Abstract
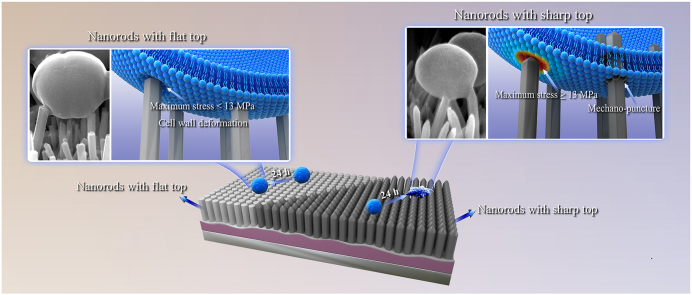
Nanorods can induce mechano-puncture of Staphylococcus aureus (S. aureus) that often impairs osseointegration of orthopedic implants, while the critical nanorod top sharpness able to puncture S. aureus and the predominant contributor between top sharpness and length to mechano-puncture activity remains elusive. Herein, we fabricated three kinds of Al2O3-wrapped nanorods patterned arrays with different lengths and top sharpness. The top-sharp nanorods have lengths of 469 and 884 nm and the shorter show a length identical to the top-flat nanorods. Driven by the equivalent adhesive force of S. aureus, the top-flat nanorods deform cell envelops, showing a bacteriostatic rate of 29% owing to proliferation-inhibited manner. The top-sharp nanorods puncture S. aureus, showing a bactericidal rate of 96% for the longer, and 98% for the shorter that simultaneously exhibits fair osseointegration in bacteria-infected rat tibias, identifying top sharpness as a predominate contributor to mechano-puncture activity. Based on finite-element simulation, such top-flat nanorod derives the maximum stress (Smax) of 5.65 MPa on cell wall, lower than its ultimate-tensile-strength (13 MPa); while such top-sharp and shorter nanorod derives Smax of 20.15 MPa to puncture cell envelop. Moreover, a critical top conical angle of 138° is identified for nanorods able to puncture S. aureus.
Keywords: Anti-bacteria, Nanorods, Top sharpness, Mechano-puncture, Finite element simulation
Graphical abstract
Highlights
-
•
Top sharpness depended mechano-puncture of nanorods against S. aureus is clarified.
-
•
Top-flat nanorods deform bacterial cell envelop to inhibit their proliferation.
-
•
Top-sharp nanorods (conical angle of 50°) puncture bacteria to intensely kill them.
-
•
138° is confirmed as critical top conical angle for nanorods to puncture S. aureus.
1. Introduction
Bacterial colonization and biofilm formation derived infection is the major cause for failure of orthopedic implants [1,2]. To solve the problem, preventing bacteria from adhesion to the implant surfaces and killing bacteria are currently used strategies, and the latter is proven to be more highly effective for anti-bacteria [1]. Prevalently, chemical bactericides, such as antibiotic [[3], [4], [5]], bactericidal ions [[6], [7], [8], [9]] and antimicrobial peptide [10] were loaded on the implant surfaces to kill bacteria via inhibiting bacterial wall synthesis and disrupting DNA replication [11,12] or to destruct cell envelop (consisting of cell wall and membrane) via inducing reactive oxidation species (ROS) [6,9]. However, these bactericides are likely able to induce cytotoxicity [9,13] to impair osseointegration, nephrotoxicity [14] or neurovirulence [15], and render multi-drug resistance bacteria or superbacteria [2,11], limiting their use and effectiveness in implants for orthopedic repair. In addition, photosensitizer incorporated coatings are widely investigated to realize antibacterial activity utilizing their photo-thermal/dynamic effects [[16], [17], [18], [19]]. Nevertheless, photo-thermal effect derived hyperpyrexia and photodynamic effect derived ROS may destroy peri-implant healthy tissues [20], moreover, the incorporated photosensitizers also may induce biotoxicity [21].
Inspired by the nanorods-patterned arrays of cicada and dragonfly wings that show bactericidal effect [22], diverse nanoscaled arrays with high length/diameter ratio, such as Si [23,24] and polymer [25] nanofibers, TiO2 and ZnO nanorods [26,27], and carbon nanotubes [28], which are in follows collectively termed as nanorods, have been constructed and proven to mechanically kill bacteria. It is clear that when cultured on nanorods, bacteria subject to bacterial gravity, van der Waals force and the adhesive force derived by bacteria-secreted extracellular polymeric substance (EPS) [2,[29], [30], [31], [32]], among which the EPS-derived adhesive force is much stronger than the former [31,32]. This adhesive force would not only induce shear force acting on cell envelop of bacteria to limit their migration over the nanorods, but also drive the nanorods to induce cell envelop deformation (mechano-deformation) or even to penetrate into the envelop (mechano-puncture) [31,33]. Given that gram-negative bacteria have thinner cell wall than gram-positive bacteria, the gravity and van der Waals force induced stretching force is only able to rupture cell envelops of gram-negative bacteria [2,34], while the EPS-derived strong adhesive force can draw nanorods to penetrate into both gram-negative and positive bacteria, leading to their death [26,27,35]. Therefore, coating nanorods patterned arrays with mechano-puncture ability is expected to protect orthopedic implants more effectively from bacteria-derived infection, which is predominately caused by Staphylococcus aureus (S. aureus) owing to its gram-positive and antibiotics-resistant feature [36,37].
Given that the mechano-puncture ability of nanorods-shaped arrays against S. aureus depends on their topographical parameters, especially on the nanorod top sharpness [37,38], various nanorods with different top sharpness were fabricated but exhibit distinct mechano-puncture efficiency. For instance, top-flat Si nanorods were unable to penetrate into S. aureus [24], while top-blunt TiO2 nanorods shallowly penetrated into S. aureus with an antibacterial rate of ∼10% [26]. Encouragingly, top-sharp Si nanorods were shown to deeply penetrate into S. aureus to effectively kill them [23]. Although these works qualitatively suggest that the mechano-puncture activity of the arrays is enhanced with sharpening of nanorod top, the concentrated stress on cell envelop derived by the nanorods with different top sharpness have not been quantified yet, leading to uncertainty of the critical nanorod top sharpness able to penetrate into S. aureus. In addition to top sharpness, nanorod length is also found to contribute to mechano-puncture efficacy [28,38], but the drawn results are contradictive. For instance, carbon nanotubes with lengths of 1 and 30 μm killed ∼67% and ∼17% of the attached S. aureus, respectively [28]. Contrarily, longer Si nanorods with sharp tops exhibited more pronounced bactericidal activity compared to the shorter ones with slightly blunt tops [38]. The reason for this controversy is that the topographical parameters, e.g. top sharpness and length of the nanorods patterned arrays in these works are concomitantly but not independently changed with each other [1,2,34,38]. These concomitant changes draw another confusion that for top sharpness and length, which is the predominate contributor to mechano-puncture activity of the nanorods. Notably, the variation of topographical parameters of the arrays is also accompanied with the changes in surface roughness, hydrophilia and zeta potential, which also influence bactericidal activity [2].
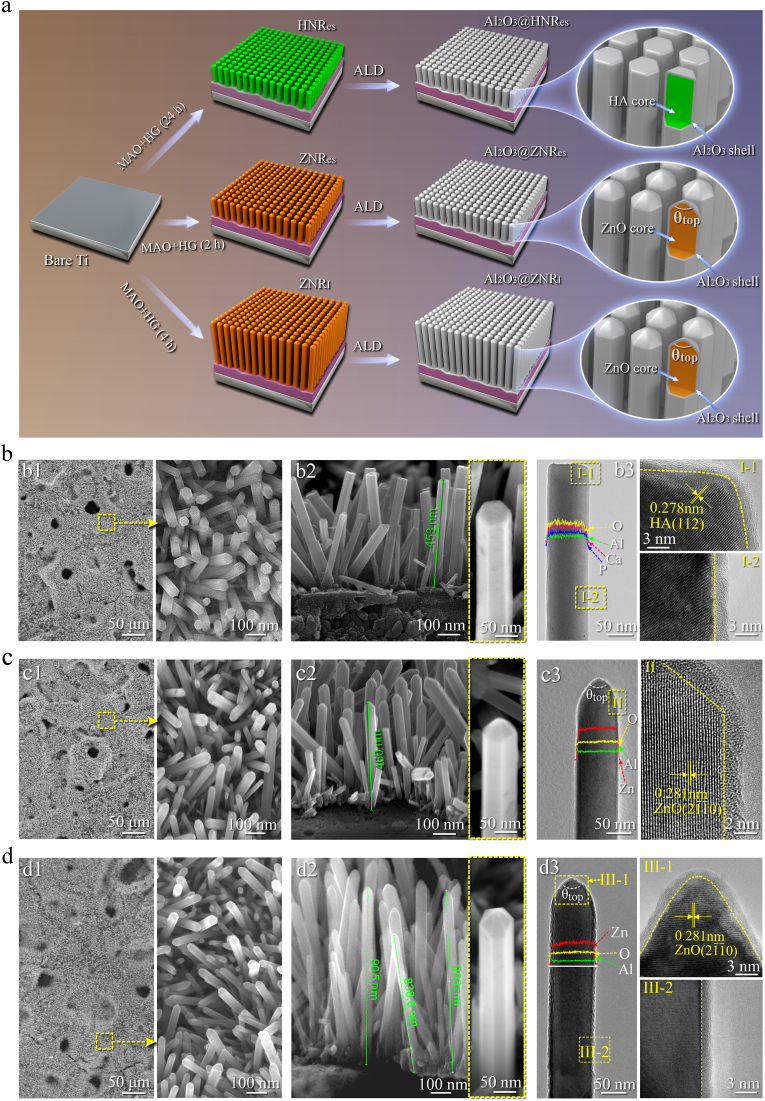
Herein, we constructed three kinds of core-shell structured nanorods patterned arrays on Ti discs, with micro-arc oxidation (MAO) and hydrothermal growth (HG) formed hydroxyapatite (HA) or ZnO as a core and atomic layer deposition (ALD) formed amorphous Al2O3 as a shell (schematically as Fig. 1a). The arrays exhibit two kinds of different nanorod top sharpness: flat top and sharp top, in which the top-sharp nanorods reveal two kinds of lengths and the short show an equivalent length to the top-flat nanorods. With these nanorods patterned arrays, we identify the critical top sharpness of nanorods able to penetrate into S. aureus and clarify the contributions of nanorod top sharpness and length to mechano-puncture derived bactericidal efficiency, providing the quantitative basis to optimize the top sharpness for achieving mechano-puncture. We also show that the top-sharp and short nanorods patterned array acts on S. aureus via unique mechano-puncture without impairing the viability of mesenchymal stem cells (MSCs), having an in vitro and in vivo antibacterial rate of more than 98%, and simultaneously exhibits fair osseointegration in S. aureus-infected rat tibia. Such antibacterial efficiency is comparable to that exhibited by the synergistic action of nanorods-derived mechano-puncture, bactericidal components and/or photo-thermal/dynamic effect [27,39,40].
Fig. 1.
(a) Schematic illustration of the fabrication process for the nanorods patterned arrays on Ti (θtop: conical angle of nanorod); SEM and TEM analyzed microstructures of (b) Al2O3@HNRes, (c) Al2O3@ZNRes and (d) Al2O3@ZNRl: (b1, c1 and d1) and (b2, c2 and d2) showing SEM surface and cross-sectional images of the nanorods patterned arrays, respectively; (b3, c3 and d3) showing TEM analyzed the ultrafine structures of individual nanorods scratched from Al2O3@HNRes, Al2O3@ZNRes and Al2O3@ZNRl, respectively; for the detailed structures of the nanorods, I-1 and I-2 showing the HRTEM images of the square-dotted micro-regions in (b3), II showing the HRTEM image of the square-dotted micro-region in (c3), and III-1 and III-2 showing the HRTEM images of the square-dotted micro-regions in (d3).
2. Experimental sections
2.1. Fabrication of nanorods patterned arrays
HA nanorods-patterned array was fabricated using MAO and hydrothermal treatment (HT), the detailed process was described in our previous work [41]. Briefly, Ti discs with size of 14 × 2 mm were micro-arc oxidized (MAOed) in an aqueous electrolyte containing calcium acetate (0.2 M) and β-glycerophosphate disodium (0.02 M) at an applied voltage of 400 V, a pulse frequency of 100 Hz and a duty ratio of 26% for 3 min. After adding 10 ml NaOH solution (0.01 M) into 60 ml volumetric Teflon-lined autoclave, each of the resultant MAOed Ti discs was immersed in the NaOH solution to receive HT at 140 °C for 24 h, consequently to form the HA nanorods-patterned array.
Commercial pure Ti discs with size of 14 × 2 mm were MAOed in zinc acetate (0.2 M) contained aqueous electrolyte at an applied voltage of 530 V, a pulse frequency of 100 Hz and a duty ratio of 26% for 1 min. Each MAOed Ti disc underwent HT in 10 mL NaOH solution (0.01 M) at 140 °C for 2 h or 4 h to form the differently longitudinal ZnO nanorods-patterned arrays.
All the resultant nanorods-arrayed Ti discs were cleaned with deionized water and dried in an oven overnight at 60 °C.
2.2. Al–O layers deposition on the nanorods and Ti by ALD
An ALD equipment (F-100-41, MNT Micro and Nanotech Co., LTD, China) was used to coat Aluminum (Al) and Oxygen (O) (Al–O) layers on the as-formed nanorods-patterned arrays. Trimethylaluminum and hydrogen peroxide (H2O2, 40 vol%) aqueous solution were used as precursors of Al and O, respectively, and high-purity N2 was used as the purged gas. The nanorods patterned Ti discs were put into chamber of the ALD equipment, followed by vacuating the chamber to 10−3 Pa and heating the chamber up to 150 °C. Subsequently, Al and O precursors and N2 were sequentially auto-injected into the chamber following the sequence of 1 s TMA expose/20 s N2 purge/0.2 s H2O2 expose/40 s N2 purge for each deposition cycle. After 100 deposition cycles, Al–O coated nanorods patterned arrays were obtained. In addition, an Al–O layer was deposited on Ti disc using the aforementioned ALD procedure, followed by annealing at 800 °C for 4 h at atmosphere. The as-deposited and annealed Al–O layers on Ti discs were used to identify phase component in the ALD-derived Al–O layer.
2.3. Structural analysis of the nanorods patterned arrays
Phase identification was carried out with an X-ray diffractometer (XRD, X'Pert PRO, Netherland) in θ-2θ geometry using Cu-Kα (λ = 0.15406 nm) radiation over a 2θ angles of 20–60° at a step of 0.02° with 40 kV scanning voltage and 40 mA scanning current, and the phases from obtained XRD spectra were identified by the ICDD cards. Field emission scanning microscope (FE-SEM, FEI QUANTA 600F, U.S.A.) was used for examining the morphologies of the nanorods patterned arrays before and after deposition of ALD-derived Al–O layers. The nanorods scratched from Al–O coated nanorods patterned arrays were examined by transmission electron microscope (TEM, JEOL JEM-2000FX, Japan) equipped energy-dispersive X-ray (EDX) operating at 200 kV. The roughness of the Al–O coated nanorods patterned arrays was examined by atomic force microscopy (AFM, SPM-9500J3, Japan).
2.4. Property characterizations of the Al–O coated nanorods patterned arrays
The detailed methods are presented in Supporting information, and all the procedures of animal experiments has been approved by the Institutional Animal Care and Use Commit-tee (IACUC) of Xi'an Jiaotong University.
2.5. Finite element simulation
The nephograms of stress distribution on cell walls of bacteria induced by top-flat and top-sharp Al–O coated nanorods patterned arrays were drawn through finite element (FE) simulation using Dynaform software (ETA, U.S.A.). The parameters used in our FE model included the thickness, Young's modulus and ultimate tensile strength (UTS) of S. aureus cell wall, internal hydrostatic pressure of S. aureus, the applied force of nanorod to cell wall and the conical angle (θtop) of nanorod, which were detailedly described in the section of results and discussion, and the critical θtop of nanorods was identified using dichotomy by the Optimization module integrated in the Isight software (Engineous, U.S.A).
2.6. Statistical analysis
The data were expressed as mean ± standard deviation (SD) from repeated independent experiments. The data were analyzed using SPSS 16.0 software (SPSS, U.S.A.). One-way ANOVA followed by a least-significant-difference (LSD) post hoc test was used to determine the level of significance. Here, p < 0.05 and 0.01 were considered to be significant and highly significant, respectively.
3. Results and discussion
3.1. The components and structures of the nanorods patterned arrays on Ti substrates
As shown in Figs. S1a and c, the HG formed nanorods on the MAO of Ti disc are composed of HA with a length of 459.9 ± 17.9 nm. This HA nanorods-arrayed coating on Ti disc is bilayer structured, comprising an inner layer of TiO2 and an outer layer of HA nanorods with flat tops. Likewise formed with MAO and HG, ZnO nanorods-arrayed coatings on Ti discs with two kinds of nanorod lengths also exhibit a bilayer structure, consisting of TiO2 as an inner layer and ZnO nanorods as an outer layer. Notably, the ZnO nanorods appear hexagonal pyramid shaped on tops (Figs. S1b and S1c). Moreover, the shorter ZnO nanorods show a length of 467.1 ± 15.5 nm, equal to that of the HA nanorods, termed as ZNRes and so did the HA nanorods as HNRes, while the longer ZnO nanorods have a length of 882 ± 20.3 nm, namely ZNRl.
Aluminum and oxygen atoms were atomic layer deposited as an Al–O ultrathin layer to wrap each nanorod of HNRes, ZNRes and ZNRl arrays. As shown in Fig. 1b–d, the SEM images reveal that the core-shell structured nanorods in the three arrays align perpendicularly or quasi-vertically to Ti substrates with an interrod spacing of ∼80 nm and a dimeter of ∼70 nm, and reveal almost identical lengths and top shape to the corresponding primitive nanorods. The high-resolution TEM (HRTEM) images and EDX profiles show that the Al–O layer around each nanorod of HNRes, ZNRes and ZNRl arrays is uniform in thickness with a value of ∼1.5 nm but lack of lattice fringe. Further identification by XRD spectra before and after annealing confirms that the Al–O layer consists of amorphous Al2O3 (Fig. S2a). Collectively, the amorphous Al2O3-wrapped nanorods are referred to as Al2O3@HNRes, Al2O3@ZNRes and Al2O3@ZNRl, respectively, in which both Al2O3@ZNRes and Al2O3@ZNRl appear hexagonal pyramid shaped on tops with a conical angle θtop of 50°, while Al2O3@HNRes are flat on tops with a θtop of 180°, having a nanorod length (∼462 nm) identical to Al2O3@ZNRes (∼469 nm) but almost a half of Al2O3@ZNRl (∼884 nm).
3.2. Surface features, Zn2+ released from the core-shell structured nanorods patterned arrays and ROS formation on these arrays
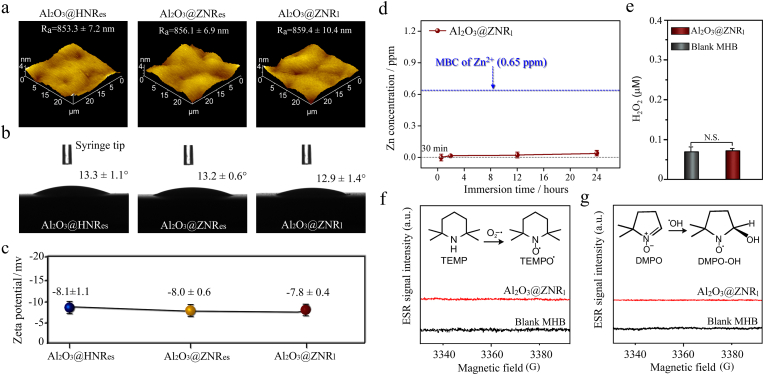
Given that surface features such as roughness [42,43], wettability [44,45] and zeta potentials [46,47] can modulate bacterial behaviors, in this context, they were tested on the three kinds of arrays as shown in Fig. 2a–c. Owing to the identical surface morphologies (Fig. 1b1-d1), Al2O3@HNRes, Al2O3@ZNRes and Al2O3@ZNRl arrays show almost equivalent values of average roughness (Ra) and root mean square roughness (Rq) measured with AFM (Table S1), moreover, contact angle and zeta potential of these arrays are also identical. It is suggested that the variations in nanorod length and top shape of the Al2O3-wrapped arrays do not induce the changes in these three parameters of surface features, excluding their contributions to the difference in antibacterial abilities of the arrays.
Fig. 2.
(a) AFM images, (b) contact angles of water droplets and (c) zeta potentials of Al2O3@HNRes, Al2O3@ZNRes and Al2O3@ZNRl; (d) Zn2+ concentrations of the bacterial culture medium, Mueller Hinton Broth (MHB) immersing Al2O3@ZNRl as a function of immersion time (MBC: minimum bactericidal concentration); (e) the concentrations of H2O2 in the blank MHB and MHB immersing Al2O3@ZNRl (N. S.: no significance); the ESR spectra of (f) superoxide (O2−•) and (g) hydroxyl radical (•OH) in the MHB immersing Al2O3@ZNRl as detected with TEMPO • and DMPO-OH spin trap agents.
Excess Zn2+ ions released from ZnO nanorods [27] and Zn2+-containing TiO2 coating [6] are known to disturb metabolic process of bacteria [27], and also enhance intracellular ROS level [6], leading to bacteria death. Moreover, ROS generated on ZnO nanorods [27] and Zn2+-containing TiO2 coating [6] in bacteria culturing medium have been reported to destroy cell envelop integrity of bacteria [27], and also upregulate intracellular ROS level to aggravate damage of cell envelop [6]. Although the nanorods made up of ZnO were demonstrated to have a mechano-puncture effect on bacteria [27], this effect induced bactericidal activity is undistinguishable owing to the concomitant Zn2+ and ROS induced bactericidal effect [6,27]. In this context, Zn2+ release from and ROS (including superoxide (O2−•), hydroxyl radical (•OH) and H2O2) formation on both the Al2O3-coated ZNRl and ZNRes arrays were tested in bacterial culture medium, Mueller Hinton Broth (MHB), together with blank MHB as a negative control. As shown in Fig. 2d–f (picking up from Al2O3@ZNRl as a representative), the Zn2+ ions released from Al2O3@ZNRl within 24 h of immersion are rare, far less than the minimum bactericidal concentration (MBC) of Zn2+. Moreover, H2O2 content of the MHB immersing Al2O3@ZNRl is similar to that of blank MHB, while ESR adsorption peaks of O2−• and •HO are invisible for the Al2O3@ZNRl immersed MHB. The results indicate that the ALD-derived amorphous Al2O3 ultrathin shells can almost completely inhibit release of ions from the inner cores (such as Zn2+ from ZnO and extendedly Ca2+ and PO43− from HA) and ROS formation on the coated ZNRl and ZNRes arrays, and amorphous Al2O3 does not induce ROS formation by itself. Further, Al2O3@HNRes, Al2O3@ZNRes, and Al2O3@ZNRl arrays were examined for the intracellular ROS levels of the adhered bacteria, and no elevated intracellular ROS levels appear compared to that displayed by the bacteria adhered on Ti as a negative control (Fig. S3). Also, amorphous Al2O3 exhibits the same bacterial adhesion and proliferation as planar Ti (Figs. S2b and c), thus lack of antibacterial activity, because Ti is known to be without antibacterial ability [6]. Therefore, the follows presented difference in antibacterial activities of Al2O3@HNRes, Al2O3@ZNRes and Al2O3@ZNRl arrays is not owing to chemical contribution.
3.3. Antibacterial activities of the core-shell structured nanorods patterned arrays
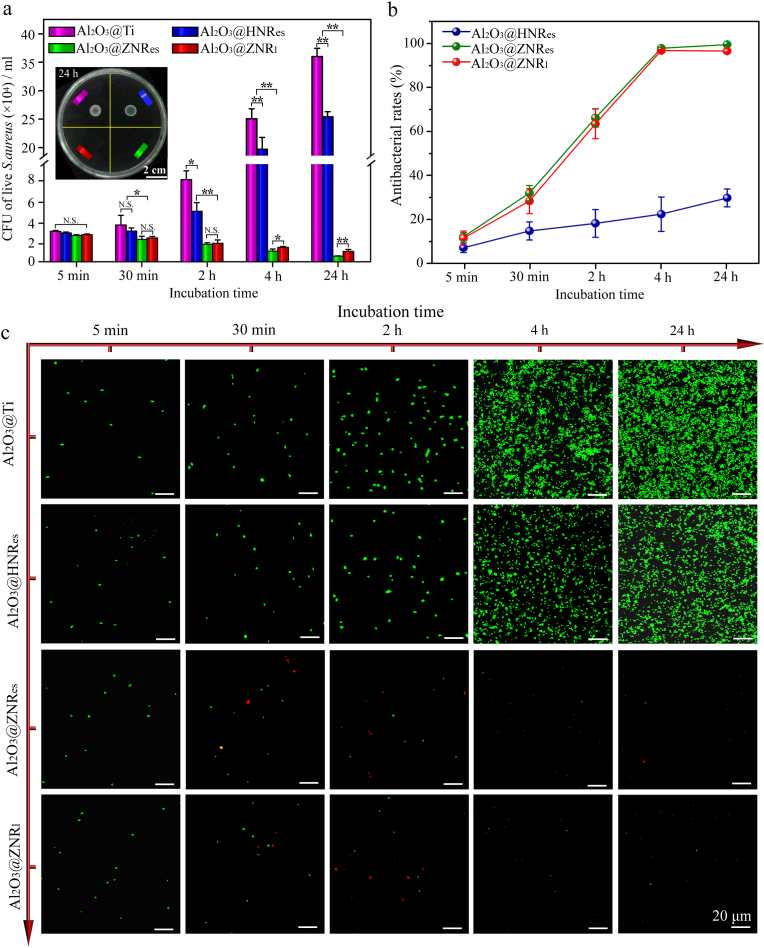
The plate-counting assessed bacterial colonies formed by dissociating S. aureus from the Al2O3-coated nanorods patterned arrays together with Al2O3 coated planar Ti (Al2O3@Ti) and re-cultivating on agar are shown in Fig. 3a. The bacterial colony numbers (BCNs) increase but with different increments on Al2O3@HNRes and Al2O3@Ti, while decrease on Al2O3@ZNRes and Al2O3@ZNRl with incubation from 30 min to 24 h. In details, at 5 min, the three kinds of Al2O3-coated nanorods patterned arrays display a comparable BCN to Al2O3@Ti. At 30 min, top-sharp Al2O3@ZNRes and Al2O3@ZNRl arrays show significantly decreased BCNs compared to top-flat Al2O3@HNRes array that has an equal BCN to Al2O3@Ti. Since then, the top-sharp arrays exhibit more pronounced decrease in BCN compared to the top-flat array. Notably, short Al2O3@ZNRes array reveals a stronger antibacterial activity than long Al2O3@ZNRl after 4 h; top-flat Al2O3@HNRes is also of a decreased BCN relative to Al2O3@Ti after 2 h. Collectively, at 24 h, top-flat Al2O3@HNRes array was measured to have an antibacterial rate of 29% (i.e., the percentage of reduction in BCNs on the array and Al2O3@Ti), however, top-sharp Al2O3@ZNRes and Al2O3@ZNRl display antibacterial rates as high as ∼ 98% and 96%, respectively (Fig. 3b). Such top-sharp Al2O3 nanorods derived antibacterial rates are much higher than those derived by top-blunt TiO2 nanorods [26], even comparable to those derived by ZnO nanorods [27] and graphdiyne-modified TiO2 nanorods [34], which kill S. aureus via the Zn2+ release [27] and photo-thermal/dynamic effect [40] besides topography-derived mechano-puncture action. It is suggested that optimizing topographical parameters of nanorods (especially top sharpness) instead of introducing antibacterial components can also achieve strong antibacterial activity.
Fig. 3.
(a) Numbers of undiluted bacteria colonies formed by dissociating S. aureus from Al2O3@Ti, Al2O3@HNRes, Al2O3@ZNRes and Al2O3@ZNRl after different incubation time and then re-cultivating on agar for 24 h, along with the inserted optical photograph of undiluted bacteria colonies formed by dissociating S. aureus from the samples after 24 h of incubation and then re-cultivating on agar for 24 h (∗p < 0.05, ∗∗p < 0.01, N. S.: no significance); (b) antibacterial rates of the nanorods patterned arrays at different incubation time points; (c) fluorescent live/dead straining images of S. aureus cultured on Al2O3@Ti and the arrays for different time.
To identify the antibacterial manner, live/dead fluorescence staining tests of S. aureus on the arrays were performed together with Al2O3@Ti, as shown in Fig. 3c. Clearly, Al2O3@HNRes array keeps all the mounted S. aureus to be viable (marked in green color) with the comparable number to Al2O3@Ti at 5 min but less than Al2O3@Ti at 2, 4 and 24 h, indicating a bacteriostatic (exactly proliferation-inhibited rather than adhesion-inhibited) action of the top-flat array on S. aureus. On contrary, although Al2O3@ZNRes and Al2O3@ZNRl arrays have the comparable number of viable S. aureus to Al2O3@HNRes and Al2O3@Ti at 5 min, they gradually evoke the mounted S. aureus dead (marked in red color) with incubation time and almost completely kill S. aureus at 24 h, indicating a bactericidal action of both the top-sharp arrays. Collecting the live/dead staining images with the plate-counting results, it is revealed that the top sharpness of the nanorods plays a critical role in anti-bacteria, and in the case of sharp top, the decrease in nanorod length further enhances bactericidal activity but to a small extent that is ascribed to nanorod length-dependent tortuosity, which is discussed next. In addition, it is needed to point out that the bactericidal rates of the top-sharp nanorods in the present work are higher than that of the nanostructured arrays mentioned in previous work, such as silicon nanocone [48], TiO2 nanorods [49] and polymer nanopillars [50]. It may be attributed to the much sharper tops of the nanorods and smaller interrod spacing of Al2O3@ZNRes and Al2O3@ZNRl compared to the aforementioned nanostructured arrays, which have been demonstrated to enhance bactericidal activity of nanostructures [38].
It has been reported that formation of biofilm is the predominant threat to induce the failure of orthopedic implants [51]. To examine biofilm formation of S. aureus after incubating on the arrays and Al2O3@Ti for 24 h, crystal violet staining were performed, as shown in Fig. S4. Both the OD values of the stained bacteria on Al2O3@Ti and Al2O3@HNRes are extremely high, revealing the formation of S. aureus biofilm on Al2O3@Ti and Al2O3@HNRes after 24 h of incubation. On contrary, almost no color appears on Al2O3@ZNRes and Al2O3@ZNRl, suggesting no biofilm forming on these arrays, which is further identified the strong bactericidal activity of the top-sharp nanorods.
3.4. Structural evolution of bacteria on the core-shell structured nanorods patterned arrays
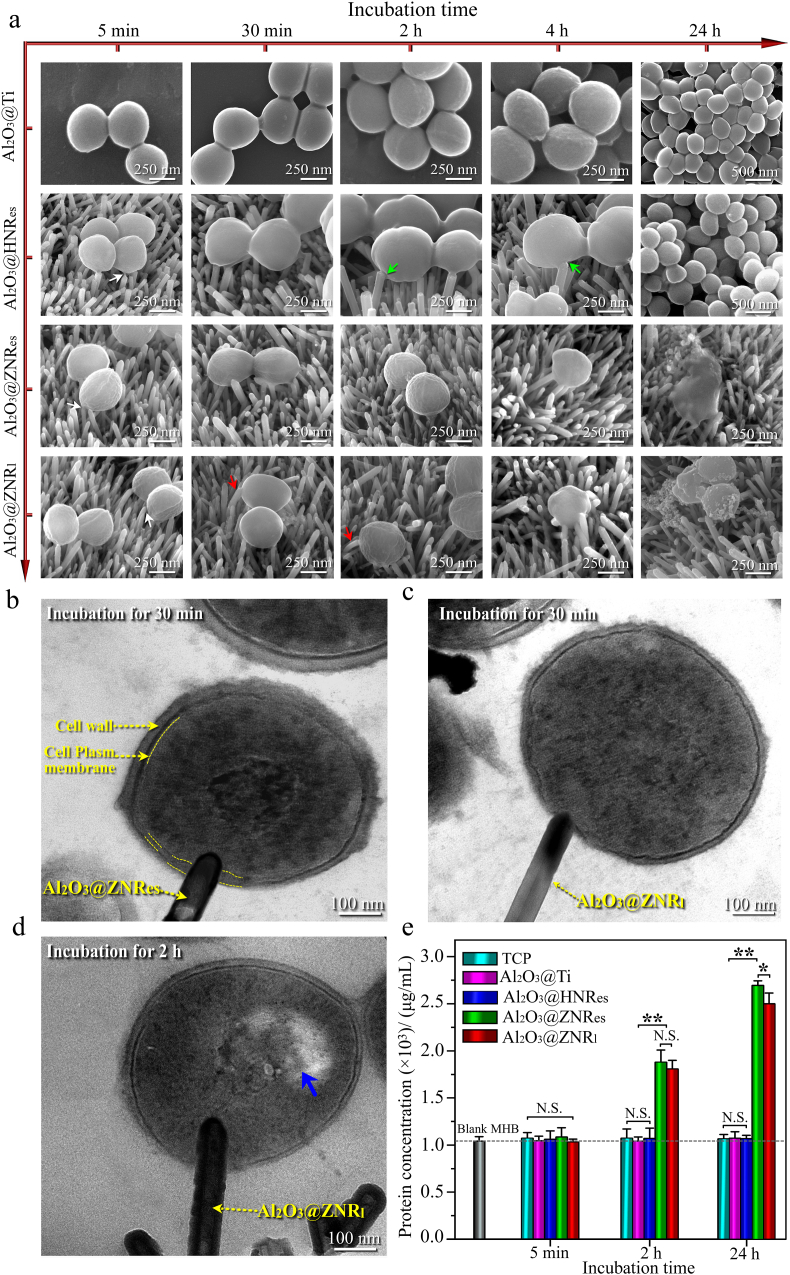
To clarify the reasons for bacteriostatic effect of top-flat Al2O3@HNRes and bactericidal effect of top-sharp Al2O3@ZNRes and Al2O3@ZNRl, the structural evolution of S. aureus on these arrays with incubation time was detailedly investigated as shown in Fig. 4. On Al2O3@Ti, S. aureus appear ball-shaped in morphology with a diameter of ∼500 nm throughout the whole incubation periods. Although S. aureus on top-flat Al2O3@HNRes are still ball-shaped at 5 and 30 min, the nanorods deform bacterial cell envelops at 2 and 4 h (as marked by green arrow in Fig. 4a). Cell envelop deformation is given to inhibit bacterial proliferation [26], consequently leading to reduction of BCNs on Al2O3@HNRes compared to Al2O3@Ti with incubation time (Fig. 3a and c).
Fig. 4.
(a) SEM images of morphological evolution of S. aureus cultured on Al2O3@Ti and Al2O3@HNRes, Al2O3@ZNRes, Al2O3@ZNRl for 5 min, 30 min, 2 h, 4 h and 24 h; TEM images of ultrafine structures of S. aureus cultured on (b) Al2O3@ZNRes and (c) Al2O3@ZNRl for 30 min as well as on (d) Al2O3@ZNRl for 2 h (white arrow: bacteria secreted extracellular polymeric substance; green arrow: cell envelope deformation; red arrow: nanorods bending; blue arrow: electron-lucent region, indicating the leakage of intracellular proteins of bacteria); (e) protein concentrations of the MHB culturing S. aureus seeded on Al2O3@Ti and Al2O3@HNRes, Al2O3@ZNRes, Al2O3@ZNRl for 5 min, 2 h, and 24 h, together with the protein concentrations of blank MHB, and the MHB culturing S. aureus seeded on tissue culture plate (TCP) as controls. (∗p < 0.05, ∗∗p < 0.01, N. S.: no significance).
Notably, S. aureus secreted EPS is clearly visible on Al2O3@HNRes, also on Al2O3@ZNRes and Al2O3@ZNRl at 5 min of incubation (as marked by white arrow in Fig. 4a). Owing to the recognition that the longer are nanorods the lower is their bending stiffness [28], EPS-induced adhesive force draws the longer nanorods of Al2O3@ZNRl to bend, as displayed at 30 min for example (Fig. 4 and Fig. S5) and nor do the shorter nanorods of Al2O3@HNRes and Al2O3@ZNRes throughout the whole incubation periods. Based on the horizontal deflection of the bended nanorods of Al2O3@ZNRl (Fig. S5), the adhesive force of S. aureus to Al2O3@ZNRl can be calculated according to Euler-Bernoulli's beam theory using the following equation, as adopted in previous work [52].
| (1) |
where F is bacterial adhesive force, δ is horizontal deflection of nanorod top, L is nanorod length, E is Young's modulus of nanorod, and I is area moment. In our work, δ is ∼33 nm in average as measured in the SEM images picked from different observation regions (Fig. S5); the nanorod length of Al2O3@ZNRl is ∼884 nm. Given that if a coated Al2O3 layer is of a thickness lower than 2 nm it would not influence the Young's modulus of a nanorod [53], based on the thickness of amorphous Al2O3 shell to be ∼1.5 nm, the Young's modulus of Al2O3@ZNRl could be considered the same as ZnO core with a value of 160 GPa [54]. Consequently, the adhesive force of S. aureus to Al2O3@ZNRl calculated based on above equation is about 27 nN. It is worth pointing out that the adhesive force of bacteria to their underlying substrate depends on the surface composition, wettability, zeta potential, and roughness of the substrate [52]. Owing to the same surface composition of Al2O3, roughness, wettability and zeta potential as well as identical interrod spacing and dimeter of the core-shell structured nanorods (Fig. 2a–c and 1b-d), it could be deduced that the adhesive forces of bacteria to Al2O3@HNRes, Al2O3@ZNRes and Al2O3@ZNRl are essentially equivalent.
Different from only deformation of bacterial cell envelop appearing on top-flat Al2O3@HNRes up to 2 h, the adhesive force drives Al2O3@ZNRes and Al2O3@ZNRl to wrinkle S. aureus surfaces at 5 min, even though the bacteria keep near-spherical shape on these top-sharp nanorods (Fig. 4a). The result indicates that the top-sharp nanorods induce higher stress on cell envelops of the adhered bacteria compared to the top-flat nanorods, owing to the fact that high stress on cell wall can distort the linkage between bacterial outer membrane and peptidoglycan to induce surface wrinkling [55]. At 30 min of incubation, both top-sharp Al2O3@ZNRes and Al2O3@ZNRl penetrate into the adhesive bacteria at a depth of ∼64 and 26 nm, respectively, as identified by TEM images in Fig. 4b and c. Moreover, the penetration elicits the structural disruption of bacterial cell envelop, which is severer for S. aureus on Al2O3@ZNRes, showing the thickening of cell envelop adjacent to nanorods (Fig. 4b). The difference in penetration depth of nanorods into S. aureus presented by the top-sharp arrays maybe ascribe the difference in nanorod length-dependent tortuosity. When bacterial adhesive force derives a nanorod bending, the axial component of the adhesive force is seemingly reduced owing to the presence of horizontal component of the force (Fig. S6), leading to a shallower penetration depth of longer Al2O3@ZNRl. The shallower penetration depth of longer Al2O3@ZNRl may lead to a slightly decreased bactericidal rate of Al2O3@ZNRl compared to shorter Al2O3@ZNRes against S. aureus after a longer incubation term (Fig. 3a and b). With prolonging incubation time to 2 h, Al2O3@ZNRl nanorods are shown to penetrate into S. aureus at a great depth of ∼125 nm (Fig. 4d). The deepened penetration of the nanorods seriously destroys the integrity of cell envelop (Fig. 4d), resulting in the leakage of intracellular proteins, as identified by the electron-lucent in Fig. 4d and the increase in the total protein concentrations of the resultant MHB in Fig. 4e. The leakage of intracellular proteins induces S. aureus collapsing and sinking into the surrounding leaks at 24 h (Fig. 4a). Given that EPS-derived adhesive force of bacteria depends on surface property of substrate [52], gram-negative bacteria, such as E. coli, may exhibit comparable adhesive force to these nanorods compared to S. aureus; together with the fact of thinner cell wall of E. coli [2,34], it can be deduced that top-sharp nanorods are also able to penetrate into E. coli to kill them.
Collectively, the aforementioned results reveal that the adhesive force of S. aureus drives the top-flat nanorods to deform cell envelop appearing bacteriostatic, but draws the top-sharp nanorods to penetrate into S. aureus appearing bactericidal. Considering essentially equivalent adhesive forces of S. aureus to Al2O3@HNRes and Al2O3@ZNRes, the different antibacterial mechanisms of the arrays may originate from the different stresses acting on cell envelops as identified next.
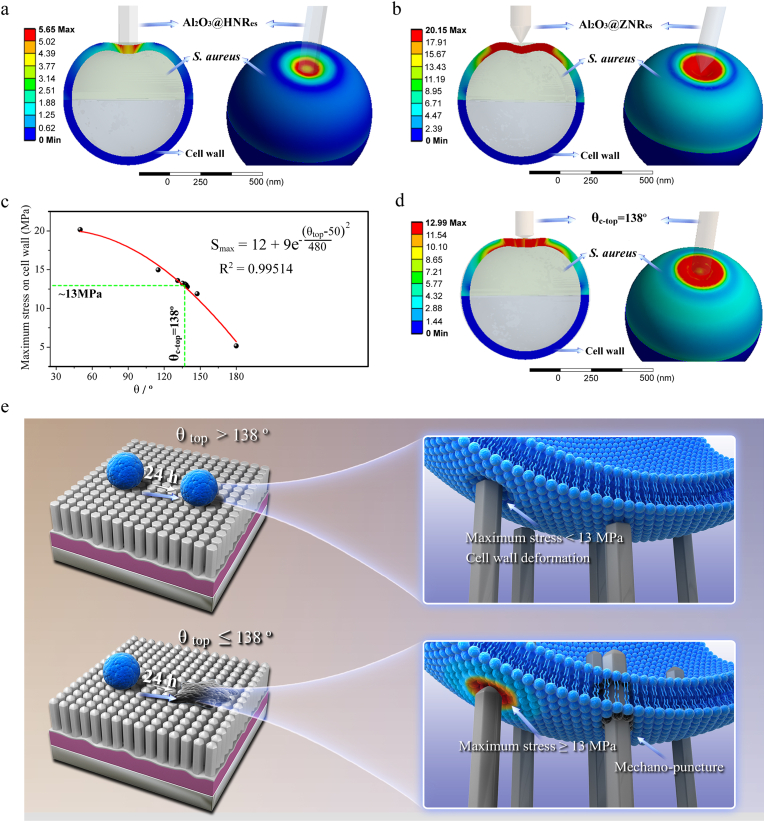
3.5. The finite element simulated stress distribution on cell wall induced by the nanorods with different top sharpness
To quantitatively demonstrate the effect of nanorod top sharpness on the stress acting on cell envelop and to identify the critical top sharpness (θc-top) of the nanorods able to penetrate into S. aureus, finite element (FE) simulation was carried out on Al2O3@HNRes with θtop of 180° and Al2O3@ZNRes with θtop of 50°, which have an identical nanorod dimeter and length and almost do not bend under the adhesive force of S. aureus throughout the whole incubation periods. Although nanorods density plays a key role in mechano-puncture activity of a nanorods patterned array against bacteria [1], Al2O3@HNRes and Al2O3@ZNRes exhibit identical interrod spacing and nanorod diameter, which result in a comparable nanorods density of the arrays, excluding its contribution to the different antibacterial activities of these arrays. Moreover, as shown in Fig. 4a, an individual nanorod with sharp top can penetrate into S. aureus. Therefore, to simplify the FE model, individual nanorods of Al2O3@HNRes and Al2O3@ZNRes were used in the FE simulation. Given that bacterial cell wall is a principal stress-bearing and shape-maintaining element relative to membrane [56], bacterial cell envelop can reasonably be simplified to cell wall in our FE model, which involves parameters including the thickness, Young's modulus and ultimate tensile strength (UTS) of S. aureus cell wall, internal hydrostatic pressure of S. aureus, the applied force of nanorod to cell wall and the θtop of nanorod. As known, the cell wall of S. aureus is usually modeled as a thin elastic shell with a thickness of ∼35 nm [24,26], the internal fluid in bacteria is usually considered to be incompressible [24] with a constant internal hydrostatic pressure of ∼10 atm for S. aureus [57], and the Young's modulus and UTS of S. aureus cell wall are ∼30 MPa and 13 MPa, respectively [58]. The adhesive force of S. aureus to each nanorod of Al2O3@HNRes and Al2O3@ZNRes provides the applied force of a nanorod to cell wall, with a value of ∼27 nN as calculated above. Based on these parameters, the stress distribution nephograms on cell walls of S. aureus on Al2O3@HNRes and Al2O3@ZNRes were FE simulated as shown in Fig. 5a and b. Clearly, one of top-flat Al2O3@HNRes derives the maximum stress of 5.65 MPa on cell wall (Fig. 5a), quite lower than UTS (13 MPa), resulting in mechano-deformation of S. aureus (Fig. 4a), while one of top-sharp Al2O3@ZNRes derives the maximum stress of 20.15 MPa on cell wall (Fig. 5b), much higher than UTS, leading to mechano-puncture into S. aureus (Fig. 4a). To further identify critical θtop, at which the nanorods-derived maximum stress on cell wall equals to its UTS of 13 MPa, dichotomy of θtop was carried out using 115° (the mean value of 180 and 50°) as a starting point which was substituted into our FE-model to obtain the maximum stress on cell wall at θtop of 115°. Subjecting to such continuous iteration to approach the maximum stress of 13 MPa, critical θtop of the nanorods was drawn to be 138° (Fig. 5c). We re-simulated the stress distribution nephogram on cell wall of S. aureus at θtop of 138° subjecting to the applied force of 27 nN using the FE-model, as shown in Fig. 5d. It is revealed that the acting stress on the same position of cell wall and the maximum stress at θtop of 138° are higher than those at 180° but lower than those at 50°. Crucially, we fitted the curve of the maximum stress (Smax) acting on cell wall versus θtop of the nanorods using dichotomy (Fig. 5c), drawing its expression formula as follow, with the correlation coefficient value R2 of 0.995:
| (2) |
which provides the quantitative basis to optimize the top sharpness of nanorods for achieving high efficacy of mechano-puncture into S. aureus. Based on the present FE stimulation, it is suggested that driven by the adhesive force of S. aureus, the nanorods deform cell envelop appearing bacteriostatic at θtop larger than 138°, while penetrate into S. aureus appearing bactericidal at θtop smaller than 138°, as schematically shown in Fig. 5e.
Fig. 5.
The finite element-simulated nephograms of stress distribution on cell walls of S. aureus on (a) Al2O3@HNRes and (b) Al2O3@ZNRes; (c) fitting curve of the maximum stress (Smax) acting on cell wall versus θtop of nanorods obtained using dichotomy and its expression formula; (d) re-simulated nephogram of stress distribution on cell wall of S. aureus at θtop of 138°; (e) schematic illustration of the antibacterial mechanisms of nanorods with different top sharpness, showing nanorods to deform cell envelop appearing bacteriostatic at θtop larger than 138°, while to penetrate into S. aureus appearing bactericidal at θtop smaller than 138°.
3.6. Anti-bacterial and osseointegrative activities of the coated pillars in vivo
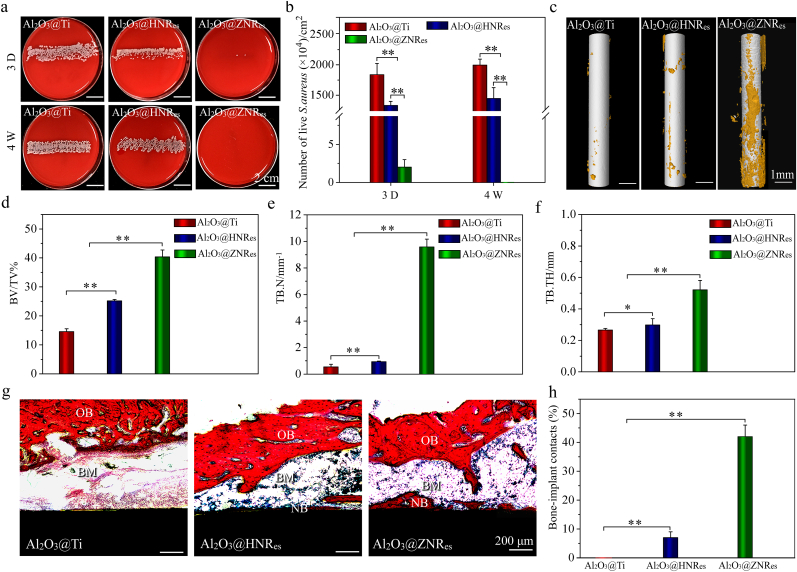
To assay the in vivo antibacterial and osseointegrative activities of the arrays, the Al2O3@HNRes and Al2O3@ZNRes patterned pillars were implanted in S. aureus-infected marrow cavities of rat tibias, together with the Al2O3@Ti pillars. Fig. 6a and b shows the photographs and corresponding counts of S. aureus colonies formed by rolling the implanted pillars on blood agar plates and re-culturing for 24 h. Although an obvious decrease in bacterial amount is displayed on top-flat Al2O3@HNRes compared to Al2O3@Ti owing to the inhibition of bacterial proliferation, there are still a mass of viable bacteria on Al2O3@HNRes at day 3 and week 4 of implantation. Conversely, almost no viable bacteria appear on top-sharp Al2O3@ZNRes at day 3 and week 4 of implantation, disclosing the in vivo bactericidal rate of Al2O3@ZNRes higher than 98%.
Fig. 6.
Histological analyses of Al2O3@Ti pillars as well as Al2O3@HNRes and Al2O3@ZNRes patterned pillars implanted in S. aureus-infected marrow cavities of rat tibias for 3 days and 4 weeks: (a) Photographs and (b) the corresponding counts of S. aureus colonies formed by rolling the implanted pillars on blood agar plates and re-culturing for 24 h; (c) Micro-CT reconstructed images of the pillars implanted for 4 weeks and quantitation of (d) bone volume fraction (BV/TV %), (e) trabecular number (TB. N) and (f) trabecular thickness (TB. TH) of the peri-implant bone based on Micro-CT assay; (g) Van Gieson's staining images of the pillars implanted for 4 weeks and (h) corresponding bone-implant contacts. Data are presented as mean ± SD, n = 6, (*) p < 0.05 and (**) p < 0.01.
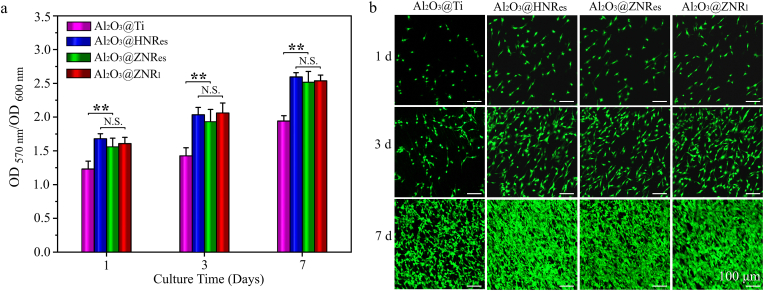
Osseointegration is primarily mediated by MSCs [59]. To understand the effect on cells, the arrays together with Al2O3@Ti were assessed on the viability and proliferation of rat bone marrow MSCs (rBMSCs) in vitro with alamar blue and fluorescent live/dead staining, respectively. Al2O3@HNRes, Al2O3@ZNRes and Al2O3@ZNRl exhibit the mitochondrial activity of rBMSCs equivalent to each other but higher than Al2O3@Ti (Fig. 7a). Moreover, these arrays significantly promote rBMSC adhesion and proliferation compared to planar Al2O3@Ti (Fig. 7b), which is ascribed to the role of nanotopography in enhancing the expression of cell integrins and formation of focal adhesions [[59], [60], [61]]. Contrary to the action on S. aureus, all the top-flat and top-sharp arrays do not impair rBMSCs, it may be attributed to the following reasons: (1) the Young's modulus of stem cells is three orders lower than that of bacterial cell envelops, ensuring sufficient flexibility of the stem cells to conform to their underlying nanorods without puncture [62,63]; (2) different from the incapable of bacteria to adapt to their underlying substrate, mammalian cells actively sense and respond to their adhered substrate through modulation of their morphology mediated by the cytoskeleton and formation of focal adhesions [49], reducing the nanorods derived stress on cell membrane; (3) the three-dimensional architecture of extracellular matrix that enables rBMSCs to withstand high stress without plastic deformation or rupture [64]. Nevertheless, further investigation are needed to clarify the accurate reasons for the contrary actions on S. aureus and rBMSCs induced by the top-sharp nanorods in the present work.
Fig. 7.
(a) Metabolic activity and (b) fluorescent live/dead straining images of rBMSCs incubated on Al2O3@Ti and Al2O3@HNRes, Al2O3@ZNRes, Al2O3@ZNRl for 1, 3 and 7 days. (∗∗p < 0.01, N. S.: no significance).
Fig. 6c–f shows the Micro-CT reconstructed images as well as the statistical bone volume fractions, and trabecular number and thickness of new bone surrounding the pillars implanted for 4 weeks. Even in bacteria-infected case, Al2O3@ZNRes patterned pillar still induces more pronounced new bone than Al2O3@HNRes patterned pillar, while Al2O3@Ti pillar rarely induces new bone. This difference is further confirmed by Van Gieson's staining images (Fig. 6g) and the statistical bone-implant contact ratios (Fig. 6h). Top-sharp Al2O3@ZNRes patterned pillar shows the quantity of de novo bone apposition and bone-implant contact ratio higher than top-flat Al2O3@HNRes patterned pillar and much higher than Al2O3@Ti pillar. Taken together, top-sharp Al2O3@ZNRes array exhibit good in vitro viability and proliferation of rBMSCs and strong in vivo bactericidal activity, leading to fair osseointegration in S. aureus-infected rat tibias.
4. Conclusions
Employing the fabricated three kinds of arrays consisting of Al2O3-wraped nanorods, which have nanorod top conical angles of 50 and 180° and nanorod lengths of about 469 and 884 nm, we show that the difference in antibacterial activities of the arrays is dependent on nanorod top sharpness rather than nanorod length without the contributions of chemical components as well as roughness, wettability and zeta potentials. Driven by the EPS-derived equivalent adhesive force of S. aureus to the assays, the top-flat nanorods just deform S. aureus cell envelops, owing to the derived maximum stress (Smax) on cell wall to be 5.65 MPa quite lower than the ultimate-tensile-strength (UTS) of cell wall, and show an antibacterial rate of 29% against S. aureus in proliferation-inhibited manner. However, the top-sharp nanorods penetrate into S. aureus, owing to the derived Smax of 20.15 MPa much higher than UTS, and show an antibacterial rate of 98% for the shorter and 96% for the longer in bactericidal manner. Moreover, the relationship between the top conical angle and nanorod derived Smax on cell wall is formularized, and a critical top conical angle of 138° is calculated for the nanorods able to penetrate into S. aureus based on the FE stimulation in the present work. Furthermore, the top-sharp but short nanorods patterned array exhibits good in vitro proliferative ability of rBMSCs and strong bactericidal activity in S. aureus-infected rat tibias, and thus simultaneously shows fair osseointegration. Our work provides quantitative basis for optimizing top sharpness of nanorods to achieve mechano-puncture derived strong bactericidal efficacy without introducing chemical bactericides to impair osseointegration.
CRediT authorship contribution statement
Jing Ye: Methodology, Investigation, Data curation, Visualization, Writing – original draft, and. Bo Li: Methodology, Investigation, Data curation, Visualization, Writing – original draft. Yufeng Zheng: Supervision. Shuilin Wu: Supervision, and. Dafu Chen: Supervision. Yong Han: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors appreciate the National Natural Science Foundation of China (Grant number 51631007, 51971171 and 3700860), and the joint project of Xi’an Jiaotong University and Beijing Research Institute of Traumatology and Orthopaedics (Contract No. 202012443) for financially supporting this work.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2021.11.028.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Modaresifar K., Azizian S., Ganjian M., Fratila-Apachitei L.E., Zadpoor A.A. Bactericidal effects of nanopatterns: a systematic review. Acta Biomater. 2019;83:29–36. doi: 10.1016/j.actbio.2018.09.059. [DOI] [PubMed] [Google Scholar]
- 2.Linklater D.P., Baulin V.A., Juodkazis S., Crawford R.J., Stoodley P., Ivanova E.P. Mechano -bactericidal actions of nanostructured surfaces. Nat. Rev. Microbiol. 2020;19:8–22. doi: 10.1038/s41579-020-0414-z. [DOI] [PubMed] [Google Scholar]
- 3.Bakhshandeh S., Gorgin-Karaji Z., Lietaert K., Fluit A.C., Boell C.H.E., Vogely H.C., Vermonden T., Hennink W.E., Weinans H., Zadpoor A.A., Amin Y.S. Simultaneous delivery of multiple antibacterial agents from additively manufactured porous biomaterials to fully eradicate planktonic and adherent Staphylococcus aureus. ACS Appl. Mater. Interfaces. 2017;9:25691–25699. doi: 10.1021/acsami.7b04950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fathi M., Akbari B., Taheriazam A. Antibiotics drug release controlling and osteoblast adhesion from titania nanotubes arrays using silk fibroin coating. Mater. Sci. Eng. C. 2019;103:109743. doi: 10.1016/j.msec.2019.109743. [DOI] [PubMed] [Google Scholar]
- 5.Hirschfeld J., Akinoglu E.M., Wirtz D.C., Hoerauf A., Bekeredjian-Ding I., Jepsen S., Haddouti E.-M., Limmer A., Giersig M. Long-term release of antibiotics by carbon nanotube-coated titanium alloy surfaces diminish biofilm formation by Staphylococcus epidermidis. Nanomed. Nanotechnol. 2017;13:1587–1593. doi: 10.1016/j.nano.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Ye J., Li B., Li M., Zheng Y., Wu S., Han Y. ROS induced bactericidal activity of amorphous Zn-doped titanium oxide coatings and enhanced osseointegration in bacteria-infected rat tibias. Acta Biomater. 2020;107:313–324. doi: 10.1016/j.actbio.2020.02.036. [DOI] [PubMed] [Google Scholar]
- 7.Zhou J., Li B., Zhao L., Zhang L., Han Y. F-doped micropore/nanorod hierarchically patterned coatings for improving antibacterial and osteogenic activities of bone implants in bacteria-infected cases. ACS Biomater. Sci. Eng. 2017;3:1437–1450. doi: 10.1021/acsbiomaterials.6b00710. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L., Guo J., Yan T., Han Y. Fibroblast responses and antibacterial activity of Cu and Zn co-doped TiO2 for percutaneous implants. Appl. Surf. Sci. 2018;434:633–642. [Google Scholar]
- 9.Makvandi P., Wang C.y., Zare E.N., Borzacchiello A., Niu L.n., Tay F.R. Metal-based nanomaterials in biomedical applications: antimicrobial activity and cytotoxicity aspects. Adv. Funct. Mater. 2020;30:1910021. [Google Scholar]
- 10.Zhang Y., Zhang L., Li B., Han Y. Enhancement in sustained release of antimicrobial peptide from dual-diameter-structured TiO2 nanotubes for long-lasting antibacterial activity and cytocompatibility. ACS Appl. Mater. Interfaces. 2017;9:9449–9461. doi: 10.1021/acsami.7b00322. [DOI] [PubMed] [Google Scholar]
- 11.Crofts T.S., Gasparrini A.J., Dantas G. Next-generation approaches to understand and combat the antibiotic resistome. Nat. Rev. Microbiol. 2017;15:422–434. doi: 10.1038/nrmicro.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brogden K.A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 13.Duewelhenke N., Krut O., Eysel P. Influence on mitochondria and cytotoxicity of different antibiotics administered in high concentrations on primary human osteoblasts and cell lines. Antimicrob. Agents Chemother. 2007;51:54–61. doi: 10.1128/AAC.00729-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morales-Alvarez M.C. Nephrotoxicity of antimicrobials and antibiotics. Adv. Chron. Kidney Dis. 2020;27:31–37. doi: 10.1053/j.ackd.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Rana M., Sharma A.K. Cu and Zn interactions with Aβ peptides: consequence of coordination on aggregation and formation of neurotoxic soluble Aβ oligomers. Metallomics. 2019;11:64–84. doi: 10.1039/c8mt00203g. [DOI] [PubMed] [Google Scholar]
- 16.Ren Y., Liu H., Liu X., Zheng Y., Li Z., Li C., Yeung K.W., Zhu S., Liang Y., Cui Z., Wu S. Photoresponsive materials for antibacterial applications. Cell Rep. Phys. Sci. 2020;1:100245. [Google Scholar]
- 17.Sun B., Zhen X., Jiang X. Development of mesoporous silica-based nanoprobes for optical bioimaging applications. Biomater. Sci. 2021;9:3603. doi: 10.1039/d1bm00204j. [DOI] [PubMed] [Google Scholar]
- 18.Wu Q., Tan L., Liu X., Li Z., Zhang Y., Zheng Y., Liang Y., Cui Z., Zhu S., Wu S. The enhanced near-infrared photocatalytic and photothermal effects of MXene-based heterojunction for rapid bacteria-killing. Appl. Catal. B Environ. 2021;297:120500. [Google Scholar]
- 19.Tian Y., Li Y., Liu J., Lin Y., Jiao J., Chen B., Wang W., Wu S., Li C. Photothermal therapy with regulated Nrf2/NF-κB signaling pathway for treating bacteria-induced periodontitis. Bioact. Mater. 2022;9:428–445. doi: 10.1016/j.bioactmat.2021.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu H., Li J., Liu X., Li Z., Zhang Y., Liang Y., Zheng Y., Zhu S., Cui Z., Wu S. Photo-sono interfacial engineering exciting the intrinsic property of herbal nanomedicine for rapid broad-spectrum bacteria killing. ACS Nano. 2021;15:18505–18519. doi: 10.1021/acsnano.1c08409. [DOI] [PubMed] [Google Scholar]
- 21.Lia C., Wang J., Wang Y., Gao H., Wei G., Huang Y., Yu H., Gan Y., Wang Y., Mei L., Chen H., Hu H., Zhang Z., Jin Y. Recent progress in drug delivery. Acta Pharm. Sin. B. 2019;9:1145–1162. doi: 10.1016/j.apsb.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivanova E.P., Hasan J., Webb H.K., Truong V.K., Watson G.S., Watson J.A., Baulin V.A., Pogodin S., Wang J.Y., Tobin M.J., Lobbe C., Crawford R.J. Natural bactericidal surfaces: mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small. 2012;8:2489–2494. doi: 10.1002/smll.201200528. [DOI] [PubMed] [Google Scholar]
- 23.Ivanova E.P., Hasan J., Webb H.K., Gervinskas G., Juodkazis S., Truong V.K., Wu A.H., Lamb R.N., Baulin V.A., Watson G.S., Watson J.A., Mainwaring D.E., Crawford R.J. Bactericidal activity of black silicon. Nat. Commun. 2013;4:2838. doi: 10.1038/ncomms3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valiei A., Lin N., Bryche J.-F., McKay G., Canva M., Charette P.G., Nguyen D., Moraes C., Tufenkji N. Hydrophilic mechano-bactericidal nanopillars require external forces to rapidly kill bacteria. Nano Lett. 2020;20:5720–5727. doi: 10.1021/acs.nanolett.0c01343. [DOI] [PubMed] [Google Scholar]
- 25.Dickson M.N., Liang E.I., Rodriguez L.A., Vollereaux N., Yee A.F. Nanopatterned polymer surfaces with bactericidal properties. Biointerphases. 2015;10 doi: 10.1116/1.4922157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenkins J., Mantell J., Neal C., Gholinia A., Verkade P., Nobbs A.H., Su B. Antibacterial effects of nanopillar surfaces are mediated by cell impedance, penetration and induction of oxidative stress. Nat. Commun. 2020;111:626. doi: 10.1038/s41467-020-15471-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J., Tan L., Liu X., Cui Z., Yang X., Yeung K.W.K., Chu P.K., Wu S. Balancing bacteria-osteoblast competition through selective physical puncture and biofunctionalization of ZnO/polydopamine/arginine-glycine-aspartic acid-cysteine nanorods. ACS Nano. 2017;11:11250–11263. doi: 10.1021/acsnano.7b05620. [DOI] [PubMed] [Google Scholar]
- 28.Linklater D.P., De Volder M., Baulin V.A., Werner M., Jessl S., Golozar M., Maggini L., Rubanov S., Hanssen E., Juodkazis S., Ivanova E.P. High aspect ratio nanostructures kill bacteria via storage and release of mechanical energy. ACS Nano. 2018;12:6657–6667. doi: 10.1021/acsnano.8b01665. [DOI] [PubMed] [Google Scholar]
- 29.Pogodin S., Hasan J., Baulin V.A., Webb H.K., Truong V.K., Phong Nguyen T.H., Boshkovikj V., Fluke C.J., Watson G.S., Watson J.A., Crawford R.J., Ivanova E.P. Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophys. J. 2013;104:835–840. doi: 10.1016/j.bpj.2012.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue F., Liu J., Guo L., Zhang L., Li Q. Theoretical study on the bactericidal nature of nanopatterned surfaces. J. Theor. Biol. 2015;385:1–7. doi: 10.1016/j.jtbi.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Bandara C.D., Singh S., Afara I.O., Wolff A., Tesfamichael T., Ostrikov K., Oloyede A. Bactericidal effects of natural nanotopography of dragonfly wing on escherichia coli. ACS Appl. Mater. Interfaces. 2017;9:6746–6760. doi: 10.1021/acsami.6b13666. [DOI] [PubMed] [Google Scholar]
- 32.Hizal F., Choi C.-H., Busscher H.J., van der Mei H.C. Staphylococcal adhesion, detachment and transmission on nanopillared Si surfaces. ACS Appl. Mater. Interfaces. 2016;8:30430–30439. doi: 10.1021/acsami.6b09437. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y., Harapanahalli A.K., Busscher H.J., Norde W., van der Mei H.C. Nanoscale cell wall deformation impacts long-Range bacterial adhesion forces on surfaces. Appl. Environ. Microbiol. 2014;80:637. doi: 10.1128/AEM.02745-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelleher S.M., Habimana O., Lawler J., O R B., Daniels S., Casey E., Cowley A. Cicada wing surface topography: an investigation into the bactericidal properties of nanostructural features. ACS Appl. Mater. Interfaces. 2016;8:14966–14974. doi: 10.1021/acsami.5b08309. [DOI] [PubMed] [Google Scholar]
- 35.Hasan J., Raj S., Yadav L., Chatterjee K. Engineering a nanostructured "super surface" with superhydrophobic and superkilling properties. RSC Adv. 2015;5:44953–44959. doi: 10.1039/C5RA05206H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitara L.D., Anywar A.D., Acullu D., Odongo-Aginya E., Aloyo J., Fendu M. Antibiotic susceptibility of Staphylococcus aureus in suppurative lesions in Lacor Hospital, Uganda. Afr. Health Sci. 2011;1:34–39. [PMC free article] [PubMed] [Google Scholar]
- 37.Modaresifar K., Kunkels L.B., Ganjian M., Tumer N., Hagen C.W., Otten L.G., Hagedoorn P.L., Angeloni L., Ghatkesar M.K., Fratila-Apachitei L.E., Zadpoor A.A. Deciphering the roles of interspace and controlled disorder in the bactericidal properties of nanopatterns against Staphylococcus aureus. Nanomaterials. 2020;10:347. doi: 10.3390/nano10020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michalska M., Gambacorta F., Divan R., Aranson I.S., Sokolov A., Noirot P., Laible P.D. Tuning antimicrobial properties of biomimetic nanopatterned surfaces. Nanoscale. 2018;10:6639–6650. doi: 10.1039/c8nr00439k. [DOI] [PubMed] [Google Scholar]
- 39.Wang W., Pei P., Chu Z., Chen B., Qian H., Zha Z., Zhou W., Liu T., Shao M., Wang H. Bi2S3 coated Au nanorods for enhanced photodynamic and photothermal antibacterial activities under NIR light. Chem. Eng. J. 2020;397:125488. [Google Scholar]
- 40.Wang R., Shi M., Xu F., Qiu Y., Zhang P., Shen K., Zhao Q., Yu J., Zhang Y. Graphdiyne- modified TiO2 nanofibers with osteoinductive and enhanced photocatalytic antibacterial activities to prevent implant infection. Nat. Commun. 2020;11:4465. doi: 10.1038/s41467-020-18267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou J., Li B., Lu S., Zhang L., Han Y. Regulation of osteoblast proliferation and differentiation by interrod spacing of Sr-HA nanorods on microporous titania coatings. ACS Appl. Mater. Interfaces. 2013;5:5358–5365. doi: 10.1021/am401339n. [DOI] [PubMed] [Google Scholar]
- 42.Truong V.K., Lapovok R., Estrin Y.S., Rundell S., Wang J.Y., Fluke C.J., Crawford R.J., Ivanova E.P. The influence of nano-scale surface roughness on bacterial adhesion to ultrafine-grained titanium. Biomaterials. 2010;31:3674–3683. doi: 10.1016/j.biomaterials.2010.01.071. [DOI] [PubMed] [Google Scholar]
- 43.Fröjd V., Chávez de Paz L., Andersson M., Wennerberg A., Davies J.R., Svensäter G. In situ analysis of multispecies biofilm formation on customized titanium surfaces. Mol. Oral Microbiol. 2011;26:241–252. doi: 10.1111/j.2041-1014.2011.00610.x. [DOI] [PubMed] [Google Scholar]
- [4.Dou X.-Q., Zhang D., Feng C., Jiang L. Bioinspired hierarchical surface structures with tunable wettability for regulating bacteria adhesion. ACS Nano. 2015;9:10664–10672. doi: 10.1021/acsnano.5b04231. [DOI] [PubMed] [Google Scholar]
- 45.Lai Y., Huang J., Cui Z., Ge M., Zhang K.-Q., Chen Z., Chi L. Recent advances in TiO2-based nanostructured surfaces with controllable wettability and adhesion. Small. 2016;12:2203–2224. doi: 10.1002/smll.201501837. [DOI] [PubMed] [Google Scholar]
- 46.Spriano S., Sarath Chandra V., Cochis A., Uberti F., Rimondini L., Bertone E., Vitale A., Scolaro C., Ferrari M., Cirisano F., Gautier di Confiengo G., Ferraris S. How do wettability, zeta potential and hydroxylation degree affect the biological response of biomaterials? Mater. Sci. Eng. C. 2017;74:542–555. doi: 10.1016/j.msec.2016.12.107. [DOI] [PubMed] [Google Scholar]
- 47.Yuan Y., Hays M.P., Hardwidge P.R., Kim J. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 2017;7:14254–14261. [Google Scholar]
- 48.Linklater D.P., Nguyen H.K.D., Bhadra C.M., Juodkazis S., Ivanova E.P. Influence of nanoscale topology on bactericidal efficiency of black silicon surfaces. Nanotechnology. 2017;28:245301. doi: 10.1088/1361-6528/aa700e. [DOI] [PubMed] [Google Scholar]
- 49.Hasan J., Jain S., Chatterjee K. Nanoscale topography on black titanium imparts multibiofunctional properties for orthopedic applications. Sci. Rep. 2017;7:41118. doi: 10.1038/srep41118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu S., Zuber F., Maniura-Weber K., Brugger J., Ren Q. Nanostructured surface topographies have an effect on bactericidal activity. J. Nanobiotechnol. 2018;16 doi: 10.1186/s12951-018-0347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Høiby N., Bjarnsholt T., Givskov M., Molin S., Ciofu O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents. 2010;35:322–332. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 52.Elbourne A., Chapman J., Gelmi A., Cozzolino D., Crawford R.J., Truong V.K. Bacterial-nanostructure interactions: the role of cell elasticity and adhesion forces. J. Colloid Interface Sci. 2019;546:192–210. doi: 10.1016/j.jcis.2019.03.050. [DOI] [PubMed] [Google Scholar]
- 53.Chen Y., Gao Q., Wang Y., An X., Liao X., Mai Y.-W., Tan H.H., Zou J., Ringer S.P., Jagadish C. Determination of Young's modulus of ultrathin nanomaterials. Nano Lett. 2015;15:5279–5283. doi: 10.1021/acs.nanolett.5b01603. [DOI] [PubMed] [Google Scholar]
- 54.Chen C.Q., Shi Y., Zhang Y.S., Zhu J., Yan Y.J. Size dependence of Young's modulus in ZnO nanowires. Phys. Rev. Lett. 2006;96 doi: 10.1103/PhysRevLett.96.075505. [DOI] [PubMed] [Google Scholar]
- 55.Greif D., Wesner D., Regtmeier J., Anselmetti D. High resolution imaging of surface patterns of single bacterial cells. Ultramicroscopy. 2010;110:1290–1296. doi: 10.1016/j.ultramic.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 56.Scheffers D.-J., Pinho M.G. Bacterial cell wall synthesis: new insights from localization studies. Microbiol. Mol. Biol. Rev. 2005;69:585. doi: 10.1128/MMBR.69.4.585-607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rojas E.R., Huang K.C. Regulation of microbial growth by turgor pressure. Curr. Opin. Microbiol. 2018;42:62–70. doi: 10.1016/j.mib.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 58.Thwaites J.J., Surana U.C. Mechanical properties of Bacillus subtilis cell walls: effects of removing residual culture medium. J. Bacteriol. 1991;173:197–203. doi: 10.1128/jb.173.1.197-203.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou J., Li B., Han Y., Zhao L. The osteogenic capacity of biomimetic hierarchical micropore/nanorod-patterned Sr-HA coatings with different interrod spacings. Nanomedicine. 2016;12:1161–1173. doi: 10.1016/j.nano.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 60.Zhou J., Han Y., Lu S. Direct role of interrod spacing in mediating cell adhesion on Sr-HA nanorod-patterned coatings. Int. J. Nanomed. 2014;9:1243–1260. doi: 10.2147/IJN.S58236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou J., Zhao L., Li B., Han Y. Nanorod diameter modulated osteogenic activity of hierarchical micropore/nanorod-patterned coatings via a Wnt/β-catenin pathway. Nanomedicine. 2018;14:1719–1731. doi: 10.1016/j.nano.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 62.Nikolaev N.I., Muller T., Williams D.J., Liu Y. Changes in the stiffness of human mesenchymal stem cells with the progress of cell death as measured by atomic force microscopy. J. Biomech. 2014;47:625–630. doi: 10.1016/j.jbiomech.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 63.Tuson H.H. Measuring the stiffness of bacterial cells from growth rates in hydrogels of tunable elasticity. Mol. Microbiol. 2012;84:874–891. doi: 10.1111/j.1365-2958.2012.08063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muiznieks L.D., Keeley F.W. Molecular assembly and mechanical properties of the extracellular matrix: a fibrous protein perspective. Biochim. Biophys. Acta. 2013;1832:866–875. doi: 10.1016/j.bbadis.2012.11.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.