Abstract
Background and purpose
There is still much debate whether bridging-therapy [intravenous thrombolysis (IVT) prior to mechanical thrombectomy (MT)] might be beneficial compared to MT alone. We investigated the effect of IVT on size and histological composition of the clots retrieved from patients undergoing bridging-therapy or MT alone.
Methods
We collected mechanically extracted thrombi from 1000 acute ischemic stroke (AIS) patients included in RESTORE registry. Patients were grouped according to the administration (or not) of IVT before thrombectomy. Gross photos of each clot were taken and Extracted Clot Area (ECA) was measured using ImageJ software. Martius Scarlett Blue stain was used to characterize the main histological clot components [red blood cells (RBCs), fibrin (FIB), platelets/other (PTL)] and Orbit Image Analysis was used for quantification. Additionally, we calculated the area of each main component by multiplying the component percent by ECA. Chi-squared and Kruskal–Wallis tests were used for statistical analysis.
Results
451 patients (45%) were treated with bridging-therapy while 549 (55%) underwent MT alone. When considering only percent histological composition, we did not find any difference in RBC% (P = 0.895), FIB% (P = 0.458) and PTL% (P = 0.905). However, bridging-therapy clots were significantly smaller than MT-alone clots [32.7 (14.8–64.9) versus 36.8 (20.1–79.8) mm2, N = 1000, H1 = 7.679, P = 0.006*]. A further analysis expressing components per clot area showed that clots retrieved from bridging-therapy cases contained less RBCs [13.25 (4.29–32.06) versus 14.97 (4.93–39.80) mm2, H1 = 3.637, P = 0.056] and significantly less fibrin [9.10 (4.62–17.98) versus 10.54 (5.57–22.48) mm2, H1 = 7.920, P = 0.005*] and platelets/other [5.04 (2.26–11.32) versus 6.54 (2.94–13.79) mm2, H1 = 9.380, P = 0.002*] than MT-alone clots.
Conclusions
Our results suggest that previous IVT administration significantly reduces thrombus size, proportionally releasing all the main histological components.
Keywords: Stroke, Mechanical thrombectomy, Bridging-therapy, Thrombus histology, Thrombus size
Introduction
Current evidence-based treatments for acute ischemic stroke (AIS) in Large Vessel Occlusion (LVO) include both intravenous thrombolysis (IVT) and mechanical thrombectomy (MT) [1–4]. Current guidelines recommend proceeding with both treatments as soon as possible. Patients may be unsuitable for IVT but still suitable for MT. While the effectiveness of MT compared to IVT alone has been extensively demonstrated [5–10], conflicting results have been reported when comparing bridging-therapy to MT thrombectomy alone [11–15]. Questions remain as to whether pre-treatment with intravenous thrombolytics significantly affects MT success and patient outcome. There are several ongoing randomized controlled clinical trials addressing this issue.
Investigation of the properties of extracted thrombi can also help in this debate, since thrombus properties like size and composition could be influenced by IVT and have clinical significance. In this regard, we recently reported that clots extracted from patients undergoing bridging-therapy are significantly smaller compared to those of patients undergoing MT alone, although there was no difference in final recanalization outcome [16]. Furthermore, several studies have reported the wide heterogeneity in thrombus composition [17–19], which is likely to be influenced by several factors, including etiology and perhaps also thrombolysis [20].
In this study, we investigated the effect of previous IVT administration in clots removed by MT on histological composition of thrombi retrieved from a larger cohort of 1000 AIS patients, both in terms of percentage composition and also as a function of clot size.
Materials and methods
Patient cohort
We collected clots from 1000 AIS patients as part of the RESTORE registry of acute ischemic stroke clots. The RESTORE Registry is a registry of thrombotic material extracted via mechanical thrombectomy from patients suffering from AIS and it accounts for clots collected during the period February 2018 to December 2019 from four stroke centres in Europe. A main aim of the Registry is to analyse clots from AIS patients and correlate clot characteristics with clinical and procedural information. For this reason, patients for which no clot could be extracted are not part of the Registry and we have no information about these patients. This multi-centre prospective study was conducted in accordance with the ethical standards of the Declaration of Helsinki and its amendments [21], by approval of the regional hospital ethics committees and National University of Ireland Galway research ethics committees (16-SEPT-08).
We included only patients > 18 years, having been treated with mechanical thrombectomy for AIS and whose thrombus material was available for analysis. For each patient an anonymized data abstraction form was collected, which contained pertinent procedural data, including if IVT was administered, occlusion location, type of device used for MT, number of passes for clot removal, suspected etiology and final modified Treatment In Cerebral Ischemia (mTICI) score [22]. Grade of reperfusion after endovascular procedure was assessed by anteroposterior/lateral Digital Subtraction Angiography and certified by at least two radiologists at the hospital site. Suspected stroke etiology was reported according to the TOAST classification system [23].
Measurement of extracted thrombus size: Extracted Clot Area
After the endovascular treatment, extracted thrombi were collected per pass and shipped in 10% formalin to NUI Galway, where a gross photo of each was taken with a Canon EOS 1300D Camera. Extracted Clot Area (ECA) was used as an estimate of the size of extracted clot and was measured as previously reported [17, 24, 25]. In brief, to measure the area of a fragment, the gross photo was opened with ImageJ software, the scale was set and the Polygon tool was used to draw a region of interest around the fragment. For passes involving multiple fragments we measured and summed the area of each fragment to obtain the ECA of each pass. Then, for cases involving multiple passes we summed the ECA of each pass to obtain the overall ECA.
Histological component analysis
After having been photographed, thrombi were placed in histological cassettes for tissue processing and were paraffin embedded. 3 µm sections were cut with a microtome. Martius Scarlett Blue (MSB) staining was performed to identify the standard clot tissue components: red blood cells (RBCs), white blood cells (WBCs), fibrin (FIB), platelets/other (PTL) and non-typical thrombus components such as collagen and calcification [26]. We used machine-learning Orbit image analysis (www.Orbit.bio) for quantification purposes [27, 28]. Briefly, we created exclusion and inclusion models to distinguish regions to be excluded (e.g. background and artefact) and regions containing the tissue components of interest, enabling quantitative assessment of the proportion of each component within each clot.
In addition, we calculated the area occupied by each main component (RBC, FIB, PTL) by multiplying the component percent by the relevant ECA. For cases involving multiple passes, we summed the values of ECAxComponent for all passes.
Statistical analysis
IBM SPSS-25 software was used for statistical analysis. Kolmogorov–Smirnov test and Shapiro–Wilk test indicated that quantitative variables did not follow a standard normal distribution. Therefore, the non-parametric chi-squared and Kruskal–Wallis tests were used to assess statistically significant difference among the groups, with a level of statistical significance set at p < 0.05 (two-sided). Results are reported as median [IQ1-IQ3] or number and % of cases.
Results
Baseline characteristics of the patients
Among the 1000 cases considered, 451 patients (45%) were treated with bridging-therapy while 549 (55%) were treated with mechanical thrombectomy alone. The total number of passes performed was 2365, with at least some clot material extracted in 1496 passes (63%), while 869 attempts were performed with no clot extraction (37%). Baseline clinical characteristics of both groups of patients are reported in Table 1. There was no significant difference between the two populations in terms of suspected etiology (X2 = 3.771, P = 0.438), first-line approach used (X2 = 2.417, P = 0.120) or revascularisation outcome (H1 = 1.189, P = 0.275); Table 1.
Table 1.
Baseline clinical characteristics of the overall cohort of patients and divided by the two groups of patients, bridging-therapy and mechanical thrombectomy alone
| Suspected etiology* | Overall cohort of patients (N = 1000) | Bridging-therapy cases (N = 451) | Mechanical thrombectomy-alone cases (N = 549) |
|---|---|---|---|
| Patients with cardioembolic suspected etiology | 346 (34.6%) | 152 (33.7%) | 194 (35.3%) |
| Patients with large artery atherosclerosis suspected etiology | 221 (22.1%) | 111 (24.6%) | 110 (20.0%) |
| Patients with other suspected etiologya | 55 (5.5%) | 22 (4.9%) | 33 (6.0%) |
| Patients with cryptogenic etiology | 255 (25.5%) | 115 (25.4%) | 140 (25.5%) |
| First line approachb | |||
| Aspiration | 657 (65.7%) | 285 (63.2%) | 372 (67.8%) |
| Stentriever | 342 (34.2%) | 166 (36.8%) | 176 (32.1%) |
| Median number of attempts performed during the endovascular procedure | 2 (1–3) | 2 (1–3) | 2 (1–3) |
| Final mTICI scorec | |||
| mTICI 0 | 17 (1.7%) | 6 (1.3%) | 11 (2.0%) |
| mTICI 1 | 12 (1.2%) | 6 (1.3%) | 6 (1.1%) |
| mTICI 2a | 62 (6.3%) | 30 (6.7%) | 32 (6.0%) |
| mTICI 2b | 257 (26.1%) | 106 (23.7%) | 151 (28.1%) |
| mTICI 2c | 207 (21.0%) | 97 (21.7) | 110 (20.5%) |
| mTICI 3 | 429 (43.6%) | 202 (45.2%) | 227 (42.3%) |
Data given as N (%) of cases or median (IQ1, IQ3)
aOther suspected etiology included: arterial dissection, pulmonary embolism, hypercoagulable states, or hematologic disorders
bIn 1 case a gooseneck snare has been used as first-line approach to remove a calcified clot
cIn 16 cases involving posterior circulation occlusion, it was not possible to assess final recanalization outcome (4 cases for bridging-therapy and 12 for MT alone)
*Suspected etiology was not recorded for 123 patients (12.3%), 51 of which were treated with bridging-therapy (11.3%) and 72 with MT alone (13.11%)
Occluded vessel location
Occluded vessel location was defined by an expert radiologist at the hospital site at the beginning of procedure. In the majority of the cases, (881, 88%) the ischemic territory was located in the anterior circulation. In 100 cases (10%), the occlusion was located in the posterior circulation, while in 17 cases (2%) both anterior and posterior territories were involved. Occlusion involved a single vessel in 78% of cases (782 single occlusions), while in 216 cases (22%), two or more vessels were occluded at the same time. Specific vessels occluded, for the overall population, and according previous IVT administration, are reported in Table 2. There was no significant difference in occlusion type observed between the bridging-therapy and mechanical thrombectomy-alone groups (anterior/posterior occlusion: X2 = 4.575, P = 0.102; singular/multiple occlusion: X2 = 0.061, P = 0.804).
Table 2.
Occluded vessels in the whole cohort of patients and in the two groups, bridging-therapy and mechanical thrombectomy alone
| Occlusion type | Occluded vessel(s) | All the cases (N = 1000) | Bridging-therapy cases (N = 451) | Mechanical thrombectomy-alone cases (N = 549) |
|---|---|---|---|---|
| Singular occlusion (N = 781) | MCA,M1 | 424 (42.4%) | 202 (44.9%) | 222 (40.4%) |
| MCA,M2 | 124 (12.5%) | 53 (11.8%) | 71 (13.0%) | |
| MCA,M3 | 5 (0.5%) | 4 (0.9%) | 1 (0.2%) | |
| MCA not specified | 10 (1.0%) | 8 (1.8%) | 2 (0.4%) | |
| Vertebro/basilar | 78 (7.8%) | 26 (5.8%) | 52 (9.5%) | |
| ICA and ICA terminus | 131 (13.1%) | 55 (12.2%) | 76 (13.9%) | |
| PCA | 8 (0.8%) | 2 (0.4%) | 6 (1.1%) | |
| ACA | 2 (0.2%) | 1 (0.2%) | 1 (0.2%) | |
| Multiple occlusion (N = 216) | Tandem occlusions | 109 (11%) | 52 (11.6%) | 57 (10.4%) |
| Other dual occlusions | 41 (4.1%) | 19 (4.2%) | 22 (4.0%) | |
| MCA, multiple segments/branches | 27 (2.7%) | 12 (2.7%) | 15 (2.7%) | |
| Three or more occlusions | 39 (3.9%) | 16 (3.6%) | 23 (4.2%) |
Data given as N (%) of cases
MCA middle cerebral artery, ICA internal carotid artery, PCA posterior cerebral artery, ACA anterior cerebral artery, CCA common carotid artery
Analysis of Extracted Clot Area
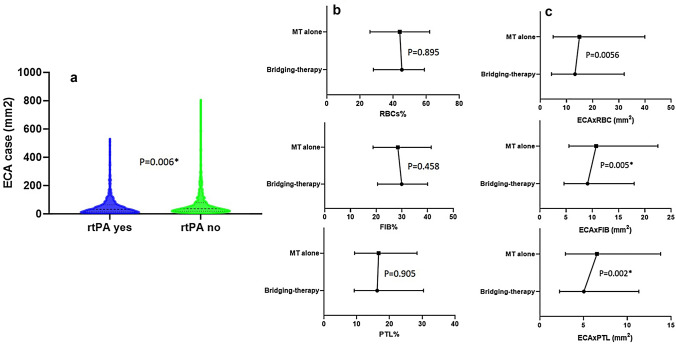
Analysis of size of extracted clot (expressed as Extracted Clot Area) showed that clots from patients undergoing bridging-therapy were statistically significantly smaller than clots retrieved from patients treated with MT alone, (Fig. 1a; median ECA was, respectively, 32.7 [14.8–64.9] versus 36.8 [20.1–79.8] mm2 N = 1000, H1 = 7.679, P = 0.006*).
Fig. 1.
Violin plot depicting variation and difference in Extracted Clot Area (a); Median values with IQ[1–3] range depicting difference in percentage of main histological components (b) and the area occupied by each main component, (expressed as ECA multiplied by component fraction) (c), between clots retrieved from patients undergoing bridging-therapy or mechanical thrombectomy alone
Bridging-therapy clots were also associated with a significantly lower number of fragments compared to MT alone (2 [1–4] versus 3 [2–5], N = 1000, H1 = 4.058, P = 0.044*).
Histological components analysis
Histological component analysis revealed no difference in main histological components between bridging-therapy and MT-only clots (Fig. 1b). Median RBCs composition was 45 [28–59]% for bridging-therapy and 44 [26–62]% for MT-alone clots (H1 = 0.018, P = 0.895); median FIB composition was 30 [21–40]% for bridging-therapy and 29 [19–41]% for MT alone (H1 = 0.552, P = 0.458); median PTL composition was 16 [9–30]% for bridging-therapy and 17 [9–28]% for MT alone (H1 = 0.014, P = 0.905).
Further analysis was then performed combining size and histological composition (Fig. 1c). The amount of RBC was lower in bridging-therapy compared to MT-only clots, although not statistically significant (median ECAxRBC was 13.25 [4.29–32.06] mm2 versus 14.97 [4.93–39.80] mm2, H1 = 3.637, P = 0.056). The amount of FIB and PTL was statistically significantly lower in bridging-therapy clots compared to MT-only clots (respectively, median ECA × FIB was 9.10 [4.62–17.98] mm2 versus 10.54 [5.57–22.48] mm2, H1 = 7.920, P = 0.005* and median ECA × PTL was 5.04 [2.26–11.32] mm2 versus 6.54 [2.94–13.79] mm2, H1 = 9.380, P = 0.002*), Fig. 1c.
Discussion
There are several studies in the literature comparing final recanalization rate and other clinical outcomes for patients treated with bridging-therapy or MT alone, although there is not yet a clear consensus of opinion [11–16]. Nevertheless, bridging-therapy, i.e. the combination of IVT and mechanical thrombectomy, is currently the standard of care for large vessel occlusion (LVO) patients with a NIHSS-score of 6 or greater [29] provided that they fulfil tPA eligibility criteria [1–4]. Although it could be very informative, there is very little published about the influence of IVT on the characteristics of the retrieved clot, such as its general composition and structural organization. Our recent study comparing the size of extracted thrombi showed that bridging-therapy clots were significantly smaller than MT-only clots [16], a finding that is reproduced in the present larger study.
In the present study, we sought to assess if thrombolysis also altered clot composition. In this respect, we investigated percentage composition of main components and we also undertook a combined size and compositional analysis. Red blood cells were the main histological component for both groups of clots, followed by fibrin and then by platelets/other. We did not find any difference between the clots retrieved from the two groups of patients in terms of percent composition of main histological components. However, when size was taken into account, bridging-therapy clots contained less RBC (mm2) and statistically significantly less fibrin and platelets/other (mm2) compared to clots from patients treated with MT alone, directly relatable to the smaller size of bridging-therapy clots. Whilst the effect on RBC (mm2) did not quite reach statistical significance, the very clear trend suggests that tPA administration reduces the content of all main components proportionally.
A previous study observed that previous IVT administration was associated with prominent changes in the architecture of the fibrin components and platelet count [30] of the retrieved clots. In particular, the phenomenon was termed as “thinning”, referring to the dissociation of superficial layers of fibrin fibres, which was remarkably notable only in clots retrieved from patients treated with bridging-therapy [30]. An earlier study observed how clots retrieved after IVT treatment were more porous and with more branching points suggesting that thrombolytic therapy is associated with significant changes in the structural composition, and in particular in the fibrin network architecture of the retrieved clot [31]. Also a recent study investigating 3D organization of AIS thrombi showed how fibrin formed a grid-like or a sponge-like pattern in clots from patients pre-treated with IVT, with thinner and less densely packed fibrin fibres [32].
However, it has been also observed that sensitivity to fibrinolytic agents might depend on original thrombus composition. In this respect, RBC-rich thrombi seem to have higher sensitivity to rtPA [33, 34] probably due to their looser architecture [35], which allows for an increased penetration of thrombolytic agents into the clot [36].
Our findings show that IVT administration, even when it does not result in the complete dissolution of the occlusive thrombi, will reduce clot size. Our findings also suggest that fibrinolytic activity of IVT causes a proportional decrease of the three main histological clot components, maintaining the percentage ratio of main components within the clot. However, we acknowledge that we have no information of clot composition before mechanical thrombectomy, which would be needed for confirmation. Our findings also showed that bridging-therapy resulted in a lower number of fragments retrieved than with mechanical thrombectomy alone. However, further study is needed before any definitive conclusion can be drawn, as many factors during thrombectomy and during clot processing could impact on the number of fragments.
Study limitations and strengths
It must be acknowledged that our study on the effect of tPA is limited to cases where mechanical thrombectomy was performed and at least part of the clot was extracted. We do not have information for patients that recanalised after IVT alone or for those that had unsuccessful thrombectomy, with no clot extracted. The two patient cohorts differ clinically regarding eligibility for tPA. MT-only patients would have been ruled ineligible for tPA treatment for reasons such as time window exclusion, risk of haemorrhage or ongoing anticoagulation for atrial fibrillation [1–4]. Also, length of time since stroke onset could influence thrombus size. Patients receiving bridging-therapy often have a shorter time window from symptoms onset to hospital admission. Further studies should address the effect of time frame on thrombus size. Furthermore, we also acknowledge the lack of complete information about eventual anticoagulation regimes of the patient, dosage and specific thrombolytic agents administered to bridging-therapy patients, although this should be addressed in future studies.
However, despite these limitations, we observed a good similarity between the two groups of patients in terms of baseline and procedural characteristics including suspected etiology, occluded vessel treated, first-line approach for endovascular procedure and final revascularisation outcome. Furthermore, the main strength of this study is the large patient population analysed, arising from four dedicated stroke centres in Europe and analysed by a specialized core laboratory, which gives robustness to our findings.
Conclusion
The present study highlighted interesting differences between AIS thrombi retrieved from patients treated with bridging-therapy or MT alone. Bridging-therapy clots are smaller and with significantly reduced fibrin and platelet/other area (mm2), but with a percentage of components similar to the MT-alone clots. These findings indicate that IVT administration significantly reduces the size of the thrombus, proportionally releasing all main histological components while carrying out its fibrinolytic activity.
Author contributions
KD obtained the funds for the study, developed the study design, coordinated its implementation and supervised the writing of the manuscript; RR participated in samples collection, composed the manuscript, performed the statistical analysis and wrote the results and discussion; SMG performed the ECA measurements, the MSB staining, scanning and quantification; AD, OMM, SF and CT participated in samples collection, the MSB staining, scanning and quantification; PB, SP, AO, GM, GT, AN, AV, KJ, PR, AN, EC, DD, JC, KP, AR and JT performed the thrombectomy at the several hospitals, evaluated the final recanalization outcome and collected samples and procedural data; KP, GT, IS, TT, AR and JT contributed to the study design and were responsible thrombus collection at the relevant stroke center; AP, MG and RM contributed to develop the study design and funding acquisition. All the authors have read and reviewed the manuscript.
Funding
Open Access funding provided by the IReL Consortium. This publication has emanated from research conducted with the financial support of Science Foundation Ireland (SFI) and is co-funded under the European Regional Development Fund under Grant Number 13/RC/2073_2. Furthermore, it was supported by Cerenovus.
Data availability
Data may be available upon reasonable request.
Code availability
Not applicable.
Declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethical approval
This study has been conducted in accordance with the ethical standards of the Declaration of Helsinki and its amendments, by approval of the regional hospital ethics committees and National University of Ireland Galway research ethics committees (16-SEPT-08).
Consent to participate
Written informed consent or a waiver of consent was obtained from the patient(s) for their anonymized information to be published in this article.
Consent for publication
Not applicable.
References
- 1.Berge E, Whiteley W, Audebert H, et al. European stroke organisation (eso) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021;6:I-LXII. doi: 10.1177/2396987321989865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powers William J, Rabinstein Alejandro A, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2019;50:e344–e418. doi: 10.1161/STROKEAHA.118.022606. [DOI] [PubMed] [Google Scholar]
- 3.Turc G, Bhogal P, Fischer U, et al. European stroke organisation (eso)-European society for minimally invasive neurological therapy (esmint) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg. 2019 doi: 10.1136/neurintsurg-2018-014569. [DOI] [PubMed] [Google Scholar]
- 4.Broderick Joseph P, Hill Michael D. Advances in acute stroke treatment 2020. Stroke. 2021;52:729–734. doi: 10.1161/STROKEAHA.120.033744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkhemer OA, Fransen PS, Beumer D, MRCLEAN Investigators et al. A randomized trial of intra-arterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 6.Campbell BC, Mitchell PJ, Kleinig TJ, EXTEND-IA Investigators et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 7.Goyal M, Demchuk AM, Menon BK, ESCAPE Trial Investigators et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 8.Saver JL, Goyal M, Bonafe A, SWIFT PRIME Investigators et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 9.Jovin TG, Chamorro A, Cobo E, REVASCAT Trial Investigators et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 10.Bracard S, Ducrocq X, Mas JL, THRACE Investigators et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15:1138–1147. doi: 10.1016/S1474-4422(16)30177-6. [DOI] [PubMed] [Google Scholar]
- 11.Katsanos AH, Malhotra K, Goyal N, et al. Intravenous thrombolysis prior to mechanical thrombectomy in large vessel occlusions. Ann Neurol. 2019;86(3):395–406. doi: 10.1002/ana.25544. [DOI] [PubMed] [Google Scholar]
- 12.Fischer U, Kaesmacher J, Molina CA, Selim MH, Alexandrov AV, Tsivgoulis G. Primary thrombectomy in tPA (tissue-type plasminogen activator) eligible stroke patients with proximal intracranial occlusions. Stroke. 2018;49(1):265–326. doi: 10.1161/STROKEAHA.117.018564. [DOI] [PubMed] [Google Scholar]
- 13.Abilleira S, Ribera A, Cardona P, et al. Outcomes after direct thrombectomy or combined intravenous and endovascular treatment are not different. Stroke. 2017;48:375–378. doi: 10.1161/STROKEAHA.116.015857. [DOI] [PubMed] [Google Scholar]
- 14.Rai AT, Boo S, Buseman C, et al. Intravenous thrombolysis before endovascular therapy for large vessel strokes can lead to significantly higher hospital costs without improving outcomes. J Neurointerv Surg. 2018;10:17–21. doi: 10.1136/neurintsurg-2016-012830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goyal N, Tsivgoulis G, Pandhi A, Malhotra K, Krishnan R, Ishfaq MF, Krishnaiah B, Nickele C, Inoa-Acosta V, Katsanos AH, Hoit D, Elijovich L, Alexandrov A, Arthur AS. Impact of pretreatment with intravenous thrombolysis on reperfusion status in acute strokes treated with mechanical thrombectomy. J Neurointerv Surg. 2019;11(11):1073–1079. doi: 10.1136/neurintsurg-2019-014746. [DOI] [PubMed] [Google Scholar]
- 16.Rossi R, Fitzgerald S, Molina S, et al. The administration of rtPA before mechanical thrombectomy in acute ischemic stroke patients is associated with a significant reduction of the retrieved clot area but it does not influence revascularization outcome. J Thromb Thrombolysis. 2021;51(2):545–551. doi: 10.1007/s11239-020-02279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzgerald S, Rossi R, Mereuta OM, et al. Per-pass analysis of acute ischemic stroke clots: impact of stroke etiology on extracted clot area and histological composition. J Neurointerv Surg. 2020 doi: 10.1136/neurintsurg-2020-016966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Douglas A, Fitzgerald S, Mereuta OM, et al. Platelet-rich emboli are associated with von Willebrand factor levels and have poorer revascularization outcomes. J NeuroInterv Surg. 2020;12:557–562. doi: 10.1136/neurintsurg-2019-015410. [DOI] [PubMed] [Google Scholar]
- 19.De Meyer SF, Andersson T, Baxter B, et al. Analyses of thrombi in acute ischemic stroke: a consensus statement on current knowledge and future directions. Int J Stroke. 2017;12(6):606–614. doi: 10.1177/1747493017709671. [DOI] [PubMed] [Google Scholar]
- 20.Jolugbo P, Ariëns RAS. Thrombus composition and efficacy of thrombolysis and thrombectomy in acute ischemic stroke. Stroke. 2021;52(3):1131–1142. doi: 10.1161/STROKEAHA.120.032810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WMA World medical association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 22.Goyal M, Fargen KM, Turk AS, et al. 2C or not 2C: defining an improved revascularization grading scale and the need for standardization of angiography outcomes in stroke trials. J Neurointerv Surg. 2014;6(2):83–86. doi: 10.1136/neurintsurg-2013-010665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.STR.24.1.35. [DOI] [PubMed] [Google Scholar]
- 24.Fitzgerald S, Rossi R, Mereuta OM, et al. Large artery atherosclerotic clots are larger than clots of other stroke etiologies and have poorer recanalization rates. J Stroke Cerebrovasc Dis. 2021;30(1):105463. doi: 10.1016/j.jstrokecerebrovasdis.2020.105463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossi R, Fitzgerald S, Mereuta OM, et al. Correlation between acute ischaemic stroke clot length before mechanical thrombectomy and extracted clot area: impact of thrombus size on number of passes for clot removal and final recanalization. Eur Stroke J. 2021 doi: 10.1177/23969873211024777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzgerald ST, Wang S, Dai D, et al. Platelet-rich clots as identified by Martius scarlet blue staining are isodense on NCCT. J Neurointerv Surg. 2019;11:1145–1149. doi: 10.1136/neurintsurg-2018-014637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fitzgerald S, Wang S, Dai D, et al. Orbit image analysis machine learning software can be used for the histological quantification of acute ischemic stroke blood clots. PLoS ONE. 2019;14:e0225841. doi: 10.1371/journal.pone.0225841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stritt M, Stalder AK, Vezzali E. Orbit image analysis: An open-source whole slide image analysis tool. PLoS Comput Biol. 2020;16:e1007313. doi: 10.1371/journal.pcbi.1007313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer BC, Lyden PD, Faha F. The modified national institutes of health stroke scale (mNIHSS): its time has come. Int J Stroke. 2009;4(4):267–273. doi: 10.1111/j.1747-4949.2009.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krajíčková D, Krajina A, Šteiner I, et al. Fibrin clot architecture in acute ischemic stroke treated with mechanical thrombectomy with stent-retrievers-cohort study. Circ J. 2018;82:866–873. doi: 10.1253/circj.CJ-17-0375. [DOI] [PubMed] [Google Scholar]
- 31.Stanford SN, Sabra A, D’Silva L, et al. The changes in clot microstructure in patients with ischaemic stroke and the effects of therapeutic intervention: a prospective observational study. BMC Neurol. 2015;15:35. doi: 10.1186/s12883-015-0289-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mereuta OM, Fitzgerald S, Christensen TA, Jaspersen AL, Dai D, Abbasi M, Puttappa T, Kadirvel R, Kallmes DF, Doyle KM, Brinjikji W. High-resolution scanning electron microscopy for the analysis of three-dimensional ultrastructure of clots in acute ischemic stroke. J Neurointerv Surg. 2020 doi: 10.1136/neurintsurg-2020-016709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi MH, Park GH, Lee JS, Lee SE, Lee SJ, Kim JH, Hong JM. Erythrocyte fraction within retrieved thrombi contributes to thrombolytic response in acute ischemic stroke. Stroke. 2018;49:652–659. doi: 10.1161/STROKEAHA.117.019138. [DOI] [PubMed] [Google Scholar]
- 34.Kim YD, Nam HS, Kim SH, et al. Time-dependent thrombus resolution after tissue-type plasminogen activator in patients with stroke and mice. Stroke. 2015;46:1877–1882. doi: 10.1161/STROKEAHA.114.008247. [DOI] [PubMed] [Google Scholar]
- 35.Gersh KC, Nagaswami C, Weisel JW. Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thromb Haemost. 2009;102:1169–1175. doi: 10.1160/TH09-03-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carr ME, Jr, Hardin CL. Fibrin has larger pores when formed in the presence of erythrocytes. Am J Physiol. 1987;253(5 Pt 2):H1069–H1073. doi: 10.1152/ajpheart.1987.253.5.H1069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data may be available upon reasonable request.
Not applicable.