Abstract
The COVID-19 pandemic has led health systems to increase the use of tools for monitoring and triaging patients remotely. In this systematic review, we aim to assess the effectiveness and safety of pulse oximetry in remote patient monitoring (RPM) of patients at home with COVID-19. We searched five databases (MEDLINE, Embase, Global Health, medRxiv, and bioRxiv) from database inception to April 15, 2021, and included feasibility studies, clinical trials, and observational studies, including preprints. We found 561 studies, of which 13 were included in our narrative synthesis. These 13 studies were all observational cohorts and involved a total of 2908 participants. A meta-analysis was not feasible owing to the heterogeneity of the outcomes reported in the included studies. Our systematic review substantiates the safety and potential of pulse oximetry for monitoring patients at home with COVID-19, identifying the risk of deterioration and the need for advanced care. The use of pulse oximetry can potentially save hospital resources for patients who might benefit the most from care escalation; however, we could not identify explicit evidence for the effect of RPM with pulse oximetry on health outcomes compared with other monitoring models such as virtual wards, regular monitoring consultations, and online or paper diaries to monitor changes in symptoms and vital signs. Based on our findings, we make 11 recommendations across the three Donabedian model domains and highlight three specific measurements for setting up an RPM system with pulse oximetry.
Introduction
Technological innovation has reshaped the modern world such that health care has become a continuous process rather than service points.1 Part of this innovation includes the possibility of monitoring patients in their home environment. The unprecedented increase of COVID-19 cases globally has overwhelmed health systems and challenged their capacities,2, 3 leading to extensive risks to patient health and wellbeing.4 However, the COVID-19 pandemic and the resulting changes in health-care delivery have encouraged the adoption of remote patient monitoring (RPM) models, and represented an opportunity to use RPM as a crucial part of health-care delivery.5 Digitally enabled health-care models that incorporate RPM could offer a more personalised approach to responding to patients’ needs.6
Evidence shows that peripheral blood oxygen saturation (SpO2) is a critical indicator of deterioration in patients with COVID-19.7 In this context, pulse oximetry is a convenient tool for monitoring patients’ SpO2 remotely and establishing whether they require hospital care or can be safely managed at home,8 and different RPM models have included pulse oximeters as part of their monitoring packages for patients with COVID-19.7, 8
RPM seems to be effective in aiding the triage of patients, enabling the prioritisation of health services for people who need them the most.9 RPM could help to prevent the unnecessary use of emergency services and to identify in a timely manner patients who are deteriorating, therefore preventing delays in treatment and extended hospital admissions.10 These RPM models are being used in several countries such as the UK, the USA, and China.7, 11, 12, 13 These models are similar in concept but differ in method, follow-up systems, clinical supervision level, and monitoring strategies and tools.11, 12, 13, 14
For example, in the UK, COVID Oximetry @home15 is a national programme of care for patients with COVID-19 in England. The programme aims to remotely monitor SpO2 in patients who are at risk of deterioration due to silent hypoxia. This model seeks to improve patient care and ensure more efficient use of National Health Service resources.7, 9, 14
Ongoing studies are investigating this RPM model using pulse oximetry in larger cohorts.14 The model could also be adapted for the remote monitoring of patients with cardiovascular or other respiratory diseases for which SpO2 concentrations could indicate deterioration, alongside other indicators.16, 17
In this systematic review, we aim to assess the effectiveness and safety of the use of pulse oximetry in the remote monitoring of patients at home with COVID-19. Specific objectives include describing RPM models that use pulse oximetry, assessing the quality of studies that use these models, and evaluating the effectiveness and safety of pulse oximetry in these models or the effect on patients’ health outcomes and health-care service use (ie, prevention of complications, decrease in the risk of developing silent hypoxia, referrals to emergency care, and decrease in critical care admissions). As a secondary aim we will identify measures and recommendations for improving the design of RPM models with pulse oximetry for patients with COVID-19, following the Donabedian model domains (structure, processes, and outcomes).18
Methods
Search strategy and selection criteria
We systematically searched the published literature in three databases accessed via OVID (MEDLINE, Embase, and Global Health), and the medRxiv and bioRxiv databases for preprints. Each database was searched independently; iterative discussions among the research team generated the specific subject headings and relevant search terms for each database. A complete list of the medical subject headings and keywords used for all databases is provided in the appendix (p 1). We followed the PRISMA guidelines when conducting this systematic review.19, 20 The study protocol is registered with PROSPERO, CRD42021254171.
All types of study were considered, including feasibility studies, clinical trials, and observational studies. Studies were considered regardless of publication status and included peer-reviewed papers and preprints, to account for the speed of research around COVID-19 and the length of time taken by peer review.
No publication date limits were applied in the search, such that resulting studies could date from database inception until April 15, 2021, when our last search was done. However, all studies were published after December, 2019, as this is when COVID-19 was first identified.
We applied a set of inclusion and exclusion criteria following the population, intervention, control, and outcome formulation.21 Studies were included if they targeted adult patients with confirmed or presumptive COVID-19; used a hand-held pulse oximeter as a remote monitoring tool for SpO2; proposed, tested, or evaluated any new or existing RPM system at home, in community-based settings, or both; and assessed the effectiveness and safety of pulse oximetry in RPM, or the effect on patients’ health outcomes (such as prevention of complications, and development of silent hypoxia) and health-care service use (such as hospital referrals, critical care admissions, or referrals to emergency care).
Studies were excluded if they exclusively targeted patients who did not have COVID-19; targeted only hospitalised patients; assessed monitoring systems that exclusively used invasive tools to measure SpO2, with no use of hand-held pulse oximeters; described an RPM system that did not include SpO2 monitoring at all; used pulse oximetry with only a proportion of study participants, and results for this subgroup were not reported individually; or assessed the accuracy of a specific pulse oximeter type or brand, without monitoring patient deterioration or health-care service use.
We also excluded studies that exclusively targeted individuals aged 18 years or younger. Commentary articles, conference abstracts without an accompanying article, and editorials were excluded as they did not assess an RPM model. However, as some of the studies that were excluded from our analysis included important relevant information, the recommendations we present are based on the literature overall.
Data screening
Studies were screened by two authors (AA and TB). Initial screening was based on the information contained in titles and abstracts, followed by full-text screening. Cohen's κ was used to measure inter-rater agreement in each screening phase.22 A κ score of greater than 0·6 was considered, on the basis of previous literature, to be a substantial agreement, and was sufficient to proceed to the next step. A third, senior author (PA) was consulted for any disagreements between the initial two authors.
Data extraction
Descriptive and technical data and outcomes were extracted from the included studies by one author (AA), and were then discussed with another author (TB). A third author (PA) was consulted for any disagreements between the initial two authors. The extracted data were discussed and agreed upon among the research team.
The descriptive data encompassed study authors, country where the study was conducted, state of publication, study period, study design, primary aim, and objectives. The characterisation (technical data and implementation methods) of the intervention included the type of oximeter used, description of the RPM system, method of recording and reporting the SpO2, measurements of SpO2 (and temperature, where provided), target population (age, gender, presence of comorbidities, and specific characteristics if relevant), and main outcomes on effectiveness and safety.
Quality assessment
We used the Newcastle–Ottawa Scale (NOS) to assess the quality of and risk of bias in the included studies. This scale uses a star system for assessment, in which nine stars is the maximum score and indicates a study of the highest quality. The NOS tool has been validated with established inter-rater reliability for assessing non-randomised studies. It evaluates three core aspects: participant selection, the comparability between the study groups, and the ascertainment of outcomes.23
One author (AA) assessed each study and discussed the outcome with a second author (TB) to reach a joint agreement. Disagreements were escalated to a third author (PA) to reach a final decision. The quality assessment of each included study was summarised and reported.
Data analysis
We conducted a narrative synthesis in which the safety and effectiveness of RPM models were described as reported in the included studies, including SpO2 measurement and reporting methods and follow-up strategies. Owing to the heterogeneity of objectives and outcomes in the included studies, a meta-analysis was not feasible.
Identifying recommendations
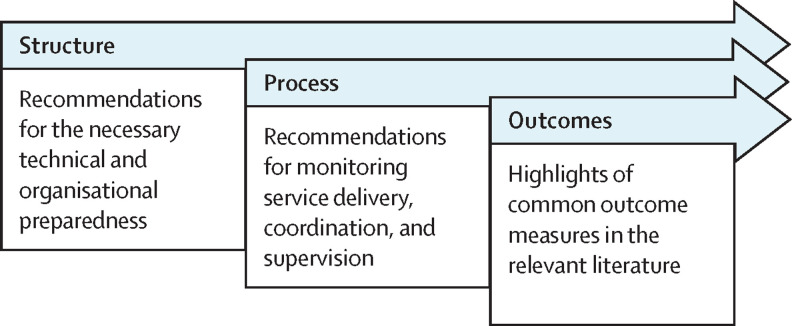
We identified common features of RPM models that were applied across the included studies that used pulse oximetry, and identified measures for improvement for similar care models on the basis of the strengths, limitations, and recommendations stated in the included studies. Future researchers and policy makers could consider these as recommendations for setting up a successful RPM model. These recommendations are presented in terms of Donabedian model domains.18 We have provided a brief description under each domain to clarify the concepts that we described within each one (figure 1 ).
Figure 1.
Use of the Donabedian model to summarise the outcome recommendations
Results
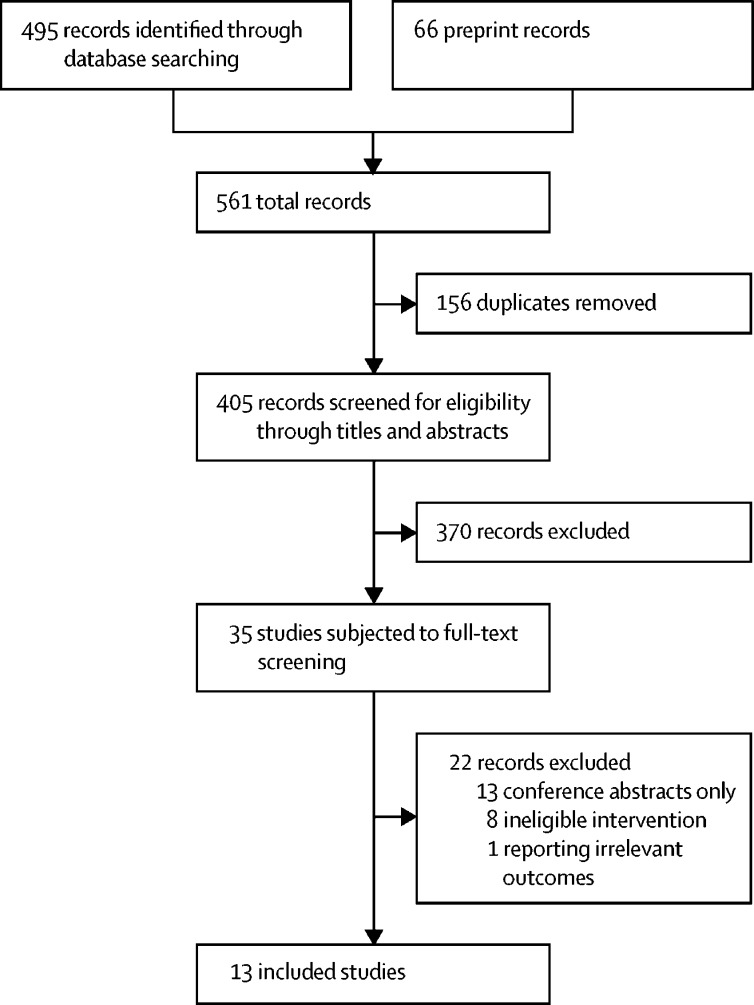
Our search identified 561 records, including 495 peer-reviewed, published results and 66 preprints (figure 2 ). We removed 156 duplicates and excluded 370 records after screening titles and abstracts, after which we reviewed the full text of 35 records. Of these 35 records, 13 were conference abstracts with little available information and were excluded.11, 12, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 The RPM models in eight studies did not include pulse oximetry as a monitoring tool for all participants but only for specific subgroups, and these studies were excluded.35, 36, 37, 38, 39, 40, 41, 42 One study was excluded owing to reporting irrelevant outcomes, focusing on therapeutic options with less emphasis on the RPM model.43
Figure 2.
PRISMA diagram showing the study selection process
We calculated the Cohen's κ score to estimate the inter-rater agreement in each screening phase. Good agreement was achieved in both phase 1 (0·71; screening titles and abstracts) and phase 2 (0·72; full-text screening).
Characteristics of the included studies
13 studies with a combined total of 2908 participants were included in our systematic review (table 1 ).14, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55 All participants had either confirmed or presumptive COVID-19, except for 12 healthy individuals who acted as a control group in one study by Motta and colleagues.45 Another study by Gordon and colleagues44 involved a control group of patients with COVID-19 who were not given pulse oximeters for remote monitoring. All the studies followed a cohort, open-label design, and 11 studies had no control group. Most of the studies were conducted during the first wave of the pandemic in each respective country.
Table 1.
Characteristics of the included studies
| Country | Date of publication | Study period | Aims and objectives | Quality assessment | |
|---|---|---|---|---|---|
| Cohort (open label, controlled) | |||||
| Gordon et al (2020)44 | USA | Nov 11, 2020 | April 8–June 10, 2020 | To describe an RPM programme for patients at home with COVID-19, with pulse oximetry, compared with patients with COVID-19 who were discharged and managed at home with no monitoring programme | ******* |
| Motta et al (2021)45 | Brazil | March 26, 2021 | NS | To develop an RPM system for patients at home with COVID-19 to detect emergencies and deterioration; a control group of healthy people was included, with no history of COVID-19, tobacco smoking, and respiratory or cardiac disease | ****** |
| Cohort (open label, uncontrolled) | |||||
| Blair et al (2021)46 | USA | Jan 5, 2021 | April 21–July 23, 2020 | To explore COVID-19 disease progression in the home setting and identify risk factors for severe disease, including silent hypoxia | ***** |
| Clarke et al (2021)14 | UK | Dec 16, 2020 | Summer 2020 (UK) | To assess the effectiveness of the use of pulse oximetry as a tool for monitoring patients at home with COVID-19 | ***** |
| Ford et al (2020)47 | USA | June 30, 2020 | March 7–April 22, 2020 | To assess the effectiveness of the use of an RPM system and virtual wards to support patients at home with suspected and confirmed COVID-19 | ***** |
| Hutchings et al (2021)48 | Australia | June 5, 2020 | March 11–29, 2020 | To describe an RPM model for monitoring patients with COVID-19 remotely at home | ***** |
| Ko et al (2020)49 | Singapore | Dec 31, 2020 | May 1–26, 2020 | To design a user-friendly RPM model with an interactive chatbot app for monitoring patients at home with COVID-19 to detect risk of deterioration | ***** |
| Kodama et al (2021)50 | USA | Dec 14, 2020 | NS | To assess the implementation and feasibility of an RPM system, with pulse oximetry as a tool for monitoring, for patients at home with COVID-19 | ***** |
| Krenitsky et al (2020)51 | USA | July 21, 2020 | March 23–April 30, 2020 | To monitor obstetric patients (pregnant and post partum) at home with confirmed or presumptive COVID-19 | **** |
| Kyriakides et al (2021)52 | UK | Jan 29, 2021 | April 1–May 30, 2020 | To assess the effect of the use of pulse oximetry for monitoring patients at home with COVID-19 to prevent hospital admissions | **** |
| Nunan et al (2020)53 | UK | Nov 17, 2020 | April 4–June 6, 2020 | To assess the feasibility of developing an RPM system for patients at home with COVID-19 | **** |
| Shah et al (2020)54 | USA | June 16, 2020 | March 20–April 22, 2020 | To assess an RPM system that uses pulse oximetry to monitor patients at home with COVID-19 | **** |
| Wilcock et al (2021)55 | UK | Jan 4, 2021 | May 14–Nov 30, 2020 | To assess the effectiveness of the use of pulse oximetry in identifying deterioration in patients monitored at home with COVID-19 | *** |
For quality assessment, we used the Newcastle–Ottawa Scale for assessing the risk of bias and the quality of the included studies; more stars means a lower risk of bias and higher quality. RPM=remote patient monitoring. NS=not specified.
Patients with different characteristics were involved in the studies, including older patients with multimorbidity,14 young people,48 and obstetric patients (pregnant and post partum).51 Some studies targeted only patients with mild and intermediate risks of deterioration, while recommending that the same model be tested among patients with more complex health needs to compare outcomes.48, 53 Ko and colleagues49 also used an RPM model to monitor the SpO2 of male migrant workers in Singapore in a specific, resource-limited setting, and the model was reported to be effective and safe.
Summary of the quality assessment
One study44 scored seven stars on the NOS tool, indicating good quality. Seven studies14,45–50 scored five or six stars, indicating fair quality, whereas five studies51, 52, 53, 54, 55 scored four or fewer stars. The absence of a control group in most of the studies negatively influenced the quality by scoring zero out of two stars in the comparability aspect of the NOS tool.23 We present the quality score for each study in table 1.
Characteristics of the RPM models
Patients were monitored for an unweighted average of 12·7 days, as reported in 11 of the 13 studies.44, 45, 46, 47, 48, 49, 50, 52, 53, 54, 55 RPM models used pulse oximeters in addition to other monitoring tools, such as thermometers, a peak flow measuring tool,45 a blood pressure cuff,51 and symptom monitoring charts. All studies offered a 24/7 (ie, 24 h every day) emergency telephone line for their patients. The studies used different SpO2 thresholds at which advanced care might be needed. An SpO2 of 92% or less was the threshold at which to escalate care in four studies.44, 46, 54, 55
Participants were trained and instructed to self-measure their SpO2 in all models. Participants in five studies received reminders by telephone call or through text message; however, the frequency of reminders varied.44, 46, 49, 53, 54 For instance, reminders were set at once per day in three models,44, 53, 54 twice per day in one model,49 and were based on a predefined schedule in other models.46, 51, 52 The rationale behind the frequency of reminders was not evident in most cases. Krenitsky and colleagues51 linked the frequency of teleconsultations, for both follow-up and reminders, with the severity of symptoms; consultations were scheduled every 24 h if symptoms were severe and every 48–72 h if symptoms were mild.
In five studies, a mobile app or an online portal was developed for patients to self-report their SpO2 readings.44, 45, 47, 48, 49 Blair and colleagues46 and Kodama and colleagues50 assessed and compared outcomes for at-rest and postexertional SpO2 (table 2 ).
Table 2.
Technical data and outcomes of the included studies
| Number of participants | Study population | RPM system | Measurement and reporting of SpO2 | Highlighted outcomes | |
|---|---|---|---|---|---|
| Blair et al (2021)46 | 118 | Patients with confirmed COVID-19 aged ≥40 years (median age: 56 years [IQR 50–63])—a total of 71 patients completed all study steps | Monitoring tools: pulse oximeter and thermometer; follow-up for 15 days; training on self-monitoring via a telephone call at day 0; follow-up telephone calls at days 0, 3, 7, 14, and 21; an in-person follow-up visit was completed between days 28 and 60 if the patient had been asymptomatic | Self-measured and reported once per day after sitting for 10 min (at-rest SpO2), and another after ambulating for 30–60 s (exertional SpO2); SpO2 threshold for hospital referral: ≤92% | Pearson's correlation r=0·61 between at-rest and exertional SpO2—a larger difference was seen at lower SpO2 concentrations; nine participants reported SpO2 ≤92%, which led to hospitalisation in five cases—the most common cause of emergency referrals; the median time to report low SpO2 was 11·5 (IQR 10–14) days at home |
| Clarke et al (2021)14 | 908 | Patients with confirmed and presumptive COVID-19 with a median age of 54 years—a total of 562 (62%) patients had comorbidities | Data were collected by different monitoring providers (no clarification of how the data were collected), including enrolment date, SpO2 at rest at enrolment, and the clinical pathway for each patient; data on emergency admission, hospital referrals, and death were collected and merged from external datasets | No data were provided on the exact method, but they were collected by different providers | A total of 52 (6%) patients needed 69 hospital referrals during the study period; in a multivariable model, the odds of hospital presentation were significantly associated with increasing age (odds ratio=1·03; p=0·018); patients enrolled after a primary care referral had higher odds of presentation to hospital than patients enrolled after discharge from accident and emergency (0·42; p=0·024) and after discharge from hospital (0·31; p=0·003) |
| Ford et al (2020)47 | 154 | Patients with confirmed and presumptive COVID-19 | Monitoring tools: Bluetooth pulse oximeter and thermometer; follow-up for 14 days; patients received reminders to update their records via an online portal and a mobile app; records were monitored virtually by trained health-care professionals | Self-measured, and automatically reported via a mobile app connected with a Bluetooth pulse oximeter, or self-reported in a personalised portal | The RPM programme led to 709 consultations done by nurses and six consultations done by physicians; 22 patients were referred for physician review, and four patients needed hospital admission; the setting of virtual wards with remote monitoring tools, including pulse oximetry, preserved resources (saved US$105 624 within 5 weeks), reduced the risk of exposure to COVID-19 by monitoring patients at home (5042 remote call consultations conducted), and provided education and emotional support to patients |
| Hutchings et al (2021)48 | 162 | Patients with confirmed COVID-19 aged <65 years with no comorbidities—patients with comorbidities and those with old age were excluded | Monitoring tools: wireless pulse oximeter and wearable temperature monitor; follow-up for 8 days (median; range 1–17); measurements were recorded on a web-based dashboard for follow-up and monitoring; the pulse oximeter was connected to a dashboard to record SpO2 readings automatically whenever used; video follow-up consultation outcomes were compared to the vital signs recorded on the dashboard | Self-measured and reported via voice or video calls and an automated dashboard to record further readings | The follow-up consultations were done remotely by video (1902 [66·4%]> of 2865) and telephone (688 [24·0%] of 2865) call, with an average duration of 15 min for video calls and 8·5 min for telephone calls; self-monitoring SpO2 at home by pulse oximetry was reported to be an effective tool for triaging patients and identifying deterioration; an ambulance was called for only five (3%) patients, of whom four (2·5%) attended emergency departments and three (1·9%) were hospitalised |
| Gordon et al (2020)44 | 181 | Adults aged >18 years with confirmed or presumptive COVID-19 | Monitoring tools: pulse oximeter and thermometer; follow-up for 12 days (median); daily morning reminders for the patients to fill their observations on an online portal; nurses and physicians monitored the portal to detect signs of deterioration | Self-measured and self-reported in an online portal | The median period of participant involvement in the RPM programme and completing the daily required survey or questionnaire was 12 (IQR 10–13) days; only 11 patients (3%) reported SpO2 <92% |
| Ko et al (2020)49 | 800 | Patients with confirmed COVID-19; all participants were male migrant workers living in Singapore with a mean (SD) age of 33 (6·8) years | Monitoring tools: pulse oximeter and thermometer; follow-up for 14 days; reminders were sent twice per day for participants to fill in their observations; records were reported and monitored through a chatbot deployed on social messaging apps that was accessible to health-care professionals through their own mobiles too; four principles were followed to initiate the RPM system: ensuring accessibility for all, safety of patients, safety of health-care staff, and cost-effectiveness | Self-measured, and self-reported via a chatbot deployed on social messaging apps | The system was self-reported as easy to use by all patients and the reporting rate was high on most days; most alerts (65%) were raised owing to SpO2 readings of <95%; 96 remote consultations were done, of which 37 were through WhatsApp messaging and 59 via WhatsApp video calls; only seven cases were referred to emergency, whereas 18 were escalated to an on-site medical review |
| Kodama et al (2021)50 | 50 | Adults aged >18 years with confirmed COVID-19; SpO2 <92% at hospital before discharging to home | Monitoring tools: pulse oximeter; remote follow-up consultations by health-care professionals; follow-up for 14 days; the ratio of staff for monitoring patients was one nurse to 50 patients; patients self-measured their SpO2 at rest, and 20 s after 60 s of exercise | Teleconsultations (telephone calls) were done twice per day (1000 h and 1900 h) to record measurements | 13 patients needed advanced care a total of 29 times, including three patients who needed emergency referrals, one of whom was hospitalised; compliance with the daily self-reporting of SpO2 measurements was high; 91% of participants who filled in an evaluation survey would highly recommend the programme to a friend or colleague |
| Krenitsky et al (2020)51 | 94 | Obstetric patients (92 pregnant and two post partum) with confirmed or presumptive COVID-19; average gestational age of 32·5 weeks (IQR 25–38) | Monitoring tools: pulse oximeter, thermometer, and blood pressure cuff; the frequency of teleconsultations was based on need: every 24 h if symptoms were severe and every 48–72 h if symptoms were mild | Self-measured and reported during the follow-up teleconsultations | 407 teleconsultations were done, including 213 (53%) via video call and 194 (47%) by telephone call; 32 patients (34%) were lost to follow-up; only 4% of teleconsultations required escalation of care; 25 patients (27%) needed to be checked in person (38 visits); hypoxia was not the main reason of hospital referral for any participating patient during the study period |
| Kyriakides et al (2021)52 | 20 | Patients with confirmed and presumptive COVID-19; mean age of 53 years; SaO2 in room air was 90–94% at rest at the time of hospital presentation; 13 patients (65%) had comorbidities | Monitoring tools: pulse oximeter. Follow-up for 7 days; emergency referral if an at-rest SpO2 of <90% was detected twice or more within the same day; follow-up calls on days 2, 5, and 7 of the programme | Self-measured 3 times per day (at 0900 h, 1300 h, and 1800 h); measurements were reported during the follow-up telephone consultations | Only three patients (15%) needed hospital referrals and admission, including one for observation and two for oxygen therapy; these three patients avoided hospital admission for a combined total of 10 days in total during the study period. The patients who needed admission had a mean age of 65 years, and all were older than 60 years |
| Motta et al (2021)45 | 24 | Patients with confirmed COVID-19; mean (SD) age of patients with COVID-19=37·2 (13·3) years, and of the control group (people with no history of COVID-19)=38·2 (15) years; n=12 for each group | Monitoring tools: pulse oximeter, thermometer, and a peak flow measuring tool. Follow-up for 30 days; a mobile app was designed to monitor the patients; the system compared the patients' inputs with preset thresholds and initiated alarms when needed | Self-measured and reported twice per day via a mobile app; SpO2 threshold for emergency referral: <92% (measured after 5 min of rest and without moving the hand to which the pulse oximeter was applied) | COVID-19 infection led to a significant reduction in SpO2 among the patients. 12 patients completed the satisfaction survey; all reported that they felt very safe using the system. 11 (92%) of 12 patients reported that pulse oximetry was very easy to use. In 30 days of follow-up, 16 alerts were initiated among the 12 patients with COVID-19 |
| Nunan et al (2020)53 | 279 | Patients with COVID-19 pneumonia confirmed by PCR, chest imaging, and SpO2 rapid walk test; mean (SD) age of 50·0 (15·3) years | Monitoring tool: pulse oximeter; follow-up for 5 days; daily follow-up calls; in case of any SpO2 readings below a preset threshold, patients were referred to emergency services | Self-measured, and reported during the daily follow-up calls by health-care professionals | 31 patients (11%) needed hospital referrals, of whom 19 were readmitted, including two who needed admission to an intensive care unit and one patient who died. The mean age of referred patients was 50·9 years (SD=16·8). Of 185 patients who completed an evaluation survey, 184 would recommend the programme to others. For all participants, a predictive cost avoidance of £106 700 per month could be achieved (a total of £640 000 over 6 months) by applying RPM |
| Shah et al (2020)54 | 77 | Adults aged ≥18 years with confirmed COVID-19; median age of 44 years (IQR 25–63); 45 patients (53%) had comorbidities | Monitoring tool: pulse oximeter; follow-up for 7 days; the research team called the patients once per day to collect data | Self-measured three times a day (0600 h, 1400 h, 2200 h); reported via a daily follow-up call | 19 patients (25%) had SpO2 readings of <92%, of whom 16 were hospitalised; at-rest SpO2<92% was significantly associated with hospitalisation (relative risk 7 [95% CI 3·4–14·5]; p<0·0001) and with admission to an intensive care unit (9·8 [2·2–44·6]; p<0·002), but not with mortality, compared with patients with SpO2 >92%; the median time to hospitalisation was 6 (IQR 4–8) days |
| Wilcock et al (2021)55 | 41 | Adult patients with confirmed COVID-19; mean (SD) age of 45·9 (8·7) years; the Charlson Comorbidity Index score was low among overall participants: 1·2 (0·9) | Monitoring tools: pulse oximeter and a symptom diary; follow-up for 14 days; measurements were self-recorded by patients and collected by the research team at the end of the study period | Self-measured and reported twice per day, separated by 12 h intervals | Only 10 participants completed the full 14-day diary; the mean (SD) number of completed follow-up days for all participants was 10·3 (1·4); a total of nine patients (22%) reported SpO2 readings of <94%, of whom three patients (7%) had SpO2 readings of <92% and were admitted to hospital |
Follow-up refers to follow-up visits with patients, either online, by video consultation, by telephone call, or through in-person visits. RPM=remote patient monitoring. SpO2=peripheral blood oxygen saturation. SaO2=arterial oxygen saturation.
Effectiveness and impact of RPM with pulse oximetry on safety outcomes
Our systematic review could not identify clear evidence for the effects of RPM with pulse oximetry on the health outcomes of patients. However, monitoring SpO2 at home was a safety net to enable identification of an early sign of deterioration: a decrease in SpO2.48, 53, 54 For instance, in one study,54 an incidental reading of low SpO2 without worsening of any other symptoms was the only reason that 50% of patients who were ultimately hospitalised returned to the emergency department.
Monitoring SpO2 helped to triage patients with COVID-19 remotely and guided care escalation. Nunan and colleagues53 considered patients to be stable if their at-rest SpO2 was greater than 94% and their decrease in SpO2 after a rapid walking test (postexertional) was 5% or less; otherwise, patients were advised to call emergency services. Shah and colleagues54 found that patients with an at-rest SpO2 of less than 92% were more likely to need hospitalisation (risk ratio 7; 95% CI 3·4–14·5; p<0·0001) than were patients with higher SpO2.
Remote monitoring reduced unnecessary contact of health-care professionals with patients with COVID-19, which could control the risk of infection transmission and enable resources to be redirected to those who need them the most.47, 48 For instance, Hutchings and colleagues48 conducted 1902 (66·4%) of 2865 consultations by video call and 688 (24·0%) by telephone. Only five of the 162 participants in this study needed in-person assessments, of whom three were admitted to hospital.48 From a cost-effectiveness perspective, Nunan and colleagues53 estimated a predictive cost avoidance of £640 000 over six months for their 279 participants by using RPM.
We identified recommendations that could help to set up successful RPM systems using pulse oximetry. These recommendations are presented in the Discussion and are in alignment with the included studies and other relevant literature.
Discussion
Our systematic review has shown that the use of pulse oximetry as a monitoring tool for patients at home with COVID-19 helped to triage patients on the basis of their SpO2 concentrations, detect the risk of deterioration, and promote patient safety. The data were insufficient to assess the effect on other proxies of effectiveness (eg, health outcomes) and dimensions of care quality compared with other care models.
Pulse oximeters were used alone or as part of remote monitoring packages. The models varied in terms of the frequency of measurement, reporting methods, and monitoring periods (12·7 days on average). The use of pulse oximetry for monitoring appeared safe and seemed applicable to different patient groups in terms of demographics, disease severity, and existence of comorbidities. Cost-effectiveness was reported and confirmed in only one study.53
There was no consensus on the SpO2 threshold at which to escalate care, or a common pathway by which to do so. At-rest SpO2 of 92% or less was considered critical in many models.44, 46, 54, 55 Relying on video consultations, telephone calls, or face-to-face visits to follow up with patients was decided on a case-by-case basis in each study. The effect on the use of health-care resources was not conclusive, because most of the studies did not include control groups to compare.
We identified recommendations for setting up an RPM system on the basis of our systematic review of the included studies and other relevant literature. The recommendations are distributed across the three domains of the Donabedian model (panel ).
Panel. Recommendations for setting up and evaluating outcomes in an at-home remote patient monitoring (RPM) system for COVID-19 patients using pulse oximetry.
Structure
Infrastructure preparedness and technology development needed for monitoring
-
•
Build an online portal, mobile app, or a monitoring platform to monitor peripheral blood oxygen saturation (SpO2) readings and link them with patient health records readings.1, 2, 3, 6, 9 The readings were self-recorded automatically via a smart system, or self-reported by patients via follow-up calls.
Providing sufficient human resources for monitoring patients (staff-to-patient ratio)
-
•
To be adjusted on the basis of severity of illness and the expected risk of deterioration among monitored patients. For instance, in the study by Kodama and colleagues,50 there was one nurse per 50 patients per shift (and for the programme in general), and in the study by Hutchings and colleagues,48 there were 25 patients per nurse per shift.
Safe delivery of pulse oximetry to the participating patients
-
•
To plan for safe delivery of pulse oximetry to patients within a convenient period of time from enrolment.5, 7, 9
Patient education on self-using pulse oximetry
-
•
To train patients before starting the programme on how to self-measure their SpO2 by pulse oximetry. This training can be via educational videos,8, 9 online calls,4 or written guidance.5 All patients should be trained well in SpO2 measurement to ensure the accuracy and effectiveness of the system, and to ensure patient safety.7, 10
Process
Continuation of monitoring for a sufficient period of time to detect deterioration
-
•
Monitoring periods varied between different models, ranging from 5 days11 up to 30 days.3 The average period of actual monitoring among all the included studies in our review was 12·7 days, and this is suggested as the minimum when developing a monitoring programme.
Standardising the method and frequency of measuring and recording SpO 2
-
•
For each remote patient monitoring (RPM) model, patients should assess their at-rest SpO2 after sitting or resting for 5–10 min, as is standard.3, 8 Postexertional SpO2 should also be monitored and measured after ambulating for 20–60 s according to Blair and colleagues46 and Kodama and colleagues.50 The frequency of SpO2 assessment varied from once per day12 to up to four times per day13 in some models.4, 5, 12
Ensuring patient safety by offering a 24/7 emergency line for participants
-
•
Participating patients should have access to a 24/7 (ie, 24 h every day) emergency telephone line dedicated to their concerns, in addition to regular medical emergency lines.4, 11, 14
Outcomes
Percentage of patients who needed escalated care after reporting a critical SpO 2 concentration
-
•
An SpO2 threshold should be predetermined to identify and escalate care for patients who might be at risk. It was set as an at-rest SpO2 of 92% or less in most models,5, 6, 8, 12 although some models set an alarm for attention when SpO2 reaches 94% or for additional safety.5 For postexertional SpO2, this threshold was set as a decrease of SpO2 of more than 3% after exertion,12 and a decrease of more than 5% in other models.11, 16
Number of online consultations needed per patient during the monitoring period
-
•
In non-emergency situations, online consultations should be considered as a first option for clinical assessment. These consultations can be escalated to a face-to-face visit or a hospital referral on a case-by-case basis.12, 17, 18
Percentage of patients who needed hospital referrals
-
•
Consider the effect of RPM systems on the health outcomes of participating patients. Future researchers and policy makers should include the percentage of patients who needed hopsital referrals as one of the outcome measures to assess the effectiveness of an RPM programme.
Median days to hospitalisation among the patients
-
•
The median days to hospitalisation must be reported to assess the effect on patients’ quality of life and their use of health-care services. In the study by Shah and colleagues,54 this value was reported as 6 days (IQR 4–8).
RPM models were designed considering various factors, such as target population characteristics, available resources, and the surrounding environment.40, 42, 48, 50, 56 We identified three measures that were highlighted across the models we reviewed. These measures can help to standardise some model criteria when pulse oximetry is used to monitor patients with COVID-19.
Firstly, both at-rest and postexertional SpO2 should be monitored in patients with COVID-19. Although most models monitored only at-rest SpO2, evidence has shown that patients with COVID-19 usually have an abrupt, not a gradual, decrease in SpO2, which could be detected early by assessing postexertional SpO2.45, 54 The safety of conducting exertional desaturation tests on patients with COVID-19 needs further assessment.57 So far, evidence has shown the safety of self-conducting exertional tests only in patients with an at-rest SpO2 of at least 96%; otherwise, medical supervision in a prepared health-care facility is needed.57
Secondly, an SpO2 of 92% or less should be considered as a minimum, preset threshold to indicate care escalation in patients with COVID-19.44, 46, 54, 55 Some models considered a higher threshold (SpO2 ≤94%), adding an extra layer of safety.52, 55 For postexertional SpO2, a decrease of 5% or more should be considered critical and indicate care escalation.36, 53 Some evidence put the threshold as a decrease of 3% or more if patients were vulnerable with multimorbidity.57 These figures might differ in some clinical conditions, such as patients with chronic obstructive pulmonary disease.53
Finally, the method by which SpO2 is self-assessed should be standardised. For example, at-rest SpO2 should be assessed after 5–10 min of rest (further recommendations to ensure accuracy of this method are below).11 Postexertional SpO2 should be measured after conducting the 1-min sit-to-stand test, which has been validated in the literature with reasonable sensitivity (88%), specificity (81%), and negative predictive value (89%).58 Other tests could be considered, such as a 6-min walk test or a 40-step walk;46, 53, 58 however, further research is needed to validate these tests.57
To our knowledge, this is the first systematic review of evidence on the use of pulse oximetry as an affordable and widely available tool for monitoring patients with COVID-19 in non-hospital settings. In 2021, a systematic review was conducted by Vindrola-Padros and colleagues42 on the adoption of general home-monitoring systems for patients with COVID-19. Their review highlighted the importance of properly training patients and supporting their involvement as a determinant of success for any home-monitoring system. Based on the findings of our systematic review, we agree with these points.36, 40, 41, 42
The use of pulse oximetry in RPM could help to alleviate the pressure on health systems during the COVID-19 pandemic; however, the risk of digital health inequalities should not be overlooked.59 Social and cultural aspects—such as technology literacy and accessibility, and financial hardship—could undermine the effect of RPM and restrain its uptake and use.9 Further qualitative research is needed to explore the potential exclusion of disadvantaged populations who might not be able to benefit from digital health services, to provide tailored care according to the patients’ needs and available resources, and to provide support to ensure health equity. The studies included in our systematic review did not highlight the inclusion of racially and ethnically diverse populations, and the number of participants was quite low in some studies. Therefore, it is recommended that trials of RPM with pulse oximetry are conducted among diverse populations.
Different models proposed different staff-to-patient ratios to support and ensure the safety of the monitored patients. A ratio of one nurse to 50 patients was reported by Kodama and colleagues,50 whereas Hutchings and colleagues48 reported a ratio of 1:25 per shift (and in the overall programme). Another model in the literature had one physician per 29 at-risk patients, defined as being older than 75 years, having a body-mass index of greater than 30 kg/m2, and having a history of chronic lung disease.40 Few studies have discussed an explicit rationale or criteria for the appropriate staff-to-patient ratio needed for RPM.56 It is mainly considered to be adjustable depending on the characteristics of the patients, the severity of illness, the monitoring technology and tools, and staff proficiency.40, 42, 48, 50, 56
The studies in the literature overall highlighted the availability, affordability, and accessibility of the monitoring tools as determinants to ensure the effectiveness of any RPM model.25, 41, 60 Some studies tested smartphone oximeters as a potential widely available option, and found clinically insignificant differences in readings compared with traditional pulse oximeters; however, further research is needed to test the validity and reliability of different types and brands.61 Researchers have developed software and mobile apps to link SpO2 measurements with patient data as a superior reporting and recording method to paper-based systems.62, 63, 64 Such a method would also boost staff capacity—a trained health-care professional would be able to monitor more patients by enabling digital care models than by use of traditional methods.50, 65
The assessment of cost-effectiveness was scarce in the literature. An analysis by Crawford and colleagues66 showed that the use of pulse oximetry to monitor moderate-to-severely ill patients at home with COVID-19 resulted in a better cost–utility outcome and increased number of quality-adjusted life-years than the standard care. There is a need for a comprehensive cost analysis of RPM models that use pulse oximetry, including the cost of oximeters, staffing, and monitoring operations, and the time consumed by professionals to maintain the system.
Few studies have assessed the effect of monitoring patients with pulse oximeters on health outcomes and the use of health-care services.37, 67 A prospective trial estimated an almost 50% reduction in unnecessary emergency and hospital readmissions among participants.44 In a study by Shah and colleagues,54 33% of participants stated that they would be more likely to visit hospitals, at least for reassurance, if they were not monitored remotely.
Various approaches for the measurement of SpO2 were noted among the different models. Some studies relied only on at-rest SpO2,14, 44, 47, 48 whereas other studies considered the change in postexertional SpO2 as an indicator of deterioration.12, 36, 46, 53, 58, 68 For at-rest SpO2, studies recommended resting for 10 min before initiating the measurement,46 observing the reading for 30–60 s to get the most accurate result, avoiding moving the finger to which the oximeter is attached, measuring the SpO2 through the index or middle finger and avoiding toes or ear lobes, removing nail polish before starting the measurement, and warming cold extremities.11 For postexertional SpO2, various tests were used: the measurements were recorded after a 1-min sit-to-stand test,12, 58 a 6-min walk,58 a 40-step walk,53 a 30-m walk,53 or exercise for 30–60 s.46 Further research might help to identify the best approach to get the most accurate, standardised, and reliable measurements of at-rest and postexertional SpO2.
We applied a rigorous set of inclusion and exclusion criteria for screening the search results. Since November, 2020, our team has been conducting a living horizon scanning of literature on the use of pulse oximetry for monitoring patients at home with COVID-19, as part of the COVID Oximetry @home programme.14, 15 The horizon scanning is updated weekly, keeping the team updated with any evolving evidence. The screening, data extraction, and quality assessment were each conducted by two authors, who had a good agreement at all stages as assessed by Cohen's κ score.22 We also present recommendations for implementing an effective RPM model with pulse oximetry based on the models applied in the included studies and in other relevant literature.
We included peer-reviewed publications and preprints in our search to ensure that all available evidence was considered. The WHO Working Group on Ethics and COVID-1969 proposed an ethical obligation for researchers to share their relevant research findings as soon as they become available, without waiting for peer review, to support the public health emergency response with evolving evidence. We acknowledged this and applied extra care in reviewing the search results to ensure the validity and accuracy of publications that had not yet undergone peer review. The final studies in our review included only two preprints, one of which70 was subsequently peer-reviewed and was published on Sept 14, 2021.
The included studies did not explicitly describe the effect of RPM with pulse oximetry on health outcomes compared with other models of care, due to relatively short monitoring periods and the absence of control groups in most of the studies. The accessibility, acceptability, and safety of using RPM models with pulsed oximetry in different populations with varied sociocultural characteristics need further research to better understand the potential risk of health inequities. Quantitative metrics cannot solely verify the success of the RPM model, but further qualitative research is needed regarding how users feel about remote-monitoring technologies, to ensure equity and effectiveness. A meta-analysis was not feasible owing to the heterogeneity of the outcomes of the included studies. Most of the studies had no control groups; as such, the data were insufficient to assess the impact of RPM with pulse oximetry and its effectiveness compared with other monitoring models. Preferences for systems through which SpO2 readings are reported were inconsistent between the studies. A similar inconsistency was observed when identifying an SpO2 threshold at which to escalate care. Further research is needed to standardise these measures to ensure best practice.
Conclusion
The COVID-19 pandemic has placed RPM as a leading interest in public health research. Given the knowledge to date about COVID-19, pulse oximetry is potentially an effective tool for monitoring deterioration and keeping patients safe at home. The model was deemed safe for application and use in some different contexts among different populations. Research into the cost-effectiveness of RPM with pulse oximetry is scarce at present, and available data about its effect on the use of health-care services are inconclusive. Further research is needed to inform the future implementation of pulse oximetry in monitoring patients with COVID-19. This research should involve more diverse populations, test the system in resource-limited settings, and assess the effect on health outcomes compared with other systems.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
This work was supported by the UK National Institute for Health Research (NIHR) under the Applied Health Research programme, Imperial Patient Safety Translational Research Centre. We acknowledge support from the Imperial NIHR Biomedical Research Centre.
Acknowledgments
Contributors
All authors contributed to the conceptualisation of the study and the protocol development. AA and TB initially screened the data and assessed quality, and PA provided senior advice. AA, TB, JMC, PA, and A-LN participated in data curation and analysis. SE, PA, AD, and A-LN advised on data validation and result presentation. AA drafted the initial full manuscript. TB, SE, JMC, AD, PA, and A-LN reviewed and provided feedback on the manuscript. All authors approved the final version of the manuscript.
Supplementary Material
References
- 1.Alotaibi YK, Federico F. The impact of health information technology on patient safety. Saudi Med J. 2017;38:1173–1180. doi: 10.15537/smj.2017.12.20631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sen-Crowe B, Sutherland M, McKenney M, Elkbuli A. A closer look into global hospital beds capacity and resource shortages during the COVID-19 pandemic. J Surg Res. 2021;260:56–63. doi: 10.1016/j.jss.2020.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iyengar K, Mabrouk A, Jain VK, Venkatesan A, Vaishya R. Learning opportunities from COVID-19 and future effects on health care system. Diabetes Metab Syndr. 2020;14:943–946. doi: 10.1016/j.dsx.2020.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rangachari P, Woods JL. Preserving organizational resilience, patient safety, and staff retention during COVID-19 requires a holistic consideration of the psychological safety of healthcare workers. Int J Environ Res Public Health. 2020;17 doi: 10.3390/ijerph17124267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taiwo O, Ezugwu AE. Smart healthcare support for remote patient monitoring during COVID-19 quarantine. Inform Med Unlocked. 2020;20 doi: 10.1016/j.imu.2020.100428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goetz LH, Schork NJ. Personalized medicine: motivation, challenges, and progress. Fertil Steril. 2018;109:952–963. doi: 10.1016/j.fertnstert.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torjesen I. COVID-19: patients to use pulse oximetry at home to spot deterioration. BMJ. 2020;371 [Google Scholar]
- 8.Shenoy N, Luchtel R, Gulani P. Considerations for target oxygen saturation in COVID-19 patients: are we under-shooting? BMC Med. 2020;18:260. doi: 10.1186/s12916-020-01735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohammadzadeh N, Safdari R. Patient monitoring in mobile health: opportunities and challenges. Med Arh. 2014;68:57–60. doi: 10.5455/medarh.2014.68.57-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noah B, Keller MS, Mosadeghi S, et al. Impact of remote patient monitoring on clinical outcomes: an updated meta-analysis of randomized controlled trials. NPJ Digit Med. 2018;1 doi: 10.1038/s41746-017-0002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luks AM, Swenson ER. Pulse oximetry for monitoring patients with COVID-19 at home: potential pitfalls and practical guidance. Ann Am Thorac Soc. 2020;17:1040–1046. doi: 10.1513/AnnalsATS.202005-418FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quaresima V, Ferrari M. More on pulse oximetry for monitoring patients with COVID-19 at home. Ann Am Thorac Soc. 2020;17 doi: 10.1513/AnnalsATS.202006-701LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jouffroy R, Jost D, Prunet B. Prehospital pulse oximetry: a red flag for early detection of silent hypoxemia in COVID-19 patients. Crit Care. 2020;24:313. doi: 10.1186/s13054-020-03036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke J, Flott K, Crespo R, et al. Assessing the safety of home oximetry for COVID-19: a multi-site retrospective observational study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-049235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Health Service COVID Oximetry @home. https://www.england.nhs.uk/nhs-at-home/covid-oximetry-at-home/
- 16.Plüddemann A, Thompson M, Heneghan C, Price C. Pulse oximetry in primary care: primary care diagnostic technology update. Br J Gen Pract. 2011;61:358–359. doi: 10.3399/bjgp11X572553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buekers J, Theunis J, De Boever P, et al. Wearable finger pulse oximetry for continuous oxygen saturation measurements during daily home routines of patients with chronic obstructive pulmonary disease (COPD) over one week: observational study. JMIR Mhealth Uhealth. 2019;7 doi: 10.2196/12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald KM, Sundaram V, Bravata DM, et al. In: Closing the quality gap: a critical analysis of quality improvement strategies. Technical Review 9. Shojania KG, McDonald KM, Wachter RM, Owens DK, editors. vol 7. Agency for Healthcare Research and Quality; Rockville, MD: 2007. Care coordination. [Google Scholar]
- 19.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339 [PMC free article] [PubMed] [Google Scholar]
- 21.Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14:579. doi: 10.1186/s12913-014-0579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med. 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]
- 23.Wells GA, Shea B, O'Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Ottawa Hospital Research Institute. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 24.Gaeta T, Chiricolo G, Mendoza C, et al. Impact of a novel telehealth follow-up protocol for at-risk emergency department patients discharged with presumptive or confirmed COVID-19. Ann Emerg Med. 2020;76(suppl):S49. (abstr). [Google Scholar]
- 25.Greenhalgh T, Knight M, Inda-Kim M, Fulop NJ, Leach J, Vindrola-Padros C. Remote management of COVID-19 using home pulse oximetry and virtual ward support. BMJ. 2021;372:n677. doi: 10.1136/bmj.n677. [DOI] [PubMed] [Google Scholar]
- 26.Greenwald P, Telehealth Working Group. Olsen E, et al. Telemedicine response to COVID-19 surge in New York City: how emergency department telemedicine changed with the curve. Ann Emerg Med. 2020;76(suppl):S78–S79. (abstr). [Google Scholar]
- 27.Gugliandolo P, Mattavelli I, Contini M, et al. Feasibility of remote home monitoring with a t-shirt wearable device in post-recovery COVID-19 patients. G Ital Cardiol. 2020;21(suppl 2):e86–e87. doi: 10.2459/JCM.0000000000001165. [DOI] [PubMed] [Google Scholar]
- 28.Heravian A, Olsen E, Kessler D, Chang B. Virtual powers of observation: a telemedicine pathway for the suspected COVID-19 patient. Ann Emerg Med. 2020;76(suppl):S80. doi: 10.5055/jem.0528. (abstr). [DOI] [PubMed] [Google Scholar]
- 29.Kyriakides J, Khani A, Kelly C, Coleman R. Analysis of an ambulatory care pathway for patients with COVID-19 utilising remote pulse oximetry. Emerg Med J. 2020;37:843. doi: 10.7861/clinmed.21-2-s48. (abstr). [DOI] [PubMed] [Google Scholar]
- 30.Maghrabi F, Bazaz R, Wilson E, et al. The development and implementation of a virtual discharge ward for patients with covid-19 pneumonia: data on the first 300 patients. Thorax. 2021;76(suppl 1):A35–A36. [Google Scholar]
- 31.Michard F, Shelley K, L'Her E. COVID-19: pulse oximeters in the spotlight. J Clin Monit Comput. 2021;35:11–14. doi: 10.1007/s10877-020-00550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patni B. A telemedicine approach for assessment, empowerment and triage for COVID-19 patients with comorbidities. Metabolism. 2021;116(suppl) [Google Scholar]
- 33.Rodriguez C. Using pulse oximetry to monitor high-risk patients with COVID-19 at home. Nursing. 2020;50:15–16. doi: 10.1097/01.NURSE.0000718376.94916.eb. [DOI] [PubMed] [Google Scholar]
- 34.Vinton D, Thomson N. Interactive home monitoring of ED patients with suspected or confirmed COVID-19. Ann Emerg Med. 2020;76(suppl):S21. (abstr). [Google Scholar]
- 35.Agarwal P, Mukerji G, Laur C, et al. Adoption, feasibility and safety of a family medicine-led remote monitoring program for patients with COVID-19: a descriptive study. CMAJ Open. 2021;9:E324–E330. doi: 10.9778/cmajo.20200174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banzi R, Sala L, Colmi A, et al. Feasibility and efficacy of home monitoring for patients with suspected or confirmed COVID-19. Recenti Prog Med. 2020;111:584–592. doi: 10.1701/3453.34418. (in Italian). [DOI] [PubMed] [Google Scholar]
- 37.Bell LC, Norris-Grey C, Luintel A, et al. Implementation and evaluation of a COVID-19 rapid follow-up service for patients discharged from the emergency department. Clin Med. 2021;21:e57–e62. doi: 10.7861/clinmed.2020-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hibberd J, Carter J, McCoy M, et al. General practice in the time of COVID-19: a mixed-methods service evaluation of a primary care COVID-19 service. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18062895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franzosa E, Gorbenko K, Brody AA, et al. “At Home, with Care”: lessons from New York City home-based primary care practices managing COVID-19. J Am Geriatr Soc. 2021;69:300–306. doi: 10.1111/jgs.16952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw JG, Sankineni S, Olaleye CA, et al. A novel large scale integrated telemonitoring program for COVID-19. Telemed J E Health. 2021;27:1317–1321. doi: 10.1089/tmj.2020.0384. [DOI] [PubMed] [Google Scholar]
- 41.Vindrola-Padros C, Sidhu MS, Georghiou T, et al. The implementation of remote home monitoring models during the COVID-19 pandemic in England. EClinicalMedicine. 2021;34 doi: 10.1016/j.eclinm.2021.100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vindrola-Padros C, Singh KE, Sidhu MS, et al. Remote home monitoring (virtual wards) for confirmed or suspected COVID-19 patients: a rapid systematic review. EClinicalMedicine. 2021;37 doi: 10.1016/j.eclinm.2021.100965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D'Amato G, Acanfora L, Delli Paoli L, D'Amato M. Preventive home therapy for symptomatic patients affected by COVID-19 and followed by teleconsultations. Multidiscip Respir Med. 2021;16:748. doi: 10.4081/mrm.2021.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon WJ, Henderson D, DeSharone A, et al. Remote patient monitoring program for hospital discharged COVID-19 patients. Appl Clin Inform. 2020;11:792–801. doi: 10.1055/s-0040-1721039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Motta LP, Silva PPFD, Borguezan BM, et al. An emergency system for monitoring pulse oximetry, peak expiratory flow, and body temperature of patients with COVID-19 at home: development and preliminary application. PLoS One. 2021;16 doi: 10.1371/journal.pone.0247635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blair PW, Brown DM, Jang M, et al. The clinical course of COVID-19 in the outpatient setting: a prospective cohort study. Open Forum Infect Dis. 2021;8 doi: 10.1093/ofid/ofab007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ford D, Harvey JB, McElligott J, et al. Leveraging health system telehealth and informatics infrastructure to create a continuum of services for COVID-19 screening, testing, and treatment. J Am Med Inform Assoc. 2020;27:1871–1877. doi: 10.1093/jamia/ocaa157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hutchings OR, Dearing C, Jagers D, et al. Virtual health care for community management of patients with COVID-19 in Australia: observational cohort study. J Med Internet Res. 2021;23 doi: 10.2196/21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ko SQ, Hooi BMY, Koo C-Y, et al. Remote monitoring of marginalised populations affected by COVID-19: a retrospective review. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-042647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kodama R, Arora S, Anand S, et al. Reengineering the discharge transition process of COVID-19 patients using telemedicine, remote patient monitoring, and around-the-clock remote patient monitoring from the emergency department and inpatient units. Telemed J E Health. 2021;27:1188–1193. doi: 10.1089/tmj.2020.0459. [DOI] [PubMed] [Google Scholar]
- 51.Krenitsky NM, Spiegelman J, Sutton D, Syeda S, Moroz L. Primed for a pandemic: implementation of telehealth outpatient monitoring for women with mild COVID-19. Semin Perinatol. 2020;44 doi: 10.1016/j.semperi.2020.151285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kyriakides J, Khani A, Coleman R, Kelly C. Analysis of an ambulatory care pathway for patients with COVID-19 utilising remote pulse oximetry at a London District General Hospital. Cureus. 2021;13 doi: 10.7759/cureus.12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nunan J, Clarke D, Malakouti A, et al. Triage into the community for COVID-19 (TICC-19) patients pathway—service evaluation of the virtual monitoring of patients with COVID pneumonia. Acute Med. 2020;19:183–191. [PubMed] [Google Scholar]
- 54.Shah S, Majmudar K, Stein A, et al. Novel use of home pulse oximetry monitoring in COVID-19 patients discharged from the emergency department identifies need for hospitalization. Acad Emerg Med. 2020;27:681–692. doi: 10.1111/acem.14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilcock J, Grafton-Clarke C, Coulson T. What is the value of community oximetry monitoring in people with SARS-CoV-2? A prospective, open-label clinical study. medRxiv. 2021 doi: 10.1101/2021.01.03.21249168. published Jan 4. (preprint). [DOI] [Google Scholar]
- 56.Davis MM, Freeman M, Kaye J, Vuckovic N, Buckley DI. A systematic review of clinician and staff views on the acceptability of incorporating remote monitoring technology into primary care. Telemed J E Health. 2014;20:428–438. doi: 10.1089/tmj.2013.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalin A, Javid B, Knight M, Inada-Kim M, Greenhalgh T. Direct and indirect evidence of efficacy and safety of rapid exercise tests for exertional desaturation in COVID-19: a rapid systematic review. Syst Rev. 2021;10:77. doi: 10.1186/s13643-021-01620-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalin A, Javid B, Knight M, Inada-Kim M, Greenhalgh T. What is the efficacy and safety of rapid exercise tests for exertional desaturation in COVID-19: a rapid review protocol. medRxiv. 2020 doi: 10.1101/2020.10.31.20223453. published online Nov 4. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crawford A, Serhal E. Digital health equity and COVID-19: the innovation curve cannot reinforce the social gradient of health. J Med Internet Res. 2020;22 doi: 10.2196/19361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galbraith M, Kelso P, Levine M, Wasserman RC, Sikka J, Read JS. Addressing silent hypoxemia with COVID-19: implementation of an outpatient pulse oximetry program in Vermont. Public Health Pract. 2021;2 doi: 10.1016/j.puhip.2021.100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Browne SH, Bernstein M, Pan SC, et al. Smartphone biosensor with app meets FDA/ISO standards for clinical pulse oximetry and can be reliably used by a wide range of patients. Chest. 2021;159:724–732. doi: 10.1016/j.chest.2020.08.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dutch M, Knott J. In response to “The Novel Use of Home Pulse Oximetry”: an Australian offer of support. Acad Emerg Med. 2020;27:792. doi: 10.1111/acem.14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Michard F, Shelley K, L'Her E. COVID-19: pulse oximeters in the spotlight. J Clin Monit Comput. 2021;35:11–14. doi: 10.1007/s10877-020-00550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O'Carroll O, MacCann R, O'Reilly A, et al. Remote monitoring of oxygen saturation in individuals with COVID-19 pneumonia. Eur Respir J. 2020;56 doi: 10.1183/13993003.01492-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Young HM, Nesbitt TS. Increasing the capacity of primary care through enabling technology. J Gen Intern Med. 2017;32:398–403. doi: 10.1007/s11606-016-3952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crawford S, Kelley M, Choy B, et al. The cost-utility of remote pulse-oximeter monitoring of COVID-19 patients. Value Health. 2021;24(suppl 1):S109. doi: 10.1016/j.jval.2021.09.008. (abstr). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tabacof L, Wood J, Mohammadi N, et al. Remote patient monitoring identifies the need for triage in patients with acute covid-19 infection. Telemed J E Health. 2021 doi: 10.1089/tmj.2021.0101. published online July 22. [DOI] [PubMed] [Google Scholar]
- 68.Izmailova ES, Reiss TF. Closer to the patient means better decisions: wearable remote monitoring of patients with COVID-19 lung disease. Clin Transl Sci. 2021;14:2091–2094. doi: 10.1111/cts.13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.WHO Working Group on Ethics and COVID-19 Ethical standards for research during public health emergencies: distilling existing guidance to support COVID-19 R&D. March 29, 2020. https://www.who.int/publications/i/item/WHO-RFH-20.1
- 70.Clarke J, Flott K, Fernandez Crespo R, et al. Assessing the safety of home oximetry for COVID-19: a multisite retrospective observational study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-049235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.