Abstract
The addition of pertuzumab (P) to trastuzumab (H) and neoadjuvant chemotherapy (NAC) has decreased the risk of distant recurrence in early stage HER2-positive breast cancer. The incidence of brain metastases (BM) in patients who achieved pathological complete response (pCR) versus those who do not is unknown. In this study, we sought the incidence of BM in patients receiving HP-containing NAC as well as survival outcome. We reviewed the medical records of 526 early stage HER2-positive patients treated with an HP-based regimen at Memorial Sloan Kettering Cancer Center (MSKCC), between September 1, 2013 to November 1, 2019. The primary endpoint was to estimate the cumulative incidence of BM in pCR versus non-pCR patients; secondary endpoints included disease free-survival (DFS) and overall survival (OS). After a median follow-up of 3.2 years, 7 out of 286 patients with pCR had a BM while 5 out of 240 non-pCR patients had a BM. The 3-year DFS was significantly higher in the pCR group compared to non-pCR group (95% vs 91 %, p = 0.03) and the same trend was observed for overall survival. In our cohort, despite the better survival outcomes of patients who achieved pCR, we did not observe appreciable differences in the incidence of BM by pCR/non-pCR status. This finding suggests that the BM incidence could not be associated with pCR. Future trials with new small molecules able to cross the blood brain barrier should use more specific biomarkers rather than pCR for patients’ selection.
Subject terms: Cancer epidemiology, Breast cancer, Breast cancer
Introduction
Central nervous system (CNS) is a common site of distant recurrence that affects prognosis and quality of life of HER2-positive breast cancer (BC) patients1. The reported cumulative incidence of brain metastases (BM) in HER2 positive BC is higher than in other subtypes suggesting that HER2 positive cancer cells have a specific tropism for the CNS2,3. The advent of different anti-HER2 agents and the implementation of local approaches such as stereotactic radiosurgery (SRS) has significantly improved the prognosis of HER2- positive BC patients with BM. However, BM still presents multiple challenges for optimal management, especially in the scenario of progression despite loco-regional therapies. New oral HER2 tyrosine kinase inhibitors (TKIs) including neratinib and tucatinib, have demonstrated CNS activity, and have been recently approved by Food and Drug Administration (FDA) in metastatic setting4,5. In early stage, neratinib is currently approved as single agent after trastuzumab-based adjuvant therapy6 and tucatinib is still under investigation in high-risk patients in combination with T-DM1 (NCT04457596).
In stages I-III BC, the CNS recurrence rate is reported around 2–4% of patients receiving trastuzumab and/or pertuzumab- based adjuvant treatments as first site of recurrence in a follow-up range of 3–5 years7–9. Few studies have reported the rate of BM in early-stage breast cancer patients treated with neoadjuvant chemotherapy (NAC). The addition of pertuzumab to trastuzumab and chemotherapy in HER2-positive BC has resulted in an improvement of pathologic complete response (pCR) rate after NAC10. Currently, the rate of BM and the predictive role of pCR on the risk of CNS seeding is unknown in patients receiving double blockade with trastuzumab and pertuzumab (HP) in preoperative setting. The interest in understanding the incidence of CNS recurrence in a real-life population arises from the necessity to shape new strategies to reduce the risk of BM in patients with early-stage BC. The aim of this study was to assess the incidence of BM in patients receiving HP-containing NAC and to compare rates of BM stratified by pCR status.
Results
Study population
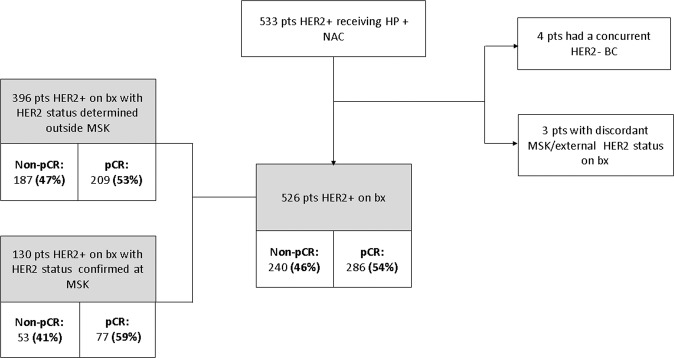
Overall, 533 patients with stage I-III HER2 positive breast cancer treated with NAC followed by surgery at MSKCC were identified. Cases with a concomitant HER2 negative BC (n = 4) and discordant HER2 status (internal versus external) (n = 3) were excluded. Among the study population (n = 526), 130 patients had preoperative HER2 status confirmed at MSKCC (Fig. 1). All clinicopathological features are described in Table 1. A pCR was achieved in 286/526 (54.4%) of cases, whereas 240/526 (45.6%) had residual disease. The majority of the patients (278/286, 97% in pCR and 226/240, 94% in non-pCR group) had a poorly differentiated breast carcinoma. The clinical stage II was the prevalent stage at the time of diagnosis (74% and 72% in pCR and non-pCR groups, respectively). In both groups, most of patients received dose dense chemotherapy with doxorubicin/cyclophosphamide followed by paclitaxel plus HP (AC-THP) as NAC (90% vs 86% in pCR and non-pCR respectively). Anthracycline-free therapy with docetaxel, carboplatin plus HP (DCbHP regimen) was administered in less than 10% of cases, in both groups. A minority of patients received vinorelbine and gemcitabine, when taxane was contraindicated (Table 1).
Fig. 1. Diagram of patients’ selection.
This schema represents a consort diagram of the study and provides the patients selection based on HER2 status on biopsy and response to neoadjuvant treatment (pCR versus non-pCR). Notes: HER2+: HER2-positive, HER2−: HER2-negative, pts: patients; BC: breast cancer; bx: biopsy.
Table 1.
Patients’ characteristics.
| Overall population (n = 526) | MSKCC HER2-status confirmed on biopsy (n = 130) | |||||
|---|---|---|---|---|---|---|
| pCR n (%) n = 286 (54.4) | non-pCR n (%) n = 240 (45.6) | p-value* | pCR n (%) n = 77 (59.2) | non-pCR n (%) n = 53 (40.7) | p-value* | |
| Median age, years (range 26–82) | 50 (24-84) | 50 (26-87) | 0.078 | 50 (28-82) | 49 (26-76) | |
| Menopausal status | 0.2 | |||||
| Pre | 145 (50.7) | 137 (57) | 38 (49) | 28 (53) | ||
| Post | 141 (49.3) | 103 (43) | 39 (51) | 25 (47) | ||
| Clinical stage | 0.010 | |||||
| I | 16 (5.6) | 3 (1) | 1 (1.3) | 36 (68) | ||
| II | 212 (74.2) | 173 (72) | 58 (75.3) | 15 (28) | ||
| III | 58 (20.2) | 64 (27) | 18 (23.4) | 2 (4) | ||
| Clinical T | 0.042 | |||||
| Tx | 6 (2) | 0 | 4 (5.2) | 0 | ||
| T1 | 47 (16.4) | 23 (9.6) | 6 (7.8) | 3 (5.7) | ||
| T2 | 171 (59.7) | 153 (63.7) | 47 (61) | 38 (71.8) | ||
| T3 | 41(14.3) | 44 (18.3) | 16 (20.8) | 9 (16.9) | ||
| T4 | 15 (5.2) | 15 (6.3) | 3 (3.9) | 2 (3.8) | ||
| T4d | 6 (2) | 5 (2.1) | 1 (1.3) | 1 (1.8) | ||
| Lymph nodes involvement (clinical staging) | 114 (39.8) | 84 (35) | 0.3 | 27 (35) | 19 (35.9) | |
| N0 | 147 (51.5) | 128 (53.4) | 42 (54.5) | 27 (50.9) | ||
| N1 | 15 (5.2) | 21 (8.7) | 6 (7.8) | 5 (9.5) | ||
| N2 | 10 (3.5) | 7 (2.9) | 2 (2.7) | 2 (3.7) | ||
| N3 | ||||||
| HER2 status on biopsy | <0.001 | |||||
| IHC 3+ | 268 (94) | 164 (68.3) | 76 (99) | 27 (51) | ||
| FISH amplified | 18 (6) | 76 (31.7) | 1 (1) | 26 (49) | ||
| HR status on biopsy | <0.001 | |||||
| Positive | 153(54) | 192 (80) | 41 (52) | 38 (70) | ||
| Negative | 133 (46) | 48 (20) | 36 (48) | 15 (30) | ||
| Histology on biopsy | NA** | |||||
| Ductal | 284 (99.3) | 236 (98.3) | 76 (99) | 52 (99) | ||
| Lobular | 2 (0.7) | 4 (1.7) | 1 (1) | 1 (1) | ||
| Differentiation | 0.2 | |||||
| Well/moderated | 8 (2.7) | 14 (5.8) | 6 (8) | 13 (24.5) | ||
| Poorly differentiated | 278 (97.3) | 226 (94.2) | 71 (92) | 40 (75.5) | ||
| NAC regimens | 0.4 | |||||
| ACTHP | 256 (89.5) | 206 (85.8) | 69 (90) | 48 (90) | ||
| DCbHP | 18 (6.3) | 22 (9.2) | 3 (4) | 2 (4) | ||
| Other | 12 (4.2) | 12 (5) | 5 (6) | 3 (6) | ||
| Type of breast surgery | 0.70 | |||||
| Mastectomy | 153(53.5) | 134 (55.8) | 41 (53) | 32 (61) | ||
| Lumpectomy | 131 (45.8) | 106 (44.2) | 35(45) | 21 (39) | ||
| Axillary dissection*** | 2 (0.7) | 0 | 1 (2) | 0 | ||
| Type of axillary surgery | <0.001 | <0.001 | ||||
| Dissection | 20 (7) | 85 (35) | 9 (12) | 22 (42) | ||
| SNLB | 266 (93) | 155 (65) | 68 (88) | 31 (58) | ||
| Radiation treatment | 0.009 | |||||
| Yes | 244 (85.3) | 223 (92.9) | 39 (50.6) | 36 (67.9) | ||
| No | 42 (14.7) | 17 (7.1) | 38 (49.4) | 17 (32.1) | ||
| Adjuvant anti-HER2 therapy | ||||||
| HP | 283 (99) | 227 (94.6) | NA | 77 (100) | 51 (96) | |
| HP → neratinib | 0 | 3 (1.3) | 0 | 0 | ||
| TDM1 | 0 | 8 (3.3) | 0 | 1 (2) | ||
| H | 3 (1) | 2 (0.8) | 1 (2) | |||
| Adjuvant endocrine treatment | HR + = 153 | HR + = 192 | NA | HR + = 41 | HR + = 38 | |
| AI | 62 (40.5) | 93 (48.4) | 20 (48.7) | 24 (63.2) | ||
| TAM | 61 (39.8) | 54 (28.2) | 15 (36.6) | 10 (26.3) | ||
| AI + LHRH | 14 (9.2) | 31 (16.2) | 1 (2.4) | 0 | ||
| TAM + LHRH | 1 (0.7) | 7 (3.6) | 0 | 0 | ||
| No ET**** | 15 (9.8) | 7(3.6) | 5 (12.3) | 4 (10.5) | ||
Bold values indicates statistically significant p values < 0.05.
L line, NAC neoadjuvant chemotherapy, ACTHP doxorubicin, cyclophosphamide, paclitaxel; trastuzumab; pertuzumab; HT hormonotherapy, DCbHP docetaxel, carboplatin, trastuzumab, pertuzumab, HR hormone receptor, AI aromatase inhibitors, TAM tamoxifen, T primary tumor, NA not applicable.
*Statistical tests performed: chi-square test of independence; t-test.
**NA due to small sample sizes in a category.
***In cases of Tx, the patients received just axillary dissection. These cases are not included in the statistical analysis because all the cases are related to the pCR group.
****No ET: patients with HR-positive tumors who did not receive endocrine treatments due to decline or clinical decision in case of low-ER and PR expression
Patients who achieved pCR compared to those did not, had more frequently HR negative tumors (46% vs 20% P < 0.001) and more often had HER2 overexpression by IHC (3 + ) (94% vs 68%, P < 0.001). In the pCR group, the proportion of patients who received axillary dissection was lower than in the non-pCR group (7% vs 35% P < 0.001). These results were consistent between overall patients and the subset of patients with HER2 status on pre-NAC biopsy verified at MSKCC (Table 1).
Disease-free survival events
After a median follow-up of 3.2 years (range 0.4–5.5), 36 DFS events occurred in the study population, 14 in the pCR group and 22 in the non-pCR group (Table 2). Among pCR patients with recurrences, 4/14 had locoregional recurrence, 9/14 had distant recurrence of which 7 had only BM, 1 visceral metastasis (lung) and 1 non-visceral metastasis (thoracic lymph nodes). One patient in the pCR group died of unknown cause. The loco-regional breast disease events included 2 contralateral breast cancer, 1 ipsilateral lymph nodal, 1 invasive breast cancer and 1 DCIS relapses. Conversely, almost the totality (17/22) of DFS events of the non-pCR group were distant relapse with 7/22 patients with visceral recurrence in the liver and adrenal glands, 5/22 skin and lymph nodes recurrence and 5/22 with a brain only recurrence.
Table 2.
Disease-free survival events: pCR versus non-pCR patients.
| Overall population (n = 526) | MSKCC HER2-status (n = 130) | |||
|---|---|---|---|---|
| pCR n = 286 | non-pCR n = 240 | pCR Tot: 77 | non-pCR Tot: 53 | |
| DFS events | 14 | 22 | 8 | 5 |
| Locoregional recurrence | 4 | 3 | 4 | 0 |
| Breast | 1 | 2 | 1 | — |
| Regional lymph nodes | 2 | — | 2 | — |
| DCIS | 1 | 1 | 1 | — |
| Distant recurrence | 9 | 17 | 4 | 5 |
| Brain only | 7 | 5 | 3 | 2 |
| Visceral disease | 1 | 7 | 1 | 2 |
| Non-visceral disease | 1 | 5 | 0 | 1 |
| Death* | 1 | 2 | 0 | 0 |
*death without prior recurrence events.
pCR pathological complete response, DCIS ductal carcinoma in situ.
Brain metastases incidence
There was a total of 7/286 (2.4%) BM events without other extracranial sites of disease in the pCR group, and 5/240 (2%) in the non-pCR group after a median follow-up of 3.2 years. We did not observe any meaningful visual differences in the cumulative incidence curves for BM for the two groups (Fig. 2). Among overall BM events (n = 14), 12 occurred as a first event of recurrence. In the pCR group the totality of patients developed BM as first events of recurrence, while in the non-pCR group 2 patients had BM as second event. The median time to development BM observed in our population was 19 (range 4–58) months and 6.5 (range 6.5–17) months in the pCR and non-pCR group, respectively.
Fig. 2. Cumulative incidence of CNS recurrence events stratified by pCR versus non-pCR.
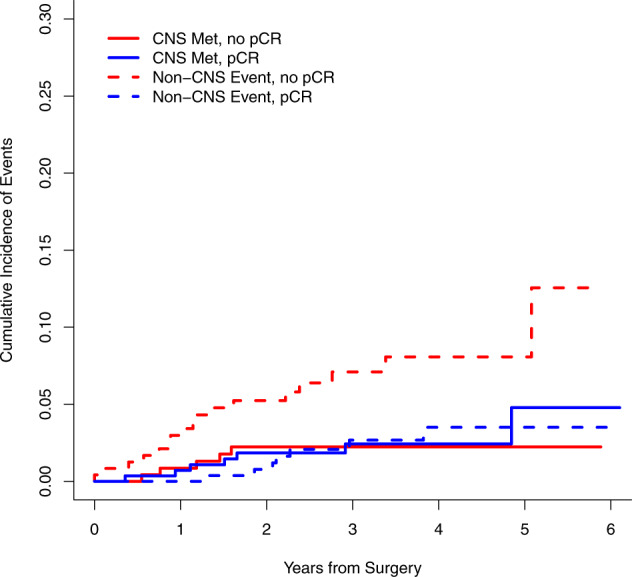
The continuous lines represent the estimated incidence of CNS events and the dashed lines the incidence of non-CNS events in non-pCR (red lines) and pCR group (blue lines), respectively.
Most of the patients had one or two brain lesions in both pCR and non-pCR groups, who underwent surgical resection followed by stereotactic radiation of the tumoral bed or radiosurgery alone. Whole brain radiation was delivered to 3 patients in the pCR group and in 1 patient in the non-pCR group. One patient of the pCR group and one of the non-pCR group had an extensive disease with severe symptoms that did not improve after local treatment. The majority of the patients received a first line systemic treatment for metastatic disease, except for two patients who continued adjuvant HP after the local treatment (Table 3). The baseline characteristic of patients who developed brain BM were homogeneous regardless the pCR status. Eleven out of 14 patients had clinical stage III disease while 3/14 stage II. In the pCR group, 5/7 patients had HR + /HER2 + disease while 3/7 patients in the non-pCR group. Only 2 patients in the pCR group and 5 patients in the non-pCR group had HR-HER2 + disease at the time of diagnosis.
Table 3.
Description of patients with brain metastases.
| Agea (yrs) | Clinical stage | HR status | Time to BM | Symptomsb | Brain as first site of recurrence (Y/N) | Need of hospitalization (Y/N)c | N of lesions as first BM event | Local Treatment | Systemic treatment | Time to 1° CNS POD or death | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pCR | 61 | cT2 N0 | + | 44 mo | seizures and visual changes | Y | Y | 1 | Surgery and SRT | Cape/Lap | 19 mo |
| 40 | cT2 N1 | + | 35 mo | cephalgia and vomiting | Y | Y | 1 | Surgery and SRT | Cape/Lap | 12 mo | |
| 36 | cT3N2 | + | 14 mo | ataxia, aphasia and fatigue | Y | Y | > 10 | WBRT | BSC | 6 mo | |
| 49 | cT1N3 | − | 4 mo | cephalgia | Y | Y | 3 | WBRT | HP | 2 mo | |
| 48 | cT4N1 | + | 11 mo | vertigo and nausea | Y | Y | > 10 | WBRT | Cape/Lap | 10 mo | |
| 66 | cT4N1 | + | 18 mo | ataxia | Y | N | > 10 | proton CSI | Cape/Lap | 11mo | |
| 55 | cT4N1 | + | 19 mo | aphasia | Y | Y | 2 | SRT | HP | NR | |
| Non-pCR | 44 | cT2 N3 | + | 15 mo | ataxia | Y | N | 1 | Surgery and SRT | LET + H | 4 mo |
| 47 | cT4d N1 | − | 9 mo | facial drop | Y | N | 2 | SRS | HP | 16 mo | |
| 61 | cT2N1 | + | 19 mo | ataxia | Y | Y | 1 | Surgery and SRT | ANA + H | 7 mo | |
| 48 | cT4N1 | − | 17 mo | seizures | Y | Y | 2 | Surgery and SRT | Cape/Lap | NR | |
| 52 | cT3N1 | − | 6 mo | vertigo | Y | Y | 1 | Surgery and SRT | BSC | 2 mo | |
| 47 | cT3N0 | + | 55 mo | nausea | N | Y | 2 | SRS | TH | 10 mo | |
| 44 | cT4N2 | − | 14 mo | cephalgia | Nd | Y | > 10 | WBRT | THP | 5 mo |
pCR pathological complete response, yrs years, mo months, BM brain metastases, HR hormone receptors, (+):ER and/or PR > 1%, (−): ER and/or PR < 1%, CNS central nervous system, POD progression of disease, H trastuzumab, HP trastuzumab and pertuzumab, Cape capecitabine, T paclitaxel, Lap lapatinib, ANA anastrozole, LET letrozole, SRT stereotactic radiotherapy, SRS stereotactic radiosurgery, NA not applicable, NR not reached, BSC best supportive care, WBRT whole brain radiation, proton CSI proton cranio-spinal irradiation.
aAge at the time of brain progression.
bBrain metastases symptoms suggesting need of brain radiological assessment.
cNeed of hospitalization for the management of neurological symptoms at the time BM relapse.
dBM have been discovered one month after the extracranial disease (liver/chest wall).
Survival outcomes
Regarding survival outcomes, the 3-year DFS was 91% (95% CI 87–95%) in the non-pCR group and 95% (95% CI 92–98%) in the pCR group (p = 0.03). The 3-years OS was 95% (95% CI 92–98) in the non-pCR group and 98% (95% CI 97–100) in the pCR group (log-rank p = 0.02) (Figs. 3 and 4).
Fig. 3. Disease-free survival stratified in pCR versus non-pCR groups.
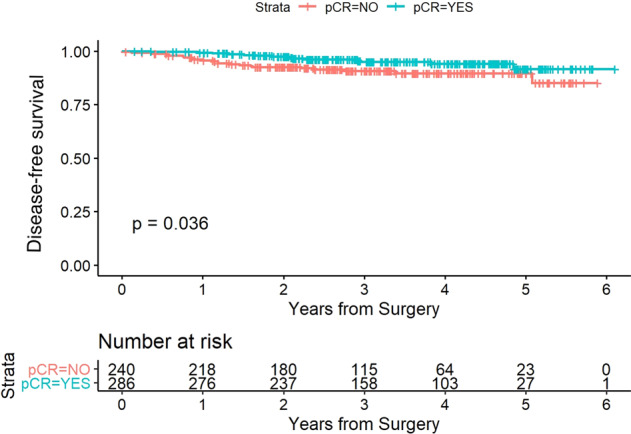
The red and blue curves show the estimated disease-free survival of the patients in the non-pCR group and pCR group, respectively.
Fig. 4. Overall survival stratified in pCR versus non-pCR groups.
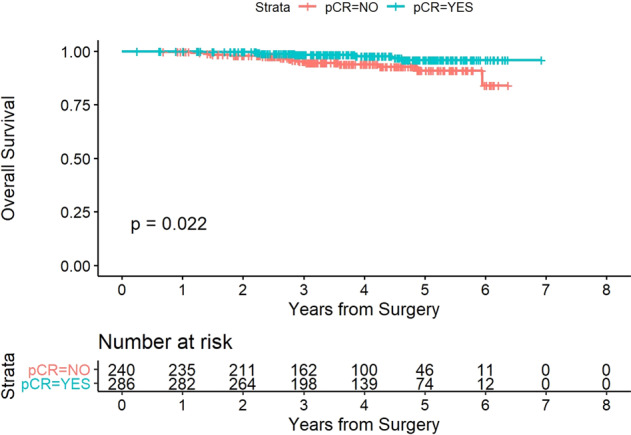
The red and blue curves show the estimated overall survival of the patients in the non-pCR group and pCR group, respectively.
Discussion
In our cohort, the absolute rate of BM was similar between pCR and non-pCR patients, 2.4% and 2%, respectively, with the median follow-up of 3.2 years. The median time of BM relapse was 19 versus 6.5 months in patients with pCR versus non-pCR, respectively. Notably, in the pCR group, 30% of DFS events were local recurrence and almost the totality of patients with distance recurrence had BM as first recurrence event. Conversely, in the non-pCR group, the majority recurrence events were distant relapse with extra-cranial sites of disease. A reasonable explanation of the predominance of brain relapse in patients who achieve pCR, could be that anti-HER2 antibodies currently used in early stage are extremely active to clear the extracranial compartments from micro-metastatic disease, inducing a selection of resistant clones with brain tropism. Patients with residual disease may have a more resistant and heterogeneous disease with more variable clonal selection.
Additionally, the combination of chemotherapy and HP was associated with pCR rate of 54% and excellent survival outcome compared with patients who did not achieve pCR, consistently with literature data10–13. Patients with HR-negative tumors and HER2 IHC 3+ were more likely to achieve pCR, as previously reported by investigators at our institution14. These data were confirmed in the subgroup of patients with HER2 status performed at MSKCC, suggesting reliability of the HER2 status of our entire population. As expected, a statistically significant difference in terms of 3-year DFS and OS was observed in favor of the pCR group.
Currently, HP dual blockade plus chemotherapy has become the standard of care in patients with early stage HER2- positive BC15. Several trials have demonstrated that the combination of HP with standard chemotherapy, can lead to a pCR rate of about 60%11,16–18. At individual level, patients with pCR have better outcomes than those with non-pCR, most notably in those with HER2-positive hormone receptor-negative and triple negative diseases19,20. In our study, the similar BM rates between pCR and non-pCR groups suggest that effective therapies that cross the blood brain barrier are needed. Our data is consistent with the results of the APHINITY trial9 that showed similar BM rate in the HP and standard arm of 1.9% and 1.8%, respectively.
In addition, there appears to be no association between NAC response and BM event rates although the absolute number of events in our population is too small to draw conclusion. Notably, a pooled analysis of GeparQuinto and GeparSixto21, which included both early HER2-positive BC treated with trastuzumab or lapatinib based-regimes and triple negative tumors, showed similar conclusions. BM as first site of metastatic disease occurred more frequently than other distant metastases in patients with the pCR (15% vs 9%), suggesting no correlation between response to NAC and BM recurrence.
The rate of BM reported in our study was consistent with the rates registered in controlled prospective trials in early stage. For instance, in the KATHERINE trial22, testing trastuzumab-DM1(T-DM1) in the post-neoadjuvant setting for patients with non-pCR, the incidence of BM after 3-years of follow-up was 5.9% and 4.3% in patients who received T-DM1 and trastuzumab, respectively. T-DM1 as well as other anti-HER2 agents approved in the treatment of early-stage BC including trastuzumab23–27 and neratinib6 appear to have no impact on the risk of CNS recurrence. In the ExteNET trial6, patients were treated with adjuvant trastuzumab alone or sequential neratinib for one year. Regardless of the assigned therapy in the trial, the rate of CNS recurrence was 1% in each arm, after a median follow-up of 5.2 years. Nevertheless, a recent unplanned subgroup analysis showed a decreased cumulative incidence of BM in neratinib arm compared to placebo arm in a subset of hormone receptor positive, HER2 positive BC patients who started neratinib ≤1 year from the end of adjuvant trastuzumab28.With regards to lapatinib, ALTTO29 and NEOALTTO studies30 showed that lapatinib alone or in combination with trastuzumab in adjuvant and neoadjuvant setting did not reduce the rate of brain recurrence.
Due to the low CNS penetrance, bioavailability or activity of the approved anti-HER2 compounds, the brain is a sanctuary for metastatic disease. More recently, tucatinib, a new TKI has demonstrated activity on BMs in patients with advanced pretreated HER2-positive BC and it has been FDA approved. In the phase I study31, the combination of tucatinib and ado-trastuzumab emtansine led to 36% of response in BM lesions. The study of Tucatinib vs. Placebo in Combination With Capecitabine and Trastuzumab in Patients With Advanced HER2 + Breast Cancer (HER2CLIMB trial)5, showed a benefit in terms of progression-free survival (PFS) and OS in patients who received tucatinib in combination with trastuzumab and capecitabine. In patients with BMs, the estimated PFS at 1 year was 24.9% (95% CI, 16.5 to 34.3) in the tucatinib-combination arm and 0% in the placebo arm. Additionally, the reported CNS-PFS was 9.9 months in the tucatinib arm versus 4.2 months in the control arm with a reduction of the risk of death by 42% in the tucatinib arm (HR: 0.58)32. These data are particularly relevant, because BM still represent a source of morbidity and mortality in patients with HER2-positive BC. Indeed, with incremental improvement with modern systemic treatment in reducing breast cancer recurrences, the management of BM has become an essential component of disease control and quality of life of patients. In the treatment of early stage disease, new escalating approaches have not been associated with a decrease in BMs6,9,22. Clinical trials in early-stage setting should explore novel drugs and strategies that may impact on BM recurrence, including with an exploratory focus on detection of BMs in asymptomatic patients. In our cohort, patients who achieved pCR, despite the better overall outcome, seem to be still at risk of brain recurrence and for this reason, they could benefit from escalating post-neoadjuvant treatment with new TKIs as well as non-pCR patients. Moving forward, trials exploring early detection of BMs in patients with early BC could help to optimize the management of brain recurrence. To date no radiological screening is recommended by ASCO33 and NCCN guidelines34 and potential benefit of early detection of asymptomatic BM is being explored in advanced setting (NCT03881605, NCT04030507, NCT03617341).
To our knowledge, this is the first sizeable analysis of patients treated with HP-based therapy, off-study, in neoadjuvant setting with a focus on BM recurrence. However, the study has several limitations including the retrospective nature, the single center setting, along with a modest number of BM events. We await a longer follow-up to see if the rate of BM increases over time in the two groups. Additionally, only a few patients with residual disease received T-DM1 as adjuvant treatment, as most of the patients were on adjuvant treatment before the approval of T-DM1 by the Food and Drug Administration in this setting. The strength of our study is that it includes a large cohort of patients who received HP based- NAC, with more than 90% receiving dose-dense AC-THP. Despite the inclusion of a large proportion of patients with external HER2 status, the analyses on the subgroup with MSKCC-verified HER2 positivity were similar for the entire population. Furthermore, this study provides a valid evidence about the incidence of brain recurrence after HP given preoperatively and continued after surgery. Indeed, neoadjuvant prospective trials did not report the BM event rates separately from extra-cranial distant recurrence events35–37.
We reported for the first time the incidence of brain recurrence in patients with pCR versus non-pCR after HP combined with chemotherapy, that seems to be not associated with response to NAC. However, a longer follow-up is awaited to confirm these results. Our findings support the investigation of new molecules with high CNS bioavailability in early stage HER2-positive BC in order to evaluate a possible role of these agents to prevent brain recurrence. Research on specific biomarkers of CNS seeding is crucial to better select the population that might benefit from an escalating post-neoadjuvant treatment. Current data do not support pCR being one of those biomarkers.
Methods
Patients’ selection
We reviewed the medical records of consecutive early stage HER2-positive breast cancer patients from the hospital cancer registry at Memorial Sloan Kettering Cancer Center (MSKCC), between September 1, 2013 to November 1, 2019. Follow-up data were obtained until June 30, 2020.
We included patients with HER2-positive breast cancer who received HP in the neoadjuvant setting. Trastuzumab and pertuzumab were administered in combination with standard chemotherapy and for at least one cycle before surgery. Surgery was performed within 6 weeks after NAC. Adjuvant treatments, including endocrine therapy and anti-HER2 therapy, were offered according to physician’s choice. Radiotherapy (RT) was offered as per standard of care. HER2 positivity was defined according to ASCO-CAP guidelines38,39, either as HER2 overexpression (3 + ) by immunohistochemistry (IHC) or gene amplification by fluorescence in situ hybridization (FISH). Hormone receptor (HR) status—estrogen receptor (ER) and progesterone receptor (PR)—was assessed by IHC and considered positive when ≥1% of cancer cell nuclei were stained40. All cases were reviewed at MSKCC by dedicated breast pathologists and the diagnosis was verified for all cases. HER2 and HR status were performed on biopsy samples at MSKCC in a limited subset of patients. For the cases tested at MSK, pre-diluted VENTANA anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody has been used to determine HER2 status. For those patients whose receptor status were based on a biopsy performed at another institution, those with HER2 assessment repeated and verified at MSKCC were included in the sensitivity analysis. During post-treatment follow-up, brain imaging with MRI was performed if there were any concerning signs or symptoms. Patients with leptomeningeal disease only were excluded. If patients experienced BM, they were treated with standard of care per physician’s discretion, including surgery and/or radiotherapy as clinically indicated.
Ethics
The study has been conducted in accordance with the Good Clinical Practice guidelines and the Declaration of Helsinki. Data has been collected and analyze after receiving approval from the MSKCC Institutional Review Board under the number 20-436. All patients reviewed in this study were consented to an institutional protocol, which gives investigators access to their clinical data for research purposes.
Objectives
The primary endpoint is the incidence of BM when it was first site of relapse in pCR and non-CR group. The pCR was defined as absence of residual invasive carcinoma in breast and axilla (ypT0/is ypN0). Disease free survival (DFS) and overall survival (OS) are secondary endpoints. DFS is defined as the interval between the date of surgery and date of any breast disease event, date of death from any cause, or, in case of non evidence of disease (NED), date of last follow-up. Breast disease events included loco-regional recurrence (ipsilateral breast recurrence of invasive carcinoma, regional-nodal recurrence, contralateral invasive breast cancer and DCIS) and distant recurrence (both CNS and non-CNS). OS, defined as the interval between diagnosis date and date of death from any cause or if alive, date of last follow-up, was also evaluated.
Statistical analysis
The incidence of BM was estimated using the cumulative incidence function41 and compared by pCR status using the Gray test42. DFS and OS were analyzed using the Kaplan-Meier method and differences assessed using the log-rank test. Differences between clinicopathological features and pCR status was evaluated using chi-square test and t-test. Any p-value less than 0.05 was deemed to be statistically significant. In a sensitivity analysis, all analyses were done on the whole population (N = 526) and on the subgroup of patients who had a verified HER2 status at MSKCC (N = 130).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
E.F. is funded by the 2019–2020 American-Italian Cancer Foundation Post-Doctoral Fellowship.
Author contributions
E.F., C.T.D. and J.S. conceived the study. R.P. and S.P. contributed to the study design. A.V.B. provided materials. E.F. and J.S. collected clinicopathological data. S.P. performed the statistical analysis. E.F., C.T., J.S., R.P. and S.P. drafted the manuscript. C.T.D., M.R., S.C., S.M., A.V.B., R.M., I.K.M., A.B., H.Y.W., E.B., A.D.S. and L.N. provided a critical interpretation of data. All authors reviewed the manuscript and provided the final approval. E.F. and J.S. contributed equally to this work.
Data availability
All data analyzed in this study are included in this manuscript. The data supporting Table 1 as well as MRI imaging data are not publicly available in order to protect patient privacy but can be made available for non-commercial use only and on reasonable request from the corresponding author. For the data sharing, an agreement with the corresponding author about data usage is required.
Competing interests
P.R. received speakers’ bureau and consultancy/advisory role from Novartis, Foundation Medicine, AstraZeneca, Epic Sciences, Inivata, Natera, Tempus and contracted research funding from Grail, Illumina, Novartis, Epic, Sciences, ArcherDx. S.M. received research support from Genentech, Daiichi-Sankyo, Astrazeneca, Seattle Genetics; speakers bureau from Genentech, Daiichi-Sankyo, Astrazeneca, Seattle Genetics; advisory honoraria form Macrogenics, Daiichi-Sankyo, Astrazeneca, Seattle Genetics. S.C. received contracted research funding from Daiichi-Sankyo, Paige.ai, Novartis, Sanofi, Lilly. A.B received a role in the scientific Advisory Board of Evren Scientific (unpaid); patents: Sloan Kettering Institute, assignee for the following United States Provisional Applications: No.: 62/258,044. November 20, 2015, No.: 10413522, awarded September 17, 2019 and No.: 63/052,139. A.D.S received honoraria as speaker and consultant from Genentech. M.E.R received honoraria from Research to Practice, Intellisphere and physicians’ Education Resource, consulting or advisory role from AstraZeneca (uncompensated), Change Healthcare, Daiichi-Sankyo (uncompensated), Epic Sciences (uncompensated), Merck (uncompensated), Pfizer (uncompensated) and research funding from AbbVie (institution), AstraZeneca (Institution), Invitae (Institution, in-kind), Merck (Institution) and Pfizer (Institution). C.T.D received research funding and personal fees from Genentech/Roche and Puma Technology. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Emanuela Ferraro, Jasmeet Singh.
Supplementary information
The online version contains supplementary material available at 10.1038/s41523-022-00380-7.
References
- 1.Lin NU, Winer EP. Brain metastases: The HER2 paradigm. Clin. Cancer Res. 2007;13:1648–1655. doi: 10.1158/1078-0432.CCR-06-2478. [DOI] [PubMed] [Google Scholar]
- 2.Kallioniemi OP, et al. Association of c-erbB-2 protein over-expression with high rate of cell proliferation, increased risk of visceral metastasis and poor long-term survival in breast cancer. Int. J. Cancer. 1991;49:650–655. doi: 10.1002/ijc.2910490504. [DOI] [PubMed] [Google Scholar]
- 3.Pestalozzi BC, et al. Identifying breast cancer patients at risk for central nervous system (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG) Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2006;17:935–944. doi: 10.1093/annonc/mdl064. [DOI] [PubMed] [Google Scholar]
- 4.Saura C, et al. Neratinib + capecitabine versus lapatinib + capecitabine in patients with HER2+ metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: findings from the multinational, randomized, phase III NALA trial. J. Clin. Oncol. 2019;37:1002–1002. doi: 10.1200/JCO.2019.37.15_suppl.1002. [DOI] [Google Scholar]
- 5.Murthy RK, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N. Engl. J. Med. 2020;382:597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- 6.Prof Miguel Martin M, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1688–1700. doi: 10.1016/S1470-2045(17)30717-9. [DOI] [PubMed] [Google Scholar]
- 7.Tonyali O, et al. Risk factors for brain metastasis as a first site of disease recurrence in patients with HER2 positive early stage breast cancer treated with adjuvant trastuzumab. Breast. 2016;25:22–26. doi: 10.1016/j.breast.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Pestalozzi BC, et al. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1-01) Lancet Oncol. 2013;14:244–248. doi: 10.1016/S1470-2045(13)70017-2. [DOI] [PubMed] [Google Scholar]
- 9.Von Minckwitz G, et al. Adjuvant pertuzumab and trastuzumab in early her2-positive breast cancer. N. Engl. J. Med. 2017;377:122–131. doi: 10.1056/NEJMoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gianni L, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 11.Schneeweiss A, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Ann. Oncol. 2013;24:2278–2284. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 12.Loibl S, et al. Dual HER2-blockade with pertuzumab and trastuzumab in HER2-positive early breast cancer: a subanalysis of data from the randomized phase III GeparSepto trial. Ann. Oncol. 2017;28:497–504. doi: 10.1093/annonc/mdw610. [DOI] [PubMed] [Google Scholar]
- 13.Swain, S. M. et al. Risk of recurrence and death in patients with early HER2-positive breast cancer who achieve a pathological complete response after different types of HER2-targeted therapy: a retrospective exploratory analysis. SABCS Meet. (2018).
- 14.Krystel-Whittemore M, Xu J, Brogi E. Pathologic complete response rate according to HER2 detection methods in HER2 positive breast cancer treated with neoadjuvant systemic therapy. Breast Cancer Res Treat. 2019;177:61–66. doi: 10.1007/s10549-019-05295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gradishar WJ, et al. Breast Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2020;18:452–478. doi: 10.6004/jnccn.2020.0016. [DOI] [PubMed] [Google Scholar]
- 16.Mette S vanRamshorst, et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1630–1640. doi: 10.1016/S1470-2045(18)30570-9. [DOI] [PubMed] [Google Scholar]
- 17.Swain SM, et al. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): a phase II, open-label, multicenter, multinational cardiac safety study. Ann. Oncol. 2018;29:646–653. doi: 10.1093/annonc/mdx773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurvitz SA, et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018;19:115–126. doi: 10.1016/S1470-2045(17)30716-7. [DOI] [PubMed] [Google Scholar]
- 19.Patricia Cortazar, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 20.Spring, L. M. et al. Pathological complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin. Cancer Res. (2020) 10.1158/1078-0432.ccr-19-3492. [DOI] [PMC free article] [PubMed]
- 21.Laakmann E, et al. Development of central nervous system metastases as a first site of metastatic disease in breast cancer patients treated in the neoadjuvant trials GeparQuinto and GeparSixto. Breast Cancer Res. 2019;21:1–8. doi: 10.1186/s13058-019-1144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Von Minckwitz G, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 2019;380:617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 23.Romond EH, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 24.Martine J. Piccart-Gebhart, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer martine. N. Engl. J. Med. 2005;353:225–237. doi: 10.1056/NEJMp058073. [DOI] [PubMed] [Google Scholar]
- 25.Spielmann M, et al. Trastuzumab for patients with axillary-node-positive breast cancer: results of the FNCLCC-PACS 04 trial. J. Clin. Oncol. 2009;27:6129–6134. doi: 10.1200/JCO.2009.23.0946. [DOI] [PubMed] [Google Scholar]
- 26.Olson EM, et al. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann. Oncol. 2013;24:1526–1533. doi: 10.1093/annonc/mdt036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin W, et al. Trastuzumab in the adjuvant treatment of HER2-positive early breast cancer patients: a meta-analysis of published randomized controlled trials. PLoS ONE. 2011;6:e21030. doi: 10.1371/journal.pone.0021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan A, et al. Final efficacy results of neratinib in HER2-positive hormone receptor-positive early-stage breast cancer from the phase III ExteNET trial. Clin. Breast Cancer. 2021;21:80–91.e7. doi: 10.1016/j.clbc.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Piccart-Gebhart M, et al. Adjuvant lapatinib and trastuzumab for early human epidermal growth factor receptor 2-positive breast cancer: Results From the randomized phase III adjuvant lapatinib and/or trastuzumab treatment optimization trial. J. Clin. Oncol. 2016;34:1034–1042. doi: 10.1200/JCO.2015.62.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Azambuja E, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): Survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014;15:1137–1146. doi: 10.1016/S1470-2045(14)70320-1. [DOI] [PubMed] [Google Scholar]
- 31.Borges VF, et al. Tucatinib combined with ado-Trastuzumab emtansine in advanced ERBB2/HER2-positive metastatic breast cancer: a Phase 1b Clinical Trial. JAMA Oncol. 2018;4:1214–1220. doi: 10.1001/jamaoncol.2018.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin NU, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J. Clin. Oncol. 2020;38:2610–2619. doi: 10.1200/JCO.20.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramakrishna N, et al. Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2–positive breast cancer and brain metastases: ASCO clinical practice guideline update. J. Clin. Oncol. 2018;36:2804–2807. doi: 10.1200/JCO.2018.79.2713. [DOI] [PubMed] [Google Scholar]
- 34.Gradishar, W. J. et al. NCCN Clinical Practice Guidelines on Oncology- Breast cancer. vol. version 3. (2020). [DOI] [PubMed]
- 35.Gianni L, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17:791–800. doi: 10.1016/S1470-2045(16)00163-7. [DOI] [PubMed] [Google Scholar]
- 36.Schneeweiss A, et al. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: Evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive ea. Eur. J. Cancer. 2018;89:27–35. doi: 10.1016/j.ejca.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 37.Hurvitz SA, et al. Neoadjuvant Trastuzumab Emtansine and Pertuzumab in Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Three-Year Outcomes From the Phase III KRISTINE Study. J. Clin. Oncol. 2019;37:2206–2216. doi: 10.1200/JCO.19.00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolff AC, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast. J. Clin. Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 39.Wolff AC, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch. Pathol. Lab. Med. 2018;142:1364–1382. doi: 10.5858/arpa.2018-0902-SA. [DOI] [PubMed] [Google Scholar]
- 40.Allison KH, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J. Clin. Oncol. 2020;38:1346–1366. doi: 10.1200/JCO.19.02309. [DOI] [PubMed] [Google Scholar]
- 41.Kalbfleisch, J. D. & Prentice, R. L. The Statistical Analysis of Failure Time Data. 2nd Edition, John Wiley and Sons, New York. 10.1002/9781118032985. (2002).
- 42.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann. Stat. 1988;16:1141–1154. doi: 10.1214/aos/1176350951. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analyzed in this study are included in this manuscript. The data supporting Table 1 as well as MRI imaging data are not publicly available in order to protect patient privacy but can be made available for non-commercial use only and on reasonable request from the corresponding author. For the data sharing, an agreement with the corresponding author about data usage is required.