Abstract
Time-kill studies examined the activities of telithromycin (HMR 3647), erythromycin A, azithromycin, clarithromycin, roxithromycin, clindamycin, pristinamycin, amoxicillin-clavulanate, and metronidazole against 11 gram-positive and gram-negative anaerobic bacteria. Time-kill studies were carried out with the addition of Oxyrase in order to prevent the introduction of CO2. Macrolide-azalide-ketolide MICs were 0.004 to 32.0 μg/ml. Of the latter group, telithromycin had the lowest MICs, especially against non-Bacteroides fragilis group strains, followed by azithromycin, clarithromycin, erythromycin A, and roxithromycin. Clindamycin was active (MIC ≤ 2.0 μg/ml) against all anaerobes except Peptostreptococcus magnus and Bacteroides thetaiotaomicron, while pristinamycin MICs were 0.06 to 4.0 μg/ml. Amoxicillin-clavulanate had MICs of ≤1.0 μg/ml, while metronidazole was active (MICs, 0.03 to 2.0 μg/ml) against all except Propionibacterium acnes. After 48 h at twice the MIC, telithromycin was bactericidal (≥99.9% killing) against 6 strains, with 99% killing of 9 strains and 90% killing of 10 strains. After 24 h at twice the MIC, 90, 99, and 99.9% killing of nine, six, and three strains, respectively, occurred. Lower rates of killing were seen at earlier times. Similar kill kinetics relative to the MIC were seen with other macrolides. After 48 h at the MIC, clindamycin was bactericidal against 8 strains, with 99 and 90% killing of 9 and 10 strains, respectively. After 24 h, 90% killing of 10 strains occurred at the MIC. The kinetics of clindamycin were similar to those of pristinamycin. After 48 h at the MIC, amoxicillin-clavulanate showed 99.9% killing of seven strains, with 99% killing of eight strains and 90% killing of nine strains. At four times the MIC, metronidazole was bactericidal against 8 of 10 strains tested after 48 h and against all 10 strains after 24 h; after 12 h, 99% killing of all 10 strains occurred.
Anaerobes are established causes of serious human infections, especially in debilitated hosts. Although infections caused by members of the Bacteroides fragilis group occur most commonly, infections caused by other gram-negative anaerobic rods, as well as by gram-positive cocci and clostridia, are increasingly encountered (1, 17). The susceptibility spectrum of clinically isolated anaerobes is changing: although β-lactamase production, and concomitant resistance to β-lactams, is the rule in the B. fragilis group, both phenomena are increasingly encountered in non-B. fragilis-group Bacteroides, Prevotella, Porphyromonas, and Fusobacterium species. β-Lactamase production has also been described in some non-Clostridium perfringens Clostridium species, and metronidazole resistance, apart from being the rule among anaerobic gram-positive non-spore-forming rods, has been reported in peptostreptococci, non-C. perfringens clostridia, and members of the B. fragilis group. Additionally, clindamycin resistance is not unusual among anaerobic gram-negative rods (1).
Previous studies from our laboratory have documented significant antianaerobic activity by agar dilution for some members of the macrolide-azalide-ketolide groups, notably HMR 3647 (telithromycin) and clarithromycin, if CO2 is excluded from the environment (5, 12–14). In order to further examine the antianaerobic activity of the macrolide-azalide-ketolide and streptogramin groups, we used a time-kill method developed in our laboratory (15, 16) to examine the activities of telithromycin, a new ketolide (2, 3), erythromycin A, azithromycin, clarithromycin, roxithromycin, clindamycin, pristinamycin, amoxicillin-clavulanate, and metronidazole. Representatives of the macrolide-azalide-ketolide-streptogramin group were compared with established drugs in the treatment of anaerobic infections, including those not caused by the B. fragilis group (see Discussion).
MATERIALS AND METHODS
Bacteria and antimicrobials.
The 11 anaerobic strains used (1 strain each of B. fragilis, Bacteroides thetaiotaomicron, Prevotella bivia, Prevotella intermedia, Prevotella disiens, Fusobacterium nucleatum, Fusobacterium necrophorum, Peptostreptococcus magnus, Peptostreptococcus asaccharolyticus, Propionibacterium acnes, and C. perfringens) were recent clinical isolates identified by standard procedures (17) and were kept frozen in double-strength skim milk at −70°C until use. β-Lactamase testing was carried out by the nitrocefin disk method (Cefinase; BBL Microbiology Systems, Inc., Cockeysville, Md.) (1). Strains were chosen so as to represent a panel of anaerobes often implicated in human infections (17).
MIC determination.
Unpublished studies have shown that the Oxyrase method cannot be reliably adapted for the testing of all antimicrobials by the microdilution method recommended by the National Committee for Clinical Laboratory Standards (4). For this reason, MICs of all macrolides were determined by using the Oxyrase agar dilution method standardized in our laboratory (12–14), as follows. To 20.5 ml of molten Wilkins-Chalgren agar were added 1.2 ml of antibiotic solution, 1.0 ml of sheep blood, and 2.3 ml of Oxyrase-for-agar solution, containing Oxyrase plus substrates (Oxyrase, Inc., Mansfield, Ohio). This mixture was then poured into OxyDish plates (Oxyrase, Inc.). After plates were inoculated with a Steers replicator, they were sealed with their lids and incubated in air. All MIC plates were incubated at 37°C for 48 h. Clavulanate was added to amoxicillin in a 1:2 ratio. Because CO2 does not influence their MICs, pristinamycin, clindamycin, amoxicillin-clavulanate, and metronidazole were tested by agar dilution (9) in an anaerobic chamber (Coy Laboratory Products, Ann Arbor, Mich.) in CO2. The lowest antibiotic concentration yielding no growth was read as the MIC. Standard quality control strains (12–16) were included in each run.
Time-kill determinations.
All 11 strains were tested in time-kill studies as follows (15, 16). A suspension equal to a 1 McFarland standard was made by suspending approximately five colonies from brucella plates in a tube containing 5 ml of prereduced brucella broth. A 100-μl aliquot was then delivered by syringe into each tube. All inocula were prepared in the chamber. Each tube was filled with 2.7 ml of brucella broth with additives (5% lysed horse blood, 5 μg of hemin/ml, 1 μg of vitamin K/ml), 1 ml of an antibiotic dilution prepared in brucella broth, 200 μl of Oxyrase solution (Oxyrase, Inc.), and 100 μl of inoculum. Amoxicillin-clavulanate was tested without Oxyrase because Oxyrase contains penicillin binding proteins which may inactivate β-lactams (4). Controls without any antibiotic were included in each run.
For each drug, concentrations three dilutions above and three dilutions below the MIC were tested. Viability counts (100 μl) were performed at 0, 6, 12, 24, and 48 h. Plates yielding 30 to 300 colonies were used to determine viability counts. Data were analyzed by expressing growth as the decrease, in log10 CFU per milliliter, compared with the count at 0 h. Bacteriostatic activity was defined as a change in bacterial concentration of 0 to <3 log10 CFU/ml, and bactericidal activity was defined as a change of ≥3 log10 CFU/ml, compared to the count at 0 h. Tests were performed in such a way as to minimize drug carryover by dilution, as described previously (15, 16). Time-kill studies were not performed for metronidazole against P. acnes.
RESULTS
β-Lactamase production was detected in all gram-negative rods except the two fusobacteria. The results of MIC testing are presented in Table 1. Macrolide-azalide-ketolide MICs ranged between 0.004 and 32.0 μg/ml. Of the latter group, telithromycin had the lowest overall MICs, especially against non-B. fragilis-group strains (MICs, 0.004 to 4.0 μg/ml), followed by azithromycin (0.06 to 1.0 μg/ml), clarithromycin (0.016 to 8.0 μg/ml), erythromycin A (0.03 to 16.0 μg/ml), and roxithromycin (0.125 to 32.0 μg/ml). Azithromycin had the lowest MICs of the macrolide-azalide-ketolide group against fusobacteria (0.25 to 0.5 μg/ml), while no member of the group was very active against the B. fragilis group (MICs, 2.0 to 32.0 μg/ml). Clindamycin was active (MIC ≤ 2.0 μg/ml) against all anaerobes except the P. magnus and the B. thetaiotaomicron strain, while pristinamycin MICs ranged between 0.06 and 4.0 μg/ml, and the addition of clavulanate to amoxicillin enhanced β-lactam activity against β-lactamase-positive strains, with MICs of 0.125 to 1.0 μg/ml. All β-lactamase-negative strains were susceptible to amoxicillin, with MICs of 0.016 to 0.25 μg/ml. Metronidazole was active (MICs, 0.03 to 2.0 μg/ml) against all strains except the P. acnes strain.
TABLE 1.
MICs of antibiotics against individual strains
| Strain | MIC (μg/ml)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Telithromycin | Erythromycin | Azithromycin | Clarithromycin | Roxithromycin | Clindamycin | Pristinamycin | Amoxicillin-clavulanate | Metronidazole | |
| B. fragilis | 8.0 | 8.0 | 8.0 | 2.0 | 16.0 | 2.0 | 4.0 | 0.5 | 0.5 |
| B. thetaiotaomicron | 4.0 | 8.0 | 4.0 | 4.0 | 32.0 | 4.0 | 4.0 | 1.0 | 1.0 |
| P. bivia | 0.25 | 1.0 | 0.5 | 0.5 | 1.0 | 0.03 | 0.5 | 1.0 | 2.0 |
| P. intermedia | 0.06 | 0.25 | 0.5 | 0.03 | 0.125 | 0.016 | 0.5 | 0.5 | 2.0 |
| P. disiens | 0.03 | 0.125 | 0.25 | 0.03 | 0.25 | 0.016 | 0.5 | 0.125 | 1.0 |
| F. necrophorum | 2.0 | 2.0 | 0.25 | 2.0 | 16.0 | 0.06 | 1.0 | 0.03 | 0.06 |
| F. nucleatum | 4.0 | 16.0 | 0.5 | 8.0 | 32.0 | 0.125 | 2.0 | 0.06 | 0.03 |
| P. magnus | 0.03 | 4.0 | 1.0 | 0.5 | 4.0 | 8.0 | 0.25 | 0.25 | 0.5 |
| P. asaccharolyticus | 0.03 | 2.0 | 1.0 | 1.0 | 4.0 | 0.125 | 0.25 | 0.25 | 1.0 |
| P. acnes | 0.004 | 0.03 | 0.06 | 0.016 | 0.125 | 0.06 | 0.06 | 0.06 | >16.0 |
| C. perfringens | 0.03 | 0.5 | 0.25 | 0.25 | 1.0 | 0.03 | 0.25 | 0.016 | 1.0 |
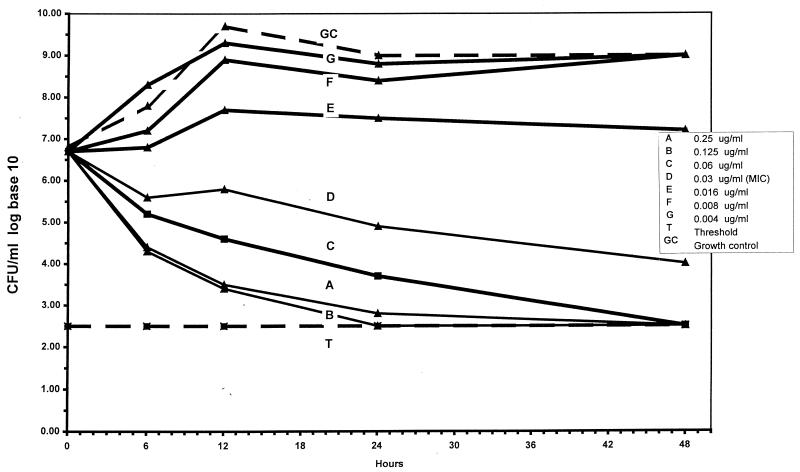
Results of time-kill studies are presented in Table 2. Visual inspection revealed that microdilution MICs for nonmacrolide compounds were all within two dilutions of agar dilution MICs. After 48 h at twice the MIC, telithromycin was bactericidal against 6 strains, with 99% killing of 9 strains and 90% killing of 10 strains. After 24 h at twice the MIC, 90, 99, and 99.9% killing of nine, six, and three strains, respectively, occurred. Lower rates of killing were seen at earlier times. Similar kill kinetics relative to the MIC were seen with erythromycin A, azithromycin, clarithromycin, and roxithromycin. After 48 h at the MIC, clindamycin was bactericidal against 8 strains, with 99 and 90% killing of 9 and 10 strains, respectively. After 24 h at the MIC, 90% killing of 10 strains occurred. The kill kinetics of pristinamycin were similar to those of clindamycin. After 48 h at the MIC, amoxicillin-clavulanate showed 99.9% killing of seven strains, with 99% killing of eight strains and 90% killing of nine strains. The kill kinetics were good compared to those of other compounds, even after 6 h. At four times the MIC, metronidazole was bactericidal against 8 of 10 strains after 48 h and against all 10 strains after 24 h; after 12 h, 99% killing of all 10 strains occurred. The bactericidal activity of telithromycin against one strain of P. magnus is depicted graphically in Fig. 1.
TABLE 2.
Results of time-kill assays
| Drug and concn | No. of strains with the indicated decrease (log10 CFU/ml) in the colony counta at:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 h
|
12 h
|
24 h
|
48 h
|
|||||||||
| −1 | −2 | −3 | −1 | −2 | −3 | −1 | −2 | −3 | −1 | −2 | −3 | |
| Telithromycin | ||||||||||||
| 8× MIC | 5 | 2 | 1 | 6 | 4 | 2 | 9 | 8 | 5 | 11 | 8 | 7 |
| 4× MIC | 4 | 2 | 1 | 6 | 4 | 2 | 9 | 8 | 4 | 11 | 9 | 6 |
| 2× MIC | 4 | 1 | 0 | 3 | 2 | 1 | 9 | 6 | 3 | 10 | 9 | 6 |
| MIC | 2 | 0 | 0 | 4 | 1 | 1 | 6 | 3 | 2 | 6 | 5 | 3 |
| 0.5× MIC | 0 | 0 | 0 | 2 | 1 | 0 | 4 | 2 | 1 | 2 | 2 | 0 |
| Erythromycin A | ||||||||||||
| 8× MIC | 6 | 2 | 1 | 8 | 3 | 1 | 10 | 9 | 5 | 10 | 10 | 9 |
| 4× MIC | 5 | 2 | 1 | 7 | 3 | 1 | 10 | 9 | 5 | 9 | 9 | 9 |
| 2× MIC | 5 | 1 | 1 | 7 | 3 | 1 | 10 | 6 | 5 | 9 | 8 | 6 |
| MIC | 5 | 1 | 1 | 6 | 2 | 1 | 7 | 6 | 4 | 6 | 6 | 5 |
| 0.5× MIC | 2 | 0 | 0 | 4 | 1 | 1 | 5 | 3 | 3 | 6 | 4 | 4 |
| Azithromycin | ||||||||||||
| 8× MIC | 5 | 2 | 1 | 7 | 4 | 3 | 9 | 7 | 4 | 11 | 9 | 7 |
| 4× MIC | 5 | 2 | 1 | 7 | 4 | 3 | 9 | 6 | 4 | 11 | 10 | 7 |
| 2× MIC | 4 | 1 | 1 | 5 | 3 | 3 | 9 | 6 | 3 | 9 | 9 | 7 |
| MIC | 3 | 1 | 1 | 3 | 2 | 1 | 6 | 4 | 2 | 6 | 6 | 2 |
| 0.5× MIC | 0 | 0 | 0 | 3 | 2 | 1 | 4 | 3 | 2 | 5 | 5 | 2 |
| Clarithromycin | ||||||||||||
| 8× MIC | 5 | 2 | 1 | 6 | 3 | 1 | 10 | 9 | 5 | 10 | 10 | 9 |
| 4× MIC | 5 | 2 | 1 | 6 | 3 | 1 | 10 | 8 | 4 | 10 | 10 | 9 |
| 2× MIC | 5 | 1 | 0 | 6 | 1 | 1 | 10 | 8 | 3 | 9 | 9 | 5 |
| MIC | 3 | 0 | 0 | 5 | 1 | 0 | 6 | 3 | 2 | 6 | 5 | 3 |
| 0.5× MIC | 0 | 0 | 0 | 2 | 1 | 0 | 4 | 3 | 2 | 5 | 4 | 2 |
| Roxithromycin | ||||||||||||
| 8× MIC | 3 | 1 | 0 | 7 | 3 | 2 | 10 | 8 | 4 | 10 | 10 | 9 |
| 4× MIC | 3 | 1 | 0 | 6 | 2 | 2 | 10 | 7 | 4 | 10 | 10 | 10 |
| 2× MIC | 2 | 1 | 0 | 6 | 2 | 1 | 9 | 6 | 4 | 9 | 8 | 8 |
| MIC | 1 | 0 | 0 | 6 | 0 | 0 | 8 | 5 | 3 | 7 | 6 | 5 |
| 0.5× MIC | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 1 | 0 | 3 | 2 | 0 |
| Clindamycin | ||||||||||||
| 8× MIC | 4 | 2 | 0 | 8 | 3 | 1 | 10 | 7 | 4 | 10 | 10 | 9 |
| 4× MIC | 4 | 2 | 0 | 8 | 2 | 1 | 10 | 7 | 4 | 10 | 10 | 8 |
| 2× MIC | 3 | 1 | 0 | 8 | 2 | 1 | 10 | 7 | 3 | 10 | 10 | 8 |
| MIC | 2 | 0 | 0 | 7 | 2 | 1 | 10 | 6 | 3 | 10 | 9 | 8 |
| 0.5× MIC | 3 | 0 | 0 | 6 | 2 | 0 | 9 | 3 | 2 | 8 | 7 | 6 |
| Pristinamycin | ||||||||||||
| 8× MIC | 7 | 4 | 0 | 8 | 5 | 2 | 11 | 7 | 4 | 11 | 10 | 7 |
| 4× MIC | 6 | 3 | 0 | 7 | 5 | 1 | 11 | 7 | 4 | 11 | 10 | 8 |
| 2× MIC | 7 | 2 | 0 | 6 | 4 | 1 | 10 | 7 | 4 | 10 | 9 | 8 |
| MIC | 5 | 2 | 0 | 7 | 4 | 2 | 10 | 6 | 4 | 10 | 8 | 7 |
| 0.5× MIC | 4 | 0 | 0 | 4 | 3 | 0 | 8 | 4 | 3 | 5 | 3 | 3 |
| Amoxicillin-clavulanate | ||||||||||||
| 8× MIC | 7 | 6 | 3 | 8 | 8 | 6 | 11 | 8 | 7 | 11 | 11 | 9 |
| 4× MIC | 7 | 5 | 2 | 8 | 7 | 4 | 11 | 8 | 7 | 11 | 10 | 8 |
| 2× MIC | 7 | 5 | 2 | 8 | 7 | 4 | 10 | 7 | 6 | 10 | 9 | 6 |
| MIC | 7 | 4 | 2 | 7 | 7 | 3 | 9 | 7 | 5 | 9 | 8 | 7 |
| 0.5× MIC | 1 | 0 | 0 | 5 | 2 | 1 | 2 | 1 | 1 | 3 | 2 | 2 |
| Metronidazole | ||||||||||||
| 8× MIC | 10b | 8 | 3 | 10 | 10 | 9 | 10 | 10 | 10 | 10 | 10 | 10 |
| 4× MIC | 10 | 7 | 4 | 10 | 10 | 9 | 10 | 10 | 10 | 10 | 10 | 8 |
| 2× MIC | 9 | 6 | 2 | 10 | 10 | 5 | 10 | 9 | 7 | 7 | 6 | 6 |
| MIC | 6 | 4 | 1 | 5 | 3 | 3 | 5 | 3 | 2 | 4 | 2 | 2 |
| 0.5× MIC | 2 | 2 | 0 | 3 | 2 | 0 | 2 | 2 | 1 | 0 | 0 | 0 |
Compared to the colony count at 0 h.
Metronidazole was not tested against P. acnes.
FIG. 1.
Kill kinetics of telithromycin against P. magnus 6165.
DISCUSSION
Telithromycin is a new ketolide active against staphylococci, streptococci (including erythromycin A-resistant Streptococcus pneumoniae and enterococci), Haemophilus influenzae, Moraxella catarrhalis, Neisseria spp., Legionella spp., Helicobacter pylori, Chlamydia spp., mycoplasmas, and nontuberculous mycobacteria (3, 6–8, 10, 11). A previous study from our laboratory (5) has documented that telithromycin possesses good antianaerobic activity against non-B. fragilis group strains. The MICs against anaerobes obtained in our study were similar to those reported by us previously (5). The relative antianaerobic activities of erythromycin A, azithromycin, clarithromycin, and roxithromycin are also in agreement with our previous report (5). The higher activity of azithromycin against fusobacteria compared to other macrolides, and differences between the azithromycin MICs at which 50 and 90% of isolates are inhibited (MIC50 and MIC90) for peptostreptococci were similar to those previously reported by our group (5).
The results of the current study show that all members of the macrolide-azalide-ketolide group showed similar kill kinetics against the anaerobes tested. However, when MICs (5) and kill kinetics were taken together, telithromycin showed the best overall activity, especially against non-B. fragilis group strains. Because of the paucity of strains and different species tested in the current study, however, these conclusions must be considered preliminary. All other nonmacrolide drugs tested showed good kill kinetics, especially after 48 h, with metronidazole killing strains rapidly.
Several prior studies performed in our laboratory have established and confirmed the usefulness of the Oxyrase method for agar dilution and E-test MIC testing of anaerobes in the absence of CO2. It has been established that, for compounds such as metronidazole which are not CO2 dependent, agar dilution MICs obtained by the Oxyrase method do not differ significantly from those obtained in CO2 (12–14). As far as macrolides are concerned, there is, in our opinion, no established method with which to compare the Oxyrase system developed in our laboratory for MIC and time-kill studies of macrolide-azalide-ketolide activity against anaerobes, because of the influence of CO2 on their MICs (12–14). In previous studies performed in our laboratory, the results of time-kill studies in the presence or absence of Oxyrase for drugs not influenced by CO2 were similar (15, 16). Additionally, multiple studies by our group using different strains as well as recognized quality control organisms (9) have demonstrated reproducible macrolide-azalide-ketolide anaerobe MICs by Oxyrase agar dilution for differing strains of the same species (5, 12–14). For these reasons, we are confident of the reliability of the Oxyrase method, as used in the present study, compared with incubation in CO2 for drugs which are not CO2 dependent.
It is recognized that a very small number of strains were tested and that accurate comparisons of differences in kill kinetics cannot be made from the results of the present study. It is also recognized that, for accurate comparison, MICs for time-kill results should all have been obtained by broth microdilution. However, because (i) the Oxyrase method is not readily adaptable to microdilution for all compounds (4), (ii) microdilution MICs for nonmacrolide compounds were all within two dilutions of agar dilution MICs (data not shown), and (iii) time-kill results for all compounds correlated well with agar dilution MICs, we believe that our results can be interpreted reliably. Because Oxyrase is produced from Escherichia coli cell membranes and binds to penicillin binding proteins, it cannot be used for β-lactam susceptibility testing (4). We also realize that interpretation of time-kill studies of strains with different MICs together is not optimal. However, the kill kinetics of all compounds relative to their respective MICs were similar. Additionally, the time-consuming nature of these time-kill experiments makes the testing of many organisms in each species impractical (15, 16). In view of these problems, we believe that conclusions of statistical analyses of our findings would be questionable, and thus such analyses were not performed. The main point of the current time-kill study, in our opinion, is that members of the macrolide-azalide-ketolide group do exhibit a degree of killing against anaerobes, compared to streptogramins, β-lactams, clindamycin, and metronidazole, especially after 48 h. In our opinion, valid statistical analyses can be performed only when many more species, and many more strains in each species, are studied.
The results of the present study, together with the spectrum of activity of telithromycin against aerobes (3, 6–8, 10, 11), suggest clinical potential in the treatment of non-life-threatening mixed anaerobic infections not caused by the B. fragilis group. Examples include infections of the ear, nose, and throat; infections of skin and soft tissue, including bite wounds; and bacterial vaginosis. Conclusions as to the biological potency of telithromycin will also depend on toxicology, pharmacokinetics (2), and animal studies.
ACKNOWLEDGMENTS
This study was supported by a grant from Hoechst-Marion-Roussel Anti-Infectives, Romainville, France.
We thank J. Copeland (Oxyrase, Inc.) for kind provision of Oxyrase and OxyDishes.
Footnotes
This study is dedicated to the memory of Sheila Spangler.
REFERENCES
- 1.Appelbaum P C, Spangler S K, Jacobs M R. Susceptibility of 539 gram-positive and gram-negative anaerobes to new agents, including RP 59500, biapenem, trospectomycin and piperacillin/tazobactam. J Antimicrob Chemother. 1993;32:223–231. doi: 10.1093/jac/32.2.223. [DOI] [PubMed] [Google Scholar]
- 2.Boswell F J, Andrews J M, Wise R. Pharmacodynamic properties of HMR 3647, a novel ketolide, on respiratory pathogens, enterococci and Bacteroides fragilis demonstrated by studies of time-kill kinetics and postantibiotic effect. J Antimicrob Chemother. 1998;41:149–153. doi: 10.1093/jac/41.2.149. [DOI] [PubMed] [Google Scholar]
- 3.Bryskier A, Agouridas C, Chantot J-F. Ketolides: new semisynthetic 14-membered-ring macrolides. In: Zinner S H, Young L S, Acar J F, Neu H C, editors. Expanding indications for the new macrolides, azalides, and streptogramins. New York, N.Y: Marcel Dekker; 1996. pp. 39–50. [Google Scholar]
- 4.Copeland, J. 1998. Personal communication.
- 5.Ednie L M, Jacobs M R, Appelbaum P C. Comparative antianaerobic activities of the ketolides HMR 3647 (RU 66647) and HMR 3004 (RU 64004) Antimicrob Agents Chemother. 1997;41:2019–2022. doi: 10.1128/aac.41.9.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein E J C, Citron D M, Gerardo S H, Hudspeth M, Merriam C V. Activities of HMR 3004 (RU 64004) and HMR 3647 (RU 66647) compared to those of erythromycin, azithromycin, clarithromycin, roxithromycin, and eight other antimicrobial agents against unusual aerobic and anaerobic human and animal bite pathogens isolated from skin and soft tissue infections in humans. Antimicrob Agents Chemother. 1998;42:1127–1132. doi: 10.1128/aac.42.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton-Miller J M T, Shah S. Comparative in-vitro activity of ketolide HMR 3647 and four macrolides against gram-positive cocci of known erythromycin status. J Antimicrob Chemother. 1998;41:649–653. doi: 10.1093/jac/41.6.649. [DOI] [PubMed] [Google Scholar]
- 8.Jones R N, Biedenbach D J. Antimicrobial activity of RU-66647, a new ketolide. Diagn Microbiol Infect Dis. 1997;27:7–12. doi: 10.1016/s0732-8893(96)00181-2. [DOI] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Methods for antimicrobial susceptibility testing of anaerobic bacteria, 4th ed. Approved standard. NCCLS publication no. M11-A4. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 10.Pankuch G A, Visalli M A, Jacobs M R, Appelbaum P C. Susceptibilities of penicillin- and erythromycin-susceptible and -resistant pneumococci to HMR 3647 (RU 66647), a new ketolide, compared with susceptibilities to 17 other agents. Antimicrob Agents Chemother. 1998;42:624–630. doi: 10.1128/aac.42.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soriano F, Fernández-Roblas E, Calvo R, García-Calvo G. In vitro susceptibilities of aerobic and facultative non-spore-forming gram-positive bacilli to HMR 3647 (RU 66647) and 14 other antimicrobials. Antimicrob Agents Chemother. 1998;42:1028–1033. doi: 10.1128/aac.42.5.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spangler S K, Appelbaum P C. Oxyrase, a method which avoids CO2 in the incubation atmosphere for anaerobic susceptibility testing of antibiotics affected by CO2. J Clin Microbiol. 1993;31:460–462. doi: 10.1128/jcm.31.2.460-462.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spangler S K, Jacobs M R, Appelbaum P C. Effect of CO2 on susceptibilities of anaerobes to erythromycin, azithromycin, clarithromycin, and roxithromycin. Antimicrob Agents Chemother. 1994;38:211–216. doi: 10.1128/aac.38.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spangler S K, Jacobs M R, Appelbaum P C. Susceptibilities of 201 anaerobes to erythromycin, azithromycin, clarithromycin, and roxithromycin by Oxyrase agar dilution and E-test methodologies. J Clin Microbiol. 1995;33:1366–1367. doi: 10.1128/jcm.33.5.1366-1367.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spangler S K, Jacobs M R, Appelbaum P C. Time-kill study of the activity of trovafloxacin compared with ciprofloxacin, sparfloxacin, metronidazole, cefoxitin, piperacillin and piperacillin/tazobactam against six anaerobes. J Antimicrob Chemother. 1997;39(Suppl. B):23–27. doi: 10.1093/jac/39.suppl_2.23. [DOI] [PubMed] [Google Scholar]
- 16.Spangler S K, Jacobs M R, Appelbaum P C. Bactericidal activity of DU-6859a compared to activities of three quinolones, three β-lactams, clindamycin, and metronidazole against anaerobes as determined by time-kill methodology. Antimicrob Agents Chemother. 1997;41:847–849. doi: 10.1128/aac.41.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Summanen P, Baron E J, Citron D M, Strong C A, Wexler H M, Finegold S M. Wadsworth anaerobic bacteriology manual. 5th ed. Belmont, Calif: Star Publishing Co.; 1993. [Google Scholar]