Abstract
Synercid and each of its components (quinupristin and dalfopristin) were examined for their activities against Toxoplasma gondii. In vitro, intracellular replication of tachyzoites was inhibited by synercid and each of its two components. The 50% inhibitory concentrations of synercid, quinupristin, and dalfopristin were 1.6, 2.7, and 6.3 μg/ml, respectively. Thus, synercid was markedly more active than its components. Treatment of acutely infected mice with 100 or 200 mg of synercid per kg of body weight per day administered intraperitoneally for 10 days resulted in survival of 50% (P = 0.0002) and 100% (P < 0.0001) of infected mice, respectively, whereas all control mice died by day 18. In contrast, treatment with 200 mg of either quinupristin and dalfopristin per kg per day alone resulted in only 20% survival; treatment with 50 mg of either drug per kg per day resulted only in the prolongation of time to death. These results suggest that synercid may be useful for treatment of toxoplasmosis in humans.
Toxoplasmosis remains a significant problem among immunocompromised patients, infected newborns, and individuals with ocular toxoplasma involvement. Treatment of acute toxoplasmosis with pyrimethamine plus sulfadiazine or pyrimethamine plus clindamycin has been successful in most patients but may be associated with considerable toxicity (11). Thus, drugs with increased potencies and lower levels of toxicity are needed to treat all forms of toxoplasmosis.
Synercid is a new drug with a mechanism of action and antibacterial spectrum similar to those of macrolides, azalides, and ketolides (12, 13) that have been found to be active against Toxoplasma gondii (4, 9). It has activity against gram-positive and select gram-negative bacteria and comprises two semisynthetic pristinamycin derivatives, quinupristin (RP 57669; derived from pristinamycin I) and dalfopristin (RP 54476; derived from pristinamycin IIA) in a 30:70 ratio (7, 8). Because of the reported antitoxoplasma activities of the macrolides (5), azalides (1), and ketolides (4), it was considered of interest to investigate synercid and its two components for their in vitro and in vivo activities against T. gondii.
Tachyzoites of the RH strain of T. gondii were obtained from the peritoneal cavities of mice that had been infected 2 days earlier; cysts of the C56 strain were from the brains of chronically infected mice (2). Outbred female Swiss Webster mice (B & K Laboratories, Fremont, Calif.) weighing approximately 20 g at the beginning of each experiment were used. Mice were given water and food ad libitum. Synercid, quinupristin, and dalfopristin were provided by Rhône-Poulenc Rorer (Collegeville, Pa.).
For in vitro studies, human foreskin fibroblast (HFF) cells (ATCC HS68) were grown in Dulbecco’s modified Eagle’s medium (Gibco BRL, Grand Island, N.Y.) containing 100 U of penicillin, 1 μg of streptomycin per ml, and 10% heat-inactivated T. gondii antibody-negative fetal bovine serum. In vitro activity was defined as the capacity of the drug to inhibit intracellular replication of T. gondii and was determined by the [3H]uracil incorporation technique as described previously (10). Briefly, HFF cells were plated at 104 cells/well in 96-well flat-bottom tissue culture microtiter plates, and the plates were incubated at 37°C in a 5% CO2 incubator. After confluence, the monolayers were infected with tachyzoites at a ratio of three tachyzoites/cell. Four hours later, the monolayers were washed, the drugs were added at concentrations of from 0.0 to 25.0 μg/ml, and the cultures were incubated as described above for 48 h. Four hours prior to harvesting of the cells, [3H]uracil (1 μCi/well) was added and its level of incorporation was determined. The cells were collected with a cell harvester, and the radioactivity was counted with a scintillation counter. The toxicities of the drugs for HFF cells were determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay with the Cell Titer 96 Kit (Promega Corp., Madison, Wis.) as described previously (10).
For in vivo studies, mice were infected orally with 10 cysts of the C56 strain. Treatment was initiated 3 days later as a single daily intraperitoneal dose and was continued for 10 days. Mice were observed for mortality and time to death for 30 days from the day of infection. At the end of the 30-day period, the brains of the surviving mice were examined for the presence of cysts of T. gondii as described previously (3). P values were obtained by the log-rank test of the Kaplan-Meier product limited-survival analysis by using survival tools for StatView, version 4.02, computer software (Abacus Concepts, Berkeley, Calif.). A P value of ≤0.05 was considered statistically significant.
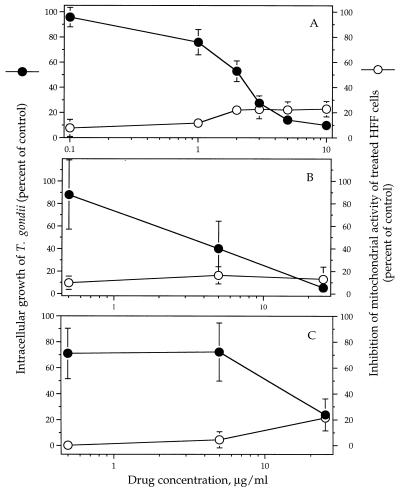
Synercid, quinupristin, and dalfopristin each inhibited the intracellular replication of T. gondii in vitro. Their 50% inhibitory concentrations after 48 h of exposure were 1.6, 2.7, and 6.3 μg/ml, respectively (Fig. 1A to C, respectively). Thus, synercid was more active than either of its components alone. None of the compounds showed toxicity for the host cells at concentrations that were highly active against T. gondii.
FIG. 1.
Effects of synercid (A), quinupristin (B), and dalfopristin (C) on intracellular replication of RH tachyzoites in vitro determined by [3H]uracil incorporation and on host HFF cells in vitro determined by the MTT assay. Results are presented as means ± standard deviations for triplicate assays.
Synercid at 100 and 200 mg/kg of body weight per day administered intraperitoneally for 10 days protected 50% (P = 0.0002) and 100% (P < 0.0001) of infected mice against death, respectively (Fig. 2A). A dosage of 50 mg/kg/day resulted only in a 2-day prolongation of the time to death that was not statistically significant. In contrast, quinupristin or dalfopristin, when administered alone, each protected only 20% (P > 0.270) of infected mice at the highest dosage tested (200 mg/kg/day) (Fig. 2B and C). Thus, synercid was fivefold more active in vivo than either of its components alone. T. gondii cysts were observed in the brains of the surviving mice in each of the groups treated with either of these drugs.
FIG. 2.
Effect of treatment with synercid (A), quinupristin (B), or dalfopristin (C) intraperitoneally for 10 days on survival of mice infected with the C56 strain of T. gondii. There were 5 mice in the control group and 10 mice in the treatment groups.
The results presented above reveal that although synercid, quinupristin, and dalfopristin had significant in vitro activities against T. gondii, only synercid had significant in vivo activity. Synercid was more potent than either of its components alone in vitro and in vivo. Although synercid significantly protected mice against death, it did not prevent colonization of tissues by T. gondii since cysts were found in the brains of surviving mice.
The mechanism of action of synercid against T. gondii is not known. However, in bacteria, quinupristin and dalfopristin bind to the RNA of the 50S ribosomal subunit and act synergistically to inhibit protein synthesis (13). Since ribosomes encoded by prokaryotic-type ribosomal genes of T. gondii are predicted to be sensitive to the lincosamide-macrolide class of antibiotics and may serve as the functional target to protein synthesis inhibitors in T. gondii and related parasites (6), synercid may be inhibiting ribosomal protein synthesis. Our results demonstrate that the combination of quinupristin and dalfopristin has an enhanced effect against T. gondii compared to the activity of either one of them alone. It is possible that such enhancement in activity may be elicited through a mechanism similar to that observed in bacteria (13).
Pharmacokinetic studies of synercid with healthy human volunteers have revealed that the maximum concentration in blood ranges from 0.95 μg/ml after a 1-h intravenous infusion of a dose of 1.4 mg/kg to 24.2 μg/ml after infusion of a dose of 29.4 mg/kg (7). However, neither of the components of synercid significantly penetrates the central nervous systems of rats, monkeys, or humans (7). The terminal half-lives of elimination for quinupristin and dalfopristin in humans were 0.93 to 0.96 and 0.39 to 0.91 h, respectively (7). Therefore, it is evident that the concentrations of synercid that were highly active against T. gondii in vitro are achievable in the serum of humans with conventional doses. Thus, synercid may be useful for the treatment of human toxoplasmosis.
Acknowledgments
This work was supported by U.S. Public Health Service grant AI-04717 and the Division of AIDS of the National Institute of Allergy and Infectious Diseases under contract NO1-AI-35174.
We thank Eddie Wehri and Eric Ho for excellent technical assistance.
REFERENCES
- 1.Araujo F G, Guptill D R, Remington J S. Azithromycin, a macrolide antibiotic with potent activity against Toxoplasma gondii. Antimicrob Agents Chemother. 1988;32:755–757. doi: 10.1128/aac.32.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araujo F G, Huskinson J, Remington J S. Remarkable in vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against tachyzoites and tissue cysts of Toxoplasma gondii. Antimicrob Agents Chemother. 1991;35:293–299. doi: 10.1128/aac.35.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araujo F G, Huskinson-Mark J, Gutteridge W E, Remington J S. In vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against the cyst form of Toxoplasma gondii. Antimicrob Agents Chemother. 1992;36:326–330. doi: 10.1128/aac.36.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araujo F G, Khan A A, Slifer T L, Bryskier A, Remington J S. The ketolide antibiotics HMR 3647 and HMR 3004 are active against Toxoplasma gondii in vitro and in murine models of infection. Antimicrob Agents Chemother. 1997;41:2137–2140. doi: 10.1128/aac.41.10.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araujo F G, Prokocimer P, Lin T, Remington J S. Activity of clarithromycin alone or in combination with other drugs for treatment of murine toxoplasmosis. Antimicrob Agents Chemother. 1992;36:2454–2457. doi: 10.1128/aac.36.11.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beckers C J M, Roos D S, Donald R G K, Luft B J, Schwab J C, Cao Y, Joiner K A. Inhibition of cytoplasmic and organellar protein synthesis in Toxoplasma gondii. J Clin Invest. 1995;95:367–376. doi: 10.1172/JCI117665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergeron M, Montay G. The pharmacokinetics of quinupristin/dalfopristin in laboratory animals and in humans. J Antimicrob Chemother. 1997;39(Suppl. A):129–138. doi: 10.1093/jac/39.suppl_1.129. [DOI] [PubMed] [Google Scholar]
- 8.Carbon C. Quinupristin/dalfopristin: a review of its activity in experimental animal models of infection. J Antimicrob Chemother. 1997;39(Suppl. A):115–119. doi: 10.1093/jac/39.suppl_1.115. [DOI] [PubMed] [Google Scholar]
- 9.Khan A A, Araujo F G. Scientific Information Guild (ed.), Recent research developments in antimicrobial agents and chemotherapy. 1st ed. Trivandrum, India: Research Signpost; 1996. Recent developments in the search for therapeutic interventions against Toxoplasma gondii infection; pp. 65–77. [Google Scholar]
- 10.Khan A A, Slifer T, Araujo F G, Remington J S. Trovafloxacin is active against Toxoplasma gondii. Antimicrob Agents Chemother. 1996;40:1855–1859. doi: 10.1128/aac.40.8.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liesenfeld O, Wong S Y, Remington J S. Toxoplasmosis in the setting of AIDS. In: Merigan T C, Bartlett J G, Bolognesi D, editors. Textbook of AIDS medicine. 2nd ed. Baltimore, Md: The Williams & Wilkins Co.; 1999. pp. 225–259. [Google Scholar]
- 12.Low D E. Quinupristin/dalfopristin: spectrum of activity, pharmacokinetics and initial clinical experience. Microb Drug Resist. 1995;1:223–234. doi: 10.1089/mdr.1995.1.223. [DOI] [PubMed] [Google Scholar]
- 13.Vannuffel P, Cocito C. Mechanism of action of streptogramins and macrolides. Drugs. 1996;51(Suppl. 1):20–30. doi: 10.2165/00003495-199600511-00006. [DOI] [PubMed] [Google Scholar]