Abstract
Multiple sclerosis (MS) is a chronic neurodegenerative disease characterized by inflammation and demyelination for which there is currently no cure; therefore, the aim of therapy is to reduce the risk of relapse and disability progression. The treatment options for MS have increased greatly in recent years with the development of several disease-modifying therapies (DMTs) and the advent of immune reconstitution therapy (IRT). IRTs are administered in short-dosing periods to produce long-term effects on the immune system. Treatment with an IRT is based on the 3Rs: reduction, repopulation, and reconstitution of lymphocytes, which leads to restoration of immune effector functions. Cladribine tablets represent a selective, high-efficacy, oral form of IRT for patients with MS that targets lymphocytes and spares innate immune cells. Patients require only two weekly treatment courses, with each course comprising two treatment weeks, in Years 1 and 2; therefore, cladribine tablets are associated with a lower monitoring burden than many other DMTs, while short dosing periods can help to improve adherence. This review provides an overview of IRT and offers the clinician’s perspective on the current MS treatment landscape, with a focus on practical advice for the management of patients undergoing treatment with cladribine tablets based on the most recent evidence available, including risks associated with COVID-19 and recommendations for vaccination in patients with MS.
Keywords: Multiple sclerosis, Cladribine, Immune reconstitution therapy, Disease-modifying therapy
Key Summary Points
| The treatment landscape for multiple sclerosis has greatly increased in recent years with the development of new, high-efficacy disease-modifying therapies. |
| Cladribine tablets are considered one such high-efficacy, immune reconstitution therapy, targeting lymphocytes and sparing innate immune cells in patients with multiple sclerosis. |
| Offering a clinician’s perspective, this review covers the current treatment landscape and provides practical advice for the management of patients. |
| The review also discusses the risks associated with COVID-19 and the current recommendations for vaccination. |
Introduction
Multiple sclerosis (MS) is a chronic neurodegenerative disease characterized by inflammation and demyelination, with over 2.8 million cases globally and approximately 133 cases per 100,000 population in Europe [1]. There is currently no cure for this disease; therefore, the aim of therapy is to reduce the risk of relapse and disability progression [1]. The heterogeneity of MS symptoms, differing rates of progression among patients, and the ongoing changes in diagnostic criteria limit direct comparisons of studies for different drugs. As such, choosing the right therapy on a per-patient basis remains a difficult task [2].
The treatment options for MS have increased greatly in recent years with the development of several disease-modifying therapies (DMTs) [3]. However, many of these options are maintenance therapies, which are dosed continuously and associated with high treatment and monitoring burdens that may adversely impact on treatment adherence [3, 4]. Recently, treatment options have continued to evolve with high-efficacy biologic and oral treatments, including cladribine tablets (3.5 mg/kg cumulative dose over 2 years). Cladribine tablets offer a high-efficacy oral treatment for patients with MS, with only two weekly treatment courses required in Years 1 and 2. The efficacy of cladribine tablets was evaluated in the CLARITY study, a 96-week placebo-controlled trial that assessed two doses of oral cladribine (cumulative dose: 3.5 or 5.25 mg/kg) compared with placebo [5]. The approved dose of cladribine tablets (3.5 mg/kg) led to a significantly reduced annualized relapse rate (0.14 vs. 0.33; P < 0.001), lower risk of 3-month sustained disability progression (hazard ratio: 0.67, 95% confidence interval: 0.48–0.93; P = 0.02), and a relative reduction in the number of T1 gadolinium-enhancing (Gd+) lesions (85.7%), active T2 lesions (73.4%), and combined unique lesions (74.4%), compared with placebo (Table 1) [5]. For the majority of patients (75%), the clinical benefits, in terms of relapse-free status, of cladribine tablets (3.5 mg/kg) given in Years 1 and 2 in CLARITY were maintained in Years 3 and 4 of the CLARITY Extension trial when patients originally treated with cladribine tablets were re-randomized to placebo for an additional 2 years [6].
Table 1.
Key efficacy results from the CLARITY and CLARITY Extension trials (licensed dose only)
| Key efficacy parameters | CLARITYa | CLARITY Extensiona | ||
|---|---|---|---|---|
| Cladribine tablets 3.5 mg/kg | Placebo | CP 3.5 mg/kg | PC 3.5 mg/kg | |
| Annualized relapse rate (CI)b,c | 0.14 (0.12–0.17) | 0.33 (0.29–0.38) | 0.15 (0.09–0.21) | 0.10 (0.07–0.13) |
| Patients relapse free, n (%) | 345 (79.7) | 266 (60.9) | 68 (75.6) | 180 (79.6) |
| Lesion activity on brain MRI, mean number | ||||
| T1 Gd+ lesions | 0.12 | 0.91 | 0.28 | 0.07 |
| Active T2 lesions | 0.38 | 1.43 | 1.42 | 1.07 |
| Combined unique lesions | 0.43 | 1.72 | NR | NR |
CI Confidence interval, CP cladribine tablets 3.5 mg/kg in CLARITY followed by placebo in CLARITY Extension, Gd+ gadolinium-enhancing, MRI magnetic resonance imaging, NR not reported, PC placebo in CLARITY followed by cladribine tablets 3.5 mg/kg in CLARITY Extension
aNon-approved cladribine tablets doses and regimens from CLARITY and CLARITY Extension are not shown
bA relapse was defined as an increase of 2 points in at least one functional system of the Expanded Disability Status Scale (EDSS) or an increase of 1 point in at least two functional systems (excluding changes in bowel or bladder function or cognition) in the absence of fever, to have lasted for at least 24 h and to have been preceded by at least 30 days of clinical stability or improvement
c95% confidence interval for CLARITY and 97.5% confidence interval in CLARITY Extension
Cladribine tablets (3.5 mg/kg; herein termed cladribine tablets) were approved in Europe in August 2017 for the treatment of adult patients with highly active relapsing MS (Box 1), followed by approval in the USA in March 2019 for relapsing–remitting multiple sclerosis (RRMS) and active secondary progressive MS in adults [7, 8], and subsequently in many other countries. This wider commercial availability has been accompanied by published data that can guide the clinical use of cladribine tablets, including post-hoc analyses of the pivotal clinical trials. Based on the evidence currently available, this review provides an overview of the principles of immune reconstitution therapy (IRT), cladribine tablets and practical advice for their use in patients with RRMS, including risks associated with COVID-19 and recommendations for vaccination in patients with MS.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Box 1 Recommendations from the European Summary of Product Characteristics (SmPC) [7].
Cladribine tablets 10 mg are given as a cumulative dose of 3.5 mg/kg body weight over 2 years, given as one treatment course of 1.75 mg/kg per year. Each treatment course comprises two treatment weeks, one at the beginning of the first month and one at the beginning of the second month in each treatment year.
Each treatment week consists of 4 or 5 days during which patients receive 10 mg or 20 mg (1 or 2 tablets) as a single daily dose (dependent on body weight). Following the completion of the two treatment courses, no further treatment with cladribine tablets is required in Years 3 and 4.
Treatment with cladribine tablets should not be initiated within the 4- to 6-week period after vaccination with live or attenuated vaccines due to the risk of active vaccine infection. Vaccination with live or attenuated live vaccines should be avoided during and after treatment with cladribine tablets as long as the patient's white blood cell counts are not within normal limits. Lymphocyte counts should be monitored until they return to normal or at least > 800 cells/mm3.
Cladribine tablets are contraindicated in patients with MS who have active malignancies. An individual benefit-risk evaluation should be performed before initiating treatment in patients with prior malignancy.
Cladribine tablets are also contraindicated in pregnant women. Based on experience with other substances inhibiting DNA synthesis in humans, cladribine could cause congenital malformations when administered during pregnancy.
Immune Reconstitution Therapy
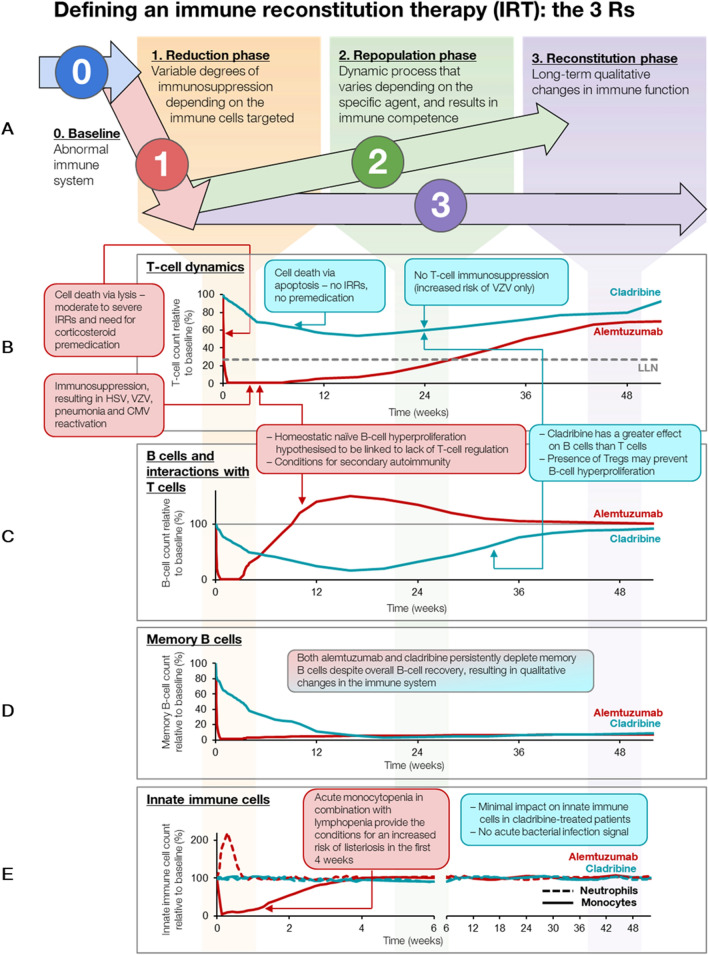
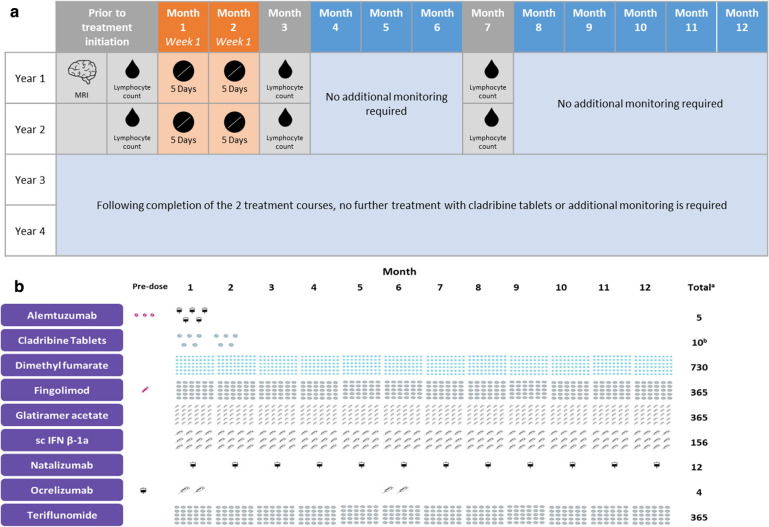
IRTs (such as cladribine tablets or alemtuzumab) are administered intermittently during short-dosing periods (up to 10 days in a year) to produce long-term effects on the immune system (Fig. 1) [3, 4, 9]. The risk of adverse events (AEs) associated with IRTs is greatest during the initial post-treatment period and decreases over time. In contrast, the risk of AEs with maintenance therapy increases proportionally with cumulative exposure [4]. All IRTs have a reduction or depletion phase, followed by a repopulation phase and then by a reconstitution phase (Fig. 1), during which the immune system recovers normal effector function in relation to recognizing and responding to infections and vaccines, both inactivated and live vaccines, and tumor surveillance. However, the reconstituted immune system is both qualitatively and quantitatively different to that before treatment, which explains sustained efficacy and/or prolonged remission. Oral IRT is associated with fewer hospital visits and a reduced treatment and monitoring burden compared with other DMTs; with the former, patients need only be treated for a maximum of 20 days over 2 years, and no further injections or infusions are required (Fig. 2a, b).
Fig. 1.
Mode of action of IRT: reduction, repopulation, and reconstitution of lymphocytes—the 3Rs. CMV Cytomegalovirus, HSV herpes simplex virus, IRR infusion-related reaction, IRT immune reconstitution therapy, LLN lower limit of normal, Tregs regulatory T cells, VZV varicella-zoster virus
Fig. 2.
a Treatment and monitoring burden of cladribine tablets. b Yearly treatment burden for cladribine tablets and other DMTs. aTotal number of administrations over the first 12 months of treatment. b10 mg tablets given as a cumulative dose of 3.5 mg/kg over 2 years, administered as 1 treatment course of 1.75 mg/kg per year for 2 years. DMTs Disease-modifying therapies, IFNβ interferon-beta, MRI magnetic resonance imaging, sc subcutaneous
Cladribine tablets and alemtuzumab are two IRTs that are currently licensed for the treatment of MS [7, 10]. Both agents deplete total B cells by at least 90% and memory B cells to a similar level (Fig. 1) [11]. Over time, however, with alemtuzumab, B cells start to recover to about 50% above baseline within the first 3 months after treatment due to homeostatic naïve B cell hyperproliferation, which potentially increases the risk of secondary autoimmunity [12], while with cladribine tablets, B cells recover more slowly (within 6 months) and there is no hyperproliferation or overshoot above baseline [13]. Furthermore, treatment with cladribine tablets is associated with consistent and lasting B cell depletion, and B cell recovery post-cladribine occurs in the presence of adequate T cell regulation [14]. Interestingly, the memory B cell population remains depleted with both alemtuzumab and cladribine tablets, which may explain the persistent efficacy of both therapies (Fig. 1) [11].
Alemtuzumab can deplete T cell numbers by > 95% [11], whereas the licensed dose of cladribine tablets (3.5 mg/kg) leads to T cell reductions of approximately 50% [13]. Importantly, during treatment with cladribine tablets, absolute T cell numbers are maintained at a level that ensures general T cell immune competency [14]. This may explain why there is no opportunistic infection signal with cladribine tablets, unlike with alemtuzumab for which Listeria monocytogenes, nocardiosis, Cytomegalovirus reactivation, Pneumocystis, and other infections have been observed [15]. Further characterization of the immunological effects of IRTs may contribute to an improved understanding of the pathogenesis of MS and further guide treatment selection [16–18].
Clinician’s Perspective
The evolving MS treatment landscape, including IRTs, provides a range of high-efficacy options for first-line treatment early in the course of MS. IRTs are preferable to maintenance therapies because they are given as short courses, with the potential for long-term remission, and are not associated with continuous immunosuppression in accordance with the 3Rs: reduction, repopulation, and reconstitution (Fig. 1). Based on these principles, IRT allows the immune system to rebuild itself, which ultimately leads to restored effector functions. Immunosuppression associated with IRT is front-loaded and essentially disappears once the immune system has reconstituted. However, with the continuous administration of maintenance immunosuppressive therapies the risk increases with time.
Cladribine Mechanism of Action and Lymphocyte Selectivity
Cladribine (2-cholorodeoxyadenosine) is an adenosine analogue pro-drug that is selectively phosphorylated intracellularly by deoxycytidine kinase (DCK) to the active moiety, CdA-5′-triphosphate (CdATP) [19, 20]. This activity is counterbalanced by 5-nucleotidase (5′NTase). Cladribine has well-defined and specifically designed activity against lymphocytes that leads to selective depletion and most importantly, cladribine only affects the adaptive immune system whilst sparing innate immune cells, thus reducing the risk of infections [4, 13, 21, 22]. The high selectivity of cladribine for lymphocytes is due to the high ratio of DCK to 5′NTase and the high ratio of DCK to adenosine deaminase that leads to preferential accumulation in these cells. Both dividing and resting lymphocytes are affected [20], which leads to transient reductions in their overall number [23]. In resting cells, cladribine gives rise to single-strand DNA breaks which lead to apoptosis, whereas in dividing cells, cladribine induces cytotoxicity by impairing DNA synthesis [23]. Importantly, cladribine at the doses used in MS exhibits only minor effects on neutrophil and platelet counts and hemoglobin and hematocrit levels [7]. Flow cytometry studies illustrated an important relationship between DCK activity and the effect of cladribine on B and T cells, according to their activation status. Observations have indicated no differences in DCK/5′NTase ratio in progenitor and mature immune cells while cladribine induced apoptosis in stimulated T and B cells and unstimulated B cells, raising the possibility that DCK activity could be studied as a potential biomarker of lymphocyte response to cladribine tablets [24].
The pathogenesis of MS is mediated by several immune cell types, including peripherally derived T cells, B cells, and myeloid cells, and resident cells of the central nervous system (CNS), including microglia and astrocytes [25, 26]. It is thought that B cells are one of the predominant drivers of immune responses within the CNS, with pathogenic B cells possessing the ability to act both centrally and peripherally [27]. Studies have indicated that cladribine has the potential to penetrate the CNS and achieve a concentration in the cerebrospinal fluid of up to 25% of its concentration in plasma [28–30], which raises the possibility that cladribine tablets may be able to reduce lymphocyte numbers within the CNS as well as those circulating in the periphery [31, 32]. However, a need exists to better understand if the potential for direct effects of cladribine within the CNS are clinically significant in terms of additional benefits over and above treatment effects on peripheral immune cells [33].
Various lymphocyte subsets are affected by treatment with cladribine tablets; however, CD19+ B cells are the most heavily and rapidly depleted cell type, reaching a nadir 13 weeks after initial administration of cladribine tablets [14]. Despite this rapid and pronounced decrease, CD19+ B cell counts recover to normal limits within 48 weeks [14]. T cells are less affected than CD19+ B cells, although both CD8+ cells and CD4+ T cells decrease following treatment with cladribine tablets [13]. Relative to CD4+ T cells, naïve T cell subtypes have been shown to decrease after treatment with cladribine tablets while the proportion of memory T cell subtypes, which provide long-term protective immunity against pathogens [34], increase [14]. Further studies using peripheral immune cell profiling have provided evidence to confirm the depletion kinetics of cladribine tablets on proinflammatory immune cell subsets, including central memory T cells and memory B cells, with a smaller effect on cells involved in immune responses to pathogens [35]. In-depth analysis combining flow cytometry with mass spectrometry has shown that MS remission after treatment with cladribine tablets is associated with an increase in CD8+ effector memory RA+ and CD8+ effector memory T cells, with a decrease in CD8+ naïve T cells and decreases in CD27+ memory B cell populations [36, 37]. It has also been reported that the number of naïve T regulatory cells dropped in patients with MS after treatment with cladribine tablets, then slowly recovered over 12 months whereas the number of activated T regulatory cells increased at 6 months after treatment initiation [38].
Induction Therapy and Re-dosing with Cladribine Tablets
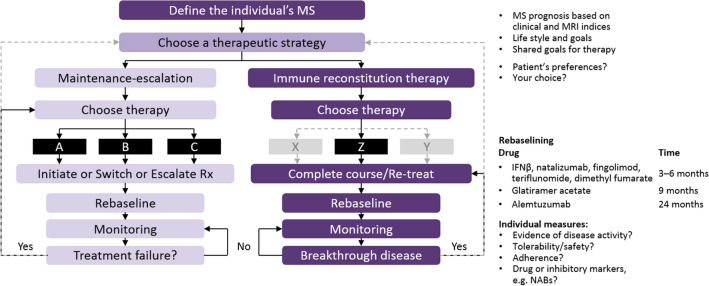
Induction therapy is based on the early use of immunosuppressive drugs, followed by maintenance treatment with immunomodulatory therapy. As licensed, cladribine tablets are not used as an induction therapy as the majority of patients experience long-term remission up to Year 4 (and possibly beyond). IRT with cladribine tablets leads to lymphocyte reduction, followed by repopulation with naïve lymphocytes. Upon treatment initiation, patients should undergo baseline assessments, as depicted in the treatment algorithm shown in Fig. 3. It is important to define each individual patient’s disease activity and level of risk, taking into account personal lifestyle goals [39].
Fig. 3.
Treat-2-target algorithm of NEDA in relapsing forms of MS (adapted from Giovannoni et al. [39]). MS Multiple sclerosis, NABs neutralizing antibodies, NEDA no evidence of disease reactivation, Rx treatment
Managing disease activity while on treatment with cladribine tablets depends on the level of disease activity and when it occurs; for example, if relevant disease activity occurs in Year 2 following the second course of cladribine tablets, retreatment is not recommended under the current label. However, based on the biology of MS and the mode of action of IRT, retreatment with cladribine tablets is an option, similar to the way in which alemtuzumab is licensed and currently used. Currently, there are no data on re-initiation of treatment with cladribine tablets after Year 4 [7].
Clinician’s Perspective
Assessing patient risk is an important aspect of therapy for MS given the potential complications of immunosuppression, such as secondary autoimmunity, opportunistic infections, and malignancy. Risk mitigation should be applied prior to initiating IRT, during active treatment, and in the monitoring phase [40]. Patients should undergo certain tests as standard before starting treatment, including full blood count, liver function tests, urea/electrolytes, and serum immunoglobulins. An infection screen should also be performed, including the following tests: hepatitis B and C; tuberculosis (ELISpot or QuantiFERON); human immunodeficiency virus (HIV) 1 and 2; syphilis; varicella zoster virus (VZV); vaginal human papillomavirus (HPV) or cervical smear testing; and screening for cutaneous warts. A vaccination review is recommended, and it may be necessary to offer patients specific vaccinations. However, the advantage of IRT is that vaccination can be delayed and given after immune reconstitution while this is not an option with maintenance immunosuppressive therapies.
Emerging Data on the Efficacy of Cladribine Tablets
Post-hoc analyses of pivotal clinical trials have provided additional insights into the efficacy of cladribine tablets that can be useful to the clinician in practice. For example, it has been shown that most baseline characteristics of patients who entered and did not enter the CLARITY Extension trial were similar, demonstrating that the Extension study did not preferentially enroll patients with less severe disease at CLARITY baseline [41]. Consequently, the findings of CLARITY Extension, including that the majority of patients (75%) remained relapse-free without need of treatment beyond Years 1 and 2 [6], are relevant for the original population enrolled in CLARITY. Post-hoc analyses have also assessed the effect of treatment with cladribine tablets on the frequency and severity of relapses in the CLARITY study and the durability of such effects in CLARITY Extension. In this analysis, all relapses included both qualifying and non-qualifying relapses (i.e., relapses reported by the evaluating physician but not confirmed as meeting strict ‘qualifying’ criteria, the latter being a 2-point increase in ≥ 1 Kurtzke Functional Systems scores [KFS] or a 1-point increase in ≥ 2 KFS scores [excluding changes in bowel or bladder function, or in cognition] in the absence of fever lasting for at least 24 h, and preceded by at least 30 days of clinical stability or improvement). The authors also considered severe relapses, which were arbitrarily defined as relapses that required either treatment with steroids or hospitalization. Results showed that patients receiving cladribine tablets had a significantly lower risk of all (and qualifying) relapses and severe relapses compared with placebo, throughout CLARITY and the extension phase. Such findings confirm that the effect of cladribine tablets in reducing the frequency and severity of relapses in CLARITY was sustained without further treatment [42].
Further evaluation of long-term disease stability after treatment with cladribine tablets in the CLARITY and CLARITY Extension studies showed that the Expanded Disability Status Scale (EDSS) score remained stable for up to 5 years from the start of CLARITY in > 50% of patients treated with cladribine tablets [43]. Treatment with cladribine tablets in CLARITY, and subsequently placebo or further cladribine tablets in CLARITY Extension also resulted in benefits for no evidence of disease activity (NEDA-3; defined as no qualifying relapse, no confirmed 6-month EDSS progression/worsening, and no T1 Gd+ or active T2 MRI lesions) being sustained during follow-up for to 6 years from CLARITY baseline [44]. It has also been shown that subgroups of patients with high disease activity at baseline showed treatment responses to cladribine tablets that were comparable with the clinical and magnetic resonance imaging (MRI) outcomes seen in the overall CLARITY population [45]. Furthermore, there were consistent treatment benefits of cladribine tablets during the CLARITY study among patients who were either previously treated with DMTs or were treatment naïve, [46] including those with high disease activity [47].
In the absence of head-to-head comparative trials, a network meta-analysis (NMA) including data from CLARITY and randomized clinical trials of other oral DMTs reported that treatment with cladribine tablets was significantly more effective in achieving NEDA-3 (defined as no clinical relapse, no 3-month confirmed disability progression, and no disease activity on MRI) than dimethyl fumarate and teriflunomide, but there was no significant difference compared with fingolimod [48]. A separate NMA showed that treatment with cladribine tablets was associated with a higher probability of sustained recovery from disability on the EDSS compared to fingolimod, natalizumab, and alemtuzumab in patients with highly active disease [49]. Using another approach, data from treatment-naïve patients in the CLARITY study were propensity score matched to data from patients in the Italian i-MuST database [50]. Cladribine tablets showed lower relapse rates compared with matched patients who started interferon, glatiramer acetate, or dimethyl fumarate. Relapse rates with cladribine tablets were similar to those of fingolimod but higher than those observed with natalizumab. The treatment effect of cladribine tablets was shown to be greatest in a subgroup of patients with high disease activity [50].
A post-hoc analysis of the ORACLE MS study on the effects of cladribine tablets in patients following a first clinical demyelinating event (i.e., early MS) reported that treatment reduced the number of lesions present on MRI scans compared to placebo by the time of the first evaluation conducted at 13 weeks after baseline. Low lesion counts were maintained with cladribine tablets throughout the 96 weeks of the study [51].
Ongoing Studies with Cladribine Tablets
There are several ongoing studies investigating the effects of cladribine tablets treatment in patients with MS, including the onset of effects after treatment which is being explored in the MAGNIFY-MS study (NCT03364036) with frequent and early MRI. Interim analysis has reported changes in combined unique active lesions during the first 6 months according to patient subgroups (based on high relapse activity or not, whether patients were treatment naïve or previously treated, or combined unique active lesion count > 0 at baseline). This study demonstrated an early onset of effect from cladribine tablets for all subgroups. In the first 6 months, combined unique active lesion counts were reduced from baseline between the first MRI scan at the end of Month 1 and the MRI scan at the end of Month 6 [52]. A separate analysis from MAGNIFY-MS reported on changes over time in peripheral immune cell subsets and IgG and IgM levels during the first 12 months of treatment with cladribine tablets. Data from 57 patients showed that effects on lymphocyte dynamics occurred from the first month and were sustained in different immune cell subpopulations. In particular, this confirmed that treatment with cladribine tablets exerted a profound effect on memory B cells, while IgG or IgM levels were unaffected over 12 months [53].
The CLASSIC-MS (NCT03961204) trial is investigating the long-term efficacy and durability of treatment with cladribine tablets beyond the two annual treatment courses in patients enrolled in the original phase 3 trials (CLARITY, CLARITY Extension, and ORACLE MS). Interim data from a population of 147 patients with a median follow-up of 10 years suggest sustained efficacy of cladribine tablets following two annual treatment courses, with a substantial proportion of patients requiring no further treatment with DMTs or with using an assistive ambulatory device [54].
The aim of the CLARIFY-MS (NCT03369665) trial is to assess the impact of cladribine tablets on health-related quality of life and treatment satisfaction in patients with highly active relapsing MS. An interim analysis involving 482 patients found that, at 6 months after starting treatment, patients were generally satisfied with cladribine tablets. The convenience and side effect profile were especially important to patients. Safety results at 6 months after treatment were reported to be consistent with the known safety profile of cladribine tablets [55]. Data from patients with MS in Italy who participated in randomized clinical trials with cladribine tablets across 17 MS centers were obtained from the Italian MS Registry, and reported by the CLARINET-MS study. This study provides an indirect measure of the long-term effectiveness of cladribine tablets and confirmed that more than half of the 80 patients analyzed did not relapse or experience disability progression during the 60 months of follow-up from the last dose administered in a clinical trial [56].
CLICK-MS (NCT03933215) and MASTER-2 (NCT03933202) are single-arm, observational studies being conducted in the USA that are evaluating the effectiveness and safety of cladribine tablets over 30 months in patients with RRMS or active secondary progressive MS who had a suboptimal response to prior injectable or infusion/oral DMT. The primary endpoint is 24-month annualized relapse rate. Key secondary endpoints include patient-reported outcomes on quality of life measures, treatment adherence, and AEs [57].
Among other studies, CLOCK-MS (NCT03963375) is a 24-month, open-label, randomized study that is aimed to improve current understanding of the mechanism of action of cladribine tablets [58], while ChariotMS (NCT04695080) is examining whether cladribine tablets can affect deterioration in people with advanced MS who are largely or completely wheelchair dependent (EDSS > 6.5) [59]. Lastly, CLOBAS (IRRID: DERR1-10.2196/24969) is a 6-year study being conducted in Australia that is collecting data on blood-based biomarkers (lymphocyte subsets, serum neurofilament light chain, DNA methylation, and RNA analysis), MRI outcomes, and cognitive performance in users of clabribine tablets. CLOBAS will be the first long-term efficacy trial of cladribine tablets to offer re-initiation of therapy after the initial two courses in Years 1 and 2. Outcomes will provide an indication of whether biomarkers can predict treatment efficacy or the need for re-initiation of cladribine tablets in patients with MS [60, 61].
Real-World Data with Cladribine Tablets
Reports of data from cohorts of patients treated with cladribine tablets under real-world prescribing conditions are also valuable to complement information from pivotal clinical trials. For example, real-world observational data for 90 patients in Australia (captured as part of the MSBase registry) suggested a disease-modifying effect from treatment with cladribine tablets, reflected by reduced disability progression and reduced frequency of relapses in a patient cohort characterized by older age and more disability than the clinical trial population [62]. Wider analyses of the MSBase registry, including patients from a range of countries, reported that between 13% and 17% of patients initiated cladribine tablets as first-line therapy. Consistent with clinical trial data, relapse rates were reduced after treatment with cladribine tablets, with few early discontinuations [63, 64].
Other reports confirming that the efficacy and safety profile of cladribine tablets in real-world prescribing are comparable to the findings reported in randomized clinical trials come from Portugal [65, 66], Scotland [67], United Arab Emirates [68], Spain [69], Italy [70, 71], and Germany [72].
Before the commercial availability of cladribine tablets, some specialist centers had experience administering the drug by injection, either intravenously or subcutaneously, to patients with MS. A retrospective study reviewed all patients treated with alemtuzumab or intravenous cladribine at the Ottawa Hospital MS Clinic (Ottawa, ON, Canada) with ≥ 2 years of follow-up and reported that alemtuzumab and cladribine achieved similar rates of NEDA-3 (no relapses, no 6-month EDSS progression, or no new activity on MRI) in long-term follow-up, with overall fewer AEs with cladribine [73]. Efficacy outcomes with subcutaneous cladribine also confirmed that efficacy was in line with data from pivotal clinical trials [74]. Recently, another study of subcutaneous cladribine (with increased cumulative maintenance dosing) reported disease stability and favorable safety over a prolonged period of follow-up (up to 20 years) in patients with relapsing MS [75].
Cladribine Tablets and the Cost of Managing Patients with MS
MS is associated with a high economic burden, and spending on DMTs and associated monitoring is increasing [76, 77]. For example, the availability of DMTs given by intravenous infusion in the hospital over recent years has resulted in increased resource use associated with treatment administration and monitoring. A simulation model assessing the efficiency of cladribine tablets or DMTs delivered by infusion from a facility perspective in the UK found that cladribine tablets could decrease the burden of hospital-based administration and monitoring [78]. However, the cost of DMTs is an important driver in many cost-assessment models, and cladribine tablets has been found to be cost effective compared to alternative treatments in modeling based on use in many country-specific settings, including Finland [79], Portugal [80], Spain [81], Saudi Arabia [82], Chile [83], Iran [84], and Poland [85].
Clinician’s Perspective
Cladribine tablets are effective in the treatment of patients with active MS who have a history of recent relapses and MRI activity (new or Gd-enhancing lesions). At present, the use of cladribine tablets is limited based on the label used in many countries. However, as real-life pharmacovigilance data emerge indicating that the cancer and infection risks highlighted in the various labels are not a problem, the use of cladribine tablets will hopefully be extended into earlier MS, i.e., after a first attack, and later in the disease course to reduce the risk of chronic immunosuppression in older patients with MS. In an era of patient choice, many patients who choose oral cladribine are doing so because of its low treatment and monitoring burden and its many other attributes, such as being good for pregnancy planning and the recent observation that it does not impact on vaccine responses.
Management of Patients Receiving Cladribine Tablets
Switching from Prior DMTs
The range of available therapies for MS means that switching treatments is a common occurrence [86]. The decision to switch is often driven collectively by physicians and patients, and may take into account various factors such as efficacy, convenience, and monitoring burden [86]. Importantly, switching DMTs should take account the dynamics of lymphocyte changes associated with both the initial and new treatment and the potential AEs associated with particular DMTs [87, 88]. A case series reported clinical experience with 17 patients who switched from the anti-CD20 DMTs rituximab or ocrelizumab to cladribine tablets after a mean of 27 weeks on treatment. The effect on lymphocytes remained predictable, despite initiating cladribine tablets at or around the lower limit of normal, with reported AEs comparable to those reported in the literature [89].
Progressive multifocal leukoencephalopathy (PML), caused by the John Cunningham virus (or JC virus), has been observed in some patients with MS, particularly in patients on natalizumab due to the decreased immunosurveillance of the CNS while on treatment [90]. As such, there is a small risk of carryover PML during the first 6–12 months of treatment in patients switched from natalizumab to an IRT [90, 91]. MRI and optional examination of cerebrospinal fluid are therefore recommended to exclude asymptomatic PML before cladribine treatment is initiated. Experience with 46 patients being followed up in Germany for between 6 and 12 months demonstrated that switching from natalizumab to cladribine tablets was safe, with no cases of PML observed [92]. Some clinicians prefer to bridge patients on a maintenance therapy, for example, fingolimod for 6 months, prior to initiating an IRT post-natalizumab [93], the rationale being the action of fingolimod can be reversed by stopping the drug. In comparison, the effect of IRTs on the immune system are irreversible and reconstitution takes many months.
Clinician’s Perspective
Further analyses of clinical trials and published data from real-world prescribing have confirmed that cladribine tablets is a high-efficacy DMT and can be used in a wide range of settings, including for treatment-naïve and previously treated patients, and those with high disease activity.
In our opinion, cladribine tablets can be used after any of the licensed DMTs with some caveats. First, switching patients at high-risk of PML from natalizumab needs to be done carefully to avoid carry-over PML. With maintenance treatments that are associated with lymphopenia, it is important to have a washout period to allow lymphocyte recovery, e.g., when switching from fingolimod we wait for the total lymphocyte count to recover to > 800 cells/mm3 and then start treatment, typically around 3–4 weeks after the last dose. We do not wait for the counts to exceed 1000 cells/mm3 because this can put patients at risk of rebound post-fingolimod [94].
In the case of anti-CD20 therapies, we would advise delaying cladribine tablets until there is evidence of B cell recovery. However, this would depend on why the patient was being switched to cladribine tablets. If the switch was due to suboptimal response to an anti-CD20 therapy, we would not recommend delaying treatment. If switching from teriflunomide, which has a long circulating half-life that may interfere with the repopulation kinetics of IRT [95], it would be advisable to use a rapid elimination protocol before initiating treatment with cladribine tablets. In the case of dimethyl fumarate, lymphocyte recovery can take much longer—in some instances many months—which can put patients at risk of increased disease activity [96].
When initiating cladribine tablets, experience from the UK has demonstrated that a nurse/pharmacy-led support program can assist patients in taking treatment as prescribed to achieve the required cumulative dose. This is achieved by providing support on treatment days together with dose interpretation advice, clinical appointment reminders, and a home-based phlebotomy service [97]. This level of patient support can reduce the potential for medication errors, improve patient safety, and allow any patients in need of additional support to be more easily prioritized.
Adverse Events and Adverse Events of Special Interest
Approximately 90% of patients treated with cladribine tablets in CLARITY completed the course, and overall AE rates were low with very few discontinuations due to AEs [5]. In CLARITY Extension, approximately 90% of patients completed treatment, with similar AE frequencies observed in the cladribine tablets and placebo groups [6]. A post-hoc analysis of CLARITY and ORACLE MS safety populations assessed the incidence of AEs with cladribine tablets or placebo within the first 12 weeks after treatment initiation. This analysis showed that 61.3% of patients treated with cladribine tablets and 55.2% treated with placebo experienced an AE within this time. The most common AEs reported with cladribine tablets were headache (7.2%), lymphopenia (6.8%), and nausea (6.0%). This study also showed that cladribine tablets were well tolerated based on a low incidence of AEs leading to treatment discontinuation [98].
Moreover, real-world data have demonstrated a consistent AE profile that echoes the results of clinical trials [66, 67, 69, 71, 89, 99]. For example, an observational study from 56 MS centers in Italy reported that the risk of Grade 3 and 4 lymphopenia with cladribine tablets was lower than that observed in randomized clinical trials, and that when Grade 3 lymphopenia did occur it was transient in most patients [99].
Skin reactions are listed in prescribing information as potential AEs during treatment with cladribine tablets [7, 8]. Reports from clinical experience in two centers in Germany indicated that within 3 months following the last dose of cladribine tablets, hair thinning, skin rash, mucositis, and pruritus can be observed [100]. Case reports of three patients who developed severe rash 3–192 days after receiving subcutaneously administered cladribine have highlighted severe skin reactions as a rare, but important, potential AE during treatment. All of these patients were effectively treated, and additional doses of subcutaneous cladribine were administered after pre-treatment with steroids and antihistamines [101]. There has also been a published case report of a patient developing visual symptoms and a large retinal cotton wool spot in association with initiation of cladribine therapy, suggesting that patients receiving cladribine tablets should be advised to seek medical advice if they develop new visual symptoms [102]. It has been suggested that the possibility of secondary autoimmunity cannot be entirely discounted in patients treated with cladribine tablets following a report of a case of anti-glomerular basement membrane antibody-mediated glomerulonephritis that occurred shortly after the second treatment in Year 2 [103]. However, this seems unlikely in view of the absence of the more common autoimmune diseases associated with alemtuzumab treatment, in particular autoimmune thyroid disease and immune thrombocytopenic purpura [104]. Finally, a published case report noted severe neutropenia occurring 1 week after the second dose of cladribine tablets in the first year of treatment. Although neutropenia resolved after 10 days with granulocyte colony-stimulating factor [105], in our opinion this case underlines the need for clinical and blood monitoring during the initial administration of cladribine tablets.
Lymphopenia
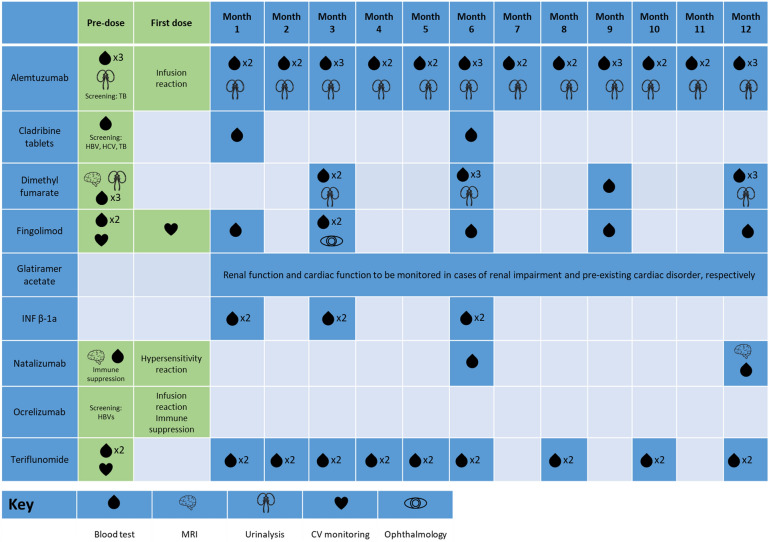
Lymphocyte counts must be determined before, during, and after treatment with cladribine tablets (Fig. 4). If the lymphocyte count is < 500 cells/mm3 (Grade 3 or 4 lymphopenia), patients should be counseled and offered antiviral prophylaxis against herpes viruses where applicable. In the CLARITY and CLARITY Extension studies, Grade 3 lymphopenia (absolute lymphocyte count [ALC]: 200–499 cells/mm3) and Grade 4 lymphopenia (ALC: < 200 cells/mm3) was experienced by 25% and < 1% of patients treated with cladribine tablets, respectively [5, 6]. Lymphocyte recovery begins soon after treatment with cladribine tablets in Years 1 and 2, returning to the normal range by 84 weeks following the first course of treatment with cladribine tablets [13]. No patient had Grade 4 lymphopenia at the end of Year 4 [6].
Fig. 4.
Yearly monitoring burden for cladribine tablets and other DMTs [7, 8, 10, 142–148]. CV Cardiovascular, DMTs disease-modifying therapies, HBV hepatitis B virus, HCV hepatitis C virus, MRI magnetic resonance imaging, TB tuberculosis
Herpes Zoster
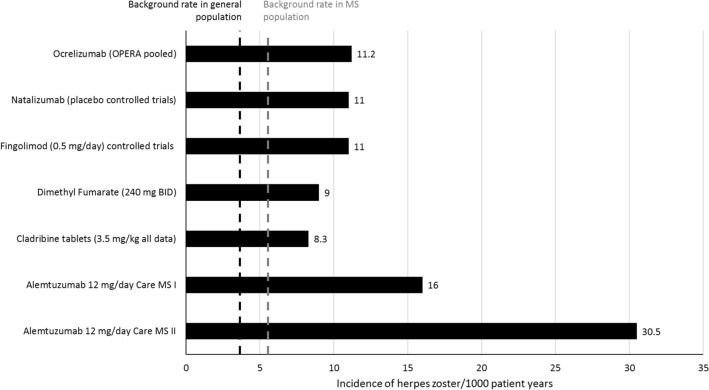
Herpes zoster is an opportunistic infection that is common in immunosuppressed patients [106]. During the CLARITY and CLARITY Extension studies, the incidence of herpes zoster was increased in patients treated with cladribine tablets versus placebo [5, 6] (Fig. 5). Moreover, the incidence of herpes zoster was observed to be higher during periods of Grade 3 or 4 lymphopenia compared with Grade 0–2 lymphopenia, and the incidences of serious and severe herpes zoster infections were higher with cladribine tablets compared with placebo (adjusted-AE per 100 patient-years: 0.09 and 0.05, respectively) [107]. Importantly, all cases were dermatomal and followed a normal disease course, with no cases of post-herpetic neuralgia; however, as a precaution, patients who are seronegative due to no previous exposure to VZV should be vaccinated prior to initiation of cladribine tablets and initiation of treatment should be postponed for 4–6 weeks.
Fig. 5.
Incidence of herpes zoster per 1000 patient years, across different multiple sclerosis treatments [107, 149, 150]. BID Twice daily, MS multiple sclerosis
Clinician’s Perspective
Case reports underline the importance of blood monitoring during treatment with cladribine tablets, and physicians should also be alert to the possibility of rare AEs. Lymphocyte counts must be normal (> 1000/mm3) before initiating cladribine tablets in Year 1 and Year 2. If necessary, the treatment course in Year 2 can be delayed for up to 6 months to allow for lymphocyte recovery; however, if lymphocytes do not recover within 6 months, no further treatment should be given. If the lymphocyte count drops to < 200 cells/mm3, anti-herpes prophylaxis according to local standard practice should be considered for the duration of Grade 4 lymphopenia. In patients with lymphocyte counts < 500 cells/mm3 (Grade 3 lymphopenia), antiviral prophylaxis should not be considered routine; however, patients should potentially be provided with rapid access to antiviral medication (e.g., acyclovir, valacyclovir, or famciclovir) with instructions on what to look out for in relation to a herpes infection. If uncertain, patients should contact their healthcare professional, and provide images for interpretation and advice. Famciclovir is generally favored over acyclovir or valacyclovir for VZV as it has a longer intracellular half-life and has been reported to be superior for VZV than acyclovir [108].
Malignancy
Cladribine tablets are contraindicated in patients with active malignancy, and careful assessment of the risk–benefit profile should be performed in patients with a prior history of malignancy. However, analysis of clinical trial and long-term safety data in a final report from the clinical development program showed there was no significant increase in the rate of malignancies with cladribine tablets compared with placebo; the incidence of malignancy was 0.26 per 100 patient-years in the treatment group compared with 0.12 per 100 patient-years in the placebo group [107, 109]. Moreover, malignancies associated with immunosuppression were not increased in patients receiving cladribine tablets. When this population of patients was compared with an external reference population (GLOBOCAN matched reference population), the rates of malignancies were similar between patients receiving cladribine tablets and the matched cohort [107, 109, 110]. The ongoing CLARION post-approval safety study (EU PAS Register number: EUPAS24484) will further characterize the real-world safety profile of cladribine tablets, including the risk of malignancy and other AEs of special interest [111].
Clinician’s Perspective
Apart from the cervical smear and/or PCR testing for vaginal HPV, malignancies are not usually screened for outside of national screening programs. IRT has the advantage of not causing continuous immunosuppression and is theoretically safer than maintenance therapies because tumor immune surveillance is generally normal after immune reconstitution. Cladribine tablets do not cause DNA mutations or translocations like other chemotherapy agents [112]; therefore, no specific malignancy signals have been observed with cladribine tablets. Furthermore, given the intermittent nature of immunosuppression following treatment with cladribine tablets, there have been no observations of the typical secondary malignancy signals that have been seen under continuous immunosuppressive therapy, such as skin cancers and hematological malignancies, and in particular lymphomas, that have been observed following fingolimod treatment [113].
Use of Cladribine Tablets During the COVID-19 Pandemic
Because of the effects of DMTs for MS on the immune system there has been a great deal of information during the SARS-CoV-2 (COVID-19) pandemic about how patients should manage their treatment [114–120]. Published data relevant to treatment with cladribine tablets were included in a study that examined outcomes reported in the Merck KGaA Global Patient Safety Database in which a total of 272 reported cases of COVID-19 among patients receiving cladribine tablets were described. These patients were found to generally not be any greater risk of serious disease and/or a severe outcome with COVID-19 compared with the general population [121]. The evaluable cohort comprised 261 patients (confirmed COVID-19, n = 160; suspected, n = 101); an additional 11 patients had symptoms compatible with COVID-19 but were not evaluated further because of negative diagnostic tests. The median time to onset of COVID-19 from the most recent treatment course of cladribine tablets was 162 days (n = 139). Outcomes for the evaluable patients were: recovered/recovering (51%); not recovered/not resolved (7%); died (0.4%); and not reported/missing/pending (41%). Of the total cohort, 15% experienced serious COVID-19 (patient hospitalized, medically significant, or fatal case). This report included two cases that were described independently in the literature [122, 123].
The study also examined the potential impact of treatment with cladribine tablets on the ability of patients to mount an antibody response to the SARS-CoV-2 virus. A serology test was reported for 17 patients treated with cladribine tablets and all had a positive result, in some cases several months after the infection. Cases appearing in the literature have reported positive serology for SARS-CoV-2 virus in some, but not all patients treated with cladribine tablets [124, 125] and that anti-SARS-CoV-2 antibody production can occur in patients with lymphopenia after treatment with cladribine tablets [126]. There are also case reports of patients treated with cladribine tablets being confirmed to have asymptomatic COVID-19 and developing anti-SARS-CoV-2 antibody responses [127]. Physicians from Spain described their experience with 14 patients with MS who had been affected by SARS-CoV-2 (with a clinical, positive PCR test, or serological diagnosis). This study showed that half of the patients mounted an antibody response and overall suggested that treatment with cladribine tablets does not appear to worsen COVID-19 disease prognosis [128]. Finally, a case series from Serbia reported that seven patients treated with cladribine tablets and who suffered from COVID-19 developed IgG antibodies 2.0–5.5 months after the last symptoms [129].
A report on clinical outcomes in patients who developed COVID-19 infection during two ongoing clinical studies of cladribine tablets (CLARIFY-MS and MAGNIFY-MS) included three patients who recovered or were recovering, with none needing mechanical ventilation [130].
Vaccinations
The risk of infectious diseases is an important consideration in managing patients with MS. Counseling about vaccinations and the risk of infections should be considered individually for each patient before starting DMTs, and reviewed regularly [131]. Patients receiving cladribine tablets should be vaccinated according to general recommendations for healthy adults [131]. A small, retrospective investigation in patients treated with cladribine tablets in the ongoing MAGNIFY-MS study reported that antibodies to seasonal influenza and VZV vaccines remained adequate in terms of providing protective immunity [132]. A sub-study of CLOCK-MS evaluated the potential impact of prior treatment with cladribine tablets on the development of antibody titers to influenza vaccination. Preliminary results showed that three patients with 4-week data all demonstrated an increase in influenza titers. Two of these patients were experiencing lymphopenia around the date of vaccination and had received treatment with cladribine tablets up to 4 months prior to vaccination [58].
Immunization against COVID-19 is strongly recommended for all patients with MS regardless of age and comorbidities [131, 133–136]. The study of Achiron et al. [136] provided evidence that cladribine treatment does not impair the humoral response to COVID-19 vaccination. These investigators measured SARS-CoV-2 IgG response in 125 patients with MS 1 month after the second dose of the Pfizer/BioNTech mRNA COVID-19 vaccine. Patients were either untreated or under treatment with cladribine tablets or other DMTs, with a group of healthy patients similarly vaccinated as control. The results showed that within 29.5–55 days after the second vaccine dose the antibody titer was high in healthy patients, untreated patients with MS, and patients with MS under cladribine treatment [136].
There are also case reports confirming that patients treated respectively with the adenoviral vector-based vaccine from AstraZeneca or an mRNA vaccine after 3 months from a second administration of cladribine tablets produced a protective antibody response despite an incomplete recovery of lymphocyte levels [137]. In the case series reported by Drulovic et al. four patients treated with cladribine tablets who were vaccinated with an mRNA vaccine all developed antibodies, as did three out of seven vaccinated with the Sinopharm vaccine [129].
Clinician’s Perspective
Patients receiving cladribine tablets should be vaccinated according to general recommendations for healthy adults and, if practical, immunizations should be given before treatment with cladribine tablets is initiated. Patients with MS receiving cladribine tablets are generally not at greater risk of serious disease and/or a severe outcome with COVID-19 compared with the general population, and decisions to initiate or continue scheduled treatment should be taken as normal, considering the benefit/risk to the individual patient. Immunization against COVID-19 is recommended for all MS patients regardless of age and comorbidities.
Pregnancy and Family Planning
Cladribine tablets are contraindicated in pregnant women; therefore, pregnancy must be excluded prior to treatment initiation [7, 8]. A report of the outcomes of pregnancies occurring during the clinical development program showed that these were consistent with epidemiological data on pregnancy outcomes for the general population or women with MS. There were no congenital malformations in pregnancies that occurred during treatment with cladribine tablets or within 6 months after the last dose [138]. Pregnancy planning can be considered from 6 months after the final dose of cladribine tablets in Year 2 [139, 140]. Cladribine tablets are eliminated relatively quickly from the body, despite having a sustained treatment effect, which is appealing for women wanting to start or extend their families. There is evidence from CLARITY Extension that showed disease control was sustained for 4 years following treatment in Years 1 and 2 [6].
At present, there are limited data available on the transfer of cladribine in human milk [141], and women taking cladribine tablets should avoid breastfeeding [7, 8].
Clinician’s Perspective
It is advised that women of childbearing age and male partners receiving cladribine tablets are given practical suggestions for contraception and family planning following completion of Year 2 dosing. If a woman falls pregnant after the first course of cladribine tablets it is advised to delay the second course until after delivery and breastfeeding. It is recommended that breastfeeding should only start or recommence 10 days after the course of cladribine tablets is complete.
Conclusions
Overall, IRT is associated with a lower risk of cumulative side effects that are often observed with maintenance therapies and chronic immunosuppression. Cladribine tablets are a high-efficacy, oral IRT for patients with RRMS that selectively target lymphocytes; due to this mechanism of action, an increase in lymphopenia is expected, but most patients’ lymphocyte levels return to normal in the long term.
Cladribine tablets have a convenient and unique posology that provides sustained efficacy following two short, weekly treatments in each of 2 years, which means there is a lower monitoring burden in daily practice compared with many other highly effective DMTs. From the patient perspective, adherence is typically not an issue compared with maintenance therapies. In addition, cladribine tablets are not associated with regular infusions or injections, which reduces the overall burden by limiting hospital visits and making treatment more comfortable. Taken together, these factors free up resources within MS services for other activities, highlighting the advantages of cladribine tablets and IRT in real-world practice.
Acknowledgements
Funding
This review and the journal’s Rapid Service Fee was supported by Merck KGaA (CrossRef Funder ID: 10.13039/100009945).
Editorial Assistance
Editorial assistance was provided by Claire Mwape and Mark O’Connor of inScience Communications, Springer Healthcare Ltd, UK, and supported by an independent medical writing grant from Merck KGaA (CrossRef Funder ID: 10.13039/100009945).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the original draft preparation and provided comments on previous versions of the manuscript. All authors have read and approved the final manuscript.
Disclosures
Gavin Giovannoni has received speaker honoraria and consulting fees from AbbVie, Actelion (Janssen/J&J), Atara Bio, Almirall, Bayer, Biogen, Celgene (BMS), FivePrime, GlaxoSmithKline, GW Pharma, Ironwood, Merck & Co., Merck, Novartis, Pfizer Inc., Protein Discovery Laboratories, Roche, Sanofi, Teva Pharmaceutical Industries Ltd, UCB, and Vertex Pharmaceuticals; and has received research support unrelated to this study from Biogen, Ironwood, Merck & Co., Merck, Novartis, and Takeda. Joela Mathews has nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1.The Multiple Sclerosis International Federation. Atlas of MS 3rd edn. 2020. https://www.msif.org/wp-content/uploads/2020/12/Atlas-3rd-Edition-Epidemiology-report-EN-updated-30-9-20.pdf. Accessed Aug 2021.
- 2.Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24(2):96–120. doi: 10.1177/1352458517751049. [DOI] [PubMed] [Google Scholar]
- 3.Giovannoni G. Disease-modifying treatments for early and advanced multiple sclerosis: a new treatment paradigm. Curr Opin Neurol. 2018;31(3):233–243. doi: 10.1097/WCO.0000000000000561. [DOI] [PubMed] [Google Scholar]
- 4.Boyko AN, Boyko OV. Cladribine tablets' potential role as a key example of selective immune reconstitution therapy in multiple sclerosis. Degener Neurol Neuromuscul Dis. 2018;8:35–44. doi: 10.2147/DNND.S161450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416–426. doi: 10.1056/NEJMoa0902533. [DOI] [PubMed] [Google Scholar]
- 6.Giovannoni G, Soelberg Sorensen P, Cook S, et al. Safety and efficacy of cladribine tablets in patients with relapsing-remitting multiple sclerosis: results from the randomized extension trial of the CLARITY study. Mult Scler. 2018;24(12):1594–1604. doi: 10.1177/1352458517727603. [DOI] [PubMed] [Google Scholar]
- 7.Merck Serono Ltd. MAVENCLAD 10 mg tablets summary of product characteristics. https://www.medicines.org.uk/emc/product/8435. Accessed Oct 2021.
- 8.EMD Serono Inc. MAVENCLAD 10 mg tablets prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022561s000lbl.pdf. Accessed Oct 2021.
- 9.AlSharoqi IA, Aljumah M, Bohlega S, et al. Immune reconstitution therapy or continuous immunosuppression for the management of active relapsing-remitting multiple sclerosis patients? A narrative review. Neurol Ther. 2020;9(1):55–66. doi: 10.1007/s40120-020-00187-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genzyme Therapeutics. LEMTRADA 12 mg concentrate for solution for infusion summary of product characterisitcs https://www.medicines.org.uk/emc/product/5409. Accessed Oct 2021.
- 11.Baker D, Herrod SS, Alvarez-Gonzalez C, Zalewski L, Albor C, Schmierer K. Both cladribine and alemtuzumab may effect MS via B-cell depletion. Neurol Neuroimmunol Neuroinflamm. 2017;4(4):e360. doi: 10.1212/NXI.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker D, Herrod SS, Alvarez-Gonzalez C, Giovannoni G, Schmierer K. Interpreting lymphocyte reconstitution data from the pivotal phase 3 trials of alemtuzumab. JAMA Neurol. 2017;74(8):961–969. doi: 10.1001/jamaneurol.2017.0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comi G, Cook S, Giovannoni G, et al. Effect of cladribine tablets on lymphocyte reduction and reconstitution dynamics in patients with relapsing multiple sclerosis. Mult Scler Relat Disord. 2019;29:168–174. doi: 10.1016/j.msard.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 14.Stuve O, Soelberg Soerensen P, Leist T, et al. Effects of cladribine tablets on lymphocyte subsets in patients with multiple sclerosis: an extended analysis of surface markers. Ther Adv Neurol Disord. 2019;12:1756286419854986. doi: 10.1177/1756286419854986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buonomo AR, Zappulo E, Viceconte G, Scotto R, Borgia G, Gentile I. Risk of opportunistic infections in patients treated with alemtuzumab for multiple sclerosis. Expert Opin Drug Saf. 2018;17(7):709–717. doi: 10.1080/14740338.2018.1483330. [DOI] [PubMed] [Google Scholar]
- 16.Cencioni MT, Mattoscio M, Magliozzi R, Bar-Or A, Muraro PA. B cells in multiple sclerosis—from targeted depletion to immune reconstitution therapies. Nat Rev Neurol. 2021;17(7):399–414. doi: 10.1038/s41582-021-00498-5. [DOI] [PubMed] [Google Scholar]
- 17.Lünemann JD, Ruck T, Muraro PA, Bar-Or A, Wiendl H. Immune reconstitution therapies: concepts for durable remission in multiple sclerosis. Nat Rev Neurol. 2020;16(1):56–62. doi: 10.1038/s41582-019-0268-z. [DOI] [PubMed] [Google Scholar]
- 18.Sellner J, Rommer PS. Immunological consequences of "immune reconstitution therapy" in multiple sclerosis: a systematic review. Autoimmun Rev. 2020;19(4):102492. doi: 10.1016/j.autrev.2020.102492. [DOI] [PubMed] [Google Scholar]
- 19.Leist TP, Vermersch P. The potential role for cladribine in the treatment of multiple sclerosis: clinical experience and development of an oral tablet formulation. Curr Med Res Opin. 2007;23(11):2667–2676. doi: 10.1185/030079907X233142. [DOI] [PubMed] [Google Scholar]
- 20.Leist TP, Weissert R. Cladribine: mode of action and implications for treatment of multiple sclerosis. Clin Neuropharmacol. 2011;34(1):28–35. doi: 10.1097/WNF.0b013e318204cd90. [DOI] [PubMed] [Google Scholar]
- 21.Biernacki T, Sandi D, Bencsik K, Vécsei L. Medicinal chemistry of multiple sclerosis: focus on cladribine. Mini Rev Med Chem. 2020;20(4):269–285. doi: 10.2174/1389557519666191015201755. [DOI] [PubMed] [Google Scholar]
- 22.Voo VTF, Butzkueven H, Stankovich J, O’Brien T, Monif M. The development and impact of cladribine on lymphoid and myeloid cells in multiple sclerosis. Mult Scler Relat Disord. 2021;52:102962. doi: 10.1016/j.msard.2021.102962. [DOI] [PubMed] [Google Scholar]
- 23.Sigal DS, Miller HJ, Schram ED, Saven A. Beyond hairy cell: the activity of cladribine in other hematologic malignancies. Blood. 2010;116(16):2884–2896. doi: 10.1182/blood-2010-02-246140. [DOI] [PubMed] [Google Scholar]
- 24.Carlini F, Ivaldi F, Gualandi F, et al. Different susceptibility of T and B cells to cladribine depends on their levels of deoxycytidine kinase activity linked to activation status. J Neuroimmune Pharmacol. 2021. 10.1007/s11481-021-09994-3. [DOI] [PMC free article] [PubMed]
- 25.Filippi M, Bar-Or A, Piehl F, et al. Multiple sclerosis. Nat Rev Dis Primers. 2018;4(1):43. doi: 10.1038/s41572-018-0041-4. [DOI] [PubMed] [Google Scholar]
- 26.Baecher-Allan C, Kaskow BJ, Weiner HL. Multiple sclerosis: mechanisms and immunotherapy. Neuron. 2018;97(4):742–768. doi: 10.1016/j.neuron.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 27.von Büdingen HC, Kuo TC, Sirota M, et al. B cell exchange across the blood–brain barrier in multiple sclerosis. J Clin Investig. 2012;122(12):4533–4543. doi: 10.1172/JCI63842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liliemark J. The clinical pharmacokinetics of cladribine. Clin Pharmacokinet. 1997;32(2):120–131. doi: 10.2165/00003088-199732020-00003. [DOI] [PubMed] [Google Scholar]
- 29.Hermann R, Karlsson MO, Novakovic AM, Terranova N, Fluck M, Munafo A. The clinical pharmacology of cladribine tablets for the treatment of relapsing multiple sclerosis. Clin Pharmacokinet. 2019;58(3):283–297. doi: 10.1007/s40262-018-0695-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kearns CM, Blakley RL, Santana VM, Crom WR. Pharmacokinetics of cladribine (2-chlorodeoxyadenosine) in children with acute leukemia. Cancer Res. 1994;54(5):1235–1239. [PubMed] [Google Scholar]
- 31.Ruggieri M, Gargano CD, Ferretta A, et al. Effect of cladribine on neuronal apoptosis: new insight of in vitro study in multiple sclerosis therapy. Brain Sci. 2020;10(8):548. doi: 10.3390/brainsci10080548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker D, Pryce G, Herrod SS, Schmierer K. Potential mechanisms of action related to the efficacy and safety of cladribine. Mult Scler Relat Disord. 2019;30:176–186. doi: 10.1016/j.msard.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 33.Correale J, Halfon MJ, Jack D, Rubstein A, Villa A. Acting centrally or peripherally: a renewed interest in the central nervous system penetration of disease-modifying drugs in multiple sclerosis. Mult Scler Relat Disord. 2021;56:103264. doi: 10.1016/j.msard.2021.103264. [DOI] [PubMed] [Google Scholar]
- 34.Jameson SC, Lee YJ, Hogquist KA. Innate memory T cells. Adv Immunol. 2015;126:173–213. doi: 10.1016/bs.ai.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moser T, Schwenker K, Seiberl M, et al. Long-term peripheral immune cell profiling reveals further targets of oral cladribine in MS. Ann Clin Transl Neurol. 2020;7(11):2199–2212. doi: 10.1002/acn3.51206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marsh-Wakefield F, Hawke S, Grau G, et al. In depth analysis of T cells in multiple sclerosis patients after treatment with cladribine. Neurology. 2020;94(15 Suppl):5301. [Google Scholar]
- 37.Marsh-Wakefield F, Juillard P, Ashhurst T, et al. In depth analysis of B cells in multiple sclerosis patients after treatment with cladribine. Mult Scler. 2020;25(3):NP25. [Google Scholar]
- 38.Verma N, O'Neill D, Al-Atiyah R, et al. The effect of cladribine upon naive and activated CD4+ T regulatory cells in MS patients. Mult Scler. 2020;26(S3):P0406. [Google Scholar]
- 39.Giovannoni G, Turner B, Gnanapavan S, Offiah C, Schmierer K, Marta M. Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult Scler Relat Disord. 2015;4(4):329–333. doi: 10.1016/j.msard.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Klotz L, Havla J, Schwab N, et al. Risks and risk management in modern multiple sclerosis immunotherapeutic treatment. Ther Adv Neurol Disord. 2019;12:1756286419836571. doi: 10.1177/1756286419836571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Comi G, Soelberg-Sorensen P, Rammohan K, et al. Efficacy outcomes in cladribine tablets-treated patients in CLARITY were similar between patients who did vs. did not enter CLARITY extension. Mult Scler. 2020;26(1S):P059. [Google Scholar]
- 42.De Stefano N, Sormani MP, Giovannoni G, et al. Analysis of frequency and severity of relapses in multiple sclerosis patients treated with cladribine tablets or placebo: the CLARITY and CLARITY extension studies. Mult Scler. 2022;28(1):111–120. doi: 10.1177/13524585211010294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giovannoni G, Comi G, Rammohan K, et al. Long-term disease stability assessed by the Expanded Disability Status Scale in patients treated with cladribine tablets 3.5 mg/kg for relapsing multiple sclerosis: an exploratory post hoc analysis of the CLARITY and CLARITY Extension studies. Adv Ther. 2021;38(9):4975–4985. doi: 10.1007/s12325-021-01865-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giovannoni G, Singer BA, Issard D, Jack D, Vermersch P. Durability of no evidence of disease activity-3 (NEDA-3) in patients receiving cladribine tablets: The CLARITY extension study. Mult Scler. 2021:13524585211049392. [DOI] [PMC free article] [PubMed]
- 45.Giovannoni G, Soelberg Sorensen P, Cook S, et al. Efficacy of cladribine tablets in high disease activity subgroups of patients with relapsing multiple sclerosis: a post hoc analysis of the CLARITY study. Mult Scler. 2019;25(6):819–827. doi: 10.1177/1352458518771875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vermersch P, Rammohan K, Damian D, Jack D, Harty G, Wong SL. The CLARITY study: efficacy outcomes among patients who received disease-modifying drugs prior to treatment with cladribine tablets. Mult Scler. 2020;26(1S):P071. [Google Scholar]
- 47.Vermersch P, Galazka A, Dangond F, et al. Efficacy of cladribine tablets in high disease activity patients with relapsing multiple sclerosis: post hoc analysis of subgroups with and without prior disease-modifying drug treatment. Curr Med Res Opin. 2021;37(3):459–464. doi: 10.1080/03007995.2020.1865888. [DOI] [PubMed] [Google Scholar]
- 48.Bartosik-Psujek H, Kaczyński Ł, Górecka M, et al. Cladribine tablets versus other disease-modifying oral drugs in achieving no evidence of disease activity (NEDA) in multiple sclerosis-A systematic review and network meta-analysis. Mult Scler Relat Disord. 2021;49:102769. doi: 10.1016/j.msard.2021.102769. [DOI] [PubMed] [Google Scholar]
- 49.Piasecka-Stryczynska K, Rolka M, Kaczynski L, et al. Cladribine tablets versus other DMT in achieving disability improvement in relapsing remitting multiple sclerosis patients-network meta-analysis. Mult Scler. 2020;26(S3):P0040. [Google Scholar]
- 50.Signori A, Saccà F, Lanzillo R, et al. Cladribine vs other drugs in MS: merging randomized trial with real-life data. Neurol Neuroimmunol Neuroinflamm. 2020;7(6):e878. doi: 10.1212/NXI.0000000000000878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Freedman MS, Coyle PK, Comi G, et al. Early MRI outcomes in participants with a first clinical demyelinating event at risk of multiple sclerosis in the ORACLE-MS study. Mult Scler J Exp Transl Clin. 2021;7(1):2055217321990852. doi: 10.1177/2055217321990852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Stefano N, Barkhof F, Montalban X, et al. Reduction in CUA MRI lesions in the first 6 months after cladribine tablets treatment for highly active relapsing mulriple sclerosis: MAGNIFY-MS subgroup analysis. Neurology. 2021;96(15 Suppl):1921. [Google Scholar]
- 53.Wiendl H, Schmierer K, Hodgkinson S, et al. Characterization of peripheral immune cell dynamics and repopulation patterns in the first 12 months of cladribine tablets treatment: MAGNIFY-MS. Neurology. 2021;96(15 Suppl):2235. [Google Scholar]
- 54.Giovannoni G, Leist T, Aydemir A, Verdun Di Cantogno E. CLASSIC-MS: long-term efficacy and real-world treatment patterns for patients receiving cladribine tablets-interim data with 8–14 years follow-up. Mult Scler. 2020;26(S3):LB1229. [Google Scholar]
- 55.Brochet B, Raymond Hupperts RH, Langdon D, et al. Treatment satisfaction in patients with highly-active relapsing multiple sclerosis treated with cladribine tablets: CLARIFY-MS study interim analysis. Mult Scler. 2020;26(S3):P1066. doi: 10.1016/j.msard.2021.103385. [DOI] [PubMed] [Google Scholar]
- 56.Patti F, Visconti A, Capacchione A, Roy S, Trojano M. Long-term effectiveness in patients previously treated with cladribine tablets: a real-world analysis of the Italian multiple sclerosis registry (CLARINET-MS) Ther Adv Neurol Disord. 2020;13:1756286420922685. doi: 10.1177/1756286420922685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miravalle AA, Katz J, Robertson D, et al. CLICK-MS and MASTER-2 Phase IV trial design: cladribine tablets in suboptimally controlled relapsing multiple sclerosis. Neurodegener Dis Manag. 2021;11(2):99–111. doi: 10.2217/nmt-2020-0059. [DOI] [PubMed] [Google Scholar]
- 58.Wu GF, Boschert U, Hayward B, Lebson LA. Evaluating the impact of cladribine tablets on the development of antibody titres: interim results from the CLOCK-MS influenza vaccine sub-study. Mult Scler. 2021;27(1S):P071. [Google Scholar]
- 59.Lieberman D, Mangat H, Allen-Philbey K, et al. Cladribine to halt deterioration in people with advanced multiple sclerosis (CHARIOTMS) Mult Scler. 2020;26(S3):P0196. [Google Scholar]
- 60.Scott JL, Maltby V, Lydon A, et al. Cladribine: A multicentre long-term efficacy biomarker Australian study (CLOBAS) Mult Scler. 2020;26(9):P-56. [Google Scholar]
- 61.Maltby VE, Lea RA, Monif M, et al. Efficacy of cladribine tablets as a treatment for people with multiple sclerosis: protocol for the CLOBAS study (cladribine, a multicenter, long-term efficacy and biomarker Australian study) JMIR Res Protoc. 2021;10(10):e24969. doi: 10.2196/24969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lizak N, Hodgkinson S, Butler E, et al. Real-world effectiveness of cladribine for Australian patients with multiple sclerosis: an MSBase registry substudy. Mult Scler. 2021;27(3):465–474. doi: 10.1177/1352458520921087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Butzkueven H, Spelman T, Hodgkinson S, et al. Real-world experience with cladribine tablets in the MSBase registry. Mult Scler. 2020;26(S3):P0907. [Google Scholar]
- 64.Hodgkinson S, Butzkueven H, Spelman T, et al. Real world experience with cladribine (Mavenclad) in MSBase registry. Mult Scler. 2020;26(9):57. [Google Scholar]
- 65.Barros A, Santos M, Sequeira J, et al. Effectiveness of cladribine in multiple sclerosis-clinical experience of two tertiary centers. Mult Scler. 2020;26(S3):P0328. [Google Scholar]
- 66.Santos M, Barros A, Sequeira J, et al. Safety and tolerability of cladribine in multiple sclerosis-clinical experience of two tertiary centers. Mult Scler. 2020;26(S3):P0387. [Google Scholar]
- 67.Suslak T, Macdougall N, Murray L. Cladribine is a safe and effective treatment for highly active relapsing-remitting multiple sclerosis. Mult Scler. 2020;26(S3):P0309. [Google Scholar]
- 68.Thakre M, Inshasi J. Real world experience of oral immune reconstitution therapy (cladribine) in the treatment of multiple sclerosis in the United Arab Emirates. Mult Scler. 2020;26(S3):P0140. [Google Scholar]
- 69.Ubago Perez R, Lvarez Sanchez RA, Nieto Gomez P, Portillo Haro S, Cabeza BJ. Preliminary cladribine effectiveness and safety results: real world data. Int J Clin Pharm. 2020;42(1):PT029. [Google Scholar]
- 70.Moccia M, Lanzillo R, Petruzzo M, et al. Single-center 8-years clinical follow-up of cladribine-treated patients from phase 2 and 3 trials. Front Neurol. 2020;11:489. doi: 10.3389/fneur.2020.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zanetta C, Sangalli F, Guerrieri S, Filippi M, Montini F, Moiola L. Efficacy/safety profile of cladribine in an Italian real-life cohort of relapsing remitting multiple sclerosis patients. Eur J Neurol. 2020;28(Suppl 1):EPR-179. [Google Scholar]
- 72.Pfeuffer S, Rolfes L, Hackert J, et al. Effectiveness and safety of cladribine in MS: real-world experience from two tertiary centres. Mult Scler. 2021:13524585211012227. [DOI] [PMC free article] [PubMed]
- 73.Bose G, Rush C, Atkins HL, Freedman MS. A real-world single-centre analysis of alemtuzumab and cladribine for multiple sclerosis. Mult Scler Relat Disord. 2021;52:102945. doi: 10.1016/j.msard.2021.102945. [DOI] [PubMed] [Google Scholar]
- 74.Allen-Philbey K, De Trane S, Stennett A, et al. Cladribine personalised dosing to treat active multiple sclerosis-follow-up in 250 patients. Experince with SC cladribine. Mult Scler. 2021;27(1S):P160. [Google Scholar]
- 75.Rejdak K, Zasybska A, Pietruczuk A, et al. Long-term safety and efficacy of subcutaneous cladribine used in increased dosage in patients with relapsing multiple sclerosis: 20-year observational study. J Clin Med. 2021;10(21):5207. doi: 10.3390/jcm10215207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elsisi Z, Hincapie AL, Guo JJ. Expenditure, utilization, and cost of specialty drugs for multiple sclerosis in the US Medicaid population, 2008–2018. Am Health Drug Benefits. 2020;13(2):74–84. [PMC free article] [PubMed] [Google Scholar]
- 77.Goudarzi MH, Eadie MJ, Hollingworth SA. Disease modifying therapies for relapsing-remitting multiple sclerosis: use and costs in Australia (1996–2019) Mult Scler Relat Disord. 2021;50:102835. doi: 10.1016/j.msard.2021.102835. [DOI] [PubMed] [Google Scholar]
- 78.Tafazzoli A, Chavan A, Harty G, Moller J, Wong SL. Efficiency model of cladribine tablets versus infusion-based disease-modifying drugs for patients with relapsing-remitting multiple sclerosis. Adv Ther. 2020;37(9):3791–3806. doi: 10.1007/s12325-020-01426-7. [DOI] [PubMed] [Google Scholar]
- 79.Mankinen P, Lundström T, Soini E, et al. Cost assessment modelling of treatments for highly active relapsing multiple sclerosis. Adv Ther. 2020;37(2):800–818. doi: 10.1007/s12325-019-01186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pinheiro B, Guerreiro R, Costa J, Miguel LS. Cost-effectiveness of cladribine tablets versus fingolimod in patients with highly active relapsing multiple sclerosis in Portugal. J Med Econ. 2020;23(5):484–491. doi: 10.1080/13696998.2020.1717499. [DOI] [PubMed] [Google Scholar]
- 81.Poveda JL, Trillo JL, Rubio-Terrés C, Rubio-Rodríguez D, Polanco A, Torres C. Cost-effectiveness of cladribine tablets and fingolimod in the treatment of relapsing multiple sclerosis with high disease activity in Spain. Expert Rev Pharmacoecon Outcomes Res. 2020;20(3):295–303. doi: 10.1080/14737167.2019.1635014. [DOI] [PubMed] [Google Scholar]
- 82.Bohlega S, Elboghdady A, Al-Johani A, et al. Economic evaluation of cladribine tablets in patients with high disease activity-relapsing-remitting multiple sclerosis in the Kingdom of Saudi Arabia. Value Health Reg Issues. 2021;25:189–195. doi: 10.1016/j.vhri.2021.03.007. [DOI] [PubMed] [Google Scholar]
- 83.Espinoza MA, Rojas R, Zaupa A, Balmaceda C. A model-based economic evaluation of cladribine versus alemtuzumab, ocrelizumab and natalizumab for the treatment of relapsing-remitting multiple sclerosis with high disease activity in Chile. Pharmacoecon Open. 2021;5:635–647. doi: 10.1007/s41669-021-00282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ayati N, Fleifel L, Sharifi S, Sahraian MA, Nikfar S. Cladribine tablets are a cost-effective and cost-saving treatment strategy for high disease activity relapsing multiple sclerosis patients in Iran. Mult Scler. 2021;27(1S):P160. doi: 10.18502/cjn.v20i3.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zieba P, Pawlik D, Wieczorek J, Wojcik R, Kaczor MP. Cladribine tablets (CT) versus other disease-modifying therapies in the treatment of relapsing-remitting multiple sclerosis (RRMS)—cost-effectiveness analysis. Value Health. 2020;23(Suppl 2):PND51. [Google Scholar]
- 86.Gross RH, Corboy JR. Monitoring, switching, and stopping multiple sclerosis disease-modifying therapies. Continuum (Minneap Minn) 2019;25(3):715–735. doi: 10.1212/CON.0000000000000738. [DOI] [PubMed] [Google Scholar]
- 87.Bigaut K, Cohen M, Durand-Dubief F, et al. How to switch disease-modifying treatments in multiple sclerosis: guidelines from the French Multiple Sclerosis Society (SFSEP) Mult Scler Relat Disord. 2021;53:103076. doi: 10.1016/j.msard.2021.103076. [DOI] [PubMed] [Google Scholar]
- 88.Radlberger RF, Sakic I, Moser T, Pilz G, Harrer A, Wipfler P. Immune phenotyping study revealing caveats regarding a switch from fingolimod to cladribine. Mult Scler Relat Disord. 2021;48:102727. doi: 10.1016/j.msard.2020.102727. [DOI] [PubMed] [Google Scholar]
- 89.O'Neill D, Hodgkinson S. Short term efficacy and safety outcomes after switching from anti-CD20 DMTS to cladribine in a real-world setting. Neurology. 2020;94(15 Suppl):5389. [Google Scholar]
- 90.Mills EA, Mao-Draayer Y. Understanding progressive multifocal leukoencephalopathy risk in multiple sclerosis patients treated with immunomodulatory therapies: a bird's eye view. Front Immunol. 2018;9:138. doi: 10.3389/fimmu.2018.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Giovannoni G, Marta M, Davis A, Turner B, Gnanapavan S, Schmierer K. Switching patients at high risk of PML from natalizumab to another disease-modifying therapy. Pract Neurol. 2016;16(5):389–393. doi: 10.1136/practneurol-2015-001355. [DOI] [PubMed] [Google Scholar]
- 92.Ziemssen T, Penner I-K, Wagner T, et al. MS disease modifying therapy sequencing – natalizumab to cladribine tablets – experience in 46 patients. Mult Scler. 2020;26(S3):P0884. [Google Scholar]
- 93.Freedman MS, Selchen D, Prat A, Giacomini PS. Managing multiple sclerosis: treatment initiation, modification, and sequencing. Can J Neurol Sci. 2018;45(5):489–503. doi: 10.1017/cjn.2018.17. [DOI] [PubMed] [Google Scholar]
- 94.Hatcher SE, Waubant E, Nourbakhsh B, Crabtree-Hartman E, Graves JS. Rebound syndrome in patients with multiple sclerosis after cessation of fingolimod treatment. JAMA Neurol. 2016;73(7):790–794. doi: 10.1001/jamaneurol.2016.0826. [DOI] [PubMed] [Google Scholar]
- 95.Kieseier BC, Benamor M. Pregnancy outcomes following maternal and paternal exposure to teriflunomide during treatment for relapsing-remitting multiple sclerosis. Neurol Ther. 2014;3(2):133–138. doi: 10.1007/s40120-014-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zecca C, Antozzi CG, Torri Clerici V, et al. Severe multiple sclerosis reactivation during prolonged lymphopenia after dimethyl fumarate discontinuation. Acta Neurol Scand. 2018;137(6):623–625. doi: 10.1111/ane.12882. [DOI] [PubMed] [Google Scholar]
- 97.Morgan K, Vernon K, Ayer M. Value-added benefits of a nurse/pharmacy-led service for patients with multiple sclerosis treated over 2 years with cladribine tablets in the UK. Mult Scler. 2020;26:P1015. [Google Scholar]
- 98.Oh J, Walker B, Giovannoni G, et al. Treatment-emergent adverse events occurring early in the treatment course of cladribine tablets in two phase 3 trials in multiple sclerosis. Mult Scler J Exp Transl Clin. 2021;7(3):20552173211024298. doi: 10.1177/20552173211024298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Margoni M, Annovazzi P, Prosperini L, et al. A multicentre, real-life study on the risk of lymphopenia and infections discloses a favourable safety profile of cladribine in ms patients. Mult Scler. 2020;26(S3):P0273. [Google Scholar]
- 100.Rolfes L, Pfeuffer S, Hackert J, et al. Skin reactions in patients with multiple sclerosis receiving cladribine treatment. Neurol Neuroimmunol Neuroinflamm. 2021;8(3):e990. doi: 10.1212/NXI.0000000000000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mateo-Casas M, Reyes S, O’Toole EA, et al. Severe skin reactions associated with cladribine in people with multiple sclerosis. Mult Scler Relat Disord. 2020;43:102140. doi: 10.1016/j.msard.2020.102140. [DOI] [PubMed] [Google Scholar]
- 102.Gummi R, Walsh RD, Ahmad B. Retinal cotton wool spot associated with cladribine therapy for multiple sclerosis. Mult Scler Relat Disord. 2021;48:102661. doi: 10.1016/j.msard.2020.102661. [DOI] [PubMed] [Google Scholar]
- 103.Schönfelder K, Schuh H, Pfister F, et al. Autoimmune glomerulonephritis in a multiple sclerosis patient after cladribine treatment. Mult Scler. 2021:13524585211022719. [DOI] [PMC free article] [PubMed]
- 104.Cossburn M, Pace AA, Jones J, et al. Autoimmune disease after alemtuzumab treatment for multiple sclerosis in a multicenter cohort. Neurology. 2011;77(6):573–579. doi: 10.1212/WNL.0b013e318228bec5. [DOI] [PubMed] [Google Scholar]
- 105.Aguirre C, Meca-Lallana V, Del Rio BJV. Severe neutropenia after cladribine first cycle in a patient with multiple sclerosis. Mult Scler. 2020;26(S3):P0391. [Google Scholar]
- 106.Ahmed AM, Brantley JS, Madkan V, Mendoza N, Tyring SK. Managing herpes zoster in immunocompromised patients. Herpes. 2007;14(2):32–36. [PubMed] [Google Scholar]
- 107.Cook S, Leist T, Comi G, et al. Safety of cladribine tablets in the treatment of patients with multiple sclerosis: An integrated analysis. Mult Scler Relat Disord. 2019;29:157–167. doi: 10.1016/j.msard.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 108.Crumpacker C. The pharmacological profile of famciclovir. Semin Dermatol. 1996;15(2 Suppl 1):14–26. [PubMed] [Google Scholar]
- 109.Leist T, Cook S, Comi G, et al. Long-term safety data from the cladribine tablets clinical development program in multiple sclerosis. Mult Scler Relat Disord. 2020;46:102572. doi: 10.1016/j.msard.2020.102572. [DOI] [PubMed] [Google Scholar]
- 110.International Agency for Research on Cancer (IARC). GLOBOCAN. Estimated cancer incidence, mortality and prevalence worldwide in 2012. 2012.
- 111.Butzkueven H, Hillert J, Sõnajalg J, et al. Disease-modifying treatment patterns of patients with multiple sclerosis and newly treated with cladribine tablets or fingolimod: an interim analysis of the CLARION study [P742] Mult Scler. 2021;27(2_suppl):134–740. doi: 10.1177/13524585211044667. [DOI] [Google Scholar]
- 112.Buttmann M, Seuffert L, Mader U, Toyka KV. Malignancies after mitoxantrone for multiple sclerosis: a retrospective cohort study. Neurology. 2016;86(23):2203–2207. doi: 10.1212/WNL.0000000000002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lebrun C, Rocher F. Cancer risk in patients with multiple sclerosis: potential impact of disease-modifying drugs. CNS Drugs. 2018;32(10):939–949. doi: 10.1007/s40263-018-0564-y. [DOI] [PubMed] [Google Scholar]
- 114.Al Jumah M, Abulaban A, Aggad H, et al. Managing multiple sclerosis in the Covid19 era: a review of the literature and consensus report from a panel of experts in Saudi Arabia. Mult Scler Relat Disord. 2021;51:102925. doi: 10.1016/j.msard.2021.102925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Alborghetti M, Bellucci G, Gentile A, et al. Drugs used in the treatment of multiple sclerosis during COVID-19 pandemic: a critical viewpoint. Curr Neuropharmacol. 2021;20:107–125. doi: 10.2174/1570159X19666210330094017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Apostolos-Pereira SL, Silva GD, Disserol CCD, et al. Management of central nervous system demyelinating diseases during the coronavirus disease 2019 pandemic: a practical approach. Arq Neuropsiquiatr. 2020;78(7):430–439. doi: 10.1590/0004-282x20200056. [DOI] [PubMed] [Google Scholar]
- 117.Bsteh G, Assar H, Hegen H, et al. COVID-19 severity and mortality in multiple sclerosis are not associated with immunotherapy: Insights from a nation-wide Austrian registry. PLoS ONE. 2021;16(7):e0255316. doi: 10.1371/journal.pone.0255316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Czarnowska A, Brola W, Zajkowska O, et al. Clinical course and outcome of SARS-CoV-2 infection in multiple sclerosis patients treated with disease-modifying therapies—the Polish experience. Neurol Neurochir Pol. 2021;55(2):212–222. doi: 10.5603/PJNNS.a2021.0031. [DOI] [PubMed] [Google Scholar]
- 119.Zheng C, Kar I, Chen CK, et al. Multiple sclerosis disease-modifying therapy and the COVID-19 pandemic: implications on the risk of infection and future vaccination. CNS Drugs. 2020;34(9):879–896. doi: 10.1007/s40263-020-00756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Inshasi J, Alroughani R, Al-Asmi A, et al. Expert consensus and narrative review on the management of multiple sclerosis in the Arabian Gulf in the COVID-19 Era: focus on disease-modifying therapies and vaccination against COVID-19. Neurol Ther. 2021:1–17. [DOI] [PubMed]
- 121.Jack D, Damian D, Nolting A, Galazka A. COVID-19 in patients with multiple sclerosis treated with cladribine tablets: an update. Mult Scler Relat Disord. 2021;51:102929. doi: 10.1016/j.msard.2021.102929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dersch R, Wehrum T, Fähndrich S, Engelhardt M, Rauer S, Berger B. COVID-19 pneumonia in a multiple sclerosis patient with severe lymphopenia due to recent cladribine treatment. Mult Scler. 2020;26(10):1264–1266. doi: 10.1177/1352458520943783. [DOI] [PubMed] [Google Scholar]
- 123.Celius EG. Normal antibody response after COVID-19 during treatment with cladribine. Mult Scler Relat Disord. 2020;46:102476. doi: 10.1016/j.msard.2020.102476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Preziosa P, Rocca MA, Nozzolillo A, Moiola L, Filippi M. COVID-19 in cladribine-treated patients with relapsing remitting multiple sclerosis: a monocentric experience. Mult Scler. 2020;26(S3):LB1154. doi: 10.1007/s00415-020-10309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gelibter S, Orrico M, Filippi M, Moiola L. COVID-19 with no antibody response in a multiple sclerosis patient treated with cladribine: implication for vaccination program? Mult Scler Relat Disord. 2021;49:102775. doi: 10.1016/j.msard.2021.102775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.De Angelis M, Petracca M, Lanzillo R, Brescia Morra V, Moccia M. Mild or no COVID-19 symptoms in cladribine-treated multiple sclerosis: two cases and implications for clinical practice. Mult Scler Relat Disord. 2020;45:102452. doi: 10.1016/j.msard.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Seferoğlu M, Ethemoğlu Ö, Turan ÖF, Siva A. MS and COVID-19 challenge: asymptomatic COVID-19 infection during treatment with cladribine. Neurol Sci. 2021;42(9):3533–3535. doi: 10.1007/s10072-021-05409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Oreja-Guevara C, Meca-Lallana V, Brieva L, et al. COVID-19 in cladribine-treated patients with multiple sclerosis. Mult Scler. 2020;26(S3):LB1165. [Google Scholar]
- 129.Drulovic J, Ivanovic J, Martinovic V, et al. Humoral response to SARS-CoV-2 and COVID-19 vaccines in patients treated with immune reconstitution therapies. Mult Scler Relat Disord. 2021;54:103150. doi: 10.1016/j.msard.2021.103150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Karan R, Roy S, Alexandri N. Clinical outcomes in patients with COVID-19 infection during phase IV studies of cladribine tablets for treatment of multiple sclerosis. Mult Scler. 2020;26(S3):LB1151. [Google Scholar]
- 131.Gold R, Fätkenheuer G, Hartung H-P, et al. Vaccination in multiple sclerosis patients treated with highly effective disease-modifying drugs: an overview with consideration of cladribine tablets. Ther Adv Neurol Disord. 2021;14:17562864211019598. doi: 10.1177/17562864211019598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Roy S, Boschert U. Analysis of influenza and varicella zoster virus vaccine antibody titers in patients with relapsing multiple sclerosis treated with cladribine tablets [P059] Mult Scler. 2021;27(1_suppl):15–122. doi: 10.1177/13524585211015908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cabreira V, Abreu P, Soares-Dos-Reis R, Guimarães J, Sá MJ. Multiple sclerosis, disease-modifying therapies and COVID-19: a systematic review on immune response and vaccination recommendations. Vaccines (Basel). 2021;9(7):773. doi: 10.3390/vaccines9070773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kelly H, Sokola B, Abboud H. Safety and efficacy of COVID-19 vaccines in multiple sclerosis patients. J Neuroimmunol. 2021;356:577599. doi: 10.1016/j.jneuroim.2021.577599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sellner J, Rommer PS. Multiple sclerosis and SARS-CoV-2 vaccination: considerations for immune-depleting therapies. Vaccines (Basel). 2021;9(2):99. doi: 10.3390/vaccines9020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord. 2021;14:17562864211012835. doi: 10.1177/17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Buttari F, Bruno A, Dolcetti E, et al. COVID-19 vaccines in multiple sclerosis treated with cladribine or ocrelizumab. Mult Scler Relat Disord. 2021;52:102983. doi: 10.1016/j.msard.2021.102983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Giovannoni G, Galazka A, Schick R, et al. Pregnancy outcomes during the clinical development program of cladribine in multiple sclerosis: an integrated analysis of safety. Drug Saf. 2020;43(7):635–643. doi: 10.1007/s40264-020-00948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Alroughani R, Inshasi J, Al-Asmi A, et al. Disease-modifying drugs and family planning in people with multiple sclerosis: a consensus narrative review from the Gulf Region. Neurol Ther. 2020;9(2):265–280. doi: 10.1007/s40120-020-00201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Varytė G, Arlauskienė A, Ramašauskaitė D. Pregnancy and multiple sclerosis: an update. Curr Opin Obstet Gynecol. 2021;33(5):378–383. doi: 10.1097/GCO.0000000000000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Datta P, Ciplea AI, Rewers-Felkins K, et al. Cladribine transfer into human milk: a case report. Mult Scler. 2021;27(5):799–801. doi: 10.1177/1352458520912173. [DOI] [PubMed] [Google Scholar]
- 142.Biogen Idec Ltd. TYSABRI 300 mg concentrate for solution for infusion summary of product characterisics https://www.medicines.or.guk/emc/product/222. Accessed Oct 2021.
- 143.Sanofi Genzyme. AUBAGIO 14 mg film-coated tablets summary of product characteristics. https://www.medicines.org.uk/emc/product/5244. Accessed Oct 2021.
- 144.Roche Products Limited. OCREVUS 300 mg concentrate for solution for infusion. https://www.medicines.org.uk/emc/product/8898. Accessed Oct 2021.
- 145.Biogen Idec Ltd. Tecfidera 120 mg gastro-resistant hard capsules. https://www.medicines.org.uk/emc/product/5256/smpc. Accessed Oct 2021.
- 146.Novartis Pharmaceuticals UK Ltd. Gilenya 0.5 mg hard capsules summary of product characterisitics https://www.medicines.org.uk/emc/product/4545. Accessed Oct 2021.
- 147.Bayer plc. Betaferon 250 microgram/ml, powder and solvent for solution for injection. https://www.medicines.org.uk/emc/product/1121. Accessed Oct 2021.
- 148.Teva Pharma B.V. Copaxone 40 mg/ml solution for injection, pre-filled syringe summary of product characteristics. https://www.medicines.org.uk/emc/product/7046/. Accessed Oct 2021.
- 149.Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- 150.Arvin AM, Wolinsky JS, Kappos L, et al. Varicella-zoster virus infections in patients treated with fingolimod: risk assessment and consensus recommendations for management. JAMA Neurol. 2015;72(1):31–39. doi: 10.1001/jamaneurol.2014.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.