Abstract
Canine rabies poses a significant risk to humans and animals in Nigeria. However, the lack of reliable tools to evaluate the performance of existing canine rabies control programs to inform public health policy decisions poses a severe obstacle. We obtained canine rabies surveillance data from the National Veterinary Research Institute (NVRI) and supplemented these data with rabies diagnoses reported in the published studies from Nigeria. To uncover contextual factors (i.e., environmental and sociodemographic) associated with canine rabies evidence at the Local Government Area (LGA) level, we classified LGAs in Nigeria into four categories based on evidence availability (i.e., LGAs with NVRI data or published studies, both, or no evidence). We described the geographical and temporal variation in coverage. We fitted a multinomial regression model to examine the association between LGA level canine rabies evidence and potential sociodemographic and ecological determinants of canine rabies evidence. The effective annual testing during the 19 years was less than one dog/100,000 Nigerian resident-year. Our results showed that 58% of Nigerian LGAs (450/774) had not been targeted by the existing national rabies surveillance or studies on rabies, including ten states capitals with high human populations. While 16% (122/774) of Nigerian LGAs concentrated in Taraba, Adamawa, and Abia had canine rabies evidence from published studies, none of these LGAs was represented in the NVRI rabies surveillance data. We also observed an increasing trend in rabies evidence over time towards the eastern part of Nigeria. Our multinomial regression model indicated that education level, poverty, population density, land use and temperature were significantly associated with canine rabies evidence at the LGA level. This study underscores the value of combining canine rabies evidence from different sources to better understand the current disease situation for targeted intervention.
Keywords: Disparity, Environmental factors, Epidemiology, Nigeria, Rabies, Lyssavirus, Socioeconomic levels, Zoonosis
Highlights
-
•
This study shows the value of combining different evidence sources to advocate for public health interventions.
-
•
Educational level, poverty, population density, land use and temperature were determinants of evidence of canine rabies.
-
•
Such evidence demonstrates the need to expand Nigeria's rabies laboratory-based surveillance to support rabies prevention.
1. Introduction
Globally, tens of millions of exposures and thousands of human deaths are attributed to rabies annually. Africa and Asia have the highest burdens, with over 95% of rabies-associated morbidity and mortality attributed to domestic dog encounters[1,2]. The 2030 Sustainable Development Goal 3 called for improvements in health to end the epidemics of neglected tropical diseases [3]. Tripartite leadership of the Food and Agriculture Organization of the United Nations (FAO), World Organization for Animal Health (OIE) and the World Health Organization (WHO) partnered to support endemic countries towards eliminating canine-mediated rabies by 2030 [4]. A One Health approach is envisioned to improve the quality of data on the occurrence of rabies in humans and animals, provide better access to postexposure prophylaxis (PEP) for dog bite victims, enhance community education, and undertake mass dog vaccination. The Stepwise Approach towards Rabies Elimination (SARE) was designed to allow rabies endemic countries to objectively measure their strengths and weaknesses, using available canine rabies evidence with scores between 0 and 5. Zero for canine rabies endemic countries and five signifying freedom from canine rabies [[5], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32]]. Several countries within the Pan-African Rabies Control Network (PARACON), such as Sierra Leone, Angola, Burkina Faso, Niger, Guinea-Bissau have a score of zero. In contrast, Namibia has the highest score of 2.5 [6]. The lack of health care infrastructure investment in vulnerable communities may result in unreported cases and unavailability of PEP for human populations at risk, consequently impacting an efficient evaluation of the progress made towards rabies prevention and control.
Efficient monitoring of rabies programs depends upon gathering accurate information on disease occurrence and regular evaluation of the representativeness of surveillance efforts for cost-effective interventions. Since the first reported human case from Nigeria in 1912, rabies has remained a significant public health problem [7]. However, in many endemic countries, the lack of reliable canine rabies evidence to evaluate the performance of the existing control program poses a significant obstacle to understanding the disease burden for targeted interventions. The current SARE score for Nigeria is 1.5, implying a lack of reliable evidence is hampering the development of a national rabies control program [5]. This figure is likely to vary across the country, and a recent scoping review unveiled substantial obstacles in developing cost-effective risk-based interventions [7].
With regional and state laboratories across Nigeria, the National Veterinary Research Institute (NVRI) is the foremost institution responsible for routine rabies testing for all suspect animals [8]. Existing rabies surveillance information can provide baseline data to measure progress towards rabies prevention and control, identify weaknesses and strengths, and make evidence-based recommendations to policymakers. However, the opportunistic nature of passive surveillance data, primarily associated with sampling design, means that surveillance data alone cannot provide a robust understanding of canine rabies for cost-effective management. For example, enhanced surveillance evidence from several published articles has been used to augment passive surveillance data to better understand the origins of rabies, its distribution and associated factors in Indonesia [9]. The commission on social determinants of health opined that social factors might influence health disparities [10]. In a recent study in El Salvador, locations prone to violence had inadequate reporting of canine rabies, with gang activities attributed as barriers to effective surveillance [11]. In Zambia, a study that used surveillance data found that regions with more evidence of dog bite incidence reported more canine rabies [12]. Areas without surveillance and control might become high-risk zones for rabies virus perpetuation. Therefore, it is crucial to understand the current scope of institutional rabies surveillance efforts combined with primary research studies and evaluate the determinants of such coverage to inform public health policy decisions in underserved communities.
This study aimed to evaluate geographical and temporal disparities in canine rabies evidence in Nigeria and quantify ecological and socioeconomic factors that might limit community access to public health services.
2. Materials and methods
2.1. Geographical classification of canine rabies evidence from NVRI and primary investigation studies
The NVRI is a parastatal organization under the Federal Ministry of Agriculture and Rural Development. It is the Nigerian institution in charge of canine rabies surveillance and diagnosis. The NVRI was established in 1913 to provide disease surveillance and diagnosis for animals and, recently, for humans (e.g., COVID-19) across the country [8]. The NVRI manages rabies surveillance in Nigeria through multiple outstations and the central diagnostic laboratory. This network consists of six zonal and eighteen state laboratories headed by a veterinarian responsible for surveillance activities across the Federal Capital Territory (FCT) and the 36 states of Nigeria [13]. All animals suspected of being rabid (e.g., animals with sudden and severe behavioural changes or that had bitten an individual without provocation) were euthanized and either submitted to a zonal or state laboratory for onward submission to the central diagnostic laboratory at Plateau State or sent directly [14]. As one limitation, either community individuals or veterinarians often bore the travel cost for samples submitted either to zonal laboratories or central diagnostic laboratories. Upon reaching the central diagnostic laboratory, the brain was extracted and subjected to Sellers stain for detection of Negri bodies [15] between 2000 and 2009 or the direct fluorescent antibody testing (DFAT) following OIE guidelines between 2009 and 2018 [16]. This study utilized all available canine rabies surveillance data recorded in hard copy at the central diagnostic laboratory of the NVRI. Canine rabies surveillance data were collected in a case report form between 2000 and 2018 (i.e., 19 years of time-series data). The dataset contained information on the street address of the origin of the offending dog/where the attack happened. We retrieved and digitalized these data into a consolidated Microsoft Excel database.
Because people must pay for sample submission, our preliminary analyses of NVRI surveillance data indicated that the data was biased towards locations close to the central laboratory. To evaluate factors associated with potential geographical biases in the NVRI canine rabies surveillance data, we supplemented official NVRI data with rabies data from systematically searched, peer-reviewed research articles on dog and human rabies involving dogs from Nigeria and published between 1990 and 2020. The detailed research protocol and the results of this systematic review were described previously [7]. In brief, we systematically reviewed published articles on rabies from Nigeria using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to search (PubMed, Web of Science, Scopus, Google scholar) and identify published articles on canine rabies across Nigeria.
We extracted information on areas where the study was conducted, determined the sample sources (where available), and contacted authors for information on sample sources where the details were not available in the text. Details of identified studies, including institutions where the study was designed (mostly different from samples sources), the number of samples collected, and the test outcome using a combination of diagnostic protocols consistent with the OIE guidelines are presented in Supplementary information file 1 S1 Table. All studies except one [17] used the current OIE recommended tests for rabies (Supplementary information file 1 S1 Table). To investigate the geographical distribution of rabies evidence across Nigerian LGAs, we overlaid the evidence from NVRI rabies surveillance and published studies onto a population density 30 arc-second (~1 km at the equator) resolution raster from the World Pop project site [18].
2.2. Environmental, socioeconomic, and epidemiological variables
Nigeria is divided into six geopolitical zones, with 774 Local Government Areas (LGAs). For this study, LGAs were considered as the spatial unit of analysis. Information on the type of rabies evidence and determinants of the type of evidence were aggregated to the LGA level. Rabies surveillance generally exhibit clustered distributions reflecting geo-temporal variation in the presence and density of susceptible and infected hosts and associated contact rates, topographic, climatic, and sociocultural factors and distribution of disease prevention and control factors [19,20]. Therefore, the availability of evidence was likely to depend on variables such as land cover types, elevation, education, poverty distribution, and public access to veterinary and health care. For this study, we obtained a poverty raster map of the proportion of people per grid square living in poverty in Nigeria at approximately 1 km resolution [21]; a literacy raster map showing the proportion of men and women aged 15–49 per grid square as literate in Nigeria at approximately 1 km resolution [22]; an urban extent grid (v.1) showing the proportion of rural and urban areas in the LGA, extracted from the Global Rural-Urban Mapping Project (GRUMP v.1, [23]; and population density 30 arc-second (~1 km at the equator) resolution from the World Pop project (https://www.worldpop.org/about). Elevation (at 10-m resolution) and mean temperature data were extracted from WorldClim (v.2). To obtain a proxy for access to dog vaccination (with the assumption that distance to the veterinary hospital was inversely related to vaccine access), we geolocated veterinary hospitals/clinics in Nigeria using Google Earth and calculated Euclidean distance from the nearest veterinary clinic (https://www.google.com/earth/) and distance to road networks (mean distance in meters) from DIVA-GIS, because, in Nigeria, dogs are generally vaccinated at veterinary hospitals/clinics. Land cover types were obtained from DIVA-GIS. We retrieved Human Influence Index grids [24], using a Global Grid of Probabilities of Urban Expansion to 2030 at a 2.5 arc-minute resolution [25]. Zonal mean values for each raster dataset were obtained at each LGA polygon using the Zonal Statistics module in the Spatial Analyst toolbox in ArcGIS software and stored in Microsoft Excel for further analysis.
2.3. Statistical analysis and variable selection
Our outcome of interest was defined by the existence of evidence of canine rabies from either the NVRI surveillance and/or published studies. This outcome was a categorical variable that classified the level of canine rabies evidence at the LGA level into four separate categories. An LGA was classified “0” were no rabies cases was reported neither from NVRI nor published studies; “1” when the LGA had canine rabies evidence from published articles only; “2” when the LGA had canine rabies evidence from NVRI only; and “3” when canine rabies evidence was reported by both NVRI and published studies. The polygon shapefile of the Nigerian LGA (that reported categories 0–3) was overlaid onto raster maps of sociodemographic and environmental variables, and information was extracted to each LGA polygon. The design matrix contained ecological, epidemiological, and sociodemographic variables (independent variables) that might explain the LGA level canine rabies evidence. We performed univariable multinomial logistic regression to select factors associated with different levels of canine rabies evidence across the country. We assessed multicollinearity and removed highly collinear variables (all correlation coefficient (r) < 0.6), that had the highest regression p-value or less epidemiological relevance. The LGAs without reported data (i.e., neither NVRI nor published evidence) were set as the reference category. All significant variables based on a liberal p-value (p < 0.2) in the univariable analysis were entered into the full additive multivariable multinomial model. The variable selection was conducted using a backward stepwise approach. We assessed confounding by checking the changes for the remaining coefficients when the target variable was removed from the model. If the coefficients for one or more of the remaining variables changed by more than 25% when the target variable was removed, then it was considered a confounder and retained in the model. Variables included in the final multivariable multinomial model with a p-value of <0.05 were deemed significant. Statistical software Stata version 15.1 (Stata Corporation, College Station, TX, U.S.A.) was used for the data analysis.
2.4. Residual spatial cluster analysis
To assess whether the variables in the final multivariable multinomial model accounted for the observed spatial autocorrelation in the LGA-level pattern of canine rabies evidence rabies in Nigeria, we carried out a cluster analysis of Pearson residuals extracted from the final multinomial model. We performed a global clustering test of residual evidence for rabies occurrence using Moran's I index [26].We used a spatial weight matrix to specify the spatial relationship of LGA. Neighbours were defined using inverse distance. Moran's negative values indicate overdispersion (neighboring LGAs are more dissimilar), and positive values indicate clustering (neighboring LGAs are similar), while zero corresponds to a spatially random distribution [26].
3. Results
3.1. Descriptive analysis and spatio-temporal distribution of canine rabies evidence in Nigeria
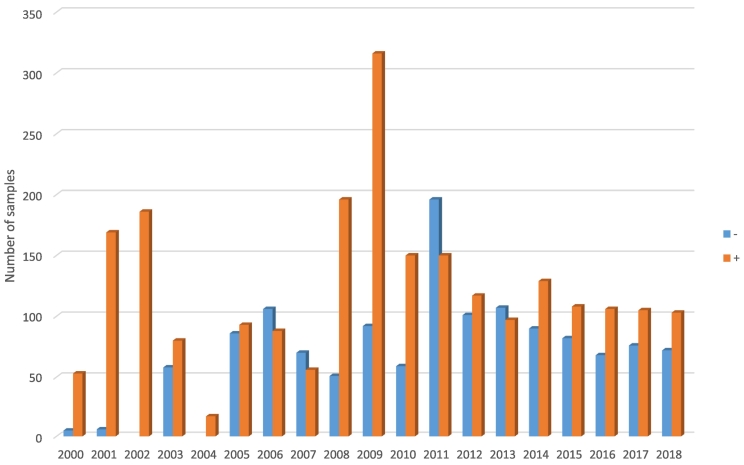
Between January 2000 and December 2018, a total of 3644 samples were submitted to NVRI. During the 19 years, less than one dog/100,000 Nigerians resident-year was submitted and processed. Most samples were offered by veterinarians, veterinary clinics, or outstations except 9% (314/3644) that community members submitted. The majority (64%, 2319/3644) of samples tested positive for rabies. Sellers stain was the diagnostic protocol between 2000 and 2004; in 2005, NVRI staff members were trained on DFAT, and a final switch to DFAT happened in 2009. Sample cases increased over the years, with a higher proportion of positive cases between 2001 (97%,169/175) and 2002 (100%,186/186). There were only 17 submissions during 2004, after which there was variation in positive and negative submissions, with peak submission occurring in 2009 at a total of 408 submissions and a steady decline afterwards (Fig. 1). Monthly trends indicated confirmed canine rabies cases peaked during April and August (Fig. 2).
Fig. 1.
Temporal distribution of submissions to NVRI for canine rabies testing and diagnostic test results.
Fig. 2.
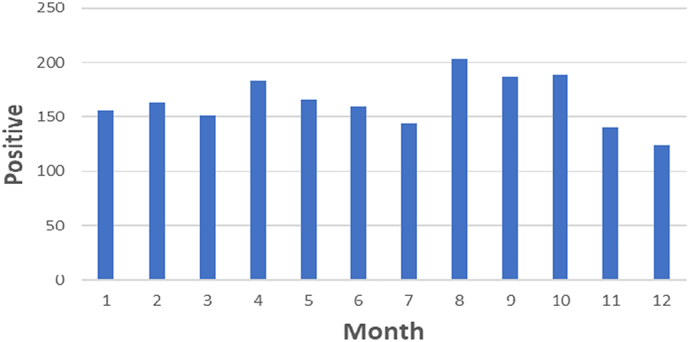
Monthly distribution of confirmed rabies cases between 2000 and 2018 with peak submissions in April and August.
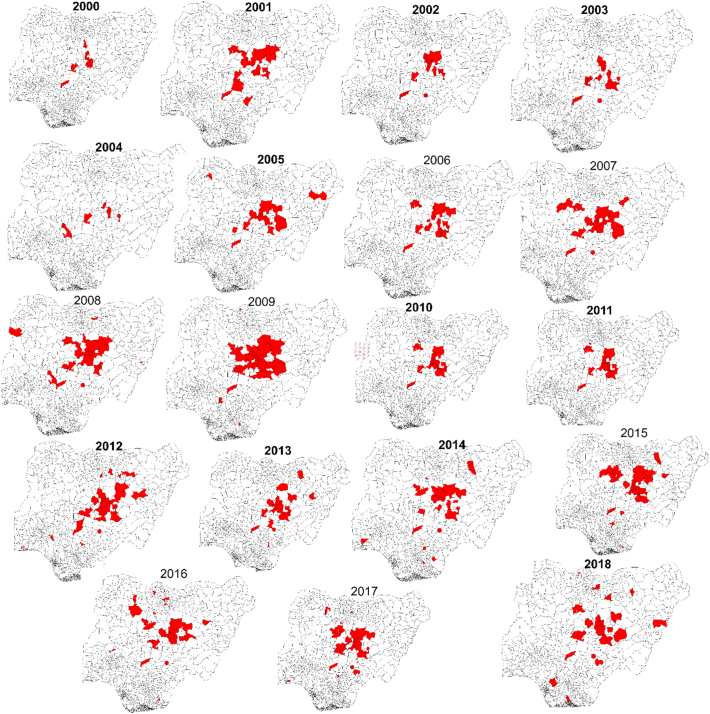
We observed spatiotemporal variation in confirmed canine rabies cases. Initially cases were concentrated in the central states, with gradual expansion between 2005 and 2008 when DFAT began to replace Sellers stain for rabies diagnosis (Fig. 3). Between 2009 and 2014, when DFAT was fully implemented at NVRI, confirmed cases expanded towards the southwest of Nigeria, especially in 2014 (Fig. 3). The trend of expanding evidence continued between 2015 onwards towards south-south and southeast LGAs, especially in 2018 (Fig. 3). Nevertheless, most LGAs (58%, 450/774), had no submission from NVRI surveillance nor published studies, notably ten state capitals with high human populations including South West (Osun, Ekiti, Oyo), South-East (Anambra, Imo), Northcentral (Niger), North East (Yobe, Adamawa) and North West (Sokoto Katsina) (Fig. 4). About 16% (122/774) of LGAs had evidence of rabies occurrence from published studies but were not captured by NVRI surveillance data, notably in Taraba, Adamawa, Benue, and Abia (Fig. 4). About 13% (97/774) of LGAs had rabies evidence from NVRI surveillance data only, mostly in Plateau, Kaduna, and Bauchi States. Published studies generally sampled dogs at different slaughter points across Nigeria. Such studies on dog rabies testing started in 1999 with a single survey conducted each year until 2010, when two studies were carried out. These continued to increase significantly during 2013 (four studies), 2014 (five studies), and 2019 (four studies)—Fig. 5.
Fig. 3.
Spatio-temporal variation in canine rabies evidence (confirmed positive cases submitted to NVRI) across Nigeria between 2000 and 2018. Evidence was generally concentrated in the central states and expanded to other regions over the years, especially between 2005 and 2018.
Fig. 4.
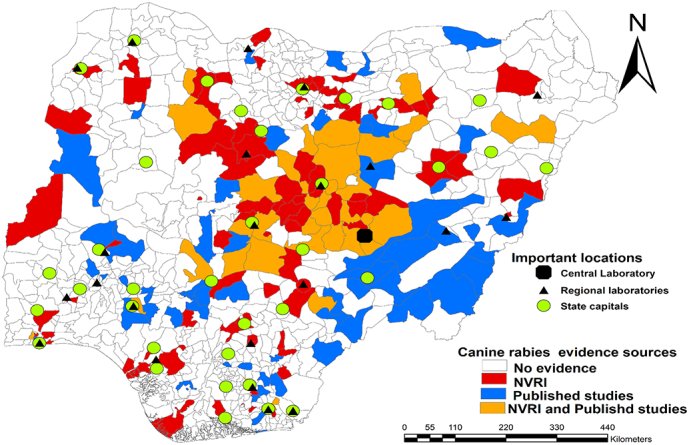
Spatial distribution of canine rabies evidence from NVRI data and published studies. A vast majority of local government areas (LGAs) are without observed canine rabies evidence, notably ten states capitals. The LGAs with evidence from published studies only are scattered across different states but concentrated in northeastern states of Taraba, Adamawa, and Abia. The map was created using ArcMap software (ESRI Inc., Redlands, CA, USA). The shapefile was retrieved from DIVA-GIS (https://www.diva-gis.org/).
Fig. 5.
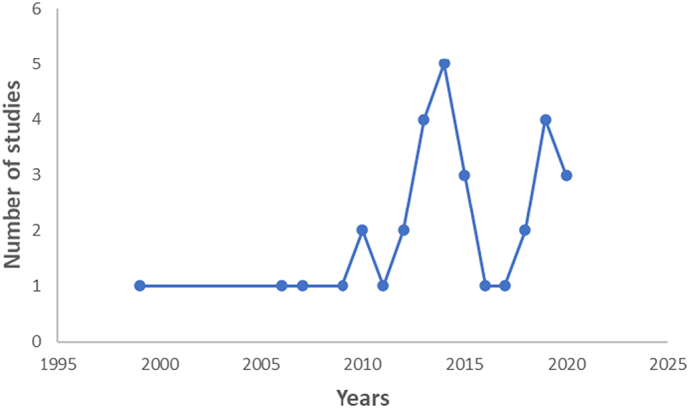
Temporal trend in the number of published studies between 1990 and 2020.
3.2. Factors associated with the presence of rabies evidence at the LGA-level in Nigeria
Our univariable multinomial models showed that LGAs with NVRI rabies surveillance data were significantly associated with population density, mosaic cropland, open broadleaved deciduous forest/woodland (>5 m) and closed to open (>15%) broadleaved or needle-leaved, evergreen or deciduous shrubland (Table 1). At the univariable level, Nigerian LGAs with published evidence of canine rabies studies were significantly associated with poverty, the level of literacy of the underlying population, urbanization, the probability of urban-expansion by 2030, human influence, distance to road and closed to open (>15%) broadleaved evergreen or semi-deciduous forest (>5 m). In our univariable multinomial analysis, LGAs reporting both NVRI and published studies were associated with distance to a veterinary hospital/clinic, distance to road, urbanization, probability of urban expansion by 2030, human influence, mosaic cropland, mosaic vegetation, closed to open (>15%) broadleaved evergreen or semi-deciduous forest (>5 m) and closed to open (>15%) broadleaved or needle-leaved, evergreen or deciduous shrubland (Table 1). We dropped distance to road because of collinearity with distance to a veterinary hospital.
Table 1.
Univariable analysis of the association between rabies virus infection evidence and risk factors at the LGA level in Nigeria.
| Variable | Rabies published studies |
NVRI surveillance data |
Published studies and NVRI data |
|||
|---|---|---|---|---|---|---|
| RRR (95%CI) | p-Value | RRR (95%CI) | p-Value | RRR (95%CI) | p-Value | |
| Sociodemographic factors | ||||||
| Distance to a veterinary hospital (Mean distance in meters) | 0.67 (0.36–1.25) | 0.206 | 0.66 (0.29–1.51) | 0.326 | 0.39 (0.15–1.00) | 0.050 |
| Distance to road (Mean distance meters) | 1.25 (1.32–1.471) | 0.025 | 1.79 (0.90–1.352) | 0.573 | 1.39 (1.70–1.71) | 0.014 |
| Poverty (mean proportion of people living in poverty) | 4.55 (1.27–16.29) | 0.020 | 2.13 (0.42–10.86) | 0.362 | 2.59 (0.47–14.37) | 0.278 |
| Literacy score (mean proportion of men and women aged 15–49) | 0.47 (0.23–0.92) | 0.028 | 1.16 (0.48–2.82) | 0.739 | 1.40 (0.55–3.57) | 0.480 |
| Population density | 1.34 (0.99–1.00) | 0.087 | 1.00 (1.40–1.85) | 0.030 | 1.00(0.99–1.79) | 0.392 |
| Proportion urban areas | 1.62 (1.09–2.44) | 0.020 | 1.15 (0.68–1.96) | 0.602 | 3.62 (1.95–6.71) | <0.001 |
| Urban by 2030 (Global Grid of Probabilities of Urban Expansion to 2030) | 2.58 (1.19–5.57) | 0.016 | 1.57 (0.51–4.78) | 0.432 | 4.97 (2.06–11.93) | <0.001 |
| Human Influence Index grids | 1.02 (1.00–1.05) | 0.026 | 1.01 (0.98–1.04) | 0.612 | 1.04 (1.01–1.08) | 0.016 |
| Environmental factors | ||||||
| Mean temperature | 1.00 (0.93–1.07) | 0.925 | 1.00 (0.89–1.13) | 0.955 | 1.20 (1.04–1.36) | 0.013 |
| Elevation | 1.00 (0.99–1.00) | 0.265 | 0.99 (0.99–1.00) | 0.740 | 1.00 (0.99–1.00) | 0.187 |
| Mosaic cropland | 1.03 (0.99–1.07) | 0.145 | 1.05 (1.00–1.09) | 0.037 | 1.09 (1.05–1.13) | <0.001 |
| Mosaic vegetation | 0.99 (0.93–1.05) | 0.679 | 0.97 (0.89–1.06) | 0.512 | 0.77 (0.63–0.95) | 0.014 |
| Closed to open broadleaved evergreen or semi-deciduous forest (>5 m) | 0.79 (0.63–1.00) | 0.050 | 0.98 (0.86–1.13) | 0.817 | 0.15 (0.04–0.62) | 0.009 |
| Open broadleaved deciduous forest/woodland (>5 m) | 1.05 (0.99–1.10) | 0.058 | 1.06 (1.01–1.12) | 0.017 | 1.00 (0.91–1.10) | 0.962 |
| Mosaic forest or shrubland/grassland | 0.94 (0.69–1.29) | 0.714 | 0.98 (0.67–1.43) | 0.921 | 1.17 (0.93–1.48) | 0.184 |
| Closed to open (broadleaved or needle leaved, evergreen, or deciduous) shrubland | 1.03 (0.98–1.07) | 0.207 | 1.06 (1.01–1.10) | 0.020 | 1.06 (1.02–1.11) | 0.004 |
| Sparse vegetation | 0.43 (0.11–1.61) | 0.208 | 0.34 (0.04–2.66) | 0.307 | 0.68 (0.19–2.43) | 0.550 |
| Water bodies | 0.64 (0.25–1.65) | 0.357 | 0.33 (0.044–2.56) | 0.292 | 0.43 (0.07–2.71) | 0.367 |
| Bare areas | 0.07 (0.001–7.40) | 0.265 | 0.76 (0.15–3.96) | 0.746 | 0.15 (0.0007–35.63) | 0.500 |
| Closed to open herbaceous vegetation | 0.91(0.79–1.05) | 0.187 | 0.95 (0.82–1.09) | 0.445 | 0.87 (0.69–1.12) | 0.293 |
After multivariable adjustment, our results indicated that LGAs with published studies on canine rabies were positively associated with the underlying population's poverty level and level of urbanization (Table 2). Those LGAs reporting NVRI canine rabies surveillance data only were positively associated with literacy level, population density and mosaic cropland/vegetation (grassland/shrubland/forest) (Table 2). Finally, LGAs reporting both NVRI surveillance data and published studies were positively associated with urbanization, probability of urban expansion by 2030, mean temperature and mosaic cropland/vegetation (grassland/scrublands/forest) (Table 2). Unsurprisingly given the large spatial areas with zero reported canine rabies evidence, our results suggested significant residual spatial autocorrelation after fitting the rabies evidence model (Moran's I = 0.684, p < 0.001), indicating that estimates of coefficient standard errors and p-values are likely biased by non-independence and some caution is needed for their interpretation [27].
Table 2.
Final multinomial analysis risk factors associated with rabies virus infection evidence at the LGA level in Nigeria.
| Variables | Rabies published studies |
NVRI surveillance data |
Published studies and NVRI data |
|||
|---|---|---|---|---|---|---|
| RRR (95%CI) | p-Value | RRR (95%CI) | p-Value | RRR (95%CI) | p-Value | |
| Sociodemographic factors | ||||||
| Literacy score (mean proportion of men and women aged 15–49) | 0.7 (0.2–1.9) | 0.4 | 4.52 (1.12–18.25) | 0.034 | 5.34 (0.84–33.98) | 0.076 |
| Poverty (mean proportion of people living in poverty) | 9.18 (1.21–69.86) | 0.032 | 12.85 (0.83–199.97) | 0.068 | 24.87 (0.63–979.09) | 0.086 |
| Proportion urban areas | 1.76 (1.07–2.88) | 0.025 | 1.07 (0.57–2.02) | 0.835 | 5.05 (1.91–13.38) | 0.001 |
| Urban by 2030 (Global Grid of Probabilities of Urban Expansion to 2030) | 2.98 (0.80–11.07) | 0.102 | 1.05 (0.16–6.77) | 0.963 | 15.44 (3.06–77.97) | 0.001 |
| Population density | 1.23 (0.99–1.00) | 0.431 | 1.6 (1.02–1.12) | 0.043 | 0.99 (0.99–1.00) | 0.973 |
| Environmental factors | ||||||
| Mean temperature | 1.00 (0.91–1.10) | 0.973 | 1.03 (0.91–1.17) | 0.583 | 1.31 (1.11–1.55) | 0.001 |
| Mosaic cropland/vegetation (grassland/shrubland/forest) | 1.02 (0.98–1.07) | 0.286 | 1.06 (1.01–1.12) | 0.010 | 1.17 (1.11–1.23) | 0.001 |
RRR -Relative Risk Ratio.
NVRI- National Veterinary Research Institute.
Cl-Confidence interval.
4. Discussion
Our results indicated significant gaps in rabies surveillance activities, with most LGAs (58%) not represented in rabies surveillance submission/reports from both NVRI and published studies. Across Nigeria, 16% of the LGAs concentrated in Taraba, Abia, and Adamawa showed rabies occurrence from published studies; the existing NVRI rabies surveillance never targeted these LGAs. This observation may be attributed to the number of investigations carried out in those states (five studies) and the fact that all are located further from the central diagnostic centres, highlighting the importance of active over passive surveillance for case detection. Furthermore, nine state capitals had no surveillance or published studies evidence. This is partly because all the states are located further from the central diagnostic laboratory.
Our findings indicated that despite two LGAs in Sokoto and Cross River having a regional diagnostic laboratory, no case was detected. This can partly be explained by the fact that regional NVRI laboratories do not carry out rabies testing. Regional NVRI laboratories should be equipped to carry out such testing. Moreover, if regional NVRI laboratories collaborate with private veterinarians and human hospitals to create awareness, it might lead to more case detection and report.
The relatively high number of reported positive cases between 2000 and 2002 can be attributed to the diagnostic protocol used at NVRI. The Sellers staining method is a histological test that allows the identification of Negri bodies within the cytoplasm of infected nerve cells [28]. Histological staining of Negri bodies is neither as sensitive nor as specific as DFAT and dRIT [28]. False-positive samples might have been processed, or only submissions from confirmed cases were processed [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50]]. Moreover, the Sellers staining method can be laborious, as evident in the number of samples processed before the introduction of DFAT, especially during 2004. Furthermore, the spatio-temporal trend of rabies evidence towards the southern part of the country might be related to primary investigations into rabies virus antigen detection among dogs from 2006 onwards [[29], [30], [31]]. Case detection in those regions and September 28 World Rabies Day activities which started in 2007, likely raised awareness about rabies by bringing together partners to enhance prevention and control efforts, resulting in more local reporting [32]. Moreover, establishing private veterinary clinics, setting up institutional rabies diagnostic centres, rabies workshops, and training are possible reasons for this gradual geographical expansion. [33]
We observed peak submissions to NVRI during April and August corresponding to the dry season, consistent with previous human hospital studies in Nigeria that reported more dog bite occurrence during the dry season [[34], [35], [36]]. The studies opined that dogs tend to be more aggressive during the dry season, which coincides with their breeding season resulting in more bites. Other factors such as heat stress and food scarcity may also contribute to aggressive behaviour, resulting in more dog bites and reporting [34]. However, some veterinary hospital studies in Nigeria reported more dog bites during the wet season [37,38]. The inconsistency between human and veterinary hospital reports can be attributed to poor record-keeping in veterinary hospitals in Nigeria, as previously reported [39]. Moreover, the lack of shelters during the wet season could have resulted in low reporting. There is a need for quantitative research into the influence of season on rabies occurrence to inform strategic interventions such as targeted education and mass vaccination campaigns in Nigeria.
The underperformance of canine rabies surveillance (i.e. an effective testing rate of less than a dog/year per 100,000 Nigerian residents) is a significant public health concern, especially during monitoring and evaluation as per the SARE guidelines towards rabies freedom. This finding is consistent with Bhutan's investigation that observed low canine rabies reporting compared to livestock [40]. The study attributed the inadequate reporting of canine rabies to difficulty tracing potential canine rabies cases and the low economic value of dogs compared to livestock [40]. However, individuals must pay for transporting samples to a regional or central diagnostic laboratory. Indeed, our univariable analysis showed that distance to a road network and veterinary clinics was significantly associated with rabies evidence. People distant from a diagnostic facility might be reluctant to send samples for diagnosis and helps to explain the spatial variation observed for NVRI surveillance data that were concentrated in states close to the central diagnostic laboratory. Therefore, Nigeria should invest in rabies surveillance by engaging surveillance officers at the LGA level and the use of point of care testing to enhance detection. Moreover, educating the public on the proper response to a rabies case, providing incentives, and covering the transportation costs for individuals who report cases directly to a rabies laboratory will support gathering of more reliable data to allow for an evidence-based evaluation of the program.
Our results demonstrated that NVRI canine rabies surveillance data was significantly associated with the literacy level of the underlying population of Nigerian LGAs. Individuals who can read and write are more likely to seek care and bear the cost of transporting samples to a laboratory for confirmation after potential rabies virus exposure than those with a lower literacy level. Previous studies indicated that illiterate people were 4.6 times more likely to delay PEP than literate people [41]. Hence, there is a need for enhanced surveillance and testing in LGAs with low literacy and improving their knowledge about rabies. Also, evidence of rabies from NVRI surveillance data was significantly associated with human population density, consistent with a study from Bhutan [40]. This finding further underscores the disparity in health care investment for rabies surveillance in LGAs with a high human population (urban areas) compared to those with a lower human population (e.g., rural areas). These results suggest that canine-rabies virus exposure in LGAs with a lower human population can go unnoticed.
Rabies evidence from published studies was significantly associated with the poverty level of the underlying population. This finding accurately represents locations where there are more rabies cases, but the NVRI surveillance system missed due to its passive nature. Authors of published studies often make substantial personal sacrifices, like sentinel surveillance offers, to obtain samples from dog markets and butchers. Enhanced surveillance, education, and health care infrastructure investment in impoverished LGAs might support rabies control in Nigeria.
Our study also demonstrates that locations with evidence from both NVRI canine rabies surveillance and published studies were significantly associated with urbanization and the probability of urban expansion by 2030. While this finding may indicate an increased occurrence of rabies virus in urbanized compared to rural settings due to increased opportunities for human-animal interactions, this finding can partly be explained by access to health care services and supportive infrastructure for rabies research. Differentiating these is critical to determine appropriate interventions. Previous studies have reported that most Nigerians keep dogs for security purposes, implying an increase in the human population would increase the dog population [42]. Urbanized LGAs may constitute a potential hotspot to rabies virus exposure since indiscriminate waste disposal near community dwellings will attract free-roaming/feral dogs in proximity to human populations [42]. Furthermore, rabies diagnostic facilities and tertiary education research centres are located primarily in urban areas [43]. Thus, metropolitan areas are more likely to have health care infrastructure and educated people with positive healthcare-seeking behaviour than rural areas. There is a need for investment in healthcare infrastructure in rural areas with insufficient evidence. Furthermore, the challenges associated with extended travel to urban areas, local dog consumption practices and transportation costs may reduce the evidence of rural regions where the rabies burden is assumed to be higher [31,44].
Distance to health care centres, landscape and weather conditions have been documented as potential risk factors associated with reported rabies occurrence locally by impacting animal distribution and movements and modulating community access to health services and the distribution of human communities at risk [45]. Understanding the relationship of different land cover types would allow a more accurate designation of areas to focus surveillance efforts for a more precise estimation of the disease burden. Our results indicated that mosaic cropland vegetation (grassland/shrubland/forest) were positively associated with local overall canine rabies evidence (i.e., NVRI data and published studies). Forest/woodland and farmland provide suitable habitats for free-roaming dogs to thrive, primarily because forests may provide suitable habitats for wildlife, including birds that may serve as prey to free-roaming/feral dogs. The use of dogs for bushmeat hunting is widespread across Nigeria [46].A previous study found that most dogs (88%) used for hunting in Nigeria were unvaccinated against rabies [47], consistent with a study from South Africa [48].The study opined that hunting dog owners might be reluctant to vaccinate, fearing it might reduce the dog's hunting ability. The use of dogs for hunting, and the decreasing chance of being vaccinated, may partly explain these findings. In Bauchi, Nigeria, a study reported a spillover of rabies virus to wildlife through hunting dogs [49]. Targeted surveillance around forest/woodland areas may increase the chance of detecting canine rabies cases in Nigeria. Our results also showed that the mean temperature was associated with canine rabies from the published studies and NVRI surveillance data. Rabies/dog bite susceptible individuals in Nigeria (male children and dog meat butchers) are more likely to be involved in outdoor activities, thereby providing an opportunity for interaction with free-roaming dogs [7]. This interaction provides an opportunity for dog bite incidents and reporting, mostly in urban areas with access to health facilities.
Our findings should be interpreted within the context of some limitations. While we reviewed the totality of the NVRI surveillance data, the storage system using hard copies might result in records going missing and lost in official archives. The noted geographical bias in the NVRI data could be due to the distance between NVRI zonal and state laboratories and the central diagnostic laboratory, which could have precluded sample submission for processing. Although we had a distance to veterinary clinics/hospitals and the distance to the road network as a proxy for health access, both factors were not retained in our final multivariable model, probably due to regression dilution bias due to the ecological nature of our modelling using aggregated rabies data at the LGA level [50]. The residual spatial autocorrelation indicates that estimates of coefficient standard errors and p-values are likely biased by non-independence, and some caution is needed for their interpretation.
In conclusion, this study has demonstrated the value of combining canine rabies evidence from different sources to better understand the current disease situation. It also underscores the need for investment in enhanced rabies surveillance to allow a more logical evaluation of Nigeria's existing rabies prevention and control program to inform public health decisions in a One Health context, particularly in highly urbanized areas and localities where published evidence exists, but no submissions have been received by NVRI identified in our study.
The following are the supplementary data related to this article.
Summary of studies reporting the prevalence of rabies virus detection among Nigerian dogs, including study institutions, locations where samples were collected, and the diagnostic tests used.
Authors contribution
PPM conceptualized the study plan, designed the study, executed the data, managed the data, analyzed the data, interpreted the results, and wrote the original manuscript. JSW, NJC and RJSM guided the conduct of the study. JSW, NJC, CER and RJSM critically reviewed the manuscript. SC, IT, DS and AAM managed the data. All authors read and approved the final manuscript for publication.
Conflicts of interest
None.
Acknowledgements
We are grateful to the NVRI for providing us with their rabies surveillance data and authors who responded to our emails for details of their sources of samples. Philip P. Mshelbwala is a recipient of the Research Training Program (RTP) scholarship funded by the Commonwealth Government, the University of Queensland, Brisbane, QLD, Australia, and Daniel Walker McLeod scholarship.
References
- 1.Hampson K., Coudeville L., Lembo T., Sambo M., Kieffer A., Attlan M., et al. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015;9(4) doi: 10.1371/journal.pntd.0003709. e0003709. Epub 2015/04/17. PubMed PMID: 25881058; PubMed Central PMCID: PMCPMC4400070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zinsstag J., Dürr S., Penny M., Mindekem R., Roth F., Gonzalez S.M., et al. Transmission dynamics and economics of rabies control in dogs and humans in an African city. Proc. Natl. Acad. Sci. 2009;106(35):14996–15001. doi: 10.1073/pnas.0904740106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nations U . United Nations, Department of Economic and Social Affairs; New York: 2015. Transforming our World: The 2030 Agenda for Sustainable Development. [Google Scholar]
- 4.Health WOfA The Global Strategic Plan to End Human Deaths from Dog-Mediated Rabies by 2030. 2018. https://www.oie.int/fileadmin/Home/eng/Media_Center/docs/Zero_by_30_FINAL_online_version.pdf Available from: [DOI] [PubMed]
- 5.Control GAfR Working to Eliminate Human Deaths from Dog Rabies by 2030. 2020. https://rabiesalliance.org/tools/planning-tools/sare/sare-faqs Available from:
- 6.Control GAfR Where We Work. 2021. https://rabiesalliance.org/about/where-we-work Available from:
- 7.Mshelbwala P.P., Weese J.S., Sanni-Adeniyi O.A., Chakma S., Okeme S.S., Mamun A.A., et al. Rabies epidemiology, prevention and control in Nigeria: scoping progress towards elimination. PLoS Negl. Trop. Dis. 2021;15(8) doi: 10.1371/journal.pntd.0009617. e0009617. Epub 2021/08/17. PubMed PMID: 34398902; PubMed Central PMCID: PMCPMC8389847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NVRI About US 2021. https://nvri.gov.ng/about Available from:
- 9.Ward M.P. Rabies in the Dutch East Indies a century ago – a spatio-temporal case study in disease emergence. Prev. Vet. Med. 2014;114(1):11–20. doi: 10.1016/j.prevetmed.2014.01.009. Epub 2014/02/04. [DOI] [PubMed] [Google Scholar]
- 10.Organization WH . 2008. Closing the Gap in a Generation: Health Equity through Action on the Social Determinants of Health - Final Report of the Commission on Social Determinants of Health. [DOI] [PubMed] [Google Scholar]
- 11.Arias-Orozco P., Bástida-González F., Cruz L., Villatoro J., Espinoza E., Zárate-Segura P.B., et al. Spatiotemporal analysis of canine rabies in El Salvador: Violence and poverty as social factors of canine rabies. PLoS One. 2018;13(8) doi: 10.1371/journal.pone.0201305. e0201305. Epub 2018/08/18. PubMed PMID: 30118490; PubMed Central PMCID: PMCPMC6097665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babaniyi O., Songolo P., Matapo B., Masaninga F., Mulenga F., Charles M.C., et al. Epidemiological characteristics of rabies in Zambia: a retrospective study (2004-2013) Clin. Epidemiol. Glob. Health. 2016;4(2):83–88. doi: 10.1016/j.cegh.2016.01.003. PubMed PMID: WOS:000399334400007. [DOI] [Google Scholar]
- 13.NVRI Zonal Laboratories 2021 [29/05/2021] https://nvri.gov.ng/contact Available from:
- 14.Mshelbwala P.P., Weese J.S. Rabies in a 3 Month Old Puppy with Human Exposure 2017. updated April, 17, 2017. https://www.wormsandgermsblog.com/2017/04/articles/animals/dogs/rabies-in-a-3-month-old-puppy-with-human-exposure/ Available from:
- 15.Tierkel E. Laboratory techniques in rabies: rapid microscopic examination for Negri bodies and preparation of specimens for biological test. Monogr. Ser. World Health Organization. 1973;23:41–55. [PubMed] [Google Scholar]
- 16.OIE OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2020. https://rabiessurveillanceblueprint.org/OIE-Manual-of-Diagnostic-Tests-and?lang=fr Available from:
- 17.Baba S. Detection of rabies virus RNA and antigen in tissues from naturally infected Nigerian dogs: in situ hybridization and. Pathol. Infect. 1999;52(2):85–91. [Google Scholar]
- 18.worldpop 2020. https://www.worldpop.org/ Available from:
- 19.Castillo-Neyra R., Zegarra E., Monroy Y., Bernedo R.F., Cornejo-Rosello I., Paz-Soldan V.A., et al. Spatial association of Canine rabies outbreak and ecological urban corridors, Arequipa, Peru. Trop. Med. Infect. Dis. 2017;2(3):38. doi: 10.3390/tropicalmed2030038. Epub 2017/08/13. PubMed PMID: 30270895; PubMed Central PMCID: PMCPMC6082090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reilly S., Sanderson W.T., Christian W.J., Browning S.R. Geographical clusters and predictors of rabies in three southeastern states. Vector Borne Zoonot. Dis. 2017;17(6):432–438. doi: 10.1089/vbz.2016.2061. Epub 2017/04/19. [DOI] [PubMed] [Google Scholar]
- 21.Tatem A., Gething P., Bhatt S., Weiss D., Pezzulo C. Oxford Uo; Southampton: 2013. Pilot High Resolution Poverty Maps. [Google Scholar]
- 22.Bosco C., Alegana V., Bird T., Pezzulo C., Bengtsson L., Sorichetta A., et al. Exploring the high-resolution mapping of gender-disaggregated development indicators. J. R. Soc. Interface. 2017;14(129) doi: 10.1098/rsif.2016.0825. Epub 2017/04/07. PubMed PMID: 28381641; PubMed Central PMCID: PMCPMC5414904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Center for International Earth Science Information Network - CIESIN - Columbia University, International Food Policy Research Institute - IFPRI, tThe World Bank, Centro Internacional de Agricultura Tropical - CIAT . NASA Socioeconomic Data and Applications Center (SEDAC); Palisades, NY: 2011. Global Rural-Urban Mapping Project, Version 1 (GRUMPv1): Urban Extents Grid. [Google Scholar]
- 24.Wildlife Conservation Society - WCS, Center for International Earth Science Information Network - CIESIN - Columbia University . NASA Socioeconomic Data and Applications Center (SEDAC); Palisades, NY: 2005. Last of the Wild Project, Version 2, 2005 (LWP-2): Global Human Footprint Dataset (Geographic) [Google Scholar]
- 25.Seto K., Güneralp B., Hutyra L.R. NASA Socioeconomic Data and Applications Center (SEDAC); Palisades, NY: 2016. Global Grid of Probabilities of Urban Expansion to 2030. [Google Scholar]
- 26.Moran P.A. The interpretation of statistical maps. J. R. Stat. Soc. Ser. B Methodol. 1948;10(2):243–251. [Google Scholar]
- 27.Pro A. Use the Spatial Autocorrelation (Moran's I) Tool to Ensure Residuals Are Not Spatially Autocorrelated. 2021. https://pro.arcgis.com/en/pro-app/latest/tool-reference/tool-errors-and-warnings/001001-010000/tool-errors-and-warnings-00851-00875-000851.htm Available from:
- 28.Prevention CfDCa . 2011. Rabies. [Google Scholar]
- 29.Hambolu S.E., Dzikwi A.A., Kwaga J.K.P., Kazeem H.M., Umoh J.U., Hambolu D.A. Rabies and dog bites cases in Lagos state Nigeria: a prevalence and retrospective studies (2006-2011) Global J. Health Sci. 2013;6(1):107–114. doi: 10.5539/gjhs.v6n1p107. (PubMed PMID: 24373270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eze U.U., Ngoepe E.C., Anene B.M., Ezeokonkwo R.C., Nwosuh C.I., Sabeta C.T. Molecular detection of rabies lyssaviruses from dogs in Southeastern Nigeria: evidence of transboundarytransmission of rabies in West Africa. Viruses. 2020;12(2):134. doi: 10.3390/v12020134. Epub 2020/01/26. PubMed PMID: 31979379; PubMed Central PMCID: PMCPMC7077224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mshelbwala P.P., Ogunkoya A.B., Maikai B.V. Detection of rabies antigen in the saliva and brains of apparently healthy dogs slaughtered for human consumption and its public health implications in abia state, Nigeria. ISRN Vet. Sci. 2013;2013:468043. doi: 10.1155/2013/468043. Epub 2014/01/15. PubMed PMID: 24416598; PubMed Central PMCID: PMCPMC3875124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prevention CfDCa World Rabies Day. 2021. https://www.cdc.gov/worldrabiesday/index.html Available from:
- 33.Africa RIW . 2021. International Conference on Rabies in West Africa. [Google Scholar]
- 34.Kale O.O. Epidemiology and treatment of dog bites in Ibadan: a 12-year retrospective study of cases seen at the University College Hospital Ibadan (1962-1973) Afr. J. Med. Med. Sci. 1977;6(3):133–140. Epub 1977/09/01. PubMed PMID: 97941. [PubMed] [Google Scholar]
- 35.Taiwo V., Antia R., Adeniran G., Adeyemi G., Alaka O., Ohore O. Rabies in dogs and cats in southwestern Nigeria: laboratory reports (1985–1995) Trop. Vet. 1998;16:9–13. [Google Scholar]
- 36.Ahmed H., Chafe U.M., Magaji A.A., Abdulqadir A. Rabies and dog bite in children: a decade of experience in Sokoto, Nigeria. Sokoto J. Vet. Sci. 2000;2:2–10. [Google Scholar]
- 37.Garba A., Umoh J., Kazeem H., Dzikwi A., Yahaya M., Zaharadeen A., et al. Hospital records (2006-2013) of Dogbite cases and laboratory confirmation of dog rabies in Niger state, Nigeria. Int. J. Anim. Vet. Adv. 2014;6(2):87–91. [Google Scholar]
- 38.Gbeminiyi Richard Otolorin JUU, Asabe Adamu DZIKWI Prevalence of rabies antigen in brain tissue of dogs slaughtered for human consumption and evaluation of vaccination of dogs against rabies in aba, Abia state Nigeria. World J. Publ. Health Sci. 2014;3(1) [Google Scholar]
- 39.Mshelbwala P., Adegbite O., Bamiselu O., Esu I., Shinkafi I., Adeyemi B., et al. One-health approach to rabies exposure surveillance within Ogun State, Nigeria: evidence of a limited collaboration between human and veterinary services. Pan. Afr. Med. J. 2019;10(10) [Google Scholar]
- 40.Tenzin Dhand N.K., Ward M.P. Anthropogenic and environmental risk factors for rabies occurrence in Bhutan. Prev Vet Med. 2012;107(1–2):21–26. doi: 10.1016/j.prevetmed.2012.05.003. Epub 2012/06/08. [DOI] [PubMed] [Google Scholar]
- 41.Wani R.T., Chowdri I.N., Dar H. Factors influencing delay in initiating post-exposure prophylaxis for rabies prevention among animal bite victims: a cross sectional study. J. Fam. Med. Prim. Care. 2020;9(9):4751–4755. doi: 10.4103/jfmpc.jfmpc_890_20. PubMed PMID: 33209795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mshelbwala P.P., Akinwolemiwa D.K., Maikai B.V., Otolorin R.G., Maurice N.A., Weese J.S. Dog ecology and its implications for rabies control in Gwagwalada, Federal Capital Territory, Abuja, Nigeria. Zoonoses Public Health. 2018;65(1):168–176. doi: 10.1111/zph.12385. Epub 2017/08/08. [DOI] [PubMed] [Google Scholar]
- 43.Mshelbwala P.P., Weese J.S. 2017. Rabies in the Developing World: Challenges & Prospects. Cliniciansbrief com. [Google Scholar]
- 44.Audu S.W., Mshelbwala P.P., Jahun B.M., Bouaddi K., Weese J.S. Two fatal cases of rabies in humans who did not receive rabies postexposure prophylaxis in Nigeria. Clin Case Rep. 2019;7(4):749–752. doi: 10.1002/ccr3.1972. Epub 2019/04/19. PubMed PMID: 30997078; PubMed Central PMCID: PMCPMC6452487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de La Rocque S., Rioux J.A., Slingenbergh J. Climate change: effects on animal disease systems and implications for surveillance and control. Rev. Sci. Tech. 2008;27(2):339–354. Epub 2008/09/30. PubMed PMID: 18819664. [PubMed] [Google Scholar]
- 46.Lifestyle A. 2019. How to Hunt Bushmeat - Nigeria Hunters Hunting Bush Meat. [Google Scholar]
- 47.Oluwayelu D.O., Adebiyi A.I., Ohore O.G. A survey of rabies virus antibodies in confined, hunting and roaming dogs in Ogun and Oyo States, Southwestern Nigeria. Asian Pac. J. Trop. Dis. 2015;5(1):17–21. doi: 10.1016/S2222-1808(14)60620-4. [DOI] [Google Scholar]
- 48.Grover M., Bessell P.R., Conan A., Polak P., Sabeta C.T., Reininghaus B., et al. Spatiotemporal epidemiology of rabies at an interface between domestic dogs and wildlife in South Africa. Sci. Rep. 2018;8(1):10864. doi: 10.1038/s41598-018-29045-x. Epub 2018/07/20. PubMed PMID: 30022116; PubMed Central PMCID: PMCPMC6052038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atuman Y., Adawa Y., Solomon A., Mshelbwala P., Ogunkoya A. Potential risks for rabies spill-over from apparently healthy dogs to wildlife in Bauchi State, Nigeria. J. Vet. Adv. 2014;4(4):493–498. [Google Scholar]
- 50.Berglund L. Regression dilution bias: tools for correction methods and sample size calculation. Ups. J. Med. Sci. 2012;117(3):279–283. doi: 10.3109/03009734.2012.668143. Epub 2012/03/10. PubMed PMID: 22401135; PubMed Central PMCID: PMCPMC3410287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of studies reporting the prevalence of rabies virus detection among Nigerian dogs, including study institutions, locations where samples were collected, and the diagnostic tests used.