SUMMARY
BACKGROUND:
Persons with hemophilia (PWH) are at risk for chronic hemophilic arthropathy (HA). Joint replacement surgery may be used to relieve intractable pain and/or restore joint function.
OBJECTIVES:
This multicenter, prospective, observational cohort study evaluated the rate of bleeding during the postoperative period after total hip (THA) or knee arthroplasty (TKA).
PATIENTS/METHODS:
We included PWH of any severity ≥18 years of age who were undergoing THA or TKA. Clinical decisions were made at the discretion of the treating physician according to local standards of care. Clinical data were prospectively recorded. Major bleeding was defined as bleeding in a critical site, bleeding that resulted in either a 2 g/dL or greater decrease in hemoglobin during any 24-hour period, or transfusion of two or more units of packed red blood cells.
RESULTS:
One hundred thirty-one procedures (98 TKA and 33 THA) were performed, 39 (29.8%) of which were complicated by major bleeding, including 46% of THA and 25% of TKA. The risk of major bleeding was increased in THA compared to TKA (OR 2.50, p=0.05), and by the presence of an inhibitor (OR 4.29, p=0.04), increased BMI (OR 4.49 and 6.09 for overweight and obese, respectively, compared to normal BMI, each p<0.01), and non-use of an antifibrinolytic medication (OR 3.00, p=0.03). Neither continuous clotting factor infusion (versus bolus infusion) nor pharmacologic thromboprophylaxis were associated with bleeding risk.
CONCLUSIONS:
The bleeding risk remains substantial after THA and TKA in PWH, despite factor replacement. Use of antifibrinolytic medications is associated with decreased risk.
Keywords: antifibrinolytics, arthroplasty, hemophilia, hemorrhage, risk factors
INTRODUCTION
Persons with hemophilia (PWH) are at risk for chronic hemophilic arthropathy (HA), a progressive disease caused by repeated intraarticular bleeding with subsequent inflammation and damage to bone, cartilage, and other joint structures. HA typically affects large joints, including knees, hips, ankles, and elbows. HA causes significant pain, disability, and impaired health-related quality of life.[1–4] The widespread adoption of prophylactic factor replacement has not conferred total protection against the development of HA; even in PWH receiving primary or secondary clotting factor prophylaxis, many still develop HA.[5–7] PWH who progress to end-stage HA are candidates for joint replacement surgery, which is considered an effective intervention for providing relief from intractable pain and restoring joint function.[8–10]
Major orthopedic surgeries, such as total knee arthroplasty (TKA) and total hip arthroplasty (THA), carry a significant risk of perioperative blood loss and need for transfusion, even in the general population.[11–13] These risks are potentially heightened in PWH due to their underlying bleeding diathesis and despite clotting factor replacement. Wang et al reported that postoperative transfusion, hospital stays, and hospital charges were greater in patients with hemophilia after TKA and THA compared to those without hemophilia.[14] Furthermore, in a recent retrospective analysis of in-hospital and readmission data, Chiasakul et al reported greater 30-day readmission rates and prolonged length of stay, in addition to more bleeding events in PWH undergoing TKA and THA than controls without hemophilia.[15]
The American College of Chest Physicians and the American Academy of Orthopaedic Surgeons recommend that patients undergoing THA or TKA receive either pharmacologic or mechanical thromboprophylaxis postoperatively to prevent venous thromboembolism (VTE).[16, 17] However, the use of pharmacologic thromboprophylaxis potentially further exacerbates the risk of bleeding in PWH. Prospective data on short-term or long-term outcomes in PWH undergoing major lower extremity orthopedic surgery are scarce. Most existing data on perioperative complications such as major bleeding and transfusion rates come from single-center, retrospective studies.[18–21] Therefore, in this study, we prospectively collected and analyzed ‘real world’ data on bleeding events and transfusion needs during post-operative hospitalization in PWH undergoing TKA or THA.
PATIENTS AND METHODS
We conducted a prospective, observational cohort study in 14 institutions to investigate outcomes associated with total hip or knee arthroplasty in PWH. Rates of postoperative VTE in the initial 46 subjects in this study were previously reported.[22] PWH scheduled for joint replacement surgery were identified prospectively by each participating center and asked to participate. We included subjects with hemophilia A or B of any severity who were at least 18 years of age, could provide informed consent and were willing to return for follow-up 4–6 weeks after surgery. The unit of analysis for this study was defined as any qualifying operative intervention (unilateral or bilateral). Individuals who participated in the study were eligible to enroll in the study again if they underwent a second qualifying procedure on a different date.
Study visits occurred immediately prior to and 4–6 weeks after surgery. For this analysis, we evaluated bleeding events that occurred during surgery or in the period of hospitalization following the procedure, as well as bleeding that led to re-hospitalization any time during the first 6 weeks after discharge. Sites also reported other clinically significant or adverse events relevant to the study. Data were recorded in real-time using a secure web-based data capture system (REDCap, Vanderbilt University). All clinical treatment decisions, including perioperative clotting factor management, postoperative rehabilitation, and VTE prophylaxis and/or treatment were made at the discretion of each treating physician according to individual local standards of care. The study was approved by each center’s institutional review board, and all subjects provided informed consent prior to enrollment.
Clinical and Outcome Variables
Clinical and outcomes data were abstracted from medical charts and obtained through patient and clinician interview. We collected information describing each subject’s type and severity of hemophilia, history or presence of inhibitors, peri-operative clotting factor product and dose, postoperative hemoglobin levels, and any VTE prophylaxis received. We considered a participant to have an active inhibitor if he received a bypass agent (recombinant activated factor VII or activated prothrombin complex concentrate) as the primary hemostatic treatment during the peri-operative period. We classified patients as having received antifibrinolytic (aminocaproic acid or tranexamic acid) or pharmacologic thromboprophylaxis (low molecular weight heparin, direct oral anticoagulants, or warfarin) if they were administered any doses of these medications during the hospitalization. Likewise, subjects were considered to have been treated with continuous infusion of clotting factor if they received factor by this method at any time during their hospitalization.
The primary outcome for this study was major bleeding during surgical procedures or the post-operative hospitalization period. Bleeding that occurred after hospital discharge was examined separately. We used a modified version of the International Society on Thrombosis and Haemostasis definition of major postoperative bleeding, defined as follows: any bleeding that occurred in a critical site (intracranial, intraspinal, intraocular, pericardial, intraarticular other than the operative site, intramuscular with compartment syndrome, or retroperitoneal) or bleeding at any site that resulted in any of the following outcomes: 1) decrease in hemoglobin of 2 g/dL or more during any 24-hour period, 2) transfusion of two or more units of packed red blood cells (pRBC) during hospitalization, or 3) death.[23] Minor bleeding was defined as any clinically overt bleeding at a site other than the operative site that did not meet the criteria for major bleeding.
Secondary outcomes included bleeding into the surgical joint space, return to the operating room, and hospital readmission within 6 weeks of discharge. In addition to information describing hemostatic and thromboprophylactic treatments, we also recorded venous or arterial thrombotic events. Additional significant clinical events, including the development of factor VIII (FVIII) or factor IX (FIX) inhibitors prior to the postsurgical follow-up visit or documented infections in the surgical joint were reported by each site.
Statistical Analyses
We analyzed the primary study outcome using both univariate and multivariable logistical regression analyses. We examined the relationships of both patient- and treatment-related variables to the primary outcome, including age, body mass index (BMI) category (<25 kg/m2, normal; 25–30 kg/m2, overweight; >30 kg/m2, obese), hemophilia type (A or B), inhibitor status, type of surgery, factor infusion strategy, use of antifibrinolytics, and use of anticoagulants. Following completion of univariate analyses, we used forward stepwise conditional multivariable logistic regression and included variables that had a p-value of 0.20 or lower in the univariate analyses. Missing data are described in Tables 1–3. We did not impute missing data for statistical analyses (complete case analysis). All statistical operations were carried out using the R and SPSS (v27) statistical packages.
Table 1:
Demographic and Clinical Characteristics of 131 THA and TKA Procedures in PWH.
| Patient Characteristic | |
|---|---|
| Age in years, mean (median, range) | 47.4 (49.6, 22.2 – 72.2) |
| Hemophilia type, n (%) | |
| A | 107 (81.7) |
| B | 22 (16.8) |
| Missing | 2 (1.5) |
| Hemophilia severity, n (%) | |
| Severe | 86 (65.6) |
| Moderate | 20 (15.2) |
| Mild | 22 (16.8) |
| Missing | 3 (2.3) |
| Active inhibitor at time of surgery, n (%) | 12 (9.2) |
| Weight (kg), mean (median, range) | 89.2 (85, 51.0 – 148.6) |
| Body mass index category, n (%) | |
| Normal / underweight (<25 kg/m2) | 38 (29.0) |
| Overweight (25–30 kg/m2) | 44 (33.6) |
| Obese (>30 kg/m2) | 43 (32.8) |
| Missing | 6 (4.6) |
| Viral infection history, n (%) | |
| HIV | 24 (18.3) |
| HCV | 95 (72.5) |
THA=total hip arthroplasty, TKA=total knee arthroplasty, PWH=persons with hemophilia, HIV=human immunodeficiency virus, HCV=hepatitis C virus
Table 3:
Clinical factors potentially related to bleeding risk in men with hemophilia A or B during hospitalization following THA or TKA.
| Variable | Number with Major Bleeding, n/total (%) |
|---|---|
| Procedure type | |
| THA | 15/33 (45.5) |
| TKA | 24/98 (24.5) |
| Hemophilia type | |
| A | 33/107 (30.8) |
| B | 6/22 (27.3) |
| Hemophilia severity | |
| Severe | 25/86 (29.1) |
| Moderate | 7/20 (35) |
| Mild | 7/22 (31.8) |
| Active inhibitor* | |
| Present | 6/12 (50) |
| Not present | 33/110 (30.0) |
| Body mass index category | |
| Normal/underweight (<25 kg/m2) | 5/38 (13.2) |
| Overweight (25–30 kg/m2) | 16/44 (36.4) |
| Obese (>30 kg/m2) | 18/43 (41.9) |
| Clotting factor administration approach | |
| Continuous infusion | 13/46 (28.3) |
| Bolus only | 24/75 (32) |
| Use of antifibrinolytic medication | |
| Yes | 8/38 (21.1) |
| No | 31/87 (35.6) |
| Use of pharmacologic thromboprophylaxis | |
| Yes (low molecular weight heparin) | 2/7 (28.6) |
| No | 37/119 (31.1) |
defined as use of bypass agent as primary hemostatic medication during / after procedure
THA=total hip arthroplasty, TKA=total knee arthroplasty
RESULTS
Between February 2010 and October 2020, there were 131 procedures at the fourteen participating sites. The mean age of subjects at the time of the surgical procedure was 47.4 years (47.8 years for THA and 47.3 years for TKA), and mean weight and BMI were 89.2 kg and 27.8 kg/m2, respectively (Table 1). Race/ethnicity were self-reported as 73.3% white, 15.3% African American, 7.6% Hispanic, 3.1% Asian, and 0.8% other/unknown. A history of FVIII or FIX inhibitor was noted in 26.7% of subjects, and 9.2% (n=12) of procedures were performed in subjects with active inhibitors, as defined above. Study procedures included 98 TKA (74.8%) and 33 THA (25.2%) (Table 2). Four individuals underwent bilateral knee replacement, which we considered as a single procedure in this analysis.
Table 2:
Procedures Performed by Hemophilia Type / Severity and Clinical Management Strategies.
| Characteristic | THA, n | TKA**, n | Total, n |
|---|---|---|---|
| Hemophilia type and severity* | |||
| A (total) | 26 | 81 | 107 |
| Severe | 16 | 47 | 63 |
| Moderate | 1 | 15 | 16 |
| Mild | 5 | 10 | 15 |
| Active inhibitor | 3 | 9 | 12*** |
| Missing severity | 1 | 0 | 1 |
| B (total) | 7 | 15 | 22 |
| Severe | 3 | 10 | 13 |
| Moderate | 1 | 3 | 4 |
| Mild | 3 | 2 | 5 |
| Clotting factor administration | |||
| Continuous infusion | 9 | 37 | 46 |
| Bolus infusions only | 23 | 52 | 75 |
| Missing | 1 | 9 | 10 |
| Antifibrinolytic therapy | |||
| Yes | 7 | 31 | 38 |
| No | 25 | 62 | 87 |
| Missing | 1 | 5 | 6 |
| Postoperative thromboprophylaxis | |||
| None | 14 | 37 | 51 |
| Pharmacologic only | 0 | 1 | 1 |
| Mechanical only | 17 | 51 | 68 |
| Pharmacologic + Mechanical | 1 | 5 | 6 |
| Missing | 1 | 4 | 5 |
Two participants missing hemophilia type.
Four participants underwent bilateral total knee arthroplasty procedures.
10 with severe hemophilia A, 2 with mild hemophilia A
THA=total hip arthroplasty; TKA=total knee arthroplasty.
Of the 131 procedures, 39 (29.8%) were complicated by major bleeding during the postoperative hospitalization period (Table 3). One unit of pRBC was transfused during hospitalization in 7 cases (5.3%), which were categorized as having minor bleeding. No other minor bleeding events were reported during this period. Of the 39 surgeries complicated by major bleeding events, 22 had a hemoglobin drop of more than 2 grams/dL in a 24-hour period, 10 had post-operative transfusion of 2 or more units of pRBCs, and 7 met both criteria (Figure 1). No subject had bleeding into a critical site or fatal bleeding.
Figure 1: Major bleeding events during hospitalization following 131 total hip and knee arthroplasty procedures in men with hemophilia A or B. No patients had fatal or critical site bleeds.
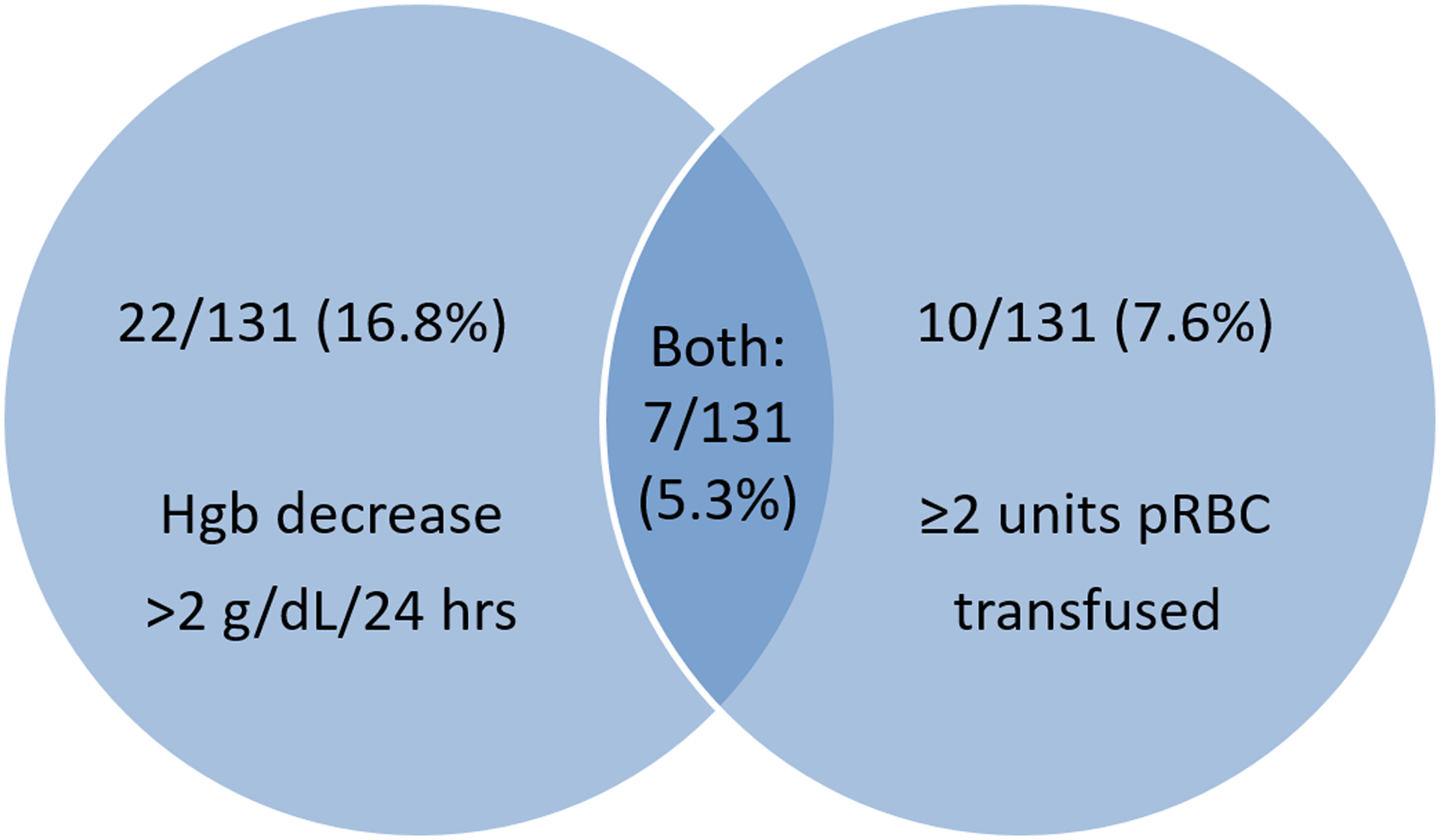
Hgb=hemoglobin, pRBC=packed red blood cells
Fourteen subjects experienced confirmed or suspected bleeding into the surgical joint space post-operatively. Thirteen out of fourteen (92.9%) of the suspected or confirmed operative joint bleeds occurred after TKA. One knee bleed required a return to the operating room for management. Four out of five operative joint bleeds diagnosed prior to discharge occurred in subjects who were also classified as having major or minor bleeding during hospitalization. The remaining 9 operative joint bleeds occurred after discharge from the hospital. Multiple possible causes were suspected for these outpatient bleeds, including missing doses of factor prior to physical therapy, sustaining injuries, and participating in routine physical activities such as walking. Nine subjects (6.9%) were readmitted to the hospital during the 6 weeks following initial discharge. Six were readmitted for management of symptoms concerning for bleeding into the operative joint. After evaluation in the hospital, 2 of these 6 were felt to be due to operative joint bleeding and 4 were felt to be due to other causes. The remaining patients were admitted for management of VTE (2 patients) and central line infection (1 patient).
Twelve patients with active inhibitors received recombinant activated factor VII (rFVIIa) prior to, during, and immediately following their surgical procedures. None received activated prothrombin complex concentrate (aPCC) initially, although one subject who was initially treated with FVIII experienced an anamnestic inhibitor response on post-operative day 6 and was treated with aPCC thereafter. Half the patients with active inhibitors experienced major bleeding (Table 3). Major bleeding occurred in 37/119 patients (31.1%) who did not receive pharmacologic thromboprophylaxis versus 2/7 (28.6%) who received low molecular weight heparin (no patient received aspirin or other anticoagulants). Of the nine participants noted to have cirrhosis or liver dysfunction at the time of surgery, one (11.1%) had major bleeding. The proportions of patients who had major bleeding with and without other treatment characteristics are summarized in Table 3.
In the multivariable analysis, factors independently associated with increased odds of major bleeding included THA (OR 2.50, 95% CI 1.00 – 6.28), having an active inhibitor at the time of surgery (OR 4.26, 95% CI 1.05 – 17.35), overweight or obesity (OR 4.49, 95% CI 1.35 – 14.91, and OR 6.09, 95% CI 1.81 – 20.52, respectively), and not using antifibrinolytic medication peri-operatively (OR 3.00, 95% CI 1.09 – 8.27).
Of the enrolled patients undergoing surgery, two (1.5%) had a family history of thrombosis, and 1 (0.8%) reported a personal history of VTE. By the 6-week follow-up visit, 5 of the 131 (3.8%) procedures were associated with symptomatic thrombotic events. Three of these events were deep vein thrombosis (DVT) – two isolated distal DVTs and one suspected event of unknown location (individual declined to have any imaging studies). One subject experienced a pulmonary embolus post-operatively.[22] In addition, there was one reported superficial venous thrombosis event. Four of the VTE events occurred after hospitalization, when factor levels had not been recently measured. One of the VTE events occurred prior to hospital discharge; the highest measured factor activity level post-operatively for this patient was 66%. While 2 of the 5 patients who developed VTE had received factor infusion by continuous infusion during hospitalization, at the time VTE was diagnosed all subjects were being treated with factor by bolus infusions. None of the VTE events occurred in subjects with inhibitors.
DISCUSSION
This is the largest prospective observational study of postoperative bleeding complications in PWH undergoing total knee or total hip arthroplasty. The rate of major bleeding in our population was 29.8%. Previous studies reported bleeding rates between 20 – 50% in patients with hemophilia undergoing TKA or THA, a wide range that includes our study’s observed rate of bleeding.[18–20] Overall, 18% of patients required post-operative red cell transfusion, with 13% receiving at least 2 units pRBCs and 5.3% receiving 1 unit of pRBC during hospitalization. These findings are in line with a large retrospective cohort of patients with hemophilia undergoing TKA or THA reported by Kapadia et al to have a 15% transfusion rate compared to 9.8% in matched patients without hemophilia.[24] Given that blood product administration is not without potentially serious risks, the increased need for transfusions in the hemophilia population warrants future mitigation efforts. It is also true, however, that thresholds for transfusion vary by institution and by provider, and postoperative transfusion could potentially be reduced by implementation of more conservative transfusion guidelines. Other approaches to reducing the need for transfusion have been described and tested in patients both with and without hemophilia, such as the multimodal blood loss prevention method, an approach that incorporates the use of intra-articular tranexamic acid, a tourniquet during the procedure, and sealing the femoral canal with bone graft. [25]
One challenge in interpreting the existing literature on bleeding after orthopedic surgery is the use of different criteria to define bleeding.[26] The 29.8% major bleed rate in PWH observed in our cohort is strikingly higher than the 0.6% incidence of major bleeding after THA and TKA reported in one study of patients without hemophilia who were receiving postoperative pharmacologic thromboprophylaxis (aspirin, enoxaparin, or rivaroxaban).[27] However, comparing the definitions of bleeding utilized in the two studies provides an illustrative example of the importance of clear, objective, and consistent measures of bleeding. Lindquist defined major bleeding using the same criteria used in our study, with a notable exception: transfusion of two or more units of blood and hemoglobin decreases of at least 2 g/dL were only considered to meet the criteria for major bleeding if these were judged to have been the result of “clinically overt” bleeding. In contrast, we did not require a subjective assessment of clinically overt bleeding; rather, the need for transfusion and rapid decline of hemoglobin were categorized in our study as being clinically relevant, regardless of whether there was clinical evidence of overt bleeding. We chose to use the term “major bleed” to signify this clinical relevance.
Furthermore, the Lindquist study’s primary outcome was “any bleeding,” which included both major bleeding as described above and clinically relevant nonmajor bleeding. The incidence of any bleeding in their study sample was 4.1%. This number does not include the large number of patients who required blood transfusions postoperatively, who represented another 23.2% of subjects.[27] Other studies of THA and TKA in the general population report transfusions in sizable proportions of patients. A single center experience found that 16.4% of patients were transfused after these procedures when a blood-conservation algorithm was not utilized; the average decline in hemoglobin observed in these patients was 4.0 g/dL after THA and 3.8 g/dL after TKA.[28] Prior to institution of more stringent blood transfusion guidelines, the rate of transfusion was even higher than that seen in our study, with up to 46% of patients receiving blood transfusions (30% autologous and 16% allogenic) after more than 9400 THA and TKA procedures performed in 1996 – 1997. On average, patients who were transfused in this study received 1.8 units of pRBC (2.1 units for those receiving allogenic transfusions).[29]
Given the differing definitions of bleeding among studies, comparing patients in the general population to those with hemophilia should be performed with caution. It should also be noted that most patients with hemophilia undergo major orthopedic surgery for a different indication (usually hemophilic arthropathy), in which the specific arthropathic changes may contribute to increased peri-operative bleeding risk. Furthermore, patients with hemophilia have an underlying predisposition to bleeding that may be mitigated but perhaps not eliminated by peri-operative treatment with clotting factor. The perceived increased bleeding risk in PWH may impact providers’ clinical management decisions, particularly regarding reducing the threshold for pRBC transfusion. Regardless of differences in bleeding rates between the two patient groups, there is room for improvement in PWH undergoing major joint replacement surgery. Whether reduction of early postoperative bleeding impacts long-term functional outcomes is unknown. The present study will continue to collect annual follow up data to address this question.
Nine of the 131 participants (6.9%) required hospital re-admission within 6 weeks after discharge. Two individuals were readmitted after being clinically diagnosed with bleeding in the surgical joint. Four readmissions occurred for evaluation of lower extremity symptoms suggestive of, but ultimately felt not to be due to bleeding within the surgical joint. Most were managed conservatively with factor replacement and analgesic medications.
One subject required surgical intervention during hospitalization for intraarticular bleeding. Bleeding into the surgical joint may have been precipitated by use of the joint (physical therapy, ambulation), but these interventions are necessary to maximize potential gains of function after surgery. Optimizing postoperative factor replacement during physical therapy and ambulation might be a focus for improving future hemostatic outcomes, especially when the frequency or timing of factor replacement may not coincide with the frequency or timing of physical therapy, thereby preventing achievement of optimal factor levels at physical therapy. When postoperative bleeding occurs, not only is optimal factor treatment required but also reduced joint activity and rest, which may slow the rehabilitation progress.
It is common practice in many institutions to utilize intra-operative antifibrinolytic agents in major lower extremity orthopedic surgery to reduce blood loss, with no apparent increased risk of postoperative VTE.[30] A number of clinical trials in the non-hemophilia population have supported this practice and shown antifibrinolytics to be safe and efficacious for preventing intra- and post-operative blood loss after THA and TKA.[31, 32] We observed in our cohort that only 21% of those who received any antifibrinolytic medication during hospitalization exhibited major bleeding, compared to 36% of those who did not receive these medications. Our findings support the routine use of antifibrinolytic agents in PWH undergoing major orthopedic surgery, though identification of the most effective and safe dosing regimens requires further investigation.
The risk of bleeding during the perioperative period must be balanced against the risk of thrombosis, particularly VTE. In 131 TKA or THA procedures, we identified 3 symptomatic deep venous thromboses (2.3%) and 1 pulmonary embolus (0.8%), despite only 7 of 131 (5.3%) participants receiving pharmacologic thromboprophylaxis. Notably, the majority of participants (57.3%) received some form of thromboprophylaxis (pharmacologic and/or mechanical) during hospitalization (Table 2).
Our ability to control for and study all clinical practices related to THA and TKA in PWH was limited by the observational nature of our study. For instance, the low number of subjects receiving pharmacologic thromboprophylaxis limited our ability to investigate the impact of this intervention on bleeding risk. Our findings must also be considered in light of differences in practice patterns in the surgical and medical management of PWH undergoing major lower extremity orthopedic surgery. It is also possible that some of the patients included in the study had joint disease due to osteoarthritis rather than hemophilic arthropathy. This study did not collect data describing number and locations of joint hemorrhages prior to hip or knee replacement, and findings from histopathologic examinations of joint tissues, if performed, were not assessed by the study. It is therefore unclear whether the underlying condition that led to the need for arthroplasty might have impacted bleeding outcomes. That said, the study’s prospective, multicenter approach to recruitment, data collection, and analysis captured a representative, real-world sample of men with hemophilia who underwent TKA or THA across 14 centers in the United States. Moreover, the variability in practice and patient characteristics observed in this study underscores the need for more uniform guidance to reduce post-surgical bleeding in PWH, as well as standard approaches to post-operative physical rehabilitation.
Although our study was not designed to establish a comparison group that would allow matching patients with hemophilia with non-hemophilic controls, other retrospective cohort studies have demonstrated that PWH undergoing TKA and THA face unique risks compared to those in the general population, and thus should be cared for by experienced multidisciplinary teams supported by on site laboratories capable of performing rapid turnaround clotting factor assays.[33–35] Future prospective research and quality improvement initiatives should focus on narrowing the gap in complication rates between patients in the general population and PWH undergoing major lower extremity orthopedic surgery.
In conclusion, this prospective, multicenter observational study of men with hemophilia undergoing major lower extremity orthopedic surgery highlights that perioperative bleeding is relatively common despite the use of factor replacement. Our data suggest that this risk may be mitigated by treating with an antifibrinolytic medication, though further study is needed. These considerations and the data from this study can help inform the shared decision-making process when PWH and their providers discuss the potential risks and benefits of joint replacement surgery.
Table 4:
Univariable analysis of major bleeding in hemophilia patients undergoing hip or knee arthroplasty.
| Variable | n | OR | 95% CI | p value |
|---|---|---|---|---|
| Procedure type: THA | 125 | 2.54 | 1.10 – 5.85 | 0.03 |
| Age (continuous) | 125 | 0.99 | 0.96 – 1.03 | 0.61 |
| Hemophilia type: A | 125 | 1.26 | 0.45 – 3.51 | 0.66 |
| Hemophilia severity | 125 | 0.90 | 0.55 – 1.47 | 0.68 |
| Active inhibitor | 122 | 2.44 | 0.73 – 8.13 | 0.15 |
| Overweight vs. normal BMI | 124 | 3.66 | 1.19 – 11.27 | 0.02 |
| Obese vs. normal BMI | 124 | 4.61 | 1.50 – 14.13 | 0.01 |
| Thromboprophylaxis | 125 | 0.88 | 0.16 – 4.72 | 0.88 |
| No antifibrinolytic used | 125 | 2.08 | 0.85 – 5.08 | 0.11 |
| Continuous factor infusion | 121 | 1.20 | 0.53 – 2.67 | 0.67 |
P values in bold (less than 0.2) indicate variables included in multivariable analysis.
OR=odds ratio, CI=confidence interval, THA=total hip arthroplasty, BMI=body mass index
includes individuals with BMI data available (see Table 1)
Table 5:
Odds of major bleeding based on patient characteristics and surgical factors: multivariable analysis (n=121).
| Variable | OR | 95% CI | p value |
|---|---|---|---|
| Procedure type: THA | 2.50 | 1.00 – 6.28 | 0.05 |
| Active inhibitor | 4.26 | 1.05 – 17.35 | 0.04 |
| BMI: | |||
| overweight vs. normal | 4.49 | 1.35 – 14.91 | 0.01 |
| obese vs. normal | 6.09 | 1.81 – 20.52 | <0.01 |
| No antifibrinolytic used | 3.00 | 1.09 – 8.27 | 0.03 |
OR=odds ratio, CI=confidence interval, THA=total hip arthroplasty, BMI=body mass index
ESSENTIALS.
Risk factors for bleeding after orthopedic surgery in patients with hemophilia are unknown.
Bleeding outcomes in adults with hemophilia were assessed in a prospective, multicenter study.
Thirty percent of patients with hemophilia had major bleeding after hip or knee arthroplasty.
Bleeding risk was impacted by inhibitor status, body mass index and use of antifibrinolytics.
Acknowledgements:
The MOSH study was funded by an investigator-initiated grant from Baxter/Shire (NSK), and the original protocol for the study was written by NSK and MEE. TWB and BK were supported by T32 HL007149. The project was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR002489. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosures:
B. Kleiboer: None
M. A. Layer: None
L. A. Cafuir: Consultant for BioMarin
A. Cuker: Consultant for Synergy and UpToDate and his institution has received research support on his behalf from Alexion, Bayer, Novartis, Novo Nordisk, Pfizer, Sanofi, Spark, and Takeda
M. Escobar: Consultant for NovoNordisk, Hemobiologics, Genentech, Biomarin, Takeda and Magellan. Advisory Board for NovoNordisk, Genentech, CSL Behring, Sanofi, Takeda, Pfizer, Kedrion, UniQure, NHF
M. E. Eyster. Research funding: Spark, Baxalta / Takeda, Novo Nordisk, Genentech
E. Kraut: Advisory board: uniQure
A. D. Leavitt: Advisory Board for BioMarin, CSL, Catalyst, Genentech, HEMA Biologics, DOVA pharmaceuticals; Consultant for Merck
S. R. Lentz: Consultant for Novo Nordisk, uniQure, Apellis, and Opko.
D. Quon: Honoraria / Consulting fees: Bayer, Biomarin, Bioverativ / Sanofi, Catalyst, Novo Nordisk, Pfizer, Roche / Genentech; Speaker’s bureau: Biomarin, Bioverativ / Sanofi, Novo Nordisk, Takeda, Roche / Genentech
M. V. Ragni: Consultant to Alnylam/Sanofi, BioMarin, Bioverativ/Sanofi, SPARK Therapeutics; and her institution has received research support on her behalf from Alnylam/Sanofi, BioMarin, Bioverativ/Sanofi, SPARK Therapeutics, and Takeda Pharmaceuticals
D. Thornhill: None
M. Wang: Advisor / Consultant: Bayer, Biomarin, Bioverativ / Sanofi, Catalyst Biosciences, CSL Behring, Hema Biologics, Roche / Genentech, Novo Nordisk, Takeda, uniQure
N. S. Key: Consultant: uniQure; Research funding: Takeda; Advisory board: Biomarin; Chair of Investigator-Initiated Studies Grants Committee: Novo Nordisk
T. W. Buckner: Consultant: uniQure, BioMarin, and Tremeau Pharmaceuticals; advisory board participation with uniQure, BioMarin, Tremeau Pharmaceuticals, Novo Nordisk, CSL Behring, Genentech, Spark, Sanofi, Bayer, Pfizer, and Takeda
REFERENCES
- 1.Batt K, Boggio L, Neff A, Buckner TW, Wang M, Quon D, Witkop M, Recht M, Kessler C, Iyer NN, Cooper DL. Patient-reported outcomes and joint status across subgroups of US adults with hemophilia with varying characteristics: Results from the Pain, Functional Impairment, and Quality of Life (P-FiQ) study. European journal of haematology. 2018;100 Suppl 1:14–24. [DOI] [PubMed] [Google Scholar]
- 2.Luck JV Jr., Silva M, Rodriguez-Merchan EC, Ghalambor N, Zahiri CA, Finn RS. Hemophilic arthropathy. The Journal of the American Academy of Orthopaedic Surgeons. 2004;12:234–45. [DOI] [PubMed] [Google Scholar]
- 3.O’Hara J, Walsh S, Camp C, Mazza G, Carroll L, Hoxer C, Wilkinson L. The impact of severe haemophilia and the presence of target joints on health-related quality-of-life. Health and quality of life outcomes. 2018;16:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varaklioti A, Kontodimopoulos N, Niakas D, Kouramba A, Katsarou O. Health-Related Quality of Life and Association With Arthropathy in Greek Patients with Hemophilia. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2018;24:815–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manco-Johnson MJ, Lundin B, Funk S, Peterfy C, Raunig D, Werk M, Kempton CL, Reding MT, Goranov S, Gercheva L, Rusen L, Uscatescu V, Pierdominici M, Engelen S, Pocoski J, Walker D, Hong W. Effect of late prophylaxis in hemophilia on joint status: a randomized trial. Journal of thrombosis and haemostasis : JTH. 2017;15:2115–24. [DOI] [PubMed] [Google Scholar]
- 6.Manco-Johnson MJ, Soucie JM, Gill JC, Joint Outcomes Committee of the Universal Data Collection USHTCN. Prophylaxis usage, bleeding rates, and joint outcomes of hemophilia, 1999 to 2010: a surveillance project. Blood. 2017;129:2368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wyseure T, Mosnier LO, von Drygalski A. Advances and challenges in hemophilic arthropathy. Seminars in hematology. 2016;53:10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva M, Luck JV Jr. Long-term results of primary total knee replacement in patients with hemophilia. The Journal of bone and joint surgery American volume. 2005;87:85–91. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Merchan EC. Surgical approaches to hemophilic arthropathy. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2019;30:S11–S3. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Merchan EC. Risks and patient outcomes of surgical intervention for hemophilic arthropathy. Expert review of hematology. 2019;12:325–33. [DOI] [PubMed] [Google Scholar]
- 11.Brookenthal KR, Freedman KB, Lotke PA, Fitzgerald RH, Lonner JH. A meta-analysis of thromboembolic prophylaxis in total knee arthroplasty. The Journal of arthroplasty. 2001;16:293–300. [DOI] [PubMed] [Google Scholar]
- 12.Cushner FD, Friedman RJ. Blood loss in total knee arthroplasty. Clinical orthopaedics and related research. 1991:98–101. [PubMed] [Google Scholar]
- 13.Dahl OE, Quinlan DJ, Bergqvist D, Eikelboom JW. A critical appraisal of bleeding events reported in venous thromboembolism prevention trials of patients undergoing hip and knee arthroplasty. Journal of thrombosis and haemostasis : JTH. 2010;8:1966–75. [DOI] [PubMed] [Google Scholar]
- 14.Wang SH, Chung CH, Chen YC, Cooper AM, Chien WC, Pan RY. Does Hemophilia Increase Risk of Adverse Outcomes Following Total Hip and Knee Arthroplasty? A Propensity Score-Matched Analysis of a Nationwide, Population-Based Study. The Journal of arthroplasty. 2019;34:2329–36 e1. [DOI] [PubMed] [Google Scholar]
- 15.Chiasakul T, Buckner TW, Li M, Vega R, Gimotty PA, Cuker A. In-Hospital Complications and Readmission in Patients with Hemophilia Undergoing Hip or Knee Arthroplasty. JB JS Open Access. 2020;5:e0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, Ortel TL, Pauker SG, Colwell CW Jr., American College of Chest P. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e278S–325S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mont MA, Jacobs JJ, Boggio LN, Bozic KJ, Della Valle CJ, Goodman SB, Lewis CG, Yates AJ Jr., Watters WC 3rd, Turkelson CM, Wies JL, Donnelly P, Patel N, Sluka P. Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. The Journal of the American Academy of Orthopaedic Surgeons. 2011;19:768–76. [DOI] [PubMed] [Google Scholar]
- 18.Siboni SM, Biguzzi E, Pasta G, Mannucci PM, Mistretta C, Fantini NN, Solimeno LP, Peyvandi F. Management of orthopaedic surgery in rare bleeding disorders. Haemophilia : the official journal of the World Federation of Hemophilia. 2014;20:693–701. [DOI] [PubMed] [Google Scholar]
- 19.Chevalier Y, Dargaud Y, Lienhart A, Chamouard V, Negrier C. Seventy-two total knee arthroplasties performed in patients with haemophilia using continuous infusion. Vox sanguinis. 2013;104:135–43. [DOI] [PubMed] [Google Scholar]
- 20.Sikkema T, Boerboom AL, Meijer K. A comparison between the complications and long-term outcome of hip and knee replacement therapy in patients with and without haemophilia; a controlled retrospective cohort study. Haemophilia : the official journal of the World Federation of Hemophilia. 2011;17:300–3. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Merchan EC. Total knee replacement in haemophilic arthropathy. The Journal of bone and joint surgery British volume. 2007;89:186–8. [DOI] [PubMed] [Google Scholar]
- 22.Buckner TW, Leavitt AD, Ragni M, Kempton CL, Eyster ME, Cuker A, Lentz SR, Ducore J, Leissinger C, Wang M, Key NS. Prospective, multicenter study of postoperative deep-vein thrombosis in patients with haemophilia undergoing major orthopaedic surgery. Thrombosis and haemostasis. 2016;116:42–9. [DOI] [PubMed] [Google Scholar]
- 23.Schulman S, Angeras U, Bergqvist D, Eriksson B, Lassen MR, Fisher W, Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on T, Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. Journal of thrombosis and haemostasis : JTH. 2010;8:202–4. [DOI] [PubMed] [Google Scholar]
- 24.Kapadia BH, Boylan MR, Elmallah RK, Krebs VE, Paulino CB, Mont MA. Does Hemophilia Increase the Risk of Postoperative Blood Transfusion After Lower Extremity Total Joint Arthroplasty? The Journal of arthroplasty. 2016;31:1578–82. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Merchan EC, Encinas-Ullan CA, Gomez-Cardero P. Intra-articular Tranexamic Acid in Primary Total Knee Arthroplasty Decreases the Rate of Post-operative Blood Transfusions in People with Hemophilia: A Retrospective Case-Control Study. HSS journal : the musculoskeletal journal of Hospital for Special Surgery. 2020;16:218–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guyatt GH, Eikelboom JW, Gould MK, Garcia DA, Crowther M, Murad MH, Kahn SR, Falck-Ytter Y, Francis CW, Lansberg MG, Akl EA, Hirsh J, American College of Chest P. Approach to outcome measurement in the prevention of thrombosis in surgical and medical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e185S–94S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindquist DE, Stewart DW, Brewster A, Waldroup C, Odle BL, Burchette JE, El-Bazouni H. Comparison of Postoperative Bleeding in Total Hip and Knee Arthroplasty Patients Receiving Rivaroxaban, Enoxaparin, or Aspirin for Thromboprophylaxis. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2018;24:1315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. The Journal of bone and joint surgery American volume. 2004;86:1512–8. [DOI] [PubMed] [Google Scholar]
- 29.Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. The Journal of bone and joint surgery American volume. 1999;81:2–10. [DOI] [PubMed] [Google Scholar]
- 30.Watts CD, Pagnano MW. Minimising blood loss and transfusion in contemporary hip and knee arthroplasty. The Journal of bone and joint surgery British volume. 2012;94:8–10. [DOI] [PubMed] [Google Scholar]
- 31.Sridharan K, Sivaramakrishnan G. Tranexamic acid in total hip arthroplasty: Mixed treatment comparisons of randomized controlled trials and cohort studies. J Orthop. 2018;15:81–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sridharan K, Sivaramakrishnan G. Tranexamic Acid in Total Knee Arthroplasty: Mixed Treatment Comparisons and Recursive Cumulative Meta-Analysis of Randomized, Controlled Trials and Cohort Studies. Basic Clin Pharmacol Toxicol. 2018;122:111–9. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava A, Santagostino E, Dougall A, Kitchen S, Sutherland M, Pipe SW, Carcao M, Mahlangu J, Ragni MV, Windyga J, Llinas A, Goddard NJ, Mohan R, Poonnoose PM, Feldman BM, Lewis SZ, van den Berg HM, Pierce GF, panelists WFHGftMoH, co a. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia : the official journal of the World Federation of Hemophilia. 2020;26 Suppl 6:1–158. [DOI] [PubMed] [Google Scholar]
- 34.Escobar M, Maahs J, Hellman E, Donkin J, Forsyth A, Hroma N, Young G, Valentino LA, Tachdjian R, Cooper DL, Shapiro AD. Multidisciplinary management of patients with haemophilia with inhibitors undergoing surgery in the United States: perspectives and best practices derived from experienced treatment centres. Haemophilia : the official journal of the World Federation of Hemophilia. 2012;18:971–81. [DOI] [PubMed] [Google Scholar]
- 35.Escobar MA, Brewer A, Caviglia H, Forsyth A, Jimenez-Yuste V, Laudenbach L, Lobet S, McLaughlin P, Oyesiku JOO, Rodriguez-Merchan EC, Shapiro A, Solimeno LP. Recommendations on multidisciplinary management of elective surgery in people with haemophilia. Haemophilia : the official journal of the World Federation of Hemophilia. 2018;24:693–702. [DOI] [PubMed] [Google Scholar]


