Abstract
Interstitial ciprofloxacin concentrations were measured by microdialysis in inflamed foot lesions of non-insulin-dependent diabetes mellitus patients following intravenous administration of 0.2 g of ciprofloxacin. Interstitial ciprofloxacin concentrations were significantly lower than corresponding serum concentrations. There was no significant difference in the penetration of ciprofloxacin into inflamed and unaffected tissue (area under the concentration-time curveinfection/area under the concentration-time curveunaffected tissue = 0.99 ± 0.15 [mean ± standard error], n = 6). Thus, inflammation appears to have little or no effect on the penetration of ciprofloxacin into tissue.
Bacterial foot infections are frequent and serious complications in patients with diabetes mellitus (19) and account for more in-hospital days than any other complication of diabetes mellitus (8). The importance of diabetic foot infections is further underlined by the fact that the necessity of amputations is often determined by the extent of inflammation (12). Thus, immediate antimicrobial chemotherapy is of crucial importance in patients with inflamed diabetic foot ulcers. In some cases, however, empiric therapy fails to be effective, despite documented in vitro susceptibility of the causative pathogen (6).
One of the antibiotic agents that is most frequently employed for diabetic foot infections is ciprofloxacin (19), with total tissue concentrations exceeding corresponding serum concentrations (5, 13). Measurement of tissue concentrations in tissue homogenates (3, 5, 7) or from skin blisters (18, 24), however, may be misleading, since the unbound, interstitial drug concentration which exerts antibacterial activity (11, 15, 20) may be substantially lower than the total tissue concentration (2, 17). Subinhibitory effect site concentrations may, thus, provide an explanation for those cases in which ciprofloxacin failed to eradicate the relevant pathogen, despite documented in vitro susceptibility (6). Moreover, an impaired target site penetration may gain particular importance if the infecting pathogens are located in poorly accessible peripheral sites or if drug penetration is hampered by interstitial diffusion barriers which may develop during inflammatory processes (20, 21).
In the present study, we aimed at measuring ciprofloxacin concentrations in infected diabetic foot ulcers by microdialysis (14, 16, 17), an innovative clinical technique, which allows for the exclusive measurement of unbound drug concentrations in the interstitial space, the relevant target compartment for antimicrobial chemotherapy (20).
The study was approved by the local ethics committee. All patients were given a detailed description of the study, and their written consent was obtained. The study population included six patients (one female, five males) with non-insulin-dependent diabetes mellitus, aged 72 ± 6 years (mean ± standard error [SE]) (weight, 70 ± 4 kg; height, 166 ± 3 cm), who presented with a diabetic foot infection severe enough to require hospital admission and parenteral antibiotic therapy.
To measure interstitial ciprofloxacin concentrations, we employed microdialysis as described previously (2, 17). Two microdialysis probes (CMA 10; CMA, Stockholm, Sweden) were inserted, one into the inflamed lesion close to the border of the primary necrosis and a reference probe into unaffected subcutaneous adipose tissue of the ipsilateral extremity, as described previously (16, 17). Following an in-vivo calibration period (17, 22), ciprofloxacin (Ciproxin; Bayer, Leverkusen, Germany) was administered as a single intravenous (i.v.) dose of 200 mg over 20 min. Ciprofloxacin concentrations in the samples were analyzed by a published high-perfusion liquid chromatography method (10).
All data are presented as means ± SE. Data were analyzed by using model-independent equations with a commercially available computer program (Kinetica; Micropharm International, Champs sur Marne, France). An a priori sample size calculation was performed according to the equation published by Stolley and Strom (23), based on a priori assumptions of α = 0.05 and β = 0.20 and an intraindividual coefficient of variation of 10% for interstitial concentration measurements (14, 16). Thus, a study with six patients had the statistical power to detect a 16% intraindividual difference in area under the concentration-time curves (AUCs). For correlations or comparisons between pharmacokinetic parameters of different compartments, Spearman rank-order correlations (rs) or Mann-Whitney U tests, respectively, were employed, as pharmacokinetic parameters were nonnormally distributed. P < 0.05 was considered the level of significance.
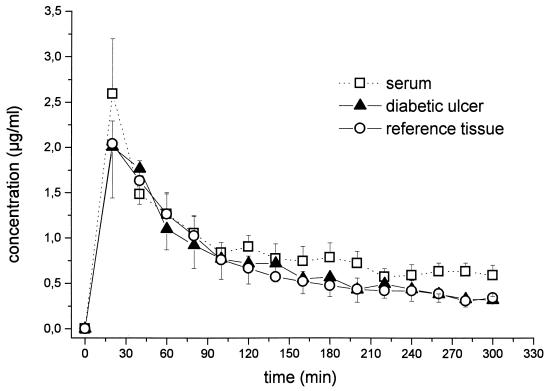
The time versus concentration profiles for ciprofloxacin in serum and in the interstitial space fluid of diabetic ulcer lesions and an unaffected reference tissue, i.e., subcutaneous adipose tissue, are shown in Fig. 1. Pharmacokinetic parameters are given in Table 1. Half-life at β phase for the serum compartment was 309 ± 121 min. There was a significant correlation between AUClesion and AUCserum (rs = 0.94, P = 0.005), with a mean AUClesion/AUCserum ratio of 0.78 ± 0.08 (range, 0.56 to 1.09). The mean AUCreference/AUCserum ratio was 0.82 ± 0.08 (range, 0.51 to 0.95; rs = 0.83, P = 0.041), and the mean AUClesion/AUCreference ratio was 0.99 ± 0.15 (range, 0.61 to 1.69; rs = 0.94; P = 0.005). There was no significant difference between AUClesion and AUCreference.
FIG. 1.
Time versus ciprofloxacin concentration profiles for serum and interstitial space fluid of inflamed diabetic foot ulcers and unaffected subcutaneous adipose tissue following administration of ciprofloxacin to patients with non-insulin-dependent diabetes mellitus (single i.v. dose of 200 mg over 20 min; n = 6). Results are presented as means ± SE. Time of administration, 0 to 20 min.
TABLE 1.
Pharmacokinetic parameters for serum and interstitial space fluid of inflamed diabetic foot lesions and unaffected subcutaneous adipose tissue following administration of ciprofloxacin to patients with non-insulin-dependent diabetes mellitusa
| Sample | Mean ± SDb
|
||
|---|---|---|---|
| Cmax | Tmax | AUC0–∞, AUC0–300 | |
| Serum | 2.83 ± 1.21 | 23 ± 8 | 527 ± 318, 253 ± 102 |
| Diabetic ulcer | 2.18 ± 1.13 | 30 ± 11 | 278 ± 182, 209 ± 121 |
| Healthy subcutis | 2.12 ± 0.55 | 23 ± 8 | 317 ± 207, 199 ± 75 |
Patients (n = 6) were given a single 200-mg dose i.v. over 20 min.
Cmax (micrograms/milliliter), maximum concentration of drug in serum; Tmax (minutes), time to maximum concentration of drug in serum; AUC0–∞ (micrograms · minute/milliliter), area under the concentration-time curve from 0 h to infinity.
Our main objective in the present study was to assess the effect of severe tissue inflammation on the penetration of drug into tissue. The rationale for this study was derived from various reports describing poor clinical outcome in up to 30% of patients suffering from soft-tissue infections (1), which were treated with ciprofloxacin, a drug of choice in polymicrobial soft-tissue infections (19). Some of these cases could be attributed to the persistence of nonsusceptible pathogens or the development of resistance (6, 13). However, in the remainder of cases, ciprofloxacin failed to be effective, despite documented in vitro susceptibility of the causative pathogen. One plausible explanation for these therapeutic failures may be an impaired penetration of ciprofloxacin to the infected target site.
In our experiments, interstitial ciprofloxacin concentrations were significantly lower than corresponding serum concentrations. This result is in line with previous reports on ciprofloxacin (2) and other antimicrobial agents (16, 17) showing that total serum concentrations and serum-derived pharmakokinetic surrogate parameters (9) may not necessarily reflect target site concentrations. The main finding of the present study, however, was that interstitial ciprofloxacin concentrations at the site of inflammation did not differ significantly from concentrations attained at an unaffected site. This does not support the hypothesis that the process of drug distribution may be significantly impaired by the development of diffusion barriers in inflamed tissue (20, 21). Our findings are in contrast to previous reports, mostly on whole-tissue biopsies, showing that ciprofloxacin may preferentially distribute via phagocytes to inflamed sites (4, 7), thereby reaching concentrations at the target site which are severalfold higher than those in the serum (13).
Although our small sample size precludes a detailed analysis, it is noteworthy that an unfavorable outcome, i.e., the requirement of a surgical intervention, was observed in our study in two of three cases (data not shown) in which an isolate was cultured that was not susceptible to ciprofloxacin. Thus, it may be speculated that, at least under the present conditions, biological properties of the relevant bacterial isolate may contribute more to overall clinical outcome than an altered penetration of the antibiotic agent to the target site.
In conclusion, the results of the present study demonstrate that inflammation has little or no effect on target site concentrations of ciprofloxacin which are in the range of free serum concentrations.
Acknowledgments
This work was supported by a grant (no. P 12659-MED) from the FWF, the Austrian Science Fund (Fonds zur Förderung der Wissenschaftlichen Forschung).
We are grateful to Edith Lackner and Ines Hertling for their contributions.
REFERENCES
- 1.Arcieri G, August R, Becker N, Doyle C, Griffith E, Gruenwaldt G, Heyd A, O’Brien B. Clinical experience with ciprofloxacin in the USA. Eur J Clin Microbiol. 1986;5:220–225. doi: 10.1007/BF02013994. [DOI] [PubMed] [Google Scholar]
- 2.Brunner M, Hollenstein U, Delacher S, Jäger D, Schmid R, Lackner E, Georgopoulos A, Eichler H G, Müller M. Distribution and antimicrobial activity of ciprofloxacin in human soft tissues. Antimicrob Agents Chemother. 1999;43:1307–1309. doi: 10.1128/aac.43.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgmann H, Georgopoulos A, Graninger W, Koppensteiner R, Maca T, Minar E, Schneider B, Stümpflen A, Ehringer H. Tissue concentration of clindamycin and gentamicin near ischaemic ulcers with transvenous injection in Bier’s arterial arrest. Lancet. 1996;348:781–783. doi: 10.1016/S0140-6736(96)07089-4. [DOI] [PubMed] [Google Scholar]
- 4.Capecchi P L, Blardi P, De Lalla A, Ceccatelli L, Volpi L, Pasini F L, Di Perri T. Pharmacokinetics and pharmacodynamics of neutrophil-associated ciprofloxacin in humans. Clin Pharmacol Ther. 1995;57:446–454. doi: 10.1016/0009-9236(95)90215-5. [DOI] [PubMed] [Google Scholar]
- 5.Dan M, Torossian K, Weissberg D, Kitzes R. The penetration of ciprofloxacin into bronchial mucosa, lung parenchyma, and pleural tissue after intravenous administration. Eur J Clin Pharmacol. 1993;44:101–102. doi: 10.1007/BF00315290. [DOI] [PubMed] [Google Scholar]
- 6.Fass R J. Treatment of skin and soft tissue infections with oral ciprofloxacin. J Antimicrob Chemother. 1986;18(Suppl. D):153–157. doi: 10.1093/jac/18.supplement_d.153. [DOI] [PubMed] [Google Scholar]
- 7.Fong I W, Ledbetter W H, Vandenbroucke A C, Simbul M, Rahm V. Ciprofloxacin concentrations in bone and muscle after oral dosing. Antimicrob Agents Chemother. 1986;29:405–408. doi: 10.1128/aac.29.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibons G W, Eliopoulous G M. Infection of the diabetic foot. In: Kozak G P, Campbell D, Hoar C S, editors. Management of diabetic foot problems. W. B. New York, N.Y: Saunders; 1984. pp. 97–102. [Google Scholar]
- 9.Hyatt J M, McKinnon P S, Zimmer J S, Schentag J J. The importance of pharmacokinetic/pharmacodynamic surrogate markers to outcome. Clin Pharmacokinet. 1995;28:143–160. doi: 10.2165/00003088-199528020-00005. [DOI] [PubMed] [Google Scholar]
- 10.Krol G J, Beck G W, Bentham T. HPLC analysis of ciprofloxacin and ciprofloxacin metabolites in body fluids. J Pharm Biomed Anal. 1995;14:181–190. doi: 10.1016/0731-7085(95)01611-2. [DOI] [PubMed] [Google Scholar]
- 11.Kunin C M, Craig W A, Kornguth M, Monson R. Influence of binding on the pharmacologic activity of antibiotics. Ann N Y Acad Sci. 1973;26:214–224. doi: 10.1111/j.1749-6632.1973.tb20483.x. [DOI] [PubMed] [Google Scholar]
- 12.Lassen N A. General discussion on occlusive arterial disease in diabetes mellitus. Scand J Clin Lab Investig. 1973;128(Suppl.):235–237. [PubMed] [Google Scholar]
- 13.Licitra C M, Brooks R G, Sieger B E. Clinical efficacy and levels of ciprofloxacin in tissue in patients with soft tissue infection. Antimicrob Agents Chemother. 1987;31:805–807. doi: 10.1128/aac.31.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lönnroth P, Jansson P A, Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol (Endocrinol Metab) 1987;16:E228–E231. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- 15.Merrikin D J, Briant J, Rolinson G N. Effect of protein binding on antibiotic activity in vivo. J Antimicrob Chemother. 1983;11:233–238. doi: 10.1093/jac/11.3.233. [DOI] [PubMed] [Google Scholar]
- 16.Müller M, Schmid R, Georgopoulos A, Buxbaum A, Wasicek C, Eichler H G. Application of microdialysis to clinical pharmacokinetics in humans. Clin Pharmacol Ther. 1995;57:371–380. doi: 10.1016/0009-9236(95)90205-8. [DOI] [PubMed] [Google Scholar]
- 17.Müller M, Haag O, Burgdorff T, Georgopoulos A, Weninger W, Jansen B, Stanek G, Agneter E, Pehamberger H, Eichler H G. Characterization of peripheral compartment kinetics of antibiotics by in vivo microdialysis in humans. Antimicrob Agents Chemother. 1996;40:2703–2709. doi: 10.1128/aac.40.12.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller M, Brunner M, Schmid R, Putz E M, Schmiedberger A, Wallner I, Eichler H G. Comparison of three different experimental methods for the assessment of peripheral compartment pharmacokinetics in humans. Life Sci. 1998;62:PL227–PL234. doi: 10.1016/s0024-3205(98)00071-x. [DOI] [PubMed] [Google Scholar]
- 19.Peterson L R, Lissack L M, Canter K, Fasching C E, Clabots C, Gerding D N. Therapy of lower extremity infections with ciprofloxacin in patients with diabetes mellitus, peripheral vascular disease, or both. Am J Med. 1989;86:801–808. doi: 10.1016/0002-9343(89)90476-2. [DOI] [PubMed] [Google Scholar]
- 20.Ryan D M, Cars O, Hoffstedt B. The use of antibiotic serum levels to predict concentrations in tissues. Scand J Infect Dis. 1986;18:381–388. doi: 10.3109/00365548609032352. [DOI] [PubMed] [Google Scholar]
- 21.Seabrook G R, Edmiston C E, Schmitt D D, Krepel C, Bandyk D F, Towne J B. Comparison of serum and tissue antibiotic levels in diabetes-related foot infections. Surgery. 1991;110:671–676. [PubMed] [Google Scholar]
- 22.Stahle L, Arner P, Ungerstedt U. Drug distribution studies with microdialysis. III. Extracellular concentration of caffeine in adipose tissue in man. Life Sci. 1991;49:1853–1858. doi: 10.1016/0024-3205(91)90488-w. [DOI] [PubMed] [Google Scholar]
- 23.Stolley P D, Strom B L. Sample size calculations for clinical pharmacology studies. Clin Pharmacol Ther. 1986;39:489–490. doi: 10.1038/clpt.1986.85. [DOI] [PubMed] [Google Scholar]
- 24.Wise R, Donovan I A. Tissue penetration and metabolism of ciprofloxacin. Am J Med. 1987;82:103–107. [PubMed] [Google Scholar]