Abstract
Endocannabinoids are traditionally thought to have an analgesic effect. However, it has been shown that while endocannabinoids can depress nociceptive signaling, they can also enhance non-nociceptive signaling. Therefore, endocannabinoids have the potential to contribute to non-nociceptive sensitization after an injury. Using Hirudo verbana (the medicinal leech), a model of injury-induced sensitization was developed in which a reproducible piercing injury was delivered to the posterior sucker of Hirudo. Injury-induced changes in the non-nociceptive threshold of Hirudo were determined through testing with Von Frey filaments and changes in the response to nociceptive stimuli were tested by measuring the latency to withdraw to a nociceptive thermal stimulus (Hargreaves apparatus). To test the potential role of endocannabinoids in mediating injury-induced sensitization, animals were injected with tetrahydrolipstatin (THL), which inhibits synthesis of the endocannabinoid transmitter 2-arachidonoylglycerol (2-AG). Following injury, a significant decrease in the non-nociceptive response threshold (consistent with non-nociceptive sensitization) and a significant decrease in the response latency to nociceptive stimulation (consistent with nociceptive sensitization) was observed. In animals injected with THL a decrease in non-nociceptive sensitization in injured animals was observed, but no effect on nociceptive sensitization was observed.
Keywords: sensitization, nociception, endocannabinoid, leech, Hirudo
Introduction
Pain is a critical neurobehavioral function that involves both sensory and emotional components (Kuner and Kuner 2021). The sensory component is often referred to as nociception, the detection of damaging or potentially damaging stimuli (Smith and Lewin 2009). Sensitization, or amplification of a response to stimuli, as a result of injury can be an adaptive process that prevents further damage to the injured area of the body. In the context of pain, sensitization can manifest as hyperalgesia, increased sensitivity/responsiveness to painful stimuli, or as allodynia, increased sensitivity/responsiveness to non-painful stimuli (Anderson 2015). Nociception exhibits considerable evolutionary conservation, so studies of invertebrates can lead to important insights relevant to the nociceptive processes throughout the animal kingdom, including modulation due to sensitization (Walters 1994; Smith and Lewin 2009; Im and Galko 2012; Burrell 2017). The terms hyperalgesia and allodynia imply both the sensory and affective components of pain, and it is not clear if emotional components of pain apply to invertebrates (Anderson and Adolphs 2014; Perry and Baciadonna 2017). Therefore, in studies using invertebrates we have restricted ourselves to the terms nociception rather than pain, nociceptive sensitization in place of hyperalgesia, and non-nociceptive sensitization in place of allodynia.
The present studies of injury–induced sensitization were conducted using Hirudo verbana, the medicinal leech. The advantages of Hirudo include a well-described central nervous system (CNS) in which the neurons that make up several functional neural circuits are known. This allows researchers to link the cellular properties of identifiable neurons and/or synapses to contributions at the behavioral level (Kristan et al. 2005; Wagenaar 2015). In particular the somatosensory neurons in Hirudo are well-characterized and have a number of properties similar to those found in the vertebrate CNS (Smith and Lewin 2009; Burrell 2017; Walters and Williams 2019). Non-nociceptive mechanical input is detected by rapidly-adapting touch-sensitive (T cells) and slow-adapting pressure-sensitive (P cells) neurons (Nicholls and Baylor 1968). Hirudo also possess mechanical and polymodal nociceptors (N cells) (Nicholls and Baylor 1968; Blackshaw et al. 1982; Pastor et al. 1996). N cells elicit a rapid withdrawal reflex, whole body shortening, when activated. P cells can also elicit whole body shortening when activated at sufficiently high frequencies (Shaw and Kristan 1995).
Previous work by our group has focused on the role of endocannabinoids in modulating nociceptive and non-nociceptive synapses. Endocannabinoids are lipid-based neurotransmitters, primarily 2-arachidonoylglycerol (2-AG) and anandamide, that are synthesized on-demand (Katona and Freund 2012). They are most often involved in retrograde signaling, where the neurotransmitter is released by the postsynaptic neuron and then binds to presynaptic receptors. These receptors include both metabotropic (e.g., cannabinoid or CB1/2) and ionotropic (transient receptor potential vanilloid or TRPV1) receptors. 2-AG, anandamide, and the enzymes involved in their synthesis and metabolism are well-represented throughout the animal kingdom (Elphick 2012; Paulsen and Burrell 2019). Therefore comparative studies of neurobehavioral modulation by endocannabinoids can help to inform the use of potential cannabinoid-based therapies, e.g. the treatment of pain.
In prior experiments, we have found that endocannabinoids had opposing effects related to nociception. They depressed transmission by nociceptive N cell synapses, but potentiated non-nociceptive P cell synapses (Yuan and Burrell 2010; Higgins et al. 2013; Wang and Burrell 2016). These opposing effects were observed at the behavioral level in vivo and using semi-intact preparations, with endocannabinoids reducing responses to nociceptive stimuli or N cell activation and potentiating responses to non-nociceptive stimuli or P cell activation (Yuan and Burrell 2013; Summers et al. 2017; Wang and Burrell 2018). The goal of this project was to develop a method for producing a persistent sensitized state in Hirudo as a result of injury in vivo and to test whether endocannabinoid signaling contributed to this injury-induced sensitization. We hypothesized that while endocannabinoids contributed to injury-induced non-nociceptive sensitization, they did not contribute to nociceptive sensitization.
Methods
The animals used are medicinal leeches, Hirudo verbana, that are ordered from Niagara Medicinal Leeches (Niagara Falls, ON) and each weigh approximately 3g. The animals are sustained in an artificial pond water (0.5g sea salt per L of Barnstead water) and kept in a 15°C incubator with a 12 hour light/dark cycle to imitate a natural setting. The needed drugs are prepared from frozen stocks that are added to Hirudo saline solution (in mM: 114 NaCl, 4 KCl, 1.8 CaCl2, 1 MgCl2, 5 NaOH, and 10 HEPES; pH = 7.4) just prior to the injection period. Lipopolysaccharide (LPS) from E. coli, dimethyl sulfoxide (DMSO), fast green, and tetrahydrolipostatin (THL) were obtained from Sigma-Aldrich, St. Louis, MO. Both LPS and THL were dissolved in DMSO prior to being frozen. 1% by weight fast green was also dissolved into the LPS final solution just prior to injection. Vehicle control experiments were performed in saline containing equivalent levels of DMSO (0.025%) and fast green when necessary.
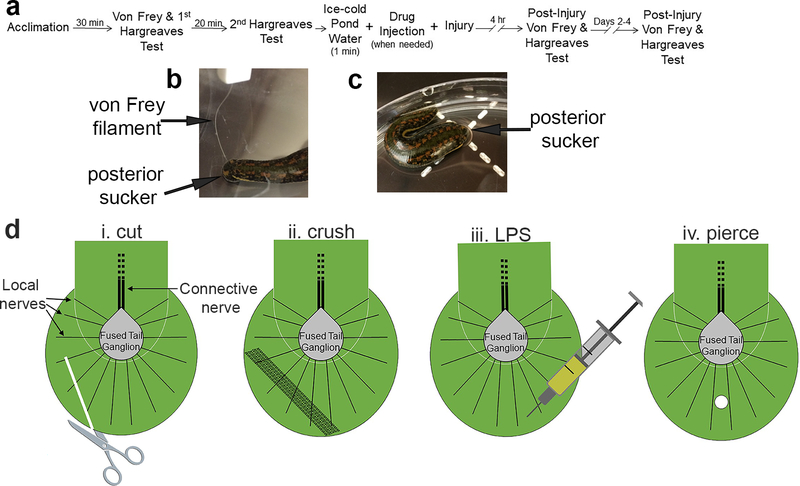
The timeline of the experiments can be seen in Fig. 1a. To begin an experiment, 50mL of pond water is poured into a 14 cm diameter petri dish at room temperature (22–23°C). Next, one animal is placed in each petri dish and the lid is then placed on top of the petri dish to prevent the animal from crawling out of the dish. Following a period of 30 minutes to allow the animals to acclimate, a baseline measure of threshold for the non-nociceptive stimulus is taken. This is done using Von Frey Filaments (Fig. 1b), which are monofilaments of varying thicknesses that each bend at a different set amount of force and deliver only that amount of force. The initial method that was used to determine the non-nociceptive threshold through Von Frey testing was the ascending method, in which the filament of the lowest force is tested first, followed by increasing levels of force until a response (a localized shortening reflex) occurs. The first filament that elicits the localized shortening is recorded as the threshold. This method was used for the cut, crush, and LPS injuries. After examining additional literature on von Frey methods, the simplified up and down (SUDO) method began to be used as described by McMackin et al. (McMackin et al. 2016). In the SUDO method a mid-range level of stimulus is identified and is the first stimulus that the animal receives, determined to be filament number 2.83, which applies 0.07g of force. The filament is applied to the posterior sucker of the leech, as seen in Fig. 1b. The response to this stimulation was either a local shortening of the posterior sucker or no response at all. If there is no response, after 60 seconds the filament of the next larger amount of force is applied. If there is movement in response to the stimulus, after 60 seconds, the filament of the next smallest amount of force applied is used. This pattern is continued until the Hirudo responds to two differing filament numbers in a row. The amount of force applied by these two filaments is averaged and recorded as the baseline threshold for the animal.
Figure 1:
Experimental methods for studying injury-induced sensitization in Hirudo. (a) Timeline for experimental protocol. (b) Example of application of a von Frey filament to the posterior sucker of Hirudo. (c) Example of Hirudo in a petri dish set over the Hargreaves apparatus (indicated by the dashed plus sign). (d) Graphics illustrating basic anatomy of the posterior sucker and the four injury-inducing protocols in the posterior sucker. The sucker is innervated by 14 local nerves (only three are labeled) that radiate from the fused tail ganglion. The tail ganglion is in the portion of the leech body that terminates just before the posterior sucker and this ganglion is connected to anterior segmental ganglia via the connective nerve (Muller et al. 1981). The terminal portion of the leech body also includes musculature and elements of the vascular system and rectum (not shown) and this is the region where the THL injections were carried out. (i) Cut protocol using surgical scissors. (ii) Crush protocol using a hemostat. (iii) LPS injection protocol. (iv) Piercing injury protocol using a T-pin.
Once the non-nociceptive threshold for the animal is determined and recorded, the animal’s nociceptive latency is determined and recorded. The nociceptive latency of the animal is determined using a Hargreaves apparatus, which emits an infrared light that produces a thermal stimulus of increasing heat to the target of interest, eventually reaching the nociceptive heat threshold of the animal (Yeomans and Proudfit 1994). The stimulus is applied to the posterior sucker of the leech, as shown in Figure 1b, and the apparatus measures the amount of time (in seconds) that the stimulus is applied before the leech responds with a whole-body movement, in which the animal removes its sucker from the site of the stimulus. The apparatus will display the amount of time that it took for the animal to withdraw, and this measure is considered to be the nociceptive heat threshold of the animal. This initial measurement is recorded. After 20 minutes or longer if the animal was still moving (no more than 30 mins), an additional measurement is taken using the same procedure. To minimize potential variability from a single behavioral response, a second response latency is measured 20 mins later and the two latencies are averaged and recorded as the baseline nociceptive latency for the animal (Carey et al. 2016). Once all baseline data measurements have been completed, the animals are sorted into the control (no injury) and experimental (injury) groups. Each animal assigned a unique identifier so that the person collecting subsequent behavioral data was blind to the animal’s control vs. experimental group status.
Prior to the testing of the effects of endocannabinoids, four different protocols were evaluated for inducing an injury that results in both non-nociceptive and nociceptive sensitization (summarized in Fig. 1di–iv). For all of these procedures, each animal was first placed in ice-cold pond water for one minute to anesthetize, reduce movement, and make the animal easier to handle. The first injury type that was tested was a cut to the posterior sucker. After baseline data was recorded, the portion of the posterior sucker was cut using a FST 14094–11 Stainless 25R scissors (Fig. 1di). The second type of injury tested was a 20 second crush to the posterior sucker with a 13 cm long hemostat (Fig. 1dii) (Summers et al. 2017). The third type of injury was injection of lipopolysaccharide (LPS), a known inflammatory agent, into the dorsal surface of the posterior sucker (Fig. 1diii). Animals were injected with 50 μL of 3 μM LPS solution along with 0.1% Fast Green to ensure that the injection was effectively entering the sucker. Control animals were injected in the posterior sucker with the vehicle control solution that included Fast Green. The final injury method that was tested was a piercing to the posterior sucker with a 4.6 cm length T pin (Fig. 1div).
For drug injections each animal in the control group was placed in ice cold pond water for one minute in order to slow their movements. Next, the vehicle control solution of 0.025% DMSO is injected into the ventral side of the leech, directly medial to the posterior sucker. The animal is then placed into a jar containing pond water. Animals in the experimental groups were injected with 50 μM of THL on their ventral side, directly medial to the posterior sucker. THL is an inhibitor of diacylglycerol lipase, and thereby prevents synthesis of the 2-AG endocannabinoid. After the injection, animals were placed back into the ice-cold pond water for one minute and then underwent the piercing injury protocol described above. Once testing was completed the animals were placed back into individual jars with pond water and held in a 15°C incubator until subsequent testing either that day or 24 hrs later. This was done so that both the pre- and post-injury tests were conducted following a similar “warm-up” period.
Approximately 3 hours and 15 minutes later, the animals are removed from the incubator and placed back into petri dishes containing 50 mL of pond water at room temperature. They are again allowed 30 minutes to adapt to the dish and to discontinue any movements. After this, the non-nociceptive threshold of each animal is re-measured, and then the nociceptive latency of each animal is re-measured through the steps described above. The measurements of the non-nociceptive threshold and nociceptive latency should be taking place at approximately four hours after the injury was delivered. This process is repeated again so that these measurements are taken at approximately 24 hour intervals after injury.
Once the final measurements have been recorded after the last day of testing after the injury, the experimenter is un-blinded to the identity of the animals, and the baseline data for each animal is compared to the data after the injury. Data for the non-nociceptive stimulus is compared using the difference between the pre- and post-injury values (delta) to analyze how the values following the injury change over time compared to the baseline values. Data for the nociceptive stimulus is compared using a percent change between pre- and post-injury values. The data from these comparisons is entered into SigmaPlot software to be graphed and for statistical analysis to be performed. Results of the behavioral data were normalized relative to baseline test results for both the non-nociceptive and nociceptive tests and are presented as means ± standard error. Statistical analyses through the use of either two-way or three-way analysis of variance (ANOVA) were performed to determine the main effects. Student-Newman-Keuls post hoc tests were used to confirm the ANOVA results. All significance was determined with an α level of at least P ≤ 0.05.
Results
Assessment of methods for injury-induced sensitization
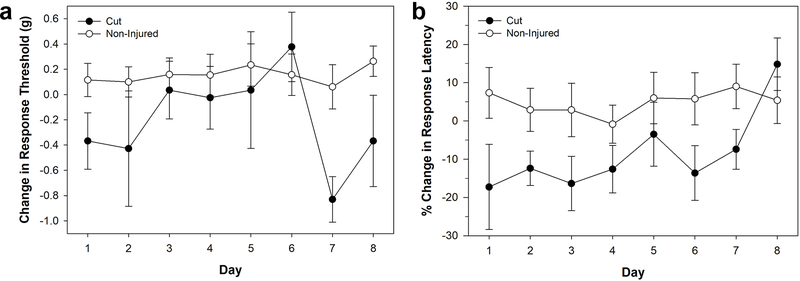
The emphasis in these experiments was to generate a reproducible method for producing injury-induced sensitization that approximated the types of injuries that Hirudo might normally obtain in encounters with potential predators, e.g. fish, amphibians and reptiles. The first injury method tested was a cut to the posterior sucker using dissecting scissors. In terms of responses to non-nociceptive stimuli, a decrease in the response threshold to von Frey filament stimulation was observed in injured animals, but it was quite variable over the seven day testing period (Fig. 2a). Nevertheless, a two-way ANOVA showed a significant decrease in non-nociceptive threshold in the cut-injured (N = 4) compared to the non-injured (N = 9) animals (F1,88 = 8.817, P < 0.01). No significant effect was observed of testing day (F7,88 = 1.491, P > 0.05) or day-injury interaction (F7,88 = 1.081, P > 0.05). In terms of nociceptive sensitization, a decrease in the response latency to the thermal nociceptive stimuli delivered by the Hargreaves apparatus was observed in the injured animals (Fig. 2b). Two-way ANOVA showed a significant difference between the nociceptive sensitization of the cut-injured (N = 4) and non-injured animals (N = 14) (F1,136 = 7.964, P < 0.01), but no significant effect of day of testing (F7,136 = 0.733, P > 0.05) or day-injury interaction (F7,136 = 0.596, P > 0.05). Despite these initial results, this approach was discarded because the effect on non-nociceptive stimuli was highly variable. This may be due, in part, because the cut to the posterior sucker actually reduced the available area for applying the Von Frey filaments. In addition, the extent of the cut to the sucker was difficult to control between animals, leading to different animals having differing levels of injury. These technical issues discouraged us from increasing the sample size of the cut injury group.
Figure 2:
Effects of cut injury to the posterior sucker. (a) Changes in response threshold to von Frey filament stimulation in injured (cut) and non-injured animals. (b) Changes in response latency to thermal stimuli in injured (cut) and non-injured animals. Data is represented as the average ± the standard error.
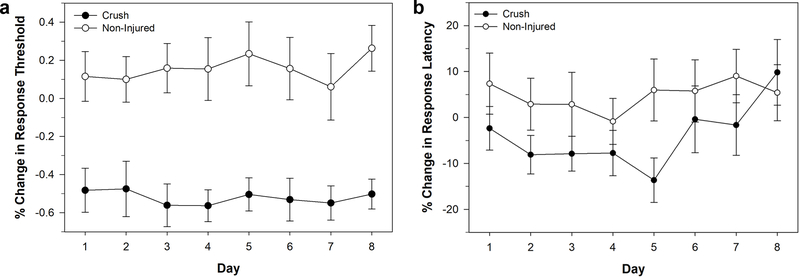
The next approach was a crush to the posterior sucker using a hemostat. The crush injury produced a robust sensitization to non-nociceptive stimuli (Fig. 3a). A two way ANOVA showed a significant decrease in von Frey response threshold in the crush-injured (N = 10) compared to the non-injured (N = 9) animals (F1,136 = 102.536, P < 0.001). There was no significant effect of day (F7,136 = 0.175, P > 0.05), and no significant day-injury interaction effect (F7,136 = 0.145, P > 0.05). There was also evidence of nociceptive sensitization following crush injury (Fig. 3b). Two-way ANOVA showed a significant decrease in the response latency between the-crush injured (N = 10) and non-injured (N=14) animals (F1,184 = 7.195, P < 0.01), but no significant effect of testing day (F7,184 = 0.864, P > 0.05), and no significant injury-day interaction effect (F7,184 = 0.524, P > 0.05). Despite the robust and consistent non-nociceptive sensitization effect and the significant nociceptive sensitization effect, this approach was also discarded. This was because the nociceptive sensitization effect was not especially robust and may have been an artifact of an increase in response latency of the non-injured, control animal, leading to questions about reproducibility of this approach. Another concern was that while it was possible to control the pressure and duration of the crush, it was difficult control for the crush area between animals.
Figure 3:
Effects of crush injury to the posterior sucker. (a) Changes in response threshold to von Frey filament stimulation in injured (crushed) and non-injured animals. (b) Changes in response latency to thermal stimuli in injured (crushed) and non-injured animals. Data is represented as the average ± the standard error.
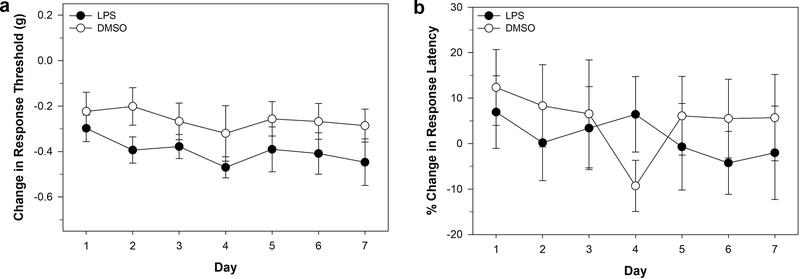
Injections of lipopolysaccharide (LPS) are often used to in pain studies given its capacity to produce inflammation that can occur as a result of injury and/or infection. The effects of LPS were tested in Hirudo via an injection into the posterior sucker. LPS injection (N = 9) produced significant decrease in response threshold to von Frey filaments compared to the vehicle control injected animals (N = 11) indicating non-nociceptive sensitization (Fig. 4a). A two-way ANOVA showed a significant effect of treatment (F1,119 = 8.585, P < 0.01), but no significant effect of testing day (F6,119 = 0.463, P > 0.05) nor treatment-day interaction effect (F6,119 = 0.0977, P > 0.05). No differences were observed in the response latency to thermal nociceptive stimuli between the LPS-injected (N = 9) and vehicle-injected (N = 10) animals, indicating that this treatment did not produce nociceptive sensitization (Fig. 4b). Two-way ANOVA showed no significant effect of treatment (F1,111 = 0.472, P > 0.05), testing day (F6,111 = 0.254, P > 0.05), and treatment-day interaction (F6,111 = 0.254, P > 0.05). The control injection group also showed a decrease in response threshold. Therefore, even though there was a statistically significant effect of LPS, we chose to not continue with this injury-producing procedure.
Figure 4:
Effects of LPS injection to the posterior sucker. (a) Changes in response threshold to von Frey filament stimulation in LPS-injected and vehicle-injected animals. (b) Changes in response latency to thermal stimuli in LPS-injected and vehicle-injected animals. Data is represented as the average ± the standard error.
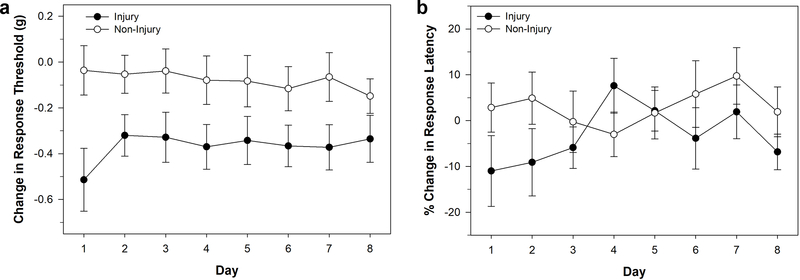
The last injury approach tested was use of a T-pin to pierce the posterior sucker. This produced a much smaller wound compared to the cut injury used in in Figure 2. In fact it was difficult to visually find the site of injury more than a few minutes after piercing, which allowed for effective blinding of experiments. This injury was also more reproducible between animals than the cut and crush injury. Piercing injury (N = 9) did result in a significant decrease in response threshold to von Frey filaments compared to control animals (N = 14) indicating non-nociceptive sensitization (Fig. 5a). A two-way ANOVA showed a significant effect of injury (F1,184 = 27.442, P < 0.001), but no significant effect of testing day (F7,184 = 0.134, P > 0.05), nor injury-day interaction effect (F7,184 = 0.225, P > 0.05). In terms of nociceptive sensitization, there was evidence of reduced response latency compared to control animals on the same day as injury (day 1), with response latency returning to control levels over days 2–8 (Fig. 5b). Two-way ANOVA showed no significant effect of injury (F1,188 = 2.311, P > 0.05), testing day (F7,188 = 0.715, P > 0.05), and injury-day interaction (F7,188 = 0.918, P > 0.05). A comparison of the changes in behavior on Day 1 between the injury and control groups showed that 78% (7 of 9) from the injury group exhibited a decrease in response latency consistent with sensitization, but only 47% (7 of 15) of the control group exhibited increases in response latency.
Figure 5:
Effects of piercing injury to the posterior sucker. (a) Changes in response threshold to von Frey filament stimulation in injured (injury) and non-injured animals. (b) Changes in response latency to thermal stimuli in injured (injury) and non-injured animals. Data is represented as the average ± the standard error.
Role of endocannabinoid signaling
In earlier experiments it was observed that endocannabinoids had opposing effects on both the behaviors elicited by non-nociceptive vs. nociceptive stimuli and the non-nociceptive and nociceptive synapses themselves. Endocannabinoids potentiated non-nociceptive P cell synapses and enhanced responses to non-nociceptive stimuli (Higgins et al. 2013; Summers et al. 2017; Wang and Burrell 2018). However, endocannabinoids depressed nociceptive N cell synapses and reduced response to nociceptive stimuli (Yuan and Burrell 2010, 2013; Summers et al. 2017). In the following experiments the potential role of endocannabinoids to injury-induced sensitization was examined. The piercing injury approach was used given that it produced a robust and reliable sensitization to non-nociceptive stimuli as well as transient sensitization to nociceptive stimuli. To test the potential role of 2-AG in mediating injury-induced sensitization, animals were injected with tetrahydrolipstatin (THL), an inhibitor of diacylglycerol lipase, the main 2-AG synthesizing enzyme.
A pilot set of experiments were carried out testing a range of THL concentrations injected into uninjured Hirudo at 100, 50, and 25 μM plus vehicle control (0.025% DMSO; see Supplemental Fig. 1a). No significant differences were observed between the three THL concentrations and the control group over five days of testing responses to von Frey filaments based on a 2-way ANOVA which detected no effect of THL concentration (F3,90=0.895, p>0.05), testing day (F4,90=1.469, p>0.05), nor concentration-day interaction (F12,90=0.413, p>0.05). There was some evidence of a decrease in response threshold in animals injected with 100 μM THL, but this did not reach statistical significance. In terms of responses to thermal nociceptive stimuli (Supplemental Fig. 1b), the pilot data did indicate that 100 μM THL did affect response latency on its own (F3,10 = 28.43, p<0.001). Based on these preliminary findings, we chose to use 50 μM THL in subsequent injury experiments.
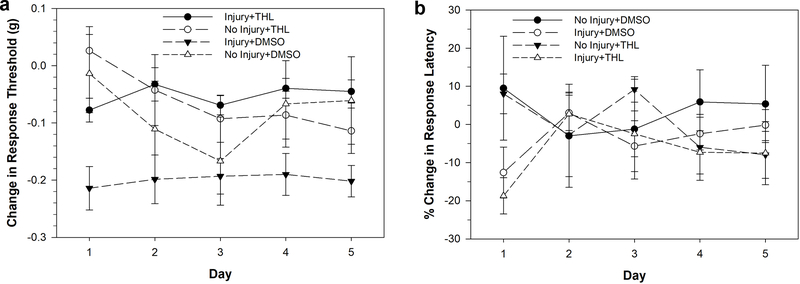
Following injection with either 50 μM THL or 0.025% DMSO as a vehicle control, Hirudo were separated into no-injury and injury groups, with piercing of the posterior sucker used to induce injury. Injury was found to induce a decrease in response threshold to von Frey filaments in the DMSO-injected animals over a five day period, characteristic of non-nociceptive sensitization (Fig. 6a; Injury+DMSO, N=12). DMSO injection without injury had no effect on response threshold (No Injury+DMSO, N=6). However, injured animals that were injected with THL (Injury+THL, N=10) did not exhibit a decrease in response threshold, indicating that non-nociceptive sensitization was blocked. THL did not affect response thresholds in non-injured animals (No Injury+THL, N=4). These results were confirmed by a three-way ANOVA that detected a significant effect of injury (F1,165=5.543, p<0.05), a significant effect of drug treatment (F1,165=13.642, p<0.001), and a significant injury-drug interaction effect (F1,165=7.458, p<0.01). No significant effect of testing day was observed (F4,165=0.699, p>0.05). A post-hoc analysis of the injury-drug interaction effect showed a significant difference between the Injury+THL and Injury+DMSO groups (p<0.001) and a significant difference between the Injury+DMSO and the No Injury+DMSO groups (p<0.001). No significant differences were observed between the No Injury+THL and No Injury+DMSO groups nor between the No Injury+THL and Injury+THL groups.
Figure 6:
Effects 50 μM THL injection on injury-induced sensitization elicited by piercing of the posterior sucker. (a) Injury+DMSO animals did experience a decrease in response threshold indicative of non-nociceptive sensitization. However, this effect of injury was not observed in the Injury+THL animals indicating that THL injection prevented injury-induced non-nociceptive sensitization. No changes in response threshold were observed in the No Injury+DMSO and No Injury-THL groups. (b) Injury+DMSO animals did experience a decrease in response latency indicative of nociceptive sensitization. This effect of injury was also observed in the Injury+THL animals indicating that THL injection had no effect on injury-induced nociceptive sensitization. No changes in response threshold were observed in the No Injury+DMSO and No Injury-THL groups. Data is represented as the average ± the standard error.
Responses to nociceptive thermal stimuli were also analyzed in the Injury+DMSO, No Injury+DMSO, Injury+THL and No Injury+THL groups (Fig, 6b). A decrease in response latency was observed in the two injury groups (DMSO+Injury and THL+Injury) on day 1. Here a statistically significant decrease in response latency was observed on the day 1 in the injury groups, indicating nociceptive sensitization. A two-way ANOVA detected a significant effect of injury (F1,23=6.922, p<0.05). No effect of THL injections (F1,23=0.167, p>0.05) nor an injury-drug interaction effect (F1,23=0.062, p>0.05) were observed. These findings suggest that inhibition of 2-AG synthesis does not have an effect on nociceptive sensitization.
Discussion
This study examined multiple methods of inducing a persistent form of injury-induced sensitization in Hirudo and the role of 2-AG signaling in mediating this sensitization. Of the multiple methods used to induce nociceptive and non-nociceptive sensitization, the piercing injury to the posterior sucker was judged to produce the most reliable results as summarized in Table 1. A cutting injury to the posterior sucker produced nociceptive sensitization, but not non-nociceptive sensitization (Fig. 2). Furthermore, this approach was judged to be difficult to reproduce between animals owing to the fact that Hirudo were only lightly anesthetized by cold and it was difficult to adequately control the animal during the cut. This procedure also likely cut multiple nerves (Fig. 1di) that may have impacted sensory and motor function during the post-injury testing period. Injection of LPS to the posterior sucker produced non-nociceptive sensitization, but not nociceptive sensitization (Fig. 4). Furthermore, vehicle control injections to the posterior sucker may have also produced a degree of non-nociceptive sensitization, based on the observed reduction in response threshold. This may be due to the injection process itself, e.g., either as a result of piercing injury by the injection needle or swelling due to the DMSO injection volume. A crush injury produced non-nociceptive sensitization and nociceptive sensitization (Fig. 3), but the increase in response latency in the non-injured animals made it unclear if this protocol would produce a reproducible nociceptive sensitization effect. The piercing injury to the posterior sucker produced non-nociceptive sensitization that lasted several days and a nociceptive sensitization that was only observed within 4 hours of injury (Fig. 5).
Table 1.
Summary of experimental methods for inducing posterior sucker injury in Hirudo.
| Posterior Sucker cut | Posterior Sucker crush | Posterior Sucker LPS injection | Posterior Sucker pierce | |
|---|---|---|---|---|
|
| ||||
| Non-nociceptive sensitization | Yes (but see below) | Yes | Yes | Yes |
|
|
||||
| Nociceptive sensitization | Yes | Yes (but see below) | No | Yes (transient) |
|
|
||||
| Reason for rejection of injury method | • Level of non-nociceptive sensitization was highly variable day-to-day. • Extent of cut difficult to standardize |
• Differences in injured vs. non-injured groups may have been due to an increase in the response latency in the non-injured (control) group | • No nociceptive sensitization • Non-nociceptive sensitization was not robust • Control-injected animals exhibited decrease in response threshold to von-Frey |
|
The most consistent form of sensitization that was observed was to non-nociceptive mechanosensory stimuli. In Hirudo, the von Frey filaments are most likely activating the P cells, which can initiate local (meaning restricted to a few body segments) withdrawal reflexes, the whole body shortening withdrawal reflex, and locomotory behaviors that could be used to escape noxious stimuli (Kristan et al. 1982; Shaw and Kristan 1995; Kristan et al. 2005). Previous studies using semi-intact preparations have shown that noxious stimuli or direct activation of the N cells elicits long-term potentiation of P cell synapses that is accompanied by an increase in the whole-body shortening produced by P cell stimulation (Wang and Burrell 2018). Increased signaling by non-nociceptive afferents is thought to be a major feature of a number of pain conditions in humans, referred to as allodynia, and is also observed experimental animal models (Liu et al. 2000; Sandkuhler 2009). Furthermore, there is considerable evidence that these sensory neurons can also have access to what are normally nociceptive-specific neural circuits in the spinal cord (Arcourt and Lechner 2015; Petitjean et al. 2015). It is not clear why, in general, non-nociceptive sensitization was more persistent and relatively easier to elicit across all of the injury procedures tested. One possibility is that when signaling by lower threshold sensory neurons is enhanced by injury, there is little adaptive need for the nociceptors to be equally facilitated at least in the methods that we used to elicit injury. This is not to say that increased signaling by nociceptors is not a critical component of injury-induced sensitization since there is considerable evidence of this being the case (Woolf and Ma 2007; Babcock et al. 2009; Smith and Lewin 2009; Crook et al. 2013). It is possible that a more extensive injury and/or an injury that produces substantial damage to the CNS is necessary to elicit nociceptive sensitization in Hirudo.
A piercing injury resulted in both non-nociceptive and nociceptive sensitization (Fig. 5). When 2-AG signaling was disrupted with an injection of THL, non-nociceptive sensitization did not occur (Fig. 6a). Nociceptive sensitization, however, was not affected by disruption of endocannabinoid signaling (Fig. 6b). Together, these results suggest that endocannabinoids do contribute to non-nociceptive, but not nociceptive sensitization. The pro-nociceptive effects of endocannabinoids are thought to be due to depression of inhibitory input to the P mechanosensory afferents, resulting in disinhibition of P synapses (Higgins et al. 2013; Wang and Burrell 2016). A similar effect on cannabinoid signaling has also been observed in the mammalian spinal cord (Pernia-Andrade et al. 2009) and disinhibition of non-nociceptive pathways is a well-recognized mechanism for mediating allodynia/non-nociceptive sensitization (Baba et al. 2003; Torsney and MacDermott 2006; Kim et al. 2012; Lu et al. 2013; Petitjean et al. 2015). Endocannabinoid-mediated disinhibition of non-nociceptive synapses may therefore represent an important, evolutionarily-conserved mechanism for injury-induced sensitization of afferent synaptic circuits.
N cell synapses are directly depressed by endocannabinoids (Yuan and Burrell 2010; Wang and Burrell 2016), explaining why injury-induced nociceptive sensitization was not affected by drugs inhibiting 2-AG synthesis. One question that could be posed is why N synapses are not subject to the same endocannabinoid-mediated disinhibition effects observed in P synapses. The answer seems to be that the N cells themselves have a more depolarized Cl- equilibrium potential and are actually depolarized by GABA(Sargent et al. 1977; Wang et al. 2015). In this way, N synapses are thought to be “protected” from disinhibition.
This study provides a useful template for studying injury-induced sensitization in Hirudo with future studies investigating the cellular mechanisms in more detail or examining how injury affects other behaviors in Hirudo, e.g., feeding, hunting, or learning and memory. The fact that Hirudo studies at both the synaptic physiological and the behavioral level show opposing effects of endocannabinoids on nociceptive vs. non-nociceptive signaling has potential comparative relevance to understanding cannabinoid-based modulation in mammals. The opposing effects of endocannabinoids we have observed in Hirudo have also been observed in rodents (Pernia-Andrade et al. 2009; Kato et al. 2012; Carey et al. 2016). Understanding these pro- and anti-nociceptive effects of endocannabinoids is critical for the effective use of cannabinoid-based therapies to treat pain. While there is clinical evidence supporting the cannabinoid-suppression of pain (Hill et al. 2017; Habib et al. 2019), the actual clinical efficacy of cannabinoids in treating pain has been seriously questioned (Kraft 2012; De Vita et al. 2018; Mücke et al. 2018; Stockings et al. 2018). A likely reason for this controversy is that cannabinoid-based therapies are applied in ways that can potentially elicit both the pro- and anti-nociceptive effects. A more complete understanding of how the nervous system endogenously uses cannabinoids to modulate nociceptive vs. non-nociceptive pathways is necessary to development of more effective cannabinoid-based therapeutics. Perhaps there are specific patterns of activity that normally elicit pro- vs. anti-nociceptive effects. Alternatively, different cannabinoid receptors or compliments of receptors may be responsible for these distinctive effects on nociception. Addressing these questions can be best accomplished by using comparative approaches to understanding the effects of endocannabinoid signaling at the cellular, physiological, and behavioral level.
Supplementary Material
Supplemental Figure 1: Effects of multiple concentrations of THL on response threshold and response latency. (b) 25 and 50 μM THL had no effect on response latency when compared to DMSO-injected animals. 100 μM THL also had no statistically significant effect, although there did appear to be a decrease in response threshold on days 4 and 5. (b) For the initial 4 hour post injury measurement of response latency, 100 μM THL significant reduced response latency compared to the other THL concentrations and the DMSO injected group (*). Data is represented as the average ± the standard error.
Acknowledgements
This work was supported by the University of South Dakota’s Summer Program for Undergraduate Research in Addiction (SPURA), which is funded by the National Institute on Drug Abuse (NIDA) grant R25-DA033674. It was also supported by a National Institute of Neural Disorders and Stroke (NINDS), NIH R01-NS092716 grant.
Abbreviations
- ANOVA
analysis of variance
- 2-AG
2-arachidonoylglycerol
- CB
annabinoid
- CNS
central nervous system
- DMSO
dimethyl sulfoxide
- LPS
ipopolysaccharide
- N cell
nociceptive cell
- P cell
pressure cell
- SUDO
simplified up and down
- THL
tetrahydrolipostatin
- T cell
touch cell
- TRPV
transient receptor potential vanilloid
Footnotes
DECLARATIONS
Conflicts of interest/Competing interests: The authors have no competing interests to declare.
Availability of data and material: Data is available upon request.
Ethics approval: No vertebrate animals were used in these experiments.
Consent to participate: not applicable
Consent to publish: not applicable
References
- Anderson David J, Adolphs R(2014) A framework for studying emotions across species. Cell 157 (1):187–200. doi: 10.1016/j.cell.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcourt A, Lechner SG (2015) Peripheral and spinal circuits involved in mechanical allodynia. Pain 156 (2):220–221. doi: 10.1097/01.j.pain.0000460818.62406.38 [DOI] [PubMed] [Google Scholar]
- Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, Woolf CJ (2003) Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell Neurosci 24 (3):818–830 [DOI] [PubMed] [Google Scholar]
- Babcock DT, Landry C, Galko MJ (2009) Cytokine signaling mediates UV-induced nociceptive sensitization in Drosophila larvae. Curr Biol: CB 19 (10):799–806. doi: 10.1016/j.cub.2009.03.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw SE, Nicholls JG, Parnas I (1982) Physiological responses, receptive fields and terminal arborizations of nociceptive cells in the leech. J Physiol 326:251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell BD (2017) Comparative biology of pain: What invertebrates can tell us about how nociception works. J Neurophysiol 117 (4):1461–1473. doi: 10.1152/jn.00600.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey LM, Slivicki RA, Leishman E, Cornett B, Mackie K, Bradshaw H, Hohmann AG (2016) A pro-nociceptive phenotype unmasked in mice lacking fatty-acid amide hydrolase. Mol Pain 12. doi: 10.1177/1744806916649192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook RJ, Hanlon RT, Walters ET (2013) Squid have nociceptors that display widespread long-term sensitization and spontaneous activity after bodily injury. J Neurosci 33 (24):10021–10026. doi: 10.1523/jneurosci.0646-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vita MJ, Moskal D, Maisto SA, Ansell EB (2018) Association of cannabinoid administration with experimental pain in healthy adults: A systematic review and meta-analysis. JAMA Psychiatry 75 (11):1118–1127. doi: 10.1001/jamapsychiatry.2018.2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elphick MR (2012) The evolution and comparative neurobiology of endocannabinoid signalling. Philos Trans R Soc Lond B Biol Sci 367 (1607):3201–3215. doi: 10.1098/rstb.2011.0394rstb.2011.0394 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib AM, Okorokov AL, Hill MN, Bras JT, Lee M-C, Li S, Gossage SJ, van Drimmelen M, Morena M, Houlden H (2019) Microdeletion in a FAAH pseudogene identified in a patient with high anandamide concentrations and pain insensitivity. Br J Anaesth 123 (2):e249–e253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins A, Yuan S, Wang Y, Burrell B (2013) Differential modulation of nociceptive versus non-nociceptive synapses by endocannabinoids. Molec Pain 9 (1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KP, Palastro MD, Johnson B, Ditre JW (2017) Cannabis and pain: a clinical review. Cannabis Cannabinoid Res 2 (1):96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im SH, Galko MJ (2012) Pokes, sunburn, and hot sauce: Drosophila as an emerging model for the biology of nociception. Dev Dyn 241 (1):16–26. doi: 10.1002/dvdy.22737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Punnakkal P, Pernia-Andrade AJ, von Schoultz C, Sharopov S, Nyilas R, Katona I, Zeilhofer HU (2012) Endocannabinoid-dependent plasticity at spinal nociceptor synapses. J Physiol 590 (Pt 19):4717–4733. doi: 10.1113/jphysiol.2012.234229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Freund TF (2012) Multiple functions of endocannabinoid signaling in the brain. Ann Rev Neurosci 35:529–558. doi: 10.1146/annurev-neuro-062111-150420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Back SK, Davies AJ, Jeong H, Jo HJ, Chung G, Na HS, Bae YC, Kim SJ, Kim JS, Jung SJ, Oh SB (2012) TRPV1 in GABAergic interneurons mediates neuropathic mechanical allodynia and disinhibition of the nociceptive circuitry in the spinal cord. Neuron 74 (4):640–647. doi: 10.1016/j.neuron.2012.02.039 [DOI] [PubMed] [Google Scholar]
- Kraft B (2012) Is there any clinically relevant cannabinoid-induced analgesia? Pharmacology 89 (5–6):237–246. doi: 10.1159/000337376 [DOI] [PubMed] [Google Scholar]
- Kristan WB Jr., Calabrese RL, Friesen WO (2005) Neuronal control of leech behavior. Prog Neurobiol 76 (5):279–327 [DOI] [PubMed] [Google Scholar]
- Kristan WB, McGirr SJ, Simpson GV (1982) Behavioural and Mechanosensory Neurone Responses to Skin Stimulation in Leeches. J of Exp Biol 96 (1):143–160 [Google Scholar]
- Kuner R, Kuner T (2021) Cellular circuits in the brain and their modulation in acute and chronic pain. Physiol Rev 101 (1):213–258 [DOI] [PubMed] [Google Scholar]
- Liu CN, Wall PD, Ben-Dor E, Michaelis M, Amir R, Devor M (2000) Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain 85 (3):503–521. doi:S0304395900002517 [pii] [DOI] [PubMed] [Google Scholar]
- Lu Y, Dong H, Gao Y, Gong Y, Ren Y, Gu N, Zhou S, Xia N, Sun YY, Ji RR, Xiong L (2013) A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J Clin Invest 123 (9):4050–4062. doi: 10.1172/JCI70026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMackin MZ, Lewin MR, Tabuena DR, Arreola FE, Moffatt C, Fuse M (2016) Use of von Frey filaments to assess nociceptive sensitization in the hornworm, Manduca sexta. J Neurosci Methods 257:139–146. doi: 10.1016/j.jneumeth.2015.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W (2018) Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev (3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller KJ, Nicholls JG, Stent GS (1981) Neurobiology of the Leech. Cold Spring Harbor Laboratory Press, Cold Spring Habbor [Google Scholar]
- Nicholls JG, Baylor DA (1968) Specific modalities and receptive fields of sensory neurons in CNS of the leech. J Neurophysiol 31 (5):740–756 [DOI] [PubMed] [Google Scholar]
- Pastor J, Soria B, Belmonte C (1996) Properties of the nociceptive neurons of the leech segmental ganglion. J Neurophysiol 75 (6):2268–2279 [DOI] [PubMed] [Google Scholar]
- Paulsen RT, Burrell BD (2019) Comparative studies of endocannabinoid modulation of pain. Philos Trans R Soc Lond B Biol Sci 374 (1785):20190279. doi: 10.1098/rstb.2019.0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernia-Andrade AJ, Kato A, Witschi R, Nyilas R, Katona I, Freund TF, Watanabe M, Filitz J, Koppert W, Schuttler J, Ji G, Neugebauer V, Marsicano G, Lutz B, Vanegas H, Zeilhofer HU (2009) Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization. Science 325 (5941):760–764. doi:325/5941/760 [pii] 10.1126/science.1171870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry CJ, Baciadonna L (2017) Studying emotion in invertebrates: what has been done, what can be measured and what they can provide. J Exp Biol 220 (21):3856–3868 [DOI] [PubMed] [Google Scholar]
- Petitjean H, Pawlowski SA, Fraine SL, Sharif B, Hamad D, Fatima T, Berg J, Brown CM, Jan LY, Ribeiro-da-Silva A, Braz JM, Basbaum AI, Sharif-Naeini R (2015) Dorsal horn parvalbumin neurons are gate-keepers of touch-evoked pain after nerve injury. Cell Rep 13 (6):1246–1257. doi: 10.1016/j.celrep.2015.09.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkuhler J (2009) Models and mechanisms of hyperalgesia and allodynia. Physiol Rev 89 (2):707–758. doi:89/2/707 [pii] 10.1152/physrev.00025.2008 [DOI] [PubMed] [Google Scholar]
- Sargent PB, Yau KW, Nicholls JG (1977) Extrasynaptic receptors on cell bodies of neurons in central nervous system of the leech. J Neurophysiol 40 (2):446–452 [DOI] [PubMed] [Google Scholar]
- Shaw BK, Kristan WB Jr. (1995) The whole-body shortening reflex of the medicinal leech: motor pattern, sensory basis, and interneuronal pathways. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 177 (6):667–681 [DOI] [PubMed] [Google Scholar]
- Smith ES, Lewin GR (2009) Nociceptors: a phylogenetic view. J Comp Physiol A Neuroeth Sen Neural Beh Physiol 195 (12):1089–1106. doi: 10.1007/s00359-009-0482-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockings E, Campbell G, Hall WD, Nielsen S, Zagic D, Rahman R, Murnion B, Farrell M, Weier M, Degenhardt L (2018) Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain 159 (10):1932–1954 [DOI] [PubMed] [Google Scholar]
- Summers T, Hanten B, Peterson W, Burrell B (2017) Endocannabinoids Have Opposing Effects On Behavioral Responses To Nociceptive And Non-nociceptive Stimuli. Sci Rep 7 (1):5793. doi: 10.1038/s41598-017-06114-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsney C, MacDermott AB (2006) Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci 26 (6):1833–1843. doi:26/6/1833 [pii] 10.1523/JNEUROSCI.4584-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenaar DA (2015) A classic model animal in the 21st century: recent lessons from the leech nervous system. J Exp Biol 218:3353–3359. doi: 10.1242/jeb.113860 [DOI] [PubMed] [Google Scholar]
- Walters ET (1994) Injury-related behavior and neuronal plasticity: an evolutionary perspective on sensitization, hyperalgesia, and analgesia. Int Rev Neurobiol 36:325–427 [DOI] [PubMed] [Google Scholar]
- Walters ET, Williams ACdC (2019) Evolution of mechanisms and behaviour important for pain. Phil Trans R So B 374 (1785):1–8. doi: 10.1098/rstb.2019.0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Burrell BD (2016) Differences in chloride gradients allow for three distinct types of synaptic modulation by endocannabinoids. J Neurophysiol 116 (2):619–628. doi: 10.1152/jn.00235.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Burrell BD (2018) Endocannabinoid-mediated potentiation of nonnociceptive synapses contributes to behavioral sensitization. J Neurophysiol 119 (2):641–651. doi: 10.1152/jn.00092.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Summers T, Peterson W, Miiller E, Burrell BD (2015) Differential effects of GABA in modulating nociceptive vs. non-nociceptive synapses. Neuroscience 298:397–409. doi: 10.1016/j.neuroscience.2015.04.040 [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Ma Q (2007) Nociceptors--noxious stimulus detectors. Neuron 55 (3):353–364. doi:S0896–6273(07)00537–5 [pii] 10.1016/j.neuron.2007.07.016 [DOI] [PubMed] [Google Scholar]
- Yeomans DC, Proudfit HK (1994) Characterization of the foot withdrawal response to noxious radiant heat in the rat. Pain 59 (1):85–94. doi: 10.1016/0304-3959(94)90051-5 [DOI] [PubMed] [Google Scholar]
- Yuan S, Burrell BD (2010) Endocannabinoid-dependent LTD in a nociceptive synapse requires activation of a presynaptic TRPV-like receptor. J Neurophysiol 104 (5):2766–2777. doi: 10.1152/jn.00491.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Burrell BD (2013) Nonnociceptive afferent activity depresses nocifensive behavior and nociceptive synapses via an endocannabinoid-dependent mechanism. J Neurophysiol 110 (11):2607–2616. doi: 10.1152/jn.00170.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Effects of multiple concentrations of THL on response threshold and response latency. (b) 25 and 50 μM THL had no effect on response latency when compared to DMSO-injected animals. 100 μM THL also had no statistically significant effect, although there did appear to be a decrease in response threshold on days 4 and 5. (b) For the initial 4 hour post injury measurement of response latency, 100 μM THL significant reduced response latency compared to the other THL concentrations and the DMSO injected group (*). Data is represented as the average ± the standard error.