Key Points
Question
Are lower (worse) left atrial (LA) function and greater LA size associated with increased risk of subsequent dementia?
Findings
In this retrospective cohort study that included 4096 participants, there were statistically significant associations between measures of lower LA function and incident dementia comparing the lowest vs highest quintile for LA reservoir strain (HR, 1.98), conduit strain (HR, 1.50), contractile strain (HR, 1.57), emptying fraction (HR, 1.87), and active emptying fraction (HR, 1.43). Measures of LA size were not significantly associated with incident dementia.
Meaning
The findings suggest that impaired LA function may be a risk factor associated with dementia.
Abstract
Importance
Atrial myopathy—characterized by alterations in left atrial (LA) function and size—is associated with ischemic stroke, independent of atrial fibrillation (AF). Electrocardiographic markers of atrial myopathy are associated with dementia, but it is unclear whether 2-dimensional echocardiographic (2DE)–defined LA function and size are associated with dementia.
Objective
To examine the association of LA function and size with incident dementia.
Design, Setting, and Participants
The Atherosclerosis Risk in Communities (ARIC) study is a community-based prospective cohort. An exploratory, retrospective analysis was conducted. ARIC centers are located in Forsyth County, North Carolina; Jackson, Mississippi; Washington County, Maryland; and suburban Minneapolis, Minnesota. For this analysis, visit 5 (2011-2013) served as the baseline. Participants without prevalent AF and stroke and who had 2DEs in 2011-2013 were included and surveilled through December 31, 2019.
Exposures
LA function (reservoir strain, conduit strain, contractile strain, emptying fraction, passive emptying fraction, and active emptying fraction), and LA size (maximal and minimal volume index) as evaluated by 2DE.
Main Outcomes and Measures
Dementia cases were identified using in-person and phone cognitive assessments, hospitalization codes, and death certificates. Cox proportional hazards models were used.
Results
Among 4096 participants (mean [SD] age, 75 [5] years; 60% women; 22% Black individuals), 531 dementia cases were ascertained over a median follow-up of 6 years. Dementia incidence for the lowest LA quintile was 4.80 for reservoir strain, 3.94 for conduit strain, 3.29 for contractile strain, 4.20 for emptying fraction, 3.67 for passive emptying fraction, and 3.27 for active emptying fraction per 100 person-years. After full-model adjustments, there were statistically significant associations between measures of LA function and dementia; the hazard ratios (HRs) from the lowest vs highest quintile for reservoir strain were 1.98 (95% CI, 1.42-2.75); for conduit strain, 1.50 (95% CI, 1.09-2.06); for contractile strain, 1.57 (95% CI, 1.16-2.14); for emptying fraction, 1.87 (95% CI, 1.31-2.65); and for active emptying fraction, 1.43 (95% CI, 1.04-1.96). LA passive emptying fraction was not significantly associated with dementia (HR, 1.26 [95% CI, 0.93-1.71]). Dementia incidence for the highest LA maximal volume index quintile was 3.18 per 100 person-years (HR for highest vs lowest quintile, 0.77 [95% CI, 0.58-1.02]) and for the highest minimal volume index quintile was 3.50 per 100 person-years (HR for the highest vs lowest quintile, 0.95 [95% CI, 0.71-1.28]). Both measures were not significantly associated with dementia. These findings were robust to sensitivity analyses that excluded participants with incident AF or stroke.
Conclusions and Relevance
In this exploratory analysis of a US community-based cohort, several echocardiographic measures of lower LA function were significantly associated with an increased risk of subsequent dementia. Measures of LA size were not significantly associated with dementia risk. These findings suggest that impaired LA function may be a risk factor associated with dementia.
This exploratory retrospective analysis evaluated the association of echocardiographic measures of left atrial function and size with incident dementia in a large community-based cohort.
Introduction
Atrial myopathy—characterized by alterations in left atrial (LA) function and size—is associated with adverse cardiovascular events.1,2,3,4,5,6 Lower (worse) LA function is associated with adverse cardiovascular outcomes in the general population and worse prognosis among those with cardiovascular disease.7 Greater maximal LA volume index (LAVI), and more recently greater minimal LAVI, are also risk factors for cardiovascular events.5,6,8,9,10
Although the evidence linking atrial myopathy to adverse cardiovascular outcomes is compelling, less is known about the association of atrial myopathy with dementia. Because lower LA reservoir function has been associated with presence of silent brain infarcts and white matter hyperintensities,11 it is possible that atrial myopathy is also associated with dementia. Furthermore, prior research has found electrocardiographic (ECG) markers of atrial myopathy to be associated with greater cognitive decline and dementia.12 However, whether abnormal LA function and size, as assessed by echocardiography, are linked to increased dementia risk is unknown.
Furthermore, given that atrial myopathy is a precursor to atrial fibrillation (AF) and the latter is associated with dementia,13 the association between atrial myopathy and dementia (if any) may be mediated by AF. Hence, in evaluating the relationship of atrial myopathy to dementia, it is essential to account for the contribution of AF. Therefore, the aim of this study was to assess the association of echocardiographic measures of LA function and size with incident dementia in the Atherosclerosis Risk in Communities (ARIC) study, a large community-based cohort. A secondary aim was to quantify the contribution of AF to the foregoing associations using mediation analysis. The hypothesis of this study was that lower (worse) LA function and larger LA size are associated with an increased risk of dementia, independent of incident AF, incident stroke, and other cardiovascular and dementia risk factors.
Methods
Study Population and Design
Institutional review boards at each center approved the study and participants provided written informed consent at each visit.
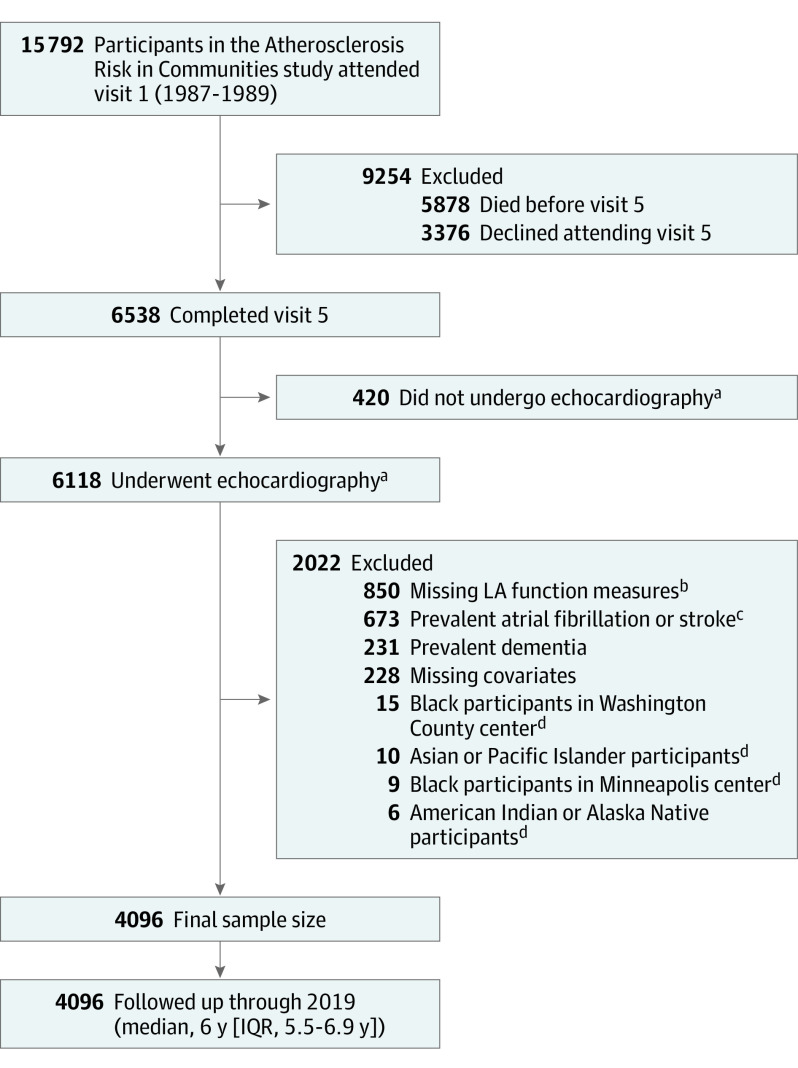
The ARIC study is a community-based cohort of predominately Black and White adults aged 45 through 64 years at baseline (1987-1989). At inception, 15 792 participants were recruited from 4 US communities: Forsyth County, North Carolina; Jackson, Mississippi; Washington County, Maryland; and the northwest suburbs of Minneapolis, Minnesota.14 Participants have attended several additional follow-up visits and are observed continuously for hospitalizations. For this analysis, visit 5 (2011-2013) served as the baseline because echocardiograms were performed at this visit (Figure 1).
Figure 1. Flow of Participants in the Atherosclerosis Risk in Communities Study, 2011-2019.
aParticipants did not have echocardiograms because they declined the echocardiogram or they opted for an abbreviated examination that did not include an echocardiogram.
bLeft atrial (LA) function measures were missing due to suboptimal echocardiogram images.
cAtrial fibrillation or stroke events were considered prevalent if they were diagnosed at or before the time of each participant’s visit 5 date.
dThose other than Black or White and Black participants from the Minneapolis and Washington County centers were excluded due to small numbers.
Participants who attended visit 5 and had an echocardiogram were included in this analysis (Table 1). Of those with echocardiograms, images that were suboptimal for LA function measurement were excluded. From speckle tracking analysis, LA phasic function was measured using volumes and strain indexes calculated as the mean of 12 segments obtained using apical 4-chamber views. Measurements were considered unreliable and not performed if more than 2 segments could not be tracked, if there were missing views, or if there was significant foreshortening of the cavity. Other exclusion criteria included those with prevalent dementia, AF, or stroke at visit 5 or missing covariates, as well as those whose race was other than Black or White and non-White individuals in the Minneapolis and Washington County centers due to small numbers.
Table 1. Baseline Participant Characteristics: Atherosclerosis Risk in Communities Study, 2011-2013a.
| No. (%) | ||
|---|---|---|
| Dementia (n = 531) | No dementia (n = 3565) | |
| Demographics | ||
| Age, mean (SD), y | 78.0 (5.2) | 74.6 (4.8) |
| Sex | ||
| Women | 310 (58.4) | 2133 (59.8) |
| Men | 221 (41.6) | 1432 (40.2) |
| Raceb | ||
| Black | 152 (28.6) | 743 (20.8) |
| White | 379 (71.4) | 2822 (79.2) |
| <High school education | 108 (20.3) | 396 (11.1) |
| Current smokers | 38 (7.2) | 190 (5.3) |
| Clinical characteristics | ||
| Body mass index, median (IQR)c | 27.4 (24.3-30.9) | 27.8 (24.9-31.2) |
| Systolic blood pressure, median (IQR), mm Hg | 130.5 (119.5-142.0) | 128.5 (117.5-140.0) |
| Antihypertensive medication used | 417 (78.5) | 2521 (70.7) |
| Diabetes | 192 (36.2) | 995 (27.9) |
| ≥1 APOE ɛ4 allelee | 194 (36.5) | 925 (25.9) |
| Coronary heart diseasef | 84 (15.8) | 436 (12.2) |
| Heart failureg | 86 (16.2) | 282 (7.9) |
| Anticoagulant medication useh | 12 (2.3) | 78 (2.2) |
| Echocardiogram measures | ||
| LA, mean (SD), % | ||
| Reservoir strain | 30.1 (8.1) | 33.2 (7.5) |
| Conduit strain | 13.1 (5.4) | 15.1 (5.6) |
| Contractile strain | 17.0 (5.8) | 18.1 (5.5) |
| Emptying fraction | 55.2 (10.5) | 58.8 (9.1) |
| Passive emptying fraction | 24.1 (10.2) | 26.8 (10.3) |
| Active emptying fraction | 41.0 (11.5) | 43.5 (10.3) |
| LA volume index, median (IQR), mL/m2 | ||
| Maximal | 33.6 (26.7-42.3) | 32.0 (25.9-39.4) |
| Minimal | 14.8 (10.5-19.9) | 12.8 (9.5-17.3) |
| LV ejection fraction, median (IQR), %i | 65.9 (61.5-69.0) | 66.1 (62.6-69.6) |
| LV mass index, median (IQR),i g/m2 | 78.0 (66.8-93.7) | 74.8 (65.2-86.7) |
Abbreviations: APOE, apolipoprotein E; LA, left atrial; LV, left ventricular.
Restricted to the analytic sample, which required participants to have an echocardiogram completed at visit 5 (2011-2013) and to not meet any exclusion criteria.
Race categories other than Black or White were excluded from this analysis because of small numbers. Race was self-selected by participants from fixed categories.
Calculated as weight in kilograms divided by height in meters squared.
Antihypertensive medications included all medications indicated for hypertension, including diuretics, calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, Angiotensin II receptor antagonist, adrenergic receptor antagonists, aldosterone receptor antagonists, and α2 adrenergic receptor agonists.
APOE ɛ4 carriers were defined based on the number of ɛ4 alleles: 0, 1, or 2.
Coronary heart disease was defined by self-reported physician-diagnosis, prior myocardial infarction by electrocardiogram (ECG), or ascertained by the Atherosclerosis Risk in Communities Study (ARIC) morbidity and mortality classification committee through follow-up calls and hospitalization records.
Heart failure was identified by Gothenburg criteria (visit 1 only), self-reported heart failure medication use within the past 2 weeks or International Classification of Diseases, Ninth Revision (ICD-9) code 428.x or ICD-10 code I50 from hospitalization records.
Anticoagulant medications included warfarin and direct oral anticoagulants, including rivaroxaban, apixaban, dabigatran, and edoxaban.
Exposure Measurements
The exposures of interest included measures of LA function (reservoir strain, conduit strain, contractile strain, emptying fraction, passive emptying fraction, and active emptying fraction) and LA size (maximal or minimal LAVI). These measures were evaluated by 2-dimensional echocardiography (2DE). At visit 5, transthoracic 2DEs were performed as previously described.15 Briefly, echocardiograms were performed by trained sonographers using Philips iE33 ultrasound systems with Vision 2011 software. Studies were transferred from each field center to a secure server at the Echocardiography Reading Center (Brigham and Women’s Hospital).
Using the Simpson method, LA time-volume curves were generated by calculating LA volume at each phase of the cardiac cycle (maximal and minimal LA volumes). Both the LAVIs were derived by indexing the volumes to body surface area.
LA function was measured using a speckle tracking vendor-dependent software using R-R gating with an autostrain algorithm (QLAB Advanced Quantification software 13.0, Philips Ultrasound Inc). Speckles were tracked during a cardiac cycle frame by frame. LA phasic function was measured using volumes and strain indices calculated as the mean of the segments obtained. For this analysis, the absolute values of LA conduit and contractile strain were used. LA pre-atrial contraction volume was defined as LA volume before atrial contraction. The following equations were used to calculate LA function measures:
| LA Emptying Fraction (Reservoir Function) = [(Maximal LA Volume −Minimal LA Volume)/Maximal LA Volume] × 100; |
| LA Passive Emptying Fraction (Conduit Function) = [(Maximal LA Volume −LA Pre-Atrial Volume)/Maximal LA Volume] × 100; |
| LA Active Emptying Fraction (Pump Function) = [(LA Pre-Atrial Volume −Minimal LA Volume)/LA Pre-Atrial Volume] × 100. |
Intrareader and interreader variability for LA reservoir strain was assessed in a sample of 40 randomly selected participants. The intraclass correlation coefficient for interreader and intrareader variability were 0.91 and 0.98, respectively.
Outcome Ascertainment
Detailed dementia ascertainment descriptions have been previously described.16 Briefly, dementia was ascertained 3 ways16: (1) Adjudicated dementia cases identified from in-person evaluations at ARIC Neurocognitive Study (ARIC-NCS) visit 6 (2016-2017) and visit 7 (2018-2019); (2) Among participants who were alive, but did not attend ARIC-NCS visits, the modified Telephone Interview of Cognitive Status (TICSm) or 6-item screener (SIS) were used to determine cognitive status or an informant interview was conducted; and (3) International Classification of Diseases (ICD) hospitalization discharge codes or death certificate codes were used to identify additional dementia cases.17
Covariate Measurements
Covariates obtained from visit 5 included age, sex, race, ARIC field center, body mass index (BMI), smoking status, diabetes, systolic blood pressure, antihypertensive medication use, coronary heart disease (CHD), heart failure (HF), and anticoagulant use. Educational level was assessed at visit 1. Incident stroke and AF were obtained after visit 5 and through visit 7. Participants self-reported their age, sex, educational attainment, and smoking status. Race was self-selected from fixed categories of American Indian or Alaska Native, Asian or Pacific Islander, Black, or White. In all analyses, a 5-level race and center variable (White participants from Minneapolis; White participants from Washington County; Black participants from Jackson; Black participants from Forsyth County; White participants from Forsyth County) was used as a covariate for adjustment. Medication use was recorded by technicians via medication bottles that participants brought to visits. Technicians measured height and weight to derive BMI. Blood pressure was measured 3 times after a 5-minute rest and the mean of the final 2 measurements was calculated. Genotyping the apolipoprotein E ɛ4 (APOE ɛ4) allele was done using the TaqMan assay (Applied Biosystems).18 Diabetes was defined as a fasting glucose level of 126 mg/dL or higher or a nonfasting glucose of 200 mg/dL or higher, antidiabetic medication use in the past 2 weeks, or a self-reported physician diabetes diagnosis.
CHD was defined by self-reported physician diagnoses at visit 1, myocardial infarction diagnosis by ECG, or adjudicated cases after visit 1.19,20 HF was identified by the Gothenburg criteria (visit 1 only), HF medication use within the past 2 weeks, or ICD-9 code 428.x or ICD-10 code I50 for HF from hospitalization records during follow-up.19 Stroke was defined as self-reported history of a stroke diagnosis (prior to visit 1) or adjudicated stroke cases, after visit 1, using criteria adapted from the National Survey of Stroke.21 AF was ascertained from ECGs conducted during study visits, ICD codes from hospitalization records, and an ambulatory ECG monitoring device. ECGs were evaluated by a trained cardiologist.22 At visit 6 (2016-2017), participants were invited to wear an ambulatory ECG monitoring device; exclusion criteria have been described.23 Briefly, participants without a history of cardiac electronic device implant or skin allergic reaction to adhesive tape were invited to wear the ambulatory ECG monitoring device for a prescribed wear time of 14 days.
Left ventricular (LV) ejection fraction and LV mass index were obtained from 2DE at visit 5. LV ejection fraction was calculated as 100 × (LV end-diastolic volume −LV end-systolic volume)/LV end-diastolic volume. LV mass index was calculated from LV linear measures and indexed to body surface area, per recommendations by the American Society of Echocardiography.24
Subclinical cerebrovascular disease, which included white matter hyperintensities volume, cerebral microbleeds, and brain infarcts, was assessed using T2 fluid-attenuated inversion recovery images and gradient-echo T2–weighted brain magnetic resonance imaging (MRI) sequences.25
Statistical Analysis
Baseline characteristics were described using means and SDs for continuous variables and frequencies and percentages for categorical variables. For the primary analysis, Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% CIs for incident dementia based on quintiles of LA measure (highest LA function and lowest LA size quintile as referent categories). In a secondary analysis, Cox proportional hazards models were used to estimate HRs per 1-SD increment or decrement in each LA measure. Follow-up time was defined as the time from visit 5 to the occurrence of incident dementia, loss to follow-up, death, or December 31, 2019, whichever occurred first. Because it was likely that dementia diagnoses had an onset prior to when they were detected by ARIC, 6 months was subtracted from date of a dementia diagnosis to account for a likely earlier dementia onset.26 Multivariable models were adjusted as follows: model 1 adjusted for age, sex, race and center, APOE ɛ4, education, BMI, smoking status, diabetes, systolic blood pressure, antihypertensive medication use, CHD, HF, and anticoagulant use; model 2 additionally adjusted for incident stroke and incident AF as time-varying covariates; and model 3 additionally adjusted for minimal LAVI (for LA function measures only), LV ejection fraction, and LV mass index. The proportional hazards assumption was assessed by including an interaction term between each LA measure and time in the fully adjusted model in the primary analysis.
To determine a possible cutoff to classify normal vs abnormal LA measures, the following approach was adopted. First, restricted cubic splines were constructed to display the fully adjusted relationship of each LA measure with dementia incidence. To determine the number of knots to use, 3 to 6 knots were assessed, and 3 knots were used because it had the lowest Akaike information criterion. Second, among LA measures that were significantly associated with dementia and demonstrated a nonlinear relationship, Cox proportional hazards models (adjusting for all covariates in model 3) were used to estimate HRs for dementia based on a range of binary cutoff values; the value that corresponded to the highest HR was determined as a possible binary cutoff to classify normal vs abnormal LA measure. Third, based on this binary cutoff, Cox proportional hazards models were used to estimate the HR of the abnormal LA measure for dementia.
To quantify the extent to which significant associations between LA measures and dementia were mediated by incident AF, mediation analyses were performed using the natural effect model.27 AF was considered a mediator if new-onset AF developed after baseline (visit 5), but before a dementia diagnosis. Cox proportional hazards models were used to estimate HRs for incident dementia by lowest vs highest quintile of each LA measure, while additive hazards models with time-independent effects were used to estimate absolute risks. Models adjusted for demographic and clinical characteristics that were potential confounders. The hypothesized associations among LA measures, dementia, AF, and confounders are shown in eFigure 1 in the Supplement.
An additional exploratory analysis was performed restricted to participants with brain MRIs from visit 5 to elucidate potential mechanisms for the associations between LA measures and dementia.
Several sensitivity analyses were performed. First, participants with incident AF or incident stroke through visit 7 were excluded. Second, an analysis excluding participants who were taking anticoagulants was performed. Third, the Fine-Gray subdistribution hazards model was used to account for the competing risk of death. Fourth, participants with moderate to severe valvular heart disease were excluded. Fifth, to assess the role of inflammation, an analysis additionally adjusting for C-reactive protein as a covariate was performed. Sixth, multiple imputation by chain equation was used to impute LA function data for participants who were missing LA function data. Clinical characteristics and echocardiographic variables were used to create 10 data sets without missing values. SAS procedures proc MI and proc MIANALYZE were used to create and analyze the imputed data sets.
Past studies have indicated disparities in regard to dementia rates, with higher rates in Black than in White individuals,28 as well as differences in etiologies of dementia by race.29 Although it is likely that these differences are based on underlying social factors, assessing consistency of associations by race can help address and clarify health disparities and inequities. Furthermore, the ARIC study was funded by the National Institutes of Health to investigate the etiology of cardiovascular disease and its clinical sequelae, and variation in cardiovascular risk factors, medical care, and disease by race and sex.30 Therefore, in line with the objectives of the ARIC study, we performed race- and sex-stratified analyses to assess consistency of associations in Black and White participants and in men and women. Multiplicative interactions by race and sex were evaluated by including cross-product terms in the fully adjusted model.
The significance threshold was defined as a 2-sided P < .05 and an interaction was considered present when an interaction term was significant at P < .10. Because of the potential for type I error due to multiple comparisons, findings for the analyses should be interpreted as exploratory. Analyses were conducted using SAS version 9.4 (SAS Institute Inc) and R version 4.1.2.
Results
A total of 6538 participants attended visit 5, of whom 6118 had echocardiograms. After all exclusions, 4096 participants were included in this analysis (Figure 1). Clinical characteristics of participants excluded from this analysis are shown in eTables 1, 2, 3, and 4 in the Supplement. At visit 5, participants were a mean (SD) age of 75 (5) years (60% women, 22% Black individuals). Over a median follow-up of 6 years, 531 incident dementia cases were ascertained. In those who developed dementia, 32% were diagnosed via in-person cognitive assessments at study visits, 41% were diagnosed from TICSm, SIS, or informant interview, and 27% were diagnosed by ICD hospitalization or death certificate codes. Those who developed dementia were more likely to be older, identify as Black, had 1 or more APOE ɛ4 allele, had lower educational attainment, higher prevalence of cardiovascular disease and risk factors, and greater adverse LA and LV remodeling (Table 1).
LA Measures and Dementia
The associations of LA function and size with incident dementia are shown in Table 2; eFigure 2 in the Supplement. The proportional hazards assumptions held for all LA measures (P > .05), except for a minimal deviation for LA emptying fraction (P = .04). For all LA function measures, the incidence of dementia was highest in the lowest quintile (4.80, reservoir strain; 3.94, conduit strain; 3.29, contractile strain; 4.20, emptying fraction; 3.67, passive emptying fraction; and 3.27, active emptying fraction per 100 person-years). The incidence rate difference per 100 person-years of LA function measures in the lowest quintile compared with the highest quintile were 3.25 (95% CI, 2.48-4.01) for reservoir strain, 2.66 (95% CI, 1.99-3.33) for conduit strain, 1.49 (95% CI, 0.83-2.14) for contractile strain, 2.70 (95% CI, 1.99-3.40) for emptying fraction, 2.18 (95% CI, 1.52-2.84) for passive emptying fraction, and 1.53 (95% CI, 0.89-2.18) for active emptying fraction. Dementia incidence was highest in the highest quintile for LA size measures (3.18 per 100 person-years for maximal LAVI and 3.50 per 100 person-years for minimal LAVI). Incidence rate differences when comparing the highest vs lowest maximal LAVI quintile was 1.06 (95% CI, 0.40-1.73) and the highest vs the lowest minimal LAVI quintile was 1.76 (95% CI, 1.07-2.40). After full-model adjustment, the lowest vs highest quintile for all LA function measures, except LA passive emptying fraction (HR, 1.26 [95% CI, 0.93-1.71]), was significantly associated with incident dementia. The HRs were 1.98 (95% CI, 1.42-2.75) for reservoir strain, 1.50 (95% CI, 1.09-2.06) for conduit strain, 1.57 (95% CI, 1.16-2.14) for contractile strain, 1.87 (95% CI, 1.31-2.65) for emptying fraction, and 1.43 (95% CI, 1.04-1.96) for active emptying fraction.
Table 2. Association of Left Atrial Measures With Incident Dementia: Atherosclerosis Risk in Communities Study, 2011-2019 (N = 4096).
| No. incident dementia/No. at risk | Incidence rate, per 100 person-yearsa | Incidence rate difference (95% CI), per 100 person-yearsa | Hazard ratio (95% CI)b | ||||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||
| LA reservoir strain, % | |||||||
| ≤26.72 | 190/819 | 4.80 | 3.25 (2.48 to 4.01) | 2.09 (1.58 to 2.76) | 2.00 (1.51 to 2.64) | 1.98 (1.42 to 2.75) | |
| 26.73-30.59 | 102/820 | 2.09 | 0.54 (0.01 to 1.07) | 1.02 (0.76 to 1.38) | 1.00 (0.74 to 1.34) | 1.01 (0.74 to 1.38) | |
| 30.60-34.41 | 86/819 | 1.72 | 0.17 (−0.33 to 0.67) | 0.93 (0.68 to 1.26) | 0.92 (0.67 to 1.25) | 0.94 (0.68 to 1.28) | |
| 34.42-39.40 | 74/819 | 1.46 | −0.09 (−0.57 to 0.39) | 0.87 (0.63 to 1.20) | 0.86 (0.63 to 1.19) | 0.87 (0.63 to 1.20) | |
| ≥39.41 | 79/819 | 1.55 | [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | |
| LA conduit strain, % | |||||||
| ≤9.90 | 167/819 | 3.94 | 2.66 (1.99 to 3.33) | 1.81 (1.34 to 2.44) | 1.75 (1.30 to 2.37) | 1.50 (1.09 to 2.06) | |
| 9.91-13.06 | 120/817 | 2.57 | 1.29 (0.73 to 1.84) | 1.45 (1.07 to 1.97) | 1.39 (1.02 to 1.89) | 1.29 (0.94 to 1.77) | |
| 13.07-15.89 | 89/821 | 1.82 | 0.54 (0.06 to 1.03) | 1.13 (0.82 to 1.56) | 1.12 (0.81 to 1.55) | 1.08 (0.78 to 1.49) | |
| 15.90-19.52 | 89/820 | 1.77 | 0.49 (0.01 to 0.97) | 1.19 (0.87 to 1.64) | 1.19 (0.86 to 1.64) | 1.17 (0.85 to 1.61) | |
| ≥19.53 | 66/819 | 1.28 | [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | |
| LA contractile strain, % | |||||||
| ≤13.47 | 143/819 | 3.29 | 1.49 (0.83 to 2.14) | 1.75 (1.33 to 2.30) | 1.70 (1.29 to 2.23) | 1.57 (1.16 to 2.14) | |
| 13.48-16.62 | 104/819 | 2.14 | 0.34 (−0.21 to 0.90) | 1.15 (0.86 to 1.53) | 1.09 (0.82 to 1.45) | 1.06 (0.79 to 1.42) | |
| 16.63-19.17 | 95/819 | 1.96 | 0.16 (−0.38 to 0.70) | 1.11 (0.83 to 1.48) | 1.11 (0.83 to 1.49) | 1.07 (0.80 to 1.44) | |
| 19.18-22.37 | 100/821 | 2.00 | 0.20 (−0.34 to 0.74) | 1.14 (0.85 to 1.52) | 1.12 (0.84 to 1.49) | 1.09 (0.82 to 1.46) | |
| ≥22.38 | 89/818 | 1.80 | [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | |
| LA emptying fraction, % | |||||||
| ≤50.92 | 176/819 | 4.20 | 2.70 (1.99 to 3.40) | 1.87 (1.42 to 2.47) | 1.78 (1.34 to 2.36) | 1.87 (1.31 to 2.65) | |
| 50.93-56.58 | 111/819 | 2.32 | 0.82 (0.28 to 1.37) | 1.19 (0.88 to 1.59) | 1.17 (0.87 to 1.58) | 1.20 (0.87 to 1.66) | |
| 56.59-61.28 | 91/820 | 1.83 | 0.33 (−0.17 to 0.84) | 1.08 (0.79 to 1.47) | 1.07 (0.79 to 1.46) | 1.09 (0.80 to 1.50) | |
| 61.29-66.17 | 77/819 | 1.55 | 0.05 (−0.43 to 0.54) | 1.01 (0.74 to 1.39) | 1.01 (0.73 to 1.38) | 1.01 (0.74 to 1.40) | |
| ≥66.18 | 76/819 | 1.50 | [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | |
| LA passive emptying fraction, % | |||||||
| ≤17.63 | 161/819 | 3.67 | 2.18 (1.52 to 2.84) | 1.49 (1.12 to 1.98) | 1.45 (1.09 to 1.92) | 1.26 (0.93 to 1.71) | |
| 17.64-23.66 | 97/819 | 2.06 | 0.57 (0.05 to 1.10) | 1.04 (0.76 to 1.41) | 1.04 (0.76 to 1.41) | 0.97 (0.71 to 1.33) | |
| 23.67-28.68 | 110/820 | 2.27 | 0.78 (0.25 to 1.33) | 1.23 (0.91 to 1.65) | 1.22 (0.91 to 1.64) | 1.15 (0.85 to 1.56) | |
| 28.69-35.20 | 87/819 | 1.77 | 0.28 (−0.22 to 0.78) | 1.04 (0.76 to 1.41) | 1.05 (0.77 to 1.43) | 1.03 (0.75 to 1.40) | |
| >35.20 | 76/819 | 1.49 | [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | |
| LA active emptying fraction, % | |||||||
| ≤35.15 | 145/819 | 3.27 | 1.53 (0.89 to 2.18) | 1.56 (1.19 to 2.05) | 1.50 (1.14 to 1.97) | 1.43 (1.04 to 1.96) | |
| 35.16-41.05 | 108/819 | 2.28 | 0.54 (−0.02 to 1.11) | 1.23 (0.92 to 1.64) | 1.18 (0.88 to 1.57) | 1.12 (0.83 to 1.51) | |
| 41.06-46.07 | 105/820 | 2.11 | 0.37 (−0.17 to 0.92) | 1.09 (0.82 to 1.45) | 1.08 (0.81 to 1.45) | 1.04 (0.77 to 1.40) | |
| 46.09-51.88 | 87/819 | 1.78 | 0.04 (−0.48 to 0.57) | 0.99 (0.73 to 1.34) | 0.99 (0.74 to 1.34) | 0.99 (0.73 to 1.33) | |
| ≥51.89 | 86/819 | 1.74 | [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | |
| Maximal LA volume index, mL/m2 | |||||||
| ≤24.69 | 103/819 | 2.12 | [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | |
| 24.70-29.77 | 88/819 | 1.80 | −0.32 (−0.87 to 0.24) | 0.75 (0.56 to 0.99) | 0.77 (0.57 to 1.02) | 0.74 (0.55 to 0.99) | |
| 29.78-34.85 | 96/820 | 1.96 | −0.16 (−0.72 to 0.41) | 0.79 (0.59 to 1.04) | 0.81 (0.61 to 1.07) | 0.76 (0.57 to 1.01) | |
| 34.86-41.65 | 100/819 | 2.07 | −0.05 (−0.62 to 0.53) | 0.78 (0.59 to 1.04) | 0.79 (0.59 to 1.04) | 0.71 (0.53 to 0.94) | |
| ≥41.66 | 144/819 | 3.18 | 1.06 (0.40 to 1.73) | 0.98 (0.75 to 1.28) | 0.94 (0.72 to 1.24) | 0.77 (0.58 to 1.02) | |
| Minimal LA volume index, mL/m2 | |||||||
| ≤8.96 | 88/819 | 1.76 | [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | |
| 8.97-11.61 | 78/819 | 1.59 | −0.17 (−0.68 to 0.35) | 0.87 (0.64 to 1.17) | 0.89 (0.65 to 1.20) | 0.86 (0.63 to 1.16) | |
| 11.62-14.56 | 92/820 | 1.86 | 0.10 (−0.43 to 0.63) | 0.85 (0.63 to 1.14) | 0.87 (0.64 to 1.17) | 0.82 (0.61 to 1.10) | |
| 14.57-19.00 | 121/819 | 2.52 | 0.76 (0.18 to 1.35) | 1.07 (0.81 to 1.42) | 1.09 (0.82 to 1.44) | 0.98 (0.74 to 1.30) | |
| ≥19.01 | 152/819 | 3.50 | 1.76 (1.07 to 2.40) | 1.23 (0.93 to 1.63) | 1.17 (0.89 to 1.55) | 0.95 (0.71 to 1.28) | |
Abbreviations: APOE, apolipoprotein E; BMI, body mass index; LA, left atrial.
Incidence rates are unadjusted.
Model 1 was adjusted for age, sex, race and center, APOE ɛ4, education, BMI, smoking status, diabetes, systolic blood pressure, coronary heart disease, heart failure, antihypertensive medications, and anticoagulant use; model 2, model 1 plus incident atrial fibrillation and incident stroke; and model 3, model 2 plus minimal LA volume index, left ventricular ejection fraction, and left ventricular mass index.
LA size, after full-model adjustment comparing the highest vs the lowest quintiles of maximal LAVI (HR, 0.77 [95% CI, 0.58-1.02]) and the minimal LAVI (HR, 0.95 [95% CI, 0.71-1.28]) was not significantly associated with incident dementia. Race- and sex-stratified results are presented in eTables 5 and 6 in the Supplement. No interactions by race or sex were noted.
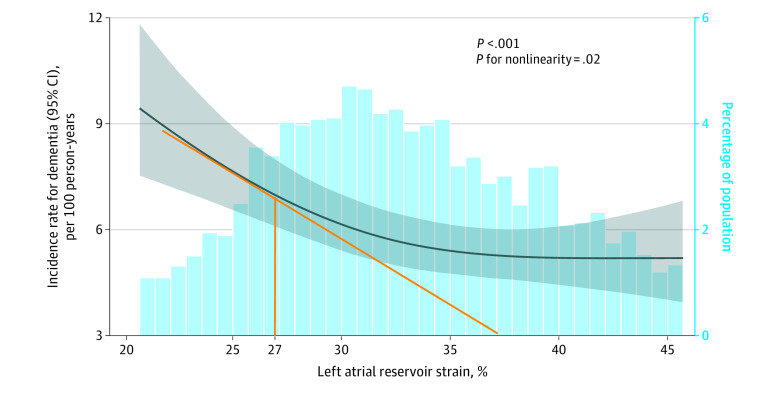
Using restricted cubic splines, a nonlinear association was observed for LA reservoir strain (Figure 2; P for nonlinearity = .02); however, other LA function measures showed a linear relationship with dementia incidence (eFigure 3 in the Supplement). Given the nonlinear relationship, a possible cutoff value of 27% for LA reservoir strain was determined by assessing several cutoff values ranging from 22% to 34% (eTable 7 in the Supplement). Of the cohort, 864 participants had LA reservoir strain lower than 27% and 3232 participants had LA reservoir strain of 27% or higher. Participants with an LA reservoir strain lower than 27% were older, more likely to identify as Black, had lower educational attainment, had more cardiovascular risk factors and disease, and had more adverse LA and LV remodeling than participants whose LA reservoir strain was 27% or higher (eTable 8 in the Supplement). Incidence rates for dementia were 4.72 per 100 person-years for participants with an LA reservoir strain less than 27% and 1.68 per 100 person-years for participants with an LA reservoir strain 27% or higher (eTable 9 in the Supplement). In the fully adjusted model, participants with LA reservoir strain less than 27% had a significantly higher risk of dementia than those with 27% or higher (HR, 2.10 [95% CI, 1.68-2.61]; eTable 9 in the Supplement).
Figure 2. Restricted Cubic Spline Plotting Dementia Incidence by Left Atrial Reservoir Strain: Atherosclerosis Risk in Communities Study, 2011-2019 (N = 4096).
The figure illustrates the nonlinear association between left atrial (LA) reservoir strain and incidence of dementia. The beginning of the steepest part of the curve (as indicated by the orange line) corresponds with LA reservoir strain of 27%. The dark blue line indicates the incidence rate for dementia; gray shading, 95% CIs; blue bars, the percentage of population. Similar analyses for other LA measures are presented in eFigure 3 in the Supplement.
Adjusted for age, sex, race and center, APOE ɛ4 allele, education, body mass index, smoking status, diabetes, systolic blood pressure, antihypertensive medications, coronary heart disease, heart failure, anticoagulant use, incident atrial fibrillation and incident stroke, minimal LA volume index, left ventricular ejection fraction, and left ventricular mass index.
Total events of dementia were 531 in a population of 4096, with median observation time per person of 6 years (IQR, 5.5-6.9 years). Median left atrial reservoir strain was 32.5% (IQR, 28.8%-38.1%). P < .001 refers to the association between LA reservoir strain and dementia incidence.
Brain MRI measures, stratified by dementia status, in participants with LA reservoir less than 27% are presented in eTable 10 of the Supplement. Of the 283 participants in this analysis, 81 developed dementia, of whom 21 (25.9%) had lacunar infarcts, 11 (13.6%) had cortical infarcts, 29 (35.8%) had any infarcts, and 30 (37.0%) had cerebral microbleeds detected by brain MRI. Among the 202 participants who did not develop dementia, 34 (16.8%) had lacunar infarcts, 23 (11.4%) had cortical infarcts, 49 (24.3%) had any infarcts, and 63 (31.2%) had cerebral microbleeds. The mean (SD) white matter hyperintensity volume was 23.4 (18.1) cm3 among those who developed dementia and 19.2 (20.4) cm3 among those who did not. Compared with participants who did not develop dementia, those who developed dementia had significantly higher prevalence of any brain infarcts (24.3% vs 35.8%, P = .04).
Mediation Analysis
Among participants included in this analysis, 44% wore the ambulatory ECG monitoring device at visit 6 (mean wear time, 12.3 [SD, 3.0]) days. There were 389 incident AF events that occurred after visit 5, of which 86% were diagnosed by ICD codes from hospitalization records and 14% were diagnosed by the ambulatory ECG monitoring device. Table 3 shows the results of mediation analysis comparing the lowest vs highest (referent) quintile of LA function measures. Incident AF mediated less than 2% of the association between each LA function measure and dementia incidence.
Table 3. Mediation (by Incident Atrial Fibrillation) Analysis of Left Atrial Measures and Dementia: Atherosclerosis Risk in Communities Study, 2011-2019.
| Exposurea | Quintile, No. (% range) | Adjusted for age, sex, race and center, education, body mass index, smoking status, diabetes, systolic blood pressure, antihypertensive medications, coronary heart disease, heart failure | |||||
|---|---|---|---|---|---|---|---|
| Quintile incidence rate (per 100 person-years)b | Hazard ratio (95% CI)c | % Mediated (95% CI)d | |||||
| Lowest | Highest | Lowest | Highest | Additional incident dementia cases (per 100 person-years)b,e | |||
| LA reservoir strain | 819 (≤26.72) | 819 (≥39.43) | |||||
| Natural direct effect | 6.64 | 4.43 | 2.21 | 2.06 (1.55 to 2.80) | 99.98 (94.72 to 104.32) | ||
| Natural indirect effect | 4.430 | 4.433 | −0.003 | 1.00 (0.97 to 1.03) | 0.02 (−4.32 to 5.28) | ||
| Total effect | 6.64 | 4.43 | 2.21 | 2.06 (1.57 to 2.78) | 100 | ||
| LA conduit strain | 819 (≤9.90) | 819 (≥19.53) | |||||
| Natural direct effect | 5.65 | 4.30 | 1.35 | 1.80 (1.35 to 2.49) | 99.62 (97.90 to 101.28) | ||
| Natural indirect effect | 4.303 | 4.299 | 0.004 | 1.00 (0.99 to 1.01) | 0.38 (−1.28 to 2.10) | ||
| Total effect | 5.65 | 4.30 | 1.35 | 1.80 (1.36 to 2.49) | 100 | ||
| LA contractile strain | 819 (≤13.47) | 818 (≥22.38) | |||||
| Natural direct effect | 5.52 | 4.31 | 1.21 | 1.68 (1.24 to 2.27) | 98.40 (89.98 to 104.33) | ||
| Natural indirect effect | 4.32 | 4.31 | 0.01 | 1.01 (0.98 to 1.04) | 1.60 (−4.33 to 10.05) | ||
| Total effect | 5.53 | 4.31 | 1.22 | 1.70 (1.27 to 2.27) | 100 | ||
| LA emptying fraction | 819 (≤50.92) | 819 (≥66.18) | |||||
| Natural direct effect | 6.01 | 4.26 | 1.75 | 1.92 (1.45 to 2.56) | 99.68 (95.21 to 103.79) | ||
| Natural indirect effect | 4.263 | 4.264 | −0.001 | 1.00 (0.98 to 1.03) | 0.32 (−3.79 to 4.79) | ||
| Total effect | 6.01 | 4.26 | 1.75 | 1.93 (1.45 to 2.57) | 100 | ||
| LA active emptying fraction | 819 (≤35.15) | 819 (≥51.89) | |||||
| Natural direct effect | 5.45 | 4.28 | 1.17 | 1.63 (1.27 to 2.19) | 98.75 (92.07 to 104.73) | ||
| Natural indirect effect | 4.29 | 4.28 | 0.01 | 1.01 (0.98 to 1.03) | 1.25 (−4.73 to 7.95) | ||
| Total effect | 5.46 | 4.28 | 1.18 | 1.64 (1.28 to 2.19) | 100 | ||
Abbreviation: LA, left atrial.
Lowest vs highest (reference) quintiles were compared.
Estimates are from the additive hazards model with time-independent effects.
Natural direct effect estimates the effect of the LA measure on dementia that does not act through the mediator, incident atrial fibrillation. Natural indirect effect estimates the effect of the LA measure on dementia that acts through the mediator, incident atrial fibrillation.
Percent mediated estimates the percent of the total LA measure effect, on the log(HR) scale, that acts through the mediator, incident atrial fibrillation.
Additional incident dementia cases (per 100 person-years) comparing lowest vs highest quintile.
Sensitivity Analyses
When 630 participants with incident AF or stroke were excluded, the lowest (vs highest) quintile of several measures of LA function (reservoir strain, contractile strain, emptying fraction, and active emptying fraction) were significantly associated with higher risk of dementia (eTable 11 in the Supplement). In an analysis excluding 90 participants who were taking anticoagulants (eTable 12 in the Supplement) and an analysis excluding 123 participants with moderate or severe valvular heart disease (eTable 13 in the Supplement), the lowest vs highest quintile of all LA function measures (except passive emptying fraction) were significantly associated with higher risk of dementia after full-model adjustment.
Results after accounting for the competing risk of death are shown in eTable 14 in the Supplement. In model 2, the lowest vs highest quintile for all LA function variables, except passive emptying fraction, were significantly associated with higher dementia risk. After additional adjustment for echocardiographic measures in model 3, LA reservoir strain and emptying fraction remained significantly associated. To account for inflammation, results from models additionally adjusting for C-reactive protein are presented in eTable 15 in the Supplement. The lowest vs highest quintile for all LA function measures, except passive emptying fraction, were significantly associated with higher risk of dementia after full-model adjustment.
eTable 16 in the Supplement shows the results using multiple imputation by chain equation. In model 3, the lowest vs highest quintile for all LA function variables, except for LA passive and active emptying fraction, were significantly associated with higher dementia risk. In all sensitivity analyses, the highest (vs lowest) quintile of minimal and maximal LAVI were not significantly associated with higher dementia risk.
Discussion
In this exploratory analysis of a US community-based cohort, several echocardiographic measures of lower LA function were significantly associated with an increased risk of dementia. Among the LA function measures that were significantly associated with dementia, the unadjusted incidence rate difference comparing the lowest vs the highest quintile of LA function measures ranged from 2.18 to 3.25 per 100 person-years. Measures of LA size were not significantly associated with dementia risk.
Prior research suggests that atrial myopathy may be associated with increased risk of dementia. For example, abnormal P-wave indices (ECG surrogates of atrial myopathy) were associated with incident dementia, independent of AF and clinical stroke.12 Another study, which used data from the Interatrial Block and Yearly Events (BAYES) registry, found that among individuals 70 years or older with structural heart disease but no AF, advanced interatrial block was associated with cognitive impairment.31 LA enlargement has also been found to be associated with cognitive decline and lower cognitive function.32,33 Furthermore, it may be plausible that subclinical cerebrovascular disease underlies the association between atrial myopathy and dementia given that reduced LA reservoir function was associated with silent brain infarcts and white matter hyperintensities.11 In this regard, in the exploratory analysis of a subset of participants with brain MRI data, results suggest that brain infarcts may underlie the association between lower LA function and dementia. Further research is warranted to confirm these findings.
To our knowledge, this is the first study to report an independent association between echocardiographic measures of LA function with dementia. LA function has prognostic value for cardiovascular events that is over and above that provided by LA size.34,35 By interrogating a comprehensive set of LA function measures—quantified by speckle tracking and volumetric analysis—this study advances the field by linking LA function measures to dementia risk for the first time. In addition, this study also suggests that LA reservoir strain may potentially be used to define abnormal LA function. However, given the exploratory nature of this study, future studies should aim to confirm the observations, clarify mechanisms (eg, brain infarcts), and to confirm possible cutoff values that can be used to define abnormal LA function so that at-risk patients can be enrolled into future clinical trials to test potential interventions (eg, anticoagulation) to prevent dementia.
Because atrial myopathy is a well-established risk factor for AF,3,36 and the latter is in turn associated with dementia,13,37 the contribution of AF needs to be accounted for when assessing the association between atrial myopathy and dementia. To this end, mediation analyses were performed to quantify the contribution of AF to the atrial myopathy–dementia relationship. Results from these analyses suggest that diagnosed AF may minimally explain the associations between LA function measures and dementia risk, the highest being only 1.60% in the association between LA contractile strain and dementia. Furthermore, in a sensitivity analysis that excluded participants with incident AF and stroke, lower LA function was associated with an elevated risk of dementia, similar to results from the primary analysis. The mediation analysis results, however, should be interpreted with the understanding that incomplete ascertainment of AF might have led to a lower than actual proportion mediated by AF. Collectively, findings from this analysis suggest that impaired LA function is a risk factor for dementia, independent of AF. However, because the ascertainment methods for AF used in this study might have missed some cases of AF, more research will be needed to confirm these findings.
This study has several strengths. In addition to assessing maximal and minimal LAVI, a comprehensive range of echocardiographic LA function measures were assessed. Assessment of LA strain allows earlier detection of LA dysfunction as changes in LA strain occur prior to that of LA volumes.38 Additionally, it has been suggested that lower LA reservoir function may indicate more advanced LA remodeling and underlying LV dysfunction than LA enlargement alone.7 Other key strengths include the prospective cohort, large sample of Black and White adults, the use of an ambulatory ECG monitoring device to reduce the likelihood of missing subclinical AF, the accounting for AF including mediation analysis, and the adjudication of dementia.
Limitations
This study has several limitations. First, this study should be interpreted as exploratory given that multiple exposures were assessed and adjustment for multiple comparisons was not performed. Second, date of dementia onset was difficult to verify, especially among those diagnosed via ICD codes. Therefore, 6 months was subtracted from estimated diagnosis dates because it was likely that some participants had dementia prior to their diagnosis.26 Third, dementia diagnoses were ascertained using methods that had different sensitivities and specificities. The TICSm had a sensitivity of 50% and specificity of 95%, while ICD codes had a sensitivity of 25% and specificity of 99%.16 Both the TICSm and the ICD codes had high specificities, but lower sensitivities; therefore, it is possible that misclassified cases might have biased the results toward the null. Fourth, the analytic sample consisted of older adults (mean age, 75 years) and results may not be generalizable to younger individuals. However, the findings from this analysis remain clinically relevant because the public health burden of atrial myopathy and dementia is greatest in older adults. Fifth, the generalizability of this study to all US individuals may be limited given that the study sample consisted of Black and White individuals from 4 US communities. Sixth, despite multiple sources of AF detection, including the use of an ambulatory ECG monitoring device that was worn by 44% of study participants, there may be additional incident subclinical AF cases that were not detected. However, the overall proportion of AF cases in this study were similar to other published studies. Seventh, based on the data available in this study, there is the inability to completely elucidate the mechanism between LA measures and dementia. Eighth, as in all observational studies, despite efforts to control for confounding, residual confounding may exist.
Conclusions
In this exploratory analysis of a US community-based cohort, several echocardiographic measures of lower LA function were significantly associated with an increased risk of subsequent dementia. Measures of LA size were not significantly associated with dementia risk. These findings suggest that impaired LA function may be a risk factor associated with dementia.
eTable 1. Visit 1 Participant Characteristics According to Participant Follow-Up Status: The ARIC Study, 1987-1989
eTable 2. Baseline Participant Characteristics (Visit 5 Participants Missing LA Function Measures): The ARIC Study, 2011-2013
eTable 3. Baseline Participant Characteristics (Visit 5 Participants without Echocardiograms): The ARIC Study, 2011-2013
eTable 4. Baseline Participant Characteristics (Visit 5 Participants with Missing Covariates): The ARIC Study, 2011-2013
eTable 5. Association of Left Atrial Measures with Incident Dementia Stratified by Race: The ARIC Study, 2011-2019
eTable 6. Association of Left Atrial Measures with Incident Dementia Stratified by Sex: The ARIC Study, 2011-2019
eTable 7. Hazard Ratios for Dementia by Different Left Atrial Reservoir Strain Cutoffs: The ARIC Study, 2011-2019
eTable 8. Baseline Participant Characteristics (by Left Atrial Reservoir Strain <27% vs. >27%): The ARIC Study, 2011-2013
eTable 9. Association of Left Atrial Reservoir Strain (Binary Variable) with Incident Dementia: The ARIC Study, 2011-2019
eTable 10. Brain MRI Measures (2011-13) Among Participants with Left Atrial Reservoir Strain <27%, Stratified by Dementia
eTable 11. Hazard Ratios for Dementia Among Participants Without Incident Atrial Fibrillation or Stroke: The ARIC Study, 2011-2019 (n=3,466)
eTable 12. Hazard Ratios for Incident Dementia Among Participants Not Taking Anticoagulants: The ARIC Study, 2011-2019 (n=4,006)
eTable 13. Hazard Ratios for Incident Dementia Among Participants Without Valvular Heart Disease: The ARIC Study, 2011-2019 (n=3,973)
eTable 14. Competing Risk Subdistribution Hazard Models for Incident Dementia: The ARIC Study, 2011-2019 (n=4,096)
eTable 15. Hazard Ratios for Incident Dementia Among Participants with CRP Measures: the ARIC Study, 2011-2019 (n=4,057)
eTable 16. Association of Left Atrial Measures with Incident Dementia Using MICE: The ARIC Study, 2011-2019 (n=4,946)
eFigure 1. Directed Acyclic Graph
eFigure 2. Association of Left Atrial Measures with Incident Dementia: The ARIC Study, 2011-2019 (n=4,096)
eFigure 3. Restricted Cubic Splines Plotting Dementia Incidence by Left Atrial Measures: The ARIC Study, 2011-2019 (n=4,096)
References
- 1.Habibi M, Zareian M, Ambale Venkatesh B, et al. Left atrial mechanical function and incident ischemic cerebrovascular events independent of AF: insights from the MESA study. JACC Cardiovasc Imaging. 2019;12(12):2417-2427. doi: 10.1016/j.jcmg.2019.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta S, Matulevicius SA, Ayers CR, et al. Left atrial structure and function and clinical outcomes in the general population. Eur Heart J. 2013;34(4):278-285. doi: 10.1093/eurheartj/ehs188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abhayaratna WP, Fatema K, Barnes ME, et al. Left atrial reservoir function as a potent marker for first atrial fibrillation or flutter in persons > or = 65 years of age. Am J Cardiol. 2008;101(11):1626-1629. doi: 10.1016/j.amjcard.2008.01.051 [DOI] [PubMed] [Google Scholar]
- 4.Hedberg P, Selmeryd J, Leppert J, Henriksen E. Long-term prognostic impact of left atrial volumes and emptying fraction in a community-based cohort. Heart. 2017;103(9):687-693. doi: 10.1136/heartjnl-2016-310242 [DOI] [PubMed] [Google Scholar]
- 5.Tsang TSM, Abhayaratna WP, Barnes ME, et al. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol. 2006;47(5):1018-1023. doi: 10.1016/j.jacc.2005.08.077 [DOI] [PubMed] [Google Scholar]
- 6.Tsang TSM, Barnes ME, Gersh BJ, et al. Prediction of risk for first age-related cardiovascular events in an elderly population: the incremental value of echocardiography. J Am Coll Cardiol. 2003;42(7):1199-1205. doi: 10.1016/S0735-1097(03)00943-4 [DOI] [PubMed] [Google Scholar]
- 7.Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol. 2014;63(6):493-505. doi: 10.1016/j.jacc.2013.10.055 [DOI] [PubMed] [Google Scholar]
- 8.Fatema K, Barnes ME, Bailey KR, et al. Minimum vs. maximum left atrial volume for prediction of first atrial fibrillation or flutter in an elderly cohort: a prospective study. Eur J Echocardiogr. 2009;10(2):282-286. doi: 10.1093/ejechocard/jen235 [DOI] [PubMed] [Google Scholar]
- 9.Hedberg P, Selmeryd J, Leppert J, Henriksen E. Left atrial minimum volume is more strongly associated with N-terminal pro-B-type natriuretic peptide than the left atrial maximum volume in a community-based sample. Int J Cardiovasc Imaging. 2016;32(3):417-425. doi: 10.1007/s10554-015-0800-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin SH, Claggett B, Inciardi RM, et al. Prognostic value of minimal left atrial volume in heart failure with preserved ejection fraction. J Am Heart Assoc. 2021;10(15):e019545. doi: 10.1161/JAHA.120.019545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russo C, Jin Z, Liu R, et al. LA volumes and reservoir function are associated with subclinical cerebrovascular disease: the CABL (Cardiovascular Abnormalities and Brain Lesions) study. JACC Cardiovasc Imaging. 2013;6(3):313-323. doi: 10.1016/j.jcmg.2012.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutierrez A, Norby FL, Maheshwari A, et al. Association of abnormal P-wave indices with dementia and cognitive decline over 25 years: ARIC-NCS (The Atherosclerosis Risk in Communities Neurocognitive Study). J Am Heart Assoc. 2019;8(24):e014553. doi: 10.1161/JAHA.119.014553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen LY, Norby FL, Gottesman RF, et al. Association of atrial fibrillation with cognitive decline and dementia over 20 years: the ARIC-NCS (Atherosclerosis Risk in Communities Neurocognitive Study). J Am Heart Assoc. 2018;7(6):e007301. doi: 10.1161/JAHA.117.007301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright JD, Folsom AR, Coresh J, et al. The ARIC (Atherosclerosis Risk In Communities) Study: JACC focus seminar 3/8. J Am Coll Cardiol. 2021;77(23):2939-2959. doi: 10.1016/j.jacc.2021.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah AM, Cheng S, Skali H, et al. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: the Atherosclerosis Risk in Communities study. Circ Cardiovasc Imaging. 2014;7(1):173-181. doi: 10.1161/CIRCIMAGING.113.000736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study. Alzheimers Dement (Amst). 2016;2:1-11. doi: 10.1016/j.dadm.2015.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alonso A, Mosley TH Jr, Gottesman RF, Catellier D, Sharrett AR, Coresh J. Risk of dementia hospitalisation associated with cardiovascular risk factors in midlife and older age: the Atherosclerosis Risk in Communities (ARIC) study. J Neurol Neurosurg Psychiatry. 2009;80(11):1194-1201. doi: 10.1136/jnnp.2009.176818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volcik KA, Barkley RA, Hutchinson RG, et al. Apolipoprotein E polymorphisms predict low density lipoprotein cholesterol levels and carotid artery wall thickness but not incident coronary heart disease in 12,491 ARIC study participants. Am J Epidemiol. 2006;164(4):342-348. doi: 10.1093/aje/kwj202 [DOI] [PubMed] [Google Scholar]
- 19.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008;101(7):1016-1022. doi: 10.1016/j.amjcard.2007.11.061 [DOI] [PubMed] [Google Scholar]
- 20.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49(2):223-233. doi: 10.1016/0895-4356(95)00041-0 [DOI] [PubMed] [Google Scholar]
- 21.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30(4):736-743. doi: 10.1161/01.STR.30.4.736 [DOI] [PubMed] [Google Scholar]
- 22.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC Jr. Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40(4):1204-1211. doi: 10.1161/STROKEAHA.108.534735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rooney MR, Soliman EZ, Lutsey PL, et al. Prevalence and characteristics of subclinical atrial fibrillation in a community-dwelling elderly population: the ARIC Study. Circ Arrhythm Electrophysiol. 2019;12(10):e007390. doi: 10.1161/CIRCEP.119.007390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 25.Knopman DS, Griswold ME, Lirette ST, et al. ; ARIC Neurocognitive Investigators . Vascular imaging abnormalities and cognition: mediation by cortical volume in nondemented individuals: atherosclerosis risk in communities-neurocognitive study. Stroke. 2015;46(2):433-440. doi: 10.1161/STROKEAHA.114.007847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gottesman RF, Albert MS, Alonso A, et al. Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol. 2017;74(10):1246-1254. doi: 10.1001/jamaneurol.2017.1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lange T, Vansteelandt S, Bekaert M. A simple unified approach for estimating natural direct and indirect effects. Am J Epidemiol. 2012;176(3):190-195. doi: 10.1093/aje/kwr525 [DOI] [PubMed] [Google Scholar]
- 28.Power MC, Bennett EE, Turner RW, et al. Trends in relative incidence and prevalence of dementia across non-Hispanic Black and White individuals in the United States, 2000-2016. JAMA Neurol. 2021;78(3):275-284. doi: 10.1001/jamaneurol.2020.4471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen C, Zissimopoulos JM. Racial and ethnic differences in trends in dementia prevalence and risk factors in the United States. Alzheimers Dement (N Y). 2018;4:510-520. doi: 10.1016/j.trci.2018.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The ARIC investigators . The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129(4):687-702. doi: 10.1093/oxfordjournals.aje.a115184 [DOI] [PubMed] [Google Scholar]
- 31.Martínez-Sellés M, Martínez-Larrú ME, Ibarrola M, et al. Interatrial block and cognitive impairment in the BAYES prospective registry. Int J Cardiol. 2020;321:95-98. doi: 10.1016/j.ijcard.2020.08.006 [DOI] [PubMed] [Google Scholar]
- 32.Zhang MJ, Norby FL, Lutsey PL, et al. Association of left atrial enlargement and atrial fibrillation with cognitive function and decline: the ARIC-NCS. J Am Heart Assoc. 2019;8(23):e013197. doi: 10.1161/JAHA.119.013197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karadag B, Ozyigit T, Ozben B, Kayaoglu S, Altuntas Y. Relationship between left atrial volume index and cognitive decline in elderly patients with sinus rhythm. J Clin Neurosci. 2013;20(8):1074-1078. doi: 10.1016/j.jocn.2012.10.021 [DOI] [PubMed] [Google Scholar]
- 34.Inciardi RM, Giugliano RP, Claggett B, et al. ; ENGAGE AF-TIMI 48 Investigators . Left atrial structure and function and the risk of death or heart failure in atrial fibrillation. Eur J Heart Fail. 2019;21(12):1571-1579. doi: 10.1002/ejhf.1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta DK, Shah AM, Giugliano RP, et al. ; Effective aNticoaGulation with factor xA next GEneration in AF-Thrombolysis In Myocardial Infarction 48 Echocardiographic Study Investigators . Left atrial structure and function in atrial fibrillation: ENGAGE AF-TIMI 48. Eur Heart J. 2014;35(22):1457-1465. doi: 10.1093/eurheartj/eht500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards JD, Healey JS, Fang J, Yip K, Gladstone DJ. Atrial cardiopathy in the absence of atrial fibrillation increases risk of ischemic stroke, incident atrial fibrillation, and mortality and improves stroke risk prediction. J Am Heart Assoc. 2020;9(11):e013227. doi: 10.1161/JAHA.119.013227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alonso A, Knopman DS, Gottesman RF, et al. Correlates of dementia and mild cognitive impairment in patients with atrial fibrillation: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). J Am Heart Assoc. 2017;6(7):e006014. doi: 10.1161/JAHA.117.006014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gan GCH, Bhat A, Chen HHL, et al. Determinants of LA reservoir strain: independent effects of LA volume and LV global longitudinal strain. Echocardiography. 2020;37(12):2018-2028. doi: 10.1111/echo.14922 [DOI] [PubMed] [Google Scholar]
- 39.Sorrentino R, Esposito R, Santoro C, et al. Practical impact of new diastolic recommendations on noninvasive estimation of left ventricular diastolic function and filling pressures. J Am Soc Echocardiogr. 2020;33(2):171-181. doi: 10.1016/j.echo.2019.08.013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Visit 1 Participant Characteristics According to Participant Follow-Up Status: The ARIC Study, 1987-1989
eTable 2. Baseline Participant Characteristics (Visit 5 Participants Missing LA Function Measures): The ARIC Study, 2011-2013
eTable 3. Baseline Participant Characteristics (Visit 5 Participants without Echocardiograms): The ARIC Study, 2011-2013
eTable 4. Baseline Participant Characteristics (Visit 5 Participants with Missing Covariates): The ARIC Study, 2011-2013
eTable 5. Association of Left Atrial Measures with Incident Dementia Stratified by Race: The ARIC Study, 2011-2019
eTable 6. Association of Left Atrial Measures with Incident Dementia Stratified by Sex: The ARIC Study, 2011-2019
eTable 7. Hazard Ratios for Dementia by Different Left Atrial Reservoir Strain Cutoffs: The ARIC Study, 2011-2019
eTable 8. Baseline Participant Characteristics (by Left Atrial Reservoir Strain <27% vs. >27%): The ARIC Study, 2011-2013
eTable 9. Association of Left Atrial Reservoir Strain (Binary Variable) with Incident Dementia: The ARIC Study, 2011-2019
eTable 10. Brain MRI Measures (2011-13) Among Participants with Left Atrial Reservoir Strain <27%, Stratified by Dementia
eTable 11. Hazard Ratios for Dementia Among Participants Without Incident Atrial Fibrillation or Stroke: The ARIC Study, 2011-2019 (n=3,466)
eTable 12. Hazard Ratios for Incident Dementia Among Participants Not Taking Anticoagulants: The ARIC Study, 2011-2019 (n=4,006)
eTable 13. Hazard Ratios for Incident Dementia Among Participants Without Valvular Heart Disease: The ARIC Study, 2011-2019 (n=3,973)
eTable 14. Competing Risk Subdistribution Hazard Models for Incident Dementia: The ARIC Study, 2011-2019 (n=4,096)
eTable 15. Hazard Ratios for Incident Dementia Among Participants with CRP Measures: the ARIC Study, 2011-2019 (n=4,057)
eTable 16. Association of Left Atrial Measures with Incident Dementia Using MICE: The ARIC Study, 2011-2019 (n=4,946)
eFigure 1. Directed Acyclic Graph
eFigure 2. Association of Left Atrial Measures with Incident Dementia: The ARIC Study, 2011-2019 (n=4,096)
eFigure 3. Restricted Cubic Splines Plotting Dementia Incidence by Left Atrial Measures: The ARIC Study, 2011-2019 (n=4,096)