Abstract
Objectives
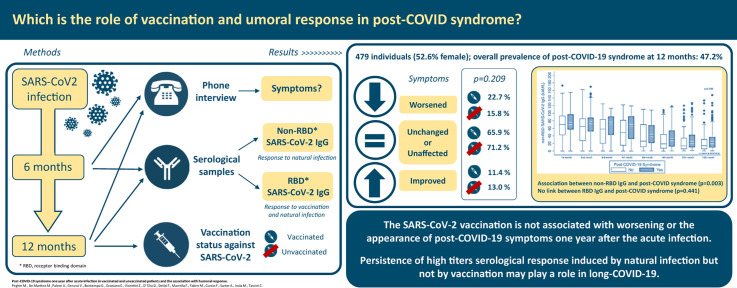
This study aimed to describe the impact of vaccination and the role of humoral responses on post–COVID-19 syndrome 1 year after the onset of SARS coronavirus type 2 (CoV-2).
Methods
This prospective study was conducted through interviews to investigate post–COVID-19 syndrome 6 and 12 months after disease onset in all adult in- and outpatients with COVID-19 at Udine Hospital (March–May 2020). Vaccination status and two different serological assays to distinguish between response to vaccination (receptor-binding domain (RBD) SARS-CoV-2 IgG) and/or natural infection (non-RBD-SARS-CoV-2 IgG) were also assessed.
Results
A total of 479 patients (52.6% female; mean age: 53 years) were interviewed 13.5 months (standard deviation: 0.6 months) after acute infection. Post–COVID-19 syndrome was observed in 47.2% of patients (n = 226) after 1 year. There were no significant differences in the worsening of post–COVID-19 symptoms (22.7% vs. 15.8%; p = 0.209) among vaccinated (n = 132) and unvaccinated (n = 347) patients. The presence of non-RBD SARS-CoV-2 IgG induced by natural infection showed a significant association with post–COVID-19 syndrome (OR: 1.35; 95% CI, 1.11–1.64; p = 0.003), and median non-RBD SARS-CoV-2 IgG titres were significantly higher in long haulers than in patients without symptoms (22 kAU/L (interquartile range, 9.7–37.2 kAU/L) vs. 14.1 kAU/L (interquartile range, 5.4–31.3 kAU/L); p = 0.009) after 1 year. In contrast, the presence of RBD SARS-CoV-2 IgG was not associated with the occurrence of post–COVID-19 syndrome (>2500 U/mL vs. 0.9–2500 U/mL; OR: 1.36; 95% CI, 0.62–3.00; p = 0.441), and RBD SARS-CoV-2 IgG titres were similar in long haulers as in patients without symptoms (50% values > 2500 U/mL vs. 55.6% values > 2500 U/mL; p = 0.451).
Discussion
The SARS-CoV-2 vaccination is not associated with the emergence of post–COVID-19 symptoms more than 1 year after acute infection. The persistence of high serological titre response induced by natural infection, but not vaccination, may play a role in long-haul COVID-19.
Keywords: COVID-19 vaccination, Hybrid immunity, Long COVID-19, Natural immunity, Post–COVID-19, SARS-CoV-2 antibodies, SARS-CoV-2 serology, SARS-CoV-2 vaccination, Unvaccinated, Vaccinated
Graphical abstract
Introduction
Post–COVID-19 syndrome is a heterogeneous, multisystemic, postacute sequelae that affects the health and quality of life of patients of all ages [[1], [2], [3]]. The potential pathophysiological mechanisms are unknown and may encompass a complex interaction between virus-specific cytopathic effects, inflammatory damage, allo- and autoimmune responses to the acute infection on one hand, and the expected sequelae of postcritical illness due to organ and microvascular damage on the other hand [4].
To date, there is still a gap on how natural and hybrid immunities, which refer to the immune-strengthening effect of exposure to infection followed by vaccination, function in post–COVID-19 [[5], [6], [7]]. A few available studies suggest both a potential improvement and deterioration of post–COVID-19 symptoms after vaccination in previously infected patients and variable associations between humoral responses and post–COVID-19 syndrome after natural infection [[8], [9], [10], [11]].
Investigating immunological mechanisms could inform both clinical and public health decisions regarding the prevention of and potential tailored treatments for long-haul COVID-19 [4]. Thus, the aim of this study was to describe post–COVID-19 syndrome 1 year after acute infection by focusing on the influence of vaccination on long-term symptoms, as well as the role of humoral responses among survivors with natural and hybrid immunities.
Methods
Study design and patients
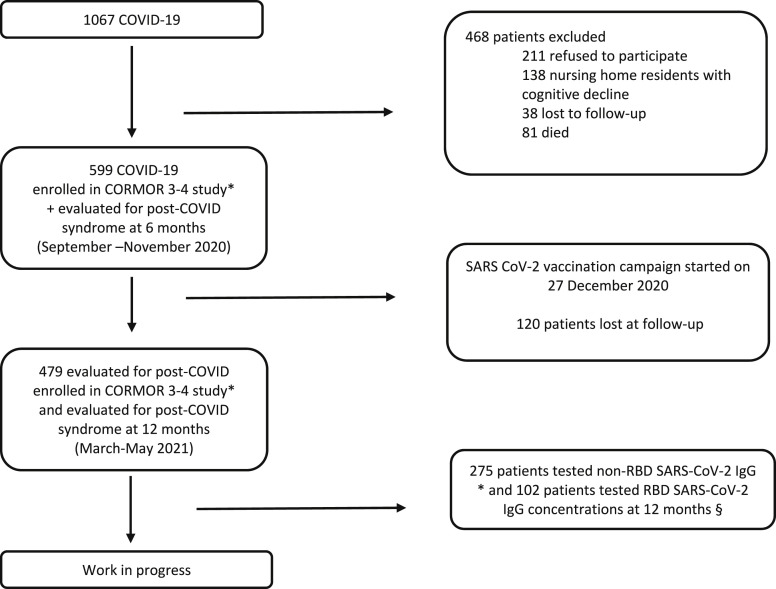
This was a prospective study [5] according to the Strengthening the Reporting of Observational Studies in Epidemiology Statement (Table S1). Patients eligible for inclusion were all adults (age ≥18 years) diagnosed with COVID-19 during the first wave (March–May 2020) and cared for at an academic hospital in all settings, followed up at 6 (September–November 2020) and 12 months (March–May 2021), and willing to participate in the study (Fig. 1 ).
Fig. 1.
Flow diagram of in- and out- COVID-19 patients included in the post-COVID-19 syndrome study at 6-12 months and serological follow-up up to May 2021. Legend: CORMOR 3-4 study.
∗ Non-RBD SARS-CoV-2 IgG antibodies (iFlash) concentrations were measured at the serological follow-up visits each month (±15 days) after symptom onset during the first four months, and every month up to 12 months (±15 days), from March 2020 to May 2021. Among the 479 patients, only 275 were evaluated at 12 months.
§ RBD SARS-CoV-2 IgG antibodies (Roche) at 12 months after the onset of symptom (±60 days). Patients were categorized as vaccinated or hybrid immunity if they had received the vaccine at least two weeks before the interview. COVID-19, Coronavirus Disease 2019; RBD, receptor binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Data collection
Demographic and clinical databases were populated at the time of enrolment and over time (Table S2). Participants were interviewed via telephone by the same trained nurses at 6 and 12 months using a homogeneous questionnaire that had been pilot-tested and previously validated [5] to investigate persistent or emerging symptoms potentially associated with COVID-19, as expressed by patients' own words ([12]. Post–COVID-19 syndrome was defined as signs and symptoms developed during or after an infection consistent with COVID-19, continuing for more than 12 weeks, and not explained by an alternative diagnosis [13]. Signs/symptoms reported by patients were classified by four independent researchers (Table S3), and then matched between the first and second interview to check changes, if any, over time. Patients were classified as unaffected when asymptomatic at both follow ups, unchanged when symptoms remained the same, worsened when new symptoms emerged, and improved when symptoms were recovered/resolved [5].
In Italy, the SARS coronavirus type 2 (CoV-2) vaccination campaign started on December 27, 2020. Vaccines approved were those with the adenovirus vector (ChAdOx1 nCoV-19 Oxford–AstraZeneca and Ad26.COV2.S Janssen COVID-19 vaccine) and the mRNA (BNT162b2 Pfizer–BioNTech and mRNA-1273 Moderna). At 12 months, patients were asked to communicate their vaccination status (yes/no), as well as the date and type of vaccine received. The data collected were matched in accuracy with electronic health records. Then, patients were categorized as vaccinated if they had received the vaccine at least 2 weeks before the interview. Those with combined immunity from natural SARS-CoV-2 infection and vaccination were considered to have hybrid immunity. Bias was prevented, as reported in Table S4.
Antibody measurement and other laboratory methods
SARS-CoV-2 antibody measurements were performed in a subgroup of patients (n = 546) who agreed to participate in a parallel study (CORMOR 3-4) [14]. Their serological data at the time of the interview (±2 months) were recorded in the database (Fig. 1). The role of serological response in post–COVID-19 syndrome was assessed using two antibody assays with different abilities to recognize the receptor-binding domain (RBD) of the Spike protein as the main target stimulated by the SARS-CoV-2 vaccination. Specifically, an IgG test that is not able to recognize the RBD SARS-CoV-2 protein (iFlash-SARS-CoV-2; IgG positivity cutoff >10.0 kAU/L) was used to follow natural humoral response (non-RBD IgG), and an IgG test of the SARS-CoV-2 S protein RBD (Elecsys Roche; IgG positivity cutoff (<0.9 U/mL and maximum value > 2500 U/mL) was used to follow both natural and vaccine-induced humoral responses to compare vaccinated and unvaccinated patients (Fig. 1). The laboratory methods used are detailed in Table S5.
Statistical analysis
Patients were divided into two groups (vaccinated, unvaccinated) at the time of the interview at 12 months. The Shapiro–Wilk test was used to assess whether data were normally or nonnormally distributed. Categorical variables were compared using the χ2 or Fisher's exact test, and quantitative variables were compared using the t or Mann–Whitney U test, as appropriate. Uni- and multivariable logistic regressions were performed to explore features associated with post–COVID-19 syndrome, estimating the OR at 95% CI (STATA 17.0).
Results
Acute COVID-19 onset and post–COVID-19 syndrome after 1 year
Overall, during the first wave of the pandemic, 1067 patients were diagnosed with COVID-19 at our hospital. Of these patients, 599 responded to the 6-month interview and 479 to the 12-month interview (Fig. 1). The baseline characteristics and clinical data from the COVID-19 onset are reported in Table 1, Table 2 . At a median of 13.5 months (standard deviation (SD): 0.6 months) after acute COVID-19 onset, the prevalence of post–COVID-19 syndrome was 47.2% (n = 226 of 479; 95% CI, 42.64–51.76), which was higher than at 6 months (40.2%; n = 241 of 599; 95% CI, 36.38–44.28; Table 2).
Table 1.
Baseline characteristics at COVID-19 onset at overall level and according to vaccination status after 12 months
| Overall (N = 479) | Vaccinated (n = 132) | Unvaccinated (n = 347) | p-value | |
|---|---|---|---|---|
| Sex, n (%) | <0.001 | |||
| Female | 252 (52.6) | 94 (71.2) | 158 (45.5) | |
| Male | 227 (47.4) | 38 (28.8) | 189 (54.5) | |
| Age group (y), n (%) | 0.061 | |||
| 18‒40 | 107 (22.3) | 33 (25.0) | 74 (21.3) | |
| 41‒60 | 205 (42.8) | 64 (48.5) | 141 (40.6) | |
| >60 | 167 (34.9) | 35 (26.5) | 132 (38.0) | |
| Ethnicity, n/N (%) | 0.360 | |||
| Native Italian | 422/457 (92.3) | 112/125 (89.6) | 310/332 (93.4) | |
| European | 327457 (7.0) | 12/125 (9.6) | 20/332 (6.0) | |
| Non-European | 3/457 (0.7) | 1/125 (0.8) | 2/332 (0.6) | |
| Smoking habit, n/N (%) | 0.295 | |||
| Nonsmoker | 310/477 (65.0) | 81/131 (61.8) | 229/346 (66.2) | |
| Smoker | 68/477 (14.3) | 24/131 (18.3) | 44/346 (12.7) | |
| Ex-smoker | 99/477 (20.7) | 26/131 (19.9) | 73/346 (21.1) | |
| Alcohol habit, n/N (%) | 0.430 | |||
| Nondrinker | 238/476 (50.0) | 70/130 (53.8) | 168/346 (48.5) | |
| Drinker | 235/476 (49.4) | 60/130 (46.2) | 175/346 (50.6) | |
| Alcohol use disorder | 3/476 (0.6) | 0/130 (0.0) | 3/346 (0.9) | |
| Work, n/N (%) | <0.001 | |||
| Health care workers | 102/443 (23.0) | 73/120 (60.9) | 29/323 (9.0) | |
| Work in contact with public | 84/443 (19.0) | 13/120 (10.8) | 71/323 (22.0) | |
| Work not in contact with public | 121/443 (27.3) | 14/120 (11.7) | 107/121 (33.1) | |
| Retired | 81/443 (18.3) | 10/120 (8.3) | 71/121 (22.0) | |
| Other | 55/443 (12.4) | 10/120 (8.3) | 45/121 (13.9) | |
| Comorbidities, n (%) | 0.160 | |||
| 0 | 230 (48.0) | 64 (48.5) | 166 (47.8) | |
| 1 | 135 (28.2) | 35 (26.5) | 100 (28.8) | |
| 2 | 66 (13.8) | 25 (18.9) | 41 (11.8) | |
| 3 | 31 (6.5) | 5 (3.8) | 26 (7.5) | |
| ≥4 | 17 (3.5) | 3 (2.3) | 14 (4.0) | |
| Comorbidities, n/N (%) | ||||
| Hypertension | 106/468 (22.6) | 25/128 (19.5) | 81/340 (23.8) | 0.323 |
| Obesity | 78 (16.3) | 22/132 (16.7) | 56/347 (16.1) | 0.889 |
| Diabetes | 25/475 (5.3) | 6/130 (4.6) | 19/345 (5.5) | 0.698 |
| Chronic respiratory diseasea | 17/475 (3.6) | 6/130 (4.6) | 11/345 (3.2) | 0.421 |
| Cardiovascular diseaseb | 7/475 (1.5) | 2/130 (1.5) | 5/345 (1.4) | 1.000 |
| Liver disease | 9/475 (1.9) | 2/130 (1.5) | 7/345 (2.0) | 1.000 |
| Psychiatric disordersc | 5 (1.0) | 1 (0.8) | 4 (1.1) | 1.000 |
| Renal impairment | 0/475 (0.0) | 0/132 (0.0) | 0/345 (0.0) | |
| Under chronic medication, n/N (%) | 0.555 | |||
| Yes | 227/473 (48.0) | 60/131 (45.8) | 167/342 (48.8) | |
| No | 246/473 (52.0) | 71/131 (54.2) | 175/342 (51.2) | |
Pulmonary disease: Asthma, chronic obstructive pulmonary disease.
Cardiovascular disease: Heart failure, ischaemic heart disease, tachyarrhythmias, valvular heart disease, venous thromboembolism.
Depression, anxiety.
Table 2.
Clinical presentation of acute COVID-19 at onset at overall level and according to vaccination status after 12 months
| Overall (N = 479) | Vaccinated (n = 132) | Unvaccinated (n = 347) | p-value | |
|---|---|---|---|---|
| Acute COVID-19 severitya, n/N (%) | 0.005 | |||
| Asymptomatic | 38/477 (8.0) | 19/132 (14.4) | 19/345 (5.5) | |
| Mild | 323/477 (67.7) | 86/132 (65.1) | 237/345 (68.7) | |
| Moderate, severe, and critical | 116/477 (24.3) | 27/132 (20.5) | 89/345 (25.8) | |
| Symptoms at onset, n (%) | 0.229 | |||
| 0 | 66 (13.8) | 26 (19.7) | 40 (11.5) | |
| 1 | 66 (13.8) | 15 (11.4) | 51 (14.7) | |
| 2 | 97 (20.2) | 25 (18.9) | 72 (20.7) | |
| 3 | 74 (15.4) | 20 (15.2) | 54 (15.6) | |
| 4 | 76 (15.9) | 23 (17.4) | 53 (15.3) | |
| ≥5 | 100 (20.9) | 23 (17.4) | 77 (22.2) | |
| Management, n (%) | 0.281 | |||
| Outpatient | 340 (71.0) | 99 (75.0) | 241 (69.4) | |
| Inpatient | ||||
| Wardb | 118 (24.6) | 30 (22.7) | 88 (25.4) | |
| Intensive care unit | 21 (4.4) | 3 (2.3) | 18 (5.2) | |
| Length of in-hospital stay (d), median (IQR) | 7 (3–11) | 6.5 (2–11) | 7 (4–12) | 0.341 |
| Viral shedding (d), median (IQR) | 19 (14–25) | 18.5 (14–26) | 20 (14–25) | 0.631 |
| Cycle threshold values, median (IQR) | 28.8 (24–33) | 28.9 (23.7–32) | 28.7 (24–33.5) | 0.611 |
| Post–COVID-19 syndrome at 6 months, n (%) | 201 (42.0) | 44 (33.3) | 157 (45.2) | 0.018 |
| Number of Post–COVID-19 symptoms at months, median (IQR) | 1 (1–2) | 2 (1–2) | 1 (1–2) | 0.084 |
IQR, interquartile range.
Asymptomatic: Mild (without pneumonia); moderate (with pneumonia); severe (with severe pneumonia); critical includes acute respiratory distress syndrome, sepsis, and/or septic shock [32].
Infectious Disease or Pneumology Department.
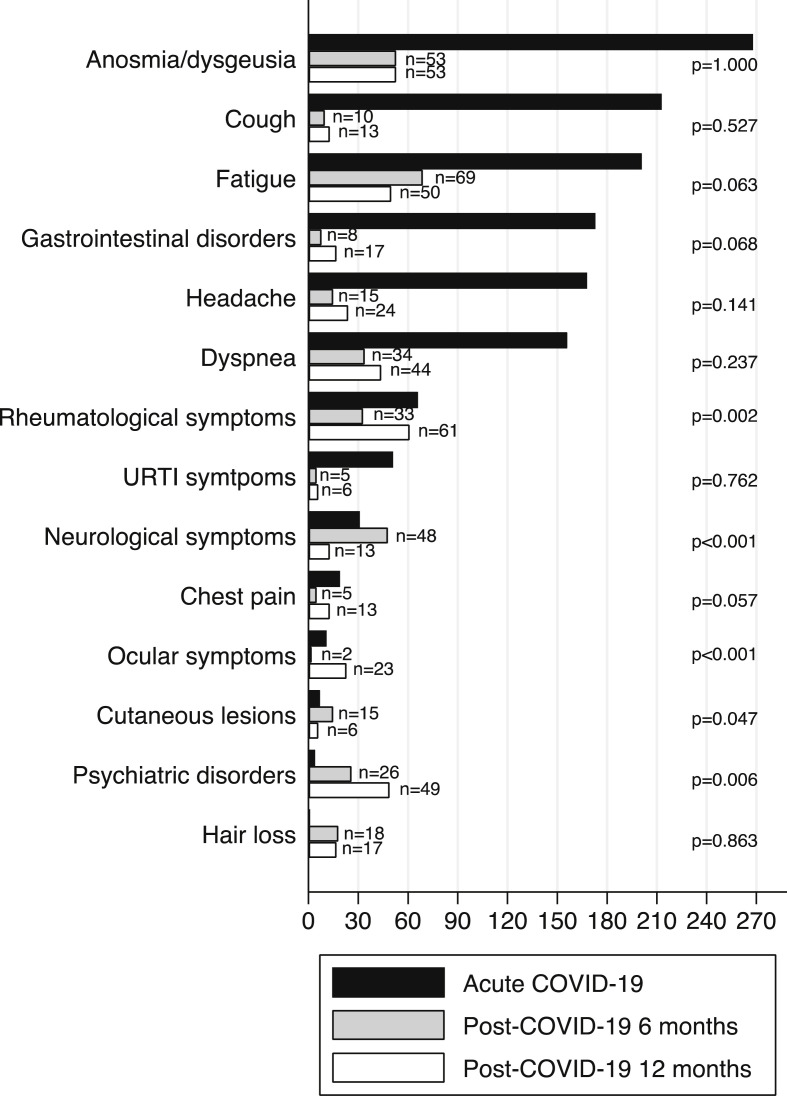
Overall, among patients reporting post–COVID-19 symptoms at 6 months (n = 201 of 479; 42.0%), 29.8% reported improvements at 12 months, and 70.2% declared unchanged symptoms. Of note, 85 patients (30.6%) reported the onset of new post–COVID-19 symptoms at 12 months. Specifically, there was a significant increase in rheumatological (6.3% vs. 12.7%; p = 0.002), ocular (0.3% vs. 23%; p < 0.001), and psychiatric symptoms (4.8% vs. 10.2%; p = 0.006), but there was a significant decrease in neurological (9.5% vs. 2.7%; p < 0.001) and cutaneous symptoms (3.5% vs. 1.2%; p = 0.047) at 12 months compared with 6 months (Fig. 2 ).
Fig. 2.
Acute– and post–COVID-19 related symptoms at 6 and 12 months. ∗ p refers to post–COVID-19 symptoms at 6 and 12 months. COVID-19, Coronavirus Disease 2019; URTI, upper respiratory tract infection.
Post–COVID-19 syndrome in vaccinated and unvaccinated patients
Overall, at the time of the interview, 347 patients (72.4%) were unvaccinated, 132 were vaccinated (27.6%) with at least one dose, and 111 had already received the second dose (all mRNA type). Patients received the first and second vaccine doses at a mean of 12.4 months (SD: 1.9 months) and 13.5 months (SD: 2.3 months), respectively, after onset of acute COVID-19. The time between vaccination (first or second dose) and interview ranged from 15 to 140 days.
As reported in Table 1, Table 2, vaccinated patients were more frequently female (n = 94 of 132; 71.2%) and health care workers (HCWs; n = 73 of 120; 60.8%) with less severe disease at acute onset (n = 105 of 132; 79.5% mild or asymptomatic). In both groups, some patients were still suffering from post–COVID-19 symptoms at 6 months, but those who were unvaccinated reported higher rates of symptoms at 6 months compared with those who were vaccinated (45.2% vs. 33.3%; p = 0.018). As reported in Table 3 , post–COVID-19 symptoms varied between 6 and 12 months according to vaccination status. In both groups, some patients had symptoms that had worsened (22.7% vs. 15.8%) or improved (11.4% vs. 13.0%), although most commonly, patients reported unchanged symptoms or were unaffected (65.9% vs. 71.2%). Overall, these differences were not statistically significant, except for the improvement in hair loss among unvaccinated patients (p = 0.033) and the worsening of ocular symptoms among vaccinated patients (p = 0.021). No significant difference in post–COVID-19 syndrome at 12 months emerged according to the vaccine received (45.8% mRNA vaccine and 12.5% adenovirus vector vaccine; p = 0.137) and vaccination status (38.1% incomplete and 45.9% complete; p = 0.507). The results of the multivariable analyses of associated post–COVID-19 syndrome risk factors are reported in Tables S6 and S7.
Table 3.
Post–COVID-19 symptoms at 12 months compared with post–COVID-19 symptoms at 6 months stratified according to vaccination status
| Vaccinated (n = 132) | Unvaccinated (n = 347) | p-value | |
|---|---|---|---|
| Vaccine, n/N (%) | |||
| Pfizer | 114/126 (90.5) | ||
| Moderna | 4/126 (3.2) | ||
| Astrazeneca | 7/126 (5.6) | ||
| Johnson & Johnson | 1/126 (0.8) | ||
| Post–COVID syndrome, n (%) | 0.209 | ||
| Unaffected + unchanged | 87 (65.9) | 247 (71.2) | |
| Worsened | 30 (22.7) | 55 (15.8) | |
| Improved | 15 (11.4) | 45 (13.0) | |
| Post–COVID symptoms, n (%) | 0.604 | ||
| 0 | 73 (55.3) | 180 (51.9) | |
| 1 | 27 (20.4) | 65 (18.7) | |
| 2 | 17 (12.9) | 42 (12.1) | |
| 3 | 7 (5.3) | 27 (7.8) | |
| 4 | 1 (0.8) | 11 (3.2) | |
| 5 | 7 (5.3) | 22 (6.3) | |
| Fatigue, n (%) | 0.616 | ||
| Unaffected + unchanged | 116 (87.9) | 294 (84.7) | |
| Worsened | 5 (3.8) | 20 (5.8) | |
| Improved | 11 (8.3) | 33 (9.5) | |
| Anosmia/dysgeusia, n (%) | 0.947 | ||
| Unaffected + unchanged | 117 (88.6) | 306 (88.2) | |
| Worsened | 8 (6.1) | 20 (5.8) | |
| Improved | 7 (5.3) | 21 (6.0) | |
| Dyspnea, n (%) | 0.965 | ||
| Unaffected + unchanged | 118 (89.4) | 311 (89.6) | |
| Worsened | 8 (6.1) | 22 (6.3) | |
| Improved | 6 (4.5) | 14 (4.1) | |
| Cough, n (%) | 0.507 | ||
| Unaffected + unchanged | 127 (96.2) | 333 (96.0) | |
| Worsened | 4 (3.0) | 7 (2.0) | |
| Improved | 1 (0.8) | 7 (2.0) | |
| Chest pain, n (%) | 0.544 | ||
| Unaffected + unchanged | 127 (96.2) | 338 (97.4) | |
| Worsened | 4 (3.0) | 8 (2.3) | |
| Improved | 1 (0.8) | 1 (0.3) | |
| Headache, n (%) | 0.175 | ||
| Unaffected + unchanged | 120 (90.9) | 330 (95.1) | |
| Worsened | 7 (5.3) | 12 (3.5) | |
| Improved | 5 (3.8) | 5 (1.4) | |
| Rheumatological disorders, n (%) | 0.104 | ||
| Unaffected + unchanged | 121 (91.6) | 298 (85.9) | |
| Worsened | 10 (7.6) | 34 (9.8) | |
| Improved | 1 (0.8) | 15 (4.3) | |
| Gastrointestinal disorders, n (%) | 0.340 | ||
| Unaffected + unchanged | 124 (93.9) | 334 (96.2) | |
| Worsened | 5 (3.8) | 10 (2.9) | |
| Improved | 3 (2.3) | 3 (0.9) | |
| Cutaneous lesions, n (%) | 0.627 | ||
| Unaffected + unchanged | 129 (97.7) | 331 (95.4) | |
| Worsened | 1 (0.8) | 4 (1.1) | |
| Improved | 2 (1.5) | 12 (3.5) | |
| Hair loss, n (%) | 0.033 | ||
| Unaffected + unchanged | 130 (98.5) | 324 (93.4) | |
| Worsened | 2 (1.5) | 10 (2.9) | |
| Improved | 0 (0) | 13 (3.7) | |
| Upper respiratory tract infection symptoms, n (%) | 0.614 | ||
| Unaffected + unchanged | 129 (97.7) | 341 (98.3) | |
| Worsened | 1 (0.8) | 4 (1.1) | |
| Improved | 2 (1.5) | 2 (0.6) | |
| Ocular symptoms, n (%) | 0.021 | ||
| Unaffected + unchanged | 127 (96.2) | 327 (94.2) | |
| Worsened | 3 (2.3) | 20 (5.8) | |
| Improved | 2 (1.5) | 0 (0) | |
| Neurological disorders, n (%) | 0.707 | ||
| Unaffected + unchanged | 120 (90.9) | 308 (88.8) | |
| Worsened | 1 (0.8) | 7 (2.0) | |
| Improved | 11 (8.3) | 32 (9.2) | |
| Psychiatric disorders, n (%) | 0.505 | ||
| Unaffected + unchanged | 117 (88.6) | 293 (84.4) | |
| Worsened | 10 (7.6) | 36 (10.4) | |
| Improved | 5 (3.8) | 18 (5.2) | |
Post–COVID-19 syndrome and antibody response after natural infection and vaccination
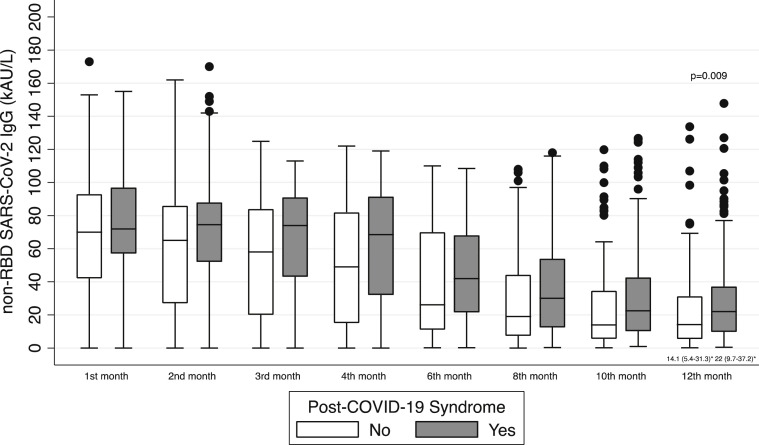
Patients included in the CORMOR 3–4 study were monitored (Fig. 1), and the antibody response of non-RBD SARS-CoV-2 IgG over time from symptom onset is shown in Fig. 3 . Overall, 275 patients completed the serological follow up with non-RBD SARS-CoV-2 IgG in proximity of the 12-month interview after onset of acute COVID-19, and 102 patients underwent a serological test with RBD SARS-CoV-2 IgG (Fig. 1).
Fig. 3.
Serological evolution against SARS-CoV-2 measured with non-RBD SARS-CoV-2 IgG in patients with or without post–COVID-19 syndrome at 12 months. COVID-19, Coronavirus Disease 2019; RBD, receptor binding domain; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2.
Approximately 153 of 275 patients (55.6%) maintained non-RBD SARS-CoV-2 IgG after 1 year. The median value of non-RBD SARS-CoV-2 IgG titre was approximately 22 kAU/L (interquartile range (IQR), 9.7–37.2 kAU/L). The presence of non-RBD IgG induced by natural infection was significantly associated with the occurrence of post–COVID-19 syndrome (OR: 1.35; 95% CI, 1.11–1.64; p = 0.003), and the median non-RBD SARS-CoV-2 IgG was significantly higher in long haulers than in patients without symptoms (22 kAU/L (IQR, 9.7–37.2 kAU/L) vs. 14.1 kAU/L (IQR, 5.4–31.3 kAU/L); p = 0.009; Fig. 3; Table 4 ).
Table 4.
SARS coronavirus type 2 RBD IgG and non-RBD IgG antibodies after natural infection and vaccination in patients with or without post–COVID-19 syndrome
| Post–COVID-19 syndrome |
p-valuea | ||||
|---|---|---|---|---|---|
| Yes (n = 153) | No (n = 122) | ||||
| Non-RBD IgG at 12 monthsb, median (interquartile range) | 22 (9.7–37.2) | 14.1 (5.4–31.3) | 0.009 | ||
| Vaccinated | Unvaccinated | Vaccinated | Unvaccinated | ||
| RBD IgG at 12 monthsc, n/N (%) | 0.451 | ||||
| <0.9 | 0/31 (0.0) | 2/23 (8.7) | 0/27 (0.0) | 0/21 (0.0) | |
| 0.9–2500 | 3/31 (9.7) | 19/23 (82.6) | 3/27 (11.1) | 21/21 (100) | |
| >2500 | 28/31 (90.3) | 2/23 (8.7) | 24/27 (88.9) | 0/21 (0) | |
RBD, receptor binding domain.
Comparison between post–COVID-19 syndrome (yes/no).
Value available for 275 patients.
Value available for 102 patients.
In contrast, the presence of RBD SARS-CoV-2 IgG in patients with hybrid immunity compared with those with natural immunity was not linked with the development of post–COVID-19 syndrome (>2500 U/mL vs. 0.9–2500 U/mL; OR: 1.36; 95% CI, 0.62–3.00; p = 0.441), and RBD SARS-CoV-2 IgG titres were similar in long haulers and patients without symptoms (50% values > 2500 U/mL vs. 55.6% values > 2500 U/mL; p = 0.451). The antibody response among vaccinated and unvaccinated patients is shown in Table 4.
Discussion
The results of this prospective study indicate that post–COVID-19 syndrome rates are high up to 1 year after acute infection, receiving the SARS-CoV-2 vaccine is not associated with worsening post–COVID-19 symptoms, and the persistence of a high titre serological response induced by natural infection but not vaccination may play a role in long-haul COVID-19. Our findings support the practice of offering SARS-CoV-2 vaccination regardless of infection history, and identifies a novel aspect of humoral response in patients with post–COVID-19 syndrome.
The high burden of long-term symptoms up to 1 year after infection in a wide range of patients, with a slight increase compared with that at 6 months, confirm both the possible fluctuation of symptoms and increased awareness of patients regarding post–COVID-19 syndrome [15,16]. Available studies have documented different rates of persistent symptoms after onset of COVID-19 in different settings [2,17]. Numerous multisystem symptoms were reported, although rheumatological, anosmia/dysgeusia, fatigue, dyspnoea, and psychiatric disorders were the most common [2,18]. Interestingly, at 12 months, we found a significant increase in rheumatological, psychiatric, and ocular symptoms compared with those reported at 6 months. In contrast, there was a significant decrease in neurological symptoms [3,18]. This delayed increase in rheumatological symptoms may confirm the role of persistent immune-mediated mechanisms, confirming the potential of SARS-CoV-2 to trigger autoimmune manifestations [2,4]. Mental health issues have been reported more frequently than in patients recovering from other infectious diseases, possibly due to the traumatic effects of the COVID-19 pandemic on mental health [16,19].
The potential immune-mediated hypotheses of long-haul COVID-19 remains uncertain [7,20]. Vaccination against SARS-CoV-2 is a leading strategy to change the course of the COVID-19 pandemic worldwide, reducing the risk of infection, severe complications, and long-term effects in case of a breakthrough infection [8,9,21]. Moreover, vaccines have shown to increase immunogenicity, antibody titres, and reactogenicity in individuals with a past infection compared with patients who have not been previously infected [22]. Vaccine hesitancy of previously infected patients and long haulers might be due to the belief that having developed a dysregulated response to a natural infection may be exacerbated by vaccination, as well as the perception that protection is acquired with previous infection, as observed in our cohort [11,23].
However, limited evidence with conflicting results is available on the potential impact of vaccination on post–COVID-19 symptoms [7,10,11]. Our findings suggest that vaccination is not associated with worsened symptoms when comparing vaccinated and unvaccinated patients, because symptoms were mostly improved or unchanged. Of interest, we only found an improvement in hair loss and worsening of ocular symptoms among unvaccinated patients. Telogen effluvium is a disorder induced by a wide variety of endogenous and exogenous factors, including COVID-19 infection [2]. Ocular morbidities in long haulers are an emerging problem that needs to be studied further [24].
Knowledge concerning humoral immune response to SARS-CoV-2 and its relationship with post–COVID-19 syndrome is still incomplete [5,6,[25], [26], [27]]. We found a significant association between growing titres of non-RBD SARS-CoV-2 antibodies after natural infection and post–COVID-19 syndrome in the prolonged follow up. In contrast, the presence and persistence of RBD IgG stimulated by the vaccine in patients with hybrid immunity were not associated with post–COVID-19 compared with patients with natural infection. The immune response induced by vaccines is a highly targeted response to the Spike protein of the SARS-CoV-2 virus, and may help the immune system tackle the possible viral reservoir, reducing the chance of nonspecific immune reactions and resetting the immune response [22,26,28,29]. In contrast, the response triggered by natural infection is broader, and may stimulate excessive or dysregulated allo- and autoimmune responses and uncontrolled inflammatory activity [22,30]. Based on our data on vaccination and humoral response, the SARS-CoV-2 vaccination should be recommended for patients with a history of previous COVID-19 infection, because further vaccine immune stimulation may not exacerbate sequalae or produce an altered humoral response. Moreover, patients with long-haul COVID-19 would benefit from vaccination to reduce their risk of further infection and avoid the risk of a vicious immune circle [31,32].
This study has several limitations. This single-centre study includes patients cared for during the first wave of the pandemic, limiting its generalizability given that the emergence of SARS-CoV-2 variants of concern may affect clinical presentations, serological responses, as well as the occurrence and severity of long-haul COVID. Moreover, a 20% drop-off rate between the 6- and 12-month interviews was observed, and only patients infected with COVID-19 were included. Thus, the study lacks a control group [16]. Furthermore, the vaccine programme in Italy prioritized HCWs and elderly patients, which might have introduced a sex bias [23]. Symptoms were self-reported, and subjectivity may have affected the findings. Additionally, the test accuracy, positivity cut-off points, and kinetics of antibodies may be assay-dependent, and measuring antibodies may be a limited indicator of immunity without the determination of cellular immunity.
In conclusion, patients with different degrees of COVID-19 severity who were cared for during the first wave of the pandemic perceive a high burden of post–COVID-19 sequelae with multi-organ clinical manifestations up to 1 year after onset. Vaccination does not seem to stimulate the appearance of symptoms, suggesting that individuals with a history of acute COVID-19 would benefit from SARS-CoV-2 vaccination. Persistently high non-RBD SARS-CoV-2 IgG titres induced by natural infection are associated with post–COVID-19 syndrome, but the presence and persistence of RBD SARS-CoV-2 IgG antibodies stimulated by the vaccine in patients with hybrid immunity are not associated with post–COVID-19 compared with those who are unvaccinated. A better understanding of the potential role of vaccination and humoral immune responses to SARS-CoV-2 is needed to inform the development of preventive and treatment strategies in the chronic phase of COVID-19.
Research ethics statement
The reference ethics committee of Friuli Venezia Giulia (CEUR-2020-OS-219 and CEUR-2020-OS-205) approved the study.
Transparency declaration
Maddalena Peghin reports receiving grants and personal fees from Pfizer, MSD, Menarini, and Dia Sorin outside of the submitted work. Carlo Tascini has received grants in the last 2 years from Correvio, Biotest, Biomerieux, Gilead, Angelini, MSD, Pfizer, Thermofisher, Zambon, Shionogi, Avir Pharma, and Hikma outside of the submitted work. The other authors have no conflicts of interest to declare.
This research was funded by PRIN 2017 n.20178S4EK9, Innovative statistical methods in biomedical research on biomarkers: from their identification to their use in clinical practice.
Author contributions
Conceptualization by MP, AP, MI, and CT. Methodology by MP, AP, MDM, MI, and CT. Software by MDM and MI. Validation by MDM and MI. Formal analysis by MDM and MI. Investigation by MP, AP, MI, MDM, and CT. Resources by MF, FC, and AS. Data curation by VG, EG, GB, DDE, FD, and FM. Writing of the original draft by MP, AP, MDM, and MI. Review and editing by MP, AP, MI, MDM, MF, FC, and CT. Visualization by MP, MI, and MDM. Supervision by MP, AP, MI, AS, and CT. Project administration by MP, AP, MI, FC, and CT. Funding acquisition by MI.
Acknowledgements
The authors thank all clinical and nursing staff who cared for the patients at the Udine Infectious Disease Clinic during hospitalization and ambulatory management. The authors are grateful to all patients for their collaboration.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2022.03.016.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Adeloye D., Elneima O., Daines L., Poinasamy K., Quint J.K., Walker S., et al. The long-term sequelae of COVID-19: an international consensus on research priorities for patients with pre-existing and new-onset airways disease. Lancet Respir Med. 2021;9:1467–1478. doi: 10.1016/S2213-2600(21)00286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nasserie T., Hittle M., Goodman S.N. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu X., Liu X., Zhou Y., Yu H., Li R., Zhan Q., et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9:747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ortona E., Malorni W. Long COVID: to investigate immunological mechanisms and sex/gender related aspects as fundamental steps for tailored therapy. Eur Respir J. 2022;59:2102245. doi: 10.1183/13993003.02245-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peghin M., Palese A., Venturini M., De Martino M., Gerussi V., Graziano E., et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin Microbiol Infect. 2021;27:1507–1513. doi: 10.1016/j.cmi.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereira C., Harris B.H.L., Di Giovannantonio M., Rosadas C., Short C.E., Quinlan R., et al. The association between antibody response to severe acute respiratory syndrome coronavirus 2 infection and post-COVID-19 syndrome in healthcare workers. J Infect Dis. 2021;223:1671–1676. doi: 10.1093/infdis/jiab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold D.T., Milne A., Samms E., Stadon L., Maskell N.A., Hamilton F.W. Are vaccines safe in patients with long COVID? A prospective observational study. MedRxiv. 2021 [Epub ahead of print] [Google Scholar]
- 8.Massey D., Berrent D., Krumholz H. Breakthrough symptomatic COVID-19 infections leading to long COVID: report from long COVID Facebook group poll. MedRxiv. 2021 [Epub ahead of print] [Google Scholar]
- 9.Antonelli M., Penfold R.S., Merino J., Sudre C.H., Molteni E., Berry S., et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis. 2022;22:43–55. doi: 10.1016/S1473-3099(21)00460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ledford H. Do vaccines protect against long COVID? What the data say. Nature. 2021;599:546–548. doi: 10.1038/d41586-021-03495-2. [DOI] [PubMed] [Google Scholar]
- 11.Strain W.D., Sherwood O., Banerjee A., van der Togt V., Hishmeh L., Rossman J. The impact of COVID vaccination on symptoms of long COVID. An international survey of people with lived experience of long COVID. Vaccines (Basel) 2022;10:652. doi: 10.3390/vaccines10050652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powers J.H., 3rd, Howard K., Saretsky T., Clifford S., Hoffmann S., Llorens L., et al. Patient-reported outcome assessments as endpoints in studies in infectious diseases. Clin Infect Dis. 2016;63:S52–S56. doi: 10.1093/cid/ciw317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institute for Health and Care Excellence COVID-19 rapid guideline: managing the long-term effects of COVID-19. https://www.nice.org.uk/guidance/ng188/resources/covid19-rapid-guideline-managing-the-longterm-effects-of-covid19-pdf-51035515742 Available at: [PubMed]
- 14.Peghin M., De Martino M., Fabris M., Palese A., Visintini E., Graziano E., et al. The fall in antibody response to SARS-CoV-2: a longitudinal study of asymptomatic to critically ill patients up to 10 months after recovery. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.01138-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cochrane Review Tool to assess risk of bias in cohort studies. https://methods.cochrane.org/sites/methods.cochrane.org.bias/files/public/uploads/Tool%20to%20Assess%20Risk%20of%20Bias%20in%20Cohort%20Studies.pdf Available at:
- 16.Matta J., Wiernik E., Robineau O., Carrat F., Touvier M., Severi G., et al. Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Intern Med. 2022;182:19–25. doi: 10.1001/jamainternmed.2021.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu T., Wu D., Yan W., Wang X., Zhang X., Ma K., et al. Twelve-month systemic consequences of COVID-19 in patients discharged from hospital: a prospective cohort study in Wuhan, China. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab703. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshee S., Vatti N., Chang C. Long-term effects of COVID-19. Mayo Clin Proc. 2022;97:579–599. doi: 10.1016/j.mayocp.2021.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martillo M.A., Dangayach N.S., Tabacof L., Spielman L.A., Dams-O'Connor K., Chan C.C., et al. Postintensive care syndrome in survivors of critical illness related to coronavirus disease 2019: cohort study from a New York City critical care recovery clinic. Crit Care Med. 2021;49:1427–1438. doi: 10.1097/CCM.0000000000005014. [DOI] [PubMed] [Google Scholar]
- 20.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., et al. COVID-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taquet M., Dercon Q., Harrison P.J. MedRxiv; 2021. Six-month sequelae of post-vaccination SARS-CoV-2 infection: a retrospective cohort study of 10,024 breakthrough infections. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangge H., Kneihsl M., Schnedl W., Sendlhofer G., Curcio F., Domenis R. Immune responses against SARS-CoV-2–questions and experiences. Biomedicines. 2021;9:1342. doi: 10.3390/biomedicines9101342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerussi V., Peghin M., Palese A., Bressan V., Visintini E., Bontempo G., et al. Vaccine hesitancy among Italian patients recovered from COVID-19 infection towards influenza and SARS-Cov-2 vaccination. Vaccines (Basel) 2021;9:172. doi: 10.3390/vaccines9020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costa I.F., Bonifacio L.P., Bellissimo-Rodrigues F., Rocha E.M., Jorge R., Bollela V.R., et al. Ocular findings among patients surviving COVID-19. Sci Rep. 2021;11:11085. doi: 10.1038/s41598-021-90482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durstenfeld M.S., Peluso M.J., Kelly J.D., Win S., Swaminathan S., Li D., et al. Role of antibodies, inflammatory markers, and echocardiographic findings in post-acute cardiopulmonary symptoms after SARS-CoV-2 infection. JCI Insight. 2022;7 doi: 10.1172/jci.insight.157053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massey D., Berrent D., Akrami A., Assaf G., Davis H., Harris K., et al. MedRxiv; 2021. Change in symptoms and immune response in people with post-acute sequelae of SARS-Cov-2 infection (PASC) after SARS-Cov-2 vaccination. [Epub ahead of print] [Google Scholar]
- 27.Garcia-Abellan J., Padilla S., Fernandez-Gonzalez M., Garcia J.A., Agullo V., Andreo M., et al. Antibody response to SARS-CoV-2 is associated with long-term clinical outcome in patients with COVID-19: a longitudinal study. J Clin Immunol. 2021;41:1490–1501. doi: 10.1007/s10875-021-01083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J., Lin H., Ye B., Zhao M., Zhan J., Dong S., et al. One-year sustained cellular and humoral immunities of COVID-19 convalescents. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab884. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra P.K., Bruiners N., Ukey R., Datta P., Onyuka A., Handler D., et al. MedRxiv; 2021. Vaccination boosts protective responses and counters SARS-CoV-2-induced pathogenic memory B cells. [Epub ahead of print] [Google Scholar]
- 30.Rosadas C., Randell P., Khan M., McClure M.O., Tedder R.S. Testing for responses to the wrong SARS-CoV-2 antigen? Lancet. 2020;396:e23. doi: 10.1016/S0140-6736(20)31830-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavanaugh A.M., Spicer K.B., Thoroughman D., Glick C., Winter K. Reduced risk of reinfection with SARS-CoV-2 after COVID-19 vaccination–Kentucky, May–June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1081–1083. doi: 10.15585/mmwr.mm7032e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bozio C.H., Grannis S.J., Naleway A.L., Ong T.C., Butterfield K.A., DeSilva M.B., et al. Laboratory-confirmed COVID-19 among adults hospitalized with COVID-19-like illness with infection-induced or mRNA vaccine-induced SARS-CoV-2 immunity–Nine states, January–September 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1539–1544. doi: 10.15585/mmwr.mm7044e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.