28.suppl_b.1. [DOI] [PubMed] [Google Scholar]
- 9.Domb A J, Linden G, Polacheck I, Benita S. Nystatin-dextran conjugates: synthesis and characterization. J Polymer Sci. 1996;34:1229–1236. [Google Scholar]
- 10.Foster D, Washington C, Davis S S. Toxicity of solubilized and colloidal amphotericin B formulation to human erythrocytes. J Pharm Pharmacol. 1988;40:325–328. doi: 10.1111/j.2042-7158.1988.tb05260.x. [DOI] [PubMed] [Google Scholar]
- 11.Gallis H A, Drew R H, Pickard W W. Amphotericin B: 30 years of clinical experience. Rev Infect Dis. 1990;12:308–329. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- 12.Graybill J R, Craven P C, Taylor R L, Williams D M, Magee W E. Treatment of murine cryptococcosis with liposome-associated amphotericin B. J Infect Dis. 1982;145:748–752. doi: 10.1093/infdis/145.2.748. [DOI] [PubMed] [Google Scholar]
- 13.Gubbins P O, Bowman J L, Penzak S R. Antifungal prophylaxis to prevent invasive mycoses among bone marrow transplantation recipients. Pharmacotherapy. 1998;18:549–564. [PubMed] [Google Scholar]
- 14.Hay R J. Liposomal amphotericin B, AmBisome. J Infect. 1994;1:35–43. doi: 10.1016/s0163-4453(94)95956-0. [DOI] [PubMed] [Google Scholar]
- 15.Hiemenz J W, Walsh T J. Lipid formulations of amphotericin B: recent progress and future directions. Clin Infect Dis. 1996;22(Suppl. 2):S133–S144. doi: 10.1093/clinids/22.supplement_2.s133. [DOI] [PubMed] [Google Scholar]
- 16.Hostetler J S, Clemons K V, Hanson L H, Stevens D A. Efficacy and safety of amphotericin B colloidal dispersion compared with those of amphotericin B deoxycholate suspension for treatment of disseminated murine cryptococcosis. Antimicrob Agents Chemother. 1992;36:2556–2560. doi: 10.1128/aac.36.12.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsen C. Dextran products, Physico-chemical and chemical aspects in relation to in vivo properties. Ph.D. dissertation. Copenhagen, Denmark: Villadsen and Christensen; 1990. [Google Scholar]
- 18.Levy M Y, Polacheck I, Barenholz Y, Benita S. Efficacy evaluation of a novel submicron amphotericin B emulsion in murine candidiasis. J Med Vet Mycol. 1993;31:202–218. doi: 10.1080/02681219380000261. [DOI] [PubMed] [Google Scholar]
- 19.Lindenbaum G, Mirgorodskaya O, Moskvichev B, Tereshin I. Study of some physicochemical and enzyme properties of polymer derivatives of terrilytin on the basis of dextran. Khim-Pharm Zh. 1977;11:81–86. [Google Scholar]
- 20.Maddux M S, Barriere S L. A review of complications of amphotericin B therapy: recommendation for prevention and management. Drug Intel Clin Pharm. 1980;14:177–181. [Google Scholar]
- 21.Maeda M, Nishimura C, Umeno D, Takagi M. Psoralen-containing vinyl monomers for conjugation of double-helical DNA with vinyl polymers. Bioconj Chem. 1994;5:527–531. doi: 10.1021/bc00030a007. [DOI] [PubMed] [Google Scholar]
- 22.Merz W G, Karp J E, Hoagland M, Jett-Goheen M, Junkins J M, Hood A F. Diagnosis and successful treatment of fusariosis in the compromised host. J Infect Dis. 1988;158:1046–1055. doi: 10.1093/infdis/158.5.1046. [DOI] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 24.Odds F C. Candida and candidosis. 2nd ed. London, United Kingdom: Bailliere Tindall; 1988. [Google Scholar]
- 25.Sabra R, Branch R A. Amphotericin B nephrotoxicity. Drug Safety. 1990;5:94–108. doi: 10.2165/00002018-199005020-00003. [DOI] [PubMed] [Google Scholar]
- 26.Stevens D A. Overview of amphotericin B colloidal dispersion (Amphocil) J Infect. 1994;28(Suppl. 1):45–49. doi: 10.1016/s0163-4453(94)95971-4. [DOI] [PubMed] [Google Scholar]
- 27.van Etten E W M, van den Heuvel de Groot C, Bakker-Woudenberg I A. Efficacies of amphotericin B-desoxycholate (Fungizone), liposomal amphotericin B (AmBisome) and fluconazole in the treatment of systemic candidosis in immunocompetent and leucopenic mice. J Antimicrob Chemother. 1993;32:723–739. doi: 10.1093/jac/32.5.723. [DOI] [PubMed] [Google Scholar]
- 28.Zar J H. Biostatistical analysis. Englewood Cliffs, N.J: Prentice-Hall; 1984. Testing for goodness of fit; pp. 40–60. [Google Scholar]