Abstract
Objective:
Proton pump inhibitors (PPIs) are among the most commonly used medications for patients with osteoarthritis (OA). Various types of PPIs have different impacts on lowering serum magnesium level that may affect knee OA progression. We aimed to compare the risk of clinically relevant endpoint of knee replacement (KR) among initiators of five different PPIs with that among histamine-2 receptor antagonist (H2RA) initiators.
Design:
Among patients with knee OA (≥50 years) in The Health Improvement Network database in the UK we conducted five sequential propensity-score matched cohort studies to compare the risk of KR over five-year among patients who initiated omeprazole (n=2,672), pantoprazole (n=664), lansoprazole (n=3,747), rabeprazole (n=751), or esomeprazole (n=827) with those who initiated H2RA.
Results:
The prevalence of PPI prescriptions among participants with knee OA increased from 12.7% in 2000 to 44.0% in 2017. Two-hundred-and-seventy-four KRs (30.8/1000 person-years) occurred in omeprazole initiators and 230 KRs (25.4/1000 person-years) in H2RA initiators. Compared with H2RA initiators, the risk of KR was 21% higher in omeprazole initiators (hazard ratio [HR]=1.21,95% confidence interval [CI]:1.01–1.44). Similar results were observed when pantoprazole use was compared with H2RA use (HR=1.38,95%CI:1.00–1.90). No such an increased risk of KR was observed among lansoprazole (HR=1.06,95%CI:0.92–1.23), rabeprazole (HR=0.97,95%CI:0.73–1.30), or esomeprazole (HR=0.83,95%CI:0.60–1.15) initiators compared with that among H2RA initiators.
Conclusions:
In this population-based cohort study, initiation of omeprazole or pantoprazole use was associated with a higher risk of KR than initiation of H2RA use. This study raises concern regarding an unexpected risk of omeprazole and pantoprazole on accelerating OA progression.
Keywords: Proton Pump Inhibitor, Knee Replacement, Osteoarthritis, Cohort
INTRODUCTION
Osteoarthritis (OA) is a leading cause of pain, disability, and socioeconomic cost worldwide1. To date, there is no effective treatment available that can halt OA progression, and the main goal of clinical management remains pain control with treatments such as oral non-steroidal anti-inflammatory drugs (NSAIDs)2. However, NSAIDs use is associated with various adverse effects3, 4, including gastrointestinal complications4, and co-prescription of proton pump inhibitors (PPIs) with NSIADs is recommended as a cost-effective therapy for OA patients with moderate or high gastrointestinal risk5–9. As a result, PPIs are currently among the most commonly used medications for patients with OA.
The prescription of PPIs has been rising in recent years, particularly among older adults10–12. While PPIs have been generally perceived to be safe and effective, an increasing concern has been raised on its potential adverse effect of hypomagnesemia10, 11, 13, 14. Histamine-2 receptor antagonist (H2RA) is another class of acid-lowering medication that are widely used in the treatment of acid-related gastrointestinal diseases. Previous studies also reported that H2RA use was associated with increased risk of hypomagnesemia compared with nonuse, but the magnitude of association was smaller than that with PPI use15–17. In addition, the effect of lowering serum magnesium also varies depending on the type of PPIs18, 19. Using the Food and Drug Administration Adverse Event Reporting System database studies have found that omeprazole, pantoprazole, or lansoprazole, but not rabeprazole users appeared to experience much higher risk of hypomagnesemia than esomeprazole users18, 19.
Magnesium is an essential ion that plays an instrumental role in supporting and sustaining health. A six-month dietary magnesium restriction significantly decreased both articular cartilage chondrocyte density and growth plate chondrocyte column formation in rats20, and local intra-articular administration of magnesium sulfate following collagenase injection in rats attenuated the development of OA21. Several observational studies in humans showed an inverse association of either dietary magnesium intake or serum magnesium levels with the prevalence of radiographic knee OA22–25, and a prospective cohort study reported lower dietary magnesium intake was associated with worse pain and function among individuals with radiographic knee OA26. To our knowledge, no study has examined the relation of various types of PPIs to the risk of knee OA progression via their different impacts on lowering serum magnesium level that is potentially associated with knee OA.
To fill this knowledge gap, we conducted five sequential propensity-score matched cohort studies to compare the risk of clinically relevant endpoint of knee replacement (KR) among individuals with knee OA who initiated one of the five PPIs, i.e., omeprazole, pantoprazole, lansoprazole, rabeprazole, or esomeprazole, with those who initiated H2RA to minimize confounding by indication using an active comparator. In addition, we also compared the risks of KR among initiators of omeprazole, pantoprazole, lansoprazole, or rabeprazole with that among esomeprazole initiators as esomeprazole may have the lowest risk of hypomagnesemia among different types of PPIs18, 19.
METHODS
Data Source
We used The Health Improvement Network (THIN), an electronic medical record database from general practitioners (GP) in the United Kingdom (UK). THIN contains health information on approximately 17 million participants from 770 general practices in the UK, and previous research has shown that THIN is representative of the UK population in terms of patient demographics and the prevalence of common illnesses27. During consultation with patients, health information is recorded on site by GP using a computerized system. The information includes socio-demographics, anthropometrics, lifestyle factors, details from GP visits, diagnoses from specialists’ referrals as well as hospital admissions, and results of laboratory tests. The Read classification system is used to code specific diagnoses and a drug dictionary in Read code and ATC code formats based on data from the Multilex classification system28, 29. This study was approved by the THIN Scientific Review Committee (18THIN073).
Study Population
Our study population included individuals aged ≥ 50 years who had a diagnosis of knee OA identified by Read codes (see Supplemental Table 1) between January 2000 and May 2018. We excluded subjects who either had a KR prior to a knee OA diagnosis, or subjects with body mass index (BMI) > 40 kg/m2, history of joint infection, or comorbidities with poor prognosis (end-stage renal disease on dialysis, severe pulmonary disease requiring supplemental oxygen, pancreatic cancer, esophageal cancer, gastric cancer, or metastatic cancer)30 as the medications under the investigation are unlikely to have an impact on the risk of KR for those people who were deemed unlikely to be a candidate for KR (i.e., the outcome of our study).
Assessment of Exposure and Active Comparators
We identified individuals who initiated either one of the five PPIs (i.e., omeprazole, pantoprazole, lansoprazole, rabeprazole, or esomeprazole) or comparative H2RA (i.e., ranitidine or cimetidine) through ATC codes (see Supplemental Table 1), limiting to oral formulations. Initiators of either PPI or comparative H2RA were required to be continuously enrolled with the general practice for ≥ one year before the first prescription date of the medication of interest (i.e., index date) and to be naive to either the specific PPI or its comparative H2RA before the index date. PPI and H2RA initiation all occurred after knee OA diagnosis.
Assessment of Outcome
The outcome of interest was incident KR identified by Read codes. Previous studies have used this approach to identify joint replacements in THIN30–32.
Sequential Propensity-score Matched Cohorts
We conducted five sequential propensity-score matched cohort studies to compare the risks of KR among initiators of omeprazole, pantoprazole, lansoprazole, rabeprazole, or esomeprazole with that among H2RA initiators, respectively. For example, to compare the risk of KR among omeprazole initiators with that among H2RA initiators, we divided time between 2000 and 2018 into 19 one-year time blocks. Within each time block, we assembled a cohort of omeprazole initiators, defined as patients who started omeprazole during that time block, and a comparator cohort of H2RA initiators, defined as patients who started H2RA during the same time block. Propensity-scores (i.e., predicted probability of omeprazole initiation) were estimated using logistic regression, separately for each time block. The variables included in the model consisted of sociodemographic factors (age at index date, sex, the Townsend Deprivation Index score33), BMI, duration of OA prior to the index date, lifestyle factors (i.e., smoking status, and alcohol use), comorbidities and medication use prior to the index date, and healthcare utilization during the two years before the index date (see Table 1). Individuals with missing values of BMI, smoking status, drinking status, or Townsend Deprivation Index were excluded when the propensity-score was calculated. For each omeprazole user, we identified a propensity-score matched H2RA user during the time block using a greedy matching algorithm34. We used the same approach to assemble the other four comparator cohorts.
Table 1.
Baseline Characteristics of Propensity-score Matched Patients with Knee Osteoarthritis (≥50 years) Initiating PPI (Omeprazole, Pantoprazole, Lansoprazole, Rabeprazole, or Esomeprazole) or H2RA
| Propensity-score Matched Study Cohorts* |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Characteristics | Omeprazole Initiators (N=2,672) | H2RA Initiators (N=2,672) | Pantoprazole Initiators (N=664) | H2RA Initiators (N=664) | Lansoprazole Initiators (N=3,747) | H2RA Initiators (N=3,747) | Rabeprazole Initiators (N=751) | H2RA Initiators (N=751) | Esomeprazole Initiators (N=827) | H2RA Initiators (N=827) |
| Demographics | ||||||||||
| Age, mean (SD), y | 70.5 (9.7) | 70.9 (9.6) | 71.0 (9.7) | 70.5 (9.8) | 70.8 (9.8) | 70.6 (9.7) | 70.5 (9.4) | 70.0 (9.2) | 69.6 (9.7) | 70.2 (9.5) |
| Male (%) | 41.7 | 42.3 | 40.1 | 42.6 | 43.1 | 42.4 | 38.1 | 38.5 | 37.6 | 39.5 |
| Socio-Economic | ||||||||||
| Deprivation Index Score**, mean (SD) | 2.7 (1.4) | 2.7 (1.4) | 2.8 (1.4) | 2.7 (1.4) | 2.8 (1.4) | 2.8 (1.4) | 2.6 (1.3) | 2.7 (1.3) | 2.7 (1.4) | 2.7 (1.4) |
| OA Duration, mean (SD), y | 7.3 (6.8) | 7.4 (6.7) | 6.6 (6.3) | 6.5 (6.1) | 7.2 (6.7) | 7.2 (6.6) | 6.5 (6.0) | 6.6 (6.2) | 7.4 (7.0) | 7.2 (6.6) |
| BMI, mean (SD), kg/m2 | 28.3 (4.7) | 28.3 (4.4) | 28.3 (4.5) | 28.3 (4.7) | 28.2 (4.6) | 28.2 (4.5) | 28.0 (4.7) | 28.2 (4.4) | 28.2 (4.7) | 28.2 (4.6) |
| Lifestyle factors | ||||||||||
| Smoking (%) | ||||||||||
| None | 56.8 | 57.1 | 57.4 | 56.3 | 56.3 | 56.4 | 59.5 | 58.3 | 57.7 | 56.2 |
| Past | 31.4 | 31.7 | 32.2 | 32.7 | 31.8 | 31.7 | 26.0 | 26.6 | 31.8 | 33.5 |
| Current | 11.8 | 11.2 | 10.4 | 11.0 | 11.9 | 11.9 | 14.5 | 15.0 | 10.5 | 10.3 |
| Alcohol Use (%) | ||||||||||
| None | 22.9 | 24.7 | 22.6 | 24.7 | 22.8 | 22.7 | 21.6 | 22.0 | 22.1 | 20.8 |
| Past | 2.9 | 2.8 | 2.7 | 1.7 | 2.9 | 2.6 | 2.8 | 2.8 | 3.4 | 3.0 |
| Current | 74.1 | 72.5 | 74.7 | 73.6 | 74.3 | 74.7 | 75.6 | 75.2 | 74.5 | 76.2 |
| Comorbidities | ||||||||||
| Charlson Index, mean (SD) | 0.8 (1.3) | 0.7 (1.3) | 0.7 (1.3) | 0.6 (1.1) | 0.7 (1.3) | 0.7 (1.3) | 0.5 (1.1) | 0.6 (1.2) | 0.8 (1.3) | 0.8 (1.3) |
| Peptic Ulcer Disease (%) | 6.8 | 7.5 | 12.3 | 12.3 | 7.0 | 7.0 | 9.7 | 8.9 | 12.2 | 10.9 |
| Gastroesophageal reflux disease (%) | 16.5 | 16.5 | 33.3 | 33.3 | 16.7 | 17.4 | 26.8 | 28.0 | 37 | 35.3 |
| Gastritis (%) | 26.9 | 25.4 | 36.6 | 36 | 26.8 | 27.6 | 32.4 | 31.0 | 43.5 | 43.9 |
| Myocardial Infarction (%) | 8.8 | 8.3 | 7.5 | 9.2 | 8.2 | 7.4 | 6.4 | 7.1 | 4.8 | 5.6 |
| Atrial Fibrillation (%) | 7.7 | 7.3 | 9.0 | 9.3 | 8.4 | 8.3 | 5.6 | 6.7 | 6.2 | 6.4 |
| Ischemic Heart Disease (%) | 21.7 | 22.0 | 22.0 | 22.3 | 21.1 | 19.8 | 19.3 | 20.1 | 16.9 | 18.4 |
| Peripheral Vascular Disease (%) | 2.6 | 2.3 | 3.2 | 3.0 | 2.1 | 2.3 | 2.7 | 2.5 | 1.9 | 2.1 |
| Congestive Heart Failure (%) | 6.0 | 5.6 | 6.2 | 6.2 | 5.9 | 5.3 | 6.0 | 5.7 | 3.9 | 4.0 |
| Valvular Heart Disease (%) | 4.7 | 4.1 | 3.0 | 3.8 | 4.1 | 3.7 | 2.3 | 2.0 | 2.3 | 3.1 |
| Transient Ischemic Attack (%) | 5.1 | 4.6 | 5.9 | 5.7 | 5.3 | 5.0 | 5.5 | 4.9 | 4.6 | 5.0 |
| Angina (%) | 13.7 | 14.1 | 14.3 | 14.8 | 13.4 | 12.6 | 14.4 | 15.2 | 12.0 | 14.0 |
| Other Circulatory Disease (%) | 36.8 | 36.0 | 40.5 | 38.7 | 36.1 | 36.1 | 34.9 | 36.0 | 37.0 | 39.4 |
| Stroke (%) | 5.2 | 4.8 | 5.4 | 3.3 | 5.9 | 5.3 | 4.0 | 3.7 | 3.1 | 3.7 |
| Hypertension (%) | 51.9 | 51.6 | 53.3 | 51.4 | 49.9 | 50.8 | 46.9 | 48.3 | 50.8 | 52.4 |
| Chronic Obstructive Pulmonary Disease (%) | 7.4 | 7.1 | 5.7 | 5.0 | 6.8 | 6.8 | 5.2 | 5.1 | 5.9 | 7.4 |
| Chronic Kidney Disease (%) | 10.8 | 9.9 | 8.7 | 8.0 | 10.3 | 9.7 | 3.1 | 3.3 | 9.8 | 9.8 |
| Liver Disease (%) | 2.7 | 2.7 | 1.4 | 1.2 | 2.6 | 3.0 | 1.7 | 2.1 | 2.8 | 2.8 |
| Diabetes (%) | 16.1 | 15.2 | 15.4 | 17.0 | 15.5 | 15.1 | 10.0 | 11.5 | 15.5 | 14.1 |
| Cancer (%) | 13.6 | 13.4 | 12.3 | 11.7 | 13.5 | 13 | 10.8 | 10.7 | 12.8 | 12.2 |
| Pneumonia or Infection (%) | 9.0 | 9.5 | 10.7 | 11.9 | 9.3 | 9.0 | 10.4 | 8.1 | 9.3 | 9.2 |
| Depression (%) | 14.0 | 13.0 | 15.8 | 16.3 | 13.4 | 14.1 | 13.6 | 13.6 | 16.4 | 18.1 |
| Venous Thromboembolism (%) | 4.3 | 4.8 | 4.1 | 4.5 | 4.2 | 4.2 | 4.3 | 3.9 | 3.7 | 3.4 |
| Varicose (%) | 15.3 | 14.3 | 16.0 | 15.8 | 14.4 | 14.2 | 12.8 | 15.4 | 15.7 | 14.8 |
| Inflammatory Conditions (%) | 12.5 | 13.1 | 11.9 | 12.3 | 13 | 12.7 | 11.1 | 11.1 | 12.6 | 12.2 |
| Falls (%) | 15.4 | 16.2 | 17.8 | 17.9 | 15.2 | 15.2 | 16.1 | 14.4 | 16.2 | 17.2 |
| Hip Fracture (%) | 2.0 | 1.9 | 2.0 | 1.4 | 1.7 | 1.7 | 1.2 | 0.8 | 1.7 | 2.1 |
| Hyperlipidemia (%) | 19.3 | 18.2 | 19.7 | 18.4 | 17.9 | 17.9 | 16.2 | 16.6 | 18.5 | 21.3 |
| Dementia (%) | 1.1 | 1.4 | 1.5 | 1.2 | 1.4 | 1.4 | 0.5 | 0.5 | 1.8 | 1.5 |
| Osteoporosis (%) | 10.4 | 11.0 | 12.8 | 15.5 | 10.9 | 10.6 | 10.5 | 10.7 | 10.8 | 12.6 |
| Seizure (%) | 0.8 | 0.8 | 0.5 | 0.5 | 0.6 | 0.7 | 0.4 | 0.4 | 0.6 | 0.2 |
| Trauma (%) | 1.1 | 1.1 | 1.4 | 1.4 | 1.2 | 1.4 | 0.8 | 0.8 | 1.0 | 1.7 |
| Medication | ||||||||||
| Nitrate (%) | 11.6 | 10.7 | 12.3 | 14.9 | 10.8 | 10.3 | 10.3 | 9.9 | 9.2 | 9.6 |
| Antihypertensive Medicine (%) | 61.3 | 60.7 | 61.6 | 62.2 | 59.5 | 60.0 | 56.7 | 59.4 | 61.3 | 61.8 |
| ACE Inhibitors (%) | 27.2 | 25.8 | 26.4 | 25.0 | 26.2 | 25.6 | 20.4 | 21.8 | 24.4 | 24.7 |
| Beta Blockers (%) | 26.8 | 26.0 | 25.9 | 25.9 | 24.8 | 24.4 | 22.2 | 24.4 | 22.1 | 23.7 |
| Calcium Channel Blockers (%) | 24.8 | 24.7 | 25.6 | 26.1 | 23.9 | 24.5 | 20.4 | 22.0 | 23.9 | 25 |
| ARBs (%) | 11.5 | 10.9 | 11.3 | 11.0 | 10.5 | 11.0 | 7.9 | 8.0 | 13.9 | 14.1 |
| Statin (%) | 36.8 | 36.6 | 36.9 | 36.9 | 36.3 | 34.9 | 23.3 | 25.0 | 35.2 | 36.3 |
| Benzodiazepines (%) | 14.7 | 14.4 | 17.8 | 17.5 | 15.3 | 15.2 | 16.0 | 17.0 | 16.6 | 15.7 |
| SSRI (%) | 9.6 | 9.2 | 10.2 | 10.4 | 9.6 | 10.7 | 7.6 | 8.5 | 11.0 | 12.0 |
| SNRI (%) | 1.8 | 1.4 | 2.0 | 2.3 | 1.8 | 1.6 | 1.2 | 1.9 | 1.9 | 1.6 |
| Aspirin (%) | 33.2 | 32.4 | 31.3 | 30.7 | 31.6 | 30.7 | 28.4 | 30 | 26.4 | 30.0 |
| NSAIDs (%) | 44.4 | 44.4 | 45.2 | 47.0 | 46.1 | 45.5 | 52.1 | 53.7 | 38.9 | 40.1 |
| Loop Diuretics (%) | 14.7 | 15.0 | 15.5 | 16.3 | 15.5 | 14.1 | 16.0 | 15.2 | 12.8 | 13.3 |
| HCTZ (%) | 20.4 | 20.1 | 19.6 | 19.0 | 18.8 | 19.8 | 22.1 | 24.0 | 19.1 | 20.7 |
| Potassium Sparing Diuretics (%) | 4.8 | 5.1 | 5.1 | 5.4 | 4.7 | 4.2 | 6.8 | 5.1 | 3.9 | 4.0 |
| DMARDs (%) | 1.8 | 1.5 | 1.5 | 1.5 | 1.7 | 1.7 | 1.5 | 1.5 | 2.2 | 2.1 |
| Glucocorticoids (%) | 11.8 | 11.6 | 12.0 | 10.2 | 12.6 | 13.1 | 10.3 | 11.1 | 11.1 | 10.5 |
| Anticoagulant (%) | 6.2 | 6.2 | 7.5 | 7.2 | 6.0 | 6.2 | 4.3 | 5.6 | 4.7 | 3.9 |
| Opioids (%) | 16.7 | 17.6 | 22.1 | 20.0 | 16.6 | 17.2 | 16.9 | 15.3 | 22.2 | 23.1 |
| Other PPIs (%) | 20.9 | 21.2 | 51.5 | 49.7 | 26.9 | 25.9 | 27.0 | 24.9 | 71.3 | 70.1 |
| Bisphosphonates (%) | 6.5 | 6.4 | 7.2 | 8.0 | 6.3 | 5.8 | 5.2 | 4.7 | 6.4 | 6.7 |
| Antidiabetic Medication | 11.3 | 10.6 | 10.2 | 11.6 | 10.8 | 10.4 | 7.5 | 9.1 | 10.8 | 10.2 |
| Health Care Utilization, mean (SD) | ||||||||||
| General Practice Visits*** | 14.0 (11.6) | 14.1 (11.6) | 15.6 (11.7) | 15.2 (11.1) | 14.2 (11.1) | 14.2 (11.0) | 13.8 (10.2) | 13.8 (10.4) | 16.2 (11.5) | 16.3 (11.9) |
| Hospitalizations*** | 0.8 (1.9) | 0.8 (1.6) | 0.8 (1.7) | 0.9 (1.8) | 0.8 (1.7) | 0.8 (1.9) | 0.4 (1.1) | 0.3 (0.9) | 0.9 (1.9) | 1.0 (1.9) |
| Referrals*** | 1.2 (1.7) | 1.2 (1.8) | 1.4 (1.7) | 1.4 (2.0) | 1.2 (1.7) | 1.2 (1.8) | 0.9 (1.5) | 0.9 (1.3) | 1.5 (1.8) | 1.6 (2.2) |
H2RAs, histamine-2 receptor antagonists; N, number; OA, osteoarthritis; BMI, body mass index; ACE, angiotensin converting enzyme; ARB, Angiotensin II receptor blocker; SSRI, Selective serotonin reuptake inhibitor; SNRI, Serotonin-norepinephrine reuptake inhibitor; NSAIDs, non-steroidal anti-inflammatory drugs; HCTZ, hydrochlorothiazide; DMARDs, disease-modifying antirheumatic drugs; PPI, proton pump inhibitor; H2RA, Histamine-2 receptor antagonist; y, years; SD, standard deviation.
A comparison initiator was randomly selected to match to each initiator within 1 -year cohort accrual block.
Socio-Economic Deprivation Index Score was measured by the Townsend Deprivation Index, which was grouped into quintiles from 1 (least deprived) to 5 (most deprived).
Frequency during the past two years
Statistical Analysis
We described the annual prevalence of prescriptions for overall PPI, overall H2RA, and each specific PPI among patients with knee OA. For a specific calendar year, the denominator of yearly prevalence was all person diagnosed with OA up to that year. Of them, we obtained the numerator of individuals who had a prescription or had prescription period covered within this year. We compared the baseline characteristics of each of the PPI cohorts with the comparison cohorts (i.e., H2RA). Participants began accruing risk time from the index date until the date of KR, death, age of 90, date of disenrollment in THIN, or the end of the fifth year of follow-up (i.e., approximately 95% of subjects took PPI for five years or less), whichever occurred first.
For each study cohort, we calculated the incidence rate of KR. We calculated the cumulative incidence rate of KR to depict risk of KR for each cohort accounting for competing risk of death35. We fitted Cox proportional hazard models to determine the relation of each specific PPI initiation vs. H2RA initiation to the risk of KR. We tested the proportional hazards assumption for each comparison cohorts using the Kolmogorov supremum test36.
We performed several sensitivity analyses to assess the robustness of our study findings. First, we performed asymmetric trimming to exclude patients whose propensity-score was below the 2.5th percentile of the propensity-score of each specific PPI cohort and above 97.5th percentile of the propensity-score of comparison cohort; thus, patients who were treated with specific PPI most contrary to prediction were excluded from the analyses to minimize potential unmeasured confounders. Second, since individuals with missing values of BMI, smoking status, drinking status, or Townsend Deprivation Index were excluded when the propensity-score was calculated, we imputed values using a sequential regression method based on a set of covariates as predictors37. Details of the missing data imputation procedure can be found in the Supplemental Text. Finally, since the analyses may not fully adjust for potential confounders we performed quantitative sensitivity analyses to assess the minimum unmeasured confounding effect that would need to explain away an association observed in previous analyses conditional on the measured covariates that have been adjusted for38.
In addition, we took the same approach to conduct another four sequential propensity-score matched cohort studies to compare the risks of KR among initiators of omeprazole, pantoprazole, lansoprazole, or rabeprazole with that among esomeprazole initiators.
All P-values were two-sided. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
As shown in Figure 1A, the prevalence of PPI prescriptions among participants with knee OA increased from 12.7% in 2000 to 44.0% in 2017. In comparison, the annual prevalence of H2RA prescription decreased slightly from 7.3% in 2000 to 4.7% in 2017. Prevalence of prescriptions of each specific PPI is depicted in Figure 1B. Omeprazole and lansoprazole were the two most commonly prescribed PPIs in the UK, and each of their prescription increased from 5.6% and 6.4% in year 2000 to 29.7% and 16.5% in year 2012, respectively, and then remained stable. The prevalence of esomeprazole prescription increased slightly but was still low during the study period (<1.6%). Pantoprazole prescription was uncommon and stable during the study period (0.5%−1.4%), and the prevalence of rabeprazole prescription was also low and appeared to decrease steadily over time (from 1.5% to 0.2%).
Figure 1. Secular Trend of Prevalence of Prescription of (A) Overall PPI and H2RA, and (B) Omeprazole, Pantoprazole, Lansoprazole, Rabeprazole, and Esomeprazole, among Patients with Knee Osteoarthritis in The Health Improvement Network Database.
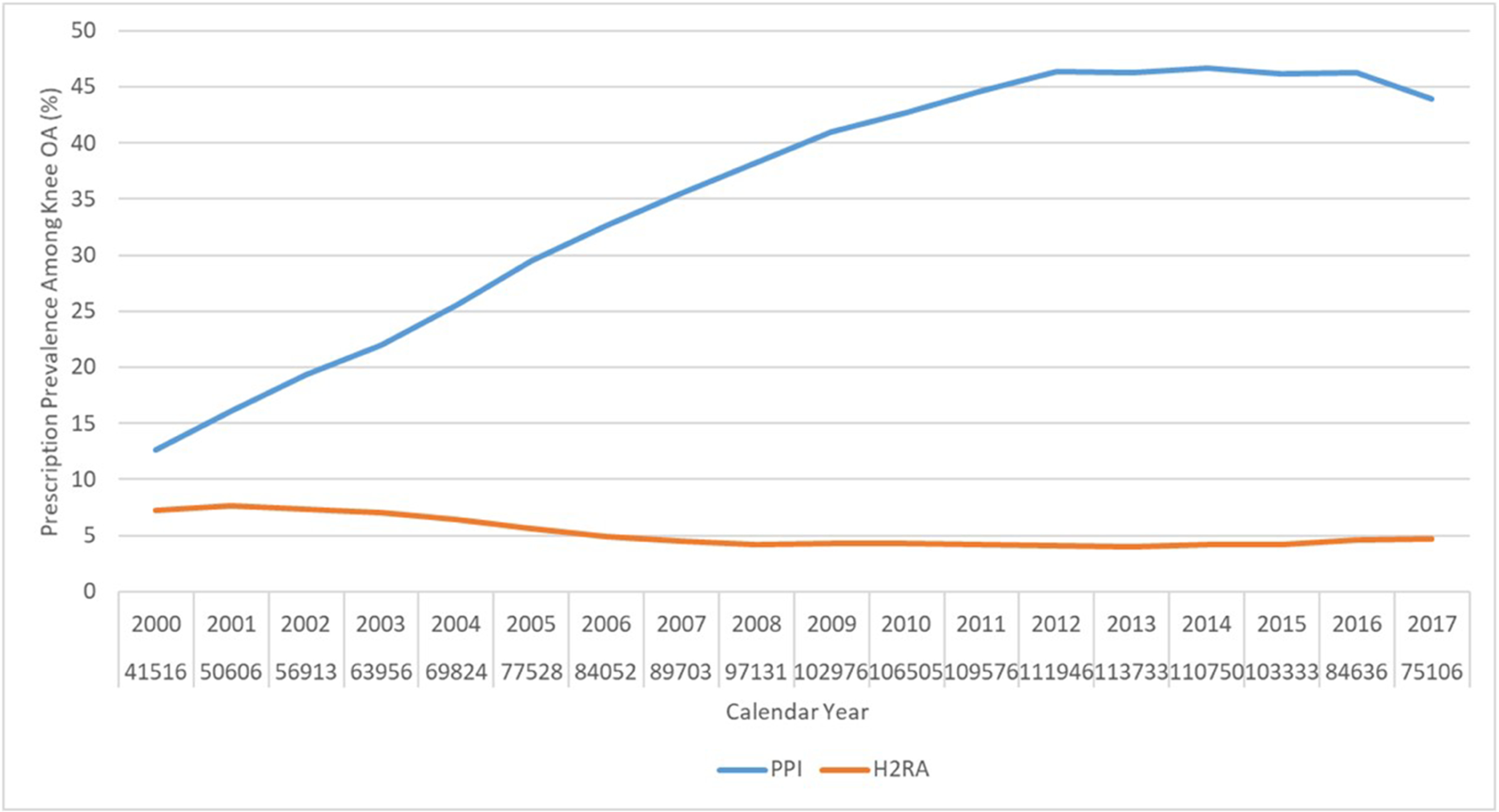
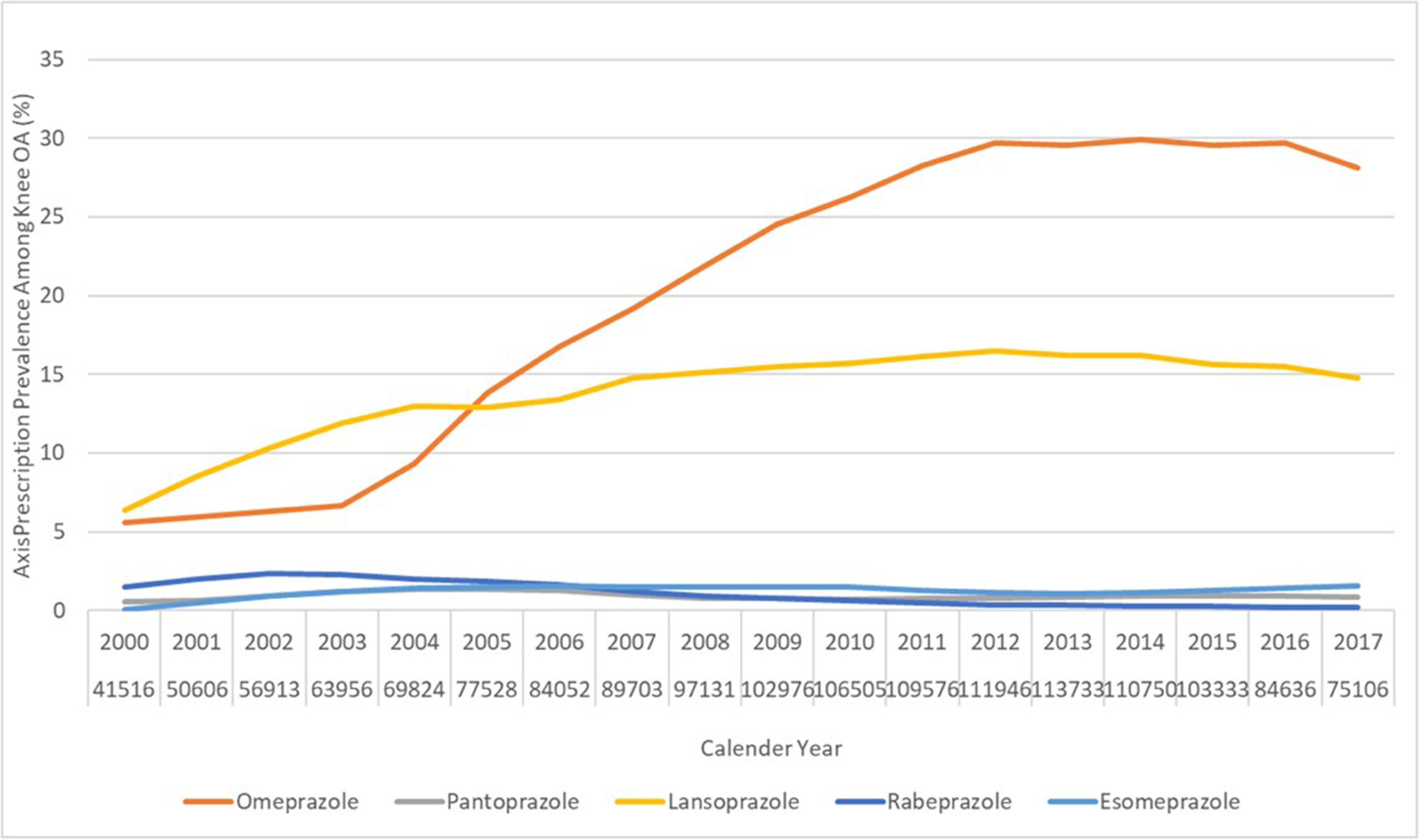
OA, osteoarthritis; PPI, proton pump inhibitor; H2RA, Histamine-2 receptor antagonist.
The selection process of included participants and the baseline characteristics of four propensity-score matched cohorts are shown in Supplemental Figure 1 and Table 1, respectively. The mean age ranged from 69.6 to 71.0 years, and approximately 40% were men. The characteristics of each specific PPI cohort and its matched comparison cohorts were well-balanced, with all standardized differences being < 0.139.
The cumulative incidence of KR was higher in the omeprazole cohort than that in the H2RA cohort (Figure 2A). As shown in Table 2, 274 KRs (30.8/1000 person-years) occurred in the omeprazole initiators and 230 (25.4/1000 person-years) occurred in the H2RA initiators during the five years follow-up period. Compared with H2RA use, the hazard ratio (HR) of KR for omeprazole use was 1.21 (95% confidence interval [CI]: 1.01 to 1.44). Similar findings were also observed when risk of KR among the pantoprazole cohort was compared with that among the H2RA cohort (HR=1.38, 95% CI: 1.00 to 1.90) (Figure 2B, Table 2). There was no apparent difference in the risk of KR between lansoprazole (HR=1.06, 95% CI: 0.92 to 1.23) or rabeprazole (HR=0.97, 95% CI: 0.73 to 1.30) cohort with its comparison H2RA cohort (Figure 2C, Figure 2D, Table 2). Esomeprazole use had a lower risk of KR (HR=0.83, 95% CI: 0.60 to 1.15) than H2RA use (Figure 2E, Table 2). In all analyses, the proportional hazards assumption for Cox-proportional model was not violated (P > 0.05).
Figure 2. Time to Knee Replacement over Five Years for the Propensity-score Matched Cohorts of Knee Osteoarthritis Patients with Initiation of (A) Omeprazole, (B) Pantoprazole, (C) Lansoprazole, (D) Rabeprazole, or (E) Esomeprazole Compared with Initiation of H2RA adjusting for Competing Risk of Death.
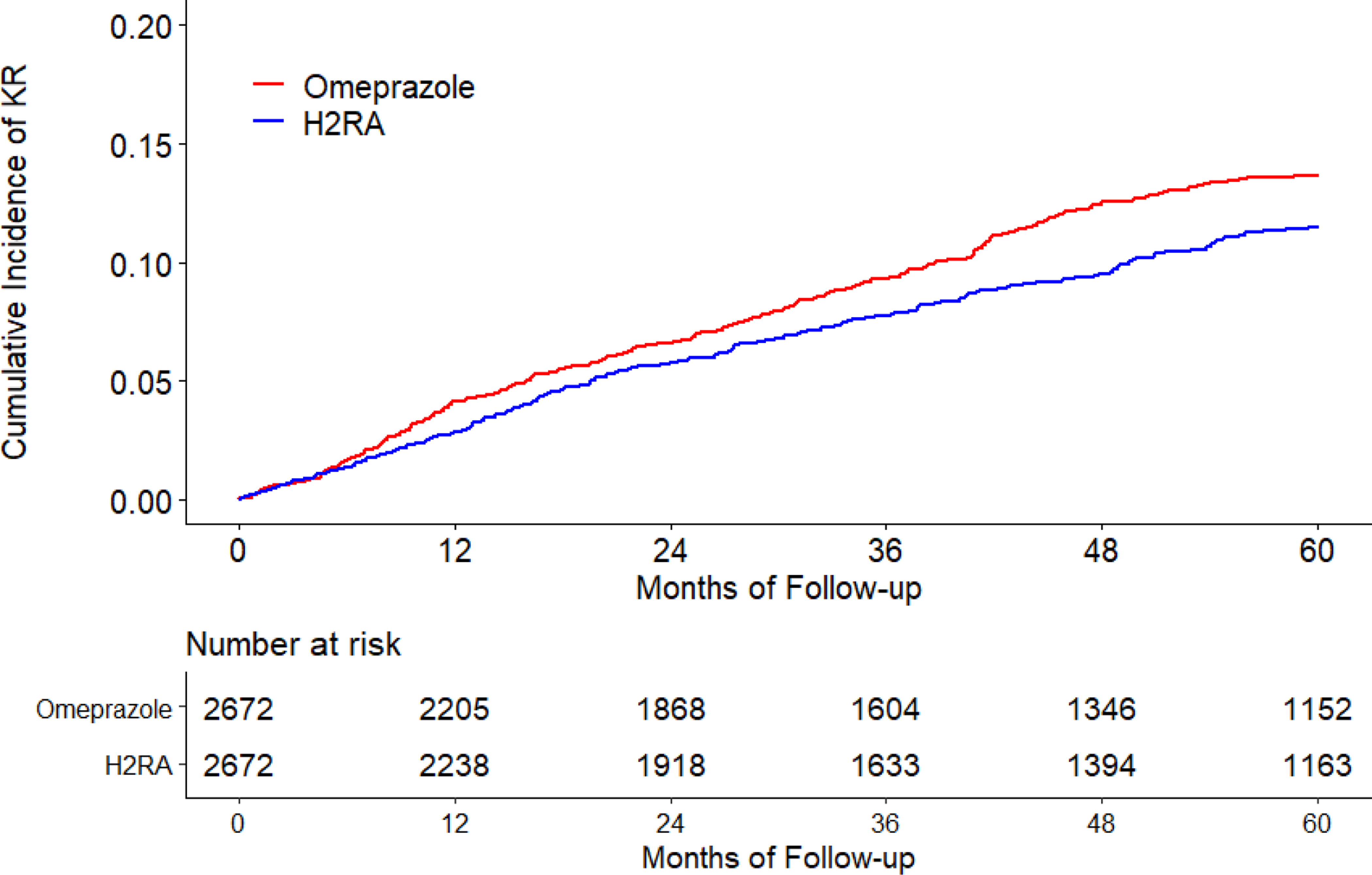
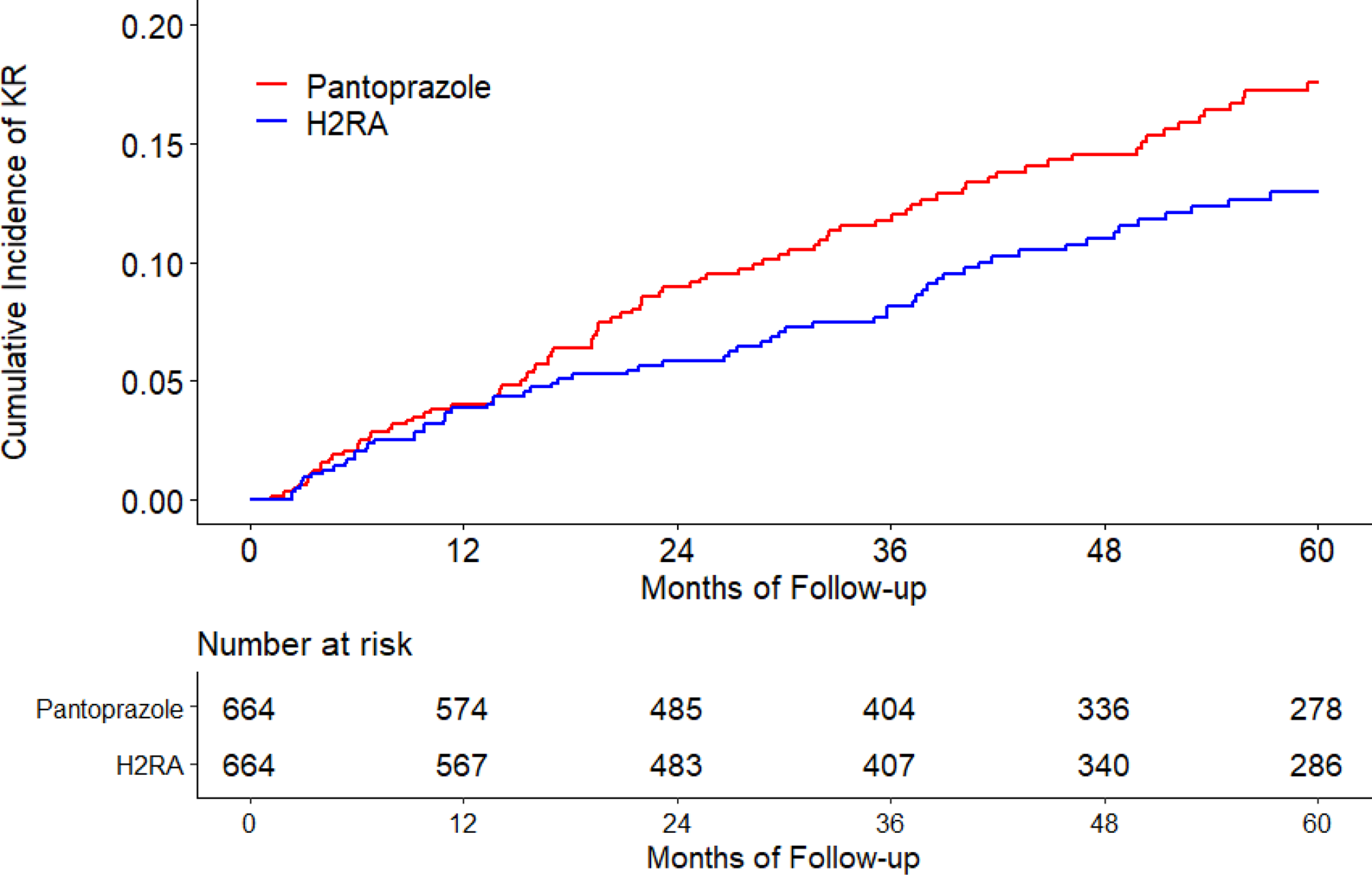
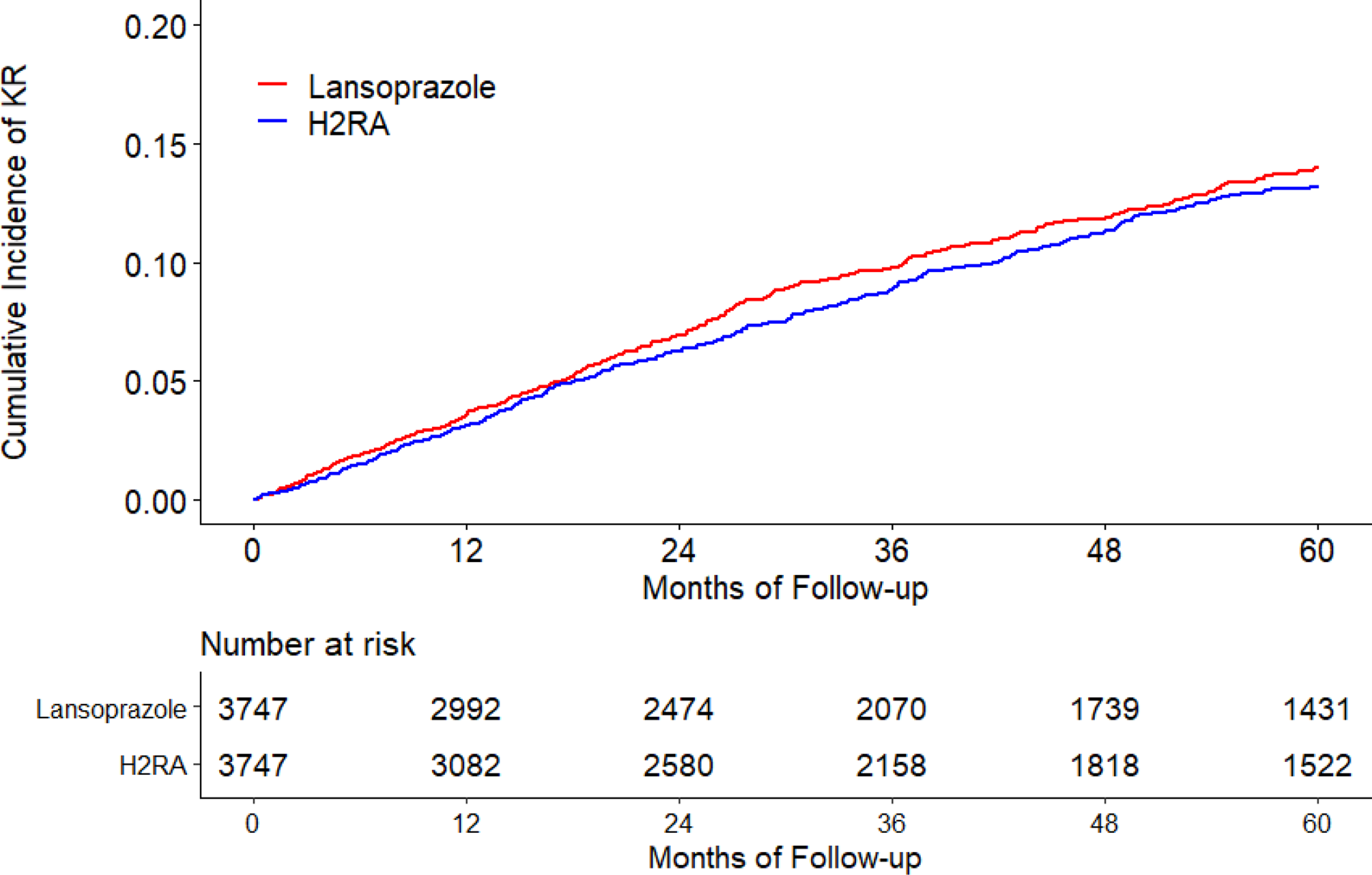
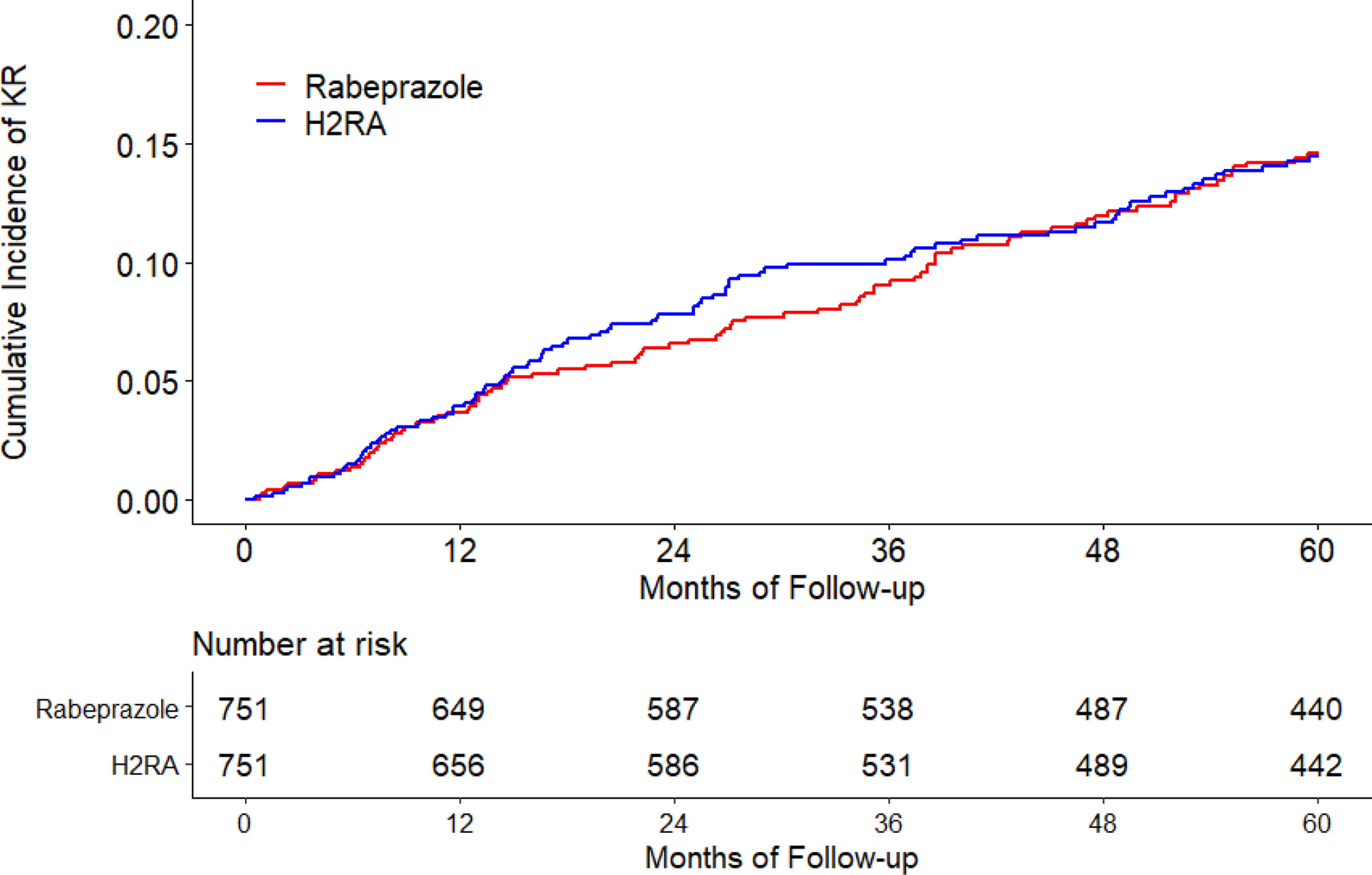
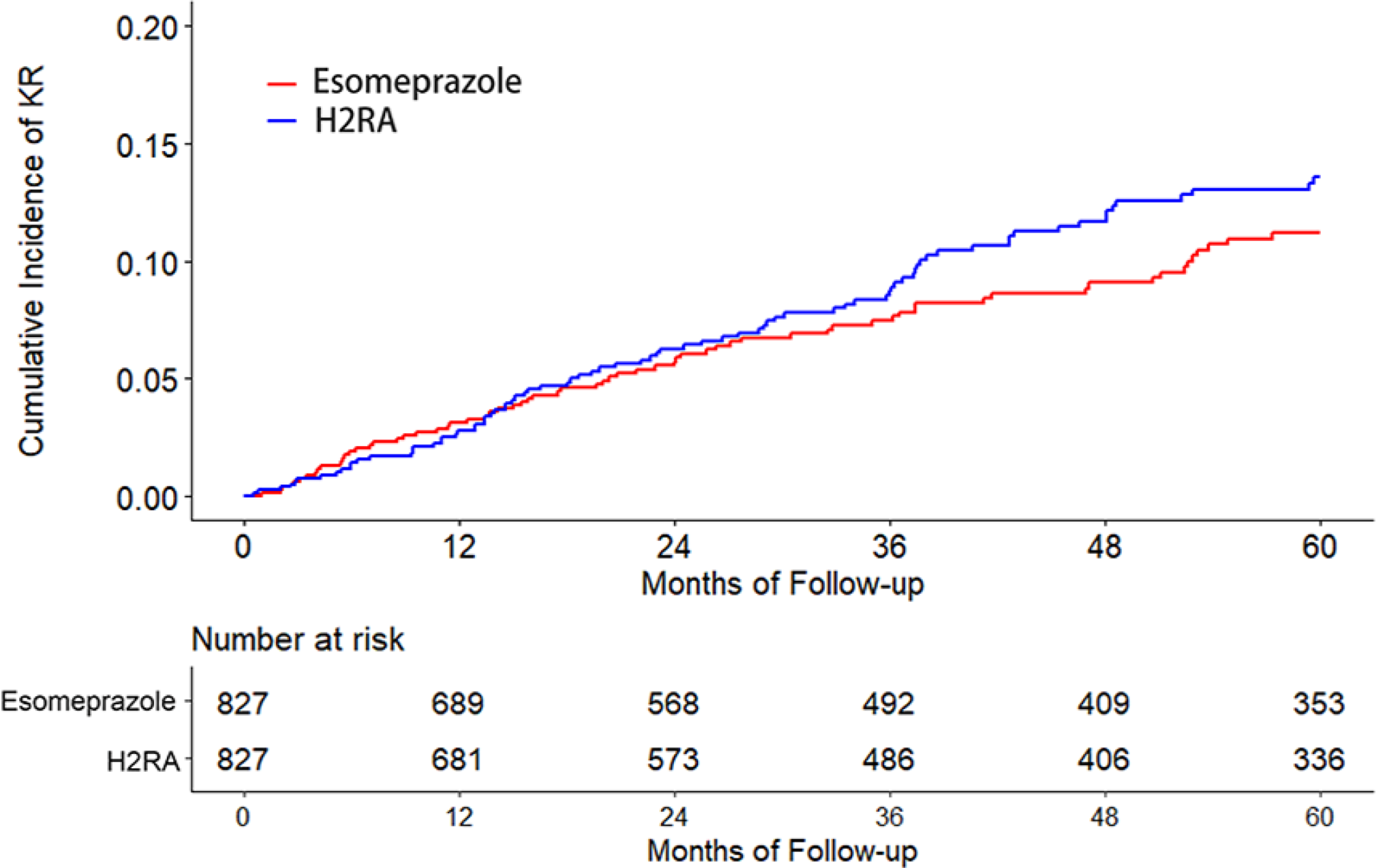
H2RA, Histamine-2 receptor antagonist.
Table 2.
Knee Replacement Surgery over Five Years Among Participants Initiating PPI (Omeprazole, Pantoprazole, Lansoprazole, Rabeprazole, or Esomeprazole) Compared with Initiation of H2RA
| Omeprazole vs. H2RA | Pantoprazole vs. H2RA | Lansoprazole vs. H2RA | Rabeprazole vs. H2RA | Esomeprazole vs. H2RA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Participant (n) | 2,672 | 2,672 | 664 | 664 | 3,747 | 3,747 | 751 | 751 | 827 | 827 |
| KR case (n) | 274 | 230 | 90 | 65 | 372 | 357 | 91 | 92 | 68 | 81 |
| Mean follow-up (year) | 3.33 | 3.39 | 3.40 | 3.42 | 3.14 | 3.26 | 3.80 | 3.80 | 3.31 | 3.28 |
| Rate (/1000 person-years) | 30.8 | 25.4 | 39.8 | 28.6 | 31.6 | 29.2 | 31.9 | 32.3 | 24.8 | 29.9 |
| 1.21 | 1.00 | 1.38 | 1.00 | 1.06 | 1.00 | 0.97 | 1.00 | 0.83 | 1.00 | |
| HR, (95% CI) # * | (1.01, 1.44) | (reference) | (1.00, 1.90) | (reference) | (0.92, 1.23) | (reference) | (0.73, 1.30) | (reference) | (0.60, 1.15) | (reference) |
| PS trimming, | 1.23 | 1.00 | 1.43 | 1.00 | 1.06 | 1.00 | 0.97 | 1.00 | 0.79 | 1.00 |
| HR, (95% CI) # * | (1.03, 1.48) | (reference) | (1.03, 1.98) | (reference) | (0.91, 1.23) | (reference) | (0.72, 1.30) | (reference) | (0.57, 1.11) | (reference) |
| Missing data imputation, | 1.18 | 1.00 | 1.38 | 1.00 | 1.15 | 1.00 | 1.09 | 1.00 | 0.87 | 1.00 |
| HR, (95% CI) # * | (1.01, 1.39) | (reference) | (1.03, 1.85) | (reference) | (0.98, 1.34) | (reference) | (0.77, 1.55) | (reference) | (0.63, 1.21) | (reference) |
n, number; KR, knee replacement; HR, Hazard Ratio; CI, confidence interval; PS, propensity score; PPI, proton pump inhibitor; H2RA, Histamine-2 receptor antagonist.
Results from propensity score matched cohort study (i.e., intent-to-treat approach).
Adjusting for competing risk of death.
The results from various sensitivity analyses are presented in Table 2. Exclusion of participants with the extreme propensity-score or missing data imputation did not change the association materially. Furthermore, the quantitative sensitivity analyses indicated that an unobserved confounder would have to be associated with both omeprazole or pantoprazole use and risk of KR by a HR of at least 1.64 above and beyond the measured confounders to explain away the association between omeprazole or pantoprazole use and risk of KR when compared with H2RA use, based on the assumption that the prevalence of this unmeasured confounder is 100% in the PPI groups.
When we compared the risk of KR among the initiators of different PPIs, the characteristics of each specific PPI cohort and its matched comparison cohort were well-balanced, with all standardized differences being < 0.1 (Supplemental Table 2)39. The HRs of KR for omeprazole and pantoprazole use were 1.57 (95% CI: 1.13 to 2.17) and 1.37 (95% CI: 1.03 to 1.81) compared with esomeprazole use, respectively. However, no significantly increased risk of KR was observed among lansoprazole (HR=1.20, 95% CI: 0.88 to 1.65) or rabeprazole (HR=1.24, 95% CI: 0.88 to 1.75) use 284 than esomeprazole use (Table 3).
Table 3.
Knee Replacement Surgery over Five Years Among Participants Initiating Omeprazole, Pantoprazole, Lansoprazole, or Rabeprazole Compared with Initiation of Esomeprazole
| Omeprazole vs. Esomeprazole | Pantoprazole vs. Esomeprazole | Lansoprazole vs. Esomeprazole | Rabeprazole vs. Esomeprazole | |||||
|---|---|---|---|---|---|---|---|---|
| Participant (n) | 699 | 699 | 1,006 | 1,006 | 862 | 862 | 657 | 657 |
| KR case (n) | 90 | 60 | 114 | 85 | 84 | 71 | 72 | 58 |
| Mean follow-up (year) | 3.58 | 3.39 | 3.45 | 3.49 | 3.44 | 3.55 | 3.98 | 3.98 |
| Rate (/1000 person-years) | 37.1 | 23.7 | 32.8 | 24.2 | 28.3 | 23.2 | 27.6 | 22.2 |
| HR, (95% CI) # * | 1.57 | 1.00 | 1.37 | 1.00 | 1.20 | 1.00 | 1.24 | 1.00 |
| (1.13, 2.17) | (reference) | (1.03, 1.81) | (reference) | (0.88, 1.65) | (reference) | (0.88, 1.75) | (reference) | |
n, number; KR, knee replacement; HR, Hazard Ratio; CI, confidence interval; PS, propensity score; PPI, proton pump inhibitor; H2RA, Histamine-2 receptor antagonist.
Results from propensity score matched cohort study (i.e., intent-to-treat approach).
Adjusting for competing risk of death.
DISCUSSION
Using data collected from THIN we found that the prescriptions of overall PPI increased dramatically whereas H2RA prescription remained low among participants with knee OA in the UK during the study period. This finding was in agreement with a previous report based on the data collected from Clinical Practice Research Datalink in the UK40. In addition, omeprazole was more commonly prescribed than lansoprazole, pantoprazole, rabeprazole, and esomeprazole. Our study showed that initiation of either omeprazole or pantoprazole was associated with an increased risk of KR when compared with initiation of either H2RA or esomeprazole.
Possible Explanations and Previous Studies
While the biological mechanisms of the current findings are not fully understood, magnesium may be the link between PPI use and the risk of KR. Animal studies have found that intra-articular magnesium sulfate attenuated the development of OA by reducing the chondrocyte apoptosis21. Serum magnesium levels were also inversely associated with prevalence of knee chondrocalcinosis41, 42, a strong predictor of OA43. In addition, both dietary and serum magnesium were inversely associated with serum C-reactive protein (a circulating inflammatory marker)44–46. Finally, magnesium is also an antagonist of N-methyl-D-aspartate receptors, which plays an important role in nociceptive transmission, modulation and sensitization of pain47. Indeed, results from meta-analyses demonstrated that systemic magnesium was effective in minimizing postoperative pain48. Thus, magnesium could be effective in OA by reducing chondrocyte apoptosis, attenuating calcification, inhibiting inflammation, and/or relieving pain.
A case-control study conducted in twins found that the odds ratio (OR) of knee radiographic OA decreased by 35% for each standard deviation increase of serum levels of magnesium (~0.08 mmol/L)22. Results from the Johnston County Osteoarthritis Project indicated subjects in the top four quintiles of dietary magnesium intake had lower risk of knee radiographic OA compared with those in the lowest quintile23. An inverse dose-response relationship between both dietary magnesium intake and serum magnesium levels with prevalent radiographic knee OA was also found among Chinese population24, 25. Recently, a prospective cohort study showed that lower dietary magnesium intake was associated with worse pain and function among subjects with radiographic knee OA26.
Several studies have shown that long-term use of PPIs reduces serum magnesium level, which is likely explained by disturbance of gastrointestinal handling of magnesium10, 13, 14, 49, 50. While hypomagnesemia seems to be a class-effect of PPIs, interestingly, severity appears to vary according to the type of PPIs18, 19. Studies reported that the risk of hypomagnesemia was higher for pantoprazole (OR=4.3), followed by omeprazole (OR=3.8) and lansoprazole (OR=1.7) than esomeprazole18, 19. Consistent with these findings18, 19, our study demonstrated that omeprazole or pantoprazole initiators had significantly higher risk of KR than esomeprazole users. In addition, we also observed a lower risk of KR among esomeprazole initiators than that among H2RA initiators. As previous studies reported that H2RA use was associated with an increased risk of hypomagnesemia compared with nonuse15–17, future studies are warranted to confirm whether our findings could be explained by the less impact of esomeprazole on lowering magnesium level than of H2RA.
Strengths and Limitations
Several strengths of our study are noteworthy. First, in adults, dietary intake or serum magnesium levels are likely to be present long before the occurrence of OA. As a result, observational studies of dietary intake or serum magnesium assessed at a single time point in relation to the risk of OA progression among subjects with OA (i.e., a potentially intermediate variable) are susceptible to selection bias (i.e., event bias or collider bias)51, 52. To mitigate this bias, we examined initiation of various PPIs after subjects have already had a diagnosis of knee OA whereby magnesium levels may be altered by the new use of these agents, thus avoiding potential selection bias due to conditioning on an intermediate variable. 345 Second, observational studies of comparing risk of KR between magnesium supplement users with non-users (or PPI users versus non-users) are susceptible to confounding by indication bias. Instead, we assembled several comparative cohorts who initiated different types of PPIs or H2RA; thus, these subjects had the similar indication for different comparative cohorts. Third, the results from various sensitivity analyses were consistent, supporting the robustness of our study findings.
Potential limitations of our study also deserve comment. First, we postulated that the observed association may be due to PPIs’ impact on the levels of serum magnesium; however, we can’t verify this mechanism owing to lack of serum magnesium data from THIN. Second, while we used several approaches to control for potential confounding bias, as in any observational study we can’t rule out residual confounding. Third, knee images were not available in THIN; thus, we were unable to examine the association between PPIs and the risk of progression of structural lesions of knee OA. However, KR has been generally accepted as a “hard” outcome in cohort studies of knee OA30, 53–55. Fourth, because use of over-the-counter PPIs or H2RA was not recorded in THIN, the exposure assessment is susceptible to misclassification bias. Such bias, if occurred, is likely to be non-differential and would dilute the observed association towards the null. In addition, since the National Health Service England provides healthcare with most services free, it is unlikely that most patients would purchase these drugs over-the-counter without a prescription, especially these medications are often used for a long period of time among elder patients with OA who are at high-risk for the development of gastrointestinal events. Last, THIN does not include data on adherence to these medications; thus, misclassification of the medication use could occur and bias the study findings.
Clinical Implications
Our findings, if confirmed by others, could have important clinical implications. PPIs were widely used among patients with knee OA (44.0% in THIN database in 2017). Of them, omeprazole was the most commonly prescribed PPIs, whereas esomeprazole only accounted for less than 1.6% of PPI prescriptions. Compared with either H2RA or esomeprazole, the risk of KR for omeprazole was higher. Although previous studies have shown that KR greatly improves the symptoms and physical function among the majority of patients with end-stage symptomatic knee OA, the surgical procedure itself is neither inexpensive nor risk-free56. Thus, reducing the number of KRs could greatly decrease the burden of OA on society. Since there is no apparent difference with respect to the safety profiles57 and cost between esomeprazole and other PPIs, esomeprazole appears to be a better choice than other PPIs for reducing gastrointestinal complications from NSAIDs while minimizing the risk of KR among patients with knee OA.
Conclusion
In this population-based cohort study, initiation of omeprazole or pantoprazole use was associated with a higher risk of KR than initiation of H2RA use. This study raises concern regarding an unexpected risk of omeprazole and pantoprazole on accelerating OA progression. Of available PPIs, esomeprazole has a lower prescription pattern, but appears to be a better option for minimizing the risk of KR among patients with knee OA.
Supplementary Material
Acknowledgements:
Everyone who contributed significantly to the work has been listed.
Funding: This work was supported by the National Institutes of Health (P60 AR047785), National Natural Science Foundation of China (81772413, 81702207, 81702206), and National Institutes of Health (K24 AR070892).
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Footnotes
Ethics Approval: Institutional Review Board approved this study, with waiver of informed consent.
Patient Consent: Not required.
Scientific approval: This study was approved by the THIN Scientific Review Committee (18THIN073).
Statement: THIN is a registered trademark of Cegedim SA in the United Kingdom and other countries. Reference made to the THIN database is intended to be descriptive of the data asset licensed by IQVIA. This work uses de-identified data provided by patients as a part of their routine primary care.
Disclaimer: The interpretation of these data is the sole responsibility of the authors.
Transparency
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Conflict of Interest Statement: No conflict of interest for any of the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Glyn-Jones S, Palmer AJR, Agricola R, Price AJ, Vincent TL, Weinans H, et al. Osteoarthritis. The Lancet. 2015;386:376–87. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Niu J. Editorial: Shifting Gears in Osteoarthritis Research Toward Symptomatic Osteoarthritis. Arthritis Rheumatol. 2016;68:1797–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA. 2006;296:1633–44. [DOI] [PubMed] [Google Scholar]
- 4.Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991;115:787–96. [DOI] [PubMed] [Google Scholar]
- 5.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care & Research. 2012;64:465–74. [DOI] [PubMed] [Google Scholar]
- 6.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:363–88. [DOI] [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence. Osteoarthritis: care and management, 2014.
- 8.Latimer N, Lord J, Grant RL, O’Mahony R, Dickson J, Conaghan PG, et al. Cost effectiveness of COX 2 selective inhibitors and traditional NSAIDs alone or in combination with a proton pump inhibitor for people with osteoarthritis. BMJ. 2009;339:b2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz JN, Smith SR, Collins JE, Solomon DH, Jordan JM, Hunter DJ, et al. Cost-effectiveness of nonsteroidal anti-inflammatory drugs and opioids in the treatment of knee osteoarthritis in older patients with multiple comorbidities. Osteoarthritis Cartilage. 2016;24:409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedberg DE, Kim LS, Yang YX. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology. 2017;152:706–15. [DOI] [PubMed] [Google Scholar]
- 11.Vaezi MF, Yang YX, Howden CW. Complications of Proton Pump Inhibitor Therapy. Gastroenterology. 2017;153:35–48. [DOI] [PubMed] [Google Scholar]
- 12.Yang YX, Metz DC. Safety of proton pump inhibitor exposure. Gastroenterology. 2010;139:1115–27. [DOI] [PubMed] [Google Scholar]
- 13.Nehra AK, Alexander JA, Loftus CG, Nehra V. Proton Pump Inhibitors: Review of Emerging Concerns. Mayo Clin Proc. 2018;93:240–46. [DOI] [PubMed] [Google Scholar]
- 14.Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, Srivali N, Edmonds PJ, Ungprasert P, et al. Proton pump inhibitors linked to hypomagnesemia: a systematic review and meta-analysis of observational studies. Ren Fail. 2015;37:1237–41. [DOI] [PubMed] [Google Scholar]
- 15.Kieboom BC, Kiefte-de Jong JC, Eijgelsheim M, Franco OH, Kuipers EJ, Hofman A, et al. Proton pump inhibitors and hypomagnesemia in the general population: a population-based cohort study. Am J Kidney Dis. 2015;66:775–82. [DOI] [PubMed] [Google Scholar]
- 16.Markovits N, Loebstein R, Halkin H, Bialik M, Landes-Westerman J, Lomnicky J, et al. The association of proton pump inhibitors and hypomagnesemia in the community setting. J Clin Pharmacol. 2014;54:889–95. [DOI] [PubMed] [Google Scholar]
- 17.Nakashima A, Ohkido I, Yokoyama K, Mafune A, Urashima M, Yokoo T. Proton Pump Inhibitor Use and Magnesium Concentrations in Hemodialysis Patients: A Cross-Sectional Study. PLoS One. 2015;10:e0143656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luk CP, Parsons R, Lee YP, Hughes JD. Response to “Proton pump inhibitor-associated hypomagnesemia: what do FDA data tell us?”. Ann Pharmacother. 2014;48:432. [DOI] [PubMed] [Google Scholar]
- 19.Tamura T, Sakaeda T, Kadoyama K, Okuno Y. Omeprazole- and esomeprazole-associated hypomagnesaemia: data mining of the public version of the FDA Adverse Event Reporting System. Int J Med Sci. 2012;9:322–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruber HE, Ingram J, Norton HJ, Wei LY, Frausto A, Mills BG, et al. Alterations in growth plate and articular cartilage morphology are associated with reduced SOX9 localization in the magnesium-deficient rat. Biotech Histochem. 2004;79:45–52. [DOI] [PubMed] [Google Scholar]
- 21.Lee CH, Wen ZH, Chang YC, Huang SY, Tang CC, Chen WF, et al. Intra-articular magnesium sulfate (MgSO4) reduces experimental osteoarthritis and nociception: association with attenuation of N-methyl-D-aspartate (NMDA) receptor subunit 1 phosphorylation and apoptosis in rat chondrocytes. Osteoarthritis Cartilage. 2009;17:1485–93. [DOI] [PubMed] [Google Scholar]
- 22.Hunter DJ, Hart D, Snieder H, Bettica P, Swaminathan R, Spector TD. Evidence of altered bone turnover, vitamin D and calcium regulation with knee osteoarthritis in female twins. Rheumatology (Oxford). 2003;42:1311–16. [DOI] [PubMed] [Google Scholar]
- 23.Qin B, Shi X, Samai PS, Renner JB, Jordan JM, He K. Association of dietary magnesium intake with radiographic knee osteoarthritis: results from a population-based study. Arthritis Care Res (Hoboken). 2012;64:1306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng C, Wei J, Li H, Yang T, Zhang FJ, Pan D, et al. Relationship between Serum Magnesium Concentration and Radiographic Knee Osteoarthritis. J Rheumatol. 2015;42:1231–36. [DOI] [PubMed] [Google Scholar]
- 25.Zeng C, Li H, Wei J, Yang T, Deng ZH, Yang Y, et al. Association between Dietary Magnesium Intake and Radiographic Knee Osteoarthritis. PLoS One. 2015;10:e0127666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shmagel A, Onizuka N, Langsetmo L, Vo T, Foley R, Ensrud K, et al. Low magnesium intake is associated with increased knee pain in subjects with radiographic knee osteoarthritis: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2018;26:651–58. [DOI] [PubMed] [Google Scholar]
- 27.Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2007;16:393–401. [DOI] [PubMed] [Google Scholar]
- 28.Stuart-Buttle CD, Read JD, Sanderson HF, Sutton YM. A language of health in action: Read Codes, classifications and groupings. Proc AMIA Annu Fall Symp. 199675–79. [PMC free article] [PubMed]
- 29.Epic. THIN data from EPIC: a guide for researchers. London: EPIC; 2007. [Google Scholar]
- 30.Neogi T, Li S, Peloquin C, Misra D, Zhang Y. Effect of bisphosphonates on knee replacement surgery. Ann Rheum Dis. 2018;77:92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Misra D, Lu N, Felson D, Choi HK, Seeger J, Einhorn T, et al. Does knee replacement surgery for osteoarthritis improve survival? The jury is still out. Ann Rheum Dis. 2017;76:140–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu N, Misra D, Neogi T, Choi HK, Zhang Y. Total joint arthroplasty and the risk of myocardial infarction: a general population, propensity score-matched cohort study. Arthritis Rheumatol. 2015;67:2771–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris R, Carstairs V. Which deprivation? A comparison of selected deprivation indexes. J Public Health Med. 1991. Nov;13:318–26. [PubMed] [Google Scholar]
- 34.Seeger JD, Williams PL, Walker AM. An application of propensity score matching using claims data. Pharmacoepidemiol Drug Saf. 2005;14:465–76. [DOI] [PubMed] [Google Scholar]
- 35.Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation. 2016;133:601–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of Martingale based residuals. Biometrika. 1993;80:557–72. [Google Scholar]
- 37.Rubin DB. Multiple imputation for nonresponse in surveys. New York: John Wiley & Sons 1987. [Google Scholar]
- 38.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann Intern Med. 2017;167:268–74. [DOI] [PubMed] [Google Scholar]
- 39.Franklin JM, Rassen JA, Ackermann D, Bartels DB, Schneeweiss S. Metrics for covariate balance in cohort studies of causal effects. Stat Med. 2014;33:1685–99. [DOI] [PubMed] [Google Scholar]
- 40.Abrahami D, McDonald EG, Schnitzer M, Azoulay L. Trends in acid suppressant drug prescriptions in primary care in the UK: a population-based cross-sectional study. BMJ Open. 2020;10:e041529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richette P, Ayoub G, Lahalle S, Vicaut E, Badran AM, Joly F, et al. Hypomagnesemia associated with chondrocalcinosis: a cross-sectional study. Arthritis Rheum. 2007;57:1496–501. [DOI] [PubMed] [Google Scholar]
- 42.Zeng C, Wei J, Terkeltaub R, Yang T, Choi HK, Wang YL, et al. Dose-response relationship between lower serum magnesium level and higher prevalence of knee chondrocalcinosis. Arthritis Res Ther. 2017;19:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang W, Doherty M, Bardin T, Barskova V, Guerne PA, Jansen TL, et al. European League Against Rheumatism recommendations for calcium pyrophosphate deposition. Part I: terminology and diagnosis. Ann Rheum Dis. 2011;70:563–70. [DOI] [PubMed] [Google Scholar]
- 44.Song Y, Ridker PM, Manson JE, Cook NR, Buring JE, Liu S. Magnesium intake, C-reactive protein, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care. 2005;28:1438–44. [DOI] [PubMed] [Google Scholar]
- 45.Almoznino-Sarafian D, Berman S, Mor A, Shteinshnaider M, Gorelik O, Tzur I, et al. Magnesium and C-reactive protein in heart failure: an anti-inflammatory effect of magnesium administration? Eur J Nutr. 2007;46:230–7. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez-Moran M, Guerrero-Romero F. Serum magnesium and C-reactive protein levels. Arch Dis Child. 2008;93:676–80. [DOI] [PubMed] [Google Scholar]
- 47.Herroeder S, Schonherr ME, De Hert SG, Hollmann MW. Magnesium--essentials for anesthesiologists. Anesthesiology. 2011;114:971–93. [DOI] [PubMed] [Google Scholar]
- 48.De Oliveira GS Jr., Castro-Alves LJ, Khan JH, McCarthy RJ. Perioperative systemic magnesium to minimize postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2013;119:178–90. [DOI] [PubMed] [Google Scholar]
- 49.Danziger J, William JH, Scott DJ, Lee J, Lehman LW, Mark RG, et al. Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int. 2013;83:692–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zipursky J, Macdonald EM, Hollands S, Gomes T, Mamdani MM, Paterson JM, et al. Proton pump inhibitors and hospitalization with hypomagnesemia: a population-based case-control study. PLoS Med. 2014;11:e1001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Niu J, Felson DT, Choi HK, Nevitt M, Neogi T. Methodologic challenges in studying risk factors for progression of knee osteoarthritis. Arthritis Care Res (Hoboken). 2010;62:1527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Neogi T, Hunter D, Roemer F, Niu J. What Effect Is Really Being Measured? An Alternative Explanation of Paradoxical Phenomena in Studies of Osteoarthritis Progression. Arthritis Care & Research. 2014;66:658–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu D, Jordan KP, Snell KIE, Riley RD, Bedson J, Edwards JJ, et al. Development and validation of prediction models to estimate risk of primary total hip and knee replacements using data from the UK: two prospective open cohorts using the UK Clinical Practice Research Datalink. Ann Rheum Dis. 2019;78:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khan T, Alvand A, Prieto-Alhambra D, Culliford DJ, Judge A, Jackson WF, et al. ACL and meniscal injuries increase the risk of primary total knee replacement for osteoarthritis: a matched case-control study using the Clinical Practice Research Datalink (CPRD). Br J Sports Med. 2018. [DOI] [PubMed]
- 55.Nielen JT, de Vries F, Dagnelie PC, van den Bemt BJ, Emans PJ, Lalmohamed A, et al. Use of thiazolidinediones and the risk of elective hip or knee replacement: a population based case-control study. Br J Clin Pharmacol. 2016;81:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skou ST, Roos EM, Laursen MB, Rathleff MS, Arendt-Nielsen L, Simonsen O, et al. A Randomized, Controlled Trial of Total Knee Replacement. N Engl J Med. 2015;373:1597–606. [DOI] [PubMed] [Google Scholar]
- 57.Miner P, Katz PO, Chen YS, Sostek M. Gastric acid control with esomeprazole, lansoprazole, omeprazole, pantoprazole, and rabeprazole: a five-way crossover study. Am J Gastroenterol. 2003;98:2616–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


