Abstract
Cell-matrix interactions play a critical role in tissue repair and regeneration. With gradual uncovering of substrate mechanical characteristics that can affect cell-matrix interactions, much progress has been made to unravel substrate stiffness-mediated cellular response as well as its underlying mechanisms. Yet, as a part of cell-matrix interaction biology, this field remains in its infancy, and the detailed molecular mechanisms are still elusive regarding scaffold-modulated tissue regeneration. This review provides an overview of recent progress in the area of the substrate stiffness-mediated cellular responses, including 1) the physical determination of substrate stiffness on cell fate and tissue development; 2) the current exploited approaches to manipulate the stiffness of scaffolds; 3) the progress of recent researches to reveal the role of substrate stiffness in cellular responses in some representative tissue-engineered regeneration varying from stiff tissue to soft tissue. This article aims to provide an up-to-date overview of cell mechanobiology research in substrate stiffness mediated cellular response and tissue regeneration with insightful information to facilitate interdisciplinary knowledge transfer and enable the establishment of prognostic markers for the design of suitable biomaterials.
Keywords: Substrate stiffness, Cellular response, Cell-matrix interaction, Mechanobiology, Tissue engineering
Graphical abstract
Highlights
-
•
Substrate stiffness physically determines cell fate and tissue development.
-
•
Rational design of scaffolds requires the understanding of cell-matrix interactions.
-
•
Substrate stiffness depends on scaffold molecular-constituent-structure interaction.
-
•
Substrate stiffness-mediated cellular responses vary in different tissues.
Abbreviations
- 2D
two-dimensional
- 3D
three-dimensional
- AFM
Atomic force microscopy
- ALP
alkaline phosphatase
- ATP
adenosine triphosphate
- BG
bioactive glass
- BMP4
bone morphogenetic protein 4
- CNT
carbon nanotube
- DHT
dehydrothermal treatment
- DRG
dorsal root ganglion
- ECs
endothelial cells
- ECM
extracellular matrix
- EDC
1-ethyl-3-(3-(dimethylamino)propyl)-carbodiimide hydrochloride
- ERK
extracellular signal-regulated kinase
- FAs
focal adhesions
- FAK
focal adhesion kinase
- GAG
glycosaminoglycans
- GelMA
gelatin methacrylate
- GTA
glutaraldehyde
- HA
hyaluronic acid
- HAp
hydroxylapatite
- H2O2
hydrogen peroxide
- HPA
hydroxyphenylpropionic acid
- HRP
horseradish peroxidase
- ICAM-1
intercellular cell adhesion molecule-1
- IL-1β
interleukin-1β
- iPSCs
induced pluripotent stem cells
- LINC
linkers of the nucleoskeleton and cytoskeleton complex
- MeHA
methacrylated hyaluronic acid
- MIF
macrophage migration inhibitory factor
- MLC
myosin light chain
- MSC
mesenchymal stem cell
- NHS
N-succinic anhydride
- NPCs
neural progenitor cells
- NSCs
neural stem cells
- PCL
polycaprolactone
- PDLSC
periodontal ligament stem cells
- PDMS
polydimethylsiloxane
- PEG
polyethylene glycol
- PEGDA
PEG diacrylate
- PGS
poly(glycerol sebacate)
- PLLA
poly(l-lactide)
- PPF-co-POSS
poly(propylene fumarate)-co-polyhedral oligomeric silsesquioxane
- PVA
polyvinyl alcohol
- RGD
arginine-glycine-aspartic acid
- ROCK
Rho-associated kinase
- SBF
simulated body fluid
- SMCs
smooth muscle cells
- TAZ
transcriptional coactivator with PDZ-binding motif
- TNFα
tumor necrosis factor-α
- Tyr
tyramine
- UV
ultraviolet radiation
- YAP
Yes associated protein
1. Introduction
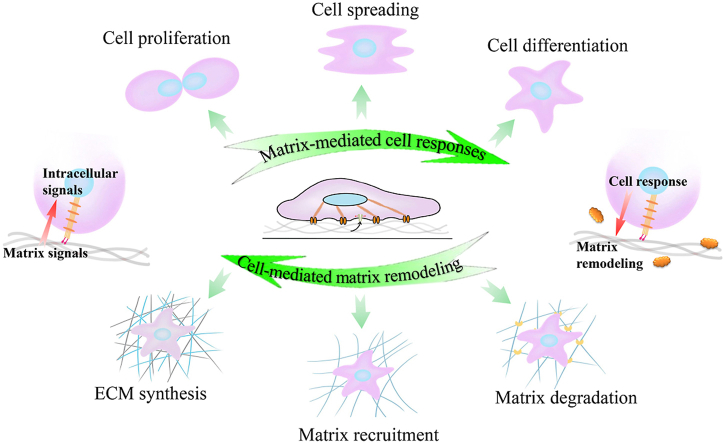
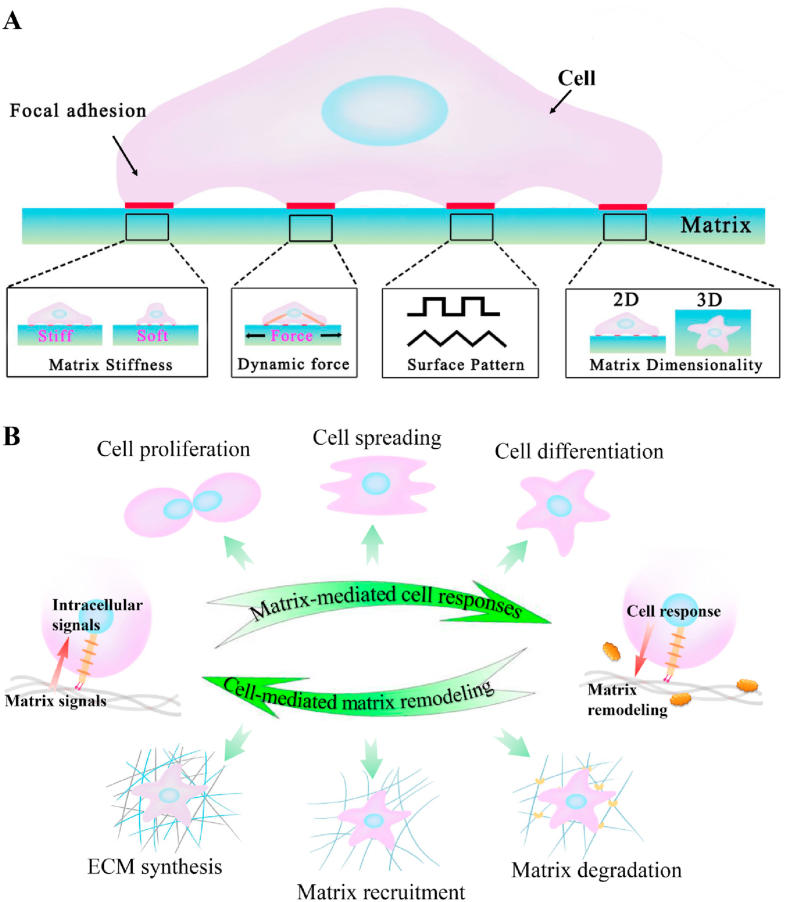
The extracellular matrix is a characteristic network-structured extracellular space that provides mechanical support for cell growth, tissue development, and buffering of body exercise load. The complex milieu (e.g., biochemical composition and biophysical cues) of ECM widely vary from brain to calcified bone in the body, and the surrounding cells constantly sense and process the complex milieu to make decisions of their fate through cell-ECM interactions [1]. As an arisen subset of regenerative medicine, the tissue engineering approach utilizing cells as the building block and providing scaffold to guide cell growth and matrix synthesis has gained increasing attention for rebuilding damaged tissues through biomimetic strategies. As discussed in the recent reviews, when placed or recruited into scaffolds, cells can sense properties of the mechanical cues (e.g., stiffness, contractility, surface pattern, and dimensionality) that usually act in concert with other physicochemical and biochemical properties through cell membrane-bound receptors (Fig. 1A) [2]. This complex array of extracellular signals then initiates intracellular signaling cascades and ultimately converts them into cellular responses, including migration, proliferation, and differentiation. Cells, in turn, exert forces on the scaffolds and remodel their structure throughout tissue regeneration, adaptation, and disease processes [2]. Therefore, understanding the interplay between ECM and cellular responses is fundamental for advancing the engineered substrates via mimicking the targeted tissue ECM's properties to actively manipulate cell behaviors and guide tissue regeneration and repair.
Fig. 1.
Cell-matrix interactions: (A) Representation of matrix mechanical cues, namely, matrix stiffness, external dynamic force, surface pattern, and matrix dimensionality, have been shown to affect numerous cellular behaviors through cell membrane-bound receptors that call FAs; (B) Cartoon depicting the interaction between ECM and surrounding cells, including matrix-mediated cell responses and cell-mediated matrix remodeling.
As a nonlinear, viscoelastic and anisotropic object, native tissue commonly exhibits complex mechanical properties in which their mechanical behaviors depend on the amount, direction as well as the time of applied deformation [3]. However, to quantify the mechanical properties practicably and provide the guidance to prepare biomimetic scaffolds, tissues are often assumed to be linear, elastic, and isotropic solids. In this simplified case, three moduli including elastic modulus, shear modulus, and bulk modulus are widely used to define the mechanical properties of tissues or scaffolds [4]. Stiffness, the extent to resist deformation in response to an applied force, is regarded as the rigidity of substrate. In the field of mechanobiology, substrate stiffness was performed generally to refer to the material moduli [3,4]. A general literature survey of the development of tissue engineering approaches to study cell-ECM interactions indicates that substrate stiffness has been highlighted as a key determinant of cell fate during the last few decades [5]. In 1997, polyacrylamide matrix was used for investigating the relationship between substrate stiffness and cellular responses of fibroblasts and epithelial cells lines [6]. Later on, other similar studies were reported on hydrogel [7], sponge [8], or fibrous substrates [3] in order to establish the strategies of fabricating various engineered scaffolds with controllable stiffness and thus to explore mechanical feedback to many other cell types, including stem cells. These studies add to a growing body of evidence that substrate stiffness-mediated cellular behavior can obviously regulate the matrix microenvironment and mechanical homeostatic actions in multiple scales from large tissue modification to cellular signaling pathway regulation. For instance, engineered scaffolds with overly stiffness, on the one hand, hinder bone regeneration through the stress shielding phenomenon [9]. On the other hand, cells within substrates sense the surrounding matrix stiffness and transduce the mechanical signals into physiological responses involving cell growth and differentiation. Such cognizance inspires us to speculate that substrates with matched stiffness in the defective tissue favor for tissue reconstruction. Regrettably, to date, limited progress has been made to regulate substrate stiffness without the change of other characteristics that are essential for fibrillar ECM functions. It indicates that appropriate biomimetic scaffolds that imitate biophysical and biochemical cues with a solely emphasis on tunable stiffness remain relatively underexplored, much less known are the pathways by which the cells sense the mechanical signal and translate them into biochemical mechanisms, as well as unveiling the initiated molecular mechanisms.
Overall, the rational design of functional scaffolds necessitates a high throughput understanding of key cellular decisions, a complete picture of the cellular mechanochemical pathways, and associated downstream signaling pathways in response to substrate stiffness. Based on the current knowledge and emerging research in the field, this review summarized the exploited studies that have been performed to explore the substrate stiffness-mediated cellular responses, aiming to provide comprehensive and effective mechanobiology information of cell-matrix interactions for tissue engineering. Three parts were mainly reviewed: Firstly, we introduced ECM of different stiffness used in tissue regeneration and how they act as an instructive cue to influence cell fate. Then, the latest advances of engineering-based approaches to achieve the controllable modulation of scaffold stiffness for the study of cellular decision-making are discussed. Finally, this review highlighted the research progress of substrate stiffness-mediated cellular responses in representative tissues. Biomaterial-based scaffolds are more than fundamental research platforms for studying cellular responses and underlying molecular mechanisms but could also be used for uncovering the role of substrate stiffness in physiological and pathological processes. We hope this review could provide insightful information and contribute to the future development of biomaterial-based scaffolds in regenerative medicine.
2. Substrate stiffness physically determines cell fate and tissue development
As the housing environment of cells, ECM transmits external mechanical loads and internal mechanical stimulation to resident cells and guides their fate through modulating cell growth and differentiation. Meanwhile, cells could constantly change the composition and macromolecular network structure by secreting matrix components and matrix metalloproteinases or exerting mechanical forces to regulate the type and arrangement of macromolecules in ECM (Fig. 1B), thus contributing to the variety of tissue stiffness differing from the soft brain to the stiff cortical bone [10,11]. Increased studies have confirmed the significant role of matrix stiffness (including native ECM and engineered substrates) in affecting cellular decisions and tissue development via tissue engineering approaches. This section demonstrated the structural components and corresponding architecture of native ECM, the mechanobiological mechanisms of cells-substrate interaction, and the strategies to design scaffolds with desired mechanical parameters.
2.1. Unique stiffness attribute of native ECM
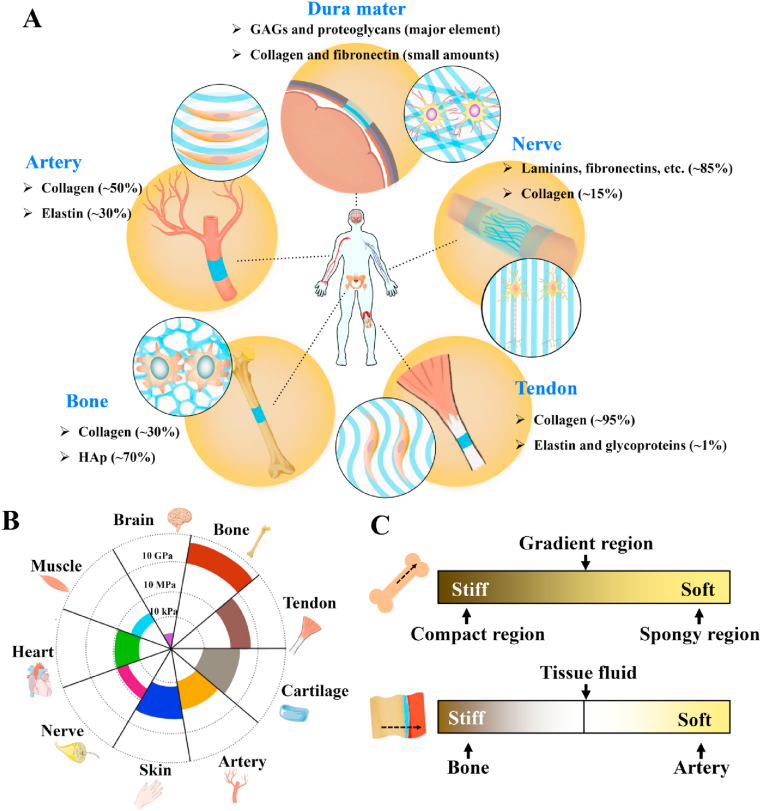
The ECM is a complex and highly dynamic network-structured extracellular space in which the molecular composition and matrix pliability are modulated by the resident cells [12]. Attributing to the intrinsic properties of ECM constituent molecules and their exclusive self-assembled hierarchical architecture (Fig. 2A), ECM stiffness (or measured as Young's Modulus) presents widely diverse spanning from the brain (1–3 kPa) [10,11] to muscle (23–42 kPa) [13], blood vessel (1.16–860 MPa) [14], tendon (136–820 MPa) [15], or bone (15–40 GPa) [16] (Fig. 2B).
Fig. 2.
Unique stiffness attribute of each native ECM: (A) composition and self-assembled hierarchical architecture of ECM to each specific tissue; (B) elastic moduli of ECM presenting widely diverse spanning from the brain to bone; (C) example of the heterogeneity of substrate stiffness showed in different regions of bone ECM.
The chemical composition and the hierarchical architecture are decisive for ECM's stiffness. From the aspect of chemical composition, ECM is mainly composed of two main classes of macromolecules: fibrous proteins (e.g., collagen, elastin, fibronectin, and laminin) and proteoglycans, with plentiful ECM-binding enzymes as cross-linkers. Fibrous proteins constitute the fibrous component of ECM, serving for energy storage, to provide tensile strength and adhesive sites for cells attachment; while viscous proteoglycans fill the interstitial space of fibrous architecture in the form of hydrated gel, acting as an energy absorber, to endow ECM with functions in buffering and bearing the external force [14,15]. As the most abundant structural fibrous protein, collagen is the basic building block of ECM architecture and widely distributes in the hierarchical structural elements to provide the tensile strength of ECM. Meanwhile, elastic proteins (e.g., elastin) and proteoglycans give ECM superior elasticity. The variation in the content of these structural fibrous proteins lays the groundwork for tissues of specific stiffness. For example, the enrichment of fibrillar collagens and mineralized inorganic component with less elastic elements contributes to the supreme rigidity of bone tissues and help to maintain physiological locomotion and load-bearing capacity [17]. For the tendon that connects muscle and bone, certain elastic components of elastin and other glycoproteins within collagen give it excellent elasticity to stretch and retract along the longitudinal direction [18]. The enrichment of elastin in the fibrous ECM is also crucial for blood vessels to sustain the tensional forces exerted by the blood flow [5]. Structurally, the upper level of hierarchical architecture is another determinant in the variation of ECM stiffness. Collagen fibers in tendon present an undulating hierarchical anisotropic architecture and thus imbue unique mechanical characteristics of substrate to experience highly complicated loading patterns [19]; In contrast, as the continuous membrane that encloses the brain and the spinal cord, the collagen fibers of dura mater are arranged irregularly within tissue and sustain the low modulus (< 1 MPa) [15].
Thus, the characteristics of ECM composition and self-assembled hierarchical architecture lead to the unique stiffness attribute of a specific tissue. Moreover, heterogeneity extends to substrate stiffness in different regions, even in the same tissue or organ. For instance, the stiffness of bone ECM rises from spongy regions to compact regions or decreases and then increases from bone to surrounding arteries (Fig. 2C) [17].
2.2. Cell mechanobiological responses to substrate
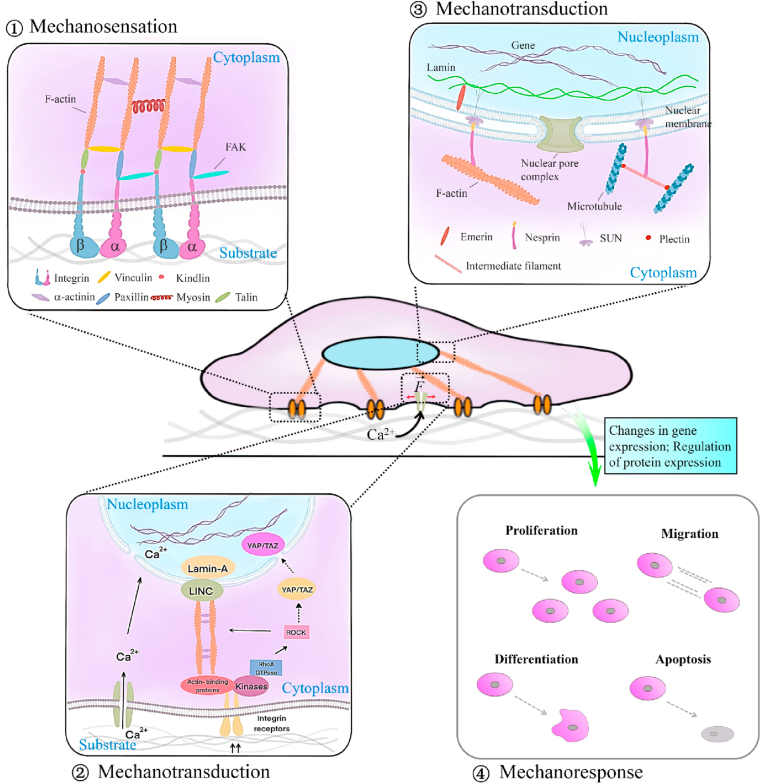
Cells constantly sense their microenvironment and make adjustments over time to control cell function and tissue remodeling. With growing importance attached to the cell-matrix interaction in tissue remodeling, cell mechanobiology has also witnessed rapid development and underpinned scientific advances in the scaffold design strategies that recapitulate the critical cues to guide tissue regeneration via tissue engineering approaches. It is now widely accepted that cells (from stem cells to mature cells) can sense and respond to the substrate stiffness by three steps: mechanosensation, mechanotransduction, and downstream mechanoresponses (Fig. 3) [3]. Briefly, when cells first contact the substrate surface, large protein complexes called FAs are formed to tether the cell cytoskeleton (e.g., integrins) to the substrate (e.g., ligands) [20]. Then the physical signals of the microenvironment are sensed and transduced into biochemical signals, activating subsequent changes in cell behaviors, including cell morphology, growth, differentiation, and death [3].
Fig. 3.
Schematic illustration of cell mechanobiological responses to substrate: ① mechanosensation process. Transmembrane protein integrins perceive substrate mechanical cues by activating the assembly of focal adhesion molecules (e.g., vinculin, talin, and paxillin) and recruiting FAK; ② mechanotransduction process from the extracellular environment to the cytoplasm. The perceived mechanical signals are transmitted from FAs to the cytoplasm through multiple mechanisms, including ion channel activation, actin remodeling, as well as RhoA/ROCK molecule pathway; ③ mechanotransduction process from the cytoplasm to the nucleoplasm. This transmission process is mainly mediated by LINC molecular complexes, which are composed of nesprin isoforms that link to cytoskeletons in the cytoplasm and inner nuclear membrane-associated SUN proteins that bind to lamins in the nucleoplasm; ④ mechanoresponse process. The interactions between Lamins and LINC result in gene and protein expression changes and then induce the adaptive cellular response.
Mechanosensation is recognized as the initiating event of cell-substrate interaction (Fig. 3①). Extracellular signal-regulated kinases (e.g., FAK and Rho) play a crucial role in the mechanosensory chain [21], while substrate geometry and polarity could also influence this process within cells. FAs are nanoscaled mechanosensors located on cell membranes that could anchor the contractile cytoskeleton to the substrate through the tripeptide sequence RGD [21] and mediate the bidirectional cell-matrix signaling from inside and outside of cells [22]. The formation of cell-adhesion sites requires a space of less than 73 nm for the extracellular ligands to bind to clustered RGD peptides [22]. Besides, evidence revealed that the size of the formed sites would increase in a stiffness-dependent manner [23]. Compared to the 2D microenvironment, the mechanosensing pathways in the 3D substrates are even more complex because cells in the 3D microenvironment pull and push substrate from any direction to gauge the stiffness [24]. However, the influence of the 3D microenvironment provided by biomimetic scaffolds on the resident cells is poorly considered in cell-based or in situ tissue regeneration studies due to its complexity.
Once cells sense the substrate features, including stiffness, a diverse set of structural motifs begin conformational change over a range of mechanical stimulation. They undergo a host of biochemical pathways such as the force-induced exposure of otherwise cryptic peptide sequences, the opening of mechanosensitive ion channels, or receptor-ligand interactions responses, known as mechanotransduction (Fig. 3②-③) [25]. The cytoskeleton, propagating deep into the cytoplasm and linking the cell nucleus to the substrate, is confirmed to transmit the sensed mechanical signals from the substrate to the nucleus by actin polymerization and microtubule assembly [26]. Alteration of enzymes activity is another common pathway that transduces mechanical signals into biochemical signals by directly regulating protein conformational changes. For instance, the increased substrate stiffness can promote ATP hydrolysis that moves along the actin filaments and microtubules to generate energy for the contractile forces of cells on the substrate [27]. Then, the sensed signals are transmitted to the nucleus via the LINC complex, lamins, and other proteins such as lamina-associated polypeptides 2 [22]. These propagated mechanical signals will regulate the expression of genes and the related nuclear protein. Another pathway of the sensed mechanical signals transmitting through the cytoplasm to the nucleus is the structural modification of cytoplasmic proteins, such as YAP and transcriptional coactivators TAZ, which are then shuttling to the nucleus to regulate gene expression and control cell behaviors [28]. Furthermore, the change of conductive state of mechanosensitive ion channels also transduces mechanical signals into biochemical signals, such as the increased open of ion channels at high tensions in response to the stiff substrate [29]. Notably, some focal-contact proteins releasing from the adhesion sites, such as paxillin and vinculin, can serve as cell signaling molecules if the receptor-ligand interactions fail on the substrate [30]. Overall, the mechanotransduction process is in a position- and time-dependent manner in cells [22].
Lastly, during mechanotransduction, gene and protein expression changes initiate the adaptive cellular responses, including cell growth, differentiation, and death [3]. These responsive processes are known as mechanoresponse (Fig. 3④). The cytoskeleton is a dynamic structure that provides 3D support to cells and is responsible for cell functions. In response to the substrate stiffness, cells can alter their internal cytoskeleton to reinforce and reorganize the cell and then activate various intracellular signaling pathways to govern cell fate for tissue growth [22]. Usually, early cell responses occur on a second to minute timescale [25]. Then they activate actin filament extension, recruit myosin molecules (the mediator of cell contraction), and upregulate the production of ECM proteins through stimulating the release of paracrine growth factors or directly triggering gene expressions of an intracellular signaling pathway [31]. Under exceptional circumstances, cells will lose their cellular responses to substrate stiffness if they contact other cells and the cell-cell binding overwrites the cell-matrix mechanobiological signaling [32]. Previous studies have shown that cell-cell interactions coordinated by specific protein complexes such as adherent junctions allow mechanical forces to propagate across tissue cells, which indicates the pivotal role of cell-cell interactions in cell mechanobiological responses to the substrate [22]. Besides, the substrate stiffness regulates gap junctions formed from two cellular hemi-channels composed of connexin proteins (membrane proteins). They protrude through the cell membrane and connect the cytoplasm of adjacent cells to permit the movement of small molecules, followed by the activation of an intracellular signaling cascade in response to substrate stiffness [33].
2.3. Stiffness as a crucial physical cue to design tissue-engineered substrate
The unique attribute of native ECM stiffness and the clarified mechanobiological pathways indicates the key role of substrate stiffness in driving a variety of instructions to cells via cell-matrix interactions. Commonly, the balance between the internal forces generated by cytoskeleton tension and substrate stiffness is required to maintain the shape and anatomical localization of cells [34], thus resulting in a more spreading area of cells on a stiffer substrate [35]. Furthermore, the substrate stiffness matched that of native ECM from neural to bone tissues leads stem cells to express typical precursor genes of the cell lineage found in those respective tissues. Other circumstances include neuron preference on soft matrix surface, whereas fibroblasts grow better on the rigid matrix surface [36]. Such results revealed the crucial role of substrate stiffness in designing biomimetic engineered substrates from the microscale aspect.
At the macroscale, substrate stiffness supports physiological activities, including the physiological loads and the ability to prevent the rupture of tissues. The macroscopic mechanical anisotropy lends a unique function to load-bearing tissues. As shown in the tendon, the aligned fibrous architecture of ECM supports tensile loads through allowing energy storage and dissipation to transmit force along the tendon during motion such as walking and running, thus decreasing the overall energy commanded in daily exercise [37]. Similar protection mechanisms against large continual strain have also been reported in the vascular system. Due to the axially aligned fibrous architecture, a small hysteresis of arteries, existing in each cardiac cycle, can effectively maintain stable blood pressure through fairly low-energy dissipation, thus preventing the resonation of the reflected pressure waves in the vasculature [38]. Certainly, alteration of the distinct stiffness of native tissues is correlated with cancerous growth and tissues lesions. Over-secretion of structural proteins by cells in pathophysiological settings can display local alteration of tissue stiffness and then stimulate the chain reaction of tissue pathological changes. For example, tumor progression resulting from the secretion of enzymes and cytokines by infiltrated immune cells could alter ECM architecture, promote fibrosis, and enhance matrix stiffness. The enhanced ECM stiffness in the tumor microenvironment, in turn, promoted tumor cell survival and growth, thus affected the malignant transition of cells and the formation of metastasis [39,40]. Fibrosis following a myocardial infarction, gliosis after a cerebrovascular insult, and collagen deposition after epithelial disruption are all related to the elevated local mechanical microenvironment of normal tissues [41], while osteoporosis associating with reduced ECM stiffness was characterized by impaired bone microarchitecture, which easily leads to bone fragility and proneness to fracture [42].
In summary, substrate stiffness is a crucial physical cue in both controlling cell fate at the microscale and remodeling tissues at the macroscale. The ability to accurately emulate the natural features of ECM is essential for designing biomimetic scaffolds and physiologically relevant platforms to study tissue development and pathology [3]. It necessitates a thorough examination of the cellular mechanobiological responses to substrate stiffness and the mechanisms underlying physiological activities and pathological disorders, thereby providing new insights into the development of tissue-engineered substitutes to improve their function and decrease potential side effects in tissue regeneration. Else, given the different responses of cells to substrate stiffness among various tissues, it remains unclear why some cells normally require a rigid surface for growth, whereas others thrive on soft surfaces. It would be interesting to exploit the unknown mechanism via tissue engineering approaches and go beyond empirical approaches to the rational design of engineering systems to control tissue development through well-defined in vitro systems with controllable stiffness.
3. Strategies to tune the stiffness of engineering-based substrates
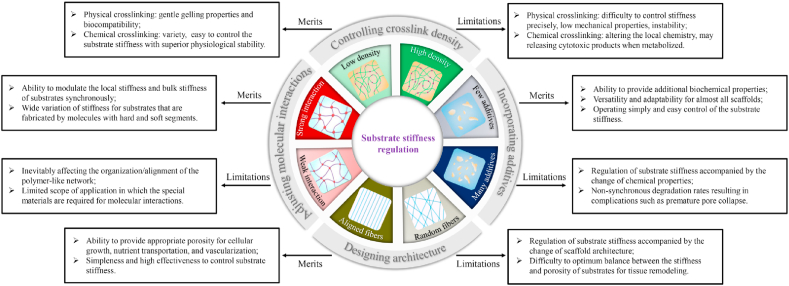
With increased awareness of the role of substrate stiffness in regulating cell behavior, numerous engineering substrates with controllable parameters have been developed (Table 1). Over the past few decades, strategies to regulate substrate stiffness could be classified into four major groups: controlling crosslink density, incorporating additional constituents, designing architecture using fabrication approaches, and adjusting molecular interactions (Fig. 4). This section introduced the recent studies of scaffold fabrication technologies for tunable stiffness.
Table 1.
Numerous methods to fabricate engineered substrates with controlled stiffness.
| Materials | Scaffold types | Mechanical parameter | Mechanical range | Changed parameter to regulate substrate stiffness | Ref. |
|---|---|---|---|---|---|
| Natural materials | |||||
| Alginate | Hydrogel | Young's modulus measured by AFM | 1.5–19.0 kPa | G content and alginate concentration | [43] |
| Gelatin | Hydrogel | Stiffness measured by rheometer | 10–1000 Pa | H2O2 concentration | [44] |
| HA | Hydrogel | Compressive modulus measured by static material tester | 5.4–11.8 kPa | H2O2 concentration | [45] |
| Silk fibroin | Hydrogel | Compressive modulus measured by mechanical testing system | 6.41–63.98 kPa | Silk protein concentration | [46] |
| Collagen | Hydrogel | Compressive modulus measured by AFM | 2–50 kPa | EDC/NHS concentration | [47] |
| Synthetic materials | |||||
| Polyacrylamide | Hydrogel | Stiffness measured by nanoindentation | 30–665 kPa | Acrylamide/bis-acrylamide concentration | [48] |
| PCL | Electrospun fibers | Tensile modulus measured by universal testing machine | 6.12–33.20 MPa | Collector rotation speed | [49] |
| PLLA | Electrospun fibers | Tensile modulus measured by universal testing machine | 696.90–944.57 MPa | Annealing temperature | [50] |
| PEGDA | Hydrogel | Compressive modulus measured by microstrain analyzer | 0.31–5.1 kPa | Altering the organization of the crosslinking sites using allyl-presenting monomer | [51] |
| GelMA | Hydrogel | Stiffness measured by AFM | 3.8–29.9 kPa | Degree of methacryloyl functionalization | [52] |
| Composite materials | |||||
| Collagen-HAp | Freeze-dried scaffolds | Compressive modulus measured in uniaxial compression | 60–1000 kPa | Mass fraction of HAp | [53] |
| PLCL-PLLA | Electrospun fibers | Young's modulus measured using universal testing machine | 14.68–2141.72 MPa | Shell-core structure of single fiber | [54] |
| Collagen-BG | Hydrogel | Compressive modulus measured using unconfined compressive testing | 37–482 kPa | Thickness of carbonated hydroxylapatite coating on scaffolds | [55] |
| PGS-gelatin | Electrospun fibers | Tensile modulus measured by universal testing machine | 1.5–54.4 MPa | Ratio of PGS to gelatin | [56] |
| PCL-gelatin | Electrospun fibers | Stiffness measured by universal testing machine | 223.06–623.33 kPa | Coaxial core diameter | [57] |
Fig. 4.
Hallmarks of the current strategies to regulate substrate stiffness.
3.1. Controlling the crosslink density of substrates for tunable stiffness
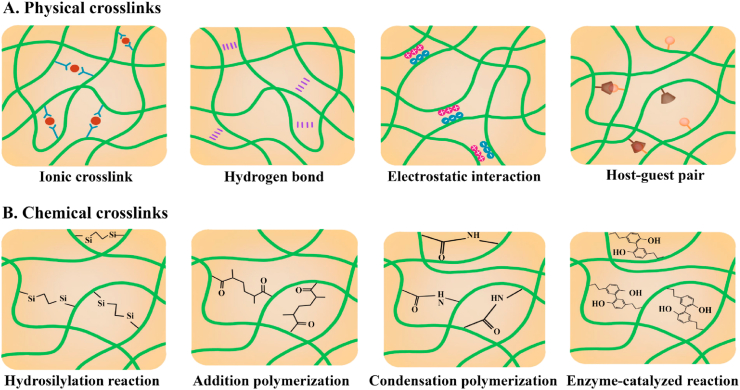
Crosslinks hold the different polymer chains together via substantial entanglement in the polymeric network of the crosslinked substrate, which helps to avoid the dissolution of hydrophilic polymer chains into the aqueous phase. Hydrogels that present excellent water-absorbing capacity is a typical example. The substrate stiffness can be controlled effectively by regulating the degree of intermolecular substantial entanglement [58]. Substantial entanglement in networks is divided into physical or chemical crosslinks based on the type of bonds between the polymer chains (Fig. 5) [7]. The design of hydrogels with tunable stiffness via controlling crosslinking density would be described and discussed in the following section.
Fig. 5.
Schematics of representative crosslinks for polymer network interactions: (A) physical crosslinks; (B) chemical crosslinks.
3.1.1. Control of physical crosslink density
Physical crosslink (i.e., reversible interaction) is useful to construct self-healing and mechanically dynamic shear-thinning hydrogels [58]. In physically crosslinked hydrogels, the molecular entanglements include but are not limited to ionic crosslinks, hydrogen bonds, electrostatic interactions, as well as host-guest or antigen-antibody pairs (Fig. 5A).
Regulating ionic crosslink density (e.g., alginate-Ca2+ [59], kappa carrageenan-K+ [60], and pectin-Fe3+ network [61]) is an effective crosslinking mode to adjust substrate stiffness. Alginate is a well-known example that consists of α-l-guluronate blocks (G-blocks) and β-d-mannuronate blocks (M-blocks), in which the carboxylic acids of G-blocks can interact with divalent cations (e.g., Ca2+, Mg2+, and Ba2+) to form a 3D gelatin network [59]. The stiffness of the alginate hydrogels can be modulated by regulating the overall fraction of G residues, the concentration of divalent cations, or the concentration of alginate [59,62]. Due to the special molecular structures, hydrogen bonds could also be used for controlling substrate stiffness. For example, adenine particles can generate intermolecular hydrogen bonds of adenine-adenine [63]; the abundant –OH of tannic acid can form hydrogen bonds with other macromolecules such as the ether groups of PEG [64]; or the induction of β-sheet conformation can be implemented to form hydrogen bonds in natural polymers including collagen and silk fibroin [47,65]. If the hydrogels are generated based on the opposite charge attributes of two materials (i.e., polycation and polyanion pairs) or ampholyte (e.g., ion-pair comonomers), the substrate stiffness can be regulated through controlling electrostatic interactions via modulating solution pH. It has been confirmed that the substrate stiffness could be directly modulated via pH by orders of magnitude [66]. Moreover, host-guest and antigen-antibody pairs could also be used as physical crosslink modes to control substrate stiffness [67,68]. Different from the above described physical crosslinks, hydrogels crosslinked by host-guest interaction show muscle-like fast self-recovery after large deformation through tuning the kinetics of the host-guest association and dissociation [67], and hydrogel crosslinked by antigen-antibody interaction can act as an effective approach to permit drug delivery in response to a specific antigen [68].
Physically crosslinked hydrogels have gained much interest in tissue engineering applications because of their recognized biocompatibility and gentle gelling properties. However, their stiffness is difficult to control precisely due to the uncontrollable crosslinks and unavoidable influences of surrounding environments, including temperature, ion concentration, and pH [69]. Besides, physically crosslinked hydrogels also have other restrictions such as inadequate mechanical properties, limiting their applications in tissue engineering [65].
3.1.2. Control of chemical crosslink density
Chemical or ‘permanent’ crosslinks, in which covalent bonds are formed between polymer chains, have gained more attention from researchers than physical crosslinks due to the variety available and easy control of the substrate stiffness with superior physiological stability [70]. The efficiency and attention of chemical crosslinked substrates have now been shifted to the development of functional alternatives with higher degrees of complexity, biomimetic extracellular matrices. According to crosslinking mechanisms, the current frequently used chemical crosslinks can be classified into hydrosilylation reaction, addition polymerization, condensation polymerization, and enzyme-catalyzed reaction (Fig. 5B).
Hydrosilylation reaction between the vinyl-terminated end groups and silicon hydride groups to form Si–C bonds is a frequently-used covalent crosslinking approach to fabricate substrates with controlled stiffness as in vitro and in vivo biomaterial models [71,72]. As a typical representative, PDMS is made up of a base and a curing agent. By changing the processing conditions (e.g., the curing temperature and time) and the ratio of the base and curing agent, the crosslink density in PDMS can be regulated to tune substrate stiffness [73]. As a result of the high protein binding capability for cell growth [71] and transparent characteristic for easy observation [74], PDMS with controlled stiffness have been widely used for exploring the cell-matrix interaction and developed as an effective platform for new diagnostic strategies. Addition polymerization is another frequently-used covalent crosslinking method to regulate substrate stiffness. One of the most widely used substrates aiming to explore cellular responses to substrate stiffness is polyacrylamide. Given that the crosslinking agent of acrylamide monomer can be directly crosslinked with bis-acrylamide, polyacrylamide stiffness can be varied within 2–665 kPa by changing the constituent concentration and the proportion of acrylamide in the final specimen [75,76]. Additionally, acrylamide has also been used as a functional group to conjugate with polymers for polymerization, including synthetic polymers (e.g., PEG, PCL) and natural molecules (e.g., gelatin, alginate, HA) molecules [69,77]. PEGDA is widely used for systematically exploring cell response to specific alterations in substrate stiffness because its hydrophilic characteristic permits the defined investigation without the interference of any bioactive moiety that conjugated or adsorbed to the substrate [77]. Another substrate that has been widely used for exploring cell response to substrate stiffness (in especial 3D cell-laden systems) is GelMA due to its biocompatibility for cell survival and the mild light-polymerizable ability into hydrogel [78]. Regrettably, the poor mechanical property of GelMA with a modulus range of 5–180 kPa limits its applications in load-bearing tissues compared to synthetic materials [52,78]. The chemical crosslink of functional groups (for instance, –OH, –NH2, and –COOH) offers other approaches to tailor substrate stiffness through condensation polymerization [47,65]. This method is mainly performed in natural materials, including collagen, silk fibroin, and HA [65,79]. Condensation polymerization can be broadly classified into ‘zero length’ crosslinkers that remain not part of the crosslinker structure post-crosslinking and ‘non-zero length’ crosslinkers where some or all of the crosslinker are incorporated, by their potential to incorporate the crosslinker directly into substrate materials [80]. The ‘zero length’ crosslinkers mainly contain EDC/NHS that crosslinks free primary amine groups and carboxylate anions [81], DHT relying upon dehydration between carboxyl and amine groups [47], and UV inducing the formation of radicals for crosslinking within molecules [80]. In contrast, the ‘non-zero length’ crosslinkers include GTA, which forms monomeric or oligomeric crosslinks between two amino acid side groups [65], and genipin, which involves a secondary amide linkage of a free amine via an SN2 nucleophilic substitution [80]. For those chemically crosslinked substrates, the chemical crosslink density can be regulated to varied substrate stiffness by altering the crosslinker concentration. Nevertheless, there is some concern with the potential of condensation polymerization. For example, ‘zero length’ crosslinkers may modify the local chemical structure of natural polymers, resulting in the diminishment of the availability of essential cell-binding motifs; and ‘non-zero length’ crosslinked substrates may release cytotoxic products when metabolized. Enzyme-mediated oxidation reactions undergo covalent crosslinking to alter substrate stiffness by using enzyme systems like tyrosinases, transferases, or lysyl oxidases as catalysts [70]. The majority of the enzymes involved in the chemical crosslinking are common to the enzyme-mediated reactions naturally occurring in our body, and the stiffness can be regulated by modulation of the enzyme activity. The representative example is that biopolymers with phenol moieties such as HPA [44], Tyr [45], and dopamine [82] can be performed to form phenol conjugates using the oxidative coupling under the catalytic action of H2O2 and HRP. The independent tuning of stiffness can be modulated by varying the ratio of H2O2 and HRP [44] or the content of basal biopolymer [82]. Additionally, enzyme-mediated oxidation reaction has been proven to present several advantages for engineered substrates, such as the avoidance of unwanted side effects (i.e., toxicity) that may occur with organic solvents and excellent mildness at normal physiological conditions that can avoid the possible loss of bioactivity of natural polymers [70].
In short, an interpenetrating network can be formed successfully as long as the individual molecule has the ability to provide adequate reactive groups for multilateral crosslinking. Based on this understanding, alternative methods that modify polymer molecules with reactive groups have been developed to hold the separate non-interacting molecules together to form a crosslinking network. For example, using DNA as a functional crosslinker to modify polymer molecules, the substrate stiffness could span from ∼100 Pa to 30 kPa through the varying length of crosslinker, monomer concentration, and level of crosslinking [83].
3.2. Incorporating additional constituents into substrates for tunable stiffness
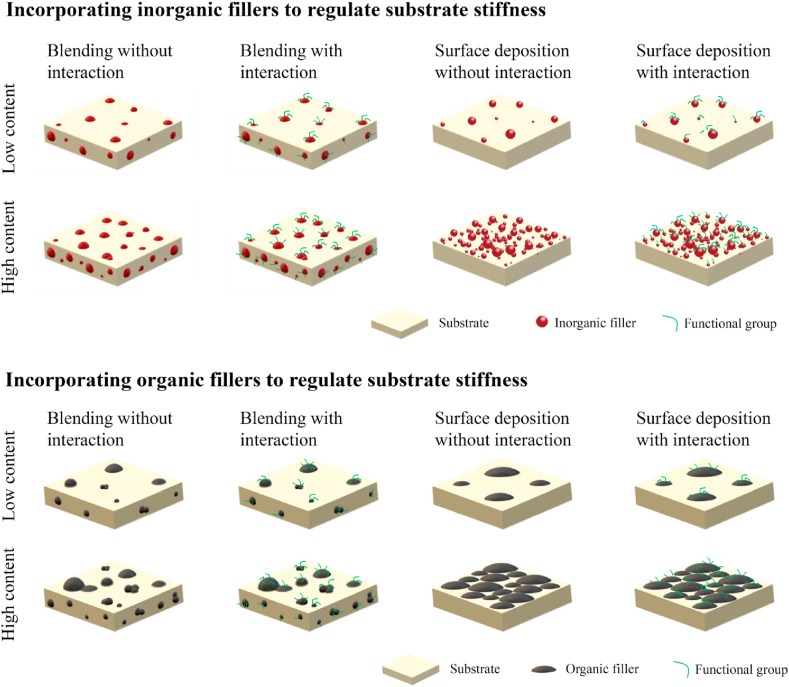
During the fabrication process of tissue-engineered substrates, incorporating additional constituents, including blending substrates with organic/inorganic fillers [84,85] or modifying substrate via surface deposition [55,86], has aroused researchers' attention and been deemed as another effective strategy to tailor substrate stiffness, attributing to its ability to simultaneously improve substrate bioactivity and promote ‘healing’ efficiency (Fig. 6). This section discussed the recent studies for tunable stiffness from the aspect of incorporating additional constituents.
Fig. 6.
Schematics of the current strategies to tailor substrate stiffness through incorporating additional constituents.
3.2.1. Inorganic filler reinforcement
Incorporation of inorganic bioactive fillers, including calcium phosphates [53,87], silicate [88], BG [89], CNT [90], and graphene [91], into polymeric substrates has been prepared with an emphasis on the fabrication of hybrid biocomposites with tunable stiffness, as well as the adjustment of biological performance. As one of the primary components of bone ECM, calcium phosphates, mainly in the form of HAp [53,92], have been widely used as osteoinductive fillers in bone substrates due to their high strength and outstanding osteoconductivity. CNT has attracted intensive attention attributing to their nanoscale dimensions and good acceptance by the biological environment [90]. Recent findings indicated the good biocompatibility of graphene with exceptional mechanical and electrical properties [93]. However, the usage of inorganic fillers has two major drawbacks: (1) easy to agglomerate in substrates, and (2) lack of specific interactions between the inorganic fillers and based materials, thereby leading to poor dispersion, compromised mechanical properties, and unfavorable cellular responses. To improve the effective use of inorganic fillers, enhancing the interactions between inorganic fillers and substrate materials in terms of physicochemical properties has carried out extensive research. For example, enhancing the hydrophobic-hydrophobic interactions between graphene oxide and silk fibroin via water/temperature annealing routines could lead to a 75% increase in Young's modulus [94]. Additionally, the introduction of external functional elements is also an effective strategy to enhance the delicate interactions for expected stiffness, such as coating inorganic fillers with mussel-inspired polymer [95] or modifying the inorganic nanoparticles with functional groups [96].
In addition to the above descriptive circumstances, the deposition of inorganic phase materials on the surface of substrates is another approach to control substrate stiffness [55,86]. For example, the soaking of substrates in SBF to uniformly precipitate mineralized layer (i.e., HAp) over the substrate surface has been frequently studied for its improved biocompatibility, changed chemistry at the surface, as well as excellent osteophilic surface to bond with the natural bone after implantation [97]. The stiffness of mineral-coated substrates can be realized through controlling the deposited mineral thickness [55]. To further enhance the regulated substrate stiffness, a novel composite polymeric scaffold has been developed by attaching minerals to substrates using an adhesive material polydopamine as a ‘‘superglue’’ [86].
3.2.2. Organic filler reinforcement
Similar to the inorganic filler reinforcement, combining two or more types of polymers in a single scaffold is a convenient strategy to modulate substrate stiffness and provide additional biochemical properties to address the specific requirements for tissue regeneration. Usually, the strength of polymeric substrates can be improved via the incorporation of stiffer polymer material, while the compliance of the polymeric substrates can be generally enhanced through incorporating more elastic polymer material. For example, insoluble elastin addition could reduce the compressive modulus of collagen scaffolds from 0.32 kPa (pure collagen scaffold) to 0.25 kPa (pure elastin scaffold) [98]; fibrous substrates made of elastomeric PGS and incorporated into gelatin scaffolds have a wide tensile modulus range of ∼1.5–54.4 MPa [56]. However, inhomogeneous mixtures and phase separation generally occur when poor compatibility exists between components, and their non-synchronous degradation rates could result in complications such as premature pore collapse [99]. Thus, a new type of hybrid system, in which the components are the same materials, has been formed (such as by incorporating silk particles in a regenerated silk substrate [100]). Additionally, enhancing the interaction in substrate components can also avoid homogeneous mixtures, thereby improving the stiffness range of substrates [101].
Coating substrates with a thin layer of polymer is also an alternative method to tune substrate stiffness. Recently, much effort has been made to directly coat polymeric material on ceramic substrates to fill the existed cracks of substrate microstructure, thus reducing brittleness through lowering the chance of crack propagation under load [102]. An example is that multiple silk coatings (5–7 times) on ceramic substrates have a significant 6-fold increase in compressive strength compared to non-coating substrates [102]. Furthermore, coating synthetic polymeric substrates with biocompatible polymers can also change the physicochemical properties for cell attachment and subsequent biological functions in addition to the substrate stiffness. More interesting, coaxial electrospinning has been developed recently to control the stiffness of biomimetic micro/nano-fibrous substrates that comprise an outer shell material and an inner core material that can be adjusted independently without altering the substrate surface chemistry and topological structure [103]. However, physical modification of polymers has insufficient stability and can easily be shed from the based matrix under physiological environments. Chemical modification has been investigated to cover those shortages. For instance, the surface modification of BG with PLLA by diisocyanate could avoid the phase separation phenomenon and adjust the tensile modulus ranging within ∼2–3.0 GPa [104]. This method has been developed to tailor the substrate stiffness without significant changes of substrate microstructure such as morphology, porosity, pore size, and pore interconnectivity [105,106]. Furthermore, an inorganic and organic mixture could also be used for regulating substrate stiffness.
3.2.3. Other fillers reinforcement
Incorporating nanofiber into substrates has been developed as a novel strategy to adjust substrate stiffness, especially in a ‘nanocomposite hydrogel’ system similar to native ECM [107]. Increasing the amounts of incorporated nanofibers into a hydrogel can significantly increase Young's modulus of the composite hydrogels. Regrettably, it simultaneously carries the risk that the incorporated nanofillers may elicit non-specific responses from surrounding cells [108]. Therefore, 3D polymeric substrates could act as a structural framework to enhance the structural integrity and substrate fracture resistance without inadvertently influencing cell responses [109]. Just as the endoskeletal system of vertebrate species, it has evolved to provide structural support and protection for tissue structures and guide their overall shape [110]. Recently, the reinforcement strategy has been widely used for overcoming the structural weakness of hydrogels that results in easy breaking and a high degree of swelling [109].
3.3. Designing substrate architecture using fabrication approaches for tunable stiffness
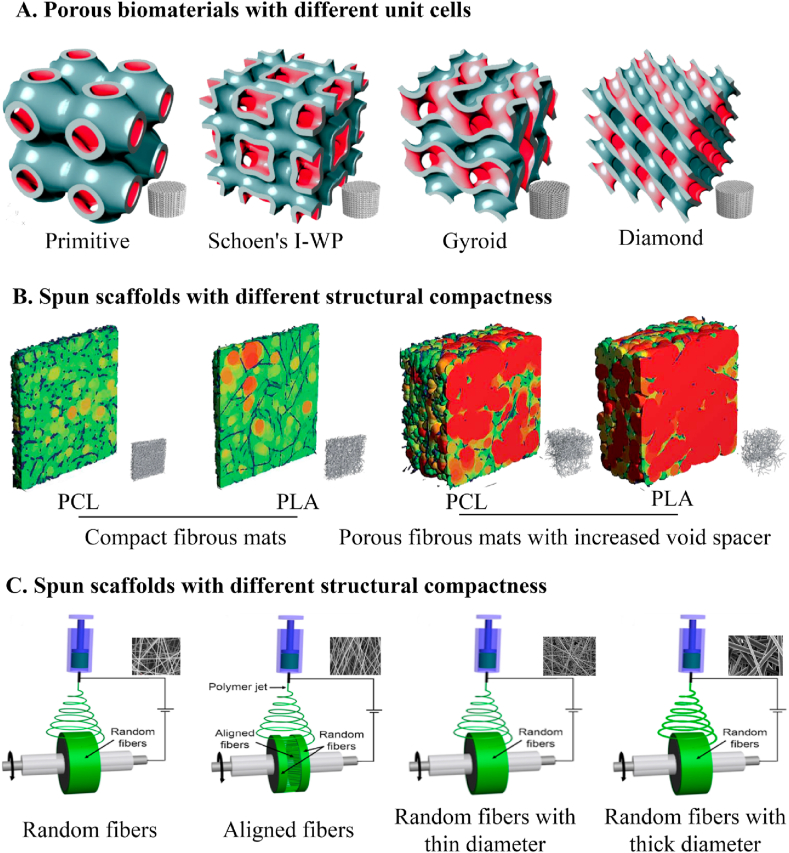
In addition to local stiffness, cells also respond to the bulk mechanical properties of surrounding substrates such as tensile and compressive loading [111]. Since native ECM are accumulated in spatial patterns to optimize structure-function relationships from the inside out [112], substrate architecture has been developed as an acute need for the design of rational tissue-engineered substrates to modulate the bulk stiffness of substrates, including porous structure design, structural compactness control, and fibrous architecture control (Fig. 7).
Fig. 7.
Schematics of the current strategies to tailor substrate stiffness through controlling substrate architecture, including designing porous biomaterials with different unit cells [113], fabricating fibrous scaffolds with different structural compactness [114], and electrospinning scaffolds with different fibrous architecture [115].
3.3.1. Porous structure design
Bulk mechanical properties of substrates are affected by the uniformity in pore geometry [116]. The excellent uniformity in defect-free pore geometry allows for greater protein absorption without compromising mechanical properties. Appropriate porosity and pore sizes are necessary to provide cells with an adequate biomechanical environment for cellular influx/growth, nutrient/metabolite transportation, and blood vessels ingrowth [116]. The respective roles of substrate porosity in the outcome of the bulk mechanical behavior have been established. As proved by experiments, together with the outer boundary conditions defining scaffolds’ inner and outer surfaces, the spatial distribution of pores within the scaffold can define the local stiffness and mechanically modulate cues such as molecular gradients via globally defined flow regimes [117]. With the increase of pores size and substrate porosity, the substrate strength usually is compromised, indicating that an optimum balance between the mechanical performance and porosity of substrates must be achieved to ensure successful long-term tissue remodeling. Current techniques for attaining porous scaffolds to regulate substrate stiffness include salt leaching, phase separation, gas foaming, and unidirectional freeze-casting [118]. Substrate porosity and pore architecture can be easily adjusted by varying the amounts and size of porogens, controlling phase separation parameters such as oil/water ratio and temperature, adjusting the depressurization rate in gas foaming, or regulating cooling rates during unidirectional freeze-casting.
Given the limitations of the above substrate fabrication techniques that only a few parameters like porosity and pore size can be tuned, rapid prototyping techniques, including selective laser sintering, fused deposition modeling, 3D printing, and stereolithography [119], have been developed due to the ability to control over the whole design of 3D porous structures. Up to date, these fabrication approaches have been performed to build a complete database on the correlation between substrate structure and the corresponding compressive stiffness based on different structural configurations, such as triply periodic minimal surfaces [113], diamond-like architecture [120], and unit cell orientation [121]. With the increased awareness of the importance of surface curvature in the tissue regeneration rate, porous biomaterials based on different triply periodic minimal surfaces (minimal surfaces with “translational symmetries in three independent directions”) have been generated with unique combinations of topological, structural, and mechanical properties [113]. Besides, diamond-like architecture was found to give porous substrates the most controllable results without any observable substantial volume contractions, as well as the breaking of the limitation of their mechanical integrity [120]. Furthermore, the functional correlation in the strut orientation, loading angles, and substrate stiffness was also tested based on the unit cells in different orientations, leading to the elastic modulus of the substrates varied in 3.4–26.3 GPa [121]. However, the studies mentioned above vary multiple parameters between groups without controlling porosity, which confounds the effects of every single parameter. Therefore, researchers explored the influence of angle and offset changes between layers on the stiffness of the scaffold, while maintaining a constant porosity of the scaffold [122]. However, the enhancement efficiency of the compressive stiffness of substrates is usually lower than that in substrates without controlling porosity, which illustrates the important role of porous structures in controlling substrate stiffness.
3.3.2. Structural compactness control
Structural compactness is used for tuning substrate stiffness due to its unique preponderances such as convenience, affordability, and time-saving. Current strategies for tunable structural compactness mainly include the variation in the concentrations of the polymer [46] and plastic compression [123]. Changes in polymer concentration typically translate into an altered mechanical environment of substrates. For example, modifying silk protein concentration within 1.5–4% under high-pressure CO2 results in hydrogels of variable compressive modulus ranging from 6.41 kPa to 63.98 kPa while retaining constant key structural parameters and β-sheet crystallinity [46]. As another alternative approach, plastic compression, which involves applying controlled compressive stress, presents simpleness and high effectiveness to improve the structural compactness of substrates, especially for hydrogels such as collagen [123] and fibrin [124]. For example, to improve the stiffness of highly hydrated collagen gels and inhibit the cell-mediated contraction of the substrates, plastic compression has been performed via the progressive reduction of thickness and the significant exclusion of water [123]. In combination with the rapid ability to improve substrate stiffness, this process has no substantial effect on cell viability within the gels [124]. However, the requirement of tissue-engineered constructs with widely stiffness range presents significant challenges for this process arising from the limitation of geometrical and architectural space.
3.3.3. Fibrous architecture control
Fibrous substrates, presenting a biomimetic architecture, have distinctive structural features compared to hydrogels and sponges. According to the structure-property relationship, the stiffness of fibrous substrates mainly depends on the properties of individual fiber (e.g., fiber diameter and material composition) [125] and the mesh architecture (e.g., interfiber spacing and relative fiber orientation) [126]. Plenty of researches have demonstrated that the variation of polymer concentrations or the process parameters of electrospinning (an attractive strategy to form nano/micro-fibers) could lead to the change of fiber diameter [115], thus giving rise to the variation of substrate stiffness. Shell-core structure of single fiber has also been used for controlling the mechanical properties of individual fiber through changing the mass fraction and the type of the core material, as described in section 3.2.2. The stiffness of fibrous substrates can also be tailored, to some extent, according to the alteration of fibrous architecture, including fiber orientation [125], fiber intersection density [127], or fiber crimp degree [128]. Changing the degree of fiber alignment could effectively regulate the anisotropic stiffness of fibrous substrates. A representative example is that the rotation speed of the drum-rotating collector can regulate the degree of fiber alignment during electrospinning, thereby affecting the stiffness of both individual fibers and bulk fibrous substrates [125,129]. In general, the modulus of a single fiber decrease with the higher collection speeds due to the drop in crystallinity at higher uptake rates, while a higher degree of fiber alignment enhances their bulk modulus attributing to the increased packing and decreased inter-fiber pore size [49]. As for constructing soft tissue, a reliable method is needed to control the inherent bending modulus. For example, the specific microstructural features that determine the flexural behavior of electrospun substrates could be regulated by the modification of processing variables (deposition mandrel translated at varying rates) or by the tuning of secondary fiber populations (introducing stiffer secondary fibers or selective dissolution of part fibers to modify fiber intersection density) [127]. Given the nonlinear mechanics related to the special biological functionality in natural tissues, a number of approaches have been tried to recreate the nonlinear mechanical properties of substrates for flexibility as well as to prevent over-extension, including the change of the crimp degree of fibers in which the stress-strain behavior is in correlation to the crimp degree of fibers [128]. It is noteworthy that these micro-scaled architecture changes not only affect the bulk stiffness of substrates but also regulate the morphology and growth of adherent cells.
3.4. Adjusting molecular interactions of substrates for tunable stiffness
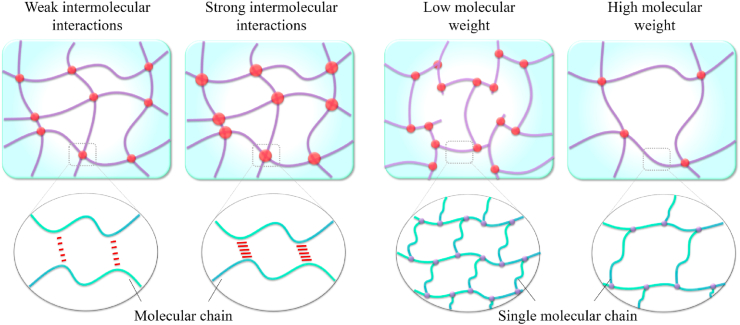
Regulation of substrate stiffness at the molecular level (adjusting molecular interactions) has become a prevailing method with an adjusting mechanism partially similar to that in crosslinked hydrogels. Compared to physical mixing strategies to control substrate stiffness, the adjustment of molecular interactions exhibits an obvious advantage in synchronously modulating the local stiffness and bulk stiffness of substrates [130]. Recently, molecular interaction has been developed as a critical factor to design substrates with tunable stiffness, including enhancing intermolecular interactions and changing molecular weight (Fig. 8).
Fig. 8.
Schematics of the current strategies to tailor substrate stiffness through adjusting molecule interactions, such as enhancing intermolecular interactions and changing molecular weight.
3.4.1. Change of intermolecular interactions
Except for the strategies above, several researchers have proposed the enhancement of the intermolecular interactions to improve substrate stiffness. Thermal annealing treatment, in which the selected annealing temperature is higher than the exothermic crystallization temperature, has been demonstrated to modulate substrate stiffness via the enhancement of molecular interactions (e.g., the formation of more hydrogen bonds by increasing crystallinity) and the introduction of inter-fiber bonding without any gross observable and ultrastructural changes [131]. Annealing processing on a polyelectrolyte complexation could also promote the conjugation of the oppositely charged polymers, resulting in the higher tensile modulus [132] or on a protein-based substrate to enhance the hydrophobic-hydrophobic interaction by modulating the content of β-sheet domains for tunable stiffness [94]. Inspired by the relationship of β-sheet crystal content and substrate stiffness, the control of silk-bound water interactions has also been developed to modulate substrate stiffness by utilizing different drying rates of the solution. Thus, the enhancement of silk-bound water interactions could maintain a more random coil structure [133]. For polymers with different secondary structures, the substrate stiffness can be modulated by regulating the content of different secondary structures in the polymeric substrates [134]. Given that divalent metal ions can form ionic bonds with the carboxylic acid groups of polymers, removing divalent metal ions was found to strengthen the electrostatic interactions between molecules by freeing the carboxylic acid group and eventually improving the stiffness of the polypeptide hydrogel [135]. In addition, microthread processing conditions, which extrude dense scaffolds into microthreads, have also been proved as an effective strategy to strengthen substrate stiffness [136]. Regrettably, this method would inevitably decrease the fiber diameter and increase the organization/alignment of the polymer-like network.
3.4.2. Adjustment of molecular structure
The molecular structure of substrates also plays a key role in substrate stiffness, such as molecular weight [137] and molecular functional groups [138]. In general, an increase in molecular weight of components brings the decreased stiffness of substrates [137]. Modification of molecular functional groups (e.g., grafting with different functional groups [138]) or regulation of the weight compositions of hard segments [139] in hybrids elastomer also gives a promising application for the generation of substrates with tunable stiffness. However, different molecular functional groups can alter the chemical composition that guides cellular behavior [138]. Thus, the interest in concurrently studying scaffolds' biochemical and mechanical properties for bioengineering applications will increase if the bioactive functional groups that can enhance the cellular behavior are chosen to tailor substrate stiffness. More interestingly, to satisfy different stiffness from hard to soft tissue replacements, a series of copolymers with various weight compositions of hard and soft segments has been introduced to fabricate substrates, which demonstrated that the substrate stiffness could vary significantly from flexible construction to stiff ones along with the increase of hard segment content in molecules [140].
3.5. Multifactorial adjustment
The stiffness range of substrates is majorly derived from the used strategies for substrate fabrication. For the needs of tissue engineering experiments, multifactorial adjustments combining two or more methods are proposed. In most cases, the substrates fabricated by the physical crosslinking methods yield weak mechanical properties in physiological conditions. Thus, a double-network hydrogel with both physical and chemical crosslinking has been developed to enlarge the mechanical range [141]. Alternatively, polymeric scaffolds with a large stiffness range can also be fabricated by changing the molecular weight of polymer and polymer concentration at the same time [7]. Certainly, multifactorial adjustment, such as modulating molecular weight and pore geometrical configurations of substrates, not only modulates the substrate stiffness but also affects the biocompatibility of the substrates for cell growth [142].
3.6. Substrate stiffness usually coupled with other physicochemical properties
As historically recognized, strategies to fabricate scaffolds with controlled substrate stiffness are usually coupled with the change of other biophysical or biochemical cues such as scaffold ultrastructure and chemical composition. Although substrate stiffness is identified as an important cue to regulate cellular responses, it is not the only factor that affects biologic activity of responding cells. Specifically, the surface geometries, protein composition, and mechanical strain inherent to substrates are also the key cues for modulating cell fate/activity and tissue regeneration [143]. Even more fascinating is the recent work which suggest that substrate stiffness would be more crucial than ligand density/peptide affinity of scaffolds for directing the behavior of both adult cells (e.g., SMCs [144]) and stem cells (e.g., NSCs [145]), whilst topological cue might be more influential than substrate stiffness cue in directing the cell morphology [54]. Therefore, it should be more cautious to implicate what has been observed when it is difficult to decouple the effects of substrate stiffness from other microenvironmental cues on cellular responses.
4. The role of substrate stiffness in material-mediated tissue regeneration
A key challenge in tissue regeneration is the precise control of cell behavior using biomimetic substrates [71]. Since one research demonstrated that 3T3 fibroblasts became less motile while increasing spreading as substrate stiffness increased [6], substrate stiffness-mediated cellular responses of different cells have been explored based on various types of scaffolds (Table 2), indicating the important role of substrate stiffness in tissue regeneration via affecting cell behavior and cell fate. As illuminated by extensive studies, softer surfaces generally enhance cell secretory activity (e.g., higher secretion of multiple paracrine factors) in vitro versus stiffer ones, whereas stiffer substrate leads to a higher ability of the cells to generate stress fibers and to enter the cell cycle, resulting in increased cell traction, spreading, and proliferation [9,146]. Immune system such as macrophage and adaptive T cell is also sensitive to the surrounding mechanical cues [147]. In fact, every scaffold, once implanted in vivo, will elicit a definitive host immune response [148]. Up to date, the macrophage plasticity was found to exhibit a reciprocal polarization behavior depending on substrate stiffness [147], and pro-inflammatory response activated by engineered materials is also likely to contribute to the disrupted tissue remodeling via regulating the secreted extracellular vesicle of relevant cells [149]. As cellular responses to substrates stiffness vary in different tissues, developing an appropriate engineered substrate for clinical applications necessitates a thorough understanding of cell-substrate stiffness relationships. This section focused on the recent development of studies about cellular response to substrate stiffness in some representative tissue engineering varying from stiff tissue to soft tissue.
Table 2.
Effects of substrate stiffness on cell functions.
| Cells | Scaffolds | Stiffness range | Research objectives | Results | Ref. |
|---|---|---|---|---|---|
| Adult cells | |||||
| MC3T3-E1 | Crosslinked PPF-co-POSS | 21.4–108.2 MPa | Simultaneously enhancing stiffness and toughness of substrates for bone repair | Stiffer substrate enhanced cell functions including cell attachment, spreading, proliferation, differentiation, and gene expression. | [130] |
| Bone-derived cells | Collagen-coated polyacrylamide substrate | 1.46–26.12 kPa | To investigate the influence of matrix stiffness on differentiated osteogenic cell lineage of bone-derived cells | Osteogenic differentiation did not vary depending on the substrate stiffness. | [150] |
| Chondrocytes | PDMS substrate | 1.4–135 kPa | To understand the effect of substrate stiffness on chondrogenic phenotype and its underlying mechanism | The cytoskeletal tension increased as substrate stiffness decreased. Chondrocyte cultured on soft substrates showed better chondrocyte functionalization via RhoA/ROCK pathway. | [151] |
| Tenocytes | PEG fibers embedded within PEG hydrogel | 53–1300 kPa | To observe the effect of fiber stiffness on tenocyte function in a tendon mimetic fiber composite hydrogel | Changes in the matrix cue influenced catabolic genes in tenocytes, while having minimal effects on tendon and homeostatic genes. | [152] |
| SMCs | Poly(urethane acrylate) mold | 11–1100 MPa | To observe the synergistic effects of matrix nanotopography and stiffness on SMCs function | Stiff substrate triggered the mechanical plasticity of SMCs resulting in a hypercontractile SMC phenotype, as observed in diabetes or hypertension. | [153] |
| ECs | PLCL-PLLA aligned fibers | 14.68–2141.72 MPa | To explore the effect of aligned fiber stiffness on structural and functional integrity of the oriented ECs | Stiff fibers exacerbated the disruption of endothelium integrity and the inflammation induced activation in the endothelial monolayer. | [103] |
| ECs | Polyacrylamide gel | 6–50 kPa | To explore the effect of substrate stiffness on tension induced by TNFα and thrombin in endothelial monolayers | Stiff substrates change intercellular junction protein localization and degradation, which may counteract the inflammation-induced increase in endothelial monolayer tension through ROCK-mediated contractility. | [154] |
| Schwann cells | Polyacrylamide gel | 4.42–12.04 kPa | To explore the influence of substrate stiffness on the behavior and functions of Schwann cells | The best cell adhesion, spreading, migration, and viability, as well as cell elongation length were observed on the 7.45 kPa. | [76] |
| DRG | Polyacrylamide hydrogel | 3.6–16.5 kPa | To explore the effect of substrate stiffness on DRG functions | DRGs on the hydrogels with elastic modulus of 5.1 kPa exhibited highest gene and protein expression of proliferation marker Epha4, Ntn4, Sema3D and differentiation marker Unc5B. | [155] |
| Stem cells | |||||
| Bone MSCs | Enzymatic mineralizable collagen hydrogel | 12.9–525.5 kPa | To investigate the effect of matrix stiffness on osteogenic differentiation of bone MSCs | The mineralized hydrogel with higher stiffness promoted the osteogenic differentiation of MSCs through inducing cytoskeletal assembly, which then enhanced the expression and nuclear colocalization of YAP and RUNX2. | [156] |
| Human MSCs | Polyacrylamide hydrogel | 1.6–40 kPa | To study the effects of matrix elasticity and cell density on human MSCs differentiation | Interplays between cell-matrix and cell-cell interactions contributed to MSCs differentiation. The promotion of osteogenic differentiation on hard matrix was mediated through the Ras pathway which is not sufficient for osteogenesis. | [157] |
| Human MSCs | Nanofibrous silk protein matrix | 4.8–7.8 GPa | To understand the chondrogenic condensation mechanisms | Soft matrix promoted MSCs adopting a rounded morphology with vinculin accumulated and less stress fibers for cell aggregates. Then the putative ‘perichondrium’-like tissue developed with smooth and more organized appearance for compact cartilage histogenesis. | [158] |
| MSCs | Electrospun fibers of PCL/polytetrahydrofuran and collagen | 4.3–6.8 MPa | Developing new electrospun nanofibers to trigger the chondrogenic differentiation of MSCs and the cartilage regeneration in vivo | Soft fibers induced the chondrogenic differentiation in vitro and cartilage regeneration in vivo more efficiently than stiff fibers by specifically blocking the NF-kappa B signaling pathway to suppress inflammation. | [159] |
| PDLSCs | PDMS substrate | 6–135 kPa | To understand the effect of substrate stiffness on PDLSCs and its underlying mechanism | PDLSC proliferation increased with substrate stiffness. Osteogenic differentiation of PDLSCs was higher on stiff substrates. Notch pathway markers were up-regulated in PDLSCs cultured on stiff substrates. | [160] |
| iPSCs | PDMS substrate | 3–1700 kPa | Using PDMS as a substrate to study EC commitment of iPSCs by a stepwise differentiation scheme | Substrate compliance guided EC commitment of iPSCs by enhancing mesoderm induction through Wnt/β-catenin signaling. | [161] |
| NSCs | Methacrylamide chitosan scaffold | <1–20 kPa | To understand the contribution of substrate stiffness to NSCs differentiation and proliferation | NSCs exhibited maximal proliferation on 3.5 kPa surfaces. Oligodendrocyte differentiation was favored on stiffer scaffolds (>7 kPa), but maturation was best on <1 kPa scaffolds. Astrocyte differentiation was only observed on <1 and 3.5 kPa surfaces and represented less than 2% of the total cell population. | [162] |
4.1. Bone regeneration: stiffer substrate enhances osteogenic differentiation in vitro but impairs bone formation in vivo
Bone is probably the hardest human tissue with Young's Modulus arranging within 15–40 GPa. Osteoblasts and MSCs are primary cell types that sense the mechanical stimulations of surrounding substrates and respond to them during the overall process of bone regeneration and remodeling [163]. In in vitro models, several studies have demonstrated that increasing substrate stiffness could trigger osteogenic commitment of these cells via TAZ-involved pathways such as Polycystins/TAZ complex, YAP/TAZ, and MIF-mediated AKT/YAP/Runx2 pathways [50,164,165]. For example, the stiffer 2D substrate (tensile modulus of ∼1.2 GPa) was found to promote the expression of the osteogenic transcription factors (e.g., Runx2) significantly and the osteoblast markers (e.g., ALP) of osteoblasts MC3T3-E1 [130,140]. Given the impairment of osteoblast-mediated bone formation and additive decrements in bone mass presenting in mice that lack polycystin-1 and TAZ, Xiao et al. have demonstrated that the stiffer substrate-guided osteogenic commitment of MC3T3-E1 was related to the stimulation of the nuclear translocation of the polycystin-1/TAZ complex at the molecular level [164]. On the stiff substrate, MSCs also tend to exhibit a polygonal morphology with a large spreading area, good proliferation [160], and increased expression of osteogenic differentiation markers through a stiffness-sensing mechanism that is mediated by integrin engagement (e.g., α2, β1, and α5β1 integrin), myosin-based contraction of the cytoskeleton [156]. Chen et al. and Hwang et al. have demonstrated that stiffer substrate could significantly promote the osteogenic differentiation of MSCs via increasing the nuclear translocation of YAP and TAZ for cytoskeletal assembly [156,165]. To further illustrate the relevant mechanisms, Yuan et al. have revealed that MSCs on stiffer substrate could increasingly activate AKT and upregulate expression of YAP target genes and the Runx2 gene. Inhibiting AKT led to the decreased expression of YAP and Runx2 and knocking down MIF resulted in the decreased AKT phosphorylation, indicating the regulated osteogenic differentiation of MSCs relying on the MIF-mediated AKT/YAP/Runx2 pathway [50].
However, once substrates are implanted in vivo, high-stiffness substrates may impair the reconstruction and the repaired quality of mass bone defect through suppressing the osteogenic maturation and stimulating pro-inflammatory response [166,167]. Hu and his colleague showed that the demineralized bone matrix with a high level of elasticity (∼0.67 MPa) could enhance the deposition of collagen fibers and the positive osteopontin/osteocalcin expression of endogenous cells in rat subcutaneous implantation experiments, as well as the bone repair of rabbit femoral condylar defect compared to scaffolds with a compressive modulus of 26.90, 66.06, and 230.93 MPa [166]. These findings suggested that elastic scaffold could allow cell-mediated contraction and support a greater level of osteogenic maturation, whereas stiff substrate may restrain cell contraction from retaining the original substrate structure, promoting a 2-fold increase of cell number [168]. Additionally, inflammation participates in the remodeling and repair of bones because it mediates endogenous cell recruitment to the injury site when substrates are implanted into bone defect sites [105,106]. It was found that macrophages encapsulated in the high-stiffness matrix were likely to polarize toward the pro-inflammatory M1 phenotype, thus negatively affecting the osteogenic differentiation of MSCs [167]. Nevertheless, the aggregation of blood vessel-like endothelial cells has been verified to be enhanced in stiffer matrices (37.70 kPa) compared to the softer matrices (13.00 kPa) [105]. These results indicate that substrate stiffness-mediated bone regeneration is associated with scaffold dimensionality and cell types (e.g., osteoclasts, macrophage, or ECs), but the exact regulation mechanisms are still elusive and need to be fully illustrated in future studies. Besides, osteoclasts, a type of bone-resorbing cells residing in bone tissues, also play a vital role in bone remodeling like bone resorption. Regrettably, few studies have been performed to delineate how substrate stiffness affects osteoclast behavior and the underlying intracellular signaling.
4.2. Cartilage regeneration: elastic substrate prefers to maintain chondrogenic phenotype in vitro but restricts cell volume expansion leading to cartilage degradation in vivo
Articular cartilage, a smooth and load-bearing tissue that facilitates the transmission of loads to the underlying subchondral bone, has moderate stiffness and high elasticity [169]. Chondrocytes are primary cell sources for cartilage remodeling as well as repair. When chondrocyte mechanotransduction appears mess, cartilage diseases initiate accompanied by the variation of cartilage ECM stiffness (e.g., osteoarthritis) [170]. It ensures the significant role of substrate stiffness in biomaterials-manipulated cartilage regeneration. Several studies have revealed that softer substrate could promote cellular aggregation of chondrocytes and MSCs and induce them to biosynthesize more chondrogenic proteins via RhoA/ROCK pathway and blocking NF-kappa B signaling pathway in in vitro studies. For example, chondrocytes cultured on stiff substrates (>16 kPa) displayed a spreading and polygonal shape with well-established actin fibers resulting in high cytoskeleton tension. In contrast, soft substrate (6 kPa) could provide chondrocytes with a proper living circumstance, leading to a small and round shape with compromised cytoskeleton tension to allow cellular aggregation and maintain chondrogenic phenotype by more production of cartilaginous matrix secretion [151,171,172]. Similarly, MSCs could also adopt a rounded morphology with fewer stress fibers but more cortical actins on the soft matrix (e.g., elastic moduli of ∼10 kPa for hydrogels, ∼10 MPa for fibrous matrix) [45,158], as well as with vinculin accumulated on the peripheral parts of MSCs for cell aggregates. Once the aggregates stabilize and gradually transform from oblong to roundish, the putative ‘perichondrium’-like tissue shall smoothly develop into a more organized appearance for compact cartilage histogenesis [158]. If the substrate stiffness increases, MSC differentiation will shift toward fibrous phenotypes that form fibrocartilage [45]. As for the underlying molecular mechanisms, a softer substrate has been demonstrated to facilitate the chondrocyte functionalization of chondrocytes via RhoA/ROCK pathway [173] while inducing the chondrogenic differentiation of MSCs through blocking the NF-kappa B signaling pathway [159].
Nevertheless, after implantation in vivo, softer substrates exhibited slower relaxation will restrict the cell volume expansion and increase the secretion of IL-1β, leading to the strong upregulation of genes associated with cartilage degradation and cell death [174]. For example, using 3D gelatin hydrogel as a model system, although soft hydrogel (shear modulus of ∼450 Pa) has been proven to promote the chondrogenic differentiation in vitro, upon ectopic implantation, only the stiffer one (shear modulus of ∼800 Pa) could show superiority in the de novo formation of cartilage tissue [175]. Interestingly, Arora et al. have demonstrated that pericellular plasma clot negates the influence of scaffold stiffness on chondrogenic differentiation, in which chondrocytes showed efficient cartilaginous matrix secretion on soft scaffolds in vitro while cartilaginous matrix secretion of cells was observed in both soft and stiff scaffolds when the scaffolds were coated with pericellular plasma clot [172]. Furthermore, given the complex mechanisms of cartilage regeneration involved in multiple types of cells, Fernandez-Muinos et al. have demonstrated that softer substrates (elastic modulus of ∼0.1 kPa) could enhance the expression of chondrogenic inductor BMP4 in mouse embryonic fibroblasts. However, such a trend disappeared when the cells were co-cultured with ECs [176]. These findings indicated that good quality cartilage formation is likely to occur in vivo in scaffolds with certain hardness (higher than the appropriate stiffness found in vitro), such as shear modulus of ∼800 Pa or elastic moduli of >10 kPa for hydrogels, elastic moduli of >10 MPa for fibrous matrix.
4.3. Tendon regeneration: engineered scaffolds with tendon-like stiffness anisotropy strengthen the tendon repair/remodeling
Tendon is composed of parallel arrangements of tightly packed collagen fibers that relay muscular forces to the skeletal structure during movement [18], with stiffness significantly lower than bone but higher than cartilage with Young's Modulus ranging from 136 MPa to 820 MPa. As important tendon cells, the surrounding tenocytes play a critical role in receiving and transmitting the mechanical stimuli as biological signals to regulate tendon remodeling or regeneration [177]. To better understand the substrate stiffness-mediated tenocytes responses in engineered scaffolds, Patel et al. developed tendon-mimetic fiber composite hydrogels in which tenocytes are seeded onto discontinuous fibers with tunable stiffness. Results suggested that changes of the fiber tensile modulus (53–1300 kPa) could affect the catabolic genes with minimal impacts on the tendon and homeostatic genes [152]. Given substrate stiffness-mediated MSC differentiation into tenogenesis, Islam et al. fabricated collagen sheet substrates with the random topographical surface while moduli ranged between 0.1 and 100 MPa, which promoted tendon differentiation of MSCs with stiffer substrates [178]. However, this does not mean that the progressive increase of substrate stiffness could translate into the repair with a stiffer or stronger tendon. As proven by Aurora and his co-workers’ work based on the spring-network model, engineered substrate with supraphysiologic stiffness could seriously impair the tendon repairs [179] because of the excessive stiff substrates, in which the shape change and the matrix displacement was limited in response to mechanical loading, weakened the influence of substrate mechanical stimulation on cell behaviors in vivo [180]. These findings suggested that future efforts may strengthen the tendon repair using engineered scaffolds with tendon-like mechanical properties.
Notably, the cytoskeletons and nuclei of tenocytes are elongated along the arrangement direction of collagen fibers in the natural tendon ECM. In such a fundamental context, the effects of substrate stiffness anisotropy on cell functions were further investigated. Results have demonstrated that MSCs seeded on substrates with varying anisotropy stiffness display a dose-dependent upregulation of tendon-related markers [181]. Furthermore, inspired by the special load-deformation curve of tendons allowing tissue flexibility while preventing over-extension, Chao et al. developed microcrimped fibers with the nonlinear mechanics to mimick this complex mechanical functionality. They have demonstrated that enhancing the degree of fiber crimp in fibrous substrates could significantly promote the ligament phenotypic gene expression of ligament fibroblasts [128]. All results indicated that good quality tendon repair requires substrates displaying microcrimped fibrous structure with proper stiffness anisotropy. However, up to date, the suitable substrate stiffness for tendon regeneration in situ and the specific underlying signaling/molecular pathways of stiffness-guided tendon repair remain poorly defined based on the microcrimped fiber model, because most of the previous efforts focused on the dynamic mechanical cues-mediated cellular responses. Given the nonnegligible roles of cell-matrix adhesion molecules (e.g., integrins) and cell-cell connections (e.g., gap junctions and cadherins) [177], future studies should focus on whether and how tenocytes interrogate the mechanical environment via the transmembrane proteins.
4.4. Vascular regeneration: the compliance mismatch of substrates leads to dysfunctions of SMC and ECs with initiated inflammatory responses
Arteries are elastic tissues that function as elastic storage reservoirs in the arterial circulation. Vascular cells (e.g., ECs and SMCs) play a homeostatic role in maintaining the function and long-term patency of the vessels, mainly attributed to the elastic recoil/contractility of SMCs alternating layers and the anti-thrombogenic ability of EC monolayer [182]. Accumulated evidence has revealed that mismatched substrates’ elasticity could simultaneously lead to the dysfunction of both SMCs and ECs via cytoskeletal organization and then initiate inflammatory responses during biomaterials-guided vascular regeneration, subsequently causing intimal hyperplasia or thrombosis. For example, the stiffer substrate (elastic moduli of ∼11 MPa for nanopatterned substrate, ∼14 MPa for aligned fibrous matrix) has been proved to promote the exerted mechanical tension of SMCs to manipulate substrate with the increased extent of F-actin bundling and the enlarged size of focal adhesions via MLC phosphorylation and integrin β3-mediated pathways [54,153], causing cells to assume a proliferative phenotype accompanied by the increased secretion of collagen rather than elastin for the disorder of ECM remodeling [183]. Such stiff substrate-triggered mechanical plasticity of SMCs to hypercontractile phenotype could further result in the homing of inflammatory cells via upregulation of inflammatory genes expression [54] or via activating inflammatory NF-κB pathway [153]. Similarly, a stiffer substrate has also been revealed to destroy the structural integrity of regenerated endothelial monolayer [184], although it could promote EC proliferation and EC monolayer formation [103]. The high stiffness of substrates (local elastic moduli of ∼14 kPa for gels) contributes to the increased heterogeneity of stress distributions in the endothelial monolayer, resulting in the disruption of endothelial cell-cell junctions and the enhancement of monolayer permeability. Structural damage of the regenerated monolayer consequently could enhance monocyte-EC adhesion and the subsequent inflammatory response through ROCK-mediated contractility [154] or NF-κB activation [185] or the occurrence of endothelial-to-mesenchymal transition [103]. Considering the more sensibility of SMCs to surface stiffness and the intrinsic stiffer cytomembrane tension of SMCs than ECs [186], an approach should be proposed to enhance the competitiveness between ECs and SMCs through modulating a complex surface stiffness of substrates. Nonetheless, there remain significant knowledge gaps regarding the underlying mechanisms that regulate the responses of vascular cells to substrate stiffness.
To fully elucidate the mechanism by which compliance mismatch leads to intimal hyperplasia, Post et al. utilized an ex vivo organ culture model to estimate changes in fluid flow and wall shear stress as a function of graft compliance. Results demonstrated that low wall shear stress of low compliance grafts was correlated well with alterations in SMC markers, ECM status, and inflammatory markers at the distal anastomoses, supporting the intrinsic link between compliance mismatch and intimal hyperplasia [187]. Besides, the progression of vascular remodeling is complicated, and the involved micro-constituents include EC layer regeneration, vascular SMC contractility, micro-calcification, graft degradation, inflammatory response, and constitutive blood equations. For example, considering the decisive role of platelet-mediated thrombogenesis in vascular remodeling, Merkle et al. have showed that increasing the substrate stiffness within 0.25–100 kPa could promote the platelet adhesion and spreading, relying on the activation of Rac1 and actomyosin activity [188]. Thus, the relationships between substrate stiffness and the factors mentioned above should be well investigated in the future in order to determine the optimal substrate stiffness for vascular regeneration.
4.5. Nerve regeneration: substrates with nerve-like stiffness stimulate neurons differentiation, whereas gliocyte mature requires substrate stiffness 10-times higher than native nerve
Native nerve is one of the softest tissues in the body, whose elastic modulus is approximately 0.5–1 kPa [11]. Neurons and gliocytes are the building blocks of the nervous system, and change of the local nerve stiffness results in a pathological context to guide nerve cells for degenerative disease and nerve injury [189]. Thus, plenty of studies have been performed to understand the substrate stiffness-mediated nerve regeneration based on neuron and gliocyte differentiation. It is well-known that substrates with nerve-like stiffness prefer promoting neuronal differentiation by facilitating neurite retraction and maintaining neuronal phenotype's intrinsic excitability. Taking DRG as an example, neurite maturation of DRG could be reduced on various stiffer substrates (e.g., agarose gels [190], fibrin gels [191], and silk hydrogels [192]), in which soft substrates expedite axon specification while stiff materials promote dendrite arborization [193]. The involved mechanism attributes to the soft stiffness-mediated weaker adhesion of neurons that facilitates the neurite retraction. It increases the frequency of “extension-retraction” events for one neurite reaching a critical length via the ERK1/2 pathway, resulting in the axon differentiation of neurons with one major apical dendrite at one side of the soma and some minor dendrites appearing from the opposite side [193,194]. On the contrary, stiffer substrates could enhance the voltage-gated Ca2+ channel currents in neurons with increased synaptic connectivity and excitatory synaptic transmission, scilicet decreasing the intrinsic excitability of the network [195]. However, the best behaviors of gliocytes, which support neurons in the peripheral nervous system through participating in axonal regeneration and nerve remyelination [196], are observed on substrates with stiffness higher than nerves. For example, the substrate stiffness (1 kPa) matching that of native nerve could facilitate the neuronal differentiation but restricte the growth of glial cell populations, whereas a peak level of the astrocytic differentiation has been observed on substrates at 10 kPa [44,79]. In the polyacrylamide gel systems with a stiffness range of 4.42–12.04 kPa, 7.45 kPa substrate in which the stiffness is approximately 10 × higher than that of nerves has been demonstrated to induce the best growth of Schwann cells, as well as their normal cell shape with appropriate cell elongation length [76].
More interestingly, substrate stiffness-mediated nerve regeneration depends on the dimensionality of the matrix. For example, Lampe et al. have demonstrated that the metabolic activity of NPCs was independent of the stiffness of 2D PEG substrates with a compressive modulus ranging from ∼1 to 300 kPa while the PEG stiffness affected both metabolic and apoptotic activity in the 3D form [197]. Such differences between 2D and 3D matrices have been revealed to involve cell-material interactions relying on integrin α5, β1, β3, and proteins present in focal adhesions [198]. It indicated that a 3D culture niche can better mimic the in vivo mechanical environment by mimicking cells’ adhesion points to interact with the material and cell-cell communication. However, essential information about cellular interactions in response to substrate stiffness was provided based on the 2D platform. Nevertheless, the neurite outgrowth of DRG was still found to show the optimal state in a 3D environment with compliance similar to that of the native nerve [199]. Together, these studies indicate that neuron differentiation requires the substrate mechanical property simulating nerve-like stiffness, whereas gliocyte maturation requires substrate stiffness 10-times higher than that of native nerve. Such contradictory phenomenon suggests the necessity to dissect the molecular mechanisms how different stiffnesses determine different lineage differentiation of neurogenic cells, further defining the optimal stiffness parameter for directing required nerve regeneration. Regrettably, these key issues remain ill-defined and should become the targets of future investigation. Furthermore, substrate stiffness-mediated cellular behaviors may be modulated by other factors in the nerve regenerative process, such as the cell-cell interactions between neurons and gliocytes, the physical and biochemical properties of substrates [192]. To facilitate the successful application of scaffolds in nerve regeneration, a novel method therefore should be developed to enable fabricating functional substrates that provide a biophysically and biochemically permissive environment. With this created permissive environment, a reproducible and native-like neural network that exhibits the interactions of neurons with gliocytes can be induced to generate within scaffolds, thus opening a way of the investigation of substrate stiffness-mediated cellular behaviors.
5. Conclusions and future directions
The tissue mechanical microenvironment where cells are positioned is determined by the composition and configuration of ECM, as well as any extrinsic mechanical loading applied to this matrix. Surrounding cells sense and respond to the mechanical environment with membrane elements, cytoskeletal components and nucleus acting as putative mechano-responders. Throughout the previous studies, an inextricable link emerged between substrates stiffness and cell response, which plays a significant role in substrate-guided tissue regeneration. Substrate stiffness can regulate cell behavior and cell fate while cells can also remodel substrate and manipulate tissue regeneration (Section 2). Fully understanding the complex mechanical interactions between cells and their extracellular matrix not only help to develop tissue engineering strategies to guide cell fate and tissue regeneration/repair, but also to facilitate the establishment of tissue engineering-based platforms, which can be used for observing other biological activities during developmental processes of tissue regeneration after trauma or disease.
First and foremost, given that the preparation of a serious of substrates with tunable stiffness is required to study cell mechanobiology, recent work involved in the areas such as crosslinking, constituents, scaffold architecture as well as molecular interactions, was performed to institute a series of biomaterials and advance the establishment of tissue-engineered scaffolds with tunable stiffness (Section 3). However, the strategies to only regulate substrate stiffness are rather limited because the alteration of matrix stiffness is usually coupled with changes of other biophysical and biochemical cues such as architecture or surface chemistry. Fabricating biomimetic materials with only stiffness variation can therefore contribute to the better understanding of material-induced tissue regeneration. In addition, the used substrates with tunable stiffness in most of previous studies (e.g., crosslinked PDMS, PA or agarose) are lack of adhesive sites for cell adhesion. This may confound the cell response to substrate stiffness and the obtained substrates are also not suitable for the candidates of implantable biomaterials for tissue engineering researches. Furthermore, in vivo results observed in 3D models differ from in vitro models, but it remains a particular challenge in the reproduction of the complex 3D cellular mechanical environment in substrates, which need to be addressed in the future. For instance, the viscoelastic and plastic properties of the matrix recently have been demonstrated to play important roles in regulating cellular responses to matrix mechanical properties, which should also be considered when engineering matrix mechanics for tissue regeneration. To sum up, the development of novel materials and new techniques are needed in order to achieve the desired and precise control over the stiffness of biomimetic 3D fibrillar matrix, together with satisfactory routes of administration and corresponding cell types.
Although work to observe the substrate stiffness-guided cellular responses in different types of engineered tissues varying from stiff tissue to soft tissue has provided clues on how cells sense and respond to varying stiffness substrates for tissue regeneration (Section 4), the underlying mechanism remains to be fully defined regarding how stiffness-mediated bioactivities within cells are orchestrated or tuned at different stages to manipulate tissue regeneration or remodel formed tissues. Further studies are also required to precisely clarify and grasp the intricacy of this cell-matrix-cell mechanical feedback loop, especially considering the impact of cell-cell interactions as well. As cell functions related to cell shape or cell spreading depends on the dimensionality of matrix, advanced understanding of the influence of 3D substrate stiffness on embedded cells will help to define the mechanical properties of implantable scaffolds to support cell growth and resist body mechanical stimulations in vivo. Given the inherently complex of in vivo environment where intrinsic and extrinsic mechanical cues and other factors (e.g., surface topology) coexist, future works are appealed for understanding how cells sense and integrate substrate stiffness with other biophysical and biochemical signals and how they respond accordingly in vivo during dynamic processes. Meanwhile, the progression of substrate stiffness-mediated tissue regeneration is also complicated and associated with multiple micro-constituents such as immune response, vascularization, graft degradation, and matrix remodeling. The current poorly elucidated relationships between substrate stiffness and the factors mentioned above also should be considered in future work.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by Fundamental Research Funds for National Key Research and Development Program of China (2018YFC1105800), China Postdoctoral Science Foundation (2020M681322), and National Natural Science Foundation of China (31870967 and 81671921).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Kreeger P.K., Strong L.E., Masters K.S. Engineering approaches to study cellular decision making. Annu. Rev. Biomed. Eng. 2018;20:49–72. doi: 10.1146/annurev-bioeng-062117-121011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez-Yague M.A., Abbah S.A., McNamara L., Zeugolis D.I., Pandit A., Biggs M.J. Biomimetic approaches in bone tissue engineering: integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2015;84:1–29. doi: 10.1016/j.addr.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy K.M., Bhaw-Luximon A., Jhurry D. Cell-matrix mechanical interaction in electrospun polymeric scaffolds for tissue engineering: implications for scaffold design and performance. Acta Biomater. 2017;50:41–55. doi: 10.1016/j.actbio.2016.12.034. [DOI] [PubMed] [Google Scholar]
- 4.Lv H., Wang H., Zhang Z., Yang W., Liu W., Li Y., Li L. Biomaterial stiffness determines stem cell fate. Life Sci. 2017;178:42–48. doi: 10.1016/j.lfs.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Macri-Pellizzeri L., De-Juan-Pardo E.M., Prosper F., Pelacho B. Role of substrate biomechanics in controlling (stem) cell fate: implications in regenerative medicine. J. Tissue Eng. Regen. Med. 2018;12:1012–1019. doi: 10.1002/term.2586. [DOI] [PubMed] [Google Scholar]
- 6.Pelham R.J., Wang Y.L. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. U.S.A. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandl F., Sommer F., Goepferich A. Rational design of hydrogels for tissue engineering: impact of physical factors on cell behavior. Biomaterials. 2007;28:134–146. doi: 10.1016/j.biomaterials.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Liu X., Rahaman M.N., Fu Q. Oriented bioactive glass (13-93) scaffolds with controllable pore size by unidirectional freezing of camphene-based suspensions: microstructure and mechanical response. Acta Biomater. 2011;7:406–416. doi: 10.1016/j.actbio.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L., Wang C., Wu S., Fan Y., Li X. Influence of the mechanical properties of biomaterials on degradability, cell behaviors and signaling pathways: current progress and challenges. Biomater. Sci. 2020;8:2714–2733. doi: 10.1039/d0bm00269k. [DOI] [PubMed] [Google Scholar]
- 10.Soza G., Grosso R., Nimsky C., Hastreiter P., Fahlbusch R., Greiner G. Determination of the elasticity parameters of brain tissue with combined simulation and registration. Int. J. Med. Robot. Comput. Assist. Surg. 2005;1:87–95. doi: 10.1002/rcs.32. [DOI] [PubMed] [Google Scholar]
- 11.Taylor Z., Miller K. Reassessment of brain elasticity for analysis of biomechanisms of hydrocephalus. J. Biomech. 2004;37:1263–1269. doi: 10.1016/j.jbiomech.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 12.Rhodes J.M., Simons M. The extracellular matrix and blood vessel formation: not just a scaffold. J. Cell Mol. Med. 2007;11:176–205. doi: 10.1111/j.1582-4934.2007.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu J., Sabharwal T., Kalyanasundaram A., Guo L., Wang G. Topographic mapping and compression elasticity analysis of skinned cardiac muscle fibers in vitro with atomic force microscopy and nanoindentation. J. Biomech. 2009;42:2143–2150. doi: 10.1016/j.jbiomech.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dey K., Roca E., Ramorino G., Sartore L. Progress in the mechanical modulation of cell functions in tissue engineering. Biomater. Sci. 2020;8:7033–7081. doi: 10.1039/d0bm01255f. [DOI] [PubMed] [Google Scholar]
- 15.Padhi A., Nain A.S. ECM in differentiation: a review of matrix structure, composition and mechanical properties. Ann. Biomed. Eng. 2020;48:1071–1089. doi: 10.1007/s10439-019-02337-7. [DOI] [PubMed] [Google Scholar]
- 16.Turner C.H., Rho J., Takano Y., Tsui T.Y., Pharr G.M. The elastic properties of trabecular and cortical bone tissues are similar: results from two microscopic measurement techniques. J. Biomech. 1999;32:437–441. doi: 10.1016/s0021-9290(98)00177-8. [DOI] [PubMed] [Google Scholar]
- 17.Hung B.P., Hutton D.L., Grayson W.L. Mechanical control of tissue-engineered bone. Stem Cell Res. Ther. 2013;4:10. doi: 10.1186/scrt158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brennan D.A., Conte A.A., Kanski G., Turkula S., Hu X., Kleiner M.T., Beachley V. Mechanical considerations for electrospun nanofibers in tendon and ligament repair. Adv. Healthc. Mater. 2018;7:1701277. doi: 10.1002/adhm.201701277. [DOI] [PubMed] [Google Scholar]
- 19.Baer E., Cassidy J.J., Hiltner A. Hierarchical structure of collagen composite systems - lessons from biology. Pure Appl. Chem. 1991;63:961–973. [Google Scholar]
- 20.van der Flier A., Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- 21.Silver F.H., Siperko L.M. Mechanosensing and mechanochemical transduction: how is mechanical energy sensed and converted into chemical energy in an extracellular matrix? Crit. Rev. Biomed. Eng. 2003;31:255–331. doi: 10.1615/critrevbiomedeng.v31.i4.10. [DOI] [PubMed] [Google Scholar]
- 22.Naqvi S.M., McNamara L.M. Stem cell mechanobiology and the role of biomaterials in governing mechanotransduction and matrix production for tissue regeneration. Front. Bioeng. Biotechnol. 2020;8:597661. doi: 10.3389/fbioe.2020.597661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bershadsky A.D., Ballestrem C., Carramusa L., Zilberman Y., Gilquin B., Khochbin S., Alexandrova A.Y., Verkhovsky A.B., Shemesh T., Kozlov M.M. Assembly and mechanosensory function of focal adhesions: experiments and models. Eur. J. Cell Biol. 2006;85:165–173. doi: 10.1016/j.ejcb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Doyle A.D., Yamada K.M. Mechanosensing via cell-matrix adhesions in 3D microenvironments. Exp. Cell Res. 2016;343:60–66. doi: 10.1016/j.yexcr.2015.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogel V., Sheetz M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 26.Harris A.R., Jreij P., Fletcher D.A. In: Dill K.A., editor. vol. 47. 2018. Mechanotransduction by the actin cytoskeleton: converting mechanical stimuli into biochemical signals; pp. 617–631. (Annual Review of Biophysics). [Google Scholar]
- 27.Sun S.X., Walcott S., Wolgemuth C.W. Cytoskeletal cross-linking and bundling in motor-independent contraction. Curr. Biol. 2010;20:R649–R654. doi: 10.1016/j.cub.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zanconato F., Cordenonsi M., Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sotomayor M., Schulten K. Molecular dynamics study of Gating in the mechanosensitive channel of small conductance MscS. Biophys. J. 2004;87:3050–3065. doi: 10.1529/biophysj.104.046045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallotton P., Gupton S.L., Waterman-Storer C.M., Danuser G. Simultaneous mapping of filamentous actin flow and turnover in migrating cells by quantitative fluorescent speckle microscopy. Proc. Natl. Acad. Sci. U.S.A. 2004;101:9660–9665. doi: 10.1073/pnas.0300552101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiquet M., Reneda A.S., Huber F., Fluck M. How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biol. 2003;22:73–80. doi: 10.1016/s0945-053x(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 32.Yeung T., Georges P.C., Flanagan L.A., Marg B., Ortiz M., Funaki M., Zahir N., Ming W.Y., Weaver V., Janmey P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 33.Salameh A., Dhein S. Effects of mechanical forces and stretch on intercellular gap junction coupling. Biochim. Biophys. Acta Biomembr. 2013;1828:147–156. doi: 10.1016/j.bbamem.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 34.Hao J., Zhang Y., Jing D., Shen Y., Tang G., Huang S., Zhao Z. Mechanobiology of mesenchymal stem cells: perspective into mechanical induction of MSC fate. Acta Biomater. 2015;20:1–9. doi: 10.1016/j.actbio.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 35.Engler A.J., Richert L., Wong J.Y., Picart C., Discher D.E. Surface probe measurements of the elasticity of sectioned tissue, thin gels and polyelectrolyte multilayer films: correlations between substrate stiffness and cell adhesion. Surf. Sci. 2004;570:142–154. [Google Scholar]
- 36.Georges P.C., Janmey P.A. Cell type-specific response to growth on soft materials. J. Appl. Physiol. 2005;98:1547–1553. doi: 10.1152/japplphysiol.01121.2004. [DOI] [PubMed] [Google Scholar]
- 37.Biewener A.A., Roberts T.J. Muscle and tendon contributions to force, work, and elastic energy savings: a comparative perspective. Exerc. Sport Sci. Rev. 2000;28:99–107. [PubMed] [Google Scholar]
- 38.Charalambous H.P., Roussis P.C., Giannakopoulos A.E. Viscoelastic dynamic arterial response. Comput. Biol. Med. 2017;89:337–354. doi: 10.1016/j.compbiomed.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 39.Peng Y., Chen Z., He Y., Li P., Chen Y., Chen X., Jiang Y., Qin X., Li S., Li T., Wu C., Yang H., You F., Liu Y. Non-muscle myosin II isoforms orchestrate substrate stiffness sensing to promote cancer cell contractility and migration. Cancer Lett. 2022;524:245–258. doi: 10.1016/j.canlet.2021.10.030. [DOI] [PubMed] [Google Scholar]
- 40.Alonso-Nocelo M., Raimondo T.M., Vining K.H., Lopez-Lopez R., de la Fuente M., Mooney D.J. Matrix stiffness and tumor-associated macrophages modulate epithelial to mesenchymal transition of human adenocarcinoma cells. Biofabrication. 2018;10 doi: 10.1088/1758-5090/aaafbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fomovsky G.M., Holmes J.W. Evolution of scar structure, mechanics, and ventricular function after myocardial infarction in the rat. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H221–H228. doi: 10.1152/ajpheart.00495.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rachner T.D., Khosla S., Hofbauer L.C. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang X., Hang R., Wang X., Lin N., Zhang X., Tang B. Matrix stiffness in three-dimensional systems effects on the behavior of C3A cells. Artif. Organs. 2013;37:166–174. doi: 10.1111/j.1525-1594.2012.01546.x. [DOI] [PubMed] [Google Scholar]
- 44.Wang L.-S., Chung J.E., Chan P.P.-Y., Kurisawa M. Injectable biodegradable hydrogels with tunable mechanical properties for the stimulation of neurogenesic differentiation of human mesenchymal stem cells in 3D culture. Biomaterials. 2010;31:1148–1157. doi: 10.1016/j.biomaterials.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 45.Toh W.S., Lim T.C., Kurisawa M., Spector M. Modulation of mesenchymal stem cell chondrogenesis in a tunable hyaluronic acid hydrogel microenvironment. Biomaterials. 2012;33:3835–3845. doi: 10.1016/j.biomaterials.2012.01.065. [DOI] [PubMed] [Google Scholar]
- 46.Floren M., Bonani W., Dharmarajan A., Motta A., Migliaresi C., Tan W. Human mesenchymal stem cells cultured on silk hydrogels with variable stiffness and growth factor differentiate into mature smooth muscle cell phenotype. Acta Biomater. 2016;31:156–166. doi: 10.1016/j.actbio.2015.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gurumurthy B., Janorkar A.V. Improvements in mechanical properties of collagen-based scaffolds for tissue engineering. Curr. Opin. Biomed. Eng. 2021;17:100253. [Google Scholar]
- 48.Engelmayr G.C., Jr., Sacks M.S. Prediction of extracellular matrix stiffness in engineered heart valve tissues based on nonwoven scaffolds. Biomech. Model. Mechanobiol. 2008;7:309–321. doi: 10.1007/s10237-007-0102-1. [DOI] [PubMed] [Google Scholar]
- 49.Thomas V., Jose M.V., Chowdhury S., Sullivan J.F., Dean D.R., Vohra Y.K. Mechano-morphological studies of aligned nanofibrous scaffolds of polycaprolactone fabricated by electrospinning. J. Biomater. Sci. Polym. Ed. 2006;17:969–984. doi: 10.1163/156856206778366022. [DOI] [PubMed] [Google Scholar]
- 50.Yuan H., Zhou Y., Lee M.-S., Zhang Y., Li W.-J. A newly identified mechanism involved in regulation of human mesenchymal stem cells by fibrous substrate stiffness. Acta Biomater. 2016;42:247–257. doi: 10.1016/j.actbio.2016.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chapla R., Abed M.A., West J. Modulating functionalized poly(ethylene glycol) diacrylate hydrogel mechanical properties through competitive crosslinking mechanics for soft tissue applications. Polymers. 2020;12:3000. doi: 10.3390/polym12123000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X., Chen S., Li J., Wang X., Zhang J., Kawazoe N., Chen G. 3D culture of chondrocytes in gelatin hydrogels with different stiffness. Polymers. 2016;8:269. doi: 10.3390/polym8080269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kane R.J., Weiss-Bilka H.E., Meagher M.J., Liu Y., Gargac J.A., Niebur G.L., Wagner D.R., Roeder R.K. Hydroxyapatite reinforced collagen scaffolds with improved architecture and mechanical properties. Acta Biomater. 2015;17:16–25. doi: 10.1016/j.actbio.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 54.Yi B., Shen Y., Tang H., Wang X., Li B., Zhang Y. Stiffness of aligned fibers regulates the phenotypic expression of vascular smooth muscle cells. ACS Appl. Mater. Interfaces. 2019;11:6867–6880. doi: 10.1021/acsami.9b00293. [DOI] [PubMed] [Google Scholar]
- 55.Marelli B., Ghezzi C.E., Mohn D., Stark W.J., Barralet J.E., Boccaccini A.R., Nazhat S.N. Accelerated mineralization of dense collagen-nano bioactive glass hybrid gels increases scaffold stiffness and regulates osteoblastic function. Biomaterials. 2011;32:8915–8926. doi: 10.1016/j.biomaterials.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 56.Kharaziha M., Nikkhah M., Shin S.-R., Annabi N., Masoumi N., Gaharwar A.K., Camci-Unal G., Khademhosseini A. PGS:Gelatin nanofibrous scaffolds with tunable mechanical and structural properties for engineering cardiac tissues. Biomaterials. 2013;34:6355–6366. doi: 10.1016/j.biomaterials.2013.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drexler J.W., Powell H.M. Regulation of electrospun scaffold stiffness via coaxial core diameter. Acta Biomater. 2011;7:1133–1139. doi: 10.1016/j.actbio.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 58.Yesilyurt V., Ayoob A.M., Appel E.A., Borenstein J.T., Langer R., Anderson D.G. Mixed reversible covalent crosslink kinetics enable precise, hierarchical mechanical tuning of hydrogel networks. Adv. Mater. 2017;29:1605947. doi: 10.1002/adma.201605947. [DOI] [PubMed] [Google Scholar]
- 59.Smidsrod O., Skjakbraek G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990;8:71–78. doi: 10.1016/0167-7799(90)90139-o. [DOI] [PubMed] [Google Scholar]
- 60.Mihaila S.M., Gaharwar A.K., Reis R.L., Marques A.P., Gomes M.E., Khademhosseini A. Photocrosslinkable kappa-carrageenan hydrogels for tissue engineering applications. Adv. Healthc. Mater. 2013;2:895–907. doi: 10.1002/adhm.201200317. [DOI] [PubMed] [Google Scholar]
- 61.Wu X., Sun H., Qin Z., Che P., Yi X., Yu Q., Zhang H., Sun X., Yao F., Li J. Fully physically crosslinked pectin-based hydrogel with high stretchability and toughness for biomedical application. Int. J. Biol. Macromol. 2020;149:707–716. doi: 10.1016/j.ijbiomac.2020.01.297. [DOI] [PubMed] [Google Scholar]
- 62.Lee K.Y., Rowley J.A., Eiselt P., Moy E.M., Bouhadir K.H., Mooney D.J. Controlling mechanical and swelling properties of alginate hydrogels independently by cross-linker type and cross-linking density. Macromolecules. 2000;33:4291–4294. [Google Scholar]
- 63.Zhang Q., Liu X., Ren X., Duan L., Gao G. Adenine-mediated adhesive and tough hydrogel based on hybrid crosslinking. Eur. Polym. J. 2018;106:139–147. [Google Scholar]
- 64.Lee H.-Y., Hwang C.-H., Kim H.-E., Jeong S.-H. Enhancement of bio-stability and mechanical properties of hyaluronic acid hydrogels by tannic acid treatment. Carbohydr. Polym. 2018;186:290–298. doi: 10.1016/j.carbpol.2018.01.056. [DOI] [PubMed] [Google Scholar]
- 65.Farokhi M., Aleemardani M., Solouk A., Mirzadeh H., Teuschl A.H., Redl H. Crosslinking strategies for silk fibroin hydrogels: promising biomedical materials. Biomed. Mater. 2021;16 doi: 10.1088/1748-605X/abb615. [DOI] [PubMed] [Google Scholar]
- 66.Thompson M.T., Berg M.C., Tobias I.S., Rubner M.F., Van Vliet K.J. Tuning compliance of nanoscale polyelectrolyte multilayers to modulate cell adhesion. Biomaterials. 2005;26:6836–6845. doi: 10.1016/j.biomaterials.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 67.Yang B., Wei Z., Chen X., Wei K., Bian L. Manipulating the mechanical properties of biomimetic hydrogels with multivalent host-guest interactions. J. Mater. Chem. B. 2019;7:1726–1733. doi: 10.1039/c8tb02021c. [DOI] [PubMed] [Google Scholar]
- 68.Hennink W.E., van Nostrum C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012;64:223–236. doi: 10.1016/s0169-409x(01)00240-x. [DOI] [PubMed] [Google Scholar]
- 69.Jeon O., Bouhadir K.H., Mansour J.M., Alsberg E. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials. 2009;30:2724–2734. doi: 10.1016/j.biomaterials.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 70.Teixeira L.S.M., Feijen J., van Blitterswijk C.A., Dijkstra P.J., Karperien M. Enzyme-catalyzed crosslinkable hydrogels: emerging strategies for tissue engineering. Biomaterials. 2012;33:1281–1290. doi: 10.1016/j.biomaterials.2011.10.067. [DOI] [PubMed] [Google Scholar]
- 71.Wang P.-Y., Tsai W.-B., Voelcker N.H. Screening of rat mesenchymal stem cell behaviour on polydimethylsiloxane stiffness gradients. Acta Biomater. 2012;8:519–530. doi: 10.1016/j.actbio.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 72.Ehlinger C., Mathieu E., Rabineau M., Ball V., Lavalle P., Haikel Y., Vautier D., Kocgozlu L. Insensitivity of dental pulp stem cells migration to substrate stiffness. Biomaterials. 2021;275:120969. doi: 10.1016/j.biomaterials.2021.120969. [DOI] [PubMed] [Google Scholar]
- 73.Lee J.N., Jiang X., Ryan D., Whitesides G.M. Compatibility of mammalian cells on surfaces of poly(dimethylsiloxane) Langmuir. 2004;20:11684–11691. doi: 10.1021/la048562+. [DOI] [PubMed] [Google Scholar]
- 74.Mata A., Fleischman A.J., Roy S. Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomed. Microdevices. 2005;7:281–293. doi: 10.1007/s10544-005-6070-2. [DOI] [PubMed] [Google Scholar]
- 75.Hsieh W.-T., Liu Y.-S., Lee Y.-h., Rimando M.G., Lin K.-h., Lee O.K. Matrix dimensionality and stiffness cooperatively regulate osteogenesis of mesenchymal stromal cells. Acta Biomater. 2016;32:210–222. doi: 10.1016/j.actbio.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 76.Gu Y., Ji Y., Zhao Y., Liu Y., Ding F., Gu X., Yang Y. The influence of substrate stiffness on the behavior and functions of Schwann cells in culture. Biomaterials. 2012;33:6672–6681. doi: 10.1016/j.biomaterials.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 77.Liao H., Munoz-Pinto D., Qu X., Hu Y., Grunlan M.A., Hahn M.S. Influence of hydrogel mechanical properties and mesh size on vocal fold fibroblast extracellular matrix production and phenotype. Acta Biomater. 2008;4:1161–1171. doi: 10.1016/j.actbio.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schuurman W., Levett P.A., Pot M.W., van Weeren P.R., Dhert W.J.A., Hutmacher D.W., Melchels F.P.W., Klein T.J., Malda J. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromol. Biosci. 2013;13:551–561. doi: 10.1002/mabi.201200471. [DOI] [PubMed] [Google Scholar]
- 79.Her G.J., Wu H.-C., Chen M.-H., Chen M.Y., Chang S.-C., Wang T.-W. Control of three-dimensional substrate stiffness to manipulate mesenchymal stem cell fate toward neuronal or glial lineages. Acta Biomater. 2013;9:5170–5180. doi: 10.1016/j.actbio.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 80.Nair M., Best S.M., Cameron R.E. Crosslinking collagen constructs: achieving cellular selectivity through modifications of physical and chemical properties. Appl. Sci. Basel. 2020;10:6911. [Google Scholar]
- 81.Sun H., Zhu F., Hu Q., Krebsbach P.H. Controlling stem cell-mediated bone regeneration through tailored mechanical properties of collagen scaffolds. Biomaterials. 2014;35:1176–1184. doi: 10.1016/j.biomaterials.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee C., Shin J., Lee J.S., Byun E., Ryu J.H., Um S.H., Kim D.-I., Lee H., Cho S.-W. Bioinspired, calcium-free alginate hydrogels with tunable physical and mechanical properties and improved biocompatibility. Biomacromolecules. 2013;14:2004–2013. doi: 10.1021/bm400352d. [DOI] [PubMed] [Google Scholar]
- 83.Jiang F.X., Yurke B., Firestein B.L., Langrana N.A. Neurite outgrowth on a DNA crosslinked hydrogel with tunable stiffnesses. Ann. Biomed. Eng. 2008;36:1565–1579. doi: 10.1007/s10439-008-9530-z. [DOI] [PubMed] [Google Scholar]
- 84.Pryadko A., Surmeneva M.A., Surmenev R.A. Review of hybrid materials based on polyhydroxyalkanoates for tissue engineering applications. Polymers. 2021;13:1738. doi: 10.3390/polym13111738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wei G.B., Ma P.X. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials. 2004;25:4749–4757. doi: 10.1016/j.biomaterials.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 86.Xie J., Zhong S., Ma B., Shuler F.D., Lim C.T. Controlled biomineralization of electrospun poly(epsilon-caprolactone) fibers to enhance their mechanical properties. Acta Biomater. 2013;9:5698–5707. doi: 10.1016/j.actbio.2012.10.042. [DOI] [PubMed] [Google Scholar]
- 87.Alshemary A.Z., Bilgin S., Isik G., Motameni A., Tezcaner A., Evis Z. Biomechanical evaluation of an injectable Alginate/dicalcium phosphate cement composites for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2021;118:104439. doi: 10.1016/j.jmbbm.2021.104439. [DOI] [PubMed] [Google Scholar]
- 88.Taufiq Musa M., Shaari N., Kamarudin S.K. Carbon nanotube, graphene oxide and montmorillonite as conductive fillers in polymer electrolyte membrane for fuel cell: an overview. Int. J. Energy Res. 2021;45:1309–1346. [Google Scholar]
- 89.Zhou L., Fan L., Zhang F.M., Jiang Y., Cai M., Dai C., Luo Y.A., Tu L.J., Zhou Z.N., Li X.J., Ning C.Y., Zheng K., Boccaccini A.R., Tan G.X. Hybrid gelatin/oxidized chondroitin sulfate hydrogels incorporating bioactive glass nanoparticles with enhanced mechanical properties, mineralization, and osteogenic differentiation. Bioactive Mater. 2021;6:890–904. doi: 10.1016/j.bioactmat.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaur T., Thirugnanam A. Tailoring in vitro biological and mechanical properties of polyvinyl alcohol reinforced with threshold carbon nanotube concentration for improved cellular response. RSC Adv. 2016;6:39982–39992. [Google Scholar]
- 91.Fauzi F., Musawwa M.M., Hidayat H., Kusumaatmaja A., Dwandaru W.S.B. Nanocomposites based on biocompatible polymers and graphene oxide for antibacterial coatings. Polym. Polym. Compos. 2021;29:S1609–S1620. [Google Scholar]
- 92.Sadat-Shojai M., Khorasani M.-T., Jamshidi A. A new strategy for fabrication of bone scaffolds using electrospun nano-HAp/PHB fibers and protein hydrogels. Chem. Eng. J. 2016;289:38–47. [Google Scholar]
- 93.Ruan J., Wang X., Yu Z., Wang Z., Xie Q., Zhang D., Huang Y., Zhou H., Bi X., Xiao C., Gu P., Fan X. Enhanced physiochemical and mechanical performance of chitosan-grafted graphene oxide for superior osteoinductivity. Adv. Funct. Mater. 2016;26:1085–1097. [Google Scholar]
- 94.Wang Y., Ma R., Hu K., Kim S., Fang G., Shao Z., Tsukruk V.V. Dramatic enhancement of graphene oxide/silk nanocomposite membranes: increasing toughness, strength, and young's modulus via annealing of interfacial structures. ACS Appl. Mater. Interfaces. 2016;8:24962–24973. doi: 10.1021/acsami.6b08610. [DOI] [PubMed] [Google Scholar]
- 95.Ryu S., Lee Y., Hwang J.W., Hong S., Kim C., Park T.G., Lee H., Hong S.H. High-strength carbon nanotube fibers fabricated by infiltration and curing of mussel-inspired catecholamine polymer. Adv. Mater. 2011;23:1971–1975. doi: 10.1002/adma.201004228. [DOI] [PubMed] [Google Scholar]
- 96.El-Fiqi A., Lee J.H., Lee E.-J., Kim H.-W. Collagen hydrogels incorporated with surface-aminated mesoporous nanobioactive glass: improvement of physicochemical stability and mechanical properties is effective for hard tissue engineering. Acta Biomater. 2013;9:9508–9521. doi: 10.1016/j.actbio.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 97.Jaiswal A.K., Chhabra H., Soni V.P., Bellare J.R. Enhanced mechanical strength and biocompatibility of electrospun polycaprolactone-gelatin scaffold with surface deposited nano-hydroxyapatite. Mater. Sci. Eng. C-Mater. Biol. Appl. 2013;33:2376–2385. doi: 10.1016/j.msec.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 98.Ryan A.J., O'Brien F.J. Insoluble elastin reduces collagen scaffold stiffness, improves viscoelastic properties, and induces a contractile phenotype in smooth muscle cells. Biomaterials. 2015;73:296–307. doi: 10.1016/j.biomaterials.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 99.Wang M. Developing bioactive composite materials for tissue replacement. Biomaterials. 2003;24:2133–2151. doi: 10.1016/s0142-9612(03)00037-1. [DOI] [PubMed] [Google Scholar]
- 100.Gil E.S., Kluge J.A., Rockwood D.N., Rajkhowa R., Wang L., Wang X., Kaplan D.L. Mechanical improvements to reinforced porous silk scaffolds. J. Biomed. Mater. Res. 2011;99A:16–28. doi: 10.1002/jbm.a.33158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jeong J.H., Liang Y., Jang M., Cha C., Chu C., Lee H., Jung W., Kim J.W., Boppart S.A., Kong H. Stiffness-modulated water retention and neovascularization of dermal fibroblast-encapsulating collagen gel. Tissue Eng. 2013;19:1275–1284. doi: 10.1089/ten.tea.2012.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li J.J., Gil E.S., Hayden R.S., Li C., Roohani-Esfahani S.-I., Kaplan D.L., Zreiqat H. Multiple silk coatings on biphasic calcium phosphate scaffolds: effect on physical and mechanical properties and in vitro osteogenic response of human mesenchymal stem cells. Biomacromolecules. 2013;14:2179–2188. doi: 10.1021/bm400303w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yi B., Shen Y., Tang H., Wang X., Zhang Y. Stiffness of the aligned fibers affects structural and functional integrity of the oriented endothelial cells. Acta Biomater. 2020;108:237–249. doi: 10.1016/j.actbio.2020.03.022. [DOI] [PubMed] [Google Scholar]
- 104.Liu A., Hong Z., Zhuang X., Chen X., Cui Y., Liu Y., Jing X. Surface modification of bioactive glass nanoparticles and the mechanical and biological properties of poly(L-lactide) composites. Acta Biomater. 2008;4:1005–1015. doi: 10.1016/j.actbio.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 105.Chen G., Dong C., Yang L., Lv Y. 3D scaffolds with different stiffness but the same microstructure for bone tissue engineering. ACS Appl. Mater. Interfaces. 2015;7:15790–15802. doi: 10.1021/acsami.5b02662. [DOI] [PubMed] [Google Scholar]
- 106.Chen G., Yang L., Lv Y. Cell-free scaffolds with different stiffness but same microstructure promote bone regeneration in rabbit large bone defect model. J. Biomed. Mater. Res. 2016;104:833–841. doi: 10.1002/jbm.a.35622. [DOI] [PubMed] [Google Scholar]
- 107.Bai S., Han H., Huang X., Xu W., Kaplan D.L., Zhu H., Lu Q. Silk scaffolds with tunable mechanical capability for cell differentiation. Acta Biomater. 2015;20:22–31. doi: 10.1016/j.actbio.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haraguchi K., Takehisa T. Nanocomposite hydrogels: a unique organic-inorganic network structure with extraordinary mechanical, optical, and swelling/de-swelling properties. Adv. Mater. 2002;14:1120–1124. [Google Scholar]
- 109.Visser J., Melchels F.P.W., Jeon J.E., van Bussel E.M., Kimpton L.S., Byrne H.M., Dhert W.J.A., Dalton P.D., Hutmacher D.W., Malda J. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat. Commun. 2015;6:6933. doi: 10.1038/ncomms7933. [DOI] [PubMed] [Google Scholar]
- 110.Cha C., Soman P., Zhu W., Nikkhah M., Camci-Unal G., Chen S., Khademhosseini A. Structural reinforcement of cell-laden hydrogels with microfabricated three dimensional scaffolds. Biomater. Sci. 2014;2:703–709. doi: 10.1039/C3BM60210A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Steward A.J., Kelly D.J. Mechanical regulation of mesenchymal stem cell differentiation. J. Anat. 2015;227:717–731. doi: 10.1111/joa.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tate M.L.K., Shvartsman S., Friedman A. Cell and tissue engineering - taking cues from nature's engineering paradigm for developing, growing, and repairing tissues. Tissue Eng. 2008;14:1459–1460. doi: 10.1089/ten.tea.2008.0333. [DOI] [PubMed] [Google Scholar]
- 113.Bobbert F.S.L., Lietaert K., Eftekhari A.A., Pouran B., Ahmadi S.M., Weinans H., Zadpoor A.A. Additively manufactured metallic porous biomaterials based on minimal surfaces: a unique combination of topological, mechanical, and mass transport properties. Acta Biomater. 2017;53:572–584. doi: 10.1016/j.actbio.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 114.Simonet M., Stingelin N., Wismans J.G.F., Oomens C.W.J., Driessen-Mol A., Baaijens F.P.T. Tailoring the void space and mechanical properties in electrospun scaffolds towards physiological ranges. J. Mater. Chem. B. 2014;2:305–313. doi: 10.1039/c3tb20995d. [DOI] [PubMed] [Google Scholar]
- 115.Pu J., Komvopoulos K. Mechanical properties of electrospun bilayer fibrous membranes as potential scaffolds for tissue engineering. Acta Biomater. 2014;10:2718–2726. doi: 10.1016/j.actbio.2013.12.060. [DOI] [PubMed] [Google Scholar]
- 116.Marew T., Birhanu G. Three dimensional printed nanostructure biomaterials for bone tissue engineering. Regen. Ther. 2021;18:102–111. doi: 10.1016/j.reth.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Anderson E.J., Knothe M.L. Design of tissue engineering scaffolds as delivery devices for mechanical and mechanically modulated signals. Tissue Eng. 2007;13:2525–2538. doi: 10.1089/ten.2006.0443. [DOI] [PubMed] [Google Scholar]
- 118.Kim T.G., Shin H., Lim D.W. Biomimetic scaffolds for tissue engineering. Adv. Funct. Mater. 2012;22:2446–2468. [Google Scholar]
- 119.Zhang X.-Y., Fang G., Zhou J. Additively manufactured scaffolds for bone tissue engineering and the prediction of their mechanical behavior: a review. Materials. 2017;10:50. doi: 10.3390/ma10010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thavornyutikarn B., Tesavibul P., Sitthiseripratip K., Chatarapanich N., Feltis B., Wright P.F.A., Turney T.W. Porous 45S5 Bioglass (R)-based scaffolds using stereolithography: effect of partial pre-sintering on structural and mechanical properties of scaffolds. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017;75:1281–1288. doi: 10.1016/j.msec.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 121.Weissmann V., Bader R., Hansmann H., Laufer N. Influence of the structural orientation on the mechanical properties of selective laser melted Ti6Al4V open-porous scaffolds. Mater. Des. 2016;95:188–197. [Google Scholar]
- 122.Ribeiro J.F.M., Oliveira S.M., Alves J.L., Pedro A.J., Reis R.L., Fernandes E.M., Mano J.F. Structural monitoring and modeling of the mechanical deformation of three-dimensional printed poly(epsilon-caprolactone) scaffolds. Biofabrication. 2017;9 doi: 10.1088/1758-5090/aa698e. [DOI] [PubMed] [Google Scholar]
- 123.Ghezzi C.E., Marelli B., Muja N., Nazhat S.N. Immediate production of a tubular dense collagen construct with bioinspired mechanical properties. Acta Biomater. 2012;8:1813–1825. doi: 10.1016/j.actbio.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 124.Haugh M.G., Thorpe S.D., Vinardell T., Buckley C.T., Kelly D.J. The application of plastic compression to modulate fibrin hydrogel mechanical properties. J. Mech. Behav. Biomed. Mater. 2012;16:66–72. doi: 10.1016/j.jmbbm.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 125.Wang S., Hashemi S., Stratton S., Arinzeh T.L. The effect of physical cues of biomaterial scaffolds on stem cell behavior. Adv. Healthc. Mater. 2021;10:2001244. doi: 10.1002/adhm.202001244. [DOI] [PubMed] [Google Scholar]
- 126.Stylianopoulos T., Bashur C.A., Goldstein A.S., Guelcher S.A., Barocas V.H. Computational predictions of the tensile properties of electrospun fibre meshes: effect of fibre diameter and fibre orientation. J. Mech. Behav. Biomed. Mater. 2008;1:326–335. doi: 10.1016/j.jmbbm.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Amoroso N.J., D'Amore A., Hong Y., Rivera C.P., Sacks M.S., Wagner W.R. Microstructural manipulation of electrospun scaffolds for specific bending stiffness for heart valve tissue engineering. Acta Biomater. 2012;8:4268–4277. doi: 10.1016/j.actbio.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chao P.-h.G., Hsu H.-Y., Tseng H.-Y. Electrospun microcrimped fibers with nonlinear mechanical properties enhance ligament fibroblast phenotype. Biofabrication. 2014;6 doi: 10.1088/1758-5082/6/3/035008. [DOI] [PubMed] [Google Scholar]
- 129.Reid J.A., Dwyer K.D., Schmitt P.R., Soepriatna A.H., Coulombe K., Callanan A. 2021. Architected Fibrous Scaffolds for Engineering Anisotropic Tissues, Biofabrication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cai L., Chen J., Rondinone A.J., Wang S. Injectable and biodegradable nanohybrid polymers with simultaneously enhanced stiffness and toughness for bone repair. Adv. Funct. Mater. 2012;22:3181–3190. [Google Scholar]
- 131.Wang Z., Cai N., Dai Q., Li C., Hou D., Luo X., Xue Y., Yu F. Effect of thermal annealing on mechanical properties of polyelectrolyte complex nanofiber membranes. Fibers Polym. 2014;15:1406–1413. [Google Scholar]
- 132.Xu J., Cai N., Xu W., Xue Y., Wang Z., Dai Q., Yu F. Mechanical enhancement of nanofibrous scaffolds through polyelectrolyte complexation. Nanotechnology. 2013;24 doi: 10.1088/0957-4484/24/2/025701. [DOI] [PubMed] [Google Scholar]
- 133.Lu Q., Zhu H., Zhang C., Zhang F., Zhang B., Kaplan D.L. Silk self-assembly mechanisms and control from thermodynamics to kinetics. Biomacromolecules. 2012;13:826–832. doi: 10.1021/bm201731e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nickels J.D., Perticaroli S., Ehlers G., Feygenson M., Sokolov A.P. Rigidity of poly-L-glutamic acid scaffolds: influence of secondary and supramolecular structure. J. Biomed. Mater. Res. 2015;103:2909–2918. doi: 10.1002/jbm.a.35427. [DOI] [PubMed] [Google Scholar]
- 135.Xing Q., Yates K., Vogt C., Qian Z., Frost M.C., Zhao F. Increasing mechanical strength of gelatin hydrogels by divalent metal ion removal. Sci. Rep. 2014;4:4706. doi: 10.1038/srep04706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Grasman J.M., Pumphrey L.M., Dunphy M., Perez-Rogers J., Pins G.D. Static axial stretching enhances the mechanical properties and cellular responses of fibrin microthreads. Acta Biomater. 2014;10:4367–4376. doi: 10.1016/j.actbio.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nii M., Lai J.H., Keeney M., Han L.-H., Behn A., Imanbayev G., Yang F. The effects of interactive mechanical and biochemical niche signaling on osteogenic differentiation of adipose-derived stem cells using combinatorial hydrogels. Acta Biomater. 2013;9:5475–5483. doi: 10.1016/j.actbio.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 138.Rivero R.E., Alustiza F., Rodriguez N., Bosch P., Miras M.C., Rivarola C.R., Barbero C.A. Effect of functional groups on physicochemical and mechanical behavior of biocompatible macroporous hydrogels. React. Funct. Polym. 2015;97:77–85. [Google Scholar]
- 139.Wang S., Kempen D.H.R., de Ruiter G.C.W., Cai L., Spinner R.J., Windebank A.J., Yaszemski M.J., Lu L. Molecularly engineered biodegradable polymer networks with a wide range of stiffness for bone and peripheral nerve regeneration. Adv. Funct. Mater. 2015;25:2715–2724. [Google Scholar]
- 140.Du Y., Yu M., Chen X., Ma P.X., Lei B. Development of biodegradable poly(citrate)-polyhedral oligomeric silsesquioxanes hybrid elastomers with high mechanical properties and osteogenic differentiation activity. ACS Appl. Mater. Interfaces. 2016;8:3079–3091. doi: 10.1021/acsami.5b10378. [DOI] [PubMed] [Google Scholar]
- 141.Liang Z., Liu C., Li L., Xu P., Luo G., Ding M., Liang Q. Double-network hydrogel with tunable mechanical performance and biocompatibility for the fabrication of stem cells-encapsulated fibers and 3D assemble. Sci. Rep. 2016;6:33462. doi: 10.1038/srep33462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Olubamiji A.D., Izadifar Z., Si J.L., Cooper D.M.L., Eames B.F., Chen D.X.B. Modulating mechanical behaviour of 3D-printed cartilage-mimetic PCL scaffolds: influence of molecular weight and pore geometry. Biofabrication. 2016;8 doi: 10.1088/1758-5090/8/2/025020. [DOI] [PubMed] [Google Scholar]
- 143.Hadden W.J., Choi Y.S. The extracellular microscape governs mesenchymal stem cell fate. J. Biol. Eng. 2016;10:792. doi: 10.1186/s13036-016-0037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vatankhah E., Prabhakaran M.P., Semnani D., Razavi S., Zamani M., Ramakrishna S. Phenotypic modulation of smooth muscle cells by chemical and mechanical cues of electrospun tecophilic/gelatin nanofibers. ACS Appl. Mater. Interfaces. 2014;6:4089–4101. doi: 10.1021/am405673h. [DOI] [PubMed] [Google Scholar]
- 145.Stukel J.M., Willits R.K. The interplay of peptide affinity and scaffold stiffness on neuronal differentiation of neural stem cells. Biomed. Mater. 2018;13 doi: 10.1088/1748-605X/aa9a4b. [DOI] [PubMed] [Google Scholar]
- 146.Guvendiren M., Burdick J.A. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat. Commun. 2012;3:792. doi: 10.1038/ncomms1792. [DOI] [PubMed] [Google Scholar]
- 147.Friedemann M., Kalbitzer L., Franz S., Moeller S., Schnabelrauch M., Simon J.-C., Pompe T., Franke K. Instructing human macrophage polarization by stiffness and glycosaminoglycan functionalization in 3D collagen networks. Adv. Healthc. Mater. 2017;6:201600967. doi: 10.1002/adhm.201600967. [DOI] [PubMed] [Google Scholar]
- 148.Hu C., Chu C., Liu L., Wang C., Jin S., Yang R., Rung S., Li J., Qu Y., Man Y. Dissecting the microenvironment around biosynthetic scaffolds in murine skin wound healing. Sci. Adv. 2021;7:abf0787. doi: 10.1126/sciadv.abf0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang C., Xing C., Li Z., Liu Y., Li Q., Wang Y., Hu J., Yuan L., Yang G. Bioinspired therapeutic platform based on extracellular vesicles for prevention of arterial wall remodeling in hypertension. Bioactive Mater. 2022;8:494–504. doi: 10.1016/j.bioactmat.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Witkowska-Zimny M., Walenko K., Wrobel E., Mrowka P., Mikulska A., Przybylski J. Effect of substrate stiffness on the osteogenic differentiation of bone marrow stem cells and bone-derived cells. Cell Biol. Int. 2013;37:608–616. doi: 10.1002/cbin.10078. [DOI] [PubMed] [Google Scholar]
- 151.Zhang T., Gong T., Xie J., Lin S., Liu Y., Zhou T., Lin Y. Softening substrates promote chondrocytes phenotype via RhoA/ROCK pathway. ACS Appl. Mater. Interfaces. 2016;8:22884–22891. doi: 10.1021/acsami.6b07097. [DOI] [PubMed] [Google Scholar]
- 152.Patel D., Sharma S., Screen H.R.C., Bryant S.J. Effects of cell adhesion motif, fiber stiffness, and cyclic strain on tenocyte gene expression in a tendon mimetic fiber composite hydrogel. Biochem. Biophys. Res. Commun. 2018;499:642–647. doi: 10.1016/j.bbrc.2018.03.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chaterji S., Kim P., Choe S.H., Tsui J.H., Lam C.H., Ho D.S., Baker A.B., Kim D.-H. Synergistic effects of matrix nanotopography and stiffness on vascular smooth muscle cell function. Tissue Eng. 2014;20:2115–2126. doi: 10.1089/ten.tea.2013.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Urbano R.L., Furia C., Basehore S., Clyne A.M. Stiff substrates increase inflammation-induced endothelial monolayer tension and permeability. Biophys. J. 2017;113:645–655. doi: 10.1016/j.bpj.2017.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang L., Han Q., Chen S., Suo D., Zhang L., Li G., Zhao X., Yang Y. Soft hydrogel promotes dorsal root ganglion by upregulating gene expression of Ntn4 and Unc5B. Colloids Surf. B Biointerfaces. 2021;199:111503. doi: 10.1016/j.colsurfb.2020.111503. [DOI] [PubMed] [Google Scholar]
- 156.Chen L., Wu C., Wei D., Chen S., Xiao Z., Zhu H., Luo H., Sun J., Fan H. Biomimetic mineralized microenvironment stiffness regulated BMSCs osteogenic differentiation through cytoskeleton mediated mechanical signaling transduction. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021;119:111613. doi: 10.1016/j.msec.2020.111613. [DOI] [PubMed] [Google Scholar]
- 157.Xue R., Li J.Y.-S., Yeh Y., Yang L., Chien S. Effects of matrix elasticity and cell density on human mesenchymal stem cells differentiation. J. Orthop. Res. 2013;31:1360–1365. doi: 10.1002/jor.22374. [DOI] [PubMed] [Google Scholar]
- 158.Ghosh S., Laha M., Mondal S., Sengupta S., Kaplan D.L. In vitro model of mesenchymal condensation during chondrogenic development. Biomaterials. 2009;30:6530–6540. doi: 10.1016/j.biomaterials.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jiang T., Kai D., Liu S., Huang X., Heng S., Zhao J., Yu Chan B.Q., Loh X.J., Zhu Y., Mao C., Zheng L. Mechanically cartilage-mimicking poly(PCL-PTHF urethane)/collagen nanofibers induce chondrogenesis by blocking NF-kappa B signaling pathway. Biomaterials. 2018;178:281–292. doi: 10.1016/j.biomaterials.2018.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu N., Zhou M., Zhang Q., Yong L., Zhang T., Tian T., Ma Q., Lin S., Zhu B., Cai X. Effect of substrate stiffness on proliferation and differentiation of periodontal ligament stem cells. Cell Prolif. 2018 doi: 10.1111/cpr.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Smith Q., Chan X.Y., Carmo A.M., Trempel M., Saunders M., Gerecht S. Compliant substratum guides endothelial commitment from human pluripotent stem cells. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1602883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Leipzig N.D., Shoichet M.S. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials. 2009;30:6867–6878. doi: 10.1016/j.biomaterials.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 163.Chang Y., Cho B., Kim S., Kim J. Direct conversion of fibroblasts to osteoblasts as a novel strategy for bone regeneration in elderly individuals. Exp. Mol. Med. 2019;51:54. doi: 10.1038/s12276-019-0251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xiao Z., Baudry J., Cao L., Huang J., Chen H., Yates C.R., Li W., Dong B., Waters C.M., Smith J.C., Quarles L.D. Polycystin-1 interacts with TAZ to stimulate osteoblastogenesis and inhibit adipogenesis. J. Clin. Invest. 2018;128:157–174. doi: 10.1172/JCI93725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hwang J.-H., Byun M.R., Kim A.R., Kim K.M., Cho H.J., Lee Y.H., Kim J., Jeong M.G., Hwang E.S., Hong J.-H. Extracellular matrix stiffness regulates osteogenic differentiation through MAPK activation. PLoS One. 2015;10 doi: 10.1371/journal.pone.0135519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hu Q., Liu M., Chen G., Xu Z., Lv Y. Demineralized bone scaffolds with tunable matrix stiffness for efficient bone integration. ACS Appl. Mater. Interfaces. 2018;10:27669–27680. doi: 10.1021/acsami.8b08668. [DOI] [PubMed] [Google Scholar]
- 167.He X.-T., Wu R.-X., Xu X.-Y., Wang J., Yin Y., Chen F.-M. Macrophage involvement affects matrix stiffness-related influences on cell osteogenesis under three-dimensional culture conditions. Acta Biomater. 2018;71:132–147. doi: 10.1016/j.actbio.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 168.Keogh M.B., O'Brien F.J., Daly J.S. Substrate stiffness and contractile behaviour modulate the functional maturation of osteoblasts on a collagen-GAG scaffold. Acta Biomater. 2010;6:4305–4313. doi: 10.1016/j.actbio.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 169.Patel J.M., Wise B.C., Bonnevie E.D., Mauck R.L. A systematic review and guide to mechanical testing for articular cartilage tissue engineering. Tissue Eng. C Methods. 2019;25:593–608. doi: 10.1089/ten.tec.2019.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zignego D.L., Hilmer J.K., Bothner B., Schell W.J., June R.K. Primary human chondrocytes respond to compression with phosphoproteomic signatures that include microtubule activation. J. Biomech. 2019;97:109367. doi: 10.1016/j.jbiomech.2019.109367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang L.-S., Du C., Toh W.S., Wan A.C.A., Gao S.J., Kurisawa M. Modulation of chondrocyte functions and stiffness-dependent cartilage repair using an injectable enzymatically crosslinked hydrogel with tunable mechanical properties. Biomaterials. 2014;35:2207–2217. doi: 10.1016/j.biomaterials.2013.11.070. [DOI] [PubMed] [Google Scholar]
- 172.Arora A., Kothari A., Katti D.S. Pericellular plasma clot negates the influence of scaffold stiffness on chondrogenic differentiation. Acta Biomater. 2016;46:68–78. doi: 10.1016/j.actbio.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 173.Zhu D., Tong X., Trinh P., Yang F. Mimicking cartilage tissue zonal organization by engineering tissue-scale gradient hydrogels as 3D cell niche. Tissue Eng. 2018;24:1–10. doi: 10.1089/ten.tea.2016.0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lee H.p., Gu L., Mooney D.J., Levenston M.E., Chaudhuri O. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat. Mater. 2017;16:1243–1251. doi: 10.1038/nmat4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sarem M., Arya N., Heizmann M., Neffe A.T., Barbero A., Gebauer T.P., Martin I., Lendlein A., Shastri V.P. Interplay between stiffness and degradation of architectured gelatin hydrogels leads to differential modulation of chondrogenesis in vitro and in vivo. Acta Biomater. 2018;69:83–94. doi: 10.1016/j.actbio.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 176.Fernandez-Muinos T., Suarez-Munoz M., Sanmarti-Espinal M., Semino C.E. Matrix dimensions, stiffness, and structural properties modulate spontaneous chondrogenic commitment of mouse embryonic fibroblasts. Tissue Eng. 2014;20:1145–1155. doi: 10.1089/ten.tea.2013.0369. [DOI] [PubMed] [Google Scholar]
- 177.Schiele N.R., Marturano J.E., Kuo C.K. Mechanical factors in embryonic tendon development: potential cues for stem cell tenogenesis. Curr. Opin. Biotechnol. 2013;24:834–840. doi: 10.1016/j.copbio.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Islam A., Mbimba T., Younesi M., Akkus O. Effects of substrate stiffness on the tenoinduction of human mesenchymal stem cells. Acta Biomater. 2017;58:244–253. doi: 10.1016/j.actbio.2017.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Aurora A., McCarron J.A., van den Bogert A.J., Gatica J.E., Iannotti J.P., Derwin K.A. The biomechanical role of scaffolds in augmented rotator cuff tendon repairs. J. Shoulder Elbow Surg. 2012;21:1064–1071. doi: 10.1016/j.jse.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 180.Boriek A.M., Rodarte J.R. Effects of transverse fiber stiffness and central tendon on displacement and shape of a simple diaphragm model. J. Appl. Physiol. 1997;82:1626–1636. doi: 10.1152/jappl.1997.82.5.1626. [DOI] [PubMed] [Google Scholar]
- 181.Islam A., Younesi M., Mbimba T., Akkus O. Collagen substrate stiffness anisotropy affects cellular elongation, nuclear shape, and stem cell fate toward anisotropic tissue lineage. Adv. Healthc. Mater. 2016;5:2237–2247. doi: 10.1002/adhm.201600284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kielty C.M., Stephan S., Sherratt M.J., Williamson M., Shuttleworth C.A. Applying elastic fibre biology in vascular tissue engineering. Phil. Trans. Biol. Sci. 2007;362:1293–1312. doi: 10.1098/rstb.2007.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Crapo P.M., Wang Y. Physiologic compliance in engineered small-diameter arterial constructs based on an elastomeric substrate. Biomaterials. 2010;31:1626–1635. doi: 10.1016/j.biomaterials.2009.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chang H., Zhang H., Hu M., Chen J.-y., Li B.-c., Ren K.-f., Martins M.C.L., Barbosa M.A., Ji J. Stiffness of polyelectrolyte multilayer film influences endothelial function of endothelial cell monolayer. Colloids Surf. B Biointerfaces. 2017;149:379–387. doi: 10.1016/j.colsurfb.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 185.Ding Y., Floren M., Tan W. High-throughput screening of vascular endothelium-destructive or protective microenvironments: cooperative actions of extracellular matrix composition, stiffness, and structure. Adv. Healthc. Mater. 2017;6:1601426. doi: 10.1002/adhm.201601426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chang H., Zhang H., Hu M., Chen X.-c., Ren K.-f., Wang J.-l., Ji J. Surface modulation of complex stiffness via layer-by-layer assembly as a facile strategy for selective cell adhesion. Biomater. Sci. 2015;3:352–360. doi: 10.1039/c4bm00321g. [DOI] [PubMed] [Google Scholar]
- 187.Post A., Diaz-Rodriguez P., Balouch B., Paulsen S., Wu S., Miller J., Hahn M., Cosgriff-Hernandez E. Elucidating the role of graft compliance mismatch on intimal hyperplasia using an ex vivo organ culture model. Acta Biomater. 2019;89:84–94. doi: 10.1016/j.actbio.2019.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Merkle V.M., Martin D., Hutchinson M., Tran P.L., Behrens A., Hossainy S., Sheriff J., Bluestein D., Wu X., Slepian M.J. Hemocompatibility of poly(vinyl alcohol)-gelatin core-shell electrospun nanofibers: a scaffold for modulating platelet deposition and activation. ACS Appl. Mater. Interfaces. 2015;7:8302–8312. doi: 10.1021/acsami.5b01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Franze K., Janmey P.A., Guck J. In: Yarmush M.L., editor. vol. 15. 2013. Mechanics in neuronal development and repair; pp. 227–251. (Annual Review of Biomedical Engineering). [DOI] [PubMed] [Google Scholar]
- 190.Balgude A.P., Yu X., Szymanski A., Bellamkonda R.V. Agarose gel stiffness determines rate of DRG neurite extension in 3D cultures. Biomaterials. 2001;22:1077–1084. doi: 10.1016/s0142-9612(00)00350-1. [DOI] [PubMed] [Google Scholar]
- 191.Man A.J., Davis H.E., Itoh A., Leach J.K., Bannerman P. Neurite outgrowth in fibrin gels is regulated by substrate stiffness. Tissue Eng. 2011;17:2931–2942. doi: 10.1089/ten.tea.2011.0030. [DOI] [PubMed] [Google Scholar]
- 192.Hopkins A.M., De Laporte L., Tortelli F., Spedden E., Staii C., Atherton T.J., Hubbell J.A., Kaplan D.L. Silk hydrogels as soft substrates for neural tissue engineering. Adv. Funct. Mater. 2013;23:5140–5149. [Google Scholar]
- 193.Sur S., Newcomb C.J., Webber M.J., Stupp S.I. Tuning supramolecular mechanics to guide neuron development. Biomaterials. 2013;34:4749–4757. doi: 10.1016/j.biomaterials.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Stukel J.M., Willits R.K. Mechanotransduction of neural cells through cell-substrate interactions. Tissue Eng. B Rev. 2016;22:173–182. doi: 10.1089/ten.teb.2015.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang Q.-Y., Zhang Y.Y., Xie J., Li C.X., Chen W.Y., Liu B.L., Wu X.a., Li S.N., Huo B., Jiang L.H., Zhao H.C. Stiff substrates enhance cultured neuronal network activity. Sci. Rep. 2014;4:6215. doi: 10.1038/srep06215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Namgung U. The role of Schwann cell-axon interaction in peripheral nerve regeneration. Cells Tissues Organs. 2014;200:6–12. doi: 10.1159/000370324. [DOI] [PubMed] [Google Scholar]
- 197.Lampe K.J., Mooney R.G., Bjugstad K.B., Mahoney M.J. Effect of macromer weight percent on neural cell growth in 2D and 3D nondegradable PEG hydrogel culture. J. Biomed. Mater. Res. 2010;94A:1162–1171. doi: 10.1002/jbm.a.32787. [DOI] [PubMed] [Google Scholar]
- 198.Cukierman E., Pankov R., Stevens D.R., Yamada K.M. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 199.Khoshakhlagh P., Moore M.J. Photoreactive interpenetrating network of hyaluronic acid and Puramatrix as a selectively tunable scaffold for neurite growth. Acta Biomater. 2015;16:23–34. doi: 10.1016/j.actbio.2015.01.014. [DOI] [PubMed] [Google Scholar]