Abstract
Ultrasounds (US) are a non-ionizing mechanical wave, with less adverse effects than conventional pharmacological or surgical treatments. Different biological effects are induced in tissues and cells by ultrasound actuation depending on acoustic parameters, such as the wave intensity, frequency and treatment dose. This non-ionizing radiation has considerable applications in biomedicine including surgery, medical imaging, physical therapy and cancer therapy. Depending on the wave intensity, US are applied as high-intensity ultrasounds (HIUS) and low-intensity pulsed ultrasounds (LIPUS), with different effects on cells and tissues. HIUS produce thermal and mechanical effects, resulting in a large localized temperature increase, leading to tissue ablation and even tumor necrosis. This can be achieved by focusing low intensity waves emitted from different electrically shifted transducers, known as high-intensity focused ultrasounds (HIFU). LIPUS have been used extensively as a therapeutic, surgical and diagnostic tool, with diverse biological effects observed in tissues and cultured cells. US represent a non-invasive treatment strategy that can be applied to selected areas of the body, with limited adverse effects. In fact, tumor ablation using HIFU has been used as a curative treatment in patients with an early-stage pancreatic tumor and is an effective palliative treatment in patients with advanced stage disease. However, the biological effects, dose standardization, benefit-risk ratio and safety are not fully understood. Thus, it is an emerging field that requires further research in order to reach its full potential.
Key words: ultrasounds, cancer therapy, high-intensity ultrasounds (HIUS), low-intensity pulsed ultrasounds (LIPUS), cancer treatment, new therapies
Introduction
Ultrasounds (US) are mechanical waves with frequencies higher than 15kHz, beyond the audible range of the human ear. Parameters such as the intensity of the acoustic waves or the ultrasound dose determine different effects on the treated samples. US have been used in medicine for therapeutic purposes for many years and are extensively used as a diagnostic tool. Low-frequency ultrasounds have been used since the 1950s in physiotherapy to treat tendinitis or bursitis.1,2 In the 1980s, they were used to treat kidney stones, and were replaced by the more aggressive technique of lithotipsy.3,4 Today, this source of energy continues to be used in the clinic for uterine fibrous ablation,5 cataract removal,6 surgical and hemostasis tissue cutting,7 transdermal drug introduction, 8,9 and bone fractures,10,11 among others.4 US are currently used as an experimental treatment, either alone or in combination with drugs, in diseases such as cancer,12,13 diabetes,9 stroke,14 and thrombosis.15
Biological effects of ultrasounds
Low-intensity pulsed ultrasounds (LIPUS) generally use 1-2 MHz frequency pulses, with a pulse width of 200 μs, repeated at 1 kHz and very low spatial average temporal intensities (<3W/cm2).16 In particular, LIPUS are used extensively in medicine as a therapeutic and diagnostic tool.17–19 The application of LIPUS has minimal thermal effects on tissues and is therefore considered a non-invasive and safe technique.20–27 Tissue properties affect the response, so each tissue requires different LIPUS parameters to achieve a specific stimulation. Therefore, it is important to evaluate the biological effects of different intensities, frequencies or application cycle of LIPUS on each specific cell type or tissue sample. LIPUS actuation stimulates stem cell growth and differentiation.20,22,28 Some studies reported mechanical angiogenesis enhancement.29,30 The effects of low-intensity continuous ultrasounds (LICUS) were studied in a recent paper31 on the inflammatory response of mouse pancreatic tumor explants. The authors found significant upregulation of IFN-γ, IL-1β and TNF-α in the pancreatic tumor explants. They assessed the LICUS effects on tumor vasculature and collagen I deposition and demonstrated that LICUS is minimally invasive to the structure and morphology of the tissue.
Low-intensity ultrasounds (LIUS) are also useful in the clinic to stimulate physiological responses or accelerate the transport of drugs through the skin.32 These waves open pores in the membrane temporarily, allowing drugs, proteins and genetic material to pass through, as described in the literature.33,34 Gene therapy and especially the action of antineoplastic drugs, require the entry of a large number of molecules into the cell to carry out their action. It has also been shown that LIUS enhance molecular transport of small molecules, macromolecules and genetic material through the membranes of living cells, in a reversible manner. However, a great heterogeneity of the effects among adjacent cell subpopulations was observed in these studies.35–41
High-intensity ultrasound (HIUS) relies on the same principles as conventional ultrasound but refer to intensities >5W/cm2, which produce thermal and mechanical effects, generating a large localized temperature increase in the tissues. The risks of this procedure are related to non-target specific sonification (when tissue surrounding the area being treated is affected by the ultrasound waves). Thus, HIFU allows a precise treatment of targeted areas without injury to the surrounding soft tissue. Therefore, biomedical applications of these powered ultrasounds always refer to HIFU. This can be achieved by focusing low intensity waves emitted from different electrically shifted transducers arranged in 1D or 2D arrays, allowing the selection of small areas of the body for application with limited adverse effects in the surroundings tissues. The waves emitted from the transducers propagate harmlessly through living tissue with a low intensity, until reaching the focused area, where the resulting acoustic energy increases and causes a local rise in temperature. One application of this tool is noninvasive ultrasound surgery, in which a HIFU beam is focused within the body to induce rapid localized heating of tumor tissues. 42 Different clinical applications of HIFU have specific requirements for the pressure levels and degree of non-linear waveform distortion at the focus. HIFU provides a completely non-invasive ablation. In contrast to ionizing radiation, HIFU treatment can be applied more than once, as there is no upper limit of tissue tolerance to repeated ultrasound exposure. Part of the energy of an ultrasonic wave travelling through a tissue is transformed into heat, which dissipates very rapidly in normal conditions and low intensities. However, if the rate of heating exceeds the rate of cooling, the tissue temperature increases as a result of the ultrasonic actuation. In fact, HIFU actuation is based on this effect and a temperature of 56°C is reported in the literature as the threshold of thermal toxicity, with reproductive failure preceding irreversible cell death through coagulative necrosis.8,43 In the context of HIFU, the temperature at the focus can rise rapidly above 80°C,44 and even short exposures should lead to effective cell killing.45
During the last decade, many novel therapeutic applications have been rapidly developed for HIFU. Even though thermal damage is the most reported effect of HIFU, non-thermal mechanical destruction may play an even more relevant role in HIFU ablation, particularly regarding the immunomodulated effect.46 Mechanical ablation rather than thermal ablation seems to result in less damage to the surrounding tissue, as the mechanical effect is not impacted by heat perfusion via blood flow, and the treated area is more precisely defined as it coincides with the ultrasound focal region.47 One of the most important differences in mechanical ablation as opposed to thermal ablation is the absence of protein coagulation necrosis. Tissue exposure to repeated short duration pulses with low duty cycles of HIFU result in mechanical disruption and the tissue is fractionated in a controlled manner. Another mechanism associated with mechanical tissue destruction in HIFU ablation is the acoustic cavitation induced by the HIUS. Cavitation results from oscillation and subsequent violent collapse and energy dispersion of microbubbles in the targeted tissue because of their interaction with the ultrasonic energy.48 Another important effect that may be achieved with HIFU is the so-called “histotripsy”, which is a mechanical fractionation and emulsification of the tissue into liquid-appearing acellular homogenates produced by high pressure ultrasound pulses.49 The HIFU approach has been already used to treat tumors in various organs: prostate,50 uterus,51 kidneys,52 liver,53 breast,54,55 pancreas,32 and bone.56 Recently, the first successful surgeries were performed on the brain for treating essential tremor using ultrasound irradiation through the skull.57 In the reported literature, cavitation has sometimes been related to vascular damage and disruption, which can cause local and potentially systemic effects in tumor treatment.58,59 Some studies have shown enhanced therapeutic effects when acoustic cavitation is combined with radiation or chemotherapy.60 The clinical applications of HIFU are summarized in Table 1 and will be discussed in detail below.
Effects of ultrasounds on gene expression and apoptosis
Various studies have shown that US have a bilateral effect on gene expression in living tissues. Firstly, Tabuchi et al. demonstrated in 2008 that US alter gene expression in vitro in cultures of differentiated monocytes originating from human lymphoma (U937 line).61 This study showed a decrease in the expression of 193 genes associated with cell growth, proliferation and development, and an increase in the expression of 201 genes related to cell movement, morphological changes and apoptosis.
Kruse et al. demonstrated in 2008 that increased apoptosis was reflected by an increase in mRNA expression of HSP-70 (heatshock protein 70) in vivo.62 Subsequent studies performed in vitro and in vivo, have shown that US at specific wave amplitudes and dose conditions induce cell death in different types of tumors, including leukemia, squamous cell carcinoma and sarcoma under certain acoustic conditions.27–34 After exposure to US, cells show more classic morphological and molecular features of apoptosis, such as condensation of the nucleus, changes in chromatin position and increased expression of membrane phosphatidylserine. Furthermore, there are changes in the expression of apoptotic BCL-2, such as BAX, BAD and Bak, which promotes the activation of the mitochondrial intrinsic apoptotic pathway releasing cytochrome C to the cytosol, resulting in the activation of caspases 3 and 9.63-71
Activation of gaseous bodies are closely related mechanisms that depend on the amplitude of the waves. They can cause bleeding in blood vessels and cell death due to different types of damage to the cell membrane, inducing the formation of surface pores.4 The application of LIUS to human cells opens pores in the membrane temporarily, allowing drugs, proteins and genetic material to pass through, as described in the literature.33,34 Gene therapy and especially the action of antineoplastic drugs, require the entry of a large number of molecules into the cell to carry out their action. It has been shown that LIUS enhance molecular transport of small molecules, macromolecules and genetic material through the membranes of living cells, in a reversible manner. However, a great heterogeneity of the effects among adjacent cell subpopulations was observed in these studies.35–41 Other relevant effects have been reported in the literature during the last decades.
The effect of ultrasounds on ABC receptors and multidrug resistance (MDR)
Overexpression of the so-called ABC channels (ATP-binding cassette) is a problem in cancer treatment patients in general. These channels actively transport a variety of drugs (most of them amphipathic) from inside of the cell to the outside, leading to the appearance of multidrug resistance (MDR), which leads to a significant decrease in the effectiveness of chemotherapeutic treatments. MDR is a phenomenon that occurs when cancer cells develop resistance to chemotherapeutic agents, whose structure is very different from one another.72–75 The development of resistance to chemotherapy is the main cause of treatment failure, affecting several types of solid tumours.76 ABC transporters are membrane proteins that possess both transmembrane domains (TMDs) and nucleotide-binding domains (NBDs). The latter generate energy obtained from ATP hydrolysis to achieve active counter-gradient transport across the membrane.72,76 Within this family, different members are distinguished according to small variations in the domains that make up the protein, with some members in cancer cells being particularly noteworthy, such as ABCB1 (P-glycoprotein), ABCC1 (MRP1, multidrug resistance-associated protein 1) and ABCG2 (MXR, BCRP). All of them can transport a wide range of substrates, such as ions, sugars, amino acids, lipids, toxins and chemotherapeutic drugs.77,78 After exposure of hepatocarcinoma cells to US, MDR cells became more sensitive to anti-tumor drugs.79 However, the mechanism of action of the US (such as heat, cavitation, etc.) to modulate the ABC transporters in the membrane in not clear. Therefore, further studies are needed in this area, which could be an important therapeutic improvement in the clinic.
The use of high-intensity focused ultrasounds in pancreatic cancer
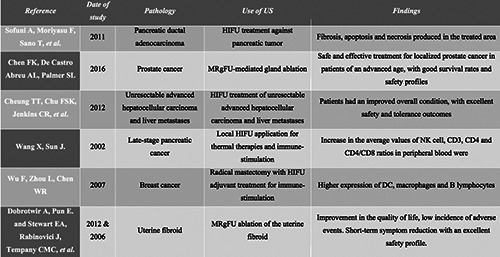
Pancreatic ductal cell adenocarcinoma (PDAC) is the most common form of pancreatic cancer, with around 367,000 new cases diagnosed each year worldwide and approximately 359,000 deaths each year.80 PDAC is associated with an extremely poor prognosis and has a mortality rate of 98%.81 A study showed that PDAC treated with HIFU resulted in fibrosis and necrosis in the treated area due to the rapid increase in temperature produced by the HIFU. Furthermore, apoptosis was induced in other damaged cells, activating the degradation of their DNA by endonuclease.82 Tumor ablation using HIFU has been shown to be curative in patients with an early-stage tumor and is also an effective palliative treatment in patients with advanced stage disease (TNM II-IV). This technique has been used and obtained good results in Asian countries such as China, Japan and South Korea, as well as in some European countries since the 1990s, although it has not been approved by the FDA in the United States.83 However, there is a varying response to this type of treatment, as some patients with wounds or scars (whether cutaneous or subcutaneous) absorb the acoustic energy to a greater extent, resulting in severe burns.84 Furthermore, there is no standardized dose of HIFU and the treatment is modified based on the empirical experience of each patient. Thus, more research is needed in this field to define the most effective application parameters that minimize adverse effects.
High-intensity focused ultrasounds and magnetic resonance- guided focused ultrasound in the treatment of prostate cancer
Prostate cancer is the most common non-cutaneous cancer in males, with nearly 1,600,000 cases and 366,000 deaths a year. In spite of the medical advances in the last years, prostate cancer remains a clinical challenge, as treatment for metastatic disease are inadequate and inefficient in many cases.85 The most important and treatment options for clinically localized prostate cancer accepted worldwide include radical prostatectomy, external beam radiation and brachytherapy. A recently discovered novel treatment option for localized prostate cancer is the use of transrectal HIFU. This therapeutic option has been optimized using a magnetic resonance imaging approach, magnetic resonance-guided focused ultrasound (MRgFUS) instead of ultrasound guidance.86 Many studies that compared conventional techniques such as wholeprostate gland surgical ablation and HIFU-mediated gland ablation, have concluded that HIFU may be a safe and effective treatment for localized prostate cancer in patients of an advanced age. In fact, the life expectancy in 97% of these individuals is around 10 years. On the other hand, MRgFUS-mediated gland ablation has some adverse effects, such as bladder outlet obstruction, urinary incontinence of diverse grades, urethral stricture, epididymitis and rectourethral fistula.50,87,88 The application of HIFU in the treatment of clinically localized prostate cancer is promising, with good survival rates and safety profiles. The majority of studies conclude further research is needed to understand this technology and to perfect the technique in this disease.
Table 1.
A summary of the clinical application of high-intensity focused ultrasounds in different tumor types and the induced effects.
High-frequency focused ultrasounds-based treatment of hepatocellular carcinoma and liver metastases
Hepatocellular carcinoma is the most frequent primary liver cancer and has been an important medical problem with limited progress in the prevention, detection, diagnosis and treatment for the last 20 years. There are 782,000 cases diagnosed and 746,000 deaths each year and this pathology is ranked as the sixth most common neoplasm and the third leading cause of cancer death. Due to the good accessibility of the liver compared with other organs, US (in this case HIFU) are a versatile tool that can be used for the treatment of both unresectable advanced hepatocellular carcinoma and liver metastases (mainly from colorectal and pancreatic tumors). Patients that received this treatment had an improved overall condition, with excellent safety and tolerance outcomes, even those with advanced cirrhosis and high-grade Child-Pugh score.89 Cheung et al. reported an interesting study in which 100 cirrhotic patients were treated with HIFU ablation, which aimed to identify predictive factors for HIFU intolerance. This study found that 13% of the patients reported complications, mainly skin burn of various grades,89 and the results suggested that age was the only predictive factor for complications. Furthermore, they concluded that HIFU might represent a well-tolerated new alternative treatment, even for high Child-Pugh score patients. In addition, HIFU are safe and effective in the treatment of tumors adjacent to major vessels, which suppose a high-risk surgery in most cases. However, the application of HIFU to the liver also has to overcome some physical obstacles such as the ribs, interposed lung parenchyma and respiratory motion of the liver itself.90 This therapy has produced some rare complications such as rib fractures, pneumothorax, pleural effusion, biliary obstruction and fistula formation.91 Although, some recent studies demonstrate the possibility to use HIFU with trans-arterial chemoembolization in the treatment of hepatocellular carcinoma, with very promising results.92
Immunomodulation effects of thermal therapies and high-intensity focused ultrasounds ablation
In the last decade, there has been a huge interest in the role of the immune system in the treatment of cancer.93 In fact, one of the most relevant and revolutionary findings is that there is a systemic response after local ablative surgery, meaning that there is a global immuno-stimulation effect from local ablative techniques, which has a huge potential in the clinic.94,95 The immune response observed after HIFU local ablation treatment is very interesting due to the effects of cavitation, which does not produce thermal destruction of the tissue and denaturation of the proteins and could be a mechanism of systemic immunostimulation.96 One theory is that the application of HIFU produces cancer cell debris after treatment that remains at the original tumor site as a source of antigens for the immune system. These antigens act as an in situ vaccine that stimulate the systemic immune response of T-cells and cytokines.97 Phagocytic cells from the cytotoxic immune system that normally reside in the damaged tissue, will capture these tumor antigens from damaged or dead cells after the local ablation. Subsequently, these cells migrate through the tumor-draining lymph nodes and passively enter the systemic circulation, where they can be taken up by lymph-node-resident phagocytic cells. This type of mechanism is essential for tumor specific immune responses,46 and different studies have shown that T-cells that recognize clonal neoantigens were detected in patients with good clinical outcomes.
This type of global immune-response (in another or similar tissue remote from the treated site) from a local treatment or ablation of a tumor tissue is called the “abscopal effect”, which was first described in 1953,98 and has since been widely reported in the literature. This “abscopal effect” plays an important role in tumor control,99–101 increasing the presence of MHC I on the tumor cell surface and the production of specific cytokines that facilitates the migration and function of effector CD8 +T cells.93,98 Furthermore, the development of antibodies aid in local tumor eradication, the control of metastases establishment and the development of an immunologic memory against cancer cells.99Wang and Sun reported a study based on the use of HIFU treatment of 15 patients with late-stage pancreatic cancer. Changes in natural killer NK cell activity and T lymphocyte and subsets were observed in 10 cases. Furthermore, the average values of NK cell, CD3, CD4 and CD4/CD8 ratios in peripheral blood were increased after HIFU application.102 In a study conducted by Wu et al.96,103, 48 females with biopsy-proven breast cancer were randomized to receive only radical mastectomy or radical mastectomy with HIFU adjuvant treatment. The results were very promising, with a significantly higher expression of DC, macrophages and B lymphocytes (measured in the primary breast tumor and also in the axillary lymph nodes) in the HIFU group. However, the immune-stimulation effects of local ablative treatments (especially with HIFU) are not fully understood and further studies on the technique are needed. Still, HIFU ablation might represent the ideal ablative technique, as it achieves mechanical ablation through different mechanisms.
High-intensity focused ultrasounds and magnetic resonance- guided focused ultrasound for the treatment of uterine fibroids
Uterine fibroids are benign smooth muscle cell tumor of the uterus and are most commonly found in female pelvic tumours104. Uterine fibroids may cause personal, social and financial problems for some women. HIFU have been used in the treatment of uterine fibroids during the last years, resulting in a reduction in pain and pressure, frequent urination and/or constipation and excessive menstrual bleeding. This treatment has fewer complications in comparison to surgery, with faster recovery times.105,106 MRgFUS is a non-invasive and relatively new therapy for the treatment of uterine fibroids based on the HIFU. In fact, this treatment has been approved by the US Food and Drug Administration in 2004 and the Therapeutics Goods Administration in 2007.107 MRgFUS is especially effective in selected patients with symptomatic uterine fibroids,106 and is carried out with conscious intravenous sedation. A focused ultrasound wave passes through the anterior abdominal wall and adjacent tissue, to focus on and deliver energy to the uterine fibroid(s),108 and ablate the uterine fibroid with multiple sonications that target different parts of the fibroid. The duration of treatment is thus dependent on the volume of the fibroid and treatment times vary with the size of the target fibroid. However, this technique has rare but well-defined complications, such as hematuria due to bladder heating, skin burns or pain after the procedure.
Dobrotwir et al. showed that HIFU were safe and effective with no significant complications reported during treatment or follow- up in a study of 100 patients with uterine fibroids.106 Stewart et al. performed a similar study, which assessed outcomes at 6 and 12 months after MRgFUS treatment for symptomatic uterine fibroids.109 In this study, 71% of females undergoing MRgFUS reached the targeted symptom reduction at 6 months and 51% at 12 months. The magnitude of improvement in the quality of life was greater than predicted in both studies. Furthermore, the incidence of adverse events was also low.109 MRgFUS treatment results in short-term symptom reduction and an improved quality of life in women with symptomatic uterine fibroids, with an excellent safety profile.
Clinical trials using ultrasound technologies
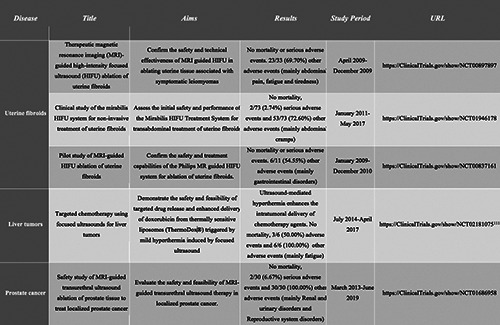
According to the ClinicalTrials.gov website (https://clinicaltrials. gov/, last access 09/06/2021), there are 145 clinical trials based on ultrasounds in diverse tumor types and pathologies, with 142 studies related with HIFU treatment (Supplementary Table 1) and 3 related to LIPUS (Supplementary Table 2). Among the clinical trials using HIFU treatment, 44 are completed studies, accounting for almost 31% (Supplementary Table 1). These trials are mainly based on prostate and breast cancer, thyroid nodules, bone metastasis and uterine fibroids. Currently, only 5 of the 44 completed studies have reported results (Table 2), representing only 11% of the completed studies and 3% of the total clinical trials using this technology. The clinical trials based on LIPU include patients with primary brain tumors and brain metastasis and no results have been reported yet. Interestingly, two trials are testing the use of the low intensity ultrasound implant device, SONOCLOUD, in glioblastoma and brain metastasis.
The future of ultrasounds in cancer therapy
Ultrasounds appear to be a very promising novel technology for the non-invasive treatment of cancer. The HIFU variant of US could be the most effective ablative technique in order to achieve an immune-stimulation effect against cancer cells and the induction of a long-term immunity against cancer. Treatments based on HIFU developed against hepatocellular carcinoma, pancreatic cancer and uterine fibroids have proved to be safe and effective, although they need optimization to exploit its therapeutic potential. However, there is still a deficiency in the knowledge of the biological and biodynamical effects that LIUS generates in human tissues and its effects on organs and the whole body. Results obtained up to now are very promising and there could be a revolution in the clinical field over the next years. The biological effects and mechanisms of action of the LIUS on tissues and cells are not well known and need to be investigated, although it has been proven that they can be very different depending on the acoustic parameters of actuation, the dose and type of tissues on which the US are applied.
In particular, LIUS may have an important role in the cancer treatment by targeting the stroma or enhancing the effect of antineoplastic drugs,110 and they offer a non-invasive treatment approach that can be applied in selected areas. The tumor stroma is composed of extracellular matrix (ECM) proteins such as collagens, fibronectin, laminin, glycoproteins, proteoglycans and glycosaminoglycans and cell types such as immune cells, cancer associated fibroblasts, endothelial cells and neuronal cells. Further studies are needed to decipher the effects of LIUS on parameters such as tumor growth and progression, cell migration and cell signaling pathways and the effects on the associated stroma cells such as cancer-associated fibroblasts, macrophages and endothelial cells. Several tumors such as breast, pancreas and colorectal cancer have a dense tumor stroma, which leads to a high resistance to the most conventional treatment options, including chemotherapy or molecular treatments such as immunotherapy. Targeting the stroma as a treatment strategy in solid aggressive tumors has gained a lot of attention recently as novel strategies are urgently required that complement the effect of chemotherapy agents by targeting tumor cells and the stroma at the same time.
Table 2.
A summary of the completed clinical trials with results based on high-intensity focused ultrasounds therapies.
Ultrasounds have shown to be a potential therapy for aggressive solid tumors, although the adverse effects produced by HIFU need to be minimized, such as skin burns, pain, hemorrhage and abdominal discomfort. Theoretically, there would be no limitation to the number of sessions that a patient can undergo as it does not produce ionizing radiation. Furthermore, US could be combined with chemotherapy agents, as cavitation of the cell membrane may enhance drug uptake and improve treatment response. US are being increasingly used in clinical trials, with many focused on the safety and feasibility of this technology as a treatment strategy. However, it still remains an emerging and incompletely unexplored field that could give provide clinicians with a new tool for the treatment of patients. The published studies demonstrate the great potential of this energy source in the clinical setting, although further research in this field is required to develop this technology for use in cancer therapy.
Funding Statement
Funding: The authors would like to acknowledge the following funding related with the effects of ultrasounds on cancer cells and tissues: Spanish National Plan project RETOS DPI 2017-90147-R. Intramural call for new research projects for clinical researchers and emerging research groups. IRYCIS. (2018/0240). Ibero-American Network CYTED-DITECROD-218RT0545.
References
- 1.Lento PH, Primack S. Advances and utility of diagnostic ultrasound in musculoskeletal medicine. Curr Rev Musculoskelet Med 2008;1:24-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ebenbichler GR, Erdogmus CB, Resch KL, et al. Ultrasound Therapy for Calcific Tendinitis of the Shoulder. N Engl J Med 1999;340:1533-8. [DOI] [PubMed] [Google Scholar]
- 3.Lingeman JE, McAteer JA, Gnessin E, et al. Shock wave lithotripsy: Advances in technology and technique. Nat Rev Urol 2009;6:660-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller DL, Smith NB, Bailey MR, et al. Overview of therapeutic ultrasound applications and safety considerations. J Ultrasound Med 2012;31:623–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tempany CMC, Stewart EA, McDannold N, et al. MR imaging- guided focused ultrasound surgery of uterine leiomyomas: A feasibility study. Radiology 2003;226:897-905. [DOI] [PubMed] [Google Scholar]
- 6.Packer M, Fishkind WJ, Fine IH, et al. The physics of phaco: A review. J Cataract Refract Surg 2005;31:424-31. [DOI] [PubMed] [Google Scholar]
- 7.Koch C, Borys M, Fedtke T, et al. Determination of the acoustic output of a harmonic scalpel. IEEE Trans Ultrason Ferroelectr Freq Control 2002;49:1522-9. [DOI] [PubMed] [Google Scholar]
- 8.Smith NB. Applications of ultrasonic skin permeation in transdermal drug delivery. Expert Opin Drug Deliv 2008;5:1107-20. [DOI] [PubMed] [Google Scholar]
- 9.Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov 2004;3:115–24. [DOI] [PubMed] [Google Scholar]
- 10.Gebauer D, Mayr E, Orthner E, et al. Low-intensity pulsed ultrasound: Effects on nonunions. Ultrasound Med Biol 2005;31:1391-402. [DOI] [PubMed] [Google Scholar]
- 11.Leighton R, Watson JT, Giannoudis P, et al. Healing of fracture nonunions treated with low-intensity pulsed ultrasound (LIPUS): A systematic review and meta-analysis. Injury 2017;48:1339-47. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer 2005;5:321–7. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy EP, Yeo CJ. The case for routine use of adjuvant therapy in pancreatic cancer. J Surg Oncol 2007;95:597–603. [DOI] [PubMed] [Google Scholar]
- 14.Sacco RL, Chong JY, Prabhakaran S, et al. Experimental treatments for acute ischaemic stroke. Lancet 2007;369:331–41. [DOI] [PubMed] [Google Scholar]
- 15.Polak JF. Ultrasound energy and the dissolution of thrombus. N Engl J Med 2004;351:2154–5. [DOI] [PubMed] [Google Scholar]
- 16.Khanna A, Nelmes RTC, Gougoulias N, et al. The effects of LIPUS on soft-tissue healing: A review of literature. Br Med Bull 2009;89:169-82. [DOI] [PubMed] [Google Scholar]
- 17.Busse JW Bhandari M Kulkarni A V.et al. The effect of lowintensity pulsed ultrasound therapy on time to fracture healing: A meta-analysis. CMAJ 2002;166:437-41. [PMC free article] [PubMed] [Google Scholar]
- 18.Siska PA, Gruen GS, Pape HC. External adjuncts to enhance fracture healing: What is the role of ultrasound? Injury. 2008;39:1095-105. [DOI] [PubMed] [Google Scholar]
- 19.Claes L, Willie B. The enhancement of bone regeneration by ultrasound. Prog Biophys Mol Biol 2007;93-384-98. [DOI] [PubMed] [Google Scholar]
- 20.Azuma Y, Ito M, Harada Y, et al. Low-intensity pulsed ultrasound accelerates rat femoral fracture healing by acting on the various cellular reactions in the fracture callus. J Bone Miner Res 2001;16:671-80. [DOI] [PubMed] [Google Scholar]
- 21.Erdoǧan O, Esen E, Ustun Y, et al. Effects of low-intensity pulsed ultrasound on healing of mandibular fractures: An experimental study in rabbits. J Oral Maxillofac Surg 2006;64:104-8. [DOI] [PubMed] [Google Scholar]
- 22.Hadjiargyrou M, McLeod K, Ryaby JP, et al. Enhancement of fracture healing by low intensity ultrasound. In: Clinical Orthopaedics and Related Research 1998;355:S216-S229. [DOI] [PubMed] [Google Scholar]
- 23.Heckman JD, Ryaby JP, McCabe J, et al. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J Bone Jt Surg - Ser A 1994;76:26-34. [DOI] [PubMed] [Google Scholar]
- 24.Khan Y, Laurencin CT. Fracture repair with ultrasound: Clinical and cell-based evaluation. In: J Bone Jt Surg – Ser A 2008;90:138-44. [DOI] [PubMed] [Google Scholar]
- 25.Padilla F, Puts R, Vico L, et al. Stimulation of bone repair with ultrasound: A review of the possible mechanic effects. Ultrasonics 2014;54:1125-45. [DOI] [PubMed] [Google Scholar]
- 26.Biankin A V, Waddell N, Kassahn KS, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012;491:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Chai Z, Zhang Y, et al. Influence of low-intensity pulsed ultrasound on osteogenic tissue regeneration in a periodontal injury model: X-ray image alterations assessed by micro-computed tomography. Ultrasonics 2014;54:1581-4. [DOI] [PubMed] [Google Scholar]
- 28.El-Bialy T, Alhadlaq A, Wong B, et al. Ultrasound effect on neural differentiation of gingival stem/progenitor cells. Ann Biomed Eng 2014;42:1406-12. [DOI] [PubMed] [Google Scholar]
- 29.Al-Daghreer S, Doschak M, Sloan AJ, et al. Effect of lowintensity pulsed ultrasound on orthodontically induced root resorption in beagle dogs. Ultrasound Med Biol 2014;40:1187-96. [DOI] [PubMed] [Google Scholar]
- 30.Young SR, Dyson M. The effect of therapeutic ultrasound on angiogenesis. Ultrasound Med Biol 1990;16:261-9. [DOI] [PubMed] [Google Scholar]
- 31.Bazou D, Maimon N, Munn LL, et al. Effects of Low Intensity Continuous Ultrasound (LICU) on mouse pancreatic tumor explants. Appl Sci 2017;7:1275. [Google Scholar]
- 32.Zhou Y. High-intensity focused ultrasound treatment for advanced pancreatic cancer. Gastroenterol Res Pr 2014;2014:205325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitragotri S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat Rev Drug Discov 2005;4:255–60. [DOI] [PubMed] [Google Scholar]
- 34.Marmottant P, Hilgenfeldt S. Controlled vesicle deformation and lysis by single oscillating bubbles. Nature 2003;423:153–6. [DOI] [PubMed] [Google Scholar]
- 35.Bao S, Thrall BD, Miller DL. Transfection of a reporter plasmid into cultured cells by sonoporation in vitro. Ultrasound Med Biol 1997;23:953–9. [DOI] [PubMed] [Google Scholar]
- 36.Fechheimer M, Denny C, Murphy RF, et al. Measurement of cytoplasmic pH in Dictyostelium discoideum by using a new method for introducing macromolecules into living cells. Eur J Cell Biol 1986;40:242–7. [PubMed] [Google Scholar]
- 37.Greenleaf WJ, Bolander ME, Sarkar G, et al. Artificial cavitation nuclei significantly enhance acoustically induced cell transfection. Ultrasound Med Biol 1998;24:587–95. [DOI] [PubMed] [Google Scholar]
- 38.Guzman HR, Nguyen DX, Khan S, et al. Ultrasound-mediated disruption of cell membranes. I. Quantification of molecular uptake and cell viability. J Acoust Soc Am 2001;110:588–96. [DOI] [PubMed] [Google Scholar]
- 39.Harrison GH, Balcer-Kubiczek EK, Gutierrez PL. In vitro mechanisms of chemopotentiation by tone-burst ultrasound. Ultrasound Med Biol 1996;22:355–62. [DOI] [PubMed] [Google Scholar]
- 40.Miller DL, Dou C. Induction of apoptosis in sonoporation and ultrasonic gene transfer. Ultrasound Med Biol 2009;35:144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saad AH, Hahn GM. Ultrasound-enhanced effects of adriamycin against murine tumors. Ultrasound Med Biol 1992;18:715–23. [DOI] [PubMed] [Google Scholar]
- 42.Dubinsky TJ, Cuevas C, Dighe MK, et al. High-intensity focused ultrasound: Current potential and oncologic applications. Am J Roentgenol 2008;190:191-9. [DOI] [PubMed] [Google Scholar]
- 43.Yarmolenko PS, Moon EJ, Landon C, et al. Thresholds for thermal damage to normal tissues: An update. Int J Hyperth 2011;27:320-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ter GR, Clarke RL, Vaughan MG, et al. Trackless surgery using focused ultrasound: Technique and case report. Minim Invasive Ther Allied Technol 1991;1. [Google Scholar]
- 45.Hill CR, Ter Haar GR. High intensity focused ultrasound - Potential for cancer treatment. Br J Radiol 1995;68:1296-303. [DOI] [PubMed] [Google Scholar]
- 46.Hoogenboom M, Eikelenboom D, den Brok MH, et al. Mechanical High-Intensity Focused Ultrasound Destruction of Soft Tissue: Working Mechanisms and Physiologic Effects. Ultrasound Med Biol 2015;41:1500-17. [DOI] [PubMed] [Google Scholar]
- 47.Dalecki D. Mechanical bioeffects of ultrasound. Annu Rev Biomed Eng 2004;6:229-48. [DOI] [PubMed] [Google Scholar]
- 48.Halpern EJ. Science to practice high-intensity focused ultrasound ablation: Will image-guided therapy replace conventional surgery? Radiology 2005;235:345-6. [DOI] [PubMed] [Google Scholar]
- 49.Mauri GA, Nicosia luc A, Xu Z, et al. Focused ultrasound: tumour ablation and its potential to enhance immunological therapy to cancer. Br J Radiol 2018;91:20170641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crouzet S, Chapelon JY, Rouviere O, et al. Whole-gland ablation of localized prostate cancer with high-intensity focused ultrasound: Oncologic outcomes and morbidity in 1002 patients. Eur Urol 2014;65:907-14. [DOI] [PubMed] [Google Scholar]
- 51.Dorenberg EJ, Courivaud F, Ring E, et al. Volumetric ablation of uterine fibroids using Sonalleve high-intensity focused ultrasound in a 3 Tesla scanner-first clinical assessment. Minim Invasive Ther Allied Technol 2013;22:73-9. [DOI] [PubMed] [Google Scholar]
- 52.Cranston D. A review of high intensity focused ultrasound in relation to the treatment of renal tumours and other malignancies. Ultrason Sonochem 2015;27:654-8. [DOI] [PubMed] [Google Scholar]
- 53.Aubry JF, Pauly KB, Moonen C, et al. The road to clinical use of high-intensity focused ultrasound for liver cancer: Technical and clinical consensus. J Ther Ultrasound 2013;1:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al-Bataineh O, Jenne J, Huber P. Clinical and future applications of high intensity focused ultrasound in cancer. Cancer Treat Rev 2012;38:346-53. [DOI] [PubMed] [Google Scholar]
- 55.Merckel LG, Bartels LW, Kohler MO, et al. MR-guided highintensity focused ultrasound ablation of breast cancer with a dedicated breast platform. Cardiovasc Intervent Radiol 2013;36:292-301. [DOI] [PubMed] [Google Scholar]
- 56.Brown MRD, Farquhar-Smith P, Williams JE, et al. The use of high-intensity focused ultrasound as a novel treatment for painful conditions - A description and narrative review of the literature. Br J Anaesth 2015;115:520-30. [DOI] [PubMed] [Google Scholar]
- 57.Elias WJ, Huss D, Voss T, et al. A Pilot Study of Focused Ultrasound Thalamotomy for Essential Tremor. N Engl J Med 2013;369:640-8. [DOI] [PubMed] [Google Scholar]
- 58.Kwok SJJ, El Kaffas A, Lai P, et al. Ultrasound-mediated microbubble enhancement of radiation therapy studied using three-dimensional high-frequency power doppler ultrasound. Ultrasound Med Biol 2013;39:1983-90. [DOI] [PubMed] [Google Scholar]
- 59.Goertz DE. An overview of the influence of therapeutic ultrasound exposures on the vasculature: High intensity ultrasound and microbubble-mediated bioeffects. Int J Hyperth 2015;31:134-44. [DOI] [PubMed] [Google Scholar]
- 60.Al-Mahrouki A, Giles A, Hashim A, et al. Microbubble-based enhancement of radiation effect: Role of cell membrane ceramide metabolism. PLoS One 2017;12:e0181951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tabuchi Y, Takasaki I, Zhao QL, et al. Genetic networks responsive to low-intensity pulsed ultrasound in human lymphoma U937 cells. Cancer Lett 2008;270:286–94. [DOI] [PubMed] [Google Scholar]
- 62.Kruse DE, Mackanos MA, O’Connell-Rodwell CE, et al. Short-duration-focused ultrasound stimulation of Hsp70 expression in vivo. Phys Med Biol 2008;53:3641–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ashush H, Rozenszajn LA, Blass M, et al. Apoptosis induction of human myeloid leukemic cells by ultrasound exposure. Cancer Res 2000;60:1014–20. [PubMed] [Google Scholar]
- 64.Feng Y, Tian Z, Wan M. Bioeffects of low-intensity ultrasound in vitro: apoptosis, protein profile alteration, and potential molecular mechanism. J Ultrasound Med 2010;29:963–74. [DOI] [PubMed] [Google Scholar]
- 65.Feril LB, Kondo T, Cui ZG, et al. Apoptosis induced by the sonomechanical effects of low intensity pulsed ultrasound in a human leukemia cell line. Cancer Lett 2005;221:145–52. [DOI] [PubMed] [Google Scholar]
- 66.Lagneaux L, de Meulenaer EC, Delforge A, et al. Ultrasonic low-energy treatment: a novel approach to induce apoptosis in human leukemic cells. Exp Hematol 2002;30:1293–301. [DOI] [PubMed] [Google Scholar]
- 67.Poff JA, Allen CT, Traughber B, et al. Pulsed high-intensity focused ultrasound enhances apoptosis and growth inhibition of squamous cell carcinoma xenografts with proteasome inhibitor bortezomib. Radiology 2008;248:485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takeuchi S, Udagawa Y, Oku Y, et al. Basic study on apoptosis induction into cancer cells U-937 and EL-4 by ultrasound exposure. Ultrasonics. 2006;44 Suppl 1:e345-8. [DOI] [PubMed] [Google Scholar]
- 69.Tang W, Liu Q, Wang X, et al. Potential mechanism in sonodynamic therapy and focused ultrasound induced apoptosis in sarcoma 180 cells in vitro. Ultrasonics 2009;49:786–93. [DOI] [PubMed] [Google Scholar]
- 70.Wang X, Liu Q, Wang Z, et al. Bioeffects of low-energy continuous ultrasound on isolated sarcoma 180 cells. Chemotherapy 2009;55:253–61. [DOI] [PubMed] [Google Scholar]
- 71.Persidis A. Cancer multidrug resistance. Nat Biotechnol 1999;17:94–5. [DOI] [PubMed] [Google Scholar]
- 72.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med 2002;53:615–27. [DOI] [PubMed] [Google Scholar]
- 73.Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol 1992;8:67–113. [DOI] [PubMed] [Google Scholar]
- 74.Sarkadi B, Homolya L, Szakacs G, et al. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev 2006;86:1179–236. [DOI] [PubMed] [Google Scholar]
- 75.Szakacs G, Hall MD, Gottesman MM, et al. Targeting the Achilles heel of multidrug-resistant cancer by exploiting the fitness cost of resistance. Chem Rev 2014;114:5753–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Higgins CF. Multiple molecular mechanisms for multidrug resistance transporters. Nature 2007;446:749–57. [DOI] [PubMed] [Google Scholar]
- 77.Hardwick LJ, Velamakanni S, van Veen HW. The emerging pharmacotherapeutic significance of the breast cancer resistance protein (ABCG2). Br J Pharmacol 2007;151:163–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robey RW, Polgar O, Deeken J, et al. ABCG2: determining its relevance in clinical drug resistance. Cancer Metastasis Rev 2007;26:39–57. [DOI] [PubMed] [Google Scholar]
- 79.Shao ZY, Zhai BJ, Zhao CL, et al. Cytotoxic effects and in vitro reversal of multidrug resistance by therapeutic ultrasound in human hepatocarcinoma cell line (HepG2). Ultrasonics 2008;48:297–302. [DOI] [PubMed] [Google Scholar]
- 80.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374–403. [DOI] [PubMed] [Google Scholar]
- 81.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 82.Sofuni A, Moriyasu F, Sano T, et al. The current potential of high-intensity focused ultrasound for pancreatic carcinoma. J Hepatobiliary Pancreat Sci 2011;18:295–303. [DOI] [PubMed] [Google Scholar]
- 83.Wang K, Zhu H, Meng Z, et al. Safety evaluation of highintensity focused ultrasound in patients with pancreatic cancer. Onkologie 2013;36:88–92. [DOI] [PubMed] [Google Scholar]
- 84.Jung SE, Cho SH, Jang JH, et al. High-intensity focused ultrasound ablation in hepatic and pancreatic cancer: complications. Abdom Imaging 2011;36:185–95. [DOI] [PubMed] [Google Scholar]
- 85.Bray F, Ferlay J, Soerjomataram I, et al. Prostate cancer statistics | World Cancer Research Fund [Internet]. CA. Cancer J Clin 2018. [Google Scholar]
- 86.Chen FK, De Castro Abreu AL, Palmer SL. Utility of ultrasound in the diagnosis, treatment, and follow-up of prostate cancer: State of the art. J Nucl Med 2016;57:13S-18S. [DOI] [PubMed] [Google Scholar]
- 87.Ganzer R, Fritsche HM, Brandtner A, et al. Fourteen-year oncological and functional outcomes of high-intensity focused ultrasound in localized prostate cancer. BJU Int 2013;112:322-9. [DOI] [PubMed] [Google Scholar]
- 88.Uchida T, Tomonaga T, Kim H, et al. Improved Outcomes with Advancements in High Intensity Focused Ultrasound Devices for the Treatment of Localized Prostate Cancer. J Urol 2015;193:103-10. [DOI] [PubMed] [Google Scholar]
- 89.Cheung TT, Chu FSK, Jenkins CR, et al. Tolerance of highintensity focused ultrasound ablation in patients with hepatocellular carcinoma. World J Surg 2012;36:2420-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu G, Luo G, He L, et al. Follow-up of high-intensity focused ultrasound treatment for patients with hepatocellular carcinoma. Ultrasound Med Biol 2011;37:1993-9. [DOI] [PubMed] [Google Scholar]
- 91.Hassanuddin A, Choi JH, Seo DW, et al. Factors affecting tumor ablation during high intensity focused ultrasound treatment. Gut Liver 2014;8:433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen B, Chen J, Luo Q, et al. Effective strategy of the combination of high-intensity focused ultrasound and transarterial chemoembolization for improving outcome of unresectable and metastatic hepatoblastoma: A retrospective cohort study. Transl Oncol 2014;7:788-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van den Bijgaart RJE, Eikelenboom DC, Hoogenboom M, et al. Thermal and mechanical high-intensity focused ultrasound: perspectives on tumor ablation, immune effects and combination strategies. Cancer Immunol Immunother 2017;66:247-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chapiro J, Geschwind JF. Science to practice: The changing face of local tumor therapies - Do we have to think systemically when treating cancer locally? Radiology 2015;276:315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rozenblum N, Zeira E, Scaiewicz V, et al. Oncogenesis: An “off-target” effect of radiofrequency ablation. Radiology 2015;276:426-32. [DOI] [PubMed] [Google Scholar]
- 96.Wu F, Zhou L, Chen WR. Host antitumor immune responses to HIFU ablation. Int J Hyperth 2007;23:165-71. [DOI] [PubMed] [Google Scholar]
- 97.Prise KM, O’Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer. 2009;9:351-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer 2016;40:25-37. [DOI] [PubMed] [Google Scholar]
- 99.Haen SP, Pereira PL, Salih HR, et al. More than just tumor destruction: Immunomodulation by thermal ablation of cancer. Clin Dev Immunol 2011;2011:160250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gravante G, Sconocchia G, Ong SL, et al. Immunoregulatory effects of liver ablation therapies for the treatment of primary and metastatic liver malignancies. Liver Int 2009;29:18-24. [DOI] [PubMed] [Google Scholar]
- 101.Sanchez-Ortiz RF, Tannir N, Ahrar K, et al. Spontaneous regression of pulmonary metastases from renal cell carcinoma after radio frequency ablation of the primary tumor: An in situ tumor vaccine? J Urol 2003;170:178-9. [DOI] [PubMed] [Google Scholar]
- 102.Wang X, Sun J. High-intensity focused ultrasound in patients with late-stage pancreatic carcinoma. Chin Med J (Engl) 2002;115:1332-5. [PubMed] [Google Scholar]
- 103.Zhou Q, Zhu XQ, Zhang J, et al. Changes in Circulating Immunosuppressive Cytokine Levels of Cancer Patients After High Intensity Focused Ultrasound Treatment. Ultrasound Med Biol 2008;34:81-7. [DOI] [PubMed] [Google Scholar]
- 104.Stewart EA. Uterine fibroids. In: Lancet 2001;2:16043. [Google Scholar]
- 105.Borah BJ, Nicholson WK, Bradley L, et al. The impact of uterine leiomyomas: A national survey of affected women. American Journal of Obstetrics and Gynecology 2013:319.e1-319.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dobrotwir A, Pun E. Clinical 24 month experience of the first MRgFUS Unit for treatment of uterine fibroids in Australia. J Med Imaging Radiat Oncol 2012;56:409–16. [DOI] [PubMed] [Google Scholar]
- 107.Hindley J, Gedroyc WM, Regan L, et al. MRI guidance of focused ultrasound therapy of uterine fibroids: Early results. Am J Roentgenol 2004;183:1713-9. [DOI] [PubMed] [Google Scholar]
- 108.Tempany CM. From the RSNA refresher courses: Imageguided thermal therapy of uterine fibroids. Radiographics. 2007;27:1819-26. [DOI] [PubMed] [Google Scholar]
- 109.Stewart EA, Rabinovici J, Tempany CMC, et al. Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril 2006;85:22–9. [DOI] [PubMed] [Google Scholar]
- 110.Gonzalez Gomez I, Ramos Fernandez A, Rodriguez-Lorenzo L, et al. Ultrasound Technology as a Novel Treatment Strategy in Pancreatic Cancer. Nov Approaches Cancer Study 2019;2. [Google Scholar]
- 111.Lyon PC, Griffiths LF, Lee J, et al. Clinical trial protocol for TARDOX: A phase I study to investigate the feasibility of targeted release of lyso-thermosensitive liposomal doxorubicin (ThermoDox®) using focused ultrasound in patients with liver tumours. J Ther Ultrasound 2017;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]


