Abstract
To investigate altitude control in honeybees, an optical configuration was designed to manipulate or cancel the optic flow. It has been widely accepted that honeybees rely on the optic flow generated by the ground to control their altitude. Here, we create an optical configuration enabling a better understanding of the mechanism of altitude control in honeybees. This optical configuration aims to mimic some of the conditions that honeybees experience over a natural water body. An optical manipulation, based on a pair of opposed horizontal mirrors, was designed to remove any visual information coming from the floor and ceiling. Such an optical manipulation allowed us to get closer to the seminal experiment of Heran & Lindauer 1963. Zeitschrift für vergleichende Physiologie 47, 39–55. (doi:10.1007/BF00342890). Our results confirmed that a reduction or an absence of ventral optic flow in honeybees leads to a loss in altitude, and eventually a collision with the floor.
Keywords: Apis mellifera, insect flight, altitude control, optic flow, motion vision, optical manipulation
1. Introduction
Flying bees, honeybees or bumblebees, are known to be particularly sensitive to the optic flow pattern generated by the contrasting features of the ground to adjust their altitude by maintaining constant ventral optic flow during terrain-following tasks [1–6]. The minimum sized section of a rectangular tunnel sets the honeybees' forward speed [5,7]; therefore, the forward and upward axes of the honeybee's flight control system can be decoupled [2]. Consequently, in a tunnel where the width is smaller than the height, the width sets the honeybees' forward speed (see also [8–11] for a description of the visuomotor modelling in honeybees). Similarly, honeybees, which have be trained to follow the tunnel ceiling, when encountering a ‘dorsal ditch’ in the middle of the tunnel configuration [4] responded to this new configuration by rising quickly and hugging the new, higher ceiling, while maintaining a similar forward speed, distance to the ceiling, and dorsal optic flow to those observed during the training step. Conversely, honeybees trained to follow the floor kept on following the floor regardless of the change in the ceiling's height [4].
The present study aims to pursue investigations about the role of dorsal and ventral visual inputs feeding the altitude control system in honeybees by manipulating or cancelling parts of the optic flow using mirrors. Inspired by Duchon & Warren [12] experiments on humans, in which the researchers designed an optical manipulation made with a pair of infinite walls in order to optically remove the floor, then by strongly decreasing visual information coming from the floor, we designed a novel optical configuration with floor and/or ceiling mirror(s) enabling a better understanding of the mechanism of altitude control in honeybees. Such an optical manipulation in which the floor appeared to be removed allowed us to mimic some of the conditions that honeybees experience when experimentally trained to fly over a natural water body and extend the seminal experiment of Heran & Lindauer [13]. Sixty years ago, these researchers trained honeybees to fly above a 247 m-long water surface. When the water surface was rippled or when a floating bridge provided a visual contrast, honeybees were able to cross the lake. However, honey bees flying over a calm water surface during foraging trips flew lower and lower until they collided with the water surface and drowned [13]. Our flight tunnel, which used a pair of mirrors placed on the floor and on the ceiling, gets closer to, and extends experimentally, a behaviour that has been observed outdoor above a water surface.
2. Methods and material
(a) . Flight tunnel
The outdoor flight tunnel is a rectangular shape (220 cm long, 71 cm high and 25 cm wide, see electronic supplementary material, figure S1 for further details), the ceiling and floor are mirrors that can be covered as in step 0 in electronic supplementary material, figure S2, the left-hand wall is solid and the right-hand wall is made of insect netting. A unique red and white striped pattern, perpendicular to the long axis of the tunnel and therefore the direction of flight, is provided on the four surfaces of the tunnel (floor, ceiling, left wall, insect netting), although it is only permanently installed on walls. The mirrors on the floor and ceiling can be covered with the same pattern as can the insect netting. In this latter case, the stripes are reproduced using a red gelatin filter (Lee Filters HT019). The tunnel is closed with white boards at each end. At one end, there is a circular entrance (5 cm in diameter) located 11.5 cm above the floor. At the other end, a square opening (3.5 cm) placed 11.5 cm above the floor gives bees access to the reward box. The tunnel's entrance and the door of the reward box were opened and closed manually by the experimenter. The flight tunnel received only indirect illumination (no direct sunlight). This video can be watched to better understand the organization of our experimental set-up: https://www.youtube.com/watch?v=KH9z8eqOBbU.
(b) . Pattern
Red stripes of two different widths (1 cm and 3 cm) form a simple 10 cm wide regularly repeated pattern. The angular subtends of the stripes ranged from 5.7° to 53° (1–10 cm-wide pattern viewed from a distance of 10 cm) and from 0.5° to 5.3° (1–10 cm-wide pattern viewed from 1 m). As honeybees do not possess red-sensitive photoreceptors [3], they perceive red stripes as grey ones. Between the red and white stripes, the Michelson contrast is 0.47 but 0.25 on the insect netting. The contrast was measured using a photodiode equipped with a green band-pass filter (Kodak Wratten n°61), the transmission spectrum of which closely matched the spectral sensitivity of the honeybee's green receptors.
(c) . Experimental procedure
See electronic supplementary material, S1.
(d) . Video recordings and flight path analysis
See electronic supplementary material, S2.
(e) . Statistical analysis
See electronic supplementary material, S3.
3. Results
(a) . Honeybees following the floor do not rely on dorsal visual information
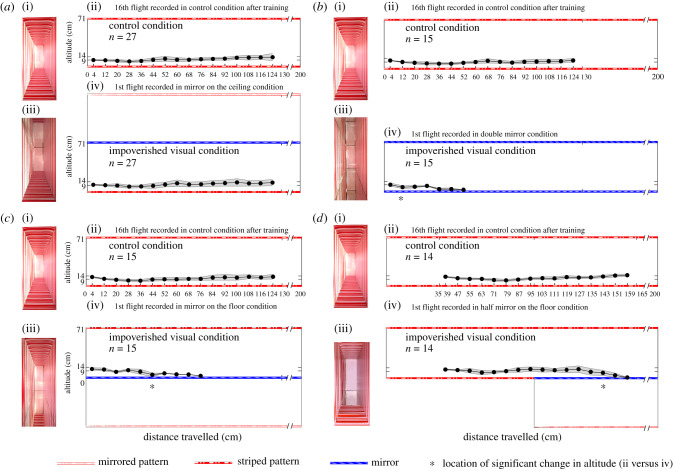
In experiment A (figure 1a), we tested the effect of a visual impoverishment in the dorsal part of the honeybees' visual field. The chronology of the procedure from step 0 to step 2 is described in electronic supplementary material, figure S2. A group of 27 honeybees were trained in the control condition (CC) (figure 1a(i,ii)). The first flight ‘in mirror on the ceiling’ condition, in which the ceiling mirror was uncovered, was recorded in an impoverished visual condition (IC) (see electronic supplementary material, figure S1). The presence of the mirror on the ceiling appearing to double the tunnel's height (142 cm) upwards (figure 1a(iii,iv)). We observed no significant change in flight behaviour in honeybees (see Exp. A in electronic supplementary material, table S1).
Figure 1.
This figure depicts the trajectories (median ± MAD) followed by honeybees in the vertical plane (x,z) in the four experimental conditions (Exp. A: a, Exp. B: b, Exp. C: c and Exp. D: d). In each panel, a comparison can be made between the trajectories produced prior to (a(ii), b(ii), c(ii) and d(ii)) and after (a(iv), b(iv), c(iv) and d(iv)) the experimental manipulations. These manipulations consisted of uncovering either a mirror on the ceiling (a(iii)), mirrors on both the floor and the ceiling (b(iii)), a mirror on the floor (c(iii)) or on half the floor (d(iii)). High-resolution pictures in the tunnel can be seen in Serres et al. [14]. Results show that while manipulating dorsal optic flow does not affect the honeybees' trajectories, manipulating ventral optic flow, whether it is a simple manipulation or a suppression, has significant consequences on the trajectories, leading to systematic collisions with the floor mirror. The abscissa marked by an asterisk symbol in (b(iv)), (c(iv)) and (d(iv)) represents the location at which we observed a significant change in height of flight before collisions (see electronic supplementary material, table S1 for comparisons of altitude distributions).
(b) . Without any ventral and dorsal visual information, bees hit the floor mirror
In experiment B (figure 1b), we tested the effect of a visual impoverishment in both the dorsal and ventral parts of the honeybees' visual field. The chronology of the procedure is described in electronic supplementary material, figure S2. A group of 15 honeybees were trained in the CC (figure 1b(i,ii)). The first flight, ‘in double mirror’ condition in which both mirrors were uncovered, was recorded in an IC (see electronic supplementary material, figure S1). The presence of mirrors on the ceiling and on the floor created an optical manipulation in which a pair of infinite walls appeared. As a result, no visual information from either the floor or ceiling was available (figure 1b(iii,iv)). We observed significant changes in the flight behaviour of honeybees (see Exp. B in electronic supplementary material, table S1) from x = 8 cm until each of the honeybees collided with the floor mirror.
(c) . Dorsal visual information does not aid in flying further over the floor mirror
In experiment C (figure 1c), we tested the effect of a visual impoverishment in the ventral part of the honeybees' visual field. The chronology of the procedure is described in electronic supplementary material, figure S2. A group of 15 honeybees were trained in the CC (figure 1c(i,ii)). The first flight, ‘in mirror on the floor’ condition in which the floor mirror was uncovered, was recorded in the IC (see electronic supplementary material, figure S1). The presence of the mirror on the floor appearing to double the tunnel's height (142 cm) downwards (figure 1c(iii,iv)) making a kind of ‘ventral ditch’ of 71 cm in depth. We observed significant changes in flight behaviour in honeybees (see Exp. C in electronic supplementary material, table S1) from x = 40 cm until each of the honeybees collided with the floor mirror. Honeybees may be visually attracted to the virtual floor 71 cm below, but then they collided with the mirror.
(d) . Covering the first half of the floor mirror does not help honeybees to fly further
In experiment D (figure 1d), we tested the effect of a visual reinforcement in the ventral part of the honeybees' visual field by covering the first half of the floor mirror with the same pattern of red and white stripes as previously used. The chronology of the procedure is described in electronic supplementary material, figure S2. A group of 14 honeybees were trained in the CC (figure 1d(i,ii)). The first flight ‘in half mirror on the floor’ condition in which the second half of the floor mirror was left uncovered, virtually doubling the tunnel height (142 cm) downwards (figure 1d(iii,iv)) making a kind of ‘ventral ditch’ of 71 cm in depth as in experiment C (figure 1c). We observed significant changes in flight behaviour in honeybees (see Exp. D in electronic supplementary material, table S1) from x = 139 cm until each of honeybees collided with the mirror. This additional texture on the floor does not help honeybees to fly further above the mirror. The travelled distance above the mirror (39 cm) was similar to that observed in experiment C (see Exp. C in electronic supplementary material, table S1).
4. Discussion and conclusion
The aim of the present study was to extend the work of Heran & Lindauer [13] by precisely manipulating parts of the optic flow. The use of a mirror on the floor (or ceiling) allowed us to manipulate the ventral (or dorsal) optic flow, thus generating a ‘ventral (or dorsal) ditch’, without having to change the orientation of the pattern stripes [1], the pattern relative velocity [2] or the tunnel geometry [4]. On the other hand, a double mirror condition allowed us to simultaneously suppress visual information coming from both the floor and ceiling, generating two infinite parallel walls.
In experiment A (figure 1a(iv)), honeybees appear to follow the floor despite this virtual ‘dorsal ditch’ because they may have learnt to follow the floor and to rely on the ventral visual information in order to regulate their flight, concurring with the results of Portelli et al. [4]. The lack of a difference between the last training flight and test trial data in experiment A suggests that the upward change in tunnel height might simply not have been perceived by the honeybees.
Conversely, each experimental manipulation that affects the ventral part of the optic flow, whether it is a total deprivation of the ventral optic flow in experiment B (figure 1b(iv)) or a reduction in the ventral part of the optic flow (figure 1c(iv),d(iv)), gave rise to a loss of altitude until the honeybee collided with the floor mirror. Interestingly our double mirror condition allowed us to get closer to the flight conditions of an open sky flight above a calm water surface as used by [13]. Our results agree with theirs insofar as the honeybees lose altitude in the absence of ventral optic flow.
In experiments C and D, the use of a mirror on the floor does not cause the suppression of the ventral part of optic flow, as the ceiling textures mirrored in the floor create a virtual ‘ventral gap’ reducing the resulting ventral optic flow. The honeybees' reduction in altitude until they collide with the mirror could result from a change in altitude intended to restore the ventral optic flow as experienced in the 15 control trials, which concurs with the results of both Portelli et al. [2] and Portelli et al. [4]. Taken together, all our results indicate unequivocally that the drowning observed in the study of Heran & Lindauer [13] reflect the propensity of honeybees to reduce their altitude, in order to restore the ventral optic flow experienced.
Results in experiments C and D (figure 1c(iv),d(iv)) reveal that a significant proportion of honeybees (half of the sample, see electronic supplementary material, table S1) can fly as far as 39 cm above the floor mirror without losing altitude showing that they perceive the ventral optic flow over a wide visual field. By picking up information over a wide visual field [6], honeybees detect and respond to changes in the environment even when lacking texture on the ground.
To conclude this study, there are different strategies of altitude control in insects. Straw et al. [15] demonstrated that flies do not use ventral optic flow in their altitude control. Our findings could be expanded or be useful in other insect species to test whether they are sensitive to ventral optic flow, or if other optical information controls their altitude [16]. Our set-up could also be re-scaled to study the effect of optic flow on altitude control in birds [17,18].
Acknowledgements
The authors would like to thank Marc Boyron and Julien Diperi for their technical assistance with designing the flight tunnel, Patrick Sainton for his video montage showing the experimental set-up, and they wish to thank David Wood (English at your Service, http://www.eays.eu/) for revising the English of the manuscript. We are grateful to the two anonymous reviewers, whose suggestions helped us improve the manuscript.
Ethics
Animal experimentation: the study involved experiments on honeybees, which were conducted according to ethical guidelines.
Data accessibility
The data that support the findings of this study are openly available in the electronic supplementary material section at https://www.biorxiv.org/content/biorxiv/early/2021/10/07/2021.09.23.461476/DC1/embed/media-1.zip?download=true.
The data are provided in the electronic supplementary material [19].
Authors' contributions
J.R.S.: conceptualization, funding acquisition, investigation, methodology, project administration, supervision, validation, writing—original draft and writing—review and editing; A.H.P.M.: conceptualization, funding acquisition, investigation, methodology, validation and writing—review and editing; C.B.: methodology, resources, software, visualization and writing—review and editing; R.M.: resources, software and writing—review and editing; G.M.: conceptualization, funding acquisition, investigation, methodology, validation and writing—review and editing; F.R.: conceptualization, funding acquisition, investigation, methodology, validation, visualization and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by Aix Marseille University and the CNRS.
References
- 1.Baird E, Srinivasan MV, Zhang S, Lamont R, Cowling A. 2006. Visual control of flight speed and height in the honeybee. In Int. Conf. on Simulation of Adaptive Behavior, pp. 40-51. Berlin, Germany: Springer. [Google Scholar]
- 2.Portelli G, Ruffier F, Franceschini N. 2010. Honeybees change their height to restore their optic flow. J. Comp. Physiol. A 196, 307-313. ( 10.1007/s00359-010-0510-z) [DOI] [PubMed] [Google Scholar]
- 3.Srinivasan MV. 2011. Honeybees as a model for the study of visually guided flight, navigation, and biologically inspired robotics. Physiol. Rev. 91, 413-460. ( 10.1152/physrev.00005.2010) [DOI] [PubMed] [Google Scholar]
- 4.Portelli G, Serres JR, Ruffier F. 2017. Altitude control in honeybees: joint vision-based learning and guidance. Sci. Rep. 7, 1-10. ( 10.1038/s41598-016-0028-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serres JR, Ruffier F. 2017. Optic flow-based collision-free strategies: from insects to robots. Arthropod. Struct. Dev. 46, 703-717. ( 10.1016/j.asd.2017.06.003) [DOI] [PubMed] [Google Scholar]
- 6.Lecoeur J, Dacke M, Floreano D, Baird E. 2019. The role of optic flow pooling in insect flight control in cluttered environments. Sci. Rep. 9, 1-13. ( 10.1038/s41598-019-44187-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Portelli G, Ruffier F, Roubieu FL, Franceschini N. 2011. Honeybees’ speed depends on dorsal as well as lateral, ventral and frontal optic flows. PLoS ONE 6, e19486. ( 10.1371/journal.pone.0019486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Portelli G, Serres J, Ruffier F, Franceschini N. 2010. Modelling honeybee visual guidance in a 3-D environment. J. Physiol. 104, 27-39. ( 10.1016/j.jphysparis.2009.11.011) [DOI] [PubMed] [Google Scholar]
- 9.Franceschini N, Ruffier F, Serres J. 2007. A bio-inspired flying robot sheds light on insect piloting abilities. Curr. Biol. 17, 329-335. ( 10.1016/j.cub.2006.12.032) [DOI] [PubMed] [Google Scholar]
- 10.Raharijaona T, Serres J, Vanhoutte E, Ruffier F. 2017. Toward an insect-inspired event-based autopilot combining both visual and control events. In 2017 3rd Int. Conf. on event-based control, communication and signal processing (EBCCSP), pp. 1-7. Piscataway, NJ:: IEEE. [Google Scholar]
- 11.Bergantin L, Harbaoui N, Raharijaona T, Ruffier F. 2021. Oscillations make a self-scaled model for honeybees’ visual odometer reliable regardless of flight trajectory. J. R. Soc. Interface 18, 20210567. ( 10.1098/rsif.2021.0567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duchon AP, Warren WH Jr. 2002. A visual equalization strategy for locomotor control: of honeybees, robots, and humans. Psychol. Sci. 13, 272-278. ( 10.1111/1467-9280.00450) [DOI] [PubMed] [Google Scholar]
- 13.Heran H, Lindauer M. 1963. Windkompensation und seitenwindkorrektur der bienen beim flug über wasser. Zeitschrift für vergleichende Physiologie 47, 39-55. ( 10.1007/BF00342890) [DOI] [Google Scholar]
- 14.Serres JR, Morice AHP, Blary C, Montagne G, Ruffier F. 2019. Honeybees flying over a mirror crash irremediably. In: 4th Int. Conf. on Invertebrate Vision (ICIV), Bäckaskog, Sweden, pp. 260. See https://www.youtube.com/watch?v=KH9z8eqOBbU. [Google Scholar]
- 15.Straw AD, Lee S, Dickinson MH. 2010. Visual control of altitude in flying Drosophila. Curr. Biol. 20, 1550-1556. ( 10.1016/j.cub.2010.07.025) [DOI] [PubMed] [Google Scholar]
- 16.Berger Dauxère A, Serres JR, Montagne G. 2021. Ecological entomology: how is Gibson's framework useful? Insects 12, 1075. ( 10.3390/insects12121075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altshuler DL, Srinivasan MV. 2018. Comparison of visually guided flight in insects and birds. Front. Neurosci. 12, 157. ( 10.3389/fnins.2018.00157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serres JR, Evans TJ, Åkesson S, Duriez O, Shamoun-Baranes J, Ruffier F, Hedenström A. 2019. Optic flow cues help explain altitude control over sea in freely flying gulls. J. R. Soc. Interface 16, 20190486. ( 10.1098/rsif.2019.0486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serres JR, Morice AHP, Blary C, Miot R, Montagne G, Ruffier F. 2022. Floor and ceiling mirror configurations to study altitude control in honeybees. FigShare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Serres JR, Morice AHP, Blary C, Miot R, Montagne G, Ruffier F. 2022. Floor and ceiling mirror configurations to study altitude control in honeybees. FigShare. [DOI] [PMC free article] [PubMed]
Data Availability Statement
The data that support the findings of this study are openly available in the electronic supplementary material section at https://www.biorxiv.org/content/biorxiv/early/2021/10/07/2021.09.23.461476/DC1/embed/media-1.zip?download=true.
The data are provided in the electronic supplementary material [19].