Abstract
Lung ischemia-reperfusion (I/R) injury poses a serious threat to human health, worldwide. The current study aimed to determine the role of lidocaine in A549 cells, in addition to the involvement of the p38 MAPK pathway. Oxygen deprivation/reoxygenation-induced A549 cells were utilized to simulate I/R injury in vitro. Cell viability and apoptosis were detected using MTT and TUNEL assays, respectively. The levels of IL-6, IL-8, TNF-α, malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase, iron and reactive oxygen species (ROS) were measured using corresponding commercial kits. The corresponding protein expression levels were also measured using western blotting. Moreover, a monolayer cell paracellular permeability assay was performed to determine the permeability of A549 cells. The results demonstrated that, whilst lidocaine had no influence on untreated A549 cells, it significantly increased the viability of hypoxia/reoxygenation (H/R)-induced A549 cells. A549 cell apoptosis and the release of inflammatory cytokines in the H/R group were decreased after the addition of lidocaine. When compared with the H/R group, increased MDA level and decreased SOD level were observed in H/R-induced A549 cells following lidocaine treatment. In addition, the permeability of H/R-induced A549 cells was markedly decreased following lidocaine treatment. Compared with the H/R group, the expression levels of tight junction and ferroptosis-related proteins were significantly upregulated by lidocaine, whereas the expression of transferrin was downregulated. However, p79350, an agonist of p38, reversed the effects of lidocaine on H/R-induced A549 cells. In conclusion, lidocaine exerted a protective role in HR-induced lung epithelial cell injury, which may serve as a potential agent for the treatment of patients with lung I/R injury.
Keywords: lidocaine, lung ischemia-reperfusion injury, ferroptosis, p38/MAPK pathway
Introduction
Ischemia-reperfusion (I/R) injury poses a great threat to the survival of grafts and recipients, leading to an increase in the morbidity and mortality of patients (1). Lung I/R injury (LIRI) usually occurs in various clinical situations, including cardiac arrest, trauma, pulmonary thrombosis, lung transplantation and cardiopulmonary bypass surgery (2,3). Respiration failure as a result of LIRI may cause related acute lung injury (ALI) or acute respiratory distress syndrome (4). At present, the most effective therapeutic targets and the most promising treatments for LIRI are still awaiting clinical trial (5). Thus, the identification and exploration of targets or drugs with high efficacy to treat LIRI is urgently required.
Lidocaine is a local anesthetic that exerts efficacy within 1–3 min after administration, with an anesthetic effect that lasts for 1–3 h (6). In clinical practice, it is used to treat arrhythmia and is the first line of treatment for patients with ventricular tachycardia and tremor (7,8). The current evidence has demonstrated that treatment with lidocaine eliminates severe arrhythmia and prevents heart death in an in vivo rat model of acute myocardial I/R (9). Chen et al (10) reported that the injection of lidocaine into the hepatoduodenal ligament of rats could protect against I/R-induced liver injury. In addition, Lei et al (11) reported that low-dose lidocaine protected against transient focal cerebral ischemia in a rat model. From these studies, it is clear that lidocaine serves a protective role in numerous types of I/R injury in different tissues. However, the effects of lidocaine on LIRI remain unclear and the methods for LIRI prevention and treatment are yet to be elucidated.
Ferroptosis is a newly discovered type of cell death, which has distinct properties and functions related to physical conditions or various diseases (12), such as tumors, kidney injury and IRI. It has been demonstrated that the inhibition of ferroptosis can effectively protect against IRI (13). Previous studies have also revealed that blocking ferroptosis greatly alleviates cardiomyopathy and that targeting ferroptosis could effectively treat fatal heart-related diseases (12,13). For example, targeting ferroptosis in cardiomyocytes can alleviate sepsis-induced cardiac injury (14). Of note, it has also been reported that lidocaine could promote ferroptosis via the microRNA (miR)-382-5p/cystine/glutamate transporter axis in ovarian and breast cancer cells (15). However, whether ferroptosis is involved in the development of LIRI and whether ferroptosis is also regulated by lidocaine during LIRI remain unclear.
The p38 MAPK signaling pathway participates in a large number of different biological effects (16). For example, the p38 pathway can respond to inflammatory stimuli to maintain cellular homeostasis in different tissues (17). The p38 pathway also regulates apoptosis and autophagy homeostasis in response to chemotherapeutic agents (18). Various studies have revealed that the regulation of p38 MAPK may partially prevent the occurrence of IRI. For instance, Rutaecarpine inhibits oxidative stress by suppressing the JNK/p38 MAPK signaling pathway, thereby attenuating renal IRI in rats (19). Apelin improves IRI-induced diabetic myocardium by suppressing apoptosis and oxidative stress through PI3K and P38-MAPK signaling pathways (20). Fibronectin-α4β1 ameliorates hepatic cold ischemia and reperfusion injury by regulating MMP-9 and MT1-MMP via the p38 MAPK pathway (21). In addition, lidocaine has been verified to protect against I/R-induced brain injury by regulating the p38 MAPK signaling pathway (22). Lidocaine also relieved LPS-induced lung injury by blocking the p38 MAPK signaling pathway in an in vivo rat model of ALI (23). Thus, it was hypothesized that lidocaine may protect against LIRI by blocking the p38 MAPK signaling pathway.
The current study aimed to determine the protective role and underlying mechanisms of lidocaine on hypoxia/reoxygenation (H/R)-induced LIRI.
Materials and methods
Cell culture and treatment
Human type II alveolar epithelial cells (A549; cat. no. BNCC337696) were purchased from the BeNa Culture Collection (Beijing Beina Chunglian Institute of Biotechnology) and cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), and 1% antibiotics (100 U/ml penicillin and 100 mg/ml streptomycin; Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a humidified atmosphere with 5% CO2. Lidocaine (cat. no. HY-B0185; MedChemExpress) at different doses (0.5, 1, 5 and 10 mM) was then administered to A549 cells at room temperature for 24 h. To explore the underlying mechanism of lidocaine (10 mM), p79350 (50 µM; Invitrogen; Thermo Fisher Scientific, Inc.), an agonist of p38, was adopted to treat A549 cells at room temperature for 1 h.
Lung I/R injury model construction
To simulate lung I/R injury in vitro, A549 cells were cultured under hypoxic conditions (1% O2, 5% CO2 and 94% N2) at 37°C for 24 h. Afterwards, cells were exposed to reoxygenation (5% CO2 and 95% air) for 4 h (24).
MTT assay
To increase the reliability of subsequent experiments, the effects of different concentrations of lidocaine on the viability of A549 cells and H/R-induced A549 cells were detected using MTT. A549 cells were inoculated into 96-well plates at a density of 1×104 cells/well for 24 h. Cells were then exposed to 10 µl MTT solution (Beyotime Institute of Biotechnology) in accordance with the manufacturer's protocol, and incubated at 37°C for an additional 4 h. Subsequently, 200 ml DMSO was added and the absorbance was detected using a microplate at a wavelength of 490 nm.
TUNEL assay
The effect of different concentrations of lidocaine (0.5, 1, 5 and 10 mM) on H/R-induced A549 cell apoptosis was assessed using a TUNEL assay (Beyotime Institute of Biotechnology) in accordance with the manufacturer's protocol. Cells (1×106 cells/well) were fixed with 4% paraformaldehyde for 30 min at room temperature, and incubated with 0.3% Triton X-100 for 5 min, followed by incubation with 50 µl TUNEL reaction reagent at 37°C for 1 h. After staining with 0.5 µg/ml of DAPI for 10 min at room temperature, TUNEL-positive cells randomly selected from five fields of view were imaged using a fluorescence microscope with antifade mounting medium (Beyotime Institute of Biotechnology; magnification, ×200).
ELISA
ELISA was employed to determine the release of various inflammatory cytokines from the cell supernatants of H/R-induced A549 cells (5×105 cells/well), including IL-6 (cat. no. SQ6000B), IL-8 (cat. no. Q8000B) and TNF-α (cat. no. STA00D; all from R&D Systems, Inc.). Optical density was recorded at 450 nm using a microplate reader (Bio-Rad Laboratories, Inc.).
Malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) measurement
The levels of MDA (cat. no. A003-1-2), SOD (cat. no. A001-3-2) and GSH-Px (cat. no. A005-1-2) in cell suspensions were measured using assay kits (all from Nanjing Jiancheng Bioengineering Institute), according to the manufacturer's specifications.
Iron and reactive oxygen species (ROS) level measurement
The intracellular Fe2+ level and ROS level in cells was detected using an iron colorimetric assay kit (cat. no. K390-100; BioVision, Inc.) (25) and a fluorescent probe 2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA; Beyotime Institute of Biotechnology) according to the manufacturer's protocol, respectively.
Monolayer cell paracellular permeability assay
The monolayer cell permeability assay was performed using Transwell apparatus. H/R-induced A549 cells (5×104 cells/well) were inoculated on Transwell polyester membrane cell culture inserts in a 24-well plate. Samples were then incubated at 37°C in a humidified atmosphere containing 5% CO2. Within 20–21 days, A549 cells with a confluence of 80–90% were obtained. Subsequently, 200 µl Hanks' balanced Salt solution containing 1 mg/ml sodium fluorescein was added to the monolayer of the upper chamber. Absorption was detected using a fluorescence microplate reader, with an excitation and absorption wavelength of 490 and 520 nm, respectively.
Western blotting
Total protein was extracted from A549 cells using RIPA lysis buffer (Sangon Biotech Co., Ltd.) and then quantified using a BCA kit (Pierce; Thermo Fisher Scientific, Inc.) in accordance with the manufacturer's instructions. After 15% SDS-PAGE electrophoresis, samples (30 µg) were transferred to PVDF membranes and subsequently blocked with 5% non-fat milk for 2 h at room temperature. Membranes were then incubated with primary antibodies against zonula occludens-1 (ZO-1; 1:1,000; cat. no. orb35080; Biorbyt), Occludin (1:1,000; cat. no. orb11181; Biorbyt), Claudin-1 (1:1,000; cat. no. orb127883; Biorbyt), ferritin heavy chain 1 (FTH1; 1:1,000; cat. no. orb49039; Biorbyt), glutathione peroxidase 4 (GPX4; 1:1,000; cat. no. orb340797; Biorbyt), transferrin (Tf; 1:1,000; cat. no. ab277635; Abcam), phosphorylated (p)-p38 (1:1,000; cat. no. ab4822; Abcam), p38 (1:2,000; cat. no. ab170099; Abcam) and GAPDH (1:2,500; cat. no. ab9485; Abcam) at 4°C overnight. Next, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit (1:2,000; cat. no. ab97051; Abcam) antibodies for 2 h at room temperature. Relative protein levels were visualized using an ECL kit (Beijing Solarbio Science & Technology Co., Ltd.) and semi-quantified using ImageJ software 1.52 (National Institutes of Health).
Statistical analysis
All the above experiments were conducted independently at least 3 times. Statistical analysis was conducted using SPSS 20.0 (IBM Corp.) and data are presented as the mean ± SD. One-way ANOVA was used to analyze data, after which Tukey's multiple comparison post hoc test was performed. P<0.05 was considered to indicate a statistically significant difference.
Results
Lidocaine increases the relative viability of H/R-induced A549 cells
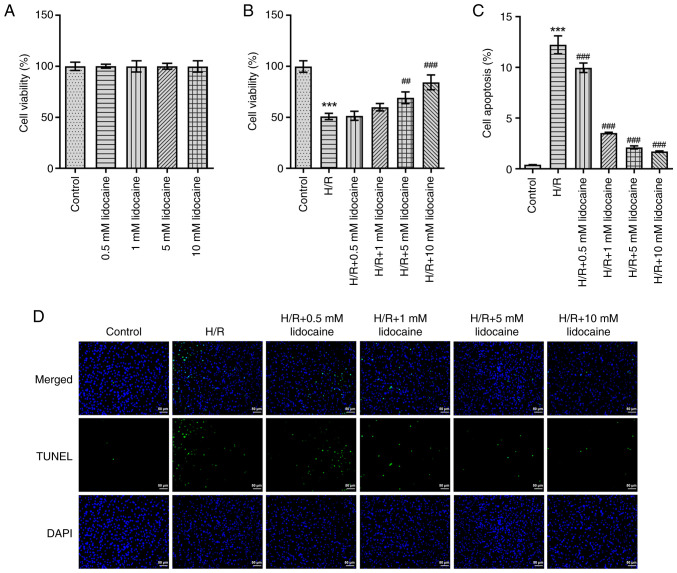
To increase the reliability of subsequent experiments, an MTT assay was employed to detect the viability of A549 and H/R-induced A549 cells. As presented in Fig. 1A, lidocaine had no marked effect on A549 cell viability. Although cell viability was greatly suppressed following H/R-induction, lidocaine treatment partly reversed this effect in a concentration-dependent manner (Fig. 1B). To further elucidate the biological role of lidocaine, H/R-induced A549 cell apoptosis was detected by using a TUNEL assay. When compared with the H/R group, the results revealed that lidocaine treatment significantly decreased the apoptosis of H/R-induced A549 cells (Fig. 1C and D).
Figure 1.
Lidocaine increases the relative viability and activation of H/R-induced A549 cells. The viability of (A) lidocaine-treated A549 cells and (B) H/R-induced A549 cells after lidocaine treatment was detected by using MTT. (C and D) Apoptosis was detected by performing a TUNEL assay. ##P<0.01 and ###P<0.001 vs. H/R; ***P<0.001 vs. control. H/R, hypoxia/reoxygenation.
Lidocaine alleviates oxidative stress and the inflammatory response in H/R-induced A549 cells
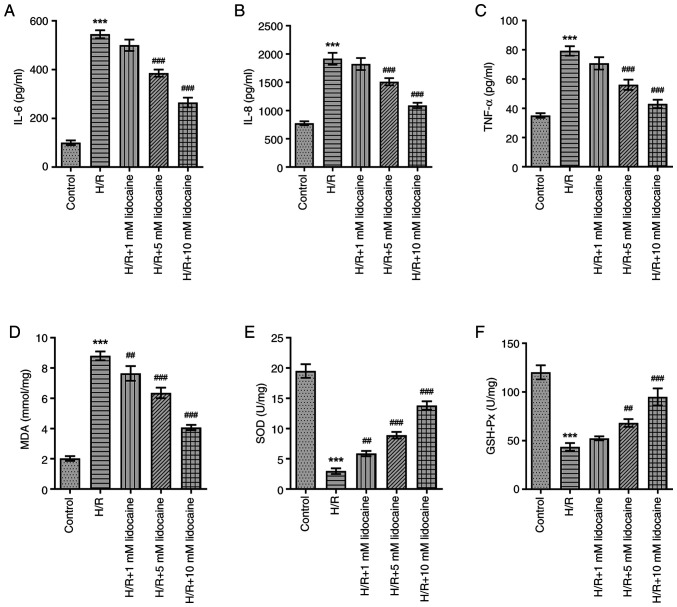
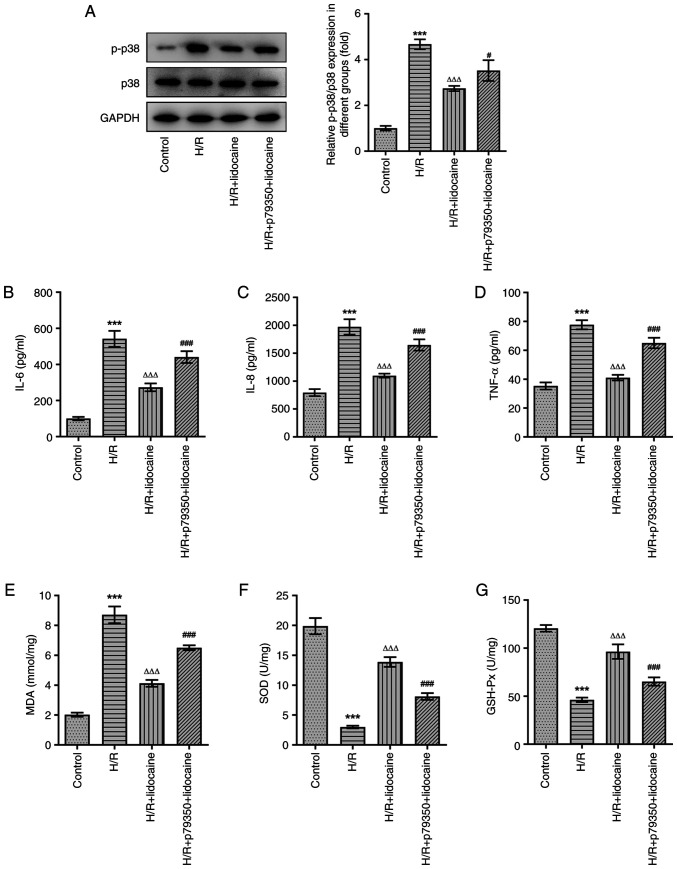
As inflammation and oxidative stress have a significant influence on IRI, the effects of lidocaine on the inflammatory response and oxidative stress were assessed in H/R-induced A549 cells. In comparison with the control group, IL-6, IL-8 and TNF-α levels were significantly increased in H/R-induced A549 cells (Fig. 2A-C). However, this effect was reversed following lidocaine treatment, particularly at the 10 mM concentration, which exhibited the most prominent inhibitory effect on the inflammatory response. H/R significantly increased MDA levels, and significantly decreased SOD and GSH-Px levels (Fig. 2D-F). However, downregulated SOD and GSH-Px, and upregulated MDA levels were reversed following lidocaine treatment in H/R-induced A549 cells. In summary, lidocaine treatment eased the inflammatory response and reduced oxidative stress.
Figure 2.
Lidocaine alleviates the inflammatory and oxidative stress response in H/R-induced A549 cells. Levels of (A) IL-6, (B) IL-8 and (C) TNF-α were detected by using ELISA. The expression levels of (D) MDA, (E) SOD and (F) GSH-Px were measured. ##P<0.01 and ###P<0.001 vs. H/R; ***P<0.001 vs. control. H/R, hypoxia/reoxygenation; MDA, malondialdehyde; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase.
Lidocaine relieves barrier dysfunction in H/R-induced A549 cells
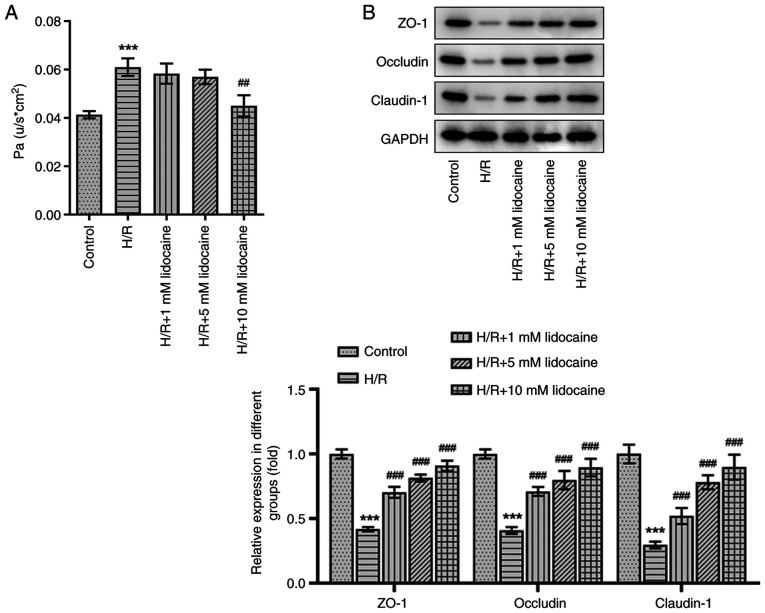
A monolayer cell paracellular permeability assay was performed to evaluate the effects of lidocaine on H/R-induced A549 cell permeability. Compared with the control group, cell permeability was increased following H/R induction, which was an effect that was diminished by 10 mM lidocaine treatment (Fig. 3A). The expression of tight junction proteins was measured via western blotting. The results revealed that the expression levels of certain proteins, including ZO-1, Occludin and Claudin-1, were decreased in H/R-induced A549 cells (Fig. 3B). However, this effect was significantly reversed by lidocaine treatment, particularly at the 10 mM dosage. The results indicated that lidocaine protected against barrier dysfunction by decreasing cell permeability and increasing tight junction protein expression in H/R-induced A549 cells.
Figure 3.
Lidocaine relieves barrier dysfunction in H/R-induced A549 cells. (A) Cell permeability was detected using a monolayer cell paracellular permeability assay. (B) The expression levels of ZO-1, Occludin and Claudin-1 were measured via western blotting. ##P<0.01 and ###P<0.001 vs. H/R; ***P<0.001 vs. control. H/R, hypoxia/reoxygenation; ZO-1, zonula occludens-1.
Lidocaine inhibits p38 MAPK-mediated ferroptosis in H/R-induced A549 cells
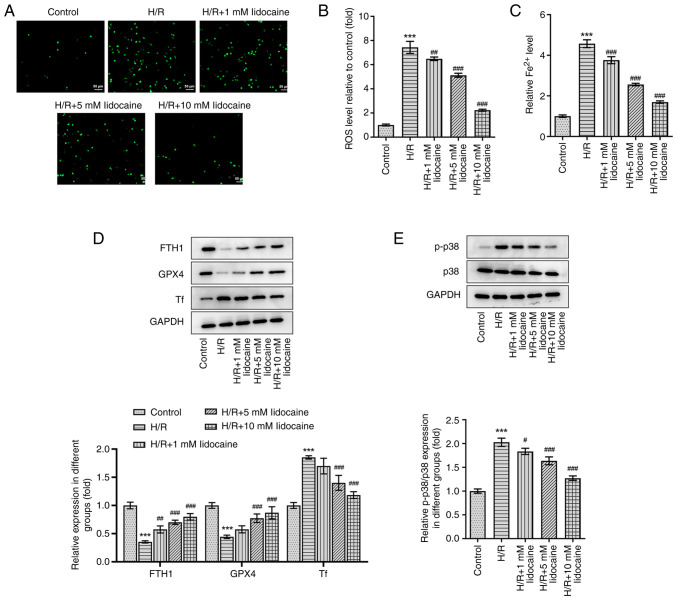
To explore the mechanism of lidocaine in ferroptosis, ROS level and iron level was first evaluated. As shown in Fig. 4A-C, ROS and iron levels were significantly elevated by H/R induction, but were then significantly reduced by lidocaine treatment in a concentration-dependent manner. Subsequently, western blotting was performed and the expression levels of p38 MAPK pathway-related and ferroptosis-related proteins were assessed, including FTH1, GPX4 and Tf. Compared with the control group, the expression levels of FTH1 and GPX4 were significantly decreased by H/R induction; however, the expression of Tf was significantly increased under the same conditions. Following lidocaine treatment at various concentrations, the H/R-induced decrease of FTH1 and GPX4, and the H/R-induced increase of Tf, was significantly reversed (Fig. 4D). Furthermore, the H/R-induced increase in p-p38, a p38 MAPK-related protein, in A549 cells was significantly downregulated following lidocaine treatment (Fig. 4E). However, p38 expression remained unchanged after lidocaine treatment and H/R induction. To conclude, lidocaine inhibited ferroptosis in H/R-induced A549 cells by regulating the p38 MAPK signaling pathway.
Figure 4.
Lidocaine inhibits p38 MAPK-mediated ferroptosis in H/R-induced A549 cells. Levels of (A and B) ROS and (C) iron were detected using their corresponding commercial kits. (D) The expression levels of FTH1, GPX4 and Tf were measured via western blotting. (E) The expression levels of p-p38 and p38 were measured via western blotting. #P<0.05, ##P<0.01 and ###P<0.001 vs. H/R; ***P<0.001 vs. control. H/R, hypoxia/reoxygenation; FTH1, ferritin heavy chain 1; GPX4, glutathione peroxidase 4; Tf, transferrin; p-, phosphorylated; ROS, reactive oxygen species.
Lidocaine suppresses oxidative stress and the inflammatory response in H/R-induced A549 cells by blocking the p38 MAPK signaling pathway
To investigate the underlying mechanism of lidocaine on the inflammatory response and oxidative stress, p79350, an agonist of p38, was used to pre-treat A549 cells. Lidocaine at a concentration of 10 mM was selected for further experiments. Compared with the H/R + lidocaine group, p-p38 protein expression was upregulated in the H/R + p79350 + lidocaine group, which demonstrated that p79350 increased the activity of p39 MAPK signaling (Fig. 5A). The results of ELISA suggested that H/R induction significantly stimulated the release of IL-6, IL-8 and TNF-α, while lidocaine treatment significantly inhibited this effect. Furthermore, when compared with the H/R + lidocaine group, IL-6, IL-8 and TNF-α levels were increased again in the H/R + p79350 + lidocaine group (Fig. 5B-D). Subsequently, the expression levels of MDA, SOD and GSH-Px were assessed. As demonstrated in Fig. 5E-G, increased MDA expression levels induced by H/R were significantly downregulated in the H/R + lidocaine group. Additionally, the H/R-induced decrease in SOD and GSH-Px expression were subsequently significantly increased in A549 cells in the H/R + lidocaine group. However, MDA expression was increased, and SOD and GSH-Px expression levels were decreased in the H/R + p79350 + lidocaine group, when compared with the H/R + lidocaine group. In summary, p79350 partially reversed the inhibitory effects of lidocaine on the inflammatory response and oxidative stress in H/R-induced A549 cells.
Figure 5.
Lidocaine suppresses oxidative stress and the inflammatory response in H/R-induced A549 cells by blocking the p38 MAPK signaling pathway. (A) The expression levels of p-p38 and p38 were measured via western blotting. Levels of (B) IL-6, (C) IL-8 and (D) TNF-α were detected by using ELISA. The expression levels of (E) MDA, (F) SOD and (G) GSH-Px were measured. ***P<0.001 vs. control; ΔΔΔP<0.001 vs. H/R; #P<0.05 and ###P<0.001 vs. H/R + lidocaine. H/R, hypoxia/reoxygenation; p-, phosphorylated; MDA, malondialdehyde; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase.
Lidocaine inhibits barrier dysfunction and ferroptosis in H/R-induced A549 cells by blocking the p38 MAPK signaling pathway
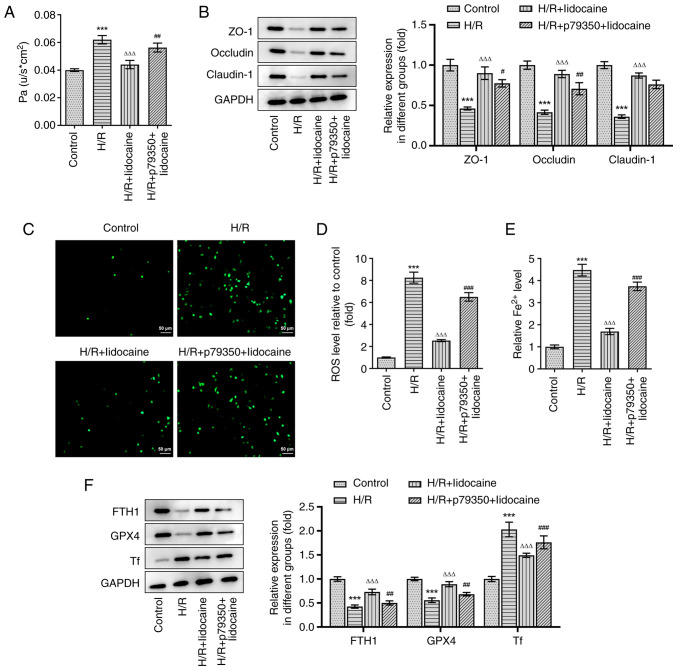
A549 cell permeability was assessed by using a monolayer cell paracellular permeability assay. As presented in Fig. 6A, the H/R-induced increase of cell permeability was significantly suppressed in the H/R + lidocaine group; however, these inhibitory effects were partially reversed in the H/R + p79350 + lidocaine group. The expression levels of tight junction proteins were measured via western blotting. In comparison with the H/R group, ZO-1, Occludin and Claudin-1 expression levels were significantly increased in the H/R + lidocaine group (Fig. 6B). However, this increase was subsequently decreased in the H/R + p79350 + lidocaine group, indicating that it counteracted the protective effects of lidocaine. In addition, the reduced ROS and iron levels in the H/R + lidocaine group were partially reversed in the H/R + p79350 + lidocaine group in A549 cells (Fig. 6C-E). Then, western blotting was also employed to measure the expression of ferroptosis-related proteins, including FTH1, GPX4 and Tf. The results revealed that H/R induction decreased the expression levels of FTH1 and GPX4, but increased the expression of Tf (Fig. 6F). FTH1 and GPX4 expression levels were significantly upregulated in the H/R + lidocaine group, and significantly downregulated Tf expression levels in A549 cells; however, these effects were reversed in the H/R + p79350 + lidocaine group. To summarize, lidocaine suppressed barrier dysfunction and ferroptosis in H/R-induced A549 cells by blocking the p38 MAPK signaling pathway.
Figure 6.
Lidocaine inhibits barrier dysfunction and ferroptosis in H/R-induced A549 cells by blocking the p38 MAPK signaling pathway. (A) Cell permeability was detected by performing a monolayer cell paracellular permeability assay. (B) The expression levels of ZO-1, Occludin and Claudin-1, were measured via western blotting. Levels of (C and D) ROS and (E) iron were detected by using their corresponding commercial kits. (F) The expression levels of FTH1, GPX4 and Tf were measured via western blotting. ***P<0.001 vs. control; ΔΔΔP<0.001 vs. H/R; #P<0.05, ##P<0.01 and ###P<0.001 vs. H/R + lidocaine. H/R, hypoxia/reoxygenation; ZO-1, zonula occludens-1; ROS, reactive oxygen species; FTH1, ferritin heavy chain 1; GPX4, glutathione peroxidase 4; Tf, transferrin.
Discussion
LIRI is a significant clinical problem that has been evident for a number of years. It usually occurs following lung transplantation, revascularization of pulmonary embolism, cardiopulmonary resuscitation, pulmonary arterioplasty, shock and various heart bypass operations (26,27). LIRI can lead to severe organ damage and various types of cell death, resulting in an increase of mortality (28). However, therapeutic methods that demonstrate high efficacy have not yet been determined (5). Thus, investigating additional effective therapeutic measures is of great importance. The current study therefore constructed an in vitro model of LIRI to investigate the protective role and underlying mechanism of lidocaine.
In the present study, lidocaine demonstrated a protective effect against LIRI. Previous studies have demonstrated that the constant infusion of lidocaine could protect against myocardial I/R injury (MIRI) and prevent cardiac death (9,29). Studies have also revealed that lidocaine administration reduced IRI (11,30). The results of the present study determined that lidocaine protected against LIRI in H/R-induced A549 cells. Evidently, lidocaine can exert protective effects on IR injury in various diseases, such as ischemic brain damage, ischemic spinal cord and myocardial injury (31–33). Of note, it has been found that lidocaine can inhibit the process of apoptosis under multiple pathological states, such as cerebral I/R injury and myocardial damage due to ischemia and reperfusion (34,35), consistent with the findings in the present study. However, lidocaine is also reported to induce the process of apoptosis in various cancer cell lines, thereby inhibiting cancer development (36,37). Therefore, the effect of lidocaine on cell apoptosis is not absolute, as lidocaine can exert proapoptotic effects on tumor cell lines or exert antiapoptotic effects on I/R-induced cell injury.
Inflammation serves an important role in the mechanism of LIRI (38), such that the regulation of inflammation has been revealed to effectively alleviate the disease (39). Previous studies have verified that IL-8 levels are markedly increased in lung tissue and systemic circulation after IR (40,41). This is problematic, as significantly higher IL-8 levels may result in higher mortality rates following lung transplantation (42). Lan et al (39) and de Perrot et al (40) reported that elevated TNF-α and IL-6 levels contributed to organ IR injury. In the present study, the results of ELISA demonstrated that IL-6, IL-8 and TNF-α levels were markedly increased in A549 cells following H/R induction. However, the application of lidocaine inhibited these effects in a concentration-dependent manner.
Oxidative stress is another critical factor in the mechanism of LIRI. MDA, the end product of lipid peroxidation, can reflect the degree of lipid peroxidation in lung tissue (43). In the present study, it was revealed that MDA levels in H/R-induced A549 cells were increased when compared with the control group, suggesting that cell membrane lipid peroxidation had increased. Subsequent lidocaine treatment decreased MDA levels, revealing that lidocaine could reduce lipid peroxidation. SOD is the main endogenous antioxidant enzyme found in lung tissue, the detection of which can reflect the degree of oxidation resistance (43). The current study determined that SOD levels were markedly decreased following H/R induction, which was an effect that could be partially abolished by lidocaine treatment. These results are in line with previous findings that antioxidants may be promising targets for LIRI (44).
As ferroptosis is a key part of LIRI, it was investigated in the current study. Ferroptosis is a newly discovered form of regulated cell death that depends on the existence of iron and is triggered by lipid ROS (45). The knockout or inactivation of GPX4 prevents cells from inhibiting lethal lipid peroxidation in phospholipid bilayers, eventually leading to cell death (46–48). The present study revealed that the expression levels of FTH1 and GPX4 were markedly downregulated in H/R-induced A549 cells, indicating that ferroptosis had occurred in LIRI, which is in line with the results of previous studies in organs such as the kidney and intestine (49,50). The adoption of lidocaine may therefore upregulate FTH1 and GPX4 expression levels, thus inhibiting ferroptosis. Of note, it is interesting that the 5 mM lidocaine concentration appeared to have similar effects to the 10 mM concentration on markers of ferroptosis and tight junction proteins. However, the effects of 10 nM lidocaine on cell viability, inflammatory response and oxidative stress were higher than those of 5 mM lidocaine. The different sensitivity of lidocaine to different cellular bioactivity may be a reason accounting for this condition.
Furthermore, it is reported that regulation of the p38 MAPK signaling pathway may be an effective target for IRI, as evidenced by a previous study that revealed MIRI could be inhibited through the regulation of the P38 MAPK signaling pathway (51). Jiang et al (22) reported that lidocaine exerted protective effects on cerebral IR injury by suppressing the activation of p38 MAPK. Additionally, through the regulation of the p38 MAPK signaling pathway, lidocaine could protect against LPS-induced lung injury (23). Therefore, it was hypothesized in the current study that lidocaine treatment could relieve LIRI by blocking the p38 MAPK signaling pathway. To test this theory, p79350, an agonist of p38, was utilized to pre-treat A549 cells in the present study. The results revealed that p79350 partially reversed the protective effect of lidocaine on H/R-induced A549 cells, indicating that regulation of the p38 MAPK signaling pathway could be an effective target underlying the protective effects of lidocaine on LIRI.
However, there are still some limitations of the present study. First, this study only explored the regulatory effect of lidocaine on ferroptosis in LIRI, but whether ferroptosis is a necessary regulatory pathway for lidocaine remains unclear. The application of ferroptosis inhibitors is worthy of further investigation to determine the involvement of ferroptosis in lidocaine-treated LIRI. Furthermore, in vivo experiments will also be conducted in our future work to further verify these findings.
In conclusion, lidocaine could regulate inflammation, oxidative stress and ferroptosis by blocking the p38 MAPK signaling pathway. Thus, lidocaine could act as a novel therapeutic treatment of patients with LIRI.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
NH designed the study. XM, WY and NH performed the experiments and analyzed the data. XM and WY drafted manuscript. NH revised the manuscript. XM and NH confirmed the authenticity of all raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sharma AK, Charles EJ, Zhao Y, Narahari AK, Baderdinni PK, Good ME, Lorenz UM, Kron IL, Bayliss DA, Ravichandran KS, et al. Pannexin-1 channels on endothelial cells mediate vascular inflammation during lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2018;315:L301–L312. doi: 10.1152/ajplung.00004.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weyker PD, Webb CA, Kiamanesh D, Flynn BC. Lung ischemia reperfusion injury: A bench-to-bedside review. Semin Cardiothorac Vasc Anesth. 2013;17:28–43. doi: 10.1177/1089253212458329. [DOI] [PubMed] [Google Scholar]
- 3.Diamond JM, Lee JC, Kawut SM, Shah RJ, Localio AR, Bellamy SL, Lederer DJ, Cantu E, Kohl BA, Lama VN, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2013;187:527–534. doi: 10.1164/rccm.201210-1865OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fei L, Jingyuan X, Fangte L, Huijun D, Liu Y, Ren J, Jinyuan L, Linghui P. Preconditioning with rHMGB1 ameliorates lung ischemia-reperfusion injury by inhibiting alveolar macrophage pyroptosis via the Keap1/Nrf2/HO-1 signaling pathway. J Transl Med. 2020;18:301. doi: 10.1186/s12967-020-02467-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laubach VE, Sharma AK. Mechanisms of lung ischemia-reperfusion injury. Curr Opin Organ Transplant. 2016;21:246–252. doi: 10.1097/MOT.0000000000000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherobin AC, Tavares GT. Safety of local anesthetics. An Bras Dermatol. 2020;95:82–90. doi: 10.1016/j.abd.2019.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berk T, Silberstein SD. The use and method of action of intravenous lidocaine and its metabolite in headache disorders. Headache. 2018;58:783–789. doi: 10.1111/head.13298. [DOI] [PubMed] [Google Scholar]
- 8.Lancaster RJ, Wren K, Hudson A, Leavitt K, Albala M, Tischaefer D. Intravenous lidocaine for chronic neuropathic pain a systematic review addressing nursing care. Pain Manag Nurs. 2020;21:194–200. doi: 10.1016/j.pmn.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Canyon SJ, Dobson GP. Protection against ventricular arrhythmias and cardiac death using adenosine and lidocaine during regional ischemia in the in vivo rat. Am J Physiol Heart Circ Physiol. 2004;287:H1286–H1295. doi: 10.1152/ajpheart.00273.2004. [DOI] [PubMed] [Google Scholar]
- 10.Chen MY, Li CH, Huang ZQ, Liu JC, Zhou NX, Huang XQ, Wang YS. Protective effects of lidocaine injected into the hepatoduodenal ligament on warm inschemia-reperfusion injury to the rat liver. Chin Med J (Engl) 2004;117:275–279. [PubMed] [Google Scholar]
- 11.Lei B, Cottrell JE, Kass IS. Neuroprotective effect of low-dose lidocaine in a rat model of transient focal cerebral ischemia. Anesthesiology. 2001;95:445–451. doi: 10.1097/00000542-200108000-00029. [DOI] [PubMed] [Google Scholar]
- 12.Fang X, Wang H, Han D, Xie E, Yang X, Wei J, Gu S, Gao F, Zhu N, Yin X, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci USA. 2019;116:2672–2680. doi: 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell. 2015;59:298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li N, Wang W, Zhou H, Wu Q, Duan M, Liu C, Wu H, Deng W, Shen D, Tang Q. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic Biol Med. 2020;160:303–318. doi: 10.1016/j.freeradbiomed.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Sun D, Li YC, Zhang XY. Lidocaine promoted ferroptosis by targeting miR-382-5p /SLC7A11 axis in ovarian and breast cancer. Front Pharmacol. 2021;12:681223. doi: 10.3389/fphar.2021.681223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yong HY, Koh MS, Moon A. The p38 MAPK inhibitors for the treatment of inflammatory diseases and cancer. Expert Opin Investig Drugs. 2009;18:1893–1905. doi: 10.1517/13543780903321490. [DOI] [PubMed] [Google Scholar]
- 17.Lee S, Rauch J, Kolch W. Targeting MAPK signaling in cancer: Mechanisms of drug resistance and sensitivity. Int J Mol Sci. 2020;21:1102. doi: 10.3390/ijms21031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sui XB, Kong N, Ye L, Han W, Zhou J, Zhang Q, He C, Pan H. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014;344:174–179. doi: 10.1016/j.canlet.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Wang CH, Hao ZY, Zhou J, Zhang L, Sun Y, Liang C. Rutae carpine alleviates renal ischemia reperfusion injury in rats by suppressing the JNK/p38 MAPK signaling pathway and interfering with the oxidative stress response. Mol Med Rep. 2017;16:922–928. doi: 10.3892/mmr.2017.6631. [DOI] [PubMed] [Google Scholar]
- 20.An ST, Wang X, Shi HR, Zhang X, Meng H, Li W, Chen D, Ge J. Apelin protects against ischemia-reperfusion injury in diabetic myocardium via in hibiting apoptosis and oxidative stress through P38K and p38-MAPK signaling pathways. Aging (Albany NY) 2020;12:25120–25137. doi: 10.18632/aging.104106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duarte S, Shen X-D, Fondevila C, Busuttil RW, Coito AJ. Fibronectin-α4β1 interactions in hepatic cold ischemia and reperfusion injury: Regulation of MMP-9 and MT1-MMP via the p38 MAPK pathway. Am J Transplant. 2012;12:2689–2699. doi: 10.1111/j.1600-6143.2012.04161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang R, Liao J, Yang MC, Deng J, Hu YX, Li P, Li MT. Lidocaine mediates the progression of cerebral ischemia/reperfusion injury in rats via inhibiting the activation of NF-kappaB p65 and p38 MAPK. Ann Transl Med. 2020;8:548. doi: 10.21037/atm-20-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen LJ, Ding YB, Ma PL, Jiang SH, Li KZ, Li AZ, Li MC, Shi CX, Du J, Zhou HD. The protective effect of lidocaine on lipopolysaccharide-induced acute lung injury in rats through NF-κB and p38 MAPK signaling pathway and excessive inflammatory responses. Eur Rev Med Pharmacol Sci. 2018;22:2099–2108. doi: 10.26355/eurrev_201804_14743. [DOI] [PubMed] [Google Scholar]
- 24.Wu SY, Li MH, Ko FC, Wu GC, Huang KL, Chu SJ. Protective effect of hypercapnic acidosis in ischemia-reperfusion lung injury is attributable to upregulation of heme oxygenase-1. PLoS One. 2013;8:e74742. doi: 10.1371/journal.pone.0074742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M, Wang X, Lu S, He C, Wang C, Wang L, Wang X, Ge P, Song D. Erastin triggers autophagic death of breast cancer cells by increasing intracellular iron levels. Oncol Lett. 2020;20:57. doi: 10.3892/ol.2020.11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beckers PAJ, Gielis JF, Van Schil PE, Adriaensen D. Lung ischemia reperfusion injury: The therapeutic role of dipeptidyl peptidase 4 inhibition. Ann Transl Med. 2017;5:129. doi: 10.21037/atm.2017.01.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saito M, Chen-Yoshikawa TF, Suetsugu K, Okabe R, Takahagi A, Masuda S, Date H. Pirfenidone alleviates lung ischemia-reperfusion injury in a rat model. J Thorac Cardiovasc Surg. 2019;158:289–296. doi: 10.1016/j.jtcvs.2018.08.098. [DOI] [PubMed] [Google Scholar]
- 29.Ebel D, Lipfert P, Frassdorf J, Preckel B, Müllenheim J, Thämer V, Schlack W. Lidocaine reduces ischaemic but not reperfusion injury in isolated rat heart. Br J Anaesth. 2001;86:846–852. doi: 10.1093/bja/86.6.846. [DOI] [PubMed] [Google Scholar]
- 30.Lei B, Popp S, Capuano-Waters C, Cottrell JE, Kass IS. Effects of delayed administration of low-dose lidocaine on transient focal cerebral ischemia in rats. Anesthesiology. 2002;97:1534–1540. doi: 10.1097/00000542-200212000-00028. [DOI] [PubMed] [Google Scholar]
- 31.Lan XY, Xu YM. Protective role of lidocaine against cerebral ischemia-reperfusion injury: An in vitro study. Exp Ther Med. 2022;23:42. doi: 10.3892/etm.2021.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu LW, Xu YF, Zhou YC, Li S, Yao J. Regionally infused lidocaine can dose-dependently protect the ischemic spinal cord in rabbits and may be associated with the EAA changes. Neurosci Lett. 2020;725:134889. doi: 10.1016/j.neulet.2020.134889. [DOI] [PubMed] [Google Scholar]
- 33.Aldakkak M, Stowe DF, Lesnefsky EJ, Heisner JS, Chen Q, Camara AKS. Modulation of mitochondrial bioenergetics in isolated guinea pig beating heart by potassium and lidocaine cardioplegia: Implications for cardioprotection. J Cardiovasc Pharmacol. 2010;54:298–309. doi: 10.1097/FJC.0b013e3181b2b842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaczmarek DJ, Herzog C, Larmann J, Gillmann HJ, Hildebrand R, Schmitz M, Westermann A, Harendza T, Werdehausen R, Osthaus AW, et al. Lidocaine protects from myocardial damage due to ischemia and reperfusion in mice by its antiapoptotic effects. Anesthesiology. 2009;110:1041–1049. doi: 10.1097/ALN.0b013e31819dabda. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Zhang J, Zan J, Zhang F, Liu G, Wu A. Lidocaine improves cerebral ischemia-reperfusion injury in rats through cAMP/PKA signaling pathway. Exp Ther Med. 2020;20:495–499. doi: 10.3892/etm.2020.8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang YC, Hsu YC, Liu CL, Huang SY, Hu MC, Cheng SP. Local anesthetics induce apoptosis in human thyroid cancer cells through the mitogen-activated protein kinase pathway. PLoS One. 2014;9:e89563. doi: 10.1371/journal.pone.0089563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu J, Han S. Lidocaine inhibits cervical cancer cell proliferation and induces cell apoptosis by modulating the lncRNA-MEG3/miR-421/BTG1 pathway. Am J Transl Res. 2019;11:5404–5416. [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Z, Chen Y, Zhou J, Li Y, Gong C, Wang X. Netrin-1 reduces lung ischemia-reperfusion injury by increasing the proportion of regulatory T cells. J Int Med Res. 2020;48:300060520926415. doi: 10.1177/0300060520926415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lan CC, Peng CK, Tang SE, Huang KL, Wu CP. Carbonic anhydrase inhibitor attenuates ischemia-reperfusion induced acute lung injury. PLoS One. 2017;12:e0179822. doi: 10.1371/journal.pone.0179822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167:490–511. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- 41.De Perrot M, Sekine Y, Fischer S, Waddell TK, McRae K, Liu M, Wigle DA, Keshavjee S. Interleukin-8 release during early reperfusion predicts graft function in human lung transplantation. Am J Respir Crit Care Med. 2002;165:211–215. doi: 10.1164/ajrccm.165.2.2011151. [DOI] [PubMed] [Google Scholar]
- 42.Ng CS, Wan S, Arifi AA, Yim AP. Inflammatory response to pulmonary ischemia-reperfusion injury. Surg Today. 2006;36:205–214. doi: 10.1007/s00595-005-3124-2. [DOI] [PubMed] [Google Scholar]
- 43.Wang F, Wang F, Li F, Wang D, Li H, He X, Zhang J. Methane attenuates lung ischemia-reperfusion injury via regulating PI3K-AKT-NFκB signaling pathway. J Recept Signal Transduct Res. 2020;40:209–217. doi: 10.1080/10799893.2020.1727925. [DOI] [PubMed] [Google Scholar]
- 44.Jiang F, Zhang Y, Dusting GJ. NADPH oxidase-mediated redox signaling: Roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev. 2011;63:218–242. doi: 10.1124/pr.110.002980. [DOI] [PubMed] [Google Scholar]
- 45.Xu Y, Li X, Cheng Y, Yang M, Wang R. Inhibition of ACSL4 attenuates ferroptotic damage after pulmonary ischemia-reperfusion. FASEB J. 2020;34:16262–16275. doi: 10.1096/fj.202001758R. [DOI] [PubMed] [Google Scholar]
- 46.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsushita M, Freigang S, Schneider C, Conrad M, Bornkamm GW, Kopf M. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J Exp Med. 2015;212:555–568. doi: 10.1084/jem.20140857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mayr L, Grabherr F, Schwärzler J, Reitmeier I, Sommer F, Gehmacher T, Niederreiter L, He GW, Ruder B, Kunz KTR, et al. Dietary lipids fuel GPX4-restricted enteritis resembling Crohn's disease. Nat Commun. 2020;11:1775. doi: 10.1038/s41467-020-15646-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muller T, Dewitz C, Schmitz J, Schröder AS, Bräsen JH, Stockwell BR, Murphy JM, Kunzendorf U, Krautwald S. Necroptosis and ferroptosis are alternative cell death pathways that operate in acute kidney failure. Cell Mol Life Sci. 2017;74:3631–3645. doi: 10.1007/s00018-017-2547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, Feng D, Wang Z, Zhao Y, Sun R, Tian D, Liu D, Zhang F, Ning S, Yao J, Tian X. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019;26:2284–2299. doi: 10.1038/s41418-019-0299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang ZH, Lu YJ, Gu KP, Xiang ZY, Huang HM. Effect of ulinastatin on myocardial ischemia-reperfusion injury through JNK and P38 MAPK signaling pathways. Eur Rev Med Pharmacol Sci. 2019;23:8658–8664. doi: 10.26355/eurrev_201910_19183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.