Abstract
The current analysis investigates genetic and environmental influences on the bidirectional relationships between temperament and general cognitive ability (GCA). Measures of GCA and three temperament factors (persistence, approach, and reactivity) were collected from 486 children ages 4–9 years (80% white, 50% female) from the Louisville Twin Study from 1976 to 1998. The results indicated a bidirectional dynamic model of temperament influencing subsequent GCA and GCA influencing subsequent temperament. The dynamic relationship between temperament and GCA arose primarily from shared genetic variance, particularly in families with higher socioeconomic status, where input from temperament contributed on average 20% to genetic variance in GCA versus 0% in lower SES families.
Neurocognitive theories of early childhood development recognize the dynamic and interactive relationships between cognitive and emotional development as described by the construct of temperament (Bell et al., 2019; Cole et al., 2004; Wolfe & Bell, 2007). Although once thought to be relatively stable over time, it is now known that the self-regulation of emotional reactivity emerges in late infancy, continues to develop throughout childhood, and is associated with control over attentional processes. At the same time, cognitive control is simultaneously developing (Bell et al., 2019; Wolfe & Bell, 2007). The trajectories of these two related and interactive systems have rarely been explored longitudinally in a genetically informed sample. The current study will explore genetic and environmental influences on the longitudinal bidirectional dynamics between specific temperament characteristics and cognitive development during childhood
Temperament has been defined as biologically based individual differences in emotional reactivity (Rothbart & Jones, 1998; Rothbart et al., 2006; Wolfe & Bell, 2007). Research has focused on three aspects of temperament thought to impact school readiness and cognitive abilities: reactivity, persistence, and approach (Chong et al., 2019; Rothbart & Bates, 2006; Smithers et al., 2018; Wolfe & Bell, 2007). Reactivity involves a child’s emotional intensity and volatility; similar and overlapping concepts include negative affectivity and anger (Chong et al., 2019; Smithers et al., 2018; Valiente et al., 2021). High emotional reactivity can interfere both with schooling and with the testing situation in which cognitive abilities are measured (Bohn-Gettler & Rapp, 2014). Persistence involves staying on task and is related to concepts such as effortful control (Bell et al., 2019; Smithers et al., 2018). Persistence and reactivity are two aspects of temperament strongly linked with self-regulation (Bell et al., 2019; Chong et al., 2019). Finally, approach—or extra-version and its opposite, shyness—indicates the degree of comfort with new situations or people and can interfere with learning and achievement (Chong et al., 2019; Eggum-Wilkens et al., 2014).
Researchers investigate the role of non-cognitive skills like these aspects of temperament because they appear to scaffold later development of cognitive skills, as evidenced in a recent meta-analysis incorporating over 500 studies (Smithers et al., 2018). Theorists emphasize, however, that emotional regulation (including temperament) and cognition are interactive processes (Cole et al., 2004; Wolfe & Bell, 2007) that “continuously influence each other across a developmental time course and in a dynamic manner” (Bell et al., 2019, p. 378). Cognitive skills such as executive function support regulation of emotion while at the same time, emotions serve to organize thinking and action (Bell et al., 2019; Cole et al., 2004). Bell and colleagues emphasize the psychobiological underpinnings of this relationship, incorporating both genetic influences and patterns of brain maturation during childhood in their model of self-regulation (Bell et al., 2019). Furthermore, evidence suggests that some portion of economic inequalities that persist in academic achievement may arise from early disadvantages in stimulation of this nexus of both cognitive and non-cognitive skills (Heckman, 2006; Smithers et al., 2018). Interventions focused on cognitive skills can impact non-cognitive outcomes in disadvantaged samples (Reynolds et al., 2003), and non-cognitive skills can mediate the relationship between socioeconomic disadvantage and cognitive outcomes (Brophy-Herb et al., 2013; Pearce et al., 2016; Schelble et al., 2010).
Understanding the nature of genetic and environmental influences on the relationship between temperament and cognition would support efforts to optimize academic achievements and social capital. An early application of co-twin comparison reported that the members of twin pairs with longer attention spans (a facet of persistence and effortful control) at 1 year also had significantly higher general cognitive ability (GCA) scores at 4 years (Matheny & Brown, 1971). Subsequent twin studies have also focused on the influence of temperament (e.g., grit, inhibition, inattention, impulsivity) on cognitive outcomes (reading ability, fluid intelligence, verbal intelligence, GCA), reporting that the covariance between temperament measures and cognitive development was driven primarily by shared genetic variance (Christopher et al., 2016; Polderman et al., 2009; Tucker-Drob et al., 2016; Zumberge et al., 2007). A study of twins age 6–14 years indicated a genetically mediated correlation between GCA and a single temperament factor combining measures of emotionality, activity, sociability, and soothability (Petrill & Thompson, 1993). A longitudinal twin study reported evidence for shared genes influencing attention difficulties at age 5 years and GCA at age 12 years (Heutink et al., 2006). None of these twin studies tested hypotheses about the bidirectional, interactive processes that may underlie the relationship between temperament and cognition (Bell et al., 2019; Cole et al., 2004). Moreover, none of these studies examined the possible role of socioeconomic disadvantage on the temperament-cognition relationship. The Scarr–Rowe effect identified in recent years by Turkheimer and colleagues (Turkheimer & Horn, 2014) indicates that heritability of GCA is much lower in children from disadvantaged backgrounds than in children from advantaged backgrounds. It is likely that a powerful environmental variable such as socioeconomic status would affect the developmental relationship between temperament and cognition as well.
The current analysis relies on temperament and cognitive data collected annually by the Louisville Twin Study (LTS; Matheny & Dolan, 1980; Wilson, 1983) from ages 4–9 years. Our aim was to evaluate dynamic, bidirectional interactions between temperament and cognition over time (Bell et al., 2019). The development of dual change score models to characterize developmental changes has facilitated specification and testing of dynamic hypotheses of this relationship (McArdle et al., 2004). The Louisville Twin Study is uniquely positioned to support direct tests of genetic and environmental influences on the bidirectional dynamic relationships between temperament and cognition. Datasets that include GCA, temperament, longitudinal data, and twins in childhood are quite rare. No previous study has examined relationships between cognitive ability and temperament using bivariate DCSM. Therefore, the analysis represents a novel and exploratory approach to a confirmatory analysis of the relationship between development of temperament and GCA. We hypothesize that the DCSM approach will replicate and expand on previous analysis of twin data by identifying bidirectional relationships between temperament and GCA. In addition, the potential role of socioeconomic disadvantage on the etiology of the longitudinal temperament-GCA relationship was investigated as part of our exploratory analyses.
METHOD
Sample
Twins enrolled in the LTS were recruited from families residing in the metropolitan Louisville, KY area. The LTS sample is a randomly selected collection of families who represent the full range of socioeconomic status, race, and ethnic diversity within the Louisville metropolitan area at the time. Approximately 80% of the participants are white, 18% are Black, and the remaining 2% are of mixed or Asian ancestry; 50% of the sample was female. Birthyear for the current sample ranged from 1972 to 1989. The mean gestational age of the sample was 37.4 weeks, which is considered premature for single children but is not uncommon for twins. Zygosity was determined by blood sera analysis made when the twins were 36 months of age or older as part of the LTS protocol. Monozygotic (MZ) twins made up 48% of the sample and dizygotic twins (DZ) were 52% of the sample (see Table 1). About half of the DZ pairs were same-sex pairs and half were opposite-sex pairs. Longitudinal data collected annually at ages 4–9 years were used in the current analyses: a total of 486 individuals contributed data.
TABLE 1.
Descriptive statistics of general cognitive ability and temperament factors
| Age in years | N | MZ/DZ pairs | General cognitive ability Mean (SD) | Persistence Mean (SD) | Approach Mean (SD) | Reactivity Mean (SD) |
|---|---|---|---|---|---|---|
| 4 | 356 | 83/94 | 90.80 (14.94) | 72.57 (12.59) | 92.62 (18.27) | 125.21 (15.04) |
| 5 | 351 | 82/93 | 95.57 (14.24) | 69.65 (13.06) | 91.09 (19.46) | 123.80 (15.98) |
| 6 | 353 | 87/89 | 98.27 (13.03) | 67.86 (12.27) | 87.20 (19.47) | 123.24 (16.78) |
| 7 | 222 | 50/61 | 97.16 (14.50) | 67.27 (12.15) | 86.21 (20.21) | 121.23 (18.25) |
| 8 | 273 | 70/66 | 99.83 (14.14) | 62.90 (9.65) | 92.60 (13.22) | 109.96 (10.76) |
| 9 | 270 | 69/66 | 102.06 (14.47) | 62.70 (9.74) | 92.34 (14.02) | 110.10 (9.40) |
Note: Higher scores on the temperament factors indicate more difficulties.
Abbreviations: DZ, dizygotic twins; MZ, monozygotic twins.
Measures
Socioeconomic status
Occupations of heads of households, converted to Duncan’s scores for socioeconomic status (Duncan, 1961), represented the entire distribution of social class. Percentage of the sample falling in each 20-point interval was 25% (0–20), 20% (20–40), 14% (40–60), 27% (60–80), and 13% (80–100). The mean score on the 100-point scale was 46.26 (SD = 27.85), which falls in the score range typical for middle-level clerical workers. The sample was divided into lower and higher SES groups at the median value of 48.
General cognitive ability
Twins were administered the age-appropriate Wechsler scales of cognitive ability individually by separate examiners at each visit to the study center. The testing schedule was arranged so that examiners did not test the same twin on successive visits. The Wechsler Preschool and Primary Scale of Intelligence (WPPSI; Wechsler, 1967) was used to assess cognitive functioning at ages 4, 5, and 6 years of age (see Table 1). Between ages 7 and 9 years, general cognitive ability was measured using the Wechsler Intelligence Scale for Children-Revised (WISC-R; Wechsler, 1974)). Full scaled scores were translated to T-score metric (mean = 50, SD = 10) based on the means and standard deviation at age 6 years (typical entry into first grade) and data points more than three standard deviations from the mean were winsorized (10 data points; 0.05%).
Temperament
Age-appropriate versions of the McDevitt and Carey scales were completed by the mother: McDevitt Style Questionnaire (McDevitt & Carey, 1978) was used at ages 4 through 7 and the Temperament in Middle Childhood Questionnaire (Hegvik et al., 1982) was used at ages 8 and 9. The questionnaires include 90 or more items coded so that higher scores indicate more difficulty. The items are reduced to nine behavioral categories: activity, persistence, approach, adaptability, mood, intensity, distress, threshold, and rhythmicity (not included in middle childhood questionnaire). Although several factor structures for these nine categories have been reported in the literature, generally they are reduced to three factors. Factor analyses were conducted separately by twin and in the full sample; because the approaches resulted in equivalent factors, results from the full sample are reported here. Oblique factor analysis at each age supported a three-factor solution: persistence (activity and persistence), approach (approach, adaptability, and mood), and reactivity (intensity, distress, and threshold). Average factor loadings and factor correlations across ages are presented in Table 2. On average, factors explained 29%, 24%, and 22% of the variance, respectively, for a total of 75% of variance explained. Factor scores were created using unit weighting and mean scores on the factors at each age are presented in Table 1. Factors were only moderately correlated with each other and correlated significantly with GCA (see Table 2) such that higher scores on the factors (more difficulties) were correlated with lower GCA scores. For these analyses, factor scores were translated to T-score metric based on the means and standard deviation at age 6 years and data points more than three standard deviations from the mean were winsorized (3 data points for persistence and reactivity, 5 data points for approach).
TABLE 2.
Factor structure for temperament measures and correlations: average across ages
| Behavioral category | Persistence | Approach | Reactivity |
|---|---|---|---|
| Factor loadings | |||
| Activity | 0.85 | −0.13 | 0.02 |
| Persistence | 0.74 | 0.13 | 0.06 |
| Approach | 0.08 | 0.58 | −0.13 |
| Adaptability | 0.23 | 0.80 | 0.04 |
| Mood | 0.38 | 0.54 | 0.17 |
| Intensity | 0.21 | 0.10 | 0.57 |
| Distress | −0.11 | −0.31 | 0.56 |
| Threshold | −0.13 | 0.08 | 0.88 |
| Correlations | |||
| Persistence | 1.00 | ||
| Approach | 0.16 | 1.00 | |
| Reactivity | 0.18** | 0.08 | 1.00 |
| GCA: Total sample | −.23** | −.22** | −.16** |
| GCA: Girls | −.24** | −.25** | −.16* |
| GCA: Boys | −.21** | −.18* | −.17* |
| GCA: Lower SES | −.29** | −.26** | −.22** |
| GCA: Higher SES | −.20** | −.28** | −.10 |
Note: Factor loadings >.50 are in bold face; GCA, general cognitive ability.
Higher scores on temperament factors indicate more difficulties.
p < .05;
p < .01.
Statistical method
Dual change score models (DCSM) were used to examine changes with age in GCA and the temperament factors both independently in univariate models and as part of bivariate relationships. Extensive discussions of the model are available (McArdle et al., 2004), as well as comparisons of DCSMs with latent growth curve models (Ghisletta & McArdle, 2012; Lövdén et al., 2005). As presented in Figure 1, the model is based on latent difference scores that create a growth curve reflecting change from one age to another age (e.g., ΔG for GCA and ΔT for the temperament factor), which is modeled as a function of both constant change (αG and αT) that accumulates over time in an additive fashion as well as proportional change (βG and βT) based on the previous score. In the DCSM, αG and αT are typically set to 1 and the parameters βG and βT differ from zero to the extent that the longitudinal change is nonlinear. The bivariate DCSM allows for a coupling mechanism (λ) where change in GCA depends on the previous value of the temperament factor (λT), and vice versa.
FIGURE 1.
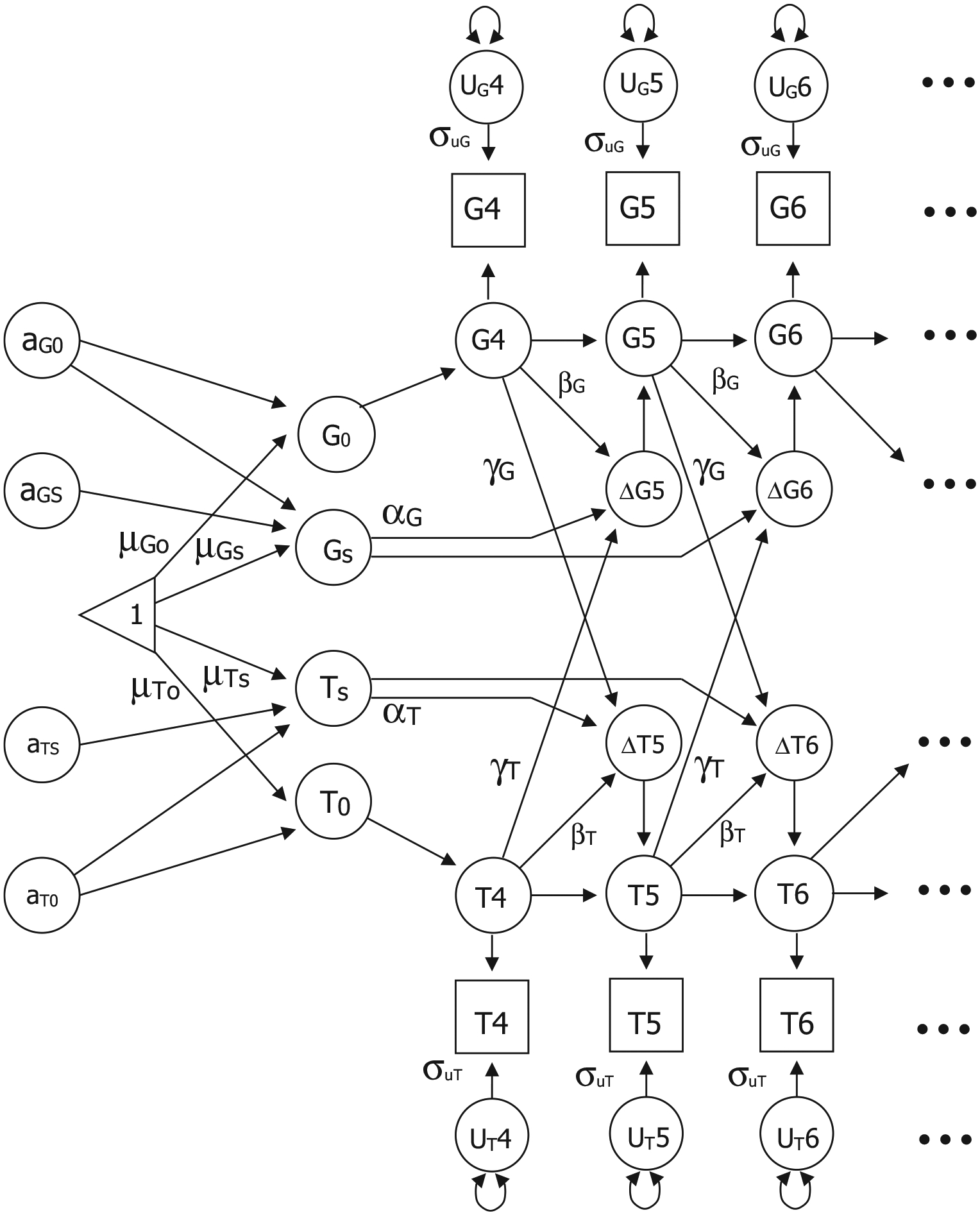
Bivariate biometric dual change score model to examine genetic and environmental influences on the relationship between age changes in two measures: G (general cognitive ability) and T (temperament factor). Error variance (σu) is assumed to be constant at each age within each factor; αG and αT represent constant change related to the slope factors Gs and Ts; βG and βT represent proportional change in G and T; cross-trait coupling is indicated by λG and λT. The model includes estimates for intercepts (G0 and T0), mean intercepts (μG0 and μT0), and mean slopes (μGs and μTs). The model includes genetic effects specific to each intercept (aG0 and aT0) and specific to each slope (aGs and aTs). The paths from aG0 to Gs and from aT0 to Ts generate the decomposition of the correlation between the intercept and slope for G and T. Note that shared environmental (cG0, cT0, cGs, and cTs) and nonshared environmental (eG0, eT0, eGs, and eTs) parameters were also included in the model but are not included in the figure
Twin data allow for the decomposition of individual variation (random effects) around the group mean intercept and slope into genetic and environmental components. The variance in any trait can be divided into three separate components: additive genetic variance (Va), shared rearing environmental variance that makes members of a family more similar to each other (Vc), and nonshared environmental variance, including measurement error, (Ve) that makes members of a family different from each other. By fitting structural models to the observed MZ and DZ covariance matrices, we can estimate the proportion of total variance accounted for by the variance in genetic factors, shared environmental factors, and nonshared environment factors. To decompose both the individual variation around the mean intercept and slope and the correlation between the intercept and slope, a standard Cholesky model was implemented (McArdle & Hamagami, 2003; Neale & Cardon, 1992). As shown in Figure 1, the model includes genetic effects specific to the intercept (aG0 and aT0) and genetic effects specific to the slope (aGS and aTS). The path from aG0 and aT0 to the slopes (Gs and Ts) generates the Cholesky decomposition of the correlation between the intercept and slope. For simplicity, only additive genetic paths are included in the figure; shared and nonshared environmental variances were also included in the model.
Univariate model fitting included three phases: fitting basic models, testing for sex differences (assuming no SES differences), and testing for SES differences (assuming no sex differences). In the basic model fitting phase, four models were fit to the data for each variable: (1) full model, (2) drop proportional change parameter β to test for nonlinear trajectory, (3) drop shared environmental variance, (4) drop additive genetic variance. Phase 2 of univariate model fitting examined sex differences by fitting four models to the data for each variable: (1) full model, (2) equating only trajectory parameters intercept, slope, and proportional change across sex, (3) equating only biometric parameters estimating genetic, shared environmental, and nonshared environmental components of variance across sex, and (4) equating all parameters across sex. Data from same-sex pairs, only (77% of the data points), were used in this phase of analysis. Phase 3 of univariate model fitting examined SES differences by fitting the same four models testing significant differences in parameters across SES groups.
Bivariate model fitting included three similar phases: fitting basic models, testing for sex differences (assuming no SES differences), and testing for SES differences (assuming no sex differences). For the purposes of the present analyses, the most important part of the bivariate DCSM is the coupling parameters: estimating the extent to which GCA impacts subsequent temperament and the extent to which temperament impacts subsequent GCA. Therefore, the first phase of bivariate model fitting focused on testing the coupling parameters: dropping all coupling (model 2) and then testing the coupling in either direction, GCA to subsequent temperament (model 3) and temperament to subsequent GCA (model 4). Phase 2 of bivariate model fitting focused on testing sex differences in the nature of coupling between GCA and temperament. Given the potential role of SES disadvantages on the dynamic relationship between temperament and cognition and its etiology, the impact of SES was explored in Phase 3.
It is important to note that one of the fundamental assumptions of DCSM is that data are missing at random. Of most importance in DCSM is demonstrating that the pattern of missing data does not differ across variables. The average number of waves of participation was 3.75 (SD = 1.83); 69% of the sample participated in three or more waves and 40% of the sample participated in five or more waves, for a total of 1825 data points for each variable. There were no sex differences in the mean number of waves of participation (t(484) = 1.40, ns); however, children from higher SES families averaged 4.03 waves versus 3.54 waves for children from lower SES families (t(484) = 2.97, p < .01).
Both univariate and bivariate biometric DCSM were fit with the structural equation modeling program Mx version 1.66b (Neale et al., 2003) using the variable-length datafile option that takes advantage of data from both complete and incomplete pairs. The raw maximum likelihood estimation procedure was used throughout. Hypotheses were tested by comparing model fit indices; nested models were compared using the difference Chi-square test obtained by taking the difference between the obtained model fits (loglikelihood) and testing its significance with the degrees of freedom equal to the difference in the number of parameters of the two models.
RESULTS
Univariate analysis
The results of the three phases of univariate model testing are presented in Table 3. A significant change in model fit indicated nonlinear change with age and significant genetic variance for all four variables: persistence, approach, reactivity, and GCA (parameter estimates can be found in Supplemental Table 1). A significant shared environmental variance was indicated for GCA, only. An estimated genetic component of variance for the intercept of the trajectory was 39%, 82%, and 63% for the three temperament factors, respectively. Estimates for intercept for GCA were 31% genetic variance, 57% shared environmental variance, and 12% nonshared environmental variance. The DCSM decomposes variance related to the age trajectory; variance specific to each time point will be estimated as error variance.
TABLE 3.
Univariate model comparisons (log-likelihood fit statistics)
| Model | Parameters | Persistence | Approach | Reactivity | General cognitive ability |
|---|---|---|---|---|---|
| Basic models | |||||
| 1. Full model | 15 | 12,660 | 12,296 | 12,120 | 12,056 |
| 2. Drop β | 14 | 12,666* | 12,313** | 12,179** | 12,167** |
| 3. Drop C | 12 | 12,663 | 12,298 | 12,123 | 12,074* |
| 4. Drop A | 12 | 12,673** | 12,329** | 12,145** | 12,108** |
| Sex differences | |||||
| 1. Full model | 30 | 9704 | 9480 | 9287 | 8935 |
| 2. Equate trajectories | 17 | 9722** | 9494** | 9290 | 8947** |
| 3. Equate biometrics | 21 | 9711 | 9491 | 9294 | 8938 |
| 4. Equate all | 15 | 9736** | 9509* | 9301 | 8956 |
| SES differences | |||||
| 1. Full model | 30 | 12,630 | 12,258 | 12,078 | 11,986 |
| 2. Equate trajectories | 17 | 12,638* | 12,258 | 12,080 | 12,022** |
| 3. Equate biometrics | 21 | 12,635 | 12,271 | 12,082 | 11,922 |
| 4. Equate all | 15 | 12,647 | 12,283* | 12,106* | 12,043** |
Model fit is significantly different from model 1 at p < .05.;
Model fit is significantly different from model 1 at p < .01.
The results of phase 2 (testing sex differences) are presented in the middle of Table 3: Although biometric parameters could be equated across sex for all four variables with no significant loss of model fit (model3), parameters estimating longitudinal trajectories could not be equated across sex without significantly reducing model fit for persistence, approach, and GCA (model 2). Longitudinal trajectories for all four variables estimated separately for boys and girls (i.e., model 1) are presented on the left in Figure 2. Although trajectories are fairly similar across sex, boys average more difficulties with persistence, fewer difficulties with approach, and slightly higher GCA scores than girls.
FIGURE 2.
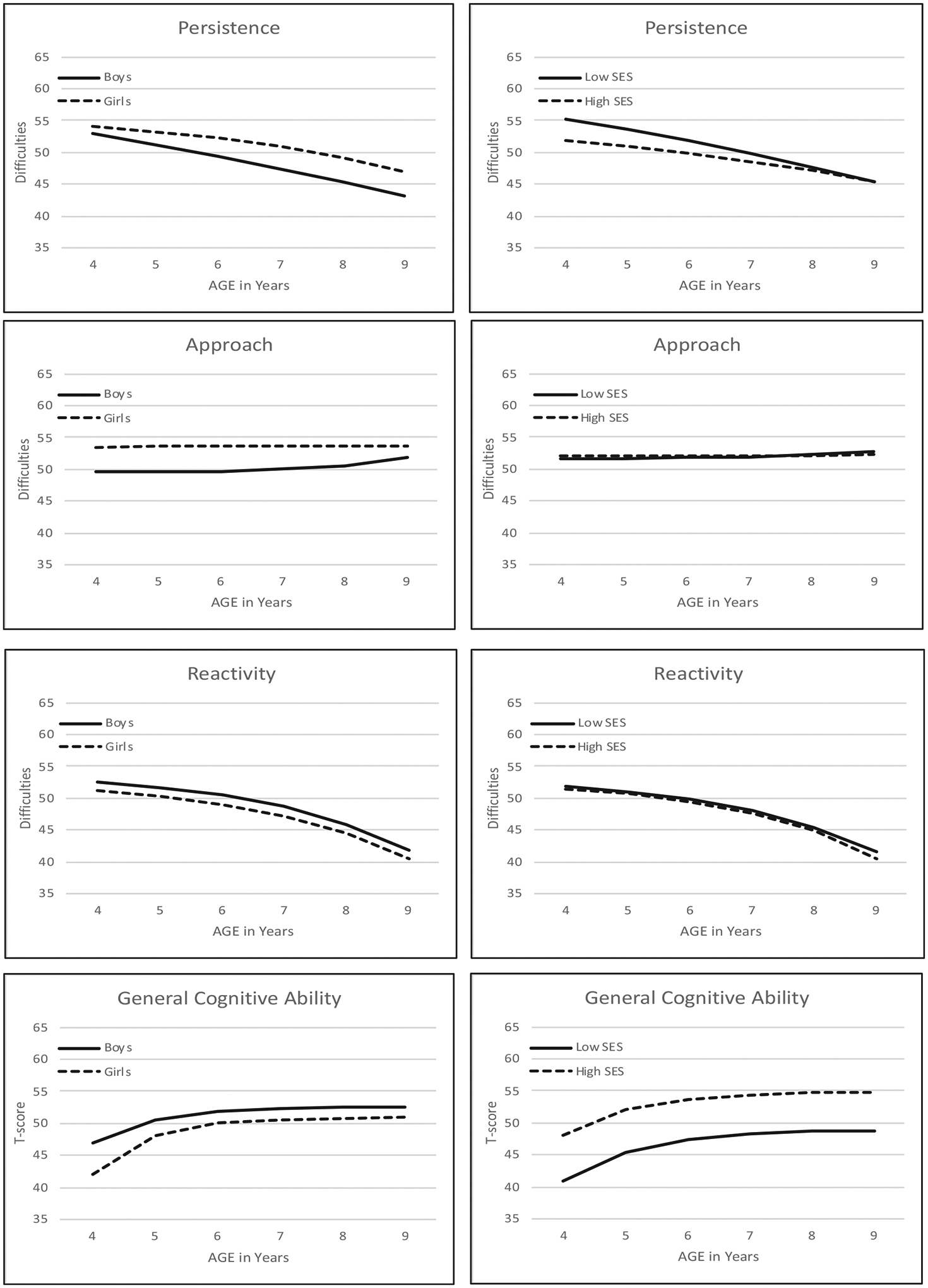
Longitudinal trajectories in means for persistence, approach, reactivity, and general cognitive ability estimated separately for boys and girls (left) and for higher and lower SES groups (right). For example, ΔG5 = αG(Gs) + βG(G4) + λT(T4). Higher scores on temperament factors indicate more difficulties
The results of phase 3 (testing SES differences) are presented at the bottom of Table 3. Differences between SES groups were indicated for all four variables. However, SES differences detected for approach and reactivity in model 4 (equate all parameters) were diffuse enough to avoid detection in models 2 or 3. Longitudinal trajectories for all four variables estimated separately for lower and higher SES groups are presented on the right side of Figure 2. Children in lower SES groups had higher mean difficulties in persistence, although the differences between SES groups decreased over age. Longitudinal trajectories in GCA showed the same pattern of increasing scores that leveled off around age 7 years for both lower and higher SES children, but mean scores were significantly lower for lower SES children.
Bivariate analysis
The results of the three phases of bivariate model testing are presented in Table 4. Model comparisons indicated significant coupling between GCA and temperament for all three temperament factors. Dropping the coupling parameter from temperament to GCA always resulted in a significant reduction in model fit, while dropping the coupling parameter from GCA to temperament resulted in a significant reduction in model fit for persistence and approach factors. Estimates of the coupling parameters are presented in Table 5. All coupling parameter estimates were negative. Negative GCA to temperament coupling parameters indicate that lower GCA scores predicted steeper increases in temperament scores at the subsequent time point. Similarly, lower temperament scores predicted greater increases in GCA at the subsequent timepoint.
TABLE 4.
Bivariate model comparisons (log likelihood fit statistics)
| Model | Parameters | Persistence | Approach | Reactivity |
|---|---|---|---|---|
| Basic models | ||||
| 1. Full model | 28 | 24,792 | 24,483 | 24,367 |
| 2. Drop all coupling parameters | 26 | 24,831** | 24,500** | 24,386** |
| 3. Drop GCA → Factor | 27 | 24,814** | 24,491** | 24,368 |
| 4. Drop factor → GCA | 27 | 24,805** | 24,491** | 24,383** |
| Sex differences | ||||
| 1. Full model | 56 | 18,713 | 18,546 | 18,390 |
| 2. Equate coupling parameters across sex | 54 | 18,714 | 18,547 | 18,390 |
| SES differences | ||||
| 1. Full model | 56 | 24,705 | 24,385 | 24,271 |
| 2. Equate coupling parameters across SES | 54 | 24,712* | 24,392* | 24,291** |
| 3. Drop GCA → Factor lower SES | 55 | 24,729** | 24,392** | 24,276* |
| 4. Drop GCA → Factor higher SES | 55 | 24,710* | 24,385 | 24,276* |
| 5. Drop factor → GCA lower SES | 55 | 24,707 | 24,387 | 24,272 |
| 6. Drop factor → GCA higher SES | 55 | 24,718** | 24,398** | 24,303** |
Abbreviations: Factor, temperament factor; GCA, general cognitive ability.
Model fit is significantly different from model 1 at p < .05;
Model fit is significantly different from model 1 at p < .01.
TABLE 5.
Coupling parameter estimates from full bivariate dual change score models (95% confidence interval)
| Parameter | Persistence | Approach | Reactivity |
|---|---|---|---|
| Total Sample | |||
| GCA → Factor | −.051 (−.076, −.029)** | −.015 (−.034, −.004)** | −.005 (−.011, .002) |
| Factor → GCA | −.128 (−.207, −.057)** | −.109 (−.187, −.033)** | −.178 (−.273, −.152)** |
| Lower SES | |||
| GCA → Factor | −.088 (−.138, −.049)** | −.018 (−.043, −.004)** | −.014 (−.030, −.002)* |
| Factor → GCA | −.063 (−.170, .026) | −.067 (−.053, .059) | −.057 (−.178, .051) |
| Higher SES | |||
| GCA → Factor | −.032 (−.065, −.002)* | .001 (−.149, .019) | .013 (.002, .027)* |
| Factor → GCA | −.187 (−.303, −.082)** | −.410 (−.632, −.170)** | −.368 (−.508, −.236)** |
Abbreviations: Factor, temperament factor; GCA, general cognitive ability.
p < .05;
p < .01.
The results of phase 2 (testing sex differences) are presented in the middle of Table 4. Equating coupling parameters across sex resulted in no significant change in model fit for any of the temperament factors, indicating that the temporal relationship between GCA and temperament was the same for boys and girls.
The results of phase 3 (testing SES differences) are presented at the bottom of Table 4. Equating coupling parameters across SES did resulted in significant reductions in model fit for all of the temperament factors. Follow-up model fitting tested the significance of each coupling parameter one at a time. The results indicated that the strongest coupling from GCA to temperament in the lower SES group and from temperament to GCA in the higher SES group. In lower SES families, lower GCA predicted greater increases in temperament scores, whereas, in higher SES families, lower temperament predicted greater increases in GCA. Parameter estimates for effects of temperament factor on GCA reported in Table 5 support this conclusion (additional parameter estimates can be found in Supplemental Table 2). Furthermore, estimates of coupling from temperament to GCA in the higher SES group are consistently the largest estimates reported in Table 5.
The results indicated that these temporal dynamics between GCA and temperament affected the biometric variance components more than they affected the mean scores (see supplemental Figure 1). Figure 3 presents the longitudinal trajectories for genetic, shared environmental, and nonshared environment variance for GCA and the temperament factors estimated with and without coupling parameters. Trajectories for the significant coupling relationships are shown with coupling from temperament to GCA in the higher SES group on the left and from GCA to temperament in the lower SES group on the right (graphs for the remaining six relationships are presented in Supplemental Figure 2). Trajectories for GCA show increasing genetic variance with age, decreasing shared environmental variance, and consistently low estimates of nonshared environmental variance that is age-related. Nonshared environmental variance is typically unique to each age and thus estimated as error variance by the longitudinal model. Longitudinal trajectories differed for the three temperament factors: increasing genetic variance for persistence but decreasing genetic variance for approach and reactivity. Comparing trajectories for full coupling and no coupling models provides an indication of the impact of temporal dynamics. Trajectories presented in Figure 3 indicate that the effect of coupling is primarily isolated to genetic variance, indicating, for example, that for the higher SES group a significant portion of the genetic variance in GCA arises from genetic variance for temperament. The same effect on a smaller scale can be seen in the lower SES group for genetic variance in temperament arising from genetic variance in GCA.
FIGURE 3.
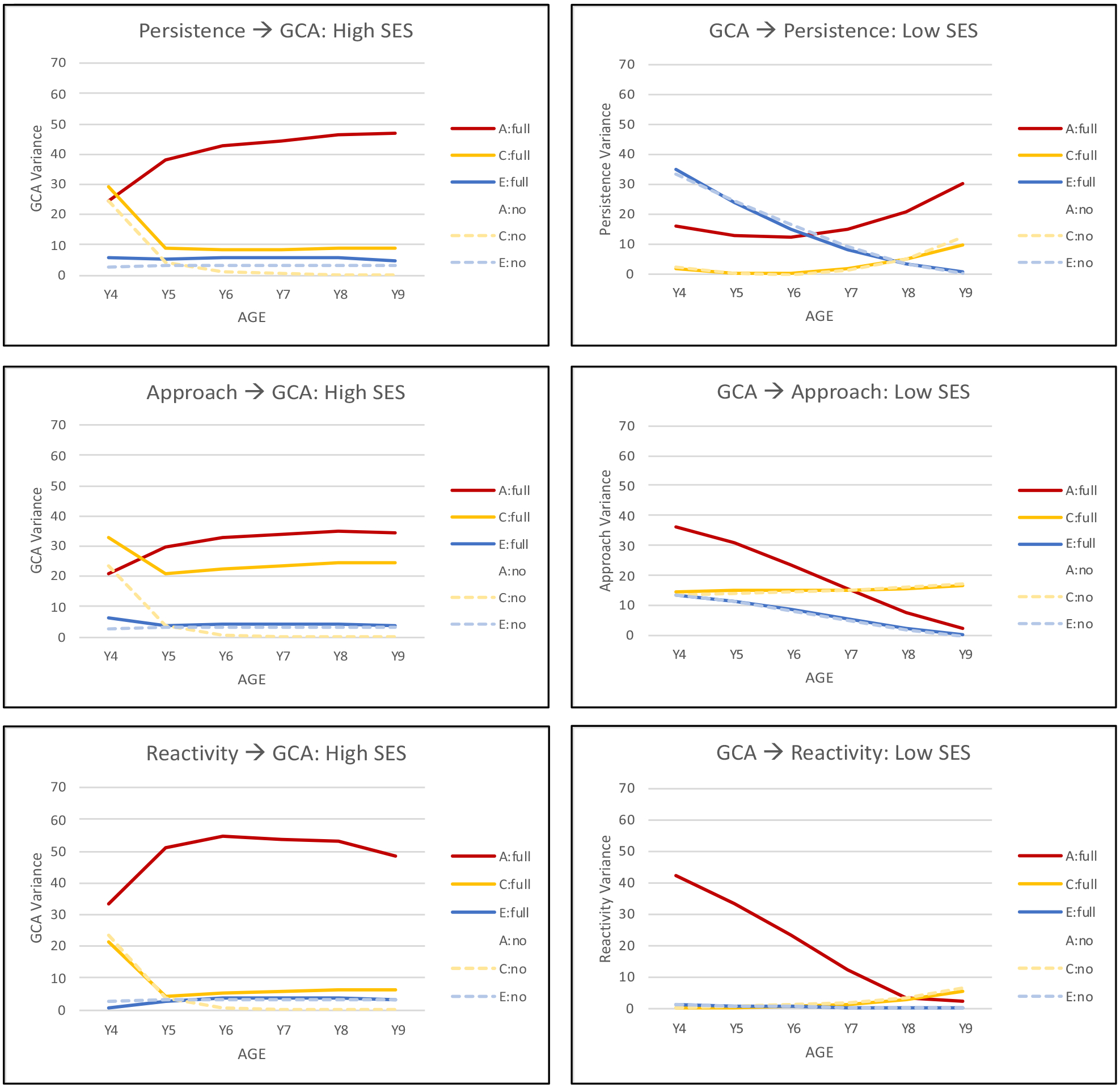
Longitudinal trajectories in proportion of genetic and environmental variance for general cognitive ability (GCA) with coupling (full) and without coupling (no) with the temperament factors in higher SES groups (left) and the temperament factors with and without coupling with GCA in lower SES groups (right). A, additive genetic variance; C, shared environmental variance; E, unique environmental variance. Calculating these trajectories incorporates all parameters from Figure 1
DISCUSSION
The aim of the current analyses was to use longitudinal twin data from the Louisville Twin Study to examine developmental theories emphasizing that temperament and cognition are related and interactive systems that support each other throughout development (Cole et al., 2004; Wolfe & Bell, 2007). For the first time, dual change score models were applied to longitudinal twin data to investigate genetic and environmental contributions to the longitudinal trajectories for measures of temperament and general cognitive ability (GCA) during childhood.
Univariate analyses
As predicted by models of development of emotional regulation (Bell et al., 2019), mean difficulties in persistence and reactivity decreased from ages 4–9 years, whereas the approach factor was more stable over that period. The mean GCA scores increased up to age 6 and then leveled off, similar to trajectories of cognition reported in the literature (McArdle et al., 2002). Model-fitting indicated significant sex differences and SES differences in intercepts for some variables, but trajectories of change with age were quite similar across groups. On average, girls averaged more difficulties in persistence and approach than boys. A recent meta-analysis of sex differences in temperament reported a modest advantage for girls in persistence and for boys in lack of shyness, but few sex differences in reactivity (Else-Quest et al., 2006). In the current analysis, the mean GCA scores were slightly lower for girls, although trajectories converged over age. Children from lower SES families average more difficulties in persistence, replicating extensive reports of associations between SES and various measures of executive function (Hackman et al., 2015). The mean GCA scores were significantly lower for low SES children, as predicted from myriad investigations of SES and cognition (Letourneau et al., 2013).
We leveraged longitudinal twin data to estimate the changes in genetic and environmental contributions to the temperament and GCA measures. The results indicated increasing genetic variance across age for GCA and persistence but decreasing genetic variance for approach and reactivity. Deater-Deckard and colleagues also reported increasing heritability for persistence in a cross-sectional study covering a similar age range (Deater-Deckard et al., 2005). Reported heritability estimates for approach or shyness tend to vary by measurement method and age (Smith et al., 2012). Similarly, heritability estimates for reactivity vary across studies, with some reporting lower heritability estimates for negative reactivity behaviors (Scott et al., 2016). The pattern of increasing genetic variance and decreasing shared environmental variance for GCA found here replicates the results of a recent meta-analysis of genetic and environmental influences on cognition (Tucker-Drob & Briley, 2014).
No systematic evidence for sex differences in the etiology of cognition is apparent in the literature (Wadsworth et al., 2014), and the same is true for temperament (Cyphers et al., 1990; Willems et al., 2019). In contrast, evidence suggests a significant impact of SES on the heritability of cognition in childhood. Estimates of genetic variance tend to be near zero in the lowest SES backgrounds and increase to 50% of total variance in the highest SES groups (Turkheimer & Horn, 2014). This “Scarr–Rowe” SES effect does not replicate in all samples; in the current analysis, SES differences in genetic contributions to the slope parameters for all four variables trended in the direction predicted by the Scarr–Rowe effect, but did not achieve significance in the univariate models. Given the potential role of SES disadvantages on the dynamic relationship between temperament and cognition and its etiology (Pearce et al., 2016; Smithers et al., 2018), the impact of SES was explored in bivariate models as well.
Bivariate analyses
Bivariate dual change score models provide a means for testing dynamic relationships between variables, and results provided support for theories emphasizing the interactive nature of temperament and cognition during the development of emotional regulation (Bell et al., 2019; Cole et al., 2004; Wolfe & Bell, 2007). For both persistence and approach temperament factors, a bidirectional relationship with GCA was indicated: age changes in temperament contributed to subsequent age changes in GCA and age changes in GCA contributed to subsequent age changes in temperament. In other words, these components of temperament are continuously influencing and being influenced by GCA across ages. The results for persistence and approach were consistent with the emotional-self regulation model (Bell et al., 2019) which posits that higher cognitive functioning provides children with more ability to appraise emotional stimuli and control the expression of temperament factors such as persistence (effortful control) and approach (shyness). In turn, these components of temperament serve to organize behavior and responses to situations requiring thinking, learning, and problem solving. In the full sample, the relationship between reactivity and GCA was unidirectional: Age changes in reactivity contributed to subsequent age changes in GCA. Reactivity and related concepts such as anger can play a role in both learning and testing situations (Bohn-Gettler & Rapp, 2014).
Although no sex differences in the longitudinal dynamic relationship between temperament and GCA were found, results indicated significant SES differences. The influence of GCA on subsequent temperament was highly significant in lower SES families (for persistence and approach), but at most moderately significant in higher SES families. In contrast, the influence of temperament on subsequent GCA did not achieve significance in lower SES families but was highly significant in higher SES families. By far the largest parameter estimates were found for the impact of temperament on subsequent GCA in higher SES families. Thus, the model results suggest that the relationship between temperament and cognition may function primarily in opposite directions in lower and higher SES children. Temperament and cognition likely develop via a complex interactive dynamic in children (Bell et al., 2019), but these analyses suggest an SES difference in dominant paths of the bivariate relationship. Extensive evidence supports the long-term noncognitive impact of early cognitive interventions in disadvantaged children (Reynolds et al., 2003). The current results support the concept of GCA as a precursor of subsequent temperament-based skills, particularly in lower SES children. In higher SES families, it is possible that parents provide the scaffolding to support the development of self-regulation and attention-regulation that can be precursors to subsequent GCA (Brophy-Herb et al., 2013; Rothbart & Posner, 2005). However, children with temperament difficulties in high SES families may not be able to take advantage of the available environmental resources in a way that children with better temperament are and, therefore, do not display the same positive increases in GCA.
Further exploration localized this SES difference in the genetic contributions to GCA and temperament in the context of the bivariate dynamic model. Comparison of estimated trajectories with and without coupling verified that the nature of the dynamic relationship between temperament and GCA arose from shared genetic variance, particularly in higher SES families. SES differences in heritability for cognition have been attributed to differential environmental opportunities to express and develop underlying genetic predispositions (Turkheimer & Horn, 2014). In other words, for disadvantaged children, there are limited intellectually stimulating environments to seek out or be shaped by. Initially, small differences in genetic predispositions can be magnified by environmental advantage or disadvantage, resulting in substantial SES differences in heritability (Beam et al., 2015). Similar processes may play a role in genetic and environmental influences on temperament (Rothbart, 2012; Rothbart & Posner, 2005). It is likely that shared genetic variance between temperament and cognition arises from genetic influences on common neural structures and circuity (Bell et al., 2019; Rothbart et al., 2006; Wolfe & Bell, 2007). For example, neural activity in the frontal lobe has been associated with approach, effortful control, and cognitive functioning in children (Bell & Fox, 1992; Fox et al., 2001). Frontal neural activity has also been associated with the activity of particular genetic variants, including the dopamine D4 receptor (Rothbart, 2012). Thus, genetic differences may contribute to different structure and functioning of neural circuity in the frontal lobe that are more likely to develop to full potential in an enriched environment, resulting in the genetic contribution to the dynamic relationship between temperament and cognition in higher SES families reported here.
Limitations
One of the primary concerns in any longitudinal study is changes in instrumentation over time, and this concern is especially relevant for a study that ran for over 40 years in the second half of the 20th century. Although the McDevitt and Carey temperament measures were state-of-the art at the time they were incorporated into the LTS protocols, revisions were made over time (Carey & McDevitt, 1978) and the instruments and methodology (parental ratings) have been critiqued (Rothbart & Hwang, 2002; Vaughn et al., 1981). Conceptual understanding of temperament has also evolved, as reflected in changes in terminology from “reactivity” to more precise terms such as “negative affectivity” and “anger.” Therefore, the results about the temperament-cognition relationship reported here should be interpreted with some caution vis-à-vis current conceptualizations of temperament. Similar caveats apply to the measure of socioeconomic status used here. Current measures of objective SES typically incorporate education, occupation, and income for both parents. However, the psycho-metric properties of the SES measure used in the current sample (Duncan, 1961) encompass the entire range of socioeconomic backgrounds.
Investigations of the relationship between temperament and cognition have focused both on general intelligence and specific cognitive abilities (Smithers et al., 2018). As the first application of bivariate dual change score models to the relationship between temperament and cognition, the current analysis focused on full-scale GCA scores. Assessing GCA from age 4 to 9 years involves changes in measurement (WPPSI to WISC) that would require some degree of harmonization to support analyses at the level of individual tests, although those harmonization attempts are ongoing with LTS data (Beam et al., 2020). Subsequent analyses will focus on specific components of cognition and their relationship with specific components of temperament. Measurement of temperament also changes with age. A constant factor structure fits the temperament data at each age, but evidence suggests that the structure of temperament may change with age (Putnam et al., 2001), changes that were not incorporated in the statistical model used here. Note that the dual change score model assumes data are missing at random, but children from lower SES families participated in somewhat fewer waves of data used here (ages 4–9) than children from higher SES families. However, the LTS staff efforts to recruit and retain lower SES families are evident in the distribution of SES scores and the fact that lower SES children averaged only one-half wave less than higher SES children.
A final consideration for any use of LTS data is the age of the database. Data collection for the LTS ran from 1957 until the late 1990s, generating arguably the most comprehensive data on the early development of U.S. twins ever collected (Rhea, 2015). Even as the LTS revisits the twins now in middle age to expand the longitudinal assessment (Beam et al., 2020), representativeness of the sample has always been a concern, in part because of little migration away from Louisville, Kentucky and the ongoing addition of new sets of twins that introduces possible cohort effects. The LTS research group is addressing these concerns directly by addressing the effect test version has on age-to-age differences in cognitive development scores (Giangrande et al., in press). Yet, despite the above limitations and the current efforts to overcome these limitations in the LTS more broadly, the current analyses were able to replicate existing research and extend understanding of the temperament-cognition relationship in the context of socioeconomic advantage.
CONCLUSIONS
In the first application of bivariate dual change score models to investigate the proposed interactive nature of temperament and GCA during child development (Bell et al., 2019; Cole et al., 2004), results support a dynamic model of temperament influencing subsequent GCA and GCA influencing subsequent temperament. The fact that SES influenced the strength of this dynamic relationship suggests that investment in both GCA and temperament in early childhood may produce dividends in multiple domains, particularly for disadvantaged children (Heckman, 2006).
Supplementary Material
ACKNOWLEDGMENT
The Louisville Twin Study is currently supported by the National Institute of Aging R01AG063949-01.
Funding information
National Institute of Aging, Grant/Award Number: R01AG063949-01
Abbreviations:
- DZ
dizygotic twins
- GCA
general cognitive ability
- LTS
Louisville Twin Study
- MZ
monozygotic twins
- WISC-R
Wechsler Intelligence Scale for Children-Revised
Footnotes
SUPPORTING IN FORM ATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
REFERENCES
- Beam CR, Turkheimer E, Dickens WT, & Davis DW (2015). Twin differentiation of cognitive ability through phenotype to environment transmission: The Louisville Twin Study. Behavior Genetics, 45(6), 622–634. 10.1007/s10519-015-9756-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam CR, Turkheimer E, Finkel D, Levine ME, Zandi E, Guterbock TM, Giangrande EJ, Ryan L, Pasquenza N, & Davis DW (2020). Midlife study of the louisville twins: connecting cognitive development to biological and cognitive aging. Behavior Genetics, 50(2), 73–83. 10.1007/s10519-019-09983-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MA, & Fox NA (1992). The relations between frontal brain electrical activity and cognitive development during infancy. Child Development, 63(5), 1142–1163. 10.2307/1131523 [DOI] [PubMed] [Google Scholar]
- Bell MA, Wolfe CD, Diaz A, & Liu R (2019). Cognition and emotion development. In LoBue V, Pérez-Edgar K, & Buss KA (Eds.), Handbook of emotional development (pp. 375–403). Springer. [Google Scholar]
- Bohn-Gettler CM, & Rapp DN (2014). Emotion during reading and writing. In Pekrun R, & Linnenbrink-Garcia L (Eds.), Educational psychology handbook series. International handbook of emotions in education (pp. 437–457). Routledge/Taylor & Francis Group. [Google Scholar]
- Brophy-Herb HE, Zajicek-Farber ML, Bocknek EL, McKelvey LM, & Stansbury K (2013). Longitudinal connections of maternal supportiveness and early emotion regulation to children’s school readiness in low-income families. Journal of the Society for Social Work and Research, 4(1), 2–19. 10.5243/jsswr.2013.1 [DOI] [Google Scholar]
- Carey WB, & McDevitt SC (1978). Revision of the infant temperament questionnaire. Pediatrics, 61(5), 735–739. [PubMed] [Google Scholar]
- Chong SY, Chittleborough CR, Gregory T, Lynch J, Mittinty M, & Smithers LG (2019). The controlled direct effect of temperament at 2–3 years on cognitive and academic outcomes at 6–7 years. PLoS One, 14(6), e0204189. 10.1371/journal.pone.0204189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher ME, Keenan JM, Hulslander J, DeFries JC, Miyake A, Wadsworth SJ, Willcutt E, Pennington B, & Olson RK (2016). The genetic and environmental etiologies of the relations between cognitive skills and components of reading ability. Journal of Experimental Psychology: General, 145(4), 451. 10.1037/xge0000146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole PM, Martin SE, & Dennis TA (2004). Emotion regulation as a scientific construct: Methodological challenges and directions for child development research. Child Development, 75(2), 317–333. 10.1111/j.1467-8624.2004.00673.x [DOI] [PubMed] [Google Scholar]
- Cyphers LH, Phillips K, Fulker DW, & Mrazek DA (1990). Twin temperament during the transition from infancy to early childhood. Journal of the American Academy of Child & Adolescent Psychiatry, 29(3), 392–397. 10.1097/00004583-199005000-00010 [DOI] [PubMed] [Google Scholar]
- Deater-Deckard K, Petrill SA, Thompson LA, & DeThorne LS (2005). A cross-sectional behavioral genetic analysis of task persistence in the transition to middle childhood. Developmental Science, 8(3), F21–F26. 10.1111/j.1467-7687.2005.00407.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan OD (1961). Occupations and social status. Free Press. [Google Scholar]
- Eggum-Wilkens ND, Valiente C, Swanson J, & Lemery-Chalfant K (2014). Children’s shyness, popularity, school liking, cooperative participation, and internalizing problems in the early school years. Early Childhood Research Quarterly, 29(1), 85–94. 10.1016/j.ecresq.2013.10.002 [DOI] [Google Scholar]
- Else-Quest NM, Hyde JS, Goldsmith HH, & Van Hulle CA (2006). Gender differences in temperament: a meta-analysis. Psychological Bulletin, 132(1), 33. 10.1037/0033-2909.132.1.33 [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, & Schmidt LA (2001). Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development, 72(1), 1–21. 10.1111/1467-8624.00262 [DOI] [PubMed] [Google Scholar]
- Ghisletta P, & McArdle JJ (2012). Latent curve models and latent change score models estimated in R. Structural Equation Modeling, 19, 651–682. 10.1080/10705511.2012.713275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangrande EJ, Womack SR, Weber R, Beam CR, Finkel D, Davis DW, & Turkheimer E (in press). Genetically informed, multilevel analysis of the flynn effect across four decades and three WISC versions. Child Development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Gallop R, Evans GW, & Farah MJ (2015). Socioeconomic status and executive function: Developmental trajectories and mediation. Developmental Science, 18(5), 686–702. 10.1111/desc.12246 [DOI] [PubMed] [Google Scholar]
- Heckman JJ (2006). Skill formation and the economics of investing in disadvantaged children. Science, 312(5782), 1900–1902. [DOI] [PubMed] [Google Scholar]
- Hegvik RL, McDevitt SC, & Carey WB (1982). The middle childhood temperament questionnaire. Journal of Developmental and Behavioral Pediatrics, 3(4), 197–200. 10.1097/00004703-198212000-00004 [DOI] [PubMed] [Google Scholar]
- Heutink P, Verhuls FC, & Boomsma DI (2006). A longitudinal twin study on IQ, executive functioning, and attention problems during childhood and early adolescence. Acta Neurologica Belgica, 106, 191–207. [PubMed] [Google Scholar]
- Letourneau NL, Duffett-Leger L, Levac L, Watson B, & Young-Morris C (2013). Socioeconomic status and child development: A meta-analysis. Journal of Emotional and Behavioral Disorders, 21(3), 211–224. 10.1177/1063426611421007 [DOI] [Google Scholar]
- Lövdén M, Ghisletta P, & Lindenberger U (2005). Social participation attenuates decline in perceptual speed in old and very old age. Psychology and Aging, 20(3), 423. 10.1037/0882-7974.20.3.423 [DOI] [PubMed] [Google Scholar]
- Matheny AP, & Brown AM (1971). Activity, motor coordination and attention: Individual differences in twins. Perceptual and Motor Skills, 32(1), 151–158. 10.2466/pms.1971.32.1.151 [DOI] [PubMed] [Google Scholar]
- Matheny AP, & Dolan AB (1980). A twin study of personality and temperament during middle childhood. Journal of Research in Personality, 14(2), 224–234. 10.1016/0092-6566(80)90030-6 [DOI] [Google Scholar]
- McArdle JJ, Ferrer-Caja E, Hamagami F, & Woodcock RW (2002). Comparative longitudinal structural analyses of the growth and decline of multiple intellectual abilities over the life span. Developmental Psychology, 38(1), 115. 10.1037/0012-1649.38.1.115 [DOI] [PubMed] [Google Scholar]
- McArdle JJ, & Hamagami F (2003). Structural equation models for evaluating dynamic concepts within longitudinal twin analyses. Behavior Genetics, 33, 137–159. 10.1023/A:1022553901851 [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Hamgami F, Jones K, Jolesz F, Kikinis R, Spiro A III, & Albert MS (2004). Structural modeling of dynamic changes in memory and brain structure using longitudinal data from the normative aging study. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 59(6), P294–P304. [DOI] [PubMed] [Google Scholar]
- McDevitt SC, & Carey WB (1978). The measurement of temperament in 3–7 year old children. Journal of Child Psychology and Psychiatry, 19(3), 245–253. 10.1111/j.1469-7610.1978.tb00467.x [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, & Maes HH (2003). Mx: Statistical modeling (6th ed.). Department of Psychiatry. [Google Scholar]
- Neale MC, & Cardon LR (1992). Methodology for genetic studies of twins and families. Springer. [Google Scholar]
- Pearce A, Sawyer AC, Chittleborough CR, Mittinty MN, Law C, & Lynch JW (2016). Do early life cognitive ability and self-regulation skills explain socio-economic inequalities in academic achievement? An effect decomposition analysis in UK and Australian cohorts. Social Science & Medicine, 165, 108–118. 10.1016/j.socscimed.2016.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrill SA, & Thompson LA (1993). The phenotypic and genetic relationships among measures of cognitive ability, temperament, and scholastic achievement. Behavior Genetics, 23(6), 511–518. 10.1007/BF01068141 [DOI] [PubMed] [Google Scholar]
- Polderman TJC, de Geus EJC, Hoekstra RA, Bartels M, van Leeuwen M, Verhulst FC, Posthuma D, & Boomsma DI (2009). Attention problems, inhibitory control, and intelligence index overlapping genetic factors: a study in 9-, 12-, and 18-year-old twins. Neuropsychology, 23(3), 381. 10.1037/a0014915 [DOI] [PubMed] [Google Scholar]
- Putnam SP, Ellis LK, & Rothbart MK (2001). The structure of temperament from infancy through adolescence. In Eliasz A, & Angleitner A (Eds.), Advances in research on temperament (pp. 164–182). Pabst Science Publishers. [Google Scholar]
- Reynolds AJ, Wang MC, & Walberg HJ (2003). Early childhood programs for a new century. Child Welfare League of America Press. [Google Scholar]
- Rhea SA (2015). Reviving the Louisville twin study: An introduction. Springer. [DOI] [PubMed] [Google Scholar]
- Rothbart MK (2012). Advances in temperament: History, concepts, and measures. In Zentner M, & Shiner RL (Eds.), Handbook of temperament (pp. 3–20). Guilford Press. [Google Scholar]
- Rothbart MK, & Bates JE (2006). Temperament. In Eisenberg N (Ed.), Handbook of child psychology: Social, emotional, and personality development (6th ed., Vol. 3, pp. 99–164). Wiley. [Google Scholar]
- Rothbart MK, & Hwang J (2002). Measuring infant temperament. Infant Behavior and Development, 25(1), 113–116. 10.1016/S0163-6383(02)00109-1 [DOI] [Google Scholar]
- Rothbart MK, & Jones LB (1998). Temperament, self-regulation, and education. School Psychology Review, 27(4), 479–491. 10.1080/02796015.1998.12085932 [DOI] [Google Scholar]
- Rothbart MK, & Posner MI (2005). Genes and experience in the development of executive attention and effortful control. New Directions for Child and Adolescent Development, 2005(109), 101–108. 10.1002/cd.142 [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Posner MI, & Kieras J (2006). Temperament, attention, and the development of self-regulation. In McCartney K, & Phillips D (Eds.), Blackwell handbook of early childhood development (pp. 338–357). Blackwell. [Google Scholar]
- Schelble JL, Franks BA, & Miller MD (2010). Emotion dysregulation and academic resilience in maltreated children. Child & Youth Care Forum, 39(4), 289–303. 10.1007/s10566-010-9105-7 [DOI] [Google Scholar]
- Scott BG, Lemery-Chalfant K, Clifford S, Tein JY, Stoll R, & Goldsmith HH (2016). A twin factor mixture modeling approach to childhood temperament: Differential heritability. Child Development, 87(6), 1940–1955. 10.1111/cdev.12561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AK, Rhee SH, Corley RP, Friedman NP, Hewitt JK, & Robinson JL (2012). The magnitude of genetic and environmental influences on parental and observational measures of behavioral inhibition and shyness in toddlerhood. Behavior Genetics, 42(5), 764–777. 10.1007/s10519-012-9551-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithers LG, Sawyer AC, Chittleborough CR, Davies NM, Smith GD, & Lynch JW (2018). A systematic review and meta-analysis of effects of early life non-cognitive skills on academic, psychosocial, cognitive and health outcomes. Nature Human Behaviour, 2(11), 867–880. 10.1038/s41562-018-0461-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob E, & Briley DA (2014). Continuity of genetic and environmental influences on cognition across the lifespan: A meta-analysis of longitudinal twin and adoption studies. Psychological Bulletin, 140(4), 949–979. 10.1037/a0035893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob E, Briley DA, Engelhardt LE, Mann FD, & Harden KP (2016). Genetically-mediated associations between measures of childhood character and academic achievement. Journal of Personality and Social Psychology, 111(5), 790. 10.1037/pspp0000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer E, & Horn EE (2014). Interactions between socioeconomic status and components of variation in cognitive ability. In Finkel D, & Reynolds CA (Eds.), Behavior genetics of cognition across the lifespan (pp. 41–68). Springer. [Google Scholar]
- Valiente C, Doane LD, Clifford S, Grimm KJ, & Lemery-Chalfant K (2021). School readiness and achievement in early elementary school: Moderation by Students’ temperament. Journal of Applied Developmental Psychology, 74, 101265. 10.1016/j.appdev.2021.101265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn BE, Taraldson BJ, Crichton L, & Egeland B (1981). The assessment of infant temperament: A critique of the Carey Infant Temperament Questionnaire. Infant Behavior and Development, 4, 1–17. 10.1016/S0163-6383(81)80003-3 [DOI] [Google Scholar]
- Wadsworth SJ, Corley RP, & DeFries JC (2014). Cognitive abilities in childhood and adolescence. In Finkel D, & Reynolds CA (Eds.), Behavior genetics of cognition across the lifespan (pp. 3–40). Springer. [Google Scholar]
- Wechsler D (1967). Wechsler preschool and primary scale of intelligence. Psychological Corporation. [Google Scholar]
- Wechsler D (1974). Wechsler intelligence scale for children-revised. Psychological Corporation. [Google Scholar]
- Willems Y, Boesen N, Li J, Finkenauer C, & Bartels M (2019). The heritability of self-control: A meta-analysis. Neuroscience & Biobehavioral Reviews, 100, 324–334. 10.1016/j.neubiorev.2019.02.012 [DOI] [PubMed] [Google Scholar]
- Wilson R (1983). The Louisville Twin Study: Developmental synchronies in behavior. Child Development, 54(2), 298–316. 10.2307/1129693 [DOI] [PubMed] [Google Scholar]
- Wolfe CD, & Bell MA (2007). The integration of cognition and emotion during infancy and early childhood: Regulatory processes associated with the development of working memory. Brain and Cognition, 65(1), 3–13. 10.1016/j.bandc.2006.01.009 [DOI] [PubMed] [Google Scholar]
- Zumberge A, Baker LA, & Manis FR (2007). Focus on words: A twin study of reading and inattention. Behavior Genetics, 37(2), 284–293. 10.1007/s10519-006-9134-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


