Correction to: Nature Communications 10.1038/s41467-020-18554-x, published online 22 September 2020.
The original version of this Article contained several errors with regards to the absolute configurations of 2’-OH and 3’-OH at ATP in the theoretical models. The errors can be found in Fig. 4a and Fig. 4b. The correct version of Fig. 4a and 4b is: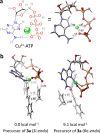
which replaces the previous incorrect version: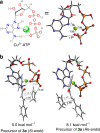
This has been corrected in both the PDF and HTML versions of the Article.
The associated Supplementary Figs. S22–S25 also contained the same error. The correct version of Supplementary Figures S22-S25 are:
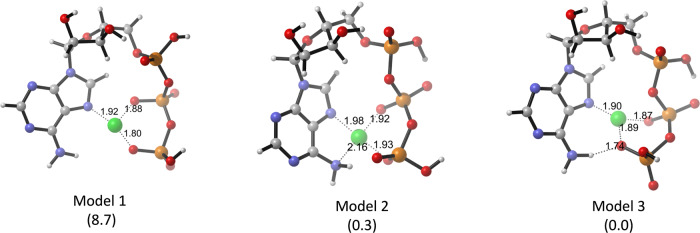
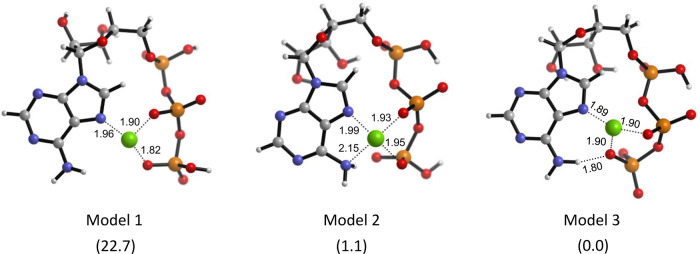
Supplementary Fig. 22. The proposed models of Cu2+⋅ATP. The relative electronic energies are in the parenthesis with a unit of kcalmol−1.
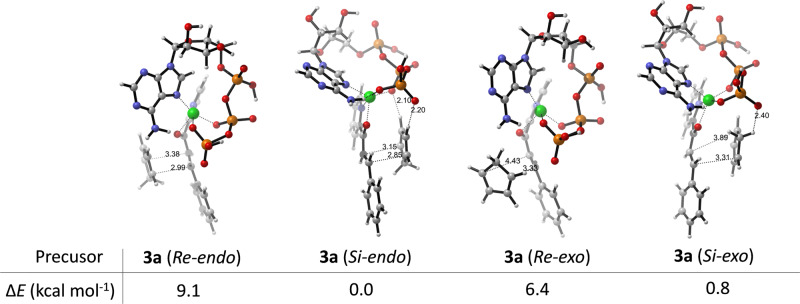
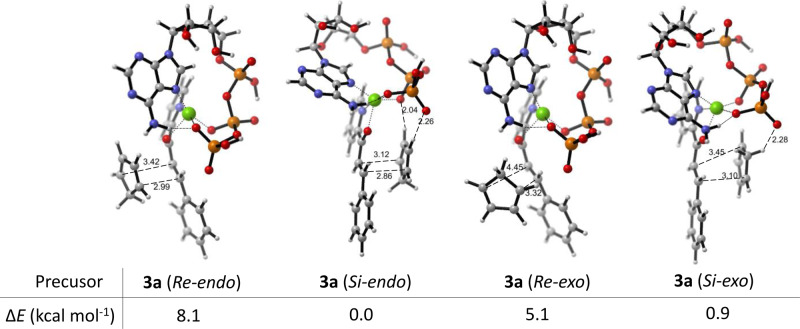
Supplementary Fig. 23. The precursors of the intermediates of 1a-Cu2+⋅ATP and 2 that yield the corresponding products 3a in different configurations. The relative electronic energies (ΔE) of the precursors are shown in the table.
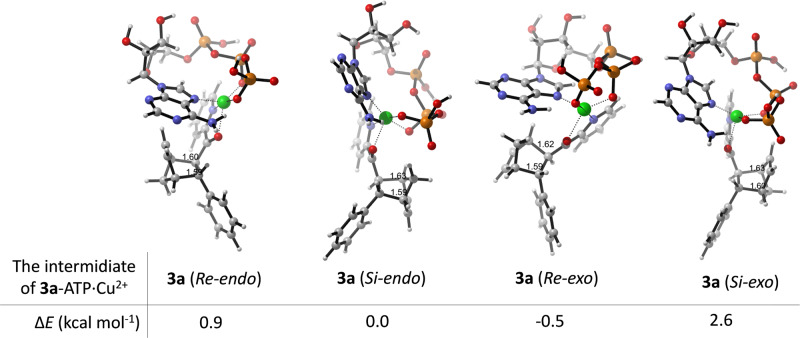
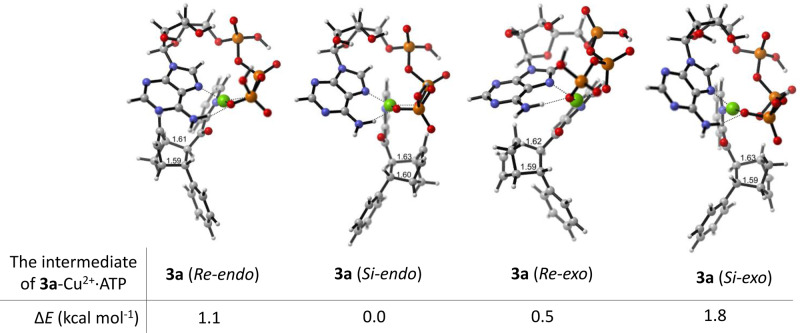
Supplementary Fig. 24. The intermediates of 3a-ATP⋅Cu2+ and their relative electronic energies (ΔE).
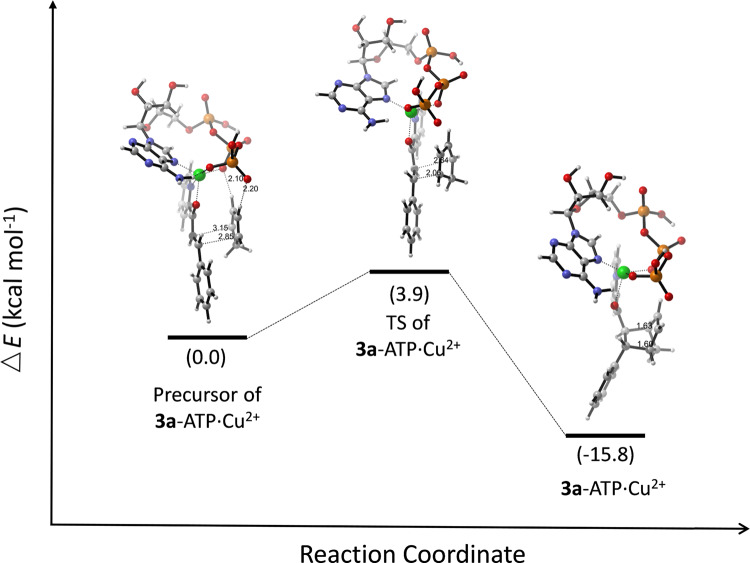
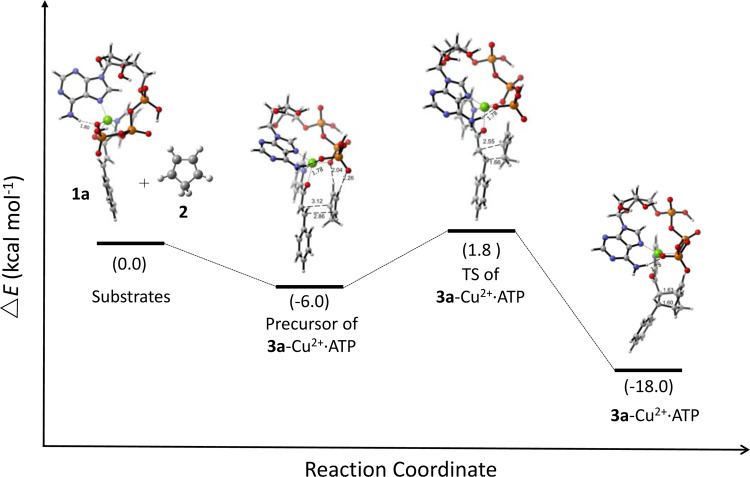
Supplementary Fig. 25. The relative electronic energy profile of the reaction path for Cu2+⋅ATP-catalyzed Diels–Alder reaction of 1a and 2 that yields 3a (endo) in the absolute configuration of 1R, 2S, 3S, 4S. TS, transition state.
which replace the previous incorrect versions:
Supplementary Fig. 22. The proposed models of Cu2+⋅ATP. The relative electronic energies are in the parenthesis with a unit of kcalmol−1.
Supplementary Fig. 23. The precursors of the intermediates of 1a-Cu2+⋅ATP and 2 that yield the corresponding products 3a in different configurations. The relative electronic energies (ΔE) of the precursors are shown in the table.
Supplementary Fig. 24. The intermediates of 3a-ATP⋅Cu2+ and their relative electronic energies (ΔE).
Supplementary Fig. 25. The relative electronic energy profile of the reaction path for Cu2+⋅ATP-catalyzed Diels–Alder reaction of 1a and 2 that yields 3a (endo) in the absolute configuration of 1R, 2S, 3S, 4S. TS, transition state.
The HTML has been updated to include a corrected version of the Supplementary Information.
Due to the above errors, the text in the original version of this Article also contained errors. Page 5, right column incorrectly reads ‘The relative electronic energy (ΔE) of the optimised Cu2+·ATP structure was 1.1 kcal mol−1 lower than that of a previously described model obtained by a molecular orbital method57 and 22.7 kcal mol−1 lower than that of the Cu2+·ATP model without a hydrogen bond (Supplementary Fig. S22).’ and ‘the ΔE value of the precursor of 1a-Cu2+·ATP and 2 that yielded 3a (endo) via the attack of the Si face was 8.1 kcal mol−1 lower than that of the precursor for the Re face attack (Fig. 4b).’ The correct version states ‘The relative electronic energy (ΔE) of the optimised Cu2+·ATP structure was 0.3 kcal mol−1 lower than that of a previously described model obtained by a molecular orbital method57 and 8.7 kcal mol−1 lower than that of the Cu2+·ATP model without a hydrogen bond (Supplementary Fig. S22).’ and ‘the ΔE value of the precursor of 1a-Cu2+·ATP and 2 that yielded 3a (endo) via the attack of the Si face was 9.1 kcal mol−1 lower than that of the precursor for the Re face attack (Fig. 4b).’
Page 6, left column incorrectly reads ‘However, the ΔE value of 3a (Re-exo) was 1.3 kcal mol−1 lower than that of 3a (Si-exo), in accordance with the experimental results (Supplementary Figs. S21, S24).’ The correct version states ‘However, the ΔE value of 3a (Re-exo) was 2.6 kcal mol−1 lower than that of 3a (Si-exo), in accordance with the experimental results (Supplementary Figs. S21, S24).’
This has been corrected in the PDF and HTML versions of the Article.
Supplementary information
Footnotes
These authors contributed equally: Changhao Wang, Qianqian Qi.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-28850-3.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.