Key Points
Question
Among children born at risk of neonatal hypoglycemia, do those who experience hypoglycemia have worse academic performance in mid-childhood?
Findings
In this prospective cohort study that included 480 participants at risk of neonatal hypoglycemia who were screened and treated if needed to maintain blood glucose concentration of at least 47 mg/dL (2.6 mmol/L), children who were and were not exposed to neonatal hypoglycemia did not significantly differ in rates of low educational performance at 9 to 10 years’ corrected age, based on standardized tests of reading comprehension or mathematics (47% vs 48%).
Meaning
Among participants at risk of neonatal hypoglycemia who were screened and treated if needed, the occurrence of neonatal hypoglycemia compared with no such exposure was not significantly associated with lower educational achievement in mid-childhood.
Abstract
Importance
Neonatal hypoglycemia is associated with increased risk of poor executive and visual-motor function, but implications for later learning are uncertain.
Objective
To test the hypothesis that neonatal hypoglycemia is associated with educational performance at age 9 to 10 years.
Design, Setting, and Participants
Prospective cohort study of moderate to late preterm and term infants born at risk of hypoglycemia. Blood and masked interstitial sensor glucose concentrations were measured for up to 7 days. Infants with hypoglycemic episodes (blood glucose concentration <47 mg/dL [2.6 mmol/L]) were treated to maintain a blood glucose concentration of at least 47 mg/dL. Six hundred fourteen infants were recruited at Waikato Hospital, Hamilton, New Zealand, in 2006-2010; 480 were assessed at age 9 to 10 years in 2016-2020.
Exposures
Hypoglycemia was defined as at least 1 hypoglycemic event, representing the sum of nonconcurrent hypoglycemic and interstitial episodes (sensor glucose concentration <47 mg/dL for ≥10 minutes) more than 20 minutes apart.
Main Outcomes and Measures
The primary outcome was low educational achievement, defined as performing below or well below the normative curriculum level in standardized tests of reading comprehension or mathematics. There were 47 secondary outcomes related to executive function, visual-motor function, psychosocial adaptation, and general health.
Results
Of 587 eligible children (230 [48%] female), 480 (82%) were assessed at a mean age of 9.4 (SD, 0.3) years. Children who were and were not exposed to neonatal hypoglycemia did not significantly differ on rates of low educational achievement (138/304 [47%] vs 82/176 [48%], respectively; adjusted risk difference, −2% [95% CI, −11% to 8%]; adjusted relative risk, 0.95 [95% CI, 0.78-1.15]). Children who were exposed to neonatal hypoglycemia, compared with those not exposed, were significantly less likely to be rated by teachers as being below or well below the curriculum level for reading (68/281 [24%] vs 49/157 [31%], respectively; adjusted risk difference, −9% [95% CI, −17% to −1%]; adjusted relative risk, 0.72 [95% CI, 0.53-0.99; P = .04]). Groups were not significantly different for other secondary end points.
Conclusions and Relevance
Among participants at risk of neonatal hypoglycemia who were screened and treated if needed, exposure to neonatal hypoglycemia compared with no such exposure was not significantly associated with lower educational achievement in mid-childhood.
This study assesses the association of neonatal hypoglycemia with low educational achievement at age 9 to 10 years among a prospective cohort of moderate to late preterm and term infants.
Introduction
Neonatal hypoglycemia is the most common metabolic disorder of infancy, affecting approximately 15% of newborns.1 It may cause permanent brain injury due to the relative dependence of the neonatal brain on glucose as an oxidative fuel.2 Most infants have transitional hypoglycemia, that is, low blood glucose due to delayed transition from placental to hepatic glucose supply, in the absence of underlying genetic or metabolic disorders or severe illness.3 Because neonatal hypoglycemia is often asymptomatic, standard practice is to screen newborns at risk and provide exogenous glucose when low blood glucose concentrations occur. However, criteria for diagnosing neonatal hypoglycemia and blood glucose targets for treated infants are debated, largely because of a paucity of long-term outcome data relating to different clinical approaches.4
The Children With Hypoglycaemia and Their Later Development (CHYLD) Study reported that among at-risk infants who were screened and treated to maintain blood glucose concentrations of at least 47 mg/dL (2.6 mmol/L), children who developed hypoglycemia had similar development at 2 years5 but increased risk of low executive function and visual-motor integration at age 4.5 years, with greater impairment in children with severe or recurrent episodes.6 Undetected and untreated episodes, identified only by masked continuous glucose monitoring, were also associated with increased risk. Similarly, in a birth cohort of newborns undergoing routine blood glucose screening, brief transitional neonatal hypoglycemia was associated with academic underachievement at age 10 years.7 Thus, additional screening and treatment of at-risk infants may be needed to prevent adverse neurological sequelae. Conversely, others have reported no evidence of later adverse outcomes after neonatal hypoglycemia and have raised concerns about the potential for harm with overtreatment,8,9 such as glucose reperfusion injury.10,11
This study investigated if the associations between neonatal hypoglycemia and adverse neurocognitive function observed at school entry persisted in mid-childhood and were associated with decreased educational performance.
Methods
Participants
Written informed consent was obtained from caregivers, along with children’s assent, and the study was approved by the New Zealand Health and Disability Ethics Committee. This was a prospective longitudinal cohort study of moderate to late preterm and term infants born at or after 32 weeks’ gestation who were recruited to 2 neonatal studies (the Babies and Blood Glucoses Influence on EEG Study [BABIES] and the Sugar Babies Study), conducted from December 2006 to November 2010 at Waikato Women’s Hospital, a regional perinatal center in Hamilton, New Zealand.12,13 Data were pooled, as the infants were recruited in a single center by the same team in overlapping time periods with similar entry criteria (gestation, risk factors) and were managed according to the same clinical protocol. Eligible infants were those born with at least 1 risk factor for neonatal hypoglycemia: infant of a mother with diabetes, preterm (<37 weeks), small (<10th percentile or <2500 g), large (>90th percentile or >4500 g), or other reason. Infants with serious congenital malformations or terminal conditions were excluded. No infants had congenital hyperinsulinism or inherited metabolic disorders. Infants were regularly screened for hypoglycemia using whole blood and a blood gas analyzer (ABL800 FLEX; Radiometer), which provides plasma equivalent glucose concentrations, commencing 1 to 2 hours after birth, then every 3 to 4 hours for the first 24 hours and every 6 to 8 hours for the following 24 hours and up to 7 days or until there were no ongoing clinical concerns. Masked continuous glucose monitoring (CGMS Gold; Medtronic Minimed) was performed for up to 7 days, with offline calibration.14,15 Hypoglycemia, defined as a blood glucose concentration less than 47 mg/dL, was treated with any combination of additional feeding, buccal dextrose gel, or intravenous dextrose, with the aim of maintaining blood glucose concentration of at least 47 mg/dL.6 A subgroup of infants (n = 237) in the Sugar Babies Study were randomized to buccal dextrose gel or placebo for initial management of hypoglycemia, but all were eligible for subsequent open-label treatment with dextrose gel.12 Demographic characteristics of infants were collected at study entry, including household socioeconomic decile16 and parent-reported ethnicity, using standard national categories (participants could select any of 8 predefined ethnicities and list “other” as free text) and prioritized as recommended and required by the New Zealand Ministry of Health.17 Neurodevelopmental outcomes at age 2 years and age 4.5 years have been reported.5,6
Study Design
The study protocol and statistical analysis plan are provided in Supplement 1 and Supplement 2. All surviving children in the cohort who had not been previously withdrawn were eligible for follow-up. At 9 to 10 years’ corrected age, children underwent in-depth assessment by trained assessors who were blinded to the neonatal glycemic history of the children. The assessments targeted 5 key domains: academic achievement, executive function, visual-motor function, psychosocial adaptation, and general health (eTable 1 in Supplement 3). The last assessment was completed in March 2020.
Academic achievement was assessed in English or Māori using a standardized curriculum-based online achievement test, the Electronic Assessment Tools for Teaching and Learning (e-asTTle), including reading comprehension/Pānui and mathematics/Pāngarau. The e-asTTle assesses students’ performance in Curriculum Levels 2 to 6 (nominally, school years [grades] 4 to 11; approximately ages 8 to 16 years), with normative data for each year and term of schooling (4 terms per year) for years 4 to 12 inclusive.18 The psychometric properties and scoring methods have been detailed elsewhere.19 In brief, a student’s achievement was rated as being “at” curriculum level if their achievement was within the range of the normative data for students in the 3 terms either side of their current school year and term, “above” if 4 or more terms above, “below” if 4 to 7 terms below, or “well below” if 8 or more terms below their school year and term. Additionally, children’s teachers were asked to rate their academic achievement against the expected curriculum level for school year and term (well below, below, at, or above) and in relation to peers (much worse, worse, about the same, better, or much better) using a structured teacher questionnaire.19
Cognitive elements of executive function were evaluated using items from the tablet-based Cambridge Neuropsychological Test Automated Battery (CANTAB) system focusing on attention and cognitive flexibility (Attention Switching Task); planning and manipulation of information (One-Touch Stockings of Cambridge, Spatial Working Memory); visual memory and learning (Paired Associate Learning); and inhibitory control (Stop Signal Task) (eTable 1 in Supplement 3).20 At 9 to 10 years of age, children are expected to have largely mastered these skills, with performance approximating 70% to 80% of adult function.21
Visual-motor integration was assessed with the Beery-Buktenica Developmental Test of Visual-Motor Integration, Sixth Edition (BBVMI-6; standardized mean score, 100 [SD, 15]).22 Motor function was assessed with the fine motor subscale of the Movement Assessment Battery for Children, Second Edition (MABC-2; standardized mean score, 10 [SD, 3])23 and the motor coordination subscale of the BBVMI-6. Visual processing was assessed with motion and form coherence thresholds, determined from random dot kinetograms of varying coherence using an adaptive staircase procedure (lower threshold indicates better processing)24 and the visual perception subscale of the BBVMI-6.
Parental questionnaires were used to screen for emotional and behavioral problems (Strengths and Difficulties Questionnaire [SDQ]; Behavior Rating Inventory of Executive Function [BRIEF]25), autism traits (Autism Spectrum Quotient: Children’s Version [AQ-Child]26,27), and functional health and well-being (Child Health Questionnaire [CHQ]27). Teachers also completed the SDQ and BRIEF. Information on the home and family environment was obtained using a structured parent questionnaire.
Exposures
Hypoglycemia was defined as at least 1 hypoglycemic event, representing the sum of nonconcurrent hypoglycemic and interstitial episodes more than 20 minutes apart.14 A hypoglycemic episode was defined as at least 1 consecutive blood glucose concentration less than 47 mg/dL and an interstitial episode as a sensor glucose concentration less than 47 mg/dL for at least 10 minutes. A severe hypoglycemia event was defined as a measurement of less than 36 mg/dL and recurrent hypoglycemia as at least 3 hypoglycemic events. The degree of hypoglycemia was defined as mild (≥36 to ≤46 mg/dL) or severe.
Outcomes
Prior to completion of data collection and analysis, the study’s steering committee changed the primary outcome from processing difficulty (protocol version 1.1, November 2016; see Supplement 1) to low educational achievement (statistical analysis plan version 1.5, June 2020; see Supplement 2), as normative data had not become available for the executive function tests. Low educational achievement was defined as an e-asTTle score below or well below the normative curriculum level in reading comprehension/Pānui or mathematics/Pāngarau.
There were 47 prespecified secondary outcomes, including individual components of the primary outcome; e-asTTle achievement z scores for year and term of school; receiving additional learning support or being older than expected for year level (≥1 year older than 95% of children in their year level); receiving additional learning support or being older than expected for year level or having low educational achievement; CANTAB items; MABC-2, BBVMI-6, SDQ, BRIEF, AQ-Child, and CHQ scores and proportion of children with borderline/abnormal scores or in the clinical range, according to normative test data; and motion and form coherence thresholds (eTable 1 in Supplement 3). Nine secondary outcomes were prespecified as critical for secondary analyses, including e-asTTle achievement z scores; receiving additional learning support or being older than expected for year level or having low educational achievement; measures of planning/working memory; MABC-2 fine motor subscale score in less than the 15th percentile; BBVMI-6 score less than 85; proportion of children with borderline/abnormal parent or teacher SDQ score; and AQ-Child score. These were selected by the steering committee as the most important end points in each test domain.
Statistical Analysis
The sample size was limited by the size of the inception cohorts. Based on previous data from this cohort, we estimated that the incidence of the primary outcome in controls would be 20%. Assuming 80% follow-up, we estimated that the study would have 90% power to detect an increase in incidence of the primary outcome from 20% in controls to 33% (risk ratio, 1.60) in children exposed to hypoglycemia (α = .05). For continuous outcomes, we estimated the study would have 90% power to detect differences of 0.3 SD.
The primary analyses compared primary and secondary outcomes between children with and without hypoglycemia using generalized linear models (maximum likelihood) with binomial, Poisson, or normal distributions and relevant link functions, as appropriate, adjusted for prespecified potential confounders (socioeconomic decile, sex, and primary risk factor for neonatal hypoglycemia). No adjustment was made for recruitment study. Models were evaluated by deviance values and parameter standard errors. Convergence difficulties were addressed by optimization techniques, increasing the maximum number of iterations, and, if necessary, collapsing or excluding covariates to achieve convergence with minimum Akaike information criteria. Associations with exposure are presented as adjusted risk ratios, risk differences, mean differences, or count ratios, as appropriate, with 95% confidence intervals. Missing data were not imputed. Secondary analyses related the primary and prespecified critical secondary outcomes to the severity, frequency, and degree of hypoglycemia, undetected low glucose concentration (detected only in masked continuous glucose monitoring), and hypoglycemic episodes in the first week after birth. Sensitivity analysis excluded children who experienced a postnatal neurological insult; who were educated outside of New Zealand; who were classified as normoglycemic but did not have neonatal continuous glucose monitoring to potentially detect low sensor glucose concentration; or who were born prior to 35 weeks’ gestation. In post hoc exploratory analysis, results for measures of executive function and low visual-motor integration, which were associated with hypoglycemia at age 4.5 years, were adjusted for scores for the same measures at age 4.5 years. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. Analysis was performed using SAS statistical software, version 9.4 (SAS Institute Inc); statistical significance was set at a 2-sided α = .05.
Results
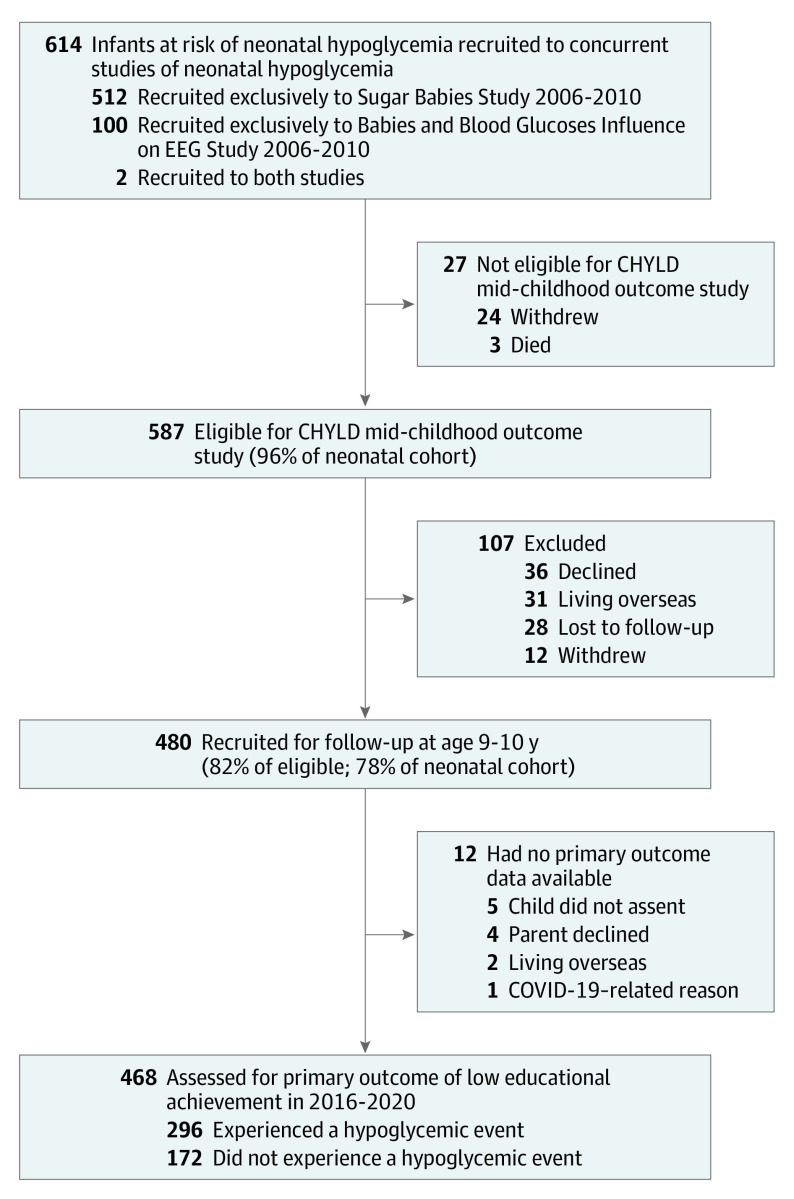
Of 614 infants recruited to this study (100 from BABIES, 512 from the Sugar Babies Study, and 2 who were in both), 24 withdrew and 3 died, leaving 587 children eligible for follow-up, of whom 480 children were assessed (82% of eligible) at age 9 to 10 years (Figure). Of 17 infants whose primary risk factor for hypoglycemia was “other,” 1 had seizures, 4 had transient tachypnea, and 12 had nonspecific concerns, such as poor feeding or hypothermia. Children who were assessed at age 9 to 10 years, compared with those who were not assessed, were more likely to be Māori and admitted to neonatal intensive care (P < .05) (eTable 2 in Supplement 3). Of those assessed at age 9 to 10 years, the primary outcome was available for 468 children (98%) and data for other administered tests were available for at least 466 children (97%).
Figure. Flow of Participants in the CHYLD Study in Mid-Childhood.
CHYLD indicates Children With Hypoglycaemia and Their Later Development.
Neonatal hypoglycemia occurred in 304 children (63%), of whom 111 (37%) had at least 1 severe event and 165 (54%) had recurrent events (198 [65%] had severe or recurrent hypoglycemia). Blood glucose concentration was measured within 2 hours of birth in 331 children (69%), of whom 148 (45%) had an initial low blood glucose concentration less than 47 mg/dL. However, only 35 children (12% of those who developed hypoglycemia) had their only episode of hypoglycemia during this period. Thus, most children who experienced neonatal hypoglycemia did so after the early physiological nadir in blood glucose. A total of 276 children (90%) had onset of hypoglycemia within 12 hours of birth, 14 (5%) had onset from 12 to 24 hours after birth, and 14 (5%) had onset 24 hours or longer after birth.
Children who experienced neonatal hypoglycemia compared with those who did not were significantly more likely to undergo continuous glucose monitoring (250/304 [82%] vs 127/176 [72%]; P = .01) and to be admitted to neonatal intensive care (173/304 [57%] vs 66/176 [38%]; P < .001) but were significantly less likely to be born to a mother with gestational diabetes (103/304 [34%] vs 72/176 [41%]; P = .02) (Table 1). Those who experienced hypoglycemia had significantly lower blood glucose concentrations and sensor glucose concentrations for 48 hours or longer after birth and were significantly more likely to receive intravenous dextrose and formula feeding in the first week (Table 1). There were no other significant differences in maternal, neonatal, or mid-childhood characteristics between participants with or without neonatal hypoglycemia (Table 1).
Table 1. Characteristics of Participants in the CHYLD Mid-Childhood Outcome Study.
| Characteristics | Hypoglycemiaa | No hypoglycemia |
|---|---|---|
| Maternal characteristics | ||
| No. of women | 280 | 166 |
| Age at entry, mean (SD), y | 30.1 (6.1) | 30.4 (6.6) |
| BMI in early pregnancy, mean (SD) | 28.8 (7.1) [n = 257] | 28.9 (7.8) [n = 160] |
| Diabetes in pregnancy, No. (%) | ||
| None | 178 (64) | 96 (58) |
| Gestational | 76 (27) | 62 (37) |
| Pregestational | 26 (9) | 8 (5) |
| Smoking in pregnancy, No./total (%) | 89/262 (34) | 41/156 (26) |
| Alcohol use in pregnancy, No./total (%) | 42/260 (16) | 16/153 (10) |
| Highest education level, No. (%) | n = 237 | n = 134 |
| Schooling incomplete | 18 (8) | 14 (10) |
| High school ≥3 y | 55 (23) | 28 (21) |
| Technical or trade | 82 (35) | 50 (37) |
| University | 82 (35) | 42 (31) |
| Neonatal characteristics | ||
| No. of infants | 304 | 176 |
| Primary risk factor for hypoglycemia, No. (%) | ||
| Infant of mother with diabetes | 103 (34) | 72 (41) |
| Preterm (<37 weeks’ gestation) | 116 (38) | 54 (31) |
| Small (birth weight <10th percentile for gestation or <2500 g) | 50 (17) | 21 (12) |
| Large (birth weight >90th percentile for gestation or >4500 g) | 22 (7) | 25 (14) |
| Otherb | 13 (4) | 4 (2) |
| Sex, No. (%) | ||
| Female | 149 (49) | 77 (44) |
| Male | 155 (51) | 99 (56) |
| Twins, No. (%) | 48 (16) | 19 (11) |
| Length of gestation, mean (SD), wk | 37.1 (2.1) | 37.6 (2.1) |
| Birth weight, mean (SD), g | 2937 (848) | 3123 (917) |
| Birth weight z score, mean (SD)c | 0.02 (1.63) | 0.20 (1.69) |
| Apgar score <7 at 5 min, No. (%) | 8 (3) | 2 (1) |
| Admitted to neonatal intensive care unit, No. (%) | 173 (57) | 66 (38) |
| Prioritized ethnicity, No. (%)d | n = 299 | n = 173 |
| European | 182 (61) | 110 (63) |
| Māori | 98 (33) | 51 (30) |
| Pacific | 4 (1) | 5 (3) |
| Other | 15 (5) | 7 (4) |
| Socioeconomic status, No. (%) | n = 303 | n = 176 |
| Most deprivede | 124 (41) | 68 (39) |
| Less deprived | 179 (59) | 108 (61) |
| Blood glucose concentration, mean (SD), mg/dL | ||
| At <12 h | 54.1 (9.0) | 66.7 (12.6) |
| At 12 to <24 h | 63.1 (12.6) | 70.3 (12.6) |
| At 24 to <48 h | 66.7 (21.6) | 72.1 (14.4) |
| At ≥48 h | 73.9 (12.6) | 82.9 (16.2) |
| Blood glucose values outside central band of 54-72 mg/dL in first 48 h, mean (SD), % | 56 (20) | 47 (27) |
| Continuous glucose monitoring, No. (%)f | 250 (82) | 127 (72) |
| Sensor glucose concentration, mean (SD), mg/dL | ||
| At <12 h | 61.3 (12.6) [n = 224] | 70.3 (16.2) [n = 116] |
| At 12 to <24 h | 64.9 (10.8) [n = 240] | 73.9 (14.1) [n = 117] |
| At 24 to <48 h | 68.5 (12.6) [n = 229] | 73.9 (16.2) [n = 108] |
| At ≥48 h | 75.7 (14.4) [n = 148] | 86.5 (18.0) [n = 47] |
| Sensor glucose values outside central band of 3-4 mmol/L in first 48 h, mean (SD), % | 50 (22) [n = 246] | 46 (32) [n = 126] |
| Feeding in the first week, No. (%) | ||
| Breast and formula milk | 194 (64) | 82 (46) |
| Breast milk only | 84 (28) | 81 (46) |
| Formula milk only | 19 (6) | 12 (7) |
| No enteral feeds | 7 (2) | 1 (1) |
| Intravenous dextrose <48 h, No. (%) | 74 (24) | 17 (10) |
| Child characteristics at age 9-10 y | ||
| Age, mean (SD), y | 9.4 (0.4) | 9.4 (0.3) |
| Main language spoken at home, No. (%) | n = 263 | n = 148 |
| English | 253 (96) | 139 (94) |
| Te reo Māori (indigenous language) | 55 (21) | 26 (18) |
| Inadequate family resources, mean (SD)g | 4 (2) [n = 262] | 1 (1) [n = 148] |
| Year (grade) at school, No. (%) | n = 303 | n = 173 |
| Year 4 | 119 (39) | 53 (31) |
| Year 5 | 170 (56) | 115 (66) |
| Year 6 | 14 (5) | 6 (3) |
| National school curriculum, No. (%)h | ||
| New Zealand (English-medium curriculum) | 286 (94) | 166 (94) |
| Te Marautanga o Aotearoa (Māori-medium curriculum) | 10 (3) | 7 (4) |
| Other (eg, Australia) | 8 (3) | 3 (2) |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; CHYLD, Children With Hypoglycaemia and Their Later Development.
SI conversion factor: To convert glucose to millimoles per liter, multiply by 0.0555.
Hypoglycemia is defined as ≥1 hypoglycemic event, representing the sum of nonconcurrent hypoglycemic and interstitial episodes more than 20 minutes apart; a hypoglycemic episode is defined as ≥1 consecutive blood glucose concentration <47 mg/dL (<2.6 mmol/L) and an interstitial episode as a sensor glucose concentration <47 mg/dL for ≥10 minutes.
Includes seizure, transient tachypnea, poor feeding, and hypothermia.
The z scores are gestation and sex specific.
Ethnicity prioritized in order of Māori, Pacific, other, and European according to New Zealand Ministry of Health protocol17; numbers with “other” ethnicity were as follows: for the hypoglycemia group, South Asian, n = 1; African, n = 2; South East Asian, n = 12; for the no hypoglycemia group, African, n = 1; South East Asian, n = 6.
Most deprived is defined as New Zealand Deprivation Index 8-10, which represents the highest 3 national deciles for household deprivation, a composite of income, employment, qualifications, home ownership, living space, transportation, and internet access.16
Continuous glucose monitoring provides a sensor reading every 5 minutes; sensor glucose concentration data shown are the mean (SD) values for the periods defined.
Inadequate family resources is defined as a total score <32 on the New Zealand Family Resource Scale questions 1 (food for 2 meals a day), 2 (house or apartment), 5 (heat for house of apartment), 6 (indoor plumbing/water), 7 (money for monthly bills), 9 (medical care for family), 11 (dependable transportation), and 12 (access to a telephone), for a maximum score of 40; scores of 32 or above indicate that resources are usually adequate for all items.
Te reo Māori is an official language in New Zealand, and New Zealand schools are required to teach either the New Zealand curriculum or the Māori curriculum; this is typically based on the primary teaching language.
Primary Analyses
Exposure to neonatal hypoglycemia compared with no exposure was not significantly associated with low educational achievement (138/296 [47%] vs 82/172 [48%], respectively; adjusted risk difference, −2% [95% CI, −11% to 8%]; adjusted risk ratio, 0.95 [95% CI, 0.78-1.15]) (Table 2). Children who were exposed to neonatal hypoglycemia, compared with those who were not, were significantly less likely to be rated by teachers as being below or well below the curriculum level for reading (68/281 [24%] vs 49/157 [31%], respectively; adjusted risk difference −9% [95% CI, −17% to −1%]; adjusted risk ratio, 0.72 [95% CI, 0.53-0.99; P = .04]) (Table 2). There were no significant differences between exposure groups in any other secondary outcomes, including low achievement in reading comprehension/Pānui or mathematics/Pāngarau, e-asTTle z scores, learning support, teacher-rated educational performance in mathematics and writing, behavioral manifestations of executive function, fine motor function, visual processing, emotional-behavioral regulation, autism traits, and overall physical and psychosocial well-being (Table 2 and Table 3). For the 2 tests that were significantly different between groups at age 4.5 years, neither were statistically different at age 9 to 10 years. For the executive function test of planning/working memory, on the CANTAB One-Touch Stockings of Cambridge test, the median number of problems solved on first choice was 8 (IQR, 5-10) vs 8 (IQR, 5-10), for an adjusted count ratio of 1.00 (95% CI, 0.93-1.07); mean latency to first choice was 12 510 ms (SD, 6567 ms) among children exposed to neonatal hypoglycemia vs 13 604 ms (SD, 7632 ms) among those with no hypoglycemia, for an adjusted mean difference of −1061 ms (95% CI, −2369 to 247 ms). For the BBVMI-6 test, scores less than 85 occurred among 109 (36%) of 299 children exposed to neonatal hypoglycemia vs 71 (41%) of 172 with no hypoglycemia, for an adjusted risk difference of −7% (95% CI, −2% to 17%) and an adjusted risk ratio of 0.88 (95% CI, 0.70-1.11).
Table 2. Neonatal Hypoglycemia and Neurocognitive Function in Mid-Childhood.
| Outcomes | Hypoglycemiaa | No hypoglycemia | Adjusted risk difference, %, or mean difference (95% CI)b | Adjusted risk ratio or count ratio (95% CI)b |
|---|---|---|---|---|
| Primary outcome | ||||
| Low educational achievement, No./total (%)c | 138/296 (47) | 82/172 (48) | −2 (−11 to 8) | 0.95 (0.78-1.15) |
| Secondary outcomes | ||||
| Educational achievement | ||||
| e-asTTled | ||||
| Low achievement in reading comprehension/Pānui, No./total (%) | 95/296 (32) | 59/172 (34) | −2 (−11 to 6) | 0.89 (0.69-1.16) |
| Low achievement in mathematics/Pāngarau, No./total (%) | 99/295 (34) | 59/171 (35) | −2 (−11 to 7) | 0.96 (0.74-1.24) |
| Achievement z score in reading comprehension/Pānui, mean (SD) | −0.21 (1.11) [n = 296] | −0.32 (1.26) [n = 172] | 0.12 (−0.09 to 0.33) | |
| Achievement z score in mathematics/Pāngarau, mean (SD) | −0.13 (0.84) [n = 295] | −0.21 (0.78) [n = 171] | 0.09 (−0.06 to 0.24) | |
| Teacher ratings, No./total (%)e | ||||
| Low achievement in reading for curriculum level | 68/281 (24) | 49/157 (31) | −9 (−17 to −1) | 0.72 (0.53-0.99) |
| Low achievement in mathematics for curriculum level | 97/281 (35) | 63/158 (40) | −7 (−16 to 2) | 0.79 (0.61-1.01) |
| Low achievement in writing for curriculum level | 121/280 (43) | 70/157 (45) | −3 (−12 to 7) | 0.96 (0.78-1.18) |
| Low achievement in reading relative to peers | 65/280 (23) | 47/158 (30) | −8 (−16 to 1) | 0.74 (0.54-1.01) |
| Low achievement in mathematics relative to peers | 84/280 (30) | 50/159 (31) | −3 (−12 to 6) | 0.90 (0.67-1.19) |
| Low achievement in writing relative to peers | 107/280 (38) | 65/158 (41) | −4 (−14 to 5) | 0.92 (0.72-1.16) |
| Received additional learning support or older than expected for year level | 140/303 (46) | 73/174 (42) | 4 (−5 to 13) | 1.13 (0.91-1.40) |
| Received additional learning support, older than expected for year level, or low educational achievement | 212/303 (70) | 120/174 (69) | 1 (−8 to 9) | 1.01 (0.89-1.14) |
| Cognitive elements of executive functionf | ||||
| Attention/cognitive flexibility: CANTAB Attention Switching Task | n = 298 | n = 173 | ||
| Switching response latency, mean (SD), ms | 842 (179) | 834 (173) | 9 (−24 to 42) | |
| Congruent response latency, mean (SD), ms | 723 (125) | 712 (122) | 11 (−12 to 34) | |
| Planning/working memory | ||||
| CANTAB One-Touch Stockings of Cambridge | n = 299 | n = 173 | ||
| No. of problems solved on first choice, median (IQR) | 8 (5-10) | 8 (5-10) | 1.00 (0.93-1.07) | |
| Latency to first choice, mean (SD), ms | 12 510 (6567) | 13 604 (7632) | −1061 (−2369 to 247) | |
| CANTAB Spatial Working Memory | n = 299 | n = 173 | ||
| Between-search errors, median (IQR) | 19 (14-23) | 18 (14-22) | 1.04 (0.99-1.09) | |
| Strategy score, mean (SD) | 9.0 (1.5) | 8.9 (1.6) | 0.1 (−0.2 to 0.3) | |
| Visual memory/learning: CANTAB Paired Associate Learning | n = 298 | n = 171 | ||
| First-attempt memory score, mean (SD) | 13.4 (3.6) | 13.7 (3.8) | −0.2 (−0.9 to 0.5) | |
| Total errors (adjusted), median (IQR) | 9 (5-14) | 7 (4-14) | 1.04 (0.98-1.10) | |
| Inhibitory control: CANTAB Stop Signal Task | n = 295 | n = 171 | ||
| Stop signal reaction time, mean (SD), ms | 339 (78) | 332 (84) | 9 (−6 to 24) | |
| Failed stop reaction time less than go reaction time, No. (%) | 289 (98) | 168 (98) | 0 (−10 to 10) | 1.00 (0.88-1.13) |
| Mean failed stop reaction time increases, No. (%) | 269 (91) | 149 (87) | 3 (−5 to 11) | 1.02 (0.95-1.10) |
| Visual-motor function | ||||
| Visual processingg | ||||
| Form coherence threshold, mean (SD) | 29.7 (15.0) [n = 293] | 29.4 (15.2) [n = 173] | 0.4 (−2.4 to 3.2) | |
| Motion coherence threshold, mean (SD) | 22.8 (17.1) [n = 295] | 23.5 (19.2) [n = 173] | −0.5 (−3.8 to 2.9) | |
| Difference between form and motion coherence thresholds, mean (SD) | −6.9 (18.5) [n = 293] | −5.9 (20.5) [n = 173] | −0.9 (−4.5 to 2.7) | |
| MABC-2 fine motor functionh | n = 299 | n = 171 | ||
| Score, mean (SD) | 7.7 (3.2) | 7.6 (3.3) | −0.1 (−0.7 to 0.5) | |
| <15th percentile, No. (%) | 130 (43) | 75 (44) | 1 (−8 to 10) | 1.00 (0.83-1.22) |
| <5th percentile, No. (%) | 85 (28) | 50 (29) | 0 (−8 to 8) | 1.00 (0.73-1.29) |
| BBVMI-6 visual-motor integrationi | n = 299 | n = 173 | ||
| Motor coordination standard score, mean (SD) | 81.2 (13.1) | 82.7 (12.3) | −2.0 (−4.3 to 0.3) | |
| Visual perception standard score, mean (SD) | 97.1 (14.5) | 98.5 (12.8) | −1.4 (−3.9 to 1.2) | |
| Visual-motor integration standard score, mean (SD) | 88.2 (11.4) | 87.3 (11.1) | 0.8 (−1.3 to 2.9) | |
| Motor coordination score <85, No. (%) | 183 (61) | 96 (55) | 8 (−2 to 17) | 1.16 (0.99-1.35) |
| Visual perception score <85, No. (%) | 55 (18) | 23 (13) | 2 (−4 to 9) | 1.39 (0.89-2.17) |
| Visual-motor integration score <85, No. (%) | 109 (36) | 71 (41) | −5 (−14 to 4) | 0.88 (0.70-1.11) |
Abbreviation: e-asTTle, Electronic Assessment Tools for Teaching and Learning.
Hypoglycemia is defined as ≥1 hypoglycemic event, representing the sum of nonconcurrent hypoglycemic and interstitial episodes more than 20 minutes apart; a hypoglycemic episode is defined as ≥1 consecutive blood glucose concentration <47 mg/dL (<2.6 mmol/L) and an interstitial episode as a sensor glucose concentration <47 mg/dL for ≥10 minutes.
Adjusted for potential confounding by sex, primary risk factor for hypoglycemia, and New Zealand Deprivation Index at study entry.
Low educational achievement is defined as an e-asTTle score below or well below the normative curriculum level in reading comprehension/Pānui or mathematics/Pāngarau.
Low achievement in reading comprehension/Pānui or mathematics/Pāngarau is defined as an e-asTTle score below or well below the normative curriculum level, which equates to a child’s learning being 4 or more terms below the expected curriculum level for their current school year and term. Achievement z score is the standardized e-asTTle score for current term of schooling.
For teacher-reported ratings, low achievement is defined as performance worse than or much worse than the expected curriculum or relative to peers, as determined by teacher global judgement. Older than expected for year level was defined as a child being 1 or more years older than 95% of children in their year level on July 1 of the year in which they were assessed; ie, being aged 9 years or older in year 4, 10 years or older in year 5, or 11 years or older in year 6.
Cognitive elements of executive function were assessed using the tablet-based Cambridge Neuropsychological Test Automated Battery (CANTAB). For continuous data, differences of ≥0.3 SD are considered clinically significant, and for counts or binary data, ratios of ≤0.80 or ≥1.20 are considered clinically significant (see eTable 1 in Supplement 3 for details).
Visual processing was assessed using random dot kinetograms of varying coherence, either for horizontal motion or geometric shape, using an adaptive staircase procedure (lower threshold indicates better processing; mean value for adults is 6).28
The fine motor subtest of the Movement Assessment Battery for Children, Second Edition (MABC-2) consists of 3 tests of manual dexterity: placing 12 pegs, threading a lace, and drawing a trail (normative mean score, 10 [SD, 3]).
The Beery-Buktenica Developmental Test of Visual-Motor Integration, Sixth Edition (BBVMI-6) includes tasks to assess visual perception (identifying a target design among choices), motor coordination (tracing a geometric shape), and visual-motor integration (copying a geometric shape) (normative mean score, 100 [SD, 15]).
Table 3. Neonatal Hypoglycemia and Psychosocial Adaptation in Mid-Childhood.
| Outcomes | Hypoglycemiaa | No hypoglycemia | Adjusted risk difference, %, or mean difference (95% CI)b | Adjusted risk ratio or count ratio (95% CI)b |
|---|---|---|---|---|
| Strengths and Difficulties Questionnairec | ||||
| Parent-rated score, mean (SD) | n = 262 | n = 145 | ||
| Total difficulties | 10.8 (7.1) | 10.3 (6.8) | 0.7 (−0.6 to 2.1) | |
| Prosocial behavior | 8.3 (1.8) | 8.3 (1.7) | 0.0 (−0.4 to 0.3) | |
| Teacher-rated score, mean (SD) | n = 285 | n = 162 | ||
| Total difficulties | 8.6 (6.7) | 8.5 (6.8) | 0.2 (−1.0 to 1.5) | |
| Prosocial behavior | 7.5 (2.4) | 7.3 (2.3) | 0.2 (−0.2 to 0.6) | |
| Combined parent and teacher ratings | ||||
| Children with borderline or abnormal total difficulties (parent score ≥14 or teacher score ≥12), No./total (%) | 122/295 (41) | 68/173 (39) | 4 (−5 to 13) | 1.09 (0.87-1.37) |
| Children with borderline or abnormal prosocial behavior (parent or teacher score ≤5), No./total (%) | 72/295 (24) | 46/173 (27) | −1 (−8 to 7) | 0.89 (0.64-1.20) |
| Behavior Rating Inventory of Executive Functiond | ||||
| Parent-rated t score, mean (SD) | n = 258 | n = 146 | ||
| Behavioral regulation | 51.1 (11.8) | 50.6 (11.8) | 0.5 (−1.8 to 2.9) | |
| Metacognition | 53.2 (12.1) | 51.7 (11.2) | 1.6 (−0.7 to 3.9) | |
| Global executive composite | 52.3 (11.8) | 51.1 (11.0) | 1.3 (−0.9 to 3.5) | |
| Teacher-rated t score, mean (SD) | n = 287 | n = 160 | ||
| Behavioral regulation | 55.6 (12.4) | 56.2 (12.6) | −0.4 (−2.8 to 1.9) | |
| Metacognition | 58.6 (13.6) | 59.5 (13.7) | −0.8 (−3.4 to 1.9) | |
| Global executive composite | 57.9 (14.2) | 58.8 (14.3) | −0.7 (−3.4 to 2.1) | |
| Combined parent and teacher ratings | ||||
| Children with global executive composite in clinical range (parent or teacher score >65), No./total (%) | 95/298 (32) | 56/172 (33) | −1 (−9 to 8) | 0.99 (0.75-1.30) |
| Autism Spectrum Quotient: Children’s Versione | n = 236 | n = 128 | ||
| Total score, mean (SD) | 54.3 (17.3) | 54.5 (15.9) | 0.2 (−3.3 to 3.7) | |
| Children with score in clinical range of >75, No. (%) | 23 (10) | 11 (9) | 1 (−5 to 7) | 1.14 (0.57-2.25) |
| Child Health Questionnaire, mean (SD)f | n = 254 | n = 145 | ||
| Physical functioning scale score | 52.8 (9.7) | 53.7 (9.2) | −0.9 (−2.8 to 1.08) | |
| Psychosocial scale score | 48.6 (11.4) | 48.4 (12.2) | 0.1 (−2.2 to 2.5) |
Hypoglycemia is defined as ≥1 hypoglycemic event, representing the sum of nonconcurrent hypoglycemic and interstitial episodes more than 20 minutes apart; a hypoglycemic episode is defined as ≥1 consecutive blood glucose concentration <47 mg/dL (<2.6 mmol/L) and an interstitial episode as a sensor glucose concentration <47 mg/dL for ≥10 minutes.
Adjusted for potential confounding by sex, primary risk factor for hypoglycemia, and New Zealand Deprivation Index at study entry.
The Strengths and Difficulties Questionnaire assesses emotional symptoms, conduct problems, hyperactivity/inattention, peer relationships, and prosocial behavior; a higher total difficulties score indicates worse emotional and behavioral regulation; boys have an expected mean score of 8.3 (SD, 7.1) and girls of 5.3 (SD, 5.7), with a possible range of 0 to 4029; a lower prosocial behavior score indicates greater social difficulties; boys have an expected mean score of 7.4 (SD, 2.5) and girls of 8.5 (SD, 2.0), with a possible range of 0 to 10.29
The Behavior Rating Inventory of Executive Function assesses behavioral manifestations of executive function; higher t scores indicate a higher level of dysfunction; the normative mean score is 50 (SD, 10; 95% range, 30-70).
The Autism Spectrum Quotient: Children’s Version assesses autism spectrum disorder traits; a higher total score indicates greater autism traits; the mean score in 4- to 11-year-old children is 41.7 (SD, 18.6), with a possible range of 0 to 150.26
The Child Health Questionnaire assesses general health, behavior, and limitation in everyday and school activities; a higher score indicates better functioning; the normative mean is 50 (SD, 10; 95% range, 30-70).
Secondary Analyses
For the primary and prespecified critical secondary outcomes, there were no significant differences between children who had experienced different severities (Table 4) or frequencies (Table 5) of hypoglycemia compared with those without any neonatal hypoglycemia. There were also no significant differences for these outcomes between children with and without hypoglycemic episodes in the first week after birth, those whose hypoglycemia was and was not detected (continuous glucose monitoring episodes but no episodes of low blood glucose concentration), or between children with no hypoglycemia and those with different degrees of hypoglycemia (eTables 3-5 in Supplement 3).
Table 4. Primary and Prespecified Critical Secondary Outcomes in Mid-Childhood by Severity of Neonatal Hypoglycemia.
| Outcomes | Hypoglycemic eventsa | Adjusted risk difference, %, or mean difference (95% CI)b | Adjusted risk ratio or count ratio (95% CI)b | ||||
|---|---|---|---|---|---|---|---|
| None | Mild | Severe or recurrent | Mild vs none | Severe or recurrent vs none | Mild vs none | Severe or recurrent vs none | |
| Low educational achievement (primary outcome), No./total (%)c | 82/172 (48) | 40/103 (39) | 98/193 (51) | −9 (−23 to 5) | 2 (−9 to 13) | 0.85 (0.62 to 1.17) | 0.99 (0.79 to 1.25) |
| e-asTTle achievement z score in reading comprehension/Pānui, mean (SD)d | −0.32 (1.26) [n = 172] | −0.02 (1.08) [n =103] | −0.31 (1.11) [n =193] | 0.27 (−0.03 to 0.58) | 0.04 (−0.22 to 0.30) | ||
| e-asTTle achievement z score in mathematics/Pāngarau, mean (SD)d | −0.21 (0.78) [n = 171] | −0.10 (0.87) [n = 102] | −0.15 (0.82) [n = 193] | 0.08 (−0.14 to 0.30) | 0.09 (−0.10 to 0.27) | ||
| Need for additional learning support, older than expected for year level, or low educational achievement, No./total (%)e | 120/174 (69) | 72/106 (68) | 140/197 (71) | −1 (−19 to 10) | 2 (−9 to 12) | 0.98 (0.82 to 1.18) | 1.02 (0.88 to 1.19) |
| CANTAB One-Touch Stockings of Cambridge (planning/working memory)f | n = 173 | n = 104 | n = 195 | ||||
| Problems solved on first choice, median (IQR) | 8 (5-10) | 8 (6-10) | 8 (5-10) | 1.01 (0.91 to 1.11) | 1.00 (0.91 to 1.08) | ||
| Latency to first choice, mean (SD), ms | 13 604 (7632) | 12 521 (6161) | 12 505 (6788) | −1133 (−3047 to 782) | −1023 (−2641 to 596) | −1133 (−3047 to 782) | −1023 (−2641 to 596) |
| MABC-2 fine motor subtest score <15th percentile, No./total (%)g | 75/171 (44) | 41/104 (39) | 89/195 (46) | −0.01 (−0.13 to 0.11) | 0.02 (−0.09 to 0.13) | 0.93 (0.69 to 1.25) | 1.05 (0.83 to 1.33) |
| BBVMI-6 score <85, No./total (%)h | 71/173 (41) | 33/104 (32) | 76/195 (39) | −25 (−62 to 13) | −6 (−34 to 22) | 0.78 (0.54 to 1.13) | 0.94 (0.71 to 1.24) |
| Borderline or abnormal total difficulties score on parent- or teacher-rated SDQ, No./total (%)i | 68/173 (39) | 37/102 (36) | 85/193 (44) | 0 (−14 to 12) | 6 (−5 to 17) | 1.02 (0.71 to 1.45) | 1.13 (0.86 to 1.48) |
| AQ-Child total score, mean (SD)j | 54.5 (15.9) [n = 128] | 51.9 (15.8) [n = 82] | 55.7 (17.9) [n = 154] | −1.8 (−6.8 to 3.3) | 1.3 (−3.0 to 5.5) | ||
Abbreviation: e-asTTle, Electronic Assessment Tools for Teaching and Learning.
Hypoglycemic event is defined as the sum of nonconcurrent hypoglycemic and interstitial episodes more than 20 minutes apart; a hypoglycemic episode is defined as ≥1 consecutive blood glucose concentration <47 mg/dL (<2.6 mmol/L) and an interstitial episode as a sensor glucose concentration <47 mg/dL for ≥10 minutes. A severe event represents a blood or sensor glucose concentration <2.0 mmol/L.
Adjusted for potential confounding by sex, primary risk factor for hypoglycemia, and New Zealand Deprivation Index at study entry.
Low educational achievement is defined as an e-asTTle score below or well below the normative curriculum level in reading comprehension/Pānui or mathematics/Pāngarau.
Achievement z score is the standardized e-asTTle score for the current term of schooling.
Older than expected for year level was defined as a child being 1 or more years older than 95% of children in their year level on July 1 of the year in which they were assessed; ie, being aged 9 years or older in year 4, 10 years or older in year 5, or 11 years or older in year 6.
The Cambridge Neuropsychological Test Automated Battery (CANTAB) One-Touch Stockings of Cambridge test assesses working memory and planning, a key executive function capacity. Greater number of problems solved (maximum, 20) and shorter latency indicate better working memory and planning; for continuous data; count ratios of ≤0.80 or ≥1.20 or mean differences in latency by 0.3 SD are considered clinically significant (see eTable 1 in Supplement 3 for further details).
The fine motor subtest of the Movement Assessment Battery for Children, Second Edition (MABC-2) consists of 3 tests of manual dexterity: placing 12 pegs, threading a lace, and drawing a trail (normative mean score, 10 [SD, 3]).
The Beery-Buktenica Developmental Test of Visual-Motor Integration, Sixth Edition (BBVMI-6) assesses integration and visual perception and coordination. Lower scores indicate worse visual-motor function.
The Strengths and Difficulties Questionnaire (SDQ) assesses emotional symptoms, conduct problems, hyperactivity/inattention, peer relationships, and prosocial behavior. A higher total difficulties score indicates worse emotional and behavioral regulation.
The Autism Spectrum Quotient: Children’s Version (AQ-Child) assesses autism spectrum disorder traits. A higher total score indicates greater autism traits; the mean in 4- to 11-year-old children is 41.7 (SD, 18.6), with a possible range of 0 to 150.26
Table 5. Primary and Prespecified Critical Secondary Outcomes in Mid-Childhood by Frequency of Neonatal Hypoglycemia.
| Outcomes | Hypoglycemic eventsa | Adjusted risk difference, %, or mean difference (95% CI)b | Adjusted risk ratio or count ratio (95% CI)b | ||||
|---|---|---|---|---|---|---|---|
| No events | 1-2 events | ≥3 events | 1-2 vs no events | ≥3 vs no events | 1-2 vs no events | ≥3 vs no events | |
| Low educational achievement (primary outcome), No./total (%)c | 82/172 (48) | 55/135 (41) | 83/161 (52) | −8 (−20 to 5) | 3 (−8 to 15) | 0.84 (0.64 to 1.11) | 1.04 (0.82 to 1.31) |
| e-asTTle achievement z score in reading comprehension/Pānui, mean (SD)d | −0.32 (1.26) [n = 172] | −0.12 (1.09) [n = 135] | −0.29 (1.13) [n = 161] | 0.19 (−0.09 to 0.48) | 0.05 (−0.22 to 0.33) | ||
| e-asTTle achievement z score in mathematics/Pāngarau, mean (SD)d | −0.21 (0.78) [n = 171] | −0.08 (0.87) [n = 134] | −0.18 (0.81) [n = 161] | 0.11 (−0.09 to 0.32) | 0.06 (−0.13 to 0.26) | ||
| Need for additional learning support, older than expected for year level, or low educational achievement, No./total (%)e | 120/174 (69) | 91/139 (65) | 121/164 (74) | −4 (−16 to 8) | 5 (−6 to 16) | 0.94 (0.79 to 1.12) | 1.07 (0.91 to 1.25) |
| CANTAB One-Touch Stockings of Cambridge (planning/working memory)f | n = 173 | n = 136 | n = 163 | ||||
| Problems solved on first choice, median (IQR) | 8 (5-10) | 8 (6-10) | 8 (5-10) | 1.02 (0.93 to 1.11) | 0.97 (0.88 to 1.07) | ||
| Latency to first choice, mean (SD), ms | 13 604 (7632) | 12 623 (6281) | 12 416 (6813) | −975 (−2746 to 796) | −1134 (−2829 to 561) | ||
| MABC-2 fine motor subtest score <15th percentile, No./total (%)g | 75/171 (44) | 53/136 (39) | 77/163 (47) | −3 (−14 to 9) | 5 (−7 to 17) | 0.88 (0.67 to 1.16) | 1.14 (0.90 to 1.45) |
| BBVMI-6 score <85, No./total (%)h | 71/173 (41) | 48/136 (35) | 61/163 (37) | −6 (−18 to 7) | 7 (−16 to 8) | 0.87 (0.66 to 1.21) | 0.90 (0.66 to 1.21) |
| Borderline or abnormal total difficulties score on parent- or teacher-rated SDQ, No./total (%)i | 68/173 (39) | 50/134 (37) | 72/161 (45) | 0 (−12 to 13) | −7 (−19 to 5) | 1.02 (0.84 to 1.23) | 0.89 (0.72 to 1.09) |
| AQ-Child total score, mean (SD)j | 54.5 (15.9) [n = 128] | 51.7 (16.5) [n = 108] | 56.6 (17.7) [n = 128] | −2.3 (−7.0 to 2.36) | 2.4 (−2.1 to 6.8) | ||
Abbreviation: e-asTTle, Electronic Assessment Tools for Teaching and Learning.
Hypoglycemic event is defined as the sum of nonconcurrent hypoglycemic and interstitial episodes more than 20 minutes apart; a hypoglycemic episode is defined as ≥1 consecutive blood glucose concentration <47 mg/dL (<2.6 mmol/L) and an interstitial episode as a sensor glucose concentration <47 mg/dL for ≥10 minutes. Recurrent events are defined as 3 or more events.
Adjusted for potential confounding by sex, primary risk factor for hypoglycemia, and New Zealand Deprivation Index at study entry.
Low educational achievement is defined as an e-asTTle score below or well below the normative curriculum level in reading comprehension/Pānui or mathematics/Pāngarau.
Achievement z score is the standardized e-asTTle score for the current term of schooling.
Older than expected for year level was defined as a child being 1 or more years older than 95% of children in their year level on July 1 of the year in which they were assessed; ie, being aged 9 years or older in year 4, 10 years or older in year 5, or 11 years or older in year 6.
The Cambridge Neuropsychological Test Automated Battery (CANTAB) One-Touch Stockings of Cambridge test assesses working memory and planning, a key executive function capacity. Greater number of problems solved (maximum, 20) and shorter latency indicate better working memory and planning; for continuous data; count ratios of ≤0.80 or ≥1.20 or mean differences in latency by 0.3 SD are considered clinically significant (see eTable 1 in Supplement 3 for further details).
The fine motor subtest of the Movement Assessment Battery for Children, Second Edition (MABC-2) consists of 3 tests of manual dexterity: placing 12 pegs, threading a lace, and drawing a trail (normative mean score, 10 [SD, 3]).
The Beery-Buktenica Developmental Test of Visual-Motor Integration, Sixth Edition (BBVMI-6) assesses integration and visual perception and coordination. Lower scores indicate worse visual-motor function.
The Strengths and Difficulties Questionnaire (SDQ) assesses emotional symptoms, conduct problems, hyperactivity/inattention, peer relationships, and prosocial behavior. A higher total difficulties score indicates worse emotional and behavioral regulation.
The Autism Spectrum Quotient: Children’s Version (AQ-Child) assesses autism spectrum disorder traits. A higher total score indicates greater autism traits; the mean in 4- to 11-year-old children is 41.7 (SD, 18.6), with a possible range of 0 to 150.26
In sensitivity analyses, results for the primary and critical secondary outcomes were not altered by excluding children who experienced a postnatal neurological insult; who were educated outside of New Zealand; who were classified as normoglycemic but did not have neonatal continuous glucose monitoring to potentially detect low sensor glucose concentrations; or who were born prior to 35 weeks’ gestation (eTables 6A-D in Supplement 3). In post hoc exploratory analysis, results for measures of executive function and low visual-motor integration, which were associated with hypoglycemia at age 4.5 years, were not altered by adjustment for scores for the same measures at age 4.5 years (eTable 7 in Supplement 3). Similarly, the primary outcome in this study was not altered by adjustment for full-scale intelligence quotient at age 4.5 years (eTable 7 in Supplement 3).
Discussion
Among children born at risk of neonatal hypoglycemia who were screened and treated if needed to maintain blood glucose concentrations of at least 47 mg/dL, children who did and did not experience neonatal hypoglycemia did not significantly differ on low educational achievement in mid-childhood. However, the upper limit of the 95% CI for the risk difference was an 8% increase in low educational achievement, and the upper limit of the 95% CI for the risk ratio was 1.15. Therefore, a small but potentially clinically relevant increase in risk cannot be ruled out.
The reason that hypoglycemia was associated with adverse neurodevelopmental outcomes in this cohort at 4.5 years of age6 but not at 9 to 10 years is unclear. Early disturbances in brain development may have diminishing effects over time due to neuroplasticity, that is, reorganization of neural networks, or delayed maturation with mid-childhood catch-up in neurocognitive function.30 It is also possible that rich preschool and school experiences could improve intelligence and academic abilities in exposed children.31
However, the very high rates of low performance at age 9 to 10 years in both groups of this cohort across a range of measures is concerning, including educational achievement, fine motor function, visual-motor integration, emotional-behavioral regulation, and behavioral manifestations of executive function. This low performance suggests that the underlying risk factors for neonatal hypoglycemia, such as late preterm birth, fetal growth restriction, and maternal diabetes, plus their socioeconomic determinants, may have a greater effect on neurodevelopmental trajectories.32,33,34 While exposure to hypoglycemia may alter the shape of the early developmental course, this study suggests that at-risk children reach similar end points by the end of primary schooling.
It is unlikely that these findings are unique to this cohort, as relatively high rates of neurodevelopmental impairment have been observed in other cohorts of infants born at risk of transitional neonatal hypoglycemia.35 Therefore, efforts to prevent and optimize adverse pregnancy conditions remain important, and developmental surveillance after birth should be considered for at-risk infants.
Strengths of this study include a sample size with sufficient power to detect clinically important associations, prospective design, detailed and accurate measures of neonatal glycemic exposure, blinded assessments, and adjustment for potential confounding.
Limitations
This study has several limitations. First, not all aspects of cognition were measured, such as processing speed. However, a broad range of targeted neurocognitive functions and educational outcomes were evaluated that have previously been reported to be affected by neonatal hypoglycemia.36 In addition, results for the primary outcome were not altered by adjusting for intelligence quotient at age 4.5 years. Second, 18% of eligible children were not assessed at 9 to 10 years of age, which could introduce bias if there were systematic differences in exposures or outcomes between those who were and were not assessed. Third, a small number of children (approximately 4%) with severe impairments at age 4.5 years were referred for developmental assessment,37 but information on the type or intensity of any treatment received is not available. Fourth, all infants in this cohort had close monitoring of blood glucose concentrations after birth and were treated with the aim of maintaining blood glucose of at least 47 mg/dL. The results of this study may not apply equally to infants receiving less intensive screening and treatment.
Conclusions
Among participants at risk of neonatal hypoglycemia who were screened and treated if needed, exposure to neonatal hypoglycemia compared with no such exposure was not significantly associated with lower educational achievement in mid-childhood.
Study Protocol
Statistical Analysis Plan
eTable 1. Assessments and Secondary Outcomes in the CHYLD Mid-Childhood Outcome Study
eTable 2. Characteristics of Children Who Did and Did Not Participate in the CHYLD Mid-Childhood Outcome Study
eTable 3. Neonatal Hypoglycaemic Episodes vs. No Hypoglycemic Episodes and Primary and Critical Secondary Outcomes at Mid-Childhood
eTable 4. Undetected vs. Detected Neonatal Hypoglycemia and Primary and Critical Secondary Outcomes at Mid-Childhood
eTable 5. Different Degrees of Neonatal Hypoglycemia and Primary and Critical Secondary Outcomes in Mid-Childhood
eTable 6A. Sensitivity Analysis of Primary and Critical Secondary Outcomes in Mid-Childhood, Excluding Children Who Experienced a Postnatal Neurological Insult
eTable 6B. Sensitivity Analysis of Primary and Critical Secondary Outcomes in Mid-Childhood, Excluding Children Who Were Educated Outside of New Zealand
eTable 6C. Sensitivity Analysis of Primary and Critical Secondary Outcomes in Mid-Childhood, Excluding Children Who Were Classified as Normoglycemic But Did Not Have Neonatal Continuous Glucose Monitoring to Potentially Detect Low Sensor Glucose Concentrations
eTable 6D. Sensitivity Analysis of Primary and Critical Secondary Outcomes in Mid-Childhood, Excluding Children Who Were Born at <35 Weeks’ Gestation
eTable 7. Neonatal Hypoglycemia and Primary Outcome, Executive Function and Visual-Motor Integration at Mid-Childhood, Adjusted for Neurocognitive Function at 4.5 Years (Exploratory Post Hoc Analysis)
eReferences
Nonauthor Collaborators. Children With Hypoglycaemia and Their Later Development (CHYLD) Study Team
References
- 1.Harding JE, Harris DL, Hegarty JE, Alsweiler JM, McKinlay CJD. An emerging evidence base for the management of neonatal hypoglycaemia. Early Hum Dev. 2017;104:51-56. doi: 10.1016/j.earlhumdev.2016.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward Platt M, Deshpande S. Metabolic adaptation at birth. Semin Fetal Neonatal Med. 2005;10(4):341-350. doi: 10.1016/j.siny.2005.04.001 [DOI] [PubMed] [Google Scholar]
- 3.McKinlay CJD, Alsweiler JM, Bailey MJ, Cutfield WS, Rout A, Harding JE. A better taxonomy for neonatal hypoglycemia is needed. J Perinatol. 2021;41(5):1205-1206. doi: 10.1038/s41372-021-01058-x [DOI] [PubMed] [Google Scholar]
- 4.Alsweiler JM, Harris DL, Harding JE, McKinlay CJD. Strategies to improve neurodevelopmental outcomes in babies at risk of neonatal hypoglycaemia. Lancet Child Adolesc Health. 2021;5(7):513-523. doi: 10.1016/S2352-4642(20)30387-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKinlay CJD, Alsweiler JM, Ansell JM, et al. ; CHYLD Study Group . Neonatal glycemia and neurodevelopmental outcomes at 2 years. N Engl J Med. 2015;373(16):1507-1518. doi: 10.1056/NEJMoa1504909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKinlay CJD, Alsweiler JM, Anstice NS, et al. ; Children With Hypoglycemia and Their Later Development Study Team . Association of neonatal glycemia with neurodevelopmental outcomes at 4.5 years. JAMA Pediatr. 2017;171(10):972-983. doi: 10.1001/jamapediatrics.2017.1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaiser JR, Bai S, Gibson N, et al. Association between transient newborn hypoglycemia and fourth-grade achievement test proficiency: a population-based study. JAMA Pediatr. 2015;169(10):913-921. doi: 10.1001/jamapediatrics.2015.1631 [DOI] [PubMed] [Google Scholar]
- 8.Tin W, Brunskill G, Kelly T, Fritz S. 15-year follow-up of recurrent “hypoglycemia” in preterm infants. Pediatrics. 2012;130(6):e1497-e1503. doi: 10.1542/peds.2012-0776 [DOI] [PubMed] [Google Scholar]
- 9.Brand PL, Molenaar NL, Kaaijk C, Wierenga WS. Neurodevelopmental outcome of hypoglycaemia in healthy, large for gestational age, term newborns. Arch Dis Child. 2005;90(1):78-81. doi: 10.1136/adc.2003.039412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burakevych N, McKinlay CJD, Harris DL, Alsweiler JM, Harding JE. Factors influencing glycaemic stability after neonatal hypoglycaemia and relationship to neurodevelopmental outcome. Sci Rep. 2019;9(1):8132. doi: 10.1038/s41598-019-44609-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest. 2007;117(4):910-918. doi: 10.1172/JCI30077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris DL, Weston PJ, Signal M, Chase JG, Harding JE. Dextrose gel for neonatal hypoglycaemia (the Sugar Babies Study): a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382(9910):2077-2083. doi: 10.1016/S0140-6736(13)61645-1 [DOI] [PubMed] [Google Scholar]
- 13.Harris DL, Weston PJ, Williams CE, et al. Cot-side electroencephalography monitoring is not clinically useful in the detection of mild neonatal hypoglycemia. J Pediatr. 2011;159(5):755-760.e1. doi: 10.1016/j.jpeds.2011.04.026 [DOI] [PubMed] [Google Scholar]
- 14.Signal M, Le Compte A, Harris DL, Weston PJ, Harding JE, Chase JG; CHYLD Study Group . Impact of retrospective calibration algorithms on hypoglycemia detection in newborn infants using continuous glucose monitoring. Diabetes Technol Ther. 2012;14(10):883-890. doi: 10.1089/dia.2012.0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris DL, Battin MR, Weston PJ, Harding JE. Continuous glucose monitoring in newborn babies at risk of hypoglycemia. J Pediatr. 2010;157(2):198-202.e1. doi: 10.1016/j.jpeds.2010.02.003 [DOI] [PubMed] [Google Scholar]
- 16.Atkinson J, Salmond C, Crampton P.. NZDep2013 Index of Deprivation. Department of Public Health, University of Otago; 2014. [Google Scholar]
- 17.Ethnicity Data Protocols for the Health and Disability Sector. Ministry of Health, New Zealand; 2004. [Google Scholar]
- 18.Hattie JAC, Brown GTL. Technology for school-based assessment and assessment for learning: development principles from New Zealand. J Educ Technol Syst. 2007;36(2):189-201. doi: 10.2190/ET.36.2.g [DOI] [Google Scholar]
- 19.Shah R, Brown G, Keegan P, Burakevych N, Harding JE, McKinlay CJ; CHYLD Study Group . Teacher rating versus measured academic achievement: implications for paediatric research. J Paediatr Child Health. 2020;56(7):1090-1096. doi: 10.1111/jpc.14824 [DOI] [PubMed] [Google Scholar]
- 20.Luciana M. Practitioner review: computerized assessment of neuropsychological function in children: clinical and research applications of the Cambridge Neuropsychological Testing Automated Battery (CANTAB). J Child Psychol Psychiatry. 2003;44(5):649-663. doi: 10.1111/1469-7610.00152 [DOI] [PubMed] [Google Scholar]
- 21.Diamond A. Executive functions. Annu Rev Psychol. 2013;64:135-168. doi: 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beery KE, Beery NA. The Beery-Buktenica Developmental Test of Visual-Motor Integration. 6th ed. Pearson; 2010. [Google Scholar]
- 23.Henderson S, Sugden D, Barnett A. Movement Assessment Battery for Children–2. Harcourt Assessment; 2007. [Google Scholar]
- 24.Chakraborty A, Anstice NS, Jacobs RJ, et al. Global motion perception is independent from contrast sensitivity for coherent motion direction discrimination and visual acuity in 4.5-year-old children. Vision Res. 2015;115(pt A):83-91. doi: 10.1016/j.visres.2015.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gioia G, Isquith P, Guy S, Kenworthy L. BRIEF: Behavior Rating Inventory of Executive Function: Professional Manual. Psychological Assessment Resources; 2000. [Google Scholar]
- 26.Auyeung B, Baron-Cohen S, Wheelwright S, Allison C. The Autism Spectrum Quotient: Children’s Version (AQ-Child). J Autism Dev Disord. 2008;38(7):1230-1240. doi: 10.1007/s10803-007-0504-z [DOI] [PubMed] [Google Scholar]
- 27.Waters E, Salmon L, Wake M, Hesketh K, Wright M. The Child Health Questionnaire in Australia: reliability, validity and population means. Aust N Z J Public Health. 2000;24(2):207-210. doi: 10.1111/j.1467-842X.2000.tb00145.x [DOI] [PubMed] [Google Scholar]
- 28.Yu TY, Jacobs RJ, Anstice NS, Paudel N, Harding JE, Thompson B; CHYLD Study Team . Global motion perception in 2-year-old children: a method for psychophysical assessment and relationships with clinical measures of visual function. Invest Ophthalmol Vis Sci. 2013;54(13):8408-8419. doi: 10.1167/iovs.13-13051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayes L. Problem behaviours in early primary school children: Australian normative data using the Strengths and Difficulties Questionnaire. Aust N Z J Psychiatry. 2007;41(3):231-238. doi: 10.1080/00048670601172715 [DOI] [PubMed] [Google Scholar]
- 30.Johnston MV, Ishida A, Ishida WN, Matsushita HB, Nishimura A, Tsuji M. Plasticity and injury in the developing brain. Brain Dev. 2009;31(1):1-10. doi: 10.1016/j.braindev.2008.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakken L, Brown N, Downing B. Early childhood education: the long-term benefits. J Res Childhood Educ. 2017;31(2):255-69. doi: 10.1080/02568543.2016.1273285 [DOI] [Google Scholar]
- 32.Levine TA, Grunau RE, McAuliffe FM, Pinnamaneni R, Foran A, Alderdice FA. Early childhood neurodevelopment after intrauterine growth restriction: a systematic review. Pediatrics. 2015;135(1):126-141. doi: 10.1542/peds.2014-1143 [DOI] [PubMed] [Google Scholar]
- 33.Adane AA, Mishra GD, Tooth LR. Diabetes in pregnancy and childhood cognitive development: a systematic review. Pediatrics. 2016;137(5):e20154234. doi: 10.1542/peds.2015-4234 [DOI] [PubMed] [Google Scholar]
- 34.Chan E, Leong P, Malouf R, Quigley MA. Long-term cognitive and school outcomes of late-preterm and early-term births: a systematic review. Child Care Health Dev. 2016;42(3):297-312. doi: 10.1111/cch.12320 [DOI] [PubMed] [Google Scholar]
- 35.Griffith R, Hegarty JE, Alsweiler JM, et al. Two-year outcomes after dextrose gel prophylaxis for neonatal hypoglycaemia. Arch Dis Child Fetal Neonatal Ed. 2021;106(3):278-285. doi: 10.1136/archdischild-2020-320305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah R, Harding J, Brown J, McKinlay C. Neonatal glycaemia and neurodevelopmental outcomes: a systematic review and meta-analysis. Neonatology. 2019;115(2):116-126. doi: 10.1159/000492859 [DOI] [PubMed] [Google Scholar]
- 37.Burakevych N, McKinlay CJ, Alsweiler JM, Wouldes TA, Harding JE. Pre-school screening for developmental and emotional health: comparison with neurodevelopmental assessment. J Paediatr Child Health. 2016;52(6):600-607. doi: 10.1111/jpc.13169 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study Protocol
Statistical Analysis Plan
eTable 1. Assessments and Secondary Outcomes in the CHYLD Mid-Childhood Outcome Study
eTable 2. Characteristics of Children Who Did and Did Not Participate in the CHYLD Mid-Childhood Outcome Study
eTable 3. Neonatal Hypoglycaemic Episodes vs. No Hypoglycemic Episodes and Primary and Critical Secondary Outcomes at Mid-Childhood
eTable 4. Undetected vs. Detected Neonatal Hypoglycemia and Primary and Critical Secondary Outcomes at Mid-Childhood
eTable 5. Different Degrees of Neonatal Hypoglycemia and Primary and Critical Secondary Outcomes in Mid-Childhood
eTable 6A. Sensitivity Analysis of Primary and Critical Secondary Outcomes in Mid-Childhood, Excluding Children Who Experienced a Postnatal Neurological Insult
eTable 6B. Sensitivity Analysis of Primary and Critical Secondary Outcomes in Mid-Childhood, Excluding Children Who Were Educated Outside of New Zealand
eTable 6C. Sensitivity Analysis of Primary and Critical Secondary Outcomes in Mid-Childhood, Excluding Children Who Were Classified as Normoglycemic But Did Not Have Neonatal Continuous Glucose Monitoring to Potentially Detect Low Sensor Glucose Concentrations
eTable 6D. Sensitivity Analysis of Primary and Critical Secondary Outcomes in Mid-Childhood, Excluding Children Who Were Born at <35 Weeks’ Gestation
eTable 7. Neonatal Hypoglycemia and Primary Outcome, Executive Function and Visual-Motor Integration at Mid-Childhood, Adjusted for Neurocognitive Function at 4.5 Years (Exploratory Post Hoc Analysis)
eReferences
Nonauthor Collaborators. Children With Hypoglycaemia and Their Later Development (CHYLD) Study Team