Abstract
Background:
Diffuse large B-cell lymphoma is a type of B-cell non-Hodgkin lymphoma with a high incidence. About one-third of patients are resistant or eventually relapse. The prognosis for patients with relapsed/resistant diffuse large B-cell lymphoma who need salvage therapy is not optimistic.
Aims:
To explore whether homebox D3 binding to lysine (K)-specific demethylase 5C promoted malignant progression of diffuse large B-cell lymphoma by decreasing p53 expression.
Study Design:
Cell culture study.
Methods:
The mRNA and protein expression of lysine (K)-specific demethylase 5C and homebox D3 in cells were respectively detected by real-time quantitative polymerase chain reaction analysis and Western blot. Real-time quantitative polymerase chain reaction analysis and Western blot were also applied to determine the transfection effects of shRNA-KDM5C or OeHOXD3 in OCI-Ly7 cells. After transfection, the cell viability, proliferation, and apoptosis were respectively analyzed by Cell Counting Kit-8 assay, EdU staining, and acridine orange—ethidium bromide staining. The interaction between homebox D3 and lysine (K)-specific demethylase 5C promoter was verified by the dual-luciferase reporter assay and chromatin immunoprecipitation (ChIP) assay.
Results:
Lysine (K)-specific demethylase 5C mRNA expression (HBL1 2.84 ± 0.29; SUDHL4 3.53 ± 0.21; OCI-Ly8 4.06 ± 0.24; OCI-Ly7 5.03 ± 0.28 vs. GM12878 1.00 ± 0.07; all P < .001) and protein expression (HBL1 1.52 ± 0.06; SUDHL4 1.77 ± 0.10; OCI-Ly8 2.34 ± 0.07; OCI-Ly7 2.78 ± 0.07 vs. GM12878 1.00 ± 0.07; all P < .001) in DLBCL cells were higher than that in GM12878 cells and showed the highest in OCI-Ly7 cells. Homebox D3 mRNA (OCI-Ly7 3.85 ± 0.17 vs. GM12878 1.00 ± 0.05; P < .001) and protein (OCI-Ly7 1.73 ± 0.10 vs. GM12878 1.00 ± 0.06; P < .001) expression were also highly expressed in OCI-Ly7 cells. Moreover, down-regulation of lysine (K)-specific demethylase 5C suppressed the viability and proliferation and enhanced the apoptosis of OCI-Ly7 cells. Knockdown of lysine (K)-specific demethylase 5C decreased the B-cell lymphoma 2 expression while increased the expression of Bax, cleaved caspase 3, cytochrome C, p53, and p21. The transcription factor homebox D3 was confirmed to interact with the lysine (K)-specific demethylase 5C promoter. Homebox D3 overexpression could reverse the regulating effect of down-regulation of lysine (K)-specific demethylase 5C on the OCI-Ly7 cells.
Conclusion:
Homebox D3 up-regulating lysine (K)-specific demethylase 5C promotes malignant progression of diffuse large B-cell lymphoma by decreasing p53 expression.
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) accounts for about 30-40% of adult non-Hodgkin’s lymphoma. Some investigations have found that 37% of B-cell tumors belong to DLBCL.1,2 DLBCL has obvious heterogeneity and invasiveness, with multiple manifestations and outcomes of pathogenic mechanisms.3 About half of the patients can be cured, but about 1/3 of the patients are refractory or eventually relapsed. The prognosis of relapsed/refractory DLBCL patients who need salvage treatment is not optimistic, only about 10% of the patients will achieve a complete cure.4
Lysine (K)-specific demethylase 5C (KDM5C) is a member of the JmjC domain protein family. KDM5C is an oncogene,5,6,7,8 which can promote the proliferation of tumor cells and metastasis to distant tissues and organs,9 and this effect seems to be achieved through specific inhibition of anti-proliferation genes.10 In domestic and foreign literatures, the research direction of KDM5C gene mainly focuses on the relationship between this gene and cancer. ELK1-activated lncRNA LBX2-AS1 aggravated ovarian cancer progression by sponging miR-4784 expression and increasing KDM5C expression.11 KDM5C was observed to be highly expressed in colon cancer tissues and promoted the proliferation of colon cancer cells.11 KDM5C expression was up-regulated in the samples of prostate cancer (PCa) and castration-resistance prostate cancer (CRPC) and an elevated expression of KDM5C could aggravate the CRPC cell proliferation by inhibiting PTEN.12 A selective KDM5A inhibitor identified in breast cancer cell lines suppressed cancer by causing cell cycle arrest and senescence.13 However, the role of KDM5C in DLBCL is still unknown.
Through JASPAR (http://jaspar.genereg.net/), the transcription factor homebox D3 (HOXD3) is predicted to bind to the KDM5C promoter. HOXD3 promoted the development of hepatocellular carcinoma through transcriptional promotion of ITGA2 to activate ERK1/2 signaling.14 The expression of tumor suppressor miR-99b-3p was decreased in gastric cancer tissues, and miR-99b-3p inhibited cell viability of gastric cancer and induced cell stagnation in the S phase.15 Vitamin D3-induced miR-99b-3p participated in its inhibitory effect on the proliferation of gastric cancer cells by targeting HOXD3.16
Above all, this paper aims to explore whether HOXD3 binding to KDM5C promotes the malignant progression of DLBCL by decreasing p53 expression.
MATERIAL and METHODS
Cell Culture
Human B lymphocyte (GM12878) and DLBC cell lines (SUDHL4, OCI-Ly8, and OCI-Ly7) were brought from Cobioer (Nanjing, China). HBL1 DLBC cell line was provided by Shanghai Yaji Biotechnology Co., Ltd (China). GM12878 was cultured in Iscove’s modified Dulbecco’s medium (IMDM) (Gibco), and SUDHL4 was cultured in RPMI 1640 medium (Gibco). Each medium contained 10% of FBS and 1% of P/S. OCI-Ly8 and OCI-Ly7 were cultured in IMDM (Gibco) (20% FBS and 1% P/S). All cells were incubated with 5% CO2 at 37°C.
Cell Transfection
OCI-Ly7 cells were cultured (4 × 105 cells) and grew to the cell confluence at 70-80% in a 6-well plate. Then, OCI-Ly7 cells were transfected with shRNA-NC, shRNA-KDM5C#1, shRNA-KDM5C#2, Oe-NC, or Oe-HOXD3 using Lipofectamine® 2000 at 37°C for 1 hour, which were incubated in a complete medium for another 48 hours. All nucleic acids used for transfection were from Shanghai Genechem Co., Ltd.
Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
Total RNA isolated from DLBC cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was used as the template for reverse transcriptional cDNA using Transcript cDNA Synthesis kit (Beijing TransGen Co., Ltd.). Then, RT-qPCRs were conducted using the SYBR Premix Ex Taq kit (Beyotime) and detected in FTC-3000P real-time PCR system (Shanghai Fengling Biotechnology Co., Ltd.). The 2−ΔΔCq method was used to calculate the relative RNA levels of the targeted genes.17
Western Blot Analysis
DLBC cells were lysed with RIPA lysis buffer (Invitrogen; Thermo Fisher Scientific, Inc.), which was centrifuged to obtain the total proteins. Proteins (20 µg) were separated via 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane which was blocked with 5% non-fat milk for 2 hours at room temperature and incubated with KDM5C (ab259913, diluted 1/1000, Abcam), B-cell lymphoma 2 (Bcl2) (ab32124, diluted 1/1000, Abcam), Bax (ab32503, diluted 1/1000, Abcam), cleaved caspase 3 (ab32042, diluted 1/500, Abcam), cytochrome-C (cyto)-C (ab133504, diluted 1/5000, Abcam), p53 (ab32389, diluted 1/1000, Abcam), p21 (ab109520, diluted 1/1000, Abcam), HOXD3 (ab190648, diluted 1/1000, Abcam), and GAPDH antibody (ab9485, diluted 1/2500, Abcam) at 4°C overnight. The membranes were washed with TBST and incubated with a goat Anti-Rabbit IgG H&L (HRP) (ab6721, diluted 1/2000, Abcam) for 1 hour at room temperature. Finally, relative protein expression was analyzed by Image-Pro Plus software (version 6.0; Media cybernetics, Inc.).
Cell Counting Kit-8 Assay
The cell viability was detected by Cell Counting Kit-8 (CCK-8; cat. no. CK04; Dojindo, Japan). After OCI-Ly7 cells (5 × 104) were incubated at 24 hours, 48 hours, and 72 hours in 96-well plates, 10 μL CCK-8 solution was added to each well for another incubation for 2 hours. A microplate reader was applied to detect the absorbance at 450 nm.
EdU Staining
After transfection for 48 hours, 60 μL OCI-Ly7 cell suspension containing 105 cells were smeared on a glass slide. The air-dried OCI-Ly7 cells were fixed, permeabilized, and labeled with EdU using the BeyoClickTM EdU-488 Cell Proliferation Assay Kit (cat.no. C0071S, Beyotime). Then, OCI-Ly7 cells were treated with Hoechst 33342 (1 μg/ml) for 15 min at 25°C to stain the nuclei, which was observed by an inverted fluorescence microscope (Olympus, IX73, Tokyo, Japan).
Acridine Orange-ethidium Bromide (AO/EB) Staining
After transfection for 48 hours, OCI-Ly7 cells (4 × 105) after cultured in a 6-well plate for 24 hours were gently washed with phosphate buffered saline (PBS) and stained with 100 μL mixture of AO/EB dye (1:1). The apoptotic cells were observed and photographed immediately using an inverted fluorescence microscope (Olympus, IX73, Tokyo, Japan).
Dual-Luciferase Reporter Assay
Luciferase reporter plasmids were pGL3 luciferase vectors containing the wild-type (WT) or mutated (MUT) KDM5C (S1/S2) promoter region. Then, OCI-Ly7 cells were co-transfected with Oe-HOXD3 or Oe-NC and luciferase reporter plasmids with Lipofectamine® 2000, which were incubated at 37°C for 48 hours. A dual-luciferase assay kit (Promega) was applied to obtain the Firefly luciferase activity and Renilla luciferase activity.
Chromatin Immunoprecipitation (ChIP) Assay
Briefly, protein/DNA complexes were obtained from OCI-Ly7 cells, which were interacted with 1% formaldehyde for 15 min at 25°C. The samples were sonicated by an ultrasonic cell disrupter and obtained DNA fragments. Then the lysate was incubated with antibodies against HOXD3 or IgG overnight at 4°C. Immunoprecipitated DNAs were analyzed by RT-qPCR.
Statistical Analysis
The experimental data were in normal distribution after the Shapiro–Wilk test and were presented as mean ± standard deviation (SD) which were analyzed using GraphPad Prism 8.0 software. Difference analysis between two groups was detected by unpaired Student’s t-test and among multiple groups was detected by the method of ANOVA followed by Tukey’s test. P value < 0.05 was considered significant.
RESULTS
KDM5C Expression Was Up-Regulated in DLBC Cell Lines and Down-Regulation of KDM5C Inhibited the Proliferation of OCI-Ly7 Cells
KDM5C mRNA expression and protein expression in DLBCL cells were higher than that in GM12878 cells, and the highest expression was in OCI-Ly7 cells (Figure 1a-b). KDM5C expression was found to be down-regulated, and KDM5C expression in shRNA-KDM5C#1 group was lower than that in shRNA-KDM5C#2 group (Figure 1c and 1d). Down-regulation of KDM5C suppressed the viability (Figure 1e) and proliferation (Figure 1f) of OCI-Ly7 cells.
Figure 1.
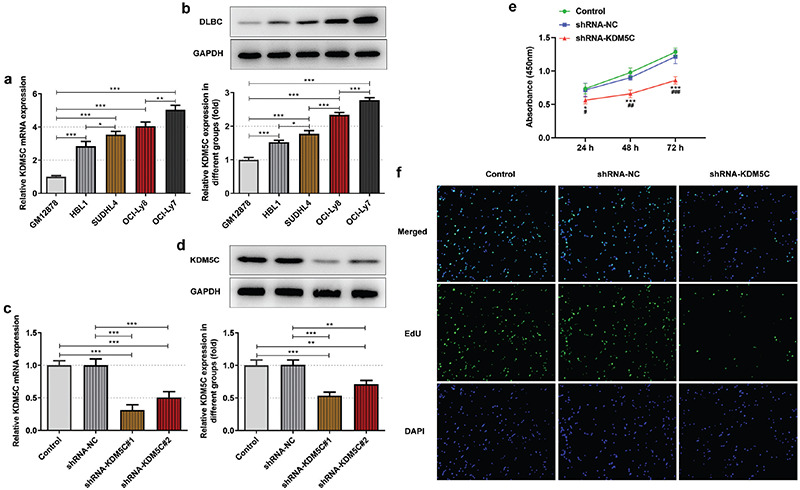
a-f. KDM5C expression was up-regulated in DLBC cell lines and down-regulation of KDM5C inhibited proliferation of OCI-Ly7 cells. (a/b) KDM5C mRNA expression and protein expression in DLBCL cells and GM12878 cells were, respectively, detected by RT-qPCR and Western blot. (c/d) KDM5C mRNA expression and protein expression in OCI-Ly7 cells transfected with shRNA-KDM5C#1/2 were, respectively, confirmed by RT-qPCR and Western blot. *P < .05, **P < .01 and ***P < .001. (e) The viability of OCI-Ly7 cells transfected with shRNA-KDM5C was analyzed by CCK-8 assay. *P < .05 and ***P < .001 vs. control group. ##P < .01 and ###P < .001 vs. shRNA-NC group. (f) The proliferation of OCI-Ly7 cells transfected with shRNA-KDM5C was analyzed by EdU staining. N = 3.
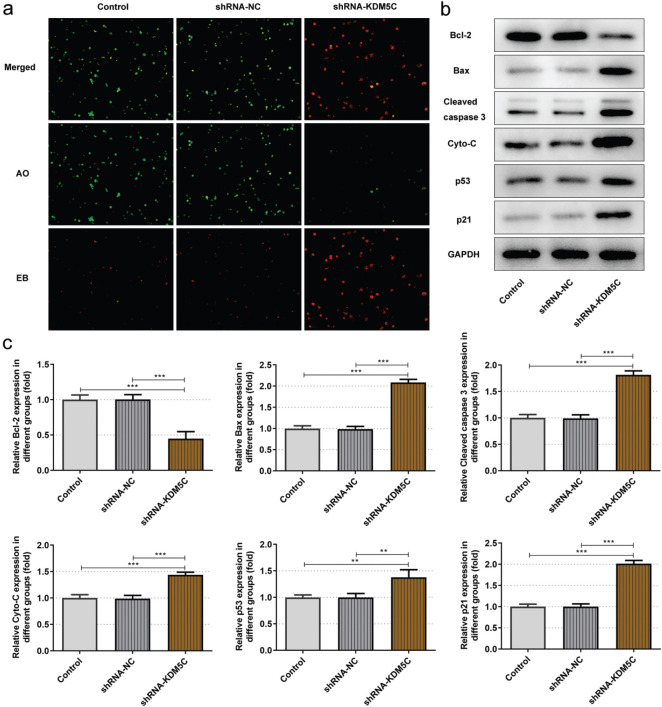
Down-Regulation of KDM5C Induced Apoptosis of OCI-Ly7 Cells and Up-Regulated p53 Expression
Down-regulation of KDM5C promoted the apoptosis of OCI-Ly7 cells (Figure 2a). Accordingly, down-regulation of KDM5C decreased the Bcl2 expression while increased the expression of Bax, cleaved caspase 3, cyto-C, p53, and p21 in OCI-Ly7 cells (Figure 2b-c).
Figure 2.
a-c. Down-regulation of KDM5C induced apoptosis of OCI-Ly7 cells and up-regulated p53 expression. (a) The apoptosis of OCI-Ly7 cells transfected with shRNA-KDM5C was analyzed by AO/EB staining. (b/c) The expression of apoptosis-related proteins in OCI-Ly7 cells transfected with shRNA-KDM5C was determined by Western blot. **P < .01 and ***P < .001. N = 3.
The Transcription Factor HOXD3 Activated the KDM5C Promoter
The binding sites between HOXD3 and KDM5C are shown in Figure 3a. HOXD3 mRNA and protein in OCI-Ly7 cells were also highly expressed in OCI-Ly7 cells compared with that in GM12878 cells (Figure 3b-c). HOXD3 mRNA and protein in OCI-Ly7 cells were up-regulated (Figure 3d-e). Relative luciferase activity was increased in OCI-Ly7 cells co-transfected with KDM5C (S1)-WT or KDM5C (S2)-WT and Oe-HOXD3 while not changed in OCI-Ly7 cells co-transfected with KDM5C (S1)-MUT or KDM5C (S2)-MUT and Oe-HOXD3 (Figure 3f). HOXD3 was confirmed to interact with KDM5C promoter (Figure 3g). shRNA-KDM5C transfection down-regulated the KDM5C mRNA expression and protein expression, which could be reversed by HOXD3 overexpression (Figure 3h-i).
Figure 3.
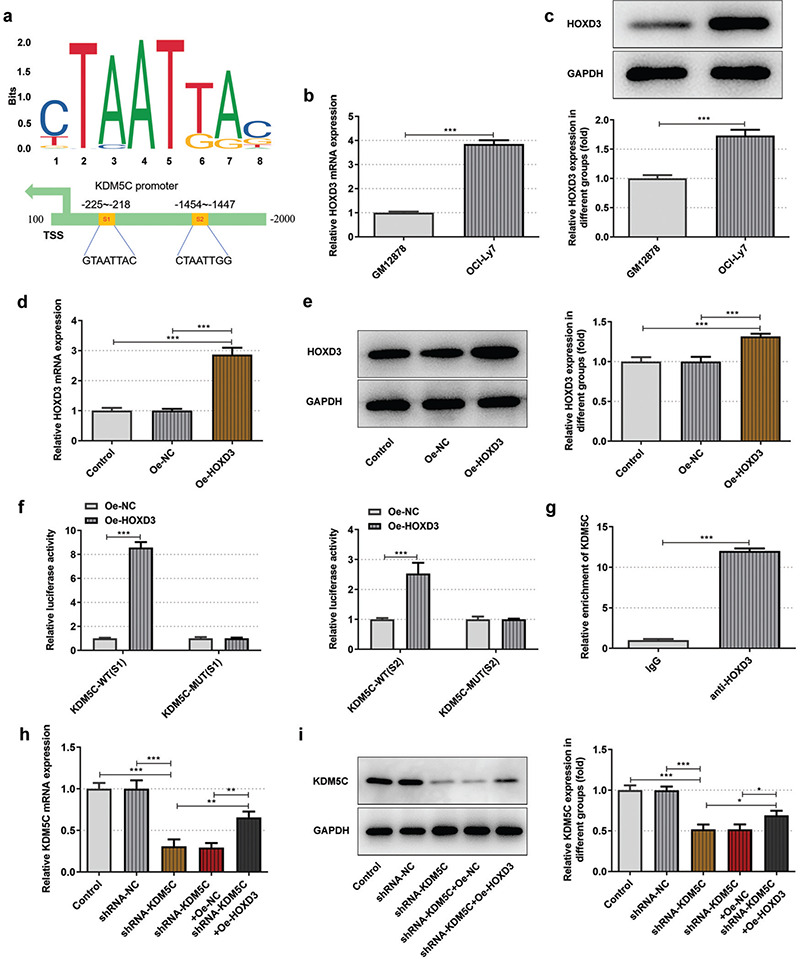
a-i. The transcription factor HOXD3 activated the KDM5C promoter. (a) The binding sites of HOXD3 and KDM5C. (b/c) HOXD3 mRNA expression and protein expression in OCI-Ly7 cells and GM12878 cells were respectively detected by RT-qPCR and Western blot. (d/e) HOXD3 mRNA expression and protein expression in OCI-Ly7 cells transfected with Oe-HOXD3 respectively confirmed by RT-qPCR and Western blot. (f) The interaction between HOXD3 and KDM5C was analyzed by dual-luciferase reporter assay. (g) The binding ability of HOXD3 to KDM5C promoter was detected by CHIP. (h/i) HOXD3 mRNA expression and protein expression in OCI-Ly7 cells transfected with shRNA-KDM5C and Oe-HOXD3 respectively confirmed by RT-qPCR and Western blot. *P < .05, **P < .01, and ***P < .001. N = 3.
High HOXD3 Expression Weakened the Regulating Effect of KDM5C Knockdown on the Proliferation and Apoptosis of OCI-Ly7 Cells
Down-regulation of KDM5C restrained the viability (Figure 4a) and proliferation (Figure 4b) and enhanced the apoptosis (Figure 4c) of OCI-Ly7 cells, which could be reversed by HOXD3 overexpression. In addition, high HOXD3 expression increased the Bcl2 expression while decreased the expression of Bax, cleaved caspase 3, cyto-C, p53, and p21 in shRNA-KDM5C transfected OCI-Ly7 cells (Figure 4d-e).
Figure 4.
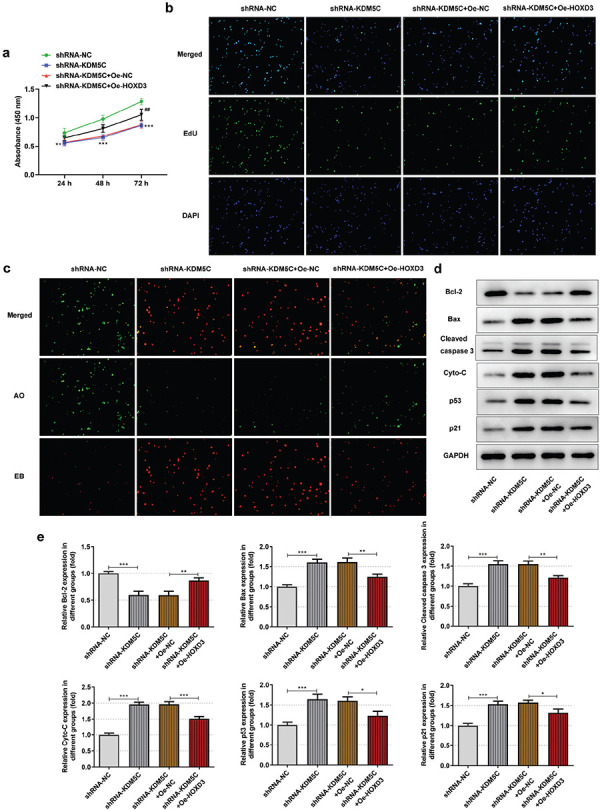
a-e. HOXD3 overexpression reversed the regulating effect of down-regulation of KDM5C on OCI-Ly7 cells. (a) The viability of OCI-Ly7 cells transfected with shRNA-KDM5C and Oe-HOXD3 was analyzed by CCK-8 assay. **P < .01 and ***P < .001 vs. control group. ##P < .01 vs. shRNA-KDM5C+Oe-NC group. (b) The proliferation of OCI-Ly7 cells transfected with shRNA-KDM5C and Oe-HOXD3 was analyzed by EdU staining. (c) The apoptosis of OCI-Ly7 cells transfected with shRNA-KDM5C and Oe-HOXD3 was analyzed by AO/EB staining. (d/e) The expression of apoptosis-related proteins in OCI-Ly7 cells transfected with shRNA-KDM5C and Oe-HOXD3 was determined by Western blot. *P < .05, **P < .01, and ***P < .001. N = 3.
DISCUSSION
The current study explored the potential role of KDM5C in DLBCL malignant progression. We demonstrated that KDM5C was up-regulated in DLBCL cells and down-regulation of KDM5C inhibited the viability and proliferation and enhanced the apoptosis of DLBCL cells. Using JASPAR prediction, luciferase activity assay, and ChIP assay, we discovered that HOXD3 could bind with KDM5C promoter in DLBCL cells. Finally, we confirmed that HOXD3 upregulating KDM5C promoted the malignant progression of DLBCL by decreasing p53 expression.
DLBCL is the most common clinically invasive B-cell lymphoma with significant clinical and molecular heterogeneity.18,19,20 Despite the fact that first-line use of R-CHOP immunochemotherapy regimens has significantly improved the rate of complete remission in DLBCL patients,21,22 about 30-40% of patients still recurred after treatment.23,24
KDM5C is a histone demethylase which can stimulate H3K4me3/me2 demethylation to H3K4me2/me1.25 The high expression of KDM5C is occurred in prostate cancer, breast cancer, ovarian cancer, and other tumor tissues and is involved in the regulation of tumor suppressor gene expression.7,26,27,28 Stein et al.7 have reported that down-regulation of KDM5C expression can lead to growth arrest of prostate cancer cells, suggesting that KDM5C can predict the tumor recurrence after prostate cancer resection. Xu et al6 have demonstrated that interference with KDM5C in gastric cancer cells can change H3K4 methylation in the promoter region of tumor suppressor gene p53 and reduce its proliferation. Wang et al.29 have found that interfering with KDM5C in breast cancer cells can also change the H3K4 methylation state in the promoter region of tumor suppressor gene BRMS1 and reduce the ability of cell metastasis. Here, KDM5C expression was found to be increased in DLBCL cells compared with that in human B lymphocytes. Furthermore, knockdown of KDM5C obviously restrained the viability and proliferation while stimulated the apoptosis of OCI-Ly7 cells.
HOXD3 can regulate cell proliferation and differentiation in the body. In recent years, it has been found that HOXD3 can promote the occurrence and development of different malignant tumors including breast, lung, and colorectal cancers.30,31,32 Chen et al.30 have found that HOXD3 expression is elevated in colorectal cancer tissues, and up-regulation of HOXD3 expression in colorectal cancer cells can significantly promote cell cycle progression and proliferation and reduce cell apoptosis, suggesting the pro-proliferation effect of HOXD3 on colorectal cancer cells. Wang et al.33 have reported that miR-203a can inhibit the proliferation and promote apoptosis of hepatocellular carcinoma cells by down-regulating HOXD3 expression. Our data revealed that HOXD3 was highly expressed in OCI-Ly7 cells and not in human B lymphocytes. And, HOXD3 could interact with KDM5C promoter to promote the KDM5C expression, thereby reversing the effects of KDM5C knockdown on OCI-Ly7 cells. However, how HOXD3 regulate the proliferation and apoptosis of DLBCL cells is worthy of being explored.
p53 is encoded by a tumor suppressor gene WT-TP53 and can monitor the integrity of cell DNA, participate in DNA repair, mediate cell cycle, block apoptosis, and prevent cell autophagy from malignant transformation.34,35 The inactivation of p53 in cells leads to the occurrence of cancer. Mutations in the TP53 gene are the main mechanism of p53 inactivation, followed by shadow noise of other proteins or non-coding RNA.36 Similar to the pro-apoptotic factor and transcriptional regulator in vivo, TP53 regulates apoptosis by activating downstream Bax, DR-5, and caspase. Mutation or overexpression of p53 exists in more than 50% of human tumors. TP53 mutation is related to the occurrence, development, and prognosis of lymphoma.37,38 The mutation rate of TP53 in DLBCL was 20%.39 The 3-year DFS and DSS of DLBCL patients in the MUT-TP53 group were significantly shortened compared with the WT-TP53 group.40 p53-mediated apoptosis pathway is an important way for p53 to exert the function of tumor suppressor gene, which can directly transcriptionally activate the expression of PUMA and then interact with the Bcl-2/Bax complex to induce the release of cyto-C in the mitochondrial membrane space and activate the caspase level. Apoptosis is eventually performed by cleaved caspase-3.41 In this study, p53 expression was increased in KDM5C knockdown OCI-Ly7 cells, which activated the expression of proapoptotic proteins (Bax, cleaved caspase 3, and cyto-C) and suppressed the antiapoptotic protein (Bcl2) expression. The above changes could be reversed by HOXD3 overexpression. However, whether there is a binding site between KDM5C and p53 that needs to be investigated and whether HOXD3 can regulate p53 expression directly remain unknown, which will be explored in the future.
p21 is a cell cycle suppressor with kinase inhibitory activity and belongs to the adult family of kinase inhibitors. It mainly participates in the negative regulation of cell cycle by down-regulating the activity of cyclin-dependent kinase (CDK).42,43 The expression of p21 can be induced by p53, which is an important downstream factor of p53 and can also regulate the cell cycle.44 p53 can activate the promoters of downstream target genes and is involved in tumor proliferation, apoptosis, DNA damage, and other important processes. Its regulation of cell senescence is accomplished through its target gene p21.45 This study showed that p21 expression was increased in KDM5C knockdown OCI-Ly7 cells, while opposite results were detected in cells over-expressing HOXD, which was regulated by p53 expression.
There is a limitation in this study. First, the study is only basing on the cell experiment. Second, only one DLBCL cell line is used to explore the role of KDM5C in DLBCL. Third, we have not demonstrated the problems: the regulated role of HOXD3 in DLBCL cells, whether there is a binding site between KDM5C and p53 and whether HOXD3 can regulate p53 expression directly.
In conclusion, HOXD3 up-regulating KDM5C could promote the malignant progression of DLBL by decreasing p53 expression, which would provide the fundamental basis for the gene targeting treatment.
Footnotes
Ethics Committee Approval: N/A.
Patient Consent for Publication: N/A.
Data Sharing Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author Contributions: Design - C.W., H.Q.; Data Collection and/or Processing - Y.C., C.L., Y.W., D.Z.; Analysis and/or Interpretation - Y.C., C.L., Y.Y.; Writing - Y.C., C.L.
Conflict of Interest: The authors have no conflicts of interest to declare.
Funding: Scientific Research Fund of Heilongjiang Provincial Education Department (No.2018-KYYWF-0932); North Medicine and Functional Food Characteristic Subject Project in Heilongjiang Province (No.2018-TSXK-02); Innovation and Entrepreneurship Training Program Project of College Students in Heilongjiang Province (No. 201910222086).
References
- 1.Miao Y, Medeiros LJ, Li Y, Li J, Young KH. Genetic alterations and their clinical implications in DLBCL. Nat Rev Clin Oncol. 2019;16:634–652. doi: 10.1038/s41571-019-0225-1. [DOI] [PubMed] [Google Scholar]
- 2.Crombie J. Classifying DLBCL subtypes for optimal treatment. Oncology. 2019;33:686504. [PubMed] [Google Scholar]
- 3.Crombie JL, Armand P. Diffuse large B-cell lymphoma and high-grade B-cell lymphoma: genetic classification and its implications for prognosis and treatment. Hematol Oncol Clin North Am. 2019;33:575–585. doi: 10.1016/j.hoc.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Li S, Young KH, Medeiros LJ. Diffuse large B-cell lymphoma. Pathology. 2018;50:74–87. doi: 10.1016/j.pathol.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Denis H, Van Grembergen O, Delatte B, et al. MicroRNAs regulate KDM5 histone demethylases in breast cancer cells. Mol Biosyst. 2016;12:404–413. doi: 10.1039/c5mb00513b. [DOI] [PubMed] [Google Scholar]
- 6.Xu L, Wu W, Cheng G, et al. Enhancement of proliferation and invasion of gastric cancer cell by KDM5C via decrease in p53 expression. Technol Cancer Res Treat. 2017;16:141–149. doi: 10.1177/1533034616629261. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Stein J, Majores M, Rohde M, et al. KDM5C is overexpressed in prostate cancer and is a prognostic marker for prostate-specific antigen-relapse following radical prostatectomy. Am J Pathol. 2014;184:2430–2437. doi: 10.1016/j.ajpath.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Wei G, Deng X, Agarwal S, Iwase S, Disteche C, Xu J. Patient mutations of the intellectual disability gene KDM5C downregulate netrin G2 and suppress neurite growth in Neuro2a cells. J Mol Neurosci. 2016;60:33–45. doi: 10.1007/s12031-016-0770-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Outchkourov NS, Muiño JM, Kaufmann K, et al. Balancing of histone H3K4 methylation states by the Kdm5c/SMCX histone demethylase modulates promoter and enhancer function. Cell Rep. 2013;3:1071–1079. doi: 10.1016/j.celrep.2013.02.030. [DOI] [PubMed] [Google Scholar]
- 10.Rondinelli B, Schwerer H, Antonini E, et al. H3K4me3 demethylation by the histone demethylase KDM5C/JARID1C promotes DNA replication origin firing. Nucleic Acids Res. 2015;43:2560–2574. doi: 10.1093/nar/gkv090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu H, Lin R, Zheng F, Zhang Q. ELK1 activated-long noncoding RNA LBX2-AS1 aggravates the progression of ovarian cancer through targeting miR-4784/KDM5C axis. J Mol Histol. 2021;52:31–44. doi: 10.1007/s10735-020-09921-5. [DOI] [PubMed] [Google Scholar]
- 12.Hong Z, Wu G, Xiang ZD, et al. KDM5C is transcriptionally regulated by BRD4 and promotes castration-resistance prostate cancer cell proliferation by repressing PTEN. Biomed Pharmacother. 2019;114:108793. doi: 10.1016/j.biopha.2019.108793. [DOI] [PubMed] [Google Scholar]
- 13.Yang GJ, Ko CN, Zhong HJ, Leung CH, Ma DL. Structure-based discovery of a selective KDM5A inhibitor that exhibits anti-cancer activity via inducing cell cycle arrest and senescence in breast cancer cell lines. Cancers. 2019;11:92. doi: 10.3390/cancers11010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Gao Y, Zhao X, et al. HOXD3 was negatively regulated by YY1 recruiting HDAC1 to suppress progression of hepatocellular carcinoma cells via ITGA2 pathway. Cell Prolif. 2020;53:e12835. doi: 10.1111/cpr.12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang S, Gao Z, Yang Y, et al. miR-99b-3p is induced by vitamin D3 and contributes to its antiproliferative effects in gastric cancer cells by targeting HoxD3. Biol Chem. 2019. doi: 10.1515/hsz-2019-0102. [DOI] [PubMed] [Google Scholar]
- 16.Yang MH, Zhao L, Wang L, et al. Nuclear lncRNA HOXD-AS1 suppresses colorectal carcinoma growth and metastasis via inhibiting HOXD3-induced integrin β3 transcriptional activating and MAPK/AKT signalling. Mol Cancer. 2019;18:31. doi: 10.1186/s12943-019-0955-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Al-Hamadani M, Habermann TM, Cerhan JR, Macon WR, Maurer MJ, Go RS. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: a longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol. 2015;90:790–795. doi: 10.1002/ajh.24086. [DOI] [PubMed] [Google Scholar]
- 19.Chan A, Dogan A. Prognostic and predictive biomarkers in diffuse large B-cell lymphoma. Surg Pathol Clin. 2019;12:699–707. doi: 10.1016/j.path.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan Y, Brem EA. Considerations for the treatment of diffuse large B cell lymphoma in the elderly. Curr Hematol Malig Rep. 2019;14:228–238. doi: 10.1007/s11899-019-00519-7. [DOI] [PubMed] [Google Scholar]
- 21.Pfreundschuh M, Kuhnt E, Trümper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12:1013–1022. doi: 10.1016/S1470-2045(11)70235-2. [DOI] [PubMed] [Google Scholar]
- 22.Pfreundschuh M, Trümper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 23.Vardhana SA, Sauter CS, Matasar MJ, et al. Outcomes of primary refractory diffuse large B-cell lymphoma (DLBCL) treated with salvage chemotherapy and intention to transplant in the rituximab era. Br J Haematol. 2017;176:591–599. doi: 10.1111/bjh.14453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–5033. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 25.Klose RJ, Kallin EM, Zhang Y. JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet. 2006;7:715–727. doi: 10.1038/nrg1945. [DOI] [PubMed] [Google Scholar]
- 26.Patani N, Jiang WG, Newbold RF, Mokbel K. Histone-modifier gene expression profiles are associated with pathological and clinical outcomes in human breast cancer. Anticancer Res. 2011;31:4115–4125. [PubMed] [Google Scholar]
- 27.Sun YX, Zhang YX, Zhang D, et al. XCI-escaping gene KDM5C contributes to ovarian development via downregulating miR-320a. Hum Genet. 2017;136:227–239. doi: 10.1007/s00439-016-1752-9. [DOI] [PubMed] [Google Scholar]
- 28.Kaiser J. Genes link epigenetics and cancer. Science. 2010;330:577. doi: 10.1126/science.330.6004.577. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q, Wei J, Su P, Gao P. Histone demethylase JARID1C promotes breast cancer metastasis cells via down regulating BRMS1 expression. Biochem Biophys Res Commun. 2015;464:659–666. doi: 10.1016/j.bbrc.2015.07.049. [DOI] [PubMed] [Google Scholar]
- 30.Chen F, Sun G, Peng J. RNAi-mediated HOXD3 knockdown inhibits growth in human RKO cells. Oncol Rep. 2016;36:1793–1798. doi: 10.3892/or.2016.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang T, Yang J, Cai YD. Novel candidate key drivers in the integrative network of genes, microRNAs, methylations, and copy number variations in squamous cell lung carcinoma. BioMed Res Int. 2015;2015:358125. doi: 10.1155/2015/358125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamada Ji J, Omatsu T, Okada F, et al. Overexpression of homeobox gene HOXD3 induces coordinate expression of metastasis-related genes in human lung cancer cells. Int J Cancer. 2001;93:516–525. doi: 10.1002/ijc.1357. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Sun H, Wang X, et al. EGR1 mediates miR-203a suppress the hepatocellular carcinoma cells progression by targeting HOXD3 through EGFR signaling pathway. Oncotarget. 2016;7:45302–45316. doi: 10.18632/oncotarget.9605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 35.Whibley C, Pharoah PD, Hollstein M. p53 polymorphisms: cancer implications. Nat Rev Cancer. 2009;9:95–107. doi: 10.1038/nrc2584. [DOI] [PubMed] [Google Scholar]
- 36.Boutelle AM, Attardi LD. p53 and tumor suppression: it takes a network. Trends Cell Biol. 2021;31:298–310. doi: 10.1016/j.tcb.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu-Monette ZY, Young KH. The TP53 tumor suppressor and autophagy in malignant lymphoma. Autophagy. 2012;8:842–845. doi: 10.4161/auto.19703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu-Monette ZY, Wu L, Visco C, et al. Mutational profile and prognostic significance of TP53 in diffuse large B-cell lymphoma patients treated with R-CHOP: report from an International DLBCL Rituximab-CHOP Consortium Program Study. Blood. 2012;120:3986–3996. doi: 10.1182/blood-2012-05-433334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young KH, Weisenburger DD, Dave BJ, et al. Mutations in the DNA-binding codons of TP53, which are associated with decreased expression of TRAILreceptor-2, predict for poor survival in diffuse large B-cell lymphoma. Blood. 2007;110:4396–4405. doi: 10.1182/blood-2007-02-072082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peroja P, Pedersen M, Mantere T, et al. Mutation of TP53, translocation analysis and immunohistochemical expression of MYC, BCL-2 and BCL-6 in patients with DLBCL treated with R-CHOP. Sci Rep. 2018;8:14814. doi: 10.1038/s41598-018-33230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen J. The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Cold Spring Harb Perspect Med. 2016;6:a026104. doi: 10.1101/cshperspect.a026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valente LJ, Gray DH, Michalak EM, et al. p53 efficiently suppresses tumor development in the complete absence of its cell-cycle inhibitory and proapoptotic effectors p21, Puma, and Noxa. Cell Rep. 2013;3:1339–1345. doi: 10.1016/j.celrep.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 43.Huang SY, Hsieh MJ, Chen CY, et al. Epstein-Barr virus Rta-mediated transactivation of p21 and 14-3-3σ arrests cells at the G1/S transition by reducing cyclin E/CDK2 activity. J Gen Virol. 2012;93:139–149. doi: 10.1099/vir.0.034405-0. [DOI] [PubMed] [Google Scholar]
- 44.Jiang L, Zhang X, Xiang C, et al. Differential cellular localization of CELSR2 and ING4 and correlations with hormone receptor status in breast cancer. Histol Histopathol. 2018;33:835–842. doi: 10.14670/HH-11-979. [DOI] [PubMed] [Google Scholar]
- 45.Rufini A, Tucci P, Celardo I, Melino G. Senescence and aging: the critical roles of p53. Oncogene. 2013;32:5129–5143. doi: 10.1038/onc.2012.640. [DOI] [PubMed] [Google Scholar]