Abstract
Background:
Multiple anal human papillomavirus (HPVs) may increase the risk of anal cancer among men who have sex with men (MSM) living with HIV. The Jaccard Similarity Index (JSI) was explored as a measure of multiple HPV persistence.
Methods:
The TRUST/RV368 cohort enrolled MSM living with and without HIV in Abuja and Lagos, Nigeria. Participants with anal swabs at baseline, 3- and/or 12-month visits were tested for high- and low-risk HPVs using a next-generation sequencing assay. Persistence of the same HPV genotypes over time was calculated using the JSI and categorized into high, medium, and low similarity tertiles. Factors associated with higher versus lower similarity were estimated with multivariable ordinal logistic regression and reported as adjusted odds ratios (aORs) and 95% confidence intervals (CIs).
Results:
Of 225 participants, median age was 25 years (interquartile range [IQR]: 22–29), 62% were living with HIV, median HPVs was 3 (IQR: 2–5), and HPV6 (28%), HPV16 (26%), HPV11 (23%), and HPV45 (20%) were most prevalent. Fifty-three percent of participants had highly similar HPVs at 3-months, and the similarity was associated with HIV (aOR:3.11, 95% CI:1.6–5.9) and recent receptive sex (1.9 [1.0–3.5]). By 12 months, 20% had highly similar HPVs and it was associated with ≥12 years since anal coital debut (6.8 [3.1–5.2]), self-reported genital warts (3.1 [1.5–6.6]), and ≤200 CD4 cells/mm3 (13.3 [2.7–65.2]) for those living with HIV.
Conclusions:
Studies evaluating the JSI as a predictor of high-grade intraepithelial lesions would further confirm its applicability as a quantitative measure of multiple HPV persistence.
Keywords: HPV, HIV, MSM, sub-Saharan Africa, persistence
Short Summary:
Multiple HPVs are a risk factor for progression to anal cancer among MSM living with HIV and may be quantified using the Jaccard similarity index.
Introduction
Approximately 93% of men who have sex with men (MSM) living with HIV are infected with anal human papillomavirus (HPV)1 and 60% have multiple high (HR) and low-risk (LR)-HPV infections.2 HPV16 is the predominant type detected in anal cancer.3–5 However, HPV16 coexists with many other HPVs in anal cancers of persons living with HIV as compared to being the sole infection in anal cancers of persons not living with HIV.6 Interestingly, HPV6, a low-risk HPV usually associated with anal warts, has been identified as the only type-specific infection in some invasive carcinomas, highlighting the non-16 types found in anal cancers.7 Moreover, anal warts are common for those who develop high-grade intraepithelial lesions8 and even if detected a decade earlier they remain a strong predictor of incident anal cancer as well as vulvar, vaginal and penile cancers.9 Therefore, evaluating the persistence of LR and HR-HPVs, may provide insight on overall HPV immunological control.
There are several analytical approaches used currently for evaluating persistently detected anal HPV. Persistent detection can be from a persistent infection or repeat infection. One approach focuses on the proportion of people with persistent detection of the most carcinogenic types HPV16 and HPV18.10 Another approach evaluates the proportion of people with persistent detection for any of the HR-HPVs.11,12 A third approach evaluates persistent detection of the HPV type-specific infections themselves13,14 with a correlation coefficient accounting for those co-occurring in a person. Lastly, marginal and mixed effects models, such as the Wei, Lin Weissfeld method, accounts for multiple HPV genotypes and correlations within a person and across time.15 Each analysis focuses on the natural history of individual type-specific infections. Therefore, a person who clears multiple infections cannot be differentiated from a person who clears one or two infections. There may be some natural history data from the infections not accounted for in the current analyses that could strengthen our understanding of who has a higher risk for HPV persistence.
A quantitative method that could account for the persistent detection of multiple infections over time is the Jaccard Similarity Index (JSI). The JSI is a proportion of similarity ranging from 0% to 100% that estimates the number of HPV types shared between two visits relative to the total unique HPV types (shared + unshared) at either visit. A higher index suggests more similarity in types detected over consecutive visits. The objective of this study was to explore the use of the JSI to characterize persistent detection of multiple type-specific infections.16 We identified those with prevalent HPV infection(s) at enrollment and estimated how similar the HPVs were over a short and longer interval of follow-up. To better understand who was at higher risk of persistent detection, we further evaluated risk factors associated with higher versus lower similarity for each of the follow-up visits.
Materials and Methods
Study Population and Sample Collection
Between March 2013 and February 2020, TRUST/RV368 recruited Nigerian MSM into a combination HIV prevention and treatment cohort study using respondent driven sampling.17,18 Participants were born male, age ≥16 years in Abuja or ≥18 in Lagos, reported receptive or insertive anal intercourse with men in the past year and provided informed consent in English or Hausa. In Abuja, 16- to 17-year olds were considered emancipated minors and exempt from parental consent.17 After enrollment, participants were seen quarterly for up to 18 months. At each visit, participants answered a structured survey instrument on demographic, behavioral and clinical characteristics and provided blood and rectal swabs for testing of HIV and other sexually transmitted infections.
Blood was tested in real-time using parallel rapid HIV antibody tests (Determine, Alere, Waltham, MA; Unigold, Trinity Biotech, Co-Wicklow, Ireland; and HIV1/2 Stat Pak, Chembio Diagnostics, Medford, NY as a tie breaker for discrepant results) according to national guidelines.19 If an HIV rapid test was positive, HIV RNA was quantified using the COBAS TaqMan HIV-1 Test (Roche Molecular Diagnostics, Pleasanton, CA) and CD4 counts were estimated using the Partec CyFlow Counter. Rectal swabs were tested for Neisseria gonorrhoeae and Chlamydia trachomatis using the Aptima Combo 2 assay (Gen-Probe, San Diego, CA). Persons with positive test results were offered antiretroviral and/or antibiotic therapy as clinically indicated.
Our longitudinal study of HPV was nested in the TRUST/RV368 cohort study. Archived rectal swab samples from participants of TRUST/RV368 who completed a baseline and two quarterly visits, aiming at 3 and 12 months of follow-up, were shipped to University of Maryland School of Medicine for HPV testing. The 3- and 12-month visits were selected to evaluate changes in persistent detection of HPV within a short and longer time interval. If the JSI was appropriately quantifying similarity in HPVs, the JSI would be expected to be higher for the shorter interval as compared to the longer interval. This study was approved by the Federal Capital Territory Health Research Ethics Committee in Nigeria, the Ministry of Defense Health Research Ethics Committee in Nigeria, the Clinical Research Committee at the University of Maryland Marlene and the Stewart Greenebaum Comprehensive Cancer Center and the IRBs at the University of Maryland, Baltimore and Walter Reed Army Institute of Research.
HPV Detection and Typing
HPV detection and typing have been described previously.20 In brief, HPV DNA was extracted using the QIAamp MinElute Media Kit (Qiagen, Valencia, CA) while blinded to participant characteristics. The BSGP5+/6+ primer set detected high and low-risk HPV genotypes using an Ion Torrent next-generation sequencing-based HPV genotyping assay.21 This assay amplifies a 140-bp fragment of the L1 consensus region. All samples were included in the sequencing pool at a standardized concentration of 500 pM. Samples without library product were included in separate pools at equal volumes. Pooled samples were purified and quantified for emulsion template preparation on the Qubit 2.0 Fluorometer (Agilent, Santa Clara, CA) and prepared using the Ion Chef (ThermoFisher, Waltham, MA). Sequencing was performed on the Ion S5 (ThermoFisher, Waltham, MA) using 400 base pair sequencing chemistry and 520 chips. Positive and negative controls, the loading of ion sphere particles (ISP), the proportion of ISPs with more than one amplicon (polyclonal reads), and average read lengths were reviewed for quality assurance and quality control.
Raw data processing was performed by the Ion Torrent server and reads at least 100 base pairs in length were mapped to the full genomic sequences of 161 HPVs downloaded from the Papillomavirus Episteme (PaVE) database. Mapping required a minimum score of AQ17. For samples with >5000 reads, HPV type-specific infections were considered positive if the mapped fragments per HPV genotype spanned >100 bases pairs in length, but no greater than 150 base pairs, and accounted for more than 1% of the total number of reads per sample. Thirteen type specific infections (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) were considered high-risk and the remainder were considered non-high risk. All samples negative for HPV were analyzed by PCR to identify the presence of an internal human control target (Isocitrate Dehydrogenase; IDH), indicative of amplifiable human cellular DNA. The IDH assay is a human PCR assay used in the same clinical lab where the HPV sequencing was performed.
Participants with a prevalent HPV infection at baseline and at least one follow-up visit within the defined time intervals were selected for the analyses. Accounting for protocol-defined allowable windows for quarterly visits, we accepted “3-month” visits within a range of 1.5 to 4.4 months elapsed since baseline and “12-month” visits within 9.5–15.4 of baseline.
Statistical analysis
The primary outcome variable was an ordinal categorization (low, medium and high) based on tertiles of the JSI. The primary independent variable was HIV status. The JSI is a ratio of the number of shared types between two visits divided by the number of unique types (shared and unshared) at either visit:
| (1) |
The JSI (presented above in equation 1) was multiplied by 100 to convert the ratio to a proportion ranging from 0% to 100%. As an example, a baseline visit with HPV types: 16, 18, 45, and 86 and a follow-up visit with HPV types: 6, 16, 35, and 86 had a numerator of 2 (shared types are 16, 86) and a denominator of 6 (shared and unshared types are 6, 16, 18, 35, 45, 86) and a similarity proportion of 33% [(2/6)×100]. The JSI was calculated for the observed HPVs at each of the follow-up visits relative to the enrollment visit. The entire sample of JSI values, irrespective of visit, was split into tertiles and then analyzed separately for the two visit intervals. The type-specific HPV composition of each tertile was described for each visit. To further explore the composition of the 12-month tertiles, Fisher’s exact tests were used to test the distribution of persistent detection of type-specific HR-HPVs with sufficient prevalence and 3 of the most abundant LR-HPVs.
Pearson’s Chi-square and Fisher’s exact tests were used to compare the distribution of covariates by exposure and outcome to identify potential confounders. Bivariate and multivariable ordinal logistic regression models were used to estimate odds ratios (ORs) with 95% confidence intervals (CIs) for the association between HIV and levels of similarity at 3-months and 12-months, separately. Candidate variables identified in bivariate analyses (P<0.10) were entered in the multivariable model using backward stepwise selection, retaining those that changed the model coefficients by more than 10%. Because number of prevalent HPV infections at baseline were incorporated into the JSI calculation, these were not included as an additional covariate in the multivariable models. Missing observations were retained in the models with indicators. In a sensitivity analysis, the analysis was restricted to individuals with all 3 visits (baseline, 3-months and 12-months). Correlation of the JSI at the two visits were quantified using Spearman’s rank correlation (RS). A two-sided P < 0.05 was considered statistically significant and a P < 0.10 was suggestive of a trend. Data were analyzed using Stata Statistical Software, Release 13 (StataCorp, College Station, TX) and Statistical Analysis Software (SAS) version 9.4 (SAS Institute, Cary, NC).
Results
Of the 255 participants with ≥2 visits for persistent detection of HPV, 7% (n=19) were excluded because of a negative HPV test at baseline and 4% (n=11) did not have follow-up visits within the 3 and 12 month visit windows. The remaining 225 participants had a median age of 25 years (interquartile range [IQR]: 22–29 and 54% (n=121) reported 7 or more years since anal coital debut. Sixty-two percent (n=140) were living with HIV and their median CD4 count was 320 cells/mm3 (IQR:250–428 cells/mm3) and 39% (54/140) had an HIV RNA <1000 copies/mL at baseline.
Participants living with HIV reported more years since anal coital debut, recent receptive anal intercourse and they had more laboratory diagnosed HPV co-infections than did participants without HIV (Table 1). For bacterial STIs, those living with HIV had fewer cases of concurrent rectal Chlamydia trachomatis as compared those without HIV (9% vs. 20%) and a similar distribution of rectal Neisseria gonorrhoeae (24% vs. 21%). Self-reported genital warts were non-significantly higher among those living with HIV as compared to those without HIV (21% vs. 12%).
Table 1.
Demographic, Behavioral and Clinical Characteristics for Participants with a Prevalent HPV Infection at TRUST/RV368 Enrollment, stratified by HIV status (N=225)
| with HIV | without HIV | ||
|---|---|---|---|
| n=140 | n=85 | ||
| Characteristics | n (column %) | n (column %) | P b |
| Age (years) | 0.29 | ||
| ≤24 | 55 (39.3) | 41 (48.2) | |
| 25–34 | 76 (54.3) | 37 (43.5) | |
| ≥35 | 9 (6.4) | 7 (8.2) | |
| Years since anal coital debut | <0.01 | ||
| ≤6 | 50 (36.8) | 50 (58.8) | |
| 7–11 | 48 (35.3) | 20 (23.5) | |
| ≥12 | 38 (27.9) | 15 (17.7) | |
| Recenta No. of anal sexual partners | 0.70 | ||
| 0–1 | 57 (42.2) | 38 (46.3) | |
| 2–3 | 47 (34.8) | 29 (35.4) | |
| ≥4 | 31 (23.0) | 15 (18.3) | |
| Recenta receptive anal sex | <0.01 | ||
| No | 30 (21.6) | 38 (45.8) | |
| Yes | 109 (78.4) | 45 (54.2) | |
| Recenta condomless sex | 0.31 | ||
| No | 58 (45.3) | 43 (52.4) | |
| Yes | 70 (54.7) | 39 (47.6) | |
| Recenta transactional sex (receiving or giving) | 0.17 | ||
| No | 65 (48.9) | 48 (58.5) | |
| Yes | 68 (51.1) | 34 (41.5) | |
| No. of HPV infections at baseline | <0.01 | ||
| 1 | 13 (9.3) | 22 (25.9) | |
| 2–5 | 88 (62.9) | 53 (62.4) | |
| 6–13 | 39 (27.9) | 10 (11.8) | |
| Rectal Neisseria gonorrhoeae | 0.54 | ||
| Negative | 106 (75.7) | 65 (79.3) | |
| Positive | 34 (24.3) | 17 (20.7) | |
| Rectal Chlamydia trachomatis | 0.02 | ||
| Negative | 128 (91.4) | 65 (80.3) | |
| Positive | 12 (8.6) | 16 (19.8) | |
| Self-report of previous genital warts | 0.07 | ||
| No | 110 (78.6) | 75 (88.2) | |
| Yes | 30 (21.4) | 10 (11.8) |
Abbreviations: No., number; HPV, human papillomavirus; P, p-value.
Time-varying variables and refers to since last visit.
Pearson’s Chi Square test.
Bold type indicates statistically significant (P<0.05) differences in characteristics by those living with and without HIV.
The median number of HPVs detected at baseline was 3 (IQR: 2–5) and a higher proportion of those living with HIV had 6 to 13 concurrent HPVs as compared to those without HIV (28% vs. 12%) (Table 1). HPV6 (28%), HPV16 (26%), HPV11 (23%), HPV45 (20%), HPV42 (17%) and HPV35 (13%) were the most prevalent HPV genotypes in rank order. The median JSI for the entire sample was 33% (IQR: 13%–56%) and once split into tertiles ranged 0%–17%, 18%–43%, and 44%–100% for the low, medium, and high categories. HPV16 increased in prevalence from the low to high tertiles both at the 3 month and 12 month visit (Supplemental Table 1). HPV6 was the predominant low risk HPV that increased in prevalence across the tertiles (Supplemental Table 1). Individual HPVs, including HPV16, that were persistently detected for a year were more abundant in the high similarity tertile as compared to the low similarity tertile (Figure 1).
Figure 1. Individuals with Poor Immunological Control of HPVs Overall have Higher Persistence of Type-Specific HPVs.
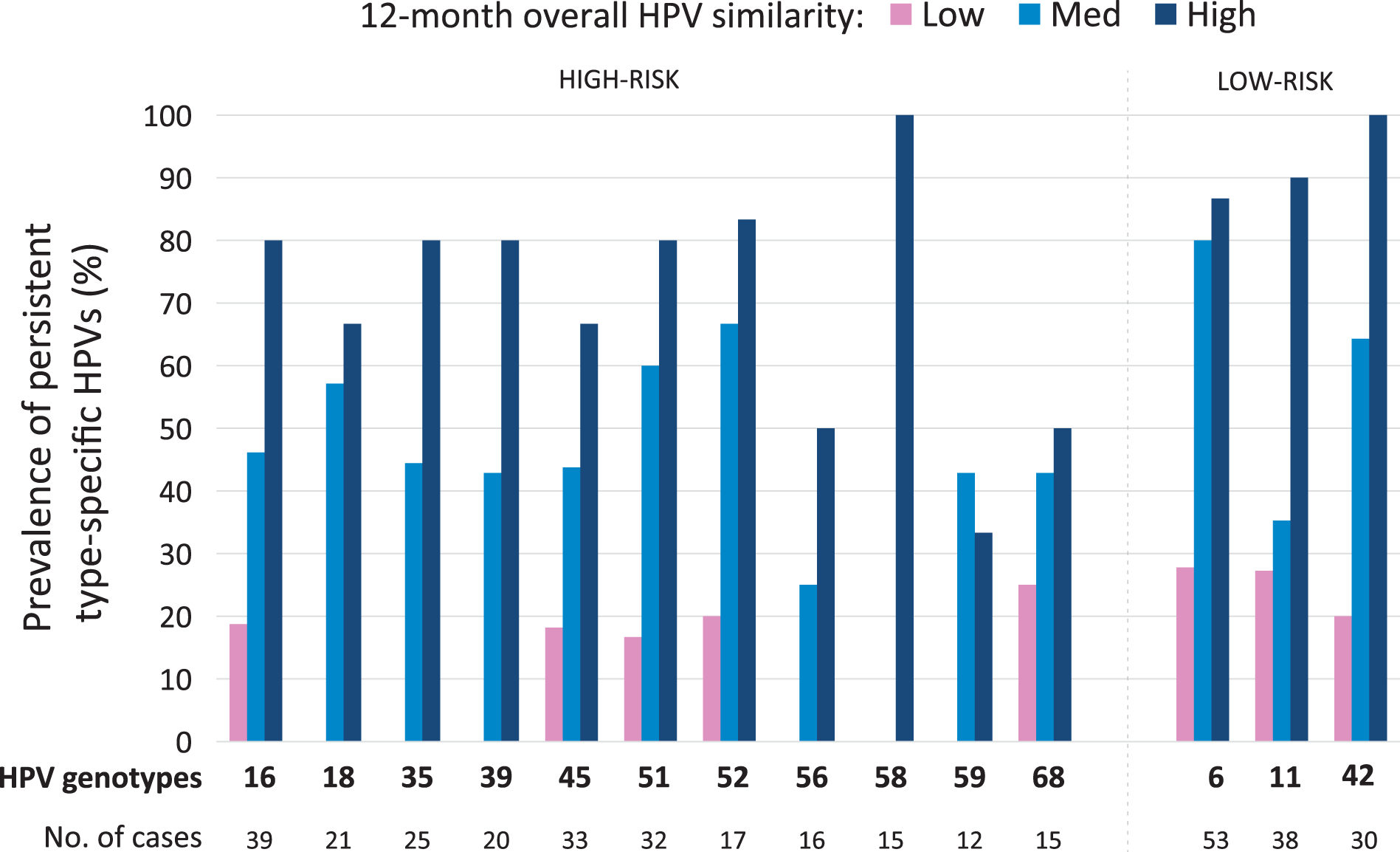
Note: Persistence for each type-specific HPV is stratified by the tertiles of the Jaccard index. If a person overall is a good controller of HPV, then they are also likely to control HPV on a type-specific level. HPV 16, 18, 35, 39, 51, 58, 6, 11, & 42 were significantly different by Jaccard Tertile using a Fisher’s exact Chi-square test (all p≤0.02). HPV 31 (n=5) and 33 (n=4) were infrequent and not evaluated.
The distribution of the JSI tertiles varied by follow-up visit. At 3 months, over half of the participants had a high similarity tertile (low:16%, medium: 31%, high: 53%) (Table 2). This distribution shifted downward at 12 months with only about 20% in the high similarity tertile (low: 44%, medium: 33%, high: 22%) (Table 3). For individuals with both follow-up visits, there was concordance in the JSI across two different time intervals as quantified by Spearman’s Rank Correlation (RS=0.36, p<0.0001). This concordance remained after stratifying by HIV status (HIV+: RS=0.31, P=0.007; HIV−: RS=0.43, P=0.004). In the unadjusted models, HIV was significantly associated with a higher odds of detecting the highest similarity tertile, relative to lower similarity tertiles, at 3 months and 12 months, respectively (Table 2, Table 3).
Table 2.
HIV and Other Factors Associated with Similarity in HPV Genotypes Repeatedly Detected at 3 Months (n=195)
| Jaccard Similarity Index | Ordinal Logistic Regression | |||||
|---|---|---|---|---|---|---|
| ≤ 17% | 18–43% | ≥44% | Unadjusted | Adjustedc | ||
| n=32 | n=60 | n=103 | ||||
| Characteristic | n (row %) | n (row %) | n (row %) | P b | OR (95% CI) | OR (95% CI) |
| HIV | <0.01 | |||||
| Negative | 23 (35.4) | 18 (27.7) | 24 (36.9) | Ref. | Ref. | |
| Positive | 9 (6.9) | 42 (32.3) | 79 (60.8) | 3.60 (2.0–6.5) | 3.15 (1.7–5.8) | |
| Follow-up Time (months) | 0.19 | |||||
| 1.5–2.4 | 0 (0.0) | 2 (18.2) | 9 (81.8) | 5.21 (1.0–26.5) | ||
| 2.5–3.4 | 26 (18.1) | 42 (29.2) | 76 (52.8) | 1.18 (0.6–2.3) | ||
| 3.5–4.4 | 6 (15.0) | 16 (40.0) | 18 (45.0) | Ref. | ||
| Years since anal coital debut | 0.42 | |||||
| ≤6 | 18 (22.0) | 25 (30.4) | 39 (47.6) | Ref. | ||
| 7–11 | 8 (15.4) | 18 (34.6) | 26 (50.0) | 1.21 (0.6–2.3) | ||
| ≥12 | 6 (10.5) | 17 (29.8) | 34 (59.7) | 1.78 (0.9–3.4) | ||
| Recenta No. of anal sexual partners | 0.96 | |||||
| 0–1 | 16 (18.8) | 25 (29.4) | 44 (51.8) | Ref. | ||
| 2–3 | 10 (14.9) | 22 (32.8) | 35 (52.2) | 1.09 (0.6–2.0) | ||
| ≥4 | 6 (14.6) | 13 (31.7) | 22 (53.7) | 1.14 (0.6–2.3) | ||
| Recenta receptive anal sex | 0.01 | |||||
| No | 17 (24.3) | 26 (37.1) | 27 (38.6) | Ref. | Ref. | |
| Yes | 15 (12.0) | 34 (27.2) | 76 (60.8) | 2.44 (1.4–4.3) | 1.84 (1.0–3.3) | |
| Rectal Neisseria gonorrhoeae | 0.88 | |||||
| Negative | 25 (17.0) | 43 (29.3) | 79 (53.7) | Ref. | ||
| Positive | 6 (15.4) | 13 (33.3) | 20 (51.3) | 0.95 (0.5–1.9) | ||
| Rectal Chlamydia trachomatis | 0.02 | |||||
| Negative | 27 (16.0) | 47 (27.8) | 95 (56.2) | Ref. | Ref. | |
| Positive | 5 (25.0) | 10 (50.0) | 5 (25.0) | 0.37 (0.2–0.8) | 0.34 (0.1–0.8) | |
| Self-report of previous genital warts | 0.09 | |||||
| No | 29 (18.7) | 50 (32.3) | 76 (49.0) | Ref. | Ref. | |
| Yes | 3 (7.5) | 10 (25.0) | 27 (67.5) | 2.24 (1.1–4.6) | 2.05 (1.0–4.4) | |
Abbreviations: No., number; HPV, human papillomavirus, OR, odds ratio, CI, confidence interval.
Time-varying variables and refers to since last visit.
Pearson’s Chi-square or Fisher’s exact test
The final model was adjusted for recent receptive anal sex, rectal Chlamydia trachomatis, and self-report of previous genital warts.
Bold type indicates statistically significant (P<0.05).
Table 3.
HIV and Other Factors Associated with Similarity in HPV Genotypes Repeatedly Detected at 12 months (n=192)
| Jaccard Similarity Index | Ordinal Logistic Regression | |||||
|---|---|---|---|---|---|---|
| ≤ 17% | 18–43% | ≥44% | Unadjusted | Adjustedc | ||
| n=85 | n=64 | n=43 | ||||
| Characteristic | n (row %) | n (row %) | n (row %) | P b | OR (95% CI) | OR (95% CI) |
| HIV | 0.09 | |||||
| Negative | 34 (55.7) | 16 (26.2) | 11 (18.0) | Ref. | Ref. | |
| Positive | 51 (38.9) | 48 (36.6) | 32 (24.4) | 1.82 (1.0–3.3) | 1.21 (0.6–2.3) | |
| Follow-up Time (months) | 0.30 | |||||
| 9.5–11.4 | 12 (33.3) | 14 (38.9) | 10 (27.8) | 1.36 (0.7–2.9) | ||
| 11.5–12.4 | 46 (51.7) | 24 (27.0) | 19 (21.4) | 0.74 (0.4–1.3) | ||
| 12.5–15.4 | 27 (40.3) | 26 (38.8) | 14 (20.9) | Ref. | ||
| Years since anal coital debut | <0.01 | |||||
| ≤6 | 42 (67.7) | 12 (19.1) | 9 (14.3) | Ref. | Ref. | |
| 7–11 | 25 (37.3) | 31 (46.3) | 11 (16.4) | 2.61 (1.3–5.2) | 2.65 (1.3–5.4) | |
| ≥12 | 16 (27.6) | 19 (32.8) | 23 (39.7) | 5.57 (2.7–11.6) | 6.83 (3.1–14.9) | |
| Recenta No. of anal sexual partners | 0.27 | |||||
| 0–1 | 28 (40.0) | 20 (28.6) | 22 (31.4) | Ref. | ||
| 2–3 | 32 (43.2) | 28 (37.8) | 14 (18.9) | 0.71 (0.4–1.3) | ||
| ≥4 | 22 (50.0) | 15 (34.1) | 7 (15.9) | 0.56 (0.3–1.1) | ||
| Recenta receptive anal sex | 0.18 | |||||
| No | 33 (51.6) | 21 (32.8) | 10 (15.6) | Ref. | Ref. | |
| Yes | 50 (40.3) | 41 (33.1) | 33 (26.6) | 1.68 (1.0– 3.0) | 1.28 (0.7–2.4) | |
| Rectal Neisseria gonorrhoeae | 0.11 | |||||
| Negative | 73 (44.5) | 52 (31.7) | 39 (23.8) | Ref. | ||
| Positive | 7 (35.0) | 11 (55.0) | 2 (10.0) | 0.99 (0.4–2.2) | ||
| Rectal Chlamydia trachomatis | 0.19 | |||||
| Negative | 70 (41.4) | 59 (34.9) | 40 (23.7) | Ref. | ||
| Positive | 9 (64.3) | 4 (28.6) | 1 (7.1) | 0.37 (0.1– 1.1) | ||
| Self-report of previous genital warts | 0.04 | |||||
| No | 77 (48.4) | 50 (31.5) | 32 (20.1) | Ref. | Ref. | |
| Yes | 8 (24.2) | 14 (42.4) | 11 (33.3) | 2.39 (1.2– 4.8) | 3.43 (1.6– 7.2) | |
Abbreviations: No., number; HPV, human papillomavirus, OR, odds ratio, CI, confidence interval.
Time-varying variables and refers to since last visit.
Pearson’s Chi-square or Fisher’s exact test
The final model was adjusted for years since anal coital debut, recent receptive anal sex, and self-report of previous genital warts.
Bold type indicates statistically significant (P<0.05).
In the multivariable analysis, those living with HIV had a higher odds of detecting the same HPV types 3 months later as compared to less similar types, after adjusting for recent receptive sex, rectal CT and self-reported genital warts (Table 2). Recent receptive anal sex and rectal CT remained independent risk factors for high similarity in persistent detection of HPVs after 3 months as compared to the less similar tertiles. In the sensitivity analysis of individuals with all 3 visits, HIV remained the strongest risk factor for persistent detection of HPV at 3 months (adjusted [a]OR: 2.9, 95% CI: 1.4–6.1). Among those living with HIV, CD4 counts and HIV RNA were not associated with repeat detection of HPVs at 3 months (Table 4).
Table 4.
Distribution of HIV-Related Immune Parameters with Similarity in HPV Genotypes at 3 and 12 Months
| Jaccard Similarity at 3 months | ||||||
|---|---|---|---|---|---|---|
| ≤17% | 18–43% | ≥44% | ||||
| n=9 | n=42 | n=79 | Unadjusted | Adjustedb | ||
| MSM with HIV | n (row %) | n (row %) | n (row %) | P a | OR (95% CI) | OR (95% CI) |
| CD4 counts (cells/mm3) | 0.57 | |||||
| ≤200 | 0 (0.0) | 4 (28.6) | 10 (71.4) | 2.50 (0.7–9.6) | 1.95 (0.8–4.8) | |
| 201–499 | 5 (5.9) | 27 (31.8) | 53 (62.4) | 1.58 (0.7–3.6) | 1.86 (0.5–7.2) | |
| ≥500 | 4 (13.3) | 10 (33.3) | 16 (53.3) | Ref. | Ref. | |
| HIV RNA (copies/mL) | 0.31 | |||||
| <1000 | 6 (6.6) | 26 (28.6) | 59 (64.8) | Ref. | Ref. | |
| ≥1000 | 3 (7.7) | 16 (41.0) | 20 (51.3) | 0.60 (0.3–1.3) | 0.70 (0.3–1.5) | |
| Jaccard Similarity at 12 months | ||||||
| ≤17% | 18–43% | ≥44% | ||||
| n=51 | n=48 | n=32 | Unadjusted | Adjustedc | ||
| n (row %) | n (row %) | n (row %) | P a | OR (95% CI) | OR (95% CI) | |
| CD4 counts (cells/mm3) | <0.01 | |||||
| ≤200 | 1 (10.0) | 2 (4.3) | 7 (70.0) | 11.85 (2.7–52.1) | 13.33 (2.7–65.2) | |
| 201–499 | 25 (35.2) | 32 (45.1) | 14 (19.7) | 1.58 (0.8–3.2) | 1.79 (0.8–3.8) | |
| ≥500 | 25 (52.1) | 13 (27.1) | 10 (20.8) | Ref. | Ref. | |
| HIV RNA (copies/mL) | 0.09 | |||||
| <1000 | 46 (42.2) | 40 (36.7) | 23 (21.1) | Ref. | Ref. | |
| ≥1000 | 5 (22.7) | 8 (36.4) | 9 (40.9) | 2.54 (1.1–6.0) | 3.91 (1.5–10.1) | |
Abbreviations: MSM, men who have sex with men; HPV, human papillomavirus, OR, odds ratio, CI, confidence interval.
Pearson’s Chi-square or Fisher’s exact test
The final model was adjusted for recent receptive anal sex, rectal Chlamydia trachomatis, and self-report of previous genital warts.
The final model was adjusted for years since anal coital debut, recent receptive anal sex, and self-report of previous genital warts.
Bold type indicates statistically significant (P<0.05).
By 12 months, HIV was no longer associated with the high similarity tertile after adjusting for years since sexual debut, recent receptive sex, and self-reported genital warts (Table 3). This null relationship was confirmed in the sensitivity analysis of individuals with all 3 visits (aOR: 0.8, 95% CI: 0.4–1.7). Other independent risk factors for detecting the same HPV types after 12 months were having 7 or more years since anal coital debut and self-reported genital warts (Table 3). These factors were confirmed in the sensitivity analysis of individuals with all 3 visits (years since sexual debut: 7–11: aOR: 2.8, 95% CI: 1.3–6.1, ≥12: aOR: 8.8, 95% CI: 3.6–21.1; self reported warts: aOR:3.6, 95% CI:1.7–7.8). Additionally, participants with HIV who had low CD4 counts had persistent detection of HPVs as compared to individuals living with HIV with higher CD4 counts (Table 4) and this association remained significant in the sensitivity analysis of individuals with all 3 visits. Individuals with high HIV RNA ≥1000 copies/mL had persistent detection of HPVs as compared to participants with HIV RNA <1000 copies/mL (Table 4) and this was significant in the sensitivity analysis of individuals with all 3 visits. Follow-up time, recent number of anal sex partners and rectal NG were not associated with the similarity tertiles.
Discussion
Multiple type-specific HPVs are common among MSM living with HIV and have been associated with increasing severity of precancer6,22 and progression to anal cancer.3–5 Despite this, measuring the persistence of multiple HPVs is not common. Our findings suggest that the JSI may provide a summary estimate of the persistent detection of many HPVs. Furthermore, the JSI does not diminish the unique oncogenic risk of HPV16, as a higher proportion of persistent HPV16 was in the highest similarity category of the JSI. Because the JSI uses the natural history data for all concurrent HPVs, it may provide insight on the host’s overall immunologic response to HPV.
HIV was associated with higher similarity at 3 months but not at 10–15 months. Other studies have also found HIV was associated with 6-month type-specific HPV persistence.12,23 As the interval of time increased to 2 to 3 years, HIV was less of a predictor of HPV 16 persistence alone (adjusted clearance rate ratio [aCRR]: 0.9, 95% CI: 0.6–1.6] but when included with other multiple HR-HPVs, HIV was associated with higher persistence (aCRR: 0.7, 95% CI: 0.6–0.9).14 Similarly, the Study of the Prevention of anal Cancer (SPANC) found HIV was not significantly associated with clearance of HPV16 (adjusted Hazard Ratio[aHR]: 0.90, 95% CI: 0.6–1.4) but for multiple HR-HPVs excluding HPV16, HIV was associated with a non-significantly higher clearance (i.e. lower persistence) (aHR: 1.13, 0.9–1.4).24 These inconsistent findings may be because of the inclusion or not of HPV16 as well as the grouping of HPVs with different infection durations. Both studies and others found that prevalent HPVs that are detected at enrollment clear slower than incident HPVs that are newly detected during follow-up.11,14,24,25 Depending on the proportion of incident relative to prevalent infections in the multiple HPV category, the clearance rates will vary. For our study and a few others,23,26,27 the analyses were restricted to baseline prevalent infections to minimize confounding from transient infections.
In our analysis, HIV together with recent receptive anal sex were independently associated with the similarity of HPV at 3 months. However, by 12 months, indicators of prior sexual history, such as time since anal coital debut and genital warts, emerged as stronger predictors. These findings parallel our earlier work where HIV and recent behavior were associated with active infections indicated by low-grade intraepithelial lesions.28 In contrast, time since anal coital debut and external warts were associated with more persistent infections indicated by high-grade intraepithelial lesions.28 This difference between recent versus past behavior on HPV natural history was also observed in a cohort of young MSM in the United States.29 In that study, recent receptive partners in the past 3 months increased the odds for prevalent and incident infections while lifetime receptive partners increased the odds for prevalent and persistently detected HPV.29 However, both our work and the cohort of young MSM in the United States had at most a year of follow-up. Most studies found no difference with recent versus past behavior on persistently detected HPV.11,23,26 Longer follow-up time of persistently detected HPV may lend insight as to whether recent versus past sexual practices could differentiate phases of HPV natural history.
For HIV-related immune parameters, immune suppression, indicted by ≤200 CD4 counts, was significantly associated with persistent detection at 12 months, but it was not related to persistent detection at 3 months. The relationship between HIV-related immune suppression and persistence is unclear with a couple of studies suggesting that nadir CD4 counts24 and current CD4 counts12 were associated with persistence. Others suggest a trend for either nadir or current CD4 counts23,27 while most have found no association with current or nadir CD4 counts.14,24,25 With HIV being a chronic infection for many years, the timing of CD4 count detection and the period observed for persistent HPV may have contributed to the inconsistency across studies. Our study also had few participants with high levels of immune suppression,17 suggesting that we may be underpowered to test this association. With longer follow-up, there may be a clearer pattern between CD4 counts and the JSI to better understand whether there is a generalized low responsiveness to long term HPV control.
This study had some limitations. First, our analysis pooled HPV16 with the other HPVs despite its unique oncogenic risk. Our objective was to explore the feasibility of using the JSI in an unsupervised approach for quantifying multiple HPVs. Second, our data did not allow for 2 negative visits for defining loss of detection.10 However, unlike many of the prior analyses, we were evaluating the repeatability of all baseline prevalent infections and therefore the change in the JSI was driven by all infections rather than a single infection with intermittent detection.27 Third, our analysis started with prevalent infections from a single timepoint and does not account for newly acquired HPVs that could become persistent. The JSI is flexible and rather than a single timepoint, it could capture a range of time (i.e. all HPVs detected within a year) to evaluate repeatability in subsequent visits. Fourth, multiple HPVs give JSI its heterogeneity and it is less sensitive for populations with few HPVs. Fifth, the HPVs detected originated from anal swabs and may not be representative of those residing in the tissue. Lastly, our study population had a high burden of HPV and other STIs and may not be generalizable to populations with a lower burden of disease.20,30 Despite our limitations, our primary objective was to assess the feasibility of the JSI to capture persistent detection of multiple HPVs.
In summary, we have demonstrated a mathematically simple approach to account for persistent detection of multiple HPV infections for up to a year among MSM living with and without HIV. Epidemiologic studies with more than a year of HPV testing could incorporate this measure to provide additional insight on its applicability for evaluating multiple HPVs over time. Moreover, studies evaluating JSI with high-grade intraepithelial lesions could further expand our understanding of the underlying biology, host immunity, and long-term persistence of multiple HPVs.
Supplementary Material
Acknowledgements:
Dr. Patti Gravitt provided valuable review of the manuscript. We also thank Jing Yin and Li Tang for their support in generating the HPV and IDH1 data as well as Talishiea Croxton for her lab support.
Conflicts of interest and Source of Funding:
JMP has stock and ownership interests in Virion Therapeutics, VIR Biotechnology; has received honoraria from Janssen Pharmaceuticals, Vaccitech,; has a consulting or advisory role for Virion Therapeutics and Vaccitech; receives research funding from Merck, and receives travel, accommodations and expenses from Merck. None of this relates to this specific work. All other authors declare no potential conflicts of interest. This work was supported by the National Cancer Institute [2P30CA134274-09S2, 5P30CA134274, K07CA225403]; the National Institutes of Health [R01 MH099001, R01 AI1209143, R01 MH110358]; the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense [W81XWH-11-2-0174, W81XWH-18-2-0040]; Fogarty Epidemiology Research Training for Public Health Impact in Nigeria program [D43TW010051]; and the President’s Emergency Plan for AIDS Relief through a cooperative agreement between the Department of Health and Human Services/Centers for Disease Control and Prevention, Global AIDS Program, and the Institute for Human Virology-Nigeria [NU2GGH002099]. The content is solely the responsibility of the authors and should not be construed to represent the positions of the National Institutes of Health, U.S. Army, the Department of Defense, the Department of Health and Human Services, or other funders. The investigators have adhered to the policies for protection of human subjects as prescribed in AR-70.
References
- 1.Machalek DA, Poynten M, Jin F, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: A systematic review and meta-analysis. Lancet Oncol 2012;13(5):487–500. [DOI] [PubMed] [Google Scholar]
- 2.del Amo J, González C, Geskus RB, et al. What drives the number of high-risk human papillomavirus types in the anal canal in HIV-positive men who have sex with men? J Infect Dis 2013;207(8):1235–1241. [DOI] [PubMed] [Google Scholar]
- 3.Hillman RJ, Garland SM, Gunathilake MPW, et al. Human papillomavirus (HPV) genotypes in an Australian sample of anal cancers. Int J Cancer 2014;135(4):996–1001. [DOI] [PubMed] [Google Scholar]
- 4.Ouhoummane N, Steben M, Coutlee F, et al. Squamous anal cancer: patient characteristics and HPV type distribution. Cancer Epidemiol 2013;37(6):807–812. [DOI] [PubMed] [Google Scholar]
- 5.Abramowitz L, Jacquard AC, Jaroud F, et al. Human papillomavirus genotype distribution in anal cancer in France: the EDiTH V study. Int J Cancer 2011;129(2):433–439. [DOI] [PubMed] [Google Scholar]
- 6.Lin C, Franceschi S, Clifford GM. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: a systematic review and meta-analysis. Lancet Infect Dis 2018;18(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornall AM, Roberts JM, Garland SM, et al. Anal and perianal squamous carcinomas and high-grade intraepithelial lesions exclusively associated with “low-risk” HPV genotypes 6 and 11. Int J Cancer 2013;133(9):2253–2258. [DOI] [PubMed] [Google Scholar]
- 8.Goddard SL, Templeton DJ, Petoumenos K, et al. Prevalence and Association of Perianal and Intra-Anal Warts with Composite High-Grade Squamous Intraepithelial Lesions Among Gay and Bisexual Men: Baseline Data from the Study of the Prevention of Anal Cancer. AIDS Patient Care STDS 2020;34(10):436–443. [DOI] [PubMed] [Google Scholar]
- 9.Blomberg M, Friis S, Munk C, et al. Genital warts and risk of cancer: a Danish study of nearly 50 000 patients with genital warts. J Infect Dis 2012;205(10):1544–1553. [DOI] [PubMed] [Google Scholar]
- 10.Patel P, Bush T, Kojic EM, et al. Prevalence, Incidence, and Clearance of Anal High-Risk Human Papillomavirus Infection among HIV-Infected Men in the SUN Study. J Infect Dis 2018;217(6). [DOI] [PubMed] [Google Scholar]
- 11.Nyitray AG, Carvalho da Silva RJ, Chang M, et al. Incidence, Duration, Persistence, and Factors Associated With High-risk Anal Human Papillomavirus Persistence Among HIV-negative Men Who Have Sex With Men: A Multinational Study. Clin Infect Dis 2016;62(11):1367–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yunihastuti E, Teeratakulpisarn N, Jeo WS, et al. Incidence, clearance, persistence and factors related with high-risk anal HPV persistence in South-East Asian MSM and transgender women. AIDS 2020;34(13):1933–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darwich L, Cañadas MP, Videla S, et al. Prevalence, clearance, and incidence of human papillomavirus type-specific infection at the anal and penile site of HIV-infected men. Sex Transm Dis 2013;40(8):611–618. [DOI] [PubMed] [Google Scholar]
- 14.Mooij SH, van Santen DK, Geskus RB, et al. The effect of HIV infection on anal and penile human papillomavirus incidence and clearance: a cohort study among MSM. AIDS 2016;30(1):121–132. [DOI] [PubMed] [Google Scholar]
- 15.Xue X, Gange SJ, Zhong Y, et al. Marginal and mixed-effects models in the analysis of human papillomavirus natural history data. Cancer Epidemiol Biomarkers Prev 2010;19(1):159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zandberg DP, Tallon LJ, Nagaraj S, et al. Intratumor genetic heterogeneity in squamous cell carcinoma of the oral cavity. Head Neck 2019;41(8):2514–2524. [DOI] [PubMed] [Google Scholar]
- 17.Charurat ME, Emmanuel B, Akolo C, et al. Uptake of treatment as prevention for HIV and continuum of care among HIV-positive men who have sex with men in Nigeria. J Acquir Immune Defic Syndr 2015;68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baral SD, Ketende S, Schwartz S, et al. Evaluating respondent-driven sampling as an implementation tool for universal coverage of antiretroviral studies among men who have sex with men living with HIV. J Acquir Immune Defic Syndr 2015;68 Suppl 2:S107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Federal Ministry of Health, National AIDS control program. Federal Republic of Nigeria National Guidelines for HIV Prevention Treatment and Care 2016. Available at: https://naca.gov.ng/wp-content/uploads/2016/11/2014-ANNUAL-REPORT-ON-HEALTH-SECTOR-HIV-and-AIDS-IN-NIGERIA.pdf. Accessed January 4, 2020.
- 20.Nowak RG, Schumaker LM, Ambulos NP, et al. Multiple HPV infections among men who have sex with men engaged in anal cancer screening in Abuja, Nigeria. Papillomavirus Res 2020;10:100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambulos NPJ, Schumaker LM, Mathias TJ, et al. Next-Generation Sequencing-Based HPV Genotyping Assay Validated in Formalin-Fixed, Paraffin-Embedded Oropharyngeal and Cervical Cancer Specimens. J Biomol Tech 2016;27(2):46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahasrabuddhe V v, Castle PE, Follansbee S, et al. Human papillomavirus genotype attribution and estimation of preventable fraction of anal intraepithelial neoplasia cases among HIV-infected men who have sex with men. J Infect Dis 2013;207(3):392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phanuphak N, Teeratakulpisarn N, Pankam T, et al. Anal human papillomavirus infection among Thai men who have sex with men with and without HIV infection: prevalence, incidence, and persistence. J Acquir Immune Defic Syndr 2013;63(4):472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poynten IM, Jin F, Garland SM, et al. HIV, Immune Dysfunction, and the Natural History of Anal High-Risk Human Papillomavirus Infection in Gay and Bisexual Men. J Infect Dis 2021;224(2):246–257. [DOI] [PubMed] [Google Scholar]
- 25.Beachler DC, D’Souza G, Sugar EA, et al. Natural history of anal vs oral HPV infection in HIV-infected men and women. J Infect Dis 2013;208(2):330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyitray AG, Carvalho da Silva RJ, Baggio ML, et al. Six-month incidence, persistence, and factors associated with persistence of anal human papillomavirus in men: the HPV in men study. J Infect Dis 2011;204(11):1711–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Critchlow CW, Hawes SE, Kuypers JM, et al. Effect of HIV infection on the natural history of anal human papillomavirus infection. AIDS 1998;12(10):1177–1184. [DOI] [PubMed] [Google Scholar]
- 28.Nowak RG, Ndembi N, Dauda W, et al. Implementation of and early outcomes from anal cancer screening at a community-engaged health care facility providing care to Nigerian men who have sex with men. J Glob Oncol 2019;2019(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glick SN, Feng Q, Popov V, et al. High rates of incident and prevalent anal human papillomavirus infection among young men who have sex with men. J Infect Dis 2014;209(3):369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keshinro B, Crowell TA, Nowak RG, et al. High prevalence of HIV, chlamydia and gonorrhoea among men who have sex with men and transgender women attending trusted community centres in Abuja and Lagos, Nigeria. J Int AIDS Soc 2016;19(1):21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


