Abstract
We estimated joint associations between having history of alcohol use disorder (AUD) (based on prior ICD-9/ICD-10 codes) and recent self-reported alcohol use and viral non-suppression (≥1 viral load measurement >20 copies/mL in the same calendar year as alcohol consumption was reported) among patients on ART enrolled in routine care, 2014–2018, in an urban specialty clinic. Among 1690 patients, 26% had an AUD, 21% reported high-risk alcohol use, and 39% had viral non-suppression. Relative to person-years in which people without AUD reported not drinking, prevalence of viral non-suppression was higher in person-years when people with AUD reported drinking at any level; prevalence of viral non-suppression was not significantly higher in person-years when people with AUD reported not drinking or person-years when people without AUD reported drinking at any level. No level of alcohol use may be “safe” for people with a prior AUD with regard to maintaining viral suppression.
Keywords: Alcohol, Alcohol Abuse, Alcohol Dependence, Alcohol Use Disorder (AUD), measurement error, viral suppression
Maintaining durable viral suppression is critically important for people with HIV to achieve optimal individual health outcomes and to prevent transmission (1–3). Yet consistently, across calendar time and time since antiretroviral therapy (ART) initiation, some people with HIV prescribed ART fail to maintain viral suppression (4–6). Heavy alcohol use is a highly prevalent exposure among persons living with HIV (7) and it is associated with poor outcomes, in particular, poor engagement in HIV care and viral non-suppression (8–10). However, the literature has been inconsistent with respect to the association between alcohol use and viral suppression. While some studies have found persons with heavy alcohol use to be more likely to have an unsuppressed viral load or worse ART adherence than persons who report no alcohol use (10–15) other studies have not found differences in viral suppression between the two groups (16–19). Understanding these discrepant results is imperative for managing alcohol use in people with HIV and increasing the prevalence of durable viral suppression.
The mixed results on whether alcohol use is associated with viral suppression could be explained in several ways, but one explanation is that persons who report not drinking and persons who report heavy alcohol use are heterogeneous groups. Persons who report abstaining from alcohol represent a mix of persons who never drank alcohol, persons who are abstaining from alcohol for a period due to personal choices, and persons who are abstaining from alcohol due to health reasons (i.e., “sick quitters”) including having an alcohol use disorder (AUD). Persons who report drinking alcohol at high-risk levels represent a mix of persons who do and do not have an AUD, and for whom the impacts of alcohol use may be quite different. An AUD is distinguished from high-risk drinking by the presence of symptoms such as (but not limited to) interference of drinking with relationships or responsibilities, an inability to stop or cut back on drinking despite negative consequences, or physical responses such as having to drink more to get the same effect, craving, or experiencing symptoms of alcohol withdrawal. In one, small study, symptoms of substance use dependence, but not specific substance use was associated with lack of viral suppression (20).
Studies of the influence of alcohol in people living with HIV would benefit from considering the intersection of AUD and alcohol use. The purpose of this study was to describe the joint associations between AUD and alcohol use and viral non-suppression in a sample of persons living with HIV who are engaged in care during the modern ART era.
Methods
Conceptual framework (Figure 1):
Figure 1.
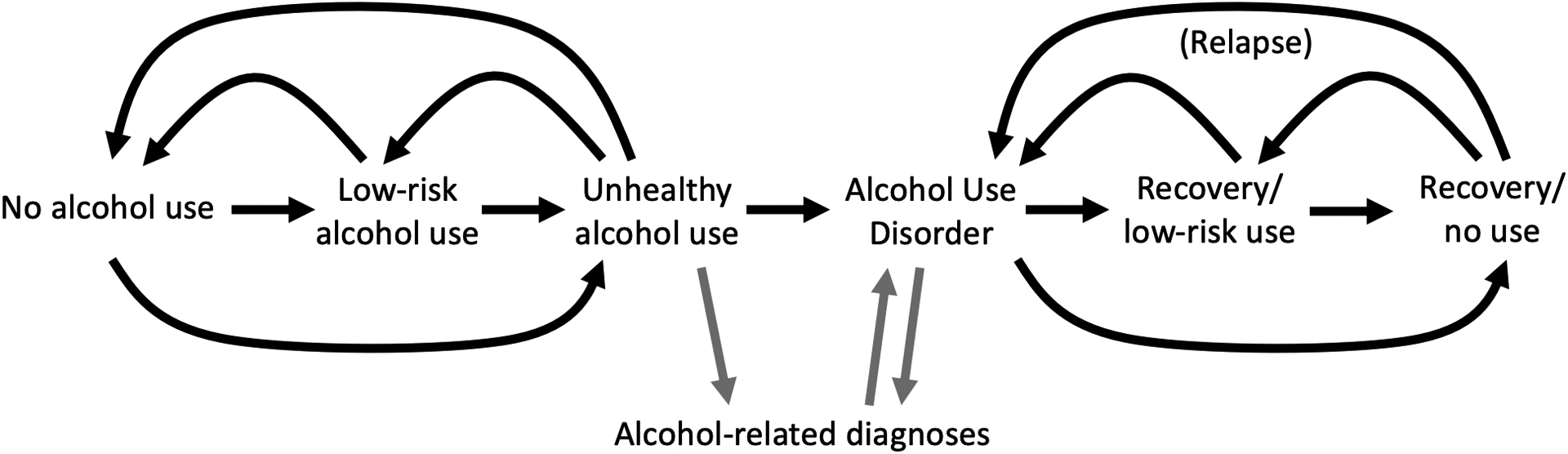
Conceptual model describing possible flow of individuals’ lifetime alcohol use and alcohol use disorder
Alcohol use and the development of AUD evolve together over a lifetime. Everyone starts out free of an AUD, most people initiate alcohol use at some point, and a subset of those who start drinking any alcohol will progress to high-risk use. A subset of persons with high-risk alcohol use will develop an AUD. Individuals with a history of AUD may continue engaging in high-risk patterns of alcohol use or may reduce or cease use of alcohol. Only a subset of individuals with an AUD will receive a clinical diagnosis of an AUD. Both the AUD itself and its clinical recognition might influence alcohol use and engagement in care (e.g., by influencing patients’ motivation to engage or by influencing the supports given patients by their physicians and other health care providers). A clinical diagnosis of an AUD in the medical record is not perfectly sensitive nor specific for an AUD based on the American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV or DSM-5 criteria. We hypothesized that individuals with a history of AUD (whether or not it was clinically diagnosed) would be more sensitive to the effects of alcohol consumption on viral non-suppression because consumption would be more likely be accompanied by symptoms that might disrupt engagement in HIV care and ART adherence. While we hypothesized that, among people who reported no recent alcohol consumption, individuals with current AUD symptoms might have a higher probability of viral non-suppression than individuals with no current AUD symptoms, we did not anticipate this would be a strong association by virtue of restricting our comparison to periods in which alcohol was not being consumed. Similarly, we hypothesized that the association between high-risk alcohol consumption and viral non-suppression would be weaker in people without an AUD and stronger in people with an AUD.
Study sample:
This study was conducted in patients enrolled in the John G. Bartlett Specialty Practice. The clinic is an infectious disease clinic serving patients in East Baltimore and the surrounding area; nutrition, pharmacy, phlebotomy, mental health, and social work services are all provided on-site. The Johns Hopkins HIV Clinical Cohort (JHHCC) includes all patients who enroll in continuity care (i.e., excludes consultations) in the John G. Bartlett Specialty Practice and who consent to share their data (>90% of patients). Full details about the cohort are available elsewhere (21). Briefly, demographics, laboratory values, diagnoses, and prescribed medications are abstracted from electronic medical record data. Additionally, a subset of patients completes structured interviews on a number of Patient Reported Outcomes (PROs), approximately every six months. PRO surveys are computer-assisted self-interviews administered on tablet computers (with assistance available for patients who have literacy challenges) in conjunction with attendance at a clinic visit.
For this study, we included all persons enrolled in the JHHCC who had initiated ART, who completed ≥1 PRO between 1 January 2014 and 31 December 2018, and who had a viral load measured in the same calendar year. For each person, we considered only the last year in which these criteria were met during the study period (i.e., we only considered one record per person).
Exposures:
Our exposure was the joint combination of “lifetime” AUD and recent alcohol consumption. We were unable to directly assess current symptoms of AUD, so we started by classifying observations according individuals’ history of a clinical diagnosis consistent with AUD, based on ICD codes (ICD-9-CM of 305.0 or 303/ICD-10-CM of F10.1 or F10.2). We discuss measurement error for AUD based on clinical diagnoses in more detail below. Here, “history” includes any diagnoses recorded between enrollment into the cohort and December 31 of the year prior to the analysis year. We used historical diagnoses to establish temporality between the AUD clinical diagnosis and alcohol use and lack of durable viral suppression, the outcome. Because there is no expiration date on prior diagnoses on which we might assume symptoms have resolved, and because competing health problems may preclude recording of additional or updated diagnoses of alcohol abuse or dependence even in the presence of ongoing symptoms, history of a clinical diagnosis consistent with AUD may or may not imply the presence of symptoms regardless of how proximal in time it was to the date of analysis. By using history of AUD rather than current symptoms, we are operating under the assumption that persons who ever experienced adverse consequences due to drinking may be differently vulnerable to any subsequent (recent) alcohol consumption; this is akin to considering lifetime AUD diagnoses rather than current AUD diagnoses.
Recent alcohol consumption was ascertained from a modified version of the US Alcohol Use Disorders Identification Test consumption questions (USAUDIT-C) (22), which is routinely completed as part of the PROs. Patients were asked about frequency and quantity of alcohol typically consumed over the last year and about instances of binge drinking. The response options for quantity and frequency questions were modified to allow for more granular collection of data (e.g., response options of “5” or “6” drinks typically consumed per occasion, rather than “5–6” drinks). From self-reported quantity and frequency of alcohol use, we calculated average drinks per week. Binge drinking was defined as ≥4 drinks on one occasion for women or ≥5 drinks on one occasion for men. We defined high-risk alcohol use in accordance with the National Institute of Alcohol Abuse and Alcoholism (NIAAA) as reporting ≥7 drinks/week for women or ≥14 drinks/week for men or reporting any instances of binge drinking. Moderate use was defined as reporting any alcohol use below the threshold for high-risk use. For each person included in the analysis, we assigned them a value for alcohol consumption consistent with the highest level of consumption reported during that year. The majority of patients in this analysis (64%) completed only one PRO and thus only reported one level of alcohol consumption. A third (33%) of patients completed two PROs and 3% of patients completed three PROs in their analysis year.
Our final exposure categories were based on combinations of having a history of AUD or not at the very beginning of the analysis year, and level of alcohol consumption reported during the analysis year (if the only PRO during the analysis year was early in the year, we are by necessity extrapolating reported consumption forward for the remainder of the year). Our referent exposure group was persons with no AUD who reported not drinking on their most recent PRO. Other exposure categories included: no AUD and moderate alcohol use; no AUD and high-risk alcohol use; AUD but no current alcohol use; AUD and moderate alcohol use; and AUD and high-risk alcohol use.
Covariates:
We adjusted analyses for variables assumed to be associated both with receiving a clinical diagnosis of AUD (developing an AUD and having it diagnosed) and alcohol consumption and with probability of viral suppression. We adjusted for sex at birth, Black race, age, HIV acquisition risk factors including being a male who has sex with men (MSM) and history of injection drug use (IDU), years in care in the Johns Hopkins HIV Clinic, mean CD4 cell count in the prior year, and history of a diagnosis of opioid use disorder (ICD-9-CM of 305.5, 304.0, or 304.7; ICD-10-CM of F11.1, F11.2, or F11.9) (because naltrexone, which is often prescribed for opioid use disorder is also clinically effective and indicated for AUD).
Other covariates that might confound the causal effect of AUD and alcohol consumption on viral non-suppression include social determinants of health (e.g., income, wealth, housing stability, and social networks), other substance use, and mental health symptoms. We did not have information on social determinants of health and there may be residual confounding by these variables. Other substance use and mental health symptoms might confound the relationship of interest, or they might mediate the effect of AUD and alcohol consumption on viral non-suppression, in which case adjusting for these variables would be inappropriate. Overall, however, our aim for this study is description (rather than causal effect estimation) and we aim to use these results to identify groups at high risk of viral non-suppression. As such, even adjustment for all correlates of AUD and alcohol use may be inappropriate as such adjustment would make our results less actionable (23).
Outcome:
We defined failure to achieve or maintain durable viral suppression as having any viral load measurement >20 copies/mL (the limit of detection of HIV RNA tests in use during the study period) during the year in which self-reported alcohol use was collected. Having all viral loads below the limits of detection was associated with better survival in a prior analysis (24). Viral load measurements are obtained as part of routine care and physicians do not see the results of the PROs (including self-reported alcohol use) when treating patients. In sensitivity analyses, we also considered a definition of viral non-suppression of having the last viral load measurement in the calendar year >20 copies/mL (which would obviate any issues with patients who are tested more frequently being more likely to have any viral load measurement >20 copies/mL). Patients had a median of one viral load test in the analysis year (interquartile range [IQR]: 1, 2).
Statistical analysis:
We estimated prevalence ratios and prevalence differences for the joint association between AUD and alcohol use and unsuppressed viral load using generalized linear (log-binomial and binomial) regression models. Estimates from these models are prevalence ratios and differences because self-reported alcohol use and viral load measurements were from approximately the same period (both were from the same year); viral load monitoring and completion of PROs do not align exactly on the same day because both are collected as part of routine care and not as part of a study conducted for the sole purposes of research. We adjusted for covariates listed above using standardization with inverse probability of exposure weights (25). Exposure weights were estimated by regressing covariates on the alcohol use/diagnosis exposure category using polytomous logistic regression; weights were the marginal probability of being in the exposure category that one was in for a person-month divided by the predicted, conditional (on covariates) probability of being in the exposure category that one was in from the model. Inverse probability of exposure weighting for adjustment gives similar results to adjusting for potential confounders by including them in the outcome model, but is more compatible with the method used to adjust for measurement error in the diagnosis of AUD (below).
Measurement error in diagnoses of AUD:
To account for the near certainty that lifetime AUD is under-diagnosed and mismeasured by history of a clinical diagnosis of AUD (i.e., ICD-9 or ICD-10 diagnoses may not be perfectly sensitive or specific for a true history of AUD as defined by DSM-IV or DSM-V criteria), we examined the plausible impact of this mismeasurement of AUD using a reparameterized imputation approach for measurement error (RIME), which is an extension of multiple imputation for measurement error that does not rely on the availability of internal validation data (26). We assumed sensitivity of an ICD-9/ICD-10 diagnosis for AUD was between 25–85% with a most likely value of 56%, and specificity was >85% with a most likely value of 98%. Two studies informed our choices for these parameters. Among 4008 randomly selected inpatients admitted to any of 4 teaching hospitals in Alberta, Canada, using chart abstraction as the gold standard, diagnosis codes were estimated to have sensitivity of 53% and specificity of 99% for alcohol abuse (27). Using a Bayesian latent class model that assumed no gold standard, but incorporated chart review for evidence of high-risk alcohol use and self-report of high-risk alcohol use on the USAUDIT as two imperfect measures of high-risk alcohol use, chart review was estimated to have sensitivity of 16–53% and specificity of 90–97% (28).
Briefly, we assumed the sensitivity of a recorded, clinical diagnosis of AUD for detecting a true AUD based on DSM criteria was drawn from a beta (5.04,3.96) distribution and specificity was drawn from beta (8.91, 0.09) distribution. We estimated the probability that patients truly had an AUD, based on covariates listed above. We created 2 copies of each record, one that assumed AUD was present and one that assumed AUD was absent, and imputed the probability of true AUD for each copy based the model and the presence of an alcohol-related diagnosis. We truncated predicted probabilities at 0.001 and 0.999. We then re-analyzed the data, as described above, weighted by these probabilities.
Results
There were 1690 patients who had initiated ART with ≥1 complete AUDIT-C and a viral load measurement in the same year between 2014 and 2018; 55% of patients’ contributed an observation in 2018. Patients were majority male (63%) and Black race (83%). Median age was 55 years (IQR: 48, 61) and median years in care before contributing to this analysis was 11 (IQR: 7, 18). Four hundred thirty-four (26%) had ever been diagnosed with AUD. Overall, 21% reported recent high-risk alcohol use; this was 29% among patients with a diagnosis of AUD (Table 1).
Table 1.
Characteristics of 1690 patients in the Johns Hopkins HIV Clinical Cohort who completed ≥1 Alcohol Use Disorders Identification Test – consumption questions survey, 2014–2018, stratified by “lifetime” prevalencea of any alcohol use disorder diagnosisb at baseline
| AUD diagnosisb | No AUD diagnosis | Total | |
|---|---|---|---|
| N | 434 | 1256 | 1690 |
| Male | 196 (68) | 775 (62) | 1071 (63) |
| Age, years | 56 (51, 61) | 55 (47, 61) | 55 (48, 61) |
| Black race | 376 (87) | 1022 (81) | 1398 (83) |
| HIV acquisition risk | |||
| IDU | 215 (50) | 345 (27) | 560 (33) |
| MSM | 81 (19) | 326 (26) | 407 (24) |
| CD4 cell count | 527 (321, 757) | 553 (355, 781) | 548 (343, 772) |
| Prior years in care | 12 (8, 17) | 11 (6, 18) | 11 (7, 18) |
| Opioid abuse/dependence diagnosis | 236 (54) | 241 (19) | 477 (28) |
| Self-reported alcohol consumption | |||
| No use | 187 (43) | 672 (54) | 859 (51) |
| Moderate use | 119 (27) | 359 (29) | 478 (28) |
| High-risk usec | 128 (29) | 225 (18) | 353 (21) |
Based on all diagnoses captured in the Johns Hopkins Hospital system for patents since enrollment in care
ICD-9-CM of 305.0 or 303, or ICD-10-CM of F10.1 or F10.2
Defined as ≥7 drinks/week for women or ≥14 drinks/week for men or any instances of binge drinking (≥4 drinks on one occasion for women or ≥5 drinks on one occasion for men)
The crude prevalence of any viral non-suppression (lack of sustained viral suppression; at least one viral load >20 copies/mL) in a year was 35% in people without an AUD diagnosis who reported not drinking. Relative to people without an AUD diagnosis who reported not drinking, high-risk drinking in the absence of an AUD diagnosis was weakly but not statistically significantly associated with higher prevalence of viral non-suppression (prevalence difference [PD]: 5.1%; 95% confidence interval [CI]: −2.0%, 12.1%). Having an AUD diagnosis but not drinking was weakly but not statistically significantly associated with higher prevalence of viral non-suppression (PD: 3.2%; 95% CI: −5.3%, 11.8%). Moderate alcohol use among patients with an AUD diagnosis and high-risk alcohol use among patients with an AUD diagnosis, however, were both strongly associated with higher prevalence of viral non-suppression, and the association was stronger among persons who reported high-risk alcohol use (PD for moderate alcohol use with AUD: 15.4%, 95% CI: 4.6%, 26.1%; PD for high-risk alcohol use with AUD: 22.8%; 95% CI: 12.8%, 32.8%) (Table 2).
Table 2.
Crude and adjusted associations between AUD diagnosis and recent, self-reported alcohol use and viral non-suppression (≥1 viral load measurement >20 copies/mL) among patients in the Johns Hopkins HIV Clinical Cohort who completed ≥1 self-reported survey about alcohol use, 2014–2018
| Crude | Adjusted for confoundinga | Adjusted for confounding and measurement errorb | |||||
|---|---|---|---|---|---|---|---|
| Prevalence of viral non-suppression | PD | PR | PD | PR | PD | PR | |
| No diagnosis | |||||||
| No use | 35.2 | 0. | 1. | 0. | 1. | 0. | 1. |
| Moderate use | 35.8 | 0.7 (−5.3, 6.6) | 1.02 (0.86, 1.20) | 1.4 (−5.1, 7.9) | 1.04 (0.87, 1.23) | 1.8 (−7.2, 10.7) | 1.05 (0.82, 1.34) |
| High-risk usec | 40.2 | 5.1 (−2.0, 12.1) | 1.14 (0.95, 1.38) | 3.2 (−5.4, 11.8) | 1.09 (0.87, 1.35) | 4.5 (−7.5, 16.5) | 1.12 (0.82, 1.53) |
| Prior diagnosis | |||||||
| No use | 38.4 | 3.2 (−5.3, 11.8) | 1.09 (0.87, 1.37) | −0.8 (−11.9, 10.3) | 0.98 (0.72, 1.33) | 2.4 (−11.2, 15.9) | 1.06 (0.74, 1.53) |
| Moderate use | 50.5 | 15.4 (4.6, 26.1) | 1.44 (1.15, 1.80) | 14.9 (1.4, 28.4) | 1.40 (1.07, 1.84) | 14.7 (−1.0, 30.4) | 1.41 (1.00, 1.98) |
| High-risk usec | 57.9 | 22.8 (12.8, 32.8) | 1.65 (1.36, 1.99) | 16.9 (4.8, 28.9) | 1.46 (1.15, 1.85) | 17.8 (1.2, 34.4) | 1.49 (1.05, 2.11) |
Abbreviations: AUD, alcohol use disorder; IDU, injection drug use; MSM, men who have sex with men; PD, prevalence difference; PR, prevalence ratio
Adjusted for birth sex, age, black race, HIV acquisition risk factors (MSM, IDU), CD4 cell count, history of an opioid use disorder, years in care
Assumes sensitivity of a diagnosis of alcohol abuse or dependence, or alcohol dependence is 56% sensitive (95% credible interval: 25%, 85%) and 98% specific (95% credible interval: 85%, 100%) for a true AUD
Defined as ≥7 drinks/week for women or ≥14 drinks/week for men or any instances of binge drinking (≥4 drinks on one occasion for women or ≥5 drinks on one occasion for men)
Patients with an AUD diagnosis were more likely than those without an AUD diagnosis to be male (68% versus 62%), report IDU as a likely route of HIV acquisition (50% versus 27%), and have a prior diagnosis of opioid abuse or dependence (54% versus 19%) (Table 1). After adjustment for differences in covariates across groups defined by history of AUD diagnosis and self-reported alcohol consumption, associations between AUD diagnoses and alcohol use and lack of durable viral suppression weakened slightly, but conclusions were similar. Relative to people without an AUD diagnosis who reported not drinking, high-risk drinking alone was not associated with prevalence of viral non-suppression (PD=3.2%; 95% CI: −5.4%, 11.8%), an AUD diagnosis in persons who reported no alcohol use was not associated with viral non-suppression (PD: −0.8%; 95% CI: −11.9%, 10.3%), and any drinking in persons with an AUD diagnosis was strongly associated with viral non-suppression (PD for moderate alcohol use with AUD: 14.9%, 95% CI: 1.4%, 28.4%; PD for high-risk alcohol use with AUD: 16.9%; 95% CI: 4.8%, 28.9%).
Adjusting for imperfect diagnosis of AUD, did not appreciably change associations between history of AUD, alcohol use, and viral non-suppression (Table 2).
When we defined viral non-suppression as last viral load in the year >20 copies/mL, rather than any viral load during the year >20 copies/mL, the prevalence of “viral non-suppression” was lower: among non-drinkers with no history of AUD, prevalence of viral non-suppression dropped from 35% (Table 2) to 21% (Supplemental Table 1). We observed similar associations between history of AUD, alcohol use, and viral non-suppression as in the main analysis. Relative to persons with no history of AUD who reported not drinking, persons with a history of AUD who reported drinking at any level had a higher prevalence of viral non-suppression. However, associations were slightly attenuated, and precision of our estimates was lower than when we used a stricter definition of viral suppression (Supplemental Table 1).
Discussion
We found the association between high-risk alcohol use and viral non-suppression was strongest among persons with an AUD, and nearly absent among persons without an AUD; among PLWH with an AUD, any level of drinking was strongly associated with lack of durable viral suppression. Additionally, we found that persons with an AUD who reported not drinking had very similar prevalence of viral non-suppression as compared to persons without an AUD. Our results suggest it may be insufficient to consider AUD diagnoses or alcohol consumption alone when attempting to identify persons with the highest probability of viral non-suppression.
Most prior research has focused on the influence of alcohol consumption alone on viral suppression in people with HIV. While the following is not an exhaustive summary of the literature on this topic, it becomes clear quickly that there is not good consensus on this relationship. Several studies have reported that relative to no alcohol use, high-level drinking and hazardous alcohol use was associated with viral non-suppression (10, 11). Because these studies did not restrict to people on ART, the degree to which alcohol use acted on access to ART versus adherence to ART is unclear. In one study, exceeding weekly quantity/frequency limits for alcohol consumption but not binge drinking was associated with viral non-suppression (14). In another study, relative to not drinking, daily drinking but not “regular drinking” (drinking less than daily) was associated with viral non-suppression (18). And in yet another study, binge drinking and moderate alcohol consumption without binge drinking were similarly associated with an increased risk of viral non-suppression relative to not drinking; the association between hazardous alcohol consumption and viral non-suppression was clinically meaningfully greater than associations with moderate alcohol consumption or binge drinking alone (15). However, several studies have failed to identify alcohol use as a risk factor for viral non-suppression in people on ART (16, 17) and people not on ART (19).
While overall, changes in alcohol consumption are relatively rare in people with HIV (especially in older cohorts) (15, 29–31), among people on ART, some studies have found both increases and decreases in drinking are associated viral non-suppression (13, 30). Another study found that only increases in drinking from abstinence (presumably, re-initiation of drinking) were associated with viral non-suppression (15). And still another found that only decreases in drinking were associated with increased probability of viral suppression (32).
In contrast to focusing only on alcohol consumption, we also considered patients’ history of an AUD and whether the influence of alcohol consumption interacted with AUD. While we believe this more nuanced view of alcohol use is a strength of our analysis – indeed, we found a meaningful interaction between AUD and alcohol consumption – there are several limitations in how we were able to operationalize our definition of AUD in this sample. First, the ideal definition of AUD for patients’ entire clinical history is unclear. The period across which we looked for diagnoses of AUD in the medical record spanned two DSM definitions of AUD: AUD prior to May 2013 would have been defined by the DSM-IV, as alcohol abuse or dependence, two distinct disorders with distinct symptoms; in May 2013, the DSM-5 integrated these two disorders into a single AUD (with mild, moderate, and severe subclassifications, that we were not able to consider in this analysis). Second, the criteria used by doctors to document AUD using ICD-9 or ICD-10 codes are unknown (33) and may not align perfectly with the DSM criteria. Third, physicians may not have been aware of specific DSM criteria for an AUD diagnosis to screen for all relevant symptoms. Although HIV providers frequently screen their patients for alcohol use, few use a standardized tool to do so (34). Finally, due to the stigma associated with carrying an AUD diagnosis, physicians may have been reluctant to ask about symptoms, or record an AUD diagnosis if present; or due to social desirability biases, patients may have been reluctant to disclose symptoms to their providers if asked (35).
A strength of this analysis was our attempt to address the resultant measurement error in capturing AUD using clinical diagnoses. A challenge we faced in adjusting our results for measurement error in clinically-diagnosed AUD was choosing a sensitivity and specificity for an ICD-9 or ICD-10 diagnosis of AUD. As described in the Methods, two studies informed our choices for these parameters. One used chart abstraction as the gold standard (27) while the other assumed no gold standard, but focused more on the presence of high-risk alcohol use as the latent construct under investigation (28). We are unaware of any studies that have considered how well routine physician diagnoses of AUD are able to capture AUD according to DSM criteria. Our analysis reflected this lack of information by assuming a wide range of potential values for both sensitivity and specificity for clinical diagnoses. Decreased precision of our estimates of association after adjusting for measurement error reflect the uncertainty in these parameters.
We classified persons as having an AUD if they ever were clinically diagnosed with AUD in the Johns Hopkins Hospital system. One strength of our approach is the very long patient histories across which we could look for AUD diagnoses; median years in care as of the analysis year was 11. While we have focused on lifetime AUD diagnoses, we are unable to comment on the association between current AUD and current alcohol consumption and viral non-suppression. If current AUD is the critical variable driving viral non-suppression, our results may underestimate the association between alcohol use and viral non-suppression in people with current AUD by including people with a history of AUD but no ongoing symptoms, effectively diluting the association of interest.
Prevalence of recent alcohol use (49%), and in particular high-risk alcohol use (21%), was high in this cohort. These estimates are in line with other estimates of the prevalence of alcohol use in people living with HIV, but higher than estimates of the prevalence of high-risk use in other cohorts of people living with HIV (36). Despite the presumed, low sensitivity of a clinical diagnosis for AUD, the “lifetime” prevalence of an AUD diagnosis in our cohort (26%) was similar to lifetime prevalence of an AUD based on face-to-face interviews and the DSM-5 classification of AUD in the non-institutionalized, civilian population in the United States (29%) (37). Our prevalence estimate is reflective only of clinical diagnoses within the Johns Hopkins Hospital system, and so may be an underestimate of true lifetime prevalence. Overall, our findings underscore the high burden of high-risk alcohol use and AUDs in people living with HIV.
Prevalence of a lack of durable viral suppression was also high in this sample (35% in people without an AUD who reported not drinking). If we considered only the last viral load value in a calendar year, similar to the Department of Health and Human Services definition for viral suppression (38), prevalence of viral non-suppression in people without AUD who reported not drinking was still fairly high (21%). In a prior analysis, we determined our primary definition of viral suppression – employing a low threshold for classifying viral load as suppressed (≤20 copies/mL), and requiring all viral load values in a year to be below this threshold – was most strongly associated with subsequent mortality compared to other potential, less stringent definitions of viral suppression (24). The prevalence of any viral suppression (e.g., any viral load measurements or last viral load measurement in the year below threshold for suppression) is by necessity higher. It is possible that our ability to detect an association between alcohol use and viral suppression may be because we used a particularly stringent definition of viral suppression. Indeed, in our sensitivity analysis with a “looser” definition of viral non-suppression, associations were slightly attenuated and less precise. That is, it is possible that people who drink alcohol and who have an AUD can achieve viral suppression for at least some portion of the year, but not maintain viral suppression for the entire year, as a result of imperfect adherence rather that non-adherence to ART. We see this focus on durable viral suppression to be a strength of this study.
While the setting of our study is unique in some ways – patients reflect the local community and are predominantly low-income, with a higher than typical proportion of people who likely acquired their HIV infection through injection drug use, a high proportion of people who are Black race, and a high prevalence of high-risk alcohol use – a strength of this study is that it is nested in routine clinical care and as such may be more likely to generalize to other settings of people with HIV. Most providers in the study clinic are not uniquely focused on alcohol use and therefore we would expect that identification of AUD by infectious disease and general internal medicine doctors who serve these patients would be similar to other clinics.
In conclusion, identifying individuals with the highest probability of viral non-suppression depended on both their history of AUD and recent alcohol use. Our results may have implications for targeting limited alcohol intervention resources for people with HIV: the most efficient strategy for improving viral suppression through interventions on alcohol use should likely consider both symptoms of AUD and patterns of alcohol consumption. The threshold for intervening on alcohol use may need to be lower in persons who have ever had an AUD and asking about AUD symptoms and identifying an AUD in people who are consuming any level of alcohol may be more helpful for identifying people in need of intervention than focusing on specific quantities of alcohol consumed. Future studies of the impact of alcohol consumption in people with HIV should also consider symptoms of AUD, but should be clear about challenges in measuring this information.
Supplementary Material
Funding:
This work was supported by grants from the National Institutes of Health (K01 AA028193; K01 AI125087; K08 MH118094; K24 AA027483; U01 DA036935; U01 AI069918; U24 AA020801 and U01 AA020793).
Footnotes
Conflicts of interest: None.
Ethics approval: This study was approved by the Johns Hopkins Medicine Institutional Review Board.
Consent to participate: All participants consented to share their data.
Code availability: Code is available upon request.
Availability of data and material:
Data are available upon request.
References
- 1.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.INSIGHT START Study Group, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Z, Purcell DW, Sansom SL, Hayes D, Hall HI. Vital Signs: HIV Transmission Along the Continuum of Care - United States, 2016. MMWR Morbidity and mortality weekly report. 2019;68(11):267–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lesko CR, Edwards JK, Moore RD, Lau B. A longitudinal, HIV care continuum: 10-year restricted mean time in each care continuum stage after enrollment in care, by history of injection drug use. AIDS. 2016;30(14):2227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yehia BR, Fleishman JA, Metlay JP, Moore RD, Gebo KA. Sustained Viral Suppression in HIV-Infected Patients Receiving Antiretroviral Therapy. JAMA. 2012;308(4):339–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crepaz N, Tang T, Marks G, Hall HI. Viral suppression patterns among persons in the United States with diagnosed HIV infection in 2014. Annals of Internal Medicine. 2017;167(6):446–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crane HM, McCaul ME, Chander G, Hutton H, Nance RM, Delaney JAC, et al. Prevalence and Factors Associated with Hazardous Alcohol Use Among Persons Living with HIV Across the US in the Current Era of Antiretroviral Treatment. AIDS Behav. 2017;21(7):1914–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vagenas P, Azar MM, Copenhaver MM, Springer SA, Molina PE, Altice FL. The Impact of Alcohol Use and Related Disorders on the HIV Continuum of Care: a Systematic Review. Current HIV/AIDS Reports. 2015;12(4):421–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams EC, Hahn JA, Saitz R, Bryant K, Lira MC, Samet JH. Alcohol Use and Human Immunodeficiency Virus (HIV) Infection: Current Knowledge, Implications, and Future Directions. Alcohol Clin Exp Res. 2016;40(10):2056–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams EC, McGinnis KA, Edelman EJ, Matson TE, Gordon AJ, Marshall BDL, et al. Level of Alcohol Use Associated with HIV Care Continuum Targets in a National U.S. Sample of Persons Living with HIV Receiving Healthcare. AIDS Behav. 2019;23(1):140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chander G, Lau B, Moore RD. Hazardous alcohol use: a risk factor for non-adherence and lack of suppression in HIV infection. Journal of acquired immune deficiency syndromes (1999). 2006;43(4):411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samet JH, Horton NJ, Meli S, Freedberg KA, Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res. 2004;28(4):572–7. [DOI] [PubMed] [Google Scholar]
- 13.Barai N, Monroe A, Lesko C, Lau B, Hutton H, Yang C, et al. The Association Between Changes in Alcohol Use and Changes in Antiretroviral Therapy Adherence and Viral Suppression Among Women Living with HIV. AIDS Behav. 2017;21(7):1836–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook R, Zhou Z, Kelso-Chichetto N, Janelle J, Morano J, Somboonwit C, et al. Alcohol consumption patterns and HIV viral suppression among persons receiving HIV care in Florida: an observational study. Addiction science & clinical practice. 2017;12(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lesko CR, Nance RM, Lau B, Fojo AT, Hutton HE, Delaney JAC, et al. Changing Patterns of Alcohol Use and Probability of Unsuppressed Viral Load Among Treated Patients with HIV Engaged in Routine Care in the United States. AIDS Behav. 2021;25(4):1072–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalichman SC, Grebler T, Amaral CM, McNerney M, White D, Kalichman MO, et al. Viral suppression and antiretroviral medication adherence among alcohol using HIV-positive adults. International journal of behavioral medicine. 2014;21(5):811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conen A, Wang Q, Glass TR, Fux CA, Thurnheer MC, Orasch C, et al. Association of Alcohol Consumption and HIV Surrogate Markers in Participants of the Swiss HIV Cohort Study. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2013;64(5):472–8. [DOI] [PubMed] [Google Scholar]
- 18.Wu ES, Metzger DS, Lynch KG, Douglas SD. Association between alcohol use and HIV viral load. Journal of acquired immune deficiency syndromes (1999). 2011;56(5):e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samet JH, Cheng DM, Libman H, Nunes DP, Alperen JK, Saitz R. Alcohol consumption and HIV disease progression. J Acquir Immune Defic Syndr. 2007;46(2):194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nolan S, Walley AY, Heeren TC, Patts GJ, Ventura AS, Sullivan MM, et al. HIV-infected individuals who use alcohol and other drugs, and virologic suppression. AIDS care. 2017;29(9):1129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore RD. Understanding the clinical and economic outcomes of HIV therapy: the Johns Hopkins HIV clinical practice cohort. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17 Suppl 1:S38–41. [DOI] [PubMed] [Google Scholar]
- 22.Higgins-Biddle JC, Babor TF. A review of the Alcohol Use Disorders Identification Test (AUDIT), AUDIT-C, and USAUDIT for screening in the United States: Past issues and future directions. Am J Drug Alcohol Abuse. 2018;44(6):578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufman JS. Statistics, Adjusted Statistics, and Maladjusted Statistics. Am J Law Med. 2017;43(2–3):193–208. [DOI] [PubMed] [Google Scholar]
- 24.Lesko CR, Chander G, Moore RD, Lau B. Variation in estimated viral suppression associated with the definition of viral suppression used. Aids. 2020;34(10):1519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology. 2003;14(6):680–6. [DOI] [PubMed] [Google Scholar]
- 26.Edwards JK, Cole SR, Fox MP. Flexibly Accounting For Exposure Misclassification with External Validation Data. American Journal of Epidemiology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quan H, Li B, Duncan Saunders L, Parsons GA, Nilsson CI, Alibhai A, et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health services research. 2008;43(4):1424–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lesko CR, Keil AP, Moore RD, Chander G, Fojo AT, Lau B. Measurement of Current Substance Use in a Cohort of HIV-Infected Persons in Continuity HIV Care, 2007–2015. Am J Epidemiol. 2018;187(9):1970–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams EC, McGinnis KA, Bobb JF, Rubinsky AD, Lapham GT, Skanderson M, et al. Changes in alcohol use associated with changes in HIV disease severity over time: A national longitudinal study in the Veterans Aging Cohort. Drug and alcohol dependence. 2018;189:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams EC, McGinnis KA, Rubinsky AD, Matson TE, Bobb JF, Lapham GT, et al. Alcohol Use and Antiretroviral Adherence Among Patients Living with HIV: Is Change in Alcohol Use Associated with Change in Adherence? AIDS Behav. 2021;25(1):203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bilal U, McCaul ME, Crane HM, Mathews WC, Mayer KH, Geng E, et al. Predictors of Longitudinal Trajectories of Alcohol Consumption in People with HIV. Alcohol Clin Exp Res. 2018;42(3):561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satre DD, Sarovar V, Leyden W, Hare CB, Catz SL, Bryant KJ, et al. Changes in Days of Unhealthy Alcohol Use and Antiretroviral Therapy Adherence, HIV RNA Levels, and Condomless Sex: A Secondary Analysis of Clinical Trial Data. AIDS Behav. 2020;24(6):1784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris AH, Kivlahan DR, Bowe T, Humphreys KN. Pharmacotherapy of alcohol use disorders in the Veterans Health Administration. Psychiatric Services. 2010;61(4):392–8. [DOI] [PubMed] [Google Scholar]
- 34.Chander G, Monroe AK, Crane HM, Hutton HE, Saag MS, Cropsey K, et al. HIV primary care providers--Screening, knowledge, attitudes and behaviors related to alcohol interventions. Drug Alcohol Depend. 2016;161:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGinnis KA, Tate JP, Williams EC, Skanderson M, Bryant KJ, Gordon AJ, et al. Comparison of AUDIT-C collected via electronic medical record and self-administered research survey in HIV infected and uninfected patients. Drug Alcohol Depend. 2016;168:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, et al. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. Journal of studies on alcohol. 2002;63(2):179–86. [DOI] [PubMed] [Google Scholar]
- 37.Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valdiserri RO, Forsyth AD, Yakovchenko V, Koh HK. Measuring what matters: development of standard HIV core indicators across the U.S. Department of Health and Human Services. Public health reports. 2013;128(5):354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request.


