Abstract
BACKGROUND:
The study of astrocytic functions in non-human primates (NHPs) has been hampered by the lack of genetic tools to selectively target astrocytes. Viral vectors with selective and efficient transduction of astrocytes could be a potent tool to express marker proteins, modulators, or sensors in NHP astrocytes, but the availability of thoroughly characterized astrocytic selective promoter sequences to use in these species remains extremely limited.
NEW METHOD:
We describe the specificity and efficiency of an astrocyte-specific promoter, GfaABC1D in the brain of the rhesus macaque, with emphasis in basal ganglia regions. AAV5-pZac2.1-GfaABC1D-tdTomato was locally injected into the globus pallidus external segment (GPe) and putamen. The extent, efficiency, and specificity of transduction was analyzed with immunohistochemistry at the light and electron microscope levels.
RESULTS:
The GfaABC1D promoter directed the expression of tdTomato in an astrocyte-specific manner in directly or indirectly targeted regions (including both segments of the globus pallidus, putamen, subthalamic nucleus and cortex).
COMPARISON WITH EXISTING METHODS:
Due to its small size, the GfaABC1D promoter is advantageous over other previously used glial fibrillary acidic protein-based promoter sequences, facilitating its use to drive expression of various transgenes in adeno-associated viruses (AAV) or other viral vectors.
CONCLUSION:
GfaABC1D is an efficient promoter that selectively targets astrocytes in the monkey basal ganglia and expands the viral vector toolbox to study astrocytic functions in non-human primates.
Keywords: Viral vector, AAV, GFAP, globus pallidus, striatum
Graphical abstract

1. Introduction
Neuroscience research in non-human primate (NHP) models commonly leverage viral vectors to express transgenes in specific cell types. One of the main factors that determine transduction specificity is the promoter sequence driving the transgene expression. In NHPs, a growing number of studies have used pan-neuronal promoters such as human synapsin (hSyn), while other studies have targeted specific neuronal subpopulations (reviewed in (Tremblay et al. 2020). However, few studies in NHPs have examined the use of promoters to drive transgene expression in non-neuronal cell types, such as astrocytes.
Astrocytes are essential for many central nervous system (CNS) functions. However, the majority of studies on astrocytes have been conducted in rodent models. Given evidence of species differences in astrocyte gene expression, morphology, and functions between primates (including humans) and rodents (Oberheim et al. 2009; Zhang et al. 2016), basic and translational research studies of astrocytes in normal and pathological conditions should be extended to primate models. Currently, the lack of efficient tools to interrogate and study astrocytes in primates is a limiting factor in expanding our knowledge of this cell type.
Many studies have used the gfa2 sequence of the glial fibrillary acidic protein (GFAP) as a promoter to target astrocytes (Lee et al. 2006, 2008). The gfa2 sequence is relatively large at 2.2 kb, encompassing nearly half of the packaging capacity of adeno-associated virus (AAV) vectors which are the most commonly used vectors in NHP neuroscience research. Despite these limitations, gfa2 remains a consistently used promoter to target astrocytes. Early truncated versions of the gfa2 promoter, such as gfa28, did not result in selective targeting of astrocytes (Lee et al. 2006). More recently, the GfaABC1D promoter, a truncated version of gfa2 (681 bp), was shown to display a higher astrocyte transduction efficiency, along with decreased non-specific targeting of neurons (Lee et al. 2008). GfaABC1D has been successfully used to target astrocytes in various studies (Lee et al. 2008; Xie et al. 2010; Poskanzer and Yuste 2016; Pignataro et al., 2017; Octeau et al. 2018; Yu et al. 2018; Nagai et al. 2019). However, most of the available studies have used the GfaABC1D promoter in mice, and recent work has demonstrated non-specific transduction and lower efficiency of the GfaABC1D promoter in rats, underscoring the need for species specific testing of viral vector tools (Taschenberger et al. 2017). Furthermore, viral efficiency and specificity often differ between NHPs and rodents, even when using the same virus and anatomical target (Jüttner et al. 2019; Albaugh et al. 2020). Thus, the specificity, efficacy, and pattern of expression for each viral vector-promoter combination must be tested in the species of interest.
To address this issue, we have characterized the efficiency and selectivity of the GfaABC1D promoter to transduce astrocytes in the monkey globus pallidus and striatum using AAV intracerebral injections. We characterized this promoter in basal ganglia regions with the long-term goal of studying the roles of astrocytes in non-human primate models of movement disorders. Our results indicate that GfaABC1D is a valuable tool to selectively target astrocytes in the monkey basal ganglia, expanding the toolbox to study astrocytic functions in non-human primates.
2. Materials and Methods
2.1. Animals
Two adult rhesus macaques (Maccaca Mulatta; MR341 male, MR348 female, both 4 years old at time of virus injection) from the Yerkes National Primate Research Center (YNPRC) colony were used in this study. Prior to enrollment in the study, blood samples from both monkeys were tested by the Yerkes Viral Core and confirmed sero-negativity for AAV5 antibodies. All procedures were approved by the Animal Care and Use Committee of Emory University (Emory IACUC, protocol number 201800051) and performed in accordance with the Guide for the Care and Use of Laboratory Animals (NRC, 2010) and the U.S. Public Health Service Policy on the Humane Care and Use of Laboratory Animals (revised 2015).
2.2. Viral vector
The pZac2.1 vector backbone and AAV5 serotype was used to express the fluorescent tag TdTomato under the GfaABC1D promoter. pZac2.1 gfaABC1D-tdTomato was a gift from Baljit Khakh (Addgene viral prep # 44332-AAV5 ; http://n2t.net/addgene:44332; RRID:Addgene_44332)(Shigetomi et al. 2013). The virus solutions were stored at −80°C and thawed the day of the injection. To produce a low titer solution, the virus was diluted using pluronic acid (Gibco-ThermoFisher, Waltham, MA) 0.001% in PBS (Gibco-ThermoFisher). The final viral titers administered are indicated below.
2.3. Surgical methods and injections of virus solutions
The two subjects differed in the method used to inject the virus solutions. MR341 was part of studies related to the electrophysiology of the GPe (not described here), which required placement of chronic recording chambers, and thus the virus was injected through a chronically implanted recording chamber. In contrast, MR348 was not enrolled in electrophysiological studies and did not receive chronic recording chambers, therefore the injections were done through burr holes in the skull during surgery.
Prior to the chamber placement surgery in monkey MR341, the animal was trained to sit in a primate chair using positive reinforcement methods. Once acclimated to the primate chair, the monkey underwent a surgery to place the chambers. The animal was sedated with ketamine (10mg/kg), intubated for isoflurane anesthesia (maintained at 1–3%), and placed in a stereotactic frame. The anesthesia was administered and monitored by a veterinary technician. Vital signs were monitored during the surgery (heart rate, respiration, oxygen saturation, and CO2). Two trephine holes were made in the skull, exposing the dura mater. The exposed dura was then covered by two stainless steel recording chambers (Crist Instruments, Hagerstown, MD; inner chamber diameter = 16 mm) positioned at a 30° angle from vertical and directed stereotaxically to the external globus pallidus (GPe) and putamen (according to Paxinos et al. 2000).The chambers were embedded into an acrylic skull cap, along with a stainless-steel head holder and bone screws. Post-operatively, the subject was treated with buprenorphine (0.03 mg/kg) and banamine (1 mg/kg) as analgesics, as well as rocephin (25 mg/kg) to prevent infection.
Before conducting the virus injections, animal MR341 was part of electrophysiological studies as mentioned above. Thus, the virus solution injections were performed 8 months after the surgery, while the animal was awake and sitting on the primate chair. First, electrophysiological mapping sessions were conducted to identify the location of the putamen and GPe in the chamber, using the characteristic firing patterns of these structures (DeLong 1971, 1973). The injections were done under electrophysiological guidance, using a custom-made probe that combined an injection tubing and a standard recording electrode (Kliem and Wichmann 2004). The injection system was positioned in the GPe using a microdrive (NAN Instruments, Israel). At least 5 minutes after reaching the target, 2 μl of AAV5-pZac2.1 gfaABC1D-tdTomato (titer 1.5 ×10e13 vg/ml) was infused at a flow rate of 0.1–0.2 μl /min (this infusion was considered the ‘high-volume’ injection for the GPe). A second deposit of 2 μl along the same track was done in the putamen (4 mm dorsal to the GPe injection). This was followed by a 20-minute wait time before withdrawal of the injection system from the brain. All recording sessions, including injection of the virus solutions, were done while the animal was awake and sitting on the primate chair, and the electrodes (or injection system) were acutely placed in the brain for each experimental session.
In monkey MR348, the injections of virus solution were performed during a surgical procedure. The subject was sedated with ketamine (10mg/kg), intubated for isoflurane anesthesia (1–3%), and placed in a stereotactic frame. Anesthesia and vital signs were monitored as indicated for subject MR341. Two craniotomies (3–5 mm in diameter) were performed (one per hemisphere), and the dura mater was punctured with a 23g needle. The coordinates for the craniotomies to target the GPe, were determined based on Paxinos et al. 2000: 16 mm anterior to the interaural line, 8.5 mm lateral to the midline and 24 mm ventral from the cortical surface. Injections were performed using a Hamilton microsyringe (Reno, NE) with a 30-ga needle and coupled to an automated pump system (Stoelting, Wood Dale, IL). Once the needle had been lowered to the appropriate depth in the GPe, we waited 5 min before starting the infusion. We delivered 1 μl of AAV5-pZac2.1 gfaABC1D-tdTomato at 0.1 μl /min. The right GPe received a titer 1.5 ×10e13 vg/ml, while the left GPe received a titer of 1.5×10e12 vg/ml (these were considered the high- and low-titer injections, respectively). For both injections, the needle was left at the injection site for 10 minutes post infusion to allow for diffusion of the virus and then the needle was withdrawn. This is a shorter post-injection waiting time compared to that used for MR341, a decision made on the fact that a much thinner injection probe was used, likely producing less tissue distortion as it was lowered into the brain. At the end of the injection, the incision was sutured in layers, and the subject recovered from anesthesia before returning to its home cage. The post-operative care and analgesic treatments were the same as those described for monkey MR341.
2.4. Perfusion and tissue preparation
The animals were perfused 9 and 4 weeks (MR341 and MR348, respectively) after the virus injection. Monkeys were deeply anesthetized with pentobarbital (100 mg/kg, i.v.), and transcardially perfused with an oxygenated Ringer’s solution followed by a fixative solution of 4% paraformaldehyde and 0.1% glutaraldehyde. Brains were extracted from the skull and placed in 4% paraformaldehyde for 24 hours. Sections were cut in 60 μm thick coronal sections using a vibrating microtome.
2.5. Immunoperoxidase labeling for light microscopy
The antibodies used in these studies are listed in Table 1. To determine the location and spread of transgene expression, every tenth section (600 μm distance) was stained for mCherry to reveal the tdTomato tag protein (thus, throughout the text, mCherry staining indicates tdTomato expression). Sections were first treated with 1% sodium borohydride, to reduce free aldehydes and Schiff bases resulting from glutaraldehyde fixation and rinsed in phosphate buffered saline (PBS; 0.01M, pH 7.4). To prevent non-specific binding and permeabilize the tissue, sections were incubated in a solution of 1% goat serum, 1% bovine serum albumin (BSA), and 0.3% Triton X-100 in PBS. Sections were then incubated overnight in the blocking/permeabilization solution containing the mCherry antibody (Table 1).
Table 1.
Antibodies
| Antibody | Vendor | Cat. No. | Lot No. | Host | Dilution | Antibody Registry ID |
|---|---|---|---|---|---|---|
| Primary antibodies | ||||||
| mCherry (to identify TdTomato expression) | Abcam (Cambridge, UK) | AB167453 | GR3358274–2 | Rabbit | 1:1000 | AB_2571870 |
| Aldh1L1 (to identify astrocytes) | NeuroMab (UC Davis, Davis, CA | 75–140 | 41–4BK-67 | Mouse | 1:3000 | AB 10673448 |
| Iba1 (to identify microglia) | Abcam | AB5076 | FR3288145–1 | Goat | 1:500 | AB_2224402 |
| NeuN (to identify neurons) | Millipore-Sigma (St. Louis, MO) | MAB377 | N61876252 | Mouse | 1:2000 | AB_2298772 |
| Sox10 (to identify oligodendrocytes) | Proteintech (Rosemont, IL) | 66786–1 | 10003287 | Mouse | 1:1000 | AB_2882131 |
| GFAP | Millipore-Sigma (St. Louis, MO) | MAB360 | 3132965 | Mouse | 1:5000 | AB 11212597 |
| Secondary antibodies | ||||||
| Biotinylated anti-rabbit | Vector Laboratories (Burlingame, CA) | BA-100 | N/A | Goat | 1:200 | AB_2323606 |
| Anti-rabbit rhodamine Red-X | Jackson Immunoresearch (West Grove, PA) | 711-295-152 | 130068 | Donkey | 1:100 | AB_2340613 |
| Anti-mouse FITC | Jackson Immunoresearch | 715-095-150 | 129083 | Donkey | 1:100 | AB_2340792 |
| DAPI | Invitrogen-Thermo Fisher | D1306 | 2031179 | N/A | 1:1000 | AB_2629482 |
Primary antibody binding sites were revealed using the avidin-biotin complex (ABC) method. Sections were treated with a biotinylated goat anti-rabbit secondary antibody for 60 minutes. Then, sections were incubated in an ABC solution (1:200; Vector Laboratories, Burlingame, CA) containing 1% BSA and 0.3% Triton X-100, for 90 minutes, followed by rinses in PBS and then TRIS buffering solution (0.05 M, pH 7.6). Sections were then incubated in a TRIS buffer solution containing 0.025% 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma, St. Louis, MO), 10 mM Imidazole and 0.05% hydrogen peroxide for 10 minutes, immediately followed by repeated PBS rinses to stop the peroxidase reaction. All incubations were done at room temperature. Sections were then mounted, cover slipped, and scanned (20× maximal magnification) with an Aperio Scanscope CS system (Leica, Wetzlar, Germany). Following imaging, the location of the injection was determined based on the intensity of the immunoperoxidase labeling (see section “Quantification of immunoperoxidase” below).
2.6. Immunoperoxidase labeling for electron microscopy
Electron microscopy (EM) studies were performed to determine the subcellular pattern of TdTomato expression. For these studies, sections from animal MR341 were first treated with 1% sodium borohydride before being placed in a cryoprotectant solution, frozen at −80°C to maximize antibody penetration and washed in serial dilutions of PBS. After blocking with 10% normal horse serum and 1% bovine serum albumin in PBS, the sections were incubated for two days at 4°C in the primary antibody. The avidin-biotin complex (ABC) method was used to amplify signal. The sections were incubated in biotinylated secondary antibodies for 2 hours, and in the ABC solution (1:100; Vectastain Standard kit, Vector) for 90 minutes. Sections were rinsed in PBS and TRIS buffer (0.05 M, pH 7.6) before being placed in DAB solution for 10 minutes (0.006% H2O2, 0.01M imidazole, 0.025% 3–3’ diaminobenzidine tetrahydrochloride). Next, sections were rinsed in PBS and kept at 4°C overnight.
The sections were then placed in PB for 10 min to desalt and postfixed in osmium tetroxide (1% in PB). They were washed in PB and dehydrated in a series of ethanol and propylene oxide. The 70% ethanol solution contained 1% uranyl acetate. The sections were embedded in resin overnight and placed on slides before being placed in the oven for 48 hours at 60°C. Two blocks of GPe, putamen, and subthalamic nucleus tissue were cut and glued on top of resin blocks. After facing, serial ultrathin sections were cut on an ultramicrotome (Leica Ultracut T2, Leica, Nussloch, Germany). Sixty nanometer thick sections from the surface of the block were collected on copper single slot grids, stained with lead citrate for 5 minutes, and examined on an electron microscope (EM Model 1011; Jeol, Peabody, MA, USA) coupled with a CCD camera (Gatan Model 785; Warrendale, PA, USA).
2.7. Immunofluorescence
After determining the approximate injection site using immunoperoxidase (above), sections at or a few mm from the injection site (see below for exact distances) were selected for cell-type specificity studies. In addition to a primary antibody against mCherry to reveal TdTomato (as described above), sections were labeled with various antibodies to identify cell types (Table 1). Binding sites were revealed using two fluorophore-conjugated secondary antibodies (Table 1). Sections were incubated in the secondary solution for 60 minutes, rinsed in PBS, mounted with Vectashield (Vector Laboratories) and cover slipped. DAPI was included in the secondary solution during incubation. Slides were imaged using a confocal microscope (Leica DM5500B) equipped with a CCD camera (Orca R2; Hamamatsu). Z-stacks were obtained at 20x with a 4μm step size at the dorsomedial edge of the GPe (bordering the internal capsule and putamen).
2.8. Analysis
2.8.1. Quantification of immunofluorescence for efficiency of transduction
To quantify the number of cells transduced at and around the site of injection, confocal Z-stacks were acquired from mCherry-immunolabeled sections that were −1.2 mm to +3.6 mm anterior-posterior to the injection site (sections were separated by 1.2 mm intervals, 5 sections per injection were used). All mCherry-positive cells in the 20x z-stack were quantified using ImageJ (Schneider et al. 2012). Data is represented as raw counts. Images and counts were completed for all injection conditions.
To determine the proportion of astrocytes that were mCherry-positive, we used sections co-labeled with mCherry and Aldh1L1 as marker of astrocytes. Z-stacks were acquired from sections −1.2 mm to +3.6 mm anterior-posterior to the injection site in 1.2 mm intervals (5 sections per injection). All mCherry-positive cells, Aldh1L1-positive cells, and co-labeled cells in the 20x z-stack were quantified using ImageJ. The proportion of transduced astrocytes was calculated as the number of Aldh1L1-positive cells that also expressed mCherry divided by the total number of Aldh1L1-positive cells in each section. Images and counts were completed for animal MR348 (low- and high-titer conditions). Since tissue sections from MR341 were also used for other studies not here reported, there were not enough sections for this case (high-volume injection) available to complete the Aldh1L1 and mCherry co-labeling studies at the appropriate distances from the injection site.
2.8.2. Quantification of immunofluorescence for specificity of transduction
To determine the specificity of transduction, the number of cells co-labeled for mCherry and each marker of interest (NeuN, Iba1, or Sox10 to identify neurons, microglia or oligodendrocytes respectively) was quantified. Z-stacks were acquired −0.5 mm to +0.5 mm anterior-posterior to the injection site in 0.5 mm intervals (3 sections per injection per marker of interest). All cells in the 20x z-stack were counted using ImageJ. The percentage of double-labeled cells for each image was calculated as the total number of mCherry-positive cells that also expressed the marker of interest over the total counts of mCherry-immunoreactive cells in the images.
2.8.3. Electron microscope analysis
Electron microscope observations were conducted to identify the type of subcellular elements identified by mCherry. Two blocks were obtained per structure (GPe, putamen, STN), and at least 1,200 μm2 of tissue was imaged and analyzed from each block. Structures containing immunoperoxidase reaction product (identifiable as a dark amorphous deposit in the tissue) were categorized as astrocytic processes based on the criteria established by (Peters et al. 1991), including: (1) irregular and tortuous outlines (as opposed to the round and smooth outline of dendrites and unmyelinated axons), (2) shape imposed by surrounding elements of the neuropil, (3) in thin processes, lack of mitochondria, endoplasmic reticulum and microtubules, (4) frequently found surrounding axonal terminals or forming end-feet with capillaries, (5) in some cases, presence of cytoplasmic fibrils (although the fibrils were most times obscured by the dense peroxidase reaction product).
3. Results
Two monkeys received injections of AAV5-pZac2.1 gfaABC1D-tdTomato (Figure 1). The monkey MR341 received 2μl (titer 1.5 ×10e13 vg/ml) in the right hemisphere GPe and a second 2μl injection in the right putamen. MR348 received 1μl (titer 1.5×10e12 vg/ml) in the left GPe and 1μl (titer 1.5×10e13 vg/ml) in the right GPe. Since three injections were done in the GPe among the 2 animals, we refer to these injection conditions as high (2 μl) vs low (1 μl) volume and low (10e12 vg/ml) vs high (10e13 vg/ml) titer.
Figure 1. Schematic of viral injections.

Virus expressing tdTomato under the GfaABC1D promoter was injected under three conditions. A) Monkey MR341R received two injections depositing in the GPe and putamen. B) Monkey MR348 received a low titer injection in the left hemisphere GPe and C) a high titer injection in the right hemisphere GPe. In all three injections, the location of the deposits occurred in the GPe approximately 16mm anterior to the interaural line.
3.1. Description of transduced cells across brain regions
mCherry labeling was identified at the injection targets (GPe, putamen) as expected. We also found mCherry labeling in the internal segment of the globus pallidus (GPi), motor thalamus, cortex, lateral habenula, and subthalamic nucleus (STN), as described below.
After the high-volume condition injection in monkey MR341, dense labeling occurred in structures near the injection sites in putamen and GPe. Dense labeling was also observed in the adjacent GPi, which likely received virus which diffused from the injection site in the GPe, Figure 2A–C). However, robust cell labeling was observed in areas remote from the injection site. Remarkably, there was significant transduction in the STN (Figure 2D). Sparser labeling also occurred in areas of cortex remote from the injection site (i. e., primary motor cortex, Figure 2F). We also identified several mCherry-positive cells in the ventral motor thalamus, the lateral habenula (localized >6 mm posterior to the injection site) and the pedunculopontine nucleus (PPN) (Figure 2E, 2G–H). We speculate that the transduction in remote regions could be a result of transport of the virus along axonal pathways (see Discussion).
Figure 2. Pattern of tdTomato expression in putative astrocytes across brain regions.
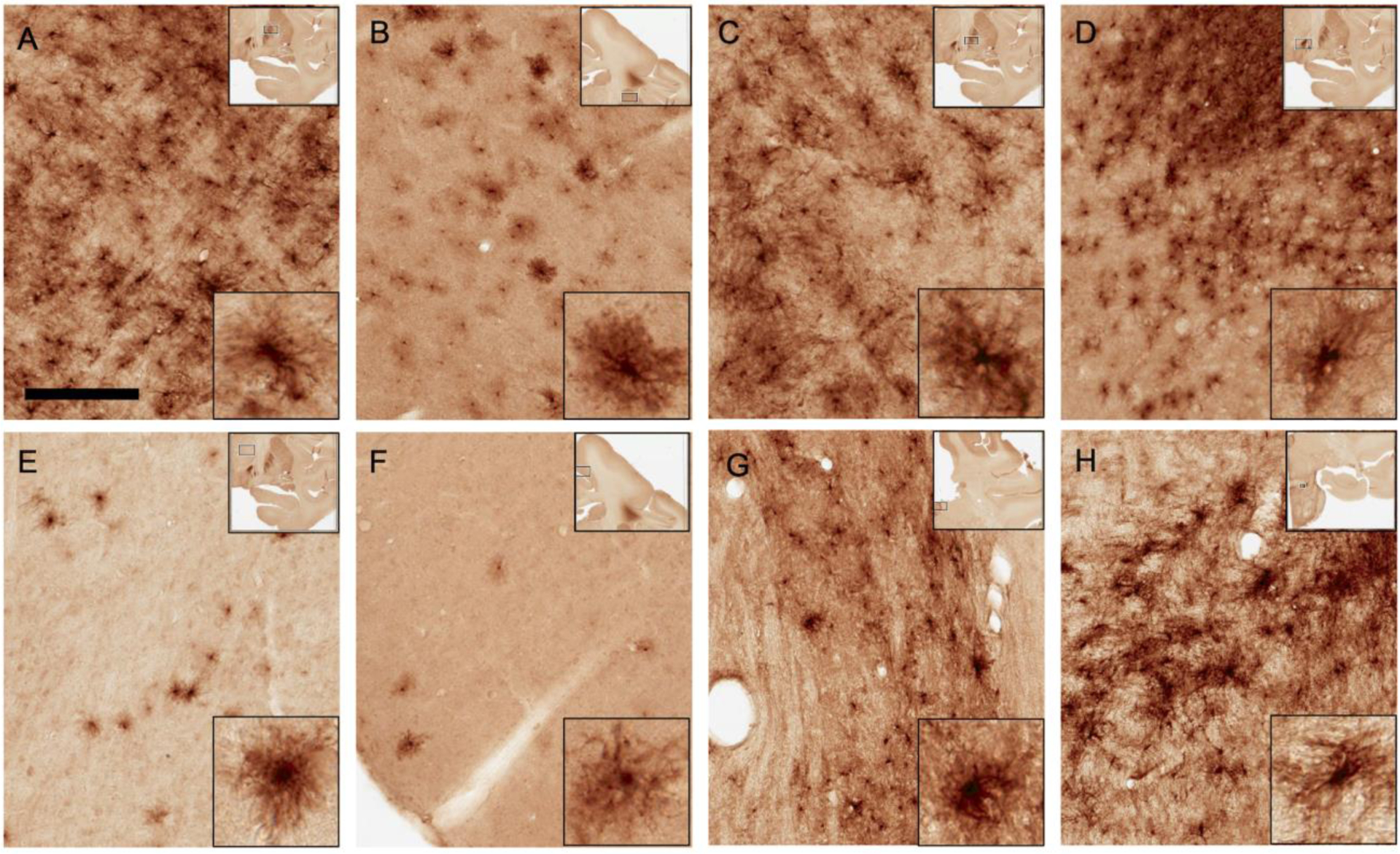
60μm sections were stained for mCherry and revealed with immunoperoxidase. A) Representative image of mCherry+ cells in the GPe. B) Representative image of mCherry+ cells in the putamen. C) Representative image of mCherry+ cells in the GPi. D) Dense transduction of cells in the subthalamic nucleus (STN). E-F) Sparse transduction of cells was also observed in the motor thalamus (E) and motor cortex (F). G-H) mCherry+ cells were also observed in the lateral habenula (G) and pedunculopontine nucleus (H). Upper and lower insets show lower and higher magnification, respectively, of the region shown in main panel. All images from monkey MR341. Scale bar corresponds to 400 μm in main images and 100 μm in lower right insets.
In monkey MR348, which received low volume injections in each GPe (with low titer and high titer in different hemispheres), the labeling was mostly restricted to the GPe with sparse labeling in GPi or putamen. However, as seen after the high-volume injection, numerous cells in the STN displayed strong labeling (data not shown). Importantly, in all regions with mCherry labeling, the morphology of transduced cells resembled that of astrocytes. Cells typically had small oval shaped somata and bushy tortuous processes (insets in Figure 2, Figure 3). Colabeling studies with the astrocyte marker Aldh1L1 demonstrated that the large majority of transduced cells in the GPe, putamen and STN were astrocytes (Figure 3). Transduced cells in these regions were also positive for GFAP (Supplementary Figure 2).
Figure 3. The GfaABC1D promoter drives specific transduction of astrocytes across brain regions.

Representative images of TdTomato+ cells stained for mCherry (first row, magenta) in the GPe (A), putamen (B), and STN (C). The sections were co-labeled with Aldh1L1 (second row, green), demonstrating that transduction occurs specifically within astrocytes (merged images, third row, white). Example images are from animal MR341 (A and C), and MR348, low titer injection (B) Scale bar corresponds to 50 μm in columns A and B, 20 μm in C.
3.2. Efficiency of the GfaABC1D Promoter
We focused the characterization of the GfaABC1D promoter in the GPe, where dense mCherry labeling was seen after all three injection conditions. MCherry-positive cells were observed up to 3.6 mm in the anterior-posterior axis from the injection sites in all conditions (Figure 4). Since the diffusion of the virus was analyzed −1.2 to 3.6 mm anterior to posterior of the injection site, the spread along that axis was at least 4.8 mm. In all three injection conditions, the number of transduced cells remained stable ±1.2 mm from the injection site and decreased from 2.4 mm onwards.
Figure 4. Efficiency of the GfaABC1D promoter.

The number of transduced cells (visualized by mCherry immunoreactivity) was counted in four sections per injection case. (A) Example of transduced cells in the GPe (monkey MR348, high titer injection, example image obtained at the injection site) labeled with mCherry (magenta). B) The same image as A, showing also the Aldh1L1 staining (green). Most cells show colocalization between mCherry and Aldh1L1. C) Number of mCherry+ cells quantified at various distances from the injection site. D) Percentage of Aldh1L1+ cells that were also mCherry+ in each section. In C and D, each dot represents the number of cells counted per section.
The percentage of transduced astrocytes (calculated as the total number of mCherry+ and Aldh1L1+ double labeled cells divided by the total number of Aldh1L1+ cells), ranged between 70–98% ± 1.2 mm from the injection site (Figure 4B). However, as expected by the number of transduced cells (Figure 4C), the proportion of transduced astrocytes significantly declined 3.6 mm from the injection site (Figure 4D). Co-labeling studies with Aldh1L1 to determine the percentage of transduced astrocytes were only assessed for both hemispheres of monkey MR348 (low titer, high titer) due to lack of remaining available tissue for MR341 (as explained in Methods). However, we were able to use counts of mCherry+ cells for Figure 4C in animal MR341 using co-labeled sections for cell-type specificity studies (sections were stained for mCherry and either NeuN, Iba1, or Sox10 as described in the next section). These results indicate that the GfaABC1D promoter transduces astrocytes with high efficiency in the GPe.
3.3. Specificity of the GfaABC1D Promoter
We then characterized the specificity of the promoter’s ability to restrict transgene expression to astrocytes and no other cell types. Sections at the injection site and ±0.5 mm away were stained for mCherry to identify transduced cells and co-stained for cell-type specific markers (NeuN: neurons, Iba1: microglia, Sox10: oligodendrocytes). DAPI was used as a general marker for cells (Figure 5). In all conditions, mCherry colocalization with Iba1 or Sox10 remained under 10% of the total number of transduced cells, calculated as the total number of double labeled mCherry+ and marker of interest+ cells divided by the total number of mCherry+ cells (Figure 5, A4, B4, C4). However, the high titer condition resulted in a slight increase in the number of transduced neurons, reaching 20% closest to the injection site (Figure 5 A4). After the injection in the putamen, we found that mCherry+ cells did not colocalize with NeuN, Iba1, or Sox10 (Supplementary Figure 1).
Figure 5. Specificity of the GfaABC1D promoter.

The number of transduced cells (visualized by mCherry immunoreactivity) that colocalized with markers of other cell types (neurons: NeuN, microglia Iba1, oligodendrocytes: Sox10) was quantified for all injection cases. A-C) Representative images obtained in the GPe (monkey MR348, high titer injection) of mCherry staining in magenta with cell-type markers in green (A: NeuN, B: Iba1, C: Sox10) and DAPI in blue. The first two rows show cell-type marker and mCherry individually, while the third row is merged. The fourth row (A4-C4) shows percentage of mCherry+ cells that colocalized with the cell-type marker of interest. Each dot indicates number of cells counted per section. Scale bar corresponds to 100 μm in columns A and 50 μm in B and C.
Overall, this data shows that the GfaABC1D promoter was highly specific to drive transgene expression exclusively in astrocytes and that titer or volume injected had little effect on specificity of transduction.
3.4. Electron Microscopy
Finally, we conducted electron microscopy (EM) studies to analyze the cellular and subcellular compartments that expressed tdTomato (revealed by mCherry) in the GPe, putamen, and STN of MR341 (high volume injection). For each structure, approximately 1,200 μm2 of tissue was analyzed. Expression of mCherry was evident even in fine astrocytic processes near synaptic terminals, dendrites, and blood vessels (Figure 6).
Figure 6. mCherry expression is restricted to astrocytic processes, as revealed by electron microscope observations.

Fine processes of mCherry+ astrocytes were visible in the GPe (A-B), STN (C-D), and putamen (E-F). Red arrows indicate mCherry+ astrocyte process, blue circles indicate dendrites, ter: terminals, per: pericyte
Astrocyte processes were often found directly bordering synaptic terminals (Figure 6A) as well as dendrites (Figure 6B), as shown in example micrographs obtained in the GPe and putamen (Figure 6E–F). Astrocyte processes were also seen in close proximity to blood vessels and pericytes (Figure 6C–D, example micrograph obtained in the STN). In all structures, the labeled elements displayed astrocytic ultrastructural features (Peters et al. 1991). Furthermore, we did not find any mCherry expression in neuronal profiles (dendrites, axonal terminals, unmyelinated axons) in all structures examined.
4. Discussion
We have evaluated the specificity and efficiency of the GfaABC1D promoter to transduce astrocytes in the NHP globus pallidus and striatum. As previously reported in rodents, the GfaABC1D promoter is highly astrocyte-specific, rarely transducing other cell types. Within the three virus conditions tested in this study (low titer, high titer, and large volume), all demonstrated high specificity for astrocytes.
4.1. Specificity and Efficiency of the Promoter
We found robust expression of the marker protein in the regions injected with the virus (GPe and putamen) and, in the case of the high-volume injection, there was also strong expression in the GPi, a structure adjacent to the GPe and in which the transgene expression was likely due to diffusion of the virus solution from the injection site in the GPe. We injected virus into the putamen in only one animal, and therefore did not provide quantification of efficiency or specificity. However, our qualitative observations indicate that the efficiency and specificity in the putamen was similar to that seen in the GPe (see Supplementary Figure 1).
Transduced cells were identified by co-expression of Aldh1L1. We chose to use Aldh1L1 as a pan-astrocytic marker to determine efficiency of the promoter. There is no known marker that labels all astrocytes, however, Aldh1l1 labels a larger proportion of astrocytes than GFAP and other commonly used markers (Cahoy et al. 2008). Transduced cells also co-expressed GFAP in most cases (see Supplementary Figure 2). Our electron microscope results further confirmed the astrocytic identity of transduced cells. The ultrastructural observations demonstrated that within individual astrocytes, tdTomato was expressed throughout fine processes, surrounding neuronal terminals and blood vessels (Fig. 5). The high proportion of transduced cells (>75% within 1 mm from injection site, Fig. 4) also supports the use of this promoter to effectively express transgenes in most astrocytes in the GPe. There were some instances in which non-astrocytic cell types were transduced. However, we found that within 0.5mm of the injection site after the “high titer” condition, nearly 20% of all transduced cells were co-labeled with NeuN (Figure 5A4). The tropism of a viral vector can vary depending on the titer, which could explain the decrease of specificity of the GfaABC1D promoter in regions that received high viral loads (Nathanson et al., 2009).
4.2. Non-injected Areas Demonstrating Transduction
In addition to expression in regions that received injections of virus solutions (or structures adjacent to them), we observed selective expression of tdTomato in cortical and white matter regions along the injection tract, probably due to leak of the virus solution as the injection system was retracted.
We also observed that the transgene tdTomato was abundantly expressed in the subthalamic nucleus (STN), and more sparsely in the motor thalamus, lateral habenula and pedunculopontine nucleus (Fig. 2). In all cases where transduction occurred in remote areas, the morphology of the transduced cells resembled astrocytes, suggesting cell-type specific transduction. In addition, in the STN we confirmed the astrocytic phenotype of transduced cells with Aldh1l1 (Fig. 3) and electron microscope observations (Fig. 5).
Although there is limited evidence for trans-synaptic retrograde and anterograde transport of AAVs (Burger et al. 2004; Markakis et al. 2010), it is possible that the viral particles were transported anterogradely along axons from the injection sites to connected regions, where the particles were released from the axon terminals and taken up by astrocytes. Retrograde transport of AAV9 and uptake by astrocytes in the non-human primate has been previously reported, though cell-type specific promoters were not used (Green et al., 2016). The STN has both afferent and efferent connections with the GPe allowing for the possibility of such transport to occur (Shink et al. 1996). Along the same line, the motor thalamus, the lateral habenula and the pedunculopontine nucleus receive projections from the GPi (Shink et al. 1997; Parent et al. 1999, 2001), thus offering a potential path for the expression of the transgene in brain regions remotely located from the injection site. It is also possible that the mCherry-positive cells in cortical regions remote to the injection track could have received the viral particles through retrograde transport from injection site in the putamen, which is known to receive wide projections from the whole cortex (Kemp and Powell 1970; McFarland and Haber 2000). It is also possible that the virus particles were taken up by axons of passage (Xu et al., 2020), however no major axonal pathways transverse in the areas injected.
It is unlikely that viral particles were transported through gap junctions in the astrocyte syncytium, due to the reported pore size of gap junctions and size of AAV vectors (Bennett et al. 2003; McIntosh et al. 2021). In addition, if virus particles were to travel through the syncytium, one would expect tiled astrocytic labelling connecting the structures (i.e., injection in the GPe and expression in the thalamus would likely need to show expression in internal capsule which lies between the basal ganglia and thalamus, but this was not observed).
4.3. Comparison of Other Methods
While the use of the GfaABC1D promoter in this study provided specific and efficient transduction of astrocytes in the rhesus macaque GPe, a variety of other promoters and transgenic lines have been used to specifically target astrocytes in rodents and marmosets. Aldh1L1 transgenic mice have commonly been used to drive transgene expression in astrocytes (Srinivasan et al. 2016). While Aldh1L1 has demonstrated specificity in transgenic mice, when packaged in an AAV, it results in significant transduction of neurons in many brain regions (Mudannayake et al. 2016; Koh et al. 2017). Given that transgenic lines are not currently available for practical reasons in NHP research, AAVs (or other viral vectors) are necessary to drive cell-type specific expression of transgenes. A study in marmosets used different fragments of the gfa2 sequence to drive expression in astrocytes, demonstrated a tradeoff between sequence length and specificity, such that a minimum sequence length of 0.3 kb was necessary to minimize non-specific transduction of neurons. This specific shortened gfa2 promoter provides a similar specificity and efficiency to GfaABC1D, but so far, the specificity has only been assessed in the cortex and cerebellum in marmosets (Shinohara et al. 2016). In addition to transgenic lines and AAVs, lentivirus has been used to transduce astrocytes in rodents. While lentiviruses have greater packaging capabilities than AAVs, their larger size restrict diffusion across large brain regions (Huda et al. 2014).
4.4. Consideration of AAV Serotype
AAV5, AAV2, and AAV9 are the most commonly used serotypes for transgene delivery in the brain. We selected the AAV5 serotype due its documented glial tropism in the rhesus macaque basal ganglia (Markakis et al. 2010). In our studies, the AAV5 serotype led to significant transduction of astrocytes with the GfaABC1D promoter. However, changes in serotype may shift expression to neurons or other cell types, and thus must be carefully considered.
4.5. Limitations
In the current study, we addressed specificity and efficiency of the GfaABC1D promoter in the GPe of rhesus macaques. It is possible that the results found in this study may not be applicable to other structures, as astrocytes demonstrate regional differences in gene expression, morphology, and other characteristics (Chai et al. 2017). However, based on our study and the possible transport or diffusion of virus particles, we did observe specific transduction in striatum, GPi, STN, cortex, motor thalamus, and lateral habenula. Our study included a limited number of subjects, and our results may not represent patterns of transduction in older or younger subjects.
4.6. Conclusion
The characterization of the GfaABC1D promoter in NHPs offers a promising new frontier in astrocyte research. Previous studies have found differences between rodent and primate astrocytes in terms of morphology and gene expression, but studies addressing functional changes have been severely limited (Oberheim et al. 2009). The use of this promoter will allow astrocyte-specific expression of DREADDs and opsins to manipulate astrocytic function, as has been done in rodent studies (Yu et al. 2020), furthering our knowledge of the functions of these cells in non-human primate models.
While a few studies have assessed astrocyte morphology in primates, further investigation has been limited by technical challenges. Techniques such as ballistic labeling and GFAP labeling are either time and resource prohibitive or unreliable, labeling an incomplete portion of a given astrocyte (Bushong et al. 2002; Oberheim et al. 2006, 2009). Our electron microscope studies showed AAV-mediated labeling in distal astrocytic processes, indicating that tag protein expression driven by the GfaABC1D promoter could be used in studies of astrocytic morphology. Furthermore, the addition of the membrane tethering Lck sequence, which has been shown to increase expression of the transgene in fine processes and leaflets, should be considered when morphological analysis is of interest (Benediktsson et al. 2005).
We have focused the characterization of the use of the GfaABC1D promoter in the macaque basal ganglia given the importance of these structures in movement-related human pathologies (Galvan et al. 2015). Specifically within the GPe, astrocytic function is altered in rodent models of parkinsonism and may contribute to motor deficits (Cui et al. 2016; Chazalon et al. 2018). Overall, the GfaABC1D promoter is a specific and efficient means of identifying astrocytes in the NHP and may be used to drive transgene expression in this cell type.
Supplementary Material
Highlights.
The promoter GfaABC1D was used to transduce astrocytes in the non-human primate (NHP) brain.
GfaABC1D is specific for astrocytes in several brain regions in the NHP assessed by colocalization of cell-type specific markers.
Transduced astrocytes reliably express the transgene of interest in fine processes as verified in the electron microscope.
Acknowledgments
The authors thank Susan Jenkins, Jean-Francois Paré, and Xing Hu for their technical assistance. This research was supported by the National Institutes of Health’s Office of the Director, Office of Research Infrastructure Programs, P51 OD011132, and grant T32NS096050 (YS and KH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT authorship contribution statement
Kate Heffernan: Conceptualization, Investigation, Methodology, Visualization, Writing – Original Draft. Kazi Rahman: Investigation. Yoland Smith: Writing – Review & Editing. Adriana Galvan: Conceptualization, Supervision, Writing – Review & Editing.
Declaration of Competing Interest
The authors declare no conflict of interest.
References
- Albaugh DL, Smith Y, Galvan A (2020) Comparative analyses of transgene expression patterns after intra-striatal injections of rAAV2-retro in rats and rhesus monkeys: A light and electron microscopic study. Eur J Neurosci 52:4824–4839. 10.1111/ejn.15027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benediktsson AM, Schachtele SJ, Green SH, Dailey ME (2005) Ballistic labeling and dynamic imaging of astrocytes in organotypic hippocampal slice cultures. Journal of Neuroscience Methods 141:41–53. 10.1016/j.jneumeth.2004.05.013 [DOI] [PubMed] [Google Scholar]
- Bennett MVL, Contreras JE, Bukauskas FF, Sáez JC (2003) New roles for astrocytes: Gap junction hemichannels have something to communicate. Trends Neurosci 26:610–617. 10.1016/j.tins.2003.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk OS, Velardo MJ, et al. (2004) Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther 10:302–317. 10.1016/j.ymthe.2004.05.024 [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH (2002) Protoplasmic Astrocytes in CA1 Stratum Radiatum Occupy Separate Anatomical Domains. J Neurosci 22:183–192. 10.1523/JNEUROSCI.22-01-00183.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, et al. (2008) A Transcriptome Database for Astrocytes, Neurons, and Oligodendrocytes: A New Resource for Understanding Brain Development and Function. J Neurosci 28:264–278. 10.1523/JNEUROSCI.4178-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai H, Diaz-Castro B, Shigetomi E, et al. (2017) Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron 95:531–549.e9. 10.1016/j.neuron.2017.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazalon M, Paredes-Rodriguez E, Morin S, et al. (2018) GAT-3 Dysfunction Generates Tonic Inhibition in External Globus Pallidus Neurons in Parkinsonian Rodents. Cell Rep 23:1678–1690. 10.1016/j.celrep.2018.04.014 [DOI] [PubMed] [Google Scholar]
- Cui Q, Pitt JE, Pamukcu A, et al. (2016) Blunted mGluR Activation Disinhibits Striatopallidal Transmission in Parkinsonian Mice. Cell Reports 17:2431–2444. 10.1016/j.celrep.2016.10.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR (1971) Activity of pallidal neurons during movement. J Neurophysiol 34:414–427. 10.1152/jn.1971.34.3.414 [DOI] [PubMed] [Google Scholar]
- DeLong MR (1973) Putamen: activity of single units during slow and rapid arm movements. Science 179:1240–1242. 10.1126/science.179.4079.1240 [DOI] [PubMed] [Google Scholar]
- Galvan A, Devergnas A, Wichmann T (2015) Alterations in neuronal activity in basal ganglia-thalamocortical circuits in the parkinsonian state. Front Neuroanat 9:5. 10.3389/fnana.2015.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Raper J, Hu X, Paré J-F, Bonaventura J, Richie CT, Michaelides M, Mueller SAL, Roseboom PH, Oler JA, Kalin NH, Hall RA, Smith Y, 2019. Ultrastructural localization of DREADDs in monkeys. Eur J Neurosci 50, 2801–2813. 10.1111/ejn.14429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green F, Samaranch L, Zhang HS, Manning-Bog A, Meyer K, Forsayeth J, Bankiewicz KS, 2016. Axonal transport of AAV9 in nonhuman primate brain. Gene Ther 23, 520–526. 10.1038/gt.2016.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huda F, Konno A, Matsuzaki Y, et al. (2014) Distinct transduction profiles in the CNS via three injection routes of AAV9 and the application to generation of a neurodegenerative mouse model. Mol Ther Methods Clin Dev 1:14032. 10.1038/mtm.2014.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jüttner J, Szabo A, Gross-Scherf B, et al. (2019) Targeting neuronal and glial cell types with synthetic promoter AAVs in mice, non-human primates and humans. Nature Neuroscience 22:1345–1356. 10.1038/s41593-019-0431-2 [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP (1970) The cortico-striate projection in the monkey. Brain 93:525–546. 10.1093/brain/93.3.525 [DOI] [PubMed] [Google Scholar]
- Kliem MA, Wichmann T (2004) A method to record changes in local neuronal discharge in response to infusion of small drug quantities in awake monkeys. J Neurosci Methods 138:45–49. 10.1016/j.jneumeth.2004.03.015 [DOI] [PubMed] [Google Scholar]
- Koh W, Park YM, Lee SE, Lee CJ (2017) AAV-Mediated Astrocyte-Specific Gene Expression under Human ALDH1L1 Promoter in Mouse Thalamus. Exp Neurobiol 26:350–361. 10.5607/en.2017.26.6.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Messing A, Su M, Brenner M (2008) GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia 56:481–493. 10.1002/glia.20622 [DOI] [PubMed] [Google Scholar]
- Lee Y, Su M, Messing A, Brenner M (2006) Astrocyte heterogeneity revealed by expression of a GFAP-LacZ transgene. Glia 53:677–687. 10.1002/glia.20320 [DOI] [PubMed] [Google Scholar]
- Markakis EA, Vives KP, Bober J, et al. (2010) Comparative transduction efficiency of AAV vector serotypes 1–6 in the substantia nigra and striatum of the primate brain. Mol Ther 18:588–593. 10.1038/mt.2009.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland NR, Haber SN (2000) Convergent inputs from thalamic motor nuclei and frontal cortical areas to the dorsal striatum in the primate. J Neurosci 20:3798–3813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh NL, Berguig GY, Karim OA, et al. (2021) Comprehensive characterization and quantification of adeno associated vectors by size exclusion chromatography and multi angle light scattering. Sci Rep 11:3012. 10.1038/s41598-021-82599-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudannayake JM, Mouravlev A, Fong DM, Young D (2016) Transcriptional activity of novel ALDH1L1 promoters in the rat brain following AAV vector-mediated gene transfer. Mol Ther Methods Clin Dev 3:16075. 10.1038/mtm.2016.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai J, Rajbhandari AK, Gangwani MR, et al. (2019) Hyperactivity with Disrupted Attention by Activation of an Astrocyte Synaptogenic Cue. Cell 177:1280–1292.e20. 10.1016/j.cell.2019.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson JL, Yanagawa Y, Obata K, Callaway EM, 2009. Preferential labeling of inhibitory and excitatory cortical neurons by endogenous tropism of AAV and lentiviral vectors. Neuroscience 161, 441–450. 10.1016/j.neuroscience.2009.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Takano T, Han X, et al. (2009) Uniquely hominid features of adult human astrocytes. J Neurosci 29:3276. 10.1523/JNEUROSCI.4707-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Wang X, Goldman S, Nedergaard M (2006) Astrocytic complexity distinguishes the human brain. Trends in Neurosciences 29:547–553. 10.1016/j.tins.2006.08.004 [DOI] [PubMed] [Google Scholar]
- Octeau JC, Chai H, Jiang R, et al. (2018) An optical neuron-astrocyte proximity assay at synaptic distance scales. Neuron 98:49–66.e9. 10.1016/j.neuron.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent M, Lévesque M, Parent A (1999) The pallidofugal projection system in primates: evidence for neurons branching ipsilaterally and contralaterally to the thalamus and brainstem. J Chem Neuroanat 16:153–165. 10.1016/s0891-0618(99)00008-3 [DOI] [PubMed] [Google Scholar]
- Parent M, Lévesque M, Parent A (2001) Two types of projection neurons in the internal pallidum of primates: single-axon tracing and three-dimensional reconstruction. J Comp Neurol 439:162–175. 10.1002/cne.1340 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang X-F, Toga A (2000) The Rhesus Monkey Brain in Stereotaxic Coordinates. Academic Press [Google Scholar]
- Peters A, Palay SL, Webster H (1991) Astrocytes. In: The Fine Structure of the Nervous System: Neurons and Their Supporting Cells, 3rd edn. pp 276–294 [Google Scholar]
- Pignataro D, Sucunza D, Vanrell L, Lopez-Franco E, Dopeso-Reyes IG, Vales A, Hommel M, Rico AJ, Lanciego JL, Gonzalez-Aseguinolaza G, 2017. Adeno-Associated Viral Vectors Serotype 8 for Cell-Specific Delivery of Therapeutic Genes in the Central Nervous System. Frontiers in Neuroanatomy 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskanzer KE, Yuste R (2016) Astrocytes regulate cortical state switching in vivo. Proc Natl Acad Sci U S A 113:E2675–E2684. 10.1073/pnas.1520759113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Bushong EA, Haustein MD, et al. (2013) Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J Gen Physiol 141:633–647. 10.1085/jgp.201210949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shink E, Bevan MD, Bolam JP, Smith Y (1996) The subthalamic nucleus and the external pallidum: two tightly interconnected structures that control the output of the basal ganglia in the monkey. Neuroscience 73:335–357 [DOI] [PubMed] [Google Scholar]
- Shink E, Sidibé M, Smith Y (1997) Efferent connections of the internal globus pallidus in the squirrel monkey: II. Topography and synaptic organization of pallidal efferents to the pedunculopontine nucleus. J Comp Neurol 382:348–363 [PubMed] [Google Scholar]
- Shinohara Y, Konno A, Takahashi N, et al. (2016) Viral Vector-Based Dissection of Marmoset GFAP Promoter in Mouse and Marmoset Brains. PLoS ONE 11:e0162023. 10.1371/journal.pone.0162023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Lu T-Y, Chai H, et al. (2016) New Transgenic Mouse Lines for Selectively Targeting Astrocytes and Studying Calcium Signals in Astrocyte Processes In Situ and In Vivo. Neuron 92:1181–1195. 10.1016/j.neuron.2016.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger G, Tereshchenko J, Kügler S (2017) A MicroRNA124 Target Sequence Restores Astrocyte Specificity of gfaABC1D-Driven Transgene Expression in AAV-Mediated Gene Transfer. Mol Ther Nucleic Acids 8:13–25. 10.1016/j.omtn.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S, Acker L, Afraz A, et al. (2020) An Open Resource for Non-human Primate Optogenetics. Neuron 108:1075–1090.e6. 10.1016/j.neuron.2020.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Wang T, Sun GY, Ding S (2010) Specific disruption of astrocytic Ca2+ signaling pathway in vivo by adeno-associated viral transduction. Neuroscience 170:992–1003. 10.1016/j.neuroscience.2010.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Holmes TC, Luo M-H, Beier KT, Horwitz GD, Zhao F, Zeng W, Hui M, Semler BL, Sandri-Goldin RM, 2020. Viral Vectors for Neural Circuit Mapping and Recent Advances in Trans-synaptic Anterograde Tracers. Neuron 107, 1029–1047. 10.1016/j.neuron.2020.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Nagai J, Khakh BS (2020) Improved tools to study astrocytes. Nature Reviews Neuroscience 21:121–138. 10.1038/s41583-020-0264-8 [DOI] [PubMed] [Google Scholar]
- Yu X, Taylor AMW, Nagai J, et al. (2018) Reducing Astrocyte Calcium Signaling In Vivo Alters Striatal Microcircuits and Causes Repetitive Behavior. Neuron 99:1170–1187.e9. 10.1016/j.neuron.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sloan SA, Clarke LE, et al. (2016) Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89:37–53. 10.1016/j.neuron.2015.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


