Abstract
As a type of elastomeric polymers, non-degradable polyurethanes (PUs) have a long history of being used in clinics, whereas biodegradable PUs have been developed in recent decades, primarily for tissue repair and regeneration. Biodegradable thermoplastic (linear) PUs are soft and elastic polymeric biomaterials with high mechanical strength, which mimics the mechanical properties of soft and elastic tissues. Therefore, biodegradable thermoplastic polyurethanes are promising scaffolding materials for soft and elastic tissue repair and regeneration. Generally, PUs are synthesized by linking three types of changeable blocks: diisocyanates, diols, and chain extenders. Alternating the combination of these three blocks can finely tailor the physio-chemical properties and generate new functional PUs. These PUs have excellent processing flexibilities and can be fabricated into three-dimensional (3D) constructs using conventional and/or advanced technologies, which is a great advantage compared with cross-linked thermoset elastomers. Additionally, they can be combined with biomolecules to incorporate desired bioactivities to broaden their biomedical applications. In this review, we comprehensively summarized the synthesis, structures, and properties of biodegradable thermoplastic PUs, and introduced their multiple applications in tissue repair and regeneration. A whole picture of their design and applications along with discussions and perspectives of future directions would provide theoretical and technical supports to inspire new PU development and novel applications.
Keywords: Biodegradable polyurethane, Thermoplastic, Elastic, Synthesis, Tissue repair
Graphical abstract
Highlights
-
•
Linear biodegradable polyurethanes with high elasticity and good biocompatibility have been utilized for soft and elastic tissue repair.
-
•
The biofunctions and characteristics of biodegradable polyurethanes can be effectively offered and tuned through designing and altering chemical structures, and they also can be easily processed into desirable architectures.
-
•
New chemistry and further evaluations are expected for future development and applications of biodegradable elastic polyurethanes in tissue repair.
1. Introduction
Tissue engineering is an interdisciplinary field dedicated to replacing or regenerating the biological functions of human tissues or organs, relying on the combination of scaffolds, cells, and bioactive signal molecules [1]. The classic tissue engineering method can construct tissue-like implants based on biomaterial matrices in a simulated physiological environment in vitro for implantation into a patient. In vivo or in situ tissue engineering directly implants an acellular biomaterial into a patient to utilize endogenous cells for tissue repair in vivo. In above tissue repair methods, biodegradable scaffolds play an important role by providing a 3D porous space, mechanical support, and microenvironment. In general, an ideal biodegradable scaffold should meet the following criteria: (i) be biocompatible to support cell adhesion, migration, and proliferation and elicit a negligible immune reaction; (ii) be biodegradable at a comparable rate to that of new tissue growth at the implanted site, and its degradation products are nontoxic and absorbable; (iii) have mechanical properties matching the native tissue, and effectively transmits forces from the environment to the growing tissue over a long period of time; and (iv) have an interconnected pore structure and high porosity to allow cell penetration and nutrient and oxygen transportation [2,3]. Synthetic biodegradable polymers are versatile biomaterials that can be processed into scaffolds with a wide range of physical, thermo, and mechanical properties [2].
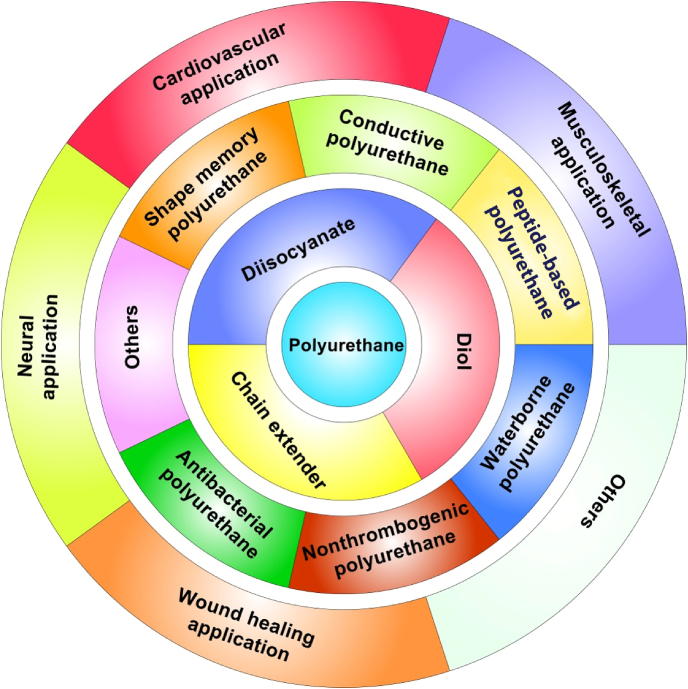
Elastic polyurethanes (PUs) are a class of important synthetic elastomeric polymers with broad biomedical applications [4]. The earliest biomedical application of non-degradable PUs dates back to the 1960s. Biomer® is the first biostable PU product for cardiovascular applications because of its durability and good mechanical properties and biocompatibility [5]. To meet the need for tissue repair and regeneration, biodegradable PUs have drawn significant interests since the 1990s. The biodegradable PU exhibits high mechanical strength, softness, and high elasticity to mimetic biomechanical behaviors of soft and elastic tissues. The flexible chemistry of PU synthesis also can generate varieties of biodegradable PUs to meet the specific needs for different tissues. Biodegradable PUs are generated by incorporating hydrolyzable bonds (e.g., ester, amide, anhydride, and carbonate) into polymer backbones [6,7]. Thermoplastic PUs are linear and can be processed into three dimensional (3D) scaffolds through a variety of fabrication techniques, and have been widely used in the biomedical field [8]. They are known as elastomers for their high elasticity and softness which make them stand out from those commonly used biodegradable polymers, such as poly(glycolide) (PGA) [9], poly(lactide) (PLA) [10], and poly(ε-caprolactone) (PCL) [11,12]. Thermoplastic PU also has numerous diversities of chemical compositions and physical-mechanical properties through alteration of its segmented blocks, which makes PU more versatile than other thermoplastic polyester based elastomers, such as polyhydroxyalkanoates (PHA) [13] and polyester-based copolymers (e.g., PLA-co-PCL (PLCL) [14], PLA-co-poly(trimethylene carbonate) (PLA-co-PTMC) [15]). Furthermore, compared with common chemically cross-linked polyester elastomers, such as poly(glycerol sebacate) (PGS) [16] and poly(1,8-octanediol-co-citric acid) [17], one important advantage of thermoplastic PUs is their good processability because the chemically cross-linked polyester elastomers are hardly re-processed [18,19]. The high elasticity, great processability, good biocompatibility, and biodegradability make the thermoplastic biodegradable PU an excellent matrix candidate for tissue repair and regeneration. In this review, we introduce the synthesis of thermoplastic biodegradable PUs and summarize and discuss the current functional biodegradable PUs and their applications in tissue repair and regeneration (Fig. 1).
Fig. 1.
Biodegradable polyurethanes: chemistry, functionalization, and tissue repair applications.
2. Biodegradable thermoplastic polyurethane synthesis
The synthesis of thermoplastic biodegradable PUs includes three Lego-block like components: a diisocyanate, a biodegradable polymer diol, and a chain extender of diol or diamine [8]. The polymer diol and chain extender are linked by a diisocyanate to form linear segmented PUs. The PUs consist hard and soft segments, which results in microphase separation [20]. The formation of the microphase-separated structure causes the PU to have high elasticity. Generally, the diisocyanate and chain extender comprise the hard segment (hard phase), and the long, linear chain of polymer diol comprises the soft segment (soft phase). In general, there exist one glass-transition temperatures (Tg) and one melting temperatures (Tm). When a crystalline soft segment is used, two TMs may be observed. The hard segment functions as a physical cross-linking element, which leads to a Tm, and a high modulus and strength of PU [21,22]. The soft segment contributes to a low Tg, and to the softness of PU [22,23]. The thermal and mechanical properties are dependent on the properties and interactions of both soft and hard segments. To achieve the degradability of the PUs, polymer diols containing hydrolyzable bonds, such as ester and amide bonds, are generally used as the soft segments [24]. Varying the three blocks and their ratios in the PU backbone can tailor its thermal and mechanical properties, and degradation profiles.
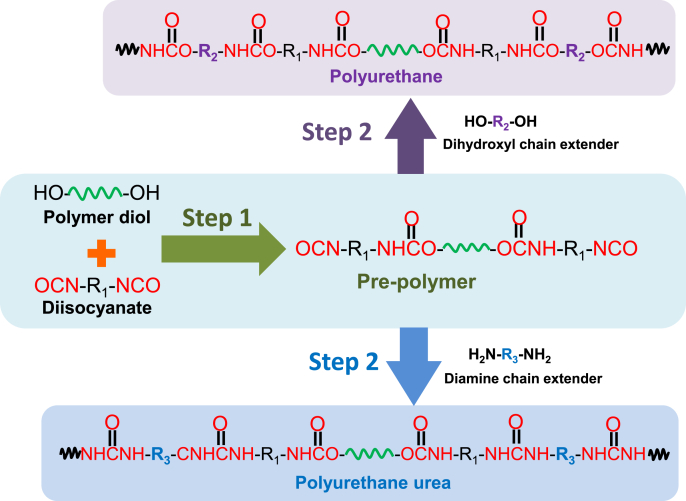
Thermoplastic PUs can be chemically synthesized via the one- or two-step method. In the one-step method, all reactants (a diisocyanate, a polymer diol, a chain extender, and catalyst) are mixed and reacted in one pot, which makes it hard to control the chemical structure of the PU [8]. The two-step solution polymerization is a popular method to synthesize thermoplastic/linear PUs (Fig. 2). The diol and excessive diisocyanate react first (first step) to form a prepolymer with two isocyanate end groups, followed by the addition of the chain extender (second step) to increase the molecular weight. The two-step method, compared with the one-pot synthesis, is more effective in controlling the structure and property of the PU [25]. To achieve a high molecular weight PU, the molar ratio of isocyanate groups to total hydroxyl or amine groups must be equivalent. Additionally, another key factor to achieve high molecular weight PUs is the absence of water, especially for small-scale synthesis, in the reaction system. To achieve a linear structure, only bifunctional monomers can be used.
Fig. 2.
Typical synthesis routine of biodegradable polyurethane and polyurethane urea via a two-step method.
Isocyanates can actively react with hydroxyls or amines to form urethane or urea bonds via a nucleophilic addition, separately. The diisocyanates for biodegradable PU synthesis are listed in Table 1. The first generation of degradable PUs is synthesized using aromatic diisocyanates, such as 4,4′-methylenebis(phenyl isocyanate) (MDI) and toluene-2, 4-diisocyanate (TDI), to link the biodegradable soft segments [26,27]. Because the potential degradation products (aromatic diamines) are toxic and carcinogenic, their usages in biodegradable PU synthesis are limited [28]. The second generation of biodegradable PUs is based on aliphatic diisocyanates (e.g., 1,4-butane diisocyanate (BDI), 1,6-hexamethylene diisocyanate (HDI), and lysine-based diisocyanate (LDI)) or cycloaliphatic diisocyanates (e.g., isophorone diisocyanate (IPDI) and 4,4′-methylene bis(cyclohexyl isocyanate) (HMDI)). HDI, BDI, and LDI are the three most used aliphatic and linear diisocyanates because of their nontoxic biodegradation products (1,4-butanediamine, 1,6-hexanediamine, and lysine) [22,[29], [30], [31], [32], [33]]. The symmetric chemical structures of HDI and BDI can result in well-ordered hard segments through hydrogen bonds, resulting in high strength and elasticity [8]. In a study of comparing HDI and BDI, biodegradable PUs were synthesized using HDI or BDI with a soft segment of PCL diol (MW = 2000) and a chain extender of butanediamine (BDA) [34]. The HDI-based PU showed higher tensile strength and breaking strain and larger plastic deformation than the BDI-based PU because of its less-ordered hard segments. The cyclohexane rings in cycloaliphatic diisocyanates, such as IPDI and HMDI, limit the flexibility of the polymer chains, and results in stiffer PUs compared with those based on aliphatic linear diisocyanates [35,36]. Furthermore, the introduction of LDI can result in a high hydrolytic degradation rate because it may affect the aggregation and hydrophilicity of hard segments due to the side group containing a hydrolyzable ester bond [32,[37], [38], [39]].
Table 1.
List of diisocyanates for biodegradable thermoplastic polyurethane synthesis.
| Chemical name | Structure | Refs |
|---|---|---|
| 1,4-butane diisocyanate (BDI) | [33,34,49,213,214,238] | |
| 1,6-hexamethylene diisocyanate (HDI) | [31,34,51,81,87,235] | |
| isophorone diisocyanate (IPDI) | 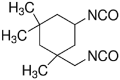 |
[35,122,132,133] |
| 4,4′- methylenebis(cyclohexyl isocyanate) (HMDI) | [36] | |
| Lysine ethyl ester diisocyanate (LDI) | 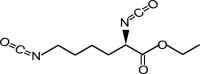 |
[32,[37], [38], [39]] |
| 4,4′-Methylenebis(phenyl isocyanate) (MDI) | [26] | |
| Tolylene-2,4-diisocyanate (TDI) | 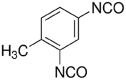 |
[27] |
The biodegradable polymer diols are commonly polyester based and used as soft segments in biodegradable PUs (Table 2). The degradation of the biodegradable PUs is majorly attributed to the hydrolytic and enzymatic degradation of these polymer diols. Most homopolymer polyester diols (e.g., PCL, poly(D,l-lactide) (PDLLA), and PGA) are synthesized via ring-opening polymerization [8,21,40]. The semicrystalline PCL diol is widely investigated in biodegradable PU synthesis because of its good biocompatibility and low Tg (−60 °C), which can result in a high flexibility of PU [41,42]. The random copolymers, such as poly(δ-valerolactone-co-ε-caprolactone) (PVCL) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate) (P3/4HB), and block polymers, such as PLA-PCL-PLA, are used as soft segments [30,[43], [44], [45]]. The PUs synthesized from polyether diols, such as poly(ethylene glycol) (PEG)/poly(ethylene oxide) (PEO), poly(propylene oxide) (PPO) and PEO-PPO-PEO, are generally considered as non-degradable PUs [[46], [47], [48]]. However, the polyether is often co-polymerized with polyester or polycarbonate to form triblock copolymers, such as PCL-PEG-PCL and PTMC-PEO-PPO-PEO-PTMC [12,46,47,[49], [50], [51]]. The introduction of a hydrophilic polyether can increase the hydrophilicity of the PU, accelerate its degradation, and change its mechanical properties. Additionally, a mixed soft segment from various biodegradable polymer diols is often used [52].
Table 2.
List of polymer diols for biodegradable thermoplastic polyurethane synthesis.
| Chemical name | Structure | Refs |
|---|---|---|
| Poly(D,l-lactide) (PDLLA) | [200,201] | |
| Poly(ε-caprolactone) (PCL) | [31,33,34,81,86,109,214] | |
| Poly(1,6-hexamethylene carbonate) (PHC) | [52,235,238] | |
| Poly(trimethylene carbonate) (PTMC) | [30] | |
| Poly[(R)-3-hydroxybutyrate] (PHB) | 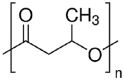 |
[61,62] |
| Poly(δ-valerolactone-co-ε-caprolactone) (PVCL) | [30,51] | |
| PVCL-PEG-PVCL | [51] | |
| Poly(ε-caprolactone)-co-poly(ethylene glycol)-co-poly(ε-caprolactone) (PCL-PEG-PCL) | [49] | |
| Polyethylene butylenenbutylene adipate (PEB) diol | [122] | |
| Poly(trimethylene carbonate)-co-poly(ethylene oxide)-co-poly(propylene oxide)-co-poly(ethylene oxide)-co-poly(trimethylene carbonate) (PTMC-PEO-PPO-PEO-PTMC) |
[47] | |
| Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) (P3/4HB) | [12,43] |
Altering the components and the molecular weight of the soft segments can significantly affect the properties of the PUs, particularly the mechanical properties and degradation. For example, a PVCL copolymer diol reached minimal crystallinity when the feeding ratio of valerolactone (VL) to caprolactone (CL) was 50/50. It resulted in a polyurethane with lower initial modulus and tensile strength than a polyurethane based on a semi-crystalline PCL diol [30,51]. In addition, increasing the block length of the amorphous PVCL diol (VL/CL = 50/50) from 2000 to 6000 significantly decreased the initial modulus and tensile strength of the PU by reducing the hard segment density. This observation is consistent with the rubber thermodynamic theory because the initial modulus of elastomers increases with the decrease of average molecular weight between the physical cross-link points [53]. Because the optimal degradation period for the implanted scaffold is unclear, the degradation rate of the tissue-engineered scaffold should be tunable to meet different requirements [52]. Slowly degradable soft segments, such as polycarbonate diol, can be introduced into the soft segment to achieve a PU with a slow degradation rate [30,47,52,54]. A blended soft segment of slowly degradable poly(1,6-hexamethylene carbonate) (PHC) and PCL would markedly reduce the hydrolytic degradation rate of the PU [52]. To reach a fast degradation rate, hydrophilic moieties (e.g., PEG) can be introduced into the soft segment in a PU backbone [49,51]. The incorporation of PEG into a PCL soft segment (PCL-PEG-PCL) significantly increased the hydrolytic degradation rate of PU [49]. However, PEG incorporation may not be able to accelerate the enzymatic degradation of the PU in a lipase/PBS solution in vitro [51,55], although the lipase can accelerate the in vitro enzymatic degradation of polymers containing ester, amide, carbonate, urea, and urethane groups [30,56,57]. One reason is that the ether groups of PEG are less susceptible to lipase than ester groups [58,59]. The other reason is that the PEG segment can lead to the increase of PU surface hydrophilicity, which reduces protein adsorption and compromises polymer enzymatic degradation [59,60]. Additionally, the crystalline degree of the soft segment may affect degradation rates of PUs. Regarding soft segments with the same chemical structures, the crystalline soft segment would lead to a slow degradation compared to amorphous soft segments. On the other hand, it is interesting to use a stiff polyester as a hard segment. For example, Li group developed a family of multi-block poly(ester urethane)s with poly[(R)-3-hydroxybutyrate] (PHB) as a hard and hydrophobic segment and PEG as a soft and hydrophilic segment [61,62].
The chain extenders are generally small molecular diols or diamines, such as 1,4-butanediol (BDO), ethylene glycol (EG), ethylenediamine (ED), 1,4-butanediamine (BDA), and peptides (Table 3). BDO and BDA are popular chain extenders [23,34,63]. One important function of the chain extender is to promote the formation of highly ordered hard segments in the PU backbone [64]. Hence, the chain extenders are usually short chains with a symmetrical structure to favor the order of hard segments. The diamine reacts with diisocyanate to form urea bonds, which, compared with urethane bonds, can significantly strengthen the PUs because of increased hydrogen bonding. The length of the chain extender also has a significant effect on the mechanical properties of PU because it directly affects the formation and strength of the hard phase in the PU. Chain extenders with hydrolyzable bonds (e.g., ester and phosphate ester) can also accelerate hard segment degradation [65,66]. A degradable ester chain extender based on lactic acid and ethylene glycol was introduced into a PU backbone, which contributed to the accelerated hydrolytic degradation of the PU in vitro [65]. Moreover, peptide-based chain extenders have been used to improve the enzyme-mediated degradation and biocompatibility of PUs [64,67]. The degradation of PU containing elastase sensitive AAK can be accelerated in an elastase solution [67].
Table 3.
List of chain extenders for biodegradable thermoplastic polyurethane synthesis.
| Chemical name | Structure | Refs |
|---|---|---|
| 1,4-Butanediol (BDO) | [121,235] | |
| Ethylene glycol (EG) |  |
[65] |
| 1,4-Butanediamine (BDA) | [33,49,51,52,67,213,214,226] | |
| Ethylenediamine (ED) |  |
[122,200,201] |
| 2-hydroxyethyl lactate | 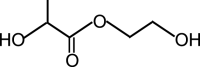 |
[65] |
| Ala-Ala-Lys (AAK) | [67] | |
| Aniline trimer | [81,86] | |
| 2-Hydroxyethyl disulfide (HDS) | [31] | |
| Sulfobetaine (SB) diol | 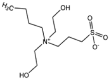 |
[167] |
| Diazeniumdiolate NO donor | SGG[K[N(O)NO-]4GGS | [183,184] |
| Collagenase sensitive peptide | GGGLGPAGGK-NH2 | [145] |
| Gemini quaternary ammonium salts (GQAS) | 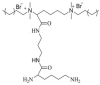 |
[166] |
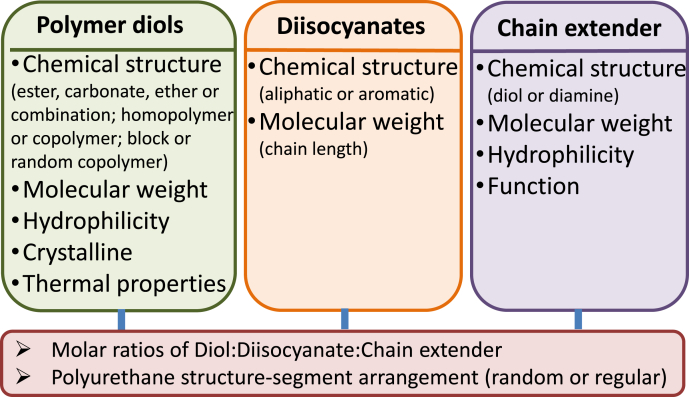
Based on these findings, we summarized the major parameters of three components, which affect the properties of biodegradable PUs (Fig. 3). Altering a single parameter, such as a component and feeding ratio, may affect synthesis and some characteristics of a biodegradable PU. As discussed above, mechanical properties and degradation of PUs are dependent on many factors including chemical structure, molecular weight, and physic microstructure (phase separation, hydrogen bonding, and crystalline et al.). Thus, to design a PU with desirable properties, it is necessary to comprehensively consider all the possible factors. Such complexity also creates opportunities to develop many attractive PUs for broad biomedical applications.
Fig. 3.
List of component parameters that affect mechanical properties and degradation of biodegradable polyurethanes.
3. Functional biodegradable thermoplastic polyurethanes
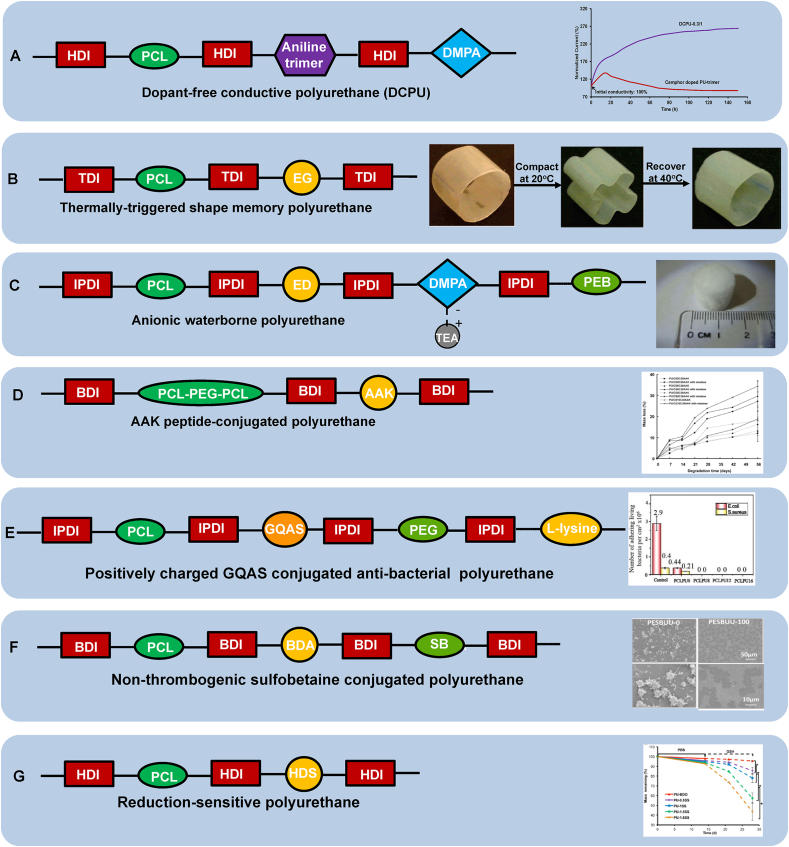
PU can be endowed with unique functions to meet the specific requirements relevant to tissue repair and regeneration. Their functions can be achieved by altering soft segments or chain extenders. According to recently published works, we primarily summarized the PUs with functions of conductivity, shape memory, and non-thrombogenicity, and peptide incorporating PUs, waterborne PUs, and antibacterial PUs (Fig. 4).
Fig. 4.
Functional biodegradable polyurethane design. (A) Dopant-free conductive polyurethane. Structural design (left); Electrical stability (right): Relationship between electrical current and incubation time in the electrical stability test of DCPU-0.3/1 film in cell culture medium. Camphor doped PU-trimer film was used as a control. Reprinted with permission from [87]. Copyright 2016 Springer Nature. (B) Thermally triggered shape-memory polyurethane. Structural design (left); Shape memory behavior (right): Polyurethane cylinder was compacted into a flower shape at 40 °C and then cooled to room temperature immediately, and it returned to the original shape when immersed in 40 °C water. Adapted with permission from [109]. Copyright 2005 American Chemical Society. (C) Anionic waterborne polyurethane. Structural design (left); Porous polyurethane sponge fabricated from polyurethane/water dispersion via freeze-drying (right). Reproduced with permission from [122]. Copyright 2014 Elsevier B.V. (D) AAK-peptide conjugated polyurethane. Structural design (left); Polyurethane enzymatic degradation manipulation by introducing elastase sensitive AAK sequence and varying the feeding ratio of polyether/polyester (PEG/PCL) in the soft segment (right). Reprinted with permission from [67]. Copyright 2005 American Chemical Society. (E) Positively charged GQAS conjugated anti-bacterial polyurethane. Structural design (left); Antibacterial activities (right): live bacteria attached on surfaces with and without GQAS and PEG. No live E. coli or S. aureus cells detected on all surfaces of the polyurethanes containing different GQASs compared with the PCLPU0 without GQAS, indicating the antibacterial property of GQAS. Reprinted with the permission from [167]. Copyright 2017 Royal Society of Chemistry. (F) Non-thrombogenic polyurethane. Structural design (left); Ovine blood platelet deposition on polyurethane films observed by scanning electron microscopy after blood contact for 2 h (right): PSBUU-0 was control group without SB content which had relatively high platelet deposition, while PSBUU-100 contained the highest SB content showing sparse platelet deposition. Reprinted with the permission from [176]. Copyright 2014 American Chemical Society. (G) Reduction sensitive polyurethane. Structural design (left); Electrospun polyurethane scaffold controllable degradation (right): Scaffolds were immersed in PBS for 14 d and then in 10 mM GSH for another 14 d, where the scaffold degradation rate increased obviously after transferring from PBS to GSH solution. * represents significantly different groups (p < 0.05). Reprinted with the permission from [31]. Copyright 2015 American Chemical Society.
3.1. Conductive polyurethanes
Conductive biomaterials, including conductive composites and polymers, have gained significant interest as smart tissue-engineered scaffolds because of their impressive performance in regulating cell behavior (e.g., adhesion, migration, proliferation, and differentiation) and in promoting electrically responsive tissue repair and regeneration (e.g., myocardium, nerve, muscle, skin, and bone) [[68], [69], [70], [71], [72]]. Conductive composites are a combination of biodegradable polymers (e.g., PLA, PCL, and PU) and non-degradable conductive polymers (e.g., polyaniline, polypyrrole, and poly(3,4-ethylenedioxythiophene (PEDOT)), or a combination of biodegradable polymers and inorganic additives (e.g., metals, carbon nanotube, or graphene). The biodegradable polymers provide mechanical behavior, and the additives provide electrical conductivity [69,[73], [74], [75]]. Biodegradable PUs have great potential to be used as conductive composite matrices because of their tunable mechanical properties, elasticity, biodegradability, biocompatibility, and ease of processing into scaffolds for tissue-engineering applications [30,51,52]. Therefore, blends of biodegradable PUs and conductive additives have been investigated [[76], [77], [78], [79], [80]]. The conductivity of the electrospun composites of biodegradable PUs and carbon black increased nearly six orders of magnitude as the carbon black amount increased from 0% to 40%. This scaffold exhibited improved PC12 neural cell proliferation and inter-cellular communication and interaction [78]. A patterned conductive PU with gold or titanium coating promoted myogenic differentiation and maturation of skeletal cells (C2C12) because of the upregulation of the myogenic regulatory factors Myf5, MyoD, and myogenin (MyoG) [79]. Although it is exciting to see positive results in cell functions on conductive composites, some concerns should be considered for clinic use. Conductive additives with PUs without covalent bonding may lead to poor controllability in mechanics and conductivity because of the immiscibility of the two materials. The residual non-degradable conductive additives may cause chronic inflammation and infection and even implant failure.
Covalently incorporating conductive oligomers (e.g., aniline oligomers) into the PU backbone to achieve a conductive polymer with the desired degradable, electrical, and mechanical properties may address these concerns from the conductive composites [[81], [82], [83], [84], [85]]. Short conductive segments, such as aniline pentamer and aniline trimer, can be introduced as chain extenders into the PU backbone. However, it is necessary to add a dopant, camphor sulfonic acid (CSA) to achieve a conductive biodegradable PU [74,75]. The polymer conductivity is dependent on conductive segment types and amounts, as well as dopant amounts [82]. A linear conductive, biodegradable PU based on PCL, HDI, and aniline trimer with CSA dopant was fabricated [81]. The conductivity of the conductive PU increased from 1.8 ± 0.6 × 10−7 S/cm to 7.3 ± 1.5 × 10−5 S/cm in a wet state (24-h of PBS immersion) with an increasing amount of CSA dopant (CSA/aniline trimer = 0.5/1–1.5/1). Although the value was relatively low, it was sufficient to conduct electrical signals in vivo [86]. Regarding these two conductive PUs, the polymer conductivity for pentamer (longer) is higher than that for trimer (shorter). However, the addition of CSA dopant significantly increased the stiffness of PU, and it also deteriorated the electrical properties (e.g., conductivity and electrical stability) and cytotoxicity of PU because the dopant leaches out with time or charging. To address this concern, a new generation of conductive PUs was designed by covalently linking the soft segment (PCL), diisocyanate (HDI), the conductive segment (aniline trimer), and a doping molecule (dimethylol propionic acid (DMPA)) into one polymer chain (Fig. 4A) [87]. This PU does not require the addition of an extra dopant and is called a dopant-free conductive PU (DCPU). The DCPU presented enhanced conductivities from 4.4 ± 0.4 × 10−7 S/cm to 4.7 ± 0.8 × 10−3 S/cm in a wet state with increasing DMPA contents (10–30%), compared with the CSA-doped PU. It is important that the chemical structure of the DCPU can markedly improve the conductivity stability compared to the CSA-doped PU because of its limited dopant mobility. The DCPU also exhibited higher tensile strength and better elasticity than the CSA-doped PU. A porous DCPU scaffold fabricated by salt leaching exhibited good tissue compatibility in vivo with extensive cell infiltration over 4 weeks in a mouse subcutaneous implantation model.
3.2. Shape-memory polyurethanes
Shape-memory polymers (SMPs) are a group of smart adaptive materials and can recover their permanent shape from their temporary shape via exposure to stimuli. These stimuli include temperature [[88], [89], [90]], magnetic field [91,92], light [93,94], pH [95], solution [[96], [97], [98]], and ultrasound [99,100]. The potential of SMP has been explored for various biomedical applications, such as self-tightening sutures [89], cardiovascular stents [90,101], dialysis needle adapters [102], and thrombectomy devices for clot removal [103]. SMPs are attractive as tissue-engineered scaffolds because of their ability to allow for minimally invasive implantation and to adapt themselves to the native physiological environment to regulate cell behavior [78].
PU-based SMPs have been developed for tissue engineering because of their good biocompatibility and elasticity and tailorable transition temperatures (Tg and Tm) [104]. Most reported tissue-engineered scaffolds based on SMPs are triggered by a thermal stimulus, in which their shape recovery temperatures are near or slightly higher than body temperature [[105], [106], [107], [108]]. The shape memory function of the PU-based SMP is majorly attributed to soft segment alteration. A SMP foam based on a PU synthesized from PCL, hydroxyapatite, castor oil, and HDI was fabricated to induce bone regeneration [105]. The SMP foam was implanted into a rabbit femoral defect with a compact shape for a minimally invasive delivery. Subsequently, the compacted SMP foam self-matched the bone defect after thermal stimulation (40 °C saline). Fast bone ingrowth and neovascularization were observed at 12-weeks post-surgery. The PU shape transition temperature, which is mainly determined by Tg or Tm, can be tuned by changing the component and the block length of the soft segments and modifying the soft-to-hard segment ratio in the PU backbone [[109], [110], [111]]. In a PCL-based PU, after changing the PCL block length from 2000 to 10,000, the lowest shape transition temperature increased from 23.5 to 48.2 °C because of different PCL crystalline statuses (Fig. 4B) [109].
Recently, interests in water-induced shape-memory PUs has grown because their shape transition can be easily triggered through body fluid (mainly water) in humans [112]. The water-actuated shape-memory PUs are commonly synthesized through combining a PU matrix with hydrophilic particles, such as PU-cellulose nanocrystals [113,114], PU-clay particles [115], and PU-poly(vinyl alcohol) particles [116]. Their shape-memory behaviors are attributed to the formation and dissociation of the hydrophilic particle network in the PU matrix upon removal of and exposure to water, respectively [113].
A multi-responsive shape-memory PU is more attractive, and it can be developed through linking multiple stimuli components using diisocyanates [117], such as thermo-photo responsive shape-memory PUs and thermo-water responsive shape-memory PUs [[118], [119], [120]]. A thermo-water responsive PU was synthesized from HDI, PEG, PHA, and BDO [121]. The hydrophilic PEG and PHA components contributed to the water-triggered shape-memory response, and the melting and formation of soft segment crystalline resulted in a thermo-triggered shape-memory response.
3.3. Waterborne polyurethanes
Waterborne PU (WBPU) is a unique category of PUs that are dispersed in water. Compared with conventional PUs, their green- and water-based synthetic process avoids the possible toxicity resulting from residual organic solvents and residual isocyanates, thus making them more biocompatible [[122], [123], [124], [125]]. The WBPU is treated as a functional PU in this article because other types of PUs are generally solid and the WBPU is rarely used in tissue repair and regeneration. WBPUs are synthesized by introducing hydrophilic ionic groups, such as carboxylic acid or tertiary amine, into the backbone [125]. In terms of the types of these ionic groups, WBPUs can be categorized as cationic, anionic, or nonionic. N-methyl diethanolamine is the most used cationic component for the synthesis of cationic WBPUs [126,127]. Anionic WBPU usually contains hydrophilic anionic moieties with ionized carboxylic acid [128]. The nonionic WBPUs normally contain nonionic hydrophilic segments, such as PEG [129]. Because of the mild polymerization condition and high emulsifying capacity of anionic hydrophilic moieties, the anionic WBPU becomes a practical choice for biomedical application [122,123,126,130,131]. A family of anionic WBPUs was synthesized from soft segments of PCL and PEG, IPDI, and a chain extender of l-lysine with a carboxyl group [132,133], and they were processed into a porous scaffold through freeze-drying. Another anionic WBPU was synthesized from PCL, polyethylene butylene adipate (PEB) diol, IPDI, 2,2-bis(hydroxymethyl)propionic acid (DMPA), ethylenediamine, and triethylamine (TEA; neutralizer), which was then processed into a porous scaffold via freeze-drying or a particulate-leaching method (Fig. 4C) [122]. Its waterborne ability is attributed to PEB diol and DMPA. This scaffold induced the chondrogenic differentiation of human-bone-marrow-derived mesenchymal stem cells (MSCs) within 7 days after induction.
3.4. Peptide-based functional polyurethanes
Peptides have been explored for various biomedical applications because of their unique physical, chemical, and biological properties [134,135]. Thus, combining peptide with the synthetic polymers is an attractive approach for biofunctional polymers. Peptide-based PUs can be classified into two main categories: PUs surface-modified with peptides and PUs containing peptides in the backbone or the pendant peptides. Many groups have reported grafting an RGD peptide, a common peptide for cell attachment to the extracellular matrix (ECM), onto the PU surface to improve cell adhesion [[136], [137], [138]]. For example, an RGD peptide was first conjugated with a PEO-MDI-PEO copolymer via sulfonyl chloride activation, and then the RGD containing polymer was physically blended with the PU to promote human umbilical vein endothelial cell (HUVEC) growth [136].
Incorporation of peptides into a PU backbone can endow the PU with improved biocompatibility and biodegradability, and create a specific biological function, such as enzyme liability or enhanced cell attachment [67,[139], [140], [141], [142], [143], [144]]. A YIGSR peptide as a chain extender was introduced into a PU backbone to enhance the endothelialization of the PU as a small-diameter vascular graft [139]. l-tyrosine-based and lysine-based chain extenders have been introduced into the PU backbone to promote polymer biocompatibility and biodegradability [64,140,141]. An Ala-Ala-Lys (AAK) peptide, which can be specifically cleaved by elastase, was used as a chain extender for PU synthesis to improve the polymer's enzymatic degradation (Fig. 4D) [67]. Furthermore, a collagenase-sensitive peptide, GGGLGPAGGK-NH2, was introduced into the PU backbone as a chain extender to obtain a collagenase labile biodegradable polymer for wound healing [145]. A dipeptide of Gly–Leu linkage is the cleavage site of various matrix metalloproteinases (MMPs), and a Gly–Leu-based chain extender was introduced into a PU to support mouse embryonic fibroblasts growth [146]. Considering above peptide-containing PUs, their functions mainly focus on degradation control and cell affinity. It is essential to expand peptide use in the PU synthesis because the peptide family has many unique biofunctions, not limited to the degradation sensitivity and cell compatibility improvement.
3.5. Antibacterial polyurethanes
A bacterial infection significantly impedes wound healing, results in disfiguration, and can be life threatening [147]. Antibacterial wound dressings can inhibit bacterial growth within the wound and on the dressing itself. PU is a promising wound dressing material because of its effective barrier properties and oxygen permeability [148,149]. Various antibacterial agents, such as silver [[150], [151], [152], [153]], gold [154], ZnO [155], quaternary ammonium salts [156], curcumin [157], N-halamine [158], chitosan [159] and antibiotics [160,161], can improve the antibacterial efficiency of PU wound dressings. These antibacterial agents can be physically blended with the PU or covalently anchored onto or conjugated with the PU.
Physical blending is an easy way to achieve the antibacterial function. An antibacterial composite scaffold of PU and nano-gold was fabricated through salt leaching [154], which showed a 99% inhibition against both S. epidermidis and Klebsiella spp. A blend of PU and mupirocin (Mu), a commonly used antibiotic for wound care, were co-electrospun into a patch, and this patch demonstrated effective antibacterial activity against Staphylococcus aureus [160]. However, the released antibacterial agents may kill healthy cells exposed in the wounded area. Furthermore, with the leaching of these agents, the material will lose antibacterial function, and the bacteria may become resistant to the diluted antibacterial agents [162,163].
To address these concerns, covalent incorporation of antibacterial agents, such as cationic components, into PU is an effective approach [[164], [165], [166], [167], [168]]. An epoxy-terminated PU prepolymer (EPU) with a soft segment of antibacterial castor oil was synthesized first, and then a mixture of EPU, a reactive bactericidal agent of glycidyltriethylammonium chloride (GTEAC), and BDA were co-cured to form a PU membrane [164]. The obtained PU membrane with 50% GTEAC demonstrated effective antibacterial activity with acceptable cell compatibility. Additionally, a family of WBPUs with gemini quaternary ammonium salt (GQAS) also showed promising antibacterial function [[166], [167], [168]]. Chitin based PUs with a chain extender curcumin also exhibited potential against selected strains of bacteria [169]. Lysine-derivative GQAS chain extenders were incorporated with PCL, PEG, IPDI and lysine to form antibacterial waterborne biodegradable PUs (Fig. 4E) [167]. Covalently introducing cationic and hydrophobic amino acids into a PU can prevent surface attachment of both Gram-positive and Gram-negative bacteria at subinhibitory concentrations and disrupt their biofilm formation without toxicity to mammalian cells [170]. In another approach, chitooligosaccharide (COS), a low-molecular-weight chitosan with antibacterial properties, was grafted onto the PU surface through the self-polymerization of dopamine [165]. The antibacterial activity of the PU membrane against Escherichia coli and Staphylococcus aureus significantly increased. However, these cationic PUs may not be good for cell growth and may induce relatively high inflammation in vivo because of its high positive charge. It is necessary to seek a balance between charge intensity for antibacterial function and cell/tissue compatibility for tissue repair.
It is notable that directly conjugating antibacterial drug into the polyurethane structure is feasible for antibacterial function [171,172]. Ciprofloxacin, a fluoroquinolone antibiotic was covalently conjugated into a polyurethane as a chain extender with 1,12-dodecane diisocyanate (DDI) and PCL (MW = 2000). The enzyme-degraded polyurethane demonstrated antibacterial activity against P. aeruginosa and E. coli [171].
3.6. Non-thrombogenic polyurethanes
Biodegradable PUs have been investigated for their use in blood-contact devices because of their attractive mechanical properties, and good biocompatibility and processability. However, common PUs easily trigger material-induced thrombosis. To enhance their nonthrombogenicity for blood contact implant use, nonthrombogenic moieties were physically or chemically combined with biodegradable PUs, such as phosphorylcholine (PC) [[173], [174], [175]], sulfobetaine [176,177], sulfate [[178], [179], [180]], PEG [181], and antithrombogenic drugs (e.g., clopidogrel, aspirin, and dipyridamole) [182]. In addition to physical blending and surface modification by nonthrombogenic moieties [175,177], these nonthrombogenic moieties can be covalently incorporated into the PU backbone or grafted onto the polymer chain as pendants. For example, biodegradable PU with pendant carboxyl groups were first synthesized by DMPA incorporation, and then PC or sulfate groups were conjugated with these pendant carboxyl groups [174,178]. The PC- or sulfate-containing PUs had effective thromboresistance in vitro. Directly incorporating those moieties into a PU backbone is also an effective way to amplify the capacity of grafted nonthrombogenic moieties. A sulfobetaine diol was introduced into the PU backbone to achieve a nonthrombogenic polymer for cardiovascular applications (Fig. 4F) [176].
Nitric oxide (NO) release also improves blood compatibility of the PU, and a NO donor can mix with the PU or can be conjugated with a PU chain [[183], [184], [185]]. NO is a natural mediator of vascular homeostasis [186]. The NO donors, N-diazeniumdiolates and S-nitrosothiols, have been used to release NO [183]. Because the leach-out and/or degradation of NO donors may cause the potential toxicity, covalently conjugating NO donors with a PU chain is a better method [183]. A nonthrombogenic polyurethane with a chain extender of a diazeniumdiolate NO donor, a lysine peptide, inhibited platelet deposition and promoted endothelial cell proliferation [187].
3.7. Others
In addition to the aforementioned PUs, other functional PUs are also interesting and attractive. The degradation rate of PUs is expected to be tunable to match the rate of new tissue growth through biological response. Poly(thioketal)urethane (PTK-UR) was synthesized by introducing thioketal bonds into the PU chain, and its degradation accelerated with a specific cell-generated reactive oxygen species (ROS) [[188], [189], [190]]. Additionally, our group developed reduction-sensitive polyurethanes containing disulfide bonds (PU-SS) that can be selectively degraded through the natural compound, glutathione (GSH) (Fig. 4G) [31]. The degradation of the PU-SS scaffold sped up on demand with the addition of GSH. Additionally, bio-adhesive PU is worth mentioning here. The chemical strategy is to synthesize the PU in the form of a prepolymer with free isocyanate end-groups, which can react with the amino groups in the biological molecules [191,192]. Another strategy is to incorporate adhesive moieties into the PU backbone. Xylose can provide four hydroxyl groups and a formyl-functional group. It reacted with 4,4-methylenebis(cyclohexyl isocyanate) (MCI) and PEG (MW = 200) to form an adhesive PU, which helped wound closure [193].
In short, the functions of the biodegradable PUs are generally gifted from functional chain extender introduction. Soft segments of biodegradable polyesters (e.g., PCL) and hydrophilic polymers (e.g., PEG) are often used to manipulate mechanical properties, degradation, and hydrophilicity, and they are hard to be replaced using a new polymer because of their recognized biosafety. The diisocyanate option is limited to aliphatic diisocyanates, such as HDI, LDI, and BDI, in terms of biosafety. Thus, their libraries are very limited compared with small molecular chain extenders. There is a huge library for chain extenders from organic diols and diamines to peptides, and it is easy to find a safe and functional molecule as a functional chain extender. Thus, utilizing or synthesizing a new chain extender is still a highly effective and efficient way to develop functional PUs.
4. Biodegradable thermoplastic polyurethane scaffolds for tissue repair
4.1. Polyurethane scaffold fabrication methods
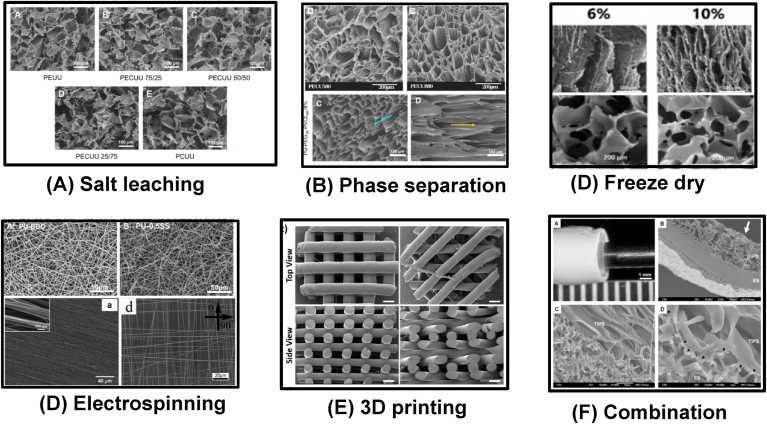
Biodegradable thermoplastic PUs can dissolve in organic solvents. This advantage allows the PUs to be processed into scaffolds by applying various techniques, such as particle leaching [52,87,194,195], thermally induced phase separation (TIPS) [49,51,196,197], freeze-drying [122,130,131,133,198,199], electrospinning [31,[200], [201], [202]], 3D printing [[203], [204], [205], [206], [207]], and their combinations [208,209]. Because of high hydrogen binding, the organic solvents for these biodegradable polyurethanes are polarized solvents, such as dimethyl sulfoxide (DMSO), dimethylformamide (DMF), and hexafluoro-2-propanol (HFIP). Six methods of PU scaffold fabrication are briefly introduced as below. (1) The particle leach is a common way to fabricate a porous PU scaffold with uniform pore size distribution (Fig. 5A). The particle is generally salt crystal and sugar. The PU solution is firstly blended with particles, and after solvent evaporation, the particles are removed through immersion in water. The pore size is easily tuned by altering the particle size, and the porosity can be tailored by changing the polymer/particle ratio. (2) The TIPS technique utilizes the changes of polymer solubility in the solvent with temperature to induce phase separation (polymer-rich phase and solvent-rich phase) and generate pores (solvent-rich phase). For example, the PU/DMSO solution at 80 °C was frozen at −80 °C to induce phase separation, and the frozen product was immersed in ethanol or water at 4 °C to remove the DMSO [196]. After freeze-drying, a porous PU scaffold can be obtained (Fig. 5B top). The pore size can be controlled by altering polymer type and concentration, temperature, coalescence time, and solvents. The polymer concentration and temperature are the most effective to control pore sizes. Because the pore formation of the TIPS scaffold is influenced by heat transition, the pore size distribution may not be uniform, and a dense outer layer may appear. Controlling heat transition from one direction can produce a porous polyurethane scaffold with aligned pores (Fig. 5B bottom) [197]. (3) Freeze-drying is not often used for PU scaffold fabrication. The WBPUs in water can be directly freeze-dried and form a porous structure [122,131,133,198,199,210]. The pore size can be tuned through altering PU concentration and temperature (Fig. 5C) [198,210], and the pore alignment also can be controlled by heat transition (Fig. 5C) [210]. (4) Three-dimension (3D) printing is an advanced manufacturing approach. Because PUs are difficult to melt, extrusion printing of biodegradable PUs is rarely reported. Recently, printable biodegradable PUs synthesized from a soft segment of a random copolymer of PLA and PCL (50/50) and a hard segment of BDI-BDO-BDI-BDO-BDI (Fig. 5D) [206,207], and WBPUs [205,211,212] exhibited promise in 3D extrusion printing. (5) Electrospinning is a popular technique to generate microscale and nanoscale fibrous scaffolds. The linear thermoplastic PU is easily dissolved in highly evaporated HFIP, thus HFIP is often used for electrospinning. The fiber diameter can be tuned by altering the polymer concentration, infuse rate, charge, distance between tips and collector, and other. Random fibers are easily achieved (Fig. 5E top). Using a high-speed rotating collector or altering the electric field of the collector can achieve aligned fibers to generate an anisotropic matrix (Fig. 5E bottom left) [213,214], and orthogonally aligned fibers (Fig. 5E bottom right) [214]. (6) The combination of multiple fabrication methods is an effective and attractive approach to generating a scaffold with new architecture and to improving properties in mechanics and functions. For example, a combination of electrospinning and TIPS was used to generate a bilayer tubular scaffold, which combined the mechanical enhancement from electrospinning and the cell loading from large porous TIPS scaffold (Fig. 5F) [208]. The selection of a fabrication technique is based on the requirement in structures and properties from the specific tissue repair of the scaffolds.
Fig. 5.
Typical morphologies of biodegradable thermoplastic polyurethane scaffolds fabricated by various methods. (A) Salt leaching. Reprinted with permission from [52]. Copyright 2010 Elsevier B.V (B) Phase separation. Top: random pores. Reprinted with permission from [196]. Copyright 2005 Elsevier B.V.; Bottom: aligned pores Reprinted with permission from [197]. Copyright 2020 American Chemical Society. (C) Freeze drying. The aligned (top) and random (bottom) scaffolds were prepared using WBPU emulsion by freeze-drying at different concentrations. Reprinted with permission from [210]. Copyright 2019 Oxford University Press. (D) Electrospinning. Top: random fibers. Reprinted with permission from [31]. Copyright 2015 American Chemical Society. Bottom left. Aligned fibers. Reprinted with permission from [213]. Copyright 2012 Elsevier B.V. Bottom right: orthogonally aligned fibers. Reprinted with permission from [214]. Copyright 2015 Wiley. (E) 3D printing. Melt extrusion printing. Reprinted with permission from [206]. Copyright 2020 Elsevier B.V. (F) Combination. A bilayer scaffold from phase separation and electrospinning. Reprinted with permission from [208]. Copyright 2010 Elsevier B.V.
4.2. Polyurethane scaffolds for cardiovascular repair
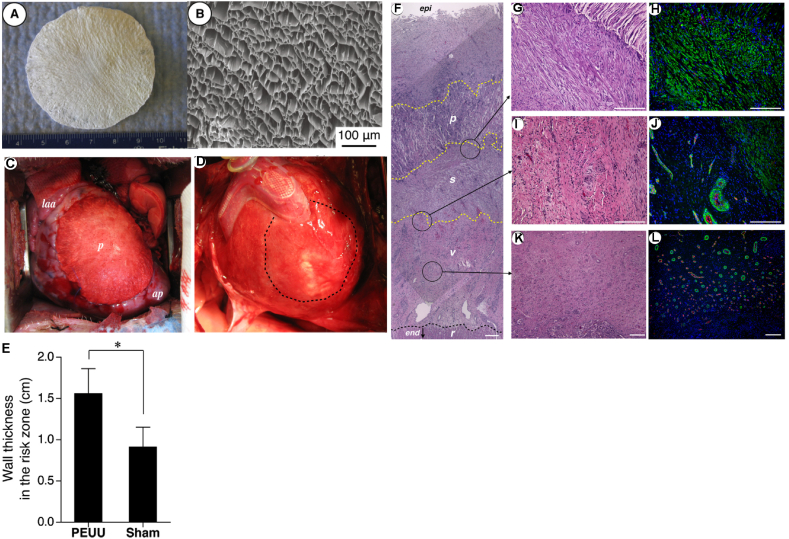
Biodegradable PUs are attractive for cardiovascular regeneration because they have good biocompatibility, and also have strong mechanical properties and high elasticity mimicking with the mechanical behaviors of heart muscle and blood vessels [[215], [216], [217]]. Biodegradable PU cardiac patches have been implanted into myocardial infarct (MI) animal models (e.g., mice, rat, and porcine) for heart repair and function restoration [[218], [219], [220], [221], [222]]. Biodegradable PUs synthesized from BDI, PCL, and BDA was fabricated into porous scaffolds by TIPS, and the scaffold was placed on a surgical defect in the right ventricular outflow tract of adult rats [222]. The PU patch allowed host fibroblast penetration and ingrowth, endocardial endothelialization, and minimal inflammation, indicating its potential for infarct heart repair. Furthermore, in a 2-week postinfarction rat model, the PU porous patches increased infarcted heart left ventricular wall thickness and smooth muscle bundles with mature contractile phenotype were observed in the infarcted area [218]. In addition (Fig. 6), a porcine MI model was used to further validate the biodegradable PU (based on BDI, PCL, and BDA) cardiac patch by observing its prevention of left ventricle dilation, preservation of contractile ability, and improvement of the retention of wall thickness of the infarcted left ventricle wall [221].
Fig. 6.
Biodegradable polyurethane cardiac patch implanted in a porcine MI model. Digital (A) and SEM (B) images of biodegradable polyurethane cardiac patch. (C) The polyurethane patched left ventricle wall (n = 7) was significantly thicker than the sham surgery wall (n = 8). *p < 0.01. (F) Hematoxylin and eosin staining and immunostaining for a-smooth muscle actin (αSMA) and CD31. The polyurethane patched wall exhibited an aSMA rich layer (s) beneath the implanted PEUU patch (p). Below the αSMA rich layer was a vascular rich layer (v) and then a myocardial remnant (r) region at the endocardial side. A higher magnification of the boundary area between the polyurethane patch and αSMA rich layer showed that the PEUU partially degraded and cellular infiltration occurred with αSMA-positive cells (G and H). (I and J) are the junction between αSMA and vascular rich layers, and (K and L) are the center of the vascular rich layer. Reprinted with permission from [221]. Copyright 2013 American Association for Thoracic Surgery.
Conductive PUs have recently become attractive options for use as cardiac patches because native cardiac muscle is an electroactive tissue that can transfer electrical signals and allows the heart to beat [223,224]. Conductive materials may support the electrical signal transfer process and improve cardiac patch contractibility. Electroactive PUs based on PCL, PEG, IPDI, and aniline pentamer were processed into porous scaffolds using a particulate leaching method [225,226]. The obtained scaffold had a conductivity at 1 ± 0.09 × 10−5 S/cm and supported neonatal cardiomyocyte adhesion and growth. Additionally, higher expressions of the cardiac genes related to muscle contraction, relaxation, and cytoskeleton alignment were observed on the electroactive PU scaffold than those on a PCL film or scaffold and a tissue culture plate.
Vascular graft is another major application of biodegradable PUs in the cardiovascular system [227,228]. Vectra® graft (Thoratec Corp, the United States) is a poly(ether urethane) urea vascular graft that obtained clearance from the United States Food and Drug Administration (FDA) in 2000 [227]. In regard to an ideal small diameter vascular graft (inner diameter <6 mm), anti-thrombosis, intimal hyperplasia inhibition, and rapid and adequate endothelialization on a graft lumen are expected. Many approaches have been used to reduce thrombogenicity and improve the intimal hyperplasia inhibition of biodegradable PUs based small diameter vascular grafts, such as surface/compositional modification with nonthrombogenic moieties [[173], [174], [175]], NO release [186,187], drug release [229,230], and endothelialization [231,232]. PUs modified with nonthrombogenic moieties such as PC, sulfate, PEG, and sulfobetaine or NO donors, are good candidates for small-diameter vascular grafts. Hong's group mixed a clinically used antithrombogenic drug dipyridamole (DPA) with a biodegradable PU, and then electrospun it into a fibrous tubular scaffold [229]. The DPA-loaded PU scaffold reduced human platelet deposition, inhibited proliferation of human aortic smooth muscle cells, and improved endothelial cell proliferation. To improve endothelialization, heparin and vascular endothelial growth factor (vEGF) were immobilized on a PU electrospun fibrous scaffold via self-polymerization and the deposition of polydopamine [231]. The result demonstrated that surface heparinization significantly inhibited platelet deposition, and vEGF immobilization markedly increased endothelialization. Additionally, stem cells, such as muscle derived stem cells and human pericytes, can be loaded into biodegradable PU based tubular bilayer scaffolds (inner layer: porous scaffold, and outer layer: electrospun fibers) as cellularized vascular grafts and have exhibited great patency and tissue remodeling in a rat aorta model [233,234]. Particularly, human pericyte loaded PU vascular grafts exhibited 100% patency and similar components with native artery after 8 week implantation in a rat aorta replacement model [233]. Another interesting work is that the biodegradable PU was electrospun simultaneously with electrosprayed smooth muscle cells to generate a cellularized vascular graft [235].
In recent years, scaffold mechanical matches with native cardiovascular tissues have gained attention because a mechanical mismatch between an implanted scaffold and a native tissue may trigger high foreign body reactions or even implant failure [236,237]. A bilayer PU scaffold was fabricated as a small diameter vascular graft with a highly porous inner layer, using a TIPS technique to allow cell penetration, and a fibrous-reinforcing outer layer by electrospinning to provide mechanical support [208]. Both layers were made of PU synthesized from PCL diol, BDI, and BDA. The resulting bilayer PU scaffold possessed mechanical properties (compliance = 4.6 ± 0.5 × 10−4 mmHg−1, β stiffness = ∼20, elastic modulus = 1.4 ± 0.4 MPa) comparable to those of healthy human coronary arteries (compliance = 14.1 ± 5.9 × 10−4 mmHg−1, β stiffness = 16.9 ± 7.1, elastic modulus = 1.4 ± 0.7 MPa) [238]. Additionally, the scaffolds were able to load muscle-derived stem cells prior to implantation in an animal model. Our group also developed relatively low-initial-modulus biodegradable PUs by varying the block length and hydrophilicity of the soft segments (PVCL or PVCL-PEG-PVCL) in the PU backbone [51]. A porous scaffold made of an optimal PU fabricated by TIPS exhibited an initial modulus (0.60 ± 0.14 MPa) similar to that of the human myocardium (0.02–0.50 MPa) [239], demonstrating its potential as a tissue-engineered cardiac patch for heart repair.
To further improve the bioactivity of PU scaffolds, natural materials, such as collagen, elastin, or elastin-like peptides (ELP), laminin, fibrin, and decellularized ECM, can be combined with PU to form a synthetic/natural material composite for cardiovascular repair and regeneration [200,217,[240], [241], [242], [243]]. A poly(carbonate urethane) synthesized from PHC, HDI, and BDO was electrospun into a fibrous scaffold [240]. ELP-4 was grafted onto the fibrous scaffold surface to enhance vascular smooth muscle cell adhesion. A PU synthesized from BDI, PCL, PHC, and BDA was co-electrospun with decellularized porcine heart ECM (hECM) into a bi-layered cardiac patch, which was implanted in a rat MI model [243]. Compared to the pure polyurethane group, this hECM-incorporated PU cardiac patch improved cardiac remodeling and function with reduced scar tissue formation and left ventricle global mechanical compliance and increased left ventricle wall thickness and vascularization.
PU is one of the most common materials used in generating tissue engineered valvular scaffold because of its good biocompatibility and high elasticity to endure repeated loading and unloading cycles. In terms of soft segment types, PUs for cardiac valve use can be categorized into three groups: poly(ether urethane), poly(ester urethane), and poly(carbonate urethane). Some commercial polyether-based PUs such as Biomer® [244], Estane® [245], and Angioflex® [246], exhibited high durability for heart valve use. However, those poly(ether urethane)s are hard to degrade with potential cracking failure [247]. Poly(ester urethane)s are biodegradable in vivo and highly elastic [248]. A biodegradable polyurethane from a soft segment of PCL-PEG-PCL triblock diol was electrospun and then combined with a PEG based hydrogel to form a composite scaffold for aortic valve tissue engineering [249]. There existed concerns about fast hydrolysis in vivo. Poly(carbonate urethane)s possess lower degradation rate in vivo compared with other biodegradable polyurethanes [52,250,251]. Mitral and aortic prosthesis made from poly(carbonate urethane)s (from Adiam Life Science, Erkelenz, Germany) exhibited high durability in vitro (up to 20 years) and advanced in vivo durability and hemodynamics but they had mild (mitral) or moderate (aortic) calcifications [252]. Wagner's group has developed a series of poly(ester urethane)s (PCL diol, BDI, and putrescine), poly(carbonate urethane)s (PHC diol, BDI and putrescine) and poly(ester carbonate) urethane (PCL/PHC diols (50/50), BDI and putrescine) and electrospun them into fibrous scaffolds with tunable degradation and mechanical properties for heart valve replacement [202,253,254]. Particularly, tricuspid valves made from the biodegradable poly(carbonate urethane) showed good acute functions in a swine model after 24 h implantation [255].
4.3. Polyurethane scaffolds for musculoskeletal applications
Several factors are important in designing skeletal-muscle-tissue-engineered scaffolds. First, scaffold stiffness plays an important role in skeletal muscle regeneration. Generally, PU scaffolds with moduli similar to healthy muscle tissue (∼12 kPa) are optimal for skeletal muscle cell growth and differentiation [[256], [257], [258]]. However, Andriani et al. hypothesized that a biomaterial with modulus in the range of tendons and bones (hundreds of MPa to several GPa) is acceptable to be used as human muscle cell culture substrates [259]. A synthesized PU with tendon-like surface modulus ranging from 150 MPa to 2.4 GPa supported the long-term in vitro culture of human myoblasts (proliferation, differentiation, and sustenance beyond 35 days). PU scaffolds with electroactivity promoted myoblast adhesion, proliferation, and differentiation [82,83,260]. Additionally, microscale and nano-scale topography of the scaffold has a major influence on skeletal muscle cell behaviors. The skeletal muscle structure is highly organized and consists of long, parallel bundles of multinucleated myotubes that formed through the differentiation and fusion of myoblast satellite cells [261]. A highly oriented fibrous PU scaffold produced by electrospinning enabled skeletal myogenesis in vitro by aiding in myoblast adhesion, myotube alignment, and noncoplanar arrangement of cells [262]. Micropatterned polyurethane films with microchannels via ultra-violet micro-embossing showed that the dimensions of the channel and wall significantly affected skeletal muscle cell elongation [263].
Biodegradable PU scaffolds were also used to manage meniscus defects in the clinic [[264], [265], [266], [267]]. A biodegradable PU scaffold implant was used for a treatment of painful irreparable partial meniscal defects with a minimum 5-year follow-up [265]. The PU meniscal implant improved knee joint function and reduced pain. However, the chondroprotective ability of the implant was questionable, and a relatively high implantation failure rate (∼40%) was noticed during the follow-up period. Schüttler et al. and Monllau et al. confirmed a functional outcome of biodegradable PU meniscal scaffolds with a 4-year and 5-year follow-up, respectively [266,267]. In Monllau's study, PU scaffold resorption and the incomplete in-growth of new meniscus-like tissue was observed [267]. Recently, the first clinical report of PU meniscal scaffolds with MSCs was published [268]. Seventeen patients (18–50 years old) with past meniscectomies were separated into two groups: acellular PU scaffold and MSC-enriched PU scaffold. After 12 months of implantation, the acellular PU scaffold maintained normal T2 mapping values in the adjacent cartilage, while the addition of MSCs did not show additional clinical benefit in articular cartilage protection.
Biodegradable PU scaffolds also show great potential for bone regeneration because of their high elasticity, enhanced calcium phosphate crystal formation (calcification) in vivo, and effective processability [269]. It was reported that scaffold hydrophilicity significantly affected calcification [270]. A poly((R)-3-hydroxybutyrate) (PHB)-based PU with improved hydrophilicity, achieved by incorporating a hydrophilic PEG segment into the soft segments, showed enhanced mineralization capability [61]. Gogolewski et al. found similar results, in which the newly formed bone in the PU scaffold with higher hydrophilicity had more bone mineral than for the scaffolds with less hydrophilicity [271]. Another way to promote calcification is the incorporation of carbon nanotubes into scaffolds to induce the nucleation of hydroxyapatite [272]. A PU foam with a surface deposition of carbon nanotubes accelerated the precipitation of calcium phosphate [273].
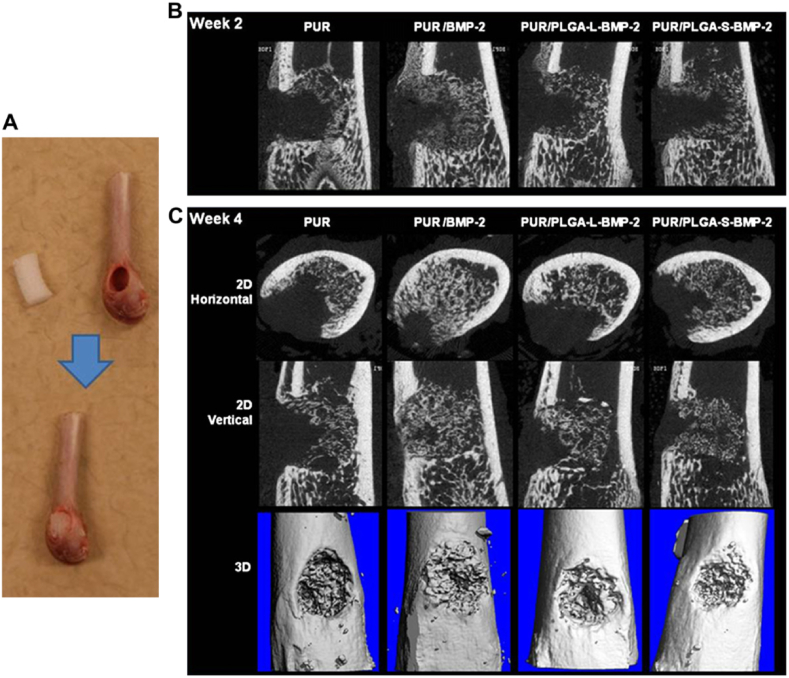
To further enhance the bioactivity of a PU scaffold for bone regeneration, bioactive materials such as native ECM [274], bioactive ceramics [275,276], and growth factors [277] can be incorporated with the PU. An electrospun PU/hydroxyapatite scaffold enhanced the viability of osteoblasts and human embryonic mesenchymal progenitor cells, calcification, and collagen deposition [275]. Recombinant human bone morphogenetic protein-2 (rhBMP-2), an osteoinductive growth factor, was physically combined with a PU scaffold (Fig. 7), and it supported bone ingrowth after 2 weeks implantation into a rat femoral plug defect [277]. New bone formation in the PU scaffold incorporating rhBMP-2 powder was more than that in the scaffold incorporating rhBMP-2 loaded PLGA microspheres at 4 weeks. Besides the above physical combination, an amino-terminated bis(l-arginine) alkylene diester extender could be covalently incorporated into a PU to promote the adhesion and proliferation of human dental pulp stem cells [278].
Fig. 7.
In vivo evaluation of the effects of polyurethane scaffold encapsulating rhBMP-2 on bone reparation in a rat femoral plug model. Four treatment groups included: PUR control (no rhBMP-2), PUR/rhBMP-2 (no PLGA microspheres), PUR/PLGA-L-rhBMP-2 (rhBMP-2 released from large PLGA microspheres), and PUR/PLGA-S-rhBMP-2 (rhBMP-2 released from small PLGA microspheres). The PUR cylinders (5 mm*3 mm) were implanted into rat femoral plug defects (A) and harvested for mCT imaging at week 2 (B) and 4 (C), respectively. All rhBMP-2 treated groups showed significantly higher new bone formation than the control (PUR) (p < 0.05). Reprinted with permission from [277]. Copyright 2009 Elsevier. Ltd.
The biodegradable PU scaffolds are also used for cartilage repair, although there are limited number of reports. A biodegradable PU from a blend of PCL/PHB (50/50) and lysine methyl ester diisocyanate supported the growth of rat chondrocytes [279]. The porous scaffolds of a Esynthesized from PCL, HDI and isosorbide diol (1,4:3,6-dianhydro-d-sorbitol) was seeded with articular chondrocytes isolated from the fetlock joints of young calves of 3–4 months of age, and up to a 42-day culture, and the scaffold supported the production of extracellular matrix proteins [280]. Bovine chondrocytes on a porous biodegradable PU membrane showed a greater rate of matrix production than on a PLA membrane within the first 10 days [281]. Furthermore, acellular biodegradable PU foam matrixes (Novosorb™, Polynovo Biomaterials Pty Ltd, Port Melbourne, Victoria, Australia) were implanted into pig ears. After 28 days, the matrix showed great integration with the auricular defect [282]. However, the PU itself was not bioactive and did not facilitate cell growth. Natural bioactive materials, such as fibrin and cellulose, were combined with the PU scaffold to support chondrogenesis of mesenchymal stem cells (MSCs) under mechanical stimuli [283,284]. It is notable that WBPUs were recently used for cartilage regeneration using 3D printing technology [205,211,212]. A water dispersed biodegradable PU was synthesized from a mixed soft segment of PCL diol (Mn = 2000) and polyethylene butylene adipate diol (Mn = 2000), IPDI, and two chain extenders of 2,2-bis(hydroxymethyl) propionic acid (DMPA) and ethylenediamine (EDA) (IPDI/oligodiols/DMPA/EDA molar ratio = 3.52:1:1:1.52) [211]. The printed scaffold was generated from a blend of the PU and hyaluronan combining with TGF-beta3 or Y27632 using a low-temperature fused deposition manufacturing (LFDM) system. The MSC-seeded PU/HA/Y27632 scaffold was implanted into a rabbit chondral defects and promoted GAG and collagen II produce at the defected area. Additionally, melt-extrusion printing was used to generate printed scaffolds using a thermoplastic PU [206,207]. The utilization of a soft segment of a random copolymer of PLA and PCL (50/50) and a hard segment of BDI-BDO-BDI-BDO-BDI significantly reduced the hydrogen bonding, which allowed the PU to melt at an appropriate temperature for extrusion printing. A teratocarcinoma derived chondrogenic cell line (ATDC5) was seeded in the printed scaffold and cultured in vitro [206]. The formed neotissue contained a high content of GAG and collagen II rich ECM with a stable chondrogenic cell phenotype.
4.4. Polyurethane scaffolds for neural applications
Biodegradable PUs can be used as nerve conduits because of its excellent elasticity and flexibility [285,286]. A conduit made from a biodegradable PU (HDI, PCL, and dianhydro-D-sorbitol) was coated with fibrin, and implanted into a 8-mm rat sciatic nerve defect model [287]. No significant differences in nerve function between the PU conduit and autologous nerve graft were observed 12 weeks after the implantation. A Y-shaped conduit from a PU (HDI, PCL, and BDA) was used for the molecular guidance cues that mediated motor and sensory neuron regeneration [288]. The common arm was filled with type I/III collagen, and two “Y” compartments were filled with collagen and neurotrophic factors loaded PLGA microspheres. In in vitro dorsal root ganglion (DRG) cultures, Y compartments filled with glial cell line-derived neurotrophic factor (GDNF) and nerve growth factor (NGF) increased the motor and sensory axon content, respectively. The Y-shaped conduit was implanted into a rat 2 mm transected sciatic nerve defect. The sensory-to-motor ratio of the regenerated axons was significantly higher in the pleiotrophin compartment of the Y-conduit when compared with a BDNF + GDNF compartment.
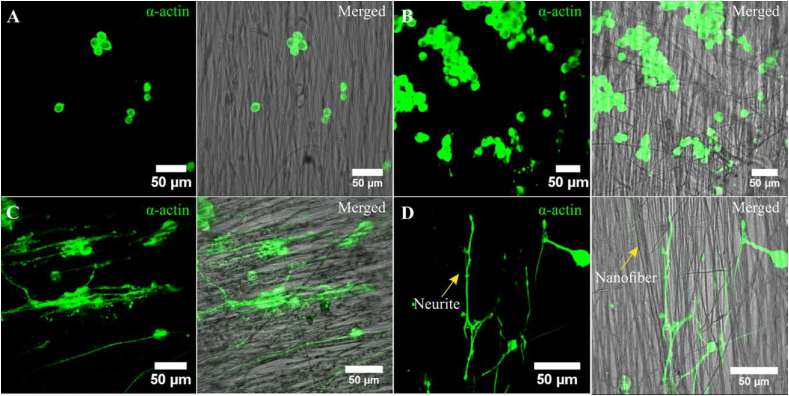
Conductive PU scaffolds have gained great attention for neural tissue engineering because neurons are electrically active cells whose activity originates from the depolarization of the plasma membrane, and scaffold conductivity may support this process [[289], [290], [291], [292]]. Aligned PU nanofibers were fabricated via electrospinning that was infused with gold nanoparticles [293]. Rat pheochromocytoma (PC-12) cells were seeded onto the PU nanofibers. It was found that the incorporation of gold nanoparticles enhanced PC-12 attachment and proliferation. Additionally, by including NGF and electrical stimulation, increased neurite outgrowth and elongation were observed (Fig. 8). Additionally, a conductive composite was prepared by a mixture of PU, poly(3,4-ethylenedioxythiophene) (PEDOT)/poly(4-styrenesulfonate) (PSS) and liquid crystal graphene oxide (LCGO). This composite supported human neural stem cell (NSC) growth and differentiation into neurons as well as neuroglia [294]. Electrical stimulation further enhanced NSC differentiation on the conductive composite.
Fig. 8.
PC12 cells growth on (A) polyurethane nanofibers; (B) gold nanoparticle decorated polyurethane nanofibers; (C) gold nanoparticle decorated aligned polyurethane nanofibers; (D) gold nanoparticle decorated aligned PU nanofibers after NGF and electrical stimulation. The neurite elongation of PC-12 cells was significantly promoted on gold nanoparticle decorated aligned PU nanofibers with the synergistic effect of NGF and electrical stimulation. Reprinted with permission from [293]. Copyright 2018 Wiley Periodicals, INC.
The waterborne biodegradable PU-based scaffolds are also attractive for neural regeneration. A series of biodegradable WBPU hydrogels based on IPDI, oligodiols (e.g., PCL, PLLA, PDLLA), DMPA and ethylenediamine were synthesized for neural regeneration [131,203]. Emulsion of WBPUs based on a blended soft segment of PCL and PLGA was freeze-dried to form porous scaffolds, which showed anti-inflammatory function when cultured with BV2 microglia cells and also promoted PC12 differentiation into neural cells [199]. Another WBPU scaffold showed great expression of the neuronal growth associated protein (GAP43) and synaptophysin, and satisfactory functional recovery in the rat traumatic brain injury model [295].
4.5. Polyurethane scaffolds for wound healing
Biodegradable PUs are promising candidates for wound healing because of their effective barrier properties and oxygen permeability [148]. However, the antibacterial activity and hydrophilicity of PUs need improvement to prevent bacterial infection and enhance cell affinity, respectively [296]. To enhance antibacterial activity, various antibacterial agents (e.g., silver, ZnO, gold, quaternary ammonium salts, curcumin, and antibiotics) can be combined with PUs. A PU scaffold incorporated with a hydrophobic vancomycin, a tricyclic glycopeptide antibiotic, significantly reduced infection in a rat femoral segmental defect model [297]. In another case, a polyurethane with soybean oil as a soft segment showed better progress of wound healing in a rat wound model compared to a non-antimicrobial dressing or a cotton gauze [298]. To improve the hydrophilicity of antibacterial polyurethanes, hydrophilic moieties such as dextran [155,299], cellulose acetate (CA) [296], and COS [165] are incorporated into PUs. PU, dextran, and antibiotic ciprofloxacin were mixed and electrospun into a fibrous scaffold [299]. The composite scaffold presented effective bacterial activity against both gram-positive and gram-negative bacteria and favorable cell-material interaction. Similar results were observed in the development of an antibacterial electrospun scaffold from a combination of PU, highly hydrophilic CA, and an antibacterial protein, zein [296].
Antioxidant moieties could also be incorporated with PUs to protect the tissue from oxidative stress during the wound healing process. Overproduction of ROS, a hallmark of inflammation and the pathogenesis of various diseases, causes oxidative stress, which may lead to tissue damage, infection, and chronic wound healing [300,301]. Ascorbic acid, an antioxidant moiety, was introduced as a chain extender into the PU backbone, and the resulting antioxidant PU scavenged free radicals and protected cardiomyocytes from oxidative stress-induced cell death [302]. Additionally, conductive PUs were also investigated for wound healing because conductive polymers can scavenge free radicals [303]. An antioxidant PU/siloxane dressing was generated based on castor oil as an antibacterial segment and aniline trimer as a conductive segment [304]. The obtained PU/siloxane dressing membrane showed effective antimicrobial activity and antioxidant efficiency, and it supported fibroblast growth. In a rat model of skin wound healing, the multifunctional dressing promoted wound contraction and collagen deposition, and enhanced vascularization in the wounded area (Fig. 9).
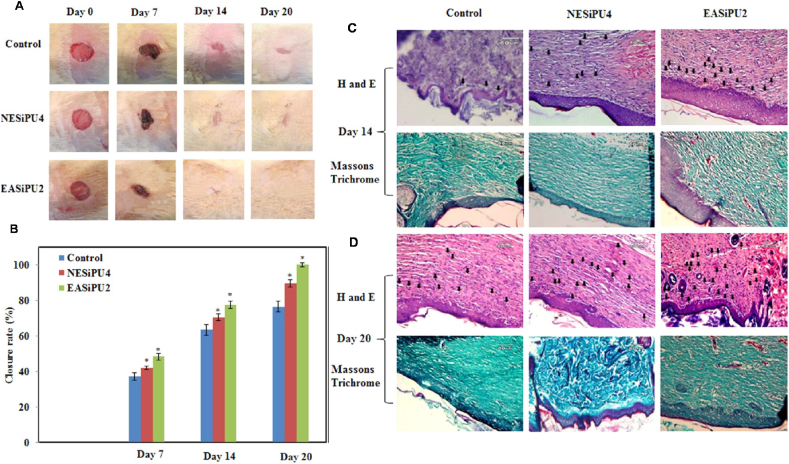
Fig. 9.
In vivo evaluation of effects of polyurethane/siloxane dressing on wound healing in a rat skin wound model. Two treatment groups include: NESiPU4 (no aniline trimer, nonconductive), and EASiPU2 (containing aniline trimer, conductive). Photographs (A) and closure rate (B) of wounds treated with gauze (control), NESiPU4, and EASiPU2 during the wound healing process for 20 days. *p < 0.05. H&E and Masson's Trichrome staining at day 14 (C) and day 20 (D). Scale bars: 60 μm. Arrows indicate capillaries. The results suggested that the electroactive wound dressing could promote fast wound healing by complete re-epithelialization of the wound, enhanced vascularization, and collagen deposition. Reprinted with permission from [304]. Copyright 2015 American Chemical Society.
5. Conclusions and perspectives
Although numerous in vivo and in vitro studies have proved the biocompatibility of biodegradable PUs, there are only a few commercial biodegradable PU products (e.g., Actifit®, Lacthane®, Artelon®, DegraPol®, and Tecoflex®) used in pharmacy or medicine. The Actifit® meniscal implant (Orteq Ltd., the United Kingdom), approved by Conformitè Europeänne (CE), is a biodegradable porous scaffold from a PU synthesized from BDI, PCL, and BDO [305]. Lacthane® (Polyganics, Netherlands), which is synthesized from BDI, polyesters, and polyethers, is applied as a wound and nasal dressing and surgical sealant [306]. Artelon® (Artimplant, Sweden) is a CE- and FDA-approved, biodegradable PU porous scaffold based on MDI, PCL, and 1.3-butanediamine for tendon and ligament reconstruction [307]. Currently, only a few biodegradable PU scaffolds have gained FDA approval in the United States. For many promising biodegradable PU products, there still exists much work prior to FDA approval. Specifically, it is important to obtain substantial evidence of the product's safety and effectiveness in vitro and in vivo. Compared with the common biodegradable polyesters, such as PLA, PCL, and PLGA, it is necessary to pay attention to fundamental research on in vitro cell biology and in vivo toxicology and immune response, and rational design and synthetic routine of biodegradable PUs.
In addition to the linear thermoplastic structure discussed in this review article, cross-linked thermoset structure cannot be neglected for biodegradable functional PU design. These biodegradable cross-linked polyurethanes contain at least one block with more than two functionalities, such as polyisocyanate, polyols, and chain extenders [21]. The cross-linked PUs are also easily integrated with desired biofunctions using appropriate functional components. Our group developed a nonthrombogenic biodegradable PUs from HDI, PCL, and dipyridamole (DPA, an antithrombogenic drug with four hydroxyl groups) via a one-pot/one-step synthesis [308]. A series of crosslinked conductive PUs from PGS and aniline pentamer are also interesting and have been used for neural cell culture [82,85]. Thus, the PU design and synthesis strategy can be flexibly utilized in terms of the required characteristics for the specific applications.
Compared with the mono-functional PUs, interest in multifunctional biodegradable PUs through a combination of multiple functional components or moieties has increased. The conductivity and shape-memory functions were combined to form electroactive shape-memory PUs [78,84,111,309]. A multifunctional PU synthesized from PCL, aniline trimer, and HDI [111] had a tunable shape transition temperature of 31–44 °C from PCL and a conductivity from aniline trimer in the polymer backbone. This polyurethane, compared with an insulated PCL, enhanced C2C12 cell proliferation, myotube formation, and relative myogenic differentiation gene expression. Even triple functions can be involved. An elastomeric poly(citric acid-co-polycaprolactone) based PU with bioactive dopamine and electroactive aniline hexamer exhibited functions of shape memory, conductivity, and bioactivity for C2C12 muscle cells although this PU is a cross-linked structure [84]. These reports can inspire novel PU designs with multiple functions through a combination of approaches, which can meet complex needs in tissue repair and regeneration.
Three-dimensional (3D) printing has become a common technique for fabricating scaffolds and devices with high structural complexity and precision for tissue engineering applications [310]. It is extremely important to design printable biodegradable PUs to meet the urgent needs of the scaffolds with regular, complex, or biomimetic structures. Because a PU has a high melting temperature due to high hydrogen bonding, it is difficult to print using the common melt extrusion printing technique, although some non-degradable PUs has been printed using melt extrusion [311,312]. As a solution, the WBPU may be a good option for printing. Hsu group developed a series of printable water-dispersible WBPUs [130,[203], [204], [205],211,212,313]. In their report [204], a WBPU based on PCL, PDLLA, IPDI, DMPA, and ethylenediamine was used for extrusion-based 3D bioprinting. It can be combined with gelatin to form a double network hydrogel utilizing chelation and thermal gelation [205]. On the other hand, new chemical structure of the biodegradable polyurethane is helpful. Recently, the PU from a random copolymer of PLA and PCL (50/50) and BDI-BDO-BDI-BDO-BDI was melted and extrusion-printed [206,207]. These cases provide evidence that it is achievable to develop printable, biodegradable PUs through molecular design.
Recent studies of designing biomaterials with “immunomodulating” capability is worthy to be noted [314]. Common strategies in the design of immunomodulatory biomaterials include alteration of material properties, incorporation of bioactive molecules, and introduction of natural macromolecules [314,315]. A recent study investigated the impact of polyurethane surface roughness on its immune response. The surface roughness in the micrometer range on polyurethane had no effect on the pro-inflammatory immune response [316]. Santerre group developed an immunomodulatory polar-hydrophobic-ionic (D-PHI) polyurethane from LDI, PHC, HEMA, MMA and MAA [[317], [318], [319]]. Its immunomodulating capability is reflected by reducing the exposure of the Fab region of immunoglobulin G (IgG) which is a strong supporter of long-term macrophage adhesion, thus attenuating pro-inflammatory monocyte activation [320]. Nanoparticles made from carboxyl-functionalized waterborne polyurethane (IPDI, PCL diol, DMPA and EDA) can induce autophagy activation and immune suppression in macrophages by transporting Ca2+ from culture medium to macrophages [321]. The strategy of combining polyurethane with natural materials, silk fibroin or chitosan, has also been reported to promote diabetic burn wound healing through immunomodulation [322,323]. Surface coating of poly-d-lysine on unsaturated PUs also effectively induced M2 polarization in vivo and resulted in a thin tissue encapsulation [324]. With these promising results, the new PU with immunomodulating function will be a new focus and a hot point in the future.
In conclusion, these biodegradable thermoplastic PUs showed tunable biological, mechanical, and physio-chemical properties through chemical design to meet the requirements of tissue repair. They can also be manufactured into a variety of scaffolds for tissue repair and regeneration. Through molecular design and novel application inspiration, these biodegradable PU-based implants are highly promising to be translated from bench to bedside.
Conflict of interest
Authors declare no conflict of interest.
Acknowledgements
We gratefully acknowledge the partial financial support from the American Heart Association (Beginning Grant-in-Aid, 14BGIA20510066, Y.H.), the National Science Foundation (Faculty Career Development (CAREER) award, #1554835 Y.H.), and the National Institutes of Health (R01HD097330, R21HD090680 and R15HL140503, Y.H.) in the United States of America.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Burdick J.A., Mauck R.L. 1 st ed. Springer-Verlag/Wien; 2011. Biomaterials for Tissue Engineering Applications: a Review of the Past and Future Trends. [Google Scholar]
- 2.O'Brien F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today. 2011;14(3):88–95. [Google Scholar]
- 3.Yang S., Leong K.F., Du Z., Chua C.K. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001;7(6):679–689. doi: 10.1089/107632701753337645. [DOI] [PubMed] [Google Scholar]
- 4.Cooper S.L., Guan J. Advances in Polyurethane Biomaterials. Elsevier; 2016. [Google Scholar]
- 5.Lamba N.M., Woodhouse K.A., Cooper S.L. CRC press; 1998. Polyurethanes in Biomedical Applications. [Google Scholar]
- 6.Zhang C., Wen X., Vyavahare N.R., Boland T. Synthesis and characterization of biodegradable elastomeric polyurethane scaffolds fabricated by the inkjet technique. Biomaterials. 2008;29(28):3781–3791. doi: 10.1016/j.biomaterials.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Pavlova M., Draganova M. Biocompatible and biodegradable polyurethane polymers. Biomaterials. 1993;14(13):1024–1029. doi: 10.1016/0142-9612(93)90196-9. [DOI] [PubMed] [Google Scholar]
- 8.Gunatillake P.A., Adhikari R. in: G. P. Felton (ed.), Biodegradable Polymers: Processing, Degradation and Applications. Nova Science Publsihers; Hauppauge, New York: 2011. Chapter 9: Biodegradable Polyurethanes: Design, Synthesis, Properties and Potential Applications; pp. 431–470. [Google Scholar]
- 9.Gentile P., Chiono V., Carmagnola I., Hatton P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014;15(3):3640–3659. doi: 10.3390/ijms15033640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta B., Revagade N., Hilborn J. Poly(lactic acid) fiber: an overview. Prog. Polym. Sci. 2007;32(4):455–482. [Google Scholar]
- 11.Labet M., Thielemans W. Synthesis of polycaprolactone: a review. Chem. Soc. Rev. 2009;38(12):3484–3504. doi: 10.1039/b820162p. [DOI] [PubMed] [Google Scholar]
- 12.Pan J., Li G., Chen Z., Chen X., Zhu W., Xu K. Alternative block polyurethanes based on poly(3-hydroxybutyrate-co-4-hydroxybutyrate) and poly(ethylene glycol) Biomaterials. 2009;30(16):2975–2984. doi: 10.1016/j.biomaterials.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Bassas-Galia M., Gonzalez A., Micaux F., Gaillard V., Piantini U., Schintke S., Zinn M., Mathieu M. Chemical modification of polyhydroxyalkanoates (PHAs) for the preparation of hybrid biomaterials. Chimia. 2015;69(10):627–630. doi: 10.2533/chimia.2015.627. [DOI] [PubMed] [Google Scholar]
- 14.Jeong S.I., Kim S.H., Kim Y.H., Jung Y., Kwon J.H., Kim B.S., Lee Y.M. Manufacture of elastic biodegradable PLCL scaffolds for mechano-active vascular tissue engineering. J. Biomater. Sci. Polym. Ed. 2004;15(5):645–660. doi: 10.1163/156856204323046906. [DOI] [PubMed] [Google Scholar]
- 15.Pego A.P., Poot A.A., Grijpma D.W., Feijen J. Physical properties of high molecular weight 1,3-trimethylene carbonate and D,L-lactide copolymers. J. Mater. Sci. Mater. Med. 2003;14(9):767–773. doi: 10.1023/a:1025084304766. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y., Ameer G.A., Sheppard B.J., Langer R. A tough biodegradable elastomer. Nat. Biotechnol. 2002;20(6):602–606. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- 17.Yang J., Webb A.R., Ameer G.A. Novel citric acid‐based biodegradable elastomers for tissue engineering. Adv. Mater. 2004;16(6):511–516. [Google Scholar]
- 18.Callister W.D., Rethwisch D.G. John Wiley & Sons; New York: 2007. Materials Science and Engineering: an Introduction. [Google Scholar]
- 19.Li Y., Thouas G.A., Chen Q.-Z. Biodegradable soft elastomers: synthesis/properties of materials and fabrication of scaffolds. RSC Adv. 2012;2(22):8229–8242. [Google Scholar]
- 20.Chu B., Gao T., Li Y., Wang J., Desper C.R., Byrne C.A. Microphase separation kinetics in segmented polyurethanes: effects of soft segment length and structure. Macromolecules. 1992;25(21):5724–5729. [Google Scholar]
- 21.Guelcher S.A. Biodegradable polyurethanes: synthesis and applications in regenerative medicine. Tissue Eng. B Rev. 2008;14(1):3–17. doi: 10.1089/teb.2007.0133. [DOI] [PubMed] [Google Scholar]
- 22.Sobczak M. Biodegradable polyurethane elastomers for biomedical applications – synthesis methods and properties. Polym. Plast. Technol. Eng. 2015;54(2):155–172. [Google Scholar]
- 23.Krol P. Synthesis methods, chemical structures and phase structures of linear polyurethanes. Properties and applications of linear polyurethanes in polyurethane elastomers, copolymers and ionomers. Prog. Mater. Sci. 2007;52(6):915–1015. [Google Scholar]
- 24.Zhou L., Yu L., Ding M., Li J., Tan H., Wang Z., Fu Q. Synthesis and characterization of pH-sensitive biodegradable polyurethane for potential drug delivery applications. Macromolecules. 2011;44(4):857–864. [Google Scholar]
- 25.Thomas S., Datta J., Haponiuk J., Reghunadhan A. Polyurethane Polymers: Composites and Nanocomposites. Elsevier; 2017. [Google Scholar]
- 26.Zhang C., Zhang N., Wen X. Improving the elasticity and cytophilicity of biodegradable polyurethane by changing chain extender. J. Biomed. Mater. Res. B Appl. Biomater. 2006;79(2):335–344. doi: 10.1002/jbm.b.30547. [DOI] [PubMed] [Google Scholar]
- 27.Panwiriyarat W., Tanrattanakul V., Pilard J.-F., Pasetto P., Khaokong C. Effect of the diisocyanate structure and the molecular weight of diols on bio-based polyurethanes. J. Appl. Polym. Sci. 2013;130(1):453–462. [Google Scholar]
- 28.Ratner B.D., Hoffman A.S., Schoen F.J., Lemons J.E. Elsevier; 2004. Biomaterials Science: an Introduction to Materials in Medicine. [Google Scholar]
- 29.Martina M., Hutmacher D.W. Biodegradable polymers applied in tissue engineering research: a review. Polym. Int. 2007;56(2):145–157. [Google Scholar]
- 30.Ma Z., Hong Y., Nelson D.M., Pichamuthu J.E., Leeson C.E., Wagner W.R. Biodegradable polyurethane ureas with variable polyester or polycarbonate soft segments: effects of crystallinity, molecular weight, and composition on mechanical properties. Biomacromolecules. 2011;12(9):3265–3274. doi: 10.1021/bm2007218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu C., Huang Y., Wu J., Tang L., Hong Y. Triggerable degradation of polyurethanes for tissue engineering applications. ACS Appl. Mater. Interfaces. 2015;7(36):20377–20388. doi: 10.1021/acsami.5b06242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hettrich W., Becker R. New isocyanates from amino acids. Polymer. 1997;38(10):2437–2445. [Google Scholar]
- 33.Guan J., Sacks M.S., Beckman E.J., Wagner W.R. Synthesis, characterization, and cytocompatibility of elastomeric, biodegradable poly(ester-urethane)ureas based on poly(caprolactone) and putrescine. J. Biomed. Mater. Res. 2002;61(3):493–503. doi: 10.1002/jbm.10204. [DOI] [PubMed] [Google Scholar]
- 34.De Groot J., De Vrijer R., Wildeboer B., Spaans C., Pennings A. New biomedical polyurethane ureas with high tear strengths. Polym. Bull. 1997;38(2):211–218. [Google Scholar]
- 35.Barikani M., Honarkar H., Barikani M. Synthesis and characterization of polyurethane elastomers based on chitosan and poly(ε-caprolactone) J. Appl. Polym. Sci. 2009;112(5):3157–3165. [Google Scholar]
- 36.Chan-Chan L.H., Vargas-Coronado R.F., Cervantes-Uc J.M., Cauich-Rodriguez J.V., Rath R., Phelps E.A., Garcia A.J., San Roman Del Barrio J., Parra J., Merhi Y., Tabrizian M. Platelet adhesion and human umbilical vein endothelial cell cytocompatibility of biodegradable segmented polyurethanes prepared with 4,4'-methylene bis(cyclohexyl isocyanate), poly(caprolactone) diol and butanediol or dithioerythritol as chain extenders. J. Biomater. Appl. 2013;28(2):270–277. doi: 10.1177/0885328212448259. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J.Y., Beckman E.J., Hu J., Yang G.G., Agarwal S., Hollinger J.O. Synthesis, biodegradability, and biocompatibility of lysine diisocyanate-glucose polymers. Tissue Eng. 2002;8(5):771–785. doi: 10.1089/10763270260424132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hassan M.K., Mauritz K.A., Storey R.F., Wiggins J.S. Biodegradable aliphatic thermoplastic polyurethane based on poly(ϵ-caprolactone) andL-lysine diisocyanate. J. Polym. Sci. Part A Polym Chem. 2006;44(9):2990–3000. [Google Scholar]
- 39.Takahara A., Hadano M., Yamaguchi T., Otsuka H., Kidoaki S., Matsuda T., Aoi K., Sasaki S. 2005 International Conference on Advanced Fibers and Polymer Materials, ICAFPM 2005. China Press; 2005. Biodegradation behavior of segmented polyurethanes prepared from amino acid-based diisocyanate. [Google Scholar]
- 40.Sawhney A.S., Hubbell J.A. Rapidly degraded terpolymers of dl-lactide, glycolide, and epsilon-caprolactone with increased hydrophilicity by copolymerization with polyethers. J. Biomed. Mater. Res. 1990;24(10):1397–1411. doi: 10.1002/jbm.820241011. [DOI] [PubMed] [Google Scholar]
- 41.Woodruff M.A., Hutmacher D.W. The return of a forgotten polymer—polycaprolactone in the 21st century. Prog. Polym. Sci. 2010;35(10):1217–1256. [Google Scholar]
- 42.Heijkants R.G., van Calck R.V., van Tienen T.G., de Groot J.H., Buma P., Pennings A.J., Veth R.P., Schouten A.J. Uncatalyzed synthesis, thermal and mechanical properties of polyurethanes based on poly(epsilon-caprolactone) and 1,4-butane diisocyanate with uniform hard segment. Biomaterials. 2005;26(20):4219–4228. doi: 10.1016/j.biomaterials.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Ou W., Qiu H., Chen Z., Xu K. Biodegradable block poly(ester-urethane)s based on poly(3-hydroxybutyrate-co-4-hydroxybutyrate) copolymers. Biomaterials. 2011;32(12):3178–3188. doi: 10.1016/j.biomaterials.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 44.Cohn D., Salomon A.H. Designing biodegradable multiblock PCL/PLA thermoplastic elastomers. Biomaterials. 2005;26(15):2297–2305. doi: 10.1016/j.biomaterials.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 45.Peponi L., Navarro-Baena I., Sonseca A., Gimenez E., Marcos-Fernandez A., Kenny J.M. Synthesis and characterization of PCL–PLLA polyurethane with shape memory behavior. Eur. Polym. J. 2013;49(4):893–903. [Google Scholar]
- 46.Korley L.T.J., Pate B.D., Thomas E.L., Hammond P.T. Effect of the degree of soft and hard segment ordering on the morphology and mechanical behavior of semicrystalline segmented polyurethanes. Polymer. 2006;47(9):3073–3082. [Google Scholar]
- 47.Wang F., Li Z., Lannutti J.L., Wagner W.R., Synthesis J. Guan. Characterization and surface modification of low moduli poly(ether carbonate urethane)ureas for soft tissue engineering. Acta Biomater. 2009;5(8):2901–2912. doi: 10.1016/j.actbio.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 48.Salehi M., Naseri-Nosar M., Ebrahimi-Barough S., Nourani M., Khojasteh A., Farzamfar S., Mansouri K., Ai J. Polyurethane/gelatin nanofibrils neural guidance conduit containing platelet-rich plasma and melatonin for transplantation of Schwann cells. Cell. Mol. Neurobiol. 2018;38(3):703–713. doi: 10.1007/s10571-017-0535-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guan J., Sacks M.S., Beckman E.J., Wagner W.R. Biodegradable poly(ether ester urethane)urea elastomers based on poly(ether ester) triblock copolymers and putrescine: synthesis, characterization and cytocompatibility. Biomaterials. 2004;25(1):85–96. doi: 10.1016/s0142-9612(03)00476-9. [DOI] [PubMed] [Google Scholar]
- 50.Cohn D., Stern T., Gonzalez M.F. J. Epstein. Biodegradable poly(ethylene oxide)/poly(epsilon-caprolactone) multiblock copolymers. J. Biomed. Mater. Res. 2002;59(2):273–281. doi: 10.1002/jbm.1242. [DOI] [PubMed] [Google Scholar]
- 51.Xu C., Huang Y., Tang L., Hong Y. Low-initial-modulus biodegradable polyurethane elastomers for soft tissue regeneration. ACS Appl. Mater. Interfaces. 2017;9(3):2169–2180. doi: 10.1021/acsami.6b15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hong Y., Guan J., Fujimoto K.L., Hashizume R., Pelinescu A.L., Wagner W.R. Tailoring the degradation kinetics of poly(ester carbonate urethane)urea thermoplastic elastomers for tissue engineering scaffolds. Biomaterials. 2010;31(15):4249–4258. doi: 10.1016/j.biomaterials.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kloczkowski A. Application of statistical mechanics to the analysis of various physical properties of elastomeric networks: a review. Polymer. 2002;43(4):1503–1525. [Google Scholar]
- 54.Christenson E.M., Dadsetan M., Wiggins M., Anderson J.M., Hiltner A. Poly(carbonate urethane) and poly(ether urethane) biodegradation: in vivo studies. J. Biomed. Mater. Res. 2004;69(3):407–416. doi: 10.1002/jbm.a.30002. [DOI] [PubMed] [Google Scholar]
- 55.Christenson E.M., Patel S., Anderson J.M., Hiltner A. Enzymatic degradation of poly(ether urethane) and poly(carbonate urethane) by cholesterol esterase. Biomaterials. 2006;27(21):3920–3926. doi: 10.1016/j.biomaterials.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 56.Ashton J.H., Mertz J.A., Harper J.L., Slepian M.J., Mills J.L., McGrath D.V., Vande Geest J.P. Polymeric endoaortic paving: mechanical, thermoforming, and degradation properties of polycaprolactone/polyurethane blends for cardiovascular applications. Acta Biomater. 2011;7(1):287–294. doi: 10.1016/j.actbio.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Z., Kuijer R., Bulstra S.K., Grijpma D.W., Feijen J. The in vivo and in vitro degradation behavior of poly(trimethylene carbonate) Biomaterials. 2006;27(9):1741–1748. doi: 10.1016/j.biomaterials.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 58.Tokiwa Y., Suzuki T. Hydrolysis of polyesters by lipases. Nature. 1977;270(5632):76–78. doi: 10.1038/270076a0. [DOI] [PubMed] [Google Scholar]
- 59.Kim Y.D., Kim S.C. Effect of chemical structure on the biodegradation of polyurethanes under composting conditions. Polym. Degrad. Stabil. 1998;62(2):343–352. [Google Scholar]
- 60.Pringle J.H., Fletcher M. Influence of substratum wettability on attachment of freshwater bacteria to solid surfaces. Appl. Environ. Microbiol. 1983;45(3):811–817. doi: 10.1128/aem.45.3.811-817.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu K.L., Choo E.S., Wong S.Y., Li X., He C.B., Wang J., Li J. Designing poly[(R)-3-hydroxybutyrate]-based polyurethane block copolymers for electrospun nanofiber scaffolds with improved mechanical properties and enhanced mineralization capability. J. Phys. Chem. B. 2010;114(22):7489–7498. doi: 10.1021/jp1018247. [DOI] [PubMed] [Google Scholar]
- 62.Loh X.J., Tan K.K., Li X., Li J. The in vitro hydrolysis of poly(ester urethane)s consisting of poly[(R)-3-hydroxybutyrate] and poly(ethylene glycol) Biomaterials. 2006;27(9):1841–1850. doi: 10.1016/j.biomaterials.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 63.Chao H., Tian N. Cray Valley USA, LLC Exton; Pennsylvania USA, PA 19341: 1998. Progress in Chain Extender Evaluation for Polyurethanes Derived from Hydroxyl-Terminated Polybutadiene Resins. [Google Scholar]
- 64.Guelcher S.A., Gallagher K.M., Didier J.E., Klinedinst D.B., Doctor J.S., Goldstein A.S., Wilkes G.L., Beckman E.J., Hollinger J.O. Synthesis of biocompatible segmented polyurethanes from aliphatic diisocyanates and diurea diol chain extenders. Acta Biomater. 2005;1(4):471–484. doi: 10.1016/j.actbio.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 65.Tatai L., Moore T.G., Adhikari R., Malherbe F., Jayasekara R., Griffiths I., Gunatillake P.A. Thermoplastic biodegradable polyurethanes: the effect of chain extender structure on properties and in-vitro degradation. Biomaterials. 2007;28(36):5407–5417. doi: 10.1016/j.biomaterials.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 66.Dahiyat B.I., Posadas E.M., Hirosue S., Hostin E., Leong K.W. Degradable biomaterials with elastomeric characteristics and drug-carrier function. React. Polym. 1995;25(2–3):101–109. [Google Scholar]
- 67.Guan J., Wagner W.R. Synthesis, characterization and cytocompatibility of polyurethaneurea elastomers with designed elastase sensitivity. Biomacromolecules. 2005;6(5):2833–2842. doi: 10.1021/bm0503322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guimard N.K., Gomez N., Schmidt C.E. Conducting polymers in biomedical engineering. Prog. Polym. Sci. 2007;32(8–9):876–921. [Google Scholar]
- 69.Dvir T., Timko B.P., Brigham M.D., Naik S.R., Karajanagi S.S., Levy O., Jin H., Parker K.K., Langer R., Kohane D.S. Nanowired three-dimensional cardiac patches. Nat. Nanotechnol. 2011;6(11):720–725. doi: 10.1038/nnano.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hirata E., Uo M., Takita H., Akasaka T., Watari F., Yokoyama A. Multiwalled carbon nanotube-coating of 3D collagen scaffolds for bone tissue engineering. Carbon. 2011;49(10):3284–3291. [Google Scholar]
- 71.Durgam H., Sapp S., Deister C., Khaing Z., Chang E., Luebben S., Schmidt C.E. Novel degradable co-polymers of polypyrrole support cell proliferation and enhance neurite out-growth with electrical stimulation. J. Biomater. Sci. Polym. Ed. 2010;21(10):1265–1282. doi: 10.1163/092050609X12481751806330. [DOI] [PubMed] [Google Scholar]
- 72.Chen M.C., Sun Y.C., Chen Y.H. Electrically conductive nanofibers with highly oriented structures and their potential application in skeletal muscle tissue engineering. Acta Biomater. 2013;9(3):5562–5572. doi: 10.1016/j.actbio.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 73.Jiang S., Zhang H., Song S., Ma Y., Li J., Lee G.H., Han Q., Liu J. Highly stretchable conductive fibers from few-walled carbon nanotubes coated on poly(m-phenylene isophthalamide) polymer core/shell structures. ACS Nano. 2015;9(10):10252–10257. doi: 10.1021/acsnano.5b04185. [DOI] [PubMed] [Google Scholar]
- 74.Li P., Sun K., Ouyang J. Stretchable and conductive polymer films prepared by solution blending. ACS Appl. Mater. Interfaces. 2015;7(33):18415–18423. doi: 10.1021/acsami.5b04492. [DOI] [PubMed] [Google Scholar]
- 75.Ma R., Kang B., Cho S., Choi M., Baik S. Extraordinarily high conductivity of stretchable fibers of polyurethane and silver nanoflowers. ACS Nano. 2015;9(11):10876–10886. doi: 10.1021/acsnano.5b03864. [DOI] [PubMed] [Google Scholar]
- 76.Sirivisoot S., Harrison B.S. Skeletal myotube formation enhanced by electrospun polyurethane carbon nanotube scaffolds. Int. J. Nanomed. 2011;6:2483–2497. doi: 10.2147/IJN.S24073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seil J.T., Webster T.J. Decreased astroglial cell adhesion and proliferation on zinc oxide nanoparticle polyurethane composites. Int. J. Nanomed. 2008;3(4):523–531. doi: 10.2147/ijn.s4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kai D., Tan M.J., Prabhakaran M.P., Chan B.Q.Y., Liow S.S., Ramakrishna S., Loh X.J. Biocompatible electrically conductive nanofibers from inorganic-organic shape memory polymers. Colloids Surf. B Biointerfaces. 2016;148:557–565. doi: 10.1016/j.colsurfb.2016.09.035. [DOI] [PubMed] [Google Scholar]
- 79.Yang H.S., Lee B., Tsui J.H., Macadangdang J., Jang S.Y., Im S.G., Kim D.H. Electroconductive nanopatterned substrates for enhanced myogenic differentiation and maturation. Adv Healthc Mater. 2016;5(1):137–145. doi: 10.1002/adhm.201500003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Javadi M., Gu Q., Naficy S., Farajikhah S., Crook J.M., Wallace G.G., Beirne S., Moulton S.E. Conductive tough hydrogel for bioapplications. Macromol. Biosci. 2018;18(2):1700270. doi: 10.1002/mabi.201700270. [DOI] [PubMed] [Google Scholar]
- 81.Xu C., Yepez G., Wei Z., Liu F., Bugarin A., Hong Y. Synthesis and characterization of conductive, biodegradable, elastomeric polyurethanes for biomedical applications. J. Biomed. Mater. Res. 2016;104(9):2305–2314. doi: 10.1002/jbm.a.35765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu Y., Wang L., Guo B., Shao Y., Ma P.X. Electroactive biodegradable polyurethane significantly enhanced Schwann cells myelin gene expression and neurotrophin secretion for peripheral nerve tissue engineering. Biomaterials. 2016;87:18–31. doi: 10.1016/j.biomaterials.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 83.Chen J., Dong R., Ge J., Guo B., Ma P.X. Biocompatible, biodegradable, and electroactive polyurethane-urea elastomers with tunable hydrophilicity for skeletal muscle tissue engineering. ACS Appl. Mater. Interfaces. 2015;7(51):28273–28285. doi: 10.1021/acsami.5b10829. [DOI] [PubMed] [Google Scholar]
- 84.Zhao X., Dong R., Guo B., Ma P.X. Dopamine-incorporated dual bioactive electroactive shape memory polyurethane elastomers with physiological shape recovery temperature, high stretchability, and enhanced C2C12 myogenic differentiation. ACS Appl. Mater. Interfaces. 2017;9(35):29595–29611. doi: 10.1021/acsami.7b10583. [DOI] [PubMed] [Google Scholar]
- 85.Wu Y., Wang L., Hu T., Ma P.X., Guo B. Conductive micropatterned polyurethane films as tissue engineering scaffolds for Schwann cells and PC12 cells. J. Colloid Interface Sci. 2018;518:252–262. doi: 10.1016/j.jcis.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 86.Niple J., Daigle J., Zaffanella L., Sullivan T., Kavet R. A portable meter for measuring low frequency currents in the human body. Bioelectromagnetics. 2004;25(5):369–373. doi: 10.1002/bem.20000. [DOI] [PubMed] [Google Scholar]
- 87.Xu C., Huang Y., Yepez G., Wei Z., Liu F., Bugarin A., Tang L., Hong Y. Development of dopant-free conductive bioelastomers. Sci. Rep. 2016;6:34451. doi: 10.1038/srep34451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bao M., Lou X., Zhou Q., Dong W., Yuan H., Zhang Y. Electrospun biomimetic fibrous scaffold from shape memory polymer of PDLLA-co-TMC for bone tissue engineering. ACS Appl. Mater. Interfaces. 2014;6(4):2611–2621. doi: 10.1021/am405101k. [DOI] [PubMed] [Google Scholar]
- 89.Lendlein A., Langer R. Biodegradable, elastic shape-memory polymers for potential biomedical applications. Science. 2002;296(5573):1673–1676. doi: 10.1126/science.1066102. [DOI] [PubMed] [Google Scholar]
- 90.Yakacki C.M., Shandas R., Lanning C., Rech B., Eckstein A., Gall K. Unconstrained recovery characterization of shape-memory polymer networks for cardiovascular applications. Biomaterials. 2007;28(14):2255–2263. doi: 10.1016/j.biomaterials.2007.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schmidt A.M. Electromagnetic activation of shape memory polymer networks containing magnetic nanoparticles. Macromol. Rapid Commun. 2006;27(14):1168–1172. [Google Scholar]
- 92.Szabo D., Szeghy G., Zrinyi M. Shape transition of magnetic field sensitive polymer gels. Macromolecules. 1998;31(19):6541–6548. [Google Scholar]
- 93.Ji S., Fan F., Sun C., Yu Y., Xu H. Visible light-induced plasticity of shape memory polymers. ACS Appl. Mater. Interfaces. 2017;9(38):33169–33175. doi: 10.1021/acsami.7b11188. [DOI] [PubMed] [Google Scholar]
- 94.Lendlein A., Jiang H., Junger O., Langer R. Light-induced shape-memory polymers. Nature. 2005;434(7035):879–882. doi: 10.1038/nature03496. [DOI] [PubMed] [Google Scholar]
- 95.Han X.J., Dong Z.Q., Fan M.M., Liu Y., li J.H., Wang Y.F., Yuan Q.J., Li B.J., Zhang S. pH-induced shape-memory polymers. Macromol. Rapid Commun. 2012;33(12):1055–1060. doi: 10.1002/marc.201200153. [DOI] [PubMed] [Google Scholar]
- 96.Wu G., Gu Y., Hou X., Li R., Ke H., Xiao X. Hybrid nanocomposites of cellulose/carbon-nanotubes/polyurethane with rapidly water sensitive shape memory effect and strain sensing performance. Polymers. 2019;11(10):1586. doi: 10.3390/polym11101586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lv H., Leng J., Liu Y., Du S. Shape‐memory polymer in response to solution. Adv. Eng. Mater. 2008;10(6):592–595. [Google Scholar]
- 98.Chen M.-C., Tsai H.-W., Chang Y., Lai W.-Y., Mi F.-L., Liu C.-T., Wong H.-S., Sung H.-W. Rapidly self-expandable polymeric stents with a shape-memory property. Biomacromolecules. 2007;8(9):2774–2780. doi: 10.1021/bm7004615. [DOI] [PubMed] [Google Scholar]
- 99.Bao M., Zhou Q., Dong W., Lou X., Zhang Y. Ultrasound-modulated shape memory and payload release effects in a biodegradable cylindrical rod made of chitosan-functionalized PLGA microspheres. Biomacromolecules. 2013;14(6):1971–1979. doi: 10.1021/bm4003464. [DOI] [PubMed] [Google Scholar]
- 100.Bao M., Tu H., Zhou Q., Dong W., Zhang Y. Ultrasound-mediated release of lysozyme from biodegradable shape-memory polymeric rods prepared from microspheres. J. Contr. Release. 2013;172(1):e110–e111. [Google Scholar]
- 101.Venkatraman S.S., Tan L.P., Joso J.F., Boey Y.C., Wang X. Biodegradable stents with elastic memory. Biomaterials. 2006;27(8):1573–1578. doi: 10.1016/j.biomaterials.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 102.Ortega J.M., Small W., Wilson T.S., Benett W.J., Loge J.M., Maitland D.J. A shape memory polymer dialysis needle adapter for the reduction of hemodynamic stress within arteriovenous grafts. IEEE Trans. Biomed. Eng. 2007;54(9):1722–1724. doi: 10.1109/TBME.2007.892927. [DOI] [PubMed] [Google Scholar]
- 103.Small Iv W., Wilson T., Benett W., Loge J., Maitland D. Laser-activated shape memory polymer intravascular thrombectomy device. Opt Express. 2005;13(20):8204–8213. doi: 10.1364/opex.13.008204. [DOI] [PubMed] [Google Scholar]
- 104.Sokolowski W., Metcalfe A., Hayashi S., Yahia L. J. Raymond. Medical applications of shape memory polymers. Biomed. Mater. 2007;2(1):S23–S27. doi: 10.1088/1748-6041/2/1/S04. [DOI] [PubMed] [Google Scholar]
- 105.Xie R., Hu J., Hoffmann O., Zhang Y., Ng F., Qin T., Guo X. Self-fitting shape memory polymer foam inducing bone regeneration: a rabbit femoral defect study. Biochim. Biophys. Acta Gen. Subj. 2018;1862(4):936–945. doi: 10.1016/j.bbagen.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 106.Yu J., Xia H., Teramoto A., Ni Q.Q. Fabrication and characterization of shape memory polyurethane porous scaffold for bone tissue engineering. J. Biomed. Mater. Res. 2017;105(4):1132–1137. doi: 10.1002/jbm.a.36009. [DOI] [PubMed] [Google Scholar]
- 107.Kai D., Prabhakaran M.P., Chan B.Q., Liow S.S., Ramakrishna S., Xu F., Loh X.J. Elastic poly(epsilon-caprolactone)-polydimethylsiloxane copolymer fibers with shape memory effect for bone tissue engineering. Biomed. Mater. 2016;11(1) doi: 10.1088/1748-6041/11/1/015007. [DOI] [PubMed] [Google Scholar]
- 108.Wang J., Kunkel R., Luo J., Li Y., Liu H., Bohnstedt B.N., Liu Y., Lee C.-H. Shape memory polyurethane with porous architectures for potential applications in intracranial aneurysm treatment. Polymers. 2019;11(4):631. doi: 10.3390/polym11040631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ping P., Wang W., Chen X., Jing X. Poly(epsilon-caprolactone) polyurethane and its shape-memory property. Biomacromolecules. 2005;6(2):587–592. doi: 10.1021/bm049477j. [DOI] [PubMed] [Google Scholar]
- 110.Liu C., Qin H., Mather P.T. Review of progress in shape-memory polymers. J. Mater. Chem. 2007;17(16):1543–1558. [Google Scholar]
- 111.Deng Z., Guo Y., Zhao X., Li L., Dong R., Guo B., Ma P.X. Stretchable degradable and electroactive shape memory copolymers with tunable recovery temperature enhance myogenic differentiation. Acta Biomater. 2016;46:234–244. doi: 10.1016/j.actbio.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 112.Yakacki C.M., Gall K. Springer; 2009. Shape-memory Polymers for Biomedical Applications, Shape-Memory Polymers; pp. 147–175. [DOI] [PubMed] [Google Scholar]
- 113.Mendez J., Annamalai P.K., Eichhorn S.J., Rusli R., Rowan S.J., Foster E.J., Weder C. Bioinspired mechanically adaptive polymer nanocomposites with water-activated shape-memory effect. Macromolecules. 2011;44(17):6827–6835. [Google Scholar]
- 114.Wu T., Frydrych M., O'Kelly K., Chen B. Poly (glycerol sebacate urethane)–cellulose nanocomposites with water-active shape-memory effects. Biomacromolecules. 2014;15(7):2663–2671. doi: 10.1021/bm500507z. [DOI] [PubMed] [Google Scholar]
- 115.Wu T., O'Kelly K., Chen B. Poly (methacrylic acid)‐grafted clay–thermoplastic elastomer composites with water‐induced shape‐memory effects. J. Polym. Sci., Part B: Polym. Phys. 2013;51(20):1513–1522. [Google Scholar]
- 116.Wu T., O'Kelly K., Chen B. Poly(vinyl alcohol) particle-reinforced elastomer composites with water-active shape-memory effects. Eur. Polym. J. 2014;53:230–237. [Google Scholar]
- 117.Ren H., Mei Z., Chen S., Zhuo H., Chen S., Yang H., Zuo J., Ge Z. A new strategy for designing multifunctional shape memory polymers with amine-containing polyurethanes. J. Mater. Sci. 2016;51(19):9131–9144. [Google Scholar]
- 118.Chan B.Q.Y., Heng S.J.W., Liow S.S., Zhang K., Loh X.J. Dual-responsive hybrid thermoplastic shape memory polyurethane. Mater. Chem. Front. 2017;1(4):767–779. [Google Scholar]
- 119.Yang J., Wen H., Zhuo H., Chen S., Ban J. A new type of photo-thermo staged-responsive shape-memory polyurethanes network. Polymers. 2017;9(7):287. doi: 10.3390/polym9070287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gu S.-Y., Chang K., Jin S.-P. A dual-induced self-expandable stent based on biodegradable shape memory polyurethane nanocomposites (PCLAU/Fe3O4) triggered around body temperature. J. Appl. Polym. Sci. 2018;135(3):45686. [Google Scholar]
- 121.Wang C., Wang H., Zou F., Chen S., Wang Y. Development of polyhydroxyalkanoate-based polyurethane with water-thermal response shape-memory behavior as new 3D elastomers scaffolds. Polymers. 2019;11(6):1030. doi: 10.3390/polym11061030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tsai M.C., Hung K.C., Hung S.C., Hsu S.H. Evaluation of biodegradable elastic scaffolds made of anionic polyurethane for cartilage tissue engineering. Colloids Surf. B Biointerfaces. 2015;125:34–44. doi: 10.1016/j.colsurfb.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 123.Xu F., Wang Y., Jiang X., Tan H., Li H., Wang K.J. Effects of different biomaterials: comparing the bladder smooth muscle cells on waterborne polyurethane or poly-lactic-co-glycolic acid membranes. Kaohsiung J. Med. Sci. 2012;28(1):10–15. doi: 10.1016/j.kjms.2011.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hao H., Deng Y., Wu Y., Liu S., Lin W., Li J., Luo F., Tan H. Synthesis of biodegradable waterborne phosphatidylcholine polyurethanes for soft tissue engineering applications. Regen Biomater. 2017;4(2):69–79. [Google Scholar]
- 125.Shin E.J., Choi S.M. in: H. J. Chun, K. Park, C. H. Kim, G. Khang (eds.), Novel Biomaterials for Regenerative Medicine. Springer; 2018. Advances in Waterborne Polyurethane-Based Biomaterials for Biomedical Applications; pp. 251–283. [DOI] [PubMed] [Google Scholar]
- 126.Li M., Liu F., Li Y., Qiang X. Synthesis of stable cationic waterborne polyurethane with a high solid content: insight from simulation to experiment. RSC Adv. 2017;7(22):13312–13324. [Google Scholar]
- 127.Wu G.H., Hsu S.H. Synthesis of water-based cationic polyurethane for antibacterial and gene delivery applications. Colloids Surf. B Biointerfaces. 2016;146:825–832. doi: 10.1016/j.colsurfb.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 128.Lei L., Zhong L., Lin X., Li Y., Xia Z. Synthesis and characterization of waterborne polyurethane dispersions with different chain extenders for potential application in waterborne ink. Chem. Eng. J. 2014;253:518–525. [Google Scholar]
- 129.Li B., Peng D., Zhao N., Mu Q., Li J. The physical properties of nonionic waterborne polyurethane with a polyether as side chain. J. Appl. Polym. Sci. 2013;127(3):1848–1852. [Google Scholar]
- 130.Hsu S.H., Hung K.C., Lin Y.Y., Su C.H., Yeh H.Y., Jeng U.S., Lu C.Y., Dai S.A., Fu W.E., Lin J.C. Water-based synthesis and processing of novel biodegradable elastomers for medical applications. J. Mater. Chem. B. 2014;2(31):5083–5092. doi: 10.1039/c4tb00572d. [DOI] [PubMed] [Google Scholar]
- 131.Hsu S.H., Chang W.C., Yen C.T. Novel flexible nerve conduits made of water-based biodegradable polyurethane for peripheral nerve regeneration. J. Biomed. Mater. Res. 2017;105(5):1383–1392. doi: 10.1002/jbm.a.36022. [DOI] [PubMed] [Google Scholar]
- 132.Jiang X., Li J., Ding M., Tan H., Ling Q., Zhong Y., Fu Q. Synthesis and degradation of nontoxic biodegradable waterborne polyurethanes elastomer with poly(ε-caprolactone) and poly(ethylene glycol) as soft segment. Eur. Polym. J. 2007;43(5):1838–1846. [Google Scholar]
- 133.Jiang X., Wang K., Ding M., Li J., Tan H., Wang Z., Fu Q. Quantitative grafting of peptide onto the nontoxic biodegradable waterborne polyurethanes to fabricate peptide modified scaffold for soft tissue engineering. J. Mater. Sci. Mater. Med. 2011;22(4):819–827. doi: 10.1007/s10856-011-4265-z. [DOI] [PubMed] [Google Scholar]
- 134.Deming T.J. Synthetic polypeptides for biomedical applications. Prog. Polym. Sci. 2007;32(8–9):858–875. [Google Scholar]
- 135.Lanza R., Langer R., Vacanti J.P. Principles of Tissue Engineering. 3rd. Academic press; Cambridge, MA: 2011. [Google Scholar]
- 136.Wang D.A., Ji J., Sun Y.H., Shen J.C., Feng L.X., Elisseeff J.H. In situ immobilization of proteins and RGD peptide on polyurethane surfaces via poly(ethylene oxide) coupling polymers for human endothelial cell growth. Biomacromolecules. 2002;3(6):1286–1295. doi: 10.1021/bm0255950. [DOI] [PubMed] [Google Scholar]
- 137.Krijgsman B., Seifalian A.M., Salacinski H.J., Tai N.R., Punshon G., Fuller B.J., Hamilton G. An assessment of covalent grafting of RGD peptides to the surface of a compliant poly(carbonate-urea)urethane vascular conduit versus conventional biological coatings: its role in enhancing cellular retention. Tissue Eng. 2002;8(4):673–680. doi: 10.1089/107632702760240580. [DOI] [PubMed] [Google Scholar]
- 138.Anderheiden D., Klee D., Hocker H., Heller B., Kirkpatrick C.J., Mittermayer C. Surface modification of a biocompatible polymer based on polyurethane for artificial blood vessels. J. Mater. Sci. Mater. Med. 1992;3(1):1–4. [Google Scholar]
- 139.Jun H.W., West J.L. Modification of polyurethaneurea with PEG and YIGSR peptide to enhance endothelialization without platelet adhesion. J. Biomed. Mater. Res. B Appl. Biomater. 2005;72(1):131–139. doi: 10.1002/jbm.b.30135. [DOI] [PubMed] [Google Scholar]
- 140.Han J., Cao R.W., Chen B., Ye L., Zhang A.Y., Zhang J., Feng Z.G. Electrospinning and biocompatibility evaluation of biodegradable polyurethanes based on L-lysine diisocyanate and L-lysine chain extender. J. Biomed. Mater. Res. 2011;96(4):705–714. doi: 10.1002/jbm.a.33023. [DOI] [PubMed] [Google Scholar]
- 141.Shah P.N., Yun Y.H. Cellular interactions with biodegradable polyurethanes formulated from L-tyrosine. J. Biomater. Appl. 2013;27(8):1017–1031. doi: 10.1177/0885328211432325. [DOI] [PubMed] [Google Scholar]
- 142.Wang R., Zhang F., Lin W., Liu W., Li J., Luo F., Wang Y., Tan H. Shape memory properties and enzymatic degradability of poly(epsilon-caprolactone)-based polyurethane urea containing phenylalanine-derived chain extender. Macromol. Biosci. 2018;18(6) doi: 10.1002/mabi.201800054. [DOI] [PubMed] [Google Scholar]
- 143.Wu G., Wang H., Xiao J., Wang L., Ke Y., Fang L., Deng C., Liao H. Blocking of matrix metalloproteinases-13 responsive peptide in poly(urethane urea) for potential cartilage tissue engineering applications. J. Biomater. Appl. 2018;32(8):999–1010. doi: 10.1177/0885328217753414. [DOI] [PubMed] [Google Scholar]
- 144.Lin H.B., Garcia-Echeverria C., Asakura S., Sun W., Mosher D.F., Cooper S.L. Endothelial cell adhesion on polyurethanes containing covalently attached RGD-peptides. Biomaterials. 1992;13(13):905–914. doi: 10.1016/0142-9612(92)90113-3. [DOI] [PubMed] [Google Scholar]
- 145.Fu H.-L., Hong Y., Little S.R., Wagner W.R. Collagenase-labile polyurethane urea synthesis and processing into hollow fiber membranes. Biomacromolecules. 2014;15(8):2924–2932. doi: 10.1021/bm500552f. [DOI] [PubMed] [Google Scholar]
- 146.Parrag I.C., Woodhouse K.A. Development of biodegradable polyurethane scaffolds using amino acid and dipeptide-based chain extenders for soft tissue engineering. J. Biomater. Sci. Polym. Ed. 2010;21(6–7):843–862. doi: 10.1163/156856209X446743. [DOI] [PubMed] [Google Scholar]
- 147.Jayakumar R., Prabaharan M., Sudheesh Kumar P.T., Nair S.V., Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011;29(3):322–337. doi: 10.1016/j.biotechadv.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 148.Lakshman L.R., Shalumon K.T., Nair S.V., Jayakumar R., Nair S.V. Preparation of silver nanoparticles incorporated electrospun polyurethane nano-fibrous mat for wound dressing. J. Macromol. Sci. 2010;47(10):1012–1018. [Google Scholar]
- 149.Rusu L.C., Ardelean L.C., Jitariu A.A., Miu C.A., Streian C.G. An insight into the structural diversity and clinical applicability of polyurethanes in biomedicine. Polymers (Basel) 2020;12(5):1197. doi: 10.3390/polym12051197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hong S.M., Kim J.W., Knowles J.C., Gong M.S. Facile preparation of antibacterial, highly elastic silvered polyurethane nanofiber fabrics using silver carbamate and their dermal wound healing properties. J. Biomater. Appl. 2017;31(7):1026–1038. doi: 10.1177/0885328216687665. [DOI] [PubMed] [Google Scholar]
- 151.Lee S.J., Heo D.N., Moon J.H., Park H.N., Ko W.K., Bae M.S., Lee J.B., Park S.W., Kim E.C., Lee C.H., Jung B.Y., Kwon I.K. Chitosan/polyurethane blended fiber sheets containing silver sulfadiazine for use as an antimicrobial wound dressing. J. Nanosci. Nanotechnol. 2014;14(10):7488–7494. doi: 10.1166/jnn.2014.9581. [DOI] [PubMed] [Google Scholar]
- 152.Wierzbicki M., Jaworski S., Sawosz E., Jung A., Gielerak G., Jaremek H., Lojkowski W., Wozniak B., Stobinski L., Malolepszy A., Chwalibog A. Graphene oxide in a composite with silver nanoparticles reduces the fibroblast and endothelial cell cytotoxicity of an antibacterial nanoplatform. Nanoscale Res. Lett. 2019;14(1):320. doi: 10.1186/s11671-019-3166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang L., Cao W., Wang X., Li P., Zhou J., Zhang G., Li X., Xing X. Biodegradable silver-loaded polycation modified nanodiamonds/polyurethane scaffold with improved antibacterial and mechanical properties for cartilage tissue repairing. J. Mater. Sci. Mater. Med. 2019;30(4):41. doi: 10.1007/s10856-019-6244-8. [DOI] [PubMed] [Google Scholar]
- 154.Tamayo L., Acuna D., Riveros A.L., Kogan M.J., Azocar M.I., Paez M., Leal M., Urzua M., Cerda E. Porous nanogold/polyurethane scaffolds with improved antibiofilm, mechanical, and thermal properties and with reduced effects on cell viability: a suitable material for soft tissue applications. ACS Appl. Mater. Interfaces. 2018;10(16):13361–13372. doi: 10.1021/acsami.8b02347. [DOI] [PubMed] [Google Scholar]
- 155.Buzarovska A., Dinescu S., Lazar A.D., Serban M., Pircalabioru G.G., Costache M., Gualandi C., Averous L. Nanocomposite foams based on flexible biobased thermoplastic polyurethane and ZnO nanoparticles as potential wound dressing materials. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;104:109893. doi: 10.1016/j.msec.2019.109893. [DOI] [PubMed] [Google Scholar]
- 156.Tran P.L., Hamood A.N., de Souza A., Schultz G., Liesenfeld B., Mehta D., Reid T.W. A study on the ability of quaternary ammonium groups attached to a polyurethane foam wound dressing to inhibit bacterial attachment and biofilm formation. Wound Repair Regen. 2015;23(1):74–81. doi: 10.1111/wrr.12244. [DOI] [PubMed] [Google Scholar]
- 157.Shababdoust A., Ehsani M., Shokrollahi P., Zandi M. Fabrication of curcumin-loaded electrospun nanofiberous polyurethanes with anti-bacterial activity. Prog Biomater. 2018;7(1):23–33. doi: 10.1007/s40204-017-0079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Peng P., Yang J., Wu Q., Wu M., Liu J., Zhang J. Fabrication of N-halamine polyurethane films with excellent antibacterial properties. E-Polymers. 2021;21(1):47–56. [Google Scholar]
- 159.Najafabadi S.A.A., Mohammadi A., Kharazi A.Z. Polyurethane nanocomposite impregnated with chitosan-modified graphene oxide as a potential antibacterial wound dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;115:110899. doi: 10.1016/j.msec.2020.110899. [DOI] [PubMed] [Google Scholar]
- 160.Chen X., Zhao R., Wang X., Li X., Peng F., Jin Z., Gao X., Yu J., Wang C. Electrospun mupirocin loaded polyurethane fiber mats for anti-infection burn wound dressing application. J. Biomater. Sci. Polym. Ed. 2017;28(2):162–176. doi: 10.1080/09205063.2016.1262158. [DOI] [PubMed] [Google Scholar]
- 161.Iga C., Agata T., Marcin L., Natalia F., Justyna K.L. Ciprofloxacin-modified degradable hybrid polyurethane-polylactide porous scaffolds developed for potential use as an antibacterial scaffold for regeneration of skin. Polymers (Basel) 2020;12(1):171. doi: 10.3390/polym12010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li P., Poon Y.F., Li W., Zhu H.Y., Yeap S.H., Cao Y., Qi X., Zhou C., Lamrani M., Beuerman R.W., Kang E.T., Mu Y., Li C.M., Chang M.W., Leong S.S., Chan-Park M.B. A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat. Mater. 2011;10(2):149–156. doi: 10.1038/nmat2915. [DOI] [PubMed] [Google Scholar]
- 163.Ziegler K., Gorl R., Effing J., Ellermann J., Mappes M., Otten S., Kapp H., Zoellner P., Spaeth D., Smola H. Reduced cellular toxicity of a new silver-containing antimicrobial dressing and clinical performance in non-healing wounds. Skin Pharmacol. Physiol. 2006;19(3):140–146. doi: 10.1159/000092594. [DOI] [PubMed] [Google Scholar]
- 164.Yari A., Yeganeh H., Bakhshi H., Gharibi R. Preparation and characterization of novel antibacterial castor oil-based polyurethane membranes for wound dressing application. J. Biomed. Mater. Res. 2014;102(1):84–96. doi: 10.1002/jbm.a.34672. [DOI] [PubMed] [Google Scholar]
- 165.Luo C., Liu W., Luo B., Tian J., Wen W., Liu M., Zhou C. Antibacterial activity and cytocompatibility of chitooligosaccharide-modified polyurethane membrane via polydopamine adhesive layer. Carbohydr. Polym. 2017;156:235–243. doi: 10.1016/j.carbpol.2016.09.036. [DOI] [PubMed] [Google Scholar]
- 166.He W., Zhang Y., Li J., Gao Y., Luo F., Tan H., Wang K., Fu Q. A novel surface structure consisting of contact-active antibacterial upper-layer and antifouling sub-layer derived from gemini quaternary ammonium salt polyurethanes. Sci. Rep. 2016;6:32140. doi: 10.1038/srep32140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang Y., He W., Li J., Wang K., Li J., Tan H., Fu Q. Gemini quaternary ammonium salt waterborne biodegradable polyurethanes with antibacterial and biocompatible properties. Mater. Chem. Front. 2017;1(2):361–368. [Google Scholar]
- 168.Zhang Y., Li Y., Li J., Gao Y., Tan H., Wang K., Li J., Fu Q. Synthesis and antibacterial characterization of waterborne polyurethanes with gemini quaternary ammonium salt. Sci. Bull. 2015;60(12):1114–1121. [Google Scholar]
- 169.Amjed N., Bhatti I.A., Zia K.M., Iqbal J., Jamil Y. Synthesis and characterization of stable and biological active chitin-based polyurethane elastomers. Int. J. Biol. Macromol. 2020;154:1149–1157. doi: 10.1016/j.ijbiomac.2019.11.097. [DOI] [PubMed] [Google Scholar]
- 170.Vishwakarma A., Dang F., Ferrell A., Barton H.A., Joy A. Peptidomimetic polyurethanes inhibit bacterial biofilm formation and disrupt surface established biofilms. J. Am. Chem. Soc. 2021;143(25):9440–9449. doi: 10.1021/jacs.1c02324. [DOI] [PubMed] [Google Scholar]
- 171.Woo G.L., Yang M.L., Yin H.Q., Jaffer F., Mittelman M.W., Santerre J.P. Biological characterization of a novel biodegradable antimicrobial polymer synthesized with fluoroquinolones. J. Biomed. Mater. Res. 2002;59(1):35–45. doi: 10.1002/jbm.1214. [DOI] [PubMed] [Google Scholar]
- 172.Woo G.L., Mittelman M.W., Santerre J.P. Synthesis and characterization of a novel biodegradable antimicrobial polymer. Biomaterials. 2000;21(12):1235–1246. doi: 10.1016/s0142-9612(00)00003-x. [DOI] [PubMed] [Google Scholar]
- 173.Hong Y., Ye S.H., Nieponice A., Soletti L., Vorp D.A., Wagner W.R. A small diameter, fibrous vascular conduit generated from a poly(ester urethane)urea and phospholipid polymer blend. Biomaterials. 2009;30(13):2457–2467. doi: 10.1016/j.biomaterials.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hong Y., Ye S.H., Pelinescu A.L., Wagner W.R. Synthesis, characterization, and paclitaxel release from a biodegradable, elastomeric, poly(ester urethane)urea bearing phosphorylcholine groups for reduced thrombogenicity. Biomacromolecules. 2012;13(11):3686–3694. doi: 10.1021/bm301158j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Soletti L., Nieponice A., Hong Y., Ye S.H., Stankus J.J., Wagner W.R., Vorp D.A. In vivo performance of a phospholipid-coated bioerodable elastomeric graft for small-diameter vascular applications. J. Biomed. Mater. Res. 2011;96(2):436–448. doi: 10.1002/jbm.a.32997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ye S.H., Hong Y., Sakaguchi H., Shankarraman V., Luketich S.K., D'Amore A., Wagner W.R. Nonthrombogenic, biodegradable elastomeric polyurethanes with variable sulfobetaine content. ACS Appl. Mater. Interfaces. 2014;6(24):22796–22806. doi: 10.1021/am506998s. [DOI] [PubMed] [Google Scholar]
- 177.Yuan J., Lin S., Shen J. Enhanced blood compatibility of polyurethane functionalized with sulfobetaine. Colloids Surf. B Biointerfaces. 2008;66(1):90–95. doi: 10.1016/j.colsurfb.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 178.Francolini I., Crisante F., Martinelli A., D'Ilario L., Piozzi A. Synthesis of biomimetic segmented polyurethanes as antifouling biomaterials. Acta Biomater. 2012;8(2):549–558. doi: 10.1016/j.actbio.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 179.Silver J.H., Hart A.P., Williams E.C., Cooper S.L., Charef S., Labarre D., Jozefowicz M. Anticoagulant effects of sulphonated polyurethanes. Biomaterials. 1992;13(6):339–344. doi: 10.1016/0142-9612(92)90037-o. [DOI] [PubMed] [Google Scholar]
- 180.Mi H.Y., Jing X., Li Z.T., Lin Y.J., Thomson J.A., Turng L.S. Fabrication and modification of wavy multicomponent vascular grafts with biomimetic mechanical properties, antithrombogenicity, and enhanced endothelial cell affinity. J. Biomed. Mater. Res. B Appl. Biomater. 2019;107(7):2397–2408. doi: 10.1002/jbm.b.34333. [DOI] [PubMed] [Google Scholar]
- 181.Wang H., Feng Y., Zhao H., Fang Z., Khan M., Guo J. A potential nonthrombogenic small-diameter vascular scaffold with polyurethane/poly(ethylene glycol) hybrid materials by electrospinning technique. J. Nanosci. Nanotechnol. 2013;13(2):1578–1582. doi: 10.1166/jnn.2013.6051. [DOI] [PubMed] [Google Scholar]
- 182.Shitole A.A., Giram P.S., Raut P.W., Rade P.P., Khandwekar A.P., Sharma N., Garnaik B. Clopidogrel eluting electrospun polyurethane/polyethylene glycol thromboresistant, hemocompatible nanofibrous scaffolds. J. Biomater. Appl. 2019;33(10):1327–1347. doi: 10.1177/0885328219832984. [DOI] [PubMed] [Google Scholar]
- 183.Coneski P.N., Schoenfisch M.H. Synthesis of nitric oxide-releasing polyurethanes with S-Nitrosothiol-Containing hard and soft segments. Polym. Chem. 2011;2(4):906–913. doi: 10.1039/C0PY00269K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Heilman B.J., Halpenny G.M., Mascharak P.K. Synthesis, characterization, and light-controlled antibiotic application of a composite material derived from polyurethane and silica xerogel with embedded photoactive manganese nitrosyl. J. Biomed. Mater. Res. B Appl. Biomater. 2011;99(2):328–337. doi: 10.1002/jbm.b.31904. [DOI] [PubMed] [Google Scholar]
- 185.Reynolds M.M., Hrabie J.A., Oh B.K., Politis J.K., Citro M.L., Keefer L.K., Meyerhoff M.E. Nitric oxide releasing polyurethanes with covalently linked diazeniumdiolated secondary amines. Biomacromolecules. 2006;7(3):987–994. doi: 10.1021/bm060028o. [DOI] [PubMed] [Google Scholar]
- 186.Jun H.-W., Taite L.J., West J.L. Nitric oxide-producing polyurethanes. Biomacromolecules. 2005;6(2):838–844. doi: 10.1021/bm049419y. [DOI] [PubMed] [Google Scholar]
- 187.Taite L.J., Yang P., Jun H.W., West J.L. Nitric oxide-releasing polyurethane-PEG copolymer containing the YIGSR peptide promotes endothelialization with decreased platelet adhesion. J. Biomed. Mater. Res. B Appl. Biomater. 2008;84(1):108–116. doi: 10.1002/jbm.b.30850. [DOI] [PubMed] [Google Scholar]
- 188.Martin J.R., Gupta M.K., Page J.M., Yu F., Davidson J.M., Guelcher S.A., Duvall C.L. A porous tissue engineering scaffold selectively degraded by cell-generated reactive oxygen species. Biomaterials. 2014;35(12):3766–3776. doi: 10.1016/j.biomaterials.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.McEnery M.A., Lu S., Gupta M.K., Zienkiewicz K.J., Wenke J.C., Kalpakci K.N., Shimko D.A., Duvall C.L., Guelcher S.A. Oxidatively degradable poly (thioketal urethane)/ceramic composite bone cements with bone-like strength. RSC Adv. 2016;6(111):109414–109424. doi: 10.1039/c6ra24642g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yao Y., Ding J., Wang Z., Zhang H., Xie J., Wang Y., Hong L., Mao Z., Gao J., Gao C. ROS-responsive polyurethane fibrous patches loaded with methylprednisolone (MP) for restoring structures and functions of infarcted myocardium in vivo. Biomaterials. 2020;232:119726. doi: 10.1016/j.biomaterials.2019.119726. [DOI] [PubMed] [Google Scholar]
- 191.Ferreira P., Pereira R., Coelho J.F., Silva A.F., Gil M.H. Modification of the biopolymer castor oil with free isocyanate groups to be applied as bioadhesive. Int. J. Biol. Macromol. 2007;40(2):144–152. doi: 10.1016/j.ijbiomac.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 192.Sheikh N., Mirzadeh H., Katbab A.A., Salehian P., Daliri M., Amanpour S. Isocyanate-terminated urethane prepolymer as bioadhesive material: evaluation of bioadhesion and biocompatibility, in vitro and in vivo assays. J. Biomater. Sci. Polym. Ed. 2001;12(7):707–719. doi: 10.1163/156856201750411611. [DOI] [PubMed] [Google Scholar]
- 193.Balcioglu S., Parlakpinar H., Vardi N., Denkbas E.B., Karaaslan M.G., Gulgen S., Taslidere E., Koytepe S., Ates B. Design of xylose-based semisynthetic polyurethane tissue adhesives with enhanced bioactivity properties. ACS Appl. Mater. Interfaces. 2016;8(7):4456–4466. doi: 10.1021/acsami.5b12279. [DOI] [PubMed] [Google Scholar]
- 194.Zhu N., Chen X. in: R. Pignatello (Ed.), Advances in Biomaterials Science and Biomedical Applications. InTech; London, UK: 2013. Chapter 12: Biofabrication of Tissue Scaffolds; pp. 315–328. [Google Scholar]
- 195.Laschke M.W., Strohe A., Menger M.D., Alini M., Eglin D. In vitro and in vivo evaluation of a novel nanosize hydroxyapatite particles/poly(ester-urethane) composite scaffold for bone tissue engineering. Acta Biomater. 2010;6(6):2020–2027. doi: 10.1016/j.actbio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 196.Guan J., Fujimoto K.L., Sacks M.S., Wagner W.R. Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials. 2005;26(18):3961–3971. doi: 10.1016/j.biomaterials.2004.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Xu C., Okpokwasili C., Huang Y., Shi X., Wu J., Liao J., Tang L., Hong Y. Optimizing anisotropic polyurethane scaffolds to mechanically match with native myocardium. ACS Biomater. Sci. Eng. 2020;6(5):2757–2769. doi: 10.1021/acsbiomaterials.9b01860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jiang X., Yu F., Wang Z., Li J., Tan H., Ding M., Fu Q. Fabrication and characterization of waterborne biodegradable polyurethanes 3-dimensional porous scaffolds for vascular tissue engineering. J. Biomater. Sci. Polym. Ed. 2010;21(12):1637–1652. doi: 10.1163/092050609X12525750021270. [DOI] [PubMed] [Google Scholar]
- 199.Du B., Yin H., Chen Y., Lin W., Wang Y., Zhao D., Wang G., He X., Li J., Li Z., Luo F., Tan H., Fu Q. A waterborne polyurethane 3D scaffold containing PLGA with a controllable degradation rate and an anti-inflammatory effect for potential applications in neural tissue repair. J. Mater. Chem. B. 2020;8(20):4434–4446. doi: 10.1039/d0tb00656d. [DOI] [PubMed] [Google Scholar]
- 200.Wong C.S., Liu X., Xu Z., Lin T., Wang X. Elastin and collagen enhances electrospun aligned polyurethane as scaffolds for vascular graft. J. Mater. Sci. Mater. Med. 2013;24(8):1865–1874. doi: 10.1007/s10856-013-4937-y. [DOI] [PubMed] [Google Scholar]
- 201.Jing X., Mi H.Y., Salick M.R., Cordie T.M., Peng X.F., Turng L.S. Electrospinning thermoplastic polyurethane/graphene oxide scaffolds for small diameter vascular graft applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015;49:40–50. doi: 10.1016/j.msec.2014.12.060. [DOI] [PubMed] [Google Scholar]
- 202.Amoroso N.J., D'Amore A., Hong Y., Rivera C.P., Sacks M.S., Wagner W.R. Microstructural manipulation of electrospun scaffolds for specific bending stiffness for heart valve tissue engineering. Acta Biomater. 2012;8(12):4268–4277. doi: 10.1016/j.actbio.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hsieh F.Y., Lin H.H., Hsu S.H. 3D bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials. 2015;71:48–57. doi: 10.1016/j.biomaterials.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 204.Ho L., Hsu S.H. Cell reprogramming by 3D bioprinting of human fibroblasts in polyurethane hydrogel for fabrication of neural-like constructs. Acta Biomater. 2018;70:57–70. doi: 10.1016/j.actbio.2018.01.044. [DOI] [PubMed] [Google Scholar]
- 205.Hsieh C.T., Hsu S.H. Double-network polyurethane-gelatin hydrogel with tunable modulus for high-resolution 3D bioprinting. ACS Appl. Mater. Interfaces. 2019;11(36):32746–32757. doi: 10.1021/acsami.9b10784. [DOI] [PubMed] [Google Scholar]
- 206.Camarero-Espinosa S., Calore A., Wilbers A., Harings J., Moroni L. Additive manufacturing of an elastic poly(ester)urethane for cartilage tissue engineering. Acta Biomater. 2020;102:192–204. doi: 10.1016/j.actbio.2019.11.041. [DOI] [PubMed] [Google Scholar]
- 207.Camarero-Espinosa S., Tomasina C., Calore A., Moroni L. Additive manufactured, highly resilient, elastic, and biodegradable poly(ester)urethane scaffolds with chondroinductive properties for cartilage tissue engineering. Mater. Today Bio. 2020;6:100051. doi: 10.1016/j.mtbio.2020.100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Soletti L., Hong Y., Guan J., Stankus J.J., El-Kurdi M.S., Wagner W.R., Vorp D.A. A bilayered elastomeric scaffold for tissue engineering of small diameter vascular grafts. Acta Biomater. 2010;6(1):110–122. doi: 10.1016/j.actbio.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Heijkants R.G.J.C., Van Tienen T.G., De Groot J.H., Pennings A.J., Buma P., Veth R.P.H., Schouten A.J. Preparation of a polyurethane scaffold for tissue engineering made by a combination of salt leaching and freeze-drying of dioxane. J. Mater. Sci. 2006;41(8):2423–2428. [Google Scholar]
- 210.Lin W., Lan W., Wu Y., Zhao D., Wang Y., He X., Li J., Li Z., Luo F., Tan H., Fu Q. Aligned 3D porous polyurethane scaffolds for biological anisotropic tissue regeneration. Regen Biomater. 2020;7(1):19–27. doi: 10.1093/rb/rbz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hung K.C., Tseng C.S., Dai L.G., Hsu S.H. Water-based polyurethane 3D printed scaffolds with controlled release function for customized cartilage tissue engineering. Biomaterials. 2016;83:156–168. doi: 10.1016/j.biomaterials.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 212.Hung K.C., Tseng C.S., Hsu S.H. Synthesis and 3D printing of biodegradable polyurethane elastomer by a water-based process for cartilage tissue engineering applications. Adv Healthc Mater. 2014;3(10):1578–1587. doi: 10.1002/adhm.201400018. [DOI] [PubMed] [Google Scholar]
- 213.Wu J., Du Y., Watkins S.C., Funderburgh J.L., Wagner W.R. The engineering of organized human corneal tissue through the spatial guidance of corneal stromal stem cells. Biomaterials. 2012;33(5):1343–1352. doi: 10.1016/j.biomaterials.2011.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mi H.Y., Salick M.R., Jing X., Crone W.C., Peng X.F., Turng L.S. Electrospinning of unidirectionally and orthogonally aligned thermoplastic polyurethane nanofibers: fiber orientation and cell migration. J. Biomed. Mater. Res. 2015;103(2):593–603. doi: 10.1002/jbm.a.35208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Holzapfel G.A., Ogden R.W. Biomechanics of Soft Tissue in Cardiovascular Systems. Springer; New York, NY: 2014. [Google Scholar]
- 216.Kucinska-Lipka J., Gubanska I., Janik H., Sienkiewicz M. Fabrication of polyurethane and polyurethane based composite fibres by the electrospinning technique for soft tissue engineering of cardiovascular system. Mater. Sci. Eng. C Mater. Biol. Appl. 2015;46:166–176. doi: 10.1016/j.msec.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 217.Boffito M., Di Meglio F., Mozetic P., Giannitelli S.M., Carmagnola I., Castaldo C., Nurzynska D., Sacco A.M., Miraglia R., Montagnani S. Surface functionalization of polyurethane scaffolds mimicking the myocardial microenvironment to support cardiac primitive cells. PLoS One. 2018;13(7) doi: 10.1371/journal.pone.0199896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Fujimoto K.L., Tobita K., Merryman W.D., Guan J., Momoi N., Stolz D.B., Sacks M.S., Keller B.B., Wagner W.R. An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction. J. Am. Coll. Cardiol. 2007;49(23):2292–2300. doi: 10.1016/j.jacc.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hashizume R., Hong Y., Takanari K., Fujimoto K.L., Tobita K., Wagner W.R. The effect of polymer degradation time on functional outcomes of temporary elastic patch support in ischemic cardiomyopathy. Biomaterials. 2013;34(30):7353–7363. doi: 10.1016/j.biomaterials.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gu X., Matsumura Y., Tang Y., Roy S., Hoff R., Wang B., Wagner W.R. Sustained viral gene delivery from a micro-fibrous, elastomeric cardiac patch to the ischemic rat heart. Biomaterials. 2017;133:132–143. doi: 10.1016/j.biomaterials.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 221.Hashizume R., Fujimoto K.L., Hong Y., Guan J., Toma C., Tobita K., Wagner W.R. Biodegradable elastic patch plasty ameliorates left ventricular adverse remodeling after ischemia-reperfusion injury: a preclinical study of a porous polyurethane material in a porcine model. J. Thorac. Cardiovasc. Surg. 2013;146(2):391–399. doi: 10.1016/j.jtcvs.2012.11.013. E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Fujimoto K.L., Guan J., Oshima H., Sakai T., Wagner W.R. In vivo evaluation of a porous, elastic, biodegradable patch for reconstructive cardiac procedures. Ann. Thorac. Surg. 2007;83(2):648–654. doi: 10.1016/j.athoracsur.2006.06.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Martins A.M., Eng G., Caridade S.G., Mano J.F., Reis R.L., Vunjak-Novakovic G. Electrically conductive chitosan/carbon scaffolds for cardiac tissue engineering. Biomacromolecules. 2014;15(2):635–643. doi: 10.1021/bm401679q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Shokraei N., Asadpour S., Shokraei S., Nasrollahzadeh Sabet M., Faridi-Majidi R., Ghanbari H. Development of electrically conductive hybrid nanofibers based on CNT-polyurethane nanocomposite for cardiac tissue engineering. Microsc. Res. Tech. 2019;82(8):1316–1325. doi: 10.1002/jemt.23282. [DOI] [PubMed] [Google Scholar]
- 225.Baheiraei N., Yeganeh H., Ai J., Gharibi R., Azami M., Faghihi F. Synthesis, characterization and antioxidant activity of a novel electroactive and biodegradable polyurethane for cardiac tissue engineering application. Mater. Sci. Eng. C Mater. Biol. Appl. 2014;44:24–37. doi: 10.1016/j.msec.2014.07.061. [DOI] [PubMed] [Google Scholar]
- 226.Baheiraei N., Yeganeh H., Ai J., Gharibi R., Ebrahimi-Barough S., Azami M., Vahdat S., Baharvand H. Preparation of a porous conductive scaffold from aniline pentamer-modified polyurethane/PCL blend for cardiac tissue engineering. J. Biomed. Mater. Res. 2015;103(10):3179–3187. doi: 10.1002/jbm.a.35447. [DOI] [PubMed] [Google Scholar]
- 227.Ercolani E., Del Gaudio C., Bianco A. Vascular tissue engineering of small-diameter blood vessels: reviewing the electrospinning approach. J. Tissue Eng. Regen Med. 2015;9(8):861–888. doi: 10.1002/term.1697. [DOI] [PubMed] [Google Scholar]
- 228.Grasl C., Bergmeister H., Stoiber M., Schima H., Weigel G. Electrospun polyurethane vascular grafts: in vitro mechanical behavior and endothelial adhesion molecule expression. J. Biomed. Mater. Res. 2010;93(2):716–723. doi: 10.1002/jbm.a.32584. [DOI] [PubMed] [Google Scholar]
- 229.Punnakitikashem P., Truong D., Menon J.U., Nguyen K.T., Hong Y. Electrospun biodegradable elastic polyurethane scaffolds with dipyridamole release for small diameter vascular grafts. Acta Biomater. 2014;10(11):4618–4628. doi: 10.1016/j.actbio.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Del Gaudio C., Ercolani E., Galloni P., Santilli F., Baiguera S., Polizzi L., Bianco A. Aspirin-loaded electrospun poly(epsilon-caprolactone) tubular scaffolds: potential small-diameter vascular grafts for thrombosis prevention. J. Mater. Sci. Mater. Med. 2013;24(2):523–532. doi: 10.1007/s10856-012-4803-3. [DOI] [PubMed] [Google Scholar]
- 231.Davoudi P., Assadpour S., Derakhshan M.A., Ai J., Solouk A., Ghanbari H. Biomimetic modification of polyurethane-based nanofibrous vascular grafts: a promising approach towards stable endothelial lining. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;80:213–221. doi: 10.1016/j.msec.2017.05.140. [DOI] [PubMed] [Google Scholar]
- 232.Ding X., Chin W., Lee C.N., Hedrick J.L., Yang Y.Y. Peptide-functionalized polyurethane coatings prepared via grafting-to strategy to selectively promote endothelialization. Adv Healthc Mater. 2018;7(5):1700944. doi: 10.1002/adhm.201700944. [DOI] [PubMed] [Google Scholar]
- 233.He W., Nieponice A., Soletti L., Hong Y., Gharaibeh B., Crisan M., Usas A., Peault B., Huard J., Wagner W.R., Vorp D.A. Pericyte-based human tissue engineered vascular grafts. Biomaterials. 2010;31(32):8235–8244. doi: 10.1016/j.biomaterials.2010.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Nieponice A., Soletti L., Guan J., Hong Y., Gharaibeh B., Maul T.M., Huard J., Wagner W.R., Vorp D.A. In vivo assessment of a tissue-engineered vascular graft combining a biodegradable elastomeric scaffold and muscle-derived stem cells in a rat model. Tissue Eng. 2010;16(4):1215–1223. doi: 10.1089/ten.tea.2009.0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Stankus J.J., Soletti L., Fujimoto K., Hong Y., Vorp D.A., Wagner W.R. Fabrication of cell microintegrated blood vessel constructs through electrohydrodynamic atomization. Biomaterials. 2007;28(17):2738–2746. doi: 10.1016/j.biomaterials.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Guilak F., Butler D.L., Goldstein S.A., Baaijens F.P. Biomechanics and mechanobiology in functional tissue engineering. J. Biomech. 2014;47(9):1933–1940. doi: 10.1016/j.jbiomech.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Xu B., Li Y., Fang X., Thouas G.A., Cook W.D., Newgreen D.F., Chen Q. Mechanically tissue-like elastomeric polymers and their potential as a vehicle to deliver functional cardiomyocytes. J. Mech. Behav. Biomed. Mater. 2013;28:354–365. doi: 10.1016/j.jmbbm.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 238.Tajaddini A., Kilpatrick D.L., Schoenhagen P., Tuzcu E.M., Lieber M., Vince D.G. Impact of age and hyperglycemia on the mechanical behavior of intact human coronary arteries: an ex vivo intravascular ultrasound study. Am. J. Physiol. Heart Circ. Physiol. 2005;288(1):H250–255. doi: 10.1152/ajpheart.00646.2004. [DOI] [PubMed] [Google Scholar]
- 239.Chen Q.-Z., Bismarck A., Hansen U., Junaid S., Tran M.Q., Harding S.n.E., Ali N.N., Boccaccini A.R. Characterisation of a soft elastomer poly (glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials. 2008;29(1):47–57. doi: 10.1016/j.biomaterials.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 240.Blit P.H., Battiston K.G., Yang M., Paul Santerre J., Woodhouse K.A. Electrospun elastin-like polypeptide enriched polyurethanes and their interactions with vascular smooth muscle cells. Acta Biomater. 2012;8(7):2493–2503. doi: 10.1016/j.actbio.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 241.Chen R., Qiu L., Ke Q., He C., Mo X. Electrospinning thermoplastic polyurethane-contained collagen nanofibers for tissue-engineering applications. J. Biomater. Sci. Polym. Ed. 2009;20(11):1513–1536. doi: 10.1163/092050609X12464344958883. [DOI] [PubMed] [Google Scholar]
- 242.Lisi A., Briganti E., Ledda M., Losi P., Grimaldi S., Marchese R., Soldani G. A combined synthetic-fibrin scaffold supports growth and cardiomyogenic commitment of human placental derived stem cells. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0034284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.D'Amore A., Yoshizumi T., Luketich S.K., Wolf M.T., Gu X., Cammarata M., Hoff R., Badylak S.F., Wagner W.R. Bi-layered polyurethane–Extracellular matrix cardiac patch improves ischemic ventricular wall remodeling in a rat model. Biomaterials. 2016;107:1–14. doi: 10.1016/j.biomaterials.2016.07.039. [DOI] [PubMed] [Google Scholar]
- 244.Gerring E.L., Bellhouse B.J., Bellhouse F.H., Haworth W.S. Long term animal trials of the Oxford aortic/pulmonary valve prosthesis without anticoagulants. Trans. Am. Soc. Artif. Intern. Organs. 1974;20 B:703–707. [PubMed] [Google Scholar]
- 245.Bernacca G.M., Mackay T.G., Wilkinson R., Wheatley D.J. Calcification and fatigue failure in a polyurethane heart valve. Biomaterials. 1995;16(4):279–285. doi: 10.1016/0142-9612(95)93255-c. [DOI] [PubMed] [Google Scholar]
- 246.Boloori Zadeh P., Corbett S.C., Nayeb-Hashemi H. In-vitro calcification study of polyurethane heart valves. Mater. Sci. Eng. C Mater. Biol. Appl. 2014;35:335–340. doi: 10.1016/j.msec.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 247.Oveissi F., Naficy S., Lee A., Winlaw D.S., Dehghani F. Materials and manufacturing perspectives in engineering heart valves: a review. Mater. Today Bio. 2020;5:100038. doi: 10.1016/j.mtbio.2019.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Stokes K., McVenes R., Anderson J.M. Polyurethane elastomer biostability. J. Biomater. Appl. 1995;9(4):321–354. doi: 10.1177/088532829500900402. [DOI] [PubMed] [Google Scholar]
- 249.Puperi D.S., Kishan A., Punske Z.E., Wu Y., Cosgriff-Hernandez E., West J.L., Grande-Allen K.J. Electrospun polyurethane and hydrogel composite scaffolds as biomechanical mimics for aortic valve tissue engineering. ACS Biomater. Sci. Eng. 2016;2(9):1546–1558. doi: 10.1021/acsbiomaterials.6b00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Salacinski H.J., Odlyha M., Hamilton G., Seifalian A.M. Thermo-mechanical analysis of a compliant poly(carbonate-urea)urethane after exposure to hydrolytic, oxidative, peroxidative and biological solutions. Biomaterials. 2002;23(10):2231–2240. doi: 10.1016/s0142-9612(01)00356-8. [DOI] [PubMed] [Google Scholar]
- 251.Tang Y., Labow R., Santerre J. Enzyme‐induced biodegradation of polycarbonate‐polyurethanes: dependence on hard‐segment chemistry. J. Biomed. Mater. Res. 2001;57(4):597–611. doi: 10.1002/1097-4636(20011215)57:4<597::aid-jbm1207>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 252.Daebritz S.H., Fausten B., Hermanns B., Franke A., Schroeder J., Groetzner J., Autschbach R., Messmer B.J., Sachweh J.S. New flexible polymeric heart valve prostheses for the mitral and aortic positions. Heart Surg. Forum. 2004;7(5):E525–532. doi: 10.1532/HSF98.20041083. [DOI] [PubMed] [Google Scholar]
- 253.D'Amore A., Luketich S.K., Hoff R., Ye S.H., Wagner W.R. Blending polymer labile elements at differing scales to affect degradation profiles in heart valve scaffolds. Biomacromolecules. 2019;20(7):2494–2505. doi: 10.1021/acs.biomac.9b00189. [DOI] [PubMed] [Google Scholar]
- 254.Hobson C.M., Amoroso N.J., Amini R., Ungchusri E., Hong Y., D'Amore A., Sacks M.S., Wagner W.R. Fabrication of elastomeric scaffolds with curvilinear fibrous structures for heart valve leaflet engineering. J. Biomed. Mater. Res. 2015;103(9):3101–3106. doi: 10.1002/jbm.a.35450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Coyan G.N., da Mota Silveira-Filho L., Matsumura Y., Luketich S.K., Katz W., Badhwar V., Wagner W.R., D'Amore A. Acute in vivo functional assessment of a biodegradable stentless elastomeric tricuspid valve. J. Cardiovasc. Transl. Res. 2020;13(5):796–805. doi: 10.1007/s12265-020-09960-z. [DOI] [PubMed] [Google Scholar]
- 256.Engler A.J., Griffin M.A., Sen S., Bonnemann C.G., Sweeney H.L., Discher D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004;166(6):877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Vannozzi L., Ricotti L., Santaniello T., Terencio T., Oropesa-Nunez R., Canale C., Borghi F., Menciassi A., Lenardi C., Gerges I. 3D porous polyurethanes featured by different mechanical properties: characterization and interaction with skeletal muscle cells. J. Mech. Behav. Biomed. Mater. 2017;75:147–159. doi: 10.1016/j.jmbbm.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 258.Liao I.C., Liu J.B., Bursac N., Leong K.W. Effect of electromechanical stimulation on the maturation of myotubes on aligned electrospun fibers. Cell. Mol. Bioeng. 2008;1(2–3):133–145. doi: 10.1007/s12195-008-0021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Andriani Y., Chua J.M., Chua B.Y., Phang I.Y., Shyh-Chang N., Tan W.S. Polyurethane acrylates as effective substrates for sustained in vitro culture of human myotubes. Acta Biomater. 2017;57:115–126. doi: 10.1016/j.actbio.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 260.Nagamine K., Sato H., Kai H., Kaji H., Kanzaki M., Nishizawa M. Contractile skeletal muscle cells cultured with a conducting soft wire for effective, selective stimulation. Sci. Rep. 2018;8(1):2253. doi: 10.1038/s41598-018-20729-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Cha S.H., Lee H.J., Koh W.G. Study of myoblast differentiation using multi-dimensional scaffolds consisting of nano and micropatterns. Biomater. Res. 2017;21(1):1. doi: 10.1186/s40824-016-0087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Riboldi S.A., Sadr N., Pigini L., Neuenschwander P., Simonet M., Mognol P., Sampaolesi M., Cossu G., Mantero S. Skeletal myogenesis on highly orientated microfibrous polyesterurethane scaffolds. J. Biomed. Mater. Res. 2008;84(4):1094–1101. doi: 10.1002/jbm.a.31534. [DOI] [PubMed] [Google Scholar]
- 263.Shen J.Y., Chan-Park M.B., Feng Z.Q., Chan V., Feng Z.W. UV-embossed microchannel in biocompatible polymeric film: application to control of cell shape and orientation of muscle cells. J. Biomed. Mater. Res. B Appl. Biomater. 2006;77(2):423–430. doi: 10.1002/jbm.b.30449. [DOI] [PubMed] [Google Scholar]
- 264.Condello V., Dei Giudici L., Perdisa F., Screpis D.U., Guerriero M., Filardo G., Zorzi C. Polyurethane scaffold implants for partial meniscus lesions: delayed intervention leads to an inferior outcome. Knee Surg. Sports Traumatol. Arthrosc. 2021;29(1):109–116. doi: 10.1007/s00167-019-05760-4. [DOI] [PubMed] [Google Scholar]
- 265.Dhollander A., Verdonk P., Verdonk R. Treatment of painful, irreparable partial meniscal defects with a polyurethane scaffold: midterm clinical outcomes and survival analysis. Am. J. Sports Med. 2016;44(10):2615–2621. doi: 10.1177/0363546516652601. [DOI] [PubMed] [Google Scholar]
- 266.Schuttler K.F., Haberhauer F., Gesslein M., Heyse T.J., Figiel J., Lorbach O., Efe T., Roessler P.P. Midterm follow-up after implantation of a polyurethane meniscal scaffold for segmental medial meniscus loss: maintenance of good clinical and MRI outcome. Knee Surg. Sports Traumatol. Arthrosc. 2016;24(5):1478–1484. doi: 10.1007/s00167-015-3759-5. [DOI] [PubMed] [Google Scholar]
- 267.Monllau J.C., Poggioli F., Erquicia J., Ramirez E., Pelfort X., Gelber P., Torres-Claramunt R. Magnetic resonance imaging and functional outcomes after a polyurethane meniscal scaffold implantation: minimum 5-year follow-up. Arthroscopy. 2018;34(5):1621–1627. doi: 10.1016/j.arthro.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 268.Olivos-Meza A., Pérez Jiménez F.J., Granados-Montiel J., Landa-Solís C., Cortés González S., Jiménez Aroche C.A., Valdez Chávez M., Renán León S., Gomez-Garcia R., Martínez-López V. First Clinical Application of Polyurethane Meniscal Scaffolds with Mesenchymal Stem Cells and Assessment of Cartilage Quality with T2 Mapping at 12 Months. Cartilage. 2019 doi: 10.1177/1947603519852415. 1947603519852415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Marzec M., Kucinska-Lipka J., Kalaszczynska I., Janik H. Development of polyurethanes for bone repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2017;80:736–747. doi: 10.1016/j.msec.2017.07.047. [DOI] [PubMed] [Google Scholar]
- 270.Thoma R., Hung T., Nyilas E., Haubold A., Phillips R. in: C. G. Gebelein (Ed.), Advances in Biomedical Polymers. Springer; Boston, MA: 1987. Metal Ion Complexation of Poly (Ether) Urethanes; pp. 131–145. [Google Scholar]
- 271.Gogolewski S., Gorna K. Biodegradable polyurethane cancellous bone graft substitutes in the treatment of iliac crest defects. J. Biomed. Mater. Res. 2007;80(1):94–101. doi: 10.1002/jbm.a.30834. [DOI] [PubMed] [Google Scholar]
- 272.Aryal Santosh, Bhattarai Shanta Raj, Remant Bahadur K.C., Khil Myung Seob, Lee Duck-Rae, Kim Hak Yong. Carbon nanotubes assisted biomimetic synthesis of hydroxyapatite from simulated body fluid. Materials Science and Engineering: A. 25 June 2006;426(1–2):202–207. doi: 10.1016/j.msea.2006.04.004. [DOI] [Google Scholar]
- 273.Zawadzak E., Bil M., Ryszkowska J., Nazhat S.N., Cho J., Bretcanu O., Roether J.A., Boccaccini A.R. Polyurethane foams electrophoretically coated with carbon nanotubes for tissue engineering scaffolds. Biomed. Mater. 2009;4(1) doi: 10.1088/1748-6041/4/1/015008. [DOI] [PubMed] [Google Scholar]
- 274.Gonzalez-Paz R.J., Lligadas G., Ronda J.C., Galia M., Ferreira A.M., Boccafoschi F., Ciardelli G., Cadiz V. Enhancement of fatty acid-based polyurethanes cytocompatibility by non-covalent anchoring of chondroitin sulfate. Macromol. Biosci. 2012;12(12):1697–1705. doi: 10.1002/mabi.201200259. [DOI] [PubMed] [Google Scholar]
- 275.Tetteh G., Khan A.S., Delaine-Smith R.M., Reilly G.C., Rehman I.U. Electrospun polyurethane/hydroxyapatite bioactive scaffolds for bone tissue engineering: the role of solvent and hydroxyapatite particles. J. Mech. Behav. Biomed. Mater. 2014;39:95–110. doi: 10.1016/j.jmbbm.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 276.Yang W., Both S.K., Zuo Y., Birgani Z.T., Habibovic P., Li Y., Jansen J.A., Yang F. Biological evaluation of porous aliphatic polyurethane/hydroxyapatite composite scaffolds for bone tissue engineering. J. Biomed. Mater. Res. 2015;103(7):2251–2259. doi: 10.1002/jbm.a.35365. [DOI] [PubMed] [Google Scholar]
- 277.Li B., Yoshii T., Hafeman A.E., Nyman J.S., Wenke J.C., Guelcher S.A. The effects of rhBMP-2 released from biodegradable polyurethane/microsphere composite scaffolds on new bone formation in rat femora. Biomaterials. 2009;30(35):6768–6779. doi: 10.1016/j.biomaterials.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 278.Zhang Y., Huang K., Yuan Q., Gu Z., Wu G. Development of arg-based biodegradable poly(ester urea) urethanes and its biomedical application for bone repair. J. Biomed. Nanotechnol. 2019;15(9):1909–1922. doi: 10.1166/jbn.2019.2818. [DOI] [PubMed] [Google Scholar]
- 279.Saad B., Neuenschwander P., Uhlschmid G.K., Suter U.W. New versatile, elastomeric, degradable polymeric materials for medicine. Int. J. Biol. Macromol. 1999;25(1–3):293–301. doi: 10.1016/s0141-8130(99)00044-6. [DOI] [PubMed] [Google Scholar]
- 280.Grad S., Kupcsik L., Gorna K., Gogolewski S., Alini M. The use of biodegradable polyurethane scaffolds for cartilage tissue engineering: potential and limitations. Biomaterials. 2003;24(28):5163–5171. doi: 10.1016/s0142-9612(03)00462-9. [DOI] [PubMed] [Google Scholar]
- 281.Chia S.L., Gorna K., Gogolewski S., Alini M. Biodegradable elastomeric polyurethane membranes as chondrocyte carriers for cartilage repair. Tissue Eng. 2006;12(7):1945–1953. doi: 10.1089/ten.2006.12.1945. [DOI] [PubMed] [Google Scholar]
- 282.Iyer K., Dearman B.L., Wagstaff M.J., Greenwood J.E. A novel biodegradable polyurethane matrix for auricular cartilage repair: an in vitro and in vivo study. J. Burn Care Res. 2016;37(4):e353–364. doi: 10.1097/BCR.0000000000000281. [DOI] [PubMed] [Google Scholar]
- 283.Venkatesan J.K., Gardner O., Rey-Rico A., Eglin D., Alini M., Stoddart M.J., Cucchiarini M., Madry H. Improved chondrogenic differentiation of rAAV SOX9-modified human MSCs seeded in fibrin-polyurethane scaffolds in a hydrodynamic environment. Int. J. Mol. Sci. 2018;19(9):2635. doi: 10.3390/ijms19092635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Cochis A., Grad S., Stoddart M.J., Fare S., Altomare L., Azzimonti B., Alini M., Rimondini L. Bioreactor mechanically guided 3D mesenchymal stem cell chondrogenesis using a biocompatible novel thermo-reversible methylcellulose-based hydrogel. Sci. Rep. 2017;7:45018. doi: 10.1038/srep45018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Robinson P.H., van der Lei B., Hoppen H.J., Leenslag J.W., Pennings A.J., Nieuwenhuis P. Nerve regeneration through a two-ply biodegradable nerve guide in the rat and the influence of ACTH4-9 nerve growth factor. Microsurgery. 1991;12(6):412–419. doi: 10.1002/micr.1920120608. [DOI] [PubMed] [Google Scholar]
- 286.Hoppen H.J., Leenslag J.W., Pennings A.J., van der Lei B., Robinson P.H. Two-ply biodegradable nerve guide: basic aspects of design, construction and biological performance. Biomaterials. 1990;11(4):286–290. doi: 10.1016/0142-9612(90)90012-f. [DOI] [PubMed] [Google Scholar]
- 287.Hausner T., Schmidhammer R., Zandieh S., Hopf R., Schultz A., Gogolewski S., Hertz H., Redl H. In: How to Improve the Results of Peripheral Nerve Surgery. Millesi H., Schmidhammer R., editors. Springer; Vienna: 2007. Nerve regeneration using tubular scaffolds from biodegradable polyurethane; pp. 69–72. [DOI] [PubMed] [Google Scholar]
- 288.Anand S., Desai V., Alsmadi N., Kanneganti A., Nguyen D.H., Tran M., Patil L., Vasudevan S., Xu C., Hong Y., Cheng J., Keefer E., Romero-Ortega M.I. Asymmetric sensory-motor regeneration of transected peripheral nerves using molecular guidance cues. Sci. Rep. 2017;7(1):14323. doi: 10.1038/s41598-017-14331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Balint R., Cassidy N.J., Cartmell S.H. Conductive polymers: towards a smart biomaterial for tissue engineering. Acta Biomater. 2014;10(6):2341–2353. doi: 10.1016/j.actbio.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 290.Shrestha S., Shrestha B.K., Lee J., Joong O.K., Kim B.S., Park C.H., Kim C.S. A conducting neural interface of polyurethane/silk-functionalized multiwall carbon nanotubes with enhanced mechanical strength for neuroregeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;102:511–523. doi: 10.1016/j.msec.2019.04.053. [DOI] [PubMed] [Google Scholar]
- 291.Cuttaz E., Goding J., Vallejo-Giraldo C., Aregueta-Robles U., Lovell N., Ghezzi D., Green R.A. Conductive elastomer composites for fully polymeric, flexible bioelectronics. Biomater. Sci. 2019;7(4):1372–1385. doi: 10.1039/c8bm01235k. [DOI] [PubMed] [Google Scholar]
- 292.Hasanzadeh E., Ebrahimi-Barough S., Mirzaei E., Azami M., Tavangar S.M., Mahmoodi N., Basiri A., Ai J. Preparation of fibrin gel scaffolds containing MWCNT/PU nanofibers for neural tissue engineering. J. Biomed. Mater. Res. 2019;107(4):802–814. doi: 10.1002/jbm.a.36596. [DOI] [PubMed] [Google Scholar]
- 293.Demir U.S., Shahbazi R., Calamak S., Ozturk S., Gultekinoglu M., Ulubayram K. Gold nano-decorated aligned polyurethane nanofibers for enhancement of neurite outgrowth and elongation. J. Biomed. Mater. Res. 2018;106(6):1604–1613. doi: 10.1002/jbm.a.36365. [DOI] [PubMed] [Google Scholar]
- 294.Javadi M., Gu Q., Naficy S., Farajikhah S., Crook J.M., Wallace G.G., Beirne S., Moulton S.E. Conductive tough hydrogel for bioapplications. Macromol. Biosci. 2018;18(2):1700270. doi: 10.1002/mabi.201700270. [DOI] [PubMed] [Google Scholar]
- 295.Wang Y.-C., Fang F., Wu Y.-K., Ai X.-L., Lan T., Liang R.-C., Zhang Y., Trishul N.M., He M., You C., Yu C., Tan H. Waterborne biodegradable polyurethane 3-dimensional porous scaffold for rat cerebral tissue regeneration. RSC Adv. 2016;6(5):3840–3849. [Google Scholar]
- 296.Unnithan A.R., Gnanasekaran G., Sathishkumar Y., Lee Y.S., Kim C.S. Electrospun antibacterial polyurethane-cellulose acetate-zein composite mats for wound dressing. Carbohydr. Polym. 2014;102:884–892. doi: 10.1016/j.carbpol.2013.10.070. [DOI] [PubMed] [Google Scholar]
- 297.Li B., Brown K.V., Wenke J.C., Guelcher S.A. Sustained release of vancomycin from polyurethane scaffolds inhibits infection of bone wounds in a rat femoral segmental defect model. J. Contr. Release. 2010;145(3):221–230. doi: 10.1016/j.jconrel.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 298.Gholami H., Yeganeh H. Vegetable oil-based polyurethanes as antimicrobial wound dressings: in vitro and in vivo evaluation. Biomed. Mater. 2020;15(4) doi: 10.1088/1748-605X/ab7387. [DOI] [PubMed] [Google Scholar]
- 299.Unnithan A.R., Barakat N.A., Pichiah P.B., Gnanasekaran G., Nirmala R., Cha Y.S., Jung C.H., El-Newehy M., Kim H.Y. Wound-dressing materials with antibacterial activity from electrospun polyurethane-dextran nanofiber mats containing ciprofloxacin HCl. Carbohydr. Polym. 2012;90(4):1786–1793. doi: 10.1016/j.carbpol.2012.07.071. [DOI] [PubMed] [Google Scholar]
- 300.Hensley K., Robinson K.A., Gabbita S.P., Salsman S., Floyd R.A. Reactive oxygen species, cell signaling, and cell injury. Free Radic. Biol. Med. 2000;28(10):1456–1462. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 301.Cui H., Cui L., Zhang P., Huang Y., Wei Y., Chen X. In situ electroactive and antioxidant supramolecular hydrogel based on cyclodextrin/copolymer inclusion for tissue engineering repair. Macromol. Biosci. 2014;14(3):440–450. doi: 10.1002/mabi.201300366. [DOI] [PubMed] [Google Scholar]
- 302.Shiekh P.A., Singh A., Kumar A. Engineering bioinspired antioxidant materials promoting cardiomyocyte functionality and maturation for tissue engineering application. ACS Appl. Mater. Interfaces. 2018;10(4):3260–3273. doi: 10.1021/acsami.7b14777. [DOI] [PubMed] [Google Scholar]
- 303.Gizdavic-Nikolaidis M., Travas-Sejdic J., Bowmaker G.A., Cooney R.P., Kilmartin P.A. Conducting polymers as free radical scavengers. Synth. Met. 2004;140(2–3):225–232. [Google Scholar]
- 304.Gharibi R., Yeganeh H., Rezapour-Lactoee A., Hassan Z.M. Stimulation of wound healing by electroactive, antibacterial, and antioxidant polyurethane/siloxane dressing membranes: in vitro and in vivo evaluations. ACS Appl. Mater. Interfaces. 2015;7(43):24296–24311. doi: 10.1021/acsami.5b08376. [DOI] [PubMed] [Google Scholar]
- 305.De Groot J. Meniscus. Springer; Berlin, Germany: 2010. Actifit, Polyurethane meniscus implant: Basic science; pp. 383–387. [Google Scholar]
- 306.van Minnen B., Stegenga B., van Leeuwen M.B., van Kooten T.G., Bos R.R. A long-term in vitro biocompatibility study of a biodegradable polyurethane and its degradation products. J. Biomed. Mater. Res. 2006;76(2):377–385. doi: 10.1002/jbm.a.30531. [DOI] [PubMed] [Google Scholar]
- 307.Gisselfält K., Edberg B., Flodin P. Synthesis and properties of degradable poly (urethane urea) s to be used for ligament reconstructions. Biomacromolecules. 2002;3(5):951–958. doi: 10.1021/bm025535u. [DOI] [PubMed] [Google Scholar]
- 308.Xu C., Kuriakose A.E., Truong D., Punnakitikashem P., Nguyen K.T., Hong Y. Enhancing anti-thrombogenicity of biodegradable polyurethanes through drug molecule incorporation. J. Mater. Chem. B. 2018;6(44):7288–7297. doi: 10.1039/C8TB01582A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Mejia M.A., Hoyos L.M., Zapata J., Restrepo L.M., Moneada M.E. Electrospinning of gelatin and SMPU with carbon nanotubes for tissue engineering scaffolds. Conf Proc IEEE Eng Med Biol Soc, IEEE. 2016:4181–4184. doi: 10.1109/EMBC.2016.7591648. [DOI] [PubMed] [Google Scholar]
- 310.Murphy S.V., Atala A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 311.Ge C., Priyadarshini L., Cormier D., Pan L., Tuber J. A preliminary study of cushion properties of a 3D printed thermoplastic polyurethane Kelvin foam. Packag. Technol. Sci. 2018;31(5):361–368. [Google Scholar]
- 312.Yang K., Grant J.C., Lamey P., Joshi-Imre A., Lund B.R., Smaldone R.A., Voit W. Diels-alder reversible thermoset 3D printing: isotropic thermoset polymers via fused filament fabrication. Adv. Funct. Mater. 2017;27(24):1700318. [Google Scholar]
- 313.Hsieh F.Y., Hsu S.H. 3D bioprinting: a new insight into the therapeutic strategy of neural tissue regeneration. Organogenesis. 2015;11(4):153–158. doi: 10.1080/15476278.2015.1123360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Franz S., Rammelt S., Scharnweber D., Simon J.C. Immune responses to implants - a review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011;32(28):6692–6709. doi: 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 315.Andorko J.I., Jewell C.M. Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine. Bioeng Transl. Med. 2017;2(2):139–155. doi: 10.1002/btm2.10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Segan S., Jakobi M., Khokhani P., Klimosch S., Billing F., Schneider M., Martin D., Metzger U., Biesemeier A., Xiong X., Mukherjee A., Steuer H., Keller B.M., Joos T., Schmolz M., Rothbauer U., Hartmann H., Burkhardt C., Lorenz G., Schneiderhan-Marra N., Shipp C. Systematic investigation of polyurethane biomaterial surface roughness on human immune responses in vitro. BioMed Res. Int. 2020;2020:3481549. doi: 10.1155/2020/3481549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Sharifpoor S., Labow R.S., Santerre J.P. Synthesis and characterization of degradable polar hydrophobic ionic polyurethane scaffolds for vascular tissue engineering applications. Biomacromolecules. 2009;10(10):2729–2739. doi: 10.1021/bm9004194. [DOI] [PubMed] [Google Scholar]
- 318.Battiston K.G., Labow R.S., Simmons C.A., Santerre J.P. Immunomodulatory polymeric scaffold enhances extracellular matrix production in cell co-cultures under dynamic mechanical stimulation. Acta Biomater. 2015;24:74–86. doi: 10.1016/j.actbio.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 319.Chan J.P., Battiston K.G., Santerre J.P. Synthesis and characterization of electrospun nanofibrous tissue engineering scaffolds generated from in situ polymerization of ionomeric polyurethane composites. Acta Biomater. 2019;96:161–174. doi: 10.1016/j.actbio.2019.06.046. [DOI] [PubMed] [Google Scholar]
- 320.Battiston K.G., Ouyang B., Honarparvar E., Qian J., Labow R.S., Simmons C.A., Santerre J.P. Interaction of a block-co-polymeric biomaterial with immunoglobulin G modulates human monocytes towards a non-inflammatory phenotype. Acta Biomater. 2015;24:35–43. doi: 10.1016/j.actbio.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 321.Huang Y.J., Hung K.C., Hsieh F.Y., Hsu S.H. Carboxyl-functionalized polyurethane nanoparticles with immunosuppressive properties as a new type of anti-inflammatory platform. Nanoscale. 2015;7(48):20352–20364. doi: 10.1039/c5nr06379e. [DOI] [PubMed] [Google Scholar]
- 322.Sen S., Basak P., Prasad Sinha B., Maurye P., Kumar Jaiswal K., Das P., Kumar Mandal T. Anti-inflammatory effect of epidermal growth factor conjugated silk fibroin immobilized polyurethane ameliorates diabetic burn wound healing. Int. J. Biol. Macromol. 2020;143:1009–1032. doi: 10.1016/j.ijbiomac.2019.09.219. [DOI] [PubMed] [Google Scholar]
- 323.Chen T.Y., Wen T.K., Dai N.T., Hsu S.H. Cryogel/hydrogel biomaterials and acupuncture combined to promote diabetic skin wound healing through immunomodulation. Biomaterials. 2021;269:120608. doi: 10.1016/j.biomaterials.2020.120608. [DOI] [PubMed] [Google Scholar]
- 324.Duan Y., Zheng H., Li Z., Yao Y., Ding J., Wang X., Nakkala J.R., Zhang D., Wang Z., Zuo X., Zheng X., Ling J., Gao C. Unsaturated polyurethane films grafted with enantiomeric polylysine promotes macrophage polarization to a M2 phenotype through PI3K/Akt1/mTOR axis. Biomaterials. 2020;246:120012. doi: 10.1016/j.biomaterials.2020.120012. [DOI] [PubMed] [Google Scholar]