Abstract
Spongistatin 1, a macrocyclic lactone from the marine sponge Hyrtios erecta, has broad-spectrum antifungal activity. Since this compound is a potent antimicrotubule agent in mammalian cells, we examined its effects on the filamentous fungus Aspergillus nidulans to determine if its antifungal effects are due to antimicrotubule activity. At 25 μg/ml (twice the MIC), spongistatin 1 caused a greater-than-twofold elevation of the chromosome and spindle mitotic indices. Immunofluorescence microscopy revealed that mitotic spindles were smaller and shorter than in control germlings. However, late-anaphase and telophase nuclei were seen occasionally, and this suggests that the spindles are capable of segregating chromosomes. Spongistatin 1 had more dramatic effects on cytoplasmic microtubules. At 30 min after initiation of treatment, 83% of germlings contained fragmented microtubules and after 2 h of treatment, microtubules had disappeared completely from 82% of germlings. In contrast, microtubules disappeared rapidly and completely from germlings treated with benomyl. We conclude that spongistatin 1 has antimicrotubule activity in A. nidulans and that its mechanism of action may involve a novel microtubule-severing activity.
Spongistatin 1 is an experimental antineoplastic agent that has recently been found to have broad-spectrum antifungal activity (11). The relative scarcity of effective antifungal drugs and the increasing incidence of serious fungal infections, particularly in immune-compromised patients, makes the existence of a new class of antifungals particularly exciting. In mammalian cells, spongistatin 1 is a potent antimitotic, antimicrotubule agent (1), and we wished to determine if its antifungal activity is due to antimicrotubule effects as well. We chose Aspergillus nidulans as our experimental organism because good immunofluorescence procedures are available for this organism and because other, related, species of Aspergillus cause severe problems in immune-compromised patients. For comparison, we have also examined the effects of the important antifungal, antimicrotubule agent benomyl on A. nidulans mitosis and microtubules.
We found that benomyl causes a complete loss of mitotic spindles and a nearly complete loss of cytoplasmic microtubules in A. nidulans. Spongistatin 1, at twice the MIC, also has substantial effects on microtubules. Rather than causing the rapid loss of microtubules, however, spongistatin 1 causes a fragmentation of cytoplasmic microtubules. These microtubule fragments disappear over time. Mitotic spindles form in spongistatin 1-treated germlings, but they do not achieve the maximum size seen in untreated material. These findings suggest that spongistatin 1 is an antimicrotubule agent in A. nidulans and that it acts by a mechanism different from that of benomyl and other antimicrotubule agents.
MATERIALS AND METHODS
Media.
YG (5 g of yeast extract per liter, 20 g of dextrose per liter) was used as a complete liquid medium. YAG (YG with 15 g of agar per liter) and FYG (YG with 25 g of Pretested Burtonite 44c [TIC Gums, Inc., Belcamp, Md.] per liter) were used as solid media. Both YAG and FYG were supplemented with trace elements (3).
Broth macrodilution susceptibility testing.
Broth macrodilution susceptibility testing of A. nidulans was performed in accordance with a proposed standardized procedure (4) with some modification. To induce conidiospore formation, A. nidulans FGSC4 (Glasgow wild type) was grown on a potato dextrose agar (PDA) slant at 35°C for 3 days. The slant was covered with 1 ml of sterile 0.05% Tween 80, and a suspension was made by gently probing the colonies with the tip of a sterile Pasteur pipette. The resulting mixture was transferred to a sterile, clear microcentrifuge tube, and heavy particles were allowed to settle for 5 min. The upper homogeneous suspension was transferred to a sterile microcentrifuge tube, vortexed for 15 s, adjusted spectrophotometrically, and diluted in sterile 0.165 M morpholinepropanesulfonic acid (MOPS)-buffered RPMI 1640 medium, pH 7.0, to yield a final inoculum of 0.5 × 103 to 2.5 × 103 CFU/ml. The inoculum was vortexed repeatedly during the broth macrodilution assay to ensure the suspension of small spores. The assay was performed in sterile plastic tubes (12 by 75 mm) containing twofold dilutions of spongistatin 1. One tube was left drug free for a turbidity control. Tubes were incubated without agitation at 35°C. The MIC was read after 24 h, when heavy growth was seen in the control. The MIC was defined as the lowest concentration of spongistatin 1 that inhibited all visible growth of A. nidulans FGSC4. The minimum fungicidal concentration (MFC) was determined by subculturing 0.1 ml from each tube with no visible growth in the broth macrodilution series onto drug-free PDA plates. The plates were incubated at 35°C for 48 h, and the MFC was defined as the lowest concentration of spongistatin 1 that completely inhibited growth on PDA plates.
Treatment with antifungal agents.
Benomyl (Sigma Chemical Co., St. Louis, Mo.) was dissolved in 95% ethanol at a concentration of 200 μg/ml. Spongistatin 1 was isolated from the marine sponge Hyrtios erecta as described elsewhere (10). Spongistatin 1 was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 2.5 mg/ml immediately before use. Conidia from A. nidulans FGSC4 (Glasgow wild type) were inoculated at a final concentration of 2.5 × 106/ml into 4-dram (1 fluidram = 3.697 ml) vials containing 1.1 ml of YG medium with 0.1% agar (agar was added to reduce clumping). Conidial suspensions were incubated for 6 h at 37°C with shaking (115 rpm), after which time most conidia had germinated. Samples (0.1 ml) were taken immediately before the addition of spongistatin 1 or benomyl and at 30-min intervals for 2 h afterwards. Spongistatin 1 was used at a final concentration of 25 μg/ml (twice the MIC). Since benomyl has been used commercially and experimentally for many years, we did not carry out macrodilution assays on multiple strains to define the MIC for benomyl. Rather, we tested FGSC4 and determined that the minimum benomyl concentration required to inhibit colonial growth on solid medium (YAG) is 1.2 μg/ml (9). This result is consistent with values previously obtained with wild-type A. nidulans strains (8, 13). Based on these data, we used benomyl at a final concentration of 2.4 μg/ml for our experiments. This concentration is twice the concentration required to inhibit colonial growth on YAG medium and should be equivalent to twice the MIC in the liquid medium we used, which differs from YAG only in that it contains 0.1% agar instead of 1.5% agar. For solvent-alone controls, DMSO or ethanol was added to give appropriate final concentrations.
Fluorescence microscopy.
Samples were fixed with 0.9 ml of freshly prepared fixative solution [8% formaldehyde in 50 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES), pH 6.7; 25 mM EGTA, pH 7.0; 5 mM MgSO4; and 5% DMSO] for 30 or 40 min. The samples were then washed twice for 10 min in 50 mM PIPES (pH 6.7). Cells from each sample were passed through a 26-gauge needle 25 times to break clumps apart. Fifty microliters of the cell suspension was spread on a coverslip, which had previously been coated with a poly-l-lysine solution (0.1% [wt/vol] in deionized water) (Sigma Chemical Co.), and allowed to dry.
To visualize DNA alone, the fixed samples were stained with 4′,6-diamidino-2-phenylindole (DAPI) at 0.015 μg/ml for 2 h, washed twice (10 min per wash) in deionized distilled water and mounted in Citifluor AF1 (Marivac, Ltd., Halifax, Nova Scotia, Canada). To visualize both DNA and microtubules, coverslips were washed in 50 mM PIPES (pH 6.7) and transferred to a solution containing Driselase (InterSpex Products, Inc., Foster City, Calif.) at 10 mg/ml, β-d-glucanase (InterSpex Products, Inc.) at 16 mg/ml, and lyticase (catalog no. 36-094; ICN Biomedicals, Inc., Costa Mesa, Calif.) at 81.4 U/ml in 50 mM sodium citrate, pH 5.8, with 50% (vol/vol) egg white. (All washes in this and subsequent steps were for 5 to 10 min.) In some experiments, β-d-Glucanase was treated to reduce protease activity by the following method (based on the method of Roncal et al. [12]). β-d-Glucanase (200 mg/ml) was dissolved in 100 mM sodium citrate, pH 4.5, and then incubated at 55°C for 5 min, kept on ice for 30 min, transferred to −70°C for storage, and used at 16 mg/ml in the digestion solution. Coverslips were incubated with digestion solution at 25°C for 1 h. They were then washed three times in PE buffer (50 mM PIPES [pH 6.7], 25 mM EGTA) and incubated in −20°C methanol for 10 min. They were washed two times in PE before incubation for 1 to 3 h in primary antibody diluted in PE with 0.5% (vol/vol) Nonidet P-40. The primary antibody was a mouse monoclonal anti-β-tubulin antibody, TU27B, from a cell line generously provided by Lester Binder (Northwestern University Medical School, Chicago, Ill.) via G. Lozano (M. D. Anderson Cancer Center, Houston, Tex.). The coverslips were washed three times in PE buffer before incubation for 1 h or overnight with a secondary antibody (CY3-conjugated goat anti-mouse immunoglobulin G [Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.]) diluted in PE containing 0.5% (vol/vol) Nonidet P-40. The diluted secondary antibody was preadsorbed against an A. nidulans acetone powder prepared by the method of Harlow and Lane (5). After three washes in 50 mM PIPES (pH 6.7), coverslips were postfixed in 2 mM 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (Sigma Chemical Co.) in 50 mM PIPES (pH 6.7) for 20 min. They were then rinsed briefly in 50 mM PIPES (pH 6.7), stained with DAPI at 0.075 μg/ml in 50 mM PIPES (pH 6.7) for 15 to 45 min, rinsed in 50 mM PIPES (pH 6.7), and mounted in Citifluor AF1.
Photographs were taken with T-MAX 400 film rated at 400 or 800 ASA using a Zeiss standard microscope. The film was developed in T-MAX developer, and negatives were scanned into Adobe Photoshop with a Polaroid SprintScan 35 Plus scanner. Images were also captured by using a Nikon Eclipse E800 microscope equipped with a Princeton Instruments MicroMax charge-coupled device camera and IPLab Spectrum software. Images were processed by using NIH Image and Adobe Photoshop. Composite images were prepared by using ClarisWorks.
RESULTS
Determination of the MIC and MFC of spongistatin 1 for A. nidulans.
Similar to those for other filamentous fungi (11), the MIC of spongistatin 1 for A. nidulans FGSC4 was 12.5 μg/ml. The MIC was unchanged after 72 h. The MFC/MIC ratio equaled 1.
Determination of CMIs.
Since microtubules are the major component of the mitotic apparatus, antimicrotubule agents cause the mitotic apparatus to fail to function normally or, in many cases, to assemble at all. The absence of a functional mitotic apparatus causes cells to become blocked in mitosis, and the percentage of cells in mitosis (the mitotic index) increases relative to that of untreated cells. We consequently determined the effects of spongistatin 1 on the chromosome mitotic index (CMI), the percentage of germlings in which nuclei have condensed chromosomes. Here it should be noted that A. nidulans is coenocytic and that the nuclei within a single cell enter mitosis together. We examined germlings at 6 to 8 h after inoculation. At this time, the great majority of germlings were a single cell.
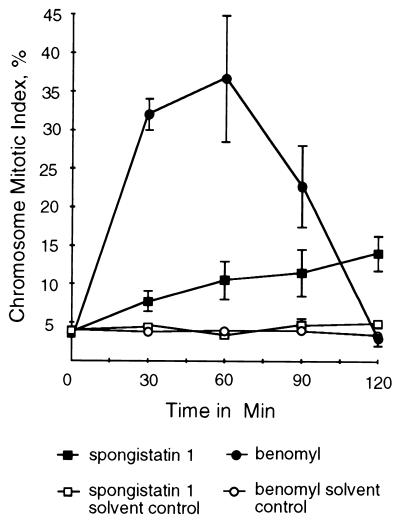
Conidia (asexual spores) were incubated for 6 h at 37°C. Spongistatin 1 was added to a final concentration of 25 μg/ml (twice the MIC). Samples were taken immediately before the addition of spongistatin 1 and at 30-min intervals thereafter. Samples were collected over 2 h during which untreated cells would pass through approximately 1.5 cell cycles (2). As controls, samples were treated with the antifungal, antimicrotubule agent benomyl at 2.4 μg/ml or with the solvents in which spongistatin 1 and benomyl were dissolved. Results are shown in Fig. 1. In spongistatin 1-treated material, the CMI rose gradually and reached a value of 14% at the 2-h time point. This value was significantly higher than that of the solvent-alone control, which maintained a CMI of under 5% for the duration of the experiment.
FIG. 1.
CMIs (percentages of germlings in which nuclei have condensed chromatin) of A. nidulans germlings treated with spongistatin 1 (25 μg/ml) and benomyl (2.4 μg/ml). Samples were taken immediately before the addition of spongistatin 1 or benomyl (zero time point) and at 30-min intervals afterward. Appropriate concentrations of the solvents used to dissolve these compounds were used as controls. Spongistatin 1 caused a gradual elevation of the CMI, whereas benomyl caused a much more rapid elevation of the CMI, which then dropped to 3.1%. Each point represents the average of three separate experiments with 400 germlings scored at each time point in each experiment. Error bars indicate standard deviations. When error bars are not shown, the standard deviations fell within the boxes.
The results obtained with benomyl were more dramatic (Fig. 1). The CMI reached 32% at 30 min after the addition of benomyl and 37% at 1 h. By 2 h, the CMI had dropped to approximately 3%. Interestingly, in benomyl-treated samples, an unusual class of nuclei was seen at 30 min and later. These nuclei did not have a typical interphase or mitotic appearance (Fig. 2A, D, and G), but rather, the nuclei had chromatin that appeared partially condensed (Fig. 3A). (Nuclei with partially condensed chromatin were not counted as mitotic for Fig. 1.) After 2 h the percentage of nuclei with partially condensed chromatin reached 37% (±20%). Such nuclei were not seen in the spongistatin-treated material and only one such nucleus was seen in over 6,000 germlings scored in the material treated with solvent only. As will be discussed below, such nuclei have been seen previously in germlings carrying a γ-tubulin disruption (7).
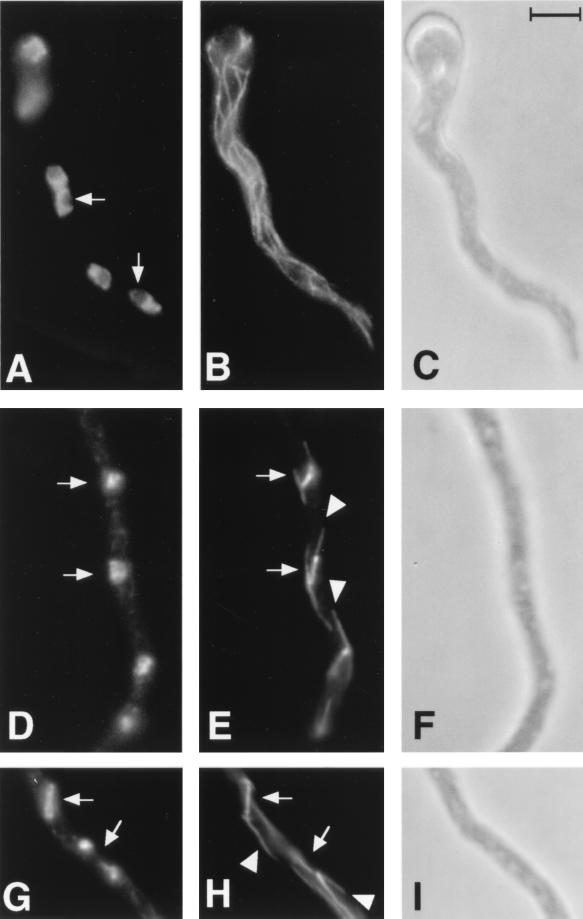
FIG. 2.
Mitotic and interphase microtubules and nuclei in control A. nidulans germlings. (A, D, and G) Chromatin as revealed by DAPI staining. (B, E, and H) Microtubules as revealed with an anti-β-tubulin antibody. (C, F, and I) Phase-contrast micrographs. (A to C) An interphase germling shows nuclei with uncondensed chromatin (A). The nucleoli (arrows) do not stain and are visible as dark regions. A normal array of cytoplasmic microtubules is present (B). (D to F) A portion of a germling in medial nuclear division shows two mitotic nuclei (arrows) with condensed chromatin and no visible nucleoli (D) and normal mitotic spindles (arrows) with astral microtubules (arrowheads) (E). (G to I) A portion of a mitotic germling with two nuclei (arrows), one in early anaphase (upper) and one in telophase (lower), is shown (G). Two spindles (arrows) with astral microtubules (arrowheads) are present (H). The lower spindle is partially out of the plane of focus. All micrographs are at the same magnification. The scale in panel C is 5 μm.
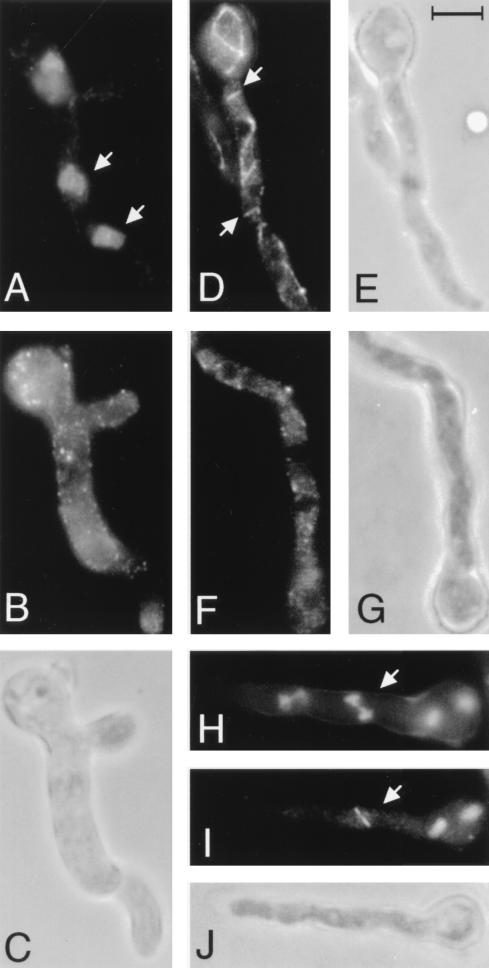
FIG. 3.
Microtubules and nuclei in A. nidulans germlings treated with benomyl (A to C) or spongistatin 1 (D to J). (A and H) Chromatin as revealed by DAPI staining. (B, D, F, and I) Microtubules as revealed with an anti-β-tubulin antibody. (C, E, G, and J) Phase-contrast micrographs. (A to C) A germling after 2 h of treatment with benomyl. (A) Nuclei (e.g., arrows) with partially condensed chromatin and no visible nucleoli. (B) Complete absence of microtubules in the germling. (D) An interphase germling after 30 min of treatment with spongistatin 1 showing fragmented microtubules (e.g., arrows). Additional fragments of microtubules are present out of the plane of focus. (F) An interphase germling after 1 h of treatment with spongistatin 1 showing complete absence of microtubules. (H to J) A mitotic germling after 1 h of treatment with spongistatin 1. (H) An anaphase nucleus (arrow) in which the chromatin has moved toward the poles. (I) A spindle (arrow) is present. Three more spindles are present but out of the plane of focus. Note the absence of astral microtubules. All micrographs are at the same magnification. The scale in panel E is 5 μm.
Spongistatin 1 effects on microtubules.
The elevation of the CMI in spongistatin 1-treated material suggested that spongistatin 1 might be inhibiting mitotic spindle function (although less completely than benomyl). To look more directly at the effects of spongistatin 1 on microtubules, we prepared germlings for immunofluorescence microscopy by using an anti-β-tubulin antibody to visualize microtubules.
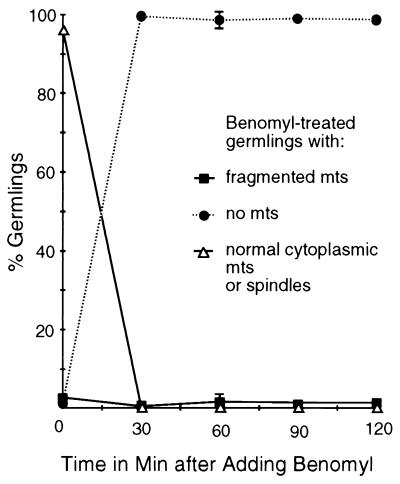
As expected, in germlings prior to treatment and in solvent-only controls, microtubules were present in virtually all germlings. Cytoplasmic microtubules were present in virtually all interphase germlings, and normal spindles were present in mitotic germlings (Fig. 2B, E, and H). Benomyl caused a rapid disassembly of nearly all microtubules (Fig. 3B and 4). Thirty minutes following the addition of benomyl, microtubules were absent from more than 99% of germlings and the number of germlings with detectable microtubules remained very low throughout the experiment (Fig. 4). Mitotic spindles were completely absent from benomyl-treated material at all time points.
FIG. 4.
Benomyl effects on microtubules. Samples were taken immediately before the addition of benomyl at 2.4 μg/ml (zero time point) and at 30-min intervals afterward. Shown are the percentages of benomyl-treated germlings with fragmented microtubules (abbreviated mts), normal cytoplasmic microtubules, spindles, or no microtubules at each time point. Benomyl caused disassembly of all microtubules in more than 99% of germlings by the first time point after addition. Spindle and normal cytoplasmic microtubules were completely absent from benomyl-treated material. There was no significant loss or fragmentation of microtubules in a solvent-only control (data not shown). Each point represents the average of three separate experiments with 400 germlings scored at each time point in each experiment. Error bars indicate standard deviations. When error bars are not shown, the standard deviations fell within the boxes.
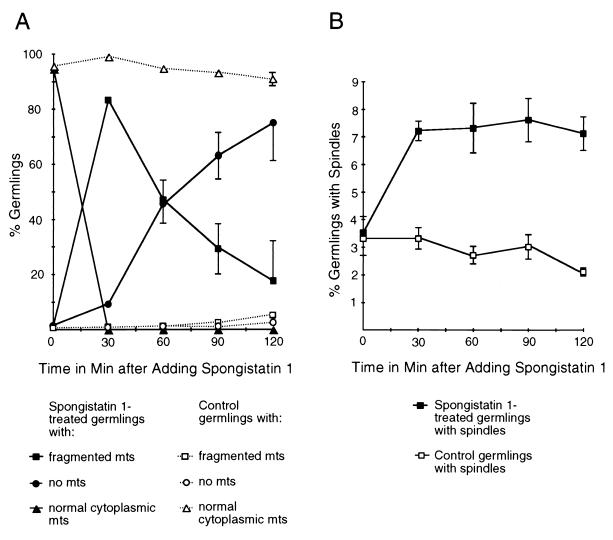
The results obtained with spongistatin 1 were quite different from those obtained with benomyl. At 30 min after the addition of spongistatin 1, the great majority of germlings contained microtubules (Fig. 5A). The microtubules were in numerous short fragments and were arranged randomly with respect to the growth axis of the germling (Fig. 3D). In control germlings, the cytoplasmic microtubules were much longer and were arranged more or less parallel to the growth axis (Fig. 2B). The fragmented microtubules gradually disappeared with time (Fig. 3F and 5A). Mitotic spindles were present at all time points but were generally small. The spindle mitotic index (SMI), the percentage of germlings with spindles, of spongistatin 1-treated material reached 7% by 30 min following the addition of spongistatin 1 and remained at similar levels over the course of the experiment (Fig. 5B). These values were about twice the SMI in solvent-only controls. Apparent late-anaphase or telophase spindles were observed occasionally (Fig. 3H to J) but were always smaller and shorter than those seen in anaphase and telophase control germlings (Fig. 2G to I). In addition, astral microtubules (microtubules extending from spindle pole bodies into the cytoplasm, which are usually seen in later stages of mitosis in control germlings [Fig. 2E and H] [6]) were completely absent in spongistatin 1-treated material (Fig. 3I).
FIG. 5.
Spongistatin 1 effects on microtubules. Samples were taken immediately before the addition of spongistatin 1 at 25 μg/ml (zero time point) and at 30-min intervals afterward. An appropriate concentration of the solvent used to dissolve this compound was used as a control. (A) Shown are the percentages of germlings with fragmented microtubules (abbreviated mts), normal cytoplasmic microtubules, or no microtubules in control or spongistatin 1-treated material. Spongistatin 1 caused the fragmentation and eventual loss of cytoplasmic microtubules, whereas they remained intact in the control. (B) SMIs (percentages of germlings with spindles) of A. nidulans germlings treated with spongistatin 1. Spongistatin 1 caused a twofold elevation of the SMI by 30 min, and the SMI remained at that level for the rest of the experiment. The SMIs in the control remained similar to those of untreated (t = 0) germlings. Each point represents the average of three separate experiments with 400 germlings scored at each time point in each experiment. Error bars indicate standard deviations. When error bars are not shown, the standard deviations fell within the boxes.
DISCUSSION
Our results demonstrate that spongistatin 1 is an antimicrotubule agent in A. nidulans, and it is reasonable to infer that it has antimicrotubule activity in other fungi as well. Spongistatin 1 appears to act differently from benomyl. Benomyl causes a relatively rapid loss of all microtubules, as shown by the fact that microtubules are completely absent from 99% of germlings by 30 min after the addition of benomyl. Spongistatin 1 does not completely block mitotic-spindle formation at twice the MIC. Spindles are present and at least partially functional because we have seen apparent telophase configurations suggesting that the spindles are capable of moving chromosomes to the poles. The spindles in spongistatin-treated material do not reach the maximum length reached by spindles in untreated material, however, and astral microtubules, cytoplasmic microtubules extending from the spindle pole body, were universally absent in spongistatin 1-treated material (Fig. 3H to J).
The most dramatic effect of spongistatin 1 is the rapid fragmentation of cytoplasmic microtubules, with 83% of germlings having fragmented cytoplasmic microtubules at 30 min after the initiation of treatment (Fig. 3D and 5A). The simplest explanation for the fragmentation of microtubules is that long interphase microtubules are simply broken into smaller fragments. It is important to note, however, that light microscopy does not afford the resolution necessary to distinguish between individual microtubules and small bundles of microtubules. The fragmentation we have seen could, in theory, result from the shortening of individual microtubules within extended bundles so that they no longer overlap. The possibility that spongistatin 1 causes breakage of microtubules is potentially important. All of the antimicrotubule agents studied to date are thought to alter the dynamics of microtubule assembly and/or disassembly by altering tubulin dimer exchange at the ends of microtubules (14). If spongistatin 1 does cause microtubule breakage, its mechanism of action would be different from that of all known antimicrotubule agents. It will be important to observe the effects of spongistatin 1 on microtubules in vitro by real-time video microscopy to determine if it really does cause microtubule severing.
The fragmented cytoplasmic microtubules disappear with time, and eventually most germlings lack any detectable microtubules (Fig. 3F and 5A). We have considered two explanations for the gradual disappearance of the microtubules. The first, and most obvious, possibility is that, after fragmentation, spongistatin 1 simply causes a gradual disassembly of cytoplasmic microtubules. The second possibility is that disassembly might be related to the entry of germlings into mitosis. In untreated germlings, cytoplasmic microtubules disassemble as cells enter mitosis and reassemble as cells complete mitosis (reviewed in reference 6). Perhaps spongistatin 1 causes cytoplasmic microtubules to fragment, but complete disassembly is a result of the normal cytoplasmic microtubule disassembly process that occurs as cells enter mitosis. If so, spongistatin 1 must prevent the reassembly of cytoplasmic microtubules after mitosis; otherwise, cytoplasmic microtubules would be present in a greater percentage of germlings at later time points. The absence of astral microtubules, cytoplasmic microtubules extending from the spindle pole bodies of mitotic nuclei, in spongistatin 1-treated material is consistent with inhibition of the reassembly of cytoplasmic microtubules. It should also be noted that the absence of astral microtubules could also be due to a direct effect of spongistatin 1 on the spindle pole body. Such an effect would have to be directed mainly at the cytoplasmic face of the spindle pole body, since spindle formation is not blocked. If spongistatin 1 severed microtubules from the cytoplasmic face of the spindle pole bodies, it might lead to some of the misorientation and fragmentation of microtubules that we have noted.
Our benomyl data also make an important point about checkpoint regulation in A. nidulans. The simplest interpretation of our data is that in the presence of benomyl, the cell cycle proceeds until germlings transit from G2 to M. At this point, chromosomes condense but benomyl prevents mitotic-spindle formation and germlings are blocked in M. The blockage is presumably due to one or more checkpoints that monitor spindle formation or other essential mitotic events and inhibit transition to G1 without successful completion of mitosis. We deduced that this checkpoint is eventually overcome because we saw a decrease in the CMI with time (Fig. 1). The decrease in the CMI does not appear to be due to the successful completion of mitosis. We have seen no evidence that mitosis is completed successfully in benomyl-treated samples (e.g., no anaphase or telophase nuclei), and it would be very surprising, indeed, if mitosis were completed successfully in the absence of mitotic spindles. Interestingly, the decondensation of the chromatin in benomyl-blocked nuclei appears to be a gradual process. The entire mitotic process normally takes less than 5 min at 37°C (2) and chromosomal decondensation only takes a fraction of this period. If decondensation occurred at a normal rate in benomyl-treated material, decondensing nuclei would be very rare, and we have seen a mean of 37% of germlings with apparently decondensing nuclei at the 2-h time point. The slow rate of decondensation does not appear to be caused directly by benomyl. Similar high percentages of nuclei with decondensing chromatin have been seen in germlings that carry a disruption of the γ-tubulin gene that prevents mitotic-spindle formation (7). It thus appears that nuclei blocked in mitosis without a mitotic spindle gradually reenter interphase without completing mitosis (i.e., the mitotic checkpoint is eventually overridden).
ACKNOWLEDGMENTS
This work was supported by grant GM31837 from the NIH and by the Arizona Disease Control Research Commission.
We thank M. Katherine Jung and C. Elizabeth Oakley for helpful discussions and critical reading of the manuscript.
REFERENCES
- 1.Bai R, Cichacz Z A, Herald C L, Pettit G R, Hamel E. Spongistatin 1, a highly cytotoxic, sponge-derived, marine natural product that inhibits mitosis, microtubule assembly, and the binding of Vinblastine to tubulin. Mol Pharmacol. 1993;44:757–766. [PubMed] [Google Scholar]
- 2.Bergen L G, Morris N R. Kinetics of the nuclear division cycle of Aspergillus nidulans. J Bacteriol. 1983;156:155–160. doi: 10.1128/jb.156.1.155-160.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cove D J. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim Biophys Acta. 1966;113:51–56. doi: 10.1016/s0926-6593(66)80120-0. [DOI] [PubMed] [Google Scholar]
- 4.Espinel-Ingroff A, Bartlett M, Bowden R, Chin N X, Cooper C, Jr, Fothergill A, McGinnis M R, Menenzes P, Messer S A, Nelson P W, Odds T C, Pasarell L, Peter J, Pfaller M A, Rex J H, Rinaldi M G, Shankland G S, Walsh T J, Weitzman I. Multicenter evaluation of a proposed standardized procedure for antifungal susceptibility testing of filamentous fungi. J Clin Microbiol. 1997;35:139–143. doi: 10.1128/jcm.35.1.139-143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. p. 633. [Google Scholar]
- 6.Jung M K, May G S, Oakley B R. Mitosis in wild-type and β-tubulin mutant strains of Aspergillus nidulans. Fungal Genet Biol. 1998;24:146–160. doi: 10.1006/fgbi.1998.1057. [DOI] [PubMed] [Google Scholar]
- 7.Martin M A, Osmani S A, Oakley B R. The role of γ-tubulin in mitotic spindle formation and cell cycle progression in Aspergillus nidulans. J Cell Sci. 1997;110:623–633. doi: 10.1242/jcs.110.5.623. [DOI] [PubMed] [Google Scholar]
- 8.Oakley B R, Morris N R. A β-tubulin mutation in Aspergillus nidulans that blocks microtubule function without blocking assembly. Cell. 1981;24:837–845. doi: 10.1016/0092-8674(81)90109-4. [DOI] [PubMed] [Google Scholar]
- 9.Ovechkina, Y. Y., and B. R. Oakley. Unpublished data.
- 10.Pettit G R, Cichacz Z A, Herald C L, Boyd M R, Schmidt J M, Hooper J N A. Isolation and structure of spongistatin 1. J Org Chem. 1993;58:1302–1304. [Google Scholar]
- 11.Pettit R K, McAllister S C, Pettit G R, Herald C L, Johnson J M, Cichacz Z A. A broad-spectrum antifungal from the marine sponge Hyrtios erecta. Int J Antimicrob Agents. 1998;9:147–152. doi: 10.1016/s0924-8579(97)00044-7. [DOI] [PubMed] [Google Scholar]
- 12.Roncal T, Ugalde U O, Barnes J, Pitt D. Production of protoplasts of Penicillium cyclopium with improved viability and functional properties. J Gen Microbiol. 1991;137:1647–1651. [Google Scholar]
- 13.Van Tuyl J M. Genetics of fungal resistance to systemic fungicides. Meded Landbouwhogesch Wageningen. 1977;2:1–137. [Google Scholar]
- 14.Wilson L, Jordan M A. Pharmacological probes of microtubule function. In: Hyams J S, Lloyd C W, editors. Microtubules. New York, N.Y: Wiley-Liss; 1994. pp. 59–83. [Google Scholar]