Abstract
LJC 11,036 is the active metabolite of L-084, a novel oral carbapenem that exhibits potent broad-spectrum activity. Antibacterial activities of LJC 11,036 against clinical isolates from respiratory infections, such as Streptococcus pneumoniae (n = 52), Streptococcus pyogenes (n = 19), Haemophilus influenzae (n = 50), Klebsiella pneumoniae (n = 53), and Moraxella catarrhalis (n = 53), and from urinary-tract infections, such as Escherichia coli (n = 53) (MICs at which 90% of the isolates were inhibited [MIC90s], 0.1, ≤0.006, 0.39, 0.05, 0.05, and 0.05 μg/ml, respectively), were 2- to 64-fold higher than those of imipenem, cefdinir, and faropenem. Moreover, against these bacterial species, except for H. influenzae, the MIC90s of LJC 11,036 were 4- to 512-fold lower than those of levofloxacin. LJC 11,036 showed bactericidal activity equal or superior to that of imipenem. Bactericidal activity against penicillin-resistant S. pneumoniae (PRSP) did not vary with the phase of growth. LJC 11,036 had potent activity against various β-lactamase-producing strains, excluding carbapenemase producers. Against renal dehydropeptidase-I, LJC 11,036 was more stable than imipenem. Furthermore, LJC 11,036 produced in vitro postantibiotic sub-MIC effects against PRSP HSC-3 (6.0 h at one-fourth the MIC) and H. influenzae LJ5 (9.2 h at one-half the MIC). LJC 11,036 showed high binding affinities for PBP1A, -1B, -2A/2X, -2B, and -3 of PRSP and for PBP1B, -2, -3A, and -3B of H. influenzae.
Carbapenems are well recognized to have broad antibacterial activities, and many derivatives have been synthesized (3, 4, 8, 12). Parenteral carbapenems such as imipenem (8), panipenem (11), and meropenem (5) have been introduced into the market; however, no oral carbapenem has been marketed as yet. Several oral carbapenem derivatives, such as GV 118819X (13), CS-834 (18), DZ-2640 (14), and CL191,121 (17) are under development. For development of an oral carbapenem, a compound should possess distinct features, e.g., broad and potent activity, high stability to β-lactamase and dehydropeptidase-I (DHP-I), and postantibiotic effect, and should achieve high oral absorption in order to prevent both ready acquisition of drug resistance in enteric bacteria and the occurrence of diarrhea. L-084 (1) is a novel oral carbapenem with a 1-(1,3-thiazolin-2-yl)azetidin-3-ylthio group at the C-2 position (Fig. 1). In this study, we evaluated the following in vitro antibacterial properties of LJC 11,036, the active metabolite of L-084, in comparison with those of imipenem, faropenem, cefdinir, and levofloxacin: (i) activity against clinical isolates, (ii) activity against β-lactamase-producing strains, (iii) correlation between the MIC and the MBC, (iv) stability to DHP-I, (v) in vitro postantibiotic sub-MIC effect, (vi) potency against bacteria at various phases of growth, and (vii) binding affinity for penicillin-binding proteins (PBPs).
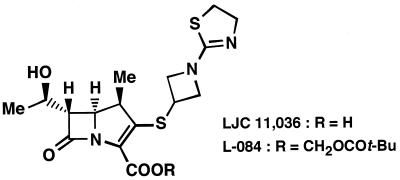
FIG. 1.
Chemical structure of LJC 11,036.
(This work was presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 24 to 27 September 1998 [1]).
MATERIALS AND METHODS
Drugs.
LJC 11,036, an active metabolite of L-084, was synthesized at the Medical Research Laboratories, Lederle (Japan), Ltd., Saitama, Japan. L-084 is not active in vitro, and all studies were performed with LJC 11,036, the active metabolite. Imipenem (Banyu Pharmaceutical Co., Ltd.), faropenem (Yamanouchi Pharmaceutical Co., Ltd./Suntory Ltd.), cefdinir (Fujisawa Pharmaceutical Co., Ltd.), and levofloxacin (Daiichi Pharmaceutical Co., Ltd.) were obtained commercially.
Organisms.
Twenty-six aerobic standard strains and 37 β-lactamase-producing strains were obtained from the stock culture collection of the Medical Research Laboratories, Lederle (Japan), Ltd. A total of 652 clinical isolates collected between 1994 and 1998 from patients at various hospitals in Japan were used for MIC determinations.
Determination of MICs.
MICs were determined by the agar dilution method (10) with Sensitivity Disk Agar-N (SDA; Nissui). SDA supplemented with 5% horse blood was used for streptococci and Moraxella catarrhalis, while SDA supplemented with 5% Fildes enrichment was used for Haemophilus influenzae. One loopful (5 μl) of an inoculum corresponding to 104 CFU per spot was inoculated on drug-containing agar plates, and the plates were incubated for 18 h at 37°C. The MIC was defined as the lowest drug concentration which inhibited visible growth of bacteria. To examine the effects of different inoculum sizes (106 to 108 CFU/ml) on the antibacterial activity of LJC 11,036, MIC tests were also performed by the agar dilution method using SDA. The stabilities of LJC 11,036 to β-lactamases were determined by drug susceptibility by using the agar dilution method against β-lactamase-producing strains.
Determination of MBCs.
MICs were determined by the broth dilution method using serial twofold dilutions. Strains were grown overnight in sensitivity test broth (STB; Nissui). Overnight cultures were diluted in fresh STB to approximately 106 CFU/ml and inoculated into STB containing various concentrations of each drug. For H. influenzae, STB supplemented with 5% Fildes enrichment was used, while STB supplemented with 5% horse serum was used for Streptococcus pneumoniae. Five microliters of cultures used for the determination of MICs from test tubes showing no visible growth of bacteria were inoculated on SDA plates. The MBC was defined as the lowest drug concentration which inhibited visible growth of bacteria.
Drug susceptibility in various phases of growth.
Growth curves obtained by monitoring the batch culture in the presence of drugs were examined. The period between the logarithmic and stationary phases was divided into three growth periods, and at each phase drugs were added. Viable counts were determined at the times indicated in Fig. 2.
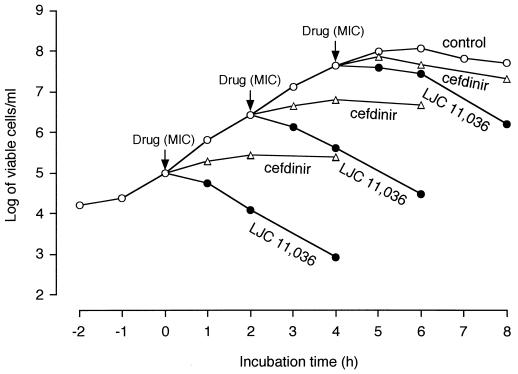
FIG. 2.
Influence of the growth phase of PRSP HSC-3 on its susceptibility to LJC 11,036. The MIC of LJC 11,036 is 0.025 μg/ml, and that of cefdinir is 1.56 μg/ml.
In vitro postantibiotic sub-MIC effect.
STB supplemented with 5% horse serum was used as the growth medium for penicillin-resistant S. pneumoniae (PRSP) HSC-3. Bacteria in the logarithmic phase of growth (approximately 108 CFU/ml) were exposed to the MIC of the test drug at 37°C for 2 h. The drug was removed by a 10−2 dilution into fresh medium containing different sub-MIC concentrations (1/16, 1/8, and 1/4 the MIC). Viable counts were determined at 0, 2, 4, 6, and 8 h after the addition of sub-MIC concentrations of drugs. The in vitro postantibiotic sub-MIC effect was calculated according to the method of Licata et al. (7). For H. influenzae LJ5, STB supplemented with 5% Fildes enrichment was used. After exposure to the drug at twice the MIC for 2 h, the drug was removed by the dilution method. Sub-MIC concentrations of one-eighth, one-fourth, and one-half the MIC were used. Viable counts were determined at 0, 2, 4, 6, 8, 10, and 12 h.
Stability to DHP-I.
The stability of LJC 11,036 to renal DHP-I was compared with that of imipenem by using recombinant human DHP-I (9). Test compounds at a final concentration of 3 mM were incubated at 30°C with DHP-I (enzyme activity, 0.6 U/ml) in 50 mM 3-(N-morpholino)propanesulfonic acid buffer (pH 7.0). After various times of incubation, the concentrations of the compounds were determined by high-performance liquid chromatography (9).
Binding assays for PBPs.
Binding of LJC 11,036 to PBPs of PRSP HSC-3 and H. influenzae LJ5 was determined by previously described competition assays (2, 15). Solubilized membrane fractions were preincubated for 10 min at 30°C with a nonradioactive compound diluted to various concentrations and then postincubated with [3H]benzylpenicillin for another 10 min at 30°C. PBPs were detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and fluorography.
RESULTS
Activity against clinical isolates.
Table 1 shows the in vitro activity of LJC 11,036 against gram-positive bacteria compared with those of imipenem, faropenem, cefdinir, and levofloxacin. The MICs at which 90% of the isolates are inhibited (MIC90s) of LJC 11,036 for methicillin-susceptible Staphylococcus aureus (MSSA), methicillin-resistant S. aureus (MRSA) (MIC of methicillin, ≥12.5 μg/ml), and Staphylococcus epidermidis were 0.025, 12.5, and 6.25 μg/ml, respectively. The activity against MSSA (MIC90) was equal to that of imipenem and 8- to 16-fold higher than those of faropenem, cefdinir, and levofloxacin. LJC 11,036 showed the highest activity against MRSA and S. epidermidis among the drugs tested. MIC90s for penicillin-susceptible S. pneumoniae (PSSP), PRSP, and Streptococcus pyogenes were ≤0.006, 0.1, and ≤0.006 μg/ml, respectively. Of the compounds used in this study, LJC 11,036 was the most active against streptococci.
TABLE 1.
Antibacterial activities of LJC 11,036 against clinical isolates of gram-positive bacteria
| Organism (no. of strains) | Drug | MICa (μg/ml)
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| S. aureus | ||||
| MSSA (53) | LJC 11,036 | ≤0.006–0.025 | 0.025 | 0.025 |
| Imipenem | 0.013–0.2 | 0.025 | 0.025 | |
| Faropenem | 0.1–0.39 | 0.2 | 0.2 | |
| Cefdinir | 0.05–3.13 | 0.39 | 0.39 | |
| Levofloxacin | 0.1–3.13 | 0.39 | 0.39 | |
| MRSA (53) | LJC 11,036 | 0.2–12.5 | 6.25 | 12.5 |
| Imipenem | 0.1–100 | 25 | 50 | |
| Faropenem | 0.78–>100 | >100 | >100 | |
| Cefdinir | 6.25–>100 | >100 | >100 | |
| Levofloxacin | 0.39–>100 | 50 | >100 | |
| S. epidermidis (53) | LJC 11,036 | 0.013–12.5 | 0.1 | 6.25 |
| Imipenem | 0.013–50 | 0.1 | 50 | |
| Faropenem | 0.05–>100 | 0.1 | >100 | |
| Cefdinir | 0.025–>100 | 0.39 | >100 | |
| Levofloxacin | 0.2–25 | 0.78 | 25 | |
| S. pneumoniae | ||||
| PSSP (24) | LJC 11,036 | ≤0.006–0.025 | ≤0.006 | ≤0.006 |
| Imipenem | ≤0.006–0.013 | ≤0.006 | 0.013 | |
| Faropenem | ≤0.006–0.05 | 0.013 | 0.025 | |
| Cefdinir | 0.025–0.39 | 0.05 | 0.2 | |
| Levofloxacin | 0.39–3.13 | 1.56 | 1.56 | |
| Benzylpenicillin | ≤0.006–0.05 | 0.025 | 0.05 | |
| PRSP (28) | LJC 11,036 | ≤0.006–0.2 | 0.05 | 0.1 |
| Imipenem | 0.013–0.78 | 0.2 | 0.39 | |
| Faropenem | 0.05–0.78 | 0.39 | 0.39 | |
| Cefdinir | 0.1–6.25 | 1.56 | 3.13 | |
| Levofloxacin | 0.78–>12.5 | 1.56 | 12.5 | |
| Benzylpenicillin | 0.1–3.13 | 0.78 | 1.56 | |
| S. pyogenes (19) | LJC 11,036 | ≤0.006 | ≤0.006 | ≤0.006 |
| Imipenem | ≤0.006 | ≤0.006 | ≤0.006 | |
| Faropenem | ≤0.006–0.025 | 0.025 | 0.025 | |
| Cefdinir | ≤0.006–0.013 | 0.013 | 0.013 | |
| Levofloxacin | 0.78–3.13 | 0.78 | 3.13 | |
Agar dilution method.
The in vitro activity of LJC 11,036 against gram-negative bacteria is shown in Table 2. Against Escherichia coli, Klebsiella pneumoniae, and M. catarrhalis, the MIC90s of this compound were 0.05 μg/ml; thus, it was 2- to 512-fold more active than the other drugs tested. Against H. influenzae, the activity of LJC 11,036 (MIC90s, 0.39 μg/ml) was four- to eightfold greater than those of the other β-lactams tested, but it was less active than levofloxacin. The MIC90s of LJC 11,036 against Enterobacter cloacae (0.2 μg/ml) and Proteus mirabilis (0.39 μg/ml) were 4- to 64-fold lower than those of imipenem and faropenem. LJC 11,036 was moderately active against Serratia marcescens (MIC50, 0.39 μg/ml; MIC90, 25 μg/ml). The activity of LJC 11,036 against Pseudomonas aeruginosa (MIC50, 6.25 μg/ml; MIC90, 100 μg/ml) was lower than those of imipenem and levofloxacin but greater than those of faropenem and cefdinir.
TABLE 2.
Antibacterial activities of LJC 11,036 against clinical isolates of gram-negative bacteria
| Organism (no. of strains) | Drug | MICa (μg/ml)
|
||
|---|---|---|---|---|
| Range | 50% | 90% | ||
| E. coli (53) | LJC 11,036 | ≤0.025–0.1 | ≤0.025 | 0.05 |
| Imipenem | 0.1–0.78 | 0.2 | 0.78 | |
| Faropenem | 0.2–3.13 | 0.78 | 3.13 | |
| Cefdinir | 0.1–50 | 0.39 | 1.56 | |
| Levofloxacin | ≤0.025–50 | 0.05 | 25 | |
| H. influenzae (50) | LJC 11,036 | ≤0.006–3.13 | 0.05 | 0.39 |
| Imipenem | 0.025–12.5 | 0.78 | 3.13 | |
| Faropenem | 0.05–12.5 | 0.39 | 3.13 | |
| Cefdinir | 0.05–3.13 | 0.39 | 1.56 | |
| Levofloxacin | ≤0.006–0.025 | ≤0.006 | 0.013 | |
| K. pneumoniae (53) | LJC 11,036 | ≤0.025–0.2 | ≤0.025 | 0.05 |
| Imipenem | 0.1–1.56 | 0.2 | 0.39 | |
| Faropenem | 0.2–6.25 | 0.78 | 1.56 | |
| Cefdinir | 0.1–0.78 | 0.2 | 0.2 | |
| Levofloxacin | ≤0.025–0.78 | 0.1 | 0.2 | |
| M. catarrhalis (53) | LJC 11,036 | ≤0.006–0.2 | 0.025 | 0.05 |
| Imipenem | 0.013–0.39 | 0.05 | 0.1 | |
| Faropenem | ≤0.006–0.78 | 0.2 | 0.78 | |
| Cefdinir | 0.013–12.5 | 0.1 | 0.78 | |
| Levofloxacin | ≤0.006–0.2 | 0.05 | 0.2 | |
| E. cloacae (53) | LJC 11,036 | 0.013–3.13 | 0.05 | 0.2 |
| Imipenem | 0.1–1.56 | 0.2 | 0.78 | |
| Faropenem | 0.39–25 | 3.13 | 12.5 | |
| Cefdinir | 0.39–>100 | >100 | >100 | |
| Levofloxacin | 0.013–50 | 0.1 | 6.25 | |
| P. mirabilis (53) | LJC 11,036 | 0.2–0.78 | 0.39 | 0.39 |
| Imipenem | 1.56–12.5 | 6.25 | 12.5 | |
| Faropenem | 3.13–12.5 | 6.25 | 12.5 | |
| Cefdinir | 0.1–12.5 | 0.2 | 0.2 | |
| Levofloxacin | 0.05–>12.5 | 0.1 | 0.39 | |
| S. marcescens (54) | LJC 11,036 | 0.05–100 | 0.39 | 25 |
| Imipenem | 0.39–>100 | 1.56 | 12.5 | |
| Faropenem | 0.78–>100 | 25 | >100 | |
| Cefdinir | 1.56–>100 | 100 | >100 | |
| Levofloxacin | 0.1–>100 | 0.78 | 50 | |
| P. aeruginosa (53) | LJC 11,036 | 3.13–>100 | 6.25 | 100 |
| Imipenem | 0.39–25 | 1.56 | 25 | |
| Faropenem | 100–>100 | >100 | >100 | |
| Cefdinir | >100 | >100 | >100 | |
| Levofloxacin | 0.2–>100 | 1.56 | 12.5 | |
Agar dilution method.
Influence of the inoculum size on the MIC.
Increasing the inoculum size from 106 to 108 CFU/ml had little or no significant effect on the in vitro activities of LJC 11,036 or imipenem against S. aureus, S. pneumoniae, E. coli, H. influenzae, and K. pneumoniae (Table 3). However, the activity of imipenem against extended-spectrum β-lactamase (ESBL)-producing K. pneumoniae (TOHO-1 type) was more affected by the inoculum size than that of LJC 11,036.
TABLE 3.
Influence of inoculum size on antibacterial activity
| Organism | Inoculum size (log10 CFU/ml) | MICa (μg/ml)
|
||
|---|---|---|---|---|
| LJC 11,036 | Imipenem | Cefdinir | ||
| S. aureus Terajima | 6 | ≤0.006 | ≤0.006 | 0.20 |
| 7 | 0.013 | 0.013 | 0.39 | |
| 8 | 0.013 | 0.013 | 0.39 | |
| S. pneumoniae | ||||
| PSSP N-6 | 6 | ≤0.006 | ≤0.006 | 0.05 |
| 7 | ≤0.006 | ≤0.006 | 0.05 | |
| 8 | ≤0.006 | ≤0.006 | 0.10 | |
| PRSP HSC-3 | 6 | ≤0.006 | 0.025 | 0.78 |
| 7 | ≤0.006 | 0.025 | 0.78 | |
| 8 | 0.013 | 0.05 | 1.56 | |
| E. coli NIHJ JC-2 | 6 | 0.013 | 0.10 | 0.39 |
| 7 | 0.013 | 0.10 | 0.39 | |
| 8 | 0.05 | 0.39 | 0.39 | |
| H. influenzae | ||||
| ATCC 35056 | 6 | 0.05 | 0.39 | 0.20 |
| 7 | 0.05 | 0.39 | 0.20 | |
| 8 | 0.10 | 0.78 | 0.39 | |
| LJ5 | 6 | 0.05 | 0.39 | 0.20 |
| 7 | 0.10 | 0.78 | 0.39 | |
| 8 | 0.10 | 0.78 | 0.39 | |
| K. pneumoniae | ||||
| PCI-602 | 6 | 0.013 | 0.10 | 0.05 |
| 7 | 0.025 | 0.20 | 0.05 | |
| 8 | 0.05 | 0.39 | 0.10 | |
| LJ11 (ESBL) | 6 | 0.05 | 0.10 | >100 |
| 7 | 0.05 | 0.78 | >100 | |
| 8 | 0.20 | 1.56 | >100 | |
Agar dilution method.
Activity against β-lactamase-producing strains.
The comparative activities of LJC 11,036 and the other drugs against various β-lactamase-producing strains are shown in Table 4. The MICs of LJC 11,036, imipenem, faropenem, and cefdinir against strains producing various types of β-lactamases, except for carbapenemase and cephalosporinase of P. aeruginosa, were ≤0.78, ≤6.25, ≤25, and 0.1 to >100 μg/ml, respectively, indicating that LJC 11,036 was the most stable among the β-lactams tested. However, like imipenem, LJC 11,036 was hydrolyzed by carbapenemase.
TABLE 4.
Antibacterial activities of LJC 11,036 against β-lactamase-producing strains
| β-Lactamasea and organism | MICb (μg/ml)
|
||||
|---|---|---|---|---|---|
| LJC 11,036 | Imi-penem | Faro-penem | Cef-dinir | Levo-floxacin | |
| PCase | |||||
| Escherichia coli | |||||
| TEM-1 | ≤0.025 | 0.20 | 0.78 | 0.20 | 0.39 |
| TEM-2 | ≤0.025 | 0.39 | 0.78 | 0.39 | 0.39 |
| OXA-1 | ≤0.025 | 0.20 | 0.78 | 0.20 | 0.39 |
| OXA-2 | 0.05 | 0.20 | 0.78 | 0.78 | 0.39 |
| PSE-1 | ≤0.025 | 0.20 | 0.39 | 0.20 | 0.39 |
| PSE-3 | ≤0.025 | 0.39 | 0.78 | 0.20 | 0.39 |
| SHV-1 | ≤0.025 | 0.39 | 0.78 | 0.39 | 0.39 |
| Staphylococcus aureus | |||||
| ML15009 | ≤0.025 | ≤0.025 | 0.05 | 0.05 | 0.20 |
| ML15009/pI258 | ≤0.025 | ≤0.025 | 0.05 | 0.05 | 0.20 |
| Klebsiella pneumoniae GN69 | ≤0.025 | 0.39 | 0.39 | 0.05 | 0.05 |
| ESBL (Klebsiella pneu-moniae LJ11c) | 0.05 | 0.10 | 3.13 | >100 | 0.78 |
| CEPase | |||||
| Escherichia coli | |||||
| GN5482 | ≤0.025 | 0.20 | 0.39 | 6.25 | 0.05 |
| No. 1501 | ≤0.025 | 0.20 | 0.20 | 0.20 | 0.05 |
| No. 96 | ≤0.025 | 0.20 | 0.39 | 6.25 | 0.05 |
| Enterobacter cloacae | |||||
| GN5797 | 0.05 | 0.39 | 1.56 | 100 | 0.10 |
| GN7467 | 0.10 | 0.39 | 1.56 | 50 | 0.05 |
| GN7471 | ≤0.025 | 0.20 | 0.39 | 100 | 0.05 |
| Citrobacter freundii | |||||
| GN346 | 0.05 | 0.39 | 1.56 | 25 | 0.05 |
| GN7391 | 0.10 | 0.78 | 6.25 | >100 | 0.20 |
| Serratia marcescens | |||||
| GN10857 | 0.78 | 6.25 | 25 | >100 | 0.20 |
| L-65 | 0.10 | 0.78 | 1.56 | 1.56 | ≤0.025 |
| L-82 | 0.05 | 0.20 | 3.13 | >100 | 0.05 |
| Providencia rettgeri | |||||
| GN4430 | 0.10 | 0.39 | 0.78 | ≤0.025 | 0.20 |
| GN4762 | 0.39 | 0.39 | 3.13 | ≤0.025 | 0.10 |
| GN5284 | 0.10 | 0.78 | 0.39 | ≤0.025 | 0.20 |
| Morganella morganii | |||||
| GN5307 | 0.10 | 1.56 | 0.78 | 1.56 | 0.05 |
| GN5375 | 0.39 | 1.56 | 0.78 | 1.56 | ≤0.025 |
| GN5407 | 0.20 | 6.25 | 0.78 | 6.25 | 0.10 |
| Proteus vulgaris | |||||
| GN76 | 0.39 | 6.25 | 6.25 | 0.10 | 0.05 |
| GN4413 | 0.39 | 6.25 | 6.25 | 50 | 0.05 |
| GN7919 | 0.39 | 0.39 | 0.78 | 3.13 | 0.05 |
| Pseudomonas aeru-ginosa | |||||
| GN918 | 6.25 | 1.56 | >100 | >100 | 1.56 |
| GN10362 | 1.56 | 1.56 | >100 | >100 | 0.39 |
| GN10367 | 3.13 | 1.56 | >100 | >100 | 0.39 |
| CBPase | |||||
| Stenotrophomonas mal-tophilia GN12873 | >100 | >100 | >100 | >100 | 0.39 |
| Pseudomonas aeru-ginosa | |||||
| LJ21d | >100 | 50 | >100 | >100 | 50 |
| LJ23d | >100 | 50 | >100 | >100 | >100 |
PCase, penicillinase; CEPase, cephalosporinase; CBPase, carbapenemase.
Agar dilution method.
TOHO-1 type.
IMP-1 type.
Correlation between the MIC and MBC.
In five of the eight strains tested, there was no difference between the MIC and the MBC for LJC 11,036 (Table 5); among the remaining strains, only a twofold difference was noted. The bactericidal activity of LJC 11,036 was the most potent, followed by those of imipenem and cefdinir, in that order.
TABLE 5.
MBCs of LJC 11,036
| Organism | MBC/MICa (ratio) of:
|
||
|---|---|---|---|
| LJC 11,036 | Imipenem | Cefdinir | |
| S. aureus Terajima | 0.025/0.025 (1) | 0.006/0.006 (1) | 0.025/0.013 (2) |
| S. pneumoniae | |||
| PSSP N-6 | 0.006/0.006 (1) | 0.006/0.006 (1) | 0.006/0.006 (1) |
| PRSP HSC-3 | 0.006/0.006 (1) | 0.013/0.006 (2) | 0.39/0.2 (2) |
| E. coli NIHJ JC-2 | 0.025/0.013 (2) | 0.1/0.1 (1) | 0.39/0.39 (1) |
| H. influenzae | |||
| LJ5 | 0.1/0.05 (2) | 0.39/0.2 (2) | 0.2/0.1 (2) |
| ATCC 35056 | 0.05/0.05 (1) | 0.2/0.2 (1) | 0.2/0.2 (1) |
| K. pneumoniae | |||
| PCI-602 | 0.013/0.013 (1) | 0.2/0.1 (2) | 0.1/0.05 (2) |
| LJ11 (ESBL) | 0.1/0.05 (2) | 0.78/0.2 (4) | >100/>100 (≥1) |
Broth dilution method.
Bactericidal activity.
A decrease of approximately 2 log10 units in the viable count was observed with LJC 11,036 against PRSP at the three different phases of growth. In contrast, cefdinir did not exhibit bactericidal activity at any of the growth phases (Fig. 2).
In vitro postantibiotic sub-MIC effect.
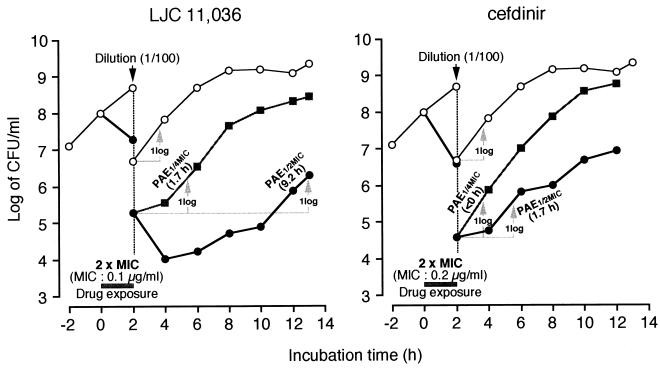
LJC 11,036, unlike cefdinir, produced a strong drug concentration-dependent postantibiotic sub-MIC effect in vitro against PRSP HSC-3 (Fig. 3). Significant postantibiotic effects (>1 h) were observed at all concentrations tested; 1.6 h at one-eighth the MIC and 6.1 h at one-fourth the MIC. Moreover, as shown in Fig. 4, against H. influenzae LJ5, significant postantibiotic effects were observed at both one-fourth the MIC (1.7 h) and one-half the MIC (9.2 h).
FIG. 3.
In vitro postantibiotic sub-MIC effect (PAE) of LJC 11,036 against PRSP HSC-3. Bacteria were exposed to the MIC from 0 to 2 h and then diluted into fresh medium containing one-fourth or one-eighth the MIC. Solid circles at 2 h represent the count of viable bacteria after exposure to the MIC, before (higher) and after (lower) 10−2 dilution. Open circles, control (no drug).
FIG. 4.
In vitro postantibiotic sub-MIC effect (PAE) of LJC 11,036 against H. influenzae LJ5. Procedures and symbols are as explained for Fig. 3, except that bacteria were exposed to twice the MIC from 0 to 2 h.
Stability to DHP-I.
After incubation at 30°C for 4 h, the residual amount of LJC 11,036 decreased by only about 11% of the initial amount, while that of imipenem decreased by 50% after only 1 h of incubation.
Binding of PBPs.
LJC 11,036 showed strong binding to PBP1A, -1B, -2A/2X, -2B, and -3 of PRSP HSC-3, with 50% inhibitory concentrations (IC50) of 0.12, 0.08, 0.16, 0.05, and 0.01 μg/ml, respectively (Fig. 5). Binding of LJC 11,036 to PBP1B, -2, -3A, and -3B of H. influenzae LJ5 was also high (IC50, 0.09, 0.01, 0.12, and 0.10 μg/ml, respectively). The MICs of LJC 11,036 against PRSP HSC-3 and H. influenzae LJ5 were 0.025 and 0.1 μg/ml, respectively.
FIG. 5.
Fluorogram of PBPs of PRSP HSC-3.
DISCUSSION
This study demonstrated that LJC 11,036 possesses greater activities than imipenem, faropenem, and cefdinir against the main causative organisms of respiratory and urinary-tract infections, i.e., S. pneumoniae, S. pyogenes, H. influenzae, K. pneumoniae, M. catarrhalis, and E. coli.
S. pneumoniae is well known to be a major causative agent of community-acquired pneumonia, bacteremia, meningitis, and acute otitis media. At present the prevalence of PRSP is increasing worldwide, which complicates treatment of these infections. A concentration of 0.2 μg of LJC 11,036/ml inhibited the growth of all S. pneumoniae strains tested, including PRSP. When this result is compared with the published antipneumococcal data for other recently developed oral carbapenems, such as GV 118819X (MIC100, 2.0 μg/ml) (13), CS-834 (MIC100, 0.5 μg/ml) (18), DZ-2640 (MIC100, 0.78 μg/ml) (14), and CL 191,121 (MIC100, 1.0 μg/ml) (17), LJC 11,036 appears to have the most potent antipneumococcal activity, which is one of its noteworthy features. As with imipenem, no significant inoculum effect was detected with LJC 11,036 against any of the strains tested. However, the MIC of imipenem against an ESBL producer, compared with those against other strains, increased much more than that of LJC 11,036. The bactericidal activity of LJC 11,036 was demonstrated by the following findings: (i) there was a good correlation between the MIC and the MBC against various strains, and (ii) the antibacterial activity against PRSP did not vary with the phase of growth tested. In addition, this compound produced longer in vitro postantibiotic sub-MIC effects against both PRSP and H. influenzae than cefdinir.
S. pneumoniae has at least five PBPs (1A, 1B, 2A, 2B, and 2X). According to Hakenbeck et al. (6), the development of drug resistance in PRSP is considered to be due both to multiple changes in PBP1A and PBP2B and to the appearance of PBP2X. However, in strain HSC-3, used in this study, separation of PBP2A and PBP2X could not be detected. As was pointed out by Hakenbeck et al. (6), this finding may be due to the lot of polyacrylamide used for the preparation of SDS-PAGE.
It is of considerable interest that LJC 11,036 shows high binding affinities for five PBPs, including 2B, which exhibits reduced affinity for oral cephalosporins (16). For LJC 11,036, the first target PBP of H. influenzae is PBP2, as has been noted with imipenem. The morphological change (induction of a spherical form) associated with PBP2 was observed (data not shown). The stability of LJC 11,036 to various β-lactamases, excluding carbapenemases, was demonstrated indirectly by way of determination of the MICs against β-lactamase-producing strains. Although LJC 11,036 was less active against P. aeruginosa than imipenem and levofloxacin, this compound exhibits strong binding to PBPs in P. aeruginosa (data not shown). Therefore, the decreased activity against P. aeruginosa may be due to poor permeation through the outer membrane. According to the results of a Phase Ia clinical study (single oral doses of 25 to 200 mg), L-084 showed a high urinary recovery rate (approximately 60 to 70%). Moreover, antibiotic-related diarrhea was not observed with any doses of L-084 (unpublished data). It is considered that the high stability of LJC 11,036 to renal DHP-I contributes to the high urinary recovery. These findings may lead to the efficacious use of L-084 against complicated urinary-tract infections caused by P. aeruginosa.
In conclusion, the specific features of L-084 discussed above warrant further investigation for its potential human clinical application. L-084 may have a potential role in the oral treatment of infections that are less responsive to the currently available oral agents, especially β-lactams.
ACKNOWLEDGMENTS
We thank M. Inoue (Kitasato University School of Medicine) for support and helpful discussion on the classification of β-lactamases, K. Ubukata (Teikyo University School of Medicine) for supplying S. pneumoniae HSC-3, and R. Testa (Wyeth-Ayerst Research) for helpful advice and comments.
REFERENCES
- 1.Abe T, Hayashi K, Mihira A, Satoh C, Tamai S, Yamamoto S, Hikida M, Kumagai T, Kitamura M. Abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. L-084, a new oral carbapenem: synthesis and structure-activity relationships of C2-substituted 1β-methylcarbapenems, abstr. F-64; p. 249. [Google Scholar]
- 2.Asahi Y, Ubukata K. Association of a Thr-371 substitution in a conserved amino acid motif of penicillin-binding protein 1A with penicillin resistance of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1998;42:2267–2273. doi: 10.1128/aac.42.9.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birnbaum J, Kahan F M, Kropp H, MacDonald J S. Carbapenems, a new class of beta-lactam antibiotics. Am J Med. 1985;78(Suppl. 6A):3–21. doi: 10.1016/0002-9343(85)90097-x. [DOI] [PubMed] [Google Scholar]
- 4.Coulton S, Hunt E. Recent advances in the chemistry and biology of carbapenem antibiotics. Prog Med Chem. 1996;33:99–145. doi: 10.1016/s0079-6468(08)70304-7. [DOI] [PubMed] [Google Scholar]
- 5.Edwards J R. Meropenem: a microbiological overview. J Antimicrob Chemother. 1995;36(Suppl. A):1–17. doi: 10.1093/jac/36.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- 6.Hakenbeck R, Tarpay M, Tomasz A. Multiple changes of penicillin-binding proteins in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980;17:364–371. doi: 10.1128/aac.17.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Licata L, Smith C E, Goldschmidt R M, Barrett J F, Frosco M. Comparison of the postantibiotic and postantibiotic sub-MIC effects of levofloxacin and ciprofloxacin on Staphylococcus aureus and Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:950–955. doi: 10.1128/aac.41.5.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitsuhashi S. In-vitro and in-vivo antibacterial activity of imipenem against clinical isolates of bacteria. J Antimicrob Chemother. 1983;12(Suppl. D):53–64. doi: 10.1093/jac/12.suppl_d.53. [DOI] [PubMed] [Google Scholar]
- 9.Mori M, Hikida M, Nishihara T, Nasu T, Mitsuhashi S. Comparative stability of carbapenem and penem antibiotics to human recombinant dehydropeptidase-I. J Antimicrob Chemother. 1996;37:1034–1036. doi: 10.1093/jac/37.5.1034. [DOI] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 2nd ed. Approved standard M7-A2. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1990. [Google Scholar]
- 11.Neu H C, Chin N X, Saha G, Labthavikul P. In vitro activity against aerobic and anaerobic gram-positive and gram-negative bacteria and beta-lactamase stability of RS-533, a novel carbapenem. Antimicrob Agents Chemother. 1986;30:828–834. doi: 10.1128/aac.30.6.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norrby S R. Carbapenems. Med Clin North Am. 1995;79:745–759. doi: 10.1016/s0025-7125(16)30037-2. [DOI] [PubMed] [Google Scholar]
- 13.Spangler S K, Jacobs M R, Appelbaum P C. MIC and time-kill studies of antipneumococcal activity of GV 118819X (sanfetrinem) compared with those of other agents. Antimicrob Agents Chemother. 1997;41:148–155. doi: 10.1128/aac.41.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka M, Hohmura M, Nishi T, Sato K, Hayakawa I. Antimicrobial activity of DU-6681a, a parent compound of novel oral carbapenem DZ-2640. Antimicrob Agents Chemother. 1997;41:1260–1268. doi: 10.1128/aac.41.6.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas R P, Jr, Bryan L E. Mechanism of resistance of an ampicillin-resistant, β-lactamase-negative clinical isolate of Haemophilus influenzae type b to β-lactam antibiotics. Antimicrob Agents Chemother. 1984;25:747–753. doi: 10.1128/aac.25.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ubukata K, Muraki T, Igarashi A, Asahi Y, Konno M. Identification of penicillin and other beta-lactam resistance in Streptococcus pneumoniae by polymerase chain reaction. J Infect Chemother. 1997;3:190–197. doi: 10.1007/BF02490033. [DOI] [PubMed] [Google Scholar]
- 17.Weiss W, Petersen P, Jacobus N, Lin Y, Bitha P, Testa R. In vitro activities of aminomethyl-substituted analogs of novel tetrahydrofuranyl carbapenems. Antimicrob Agents Chemother. 1999;43:454–459. doi: 10.1128/aac.43.3.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaguchi K, Domon H, Miyazaki S, Tateda K, Ohno A, Ishii K, Matsumoto T, Furuya N. In vitro and in vivo antibacterial activities of CS-834, a new oral carbapenem. Antimicrob Agents Chemother. 1998;42:555–563. doi: 10.1128/aac.42.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]