Abstract
Enrofloxacin (2.5 mg/kg of body weight) and danofloxacin (1.25 mg/kg) were administered subcutaneously to ruminating calves (n = 8) fitted with subcutaneous tissue cages. Concentrations of enrofloxacin, its metabolite ciprofloxacin, and danofloxacin in blood (plasma), tissue cage exudate (following intracaveal injection of 0.3 ml of 1% [vol/wt] carrageenan), and bronchial secretions were measured by high-performance liquid chromatography (HPLC) and microbiological assay (enrofloxacin plus ciprofloxacin and danofloxacin). Mean maximum concentrations (Cmax) ± standard deviations of enrofloxacin (0.24 ± 0.08 μg/ml), ciprofloxacin (0.11 ± 0.03 [total, 0.34 ± 0.10] μg/ml), and danofloxacin (0.23 ± 0.05 μg/ml) were detected in the plasma of calves by HPLC. The Cmax were 0.49 ± 0.17 μg/ml (enrofloxacin equivalents) and 0.24 ± 0.03 μg/ml (danofloxacin) when they were measured by microbiological assay. Mean Cmax in exudate (HPLC) were 0.18 ± 0.07 μg/ml (enrofloxacin), 0.10 ± 0.04 μg/ml (ciprofloxacin), 0.27 ± 0.09 μg/ml (enrofloxacin plus ciprofloxacin), and 0.19 ± 0.05 μg/ml (danofloxacin), and concentrations in exudate exceeded those in plasma from 8 h (enrofloxacin and ciprofloxacin) or 6 h (danofloxacin) after drug administration. The Cmax were 0.34 ± 0.09 μg/ml (enrofloxacin equivalents) and 0.22 ± 0.04 μg/ml (danofloxacin) in exudate when they were measured by the microbiological assay. The maximum mean concentration achieved in bronchial secretions (HPLC) were 0.07 ± 0.04 μg/ml (enrofloxacin), 0.04 ± 0.07 μg/ml (ciprofloxacin), 0.10 ± 0.05 μg/ml (enrofloxacin plus ciprofloxacin), and 0.12 ± 0.09 μg/ml (danofloxacin). The maximum mean concentration in bronchial secretions from a limited number of animals from which samples were available for microbiological assay were 0.27 ± 0.11 μg/ml (n = 4 [enrofloxacin equivalents]) and 0.14 ± 0.02 μg/ml (n = 3 [danofloxacin]). With predictive models of efficacy (Cmax/MIC and area under the concentration-time curve/MIC ratios in plasma) for Pasteurella multocida (MIC of enrofloxacin, 0.06 μg/ml [24]; MIC of danofloxacin, 0.06 μg/ml [6]), enrofloxacin produced scores of 8.17 and 52.00, respectively, compared to those of danofloxacin, which were 4.02 and 23.05, respectively. With the dosing rates recommended in some markets by manufacturers, enrofloxacin and danofloxacin achieved concentrations above the MICs for important pathogenic organisms in plasma, tissue cage exudate, and bronchial secretion. Since fluoroquinolones display concentration-dependent activities, Cmax/MIC ratios may be critical to efficacy. In the United States enrofloxacin is currently the only fluoroquinolone licensed for food animals and dosages for acute respiratory disease are 2.5 to 5 mg/kg for 3 days or 7.5 to 12.5 mg/kg once. The higher dosages on a single occasion are likely to confer Cmax/MIC ratios that are associated with greater clinical efficacy.
Enrofloxacin and danofloxacin are fluoroquinolones which are licensed for use in cattle in many countries throughout the world. Enrofloxacin is indicated for the treatment of respiratory and alimentary tract diseases of bacterial or mycoplasmal origins at a daily dose rate of 2.5 mg/kg of body weight by subcutaneous injection for three to five consecutive days. Recommendations have been made to increase the dose to 5 mg/kg for some bacterial infections. In the United States enrofloxacin is used in beef breed cattle with acute respiratory disease caused by Pasteurella haemolytica, Pasteurella multocida, and Haemophilus somnus at dosage rates of 2.5 to 5 mg/kg for 3 days or 7.5 to 12.5 mg/kg on a single occasion. Enrofloxacin is known to be partially metabolized to ciprofloxacin in cattle, and ciprofloxacin achieves 25 to 35% of the concentration of the parent drug in blood (13, 19). Danofloxacin is recommended for bovine respiratory disease at a daily dose of 12.5 mg/kg by subcutaneous injection; it is not recommended for use in the United States.
The fluoroquinolones are antimicrobial drugs which generally have very good activities against a broad spectrum of aerobic bacteria, including Pasteurella spp., and against mycoplasma (6, 9, 10). The in vitro activities (MICs at which 90% of the isolates are inhibited [MIC90s]) of ciprofloxacin (0.02 μg/ml), danofloxacin (0.06 μg/ml), and enrofloxacin (0.06 μg/ml) (6, 18, 24) against relevant veterinary isolates of P. multocida have been determined. Fluoroquinolones have been shown to achieve high concentrations in lung tissue (16, 20) and in bronchial secretions (4), and the latter concentrations have been attributed to an active process of transport across the airway epithelium. Generally, fluoroquinolones are characterized by high volumes of distribution and high bioavailabilities (6, 13, 15). In cattle, danofloxacin has a relatively large volume of distribution at steady state (2.5 liters/kg) and is 100% bioavailable when it is given by the intramuscular route (6). The concentrations of enrofloxacin and danofloxacin in inflammatory exudate and bronchial secretions may provide information upon which predictions of efficacy can be made that is more realistic than the information provided by concentrations in plasma, since these may be the sites where bacteria establish and multiply. Tissue cages may be used to obtain extracellular fluid samples (transudate) in animals, and the granulation tissue which infiltrates the cages may be inflamed by the intracaveal administration of mild irritants such as carrageenan (11). Following administration of the inflammatory stimulus, exudate may be collected from the cages.
It is also possible to serially sample bronchial secretions in cattle with absorbent lint plugs delivered to the bronchi in a nasogastric tube passed through the nasal passage (4).
The purpose of this study was to determine the pharmacokinetics of enrofloxacin and danofloxacin in plasma, inflammatory exudate, and bronchial secretions of ruminating calves following administration at the dose rates and routes of administration recommended by manufacturers in some markets.
(This work was presented in abstract form at the 7th European Association for Veterinary Pharmacology and Toxicology International Congress, Madrid, Spain, in 1997.)
MATERIALS AND METHODS
Experimental design.
Eight calves were allocated by ballot into two groups of four, groups 1 and 2. Following a 21- to 23-day acclimatization period, a 4-cm-diameter spherical (volume, 33.5 ml3) tissue cage made of smooth plastic was implanted under general anesthesia in the lateral aspect of each side of the neck of each animal. Forty-eight to 50 days after implantation of the tissue cages, animals in group 1 were administered enrofloxacin (Baytril, 10% injection; Bayer plc, Bury St. Edmunds, Suffolk, United Kingdom) at 2.5 mg/kg by the subcutaneous route and animals in group 2 were administered danofloxacin (Advocin, 2.5% injection; Pfizer Ltd., Sandwich, Kent, United Kingdom) at 1.25 mg/kg by the subcutaneous route (crossover 1). Twenty minutes prior to administration of the antimicrobial, an intracaveal injection of carrageenan (0.3 ml of 1% [vol/wt]) was made in the left-side tissue cage to stimulate a mild acute inflammatory response. Plasma (collected at 0, 0.25, 0.50, 1, 2, 4, 6, 8, 12, 24, and 36 h), tissue cage exudate from the left-side tissue cage (collected at the same times as for plasma except at 0.25 h), and bronchial secretions (collected at 0, 1, 4, 8, 12, 24, and 36 h) were collected at predetermined times following administration of the antimicrobials. Three weeks (21 days) after the first crossover, the experiment was repeated (crossover 2) with the group 2 animals receiving enrofloxacin and the group 1 animals receiving danofloxacin. On the second crossover occasion the opposite tissue cage (right side) was used to collect exudate.
Animals and husbandry.
The calves were Friesian cross males (castrated prior to crossover 1) and were identified by unique ear tags. They were 10.13 ± 1.73 (mean ± standard deviation [SD]) months old at the time of crossover 2. The calves were weighed and given a veterinary health inspection prior to the day of drug administration for each crossover. The calves weighed 256.13 ± 40.50 kg at crossover 1 and 274.75 ± 40.75 kg at crossover 2. The calves were fed approximately 2 kg of calf-rearing mixture or 2 kg of bruised barley and ad libitum silage daily. All animals had ad libitum access to water.
Drug administration and sampling procedure.
Enrofloxacin and danofloxacin volumes for administration were calculated according to the weights of the animals taken within 24 h before drug administration, and subcutaneous administration of each drug was made by a 20-gauge 1-in. needle at a site on the thorax clipped for the purpose. Injections were made on different sides of the thorax for each crossover and were contralateral to the tissue cage from which exudate was collected. Blood samples (20 ml) were collected by jugular venipuncture into lithium-heparin beaded tubes (monovette; Sarstedt Ltd.) with a 20-gauge 1-in. needle. Blood was centrifuged at 1,850 × g, and plasma was harvested and divided into two aliquots, which were stored at −20°C until estimation of drug concentration. Tissue cage exudate was collected by intracaveal puncture with an 18-gauge 1-in. needle and withdrawn into sterile plastic 10-ml syringes. Tissue cage fluid was immediately transferred to heparinized glass tubes, which were centrifuged at 1,850 × g to remove cells. The supernatant tissue cage fluid was harvested and stored at −20°C until estimation of drug concentration. For the collection of bronchial secretions, an absorbent plug of lint was fixed to the tip of a solid flexible polyethylene rod and the rod was placed inside a stomach tube and inserted into the trachea of a calf via the nostril. At the tracheal bifurcation the rod was pushed a further 10 cm into the principal bronchi. After 2 to 5 min, the absorbent plug was withdrawn into the stomach tube and the device was removed. The plug was cut from the rod and placed in a syringe, in which it was compressed, and the bronchial fluid thus expressed was collected in a glass tube which was subsequently stored at −20°C until it was analyzed for drug concentration.
Analysis of antimicrobials. (i) HPLC.
A high-performance liquid chromatography (HPLC) system similar to that used by Küng et al. (15) was used to determine concentrations of enrofloxacin, ciprofloxacin, and danofloxacin. This method used a Gilson model 302 delivery pump and a Gilson automatic sampler injector. The HPLC column was a Waters Nova Pack C18 4-μm-particle-size column (3.9 by 150 mm), and detection was with a Perkin-Elmer fluorescence spectrometer (LS4) set at an excitation wavelength of 280 nm and an emission of 440 nm. The mobile phase comprised 16% acetonitrile-methanol (13:1, vol/vol) and 84% water containing 0.4% triethylamine and 0.4% phosphoric acid (35%) and was delivered at a flow rate of 0.6 ml/min. The approximate retention times for enrofloxacin, ciprofloxacin, and danofloxacin were 12, 8, and 10 min, respectively. For enrofloxacin, assay validation indicated a percentage of recovery of 92.33% (n = 32), intra-assay variation (coefficient of variation [CV]) of 4.36 (n = 16), inter-assay CV of 5.32 (n = 16), linearity (r2) of 0.9997, and limit of quantification of 0.005 μg/ml. For ciprofloxacin the percentage of recovery was 64.41% (n = 32), intra-assay CV was 6.91 (n = 16), interassay CV was 7.71 (n = 16), linearity (r2) was 1.0, and limit of quantification was 0.005 μg/ml, and for danofloxacin the percentage of recovery was 93.33% (n = 32), intra-assay CV was 8.41 (n = 16), interassay CV was 12.10 (n = 16), linearity (r2) was 0.9995, and limit of quantification was 0.02 μg/ml. For all assays, spike concentrations for determination of recovery were made up in blank plasma, and for exudate assays, trial spike concentrations in blank exudate were made up to determine if recoveries were similar to those obtained for plasma. Insufficient blank bronchial secretion samples were available to determine recoveries, and for bronchial secretions, concentrations were determined against an internal standard (for enrofloxacin and ciprofloxacin, danofloxacin was used, and for danofloxacin, enrofloxacin was used).
(ii) Microbiological assay.
A microbiological assay similar to that used by Walker et al. (23) was used to determine enrofloxacin equivalents (antimicrobial activities associated with enrofloxacin and its metabolite) and danofloxacin. The assay was carried out on glass plates (30 by 30 cm) with iso-balanced sensitivity test agar culture medium inoculated with Escherichia coli ATCC 25922 from a 1013-CFU stock culture. The wells were 9.00 mm in diameter, and a 100-μl volume of standard, spiked, or test sample was used. Plates were incubated at 37°C for 18 h, and zones of inhibition were read with vernier digital callipers (CP Instruments). For enrofloxacin (or its equivalents), assay validation indicated a percentage of recovery of 95.77% (n = 40), intra-assay CV of 2.46 (n = 20), and interassay CV of 4.96 (n = 20), linearity (r2 of 0.9974), and limit of quantification of 0.06 μg/ml, and for danofloxacin, assay validation indicated a percentage of recovery of 97.19% (n = 40), intra-assay CV of 2.69 (n = 20), and interassay CV of 4.20 (n = 20), linearity (r2 of 0.9997), and limit of quantification of 0.06 μg/ml. For all assays, spike concentrations for determination of recovery were made in blank plasma. For exudate and bronchial secretion assays, trial spike concentrations were made up in blank exudate and bronchial secretion to determine if recoveries were similar for plasma, exudate, and bronchial secretions.
(iii) Pharmacokinetic analysis.
Pharmacokinetic analysis was carried out with the concentration-time data from individual animals with Software for the Statistical Analysis on Non-linear Models on Micros (PC NONLIN, version 4.0 [1992]) and standard pharmacokinetic equations (5). The model determined the maximum concentration (Cmax) and time to Cmax of a drug in serum from observed data. The area under the plasma concentration-time curve (AUC) and area under the first moment curve were determined by the trapezoidal rule, and mean residence time was determined by noncompartmental analysis. The PC NONLIN program attempted to determine the rate constant (β) associated with the terminal elimination phase with algorithms as described by Dunne (2). Where β was estimated, the noncompartmental parameters were extrapolated to infinity. Extrapolated data were used only where the difference between the AUC determined to the last measured data point (AUCLAST) and the AUC for data extrapolated to infinity was less than 10%.
(iv) Statistical analysis.
The data were analyzed with Genstat 5, release 3.2. An analysis of variance was carried out by fitting the following model to the data: Sequence/Subject + Period + Period · Sequence, where Sequence represents the sequence in which animals received the different drugs, Subject represents the individual animal (nested within Sequence), and Period represents the period in which the observation was recorded. By this analysis, in our experiment the carryover effect of a drug administration to the second crossover is equivalent to the Sequence term, the period effect is equivalent to the Period term, the treatment effect is equivalent to the Sequence-times-Period interaction.
RESULTS
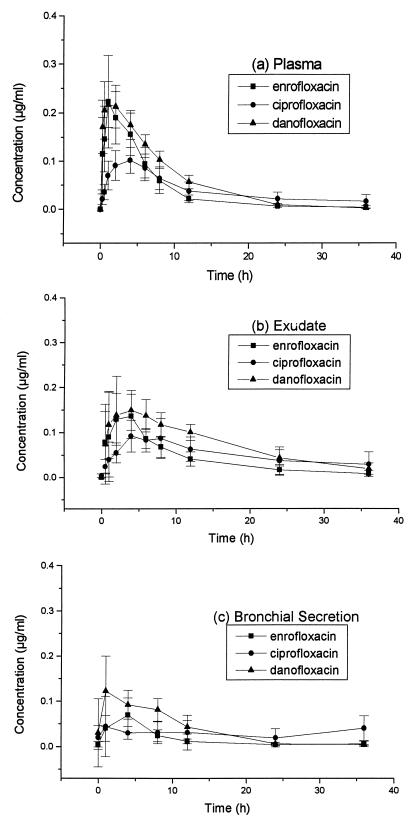
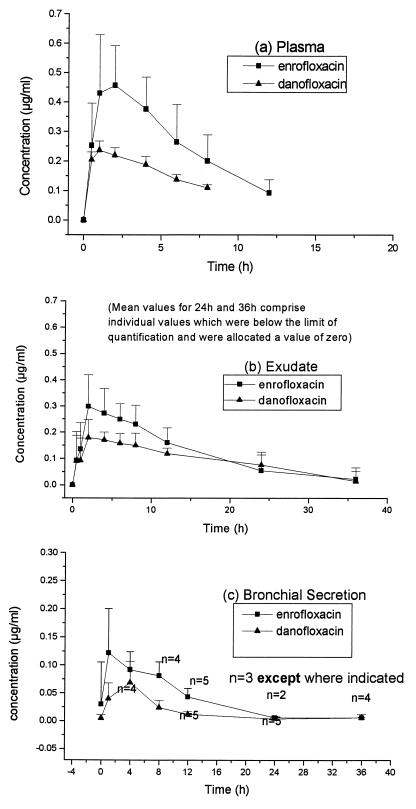
The mean concentrations of enrofloxacin, ciprofloxacin, and danofloxacin determined by HPLC for each tissue fluid are given in Fig. 1. Mean concentrations of enrofloxacin (or its equivalents) and danofloxacin determined by microbiological assay are given in Fig. 2. Pharmacokinetic data from plasma and exudate are given in Table 1; the small number of bronchial secretions samples which were taken and the missing samples meant that pharmacokinetic data could not be determined from the bronchial secretions. There were no statistically significant (P > 0.05) carryover effects on any parameter tested, indicating that the washout period between crossovers was appropriate.
FIG. 1.
Mean concentrations ± SDs of enrofloxacin, ciprofloxacin, and danofloxacin in plasma (a), exudate (b), and bronchial secretions (c) of calves determined by HPLC.
FIG. 2.
Mean concentrations + SDs of enrofloxacin (or its equivalents) and danofloxacin in plasma (a), exudate (b), and bronchial secretions (c) of calves determined by microbiological assay.
TABLE 1.
Pharmacokinetic data from assays of plasma and exudate from calves administered enrofloxacin (2.5 mg/kg) and danofloxacin (1.25 mg/kg) by subcutaneous injectiona
| Assay | Substance | Antimicrobial(s) | Tmax (h) | Cmax (μg/ml) | AUCLAST (μg · h/ml) | AUMCLAST (μg · h/ml) | MRTLAST (h) |
|---|---|---|---|---|---|---|---|
| HPLC | Plasma | Enrofloxacin | 1.75 ± 1.04 | 0.242 ± 0.084 | 1.397 ± 0.245 | 7.569 ± 2.465 | 5.522 ± 2.028 |
| Ciprofloxacin | 3.25 ± 1.04 | 0.110 ± 0.029 | 1.359 ± 0.474 | 15.338 ± 8.693 | 10.623 ± 3.575 | ||
| Danofloxacin | 1.25 ± 0.66 | 0.229 ± 0.050 | 1.643 ± 0.227 | 7.685 ± 1.084 | 4.689 ± 0.433 | ||
| Enrofloxacin + ciprofloxacin | 2.13 ± 1.25 | 0.338 ± 0.103 | 2.506 ± 0.427 | 16.879 ± 6.742 | 6.753 ± 2.768 | ||
| Microbiological | Plasma | Enrofloxacin | 2.25 ± 1.17 | 0.491 ± 0.165 | 3.116 ± 0.980 | 14.141 ± 5.983 | 4.482 ± 0.922 |
| Danofloxacin | 1.38 ± 0.52 | 0.241 ± 0.030 | 1.383 ± 0.153 | 4.900 ± 0.551 | 3.543 ± 0.089 | ||
| HPLC | Exudate | Enrofloxacin | 2.94 ± 1.86 | 0.179 ± 0.065 | 1.478 ± 0.417 | 13.578 ± 6.925 | 8.885 ± 2.429 |
| Ciprofloxacin | 5.25 ± 1.83 | 0.104 ± 0.039 | 1.849 ± 0.927 | 26.829 ± 17.966 | 13.744 ± 3.092 | ||
| Danofloxacin | 3.25 ± 2.49 | 0.192 ± 0.053 | 2.508 ± 0.658 | 28.167 ± 12.966 | 10.720 ± 3.499 | ||
| Enrofloxacin + ciprofloxacin | 3.63 ± 1.51 | 0.267 ± 0.085 | 3.330 ± 1.206 | 43.523 ± 21.744 | 12.661 ± 2.248 | ||
| Microbiological | Exudate | Enrofloxacin (n = 7) | 4.00 ± 2.31 | 0.337 ± 0.087 | 4.062 ± 1.990 | 43.146 ± 34.339 | 9.254 ± 3.546 |
| Danofloxacin | 3.668 ± 2.94 | 0.217 ± 0.039 | 3.417 ± 0.931 | 43.414 ± 22.116 | 11.981 ± 3.408 |
All values are means ± SDs, and the number of samples was 8 unless otherwise indicated. Tmax, time until the maximum concentration was achieved; AUMCLAST, area under the first moment of the concentration-time curve determined to the last measured data point; MRTLAST, mean residence time using data to the last measured concentration.
Mean concentrations of each of the antimicrobials administered and of the ciprofloxacin metabolite were initially higher in plasma than in exudate; however, from 8 h (enrofloxacin and ciprofloxacin) or 6 h (danofloxacin) after administration, concentrations were higher in exudate than in plasma and this was reflected in larger AUC values in exudate than in plasma for each antimicrobial. As determined from HPLC-derived data, the differences between AUCs in plasma and exudate were statistically significant only for danofloxacin (P = 0.001). Concentrations of all measured antimicrobials were higher in plasma and exudate than in bronchial secretions throughout the sampling periods.
The concentrations (AUCs) of the parent enrofloxacin were lower (but not significantly so) than those of danofloxacin when they were measured in plasma and exudate by HPLC; however, when concentrations of enrofloxacin and its metabolite ciprofloxacin were summated, their total Cmaxs and AUCs in plasma were larger (P = 0.002 and P < 0.001, respectively) than those of danofloxacin. The concentrations of enrofloxacin equivalents in plasma as measured by the microbiological assay were also higher (Cmax, P = 0.004; AUC, P = 0.003) than those of danofloxacin. Concentrations of enrofloxacin equivalents measured by the microbiological assay were higher (P = 0.002) than those of enrofloxacin plus ciprofloxacin measured by HPLC (Cmax, 0.491 ± 0.165 versus 0.338 ± 0.103 μg/ml in plasma).
Insufficient bronchial secretion was available for us to carry out the microbiological assay for antimicrobials on many of the sample occasions. When samples were available, the concentrations of enrofloxacin equivalents and danofloxacin were determined and are given in Fig. 2c.
Concentrations of enrofloxacin equivalents measured by the microbiological assay were higher than concentrations of danofloxacin in bronchial secretions throughout the sampling period.
DISCUSSION
The tissue cage and bronchial secretion sample systems used in this study proved highly successful and allowed serial samples of cellular exudate and bronchial secretions to be collected. Determination of antimicrobial concentrations in inflammatory exudate and in lung secretions is likely to permit more accurate predictions of the efficacy of an antimicrobial in infections of peripheral tissue and lung, respectively, than determination of concentrations in plasma. Both sample types represent extracellular fluid, where many bacterial species are known to multiply (14); however, these measurements may not accurately predict efficacy against intracellular organisms, although this may not be a clinical concern, since a marked accumulation of fluoroquinolones intracellularly has been shown to occur (1).
In the present study enrofloxacin and danofloxacin were administered to cattle at dose rates and by routes of administration recommended in clinical practice in some markets, although these dosages do not fully reflect those recommended in the United States or those which might be anticipated to be most effective for drugs with concentration-dependent pharmacodynamics. Both antimicrobials achieved concentrations in plasma, exudate, and bronchial secretions which were above the MIC90s for common bovine pathogens, including P. multocida, for which the MIC90 of both enrofloxacin and danofloxacin has been reported to be 0.06 μg/ml (6, 24). It has been suggested (for ciprofloxacin) that a Cmax/MIC ratio of 10 or greater is predictive of a successful clinical outcome (21) or, alternatively, that an AUC for a 24-h dosing period divided by the MIC of 125 or greater is predictive of bacterial eradication in pneumonic patients (3). With these predictive models and by incorporating Cmax and AUC data derived from plasma in the microbiological assay in this study together with an MIC90 of 0.06 μg/ml for P. multocida, the following values were obtained. The Cmax/MIC ratios for enrofloxacin and for danofloxacin were 8.17 and 4.02, respectively, and the AUC/MIC ratios for enrofloxacin and danofloxacin were 52.00 and 23.05, respectively. These values fall short of the predicted effective values referred to above; however, it must be recognized that the breakpoints described were determined with models for humans and small animals either naturally compromised by neutropenia or with experimentally induced neutropenia. Appropriate breakpoints for success are likely to be quite different for immunocompetent cattle, and this possibility has been borne out by the successful use of both drugs at recommended doses in clinical field trials (8, 12, 22). The bovine model used in the present study could be adapted by experimental infection (7) to provide an accurate method by which the Cmaxs and AUCs in plasma exudate and bronchial secretions could be titrated against the MIC for the infecting organism to determine accurate optimal dosing strategies.
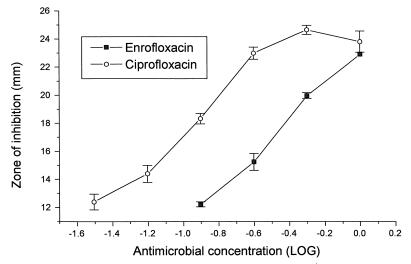
In the present study the concentrations of enrofloxacin plus those of its metabolite ciprofloxacin measured by HPLC were lower than the concentrations of enrofloxacin (or its equivalents) as determined by the microbiological assay, in which pure enrofloxacin standards were used. It is possible that enrofloxacin and ciprofloxacin are synergistic when they are applied to microbiological systems, although the application of serially increasing concentrations of enrofloxacin together with serially decreasing concentrations of ciprofloxacin over the concentration range of 0 to 0.5 μg/ml indicates that the two antimicrobials are simply additive against the test organism E. coli ATCC 25922 (Table 2). This conclusion supports the work of Pirro et al. (17). It is more likely that the test organism is more sensitive to the ciprofloxacin component than the enrofloxacin component in a mixture of enrofloxacin and ciprofloxacin. This possibility has been confirmed by assaying plasma samples containing enrofloxacin and ciprofloxacin against pure enrofloxacin and pure ciprofloxacin standards (Fig. 3). Convergence of the curves for enrofloxacin and ciprofloxacin at the higher concentrations shown in Fig. 3 explain the small differences observed in inhibitory zones at concentrations higher than those indicated in Table 2.
TABLE 2.
Sizes of inhibitory zones produced by enrofloxacin and ciprofloxacin administered alone or in combination
| Antimicrobial(s) | Concn (μg/ml) | Observed (predicteda) size of inhibitory zone (mm) |
|---|---|---|
| Enrofloxacin | 0.5 | 18.71 |
| 0.25 | 13.70 | |
| 0.125 | 10.97 | |
| Ciprofloxacin | 0.5 | 18.82 |
| 0.25 | 14.89 | |
| 0.125 | 13.25 | |
| Enrofloxacin + ciprofloxacin | 0.25 (each) | 18.53 (18.77) |
| 0.125 (each) | 14.83 (14.30) | |
| 0.065 (each) | 12.27 (12.11) |
Predicted from the means of results from the individual administrations combined.
FIG. 3.
Mean sizes of zones of inhibition ± SDs for enrofloxacin assayed against ciprofloxacin standards and for ciprofloxacin assayed against enrofloxacin standards.
These assays highlight the potential inaccuracies and misinterpretations which may arise when microbiological assays are used to determine the concentrations of antimicrobials which produce active metabolites in vivo. On the other hand, bioassays measure the total microbiological activity of an antimicrobial, which may be a more accurate predictor of in vivo activity than HPLC.
ACKNOWLEDGMENTS
We are very grateful to Iain McKentrick of BioSS for assistance with statistical comparisons.
This work was supported by Bayer AG.
REFERENCES
- 1.Boothe D, Hawkins E, Guinn A, Aucoin D. Accumulation of enrofloxacin and its active metabolite ciprofloxacin in lung tissues of the dog. J Vet Intern Med. 1996;10:165. [Google Scholar]
- 2.Dunne A. JANA: a new iterative polyexponential curve stripping program. Comput Methods Programs Biomed. 1985;20:269–275. doi: 10.1016/0169-2607(85)90085-9. [DOI] [PubMed] [Google Scholar]
- 3.Forrest A, Nix D E, Ballow C H, Goss T F, Birmingham M C, Schentag J J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–1081. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friis C. Penetration of danofloxacin into the respiratory tract tissues and secretion in calves. Am J Vet Res. 1993;53:1122–1127. [PubMed] [Google Scholar]
- 5.Gibaldi M, Perrier D. Pharmacokinetics. New York, N.Y: Marcel Dekker; 1982. [Google Scholar]
- 6.Giles C J, Magonigle R A, Grimshaw W T R, Tanner A C, Risk J E, Lynch M J, Rice J R. Clinical pharmacokinetics of parenterally administered danofloxacin in cattle. J Vet Pharmacol Ther. 1991;14:400–410. doi: 10.1111/j.1365-2885.1991.tb00854.x. [DOI] [PubMed] [Google Scholar]
- 7.Gilmour N J L. The experimental production of pneumonic pasteurellosis in sheep and calves. In: Keusch G, Wadstrom T, editors. Experimental bacterial and parasitic infections. New York, N.Y: Elsevier; 1983. pp. 269–275. [Google Scholar]
- 8.Grimshaw W T R, Giles C J, Cooper A C, Shanks D J. The efficacy of danofloxacin in the therapy of pneumonia associated with Pasteurella species in housed calves. Dtsch Tieraerztl Wochenschr. 1990;97:529–532. [PubMed] [Google Scholar]
- 9.Gutierrez Martin C B, Rodriguez Ferri E F. In vitro susceptibility of Pastuerella multocida subspecies multocida strains isolated from swine to 42 antimicrobial agents. Zentbl Bakteriol. 1993;270:387–393. doi: 10.1016/s0934-8840(11)80371-3. [DOI] [PubMed] [Google Scholar]
- 10.Hannan P C T, Windsor G D, de Jong A, Schmeer N, Sregemann M. Comparative susceptibilities of various animal-pathogenic mycoplasmas to fluoroquinolones. Antimicrob Agents Chemother. 1997;41:2037–2040. doi: 10.1128/aac.41.9.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins A J, Lees P, Wright J A. A tissue cage model for the collection of inflammatory exudate in ponies. Res Vet Sci. 1984;36:284–289. [PubMed] [Google Scholar]
- 12.Highland R, Copeland D, Davidson J, TerHune T, Lechtenberg K, Johnson E, Miles D, Apley M, Wray M. Proceedings of the 18th World Buiatrics Congress. 1994. Dose determination and clinical evaluation of the efficacy of enrofloxacin injectable solution in the treatment of bovine respiratory disease; pp. 627–630. [Google Scholar]
- 13.Kaartinen L, Salonen M, Alli L, Pyorala S. Pharmacokinetics of enrofloxacin after single intravenous, intramuscular and subcutaneous injections in lactating cows. J Vet Pharmacol Ther. 1995;18:357–362. doi: 10.1111/j.1365-2885.1995.tb00604.x. [DOI] [PubMed] [Google Scholar]
- 14.Kissane J M. Bacterial diseases. In: Anderson W A D, Kissane J M, editors. Pathology. 7th ed. St. Louis, Mo: Mosby; 1997. pp. 369–414. [Google Scholar]
- 15.Küng K, Riond J-L, Wolffram S, Wanner M. Comparison of an HPLC and bioassay method to determine antimicrobial concentrations after intravenous and oral administration of enrofloxacin in four dogs. Res Vet Sci. 1993;54:247–248. doi: 10.1016/0034-5288(93)90065-n. [DOI] [PubMed] [Google Scholar]
- 16.McKellar Q A, Gibson I F, McCormack R Z. Pharmacokinetics and tissue disposition of danofloxacin in sheep. Biopharm Drug Dispos. 1998;19:123–129. doi: 10.1002/(sici)1099-081x(199803)19:2<123::aid-bdd89>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 17.Pirro F, Scheer M, de Jong A. Proceedings of the 14th Annual Congress of the European Society of Veterinary Dermatology and the European College of Veterinary Dermatology. 1997. Additive in vitro activity of enrofloxacin and its main metabolite ciprofloxacin; p. 199. [Google Scholar]
- 18.Prescott J F, Baggot J D. Fluoroquinolones. In: Prescott J F, Baggot J D, editors. Antimicrobial therapy in veterinary medicine. Ames: Iowa State University Press; 1993. pp. 252–262. [Google Scholar]
- 19.Richez P, Dellac B, Froyman R, de Jong A. Pharmacokinetics of enrofloxacin in calves and adult cattle after single and repeated subcutaneous injections. In: Lees P, editor. 6th European Association for Veterinary Pharmacology and Toxicology. Edinburgh, United Kingdom: Blackwell Scientific Publications; 1994. pp. 232–234. [Google Scholar]
- 20.Scheer M, de Jong A. Concentrations of fluoroquinolones in intestinal tract tissues after intramuscular administration to calves. In: Anadon A, McKellar Q, editors. 7th European Association for Veterinary Pharmacology and Toxicology. Edinburgh, United Kingdom: Blackwell Scientific Publications; 1997. pp. 50–51. [Google Scholar]
- 21.Sullivan M C, Cooper B W, Nightingale C H, Quintiliani R, Lawlor M T. Evaluation of the efficacy of ciprofloxacin against Streptococcus pneumoniae by using a mouse protection model. Antimicrob Agents Chemother. 1993;37:234–239. doi: 10.1128/aac.37.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vancutsem P M, Babish J G, Schwark W S. The fluoroquinolone antimicrobials: structure antimicrobial activity, pharmacokinetics, clinical use in domestic animals and toxicity. Cornell Vet. 1990;80:173–186. [PubMed] [Google Scholar]
- 23.Walker R D, Stein G F, Hauptman J G, Macdonald K H. Pharmacokinetic evaluation of enrofloxacin administered orally to healthy dogs. Am J Vet Res. 1992;53:2315–2319. [PubMed] [Google Scholar]
- 24.Watts J L, Yancey R J, Jr, Case C A. Abstracts of the 92nd General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1992. Resistance trends in bovine respiratory disease isolates, abstr. C-352; p. 479. [Google Scholar]