Abstract
Novel COVID-19 is a public health emergency that poses a serious threat to people worldwide. Given the virus spreading so quickly, novel antiviral medications are desperately needed. Repurposing existing drugs is the first strategy. Anti-parasitic drugs were among the first to be considered as a potential treatment option for this disease. Even though many papers have discussed the efficacy of various anti-parasitic drugs in treating COVID-19 separately, so far, no single study comprehensively discussed these drugs. This study reviews some anti-parasitic recommended drugs to treat COVID-19, in terms of function and in vitro as well as clinical results. Finally, we briefly review the advanced techniques, such as artificial intelligence, that have been used to find effective drugs for the treatment of COVID-19.
Keywords: : anti-parasitic drugs, artificial intelligence, COVID-19, drug repositioning, epidemic, pandemic, SARS-CoV-2
Over the past 20 years, the world encountered two coronavirus-related epidemics: severe acute respiratory syndrome (SARS), which emerged in China at the end of 2003, and Middle East respiratory syndrome (MERS), which was first reported in Saudi Arabia in 2012. At the end of December 2019, the novel SARS-CoV-2 first appeared in Wuhan, Hubei Province, China, and rapidly spread to around the globe in a few weeks. On 30 January 2020, COVID-19, the disease caused by this virus, was announced a pandemic by the WHO [1]. Similar to previous pathogenic coronaviruses, SARS-CoV-2 causes pneumonia and severe respiratory syndrome [2,3].
The COVID-19 outbreak, the second global pandemic after MERS, has had a devastating impact on not just the healthcare system but also the global economy [4,5]. SARS-CoV-2 is an enveloped and positive-stranded RNA virus transmitted from humans to humans by exposure to respiratory droplets containing viral particles or touching contaminated surfaces. Because of the high inter-transmission rate among humans, physical distancing, wearing a face mask and following health instructions are the best-recommended ways to stop the spread of this disease [6].
During the second year of this pandemic, new variants of the SARS-CoV-2, including Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Kappa, Delta (B.1.617.1 and B.1.617.2) and Delta Plus (AY. 1) were identified and reported across the world. These new variants are named ‘variants of concern’ (VOCs), which have caused global health havoc [7,8]. The Delta variant has attracted significant attention due to a faster transmission rate, especially among children; being more contagious than other variants; causing a more severe illness in patients; and being less responsive to treatment [9]. Despite the resistance of emerging Delta variants to monoclonal antibody cocktail treatments, antibody therapy could still be a possible therapeutic way to control Delta and other VOCs [10]. In this regard, a study conducted in Vero and BEAS-2B cell lines indicated that meplazeumab, a humanized anti-CD147 antibody, could block the cellular entry of VOCs, with noticeable inhibition rates [11]. In addition, sotrovimab appears to maintain its activity against all VOCs [12]. S1 receptor-binding domain (RBD)-targeted therapy, endosomal formation prevention and VOCs genome interruption have been proposed as potential treatment options against the new SARS-CoV-2 VOCs. However, recent findings regarding the use of monoclonal antibodies, convalescent serum and vaccines have raised concerns about using these agents for future variants [13]. These have prompted scientists to look for other safe and secure treatment strategies for COVID-19.
‘Herd immunity’ to COVID-19 has recently become a highly debated topic. When a large portion of a community develops ‘natural immunity’ to an infectious disease the term ‘herd immunity’ is used, however, achieving herd immunity for 50–66% of the population takes a long time, may result in many avoidable deaths and causes irreversible damage to the health system [13–15]. In contrast, immune-modulatory drugs and vaccination quickly achieve protective immunity against the infectious agent without devastating side effects. Accordingly, positive steps were taken by some companies to manufacture effective vaccines, some of which are now licensed for emergency use.
Although there is no effective treatment to combat SARS-CoV-2 infection, many efforts were made to reuse existing therapeutic agents, particularly anti-parasitic ones. Recent findings suggest that the anthelmintic agent albendazole appears to have a protective effect in COVID-19 patients with hydatid cysts caused by the tapeworm Echinococcus granolusus [16]. These intriguing results prompted us to review a few anti-parasitic drugs to evaluate their effectiveness against SARS-CoV-2. In addition, at the end of this review, we add brief descriptions about the application of new technologies such as artificial intelligence (AI) for developing new effective therapies against COVID-19. As far as we know, this is the first comprehensive review article that exclusively addresses anti-parasitic agents as potential therapies against COVID-19.
Genome organization of SARS-CoV-2
The genomic sequences of SARS-CoV-2 and two bat coronaviruses, SLCoVZC45 and SL-CoVZXC21 (89–96.3% similarity), as well as human SARS-CoV (79–82%), are strikingly similar [17]. SARS-CoV-2 genome consists of 30 kb RNA that is protected at both ends by unique structures, including a 5’-cap and a 3’poly-A tail [18]. The novel coronavirus genome contains 14 open-reading frames (ORFs), which encode structural and nonstructural proteins. Out of them, 16 nonstructural proteins (nsp1–nsp16) are organized into two gene loci called ORF1a and ORF1b, which comprise 67% of the genome. They are located at the 5’ end of the genome and collectively mediate virus replication and possibly immune system evasion [19,20]. Furthermore, structural and accessory proteins are encoded by the remaining ORFs, which are located at the 3’ terminus of the genome [21]. There are four main structural proteins, including spike glycoprotein (S), envelope protein (E), membrane protein (M) and nucleocapsid protein (N). Two main subunits of spike surface glycoprotein, S1 and S2, are involved in the fusion of viral and host cellular membranes [22]. Therefore, a few studies indicated that the mutations in SARS-CoV-2 genome that include S, nsp-1, nsp-3 and nsp-15 may affect the virus’s interaction with the host [23,24].
COVID-19 potential therapeutic strategies
To control the COVID-19 pandemic, considerable efforts were made to find suitable drugs [25]. Based on the virus’s life cycle, which includes assembly, budding and envelope formation, as well as pathogenesis [26], potential therapeutic strategies can be classified into the following categories: Blocking host–virus interaction using monoclonal antibodies or chemical agents that inhibit the virus from binding to host receptors [27], Blocking virus entry to host cells through inhibition of the clathrin-mediated endocytosis process [28–30], Neutralizing viral particles by disrupting viral enzyme activity and critical functional proteins involved in viral replication and multiplication [31–33] and Targeting viral structural proteins, including membrane, envelope and nucleocapsid proteins [34–36].
The first step of virus entry to host cells is the binding of the RBD of the viral S protein (S1) to angiotensin-converting enzyme 2 (ACE2) receptors (Figure 1), which is abundant in humans [4]. The viral spike glycoproteins are activated when they are cleaved by a serine protease. This facilitates virus–host cell membrane fusion necessary for virus entry, replication and distribution. Virus entry depends on the host’s trans-membrane serine protease, TMPRSS2 [37], which is proposed as a potential target for antiviral drug design. Therefore, the interaction between ACE2 and S protein can be targeted as an effective treatment strategy to combat the novel coronavirus [38]. In addition to the ACE2 receptor, host cellular serine protease is involved in facilitating the viral entry to host cells [39]; hence, TMPRSS2 inhibitors can significantly reduce infection as shown in cell lines with human lung origin (second strategy; Figure 2) [40–42]. A third strategy, potential antiviral drugs such as remdesivir can inhibit viral RNA-dependent RNA polymerase enzyme by being incorporated into the nascent viral RNA strands, which in turn causes premature transcription termination [43,44]. A fourth strategy that may be overlooked is to disrupt cellular packaging by interfering with the structural proteins of the virus. Among these proteins, N protein binds to the viral genome through its N-terminal and forms a ribonucleoprotein complex which has a principal role in viral replication and transcription [45]. Therefore, developing a potential drug candidate targeting this cross-linking prevents attachment of the N-terminal to viral RNA and may halt the viral replication and transcription [4,46]. It is well established that the structural proteins suppressing the host’s immune system play a central role in organizing the coronavirus assembly [47]. Moreover, the S protein, which is assembled on the surface of the viral particle, mediates binding and fusion to host cells and facilitates virus entry into the host cells [48]. Hence, M and S proteins can be potential therapeutic targets. E protein is another structural protein containing 76–109 amino acid and is essential in different stages of the virus life cycle such as envelope formation, pathogenesis, budding and virus assembly. Some studies have recently reported that E protein depletion results in an attenuated viral particle, which supports the idea that E protein may also be one of the potential therapeutic targets [49].
Figure 1. General mechanism of virus entry into the target cell.

The RBD in the S1 subunit of the virus surface facilitates SARS-CoV-2 entry into host cells through the ACE2 receptor. The S2 subunit is involved in the fusion of the virus and target cell membranes and the following delivery of viral RNA into the cytoplasm. Following the entry and release of the viral genome into the host cell, the translated nonstructural polypeptides from two large ORFs form replication and transcription machinery to produce new virions. In the next step, translated structural proteins, including Spike, Membrane and Envelope, translocate to the ER and then from ER to Golgi intermediate complex so-called the ERGIC. Finally, encapsidated genomic RNA by Nucleocapsid assembles with structural proteins and is ready to release from infected cells through exocytosis.
ER: Endoplasmic reticulum; ERGIC: Endoplasmic reticulum-Golgi intermediate compartment; E: Envelope; M: Membrane; N: Nucleocapsid; ORF: Open-reading frame; RBD: Receptor-binding domain; SARS-CoV-2: Severe acute respiratory syndrome coronavirus-2; S: Spike.
Figure 2. General mechanism of SARS-CoV-2 treatment: the role of type 2 transmembrane serine proteases.
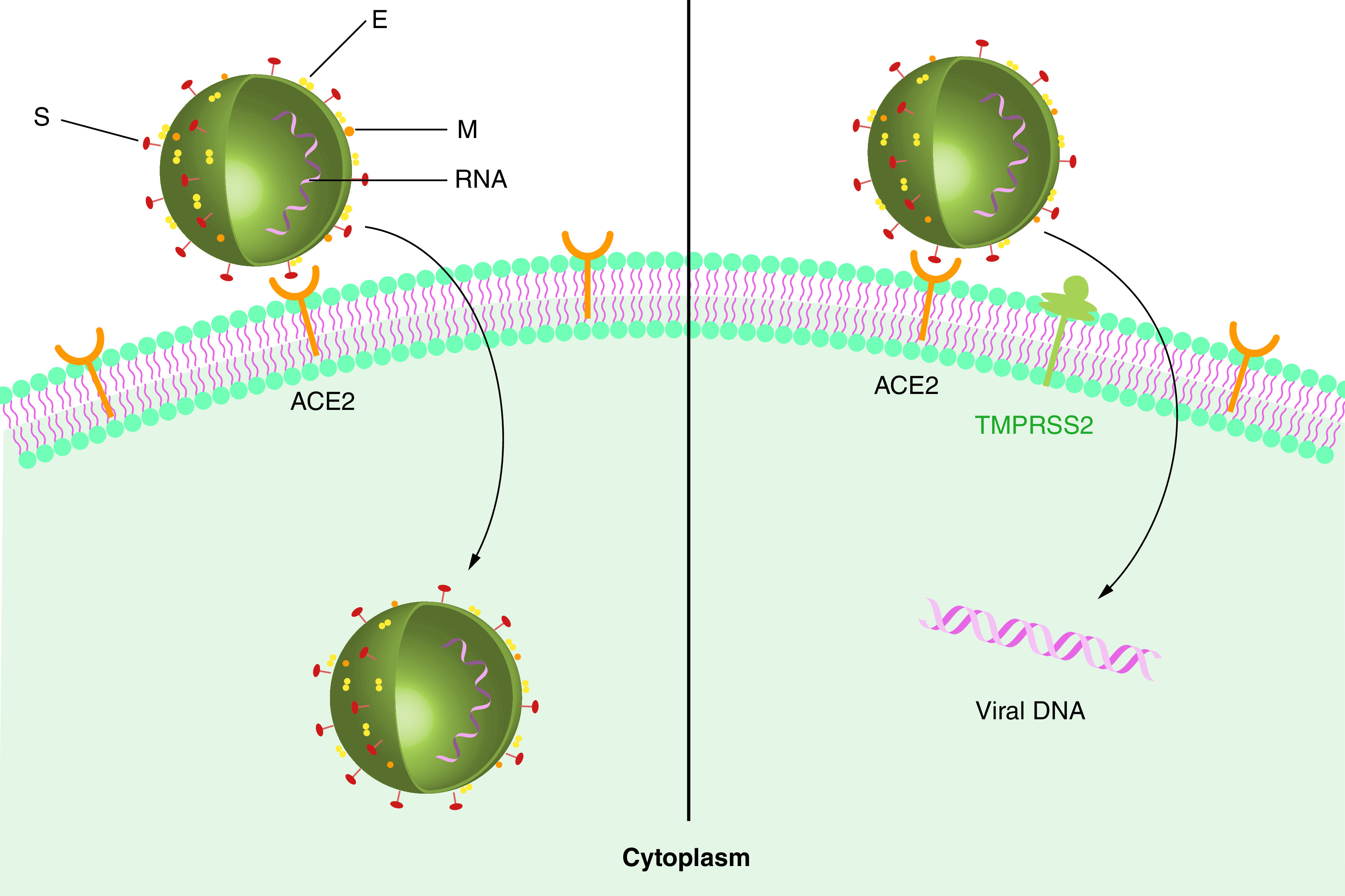
The TMPRSS2 found on host cells are involved in facilitating the viral entry into host cells, and TMPRSS2 inhibitors can significantly reduce infection.
E: Envelope protein; M: Membrane protein; S: Spike protein; TMPRSS2: Type 2 transmembrane serine protease.
Repurposed drugs to harness SARS-CoV-2
Only few countries were able to control the SARS-CoV-2 outbreak to some extent. Therefore, there is an urgent need to find new medications to protect humans against this disastrous pandemic. Given the urgency, repurposing existing the US FDA-approved medications is the most effective option for combating SARS-CoV-2. In this regard, some anti-parasitic agents have been suggested for the treatment of COVID-19 due to their potential effects in inhibiting SARS-CoV-2 [50] through inhibiting viral protease [51]. For instance, ivermectin (IVM) is an anti-parasitic agent that can reduce SARS-CoV-2 RNA load 5000-fold in 48 h post-infection in vitro [52]. Such reduction can potentially point out to the efficacy of IVM in treating COVID-19 with adequate dosing.
One of the most common clinical problems with the concomitant use of drugs is the problems caused by drug–drug interactions. For instance, coadministration of chloroquine (CQ) and paracetamol enhances the maximum serum concentration of paracetamol, which can cause side effects such as heart attack, stomach bleeding and kidney failure and so on. Also, concomitant administration of CQ and antacids causes a reduction in the absorption rate of CQ [53]. Furthermore, a growing body of evidence indicates that CQ and hydroxychloroquine (HCQ) coadministration cause QT-prolongation and arrhythmias. Although combination therapy is more effective than monotherapy, caution must be exercised in the concomitant use of these agents with antiviral drugs such as iopinavir/ritonavir, atazanavir, remdesivir and azithromycin (AZT) due to the increased risk of cardiac death [54].
Furthermore, according to the FDA fact sheet report, HCQ and CQ concomitant administration with remdesivir reduces antiviral activity of remdesivir [55]. This may be attributed to these agents being metabolized by the same cytochrome P450 (CYPs) isoenzymes. Studies have shown that IL-6, a proinflammatory cytokine increased in COVID-19 patients, inhibits the expression and activity of hepatic CYP isoenzymes, resulting in increased levels of drugs such as HCQ, CQ and remdesivir [56].
In the following sections, two main anti-parasitic drug categories are described, antimalarial and anthelmintic drugs, which are proposed as possible therapeutic options for SARS-CoV-2.
Antimalarial drugs
Currently approved antimalarial medications apply two strategies to control SARS-CoV-2 outbreaks: those that aim to reduce the symptoms of the disease and those that prevent viral replication [57]. The potential antimalarial drugs against COVID-19 infection included CQ, HCQ, artesunate (ART), artefenomel (OZ439) and atovaquone (AV) [58].
Chloroquine & hydroxychloroquine
CQ and HCQ are quinine analogs that are derived from the Cinchona officinalis tree. HCQ is derived from CQ by introducing a hydroxyl group at the end of the side chain and was found to have better therapeutic effects on malaria due to a better safety profile, longer half-life, high levels of accumulation in cells and lesser drug–drug interactions [59]. They belong to 4-aminoquinolines class of medications that inhibit DNA and RNA polymerase have been used to treat malaria for the last 70 years [60]. Apart from malaria, CQ and HCQ are currently used to treat several viral infections in humans, as well as autoimmune diseases like systemic lupus erythematosus and rheumatoid arthritis [57,61]. These agents were proposed as a potential treatment for the novel coronavirus. CQ and HCQ share very similar structures and mechanisms of action, so both drugs may be useful to treat SARS-CoV-2 infection based on several in vitro studies [62,63]. Several modes of action were proposed to explain the therapeutic effects of CQ/HCQ, however, a precise underlying mechanism remains unknown. One possible mechanism is the inhibition of endosomal/lysosomal acidification by preventing pH reduction to prevent the release of the viral genome into the host cell cytoplasm [64]. In COVID-19, it is speculated that CQ and HCQ impede the virus entry into the cells by interfering in ACE2 glycosylation and give rise to decrease ACE2 affinity for the coronavirus S protein [65]. In the confirmation of this notion, the study on ACE2 high-expressed HEK293T cells (ACE2h cells) showed that the entrance of COVID-19 spike pseudotyped virus into ACE2h cells was suppressed by CQ and HCQ. Their results also showed that HCQ is slightly more toxic to ACE2h cells than CQ [66]. Furthermore, CQ/HCQ are known to interfere with the Toll-like receptor (TLR) pathway involved in proinflammatory cytokine signaling [67]; therefore, they suppress the immune system activation by down-regulating cytokine production and TLR–ligand interaction inhibition [68]. Both drugs can indirectly inhibit the production of IL-1, IL-6, TNFα, IFNα, MIP1β and IFNγ by various cell types with RNA-containing immune complexes. Hence, both drugs can interfere with antigen processing for MHC-II presentation through antigen-processing cells [69]. The detailed mechanism of CQ/HCQ action is presented in Figure 3.
Figure 3. Possible mechanism of action of chloroquine and hydroxychloroquine in the intracellular space.
In COVID-19, CQ and HCQ impede the virus entry into the cells by interfering in ACE2 glycosylation and decreasing ACE2 affinity for the coronavirus S protein. Furthermore, CQ/HCQ is known to interfere with the TLR pathway involved in proinflammatory cytokine signaling. Inhibition of endosomal/lysosomal acidification by preventing pH reduction to prevent the release of the viral genome into the host cell cytoplasm is suggested to be another mechanism.
ACE2: Angiotensin-converting enzyme 2; cGAS: Cyclic GMP–AMP synthase; CQ: Chloroquine; HCQ: Hydroxychloroquine; S: Spike; STING: Stimulator of interferon genes; TLR: Toll-like receptor.
CQ/HCQ safety was determined in malaria and individuals with autoimmune disease but not in COVID-19 patients [70]. In patients with COVID-19, especially those who have comorbidities such as endocrine disorders or cardiovascular diseases, CQ and HCQ use was reported to be challenging [71]. There are several studies, in vitro and in vivo, that show CQ and HCQ which affect various viruses, including influenza, HIV as well as SARS coronaviruses. In SARS coronaviruses their effects are attributed to impaired ACE2 receptor glycosylation [72–76]. In an in vitro study by Hu et al. for the evaluation of cytotoxicity and anti-SARS-CoV-2 effects of CQ and HCQ using Vero E6 cells 273.20 and 249.50 μm were indicated to be cytotoxic concentration, respectively. Thus, the dose-response curves of both drugs showed a noticeable effect on SARS-CoV-2, although the antivirus activity of CQ was higher than that of HCQ. Moreover, they were shown to inhibit the entry of the virus into host cells [72]. A similar study using the same cell line by Wang et al. showed the inhibitory effects of CQ alone or in combination with remdesivir; although the CQ itself was more effective than its combined form to control COVID-19 infection in vitro [75]. Using in vitro data and analysis by a specific computational method, Yao et al. conducted a study to determine the optimal dose of HCQ and CQ to use in the clinic. They found that HCQ has a more potent effect compared with CQ (EC50 = 0.72 vs 5.47 μm) on SARS-CoV-2 infection in vitro. Based on their analysis, a 400-mg HCQ and 200-mg CQ twice daily for 4 days is the optimal range [76].
Unlike in vitro studies, several clinical trials or observational studies have recently reported contradictory results regarding the safety and efficacy of CQ and HCQ in COVID-19 patients (see Table 1). For instance, a multicenter prospective study conducted by Huang et al. in China on 197 COVID-19 patients older than 18 showed no serious side effects in a full dose of CQ, while the patients who were treated with a half dose of CQ experienced a lower rate of adverse effects [77]. In contrast, a comparative study carried out in Brazil indicated a higher lethality and fatality rate for higher CQ dosages in a randomized, double-blind, parallel-group [78].
Table 1. Studies on some anti-parasitic drugs (chloroquine, hydroxychloroquine, ivermectin and nitazoxanide) in COVID-19 treatment.
| Country | Date | Drug | Cell line or treatment | Sample size | Main findings | Ref. |
|---|---|---|---|---|---|---|
| In vitro studies | ||||||
| China | February 2020 | CQ and remdesivir | Vero E6 cells | Triplicates/different doses MOI | EC50 = 1.13 μm and CC50 >100 μm, High inhibition at low concentration | [75] |
| China | March 2020 | CQ and HCQ | Vero E6 cells | Triplicates/different doses | CQ more potent than HCQ | [76] |
| China | July 2020 | CQ and HCQ | HEK293T cells, HSAEpC cells, AT2 cells and EOL-1 cells | Both drugs at concentration of (0–400 μm), the toxicity and autophagy effects of drugs were evaluated on ACE2 high-expressed HEK293T cells | The entrance of COVID-19 spike pseudotype virus into ACE2h cells was suppressed by both CQ and HCQ | [66] |
| China | August 2020 | CQ and HCQ | Vero E6 cells | Vero cells were infected at a multiplicity of infection of 0.01 (100 plaque-forming units/well) | HCQ more potent than CQ full-time entry, as well as postentry steps were inhibited by CQ and remdesivir | [72] |
| Germany | October 2020 | Artemisinin-based treatments | VeroE6 and Huh7.5 cells | Vero cells were incubated in the presence of tenfold serial dilutions of the artemisinin derivatives for 15, 30, 60 or 120 min, before the virus was added at a concentration of 200 PFU per well for 120 min | Artesunate was the most effective inhibitor on SARS-COV-2 | [131] |
| Australia | March 2020 | IVM | Vero-hSLAM cells | Cells were seeded into 12-well tissue culture plates 24 h prior to infection with SARS-COV-2 | Approximately 5000-fold reduction in viral RNA at 48 h | [52] |
| Clinical trials | ||||||
|---|---|---|---|---|---|---|
| Brazil | January 2020 | CQ | High dose: 600 mg CQ/BD/10 days or total dose 12 g; low dose: 450 mg/5 days, twice daily only on the first day or total dose 2.7 g | 440 patients | High lethality and fatality at the higher dosage | [78] |
| China | February 2020 | HCQ | 1200 mg/day × 3 days then followed by a maintained dose of 800 mg/day daily/2 or 3 weeks | 150 patients | Higher adverse events in HCQ recipients | [83] |
| China | February 2020 | HCQ | 400 mg/day up to 5 days | 62 patients | HCQ could significantly reduce TTCR and promote the absorption of pneumonia | [84] |
| China | February 2020 | CQ | CQ 500 mg twice daily up to 10 days + SOC | 100 patients | Apparent efficacy and acceptable safety of CQ | [87] |
| China | March 2020 | HCQ | 400 mg/day/5 days | 30 patients | No significant difference of the cure rate in case and control groups | [81] |
| France | March 2020 | HCQ, AZT | 600 mg/day/10 days | 36 patients | 12 cases were treated, enhanced effects in combination with AZT | [91] |
| France | March 2020 | HCQ, AZT | HCQ 600 mg/day/10 days AZ 500 mg/day 1 and 250 mg/days 2–5 |
11 patients | No evidence of efficacy of the combination of HCQ and AZT for the treatment of hospitalized patients | [92] |
| USA | March–April 2020 | HCQ | Exposure was defined as a prescription written for the drug as found in the electronic health record | 1274 outpatients | Exposure to HCQ decreased the rate of hospitalization from COVID-19 outpatients (OR: 0.53; 95% CI: 0.29, 0.95) | [107] |
| Spain | March–April 2020 | HCQ | Case arm: HCQ 800 mg/day 1, then 400 mg/day for 6 days Control arm: receive no specific therapy |
Case arm: 1116 patients control arm: 1198 patients | No positive effect of postexposure therapy | [98] |
| Pakistan | April 2020 | HCQ | Case group: HCQ 400 mg/BD/day 1, then 200 mg/BD/4 days Control group: SOC (Vit C, Vit D and zinc only) |
500 patients with mild COVID-19 (control group: 349 patients received SOC, case group: 151 received HCQ) | HCQ combined with SOC in mild COVID-19 neither prevents disease progression nor is it significantly associated with successive PCR negativity on day 7 | [100] |
| France | April 2020 | CQ, AZT | HCQ 200 mg three-times/day/10 days AZT 500 mg/day 1 followed by 250 mg/day up to 4 days |
80 patients | HCQ may be in force in lowering respiratory viral load and patient carrying duration, augmented by AZT | [90] |
| France | May 2020 | HCQ | 600 mg/day/21 days | Treatment group: 84 Control group: 89 |
Do not support drug use in hospitalized patients | [93] |
| USA | May 2020 | HCQ, AZT | HCQ 600 mg/BD/day 1, then 400 mg/day/4 days | 1376 patients | HCQ use was not related to noticeable lower or higher hazard of death or intubation | [94] |
| China | May 2020 | HCQ | 200 mg/BD/7–10 days | Case group: group: 48 Control group: 520 |
The mortality and inflammatory cytokine IL-6 decreased in case group | [85] |
| USA | May 2020 | HCQ, AZT | HCQ 200–600 mg AZT 200–500 mg once alone or at combination |
1438 patients | Treatment with HCQ, AZT alone or both was not decreased the mortality of hospitalized patients | [95] |
| Korea | June 2020 | HCQ | 400 mg/day/14 days | 189 patients | Safety results in postexposure prophylaxis | [80] |
| England | June 2020 | HCQ | 800 mg/BD/day 1, then 400 mg/BD/9 days | 1561 | Severity and comorbidities | [101] |
| China | September 2020 | CQ | 500 mg once (half dose) or twice (full dose) daily | 197 | No adverse side effects, especially in half dose | [79] |
| China | February 2020 | CQ, HCQ | CQ = 40, HCQ = 40 Control group = 20 |
Phase 0 | ChiCTR2000030054 | |
| Egypt | March 2020 | CQ, HCQ | 200 participants | Phase II, III | NCT04353336 | |
| England | April 2020 | CQ, HCQ | 40,000 participants | Phase not applicable | NCT04303507 | |
| Egypt | June 2020 | CQ, HCQ vs remedesivir | 120 patients | Phase II, III | NCT04345419 | |
| Bangladesh | June 2020 | IVM-doxycycline and HQC-AZT | Group A: IVM 200 μg/kg single dose + doxycycline 100 mg BD for 10 days Group B: HQC 400 mg first day, then 200 mg BD for 9 days + AZT 500 mg |
Group A: 60 patients Group B: 56 patients |
IVM-doxycycline had better effect on reducing symptoms, recovery duration time and adverse effects compared with group B | NCT04434144 [86] |
| USA | August 2020 | IVM | IVM at 200 μg/kg repeated at day 7 | Unmatched cohort: 280 patients Matched cohort: 196 |
IVM therapy is associated with lower mortality, especially in patients needed oxygen and ventilatory support | [152] |
| Iraq | October 2020 | IVM-doxycycline | IVM at 200 μg/kg/day/2–3 days + 100 mg doxycycline/BD/5–10 days | 70 COVID-19 patients (48 mild–moderate, 11 severe and 11 critical patients) | Reducing recovery time and progress to more advanced stage; Significantly reducing mortality rate in severe patients |
[154] |
| Egypt | June 2020 | IVM | IVM: 3 successive days/three-times a day, started within 48 h of symptoms | 100 participants | Phase II, III | [NCT04445311] |
| Colombia | October 2020 | IVM | IVM 400 μg/kg (2 drops per kg) orally in a single dose | 50 patients | Phase II | [NCT04602507] |
| Iraq | April 2020 | IVM, HCQ AZT |
IVM: Single dose 0.2 mg/kg HCQ: 400 mg BD in first day then 200 mg BD/5 days AZT: 500 mg in first day then 250 mg/5 days |
16 participants | Phase I | [NCT04343092] |
| Argentina | May 2020 | IVM | IVM: 600 μg/kg once daily plus standard care | 45 participants | Phase II | [NCT04381884] |
| USA | May 2020 | IVM | Days 1–2: weight <75 kg: four tabs (12 mg total daily dose) Days 1–2: weight >75 kg: five tabs (15 mg total daily dose) | 240 participants | Phase II | [NCT04374019] |
| Bangladesh | August 2020 | IVM, doxycycline | IVM: 6 mg Doxycycline: 100 mg BD/5 days |
400 participants | Phase III | [NCT04523831] |
| Colombia | March 2021 | IVM | 400 patients 300 μg/kg of body weight per day for 5 days (n = 200) or placebo (n = 200) | Did not improve the time to resolution of symptoms Do not support the use of IVM for mild COVID-19 |
[155] | |
| Bangladesh | February 2021 | IVM, IVM-doxycycline | Oral IVM alone 12 mg once daily for 5 days 12 mg IVM single dose and 200 mg doxycycline on day 1, followed by 100 mg every 12 h for the next 4 days |
Earlier virological clearance in IVM group | [157] | |
| Brazil | December 2020 | NTZ, Placebo | NTZ or placebo: 500 mg/3-times/5 days | 392 patients | Symptom resolution did not differ between two groups, significantly reduced viral load | [169] |
| Egypt | April 2020 | IVM plus NTZ | IVM: 200 μg/kg once + NTZ: 500 mg/BD/6 days | 100 participants | Phase II/III | [NCT04360356] |
| Egypt | May 2020 | NTZ | – | 160 participants | Phase III | [NCT04382846] |
| Egypt | February 2021 | NTZ–ribavirin-IV-zinc | NTZ500 mg rapid release formula/6 h ribavirin 1200 mg (400 mg divided doses) IVM weight dependent zinc 30 mg twice daily |
113 patients | Decreasing the duration of viral nasopharyngeal clearance | [170] |
| Brazil | July 2021 | AZT plus NTZ, IVM or HQC | AZT 500 mg daily for 5 days for all patients, in association with one of the following: HCQ 400 mg daily for 5 days, NTZ 500 mg twice a day for 6 days, or IVM 0.2 mg/kg/day in a single daily dose for 3 days |
722 patients | There were no actual effective options for early COVID-19, in other words, that none of the drugs would confer any protection | [175] |
| Nigeria | 6 October 2020 | NTZ, atazanvir, aitonavir | 1000 mg of NTZ twice daily orally and 300/100 mg of atazanvir/ritonavir once daily orally | 98 Patients | Phase II | [176] |
| Egypt | June–July 2020 | AZT, doxycycline, NTZ | AZT was 500 mg once daily for 5 days; doxycycline 100 mg daily for 10 days; NTZ was 600 mg twice daily for 5 days | 80 patients | Symptomatic improvement of mild-to-moderate subjects was seen on the fifth or seventh day after starting treatment. Both NTZ and doxycycline have great therapeutic potential against COVID-19 |
[177] |
| Mexico | 1 May and 20 July 2020 | NTZ | NTZ 500 mg orally, every 6 h for 2 days and then 500 mg twice a day for 4 days | 150 Patients | NTZ prove to be useful against SARS-CoV-2 as an early intervention to avoid complications | [178] |
| Argentina | July–December 2020 | NTZ | Patients receive NTZ orally with food for 14 days | 46 patients | The ratio of patients with a viral load reduction ≥35% from baseline up to day 7 of treatment was significantly greater for NTZ compared with placebo | [179] |
AZT: Azithromycin; CQ: Chloroquine; HCQ: Hydroxychloroquine; IVM: Ivermectin; NTZ: Nitazoxanide; OR: Odds ratio; SOC: Standard of care; TTCR: Time to clinical recovery; Vit: Vitamin.
In addition, the prophylactic or postexposure prophylactic administration of CQ and HCQ has not shown any efficacy in COVID-19 patients [79,80]. Furthermore, a systematic review analysis indicated that treatment of hospitalized COVID-19 with CQ/HCQ might not reduce the risk of death and infection rate [81,82]. Another recent systematic review illustrated no obvious profit in CQ/HCQ use for the treatment of COVID-19 or prophylaxis against this disease [83].
As a CQ derivative, HCQ is believed to be effective in mild-to-moderate cases of SARS-CoV-2 patients when used as monotherapy or in combination with CQ or AZT. In this regard, Chen et al. conducted a randomized clinical trial study to evaluate HCQ efficacy in 30 adult COVID-19 patients in comparison to a control group including patients with liver abnormalities, anemia and renal dysfunction. Although the prognosis of these COVID-19 patients was good in this small pilot study, the symptom improvement and cure rate in the COVID-19 group did not differ from the control group [84]. In another study, mortality rate and inflammatory cytokine IL-6 levels decreased versus HCQ-treated group [85]. In a recent trial conducted on mild-to-moderately ill patients without comorbidities or with near-normal chest radiographs, HCQ-AZT combination therapy was able to reverse PCR results within 7 days and abolish all symptoms by 9 days [86]. Another clinical trial study by Gao et al. was conducted to evaluate the efficacy and safety of CQ phosphate in 100 hospitalized COVID-19 patients who also suffered from pneumonia. Following the treatment, the severity of pneumonia was decreased in patients without any severe adverse reactions. Given these results, the authors recommended using this drug for the prevention and treatment of pneumonia caused by COVID-19 [87,88].
In an randomized clinical trial (RCT) study, a randomized and parallel control group study in Renmin Hospital of Wuhan University, China, the efficacy of HCQ was evaluated in 62 COVID-19 patients, which resulted in a shortening of the recovery time in the patients who received HCQ [89]. Although there are some concerns about the arrhythmogenic (torsadogenic) effect of CQ/HCQ alone, it was shown that coadministration of AZT with CQ reduces this effect [90]. A small-scale nonrandomized open-label trial study investigated HCQ effect alone or combined with AZT on respiratory system viral load. The results showed that HCQ combined with AZT versus HCQ alone was able to treat 100% of patients [91]. Unfortunately, despite the reduction in viral load, the authors overlooked the assessment of QT prolongation when both drugs are coadministrated. Furthermore, a systematic review and meta-analysis study by Fiolet et al. [92] and others found that HCQ with AZT increased the mortality rate in hospitalized patients compared with HCQ only [93]. In observational study by Geleris et al., they did find no association with either a greatly lowered or an increased risk of the composite end point of intubation or death [94]. In another research, investigators did also not find significant effect on mortality rates among patients hospitalized with COVID-19 treated with HCQ, AZT or both [95].
Continuing to investigate HCQ/CQ as a potential treatment for COVID-19, Hussein and Elkhair conducted a molecular docking survey to improve CQ and HCQ efficiency. Using molecular docking and molecular dynamics methodologies, they showed that the addition of zinc compounds to CQ/HCQ enhances their activity as potential inhibitors of COVID-19 main protease [96].
Despite positive results of some studies, some others stood in contrast and reported the opposite results regarding CQ/HCQ efficacy to treat COVID-19 patients [97,98]. In a multicenter study conducted in France, following administration of HCQ (600 mg/day), it was reported that administration of HCQ had no effect in COVID-19 patients who were admitted to the hospital [99]. Moreover, in a randomized trial involving 491 outpatients with mild disease in early stages, HCQ was not effective in reducing symptom severity [100]. Another large-scale randomized trial involving more than 4000 hospitalized patients showed no difference between patients who received HCQ and those patients undergoing usual care at 28 days in death incidence [101].
Hypothetically, HCQ can fight SARS-CoV-2 infection due to its mode of action. HCQ interferes with viral entry into host cells [102]. Based on another hypothesis, HCQ can prevent SARS-CoV-2 infection in healthy persons who are exposed to PCR-positive patients. Mitja et al. conducted a large-scale open-label cluster-randomized clinical trial in Spain to test this hypothesis. After analysis, they concluded that postexposure therapy with HCQ does not prevent infection in healthy contacts [103]. A similar study to test this hypothesis was conducted on participants who had a high risk of exposure to COVID-19 in a house or occupational office. Similar to the previous study, they did not find any preventive effects of HCQ [104]. A study designed by Xie et al. to evaluate the efficacy and safety of HCQ in 150 hospitalized adult patients with COVID-19 showed higher adverse events in HCQ plus standard-of-care (SOC) recipients than in HCQ nonrecipients. The authors concluded no significant difference between HCQ and SOC groups in conversion of mild-to-moderate condition [105]. In agreement with the aforementioned results, another study confirmed that the addition of HCQ to SOC in mild COVID-19 cases neither stops disease progression nor helps with early and sustained viral clearance [106].
However, the results of a recent study conducted by Ip et al. showed that although HCQ has not been associated with improved survival among hospitalized COVID-19 patients, there is an association between HCQ administration and a decreased rate of hospitalization of COVID-19 patients who are mildly symptomatic. According to their results, the subsequent hospitalization rate was declined with HCQ exposure [107].
All clinical trials related to CQ/HCQ presented here have been collected from the clinicaltrials.gov database until 20 May 2021 (https://clinicaltrials.gov/ct2/results/details?cond=COVID-19). The database search resulted in 89 and 22 completed clinical trials for HCQ and CQ, respectively. During this period, 180 and 40 clinical trials have been registered that have not yet been completed. Some of them demonstrated good virological and clinical outcomes with HCQ and CQ alone or in combination with other anti-COVID-19 agents. Nevertheless, most trials had varying degrees of methodological limitations. In contrast, some studies either showed a negative result or did not show any changes with CQ/HCQ exposure compared with the control groups. So far, numerous studies have reported serious adverse effects caused by CQ and HCQ, although uncommon [108–110]. These include hemolysis [111], cardiac toxicity in the form of cardiomyopathy [109], and prolonged QTc interval [110,112]. Van den Broek et al. studied the degree of CQ-induced QTc interval prolongation in hospitalized COVID-19 patients. In a total of 95 patients who were suspected of COVID-19 infection, ECG was collected pre-/post-treatment with CQ. About 23% of patients showed a QTc interval of more than 500 ms during CQ therapy, which represents a significant role of CQ in prolonging QTc interval in a clinically relevant manner [110].
Based on the NIH panel, the combined use of HCQ plus AZT and high-dose CQ (600 mg twice daily for 10 days) due to the potential for toxicity has not been recommended [113]. Given these controversial results, the WHO removed these drugs from joint international trials because they were not effective versus SOC as recommended by steering committee’s recommendation [114].
In general, as mentioned above, different studies have got contradictory results considering CQ/HCQ efficacy in COVID-19 patients [115], which may be in part attributed to various study designs, sample sizes and various statistical procedures as well as population genetics. Therefore, it may be necessary to carry out clinical trials on a larger scale taking into account different populations, races, demographics and patient-related factors; for example, are the patient hospitalized or not [88,116]?
Artesunate
ART, one of the semisynthetic derivatives of artemisinin, is a vital sesquiterpene lactone obtained from Artemisia annua leaves. As one of the biologically active compounds against malaria, and taking into consideration its low toxicity, rapid distribution, high efficiency, high solubility in water and how well it is tolerated, ART was proved to be a standard treatment for cerebral malaria and other severe forms of malaria [117]. In traditional Chinese medicine, it was used for the treatment of several diseases for more than 2000 years [118]. Furthermore, it is indicated that ART has broad antiviral activity against certain viruses, such as human cytomegalovirus, hepatitis B and C virus, herpes virus and bovine viral diarrhea virus [119,120]. ART was recommended by some investigators to be used for the treatment of patients infected with SARS-CoV-2; however, the exact molecular mechanisms by which ART can affect SARS-CoV-2 have not been elucidated. Antiviral properties of ART may be attributed to inhibition of transcription NF-κB expression and disrupting viral protein synthesis as well as to blocking the early steps of viral replication [120,121]. It is known that the NF-κB is the master regulator of host immune and inflammatory responses against invasive pathogens [122,123]. SARS-CoV-2, like other families of Coronaviridae, primarily targets the upper respiratory tract [124], so it causes excessive proinflammatory host responses that induce immune pathology and can harm lung tissue [125]. The idea that ART can affect COVID-19 comes from the fact that it can inhibit the production of IL-1B, IL-6 and IL-8 by inhibiting NF-κB translocation in a dose-dependent manner in vitro [126]. Elevated IL-6 serum levels in COVID-19 patients may be a sign of cytokine release syndrome, suggesting that controlling IL-6 could decrease the natural course of the disease [127]. Recent studies have shown that ART is active against SARS-CoV-2 [128–131]. For instance, Gilmore et al. used artemisinin and its synthetic derivative as inhibitors of SARS-CoV-2 in vitro [131]. Their results showed that ART is a more effective inhibitor of SARS-CoV-2 compared with A. annua extracts [131,132]. In another study, Cao et al. evaluated the anti-SARS-CoV-2 potential of nine artemisinin-related compounds in vitro. Their results highlight that the artemisinins could be considered potential anti-SARS-CoV-2 candidates in drug research and development [128,133,134]. In addition, positive clinical effects of artemisinin have been shown recently. The drug hampers the progression of the symptoms in mild/moderate form of COVID-19 [135].
Atovaquone
Atovaquone, an analog of ubiquinone, is a highly lipophilic hydroxynaphthoquinone. It is used against Plasmodium falciparum and Pneumocystis carinii, which cause malaria and pneumonia, respectively. It selectively inhibits the parasite’s mitochondrial cytochrome bc1 complex (complex III) [136]. However, the underlying molecular mechanism against P. carinii has not been fully elucidated.
A clinical trial is underway by HonorHealth (AZ, USA) to answer the question of whether coadministration of atovaquone and AZT have more advantage in COVID-19 patients over other treatments? Their results showed fewer cardiac side effects; however, available data are insufficient to draw judgments about the effects of atovaquone as a COVID-19 treatment [137].
Although clinical trials on the function of atovaquone in COVID-19 patients are limited, however, some in silico studies have proposed that it may be suitable to control this pandemic.
In this regard, molecular docking analysis based on the docking score as well as its binding energy among 129 drugs showed that atovaquone could be suitable to control the novel coronavirus disease [138]. A similar study published in preprint server, which used structure-based drug modeling design, found similar results and demonstrated that atovaquone was among the top three candidates [139]. In contrast, an in silico survey among 13 approved antimalarial drugs gathered by docking analysis against two specific targets, spike antigen and main protease of the SARS-CoV-2, indicated that the atovaquone is one of the moderately effective drugs based on the g-score [58]. Another computational study showed a good connection between the IVM, atovaquone and some antimicrobial agents with SARS-CoV-2 protease enzymes [140]. More experimental research is needed to confirm the sensitivity and specificity of this agent [138,140].
Recently, a study showed the potential antiviral effect of atovaquone on emerging VOCs of SARS-CoV-2 in vitro by interfering in viral replication at the postentry phase. However, they concluded that there is a need to conduct additional clinical studies with using either atovaquone alone or in combination with other recommended drugs for COVID-19 [141].
Antihelminthic drugs on COVID-19
Ivermectin
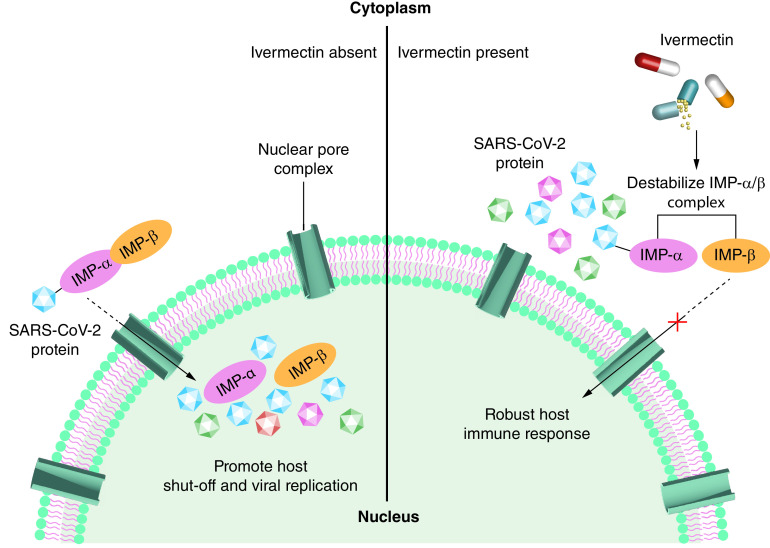
IVM is frequently used as an antistrongyloidiasis, ascariasis and onchocerciasis drug. It was confirmed as an appropriate therapy for some other parasitic micro-organisms [142,143]. Apart from its conventional use, IVM clearly has shown anti-COVID-19 properties in vitro. It seems that its mode of action may be through inhibiting the translocation of viral components into the nucleoplasm, which is mediated by ‘importin α/β1’ (Figure 4) [52]. An in silico study conducted for decrypting the binding mode of IVM interaction with potential drug targets associated with COVID-19 showed that IVM interacts with a strong and moderate affinity to Nsp9 and IMPα, respectively [144]. By the same mechanism, IVM also inhibits replication of other RNA viruses like HIV, influenza, yellow fever, etc. [145–148]. Besides, it was suggested that the accumulation of two IVM molecules together onto the virus capsid causes the formation of an ionophore structure that osmotically causes the virus disintegration [149]. However, these hypotheses should be tested before using this agent as a therapeutic arm against COVID-19.
Figure 4. Mode of action of ivermectin in the intracellular space.
IVM destabilizes the IMP-α/β complex and robust host immune response. In the absence of IVM, this complex enters the nucleus resulting in viral replication.
IVM: Ivermectin; NPC: Nuclear pore complex.
Despite the significant effects of IVM in vitro [52], due to its neurotoxic side effects at high concentrations in humans, its use for clinical trials requires careful risk–benefit considerations, especially in critically ill patients. However, the presence of higher levels of the drug in lung tissue than plasma after 1 week of oral administration has raised hopes to bypass its dose-dependent side effects [146]. In comparison to HCQ with side effects including retinal damage and elevated QT interval, IVM has fewer side effects [150]. As usual, several clinical trials are ongoing to test the potency of IVM as an anti-parasitic drug against COVID-19. For instance, in a randomized and comparative clinical study of IVM-doxycycline and HCQ-AZT therapy for COVID-19, findings showed that the combination of IVM-doxycycline had an even higher trend of superiority to the HCQ-AZT in low-risk patients. However, these differences are not statistically significant [86]. A randomized controlled trial study showed that in the early stages of the disease, IVM with doxycycline treatment has clearly prevented the progression of the disease to higher stages and significantly decreased mortality rates in severe patients [151]. It was suggested that the administration of IVM and HCQ together could have synergistic effects in COVID-19 patients [102]. HCQ fights the virus by preventing it from entering the host cell, and IVM combats the virus by inhibiting viral replication. However, basic and clinical studies are needed to reveal the possibility of this combination being used as an acceptable COVID-19 treatment [102]. Although IVM treatment of 173 hospitalized COVID-19 patients resulted in lower mortality in both severe and nonsevere groups with pulmonary involvement compared with nontreated groups [152]. However, this study and a similar study [145] carried out by the same team suggested that more studies, including randomized controlled trials, are needed to clarify the effect of this drugs on SARS-CoV-2 in comparison to control groups [152,153]. A randomized double-blind placebo-controlled small-scale trial also revealed that 5 days of treatment with IVM increased the virological clearance in hospitalized adult COVID-19 patients with mild disease [154]. In contrast, another similar study on adults with mild COVID-19 who were treated with a 5-day course of IVM showed that the time of symptom resolution was not affected significantly. They suggested that IVM is not a good treatment for mild cases [155] (for more details, see Table 1).
Nevertheless, a recent meta-analysis study revealed that IVM reduced the risk of death compared with no IVM [156]. Additionally, in silico study by using AI-based and classical simulation methods indicated positive interaction between IVM and viral protein targets; however, it requires evidence from clinical studies to support its use [157]. In confirming the significance of IVM, a web-based reported meta-analysis of 65 studies revealed that there is an statistically significant improvements for mortality, ventilation, ICU admission, hospitalization, recovery, cases and viral clearance by using IVM [158].
While in March 2021, the WHO recommended that IVM could be used in clinical trials, new reports indicate that COVID-19 patients may die from its poisoning. Therefore, larger clinical trials may be needed to uncover the efficiency of IVM in COVID-19 patients.
Nitazoxanide
Nitazoxanide (NTZ), as a pyruvate-ferredoxin oxidoreductase inhibitor, inhibits a wide range of RNA and DNA virus replication, including influenza, parainfluenza, rotavirus, coronavirus and so on [159–162]. The antiviral activity of NTZ has already been demonstrated on MERS by inhibiting the production of nucleocapsid protein in vitro [163]. Recently, it has also been shown that it can prevent the SARS-CoV-2 growth in the vero cell model [75].
At least one study has suggested that dual therapy can be highly effective against SARS-CoV-2 by a multitude of cellular and molecular mechanisms. HCQ prevents the virus from entering the cell; on the other hand, NTZ increases the innate interferon-dependent immune responses [164]. Furthermore, NTZ promotes and upregulates other innate immune components, including MDA5, RIG-1, MAVS [165]. Moreover, due to its specific antiviral effects, NTZ may also have anti-inflammatory activities by reducing IL-6 and TNF-α. Considering hyperinflammation and cytokine storm caused by SARS-CoV-2, it may improve patient prognosis even though direct evidence is lacking [166,167]. It is well established that NTZ and niclosamide (NIC), two potent TMEM16A antagonists, possess bronchodilatory properties which may effectively improve respiratory symptoms, decrease shortness and tightness of breath, and facilitate respiration and pulmonary ventilation in COVID-19 patients [168]. Despite the several above-mentioned useful effects, unfortunately, due to existing confounding data in the literature, limited clinical trials have been ongoing to evaluate NTZ efficacy on COVID-19 patients (Table 1) [164]. For instance, in a double-blind randomized multicenter clinical trial, early-stage treatment of 194 COVID-19 patients by NTZ resulted in significantly reduced viral load but did not affect symptom resolution compared with the placebo group. This study also showed that neither were the disease markers significantly affected nor were significant side effects observed [169]. In contrast, a combination therapy including NTZ, ribavirin, IVM plus zinc resulted in significantly increased SARS-CoV-2 clearance from the nasopharynx compared with symptomatic treatment [170]. Another set of clinical trials is under design with NTZ monotherapy or its combination with other therapies, including HCQ or AZT [164,171].
According to the findings of a systematic review, NTZ is considered safe at the approved doses. However, further evidence is required for its cardiovascular, hepatorenal and teratogenic consequences [172]. Dose predictions were performed to achieve appropriate plasma and lung concentrations effective against SARS-CoV-2 according to its specific EC90, and adjustments were made for subsequent clinical trials [173]. Further studies are given in Table 1 [174–179].
Niclosamide
NIC is an anthelmintic drug with anti-inflammatory and immune regulatory effects and acts by interfering with the oxidative phosphorylation pathway. Besides, it has recently been used as a potential anticancer, antibacterial and antiviral agent in addition to what has been stated [180]. Its inhibitory effects on Zika virus replication, an RNA virus responsible for infection of astrocytes and neural progenitor cells were identified [181]. Furthermore, NIC has previously been shown to have anti-SARS-CoV-2 effects by inhibiting viral replication through blocking virus spike and nucleocapsid antigens production [182,183]. It has also recently been shown that NIC has anti-SARS-CoV-2 and anti-MERS-COV properties in vitro [184]. In an experiment, two stable forms of NIC (C1 and C2) were assessed to determine their solvation energy and reactivity with COVID-19 proteins in different media (gas and aqueous media) compared with eleven other antivirals. C1 and C2 forms showed higher reactivity with COVID-19 proteins, despite their lower solvation energy which is attributed to the NO2 and Cl groups in the active site of their structures [185]. NIC also ranked at the top of the list of 1553 FDA-approved and 7012 off-market drugs since it has low binding energy and high affinity for COVID-19 protease. Possible other modes of action, including prevention of endocytosis and deactivation of S-phase kinase-associated protein 2 (SKP2), may contribute to NIC anti-SARS-CoV-2 activity [186,187].
Hypothetically SARS-CoV-2 infection declines autophagy; hence, the addition of autophagy-inducing compounds can be considered a treatment goal against COVID-19. Autophagy inhibition could increase the replication of the virus by stopping the segmentation of viral antigens. In this regard, an in vitro study suggested that NIC as an autophagic cell death inducer agent can be used as prophylactic treatment during SARS-CoV-2 infection [188]. Furthermore, molecular docking analysis indicated that the NIC and other antihelminth drugs such as primaquine, mepacrine, artemisinin could bind to the active site of the SARS-CoV-2 protease. This study has also shown that the type of interaction between ligands and virus protein is important in therapy [189]. A similar computational study confirmed that NIC could bind with SARS-CoV-2 protease with high affinity [185]. Recently, several clinical trials have been ongoing to test the efficacy of NIC alone or combined with HCQ against COVID-19 (Table 1). For instance, the intranasal and inhalation form of NIC was evaluated for their safety and possible side effects. The results indicated that this solution might serve as the primary eradicator of SARS-CoV-2 from the upper respiratory tract without adverse effects other than temporary mild stimulation [190].
Mebendazole
As an anthelminthic drug, mebendazole (MBZ), along with other benzimidazoles, was found to have antiviral properties and to be efficient against certain viruses such as HSV-1, CVB-2 and Zika virus [181,191,192]. According to molecular docking analysis, MBZ with two other compounds, atovaquone and ouabain, showed anti-SARS-CoV-2 properties in vitro [193]. In a single-cell RNA sequencing dataset analysis obtained from mild-to-severe cases of COVID-19 patients, MBZ also ranked in the top ten compounds based on connectivity score. This study also indicated that the immune profile of patients in response to different viral infections impacts the efficacy of different compounds against those infections [194]. Based on the calculated binding energy and affinity, MBZ has also been ranked in the top 30 FDA-approved drugs that interfere with SARS-CoV-2 activity [186]. MBZ and similar structure drugs such as albendazole, and oxibendazole may act against COVID-19 by compromising cellular microtubule integrity, which interferes with the cellular trafficking of the virus [195]. It was shown that MBZ is one of the BCG mimics, which promotes innate immune responses and is effective against new infections like SARS-CoV-2 [196].
AI, a state-of-the-art technique for finding new drugs
The emergence of the SARS-CoV-2 virus and COVID-19, the disease it causes, in the world has led to a widespread effort to find suitable treatments for this aggressive pathogen. Considering the urgency and rapid transmission, examining existing medications that are approved for human use will be the right strategy to combat the virus. Unfortunately, there is currently no certain effective treatment, and according to the recent solidarity study conducted by the WHO [194,197], compared with placebo, none of the retasked drugs, including remdesivir, HCQ and IFN-β as a single, as well as IFN-β plus lopinavir and lopinavir coadministered with ritonavir as a combinatorial therapy, were effective in survival, initiation of ventilation and hospitalization length in COVID-19 patients. Therefore, finding suitable candidates for new medications is still ongoing.
One of the most important translational research activities contributing to human well-being and health is drug discovery and design [198]. While the physicians’ resort to trial-and-error techniques in hospitals due to the inefficiency of the lab-based high-throughput screenings, virtual screening and molecular docking have emerged as a common tool to discover powerful compounds against SARS-CoV-2 [199–205]. In general, this computational approach applies chemically and biologically algorithms in large chemical libraries to find appropriate hits based on the known structure of the target or the ligands. Although this method has had a lot of success in recent years in discovering new drugs such as nelfinavir and zanamivir, it still faces various challenges such as sampling various conformations of flexible molecules and calculating binding energy between receptor and ligand [206], as well as simulation cost and exhaustive similarity searching [207]. With traditional methods, in silico HTS simulation performance is not simple because of intensive computational model calculations and taking an incredible running time [208].
We have recently come across an unprecedented wealth of data in the chemical and pharmacological fields that can feed state-of-the-art methods such as AI algorithms in drug discovery or design. Unlike traditional methods, this method does not rely on developing complicated physical and chemical concrete principles and only turns the large amount of medical data currently available into reusable knowledge [209]. In different phases of the drug development process, including target validation, assay development, HTS, hit to lead, lead optimization, preclinical and clinical development as well as drug repurposing, AI-based approaches are increasingly being used to boost time and cost-efficiency [198].
There is still no consensus definition for AI. In general, it is a branch of computer science whose main purpose is to produce intelligent machines capable of performing tasks that require human intelligence. AI applications cover a wide range of disciplines, including computer vision, voice recognition, language understanding and digital pathology, as well as recently, drug discovery and vaccine development [199,210]. Accordingly, a systematic review conducted by Carla Pires on the contribution of AI in the development of therapies for COVID-19 concluded that the AI methods accelerate drug repurposing against COVID-19 [211].
To this end, recently, many studies are being conducted to determine the effective drugs against the novel coronavirus, SARS-CoV-2, each of which has proposed some older drugs as effective drugs using different AI models, some of which are currently undergoing clinical trials [210,212–216]. For instance, in an effort, Benevolent AI (London, UK) introduced baricitinib on 4 February 2020, as the potential treatment for COVID-19 by using AI methods [217,218]. Nine months later, baricitinib received emergency use authorization from FDA in hospitalized patients. Since then, a few clinical trials have been conducted to test its effectiveness in improving the clinical status of COVID-19 patients [219–221].
Many companies worldwide have been founded based on AI strategies for drug discovery in recent years. For instance, Evotec (Hamburg, Germany) has started a joint venture with Exscientia (Oxford, UK), an AI-based company, on developing a small molecule, an A2a receptor antagonist, to help T-cell combat solid tumors. This first AI-designed immuno-oncology drug has entered a phase I clinical trial by Evotec. Another candidate drug developed with the help of AI models by Exscientia in partnership with Sumitomo Dainnipon Pharma (Osaka, Japan) is a selective serotonin reuptake inhibitor designed to cure obsessive-compulsive disorder that has entered human clinical trial in Japan. In recent years, many companies, including Benevolent AI, AstraZeneca (Cambridge, UK), Gilead (CA, USA), Insitro (CA, USA), Schrödinger (NY, USA) and so on, have started investing in drug discovery with the help of AI [222].
Although AI and its subdisciplines, such as machine learning and deep learning, have impacted clinical pharmacology in recent years, they also face certain challenges [223]. Some of these challenges include data heterogeneity and low quality, insufficient data shared by companies about candidate drugs or their combinations, and security and interpretability of the models [223,224]. Collectively, despite the problems in the field, AI and its subdisciplines will support the response against COVID-19 in a wide range of areas, including molecular perspectives such as drug discovery and development, clinical perspectives such as diagnosis, clinical outcome prediction and societal perspectives such as epidemiology [225].
Conclusion
The fact is that the COVID-19 pandemic has disrupted the foundation of healthcare and the economy of our planet. Thus, preventive strategies such as quarantine, dissociation of people in social sites and contact tracing were employed by agencies due to a lack of effective treatment. We reviewed the prophylactic or therapeutic effects of some FDA-approved antiprotozoal and antihelminthic drugs on COVID-19. Despite in vitro and in vivo success of these drugs, it seems that there still is not any confirmed therapeutic agent for COVID-19.
Even though several millions of doses of vaccines were produced and distributed worldwide, many countries, especially poor ones, still do not have access to it. Vaccines are indeed a good candidate for controlling pandemics like the one the world has been dealing with in the last 2 years; however, the emergence of new variants could affect the effectiveness of vaccines. Furthermore, vaccine preparation is a time-consuming and lengthy process. Hence, we still think there is an urgent need to find strong and effective medications or vaccines that can be prepared easily and inexpensively.
Future perspective
Considering it is not possible to experimentally test all available drugs in terms of potency against the SARS-CoV-2 in a limited time, leveraging computational methods in the future can speed up this discovery. Machine intelligence methods have revolutionized virtual screening methods for the designing and finding of efficient drugs among massive databases of molecules. AI-based methods, despite some disadvantages, have many advantages, which can lead to their use in a wide range of COVID-19 management aspects. Moreover, it can be applied in all areas of coronavirus research, including virus detection in human samples, detection of the COVID-19 through analyzing medical images, and even the detection of target molecules. For instance, AI technology can be used to scan the viral genome for an effective target locus or target molecule. These new loci and target molecules can then be used not only to find suitable agents among the available drugs but also to design new ones.
Executive summary.
Repurposed drugs to harness SARS-CoV-2
For controlling the COVID-19 outbreak, there is an urgent need to find new strategies such as antiviral drugs to protect human beings against this disaster.
Antimalarial drugs
Although a large number of antimalarial drugs are under investigation against COVID-19, there is however no certain efficacy reported.
Ivermectin
Ivermectin appears to be effective on COVID-19 and seems to exert efficacy by suppressing the viral protease.
Artificial intelligence is a state-of-the-art technique to find new drugs
Recently, artificial intelligence-based approaches have been increasingly used to increase time and cost-efficiency in finding effective drugs against severe acute respiratory syndrome.
Molecular docking has emerged as a common tool to discover powerful compounds against SARS-CoV-2.
Future perspective
Even though studies to date have shown the paradoxical effect of anti-parasitic drugs on COVID-19, further investigations with a large-scale population in different geographical areas seem to be necessary.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.WHO. COVID-19: One year later – WHO Director-General’s new year message. http://www.who.int/News/Item/30-12-2020-COVID-19-Anniversary-and-Looking-Forward-to-2021
- 2.The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 5(4), 536–544 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng J, Wang X, Nie T et al. A novel electrochemical sensing platform for detection of dopamine based on gold nanobipyramid/multi-walled carbon nanotube hybrids. Anal. Bioanal. Chem. 412(11), 2433–2441 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Boopathi S, Poma AB, Kolandaivel P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J. Biomol. Struct. Dyn. 39(9), 3409–3418 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hui DSC, Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect. Dis. Clin. North Am. 33(4), 869–889 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo ZD, Wang ZY, Zhang SF et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg. Infect. Dis. 26(7), 1583–1591 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khateeb J, Li Y, Zhang H. Emerging SARS-CoV-2 variants of concern and potential intervention approaches. Crit. Care 25(1), 244 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z, Nie K, Li K et al. Genome characterization of the first outbreak of COVID-19 Delta Variant B.1.617.2 - Guangzhou City, Guangdong Province, China, May 2021. China CDC Wkly 3(27), 587–589 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alexandar SRM, Kumar Rs, Jakkan K. . A comprehensive review on COVID-19 Delta variant. Int. J. Pharmacol. Clin. Res. 5, 83–85 (2021). [Google Scholar]
- 10.Planas D, Veyer D, Baidaliuk A et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 596(7871), 276–280 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Geng J, Chen L, Yuan Y et al. CD147 antibody specifically and effectively inhibits infection and cytokine storm of SARS-CoV-2 and its variants Delta, Alpha, Beta, and Gamma. Signal. Transduct. Target Ther. 6(1), 347 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.An EUA for sotrovimab for treatment of COVID-19. Med. Lett. Drugs Ther. 63(1627), 97–98 (2021). [PubMed] [Google Scholar]
- 13.Li M, Lou F, Fan H. SARS-CoV-2 variants of concern Delta: a great challenge to prevention and control of COVID-19. Signal. Transduct. Target Ther. 6(1), 349 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • It describes the new variants of SARS-CoV-2, variants of concern and emphasize the importance of continuous molecular surveillance.
- 14.Desai AN, Majumder MS. What is herd immunity? JAMA 324(20), 2113 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Papachristodoulou E, Kakoullis L, Parperis K, Panos G. Long-term and herd immunity against SARS-CoV-2: implications from current and past knowledge. Pathog. Dis. 78(3), (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matin S, Talei S, Dalimi A, Dadkhah M, Ghorbani M, Molaei S. COVID-19 and hydatidosis infections: is there any relationship? Iran J. Parasitol. 16(2), 343–345 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paraskevis D, Kostaki EG, Magiorkinis G, Panayiotakopoulos G, Sourvinos G, Tsiodras S. Full-genome evolutionary analysis of the novel corona virus (2019-nCoV) rejects the hypothesis of emergence as a result of a recent recombination event. Infect. Genet. Evol. 79, 104212 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mousavizadeh L, Ghasemi S. Genotype and phenotype of COVID-19: their roles in pathogenesis. J. Microbiol. Immunol. Infect. 54(2), 159–163 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 92(4), 418–423 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar SNR, Maurya VK Saxena SK. Coronavirus disease 2019 (COVID-19), epidemiology, pathogenesis, diagnosis, and therapeutics. In: Morphology, Genome Organization, Replication, and Pathogenesis of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Saxena SK (Ed.). Centre for Advanced Research, King George’s Medical University, Lucknow, India, 23–31 (2020). [Google Scholar]
- 21.Wu A, Peng Y, Huang B et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe 27(3), 325–328 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 7(6), 439–450 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maier HJ, Bickerton E, Britton P. Preface. Coronaviruses. Methods Mol. Biol. 1282 v (2015). [DOI] [PubMed] [Google Scholar]
- 24.Wen F, Yu H, Guo J, Li Y, Luo K, Huang S. Identification of the hyper-variable genomic hotspot for the novel coronavirus SARS-CoV-2. J. Infect. 80(6), 671–693 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lou J, Tian SJ, Niu SM et al. Coronavirus disease 2019: a bibliometric analysis and review. Eur. Rev. Med. Pharmacol. Sci. 24(6), 3411–3421 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J. 16(1), 69 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salata C, Calistri A, Alvisi G, Celestino M, Parolin C, Palù G. Ebola virus entry: from molecular characterization to drug discovery. Viruses 11(3), 274 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delvecchio R, Higa LM, Pezzuto P et al. Chloroquine, an endocytosis blocking agent, inhibits Zika virus infection in different cell models. Viruses 8(12), 322 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Omotade TO, Roy CR. Manipulation of host cell organelles by intracellular pathogens. Microbiol. Spectr. 7(2), 6484 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Praveen D, Chowdary PR, Aanandhi MV. Janus kinase inhibitor baricitinib is not an ideal option for management of COVID-19. Int. J. Antimicrob. Agents 55(5), 105967 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Algaissi A, Hashem AM. Evaluation of MERS-CoV neutralizing antibodies in sera using live virus microneutralization assay. Methods Mol. Biol. 2099, 107–116 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goo J, Jeong Y, Park YS et al. Characterization of novel monoclonal antibodies against MERS-coronavirus spike protein. Virus Res. 278, 197863 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinto D, Park YJ, Beltramello M et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 583(7815), 290–295 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Hijikata A, Shionyu-Mitsuyama C, Nakae S et al. Knowledge-based structural models of SARS-CoV-2 proteins and their complexes with potential drugs. FEBS Lett. 594(12), 1960–1973 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mirza MU, Froeyen M. Structural elucidation of SARS-CoV-2 vital proteins: computational methods reveal potential drug candidates against main protease, Nsp12 polymerase and Nsp13 helicase. J. Pharm. Anal. 10(4), 320–328 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saha P, Banerjee AK, Tripathi PP, Srivastava AK, Ray U. A virus that has gone viral: amino acid mutation in S protein of Indian isolate of coronavirus COVID-19 might impact receptor binding, and thus, infectivity. Biosci. Rep. 40(5), (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer D, Sielaff F, Hammami M, Böttcher-Friebertshäuser E, Garten W, Steinmetzer T. Identification of the first synthetic inhibitors of the type II transmembrane serine protease TMPRSS2 suitable for inhibition of influenza virus activation. Biochem. J. 452(2), 331–343 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Wrapp D, Wang N, Corbett KS et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367(6483), 1260–1263 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmann M, Kleine-Weber H, Schroeder S et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2), 271–280.e278 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahman N, Basharat Z, Yousuf M, Castaldo G, Rastrelli L, Khan H. Virtual screening of natural products against type II transmembrane serine protease (TMPRSS2), the priming agent of coronavirus 2 (SARS-CoV-2). Molecules 25(10), 2271 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shirato K, Kawase M, Matsuyama S. Middle East respiratory syndrome coronavirus infection mediated by the transmembrane serine protease TMPRSS2. J. Virol. 87(23), 12552–12561 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sternberg A, Mckee DL, Naujokat C. Novel drugs targeting the SARS-CoV-2/COVID-19 machinery. Curr. Top. Med. Chem. 20(16), 1423–1433 (2020). [DOI] [PubMed] [Google Scholar]
- 43.Cao YC, Deng QX, Dai SX. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: an evaluation of the evidence. Travel Med. Infect. Dis. 35, 101647 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Götte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 295(15), 4773–4779 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mcbride R, Van Zyl M, Fielding BC. The coronavirus nucleocapsid is a multifunctional protein. Viruses 6(8), 2991–3018 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarma P, Shekhar N, Prajapat M et al. In-silico homology assisted identification of inhibitor of RNA binding against 2019-nCoV N-protein (N terminal domain). J. Biomol. Struct. Dyn. 39(8), 2724–2732 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bojkova D, Klann K, Koch B et al. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature 583(7816), 469–472 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Discusses about the mechanism of viral replication in host cell, involved targets and development of new therapy strategies for the treatment of COVID-19.
- 48.Kirchdoerfer RN, Cottrell CA, Wang N et al. Pre-fusion structure of a human coronavirus spike protein. Nature 531(7592), 118–121 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gupta MK, Vemula S, Donde R, Gouda G, Behera L, Vadde R. In-silico approaches to detect inhibitors of the human severe acute respiratory syndrome coronavirus envelope protein ion channel. J. Biomol. Struct. Dyn. 39(7), 2617–2627 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lundberg L, Pinkham C, Baer A et al. Nuclear import and export inhibitors alter capsid protein distribution in mammalian cells and reduce Venezuelan Equine Encephalitis Virus replication. Antiviral Res. 100(3), 662–672 (2013). [DOI] [PubMed] [Google Scholar]
- 51.Tu YF, Chien CS, Yarmishyn AA et al. A review of SARS-CoV-2 and the ongoing clinical trials. Int. J. Mol. Sci. 21(7), 2657 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 178, 104787 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rezaee H, Pourkarim F, Pourtaghi-Anvarian S, Entezari-Maleki T, Asvadi-Kermani T, Nouri-Vaskeh M. Drug-drug interactions with candidate medications used for COVID-19 treatment: an overview. Pharmacol. Res. Perspect. 9(1), e00705 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Talukdar D, Jain V, Balaramnavar V, Srivastava SP, Sivanand P, Gupta MM. Potential drugs for COVID-19 treatment management with their contraindications and drug-drug interaction. Preprints (2021) (Epub ahead of print). [Google Scholar]
- 55.Yang K. What do we know about remdesivir drug interactions? Clin. Transl. Sci. 13(5), 842–844 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar D, Trivedi N. Disease-drug and drug-drug interaction in COVID-19: risk and assessment. Biomed. Pharmacother. 139, 111642 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hashem AM, Alghamdi BS, Algaissi AA et al. Therapeutic use of chloroquine and hydroxychloroquine in COVID-19 and other viral infections: a narrative review. Travel Med. Infect. Dis. 35, 101735 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • It describes the probably potential therapeutic effect of chloroquine/hydroxychloroquine in COVID-19 infection.
- 58.Sachdeva C, Wadhwa A, Kumari A, Hussain F, Jha P, Kaushik NK. In silico potential of approved antimalarial drugs for repurposing against COVID-19. Omics 24(10), 568–580 (2020). [DOI] [PubMed] [Google Scholar]
- 59.Weniger H, World Health Organization. Review of side effects and toxicity of chloroquine / by H. Weniger. (WHO/MAL/79.906) (1979). https://apps.who.int/iris/handle/10665/65773
- 60.Goel PGV. Chloroquine. StatPearls Publishing, FL, USA: (2019). [Google Scholar]
- 61.Freedman A, Steinberg VL. Chloroquine in rheumatoid arthritis; a double blindfold trial of treatment for one year. Ann. Rheum. Dis. 19(3), 243–250 (1960). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biot C, Daher W, Chavain N et al. Design and synthesis of hydroxyferroquine derivatives with antimalarial and antiviral activities. J. Med. Chem. 49(9), 2845–2849 (2006). [DOI] [PubMed] [Google Scholar]
- 63.Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents 55(4), 105932 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pahan P, Pahan K. Smooth or risky revisit of an old malaria drug for COVID-19? J. Neuroimmune Pharmacol. 15(2), 174–180 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vincent MJ, Bergeron E, Benjannet S et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2, 69 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang N, Han S, Liu R et al. Chloroquine and hydroxychloroquine as ACE2 blockers to inhibit viropexis of 2019-nCoV Spike pseudotyped virus. Phytomedicine 79, 153333 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 16(3), 155–166 (2020). [DOI] [PubMed] [Google Scholar]
- 68.Rainsford KD, Parke AL, Clifford-Rashotte M, Kean WF. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology 23(5), 231–269 (2015). [DOI] [PubMed] [Google Scholar]
- 69.Roche PA, Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat. Rev. Immunol. 15(4), 203–216 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khatooni EBF, Abdi Z. Safety and efficacy of hydroxychloroquine and chloroquine in treatment of COVID-19: a rapid review of evidence. Health Tech. Asmnt. Act 4(1), e5864 (2020). [Google Scholar]
- 71.Pereira BB. Challenges and cares to promote rational use of chloroquine and hydroxychloroquine in the management of coronavirus disease 2019 (COVID-19) pandemic: a timely review. J. Toxicol. Environ. Health B Crit. Rev. 23(4), 177–181 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu J, Cao R, Xu M et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 6, 16 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Naghipour S, Ghodousi M, Rahsepar S, Elyasi S. Repurposing of well-known medications as antivirals: hydroxychloroquine and chloroquine – from HIV-1 infection to COVID-19. Expert Rev. Anti Infect. Ther. 18(11), 1119–1133 (2020). [DOI] [PubMed] [Google Scholar]
- 74.Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect. Dis. 6(2), 67–69 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang M, Cao R, Zhang L et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 30(3), 269–271 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yao X, Ye F, Zhang M et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 71(15), 732–739 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang M, Li M, Xiao F et al. Preliminary evidence from a multicenter prospective observational study of the safety and efficacy of chloroquine for the treatment of COVID-19. Natl Sci. Rev. 7(9), 1428–1436 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Borba MDaVF, Sampaio Vs, Alexandre Ma et al. Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with severe respiratory syndrome in the context of coronavirus (SARS-CoV-2) infection: preliminary safety results of a randomized, double-blinded, Phase IIb clinical trial (CloroCOVID-19 study). MedRxiv (2020) (Epub ahead of print). [Google Scholar]
- 79.Lee SH, Son H, Peck KR. Can post-exposure prophylaxis for COVID-19 be considered as an outbreak response strategy in long-term care hospitals? Int. J. Antimicrob. Agents 55(6), 105988 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ullah W, Abdullah HM, Roomi S et al. Safety and efficacy of hydroxychloroquine in COVID-19: a systematic review and meta-analysis. J. Clin. Med. Res. 12(8), 483–491 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care 57, 279–283 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Patil VM, Singhal S, Masand N. A systematic review on use of aminoquinolines for the therapeutic management of COVID-19: efficacy, safety and clinical trials. Life Sci. 254, 117775 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes the mechanism of action, safety, side effects, toxicity and also details of clinical trials for chloroquine/hydroxychloroquine to benefit the clinicians, medicinal chemist, pharmacologist actively involved in controlling and treatment of COVID-19 infection.
- 83.Saghir SaM, Algabri NA, Alagawany MM et al. Chloroquine and hydroxychloroquine for the prevention and treatment of COVID-19: a fiction, hope or hype? An updated review. Ther. Clin. Risk Manag. 17, 371–387 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen J, Liu D, Liu L et al. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19. Zhejiang Da Xue Xue Bao Yi Xue Ban 49(2), 215–219 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu B, Li C, Chen P et al. Low dose of hydroxychloroquine reduces fatality of critically ill patients with COVID-19. Sci. China Life Sci. 63(10), 1515–1521 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chowdhury AT, Shahbaz M, Karim R, Islam J, Guo D, He S. A comparative study on ivermectin-doxycycline and hydroxychloroquine-azithromycin therapy on COVID-19 patients. EJMO 5(1), 63–70 (2020). [Google Scholar]
- 87.Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends 14(1), 72–73 (2020). [DOI] [PubMed] [Google Scholar]
- 88.Spinelli FR, Ceccarelli F, Di Franco M, Conti F. To consider or not antimalarials as a prophylactic intervention in the SARS-CoV-2 (COVID-19) pandemic. Ann. Rheum. Dis. 79(5), 666–667 (2020). [DOI] [PubMed] [Google Scholar]
- 89.Chen ZHJ, Zhang Z, Jiang S et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. Medrxiv (2020) (Epub ahead of print). [Google Scholar]
- 90.Fossa AA, Wisialowski T, Duncan JN, Deng S, Dunne M. Azithromycin/chloroquine combination does not increase cardiac instability despite an increase in monophasic action potential duration in the anesthetized guinea pig. Am. J. Trop. Med. Hyg. 77(5), 929–938 (2007). [PubMed] [Google Scholar]
- 91.Gautret P, Lagier JC, Parola P et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 56(1), 105949 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 92.Fiolet T, Guihur A, Rebeaud ME, Mulot M, Peiffer-Smadja N, Mahamat-Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: a systematic review and meta-analysis. Clin. Microbiol. Infect. 27(1), 19–27 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ghazy RM, Almaghraby A, Shaaban R et al. A systematic review and meta-analysis on chloroquine and hydroxychloroquine as monotherapy or combined with azithromycin in COVID-19 treatment. Sci. Rep. 10(1), 22139 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Geleris J, Sun Y, Platt J et al. Observational study of hydroxychloroquine in hospitalized patients with COVID-19. N. Engl. J. Med. 382(25), 2411–2418 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rosenberg ES, Dufort EM, Udo T et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA 323(24), 2493–2502 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hussein RK, Elkhair HM. Molecular docking identification for the efficacy of some zinc complexes with chloroquine and hydroxychloroquine against main protease of COVID-19. J. Mol. Struct. 1231, 129979 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chowdhury MS, Rathod J, Gernsheimer J. A rapid systematic review of clinical trials utilizing chloroquine and hydroxychloroquine as a treatment for COVID-19. Acad. Emerg. Med. 27(6), 493–504 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mahévas M, Tran VT, Roumier M et al. No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID-19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial. Medrxiv (2020) (Epub ahead of print). [Google Scholar]
- 99.Mahévas M, Tran VT, Roumier M et al. Clinical efficacy of hydroxychloroquine in patients with COVID-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ 369, m1844 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Skipper CP, Pastick KA, Engen NW et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann. Intern. Med. 173(8), 623–631 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Horby P, Mafham M, Linsell L et al. Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial. MedRxiv (2020) (Epub ahead of print). [Google Scholar]
- 102.Patrì A, Fabbrocini G. Hydroxychloroquine and ivermectin: a synergistic combination for COVID-19 chemoprophylaxis and treatment? J. Am. Acad. Dermatol. 82(6), e221 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mitjà O, Corbacho-Monné M, Ubals M et al. A cluster-randomized trial of hydroxychloroquine for prevention of COVID-19. N. Engl. J. Med. 384(5), 417–427 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boulware DR, Pullen MF, Bangdiwala AS et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for COVID-19. N. Engl. J. Med. 383(6), 517–525 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tang W, Cao Z, Han M et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ 369, m1849 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kamran SM, Moeed HA, Mirza ZE et al. Clearing the fog: is hydroxychloroquine effective in reducing coronavirus disease-2019 progresression? A randomized controlled trial. Cureus 13(3), e14186 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ip A, Ahn J, Zhou Y et al. Hydroxychloroquine in the treatment of outpatients with mildly symptomatic COVID-19: a multi-center observational study. BMC Infect. Dis. 21(1), 72 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oymak Y, Karapinar TH, Devrim İ. Why G6PD deficiency should be screened before COVID-19 treatment with hydroxychloroquine? J. Pediatr. Hematol. Oncol. 43(1), 35–36 (2021). [DOI] [PubMed] [Google Scholar]
- 109.Tleyjeh IM, Kashour Z, Aldosary O et al. Cardiac toxicity of chloroquine or hydroxychloroquine in patients with COVID-19: a systematic review and meta-regression analysis. Mayo Clin. Proc. Innov. Qual. Outcomes 5(1), 137–150 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Van den Broek MPH, Möhlmann JE, Abeln BGS, Liebregts M, Van Dijk VF, Van De Garde EMW. Chloroquine-induced QTc prolongation in COVID-19 patients. Neth. Heart J. 28(7–8), 406–409 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vick DJ. Glucose-6-phosphate dehydrogenase deficiency and COVID-19 infection. Mayo Clin. Proc. 95(8), 1803–1804 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chorin E, Wadhwani L, Magnani S et al. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm 17(9), 1425–1433 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Song Y, Zhang M, Yin L et al. COVID-19 treatment: close to a cure? A rapid review of pharmacotherapies for the novel coronavirus (SARS-CoV-2). Int. J. Antimicrob. Agents 56(2), 106080 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.WHO. WHO discontinues hydroxychloroquine and lopinavir/ritonavir treatment arms for COVID-19. http://www.who.int/news/item/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-COVID-19
- 115.Ho TC, Wang YH, Chen YL et al. Chloroquine and hydroxychloroquine: efficacy in the treatment of the COVID-19. Pathogens 10(2), 217 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Parperis K. To consider or not antimalarials as a prophylactic intervention in the SARS-CoV-2 (COVID-19) pandemic. Ann. Rheum. Dis. 80(1), e8 (2021). [DOI] [PubMed] [Google Scholar]
- 117.Li G, Li Y, Li Z, Zeng M. Artemisinin-based and Other Antimalarials: Detailed Account of Studies by Chinese Scientists Who Discovered and Developed Them. Academic Press; London, UK, 1–736 (2017). [Google Scholar]
- 118.Romero MR, Efferth T, Serrano MA et al. Effect of artemisinin/artesunate as inhibitors of hepatitis B virus production in an “in vitro” replicative system. Antiviral Res. 68(2), 75–83 (2005). [DOI] [PubMed] [Google Scholar]
- 119.Canivet C, Menasria R, Rhéaume C, Piret J, Boivin G. Valacyclovir combined with artesunate or rapamycin improves the outcome of herpes simplex virus encephalitis in mice compared to antiviral therapy alone. Antiviral Res. 123, 105–113 (2015). [DOI] [PubMed] [Google Scholar]
- 120.Efferth T, Romero MR, Wolf DG, Stamminger T, Marin JJ, Marschall M. The antiviral activities of artemisinin and artesunate. Clin. Infect. Dis. 47(6), 804–811 (2008). [DOI] [PubMed] [Google Scholar]
- 121.Drouot E, Piret J, Boivin G. Artesunate demonstrates in vitro synergism with several antiviral agents against human cytomegalovirus. Antivir. Ther. 21(6), 535–539 (2016). [DOI] [PubMed] [Google Scholar]
- 122.Dediego ML, Nieto-Torres JL, Regla-Nava JA et al. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J. Virol. 88(2), 913–924 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee JY, Bae S, Myoung J. Middle East respiratory syndrome coronavirus-encoded accessory proteins impair MDA5-and TBK1-mediated activation of NF-κB. J. Microbiol. Biotechnol. 29(8), 1316–1323 (2019). [DOI] [PubMed] [Google Scholar]
- 124.Brian DA, Baric RS. Coronavirus genome structure and replication. Curr. Top. Microbiol. Immunol. 287, 1–30 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Catanzaro M, Fagiani F, Racchi M, Corsini E, Govoni S, Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal. Transduct. Target Ther. 5(1), 84 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xu H, He Y, Yang X et al. Anti-malarial agent artesunate inhibits TNF-alpha-induced production of proinflammatory cytokines via inhibition of NF-kappaB and PI3 kinase/Akt signal pathway in human rheumatoid arthritis fibroblast-like synoviocytes. Rheumatology (Oxford) 46(6), 920–926 (2007). [DOI] [PubMed] [Google Scholar]
- 127.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science 368(6490), 473–474 (2020). [DOI] [PubMed] [Google Scholar]
- 128.Cao R, Hu H, Li Y et al. Anti-SARS-CoV-2 potential of artemisinins in vitro. ACS Infect. Dis. 6(9), 2524–2531 (2020). [DOI] [PubMed] [Google Scholar]
- 129.Nie C, Trimpert J, Moon S et al. In vitro efficacy of Artemisia extracts against SARS-CoV-2. Virol. J. 18(1), 182 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Russo M, Moccia S, Spagnuolo C, Tedesco I, Russo GL. Roles of flavonoids against coronavirus infection. Chem. Biol. Interact. 328, 109211 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhou Y, Gilmore K, Ramirez S et al. In vitro efficacy of artemisinin-based treatments against SARS-CoV-2. Sci. Rep. 11(1), 14571 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nair MS, Huang Y, Fidock DA et al. Artemisia annua L. extracts inhibit the in vitro replication of SARS-CoV-2 and two of its variants. J. Ethnopharmacol. 274, 114016 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hu Y, Liu M, Qin H et al. Artemether, artesunate, arteannuin B, echinatin, licochalcone B and andrographolide effectively inhibit SARS-CoV-2 and related viruses in vitro. Front. Cell. Infect. Microbiol. 11, 680127 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Krishna S, Augustin Y, Wang J et al. Repurposing antimalarials to tackle the COVID-19 pandemic. Trends Parasitol. 37(1), 8–11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Uckun FM, Saund S, Windlass H, Trieu V. Repurposing anti-malaria phytomedicine artemisinin as a COVID-19 drug. Front. Pharmacol. 12, 649532 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fry MPM. Site of action of the antimalarial hydroxynaphthoquinone, 2-[trans-4-(4’-chlorophenyl) cyclohexyl]-3-hydroxy-1, 4-naphthoquinone (566C80). Biochem. Pharmacol. 43(7), 1545–1553 (1992). [DOI] [PubMed] [Google Scholar]
- 137.Atovaquone and azithromycin combination for confirmed COVID-19 infection. NCT04339426. https://clinicaltrials.gov/ct2/show/NCT04339426
- 138.Singh S, Florez H. Coronavirus disease 2019 drug discovery through molecular docking. F1000Res 9, 502 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Farag A, Wang P, Boys In et al. Identification of atovaquone, ouabain and mebendazole as FDA approved drugs targeting SARS-CoV-2 (version 4). ChemRxiv (2021) (Epub ahead of print). [Google Scholar]
- 140.Marak BN, Dowarah J, Khiangte L, Singh VP. Step toward repurposing drug discovery for COVID-19 therapeutics through in silico approach. Drug Dev. Res. 82(3), 374–392 (2021). [DOI] [PubMed] [Google Scholar]
- 141.Carter-Timofte ME, Arulanandam R, Kurmasheva N et al. Antiviral potential of the antimicrobial drug atovaquone against SARS-CoV-2 and emerging variants of concern. ACS Infect. Dis. 7(11), 3034–3051 (2021). [DOI] [PubMed] [Google Scholar]
- 142.González Canga A, Sahagún Prieto AM, Diez Liébana MJ, Fernández Martínez N, Sierra Vega M, García Vieitez JJ. The pharmacokinetics and interactions of ivermectin in humans – a mini-review. AAPS J 10(1), 42–46 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Laing R, Gillan V, Devaney E. Ivermectin - old drug, new tricks? Trends Parasitol. 33(6), 463–472 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Azam F, Taban IM, Eid EEM et al. An in-silico analysis of ivermectin interaction with potential SARS-CoV-2 targets and host nuclear importin α. J. Biomol. Struct. Dyn., 1–14 (2020) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Azeem S, Ashraf M, Rasheed MA, Anjum AA, Hameed R. Evaluation of cytotoxicity and antiviral activity of ivermectin against Newcastle disease virus. Pak. J. Pharm. Sci. 28(2), 597–602 (2015). [PubMed] [Google Scholar]
- 146.Chaccour C, Hammann F, Ramón-García S, Rabinovich NR. Ivermectin and COVID-19: keeping rigor in times of urgency. Am. J. Trop. Med. Hyg. 102(6), 1156–1157 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Götz V, Magar L, Dornfeld D et al. Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import. Sci. Rep. 6, 23138 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lv C, Liu W, Wang B et al. Ivermectin inhibits DNA polymerase UL42 of pseudorabies virus entrance into the nucleus and proliferation of the virus in vitro and vivo. Antiviral Res. 159, 55–62 (2018). [DOI] [PubMed] [Google Scholar]
- 149.Rizzo E. Ivermectin, antiviral properties and COVID-19: a possible new mechanism of action. Naunyn Schmiedebergs Arch. Pharmacol. 393(7), 1153–1156 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gupta D, Sahoo AK, Singh A. Ivermectin: potential candidate for the treatment of Covid 19. Braz. J. Infect. Dis. 24(4), 369–371 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hashim HA, Maulood MF, Rasheed AM, Fatak DF, Kabah KK, Abdulamir AS. Controlled randomized clinical trial on using Ivermectin with doxycycline for treating COVID-19 patients in Baghdad, Iraq. MedRxiv (2020) (Epub ahead of print). [Google Scholar]
- 152.Rajter JC, Sherman MS, Fatteh N, Vogel F, Sacks J, Rajter JJ. Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ivermectin in COVID nineteen study. Chest 159(1), 85–92 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rajter JC, Sherman MS, Fatteh N, Vogel F, Sacks J, Rajter JJ. ICON (ivermectin in covid nineteen) study: use of ivermectin is associated with lower mortality in hospitalized patients with COVID-19. Chest 159(1), 85–92 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ahmed S, Karim MM, Ross AG et al. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int. J. Infect. Dis. 103, 214–216 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.López-Medina E, López P, Hurtado IC et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA 325(14), 1426–1435 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bryant A, Lawrie TA, Dowswell T et al. Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines. Am. J. Ther. 28(4), e434–e460 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • The therapeutic effect of anti-parasitic ivermectin drug in COVID-19 infection has been reported.
- 157.Kaur H, Shekhar N, Sharma S, Sarma P, Prakash A, Medhi B. Ivermectin as a potential drug for treatment of COVID-19: an in-sync review with clinical and computational attributes. Pharmacol. Rep. 73(3), 736–749 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ivermectin for COVID-19: real-time meta analysis of 65 studies. https://ivmmeta.com
- 159.Altay O, Mohammadi E, Lam S et al. Current Status of COVID-19 therapies and drug repositioning applications. iScience 23(7), 101303 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Haffizulla J, Hartman A, Hoppers M et al. Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: a double-blind, randomised, placebo-controlled, phase IIb/III trial. Lancet Infect. Dis. 14(7), 609–618 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rossignol JF. Nitazoxanide: a first-in-class broad-spectrum antiviral agent. Antiviral Res. 110, 94–103 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tilmanis D, Van Baalen C, Oh DY, Rossignol JF, Hurt AC. The susceptibility of circulating human influenza viruses to tizoxanide, the active metabolite of nitazoxanide. Antiviral Res. 147, 142–148 (2017). [DOI] [PubMed] [Google Scholar]
- 163.Rossignol JF. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J. Infect. Public Health 9(3), 227–230 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Calderón JM, Zerón HM, Padmanabhan S. Treatment with hydroxychloroquine vs hydroxychloroquine + nitazoxanide in COVID-19 patients with risk factors for poor prognosis: a structured summary of a study protocol for a randomised controlled trial. Trials 21(1), 504 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Padmanabhan S, Padmanabhan K. “The devil is in the dosing” – targeting the interferon pathway by repositioning nitazoxanide against COVID-19. (2020) (Epub ahead of print).
- 166.Hong SK, Kim HJ, Song CS, Choi IS, Lee JB, Park SY. Nitazoxanide suppresses IL-6 production in LPS-stimulated mouse macrophages and TG-injected mice. Int. Immunopharmacol. 13(1), 23–27 (2012). [DOI] [PubMed] [Google Scholar]
- 167.Mehta P, Mcauley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395(10229), 1033–1034 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Miner K, Labitzke K, Liu B et al. Drug repurposing: the anthelmintics niclosamide and nitazoxanide are potent TMEM16A antagonists that fully bronchodilate airways. Front. Pharmacol. 10, 51 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rocco PRM, Silva PL, Cruz FF et al. Early use of nitazoxanide in mild COVID-19 disease: randomised, placebo-controlled trial. Eur. Respir. J. 58(1), 2003725 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Elalfy H, Besheer T, El-Mesery A et al. Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-19. J. Med. Virol. 93(5), 3176–3183 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kelleni MT. NSAIDs and Kelleni’s protocol as potential early COVID-19 treatment game changer: could it be the final countdown? .Inflammopharmacology , 1–6 (2021) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pepperrell T, Pilkington V, Owen A, Wang J, Hill AM. Review of safety and minimum pricing of nitazoxanide for potential treatment of COVID-19. J. Virus Erad. 6(2), 52–60 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • The possibility safe and affordable effect of nitazoxanide in COVID-19 infection has been discussed.
- 173.Rajoli RKR, Pertinez H, Arshad U et al. Dose prediction for repurposing nitazoxanide in SARS-CoV-2 treatment or chemoprophylaxis. Br. J. Clin. Pharmacol. 87(4), 2078–2088 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Blum VF, Cimerman S, Hunter JR et al. Nitazoxanide superiority to placebo to treat moderate COVID-19 – a pilot prove of concept randomized double-blind clinical trial. EClinicalMedicine 37, 100981 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cadegiani FA, Goren A, Wambier CG, Mccoy J. Early COVID-19 therapy with azithromycin plus nitazoxanide, ivermectin or hydroxychloroquine in outpatient settings significantly improved COVID-19 outcomes compared to known outcomes in untreated patients. New Microbes New Infect. 43, 100915 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Olagunju A, Fowotade A, Olagunoye A et al. Efficacy and safety of nitazoxanide plus atazanavir/ritonavir for the treatment of moderate to severe COVID-19 (NACOVID): a structured summary of a study protocol for a randomised controlled trial. Trials 22(1), 3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sayed AM, Khalaf AM, Abdelrahim MEA, Elgendy MO. Repurposing of some anti-infective drugs for COVID-19 treatment: a surveillance study supported by an in silico investigation. Int. J. Clin. Pract. 75(4), e13877 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.MendietaZerón H, Meneses Calderón J, Paniagua Coria L et al. Nitazoxanide as an early treatment to reduce the intensity of COVID-19 outbreaks among health personnel. World Acad. Sci. J. 3(3), 23 (2021). [Google Scholar]
- 179.Efficacy of Nitazoxanide in reducing the viral load in COVID-19 patients. Randomized, placebo-controlled, single-blinded, parallel group, pilot study. MedRxiv (2021) (Epub ahead of print). [Google Scholar]
- 180.Chen W, Mook RA Jr, Premont RT, Wang J. Niclosamide: beyond an antihelminthic drug. Cell. Signal. 41, 89–96 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Xu M, Lee EM, Wen Z et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat. Med. 22(10), 1101–1107 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wu CJ, Jan JT, Chen CM et al. Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrob. Agents Chemother. 48(7), 2693–2696 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Xu J, Shi PY, Li H, Zhou J. Broad spectrum antiviral agent niclosamide and its therapeutic potential. ACS Infect. Dis. 6(5), 909–915 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ko M, Chang SY, Byun SY et al. Screening of FDA-approved drugs using a MERS-CoV clinical isolate from South Korea identifies potential therapeutic options for COVID-19. Viruses 13(4), 651(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Romani D, Noureddine O, Issaoui N, Brandán SA. Properties and reactivities of niclosamide in different media, a potential antiviral to treatment of COVID-19 by using DFT calculations and molecular docking. Biointerface Res. Appl. Chem 10(6), 7295–7328 (2020). [Google Scholar]
- 186.Gao K, Nguyen DD, Chen J, Wang R, Wei GW. Repositioning of 8565 existing drugs for COVID-19. J. Phys. Chem. Lett. 11(13), 5373–5382 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pindiprolu S, Pindiprolu SH. Plausible mechanisms of niclosamide as an antiviral agent against COVID-19. Med. Hypotheses 140, 109765 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gassen NC, Papies J, Bajaj T et al. Analysis of SARS-CoV-2-controlled autophagy reveals spermidine, MK-2206, and niclosamide as putative antiviral therapeutics. BioRxiv (2020) (Epub ahead of print). [Google Scholar]
- 189.Das S, Sarmah S, Lyndem S, Singha Roy A. An investigation into the identification of potential inhibitors of SARS-CoV-2 main protease using molecular docking study. J. Biomol. Struct. Dyn. 39(9), 3347–3357 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Backer V, Sjöbring U, Sonne J et al. A randomized, double-blind, placebo-controlled phase I trial of inhaled and intranasal niclosamide: a broad spectrum antiviral candidate for treatment of COVID-19. Lancet Reg. Health Eur. 4, 100084 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pascoalino BS, Courtemanche G, Cordeiro MT, Gil LH, Freitas-Junior L. Zika antiviral chemotherapy: identification of drugs and promising starting points for drug discovery from an FDA-approved library. F1000Res 5, 2523 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tonelli M, Simone M, Tasso B et al. Antiviral activity of benzimidazole derivatives. II. Antiviral activity of 2-phenylbenzimidazole derivatives. Bioorg. Med. Chem. 18(8), 2937–2953 (2010). [DOI] [PubMed] [Google Scholar]
- 193.Farag A, Wang P, Boys IN et al. Identification of atovaquone, ouabain and mebendazole as FDA approved drugs targeting SARS-CoV-2 (Version 4). Chemrxiv (2020) (Epub ahead of print). [Google Scholar]
- 194.Wang Z, Guo K, Gao P et al. Identification of repurposable drugs and adverse drug reactions for various courses of COVID-19 based on single-cell RNA sequencing data. ArXiv, (2020) (Epub ahead of print). [Google Scholar]
- 195.Law JN, Akers K, Tasnina N et al. Interpretable network propagation with application to expanding the repertoire of human proteins that interact with SARS-CoV-2. GigaSci. 10(12), (2021) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hajjo R, Tropsha A. A systems biology workflow for drug and vaccine repurposing: identifying small-molecule BCG mimics to reduce or prevent COVID-19 mortality. Pharm. Res. 37(11), 212 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Repurposed antiviral drugs for COVID-19 – interim WHO solidarity trial results. N. Engl. J. Med. 384(6), 497–511 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chan HCS, Shan H, Dahoun T, Vogel H, Yuan S. Advancing drug discovery via artificial intelligence. Trends Pharmacol. Sci. 40(8), 592–604 (2019). [DOI] [PubMed] [Google Scholar]
- 199.Alsafi MA, Hughes DL, Said MA. First COVID-19 molecular docking with a chalcone-based compound: synthesis, single-crystal structure and Hirshfeld surface analysis study. Acta Crystallogr. C Struct. Chem. 76(Pt 12), 1043–1050 (2020). [DOI] [PubMed] [Google Scholar]
- 200.Barros RO, Junior F, Pereira WS, Oliveira NMN, Ramos RM. Interaction of drug candidates with various SARS-CoV-2 receptors: an in silico study to combat COVID-19. J. Proteome Res. 19(11), 4567–4575 (2020). [DOI] [PubMed] [Google Scholar]
- 201.Hall DC Jr, Ji HF. A search for medications to treat COVID-19 via in silico molecular docking models of the SARS-CoV-2 spike glycoprotein and 3CL protease. Travel Med. Infect. Dis. 35, 101646 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Keshavarzi Arshadi A, Webb J, Salem M et al. Artificial intelligence for COVID-19 drug discovery and vaccine development. Front. Artif. Intell. 3, 65 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Peele KA, Potla Durthi C, Srihansa T et al. Molecular docking and dynamic simulations for antiviral compounds against SARS-CoV-2: a computational study. Inform. Med. Unlocked 19, 100345 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Podlogar BL, Muegge I, Brice LJ. Computational methods to estimate drug development parameters. Curr. Opin. Drug Discov. Devel. 4(1), 102–109 (2001). [PubMed] [Google Scholar]
- 205.Lestari K, Sitorus T, Instiaty Megantara S, Levita J. Molecular docking of quinine, chloroquine and hydroxychloroquine to angiotensin converting enzyme 2 (ACE2) receptor for discovering new potential COVID-19 antidote. J. Adv. Pharm. Res. 10(2), 1–4 (2020). [Google Scholar]
- 206.Shoichet BK. Virtual screening of chemical libraries. Nature 432(7019), 862–865 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Melville JL, Burke EK, Hirst JD. Machine learning in virtual screening. Comb. Chem. High Throughput Screen 12(4), 332–343 (2009). [DOI] [PubMed] [Google Scholar]
- 208.Carpenter KA, Huang X. Machine learning-based virtual screening and its applications to Alzheimer’s drug discovery: a review. Curr. Pharm. Des. 24(28), 3347–3358 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhong F, Xing J, Li X et al. Artificial intelligence in drug design. Sci. China Life Sci. 61(10), 1191–1204 (2018). [DOI] [PubMed] [Google Scholar]
- 210.Ahuja AS, Reddy VP, Marques O. Artificial intelligence and COVID-19: a multidisciplinary approach. Integr. Med. Res. 9(3), 100434 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Pires C. A systematic review on the contribution of artificial intelligence in the development of medicines for COVID-2019. J. Pers. Med. 11(9), (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes the key role of artificial intelligence in designing and finding efficient drug for COVID-19.
- 212.Ke YY, Peng TT, Yeh TK et al. Artificial intelligence approach fighting COVID-19 with repurposing drugs. Biomed. J. 43(4), 355–362 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kowalewski J, Ray A. Predicting novel drugs for SARS-CoV-2 using machine learning from a >10 million chemical space. Heliyon 6(8), e04639 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Avchaciov K, Burmistrova O, Fedichev P. AI for the repurposing of approved or investigational drugs against COVID-19. ScienceOPEN (2020) (Epub ahead of print). [Google Scholar]; •• Shows use a deep neural network to identify experimental and approved drugs against SARS-CoV-2.
- 215.Gysi Dm DVÍ, Zitnik M et al. Proceedings of the national academy of sciences network medicine framework for identifying drug-repurposing opportunities for COVID-19. Proc. Natl Acad. Sci. 118(19), (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Moskal M, Beker W, Roszak R et al. Suggestions for second-pass anti-COVID-19 drugs based on the artificial intelligence measures of molecular similarity, shape and pharmacophore distribution. ChemRxiv (2020) (Epub ahead of print). [Google Scholar]
- 217.Richardson P, Griffin I, Tucker C et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 395(10223), e30–e31 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Stebbing J, Phelan A, Griffin I et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 20(4), 400–402 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kalil AC, Patterson TF, Mehta AK et al. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N. Engl. J. Med. 384(9), 795–807 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Marconi VC, Ramanan AV, De Bono S et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase III trial. Lancet Respir. Med. 9(12), 1407–1418 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Stebbing J, Sánchez Nievas G, Falcone M et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci. Adv. 7(1), eabe4724 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Savage N. Tapping into the drug discovery potential of AI. http://www.nature.com/articles/d43747-021-00045-7
- 223.Zhavoronkov A, Vanhaelen Q, Oprea TI. Will artificial intelligence for drug discovery impact clinical pharmacology? Clin. Pharmacol. Ther. 107(4), 780–785 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bhhatarai B, Walters WP, Hop C, Lanza G, Ekins S. Opportunities and challenges using artificial intelligence in ADME/Tox. Nat. Mater. 18(5), 418–422 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bullock J, Luccioni A, Pham KH, Lam CSN, Luengo-Oroz M. Mapping the landscape of artificial intelligence applications against COVID-19. J. Artif. Intell. Res. 69, 807–845 (2020). [Google Scholar]