Abstract
The effects of therapeutically relevant concentrations of the human immunodeficiency virus (HIV) proteinase inhibitors saquinavir and indinavir on the in vitro proteinase activity of Candida albicans were investigated with isolates from HIV-infected and uninfected patients with oral candidiasis. After exposure to the HIV proteinase inhibitors, proteinase activity was significantly reduced in a dose-dependent manner. These inhibitory effects, which were similar to that of pepstatin A, and the reduced virulence phenotype in experimental candidiasis after application of saquinavir indicate the usefulness of these HIV proteinase inhibitors as potential anticandidal agents.
Candida albicans is an opportunistic pathogen which commonly colonizes human mucosal surfaces such as the oral cavity. Under certain circumstances, usually linked to a compromised host immune system, C. albicans causes infections which may be restricted to the mucosa or, in severe cases of immunodepression, progress to systemic invasion (27). Especially in human immunodeficiency virus (HIV)-infected patients, C. albicans has been recognized as the most frequent cause of opportunistic infections. Up to 90% of HIV-positive patients suffer from mucosal candidiasis at least once in the course of their disease (29). Recently, fungal infections including clinically apparent oral candidiasis have become rarer in HIV-infected patients because of the introduction of new anti-HIV drugs of the proteinase inhibitor type (13). These agents are directed against HIV proteinases. They render the enzyme nonfunctional and lead to the release of immature, noninfectious viral particles (9, 26). Among the various potential virulence factors proposed for C. albicans infections, the secreted aspartyl proteinases (Saps) encoded by a gene family with at least nine members (SAP1 to SAP9) are of key importance (17, 19, 20, 24, 34, 35). The enzymatic activities of Saps have received considerable attention in several in vitro studies (2, 3, 7, 8, 16, 28) and studies with animals (10, 11, 22, 23, 31). Inhibition of Sap activity by prophylactic treatment with the proteinase inhibitor pepstatin A resulted in reduced adherence or virulence, suggesting that Sap isoenzymes are important for pathogenesis (2, 3, 10, 28, 31). It has been shown that pepstatin A and its synthetic analogs are active against both the Saps of C. albicans and HIV proteinase (21). However, pepstatin-like drugs are not used clinically because of their metabolism in the liver and rapid clearance from blood (33).
In 1996 a single case report described an HIV-infected patient who had oral candidiasis that was refractory to treatment with fluconazole, itraconzole, amphotericin B, and nystatin and whose infection finally resolved after initiation of therapy with an antiretroviral agent combined with the HIV proteinase inhibitor saquinavir (SQV) (39). The investigator explained the therapeutic success to be the result of an improvement in the patient’s immune status (39). A retrospective study of HIV-infected patients with oral candidiasis has demonstrated a beneficial influence on the frequency and/or severity of the mucosal infections after treatment with HIV proteinase inhibitors (13). Those investigators speculated that the effects were a result not only of the improved immune status but also of direct inhibition of Saps by the HIV proteinase inhibitor. In the present study, we studied the inhibitory capabilities of SQV and indinavir (IDV), two novel HIV proteinase inhibitors, against the Saps of C. albicans isolates in an in vitro assay. These results were compared with the inhibitory effect of pepstatin A.
To assess a possible influence of the HIV proteinase inhibitors SQV and IDV on Saps, the inhibitory effect was analyzed with five clinical C. albicans isolates and was compared to that of pepstatin A. SQV was obtained from Hoffmann-La Roche AG, Grenzach-Wyhlen, Germany; IDV was from Merck Sharp & Dohme GmbH, Haar, Germany; and pepstatin A was from Sigma Chemical Company, St. Louis, Mo. Samples were removed from oral mucosal lesions of five volunteer patients (one non-HIV-infected and four HIV-infected patients) by standard clinical procedures. Characterization of the isolates as C. albicans was performed by assessing colony morphology, the germ tube test with normal human serum, and, additionally, biochemical identification of C. albicans based on the use of a ready-made system (ATB 32 C; API System, bio Mérieux, La Balme-les-Grottes, France) (4). Each C. albicans strain was grown in Sabouraud-dextrose broth (Difco Laboratories, Detroit, Mich.) in an incubator (Heraeus, Hanau, Germany) for 48 h at 27°C. To induce the secretion of Saps, 100 μl of C. albicans suspension was added to 10 ml of bovine serum albumin (BSA)-Remold medium composed of 2% glucose (Merck, Darmstadt, Germany), 0.1% KH2PO4 (Merck), 0.5% MgSO4 (Merck), 1.25 ml of 100× sterile-filtered minimum essential medium vitamins (Sigma), and 1% BSA (Sigma); and the mixture was incubated for 7 days at 27°C in a shaker at 150 rpm. Thereafter, the numbers of CFU were determined and the yeasts were removed by centrifugation at 1,500 × g for 30 min. The supernatants were adjusted to pH 6.5 with NaOH to limit autodegradation and were frozen at −20°C after filter sterilization to give the final crude enzyme preparation (Stericup, 500 ml; pore size, 0.22 μm; Millipore Corporation, Bedford, Mass.). The mean proteinase activity of the preparations was calculated to be 1,351 U/liter · h. SQV and IDV were dissolved in absolute methanol at 1 μM for SQV and 100 μM for IDV. Dilutions with concentrations of 0.075, 0.05, 0.025, 0.01, and 0.001 μM for SQV and 20, 10, 7.5, 5, 2.5, 1, and 0.1 μM for IDV were obtained with 0.2 M sodium citrate-HCl buffer (pH 4.5) (Merck). These concentrations are comparable to those obtained under clinical conditions (9, 15, 30). Following administration of SQV at 600 mg three times daily, the geometric mean maximum concentration of drug in serum (Cmax) ranged from 0.28 to 0.4 μM (9, 15, 30). The geometric mean Cmax following administration of IDV at 800 mg three times daily was 11 μM (9, 15). As a control inhibitor, pepstatin A was prepared in the same way at concentrations of 100, 10, 1, 0.075, 0.5, 0.25, and 0.1 μM. Studies with different substrates such as bovine hemoglobin (Sigma), BSA (Sigma), and human stratum corneum (Sigma) were carried out. Test tubes were each filled with 750 μl of 0.2 M sodium citrate-HCl buffer, 750 μl of fresh substrate solution (1% substrate in the same buffer), 250 μl of each sample, and 250 μl of SQV, IDV, or pepstatin A. To investigate the effect of serum on the inhibitory potency of SQV, experiments were also performed in the presence of human serum. Control experiments without inhibitor were run in parallel. The preparations were incubated at 37°C for 60 min (T60) in a shaker. Three duplicate samples were used for each experiment. The reactions were stopped with 500 μl of trichloroacetic acid, and the specimens were put on ice. For each reaction mixture an additional control was prepared by adding all ingredients plus 20% trichloroacetic acid simultaneously at T0. All specimens were centrifuged at 3,000 × g for 30 min at 4°C. A total of 160 μl of each clear supernatant was added to 40 μl of dye reagent concentrate (Coomassie brilliant blue G-250; Bio-Rad Laboratories, Munich, Germany). This protein assay is based on the observed shift in the maximum absorbance when the dye reagent reacts with protein. The protein assay (Bio-Rad Laboratories) was used to determine the Sap concentration at 595 nm with a spectrophotometer (MR 4000; Dynatech, Dinkendorf, Germany). Activity was measured as the change in optical density: sample (T60) − control (T0). One activity unit was defined as an increase of 0.100/60 min at 595 nm (29). The activities were calculated for 1 liter of Remold medium at a yeast density of 108 cells per ml. Relative proteinase activities in the presence of SQV, IDV, and pepstatin A were expressed as a percentage of the activity measured in the controls without inhibitor. Inhibition in the presence of SQV, IDV, and pepstatin A was compared by analysis of variance, and the significance of all differences was calculated by the least-significance-difference (LSD) test. For the LSD test the lowest Sap activities of each C. albicans strain were taken for SQV, IDV, and pepstatin A, and the efficacies of inhibition of all three drugs were compared. Due to the exploratory character of the study, the level of significance was set at a P value of 0.05. The effect of SQV was tested in an established in vitro model of oral candidiasis based on reconstituted human epithelium (RHE) (35). SQV was dissolved in 0.2 M sodium citrate-HCl buffer (pH 4.5) (Merck) and was administered in 50 μl of phosphate-buffered saline (PBS) containing 2 × 106 C. albicans SC5314 yeast cells at a final concentration of 0.3 μM. Controls contained 50 μl of PBS with 2 × 106 C. albicans SC5314 yeast cells and 0.3 μM SQV in 0.2 M sodium citrate-HCl buffer alone. RHE was incubated at 37°C with 5% CO2 at 100% humidity for 12 h. Semithin sections (1 μm) were studied with a light microscope after staining with 1% toluidine blue and 1% pyronine G (Merck, Darmstadt, Germany). The sections were viewed at a magnification of ×400.
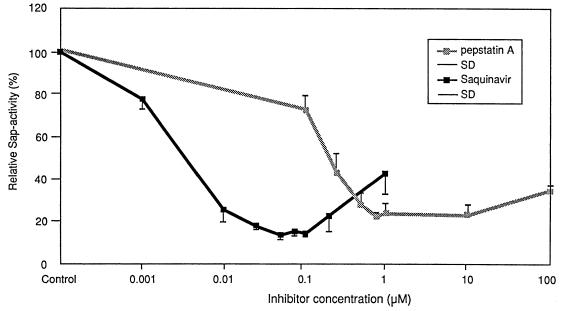
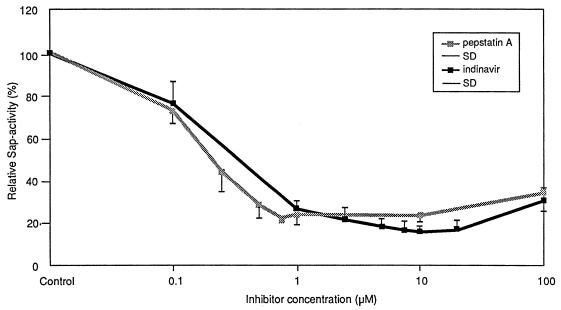
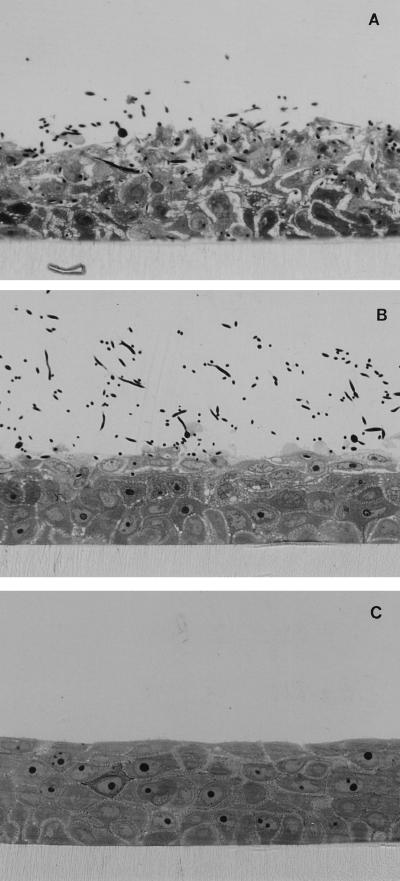
Inhibition of Sap activity by SQV, IDV, and pepstatin A was observed for all three substrates in a dose-dependent manner (data not shown). Hemoglobin was the substrate that was the most efficiently degraded by Sap activity (data not shown). In the following, exact inhibition data for hemoglobin are demonstrated in detail. SQV at concentrations ranging from 0.001 to 1 μM inhibited Saps by approximately 22 to 85%. The best inhibitory effect was seen with SQV concentrations ranging from 0.05 to 0.1 μM (Fig. 1). IDV at concentrations ranging from 0.1 to 100 μM inhibited Saps by approximately 24 to 83%. The inhibitory potency of SQV was not significantly affected by the addition of human serum. The best inhibitory effect was seen at an IDV concentration of 10 μM (Fig. 2). Similar test results were obtained for pepstatin A at concentrations ranging from 0.1 to 100 μM. The best inhibitory effect with pepstatin A was seen at concentrations ranging from 0.75 to 10 μM (Fig. 1 and 2). The inhibitory effects of the three medications were similar, with no significant difference (data not shown). Statistical analysis of Sap activity with and without a proteinase inhibitor showed a high degree of significance for each of the three agents (data not shown). An inhibitory maximum for pepstatin A was seen from 0.75 to 10 μM, with a slightly decreased inhibition at 100 μM (Fig. 1 and 2). This corresponds to the results of Ollert et al. (28), who demonstrated a similar effect in their in vitro adherence model. This slight decrease in inhibitory effect was also apparent at higher inhibitory concentrations of SQV and IDV (Fig. 1 and 2). Evidence for a direct protective role of SQV during experimental oral candidiasis could be demonstrated by a strong attenuated histological phenotype. Without SQV, at 12 h after infection with SC5314 a strong epithelial lesion was observed with vacuolation, edema, and detachment of all epithelial layers, and C. albicans was able to invade the epithelium (Fig. 3A). These morphological alterations were strongly reduced when SQV was added at a concentration of 0.3 μM (Fig. 3B). Incubation with SQV alone at a concentration of 0.3 μM demonstrated no histological alterations (Fig. 3C). The attenuated virulence phenotype in the presence of SQV suggests that Saps contribute to the virulence in this model of oral candidiasis and implies a direct anticandidal effect. Complete inhibition of the proteolytic activity was not seen in our study. This is supported by several previously published in vitro studies and studies with animals (5, 6, 10, 25, 29, 31–33) in which pepstatin A was used to inhibit Sap activity. The majority of the previous investigations demonstrated reduced virulence but not complete avirulence of C. albicans under the influence of pepstatin A. This may be due to an incomplete inhibition of all Sap isoenzymes by pepstatin A or the activities of factors other than proteinases. Since the introduction of HIV proteinase inhibitors, the frequency of clinically apparent oral candidiasis in HIV-infected patients has been reduced. This change appears to be correlated with elevated CD4-cell counts and improved immune function (13). Another reason for the resolution might also be a direct effect of the HIV proteinase inhibitor on Sap isoenzymes, which are major virulence factors for C. albicans infections (14). This hypothesis is supported by a recently published case report (12). In that report a patient had suffered from persistent oral pseudomembranous candidiasis. The C. albicans isolates proved to be resistant to azole derivates in vitro and in vivo. After starting antiretroviral combination therapy that included a proteinase inhibitor, the patient’s clinical appearance became more erythematous and the patient’s symptoms improved. A marked increase in the CD4-cell count was not observed (12), suggesting a direct action on oral candidiasis by the drug treatment.
FIG. 1.
Percent activity of Saps as a function of inhibitor (SQV and pepstatin A) concentrations. Each point represents the mean ± standard deviation (SD) for three duplicate determinations for five C. albicans isolates.
FIG. 2.
Percent activity of Saps as a function of inhibitor (IDV and pepstatin A) concentrations. Each point represents the mean ± standard deviation (SD) for three duplicate determinations for five C. albicans isolates.
FIG. 3.
Light micrographs of RHE. (A) RHE 12 h after infection with C. albicans SC5314 cells showing mucosal erosion with severe acantholysis, edema, and enlarged intercellular spaces of all keratinocyte layers. Vacuolation of the epithelium is also shown. (B) RHE 12 h after infection with C. albicans SC5314 cells in the presence of SQV at 0.3 μM. Vacuolation and edema are seen only within the two uppermost epithelial layers. (C) RHE 12 h after inoculation of SQV at 0.3 μM. Stratified keratinocytes without stratum corneum are shown. Marked alterations of the epithelium are not visible. Magnifications, ×400.
HIV proteinase and Saps of C. albicans belong to the same class of aspartyl proteinases and are inhibited by the classical inhibitor pepstatin A (21). In contrast to the very small and structurally simplified HIV proteinase, Saps are larger and more complex (1, 21). They possess a relatively large active site which might be responsible for the broader substrate specificity and also their susceptibility to aspartyl proteinase inhibitors (1). Our results confirm the inhibition of Saps by pepstatin A and suggest that SQV and IDV have inhibitory effects similar to that of pepstatin A. According to the results of in vitro gene expression studies (18, 19, 38), the proteolytic activity measured in our study reflected mainly the activities of Sap2, Sap3, and Sap8. Experimental infections and adhesion assays with epithelial cells demonstrated that SAP1 to SAP3 and their corresponding isoenzymes may be important for the pathogenesis of mucosal candidiasis (8, 36, 37). Thus, the inhibition of these proteinases by SQV and IDV would reduce the virulence of C. albicans. We have shown that SQV and IDV are as effective in vitro as pepstatin A in inhibiting candidal proteinases when the drugs are used at concentrations which have been measured in humans during treatment of HIV infections (9, 15, 30). A direct anticandidal effect of SQV was demonstrated by a reduced virulence phenotype in an established model of oral candidiasis (35). Therefore, it is likely that SQV and IDV also act as Sap inhibitors and anticandidal agents in vivo. In contrast, pepstatin A is a very effective inhibitor of Saps in vitro but is likely to be rapidly cleared in vivo and thus an ineffective anticandidal agent (10, 33). Effective inhibition of a prominent virulence factor of C. albicans by SQV and IDV in a dose-dependent manner has been demonstrated. The effect was seen in a therapeutically relevant dose range. The result justifies the development of SQV and IDV as potential anticandidal agents even in the absence of HIV infection.
Acknowledgments
We thank K. Fanderl, J. Laude, E. Januschke, and A. Kerschnitzki for excellent technical assistance and W. Burgdorf for critical reading of the manuscript.
ADDENDUM IN PROOF
An independent and similar study about inhibition of Saps by HIV proteinase inhibitors has been carried out by A. Cassone, F. De Bernardis, A. Torosantucci, E. Tacconelli, M. Tumbarello, and R. Cauda (J. Infect. Dis., in press).
REFERENCES
- 1.Abad-Zapatero C, Goldman R, Muchmore S W, Hutchins C, Stewart K, Navaza J, Payne C D, Ray T L. Structure of a secreted aspartic protease from C. albicans complexed with a potent inhibitor: implication for the design of antifungal agents. Protein Sci. 1996;5:640–652. doi: 10.1002/pro.5560050408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borg-von Zepelin M, Beggah S, Boggian K, Sanglard D, Monod M. The expression of the secreted aspartyl proteinases Sap4 to Sap6 from Candida albicans in murine macrophages. Mol Microbiol. 1998;28:543–554. doi: 10.1046/j.1365-2958.1998.00815.x. [DOI] [PubMed] [Google Scholar]
- 3.Borg-von Zepelin M, Rüchel R. Expression of extracellular acid proteinase by proteolytic Candida spp. during experimental infection of oral mucosa. Infect Immun. 1988;56:626–631. doi: 10.1128/iai.56.3.626-631.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caniaux I, Villard J, Gayral J P. Détection automatisée de l’assimilation des sources de carbone par les levures: etude préliminaire. Bull Soc Franc Mycol Med. 1985;14:269–276. [Google Scholar]
- 5.Colina A R, Aumont F, Deslauriers N, Belhumeur P, De Repenigny L. Evidence for degradation of gastrointestinal mucin by Candida albicans secretory aspartyl proteinase. Infect Immun. 1996;64:4514–4519. doi: 10.1128/iai.64.11.4514-4519.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Bernardis F, Boccanera M, Adriani D, Spreghini E, Santoni D, Cassone A. Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginitis in rats. Infect Immun. 1997;65:3399–3405. doi: 10.1128/iai.65.8.3399-3405.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Bernardis F, Boccanera M, Rainaldi L, Guerra C E, Quinti I, Cassone A. The secretion of aspartyl proteinase, a virulence enzyme, by isolates of Candida albicans from the oral cavity of HIV-infected subjects. Eur J Epidemiol. 1992;8:362–367. doi: 10.1007/BF00158569. [DOI] [PubMed] [Google Scholar]
- 8.De Bernardis F, Cassone A, Sturvetant J, Calderone R. Expression of Candida albicans SAP1 and SAP2 in experimental vaginitis. Infect Immun. 1995;63:1887–1892. doi: 10.1128/iai.63.5.1887-1892.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeks S G, Schmith M, Holodniy M, Kahn J O. HIV-1 protease inhibitors. A review for clinicians. JAMA. 1997;277:145–153. [PubMed] [Google Scholar]
- 10.Fallon K, Bausch K, Noonan J, Huguenel E, Tamburini P. Role of aspartic proteases in disseminated Candida albicans infection in mice. Infect Immun. 1997;65:551–556. doi: 10.1128/iai.65.2.551-556.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghannoum M, Abu Elteen K. Correlative relationship between proteinase production, adherence and pathogenicity of various strains of Candida albicans. J Med Vet Mycol. 1986;24:407–413. doi: 10.1080/02681218680000621. [DOI] [PubMed] [Google Scholar]
- 12.Hoegl L, Thoma-Greber E, Röcken M, Korting H C. Shift from persistent oral pseudomembranous to erythematous candidosis in a human immunodeficiency virus (HIV)-infected patient upon combination treatment with an HIV protease inhibitor. Mycoses. 1998;41:213–217. doi: 10.1111/j.1439-0507.1998.tb00326.x. [DOI] [PubMed] [Google Scholar]
- 13.Hoegl L, Thoma-Greber E, Röcken M, Korting H C. HIV protease inhibitors influence the prevalence of oral candidosis in HIV-infected patients: results of a study over a period of 2 years. Mycoses. 1998;41:321–325. doi: 10.1111/j.1439-0507.1998.tb00345.x. [DOI] [PubMed] [Google Scholar]
- 14.Hoegl L, Ollert M W, Korting H C. The role of Candida albicans secreted aspartic proteinase in the development of candidoses. J Mol Med. 1996;74:135–142. doi: 10.1007/BF01575445. [DOI] [PubMed] [Google Scholar]
- 15.Hoetelmans R M, Meenhorst P L, Mulder J W, Burger D M, Koks C H, Beijnen J H. Clinical pharmacology of HIV protease inhibitors: focus on saquinavir, indinavir, and ritonavir. Pharm World Sci. 1997;19:159–175. doi: 10.1023/a:1008629608556. [DOI] [PubMed] [Google Scholar]
- 16.Homma M, Kanabe T, Hiroji C, Tanak K. Detection of intracellular forms of secretory aspartic proteinase in Candida albicans. J Gen Microbiol. 1991;138:627–633. doi: 10.1099/00221287-138-3-627. [DOI] [PubMed] [Google Scholar]
- 17.Hube B. Candida albicans secreted aspartyl proteinases. Curr Top Med Mycol. 1996;7:55–69. [PubMed] [Google Scholar]
- 18.Hube B, Monod M, Schofield D A, Brown A J P, Gow N A R. Expression of seven members of the gene family encoding secretory aspartyl proteinase in Candida albicans. Mol Microbiol. 1994;14:87–99. doi: 10.1111/j.1365-2958.1994.tb01269.x. [DOI] [PubMed] [Google Scholar]
- 19.Hube B, Sanglard D, Monod M, Schofield D A, Brown A J P, Gow N A R. Extracellular proteolytic activity of Candida species. In: Vanden Bossche H, Stevens D A, Odds F C, editors. Host-fungus interplay. Proceedings of the Fifth Symposium on Topics in Mycology 1995. Bethesda, Md: National Foundation for Infectious Diseases; 1997. pp. 109–122. [Google Scholar]
- 20.Hube B, Sanglard D, Odds F C, Hess D, Monod M, Schäfer W, Brown A J P, Gow N A R. Gene disruption of each of the secreted aspartyl proteinase genes SAP1, SAP2, and SAP3 in Candida albicans attenuates virulence. Infect Immun. 1997;65:3529–3538. doi: 10.1128/iai.65.9.3529-3538.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato I, Yasunuga T, Ikawa Y, Yoshinaka Y. Inhibition of retroviral protease activity by an aspartyl proteinase inhibitor. Nature. 1987;329:654–656. doi: 10.1038/329654a0. [DOI] [PubMed] [Google Scholar]
- 22.Kwon-Chung K J, Lehman D, Good C, Magee P T. Genetic evidence for role of extracellular proteinase in virulence of Candida albicans. Infect Immun. 1985;49:571–575. doi: 10.1128/iai.49.3.571-575.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacDonald F, Odds F C. Virulence for mice of a proteinase-secreting stain of Candida albicans and a proteinase-deficient mutant. J Gen Microbiol. 1983;129:431–438. doi: 10.1099/00221287-129-2-431. [DOI] [PubMed] [Google Scholar]
- 24.Monod M, Togni G, Hube B, Hess D, Sanglard D. Cloning, sequencing and expression of two new members of the secreted aspartyl proteinase family of Candida albicans. Microbiology. 1998;144:2731–2737. doi: 10.1099/00221287-144-10-2731. [DOI] [PubMed] [Google Scholar]
- 25.Morschhäuser J, Virkola R, Korhonen T K, Häcker J. Degradation of human subendothelial extracellular matrix by proteinase-secreting Candida albicans. FEMS Microbiol Lett. 1997;153:349–355. doi: 10.1111/j.1574-6968.1997.tb12595.x. [DOI] [PubMed] [Google Scholar]
- 26.Moyle G. Resistance to antiretroviral compounds. London, United Kingdom: Mediscript; 1994. [Google Scholar]
- 27.Odds F C. Candida and candidosis. 2nd ed. London, United Kingdom: Ballière Tindall; 1988. [Google Scholar]
- 28.Ollert M W, Söhnchen R, Korting H C, Ollert U, Bräutigam S, Bräutigam W. Mechanisms of adherence of Candida albicans to cultured human epidermal keratinocytes. Infect Immun. 1993;61:4560–4568. doi: 10.1128/iai.61.11.4560-4568.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ollert M W, Wende C, Görlich M, McMullan-Vogel C G, Borg-von Zepelin M, Vogel C-W, Korting H C. Increased expression of Candida albicans secretory proteinase, a virulence factor, in isolates from human immunodeficiency virus-positive patients. J Clin Microbiol. 1995;33:2543–2549. doi: 10.1128/jcm.33.10.2543-2549.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry C M, Noble S. Saquinavir soft-gel capsule formulation. Drugs. 1998;55:461–486. doi: 10.2165/00003495-199855030-00014. [DOI] [PubMed] [Google Scholar]
- 31.Ray T L, Payne C D. Scanning electron microscopy of epidermal adherence and cavitation in murine candidiasis: a role for Candida acid proteinase. Infect Immun. 1988;56:1942–1949. doi: 10.1128/iai.56.8.1942-1949.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rüchel R. Properties of a purified proteinase from the yeast Candida albicans. Biochim Biophys Acta. 1981;659:99–113. doi: 10.1016/0005-2744(81)90274-6. [DOI] [PubMed] [Google Scholar]
- 33.Rüchel R, Ritter B, Schaffrinski M. Modulation of experimental systemic murine candidosis by intravenous pepstatin A. Zentbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig. 1990;273:391–403. doi: 10.1016/s0934-8840(11)80443-3. [DOI] [PubMed] [Google Scholar]
- 34.Sanglard D, Hube B, Monod M, Odds F C, Gow N A R. A triple deletion in SAP4, SAP5, and SAP6 secretory aspartyl proteinase genes of Candida albicans causes attenuated virulence. Infect Immun. 1997;65:3539–3546. doi: 10.1128/iai.65.9.3539-3546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaller M, Schäfer W, Korting H C, Hube B. Differential expression of secreted aspartyl proteinases in a model of human oral candidosis and in patient samples from the oral cavity. Mol Microbiol. 1998;29:605–616. doi: 10.1046/j.1365-2958.1998.00957.x. [DOI] [PubMed] [Google Scholar]
- 36.Schaller, M., W. Schäfer, H. C. Korting, and B. Hube. Unpublished data.
- 37.Watts H J, Cheah F S H, Hube B, Sanglard D, Gow N A R. Altered adherence in strains of Candida albicans harbouring null mutations in secreted aspartic proteinases genes. FEMS Microbiol Lett. 1998;159:129–135. doi: 10.1111/j.1574-6968.1998.tb12851.x. [DOI] [PubMed] [Google Scholar]
- 38.White T C, Agabian N. Candida albicans secreted aspartyl proteinases: isoenzyme pattern is determined by cell type, levels are determined by environmental factors. J Bacteriol. 1995;177:5215–5221. doi: 10.1128/jb.177.18.5215-5221.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zingman B S. Resolution of refractory AIDS-related mucosal candidiasis after initiation of didanosine plus saquinavir. N Engl J Med. 1996;334:1674–1675. doi: 10.1056/NEJM199606203342516. [DOI] [PubMed] [Google Scholar]