Abstract
Objective:
In patients with Parkinson Disease (PD), self-imitated or internally cued (IC) actions are thought to be compromised by the disease process, as exemplified by impairments in action initiation. In contrast, externally-cued (EC) actions which are made in response to sensory prompts can restore a remarkable degree of movement capability in PD, particularly alleviating freezing-of-gait. This study investigates the electrophysiological underpinnings of movement facilitation in PD through visuospatial cuing, with particular attention to the dynamics within the posterior parietal cortex (PPC) and lateral premotor cortex (LPMC) axis of the dorsal visual stream.
Methods:
Invasive cortical recordings over the PPC and LPMC were obtained during deep brain stimulation lead implantation surgery. Thirteen PD subjects performed an action selection task, which was constituted by left or right joystick movement with directional visual cuing in the EC condition and internally generated direction selection in the IC condition. Time-resolved neural activities within and between the PPC and LPMC were compared between EC and IC conditions.
Results:
Reaction times (RT) were significantly faster in the EC condition relative to the IC condition (paired t-test, p=0.0015). PPC-LPMC inter-site phase synchrony within the β-band (13–35 Hz) was significantly greater in the EC relative to the IC condition. Greater PPC-LPMC β debiased phase lag index (dwPLI) prior to movement onset was correlated with faster reaction times only in the EC condition. Multivariate granger causality (GC) was greater in the EC condition relative to the IC condition, prior to and during movement.
Conclusion:
Relative to IC actions, we report relative increase in inter-site phase synchrony and directional PPC to LPMC connectivity in the β-band during preparation and execution of EC actions. Furthermore, increased strength of connectivity is predictive of faster RT, which are pathologically slow in PD patients. Stronger engagement of the PPC-LPMC cortical network by an EC specifically through the channel of β-modulation is implicated in correcting the pathological slowing of action initiation seen in Parkinson’s patients.
Significance:
These findings shed light on the electrophysiological mechanisms that underlie motor facilitation in PD patients through visuospatial cuing.
Keywords: Dorsal Visual Stream, Posterior Parietal Cortex, Premotor Cortex, Choice task, Action selection, Electrocorticography
1. Introduction
Humans exhibit a deep capacity for interacting with their environment in rich and complex ways through both self-initiated or internally-cued (IC) actions as well as externally-cued (EC) actions made in response to environmental stimuli.(Goldman et al., 2018, Helmich et al., 2009, Hoffstaedter et al., 2013, Obeso et al., 2017, Wu et al., 2015, Wylie et al., 2009) In patients with Parkinson Disease (PD), IC actions are thought to be compromised by the disease process, as exemplified by impairments in action initiation. In contrast, EC movement facilitation through sensory prompts can restore a remarkable degree of movement capability in PD, particularly alleviating freezing-of-gait.(DeFelipe et al., 2013, Jahanshahi et al., 1995, Lee et al., 2012, Lim et al., 2005, Redgrave et al., 2010, Spaulding et al., 2013, Velu et al., 2014) Although it has received significant interest, a knowledge gap exists regarding neural mechanisms underlying movement facilitation in PD, with a particular lack of insight into the electrophysiologic mechanisms.(Goldberg, 1985, Redgrave et al., 2010, Sheth and Young, 2016)
In a healthy brain, the supplementary motor area (SMA) and pre-supplementary motor area (preSMA) are involved in the selection and execution of internally guided actions.(Goldberg, 1985, Haggard, 2019, Hoffstaedter et al., 2013) EC actions are facilitated by the posterior parietal cortex (PPC) input to dorsal lateral premotor cortex (LPMC) at the terminal end of the canonical dorsal visual stream.(Goldberg, 1985, Haggard, 2008, Sheth and Young, 2016) The dorsal visual stream is integral for spatial aspects of visually guided movement,(Buneo and Andersen, 2006, Desmurget and Grafton, 2000, Goodale, 1998, Rizzolatti and Matelli, 2003) with dominant directionality of bottom-up information flow from PPC to LPMC.(Desmurget et al., 1999, Kravitz et al., 2011, Pisella et al., 2000, Rossetti et al., 2005) Multiple primate studies have shown that processing in the β frequency band (13–30 Hz) is particularly important within this visuo-motor network.(Brovelli et al., 2004, Chakrabarti et al., 2014, MacKay and Mendonca, 1995, Stetson and Andersen, 2014)
Dysregulation of the IC and EC pathways is thought to contribute to the akinesia and bradykinesia seen in PD, wherein underactivity of SMA/pre-SMA from basal ganglia-thalamocortical (BGTC) pathology may be accompanied by compensatory parietal/pre-motor cortical overactivity.(Grafton, 2004) This compensation may underlie the utilization of external cues to correct pathological bradykinesia and impairments in action initiation.(Lim et al., 2005, Rubinstein et al., 2002) However, the literature is inconsistent. Some studies of cued movement facilitation have been unable to detect significant differences in cortical activity with facilitation.(Cunnington et al., 2002, Jahanshahi et al., 1995) Meanwhile, other studies using BOLD fMRI or PET have implicated PPC and LPMC in mediating sensory-based facilitation although the electrophysiological underpinnings of this phenomenon remain uncharacterized.(Debaere et al., 2003, Hanakawa et al., 1999, Herz et al., 2014, Hoffstaedter et al., 2013)
PD patients have been shown to benefit from sensory cues that dictate both timing and visuospatial components of action selection, also referred to as the “When” and “What” components of action, respectively.(Brass and Haggard, 2008, Hoffstaedter et al., 2013, Lim et al., 2005, Rubinstein et al., 2002) Cues that dictate action timing and visuospatial selection may engage distinct neural networks.(Hoffstaedter et al., 2013, Zapparoli et al., 2018) Consequently, movement facilitation in PD may be mediated by unique underlying mechanisms contingent on the type of cue provided. Here, we focus on the “What” or visuospatial component of action selection, and investigate the electrophysiological dynamics exhibited by the PPC-LPMC axis of the dorsal visual stream during IC and EC action selection. We hypothesize that the PPC-LPMC axis should be engaged preferentially during EC action, specifically with causal information flow from PPC to LPMC, and that the strength of this connectivity should be associated with improved motor responses. We further hypothesize that PPC-LPMC stream should be less engaged in execution of IC actions. To test these hypotheses and assess differential patterns of cortical neurophysiology, we implemented an intraoperative two choice selection task, designed to elicit both motor actions through internal and external cueing, during surgeries to implant deep brain stimulator (DBS) electrodes for PD.
2. Methodology
2.1. Subjects
Thirteen subjects (8 males) with idiopathic PD undergoing DBS lead implantation of the subthalamic nucleus (STN) or globus pallidus internus (GPi) provided written informed consent to participate in this study. The cohort mean ± standard deviation (SD) for age was 62.1 ± 6.7 years. Prior to surgery, Unified Parkinson’s Disease Rating Scale Part III (UPDRS III) scores (28.1± 10.5) were available for 12 subjects in the off-medication state. The study protocol was approved by the institutional review board at the University of California, Los Angeles, CA, USA. After completion of awake intraoperative clinical testing, electrocorticography (ECoG) signals were recorded from frontal and parietal cortex during a motor selection task. All patients underwent the surgical procedure in the off-medication state: all long-acting and short-acting medications were withdrawn at least 12 h prior to the surgical procedure.
2.2. Task
A schematic of the task is shown in Figure 1. Subjects performed left or right joystick (Thrustmaster T.16000M, Guillemot Corporation, France) movements with the hand contralateral to the hemisphere from which ECoG was recorded (L in n=5) in response to arrow stimuli presented on a monitor through an experimental laptop running Psychophysics Toolbox (version 3).(Brainard, 1997) Depending on clinically defined operative laterality, subjects performed the task with either dominant hand (n=6) or non-dominant hand (n=7). Each trial began with a black screen, shown for a duration of 1.0–1.1s, (random jitter of 0.1s). Then, a white fixation cross appeared in the center of the screen for 1.0–1.1s (random jitter of 0.1s). This was followed by an action selection cue comprised of combinations of 4 left or right pointing arrows arranged horizontally on the screen. The arrows remained on the screen for 1.5s, followed by a black screen for an additional 0.5s, during which response continued to be recorded. In the EC condition, all arrows pointed in the same direction (“<< <<” or “>> >>”) and subjects were instructed to move the joystick as quickly as possible in the corresponding direction. In the IC condition, arrows pointed in opposite directions (“<< >>”) and the subjects were instructed to freely choose either response direction. Thus, although the timing of the action was cued in all trials, the specific action performed was either externally cued or internally chosen. Reaction time (RT) was determined as the difference between cue onset and movement onset time, with movement onset defined as joystick displacement of 5% of the trial maximum.
Figure 1.
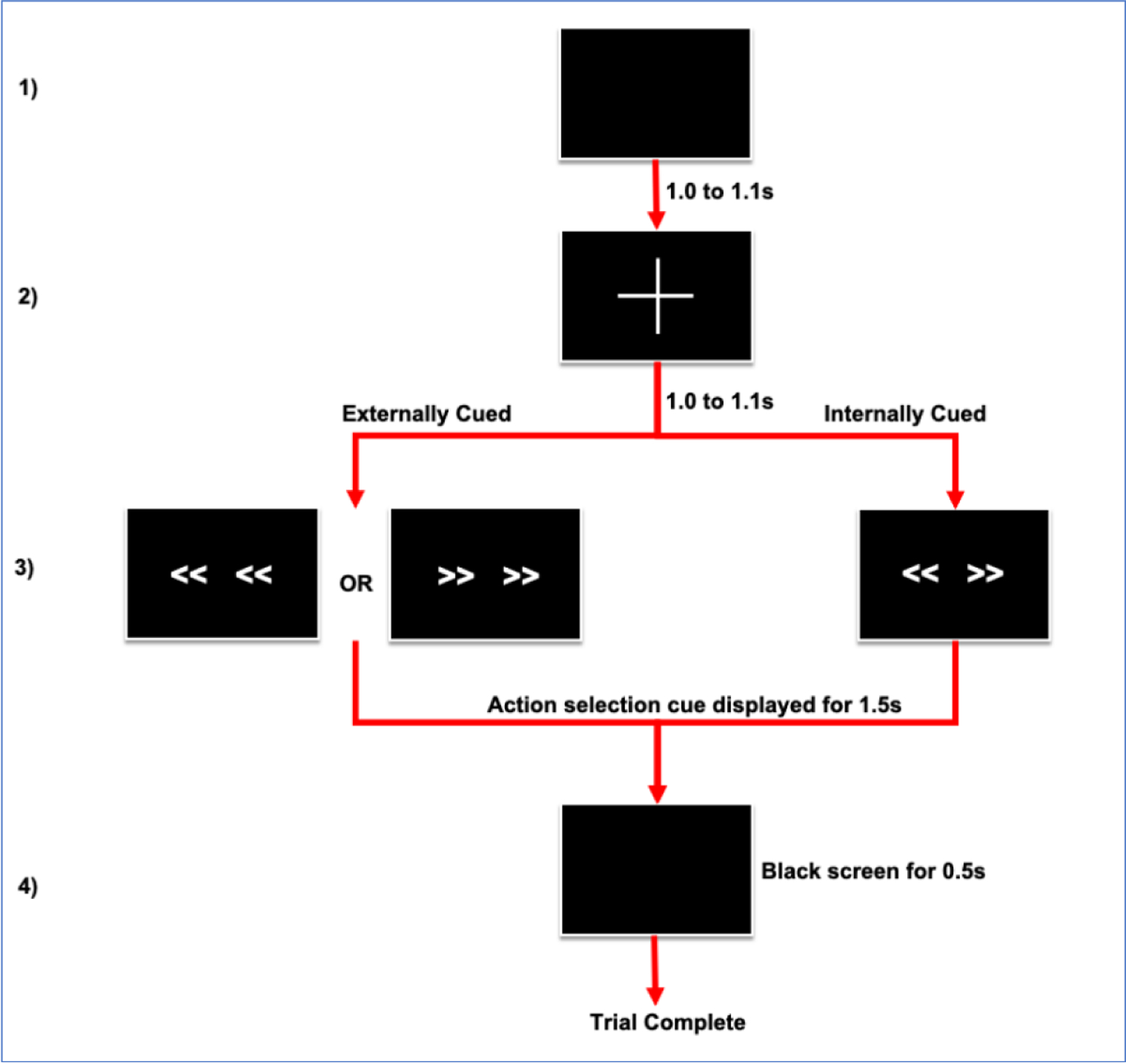
Schematic of task design. 1) Black screen for duration of 1.0 to 1.1 seconds (s) during which patient is resting. 2) Fixation cross for duration of 1.0 to 1.1s indicates that cue to move is impending. 3) Onset of action selection cue indicates patient should move joystick. In the internally cued (IC) condition bidirectional arrows are displayed indicating that patient may move joystick left or right. In the externally cued condition (EC) uniform right or left pointing arrows are displayed, instructing action selection. Cue is displayed for 1.5s or until response is recorded. 4) Response is recorded for additional 0.5s during display of black screen. In total, response is recorded for 2s following action selection cue display. After the completion of a trial, a new trial begins.
Of the 52 trials in each block, 26 were congruent with either all right or left arrows (1:1 ratio) for the EC condition, and 26 were the opposite arrows for the IC condition. To prevent the bias in IC direction selection, patients were instructed to attempt to move the joystick in both directions equally. All trials in each block were performed in random order, and each experiment consisted of 2 blocks. There was a short break (~30 sec) after completion of the 1st block. A second block of experiments was collected in those participants in whom fatigue did not preclude so.
2.3. ECoG Recording and localization
During DBS lead implantation surgery, an 8-channel subdural ECoG strip (AdTech Medical, 4mm contacts with 1cm spacing) was placed posteriorly through the same burr hole used for clinical lead implantation. The ECoG strip was oriented anterior-posteriorly across the central sulcus. ECoG strips were placed temporarily for research purposes and removed after the task and prior to completion of the surgery. ECoG recordings were obtained using Simulink in MATLAB (Version 9.8, The Mathworks Inc., Natick, MA, United States), with g.USBamp2 amplifiers (g.Tec, Austria, sampling rate: 4800 Hz, 0.1 Hz highpass filter, 60 Hz notch filter, scalp ground and reference).
The details of imaging, DBS lead targeting, and anatomical localization of ECoG strip were adapted from Randazzo et al. and are provided in prior publications.(Malekmohammadi et al., 2018, O’Keeffe et al., 2020, Randazzo et al., 2016) Briefly, all subjects underwent clinical pre- and postoperative imaging. Preoperative imaging included T1-weighted magnetization prepared rapid acquisition gradient echo images (slice thickness = 1 mm, repetition time = 2,100 ms, echo time = 2.98 ms, flip angle = 15°, 3T, Siemens Skyra). Pre and postoperative CT scans were co-registered to preoperative structural MRI. The cortical surface was reconstructed from the MRI using FreeSurfer. A 3D surface of skull and stereotactic frame was rendered from co-registered preoperative CT scan. DBS electrodes were reconstructed from the postoperative CT scan and stereotactic frame landmarks were identified on the co-registered preoperative CT scan. The 2D intraoperative fluoroscopic image and 3D skull surface were visually inspected and fused. Reconstructed DBS leads and stereotactic frame were used as fiducials for accurate 3D/2D fusion to the fluoroscopic image. ECoG contacts were manually marked on the fused images and visualized on the reconstructed cortical surface.
Channel locations were projected into standard Montreal Neurological Institute (MNI) space by aligning subject T1 MRI to a standard MNI anatomical atlas by non-linear transformation using Fieldtrip toolbox.(Oostenveld et al., 2011) For the analyses of ECoG signals, bipolar re-referencing was performed in order to focus on local oscillatory activity and reduce contributions from volume conduction from distant sources. The electrode pair immediately anterior to the motor cortex was used for the analysis of LPMC activity. The electrode pair immediately posterior to the somatosensory cortex was used for analysis PPC activity. The density of electrode contribution on the surface of cortex was computed using a distance-based inverse exponential kernel with a 1cm spatial extent that mapped relative contribution from electrodes to grid points on a finite element model of the MNI cortical surface (Figure 2).
Figure 2.
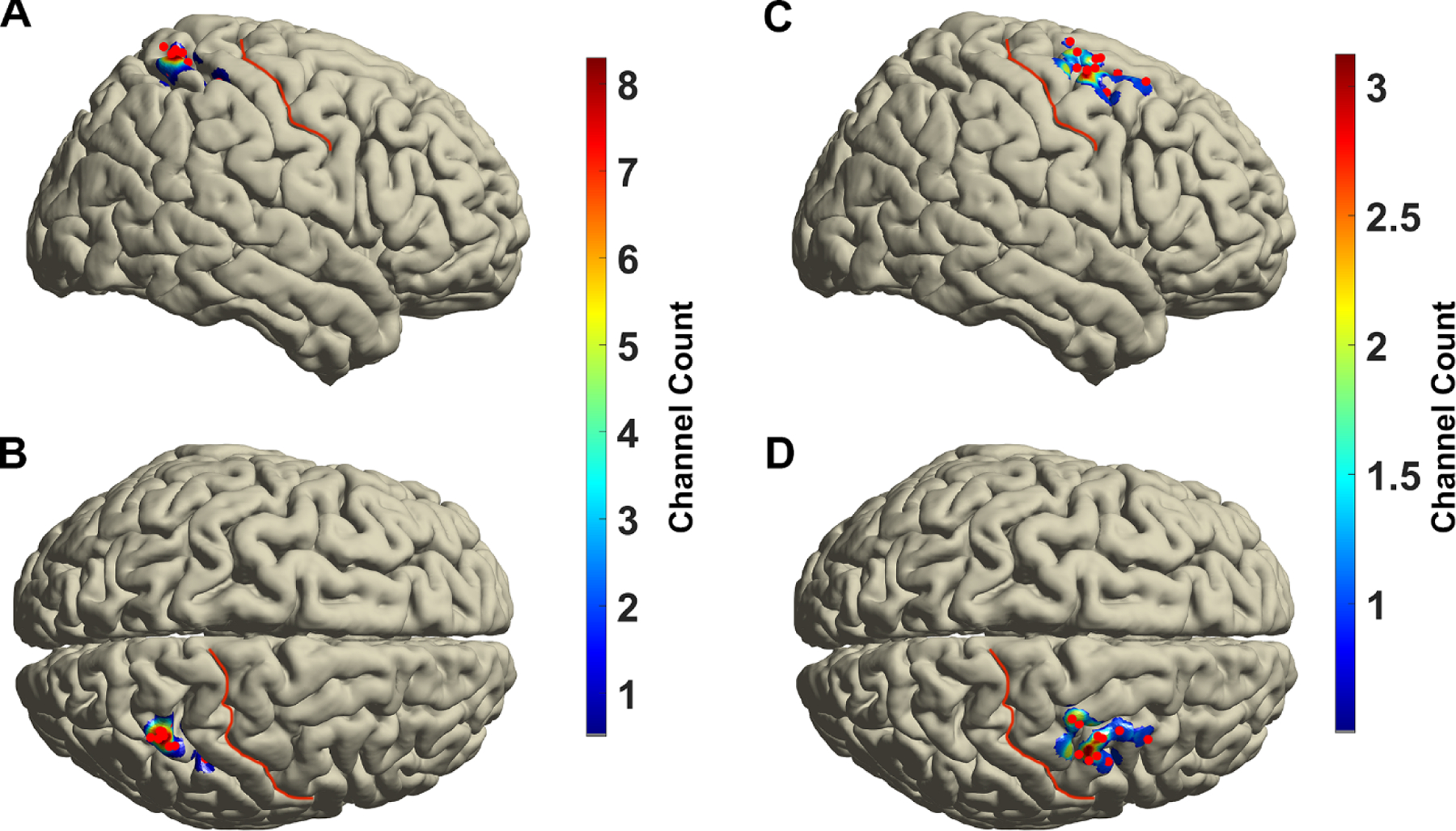
Group averaged projection of posterior parietal cortex (A, B) and lateral premotor cortex (C,D) electrode recording area over standard Montreal Neurological Institute standard brain in lateral (A,C) and dorsal (B,D) views. Red points demonstrate the position of electrodes. Central sulcus is marked with red line.
2.4. Data preprocessing
All signal analyses and subsequent statistics were performed using custom-written scripts in MATLAB, and the open source toolboxes, Fieldtrip(Oostenveld et al., 2011) and EEGLAB.(Delorme and Makeig, 2004) A two-way least-squares FIR bandpass filter (1~300 Hz, filter order: 3 times the sampling rate, “eegfilt.m” EEGLAB) and bandstop filter (60 Hz harmonics: 120, 180, and 240 Hz with 2 Hz bandwidth) were applied to ECoG recordings in order to reduce background noise and power-line interference. Filtered signals were then down-sampled to 1200 Hz. Single-trials were segmented relative to the cue onset for each task condition, including 3 seconds before and after the cue. Signals were z-normalized relative to mean signal amplitude over the whole trial.
On average, 87.8 ± 25.2 trials were potentially available for analysis for each subject. Trials with drift, discontinuity, or the transient activity with extremely high amplitude from electrical noise were excluded by visual inspection. In order to ensure that the trials used represent the behavior of interest, trials were excluded based on the task performance. Specifically, we excluded trials with incorrect responses (for EC trials), lack of response, and extremely slow (>1.5 s) or fast (<0.1 s) responses, as well as any trial in which movement onset time exceeded the mean ± 3 SD of that seen in other trials. After these steps, 76.5±25.8 trials remained per subject, including 38.5 ± 12.6 and 37.9 ± 13.4 in the EC and IC conditions, respectively.
2.5. Spectral power and Phase Based Connectivity
To obtain magnitude and instantaneous phase information in the frequency domain, complex Morlet wavelet convolution of the single-trial recordings was performed. The wavelet family was defined as a set of Gaussian-windowed complex sine waves at 299 logarithmically spaced frequencies between 2 Hz and 300 Hz. Wavelet width was increased from 4 to 10 cycles in logarithmically spaced steps to emphasize temporal precision. The resulting complex analytic signals provided the input for subsequent power and phase coherence.(Cohen, 2014) Spectral power was calculated by taking the square of the absolute value of instantaneous complex voltage amplitude at each time-frequency point. Debiased weighted phase lag index (dwPLI) was used to quantify phase coupling between PPC and LPMC according to Vinck et al. (Vinck et al., 2011) This method is largely unaffected by fluctuations in power (unlike spectral coherence). By weighting the magnitude of the imaginary part of phase synchrony, dwPLI minimizes susceptibility to volume conduction. dwPLI was calculated over 400 ms moving windows in steps of 10ms at each frequency for each single trial. 400 ms windows were chosen to include at least 3 cycles of the lowest analyzed frequency (8 Hz) as a balance of temporal precision and signal to noise ratio of estimated parameters.(Cohen, 2014)
All time-frequency power or dwPLI points were z-normalized at each frequency with reference to a baseline period −0.8 to −0.2s prior to onset of action selection cue (i.e. fixation cross period) concatenated over all trials within each condition.(Ciuparu and Muresan, 2016) Time-frequency analyses were performed for both stimulus-locked segments (from 0.2 s before to 1.8 s after cue onset) and response-locked segments (from 1s before to 1 s after movement onset).
2.6. Granger Causality Analysis
To evaluate the modulation of PPCàLMPC directional functional connectivity, multivariate autoregressive (MVAR) modeling was used to estimate the Granger causality (GC).(Chen et al., 2006, Granger, 1969) To optimize the selection of model order for the MVAR model, we first downsampled the preprocessed data to 300 Hz.(Malekmohammadi et al., 2019, Schlogl and Supp, 2006, Zavala et al., 2014) Each trial was treated as an independent realization of a statistically stationary process. To minimize non-stationarities across trials, the mean of the evoked response (averaged across all trials) was calculated and each corresponding time point was subtracted by the appropriate values corresponding to that time point in the evoked response.(Ding et al., 2000)
The appropriate model order (i.e., the number of time lags to include when generating the MVAR model matrix) was then calculated for each condition, each subject, and each time window using the Bayesian information criterion (BIC). Optimal model order ranged from 6.7 to 93.3 ms, with a mean ± SD of 24.4 ± 24.9 ms. In general, there is no optimal order for all conditions, times segments, and subjects. However, BIC calculations provide a range of orders over which an appropriate order may be extracted.(Cohen, 2014) Resolution of GC in the frequency domain is determined by the model order. Wherein, a higher order parameter enables consideration of more cycles per frequency which will improve the accuracy of frequency specific GC. However, unlike calculations of phase-locking based connectivity where a minimum of three cycles per frequency is recommended, with GC too few or too many time points will lead to poor MVAR coefficient estimates.(Cohen, 2014) Thus, for spectral GC it is recommended to lean toward having a higher order parameter, although not so high as to make the model poor fit to the data. Based on the mean mean optimal order identified with BIC and frequency band of interest, 30 ms order was selected. This model order is consistent with the model orders others have used to resolve frequencies in the β band.(Zavala et al., 2014) MVAR was calculated using the ‘armorf’ function from MATLAB bsmart toolbox,(Cui et al., 2008) over 400 ms moving windows in steps of 10ms (consistent with the dwPLI analysis). The MVAR matrixes were averaged across trials and converted into the frequency domain using the “‘cca_pwcausal” function of the Granger Causal Connectivity Analysis toolbox.(Seth, 2010) As estimates of GC are inaccurate at the single trial level, Welding normalization could not be performed.(Ciuparu and Muresan, 2016) Instead, GC was averaged over all trials prior to baseline z-normalization to reference baseline period −0.8 to −0.2s prior to onset of cue.
2.7. Power Statistics
Non-parametric cluster-based permutation testing was performed to assess the statistical significance of the mean power change relative to baseline period.48 The null hypothesis was that power following cue was equal to the baseline period, (z-power = 0). To test this hypothesis, power was first averaged over trials in a single condition for each subject. These maps were then averaged over subjects, to produce a single group level TF-power map for each condition. This step was repeated 1000 times with inversion of the sign of a random subset of individual’s mean time-frequency (TF) power map. A TF-Z value map was created by comparing the true power map, to the randomly permuted null distribution. A threshold was applied to this resultant map, so that TF-pixels with an absolute value Z < 1.65 were removed. Significant clusters of pixels were then corrected for multiple comparisons using exceedance mass testing.(Maris and Oostenveld, 2007) Exceedance mass testing involves integrating the excess mass of suprathreshold clusters each permuted spectrogram and recording the largest per iteration.(Maris and Oostenveld, 2007) The top 5% of clusters in this distribution determined the corrected threshold for image wise significance.
To test power difference between EC and IC conditions, mean difference between conditions was calculated for each subject, then averaged over subjects to create a group average power difference map. This process was repeated 1000 times, with random permutations of condition labels for each subject. A TF-Z value map was created by comparing the true power difference map, to the randomly permuted null distribution. This map was then thresholded and corrected for multiple comparisons using exceedance mass testing.(Maris and Oostenveld, 2007)
2.8. Connectivity Statistics
To assess the statistical significance of the mean induced β-band z-dwPLI difference between EC and IC trials, we first calculated subject average z-dwPLI over all trials for each condition. We then averaged z-dwPLI over the β-band (13–30 Hz) at each time point for each condition. We then determined the group averaged difference across conditions at each time point. This was repeated 1000 times while randomly permuting condition labels for each subject. Average Z-value relative to the null distribution was calculated, thresholded, then corrected using exceedance mass testing.(Maris and Oostenveld, 2007) Similar steps were repeated for calculation of cross-conditional differences in z-GC.
2.9. Connectivity-Behavioral Correlation
As connectivity was modulated by cue type, we next assessed the relationship between phase-based connectivity and behavior. We performed cross subject spearman correlation between connectivity (β-band z-dwPLI, β-band GC) and RT at each time point for each condition. This yielded a condition specific R value at each time point. We repeated this step 1000 times with random assignment of RT to subjects. At each permutation, the largest time period cluster of significant R values were recorded. Again, exceedance mass testing was used to correct for multiple comparisons across time.(Maris and Oostenveld, 2007) Finally, each significant time point was compared to the opposite condition using a Fisher-Z transform test.
3. Results
3.1. Patients respond faster during externally compared to internally cued movement
Task performance is summarized in Table 1. Eleven of 13 subjects demonstrated faster RT in the EC condition relative to the IC condition. This difference was statistically significant in 5 subjects using two sample t-tests. At the group level, RT was significantly faster in the EC condition relative to the IC condition (paired t-test, p = 0.0015). RT was similar whether dominant hand (EC RT = 0.68 ± 0.19 s, IC RT = 0.73 ± 0.20 s) or non-dominant hand (EC RT = 0.71 ± 0.15 s, IC RT = 0.79 ± 0.20 s) was used (two sample t-test, EC p = 0.76, IC p = 0.58). There were no significant correlations between UPDRS scores and EC RT (Spearman R = 0.091, p = 0.78) or IC RT (Spearman R = 0.11, p = 0.74). On average, proportion of leftward joystick selection was 46.9 ± 6.8% and 46.5 ± 19.1% in the EC and IC conditions, respectively. The average proportion of trials with left sided movement was not significantly different between conditions, (paired t-test, p = 0.94).
Table 1 –
Summary of cohort characteristics and task performance. Eleven subjects demonstrated faster mean reaction times (RT) in the externally cued (EC) relative to the internally cued (IC) condition. This difference was statistically significant in 5 subjects using two sample t-tests. At the group level, RT was significantly faster in the EC condition relative to the IC condition using paired t-test.
| Subject | Gender | Age | UPDRS-III Off | H & Y Stage | Dominant Hand | Dominant vs Test Hand Congruence | EC Trial Count, n | EC RT, seconds, mean | IC Trial Count, n | IC RT, seconds, mean | EC vs IC RT, t-test, P-Val |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 57 | 14 | 2 | R | No | 25 | 0.52 | 24 | 0.61 | 0.01 |
| 2 | M | 71 | 21 | 2.5 | R | Yes | 49 | 0.51 | 50 | 0.62 | <0.01 |
| 3 | M | 68 | 32 | 2.5 | L | Yes | 25 | 0.59 | 22 | 0.63 | 0.23 |
| 4 | F | 52 | 25 | 2 | R | No | 44 | 0.55 | 48 | 0.59 | 0.15 |
| 5 | M | 57 | 22 | 3 | R | No | 25 | 0.78 | 23 | 0.82 | 0.37 |
| 6 | M | 59 | 28 | 1.5 | R | Yes | 52 | 0.67 | 51 | 0.72 | 0.11 |
| 7 | M | 57 | 29 | 2 | R | Yes | 51 | 0.49 | 50 | 0.48 | 0.80 |
| 8 | F | 60 | 49 | 2 | R | No | 50 | 0.74 | 49 | 0.74 | 0.93 |
| 9 | F | 61 | 44 | 3 | R | No | 18 | 0.88 | 17 | 1.13 | 0.01 |
| 10 | M | 67 | NA | 2 | R | Yes | 30 | 0.85 | 24 | 0.89 | 0.34 |
| 11 | F | 56 | 35 | 2 | L | No | 51 | 0.61 | 47 | 0.67 | 0.04 |
| 12 | M | 68 | 18 | 2 | R | No | 48 | 0.87 | 45 | 0.95 | 0.02 |
| 13 | M | 74 | 21 | 3 | L | Yes | 33 | 0.96 | 43 | 1.02 | 0.26 |
| All Subjects, mean (SD) | - | 62.0 (6.7) | 28.1 (10.5) | 2.3 (0.50) | 38.5 (12.7) | 0.69 (0.16) | 37.9 (13.4) | 0.76 (0.19) | <0.01 |
EC, externally cued; H & Y, Hoehn and yahr Stage; IC, internally cued; L, left; n, number; NA, not available; RT, reaction time; UPDRS III Off, Part III of the Unified Parkinson’s Disease Rating Scale off-medications;
P <0.05, t-test
3.2. Beta power suppression in the PPC-LPMC network
In the PPC, β-power was significantly suppressed in the IC condition, starting immediately after the action selection cue (Figure 3). A similar pattern of β-power suppression was observed in the EC condition, but was of lower magnitude and did not reach statistical significance. Still, there was no significant difference between EC and IC. Equivalent results were observed in the same analysis when time-locked to movement (Supplementary Figure 3S).
Figure 3.
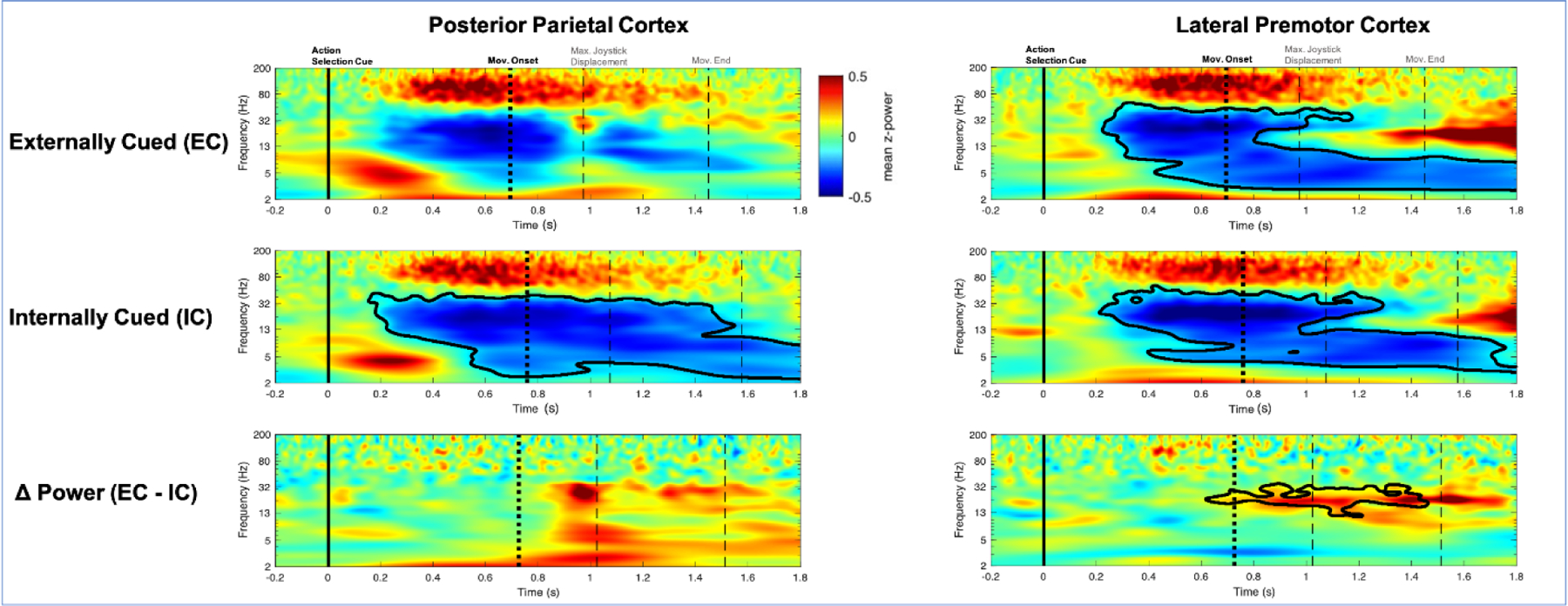
Cue locked power in the posterior parietal cortex (PPC) and lateral premotor cortex (LPMC). Movement-related beta suppression was observed in both PPC and LPMC. This suppression was not cluster significant in the PPC in the EC condition. In the PPC, there were no significant power differences between externally cued (EC) and internally cued (IC) conditions. In the LPMC there was significantly greater beta suppression toward the end of the trial. Black contour indicates cluster significance. X-axis is time from cue. Y-axis is frequency. Color indicates group averaged z-power. Solid vertical line indicates action selection cue onset, first dotted vertical line indicates movement onset, reaction time (RT). Dashed vertical lines indicates maximal joystick displacement and movement end. Hz, hertz; Mov, movement; s, seconds.
In the LPMC, β-power was significantly suppressed in both the EC and IC conditions (Figure 3). In cue locked analysis only, there was significantly greater β-power suppression in the IC condition relative to the EC condition late in movement. In the movement locked analysis, there were no significant differences between conditions, suggesting the later return to baseline is likely due to slower RT in the IC condition (Supplementary Figure 3S).
3.3. PPC-LPMC beta band functional connectivity differentiates internally and externally cued actions
β-band PPC-LPMC dwPLI significantly differed between EC and IC conditions (Figure 4A, Supplementary Figure 4SA) beginning just prior to movement onset and sustained through early movement. To determine if observed differences in connectivity are related to behavior, we examined whether variability in subject RT was related to dwPLI. Greater PPC-LPMC β-dwPLI prior to movement onset was correlated with faster reaction times in only the EC condition (Figure 4B, Supplementary Figure 4SB). That is, subjects with greater inter-regional β phase locking ~300 to 100ms prior to movement demonstrated faster RT when externally cued. Correlation between PPC-LPMC β-dwPLI and RT was not observed in the IC condition. Comparison of correlations across conditions using Fisher-z tests demonstrated that R values during EC were significantly different from those associated with the IC condition. There were no significant cross-subject correlations between β-band dwPLI and UPDRS III scores.
Figure 4.
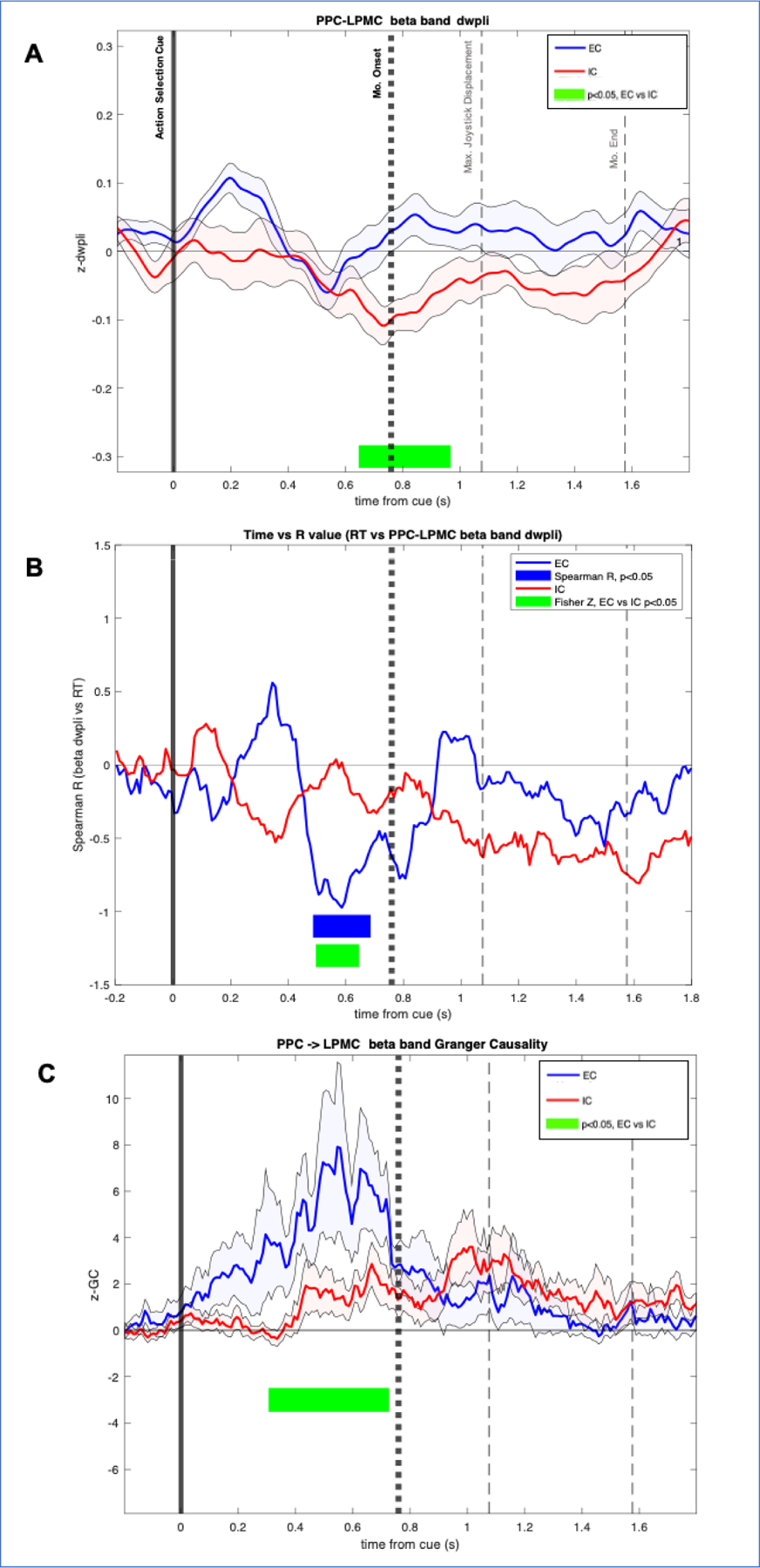
A) Cue locked beta-band debiased weight phase lag index (dwPLI) between posterior parietal cortex (PPC) and lateral premotor cortex (LPMC). Significant differences were observed between externally cued (EC) and internally cued (IC) conditions, occurring around the time of movement onset, and sustained until peak joystick displacement. X-axis is time from cue. Y axis is z-dwPLI. Time periods with significant dwPLI differences between conditions are highlighted with green. Solid vertical line indicates timing of action selection cue. Bold dotted vertical line indicates group averaged movement onset, reaction time (RT). Gray dashed vertical lines indicate maximal joystick displacement and movement end. B) Spearman R values over time. Y axis is cross-subject spearman R value correlating beta-band PPC-LPMC dwPLI at each time point versus RT. Significant R values in the EC condition are highlighted in blue. No significant R values were observed in the IC condition. Significant differences in R values across EC and IC conditions are highlighted in green. C) Cue locked Beta-band Granger Causality (GC) in PPC and LPMC. Y-axis is z-GC. Significant differences between EC and IC conditions are highlighted in green. Max, maximum; Mo, movement; S, seconds.
To test our hypothesis that bottom-up flow from PPC to LPMC supports externally cued movement, we used GC to examine the directionality of PPC-LPMC interactions. PPCàLPMC β-band GC was significantly greater in the EC condition relative to the IC condition prior to movement (Figure 4C). Similar pattern of results was seen in the movement-locked analysis, though it did not reach significance (Supplementary Figure 4SC). There were no significant cross-subject correlations between β-band GC and reaction times. To test frequency band specificity, these connectivity analyses were repeated using α-band frequency (8–12 Hz). There were no significant differences in dwPLI and GC across EC and IC trials. There were no significant correlations between measures of α-connectivity and RT.
4. Discussion
Externally and internally cued movement are distinguished by unique patterns of inter-regional connectivity that are related to behavioral performance, despite lack of statistical differences in power spectral changes in PPC and LPMC between EC and IC conditions. Relative to IC actions, we report relative increase in directional PPC to LPMC connectivity in the β-band during preparation and execution of EC actions. Furthermore, increased strength of inter-site phase synchrony is predictive of faster reaction times, which are pathologically slow in PD patients.(Bloxham et al., 1987) Thus, integration of external cues into immediate action likely involves network level engagement of the PPC-LPMC as indicated by inter-site phase synchrony and directional information flow. Taken together, these findings shed light on the electrophysiological mechanisms that underlie motor facilitation in PD patients, providing support for the various regions that have been implicated by previous fMRI studies,(Debaere et al., 2003, Herz et al., 2014, Jahanshahi et al., 1995, Michely et al., 2015) and confirming findings of electrophysiological dynamics in the dorsal visual stream that have previously been characterized in non-human primates.(Brovelli et al., 2004, Stetson and Andersen, 2014) Moreover, these results highlight that analyses of regional spectral power may be insufficient to distinguish unique network engagement across tasks.
4.1. Power
Although appearing to be different in magnitude, movement-related β-desynchronization was generally statistically indistinguishable in PPC and LPMC between EC and IC conditions. The lack of condition differences in spectro-temporal activity profile during processing of the cue and early movement suggests β-power changes may reflect basic sensory and motor features, which were equivalent across conditions, rather than decision or selection processes dictated by the different cue types. Prior fMRI studies of EC action selection have demonstrated both increased(Debaere et al., 2003) and decreased(Hoffstaedter et al., 2013) BOLD activity in the superior parietal and premotor areas relative to IC tasks. The lack of significant differences in localized power does not settle disagreements in previous reports. Additionally, local metabolic rate may be driven by changes in cross-region synchrony that are not evident in isolated power spectral analysis.
Despite significantly earlier movement onset in the EC condition, the similar onset time of β-desynchronization across conditions shows that movement is delayed relative to β-desynchronization in the IC condition. Thus, there may be other nodes within the IC network, such as the GPi, STN, or medial prefrontal cortex (mPFC), which gate or stop motor behavior, despite cortical “readiness” for movement.(Bichsel et al., 2018, Choi et al., 2020, Rae et al., 2014)
In the LPMC, β-desynchronization was more prolonged in the cue-locked IC condition, leading to significant difference in EC versus IC power late in movement period. In RT locked analyses, the conditional LMPC power difference was not significant, suggesting that the observed power difference was related to faster RT in the EC condition.
4.2. Connectivity
The reliance of the action generated by LPMC on spatial goal-oriented information encoded in the PPC during the EC task(Andersen et al., 2019, Pisella et al., 2000) should elicit a directional increase in communication strength from PPC to LPMC during EC when compared to IC condition as indexed by the ECoG signal from both regions.(Brovelli et al., 2004, Stetson and Andersen, 2014) The increase in directional PPC to LPMC GC connectivity in the 400ms preceding movement onset in the EC compared to IC condition supports this hypothesis, suggesting that sensory cue information encoded by the PPC was indeed more strongly driving preparatory motor-related activity in LPMC in the physiologically relevant β-band. Interestingly, dwPLI between PPC and LPMC on average showed no significant differences during this time, suggesting that the increased β-band GC-connectivity is partially independent of instantaneous phase interactions. Nevertheless, significant relative suppression of dwPLI is present after movement onset in the IC condition and could potentially reflect inflight motor control. For example, LPMC synchronization with the basal ganglia, rather than the dorsal stream, has been described in situations where inflight actions are based on internal specification rather than external targets.(Brittain et al., 2012) Furthermore, internally specified movement requires simultaneous selection and inhibition of competing motor programs, with potential involvement of top-down executive cognitive control processes.(Hoffstaedter et al., 2013, Redgrave et al., 1999) Thus, it is possible that internally generated choice engages similar network nodes as those commonly described in conflict tasks (e.g. STN, mPFC).(Frank, 2006, Hoffstaedter et al., 2013, Mosher et al., 2021, van den Wildenberg et al., 2006) The observed decrease in direct sensory-motor synchrony in the IC condition may represent sensory integration cortices preferentially disengaging with the LPMC in favor of other conflict related inhibitory nodes. Concurrent subcortical and prefrontal recordings must be conducted to clarify these possible mechanisms further. By contrast, the relative maintenance of PPC-LPMC dwPLI during movement execution in the EC condition may be indicative of a direct neural pathway for integration of sensory information into action. Given relative sparing of lateral cortical circuits in PD, bottom-up activation of motor representations may underlie movement facilitation by visual cues as previously proposed.(Grafton, 2004, Lim et al., 2005, Rubinstein et al., 2002)
4.3. Synchrony Predicts Behavior
The observed differences in functional and effective cortical connectivity were, crucially, also associated with improvement in task performance by the patients. Temporally-resolved spearman rank correlation analysis revealed a negative correlation in the β-band dwPLI in the 400ms prior to movement onset, indicating that increasing PPC-LPMC phase synchrony during the premovement period is strongly associated with a decrease in reaction time, thus tying the strength of dorsal stream input to motor cortex to the ability of the external cue to facilitate movement in PD patients. This finding is consistent with the previous dwPLI analysis, indicating that maintaining a higher dwPLI in the EC condition is associated with faster task-execution, whereas this PPC-LPMC synchrony appears to be disposable in the IC condition at no expense to behavioral performance. While the greatest effect of phase-synchrony on behavior occurs during preparation before movement onset, the timing of dwPLI difference across conditions occurs later, during movement. This would suggest that most subjects demonstrate relatively increased phase-synchrony by the time of movement onset in the EC condition; however, only a subset of more rapidly reacting subjects who are more receptive to visual cuing phase-synchronize well before movement onset.
4.4. Limitations
The relatively infrequent opportunity to conduct such intra-operative recordings necessarily leads to a small number of patients (n=13), and thus a susceptibility to false-negative (Type II) error. Given the invasive nature of these experiments, it is not possible to have a healthy control group with which to compare these findings. Future studies exploring non-PD neurosurgical patients are warranted to evaluate whether these findings extend to other populations, or represent PD-specific compensatory mechanisms. The motor task employed in this study is one of several potential formulations for comparison of IC versus EC movement. Notably, movement in the IC condition was not entirely self-initiated as an external imperative cue instructed subjects when to move. An alternative design in which IC actions occur without a visual timing cue make it impossible to measure RT and the behavioral benefits of visuospatial cuing. Furthermore, tasks without timing cue would confound the internal selection of the specific action (e.g. left or right movement) with the decision to move (e.g. when to initiate the action), complicating interpretation of electrophysiological differences from the EC condition in which both the timing and direction of action are specified. Also, this motor task is qualitatively very different from the rhythmic tasks (e.g. walking) in which improvement with cueing is classically present due to limitations inherent in the intraoperative environment; non-invasive techniques are required for evaluation during more ecologically valid tasks. Finally, PD patients were only studied when in an off medication state. The effect of dopaminergic medications on neurophysiological as well as behavioral measures related to cuing require further clarification.
5. Conclusions
Taken together, these results demonstrate that stronger engagement of the PPC-LPMC cortical network by an external cue specifically through the channel of β-modulation is implicated in correcting the pathological slowing of action initiation seen in Parkinson’s patients.(Lim et al., 2005, Rubinstein et al., 2002) Many outstanding questions remain, including the causal role of this PPC-LPMC connection in correcting PD motor slowing with external cues, and the role of high and low β-band segregation in this network, a distinction that is becoming increasingly relevant for understanding the pathophysiology of BGTC dysregulation in PD(Choi et al., 2020) and devising appropriate therapy through DBS.(Wang et al., 2018)
Supplementary Material
Supplementary Figure 3S. Movement onset locked power in posterior parietal cortex (PPC) and lateral premotor cortex (LPMC). Movement related beta-suppression was observed in both PPC and LPMC. This suppression was not cluster significant in the PPC in the externally cued (EC) condition. There were no significant power differences between EC and internally cued (IC) conditions. Black contour indicates cluster significance. X-axis is time from cue. Y-axis is frequency. Color indicates group averaged z-power. Solid vertical line indicates cue onset. Dotted vertical line indicates movement onset, reaction time (RT). Dashed vertical lines indicates maximal joystick displacement and movement end. Hz, hertz; Mov, movement; s, seconds.
Supplementary Figure 4S. A) Movement onset locked beta-band debiased weight phase lag index (dwPLI) between posterior parietal cortex (PPC) and lateral premotor cortex (LPMC). X-axis is time from cue. Y axis is z-dwPLI. Significant differences between EC and IC conditions are highlighted in green. Solid vertical line indicates timing of action selection cue. Bold dotted vertical line indicates group averaged movement onset, reaction time (RT). Gray dashed vertical lines indicate maximal joystick displacement and movement end. B) Spearman R values over time. Y axis is cross-subject spearman R value correlating beta-band PPC-LPMC dwPLI at each time point versus RT. Significant R values in the EC condition are highlighted in blue. No significant R values were observed in the IC condition. C) Cue locked Beta-band Granger Causality (GC) in PPC and LPMC. Y-axis is z-GC. Differences in GC were not cluster significant. Max, maximum; Mo, movement; S, seconds.
Highlights.
Reaction times in Parkinson Disease (PD) patients are faster when action selection is externally cued (EC) relative to self-initiated or internally cued (IC).
Posterior parietal cortex and lateral premotor cortex connectivity is greater during EC relative to the IC action selection.
Delayed action initiation seen in PD patients may be corrected with visuospatial cuing through engagement of dorsal visual stream.
Funding
This work was partially supported by the Dean’s Leadership in Health and Science Scholarship at the David Geffen School of Medicine at UCLA and by R01 NS097782 (NP). Study sponsors were not directly involved in the production of this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest
None of the authors have potential conflicts of interest to be disclosed.
References
- Andersen RA, Aflalo T, Kellis S. From thought to action: The brain-machine interface in posterior parietal cortex. Proc Natl Acad Sci U S A 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichsel O, Gassert R, Stieglitz L, Uhl M, Baumann-Vogel H, Waldvogel D, et al. Functionally separated networks for self-paced and externally-cued motor execution in Parkinson’s disease: Evidence from deep brain recordings in humans. Neuroimage 2018;177:20–9. [DOI] [PubMed] [Google Scholar]
- Bloxham CA, Dick DJ, Moore M. Reaction times and attention in Parkinson’s disease. J Neurol Neurosurg Psychiatry 1987;50(9):1178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 1997;10(4):433–6. [PubMed] [Google Scholar]
- Brass M, Haggard P. The what, when, whether model of intentional action. Neuroscientist 2008;14(4):319–25. [DOI] [PubMed] [Google Scholar]
- Brittain JS, Watkins KE, Joundi RA, Ray NJ, Holland P, Green AL, et al. A Role for the Subthalamic Nucleus in Response Inhibition during Conflict. J Neurosci 2012;32(39):13396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brovelli A, Ding M, Ledberg A, Chen Y, Nakamura R, Bressler SL. Beta oscillations in a large-scale sensorimotor cortical network: directional influences revealed by Granger causality. Proc Natl Acad Sci U S A 2004;101(26):9849–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buneo CA, Andersen RA. The posterior parietal cortex: sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia 2006;44(13):2594–606. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Martinez-Vazquez P, Gail A. Synchronization patterns suggest different functional organization in parietal reach region and dorsal premotor cortex. J Neurophysiol 2014;112(12):3138–53. [DOI] [PubMed] [Google Scholar]
- Chen YH, Bressler SL, Ding MZ. Frequency decomposition of conditional Granger causality and application to multivariate neural field potential data. J Neurosci Meth 2006;150(2):228–37. [DOI] [PubMed] [Google Scholar]
- Choi JW, Malekmohammadi M, Sparks H, Kashanian A, Cross KA, Bordelon Y, et al. Altered Pallidocortical Low-Beta Oscillations During Self-Initiated Movements in Parkinson Disease. Front Syst Neurosci 2020;14:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuparu A, Muresan RC. Sources of bias in single-trial normalization procedures. Eur J Neurosci 2016;43(7):861–9. [DOI] [PubMed] [Google Scholar]
- Cohen MX. Analyzing neural time series data: theory and practice Cambridge, Mass; London: MIT Press, 2014. [Google Scholar]
- Cui J, Xu L, Bressler SL, Ding M, Liang H. BSMART: a Matlab/C toolbox for analysis of multichannel neural time series. Neural Netw 2008;21(8):1094–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. Neuroimage 2002;15(2):373–85. [DOI] [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Internal vs external generation of movements: differential neural pathways involved in bimanual coordination performed in the presence or absence of augmented visual feedback. Neuroimage 2003;19(3):764–76. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Lopez-Cruz PL, Benavides-Piccione R, Bielza C, Larranaga P, Anderson S, et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci 2013;14(3):202–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 2004;134(1):9–21. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Epstein CM, Turner RS, Prablanc C, Alexander GE, Grafton ST. Role of the posterior parietal cortex in updating reaching movements to a visual target. Nat Neurosci 1999;2(6):563–7. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends Cogn Sci 2000;4(11):423–31. [DOI] [PubMed] [Google Scholar]
- Ding MZ, Bressler SL, Yang WM, Liang HL. Short-window spectral analysis of cortical event-related potentials by adaptive multivariate autoregressive modeling: data preprocessing, model validation, and variability assessment. Biol Cybern 2000;83(1):35–45. [DOI] [PubMed] [Google Scholar]
- Frank MJ. Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making. Neural Netw 2006;19(8):1120–36. [DOI] [PubMed] [Google Scholar]
- Goldberg G. Supplementary Motor Area Structure and Function - Review and Hypotheses. Behav Brain Sci 1985;8(4):567–88. [Google Scholar]
- Goldman JG, Vernaleo BA, Camicioli R, Dahodwala N, Dobkin RD, Ellis T, et al. Cognitive impairment in Parkinson’s disease: a report from a multidisciplinary symposium on unmet needs and future directions to maintain cognitive health. NPJ Parkinsons Dis 2018;4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale MA. Visuomotor control: where does vision end and action begin? Curr Biol 1998;8(14):R489–91. [DOI] [PubMed] [Google Scholar]
- Grafton ST. Contributions of functional imaging to understanding parkinsonian symptoms. Curr Opin Neurobiol 2004;14(6):715–9. [DOI] [PubMed] [Google Scholar]
- Granger CWJ. Investigating Causal Relations by Econometric Models and Cross-Spectral Methods. Econometrica 1969;37(3):424–38. [Google Scholar]
- Haggard P. Human volition: towards a neuroscience of will. Nat Rev Neurosci 2008;9(12):934–46. [DOI] [PubMed] [Google Scholar]
- Haggard P The Neurocognitive Bases of Human Volition. Annu Rev Psychol 2019;70:9–28. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Fukuyama H, Katsumi Y, Honda M, Shibasaki H. Enhanced lateral premotor activity during paradoxical gait in Parkinson’s disease. Ann Neurol 1999;45(3):329–36. [DOI] [PubMed] [Google Scholar]
- Helmich RC, Aarts E, de Lange FP, Bloem BR, Toni I. Increased dependence of action selection on recent motor history in Parkinson’s disease. J Neurosci 2009;29(19):6105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz DM, Eickhoff SB, Lokkegaard A, Siebner HR. Functional neuroimaging of motor control in Parkinson’s disease: a meta-analysis. Hum Brain Mapp 2014;35(7):3227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstaedter F, Grefkes C, Zilles K, Eickhoff SB. The “what” and “when” of self-initiated movements. Cereb Cortex 2013;23(3):520–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain 1995;118:913–33. [DOI] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, Mishkin M. A new neural framework for visuospatial processing. Nat Rev Neurosci 2011;12(4):217–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Yoo JY, Ryu JS, Park HK, Chung SJ. The effects of visual and auditory cues on freezing of gait in patients with Parkinson disease. Am J Phys Med Rehabil 2012;91(1):2–11. [DOI] [PubMed] [Google Scholar]
- Lim I, van Wegen E, de Goede C, Deutekom M, Nieuwboer A, Willems A, et al. Effects of external rhythmical cueing on gait in patients with Parkinson’s disease: a systematic review. Clin Rehabil 2005;19(7):695–713. [DOI] [PubMed] [Google Scholar]
- MacKay WA, Mendonca AJ. Field potential oscillatory bursts in parietal cortex before and during reach. Brain Res 1995;704(2):167–74. [DOI] [PubMed] [Google Scholar]
- Malekmohammadi M, Price CM, Hudson AE, DiCesare JAT, Pouratian N. Propofol-induced loss of consciousness is associated with a decrease in thalamocortical connectivity in humans. Brain 2019;142(8):2288–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malekmohammadi M, Sparks H, AuYong N, Hudson A, Pouratian N. Propofol Anesthesia Precludes LFP-Based Functional Mapping of Pallidum during DBS Implantation. Stereotact Funct Neurosurg 2018;96(4):249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 2007;164(1):177–90. [DOI] [PubMed] [Google Scholar]
- Michely J, Volz LJ, Barbe MT, Hoffstaedter F, Viswanathan S, Timmermann L, et al. Dopaminergic modulation of motor network dynamics in Parkinson’s disease. Brain 2015;138:664–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher CP, Mamelak AN, Malekmohammadi M, Pouratian N, Rutishauser U. Distinct roles of dorsal and ventral subthalamic neurons in action selection and cancellation. Neuron 2021;109(5):869–81 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keeffe AB, Malekmohammadi M, Sparks H, Pouratian N. Synchrony Drives Motor Cortex Beta Bursting, Waveform Dynamics, and Phase-Amplitude Coupling in Parkinson’s Disease. J Neurosci 2020;40(30):5833–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso JA, Stamelou M, Goetz CG, Poewe W, Lang AE, Weintraub D, et al. Past, present, and future of Parkinson’s disease: A special essay on the 200th Anniversary of the Shaking Palsy. Mov Disord 2017;32(9):1264–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011;2011:156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisella L, Grea H, Tilikete C, Vighetto A, Desmurget M, Rode G, et al. An ‘automatic pilot’ for the hand in human posterior parietal cortex: toward reinterpreting optic ataxia. Nat Neurosci 2000;3(7):729–36. [DOI] [PubMed] [Google Scholar]
- Rae CL, Hughes LE, Weaver C, Anderson MC, Rowe JB. Selection and stopping in voluntary action: a meta-analysis and combined fMRI study. Neuroimage 2014;86:381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo MJ, Kondylis ED, Alhourani A, Wozny TA, Lipski WJ, Crammond DJ, et al. Three-dimensional localization of cortical electrodes in deep brain stimulation surgery from intraoperative fluoroscopy. Neuroimage 2016;125:515–21. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. The basal ganglia: a vertebrate solution to the selection problem? Neuroscience 1999;89(4):1009–23. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, et al. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci 2010;11(11):760–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Matelli M. Two different streams form the dorsal visual system: anatomy and functions. Exp Brain Res 2003;153(2):146–57. [DOI] [PubMed] [Google Scholar]
- Rossetti Y, Revol P, McIntosh R, Pisella L, Rode G, Danckert J, et al. Visually guided reaching: bilateral posterior parietal lesions cause a switch from fast visuomotor to slow cognitive control. Neuropsychologia 2005;43(2):162–77. [DOI] [PubMed] [Google Scholar]
- Rubinstein TC, Giladi N, Hausdorff JM. The power of cueing to circumvent dopamine deficits: a review of physical therapy treatment of gait disturbances in Parkinson’s disease. Mov Disord 2002;17(6):1148–60. [DOI] [PubMed] [Google Scholar]
- Schlogl A, Supp G. Analyzing event-related EEG data with multivariate autoregressive parameters. Prog Brain Res 2006;159:135–47. [DOI] [PubMed] [Google Scholar]
- Seth AK. A MATLAB toolbox for Granger causal connectivity analysis. J Neurosci Methods 2010;186(2):262–73. [DOI] [PubMed] [Google Scholar]
- Sheth BR, Young R. Two Visual Pathways in Primates Based on Sampling of Space: Exploitation and Exploration of Visual Information. Front Integr Neurosci 2016;10:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaulding SJ, Barber B, Colby M, Cormack B, Mick T, Jenkins ME. Cueing and gait improvement among people with Parkinson’s disease: a meta-analysis. Arch Phys Med Rehabil 2013;94(3):562–70. [DOI] [PubMed] [Google Scholar]
- Stetson C, Andersen RA. The parietal reach region selectively anti-synchronizes with dorsal premotor cortex during planning. J Neurosci 2014;34(36):11948–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Wildenberg WP, van Boxtel GJ, van der Molen MW, Bosch DA, Speelman JD, Brunia CH. Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson’s disease. J Cogn Neurosci 2006;18(4):626–36. [DOI] [PubMed] [Google Scholar]
- Velu PD, Mullen T, Noh E, Valdivia MC, Poizner H, Baram Y, et al. Effect of visual feedback on the occipital-parietal-motor network in Parkinson’s disease with freezing of gait. Front Neurol 2014;4:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck M, Oostenveld R, van Wingerden M, Battaglia F, Pennartz CM. An improved index of phase-synchronization for electrophysiological data in the presence of volumeconduction, noise and sample-size bias. Neuroimage 2011;55(4):1548–65. [DOI] [PubMed] [Google Scholar]
- Wang DD, de Hemptinne C, Miocinovic S, Ostrem JL, Galifianakis NB, San Luciano M, et al. Pallidal Deep-Brain Stimulation Disrupts Pallidal Beta Oscillations and Coherence with Primary Motor Cortex in Parkinson’s Disease. J Neurosci 2018;38(19):4556–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Hallett M, Chan P. Motor automaticity in Parkinson’s disease. Neurobiol Dis 2015;82:226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SA, van den Wildenberg WP, Ridderinkhof KR, Bashore TR, Powell VD, Manning CA, et al. The effect of Parkinson’s disease on interference control during action selection. Neuropsychologia 2009;47(1):145–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapparoli L, Seghezzi S, Scifo P, Zerbi A, Banfi G, Tettamanti M, et al. Dissecting the neurofunctional bases of intentional action. Proc Natl Acad Sci U S A 2018;115(28):7440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala BA, Tan HL, Little S, Ashkan K, Hariz M, Foltynie T, et al. Midline Frontal Cortex Low-Frequency Activity Drives Subthalamic Nucleus Oscillations during Conflict. J Neurosci 2014;34(21):7322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 3S. Movement onset locked power in posterior parietal cortex (PPC) and lateral premotor cortex (LPMC). Movement related beta-suppression was observed in both PPC and LPMC. This suppression was not cluster significant in the PPC in the externally cued (EC) condition. There were no significant power differences between EC and internally cued (IC) conditions. Black contour indicates cluster significance. X-axis is time from cue. Y-axis is frequency. Color indicates group averaged z-power. Solid vertical line indicates cue onset. Dotted vertical line indicates movement onset, reaction time (RT). Dashed vertical lines indicates maximal joystick displacement and movement end. Hz, hertz; Mov, movement; s, seconds.
Supplementary Figure 4S. A) Movement onset locked beta-band debiased weight phase lag index (dwPLI) between posterior parietal cortex (PPC) and lateral premotor cortex (LPMC). X-axis is time from cue. Y axis is z-dwPLI. Significant differences between EC and IC conditions are highlighted in green. Solid vertical line indicates timing of action selection cue. Bold dotted vertical line indicates group averaged movement onset, reaction time (RT). Gray dashed vertical lines indicate maximal joystick displacement and movement end. B) Spearman R values over time. Y axis is cross-subject spearman R value correlating beta-band PPC-LPMC dwPLI at each time point versus RT. Significant R values in the EC condition are highlighted in blue. No significant R values were observed in the IC condition. C) Cue locked Beta-band Granger Causality (GC) in PPC and LPMC. Y-axis is z-GC. Differences in GC were not cluster significant. Max, maximum; Mo, movement; S, seconds.


