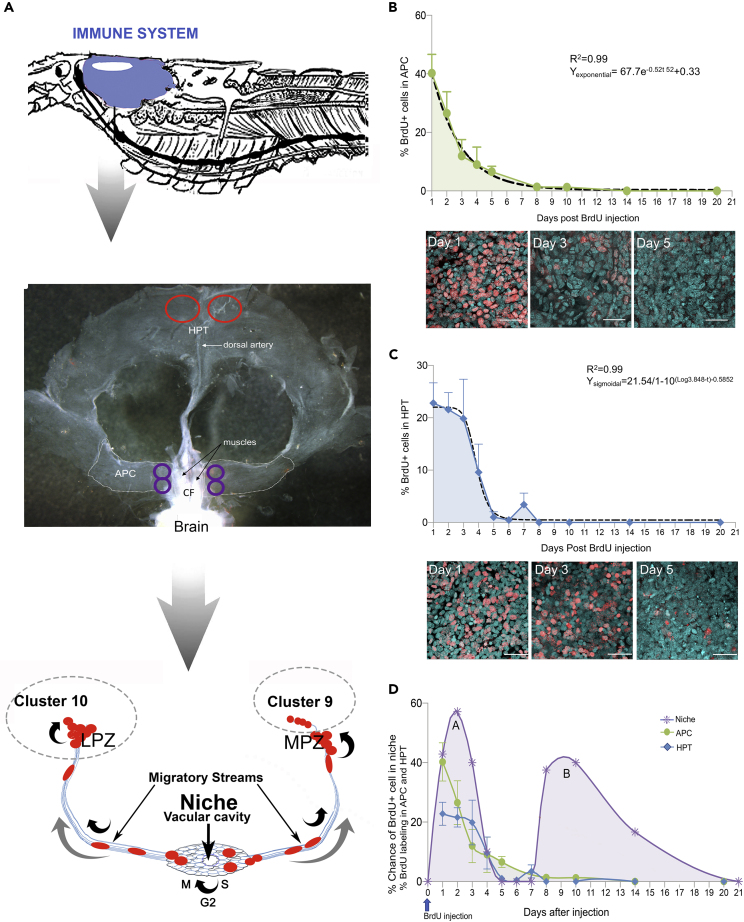
Figure 1.
Cellular dynamics in the immune system tissues are compared
An injection of BrdU was given to a group of crayfish on day 0 and animals euthanized on the days specified in the graphs. Average percentages of BrdU-labeled APC (B) and HPT (C) cells and standard deviations are shown (B, C, D).
(A) (Top) The immune system (blue) is located in the anterior, dorsal region of crayfish, surrounding the stomach. (Middle) Illustration of dissected tissue regions and sampling sites in the two parts of the hematopoietic system: the APC and HPT. The APC is located just posterior to the brain, surrounding and extending away from the cor frontale (CF). In the APC, four images were taken per animal, bilaterally at regions adjacent to the muscles of the CF (locations circled in purple). In the HPT, which is located posterior to the APC, two images were taken per animal (locations circled in red) at the posterior margin of the HPT, adjacent to the dorsal artery. (Bottom) Neurogenic niches that generate adult-born neurons are located bilaterally on the ventral surface of the brain, just beneath the sheath that surrounds the brain. One niche is represented diagrammatically, illustrating the position of the niche and migratory streams, and locations of some of the mitotic divisions in the neural lineage (curved black arrows). The direction of movement of the neural precursors in the migratory streams, toward brain Clusters 9 and 10, is indicated (grey arrows).
(B) An average of 40% of APC cells in the sampled regions were BrdU-positive on day 1. The mean percentage of BrdU-labeled cells in the APC (green-shaded graph area) then decreased exponentially (dashed regression line) over the next several days, reaching zero by 14 days post-injection. Confocal images are representative of APC labeling on days 1, 3, and 5. Propidium iodide (cyan); BrdU (red). N = # crayfish, each with multiple counting areas in each animal (see Methods): sampling days 1–2, n = 8; day 3, n = 6; day 4, n = 5; day 5, n = 6; day 8, n = 3; day 10, n = 4; day 14, n = 4; day 21, n = 4. R2 and Y exponential functions are indicated. Error bars indicate standard deviations.
(C) In the HPT, the average percentage of BrdU-labeled cells remains at ∼20% for the first three sampling days following BrdU injection, then falls to near zero between days 3 and 5. The dashed line represents a sigmoidal decay, which best fits these data. Images show BrdU labeling on days 1, 3, and 5 post-BrdU injection. Propidium iodide (cyan) and BrdU (red). N = # crayfish, with multiple counting areas in each animal (see Methods): sampling day 1, n = 5; day 2, n = 4; day 3, n = 3; day 4, n = 5; day 5, n = 4; day 6, n = 5; day 7, n = 6; day 8, n = 2; day 10, n = 2; day 14, n = 3; day 20, n = 4. R2 and Y exponential functions are indicated. Error bars indicate standard deviations.
(D) The % chance of finding a BrdU-labeled cell in the neurogenic niche after a single injection of BrdU on day 0 is plotted (purple shaded graph; adapted from Benton et al., 2014). The proliferation dynamics (% BrdU-labeling) in the APC (green; shown also in Figure 1B) and HPT (blue; shown also in Figure 1C) are overlaid on the niche graph. Two separate groups of animals were used to generate the niche graph (purple), and APC/HPT graphs (green and blue). BrdU-labeled cells are found in the niche (purple) on days 1–4 (peak A) and again on days 8–14 post-BrdU injection (see Benton et al., 2014 for details). The % of BrdU-labeled cells in the APC and HPT decreases as cells from these tissues are released into the circulation. Scale bars: (B) and (C) 30 μm.