Abstract
Background
The first clinical CT system to use photon-counting detector (PCD) technology has become available for patient care.
Purpose
To assess the technical performance of the PCD CT system with use of phantoms and representative participant examinations.
Materials and Methods
Institutional review board approval and written informed consent from four participants were obtained. Technical performance of a dual-source PCD CT system was measured for standard and high-spatial-resolution (HR) collimations. Noise power spectrum, modulation transfer function, section sensitivity profile, iodine CT number accuracy in virtual monoenergetic images (VMIs), and iodine concentration accuracy were measured. Four participants were enrolled (between May 2021 and August 2021) in this prospective study and scanned using similar or lower radiation doses as their respective clinical examinations performed on the same day using energy-integrating detector (EID) CT. Image quality and findings from the participants’ PCD CT and EID CT examinations were compared.
Results
All standard technical performance measures met accreditation and regulatory requirements. Relative to filtered back-projection reconstructions, images from iterative reconstruction had lower noise magnitude but preserved noise power spectrum shape and peak frequency. Maximum in-plane spatial resolutions of 125 and 208 µm were measured for HR and standard PCD CT scans, respectively. Minimum values for section sensitivity profile full width at half maximum measurements were 0.34 mm (0.2-mm nominal section thickness) and 0.64 mm (0.4-mm nominal section thickness) for HR and standard PCD CT scans, respectively. In a 120-kV standard PCD CT scan of a 40-cm phantom, VMI iodine CT numbers had a mean percentage error of 5.7%, and iodine concentration had root mean squared error of 0.5 mg/cm3, similar to previously reported values for EID CT. VMIs, iodine maps, and virtual noncontrast images were created for a coronary CT angiogram acquired with 66-msec temporal resolution. Participant PCD CT images showed up to 47% lower noise and/or improved spatial resolution compared with EID CT.
Conclusion
Technical performance of clinical photon-counting detector (PCD) CT is improved relative to that of a current state-of-the-art CT system. The dual-source PCD geometry facilitated 66-msec temporal resolution multienergy cardiac imaging. Study participant images illustrated the effect of the improved technical performance.
© RSNA, 2022
Online supplemental material is available for this article.
See also the editorial by Willemink and Grist in this issue.
Summary
The first clinical photon-counting detector CT system demonstrated superior spatial resolution relative to current CT systems and showed improved noise properties and multienergy temporal resolution relative to similarly configured energy-integrating detector CT.
Key Results
■ The high-spatial-resolution mode of photon-counting detector (PCD) CT demonstrated 125-µm in-plane spatial resolution and 0.34-mm longitudinal resolution, the smallest reported to date for a clinical CT system, to our knowledge.
■ The PCD CT system provided 66-msec temporal resolution multienergy imaging in dual-source mode.
■ Noise reduction (up to 47%) or dose reduction (up to 30%) was achieved in study participants with use of the PCD CT system relative to a similar CT system equipped with conventional detector.
Introduction
Photon-counting detector (PCD) CT systems have demonstrated suppression of electronic noise (1); simultaneous single-tube-potential, multienergy imaging (2–4); high spatial resolution without loss of dose efficiency (5–7); radiation dose reduction compared with energy-integrating detector (EID) CT for the same image quality (7,8); and improved iodine contrast-to-noise ratio (9). PCDs directly convert x-rays to electrical signal, facilitating small detector pixel designs without loss of geometric dose efficiency and allowing simultaneous multienergy CT with the use of multiple energy thresholds. Advances in detector technology have facilitated translation of PCDs to human imaging at clinical doses and dose rates (10,11). Investigational PCD CT systems with 0.15–0.27-mm detector pixel sizes (isocenter) and two to eight energy thresholds have been reported, with human imaging studies demonstrating potential clinical benefits (12–14).
With the introduction of the first clinical PCD CT system, the beneficial characteristics of PCDs can be realized in clinical practice. However, a comprehensive performance assessment is needed to confirm that current clinical requirements are met and to provide performance benchmarks relative to EID CT. The purpose of this study was to assess the technical performance of a clinical PCD CT system by using phantoms and representative images of study participants.
Materials and Methods
Siemens Healthineers provided support for this study under a sponsored research agreement with the Mayo Clinic. Authors employed by the Mayo Clinic, who did not receive financial support from Siemens, maintained control of all data and information presented in this article.
Approval from the institutional review board (Mayo Clinic; Rochester, Minn) and written informed consent from study participants were obtained.
PCD CT System
The first clinical PCD CT system (Naeotom Alpha, Siemens Healthineers) uses a dual-source geometry and 0.25-second gantry rotation time to provide 66-msec temporal resolution (isocenter). The primary PCD array has a 50-cm field of view, and the secondary PCD array, used only for cardiac or high–helical pitch (up to 3.2) scanning, has a 36-cm field of view (Fig E1 [online]). Additional technical specifications of the PCD CT system are listed in Table E1 (online).
The most current EID dual-source CT system (Somatom Force, Siemens Healthineers) operates its two source-detector pairs at the same tube potential to achieve 66-msec cardiac temporal resolution or high–helical pitch scanning or at two different tube-potential/tin-filter combinations for cardiac dual-energy acquisition at 125-msec temporal resolution. The energy-resolving detectors of the PCD CT system acquire multienergy data with use of a single PCD array and single tube potential. In dual-source cardiac mode, multienergy data are acquired using both PCD arrays at 66-msec temporal resolution over a 36-cm field of view. Detector pixel and energy threshold configurations are described in Appendix E1 and Figure E1 (online).
Quantitative Evaluation of Image Quality in Phantoms
Standard and advanced image quality performance metrics were assessed for PCD CT by using phantoms (Table 1) and were compared with published results for EID CT (15,16).
Table 1:
Experimental Details for Image Quality Performance Assessment
Evaluation of standard-image quality.—CT number accuracy and uniformity, section thickness, and low-contrast and high-contrast spatial resolution were evaluated using the American College of Radiology CT accreditation phantom (CT ACR 464, Sun Nuclear), and measurements were compared with published limits (17).
Advanced measures of spatial resolution.—With use of standard and high-spatial-resolution (HR) collimations, a tungsten wire was scanned and images reconstructed with the sharpest available kernels. The modulation transfer function (MTF), which characterizes in-plane spatial resolution as a function of spatial frequency, was calculated using previously validated methods (18).
For standard and HR collimations, a gold foil was scanned and images were reconstructed with the smallest section thicknesses, with 50% overlapping increment. Section sensitivity profiles were plotted and full width at half maximum values calculated to quantify longitudinal spatial resolution (18).
Image noise and noise power spectra.—Image noise (standard deviation of pixel values in a 5-cm-diameter region of interest, averaged across 15 images) and noise power spectra were measured in a water phantom for standard and HR collimations with use of weighted filtered back-projection (WFBP) and quantum iterative reconstruction algorithm (QIR, Siemens Healthineers) images (19). Low-energy threshold 120-kV images (referred to as T3D by the manufacturer) were reconstructed, which include photon energies ranging from 20 to 120 keV. The shape and peak spatial frequencies of the WFBP and QIR noise power spectra were compared to quantify any noise texture differences.
Quantitative multienergy CT performance.—A 40-cm water phantom containing solid iodine inserts (inserts from Multi-Energy CT Phantom, Sun Nuclear) with concentrations of 2, 5, 10, and 15 mg/cm3 was scanned. Virtual monoenergetic images (VMIs) at energies ranging from 40 to 140 keV were synthesized, and iodine VMI CT numbers were compared against the phantom manufacturer’s reference values. In addition, iodine maps were generated, and root mean squared error of the estimated iodine concentrations was calculated. Virtual noncontrast images were generated, and mean CT numbers were measured.
Proof-of-Principle Study Involving Participants
Participants undergoing clinically indicated EID CT were prospectively enrolled. Four clinical indications (CT angiography of the coronary arteries and of the abdomen and pelvis, whole-body low-dose CT for skeletal surveillance, and temporal bone imaging) were selected to illustrate in humans the effects of the improved technical performance of PCD CT shown in phantoms. PCD CT scans were obtained at the same or lower radiation dose relative to the clinical EID CT examination performed on the same date (Table 2).
Table 2:
Protocol Parameters Used for Each Participant’s Clinical EID CT and Research PCD CT Examinations
Results
Quantitative Evaluation of Image Quality in Phantoms
Evaluation of standard-image quality.—Standard-image quality measures conformed with limits set by the American College of Radiology’s CT accreditation program and the state of Minnesota. Measurements and images are shown in Figure E2 and Table E2 (online).
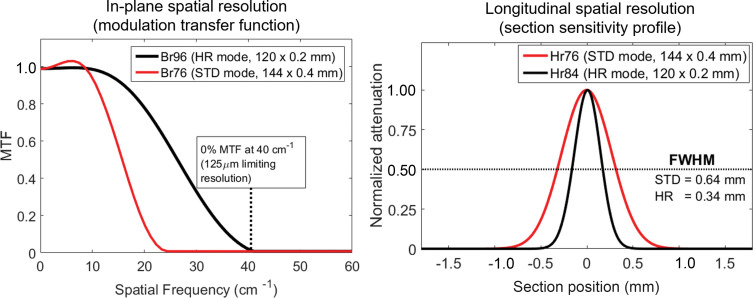
Advanced measures of spatial resolution.—MTF curves showed cutoff frequencies of 40 line pairs per centimeter (lp/cm) for the Br96 (body-regular, sharpness level 96) kernel (125-µm limiting in-plane resolution) for HR PCD CT and 24 lp/cm for the Br76 kernel (208-µm limiting in-plane resolution) for standard PCD CT, compared with 18 lp/cm (Br69, 278-µm in-plane limiting resolution) for EID CT (16). Section sensitivity profile full width at half maximum values for the smallest available section thicknesses were 0.34 mm (0.2 mm nominal), 0.64 mm (0.4 mm nominal), and 0.72 mm (0.6 mm nominal) for HR PCD, standard PCD, and EID (16) scans, respectively (Fig 1).
Figure 1:
Graphs show modulation transfer functions (MTFs) (left) and section sensitivity profiles (right) for the standard (STD) and high-spatial-resolution (HR) collimations of the photon-counting detector CT system. Limiting in-plane spatial resolution of 125 µm and full width at half maximum (FWHM) value of the section sensitivity profile of 0.34 mm were achieved using HR collimation. Br = body-regular reconstruction kernel, Hr = head-regular reconstruction kernel.
Image noise and noise power spectra.—HR images showed 9.51% (4.85 HU, WFBP) lower image noise than standard images (5.36 HU, WFBP) acquired using the same dose (Fig 2A). QIR images with strengths 1 and 3 had 11.5% and 34.5% lower image noise than WFBP, respectively, for both collimations (Table E3 [online]). Noise power spectrum curves showed 22% and 58% lower noise power values at peak frequency at QIR strengths 1 and 3, respectively, relative to WFBP in both collimations, and shape and peak frequency were unchanged from WFBP (Fig 2B, 2C).
Figure 2:
Image noise was reduced by 11.5% and 34.5% for quantum iterative reconstruction (QIR, Siemens) images using strengths of 1 and 3, respectively (labeled QIR1 and QIR3), relative to weighted filtered back projection (WFBP) using Hr40 (head-regular, sharpness level 40) kernel. (A) Bar graph has horizontal dotted lines to assist the reader in comparing noise levels between the collimation settings. (B, C) Noise power spectra (NPS) curves for standard (STD) (B) and high-spatial-resolution (HR) collimations (C) demonstrate that QIR reduced noise magnitude (height of curve) compared with WFBP images for both collimations and QIR strengths without shifting spatial frequency of the peak of the noise power spectrum curves, indicating that only the amount of noise, and not noise texture, is affected with use of QIR.
Quantitative multienergy CT performance.—Measured iodine VMI CT numbers for PCD CT matched expected values (Fig E3 [online]), with a mean percentage error of 5.7% compared with 5% at dual-source EID CT (15). Root mean squared error for iodine concentration was 0.5 mg/cm3, compared with 0.1–0.4 mg/cm3 root mean squared error for EID CT (15). The mean CT number in virtual noncontrast images corresponding to iodine inserts was –7 HU.
Proof-of-Principle Study Involving Participants
Four participants were scanned using PCD CT after their routine EID CT examination at the Mayo Clinic between May 2021 and August 2021. The resulting images provide a visual connection between phantom measurements and the appearance of participant images.
PCD coronary CT angiography images in a 71-year-old man (Fig 3) demonstrated multienergy, 66-msec temporal resolution cardiac imaging. The 45- and 55-keV VMIs showed higher iodine signal (45 keV showed 1164 HU; 55-keV showed 800 HU) compared with EID CT at 90 kV (724 HU) that used 22% more iodine contrast material (90 mL with PCD CT vs 110 mL with EID CT). PCD CT iodine maps showed clear delineation of the left coronary artery without motion blur. VMIs, iodine maps, and virtual noncontrast images could not be created from the 66-msec temporal resolution EID CT data because of the lack of multienergy information.
Figure 3:
Images in a 71-year-old man scanned with (A) energy-integrating detector (EID) CT and (B, C) photon-counting detector (PCD) CT with dual-source geometry to achieve 66-msec temporal resolution. Axial images are shown in left column, and oblique coronal images are shown in right column. While the EID CT examination is limited to single-energy data (A) at this temporal resolution, the multienergy capabilities of the PCD CT system allowed creation of low-energy (45, 55 keV) virtual monoenergetic images (VMIs) (B), which showed increased iodine signal (shown as mean Hounsfield unit measurements in regions of interest) compared with EID CT despite an 18% decrease (A: 110 mL vs B, C: 90 mL iohexol [Omnipaque 350, GE Healthcare]) in volume of administered contrast material (mean CT numbers for the black circular regions of interest are given in the left column of images). The use of VMIs adds to the inherently higher iodine contrast-to-noise ratio possible with PCD CT and provides clearer delineation of a branch of the left coronary artery (arrowheads). Increasing the VMI energy (65 keV or higher) decreased calcium blooming relative to EID CT (arrows in A and B). Absolute iodine concentration was measured using the iodine map images (mean concentration in mg/cm3 unit shown in region of interest) and virtual noncontrast (VNC) images used to visualize calcifications (arrow in C) with attenuation similar to that of iodinated blood. Reconstruction kernels used were as follows: Bv40 (body-vascular, sharpness level 40) (EID CT 90-kV, in A), Bv48 (PCD CT VMIs in B), Qr40 (quantitative-regular, sharpness level 40) (PCD CT iodine map and VNC images in C) Display windows and levels were as follows: 2000 HU and 200 HU for EID CT and PCD CT VMIs, 30/15 mg · mL−1 for iodine map, and 1000 HU and 100 HU for VNC image.
For abdominopelvic CT angiographic images in an 85-year-old man (Fig 4), HR PCD CT images reconstructed with a Br44 kernel (1.0-mm thickness) showed image noise similar to that of EID CT images reconstructed with a similar Br43 kernel (2.0-mm thickness). PCD CT allowed sharper reconstructions (Br72 kernel) than EID CT because of the inherently smaller detector elements. Given the higher spatial frequencies (0% MTF = 17 lp/cm), PCD CT Br72 image noise was higher (124.7 HU) than that of PCD CT Br44 (31.5 HU; 0% MTF = 9.6 lp/cm).
Figure 4:
Axial images in an 85-year-old man with aortoiliac stent graft scanned for angiography with energy-integrating detector (EID) CT at 100 kV (left column) and high-spatial-resolution photon-counting detector (PCD) CT (low-energy threshold, or T3D, images; includes photon energies from 20 to 120 keV) (center and right columns). Both scans were obtained with similar radiation doses (approximately 10 mGy). Images in the top row correspond to enlarged view of the rectangular region of interest displayed in the bottom row. PCD CT image in right column was created using sharper resolution (Br72 [body-regular, sharpness level 72] kernel) than is possible to achieve with EID CT, which reduces stent blooming and improves delineation of struts at the expense of increased image noise (shown as standard deviation of pixel values in the white circular region of interest [SD]). Display window = 1800 HU; display level = 440 HU.
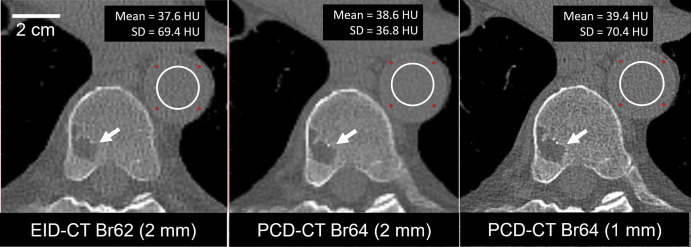
A low-dose CT skeletal survey for multiple myeloma in a 74-year-old man showed a lytic lesion in the vertebral body (Fig 5). At the same radiation dose and section thickness, PCD CT images showed 47% lower noise than EID CT images (36.8 HU vs 69.4 HU). Increasing matrix size to 1024 × 1024 and reducing section thickness to 1 mm yielded similar image noise between PCD CT and EID CT images (69 HU vs 70 HU) but further improved lesion conspicuity.
Figure 5:
Axial images in a 74-year-old man clinically indicated for whole-body low-dose CT skeletal survey for multiple myeloma. Energy-integrating detector (EID) CT scan (left) and high-spatial-resolution photon-counting detector (PCD) CT scans (center and right) were acquired using the same radiation dose (4.2 mGy). PCD CT image (center) using same section thickness (2 mm) as EID CT image (left) showed 47% lower noise (36.8 HU vs 69.4 HU within the white circular regions of interest). Use of thinner (1-mm) section thickness (right) and 1024 × 1024 matrix resulted in image noise similar to that of the EID CT image and improved delineation of vertebral lesion (arrows). Display window = 1500 HU; display level = 150 HU. Br = body-regular reconstruction kernel, SD = standard deviation of pixel values in region of interest.
Right temporal bone images in a 72-year-old man reconstructed using similar kernels (EID CT, Ur77 [ultra-high-resolution-regular, sharpness level 77]; PCD CT, Hr76 [head-regular, sharpness level 76]) showed improved stapes visualization for PCD CT (0.2-mm thickness) compared with EID CT (0.4-mm thickness) (Fig 6). At sharper kernels, EID CT Ur85 showed 124% higher noise than EID CT Ur77 (187.2 HU vs 83.5 HU), impeding visualization of the stapes and surrounding structures. PCD CT Hr84 images showed 58% higher image noise versus PCD CT Hr76 (144.8 HU vs 91.7 HU) that did not substantially deteriorate stapes visualization.
Figure 6:

Oblique axial images in a 72-year-old man scanned with (A, B) energy-integrating detector (EID) CT and (C, D) photon-counting detector (PCD) CT for temporal bone examination. Right temporal bone images acquired with use of EID CT were reconstructed using ultra-high-resolution-regular (Ur) kernels (Ur77 and Ur85) at 0.4-mm section thickness and PCD CT using head-regular (Hr) kernels (Hr76 and Hr84) at 0.2-mm section thickness. PCD CT images show better trabecular detail and delineation of the stapes (arrows) compared with EID CT (images C and D vs A and B), despite use of 30% lower dose (32.6 mGy vs 46.4 mGy) for PCD CT. Display window = 3800 HU; display level = 700 HU. Mean and standard deviation (SD) of CT numbers were measured in the white circular regions of interest.
Discussion
The evaluated photon-counting detector (PCD) CT system demonstrated 125-µm limiting in-plane spatial resolution, the smallest available for a clinical CT system, to our knowledge. Iodine CT numbers in virtual monoenergetic images matched expected values (5.7% mean percentage error), and a root mean squared error of 0.5 mg/cm3 was measured for iodine concentrations. Participant PCD CT images illustrated improved spatial resolution and the potential for lower radiation dose (30% in a temporal bone examination with 50% reduction of section thickness) and/or lower image noise (47% lower in a skeletal survey examination with matched radiation dose) compared with a similarly configured energy-integrating detector CT scanner.
Combining dual-source geometry and 0.25-second rotation time with PCDs enabled multienergy data acquisition at 66-msec temporal resolution (isocenter). Multienergy image types, including VMIs, virtual noncontrast images, and iodine maps, were therefore available without sacrificing temporal resolution (with dual-source EID CT, dual-energy acquisitions require each source or detector pair to be operated at a different tube potential, limiting the temporal resolution to 125 msec). Low–kiloelectron volt VMIs—which enable lower contrast material doses for patients with renal insufficiency (20), improve iodine signal in data acquired with suboptimal timing (21), and provide better visualization of small low-contrast structures (20)—can thus be obtained at the best temporal resolution reported to date for cardiac CT. Dual-source PCD CT also allowed multienergy imaging using helical pitch values up to 3.2, which can reduce patient dose by up to a factor of two (22).
PCD CT HR collimation can be used to generate thinner image sections with image noise similar to that of thicker EID CT sections. Alternatively, sharper kernels can be used to improve visualization of fine detail, including stents. For high-contrast applications (eg, temporal bone), PCD-specific kernels and 0.2-mm section thickness improve spatial resolution without substantially increasing image noise.
For screening or other examinations performed at reduced doses relative to routine applications, PCD HR collimation can be leveraged to reduce image noise at a prescribed spatial resolution (7,8,23). Alternatively, the 0.2-mm detector pixels can be used to improve anatomic conspicuity by using reduced section thicknesses and/or larger image matrices to reduce partial volume effects without increasing image noise relative to EID CT. Low-dose CT examinations also benefit from the intrinsic ability of PCD CT to eliminate electronic noise (1).
A clinical EID CT system with 0.25 × 0.25 mm detector pixel (isocenter), the smallest among current EID CT systems, is reported to have a 10% MTF of 19.6 lp/cm using the sharpest reconstruction kernel assessed (24), compared with a 10% MTF value of 36.1 lp/cm on the evaluated PCD CT scanner. The minimum section sensitivity profile full width at half maximum of this EID CT system is reported to be 0.45 mm (0.25-mm nominal section thickness) (25), compared with 0.34 mm (0.20-mm nominal thickness) on the evaluated PCD CT scanner. PCD CT also allows simultaneous single-tube-potential multienergy imaging, count weighting rather than energy weighting of detected photons (resulting in improved iodine contrast-to-noise ratio), and higher geometric dose efficiency by avoiding the need for inter–detector pixel septa.
Our study had the following limitations. PCD CT data were acquired using only the manufacturer-recommended tube potential. Studies at additional tube potentials are needed to determine optimal settings for different clinical tasks. Because our purpose was to assess the technical performance of the PCD CT system by using phantoms, a reader study using patient images was not performed. Instead, representative PCD CT images of participants are shown to provide a visual correlation to phantom results. Reader studies to assess diagnostic performance, reader confidence, and clinical impact for different diagnostic tasks and clinical cohorts are necessary.
In conclusion, the first clinical photon-counting detector CT system demonstrated superior spatial resolution, improved noise properties, and better multienergy temporal resolution relative to similarly configured energy-integrating detector CT. Visual correlation of phantom results was shown in humans for four clinical applications.
Acknowledgments
Acknowledgments
The authors thank Yong Lee, PhD, CCRP, and Boleyn Andrist, CCRP, for recruitment of study participants; Nikkole Weber, RT (radiography), and Holly Kasten, RT (radiography), for participant scanning; Jeffrey Marsh, BS, and Jamison Thorne, BS, for phantom scanning; and Kristina Nunez, MLIS, for manuscript submission.
Supported by the National Institutes of Health under award number R01-EB028590 and supported in kind by Siemens Healthineers, which owns the evaluated system under the terms of a sponsored research agreement with the Mayo Clinic.
Disclosures of conflicts of interest: K.R. No relevant relationships. M.P. No relevant relationships. A.H. No relevant relationships. E.R.S. No relevant relationships. B.S. No relevant relationships. T.G.F. No relevant relationships. A.F. No relevant relationships. F.B. No relevant relationships. F.E.D. Support for attending meetings and/or travel from the Spine Intervention Society. L.Y. License to Siemens Healthcare for intellectual property rights owned by the Mayo Clinic for which author is an inventor. P.R. Royalties from Elsevier. J.G.F. License to Siemens Healthcare for intellectual property rights owned by the Mayo Clinic for which author is an inventor; grant support from Siemens Healthineers. S.L. License to Siemens Healthcare for intellectual property rights owned by the Mayo Clinic for which author is an inventor. C.H.M. Grant support from Siemens Healthineers; license to Siemens Healthcare for intellectual property rights owned by the Mayo Clinic for which author is an inventor; board member of the International Society of Computed Tomography.
Abbreviations:
- EID
- energy-integrating detector
- HR
- high spatial resolution
- MTF
- modulation transfer function
- PCD
- photon-counting detector
- VMI
- virtual monoenergetic image
- WFBP
- weighted filtered back projection
References
- 1. Yu Z , Leng S , Kappler S , et al . Noise performance of low-dose CT: comparison between an energy integrating detector and a photon counting detector using a whole-body research photon counting CT scanner . J Med Imaging (Bellingham) 2016. ; 3 ( 4 ): 043503 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Muenzel D , Daerr H , Proksa R , et al . Simultaneous dual-contrast multi-phase liver imaging using spectral photon-counting computed tomography: a proof-of-concept study . Eur Radiol Exp 2017. ; 1 ( 1 ): 25 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sartoretti T , Eberhard M , Nowak T , et al . Photon-counting multienergy computed tomography with spectrally optimized contrast media for plaque removal and stenosis assessment . Invest Radiol 2021. ; 56 ( 9 ): 563 – 570 . [DOI] [PubMed] [Google Scholar]
- 4. Cormode DP , Roessl E , Thran A , et al . Atherosclerotic plaque composition: analysis with multicolor CT and targeted gold nanoparticles . Radiology 2010. ; 256 ( 3 ): 774 – 782 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leng S , Rajendran K , Gong H , et al . 150-μm spatial resolution using photon-counting detector computed tomography technology: technical performance and first patient images . Invest Radiol 2018. ; 53 ( 11 ): 655 – 662 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pourmorteza A , Symons R , Henning A , Ulzheimer S , Bluemke DA . Dose efficiency of quarter-millimeter photon-counting computed tomography: first-in-human results . Invest Radiol 2018. ; 53 ( 6 ): 365 – 372 . [DOI] [PubMed] [Google Scholar]
- 7. Rajendran K , Voss BA , Zhou W , et al . Dose reduction for sinus and temporal bone imaging using photon-counting detector CT with an additional tin filter . Invest Radiol 2020. ; 55 ( 2 ): 91 – 100 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klein L , Dorn S , Amato C , et al . Effects of detector sampling on noise reduction in clinical photon-counting whole-body computed tomography . Invest Radiol 2020. ; 55 ( 2 ): 111 – 119 . [DOI] [PubMed] [Google Scholar]
- 9. Gutjahr R , Halaweish AF , Yu Z , et al . Human imaging with photon counting-based computed tomography at clinical dose levels: contrast-to-noise ratio and cadaver studies . Invest Radiol 2016. ; 51 ( 7 ): 421 – 429 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leng S , Bruesewitz M , Tao S , et al . Photon-counting detector CT: system design and clinical applications of an emerging technology . RadioGraphics 2019. ; 39 ( 3 ): 729 – 743 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Willemink MJ , Persson M , Pourmorteza A , Pelc NJ , Fleischmann D . Photon-counting CT: technical principles and clinical prospects . Radiology 2018. ; 289 ( 2 ): 293 – 312 . [DOI] [PubMed] [Google Scholar]
- 12. Pourmorteza A , Symons R , Sandfort V , et al . Abdominal imaging with contrast-enhanced photon-counting CT: first human experience . Radiology 2016. ; 279 ( 1 ): 239 – 245 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajendran K, Marsh J, Petersilka M, et al. High resolution, full field-of-view, whole body photon-counting detector CT: system assessment and initial experience. In: Editor A, Editor B, eds.Proceedings of SPIE: medical imaging 2021.Bellingham, Wash:International Society for Optics and Photonics,2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Si-Mohamed S , Boccalini S , Rodesch PA , et al . Feasibility of lung imaging with a large field-of-view spectral photon-counting CT system . Diagn Interv Imaging 2021. ; 102 ( 5 ): 305 – 312 . [DOI] [PubMed] [Google Scholar]
- 15. Leng S , Zhou W , Yu Z , et al . Spectral performance of a whole-body research photon counting detector CT: quantitative accuracy in derived image sets . Phys Med Biol 2017. ; 62 ( 17 ): 7216 – 7232 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rajendran K , Petersilka M , Henning A , et al . Full field-of-view, high-resolution, photon-counting detector CT: technical assessment and initial patient experience . Phys Med Biol 2021. ; 66 ( 20 ): 205019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Phantom Testing: CT. American College of Radiology . https://accreditationsupport.acr.org/support/solutions/articles/11000056197-acr-ct-phantom-scanning-instructions. Published 2019. Updated December 13, 2019. Accessed October 8, 2021.
- 18. Fan M , Thayib T , Ren L , et al . A web-based software platform for efficient and quantitative CT image quality assessment and protocol optimization . In: Editor A , Editor B , eds. Proceedings of SPIE: medical imaging 2021 . Bellingham, Wash: : International Society for Optics and Photonics, 2021. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Siewerdsen JH , Cunningham IA , Jaffray DA . A framework for noise-power spectrum analysis of multidimensional images . Med Phys 2002. ; 29 ( 11 ): 2655 – 2671 . [DOI] [PubMed] [Google Scholar]
- 20. Albrecht MH , Vogl TJ , Martin SS , et al . Review of clinical applications for virtual monoenergetic dual-energy CT . Radiology 2019. ; 293 ( 2 ): 260 – 271 . [DOI] [PubMed] [Google Scholar]
- 21. Leithner D , Wichmann JL , Vogl TJ , et al . Virtual monoenergetic imaging and iodine perfusion maps improve diagnostic accuracy of dual-energy computed tomography pulmonary angiography with suboptimal contrast attenuation . Invest Radiol 2017. ; 52 ( 11 ): 659 – 665 . [DOI] [PubMed] [Google Scholar]
- 22. Flohr TG , Leng S , Yu L , et al . Dual-source spiral CT with pitch up to 3.2 and 75 ms temporal resolution: image reconstruction and assessment of image quality . Med Phys 2009. ; 36 ( 12 ): 5641 – 5653 . [DOI] [PubMed] [Google Scholar]
- 23. Kachelriess M , Kalender WA . Presampling, algorithm factors, and noise: considerations for CT in particular and for medical imaging in general . Med Phys 2005. ; 32 ( 5 ): 1321 – 1334 . [DOI] [PubMed] [Google Scholar]
- 24. Hernandez AM , Wu P , Mahesh M , Siewerdsen JH , Boone JM . Location and direction dependence in the 3D MTF for a high-resolution CT system . Med Phys 2021. ; 48 ( 6 ): 2760 – 2771 . [DOI] [PubMed] [Google Scholar]
- 25. Oostveen LJ , Boedeker KL , Brink M , Prokop M , de Lange F , Sechopoulos I . Physical evaluation of an ultra-high-resolution CT scanner . Eur Radiol 2020. ; 30 ( 5 ): 2552 – 2560 . [Published correction appears in Eur Radiol 2020;30(8):4709-4710.] [DOI] [PMC free article] [PubMed] [Google Scholar]





![Images in a 71-year-old man scanned with(A) energy-integrating detector (EID) CT and (B, C) photon-counting detector (PCD) CT with dual-source geometry to achieve 66-msec temporal resolution. Axial images are shown in left column, and oblique coronal images are shown in right column. While the EID CT examination is limited to single-energy data (A) at this temporal resolution, the multienergy capabilities of the PCD CT system allowed creation of low-energy (45, 55 keV) virtual monoenergetic images (VMIs) (B), which showed increased iodine signal (shown as mean Hounsfield unit measurements in regions of interest) compared with EID CT despite an 18% decrease (A: 110 mL vs B, C: 90 mL iohexol [Omnipaque 350, GE Healthcare]) in volume of administered contrast material (mean CT numbers for the black circular regions of interest are given in the left column of images). The use of VMIs adds to the inherently higher iodine contrast-to-noise ratio possible with PCD CT and provides clearer delineation of a branch of the left coronary artery (arrowheads). Increasing the VMI energy (65 keV or higher) decreased calcium blooming relative to EID CT (arrows in A and B). Absolute iodine concentration was measured using the iodine map images (mean concentration in mg/cm3 unit shown in region of interest) and virtual noncontrast (VNC) images used to visualize calcifications (arrow in C) with attenuation similar to that of iodinated blood. Reconstruction kernels used were as follows: Bv40 (body-vascular, sharpness level 40) (EID CT 90-kV, in A), Bv48 (PCD CT VMIs in B), Qr40 (quantitative-regular, sharpness level 40) (PCD CT iodine map and VNC images in C) Display windows and levels were as follows: 2000 HU and 200 HU for EID CT and PCD CT VMIs, 30/15 mg · mL−1 for iodine map, and 1000 HU and 100 HU for VNC image.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/ba74/8961720/2ba4137b8585/radiol.212579fig3.jpg)
![Axial images in an 85-year-old man with aortoiliac stent graft scanned for angiography with energy-integrating detector (EID) CT at 100 kV (left column) and high-spatial-resolution photon-counting detector (PCD) CT (low-energy threshold, or T3D, images; includes photon energies from 20 to 120 keV) (center and right columns). Both scans were obtained with similar radiation doses (approximately 10 mGy). Images in the top row correspond to enlarged view of the rectangular region of interest displayed in the bottom row. PCD CT image in right column was created using sharper resolution (Br72 [body-regular, sharpness level 72] kernel) than is possible to achieve with EID CT, which reduces stent blooming and improves delineation of struts at the expense of increased image noise (shown as standard deviation of pixel values in the white circular region of interest [SD]). Display window = 1800 HU; display level = 440 HU.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/ba74/8961720/a1ef334fdd4c/radiol.212579fig4.jpg)