Abstract
Objectives
Most SARS-CoV-2 seroprevalence studies have focussed on adults and high-risk populations, and little is known about young adults. The objective of the present study was to provide evidence on the SARS-CoV-2 seroprevalence among young adults in Germany and to explore determinants associated with seropositivity in general and, specifically, with previously undetected infections.
Study design
This was a population-based SARS-CoV-2 seroprevalence study.
Methods
In November 2020, a population-based study on SARS-CoV-2 seroprevalence in young adults (aged 18–30 years) was conducted in a large German city. Serum samples were obtained to analyse the SARS-CoV-2 antibody status using the Elecsys Anti-SARS-CoV-2 immunoassay. Descriptive statistics and odds ratios (ORs) of seropositivity and of previously undetected infections in relation to different determinants were calculated.
Results
Among 2186 participants, SARS-CoV-2 antibodies were detected in 72 individuals, equalling a test performance-adjusted seroprevalence of 3.1% (95% confidence interval [CI]: 2.4–4.0). Based on reported COVID-19 cases to the public health authority, a moderate underascertainment rate of 1.7 was calculated. Seropositivity was higher among individuals who sought COVID-19-related information from social media (OR: 1.83, 95% CI: 1.2–3.1), and undetected COVID-19 infections were more prevalent among men and those not adhering to social distancing.
Conclusions
The results show a substantial underascertainment of SARS-CoV-2 infections among young adults and indicate that seroprevalence is likely to be much higher than the reported COVID-19 prevalence based on confirmed COVID-19 cases in Germany. Preventive efforts should consider the heterogeneity of risk profiles among the young adult population.
Keywords: Seroprevalence, SARS-CoV-2 antibodies, COVID-19, Young adults
Introduction
In Germany, the new coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), first appeared in January 2020. At the beginning of the pandemic, high incidence rates were primarily reported among the elderly population; however, the age distribution of those infected with COVID-19 began to shift when numbers started to increase significantly among the younger population in late summer 2020.1, 2, 3 Since the start of the pandemic, there have been ongoing debates about COVID-19 susceptibility in young adults; however, empirical evidence remains scarce and often inconclusive.4, 5, 6
In this context, the use of seroprevalence studies, which assess the number of people in a population who test positive for a specific disease based on blood serum, can help to determine the number of infections at the population level and to identify the magnitude of undetected cases. Currently, however, most SARS-CoV-2 seroprevalence studies have focussed on the general population or on specific high-risk groups (e.g. hospital staff),7 , 8 and only a few studies have considered young adults.4 In some studies, the sample size was too small to enable age group–specific evaluations, and in the larger studies, young adults were frequently not considered as a separate group.9, 10, 11 In studies that did evaluate young adults, only descriptive analyses were conducted;8 , 12 for example, findings from Europe determined the seroprevalence for individual age groups and found that it was highest among 20- to 34-year-olds.8 , 12 To the best of our knowledge, to date, more in-depth studies among young adults have not been carried out.
Many previous studies were conducted during the first pandemic wave, at a time when the COVID-19 incidence was relatively low among young people. From a public health perspective, data on SARS-CoV-2 seroprevalence among young adults are of great importance for several reasons, including (1) young adults are characterised by a high number of asymptomatic cases, which may contribute to the undetected transmissions of the disease;13 (2) young adults are characterised by distinct determinants, including low-risk perception and high mobility;13 , 14 and (3) although most young adults experience a mild disease course, there are increasing concerns of long-term adverse health effects15 and identifying factors linked to infection risk (both detected and undetected) can help to understand SARS-CoV-2 transmission dynamics and consequently support the development of targeted prevention measures. The present study aims to provide evidence on SARS-CoV-2 seroprevalence among young adults in Germany in November 2020 (i.e. during the second pandemic wave in Germany) and to explore determinants associated with seropositivity in general and, specifically, with previously undetected infections.
Methods
The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist for reporting cross-sectional studies was followed in the present study.16
Study design, population and sampling
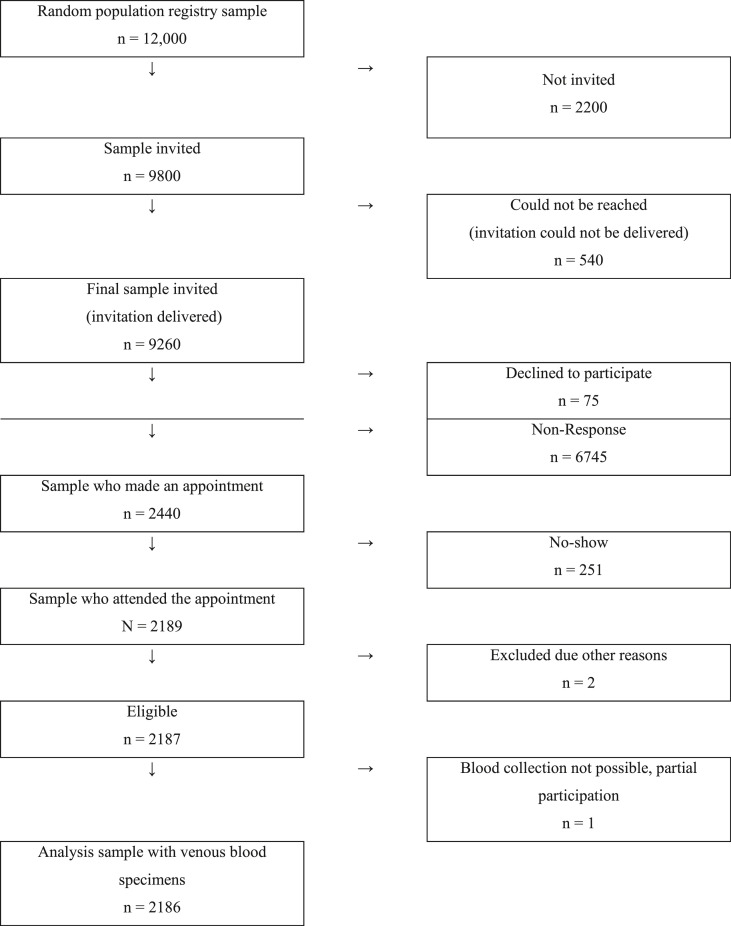
SERODUS is a population-based, cross-sectional seroepidemiological study, which was conducted in Düsseldorf (Germany) between 2 and 27 November 2020. The sampling frame consisted of all individuals aged 18–30 years who were registered in Düsseldorf in October 2020 (n = 106,449). The study sample was selected via random sampling through the population registry (Fig. 1 ). The minimum sample size was calculated to be 1600–2000 individuals, with 95% confidence limits and assuming an anticipated seroprevalence of 1–6% or lower. The anticipated seroprevalence of 1–6% was chosen based on earlier reports that suggested a SARS-CoV-2 seroprevalence of between 1% and 6% in the general adult population.17, 18, 19 To estimate even simple group differences with acceptable error intervals, the target corridor was a net sample of 2000 ± 400 individuals. Inclusion criteria were (1) permanent residence in Düsseldorf, (2) aged 18–30 years at that date when the serum sample was taken and (3) providing written informed consent. All potential participants were invited through written personal postal invitation letters (step 1) sent to their private mailboxes, including information about the study, a personal invitation number and a link to the study website. If interested in participation, an appointment could be made via the study hotline. Given the short recruitment phase of 14 days, reminder letters were sent out 5 days after the initial invitation (step 2).
Fig. 1.
Flow diagram of participant recruitment, enrolment, and study completion.
Informed consent
Participants provided written and informed consent.
Measures
The primary study outcome was SARS-CoV-2 seroprevalence, and the secondary outcome was determinants associated with seropositivity. After providing written and informed consent, study participants completed a self-administered questionnaire, and a blood sample was taken.
Established survey instruments were used to obtain information on participants' sociodemographics (e.g. age and educational level), COVID-19-related symptoms (e.g. fever), chronic conditions (e.g. diabetes), exposure (e.g. participation in festivities) and behaviour-related factors (e.g. adherence to COVID-19 public health measures). Questions relating to sociodemographic characteristics were taken from the demographic standards defined by the German federal statistics office.20 Questions concerning adherence to and support of COVID-19-related public health measures were taken from a COVID-19 questionnaire provided by the Robert Koch Institute, Germany's central public health authority.21 General adherence to public health measures was assessed by asking respondents, “To what extent do you adhere to the coronavirus containment measures, which came into effect on March 18, 2020?” Specific public health measures investigated included social distancing (in private and public settings) and wearing a face mask in public. The type and frequency of the source used to retrieve COVID-19-related information were investigated by asking respondents, “How often do you use social media for COVID-19-related information?” All study material was provided in German, English, Turkish and Arabic languages.
Laboratory analysis and assays
Serum samples were tested for antibodies (including IgG, IgA and IgM) against the nucleocapsid antigen of SARS-CoV-2 (N) using the Elecsys Anti-SARS-CoV-2 immunoassay (Roche Diagnostics, Mannheim, Germany). The assay was performed on a Cobas e801 analyser (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions. The results showing a cut-off index (COI) of <1.0 were classified as negative, and a COI ≥1.0 was deemed positive for anti-SARS-CoV-2 antibodies (hereinafter referred to as seropositive). According to internal study data of Roche Diagnostics, the overall clinical specificity of the Elecsys Anti-SARS-CoV-2 immunoassay was 99.8% containing no cross-reactivity to the common cold coronaviruses, and additionally, a clinical sensitivity of 99.5% was calculated ≥14 days post polymerase chain reaction (PCR) confirmation. Serum samples with positive results were subject to SARS-CoV-2 neutralisation assay to detect SARS-CoV-2 neutralising antibodies with a titre of ≥1:10 being considered positive.22
Data analysis
Data analysis followed a three-step approach.
First, descriptive statistics, including the calculation of frequencies and percentages, were performed to describe the sample and the seroprevalence among young adults.
Second, the underascertainment rate of SARS-CoV-2 infections was calculated. This was based on the ratio of two population proportions: the proportion of SARS-CoV-2 infections calculated from our study and the cumulative incidence of non-fatal PCR-positive cases in the young adult population of Düsseldorf. These estimates were adjusted for test sensitivity (99.5%) and specificity (99.8%) of the Roche Cobas Elecsys Anti-SARS-CoV-2 test.23
Third, to investigate the association between seropositivity and possible risk factors, a series of logistic regressions to calculate odds ratios (ORs) were performed. Specifically, the OR for seropositivity was estimated separately for each main exposure of interest (e.g. adherence to public health measures, travelling outside the European Union [EU] etc.; Model 1). In Model 2, each estimate was adjusted for age and sex (the estimate for age is adjusted only for sex, and the estimate for sex is adjusted only for age).
Analyses were performed using Stata version 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC). All statistical models fit the data well according to the Hosmer–Lemeshow goodness-of-fit test (see supplementary material Table S1).
Results
A total of 2189 individuals attended the study centre (Fig. 1). Three observations were excluded because the individuals could not be tested (e.g. syncope). This yielded an analytic sample of 2186 individuals (24% of those invited) with complete records (i.e. informed consent, questionnaire and laboratory results; Fig. 1).
Sample characteristics
Table 1 shows the sociodemographic characteristics, and Table 2 shows the health- and behaviour-related characteristics of the study participants. Of 2186 participants, 60.7% were female, 37.2% were aged 20–25 years, and 16.9% had a migration background (Table 1). Most participants rated their health as good (81%) and chronic conditions were rare, with only 9.2% of participants reporting having one or more chronic conditions (Table 2).
Table 1.
Sociodemographic characteristics of study participants (n = 2186).
| Characteristics | Participants, n (%) |
|---|---|
| Sex | |
| Female | 1327 (60.7) |
| Male | 857 (39.2) |
| Missing | 2 (0.1) |
| Age group | |
| <20 years | 139 (6.4) |
| 20–25 years | 813 (37.2) |
| 26–30 years | 1218 (55.7) |
| Missing | 16 (0.7) |
| Household size | |
| 1 person | 575 (26.3) |
| 2 persons | 1027 (47.0) |
| ≥3 persons | 567 (25.9) |
| Missing | 17 (0.8) |
| Educational levela | |
| Lower and middle | 171 (7.8) |
| Higher | 1921 (87.9) |
| Still a student or other type of education | 81 (3.7) |
| Missing | 13 (0.6) |
| Employment status | |
| Not workingb | 110 (5.0) |
| Part-time and short-term (reduced working hours) | 164 (7.5) |
| Full time | 1180 (54.0) |
| Student | 703 (32.2) |
| Missing | 29 (1.3) |
| Migration background | |
| Yes | 370 (16.9) |
| No | 1803 (82.5) |
| Missing | 13 (0.6) |
Participants' education level was assessed according to the 2011 version of the International Standard Classification of Education (ISCED) and grouped into low/middle education (e.g. primary education) and higher education.
This also includes pensioners and parents on maternity leave.
Table 2.
Health-related characteristics and social determinants of study participants (n = 2186).
| Characteristics | Participants, n (%) |
|---|---|
| Self-reported health | |
| Very good/excellent | 1771 (81.0) |
| Fair/bad | 385 (17.6) |
| Missing | 30 (1.4) |
| Chronic condition | |
| No | 1975 (90.3) |
| Yes | 201 (9.2) |
| Missing | 10 (0.5) |
| PCR test since February 2020 | |
| Yes | 784 (35.9) |
| No, no test needed | 1126 (51.5) |
| No, but I thought about getting tested | 194 (8.9) |
| No, I asked for a test but did not get one | 72 (3.3) |
| Missing | 10 (0.5) |
| Self-reported COVID-19 | |
| Yes | 46 (2.1) |
| No | 728 (33.3) |
| I do not know | 10 (0.4) |
| Symptoms since February 1a | |
| Fever ≥38 °C | 225 (10.3) |
| Cough | 648 (29.8) |
| Pneumonia | 7 (0.3) |
| Dyspnoea/shortness of breath | 121 (5.6) |
| Pain when breathing | 112 (5.2) |
| Congested/running nose | 848 (39.0) |
| Sore throat | 854 (39.1) |
| Loss of smell or taste | 100 (4.6) |
| No symptoms | 511 (23.5) |
| Contact to a confirmed COVID-19 case | |
| No | 1711 (78.3) |
| Yes, with a distance ≥1.5 m | 179 (8.2) |
| Yes, with a distance <1.5 m | 285 (13.0) |
| Missing | 11 (0.5) |
| Other exposuresa | |
| Working with patients | 262 (12.0) |
| Working with customers | 340 (15.6) |
| Participated in an event with ≥50 persons | 850 (39.1) |
| Travelled outside the EU | 246 (11.3) |
| Travelled within the EU | 1068 (49.0) |
| General adherence to public health measuresb | |
| Adheres completely to public health measures | 1243 (56.9) |
| Adheres partly to public health measures | 919 (42.0) |
| Adheres little to public health measures | 9 (0.4) |
| Does not adhere to public health measures at all | 0 (0.0) |
| Missing | 15 (0.7) |
| COVID-19 information resource: social media | |
| Rarely/never | 1488 (68.1) |
| Often/always | 686 (31.4) |
| Missing | 12 (0.5) |
| Trust in COVID-19 information from social media influencer | |
| Trust | 277 (12.7) |
| No trust | 1891 (86.5) |
| Missing | 18 (0.8) |
| Supporting social distancing in private (contact restriction) | |
| Yes | 1968 (90.0) |
| No | 204 (9.4) |
| Missing | 14 (0.6) |
| Supporting social distancing in public (at least 1.5 m distance) | |
| Yes | 2126 (97.3) |
| No | 488 (2.2) |
| Missing | 12 (0.5) |
| Supporting wearing a face mask in public spaces | |
| Yes | 2165 (99.0) |
| No | 13 (0.6) |
| Missing | 8 (0.4) |
| Supporting the travel restriction | |
| Yes | 1991 (91.1) |
| No | 169 (7.7) |
| Missing | 26 (1.2) |
PCR, polymerase chain reaction.
Multiple replies were possible.
In the following analyses, the categories "partly" and "little" were categorised as "partly, adherence to public health measures was assessed by asking respondents.
Seroprevalence
Of 2186 young adults who participated in the present study, a total of 72 individuals were seropositive, representing a crude prevalence rate of 3.3% (95% confidence interval [CI]: 2.6–4.1) and a test-adjusted prevalence of 3.1% (95% CI: 2.4–4.0; Table 3 ). The cumulative incidence of reported cases in the population aged 18–30 years was 1.8% (as of November 2020). Based on the estimated test-adjusted seroprevalence of 3.1% and the cumulative incidence of 1.8%, it is estimated that approximately 1.7-fold more infections occurred than were ascertained by confirmed case counts. Only 31 (43.1%) of individuals with antibodies had tested positive for COVID-19 by PCR before the present study. Consequently, the within-study ‘true’ rate of unreported COVID-19 cases in this population is 2.3. Among the 72 seropositive individuals, neutralising antibodies were detected in 66 individuals (91.7%; Table 3).
Table 3.
Comparison of different prevalence measures of SARS-CoV-2 antibodies (n = 2186).
| Prevalence measure | SARS-CoV-2 seroprevalence (unadjusted, n = 72), % (95% CI)] | SARS-CoV-2 seroprevalence (adjusteda, n = 72), % (95% CI) | Participants with neutralising antibodies titresb (n = 66), % (95% CI) |
|---|---|---|---|
| Overall seroprevalence | 3.3 (2.6–4.1) | 3.1 (2.4–4.0) | 3.0 (2.4–3.8) |
| Percentage of those who are seropositive and had a positive PCR before study | 2.1 (1.6–2.8) | 1.9 (1.4–2.6) | N/A |
| Percentage of those with neutralising antibody titres and a positive PCR before study | 45.5 (34.0–57.4) | 45.0 (34.1–57.6) | N/A |
CI, confidence interval; N/A, not applicable.
Adjusted for Roche Cobas Elecsys Anti-SARS-CoV-2 test sensitivity and specificity.
Only in case of a positive Roche Cobas Elecsys Anti-SARS-CoV-2 test (n = 66) neutralising antibody titre assay was performed.
Determinants of a SARS-CoV-2 infection
Table 4 and Table S2 provide a detailed presentation of seroprevalence by sociodemographic and health-related characteristics. The proportion of men with positive SARS-CoV-2 antibodies (3.8%) was slightly higher than the proportion of women (2.7%). The odds of being seropositive was significantly higher among those with self-reported COVID-19 symptoms, such as loss of smell (OR: 55.6, 95% CI: 30.7–99.0), loss of taste (OR: 40.8, 95% CI: 23.0–75.5), fever ≥38 °C (OR: 5.06, 95% CI: 3.0–8.6), dyspnoea and shortness of breath (OR: 4.15, 95% CI: 2.1–7.6; Table S2). The odds of being seropositive were also almost two times higher for those who seek COVID-19-related information from social media (Table S2; OR: 1.83, 95% CI: 1.2–3.1). An increasing trend was seen in individuals who trust information from social media influencers (OR: 1.78, 95% CI: 0.9–3.3) and who do not support social distancing (Table S2; OR: 1.81, 95% CI: 0.9–3.7). Unexpectedly, we did not find evidence for a significant association between low self-reported general adherence to COVID-19 public health measures and seroprevalence (Table S2; OR: 0.91, 95% CI: 0.5–1.5) or for participants who had travelled both within (Table S2; OR: 0.98, 95% CI: 0.6–1.6) and outside (Table S2; OR: 0.72, 95% CI: 0.3–1.8) the EU. Interestingly, no significant associations were found for those aged 20–25 years (OR: 1.27; 95% CI: 0.4–3.9), individuals with a secondary school education (OR: 1.15; 95% CI: 0.4–2.9) and those who worked with patients (OR: 1.12, 95% CI: 0.5–2.4) and customers (OR: 1.36, 95% CI: 0.78–2.38).
Table 4.
SARS-CoV-2 seroprevalence in young adults and determinants of infection (n = 2186).
| Characteristic | Distribution among seropositive participants |
OR for being seropositive unadjusted |
OR for being seropositive adjusted for age and sexa |
Distribution among seropositive participants, but without prior self-reported SARS-CoV-2 infection (positive by PCR)b |
|---|---|---|---|---|
| %c [95% CI]; n | OR [95% CI] | OR [95% CI] | %c [95% CI]; n | |
| Sex | ||||
| Male | 3.8% [2.7–5.3]; 34 | Refd | Ref | 2.6% [1.7–3.9]; 23 |
| Female | 2.7% [1.9–3.7]; 38 | 0.70 [0.42–1.15] | 0.70 [0.42–1.16] | 1.3% [0.7–2.0]; 18 |
| Missing (n) | 0 | – | – | 0 |
| Age group | ||||
| <20 years | 2.7% [0.9–7.0]; 4 | Ref | Ref | 2.0% [0.6–6.2]; 3 |
| 20–25 years | 3.4% [2.3–4.9]; 29 | 1.27 [0.4–3.9] | 1.30 [0.6–2.0] | 2.2% [1.13–3.5]; 19 |
| 26–30 years | 2.9% [2.0–4.0]; 37 | 1.07 [0.4–3.3] | 0.94 [0.4–2.0] | 1.3% [0.8–2.2]; 18 |
| Missing (n) | 2 | – | – | 1 |
| Household size | ||||
| 1 person | 3.1% [1.9–4.9]; 19 | Ref | Ref | 1.6% [0.8–3.1]; 10 |
| 2 persons | 3.2% [2.3–4.6]; 35 | 1.03 [0.6–1.9] | 1.06 [0.6–2.0] | 1.8% [1.1–2.9]; 20 |
| ≥3 persons | 2.8% [1.7–4.6]; 17 | 0.90 [0.4–1.8] | 0.94 [0.6–2.0] | 1.6% [0.8–3.1]; 10 |
| Missing (n) | 1 | – | – | 1 |
| Educational level | ||||
| Lower/middle | 2.7% [1.1–6.5]; 5 | Ref | Ref | 1.0% [0.1–4.2]; 2 |
| Higher | 3.2% [2.4–4.1]; 64 | 1.15 [0.4–2.9] | 1.52 [0.50–4.63] | 1.9% [1.2–2.5]; 37 |
| Still a student/other degree | 2.3% [0.5–8.4]; 2 | 0.83 [0.1–5.0] | 1.10 [0.17–7.18] | 2.3% [0.5–8.4]; 2 |
| Missing (n) | 1 | – | – | 0 |
| Employment status | ||||
| Full time | 3.1% [2.2–4.3]; 39 | Ref | Ref | 1.7% [1.1–2.7]; 22 |
| Not working | 5.3% [2.3–11.3]; 6 | 1.73 [0.7–4.3] | 1.88 [0.7–4.7] | 2.7% [0.8–8.1]; 3 |
| Part-time or reduced working hours | 4.1% [1.9–8.4]; 7 | 1.32 [0.6–3.1] | 1.43 [0.6–3.4] | 12 |
| Student | 2.2% [1.3–3.7]; 17 | 0.71 [0.4–1.32] | 0.71 [0.4–1.3] | 1.7% [0.9–3.1]; 13 |
| Missing | 3 | – | – | 4.4% [2.3–21.7]; 1 |
| Migration background | ||||
| No | 3.2% [2.4–4.1]; 60 | Ref | Ref | 1.6% [1.1–4.1]; 32 |
| Yes | 2.5% [1.3–4.7]; 10 | 0.79 [0.4–1.62] | 0.81 [0.4–1.65] | 1.8% [0.7–3.8]; 7 |
| Missing (n) | 2 | – | – | 2 |
CI, confidence interval; OR, odds ratio.
The estimates for household size, education, employment status and migration background are adjusted for age and sex, the estimate for age is adjusted only for sex and the estimate for sex is adjusted for age only.
For the analysis of subjects with antibodies but without self-reported SARS-CoV-2 infection, subjects with positive PCR test (n = 46) or unknown PCR result (n = 10) were excluded, resulting in a sample size of 2130 participants.
This is the test-adjusted seroprevalence; row-percentages.
Ref = reference category.
Determinants of an undetected SARS-CoV-2 infection
Table 4 and Table S2 also show a subgroup analysis of participants who were seropositive but did not report a prior SARS-CoV-2 infection (right column). Because of the small sample size, ORs were not calculated, and the interpretation is based exclusively on the 95% CIs. The analysis showed that undetected infections are about twice as likely in men (2.6%, 95% CI: 1.7–3.99) than in women (1.3%, 95% CI: 0.7–2.0). The seroprevalence estimate was also considerably higher among individuals who did not adhere to social distancing (2.9%, 95% CI: 1.2–6.3) compared with individuals who adhered to social distancing (1.6%, 95% CI: 1.1–2.3) and among those who seek COVID-19-related information from social media (2.5%, 95% CI: 1.6–4.3) compared with those who did not seek COVID-19-related information from social media (1.3%, 95% CI: 0.8–2.1). The seroprevalence estimate was also higher in individuals who had considered SARS-CoV-2 testing in the past but ultimately did not go ahead with testing. Specifically, while 1.7% of total participants had an undetected infection, 5.0% (95% CI: 2.6–9.1) of those who had considered being tested and 5.4% (95% CI: 2.0–13.3) of those who had not received a test despite requesting one were seropositive. Higher prevalence estimates were also found for individuals who attended an event with >50 people (2.4%, 95% CI: 1.6–3.8) and who travelled outside the EU (2.3%, 95% CI: 0.9–5.1).
Discussion
In the present study, a test-adjusted SARS-CoV-2 seroprevalence of 3.1% and an underascertainment factor of 1.7 were found among young adults in Germany. Factors significantly associated with SARS-CoV-2 seroprevalence included self-reported symptoms (e.g. loss of smell) and seeking COVID-19-related information from social media. Factors significantly associated with an undetected SARS-CoV-2 infection were being male, not adhering to social distancing and seeking COVID-19-related information from social media.
Compared with estimates from previous national and international studies, the reported seroprevalence and underascertainment rate in the present study are lower than in the general adult population.24 , 25 For example, a study from the United States determined a test-adjusted seroprevalence as high as 23% in some counties,24 and in Germany, Neuhauser et al. reported an underascertainment factor between 2 and 6.25 There are at least two possibilities that might explain the observed differences. First, most studies focussed on different age groups and specific population groups, such as healthcare personnel.7 , 26 , 27 Furthermore, younger people often experience an asymptomatic or mild SARS-CoV-2 infection, which is associated with lower serum titres.28 Consequently, it is possible that younger individuals have titres that fall below the threshold of serological assays, and therefore, previous infection may be less frequently detected. Second, the majority of studies were conducted at different times of the pandemic and often in hotspot areas;10 , 17 , 29 , 30 it should be noted that the timing of the study is crucial, as seroprevalence rates may vary between different pandemic phases. Specifically, many previous seroprevalence studies were performed during the first pandemic wave in spring 2020. In Germany, during this time, significantly fewer PCR tests were performed than during the second wave (starting in November 2020), and therefore, it is likely that at the beginning of the pandemic, a much higher rate of COVID-19 cases remained undetected. Furthermore, it is important to note that to correct the seroprevalence for test sensitivity and specificity, this study applied a conservative approach by using very high sensitivity and specificity values; thus, the corrected seroprevalence is lower than the uncorrected seroprevalence. However, if sensitivity and specificity were below the manufacturer's specification, seroprevalence would be higher and the underreporting rate more distinct. In addition, it has been suggested that antibody titres decline over time.31 We cannot exclude the possibility that some antibody responses no longer existed by the time of the survey, which would consequently underestimate the seroprevalence.
Potential determinants for seropositivity and undetected infection included self-reported symptoms and seeking COVID-19-related information from social media.
The findings regarding self-reported symptoms are in line with previous reports, which show that a loss of smell and taste, as well as a fever ≥38 °C were the most strongly associated factors with SARS-CoV-2 seropositivity.26 , 32 , 33 Martínez et al.,33 for instance, found that fever was among the most frequently described symptoms by seropositive individuals.
The results regarding social media use are novel. Currently, most studies investigating social media use in times of COVID-19 focussed on preventive behaviour in general34 , 35 but have not associated it with the actual SARS-CoV-2 seropositivity. Nonetheless, the results are somewhat contradictory to the findings of the present study. For example, Mahmood et al.34 found that social media use predicts self-efficacy and perceived threat of COVID-19, which, in turn, predicts preventive behaviour. However, in the present study, participants who sought COVID-19-related information from social media were found to be significantly more likely to be seropositive, and undetected cases in this group were almost twice as likely. It is possible that seropositive individuals in the present study viewed content that downplayed the COVID-19 infection risk, which may have led to lower adherence levels to public health measures. However, this remains speculative, as these interrelations could not be detected in the present study. Furthermore, it is important to note that these results may vary over time, might be wave sensitive (i.e. severity of this specific pandemic wave) and depend on information available and consequent knowledge about COVID-19. Nevertheless, social media platforms can play a critical role in the spread of information, particularly in times of crisis, and might impact adherence to public health recommendations.36 , 37 Furthermore, given that social media platforms are often the main vehicle for communication among young adults, understanding the dynamics between content consumption and social behaviour may help design more effective communication strategies.
No statistically significant results were found for the association between general adherence to COVID-19 public health measures and seropositivity. However, there was a tendency for seropositivity among individuals who reported that they were in support of social distancing. This may seem surprising at first but could be explained by the fact that adherence to prevention measures was retrospectively assessed. Therefore, information, recall bias and social desirability bias cannot be ruled out.27 Nonetheless, given that social distancing effectively reduces the risk of transmission and that young adults, despite their often mild disease course, can still spread the virus, they represent a subgroup of the population requiring additional attention from public health campaigns.38
The findings suggest that large-scale COVID-19 testing can help to detect more COVID-19 cases. The expansion and continuous development of testing are recommended to secure the containment of the pandemic. Furthermore, vaccination is also essential in younger age groups, especially as recent cross-sectional data from adolescents and young adults found evidence of new or persisting COVID-19 symptoms, such as fatigue, insomnia, headache, concentration difficulties, exercise intolerance and chest pain, 1-month postdiagnosis.39 , 40 Vaccinating young adults may, therefore, help prevent the long-term health effects of COVID-19. Furthermore, recent evidence also suggests that vaccination can help reduce the transmission of the disease.41 Given the high mobility of young adults, increasing immunisation in this population group can help prevent transmission, thereby also protecting vulnerable groups, such as the elderly.
The present study has some limitations that must be noted.
First, we aimed to explore the association between specific (social) determinants and seropositivity, but the subgroups were relatively small, which unfortunately precluded detailed and robust statistical analysis.
Second, the response rate of 24% was lower than that of other seroprevalence studies17 , 25 but higher than an epidemiological study conducted in Germany (i.e. NAKO).42 Nonetheless, the response rates of the other seroprevalence studies are only comparable to a limited extent because different groups were investigated and many studies focussed on hotspot areas.30 The recruitment period of the present study was short (14 days), resulting in a limited number of individuals being available to participate (i.e. recruitment could not be fully completed in every format [e.g. telephone recruitment attempts]); however, it did enable calculation of SARS-CoV-2 seroprevalence at a specific point in time.
Third, it is impossible to gauge whether study participants were subject to systematic bias because contact could not be established with some invitees. A comparison with the age and gender structure of the population reveals that the net sample is well represented in terms of age, but not gender. Similar to other seroprevalence studies, men were underrepresented.10 , 43 However, because men often have a higher seroprevalence and more undetected infections, it is possible that the overall seroprevalence is underestimated in the present study.
Finally, sampling bias may have also occurred. Specifically, the prevalence of COVID-19 infection was higher in this study than the officially reported cumulative prevalence. While the official reported cumulative prevalence among young adults was 1.8%, in the present study, 2.1% of participants reported a prior COVID-19 infection. It is likely that individuals with a prior infection were more sensitised and had an increased interest in participating.
In conclusion, the findings of this study suggest that the SARS-CoV-2 seroprevalence is likely to be much higher than the reported prevalence rates based on confirmed COVID-19 cases. The results further suggest that social media play an important role in young adults’ risk perception. Given the limited amount of seroepidemiological data for young adults, a follow-up study to improve the understanding of SARS-CoV-2 seroprevalence and to better identify determinants of a SARS-CoV-2 infection is needed.
Author statements
Acknowledgements
The authors wish to thank the study participants for their contribution to the research. They also wish to thank the personnel, and past staff, at the Mitsubishi Electric Hall and the MedLog24 team.
Ethical approval
The study was approved by the institutional ethics committees of the Medical Faculty of the Heinrich Heine University (reference number: IT-TEMP-2020-1042) and was conducted in line with the Declaration of Helsinki. Study participation was voluntary and could be withdrawn at any time; refusal to participate had no consequences. All participants provided informed written consent.
Funding
This work was supported by the Ministry of Labor, Health and Social Affairs of North Rhine-Westphalia. Methodological support for this study was provided by MethodCov "Method Network to Support Covid-19 Research Projects in Measuring Social and Contextual Factors" – funded by the BMBF as part of the National Network University Medicine initiative. The funder did not have a role in the design, collection, analysis, interpretation of data and/or writing of this manuscript.
Competing interests
None declared.
Availability of data and material
The data sets used and/or analysed during the present study can be made available on reasonable request and following the approval of data protection officer of the Heinrich Heine University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.puhe.2022.03.009.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Aleta A., Moreno Y. Age differential analysis of COVID-19 second wave in Europe reveals highest incidence among young adults. medRxiv. 2020:2020. 11.11.20230177. [Google Scholar]
- 2.Leidman E. MMWR Morb Mortal Wkly Rep; 2021. COVID-19 trends among persons aged 0–24 Years — United States, March 1–December 12, 2020; p. 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koch-Institut Robert. 2020. Täglicher Lagebericht des RKI zur Coranavirus-Krankheit-2019 (COVID-19) 8.12.2020. [Google Scholar]
- 4.Rumain B., Schneiderman M., Geliebter A. Prevalence of COVID-19 in adolescents and youth compared with older adults in states experiencing surges. PLoS One. 2021;16 doi: 10.1371/journal.pone.0242587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boehmer T.K. Changing age distribution of the COVID-19 pandemic — United States, May–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69 doi: 10.15585/mmwr.mm6939e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monod M., Blenkinsop A., Xi X., Hebert D., Bershan S., Tietze S., et al. Age groups that sustain resurging COVID-19 epidemics in the United States. Science. 2021 doi: 10.1126/science.abe8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin C.A., Patel P., Goss C., Jenkins D.R., Price A., Barton L., et al. Demographic and occupational determinants of anti-SARS-CoV-2 IgG seropositivity in hospital staff. J Public Health. 2020:fdaa199. doi: 10.1093/pubmed/fdaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollán M., Pérez-Gómez B., Pastor-Barriuso R., Oteo J., Hernán M.A., Pérez-Olmeda M., et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Havers F.P., Reed C., Lim T., Montgomery J.M., Klena J.D., Hall A.J., et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020. JAMA Intern Med. 2020;180:1576–1586. doi: 10.1001/jamainternmed.2020.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stringhini S., Wisniak A., Piumatti G., Azman A.S., Lauer S.A., Baysson H., et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396:313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bajema K.L., Wiegand R.E., Cuffe K., Patel S.V., Iachan R., Lim T., et al. Estimated SARS-CoV-2 seroprevalence in the US as of September 2020. JAMA Intern Med. 2021;181:450–460. doi: 10.1001/jamainternmed.2020.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knabl L., Mitra T., Kimpel J., Rössler A., Volland A., Walser A., et al. High SARS-CoV-2 seroprevalence in children and adults in the Austrian Ski Resort Ischgl. medRxiv. 2020 doi: 10.1038/s43856-021-00007-1. 2020.08.20.20178533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies N.G., Klepac P., Liu Y., Prem K., Jit M., Eggo R.M. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26:1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- 14.Nivette A., Ribeaud D., Murray A., Steinhoff A., Bechtiger L., Hepp U., et al. Non-compliance with COVID-19-related public health measures among young adults in Switzerland: insights from a longitudinal cohort study. Soc Sci Med. 2021;268:113370. doi: 10.1016/j.socscimed.2020.113370. 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Björkander S., Du L., Zuo F., Ekström S., Wang Y., Wan H., et al. SARS-CoV-2–specific B- and T-cell immunity in a population-based study of young Swedish adults. J Allergy Clin Immunol. 2022;149:65–75.e8. doi: 10.1016/j.jaci.2021.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.E von E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 17.Santos-Hövener C., Neuhauser H.K., Rosario A.S., Busch M., Schlaud M., Hoffmann R., et al. Serology- and PCR-based cumulative incidence of SARS-CoV-2 infection in adults in a successfully contained early hotspot (CoMoLo study), Germany, May to June 2020. Euro Surveill. 2020;25:2001752. doi: 10.2807/1560-7917.ES.2020.25.47.2001752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robert-Koch-Institut Serologische Unterschungen von Blutspenden auf Antikörper gegen SARS-CoV-2 - SeBluCo-Studie. Epidemiol Bull. 2020;29:14. [Google Scholar]
- 19.Santos-Hövener C., Busch M.A., Koschollek C., Schlaud M., Hoebel J., Hoffmann R., et al. Seroepidemiologische Studie zur Verbreitung von SARS-CoV-2 in der Bevölkerung an besonders betroffenen Orten in Deutschland – Studienprotokoll von CORONA-MONITORING lokal. 2020. [DOI]
- 20.Statistisches Bundesamt (Destatis) Demografische standards. Statistisches Bundesamt. 2020. https://www.destatis.de/DE/Methoden/Demografische-Regionale-Standards/textbaustein-demografische-standards.html
- 21.Santos-Hövener C., Busch M.A., Koschollek C., Schlaud M., Hoebel J., Hoffmann R., et al. Seroepidemiological study on the spread of SARS-CoV-2 in populations in especially affected areas in Germany – study protocol of the CORONA-MONITORING lokal study. 2020. [DOI] [PMC free article] [PubMed]
- 22.Müller L., Ostermann P.N., Walker A., Wienemann T., Mertens A., Adams O., et al. Sensitivity of anti-SARS-CoV-2 serological assays in a high-prevalence setting. Eur J Clin Microbiol Infect Dis. 2021;40(5):1063–1071. doi: 10.1007/s10096-021-04169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogan W.J., Gladen B. Estimating prevalence from the results OF a screening test. Am J Epidemiol. 1978;107:71–76. doi: 10.1093/oxfordjournals.aje.a112510. [DOI] [PubMed] [Google Scholar]
- 24.Bajema K.L., Wiegand R.E., Cuffe K., Patel S.V., Iachan R., Lim T., et al. Estimated SARS-CoV-2 seroprevalence in the US as of September 2020. JAMA Intern Med. 2020;181(4):450–460. doi: 10.1001/jamainternmed.2020.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuhauser H., Thamm R., Buttmann-Schweiger N., Fiebig J., Offergeld R., Poethko-Müller C., et al. 2020. Ergebnisse seroepidemiologischer Studien zu SARS-CoV-2 in Stichproben der Allgemeinbevölkerung und bei Blutspenderinnen und Blutspendern in Deutschland. (Stand 03.12.2020) [DOI] [Google Scholar]
- 26.Rudberg A.-S., Havervall S., Månberg A., Jernbom Falk A., Aguilera K., Ng H., et al. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun. 2020;11:5064. doi: 10.1038/s41467-020-18848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roederer T., Mollo B., Vincent C., Nikolay B., Llosa A.E., Nesbitt R., et al. Seroprevalence and risk factors of exposure to COVID-19 in homeless people in Paris, France: a cross-sectional study. Lancet Public Health. 2021;6:E202–E209. doi: 10.1016/S2468-2667(21)00001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bläckberg A., Fernström N., Sarbrant E., Rasmussen M., Sunnerhagen T. Antibody kinetics and clinical course of COVID-19 a prospective observational study. PLoS One. 2021;16 doi: 10.1371/journal.pone.0248918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shields A., Faustini S.E., Perez-Toledo M., Jossi S., Aldera E., Allen J.D., et al. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020;75:1089–1094. doi: 10.1136/thoraxjnl-2020-215414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Streeck H., Schulte B., Kümmerer B.M., Richter E., Höller T., Fuhrmann C., et al. Infection fatality rate of SARS-CoV-2 infection in a German community with a super-spreading event. medRxiv. 2020 doi: 10.1038/s41467-020-19509-y. 2020.05.04.20090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marot S., Malet I., Leducq V., Zafilaza K., Sterlin D., Planas D., et al. Rapid decline of neutralizing antibodies against SARS-CoV-2 among infected healthcare workers. Nat Commun. 2021;12:844. doi: 10.1038/s41467-021-21111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stefanelli P., Bella A., Fedele G., Pancheri S., Leone P., Vacca P., et al. Prevalence of SARS-CoV-2 IgG antibodies in an area of northeastern Italy with a high incidence of COVID-19 cases: a population-based study. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martínez P.T., García P.D., Salas M.R., Sánchez R.R., Avendaño-Ortíz J., Guerrero-Monjo S., et al. SARS-CoV-2 IgG seropositivity in a cohort of 449 non-hospitalized individuals during Spanish COVID-19 lockdown. Sci Rep. 2021;11(1):21612. doi: 10.1038/s41598-021-00990-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahmood Q.K., Jafree S.R., Mukhtar S., Fischer F. Social media use, self-efficacy, perceived threat, and preventive behavior in times of COVID-19: results of a cross-sectional study in Pakistan. Front Psychol. 2021;12:2354. doi: 10.3389/fpsyg.2021.562042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu P.L. COVID-19 information on social media and preventive behaviors: managing the pandemic through personal responsibility. Soc Sci Med. 1982;2021:277. doi: 10.1016/j.socscimed.2021.113928. 113928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cinelli M., Quattrociocchi W., Galeazzi A., Valensise C.M., Brugnoli E., Schmidt A.L., et al. The COVID-19 social media infodemic. Sci Rep. 2020;10:16598. doi: 10.1038/s41598-020-73510-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrara E., Cresci S., Luceri L. Misinformation, manipulation, and abuse on social media in the era of COVID-19. J Comput Soc Sci. 2020;3:271–277. doi: 10.1007/s42001-020-00094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chu D.K., Akl E.A., Duda S., Solo K., Yaacoub S., Schünemann H.J., et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walsh-Messinger J., Manis H., Vrabec A., Sizemore Bs J., Bishof K., Debidda M., et al. The kids are not alright: a preliminary report of Post-COVID syndrome in university students. J Am Coll Health J ACH. 2021:1–7. doi: 10.1080/07448481.2021.1927053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buonsenso D., Munblit D., De Rose C., Sinatti D., Ricchiuto A., Carfi A., et al. Preliminary evidence on long COVID in children. Acta Paediatr. 2021;110:2208–2211. doi: 10.1111/apa.15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Gier B., Andeweg S., Backer J.A. RIVM COVID-19 surveillance and epidemiology team, Hahné SJ, van den Hof S, et al. Vaccine effectiveness against SARS-CoV-2 transmission to household contacts during dominance of Delta variant (B.1.617.2), The Netherlands, August to September 2021. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.44.2100977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schipf S., Schöne G., Schmidt B., Günther K., Stübs G., Greiser K.H., et al. Die Basiserhebung der NAKO Gesundheitsstudie: Teilnahme an den Untersuchungsmodulen, Qualitätssicherung und Nutzung von Sekundärdaten. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 2020;63:254–266. doi: 10.1007/s00103-020-03093-z. [DOI] [PubMed] [Google Scholar]
- 43.Shook-Sa B.E., Boyce R.M., Aiello A.E. Estimation without representation: early severe acute respiratory syndrome coronavirus 2 seroprevalence studies and the path forward. J Infect Dis. 2020;222:1086–1089. doi: 10.1093/infdis/jiaa429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets used and/or analysed during the present study can be made available on reasonable request and following the approval of data protection officer of the Heinrich Heine University.