Abstract
Submucosal injection material has shown protective effect against gastrointestinal injury during endoscopic surgery in clinic. However, the protective ability of existing submucosal injection material is strictly limited by their difficult injectability and short barrier time. Herein, we report a shear-thinning gellan gum hydrogel that simultaneously has easy injectability and long-lasting barrier function, together with good hemostatic property and biocompatibility. Shear-thinning property endows our gellan gum hydrogel with excellent endoscopic injection performance, and the injection pressure of our gellan gum hydrogel is much lower than that of the small molecule solution (50 wt% dextrose) when injected through the endoscopic needle. More importantly, our gellan gum hydrogel shows much stronger barrier retention ability than normal saline and sodium hyaluronate solution in the ex vivo and in vivo models. Furthermore, our epinephrine-containing gellan gum hydrogel has a satisfactory hemostatic effect in the mucosal lesion resection model of pig. These results indicate an appealing application prospect for gellan gum hydrogel utilizing as a submucosal injection material in endoscopic surgery.
Keywords: Shear-thinning, Injectable hydrogel, Gellan gum, Long-lasting barrier, Endoscopic surgery
Graphical abstract
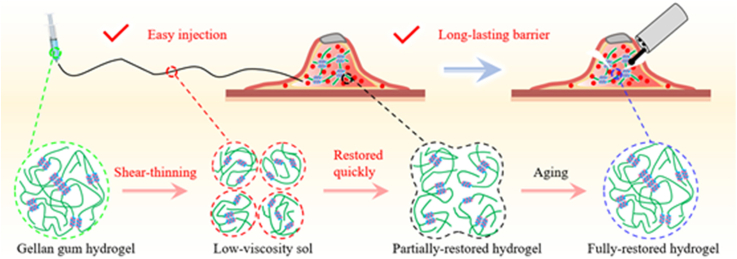
GGH formed by double-helix aggregation has shear-thinning characteristic. During injection, the hydrogel is quickly transformed into low-viscosity sol, providing easy endoscopic injectability; after injection, the hydrogel network is quickly and partially restored and then entirely restored, providing long-lasting barrier cushion.
Highlights
-
•
Submucosal injection materials are widely used in endoscopic surgery to protect against gastrointestinal injury.
-
•
Gellan gum hydrogel with shear-thinning character is a novel submucosal injection material.
-
•
Gellan gum hydrogel simultaneously has easy injectability and long-lasting barrier performance in vivo.
-
•
Epinephrine-containing gellan gum hydrogel has a satisfactory hemostatic effect.
1. Introduction
According to the global cancer statistics, an estimated 19.3 million new cancer cases occurred worldwide in 2020 [1]. The two most common types of gastrointestinal cancers are gastric cancer and colorectal cancer, ranking as the third and sixth commonly diagnosed cancers, respectively [1]. Actually, the wall of gastrointestinal tract can be divided into four layers, including mucosa, submucosa, muscularis propria, and serosa [2]. Early gastrointestinal tumor is defined as the lesion that only infiltrates within the submucosa and can be resected by endoscopic surgery [3,4]. Benefiting from the screening strategy toward gastrointestinal lesion, more and more early-stage tumors can be found and treated with endoscopic resection [[5], [6], [7], [8], [9], [10]]. Endoscopic resection has been indicated to be a popular and effective method for the treatment of early-stage gastrointestinal tumors.
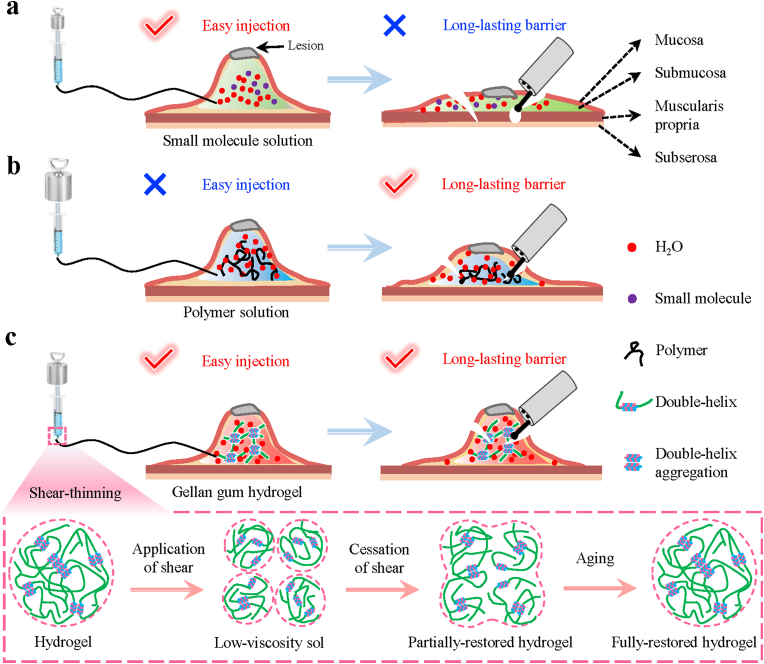
Due to the foldable and thin features of gastrointestinal tract, the resection of lesion during endoscopic surgery still faces huge challenges such as positive margin, perforation, and bleeding [[11], [12], [13]]. The application of submucosal injection material (SIM) to form submucosal barrier is a crucial method to satisfy the demand of endoscopic resection [5,6]. SIM is able to elevate lesion by producing a thick liquid cushion, which creates adequate operation spaces between the mucosa and muscularis propria, and shields the electrical and thermal damage generated during the endoscopic resection procedure [14,15]. Normal saline (NS) has been used to elevate mucosal lesion after submucosal injection through the slender tract of endoscope with proper injection pressure [16]. However, the submucosal cushion height of NS will quickly reduce after injection because of the rapid diffusion of small molecules, and result in a fast weakening of the barrier function, which can increase the difficulty of operation and the risk of perforation and bleeding (Fig. 1a) [[17], [18], [19]]. Therefore, new SIM with long-lasting barrier function to expose the edge of tumor and prevent muscularis propria damage is in urgent need.
Fig. 1.
Schematic illustration of injectability and barrier function of submucosal injection materials (SIMs) for endoscopic injection. a) Conventional small molecule solution could be easily injected into the submucosa through the slender endoscopic needle, but could not maintain the submucosal cushion for a long time to provide long-lasting barrier function. b) Although conventional polymer solution is difficult to be injected into the submucosa with the endoscopic needle, it could offer a more lasting barrier function than the small molecule solution. c) Benefitting from shear-thinning characteristic, gellan gum hydrogel could be easily injected into the submucosa through the slender endoscopic needle and form long-lasting submucosal cushion simultaneously. Thus, it could elevate lesion satisfactorily and provide durable barrier effect to reduce the risk of perforation and bleeding. The shear-thinning characteristic could be explained as follows: when a shear force is applied, the hydrogel is broken into particles, leading to formation of low-viscosity sol; once the shear is ceased, the gel network is partially restored and then fully restored after a period of aging.
Recently, progress has been made to achieve long-lasting barrier function through the injection of hypertonic liquids, polymer solutions, and hydrogels. However, most SIMs will encounter two bottleneck problems. On one hand, the effect of maintaining submucosal cushion height by injecting hypertonic liquids such as dextrose solution (DS) is unsatisfactory; the height will be quickly reduced to less than 50% within 30 min, and the hypertonic property can cause serious tissue damage (Fig. 1a) [[20], [21], [22]]. On the other hand, most polymer solutions with high viscosity such as sodium hyaluronate (SH) could create more lasting barrier, but they are difficult to inject due to their high viscosity (Fig. 1b) [[23], [24], [25], [26], [27], [28], [29], [30]]. Similarly, some hydrogels are found to provide moderate submucosal cushion height because of their high-water content and stiffness, but the inconvenient operation still restricts their applications [31]. For example, the hydrogels formed by two or more components are inconvenient to be simultaneously injected [5,32,33], and photocurable hydrogels require ultraviolet light sources, making the operation difficult under the endoscope [[34], [35], [36], [37]]. What's worse, the temperature-sensitive hydrogels have the risk of clogging [[38], [39], [40], [41], [42]]. Recently, few studies began to focus on the ex vivo barrier function of shear-thinning materials, but their biocompatibility and operability of endoscope are still unknown [6,43]. Therefore, how to balance the easy operability and long-lasting barrier property still remains a great challenge in SIMs.
In this work, we successfully develop a shear-thinning gellan gum hydrogel (GGH) with excellent biocompatibility, easy endoscopic injectability, long-lasting in vivo barrier function, and good hemostatic property (Fig. 1c). The GGH is formed by the double-helix aggregation as cross-linking point and thus presents a shear-thinning hydrogel characteristic. Once applying the shear, the hydrogel can be quickly transformed into low viscosity sol, providing easy endoscopic injectability; once the shear is ceased after completing submucosal injection, the hydrogel can entirely restore the robust hydrogel network, providing long-lasting barrier cushion. We find that the injection pressure of the shear-thinning GGH is significantly lower than that of the SH polymer solution and even lower to that of the small molecule solution (50 wt% dextrose). As such, GGH is easily injected into the stomach of a pig through the endoscope. The injected GGH with a robust restored network can create a high submucosal cushion, and perform very well as a submucosal barrier cushion for more than 30 min, a time enough for most endoscopic resection procedures. Our GGH contains a hemostatic agent (epinephrine) and then demonstrates satisfactory hemostatic activity in the mucosal lesion resection model of pig. Moreover, our GGH has good biocompatibility and does not inhibit cell growth, demonstrating a potential to heal wound after resection. We hope that our shear-thinning hydrogel could be an ideal SIM for clinical endoscopic surgery.
2. Results and discussion
Gellan gum is a natural polymer produced by the bacterium Sphingomonas elodea, and approved for food and medical usage by US FDA and European Union (E418) [44,45]. Our GGH was facilely obtained by heating a high acyl gellan gum solution at 170 °C, followed by cooling down to room temperature. The high acyl gellan gum is a type of linear polysaccharides with a repeating unit of tetra-saccharide (Fig. S1, Supporting Information) [46]. It could exist in the form of random coils above 85 °C [47]; the coils turn into double-helixes firstly during cooling, and then the helixes aggregate to form a three-dimensional hydrogel network, which could come apart at low stress, leading to a great shear-thinning property [45,48,49].
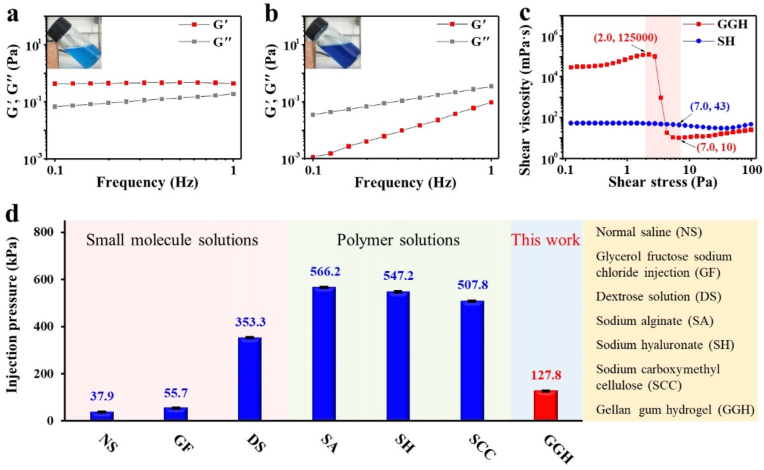
The as-obtained GGH is a “weak gel”, which has the pourable property of viscoelastic fluid but maintains the network of the gel [[47], [48], [49]]. Considering that 0.4 wt% SH was widely used as a SIM in endoscopic treatments [19,21,28], we used it as the control sample. The rheological and viscosity properties of GGH were studied to investigate its gel characteristics and shear-thinning property [[50], [51]]. In order to determine whether the sticky gellan gum liquid is a hydrogel, the frequency sweep measurement was done. It was found that in the low frequency range of 0.1–1.0 Hz, storage modulus (G′) of GGH was always greater than loss modulus (G″) (Fig. 2a), while G′ of SH was always smaller than G″ (Fig. 2b), indicating that GGH was indeed a hydrogel (G′ > G″) whereas SH was a solution (G′ < G″) [47]. Shear viscosity test was then used to indicate GGH presented a shear-thinning characteristic. As shown in Fig. 2c, when shear force increased from 2.0 to 7.0 Pa, shear viscosity of GGH dropped rapidly from 125000 to 10 mPa s, which was reduced by 12500 times. More importantly, under shear force of 7.0 Pa, the viscosity of the shear thinned GGH (10 mPa s) was 4.3 times as small as that of SH (43 mPa s). As a result, the injection pressure of GGH was as low as 127.8 kPa, which was significantly smaller than those of polymer solutions including sodium alginate (SA, 566.2 kPa), SH (547.2 kPa) and sodium carboxymethyl cellulose (SCC, 507.8 kPa), and even comparable to those of small molecule solutions including NS (37.9 kPa), glycerol fructose sodium chloride injection (GF, 55.7 kPa) and DS (353.3 kPa), when injected through a 22-gauge endoscopic injection needle (Fig. 2d). Obviously, such a low injection pressure could greatly decrease the operation difficulty of endoscopic injection when utilizing GGH as a SIM.
Fig. 2.
Rheological properties, shear-thinning characteristic, and injection pressure of submucosal injection materials. Oscillatory frequency sweeps and digital photos (inset) of a) GGH and b) SH. c) Shear viscosity-stress curve of GGH and SH. d) Comparison of GGH with other SIMs in terms of injection pressure.
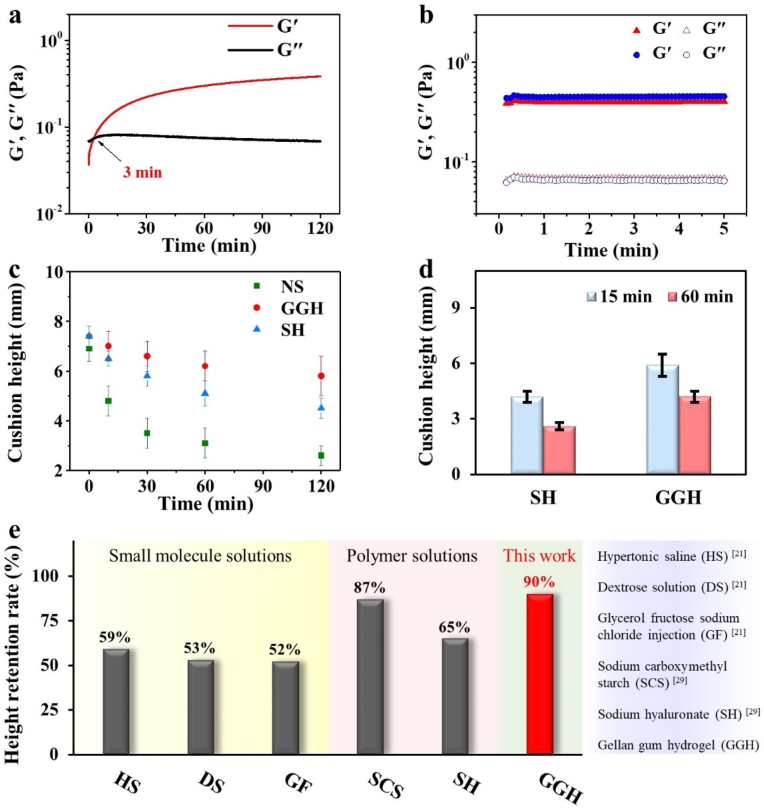
In addition to the easy injection performance, our injected GGH also demonstrated a strong shape retention function, because the hydrogel could entirely restore the robust hydrogel network once the injection shear was ceased. As shown in Fig. 3a–b, GGH rapidly underwent sol-gel transition and then G′ went back to the original modulus before injection. The result indicated that GGH had a robust restored network and could create a high submucosal cushion after submucosal injection. As shown in Fig. 3c, when GGH and control samples (NS and SH) were injected into the ex vivo porcine stomach, their submucosal cushion height was very close at 0 min. However, the submucosal cushion height of NS and SH quickly decreased to 50% and 80% at 30 min, respectively, while our GGH still kept 80% of submucosal cushion height after 120 min (Fig. 3c). Furthermore, to the best of our knowledge, the height retention rate (90%) of GGH is higher than those of reported small molecule solutions and polymer solutions at 30 min after submucosal injection [21,29] (Fig. 3e). The superior cushion retention ability of GGH was also confirmed by in vivo rat model. After 1.0 mL of GGH was subcutaneously injected into the back of rat, the cushion height of GGH was still up to 5.9 and 4.2 mm at 15 and 60 min, respectively (Fig. 3d). In contrast, the height of SH quickly dropped to 4.2 and 2.6 mm at 15 and 60 min, respectively (Fig. 3d). The ex vivo and in vivo cushion retention comparisons clearly revealed our GGH had long-lasting barrier performance as a SIM.
Fig. 3.
Rheological properties and shape retention ability of GGH. a) Oscillatory time sweep of GGH after injection through the endoscopic needle. b) Comparison of G′ and G″ for GGH before (blue) and after (red) injection for 2 h. c) Submucosal cushion height as a function of time for NS (green), SH (blue) and GGH (red) in the ex vivo model. d) Comparison of subcutaneous cushion height for SH and GGH at 15 and 60 min in the in vivo model. e) Comparison of GGH with the reported SIMs in terms of height retention rate.
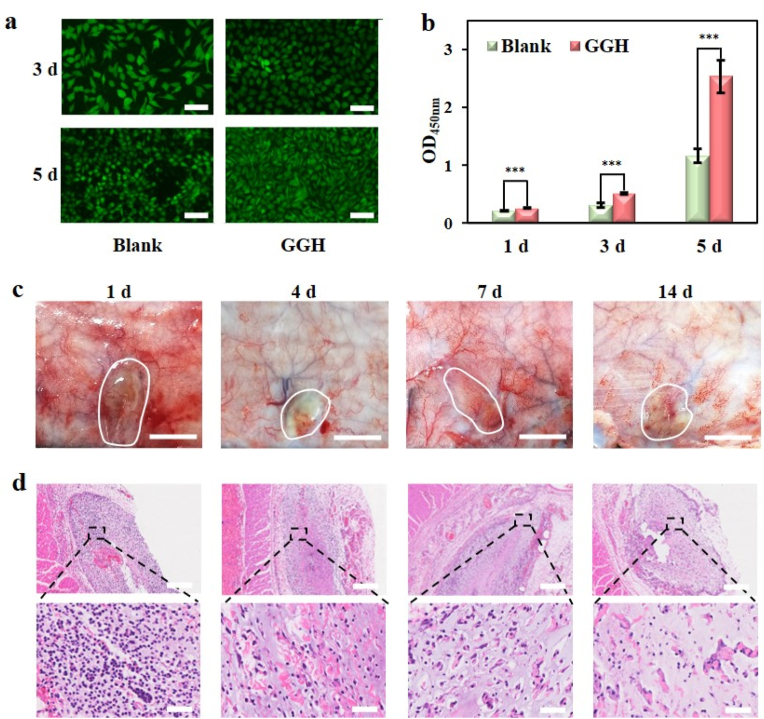
The biocompatibility of SIM is an important concern for their biomedical application. The live/dead cell double staining kit experiment exhibited that after being cultured with our GGH, L929 fibroblast cells grew well and maintained their normal spindle shape (Fig. 4a). Cell Counting Kit-8 (CCK-8) assay indicated that L929 fibroblast cells in our GGH group grew better than the blank group (Fig. 4b), demonstrating the good biocompatibility and no inhibition of cell proliferation for GGH. To further illustrate the biocompatibility in vivo, GGH was injected subcutaneously on the back of the rat to form a cutaneous hillock about 2.0 cm in diameter. It was found that there was no tissue edema, ulcer, purulent secretion, and other obvious foreign body reaction in the surrounding area of GGH within 14 d (Fig. 4c). The subcutaneous tissue containing GGH was cut into slices and assessed by haematoxylin and eosin (H&E) staining. Local infiltration of inflammatory cells was found inside and around the hydrogel on day 1; however, the inflammatory response attenuated to slight level on day 4, and a very few inflammatory cells could be found on day 7 and day 14 (Fig. 4d). The result was similar to that of previous studies [[52], [53], [54], [55]], confirming the acceptable biocompatibility in vivo.
Fig. 4.
Biocompatibility of GGH. a) Inverted fluorescence microscope images of L929 fibroblasts after incubation for 3 and 5 d (bar = 100 μm). b) CCK-8 assay about the cytotoxicity of GGH to L929 fibroblasts after incubation for 1, 3 and 5 d. c) Images of subcutaneous tissues around the injection site of GGH at different times after subcutaneous injection (bar = 1 cm). d) H&E staining of GGH at different times (upper bar = 500 μm, bottom bar = 50 μm).
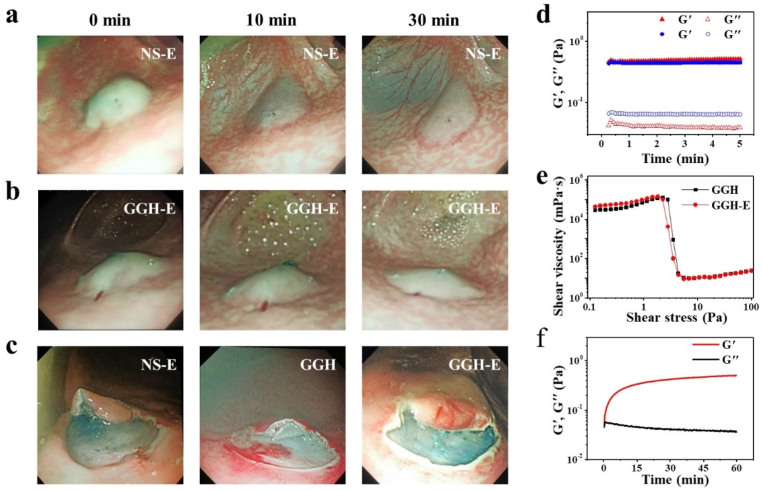
In order to assess in vivo submucosal barrier ability of our GGH, the submucosal injection model was performed by endoscopic procedure in the stomach of pig. 3.0 mL of GGH or NS containing 10 mg/L of epinephrine hemostatic agent (denoted as GGH-E or NS-E) was injected into gastric submucosa through the endoscopic equipment. It was found that a good submucosal cushion was formed after injection of NS-E or GGH-E; the cushion of NS-E flattened dramatically within 10 min and almost completely flattened at 30 min, while that of GGH-E remained nearly unchanged in 10 min and still retained an acceptable height at 30 min (Fig. 5a–b). In addition to the barrier function, hemostasis is another necessary index for SIMs during the endoscopic resection. To explore the effect of GGH on the release rate of epinephrine, an in vitro drug release experiment was conducted. It was found that there was no significant difference in the release rate of epinephrine between NS-E and GGH-E (Fig. S4, Supporting Information). The result indicated that the epinephrine in GGH could be released smoothly to play a hemostatic effect in time. In order to verify the actual hemostatic effect of GGH-E, we performed a simulated endoscopic resection in a pig stomach model. 5.0 mL of GGH, GGH-E or NS-E was injected into the gastric submucosa and the mucosa was resected by endoscope electrosurgical system. According to Fig. 5c, as compared to GGH without epinephrine, both GGH-E and NS-E showed satisfy hemostatic effect during endoscopic resection of mucosa. It is worth mentioning that addition of epinephrine had no adverse effect on the gel properties and shear-thinning characteristic of GGH; there was no significant difference in comparison of G′ and G″ for GGH and GGH-E, their shear viscosity-stress curve almost coincide, and GGH-E also could quickly undergo sol-gel transition after injection with endoscopic needle (Fig. 5d–f). These results suggested that our epinephrine-containing GGH could generate long-lasting barrier in stomach of big animal to protect stomach from perforation and bleeding during endoscopic surgery, and thus might be an ideal material for gastrointestinal endoscope procedure in clinic.
Fig. 5.
Submucosal barrier ability of GGH in vivo. Endoscopic images of submucosal cushions developed by a) NS-E and b) GGH-E at 0, 10 and 30 min after endoscopic injection. c) Comparison of hemostatic effect for submucosal cushions generated by NS-E, GGH, and GGH-E during resection by endoscope electrosurgical system. d) Comparison of G′ and G″ for GGH (blue) and GGH-E (red). e) Shear viscosity-stress curve for GGH and GGH-E. f) Oscillatory time sweep of GGH-E after injection through the endoscopic needle.
3. Conclusion
In summary, we report the development and application of GGH, which is a shear-thinning hydrogel, facilely prepared by heating high acyl gellan gum polysaccharide solution and then cooling down. Benefiting from the shear-thinning characteristic, the as-obtained easily-injectable GGH shows long-lasting submucosal barrier performance in vivo as a SIM, and simultaneously has good hemostatic property and biocompatibility. Moreover, GGH does not inhibit cell proliferation and growth, demonstrating a potential to heal wound. Based on these characteristics, our GGH could be a promising hydrogel material for broad application in endoscopic resection techniques, and potential development in luminal constriction, drug delivery, and tissue engineering.
4. Materials and methods
4.1. Materials
Sodium chloride (>99.5%), sodium alginate (90%), sodium hyaluronate (>99.0%, Mw = 450 kDa) and high acyl gellan gum (>99.0%) were purchased from Macklin, China. Sodium carboxymethyl cellulose was purchased from Guangzhou Qihua Medical Equipment Co., Ltd., China. Dextrose monohydrate (>99.7%) was purchased from Guangzhou Chemical Reagent Factory, China. Glycerol fructose sodium chloride injection was purchased from Jiangsu Chia Tai Fenghai Pharmaceutical Co., Ltd., China. Epinephrine hydrochloride injection (1 mg/mL) was purchased from Grandpharma Co., Ltd., China. Methylene blue injection (10 mg/mL) was purchased from Jiangsu Jichuan Pharmaceutical Co., Ltd., China. 10% chloral hydrate was purchased from Jin Clone Biotechnology Co., Ltd., China. Xylazine hydrochloride was purchased from Jilin Huamu Animal Health Product Co., Ltd., China. Propofol was purchased from Xi'an Libang Pharmaceutical Co., Ltd., China. MTT reagent was provided by Thermo Fisher Scientific, USA. Phosphate buffered saline (PBS) was purchased from Corning, USA. Dulbecco's modified eagle's medium (DMEM), fetal bovine serum (FBS), penicillin–streptomycin solution (PSS), and trypsin-ethylene diamine tetraacetic acid (EDTA) were obtained from Gibco, USA. Calcein-AM (2 μM) and propidium iodide (8 μM) were obtained from Sigma, USA.
4.2. Preparation of SIMs
Preparation of GGH: Firstly, 1.0 mL of methylene blue injection (10 mg/mL) was dissolved in 1 L of deionized water to obtain a methylene blue stock solution with a concentration of 10 mg/L. Subsequently, 60 mg of high acyl gellan gum was added into 100 mL of methylene blue stock solution, and then the mixture was sealed and heated at 170 °C for 1 h with a heating stirrer (LC-DMS-H, Lichen, China). Thereafter, GGH with a concentration of 0.06 wt% was obtained by naturally cooling down the solution to room temperature.
SH (0.4 wt%) or NS (0.9 wt%) was prepared by dissolving 400 mg of sodium hyaluronate or 900 mg of sodium chloride in 100 mL of methylene blue stock solution, and then underwent the same sterilization process as GGH. DS with a concentration of 50 wt% was made by dissolving 11 g of dextrose monohydrate into 9 g of deionized water. SA with a concentration of 0.4 wt% was made by dissolving 400 mg of sodium alginate into 100 mL of deionized water. SCC with a concentration of 0.4 wt% was made by dissolving 400 mg of sodium carboxymethyl cellulose into 100 mL of deionized water.
For preparation of GGH-E, 60 mg of high acyl gellan gum was added into 100 mL of methylene blue stock solution, and then the mixture was purged with N2 for 30 min to remove O2. Next, the mixture was sealed and heated at 170 °C for 1 h with the heating stirrer, and then 1.0 mL of epinephrine hydrochloride injection (1 mg/mL) was added into the mixture. Thereafter, the obtained solution was naturally cooled down to room temperature. On the other hand, NS-E was prepared by dissolving 900 mg of sodium chloride into 100 mL of methylene blue stock solution, and then underwent the same preparation process as GGH-E.
4.3. Measurement of rheological and viscosity properties for SIMs
Shear viscosity, and oscillatory time, frequency, and strain sweeps were performed using a rotational rheometer (Kinexus pro+, Malvern, UK) with a set of DIN standard C25 coaxial cylinder measuring system. According to the guidelines of instrument, pre-prepared SIMs were put into cylinder. All rheological and viscosity tests were performed at 25 °C. Oscillation frequency sweep was conducted at the strain of 1.0% and frequency range of 0.1–1.0 Hz. Additionally, oscillation time sweep experiment was performed to record G′ and G″ of SIMs after injection through the endoscopic needle. Frequency and shear strain were set to 0.1 Hz and 1.0%, respectively. Shear viscosity-stress curve was obtained through a yield viscosity measurement. Shear stress and action time were set as 0.1–100.0 Pa and 10 min, respectively.
4.4. Measurement of injection pressure for SIMs
Injection pressure of each SIM was evaluated with a system, in which an endoscopic injection needle (22-gauge, 1.8 m-long, NET2422-C4, Endo-Flex, Germany), a digital pressure gauge (HT-1895, Xinsite, China) and a syringe were connected to a three-way stopcock, followed by fixing the syringe with a syringe pump (LSP01-1A, Longer, China) to set injection speed (Fig. S2, Supporting Information) [17]. Injection pressure was measured by a 5 mL syringe at an injection speed of 0.1 mL/s, when the pressure value was stable for 3 s. Six independent injection pressure measurements were performed for each SIM, and the obtained results were expressed as the mean and standard deviation (Table S1, Supporting Information).
4.5. Measurement of submucosal cushion height in resected porcine stomach
Submucosal cushion height was measured with a flat head spiral micrometer (S5210–25B, Wenzhou Weidu Electronics Co., Ltd., China) (Fig. S3, Supporting Information). Gastric specimen (5 cm × 5 cm) was collected from the upper third of the fresh porcine stomach, whose thickness was close to that of the human stomach [17]. 2.0 mL of GGH or control groups (SH and NS) was injected into submucosa from center of specimen by using a 5 mL syringe with a 23-gauge needle. Cushion height was measured with the spiral micrometer at 0, 10, 30, 60 and 120 min after injection. Seven independent measurements were performed, and the obtained results were expressed as the mean and standard deviation (Table S2, Supporting Information). Height retention rate of submucosal cushion at different times after injection was calculated, according to the following equation:
4.6. Biocompatibility of GGH injected subcutaneously in rat model
The Sprague-Dawley (SD) rats (200–240 g) were pursed from the Laboratory Animal Center of Sun Yat-sen University, China. All the experimental operations involved in animals were approved by the guidelines of the Animal Ethics Committee for Guangzhou Huateng Biomedical Technology Co., Ltd., China. All the rats were treated following the Laboratory Animal Care and Use Guidelines strictly. Anesthesia was induced with intraperitoneal injection of 10% chloral hydrate. Firstly, back of rat was prepared for subcutaneous injection by clipping the hair and cleaning the operative area with medicinal alcohol solution. Subsequently, 1.0 mL of GGH or SH was injected into the back skin under sterile condition. Next, skin of whole injection area with subcutaneous tissue was dissected at 15 min, 60 min, 1 d, 4 d, 7 d, and 14 d after injection. After placing a ruler next to cushion, it was photographed to record, and then cushion height was measured with the ruler. Four rats in each group were tested in parallel, and the obtained results were expressed as the mean and standard deviation (Table S3, Supporting Information).
4.7. Epinephrine-release profile in vitro
The release of epinephrine from NS-E and GGH-E groups was determined by a dialysis method in vitro [56]. 20 mL of NS-E or GGH-E was placed in dialysis bag (3500 Da, Shanghai yuanye Bio-Technology Co., Ltd., China). Then the dialysis bag was incubated in 80 mL of NS (pH 5–6) at 37 °C with 120 rpm shaking. 5 mL of incubation medium was entirely replaced by the same volume of fresh NS at predetermined time points. Epinephrine was determined with high-performance liquid chromatography (HPLC) [57,58]. A HPLC system (Waters, USA) equipped with a Waters 1525 binary pump, a Waters 2489 UV–visible detector and a Waters 2707 autosampler was used. The separation was done on a C18 column (Diamonsil Plus 5 μm C18–B, DIKMA, China). The elution was performed on isocratic solvent system with formic acid solution (0.1% (v/v)) and acetonitrile as 80% and 20%, respectively, as a mobile phase at a flow rate of 0.5 mL/min. The UV–visible detector was set at the wavelength of 279 nm and injection volume was 20 μL for every sample.
4.8. Performance of GGH in pig model
A Tibet miniature pig (16–22 kg) was provided by Dongguan Songshanhu Experimental Animal Technology Co., Ltd., China. All the experimental procedures in this study were approved by the guidelines of the Animal Ethics Committee for Yin She Guangzhou Medical Technology Co., Ltd., China. The pig was treated following the Laboratory Animal Care and Use Guidelines strictly. The pig was only allowed to drink water at 2 d before experiment. The pig was anesthetized by xylazine hydrochloride and propofol, endotracheal intubation was performed with continuous oxygen intake of 2 L/min. An endoscope was entered into the pig's stomach for observation. Subsequently, 3.0 mL of GGH-E or NS-E was injected into submucosa with an endoscopic needle (NET2422-C4, Endo-Flex, Germany) at front wall or back wall of the gastric antrum. Thereafter, shape change of submucosal cushion was observed by the endoscope at 0, 10 and 30 min after endoscopic injection, and the obtained images were recorded and analyzed. On the other hand, 5.0 mL of NS-E, GGH or GGH-E was injected into submucosa and then mucosa was resected by an electrosurgical knife (KD-655L, Olympus, Japan). The obtained images were recorded to observe the hemostatic effect.
4.9. Biocompatibility assessments of GGH in vitro
Proliferation of L929 fibroblast cells (Chinese Academy of Sciences, China) was assessed by Counting Kit-8 (CCK-8) method. Briefly, experiment was divided into blank and GGH groups. In blank group, L929 fibroblast cells were seeded into 96-well plates (Corning, USA) with a density of 3000 cells/100 μL/well and incubated for 1, 3 and 5 d at 37 °C in a 5% CO2 humidified incubator (Thermo, USA) to obtain a monolayer of cells. As for GGH group, 50 μL of GGH (without methylene blue) was additionally added into 96-well plate after seeding cells, and the cells were then incubated for 1, 3 and 5 d under the same conditions as blank group. After incubation, culture medium was removed, and 100 μL of fresh medium (10 μL of CCK-8 reagent) was added into each well and then incubated at 37 °C for 2 h. Thereafter, absorbance of sample was measured by a microplate reader (Thermo, USA) at 450 nm.
As for live/dead cell double staining kit experiment, two groups were divided as above. For blank group, L929 fibroblast cells were seeded into 12-well plates (Corning, USA) with a density of 20000 cells/1 mL/well and incubated for 3 and 5 d at 37 °C in the 5% CO2 humidified incubator. As for GGH group, 200 μL of GGH (without methylene blue) was additionally added into the plates after seeding cells, and then the cells were incubated for 3 and 5 d under the same conditions as blank group. During the incubation, all the culture mediums were replaced at 3 d after seeding cells. After incubation, culture medium was removed and every well was washed with PBS for three times, and then 500 μL of solution including calcein-AM (2 μM) and propidium iodide (8 μM) was added into each well and incubated for 30 min. Thereafter, each well was washed by PBS and observed by an inversed fluorescent microscope (IX73, Olympus, Japan).
4.10. Statistical analysis
All statistical analyses were conducted using Excel software (Microsoft). Data were expressed as mean ± standard deviation. Statistical differences were determined using one-way analysis of variance (ANOVA). A p-value of less than 0.05 was considered statistically significant, and the levels of significance were labeled with *p < 0.05, **p < 0.01, ***p < 0.001.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information file.
Credit authorship contribution statement
Yinxiang Tang: Preparation and characterization of materials, Ex vivo test, Writing - original draft. Minhui Hu: Writing - original draft, ESD pig model, In vivo tests, Writing. Fuxin Tang: Cell culture and toxicity analysis. Rongkang Huang: Conceptualization, Supervision, ESD pig model, Writing - original draft, Writing - review & editing. Hui Wang: Conceptualization, Supervision, Writing - review & editing. Dingcai Wu: Conceptualization, Supervision, Writing - review & editing. Ping Lan: Conceptualization, Supervision, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the project of the National Key Research and Development Program of China (2017YFC1308800); the National Natural Science Foundation of China (51925308 and 51872336); and the Technical and Innovation Talents of Guangdong Special Support Program (2017TX04C248).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2021.11.026.
Contributor Information
Rongkang Huang, Email: huangrk3@mail.sysu.edu.cn.
Hui Wang, Email: wang89@mail.sysu.edu.cn.
Dingcai Wu, Email: wudc@mail.sysu.edu.cn.
Ping Lan, Email: lanping@mail.sysu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA. Cancer. J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Hussey G.S., Cramer M.C., Badylak S.F. Extracellular matrix bioscaffolds for building gastrointestinal tissue. Cell. Mol. Gastroenterol. Hepatol. 2018;5(1):1–13. doi: 10.1016/j.jcmgh.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shussman N., Wexner S.D. Colorectal polyps and polyposis syndromes. Gastroenterol. Rep (Oxf). 2014;2(1):1–15. doi: 10.1093/gastro/got041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nie R.C., Yuan S.Q., Li Y.F., et al. Additional gastrectomy in early-stage gastric cancer after non-curative endoscopic resection: a meta-analysis. Gastroenterol. Rep (Oxf). 2019;7(2):91–97. doi: 10.1093/gastro/goz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan C., Xu K., Huang Y., Liu S., Wang T., Wang W., Hu W., Liu L., Xing M., Yang S. Viscosity and degradation controlled injectable hydrogel for esophageal endoscopic submucosal dissection. Bioact. Mater. 2021;6(4):1150–1162. doi: 10.1016/j.bioactmat.2020.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pang Y., Liu J., Moussa Z.L., Collins J.E., McDonnell S., Hayward A.M., Jajoo K., Langer R., Traverso G. Endosopically injectable shear-thinning hydrogels facilitating polyp removal. Adv. Sci. 2019;6(19):1901041. doi: 10.1002/advs.201901041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunders B.P., Tsiamoulos Z.P. Endoscopic mucosal resection and endoscopic submucosal dissection of large colonic polyps. Nat. Rev. Gastroenterol. Hepatol. 2016;13(8):486–496. doi: 10.1038/nrgastro.2016.96. [DOI] [PubMed] [Google Scholar]
- 8.Waterhouse D.J., Fitzpatrick C., di Pietro M., Bohndiek S.E. Emerging optical methods for endoscopic surveillance of Barrett's oesophagus. Lancet. Gastroenterol. Hepatol. 2018;3(5):349–362. doi: 10.1016/S2468-1253(18)30030-X. [DOI] [PubMed] [Google Scholar]
- 9.Yeh J.H., Tseng C.H., Huang R.Y., Lin C.W., Lee C.T., Hsiao P.J., Wu T.C., Kuo L.T., Wang W.L. Long-term outcomes of orimary endoscopic resection vs surgery for T1 colorectal cancer: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2020;18(12):2813–2823. doi: 10.1016/j.cgh.2020.05.060. [DOI] [PubMed] [Google Scholar]
- 10.Visaggi P., Barberio B., Ghisa M., Ribolsi M., Savarino V., Fassan M., Valmasoni M., Marchi S., de Bortoli N., Savarino E. Modern diagnosis of early esophageal cancer: from blood biomarkers to advanced endoscopy and artificial intelligence. Cancers. 2021;13(13):3162. doi: 10.3390/cancers13133162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du C., Chai N.L., Linghu E.Q., Li H.K., Zhai Y.Q., Li L.S., Tang X.W., Wang H.B., Tang P. Clinical outcomes of endoscopic resection for the treatment of gastric gastrointestinal stromal tumors originating from the muscularis propria: a 7-year experience from a large tertiary center in China. Surg. Endosc. 2021 doi: 10.1007/s00464-021-08443-9. [DOI] [PubMed] [Google Scholar]
- 12.Takamaru H., Saito Y., Yamada M., Tsuruki E.S., Kinjo Y., Otake Y., Sakamoto T., Nakajima T., Matsuda T. Clinical impact of endoscopic clip closure of perforations during endoscopic submucosal dissection for colorectal tumors. Gastrointest. Endosc. 2016;84(3):494–502. doi: 10.1016/j.gie.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Inoue H., Fukami N., Yoshida T., Kudo S.E. Endoscopic mucosal resection for esophageal and gastric cancers. J. Gastroenterol. Hepatol. 2002;17(4):382–388. doi: 10.1046/j.1440-1746.2002.02732.x. [DOI] [PubMed] [Google Scholar]
- 14.Yeh R.W., Triadafilopoulos G. Submucosal injection: safety cushion at what cost? Gastrointest. Endosc. 2005;62(6):943–945. doi: 10.1016/j.gie.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira A.O., Moleiro J., Torres J., Dinis-Ribeiro M. Solutions for submucosal injection in endoscopic resection: a systematic review and meta-analysis. Endosc. Int. Open. 2016;4(1):E1–E16. doi: 10.1055/s-0034-1393079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yandrapu H., Desai M., Siddique S., Vennalganti P., Vennalaganti S., Parasa S., Rai T., Kanakadandi V., Bansal A., Titi M., Repici A., Bechtold M.L., Sharma P., Choudhary A. Normal saline solution versus other viscous solutions for submucosal injection during endoscopic mucosal resection: a systematic review and meta-analysis. Gastrointest. Endosc. 2017;85(4):693–699. doi: 10.1016/j.gie.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida T., Hirose R., Naito Y., Inoue K., Dohi O., Yoshida N., Kamada K., Uchiyama K., Ishikawa T., Takagi T., Konishi H., Nakaya T., Itoh Y. Viscosity: an important factor in predicting the performance of submucosal injection materials. Mater. Des. 2020;195:109008. [Google Scholar]
- 18.Castro R., Libânio D., Pita I., Dinis-Ribeiro M. Solutions for submucosal injection: what to choose and how to do it, World. J. Gastroenterol. 2019;25(7):777–788. doi: 10.3748/wjg.v25.i7.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uraoka T., Saito Y., Yamamoto K., Fujii T. Submucosal injection solution for gastrointestinal tract endoscopic mucosal resection and endoscopic submucosal dissection. Drug Des. Dev. Ther. 2009;2(6):131–138. doi: 10.2147/dddt.s3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katsinelos P., Kountouras J., Paroutoglou G., Chatzimavroudis G., Zavos C., Pilpilidis I., Gelas G., Paikos D., Karakousis K. A comparative study of 50% dextrose and normal saline solution on their ability to create submucosal fluid cushions for endoscopic resection of sessile rectosigmoid polyps. Gastrointest. Endosc. 2008;68(4):692–698. doi: 10.1016/j.gie.2008.02.063. [DOI] [PubMed] [Google Scholar]
- 21.Fujishiro M., Yahagi N., Kashimura K., Mizushima Y., Oka M., Enomoto S., Kakushima N., Kobayashi K., Hashimoto T., Iguchi M., Shimizu Y., Ichinose M., Omata M. Comparison of various submucosal injection solutions for maintaining mucosal elevation during endoscopic mucosal resection. Endoscopy. 2004;36(7):579–583. doi: 10.1055/s-2004-814517. [DOI] [PubMed] [Google Scholar]
- 22.Fujishiro M., et al. Tissue damage of different submucosal injection solutions for EMR. Gastrointest. Endosc. 2005;62(6):933–942. doi: 10.1016/j.gie.2005.07.052. [DOI] [PubMed] [Google Scholar]
- 23.Fasoulas K., et al. Endoscopic mucosal resection of giant laterally spreading tumors with submucosal injection of hydroxyethyl starch: comparative study with normal saline solution. Surg. Laparosc. Endosc. Percutaneous Tech. 2012;22(3):272–278. doi: 10.1097/SLE.0b013e318251553c. [DOI] [PubMed] [Google Scholar]
- 24.Eun S.H., Cho J.Y., Jung I.S., Ko B.M., Hong S.J., Ryu C.B., Kim J.O., Jin S.Y., Lee J.S., Lee M.S., Shim C.S., Kim B.S. Effectiveness of sodium alginate as a submucosal injection material for endoscopic mucosal resection in animal. Gut. Liver. 2007;1(1):27–32. doi: 10.5009/gnl.2007.1.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamasaki M., Kume K., Yoshikawa I., Otsuki M. A novel method of endoscopic submucosal dissection with blunt abrasion by submucosal injection of sodium carboxymethylcellulose: an animal preliminary study. Gastrointest. Endosc. 2006;64(6):958–965. doi: 10.1016/j.gie.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 26.Lenz L., Di Sena V., Nakao F.S., Andrade G.P., Rohr M.R., Ferrari A.J. Comparative results of gastric submucosal injection with hydroxypropyl methylcellulose, carboxymethylcellulose and normal saline solution in a porcine model. Arq. Gastroenterol. 2010;47(2):184–187. doi: 10.1590/s0004-28032010000200013. [DOI] [PubMed] [Google Scholar]
- 27.Dovedytis M., Liu Z.J., Bartlett S. Hyaluronic acid and its biomedical applications: a review, Engineered. Regeneration. 2020;1:102–113. [Google Scholar]
- 28.Matsui Y., Inomata M., Izumi K., Sonoda K., Shiraishi N., Kitano S. Hyaluronic acid stimulates tumor-cell proliferation at wound sites. Gastrointest. Endosc. 2004;60(4):539–543. doi: 10.1016/s0016-5107(04)01890-5. [DOI] [PubMed] [Google Scholar]
- 29.Dai M.S., Hu K.W., Wu W., Yin G.J., Hu D.M. EndoClot® SIS polysaccharide injection as a submucosal fluid cushion for endoscopic mucosal therapies: results of ex vivo and in vivo studies. Dig. Dis. Sci. 2019;64(10):2955–2964. doi: 10.1007/s10620-019-05686-4. [DOI] [PubMed] [Google Scholar]
- 30.Toshiyuki M., Akihiko O., Yuuki K., Sho M., Takayuki H. Cellulose nanofiber dispersion as a new submucosal injection material for endoscopic treatment: preliminary experimental study. Endosc. Int. Open. 2020;8(5):E623–E627. doi: 10.1055/a-1119-6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishihara M., Kumano I., Hattori H., Nakamura S. Application of hydrogels as submucosal fluid cushions for endoscopic mucosal resection and submucosal dissection. J. Artif. Organs. 2015;18(3):191–198. doi: 10.1007/s10047-015-0843-z. [DOI] [PubMed] [Google Scholar]
- 32.Hirose R., Nakaya T., Naito Y., Yoshida T., Bandou R., Daidoji T., Inoue K., Dohi O., Yoshida N., Itoh Y. An innovative next-generation endoscopic submucosal injection material with a 2-step injection system (with video) Gastrointest. Endosc. 2021;93(2):503–513. doi: 10.1016/j.gie.2020.06.031. [DOI] [PubMed] [Google Scholar]
- 33.Xu X., Xia X., Zhang K., Rai A., Li Z., Zhao P., Wei K., Zou L., Yang B., Wong W.-K., Chiu P.W.-Y., Bian L. Bioadhesive hydrogels demonstrating pH-independent and ultrafast gelation promote gastric ulcer healing in pigs. Sci. Transl. Med. 2020;12(558) doi: 10.1126/scitranslmed.aba8014. [DOI] [PubMed] [Google Scholar]
- 34.Ishizuka T., Hayashi T., Ishihara M., Yoshizumi Y., Aiko S., Nakamura S., Yura H., Kanatani Y., Nogami Y., Maehara T. Submucosal injection, for endoscopic mucosal resection, of photocrosslinkable chitosan hydrogel in DMEM/F12 medium. Endoscopy. 2007;39(5):428–433. doi: 10.1055/s-2007-966393. [DOI] [PubMed] [Google Scholar]
- 35.Zhao X., Li S., Du X., Li W., Wang Q., He D., Yuan J. Natural polymer-derived photocurable bioadhesive hydrogels for sutureless keratoplasty. Bioact. Mater. 2021;6(8):196–209. doi: 10.1016/j.bioactmat.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumano I., Ishihara M., Nakamura S., Kishimoto S., Fujita M., Hattori H., Horio T., Tanaka Y., Hase K., Maehara T. Endoscopic submucosal dissection for pig esophagus by using photocrosslinkable chitosan hydrogel as submucosal fluid cushion. Gastrointest. Endosc. 2012;75(4):841–848. doi: 10.1016/j.gie.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 37.Wu X., Zhou M., Jiang F., Yin S., Lin S., Yang G., Lu Y., Zhang W., Jiang X. Marginal sealing around integral bilayer scaffolds for repairing osteochondral defects based on photocurable silk hydrogels. Bioact. Mater. 2021;6(11):3976–3986. doi: 10.1016/j.bioactmat.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia Y.P., Shi K., Yang F., Liao J.F., Han R.X., Yuan L.P., Hao Y., Pan M., Xiao Y., Qian Z.Y., Wei X.W. Multifunctional nanoparticle loaded injectable thermoresponsive hydrogel as NIR controlled release platform for local photothermal immunotherapy to prevent breast cancer postoperative recurrence and metastases. Adv. Funct. Mater. 2020;30(25):2001059. [Google Scholar]
- 39.Yu L., Xu W., Shen W.J., Cao L.P., Liu Y., Li Z.S., Ding J.D. Poly(lactic acid-co-glycolic acid)-poly(ethylene glycol)-poly(lactic acid-co-glycolic acid) thermogel as a novel submucosal cushion for endoscopic submucosal dissection. Acta Biomater. 2014;10(3):1251–1258. doi: 10.1016/j.actbio.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 40.Yusaku T., Toshio U., Takefumi N., Shunji Y., Naohisa Y. Potential of temperature-response collagen-genipin sols as a novel submucosal injection material for endoscopic resection. Endosc. Int. Open. 2019;7(4):E561–E567. doi: 10.1055/a-0867-9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tao S.C., Huang J.Y., Gao Y., Li Z.X., Wei Z.Y., Dawes H., Guo S.C. Small extracellular vesicles in combination with sleep-related circRNA3503: a targeted therapeutic agent with injectable thermosensitive hydrogel to prevent osteoarthritis. Bioact. Mater. 2021;6(12):4455–4469. doi: 10.1016/j.bioactmat.2021.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu X., Wang X., Chen X., Yang X., Ma Q., Xu G., Yu L., Ding J. Injectable and thermosensitive hydrogels mediating a universal macromolecular contrast agent with radiopacity for noninvasive imaging of deep tissues. Bioact. Mater. 2021;6(12):4717–4728. doi: 10.1016/j.bioactmat.2021.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishioka . 2014. Submucosal Elevation Agent. CN103957954A. [Google Scholar]
- 44.Ferris C.J., in het Panhuis M. Conducting bio-materials based on gellan gum hydrogels. Soft Matter. 2009;5(18):3430. [Google Scholar]
- 45.Mahdi M.H., Conway B.R., Mills T., Smith A.M. Gellan gum fluid gels for topical administration of diclofenac. Int. J. Pharm. 2016;515(1–2):535–542. doi: 10.1016/j.ijpharm.2016.10.048. [DOI] [PubMed] [Google Scholar]
- 46.Grasdalen H., Smidsrød O. Gelation of gellan gum. Carbohydr. Polym. 1987;7(5):371–393. [Google Scholar]
- 47.Morris E.R., Nishinari K., Rinaudo M. Gelation of gellan – a review. Food Hydrocolloids. 2012;28(2):373–411. [Google Scholar]
- 48.Norton I.T., Jarvis D.A., Foster T.J. A molecular model for the formation and properties of fluid gels. Int. J. Biol. Macromol. 1999;26(4):255–261. doi: 10.1016/s0141-8130(99)00091-4. [DOI] [PubMed] [Google Scholar]
- 49.Mahdi M.H., Conway B.R., Smith A.M. Evaluation of gellan gum fluid gels as modified release oral liquids. Int. J. Pharm. 2014;475(1–2):335–343. doi: 10.1016/j.ijpharm.2014.08.044. [DOI] [PubMed] [Google Scholar]
- 50.Shin J., Lee J.S., Lee C., Park H.J., Yang K., Jin Y., Ryu J.H., Hong K.S., Moon S.H., Chung H.-M., Yang H.S., Um S.H., Oh J.W., Kim D.I., Lee H., Cho S.W. Tissue adhesive catechol-modified hyaluronic acid hydrogel for effective, Minimally Invasive Cell Therapy. Adv. Funct. Mater. 2015;25(25):3814–3824. [Google Scholar]
- 51.Peng X., Xia X., Xu X., Yang X., Yang B., Zhao P., Yuan W., Chiu P.W.Y., Bian L. Ultra-fast self-gelling powder mediates robust wet adhesion to promote healing of gastrointestinal perforations. Sci. Adv. 2021;7(23):eabe8739. doi: 10.1126/sciadv.abe8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choi J.H., Park A., Lee W., Youn J., Rim M.A., Kim W., Kim N., Song J.E., Khang G. Preparation and characterization of an injectable dexamethasone-cyclodextrin complexes-loaded gellan gum hydrogel for cartilage tissue engineering. J. Contr. Release. 2020;327(10):747–765. doi: 10.1016/j.jconrel.2020.08.049. [DOI] [PubMed] [Google Scholar]
- 53.Xu L., Bai X., Yang J., Li J., Xing J., Yuan H., Xie J., Li J. Preparation and characterisation of a gellan gum-based hydrogel enabling osteogenesis and inhibiting Enterococcus faecalis. Int. J. Biol. Macromol. 2020;165:2964–2973. doi: 10.1016/j.ijbiomac.2020.10.083. [DOI] [PubMed] [Google Scholar]
- 54.Song J.E., Lee S.E., Cha S.R., Jang N.K., Tripathy N., Reis R.L., Khang G. Inflammatory response study of gellan gum impregnated duck's feet derived collagen sponges. J. Biomater. Sci. Polym. Ed. 2016;27(15):1495–1506. doi: 10.1080/09205063.2016.1213218. [DOI] [PubMed] [Google Scholar]
- 55.Liao Z., Dong L., Wang C. Modulating, instead of suppressing, foreign body responses for biomaterials design. Eng. Regen. 2021;2:91–95. [Google Scholar]
- 56.Zhou X., He X., Shi K., Yuan L., Yang Y., Liu Q., Ming Y., Yi C., Qian Z. Injectable thermosensitive hydrogel containing erlotinib-loaded hollow mesoporous silica nanoparticles as a localized drug delivery system for NSCLC therapy. Adv. Sci. 2020;7(23):2001442. doi: 10.1002/advs.202001442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang J., Chen C., Li A., Jing W., Sun P., Huang X., Liu Y., Zhang S., Du W., Zhang R., Liu Y., Gong A., Wu J., Jiang X. Immunostimulant hydrogel for the inhibition of malignant glioma relapse post-resection. Nat. Nanotechnol. 2021;16(5):538–548. doi: 10.1038/s41565-020-00843-7. [DOI] [PubMed] [Google Scholar]
- 58.Kongkiatpaiboon S., Duangdee N., Chewchinda S., Poachanukoon O., Amnuaypattanapon K. Development and validation of stability indicating HPLC method for determination of adrenaline tartrate. J. King Saud Univ. Sci. 2019;31(1):48–51. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information file.