To the Editor –
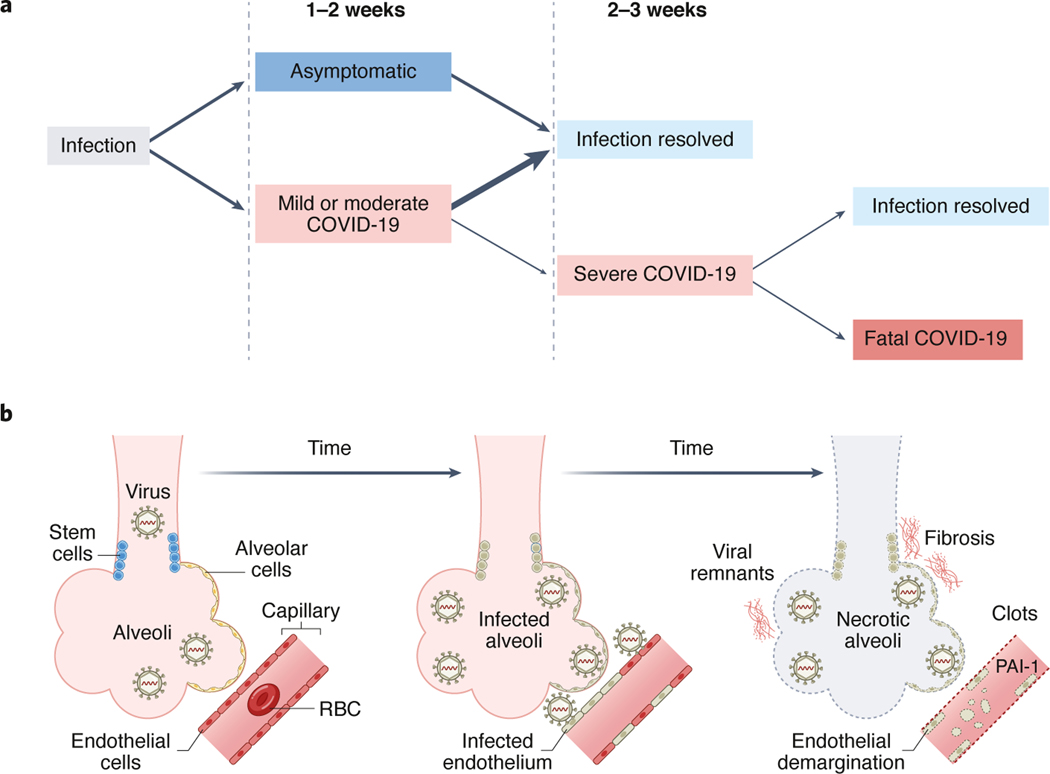
Since SARS-CoV-2 began spreading in late 2019, a total of 270 million cases and 5.3 million deaths have been reported, with the case-fatality rate of COVID-19 remaining steady at about 1–2% worldwide. Following infection, most people with COVID-19 experience asymptomatic, mild, or moderate forms of illness, and recovery without apparent complications. Some people however, roughly two to three weeks after infection, develop severe forms of COVID-19, with progressive respiratory failure, multisystem damage, and high morbidity and mortality (Figure 1A).
Figure 1. Time course of SARS-CoV-2 infection.
Typical time course from initial SARS-COV-2 infection to severe COVID-19 (A) and schematic (B) depicting the time course of SARS-CoV-2 infection within lung acinar units. End-stage pulmonary disease is associated with dead alveolar cells, dead epithelial stem cells, demarginated endothelial cells and neutrophils expressing increased PAI-1, widespread clotting, and severe pulmonary fibrosis.
Numerous studies that purport so-called cytokine storm and immune dysregulation have sustained the rationale for treating severe forms of COVID-19 with various immune system modulators (e.g., corticosteroids, anti-interleukin monoclonals, and anti-cytokine pathway inhibitors) and assorted anticoagulants. Some of these therapeutic approaches have been shown to improve clinical outcome, but others do not, and additional therapeutics based on an understanding of COVID-19 pathogenesis are still critically needed.
Two years into the pandemic, studies of the underlying mechanisms of COVID-19 pathogenesis remain surprisingly scarce. To understand the mechanism by which SARS-CoV-2 causes severe disease, the medical research community must prioritize direct tissue studies from autopsy. Several recent pathology studies have shed some light on the complex mechanisms of severe COVID-19 but there remain multiple current unknowns, which pose important questions to motivate further work.
Lung tissues from patients with fatal COVID-19 have revealed pathology cascades that include progressive diffuse alveolar damage, alveolar-capillary barrier damage, surfactant production loss, and increased vascular permeability. Such findings typify those of severe acute respiratory distress syndrome (ARDS). However, based on the detection of viral protein and RNA remnants, the same lung tissues also reveal novel pathology cascades that are distinct from pandemic influenza and SARS-CoV, and which arise from direct SARS-CoV-2 infection of respiratory epithelial and basal cells, alveolar type 1 and 2 cells, and capillary endothelial cells. Infection triggers pathways involved in epithelial and endothelial cell senescence, impaired epithelial repair, increased procoagulants, e.g., plasminogen activator inhibitor-1 (PAI-1 or SERPINE1) in endothelial cells and neutrophils, coagulopathies from inhibition of fibrinolysis, and pulmonary fibrosis (Figure 1B). The associated alveolitis and endotheliopathy culminates in dead lung acinar units that cannot replenish cells and thereby recover.1
In another study, lung tissues from 3 patients with severe COVID-19 (who required and recovered after lung transplantation) have revealed similar pathology cascades, including the clearance of SARS-CoV-2 infection within lungs and concomitant progression to irreversible pulmonary fibrotic disease. This study also showed that lung macrophages and fibroblasts expressed growth factors that drive self-sustaining cycles of cellular proliferation and fibrin deposition that resemble those of idiopathic pulmonary fibrosis.2 Brain tissues from a series of patients with SARS-CoV-2 infection revealed that microvascular cell damage is due to direct viral infection of brain endothelial cells. As demonstrated by a combination of human autopsy and animal-model studies, the main protease of SARS-CoV-2, Mpro, cleaves and ablates NEMO, an essential modulator of nuclear factor-κB, which, in turn, causes the death of human brain endothelial cells. The resulting central nervous system microvascular endotheliopathy damages the blood-brain barrier and may explain the neurological and psychological complications of COVID-19, including anosmia, epileptic seizures, encephalopathies and strokes.3
Studies using blood sampling are also providing clues that can be linked to pathologic findings. Plasma markers of endothelial cell injury and platelet activation were significantly increased in patients with severe COVID-19 as compared to less-severely ill COVID-19 patients. Increases of PAI1 and von Willebrand Factor (VWF) were further associated with increases of soluble P-selectin and soluble CD40 ligand, which supports an endotheliopathy, as observed in the above pathology studies. In addition, increased concentrations of VWF and soluble thrombomodulin suggest that such markers may be used to assess the severity of endothelial damage and predict morbidity and mortality.4 Yet clinical trials that compared therapeutic anticoagulation to standard prophylactic therapies have not demonstrated benefits.5 Conversely, more aggressive forms of anticoagulation have been associated with more adverse bleeding events in hospitalized patients, which calls for a better understanding of the underlying mechanisms of COVID-19 coagulopathies.6
Several multiomics reports, based on serial blood samples from COVID-19 patients, stratified disease outcomes with a broad array of metabolic and immunologic markers, including plasma protein and metabolite levels, secreted and cell surface protein levels, white blood cell transcriptomes, receptor gene sequences, and single-cell RNA sequencing. This identified metabolic and immunologic profiles that shift with increasing severity of COVID-19.7,8 Two other recent blood sampling studies using proteomics and single cell sequencing emphasized the hypercytokinemia in COVID-19 and the critical role of monocyte and neutrophil activation in severe COVID-19, as confirmed by autopsy studies.9,10 Collectively, these data suggest a myeloid-associated immunopathology as a key component of disease severity.
The studies highlighted above employ molecular pathology, clinical trial, and systems biology approaches to provide various snapshots of lung, brain, vascular, and immune damage. Although they suggest a time course of dysregulation, dysfunction, and destruction by progressively severe COVID-19, they further invite important and intriguing questions (Table 1). Fundamentally, why some people progress to severe forms of COVID-19 after a two-to-three-week period remains unclear, as is the underlying role of known risk factors such as obesity, diabetes, and age. Such questions can only be answered with autopsy studies.
Table 1.
Outstanding questions for pathology studies of COVID-19
| 1. Can biomarkers be identified and used to predict progression to severe COVID-19? |
| 2. Are COVID-19 coagulopathies due to pre-existing systemic endotheliopathies or a cause of them? |
| 3. Are there thresholds that pertain to viral loads, viral byproducts and/or respiratory epithelial tissue damage beyond which severe or fatal disease progresses? |
| 4. What therapies might be identified to preempt or blunt damage from COVID-19-related damage cascades initiating downstream severe or fatal endotheliopathies and coagulopathies? |
| 5. After apparent recovery from COVID-19, will recovered individuals show an increased risk of strokes, pulmonary, renal, and cardiac failures in the following years? |
Clinician-scientists and their colleagues must deal with many challenges, many of which have been amplified during the pandemic. For airborne infectious diseases such as SARS-CoV-2, we believe the hesitation to perform autopsies and tissue retrievals presents one of the foremost challenges. Such undertakings require planning, consent, and appropriate biosafety precautions in place that few pathology departments at hospitals are equipped to accomplish. Yet without tissue-based studies, it will be all the more challenging to address the questions posed in Table 1. The lack of well-developed animal models for experimental pathogenesis studies further supports the need for additional autopsy studies in humans.
Vaccines remain the best option for controlling the COVID-19 pandemic, but variants of concern such as Omicron continue to emerge that will likely decrease vaccine efficacy. COVID-19 has both acute- and post-acute manifestations (sometimes called long COVID); given the unknown outcomes of endotheliopathies and coagulopathies in COVID-19, we may face “time bomb” manifestations of COVID-19 where unforeseen clinical events such as increased incidence of cerebrovascular, myocardial, and kidney disease appear months after recovering from initial infection. Better, practical diagnostics and treatments for COVID-19 are desperately needed, and tissue-based pathology studies are vital to provide direct clues of how to understand the pathogenesis of the virus, and how to mitigate its impact.
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, NIH.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.D’Agnillo F, et al. Lung epithelial and endothelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in fatal COVID-19. Sci Transl Med 13, eabj7790 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharat A, et al. Lung transplantation for patients with severe COVID-19. Sci Transl Med 12(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wenzel J, et al. The SARS-CoV-2 main protease M(pro) causes microvascular brain pathology by cleaving NEMO in brain endothelial cells. Nat Neurosci 24, 1522–1533 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goshua G, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol 7, e575–e582 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopes RD, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet 397, 2253–2263 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spyropoulos AC & Bonaca MP Studying the coagulopathy of COVID-19. Lancet (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su Y, et al. Multi-Omics Resolves a Sharp Disease-State Shift between Mild and Moderate COVID-19. Cell 183, 1479–1495 e1420 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woo MS, et al. Multi-dimensional and longitudinal systems profiling reveals predictive pattern of severe COVID-19. iScience 24, 102752 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinha S, et al. Dexamethasone modulates immature neutrophils and interferon programming in severe COVID-19. Nat Med (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanderbeke L, et al. Monocyte-driven atypical cytokine storm and aberrant neutrophil activation as key mediators of COVID-19 disease severity. Nat Commun 12, 4117 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]