Abstract
Introduction:
The receptor for AGEs (RAGE) is a multiligand member of the immunoglobulin superfamily of cell surface receptors expressed in many organs, among them, the kidneys. When activated, RAGE leads to a sequence of signaling that results in inflammation and oxidative stress, both involved in kidney disease pathogenesis. Gamma-oryzanol (γOz) comprises a mixture of ferulic acid (FA) esters and phytosterols (sterols and triterpene alcohols) mainly found in rice, with antioxidant and anti-inflammatory activities.
Aim:
To evaluate the effect of γOz to reduce renal inflammation and oxidative stress by modulating AGEs/RAGE axis in animals submitted to a high sugar-fat diet.
Methods:
Male Wistar rats (±187g) were randomly divided into two experimental groups: control (n = 7 animals) and high sugar-fat diet (HSF, n = 14 animals) for 20 weeks. After this period, when the presence of renal disease risk factors was detected in the HSF group (insulin resistance, dyslipidemia, increased systolic blood pressure and obesity), the HSF animals were divided to begin the treatment with γOz or continue receiving only HSF for 10 more weeks.
Results:
No effect of γOz on obesity and metabolic parameters was observed. However, kidney inflammation and oxidative stress decreased as soon as RAGE levels were reduced in HSF + γOz.
Conclusion:
It is possible to conclude that the gamma- oryzanol was effective in reducing inflammation and oxidative stress in the kidney by modulating the AGEs/RAGE axis.
Keywords: AGE receptor, Inflammation, Antioxidants, Kidney, Obesity
Resumo
Introdução:
O receptor para AGEs (RAGE) é um membro multiligante da superfamília das imunoglobulinas dos receptores de superfície celular expresso em muitos órgãos, entre eles, os rins. Quando ativado, o RAGE leva a uma sequência de sinalização que resulta em inflamação e estresse oxidativo, ambos envolvidos na patogênese de doenças renais. O gama-orizanol (γOz) compreende uma mistura de ésteres de ácido ferúlico (AF) e fitoesteróis (esteróis e álcoois triterpenos) encontrados principalmente no arroz, com atividades antioxidantes e anti-inflamatórias.
Objetivo:
Avaliar o efeito do γOz para reduzir a inflamação renal e o estresse oxidativo pela modulação do eixo RAGE/AGEs em animais submetidos a uma dieta rica em gordura e açúcar.
Métodos:
Ratos Wistar machos (±187g) foram divididos aleatoriamente em dois grupos experimentais: controle (n = 7 animais) e dieta rica em gordura e açúcar (HSF, do inglês high sugar-fat diet, n = 14 animais) por 20 semanas. Após este período, quando foi detectada a presença de fatores de risco de doença renal no grupo HSF (resistência à insulina, dislipidemia, aumento da pressão arterial sistólica e obesidade), os animais HSF foram divididos para iniciar o tratamento com γOz ou continuar recebendo apenas HSF por mais 10 semanas.
Resultados:
Não foi observado nenhum efeito do γOz na obesidade e nos parâmetros metabólicos. No entanto, a inflamação e o estresse oxidativo renais diminuíram assim que os níveis de RAGE foram reduzidos em HSF + γOz.
Conclusão:
É possível concluir que o gama- orizanol foi eficaz em reduzir a inflamação e o estresse oxidativo no rim pela modulação do eixo RAGE/AGEs.
Descritores: Receptor RAGE, Inflamação, Antioxidante, Rim, Obesidade
INTRODUCTION
Protein glycation is a complex series of sequential reactions collectively called the Maillard reaction that result in advanced glycation end products (AGEs) formation. Endogenous sources of AGEs can be found in all tissues and fluids where glucose concentration is enough to react with proteins, such as in conditions of hyperglycaemia and diabetes1. Moreover, the degradation of glycated proteins, glycolytic intermediates, and degradation of aldoses and ketoses result in the formation of reactive carbonyl species such as glyoxal (G), methylglyoxal (MG), and 3-deoxyglucosone, which are also able to react with proteins to form more AGEs directly2. AGEs are also present in ingested food, characterizing exogenous AGEs sources. The content depends on the nutrient composition and how the food is processed (for example, high levels of AGEs are found in roasted, smoked, and baked foods)1 , 3.
At the cellular level, the damaging effects of AGEs have been attributed to several AGE-binding proteins, such as RAGE (receptor for AGEs), AGEs receptor (AGER) 1, R2, R3, and scavenger receptors such as CD-3647 and SCR-II 4. It is important to emphasize that these cell surface receptors can bind to advanced glycation end products, and also can interact with multiple ligands called multi-ligand receptor, among them: high-mobility group protein (B)1 (HMGB1), S-100 calcium-binding protein, amyloid-β-protein, Mac-1, and phosphatidylserine5. Among AGEs receptors, the RAGE is the most notable one, and it triggers oxidative stress and inflammation in both acute and chronic diseases. Specifically, the binding of RAGE leads to a sequence of signaling with the activation of the transcription factor nuclear factor kappa-B (NFκB) resulting in proinflammatory cytokines production, among them tumoral necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1)6.
RAGE activation leads to oxidative stress by inducing nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase (NOX), especially NOX-4. Oxidation driven by the AGEs/RAGE axis induces protein and lipid oxidation leading to the formation of protein carbonyls and lipid-peroxidation, being responsible for lipid-derived reactive carbonyl species which in turn form protein carbonyl adducts (ALEs) which are also RAGE binders, thus sustaining the RAGE activation7. Thus, according to the information above described, it is possible to note that the AGEs/RAGE axis is an interface between oxidative stress and inflammation, which are pillars for the development of several diseases, especially in organs that express these receptors for AGEs, as brain, heart, and kidneys8.
The podocyte is the main RAGE expressing cell in the renal glomerulus1 , 9. It has been described in the literature that RAGE-dependent signal transduction in podocytes leads to apoptosis, production of monocyte chemoattractant protein-1, inflammatory mediators, and oxidative stress via NOX-4, causing renal structural changes, resulting in increased proteinuria and reduced glomerular filtration rate (GFR)9. Once GFR is the main way to excrete AGEs, the exacerbation of kidney injury contributes to accumulating AGEs, characterizing a vicious positive feedback of AGEs accumulation through AGEs/RAGE-induced oxidative stress in the course of CKD progression1.
In this way, the search for strategies to lower the ligand burden (AGEs and other RAGE ligands) and strategies to dampen RAGE activation have received attention, such as the use of natural compounds as a promising pool of substances to treat diseases10 , 11. Gamma-oryzanol (γOz) comprises a mixture of ferulic acid (FA) esters and phytosterols (sterols and triterpene alcohols) mainly found in rice, a very important grain in the human diet. A great variety of biological effects have been attributed to γOz, such as antidiabetic, antioxidant, anti-inflammatory, and anti-obesity effects12. Other studies have already demonstrated a positive effect of γOz to prevent cardiorenal metabolic syndrome13, improve renal disease10, and increase muscle growth and sports performance14.
Thus, since the AGEs/RAGE ligation is able to induce kidney inflammation and oxidative stress and given the lack of studies that evaluate the effect of γOz in renal AGEs/RAGE modulation, the aim of this study was to evaluate the effect of γOz in reducing renal inflammation and oxidative stress by modulating AGEs/RAGE axis in animals submitted to a high sugar-fat diet. The rationale for using the γOz in this animal model is sustained by the recent literature demonstrating that this class of compounds, besides having a well-established antioxidant activity, exerts a direct antiglycation effect15.
MATERIAL AND METHODS
EXPERIMENTAL PROTOCOL
All the experiments and procedures were approved by the Animal Ethics Committee of Botucatu Medical School (1150/2015) and were performed in accordance with the National Institute of Health's Guide for the Care and Use of Laboratory Animals. Male Wistar rats (±187 g) were housed in individual cages in an environment-controlled room (22±3 °C; 12 h light-dark cycle, and relative humidity of 60±5 %) and randomly divided into two experimental groups: control (n = 7 animals) and high sugar-fat diet (HSF, n = 14 animals) for 20 weeks. At the 20th week, when the presence of renal disease risk factors was detected in HSF group10 , 13 (insulin resistance, dyslipidemia, increased systolic blood pressure, and obesity), the animals were divided to begin the treatment with γOzor continue receiving only HSF for 10 more weeks: HSF, n=7 animals and HSF + γOz, n= 7 animals. The HSF diet contained soybean meal, sorghum, soybean peel, dextrin, sucrose, fructose, lard, vitamins, and minerals, plus 25 % sucrose in drinking water; the control diet contained soybean meal, sorghum, soybean peel, dextrin, soy oil, vitamins, and minerals. The nutrients and nutritional composition of each diet was described in our previous study13.
GAMMA- ORYZANOL
The compound was purchased from Tokyo Chemical Industry Co., Ltd. (Toshima, Kita-ku, Tokyo) (lot.5ZZYLPJ). The γOz used in this study was added in the chow (0.5 w/w) in line with our previous study13 in order to simulate the daily consumption of rice of an adult individual in Brazil, according to data from the Family Budget Survey (POF) 2008-200916.
NUTRITIONAL PARAMETERS AND OBESITY- RELATED DISORDERS EVALUATION
The nutritional profile considered: final body weight (FBW), adiposity index (AI), insulin resistance, triglycerides levels, and systolic blood pressure (SBP). Body weight was measured weekly. After euthanasia, the fat deposits (visceral (VAT), epididymal (EAT), and retroperitoneal (RAT)) were used to calculate the adiposity index (AI) by the following formula: VAT+EAT+RAT /FBW ×10017.
After 12 h fasting, blood was collected and the plasma was used to measure insulin and biochemical parameters. Blood from fasted animals was collected in tubes containing EDTA and centrifuged at 3500 rpm and the plasma was collected for analysis. Glucose concentration was determined using a glucometer (Accu-Chek Performa, Roche Diagnostics Brazil Limited); triglycerides were measured with an automatic enzymatic analyzer system (Chemistry Analyzer BS-200, Mindray Medical International Limited, Shenzhen, China). The insulin levels were measured using the enzyme-linked immunosorbent assay (ELISA) method using commercial kits (EMD Millipore Corporation, Billerica, MA, USA). The homeostatic model of insulin resistance (HOMA-IR) was used as an insulin resistance index, calculated according to the following formula: HOMA-IR= (fasting glucose (mmol/L) × fasting insulin (µU/mL)) / 22.518.
Systolic blood pressure (SBP) evaluation was assessed in conscious rats by the non-invasive tail-cuff method with a Narco Bio-Systems® electrosphygmomanometer (International Biomedical, Austin, TX, USA). The animals were kept in a wooden box (50×40 cm) between 38 and 40 °C for 4-5 minutes to stimulate arterial vasodilation19. After this procedure, a cuff with a pneumatic pulse sensor was attached to the tail of each animal. The cuff was inflated to 200 mmHg pressure and subsequently deflated. The blood pressure values were recorded on a Gould RS 3200 polygraph (Gould Instrumental Valley View, Ohio, USA). The average of three pressure readings was recorded for each animal.
RAGE LEVELS
Renal tissue (±150 mg) was homogenized (ULTRA-TURRAX® T 25 basic IKA® Werke, Staufen, Germany) in 1.0 mL of phosphate-buffered saline (PBS) pH 7.4 cold solution and centrifuged at 800 g at 4 °C for 10 min. The supernatant (100 µL) was used in analysis. Receptors for advanced glycation end products (RAGE) levels were measured using the enzyme-linked immunosorbent assay (ELISA) method using commercial kits from R&D System, Minneapolis, USA (DY- 1616; 4000- 31.3 pg/mL of detection). The results were corrected according to the protein amount.
AGES LEVELS
Most AGEs have a characteristic fluorescence. Thus, the determination of AGEs was based on spectrofluorometric detection according to Henle et al.(1991)20 and Münch et al. (1997)21. Plasma and urine were diluted 1:20 with PBS (phosphate buffer) pH 7.4 and fluorescence intensity was recorded in emission maximum (440 nm) upon excitation at 370 nm (spectrofluorometer Fluoromax-3, Jobin Yvon Horiba, USA). Fluorescence intensity is expressed in arbitrary units (UF/mg protein).
RENAL INFLAMMATORY PARAMETERS
Inflammation itself is a risk factor for renal function loss22. The activation of RAGE leads to a sequence of signaling with activation of inflammatory response1.
Renal tissue (±150 mg) was homogenized (ULTRA-TURRAX® T 25 basic IKA® Werke, Staufen, Germany) in 1.0 mL of phosphate-buffered saline (PBS) pH 7.4 cold solution and centrifuged at 800 g at 4°C for 10min. The supernatant (100 µL) was used in the analysis. Tumoral necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1) levels were measured by ELISA method using commercial kits from R&D Systems, Minneapolis, USA (TNF-α: DY 510; IL-6: DY 506; MCP-1). The TNF-α limit of detection was 4000-62.5 pg/mL, IL-6 limit of detection was 8000-125 pg/mL, and the MCP-1 limit was 1000-15.6 pg/mL. The supernatant (100 µL) was used for analysis, and the results were corrected according to the protein amount.
RENAL PROTEIN CARBONYLATION
Carbonylation is an irreversible protein modification induced by reactive oxygen species (ROS). It can be produced by oxidative cleavage of the backbone of the protein or by an attack by ROS radicals on some specific amino acids in the side chains such as lysine, arginine, proline, or threonine. Protein carbonyls are the most widely used markers to measure oxidative protein damage23.
The supernatant described above was used for renal protein carbonylation analysis. Carbonylated proteins were measured by an unspecific method that uses DNPH (2,4-dinitrophenylhydrazine derivatizing agent) and photometric detection of any modified protein by carbonylation24. Carbonylated protein levels are expressed in nmol of DNPH/mg of protein.
RENAL NOX-4 GENE EXPRESSION
Of the NOX isoforms, Nox4 is abundantly expressed in the kidney and is an important source of renal ROS triggered by RAGE25. Total RNA was extracted from renal tissue using the reagent TRIzol (Invitrogen). The SuperScript II First-Strand Synthesis System for RT-PCR (Invitrogen) kit was utilized for the synthesis of 20 mL of complementary DNA from 1000 ng of total RNA. The mRNA levels of NOX-4 (assay Rn 00585380_m1; Applied Biosystems) were determined by real-time PCR. Quantitative measurements were made with a commercial kit (TaqMan qPCR; Applied Biosystems) in a detection system (StepOne Plus; Applied Biosystems). Cycling conditions were as follows: enzyme activation at 50 ºC for 2 min, denaturation at 95 ºC for 10 min; complementary DNA products were amplified for forty cycles of denaturation at 95 ºC for 15 s and annealing/extension at 60 ºC for 1 min. Gene expression was quantified in relation to the values of the Control group after normalization by an internal control (cyclophilin: assay Rn 00690933_m1; Applied Biosystems) by the method 22DDCT, as described previously26.
RENAL FUNCTION
After the collection of 24-h urine from the metabolic cages, the renal function was evaluated considering the glomerular filtration rate (GFR = (urine creatinine × flux)/plasma creatinine)10 and the protein/creatinine ratio, since it reflects proteinuria and is considered a marker of kidney function27.
STATISTICAL ANALYSIS
Results are reported as means ± standard deviation (SD) or median (interquartile range). Differences among the groups were determined by one-way analysis of variance. Statistically significant variables were subjected to the Tukey post-hoc test to compare all the groups. Statistical analyses were performed using Sigma Stat for Windows Version 3.5 (Systat Software Inc., San Jose, CA, USA). A p value of 0.05 was considered statistically significant.
RESULTS
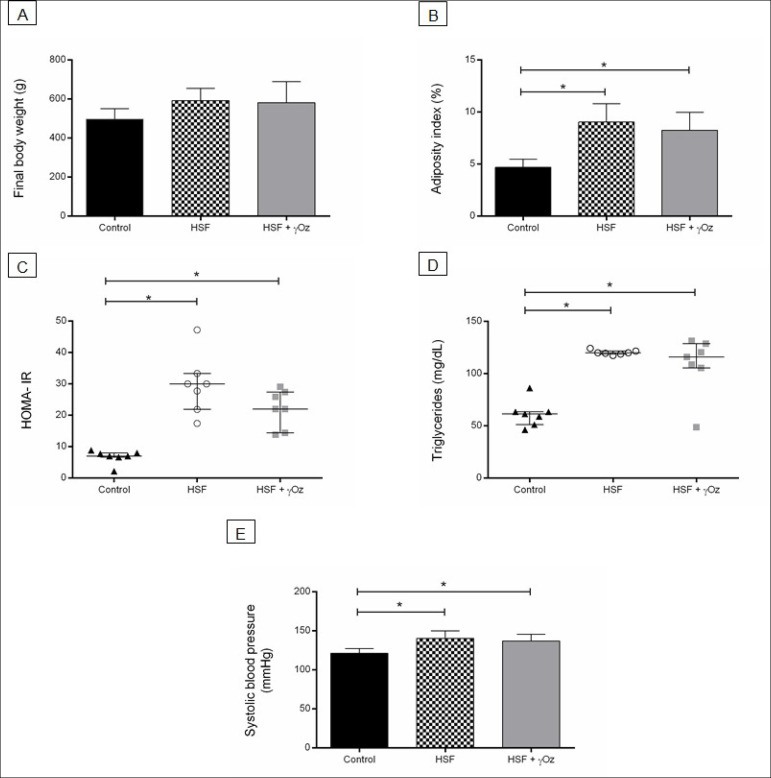
Figure 1 presents the nutritional parameters of groups. It is possible to verify that both groups that received HSF diet (HSF and HSF + γOz groups) presented increased adiposity index, insulin resistance, dyslipidemia, and systolic blood pressure compared to control group. No effect of γOz on these parameters was observed.
Figure 1. Nutritional and obesity-related disorders parameters at the end of 30 weeks. (A) Final body weight (g); (B) Adiposity index (%); (C) HOMAIR; (D) Plasma triglycerides levels (mg/dL); (E) Systolic blood pressure (mmHg). Data are expressed in means ± standard deviations or medians and interquartile ranges (n = 7 animals/group). Comparison by one-way ANOVA with Tukey post-hoc. *p < 0.05. HSF: high sugar-fat diet; HSF + γOz: high sugar-fat diet + gamma-oryzanol.
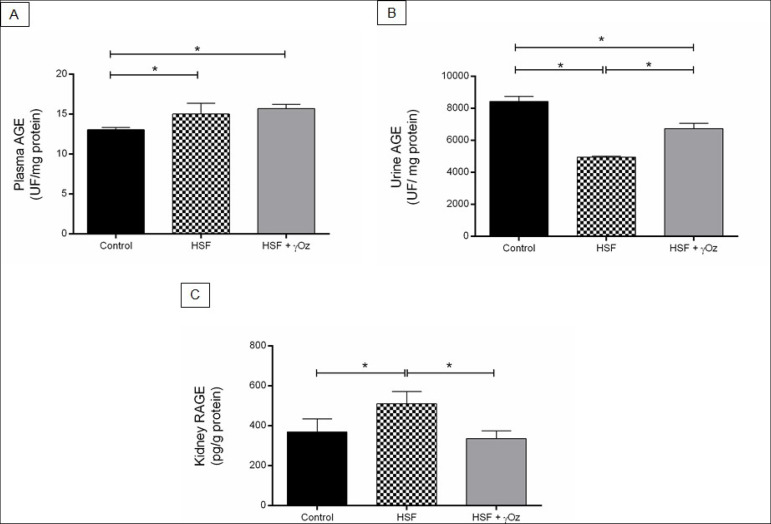
The plasma AGEs levels were the same in the HSF and HSF + γOz groups and higher than the control group. The HSF group presented lower urine AGE and increased kidney RAGE compared to the control group. The HSF + γOz group presented increased AGEs levels in urine and reduced RAGE levels compared to the HSF group (Figure 2).
Figure 2. AGEs and RAGE levels. (A) Plasma AGEs levels (UF/mg protein); (B) Urine AGEs levels (UF/mg protein); (C) Kidney RAGE levels (pg/g protein) (C). Data are reported in means ± standard deviations (n = 7 animals/group). Comparison by one-way ANOVA with Tukey post-hoc test. *p < 0.05. HSF: high sugar-fat diet; HSF + γOz: high sugar-fat diet + gamma- oryzanol.
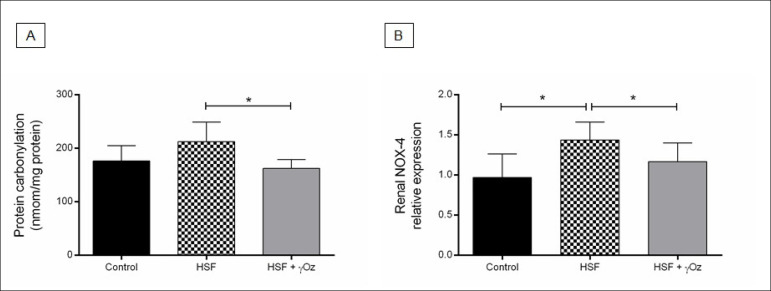
Kidney oxidative stress parameters are presented in Figure 3. The HSF group presented increased NOX-4 gene expression compared to the control group. The treatment with gamma-oryzanol was effective to reduce carbonylation and NOX-4 gene expression in the HSF + γOz compared to the HSF group.
Figure 3. Kidney oxidative stress parameters. (A) Kidney protein carbonylation (nmol/mg protein); (B) NOX-4 relative gene expression. Data are reported in means ± standard deviations (n = 7 animals/group). Comparison by one-way ANOVA with Tukey post-hoc test. *p < 0.05. HSF: high sugar-fat diet; HSF + γOz: high sugar-fat diet + gamma-oryzanol.
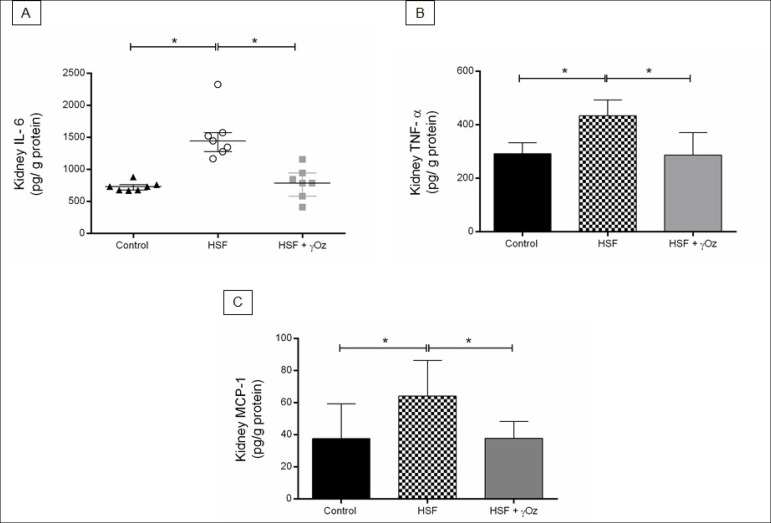
Figure 4. Kidney inflammatory parameters. (A) Interleukin-6 (IL-6, pg/g protein); (B) Tumoral necrosis factor alpha (TNF-α, pg/g protein); (C) Monocyte chemoattractant protein -1 (MCP-1, pg/g protein). Data are reported in means ± standard deviations or medians and interquartile ranges (n = 7 animals/group). Comparison by one-way ANOVA with Tukey post-hoc test. *p < 0.05. HSF- high sugar-fat diet; HSF + γOz- high sugar-fat diet + gamma-oryzanol.
Figure 4. shows the renal inflammatory parameters. The HSF group presented increased pro-inflammatory parameters levels in comparison to the control group while the HSF + γOz group presented lower IL-6, TNF-α, and MCP-1 levels than the HSF group.
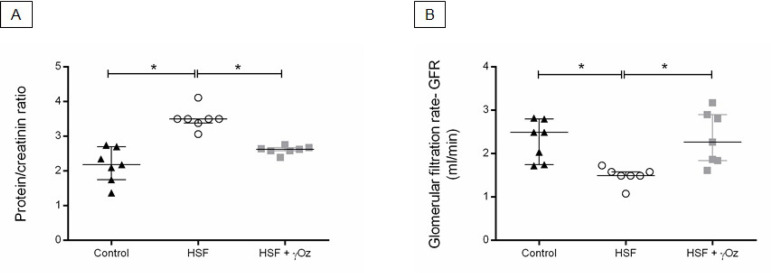
The renal function parameters are presented in Figure 5. The HSF group presented increased protein/creatinine ratio and lower GFR compared to the control group. Otherwise, the HSF + γOz group presented improvement in kidney function, characterized by increased glomerular filtration rate. This group also presented reduction in kidney injury, with lower proteinuria (protein/ creatinine ratio) compared to the HSF.
Figure 5. Renal function parameters. (A) Urine protein/creatinine ratio; (B) Glomerular filtration rate (GFR, mL/min). Data are reported in medians and interquartile ranges (n = 7 animals/group). Comparison by one-way ANOVA with Tukey post-hoc test. *p < 0.05. HSF: high sugar-fat diet; HSF + γOz: high sugar-fat diet + gamma-oryzanol.
DISCUSSION
The aim of this study was to evaluate the effect of γOz in reducing renal inflammation and oxidative stress by modulating the AGEs/RAGE axis in animals submitted to a high sugar-fat diet. Obesity is a condition associated with several disorders considered risk factors for renal disease development, among them, central obesity, increased triglyceride levels, low high-density lipoproteins, hypertension, and elevated fasting glucose28. Some possible pathways for inducing kidney disease are insulin resistance and chronic inflammation, a major contributor to microvascular remodeling; dyslipidemia and excessive nutrient availability that may induce mitochondrial dysfunction; adipokines unbalance; the renin-angiotensin system; and oxidative stress. Our results showed no effect of γOz on the metabolic parameters, in contrast to the literature which shows positive effects, especially on glucose levels and dyslipidemia. This divergence can be explained by the use of different animal models, dose of γOz, and time of treatment. Our study treated male Wistar rats with 0.5% γOz in the chow for 10 weeks, a dose correspondent to an average consumption of 50 mg/day of γOz. Wang et al. (2015)29. fed male Sprague Dawley rats with a high-fat and high-fructose diet supplemented with 0.05% of FA or 0.16% of γOz for 13 weeks and they found that FA and γOz exhibited similar effects in alleviating obesity, hyperlipidemia, hyperglycemia, and insulin resistance. Cheng et al. (2013)30 studied the effect of a diet with 15% of palm oil with the addition of 5.25 g of gamma-oryzanol for 5 weeks in male Wistar rats with type 2 diabetes induced by streptozotocin. No effect of γOz was observed on body fat, glucose, and insulin; however, insulin resistance (area under the curve), triglycerides, and LDL cholesterol levels were reduced with the compound.
The role of inflammation in chronic kidney disease (CKD) pathogenesis and progression has been recognized since the late 1990s when IL-1 levels were associated with major complications and increased rate of mortality in patients undergoing chronic dialysis. Following studies have demonstrated that persistent inflammation is able to promote adverse consequences to kidneys31. Evidence shows that inflammation and inflammatory reactions from any cause can modify or interfere with the intrarenal microcirculatory regulation and perfusion distribution and can induce renal damage, thus enhancing kidney disease progression31. Our results showed that the inflammatory response in the kidney was attenuated in the HSF group treated with γOz , corroborating the anti-inflammatory effect of the compound.
Oxidative stress pathogenesis in CKD patients has been well documented in the current literature32. Excessive production of ROS results in the activation of several enzymatic systems such as nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and the mitochondrial respiratory chain, and, together with impaired antioxidant defense mechanisms, are the main factors for the oxidative stress condition that occurs in CKD, which leads to oxidation of macromolecules, tissue damage, and dysfunction. Therefore, excess generation of ROS has been directly linked to disease mechanisms and processes associated with CKD initiation and progression, including proteinuria, arterial hypertension, and diabetes mellitus. Moreover, the association among oxidative stress and chronic and endothelial dysfunction maintain and perpetuate the vicious circle where chronic kidney damage generates more kidney injury and systemic complications of CKD, as cardiovascular dysfunction. Evidence shows that oxidative stress is already present even in the early stages of CKD with increased NADPH oxidase production, especially NADPH subunit NOX-4. Oxidized lipoprotein particles, as carbonylated proteins, have been shown to accumulate in CKD as renal dysfunction progresses 33. Thus, since our results show that the HSF + γOz group presented reduced protein carbonylated and NOX-4 levels compared to the HSF group, we can confirm the antioxidant effect of γOz.
The metabolic changes present in the HSF groups are able to lead to renal inflammation and oxidative stress activation pathways. However, even with no effect of γOz, the treated animals showed reduced inflammation and oxidative stress. This can be explained by the effect of γOz on modulating the AGEs/RAGE axis in the HSF + γOz animals. AGEs are complex fluorescent products and they are formed through different pathways involving a direct glycation followed by re-arrangements or by the reaction of reactive carbonyl species (RCS) such as glyoxal, methylglyoxal, and 3-deoxyglucosone, which are formed through degradation pathways of sugars. Based on the AGEs formation mechanism, we can consider that in the present animal model, AGEs are formed by the increased sugars present in the diet leading to a direct protein glycation and then fluorescent products by re-arrangement reactions. AGEs induce oxidative stress through RAGE activation as demonstrated here by NOX-4 upregulation, which further sustains AGEs formation by forming RCS species through reducing sugar oxidation. Oxidative stress also promotes protein carbonylation and advanced lipoxidation end products (ALEs) formation, which result either from a direct oxidation or by RCS from lipid peroxidation, such as 4- hydroxynonenal (HNE), MDA, and acrolein34. The above-mentioned events were confirmed in the study, and in particular we found that the increase of RAGE and NOX-4 expression in the kidney were accompanied by an increase of protein carbonylation.
AGEs were found to be reduced in the urine of HSF in comparison to control animals, and this can be explained by considering a reduced excretion of these protein adducts. The mechanism explaining this reduction is being evaluated, and one explanation could be that the AGEs are trapped by RAGE whose content has increased in the kidney of HSF animals, thus reducing the urinary content of the AGEs. Hence in HSF animals the AGEs/RAGE axis is activated leading to an oxidative stress response, which promotes an inflammatory condition as observed by finding an increase of IL-6, TNF-α, and MCP-1. γOz was found to significantly reduce all the above-mentioned events and in particular the AGEs/RAGE axis by increasing the urinary excretion of AGEs and reducing RAGE expression, and the oxidative damage, by reducing protein oxidation and finally the anti-inflammatory response35. The present paper does not indicate through which mechanisms γOz elicits such a protective mechanism but can be speculated based on the literature. Very recently, Sobhy et al. (2020)15 found that oryzanols inhibit glycation and AGEs formation by a direct anti-glycation effect and by scavenging the free radicals generated during the glycation reactions. Furthermore, the antioxidant activity of γOz has been reported in both in vitro and in vivo experiments by several groups10 , 36 , 37.
In summary, this study found that the group treated with gamma-oryzanol showed reduced kidney RAGE levels, inflammation, and oxidative stress and increased renal AGEs excretion probably due the positive effect on glomerular filtration rate. Thus, it is possible to conclude that the gamma-oryzanol was effective in reducing inflammation and oxidative stress in the kidney by modulating the AGEs/RAGE axis.
It is important to report some limitations of this study. Only general, and not specific AGEs or RAGE, were analyzed, more oxidative stress and inflammation markers could be included in the parameters analyzed, the immunohistochemical analysis for RAGE could have been performed, as well as histological analysis of the kidneys to demonstrate possible damage to structures.
ACKNOWLEDGMENTS
The authors thank to Universitá degli Studi di Milano and the funding by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) - process #2015/10626-0 e #2018/15294-3- and CAPES (PDSE - 88881.132505/2016-01).
REFERENCES
- 1.Gugliucci A, Menini T. In: Advances in experimental medicine and biology. Crusio WE, Dong H, Radeke HH, Rezaei N, Steinlein O, Xiao J, editors. Vol. 824. Amsterdam: Springer; 2014. The axis AGE-RAGE-soluble RAGE and oxidative stress in chronic kidney disease; pp. 191–208. [DOI] [PubMed] [Google Scholar]
- 2.Thornalley PJ, Rabbani N. Progress in uremic toxin research: highlights and hotspots of protein glycation in end-stage renal disease. Semin Dial. 22(4):400–404. doi: 10.1111/j.1525-139X.2009.00589.x. [DOI] [PubMed] [Google Scholar]
- 3.Vlassara H, Torreggiani M, Post JB, Zheng F, Uribarri J, Striker GE. Role of oxidants/inflammation in declining renal function in chronic kidney disease and normal aging. Kidney Int. 2009 Dec;76(Suppl 114):S3–S11. doi: 10.1038/ki.2009.401. [DOI] [PubMed] [Google Scholar]
- 4.Luevano-Contreras C, Garay-Sevilla ME, Chapman-Novakofski K. Role of dietary advanced glycation end products in diabetes mellitus. J Evidence-Based Complement Altern Med. 2013;18(1):50–66. doi: 10.1177/2156587212460054. [DOI] [Google Scholar]
- 5.Lee EJ, Park JH. Receptor for advanced glycation endproducts (RAGE), its ligands, and soluble RAGE: potential biomarkers for diagnosis and therapeutic targets for human renal diseases. Genomics Inform. 2013 Dec;11(4):224–229. doi: 10.5808/gi.2013.11.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agati VD, Schmidt AM. RAGE and the pathogenesis of chronic kidney disease. Nat Rev Nephrol. 2010 Apr;6:352–360. doi: 10.1038/nrneph.2010.54. [DOI] [PubMed] [Google Scholar]
- 7.Mol M, Degani G, Coppa C, Baron G, Popolo L, Carini M, et al. Advanced lipoxidation end products (ALEs) as RAGE binders: mass spectrometric and computational studies to explain the reasons why. Redox Biol. 2019 May;23:101083–101083. doi: 10.1016/j.redox.2018.101083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhuri J, Bains Y, Guha S, Kahn A, Hall D, Bose N, et al. The role of advanced glycation end products in aging and metabolic diseases: bridging association and causality. Cell Metab. 2018 Sep;28(3):337–352. doi: 10.1016/j.cmet.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busch M, Franke S, Rüster C, Wolf G. Advanced glycation end-products and the kidney. Eur J Clin Invest. 2010 Jul;40(8):742–755. doi: 10.1111/j.1365-2362.2010.02317.x. [DOI] [PubMed] [Google Scholar]
- 10.Francisqueti FV, Ferron AJT, Hasimoto FK, Alves PHR, Garcia JL, Santos KC, et al. Gamma oryzanol treats obesity-induced kidney injuries by modulating the adiponectin receptor 2/PPAR-α axis. Oxid Med Cell Longev. 2018;2018:1278392–1278392. doi: 10.1155/2018/1278392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferron AJT, Aldini G, Francisqueti-Ferron FV, Silva CCVA, Bazan SGZ, Garcia JL, et al. Protective effect of tomato-oleoresin supplementation on oxidative injury recoveries cardiac function by improving β-adrenergic response in a diet-obesity induced model. Antioxidants. 2019 Sep;8(9):368–368. doi: 10.3390/antiox8090368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minatel IO, Francisqueti FV, Corrêa CR, Pace G, Lima P. Antioxidant activity of γ-oryzanol: a complex network of interactions. Int J Mol Sci. 2016 Aug;17(8):1107–1107. doi: 10.3390/ijms17081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francisqueti FV, Minatel IO, Ferron AJT, Bazan SGZ, Silva VS, Garcia JL, et al. Effect of gamma-oryzanol as therapeutic agent to prevent cardiorenal metabolic syndrome in animals submitted to high sugar-fat diet. Nutrients. 2017 Dec;9(12):1299–1299. doi: 10.3390/nu9121299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eslami S, Esa NM, Marandi SM, Ghasemi G, Eslami S. Effects of gamma oryzanol supplementation on anthropometric measurements & muscular strength in healthy males following chronic resistance training. Indian J Med Res. 2014 Jun;139(6):857–863. [PMC free article] [PubMed] [Google Scholar]
- 15.Sobhy R, Zhan F, Mekawi E, Khalifa I, Liang H, Li B. The noncovalent conjugations of bovine serum albumin with three structurally different phytosterols exerted antiglycation effects: a study with AGEs-inhibition, multispectral, and docking investigations. Bioorg Chem. 2020 Jan;94:103478–103478. doi: 10.1016/j.bioorg.2019.103478. [DOI] [PubMed] [Google Scholar]
- 16.Instituto Brasileiro de Geografia e Estatística. Coordenação de Trabalho e Rendimento . Pesquisa de orçamentos familiares: 2008-2009. Análise do consumo alimentar pessoal no Brasil. Brasília (DF): IBGE; 2011. [Google Scholar]
- 17.Luvizotto RAM, Nascimento AF, Imaizumi E, Pierine DT, Conde SJ, Correa CR, et al. Lycopene supplementation modulates plasma concentrations and epididymal adipose tissue mRNA of leptin, resistin and IL-6 in diet-induced obese rats. Br J Nutr. 2016;110(10):1803–1809. doi: 10.1017/S0007114513001256. [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Santos PP, Rafacho BPM, Gonçalves AF, Jaldin RG, Nascimento TB, Silva MAB, et al. Vitamin D induces increased systolic arterial pressure via vascular reactivity and mechanical properties. PLoS One. 2014;9(6):e98895. doi: 10.1371/journal.pone.0098895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henle T, Deppisch R, Beck W, Hergesell O, Hänsch GM, Ritz E. Advanced glycated end-products (AGE) during haemodialysis treatment: discrepant results with different methodologies reflecting the heterogeneity of AGE compounds. Nephrol Dial Transplant. 1999 Aug;14(8):1968–1975. doi: 10.1093/ndt/14.8.1968. [DOI] [PubMed] [Google Scholar]
- 21.Münch G, Keis R, Wessels A, Riederer P, Bahner U, Heidland A, et al. Determination of advanced glycation end products in serum by fluorescence spectroscopy and competitive ELISA. Eur J Clin Chem Clin Biochem. 1997 Sep;35(9):669–677. doi: 10.1515/cclm.1997.35.9.669. [DOI] [PubMed] [Google Scholar]
- 22.Silva GB, Junior, Bentes ACSN, Daher EF, Matos SMA. Obesity and kidney disease. J Bras Nefrol. 2017 Jan-Mar;39(1):65–69. doi: 10.5935/0101-2800.20170011. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz-Ojeda FJ, Olza J, Gil Á, Aguilera CM. In: Obesity: oxidative stress and dietary antioxidants. Del Moral AM, García CMA, editors. Cambridge: Academic Press; 2018. Oxidative stress and inflammation in obesity and metabolic syndrome; pp. 1–15. [DOI] [Google Scholar]
- 24.Mesquita CS, Oliveira R, Bento F, Geraldo D, Rodrigues J V, Marcos JC. Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal Biochem. 2014;458:69–71. doi: 10.1016/j.ab.2014.04.034. [DOI] [PubMed] [Google Scholar]
- 25.Thallas-Bonke V, Jandeleit-Dahm KAM, Cooper ME. Nox-4 and progressive kidney disease. 2015 Jan;24(1):74–80. doi: 10.1097/MNH.0000000000000082. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001 Dec;25(2):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Anna VO, Mátyus J, Sárkány E, Horváth A, Fodor B. New trends in the laboratory diagnostics of proteinuria and albuminuria. Orv Hetil. 2010 May;151(21):28891–28891. doi: 10.1556/OH.2010.28891. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Lerman LO. The metabolic syndrome and chronic kidney disease. Transl Res. 2016 May;183:14–25. doi: 10.1016/j.trsl.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang O, Liu J, Cheng Q, Guo X, Wang Y, Zhao L, et al. Effects of ferulic acid and γ-oryzanol on metabolic syndrome in rats. PLoS One. 2015;10(2):e0118135. doi: 10.1371/journal.pone.0118135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng HH, Ma CY, Chou TW, Chen YY, Lai MH. Gamma-oryzanol ameliorates insulin resistance and hyperlipidemia in rats with streptozotocin/nicotinamide-induced type 2 diabetes. Int J Vitam Nutr Res. 2013 Jan;80(1):45–53. doi: 10.1024/0300-9831/a000005. [DOI] [PubMed] [Google Scholar]
- 31.Mihai S, Codrici E, Popescu ID, Enciu AM, Albulescu L, Necula LG, et al. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J Immunol Res. 2018;2018:2180373–2180373. doi: 10.1155/2018/2180373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ling XC, Kuo KL. Oxidative stress in chronic kidney disease. Ren Replace Ther. 2018 Dec;4:53–53. [Google Scholar]
- 33.Duni A, Liakopoulos V, Roumeliotis S, Peschos D, Dounousi E. Oxidative stress in the pathogenesis and evolution of chronic kidney disease: untangling Ariadne's thread. Int J Mol Sci. 2019;20(15):3711–3711. doi: 10.3390/ijms20153711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Lin X, Bu C, Zhang X. Role of advanced glycation end products in mobility and considerations in possible dietary and nutritional intervention strategies. Nutr Metab (Lond) 2018 Oct;15:72–72. doi: 10.1186/s12986-018-0306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J, Chen LJ, Yu J, Wang HJ, Zhang F, Liu Q, et al. Involvement of advanced glycation end products in the pathogenesis of diabetic retinopathy. Cell Physiol Biochem. 2018;48:705–717. doi: 10.1159/000491897. [DOI] [PubMed] [Google Scholar]
- 36.Rungratanawanich W, Abate G, Serafini MM, Guarienti M, Catanzaro M, Marziano M, et al. Characterization of the antioxidant effects of γ-oryzanol: involvement of the Nrf2 pathway. Oxid Med Cell Longev. 2018;2018:2987249–2987249. doi: 10.1155/2018/2987249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bumrungpert A, Chongsuwat R, Phosat C, Butacnum A. Rice bran oil containing gamma-oryzanol improves lipid profiles and antioxidant status in hyperlipidemic subjects: a randomized double-blind controlled trial. J Altern Complement Med. 2019 Mar;25(3):1–6. doi: 10.1089/acm.2018.0212. [DOI] [PubMed] [Google Scholar]