Abstract
Following the modern synthesis, mating signals were thought of principally as species recognition traits, a view later challenged by a burgeoning interest in sexual selection—specifically mate choice. In the 1990s, these different signal functions were proposed to represent a single process driven by the shape of female preference functions across both intra- and interspecific signal space. However, the properties of reliable ‘recognition’ signals (stereotyped; low intraspecific variation) and informative ‘quality’ signals (condition dependent; high intraspecific variation) seem at odds, perhaps favouring different signal components for different functions. Surprisingly, the idea that different components of mating signals are evaluated in series, first to recognize generally compatible mates and then to select for quality, has never been explicitly tested. Here I evaluate patterns of (i) intraspecific signal variation, (ii) female preference function shape and (iii) phylogenetic signal for male cricket call components known to be processed in series. The results show that signal components processed first tend to have low variation, closed preference functions and low phylogenetic signal, whereas signal components processed later show the opposite, suggesting that mating signal evaluation follows an ‘order-of-operations'. Applicability of this finding to diverse groups of organisms and sensory modalities is discussed.
Keywords: preference functions, phylogenetic signal, Gryllus, crickets
1. Introduction
Over the past approximately 50 years, there has been an enormous resurgence of interest in sexual selection and mating signal evolution. During that time, two patterns have become firmly established in the literature: (i) mating signals are typically species-specific and (ii) components of such displays are typically highly exaggerated and often exceed what would seem necessary if sexual signalling favoured simple efficiency of communication. These two features led some authors to suppose that sexual signals serve dual functions: species recognition and sexual selection [1,2]. As early as 1977, Popov & Shuvalov distinguished between ‘essential recognition features' and ‘motivational features’ in acoustic signals [3]; this idea was formalized and extended by Gerhardt's characterization of signals as ‘static’ or ‘dynamic’ [4] in which static traits have low variability, and so could serve as indicators of species identity, whereas dynamic traits have high variability, and so could reveal signaller motivation or quality (see also [5]). Logically this suggested that mating signals could be assessed by receivers in a hierarchy: potential mates were those individuals recognized as conspecific via species recognition traits; high-value mates were the subset of potential mates who had extreme values of dynamic, often condition-dependent, signal traits.
Since that time, both the hierarchical nature of mate assessment and the very concept of ‘species recognition’ have been challenged. In a series of influential papers, Ryan and colleagues have argued that there is no duality of species recognition and mate assessment; rather mate discrimination is a single unified process in which mating signals are evaluated by preference functions spanning both intra- and interspecific variation [6–8]. Their arguments are bolstered by compelling experiments with the Túngara frog, which show that conspecific preference via a supposed ‘species recognition’ signal element, the whine, can be reversed via the addition of an attractive second signal element, the chuck, appended to the whine (see especially [6]). Mendelson & Shaw [9] argue that ‘species recognition’ is too poorly defined to have conceptual utility, that researchers should not conflate taxonomic identity with genetic mate compatibility (at least not too narrowly), and, most germane here, that there is no evidence that animals process mating signals in a sequential manner, from ‘species’ to ‘quality'. They go on to write (p. 423), ‘whether compatibility indicators are processed first in a sequence, or even afforded the greatest weight among potentially multiple indicators of quality in a complex signal, is an intriguing hypothesis that has not, to the best of our knowledge, been empirically tested’.
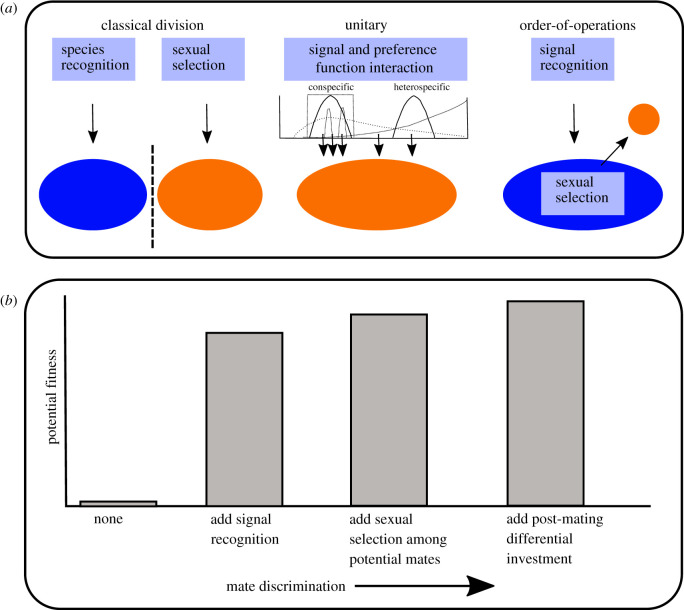
This last point forms the basis for this study: testing whether or not mating signals and receiver signal processing operate in series, first to assess broad genetic compatibility at a scale consistent with conspecific identity (signal recognition), and second to assess mate quality from among the recognized subset of broadly compatible potential mates (mate choice). I refer to such a processing scheme as ‘serial’ processing to indicate the priority of information, which could result from a literal temporally separated series, or from neural gating such that ‘attractiveness’ information is only processed if accompanied by ‘recognition’ input (e.g. via disinhibition; see [10], fig. 1a3). I refer to this as the order-of-operations model of sexual signal processing (figure 1). The order-of-operations model recognizes a simple and obvious truth: the typical marginal fitness gain from mating with a conspecific, compared to indiscriminate attempted mating, greatly exceeds the typical marginal fitness gain from discrimination among conspecifics. Indiscriminate attempts at reproduction do occur (e.g. wind pollination [11] or broadcast spawning [12]), but among animals with mating signals, the potential fitness gained from accurate signal recognition is best illustrated by cases in which correct recognition of generally compatible potential mates fails. This can occur in several ways, such as via sexual attraction to abiotic items (e.g. male Julodimorpha bakewelli beetles copulating with beer bottles [13]) or sexual attraction to biotic but entirely non-compatible organisms (e.g. males of several species of Hymenoptera are attracted to sexually deceptive orchids; reviewed by [14]), or even fatal sexual attraction to predators (e.g. aggressive sexual mimicry by female Photuris fireflies resulting in death for duped male Photinus fireflies [15], or males of several species of cicadas lured to death by the predatory katydid Chlorobalius leucoviridis, which mimics the replies of sexually receptive female cicadas [16]). In general, genetically compatible potential mates will be conspecifics, despite some well-known examples of adaptive hybridization (e.g. [17]). The first step in the continuum of mate discrimination should therefore be compatible mate recognition (sensu Ryan & Rand [7], p. 648), followed by expression of mate preference (i.e. choice) among recognized potential mates, sometimes followed by post-mating differential investment [18,19], which could include post-copulatory elimination of unsuitable mates.
Figure 1.
Conceptual models of mate discrimination; (a) shows process in pale rectangles leading to either a pool of recognized potential mates (blue ellipses) or preferred mates (orange ellipses); (b) shows potential fitness as a function of increasing investment in mate discrimination. Total fitness increases with each added level of mate discrimination, but the marginal fitness gain from signal recognition (i.e. selection of a generally compatible mate) far exceeds the marginal fitness gain from sexual selection among recognized potential mates. (Online version in colour.)
Serial processing of mating signals is a prerequisite condition of this model, but on its own does not demonstrate order-of-operations. Rather the critical feature is that the signal properties given first priority are those likely to be strongly correlated with species identity, whereas those evaluated secondarily are those likely to be correlated with intra-specific attractiveness. Thus, in addition to serial processing of signal components, the order-of-operations model makes a number of inter-related and testable predictions. We would expect that signal components processed first (i.e. ‘recognition traits’) (i) will be ‘static’ traits with low among individual intra-specific variation, (ii) will be subject to closed preference functions characteristic of a band pass filter [20,21] and (iii) will often change during speciation such that resulting phylogenetic signal may be low [22,23], especially so if the taxa are sympatric. Conversely signal components processed secondarily (iv) will be ‘dynamic’ traits with relatively high among individual intra-specific variation, (v) will be subject to open preference functions characteristic of a high pass filter and (vi) may be resistant to changes during speciation, and so may track phylogeny such that resulting phylogenetic signal may be high. In this paper, I examine sexual signalling in field crickets to address each of these predictions and then more broadly discuss applicability of the order-of-operations model to other classic examples of mate choice.
(a) . Serial processing: acoustic communication and signal recognition in crickets
Communication in field crickets has been extensively studied at both proximate and ultimate levels [24–27]. Adult male field crickets produce loud broadcast calls to attract sexually receptive females from a distance [28]. Those calls are species-specific, at least within a regional fauna [29–31]. Serial processing of mating signal components is central to the order-of-operations model; in the case of crickets at least, temporally separated serial processing is well established empirically [27,32,33]. Mechanistically, female crickets recognize male calls using a series of sensory and neural filters at both the peripheral and central auditory systems [27]. Frequency is filtered first, both mechanically by the peripheral auditory system (tympanal tuning) and by best frequency tuning of the auditory omega neurons (ON1) and of the ascending auditory neuron (AN1). Importantly AN1 is the only auditory afferent neuron which carries temporal calling song information to the brain [33], therefore serial encoding is strictly enforced—no alternate parallel pathway for acoustic calling song signal integration exists. The combination of tympanal and neural frequency filtering results in a band pass filter such that (at relevant amplitudes) only sound pulses of the correct frequency become temporally encoded within AN1 and are passed to the brain where the pulse pattern is next evaluated.
The pulse pattern recognition filter of the cricket brain is also well characterized, at least in Gryllus bimaculatus [34,35]. Pulse rate recognition is via a neural network of AN1 and four local brain neurons (LN2 – LN5) which constitute a delay line (LN2 + LN5) and a coincidence detector (LN3). If the direct input to LN3 via AN1 (from sound pulse 2) matches the delay line input to LN3 via AN1 + LN2 + LN5 (from delayed sound pulse 1), then LN3 neural spiking is sufficient to overcome inhibition of LN4, resulting in LN4 spiking; LN4 spiking matches the behavioural phonotaxis of female crickets in response to pulse rate variation [36]. Although this network has been characterized only in G. bimaculatus, computational modelling reveals that such a network could produce the full range of pulse pattern recognition known in crickets [37]. Thus, in crickets, the essential signal recognition features are frequency followed by pulse pattern (pulse rate or duty cycle) on a short time scale (less than 100 ms).
In crickets, pulses are typically arranged in groups, variously called ‘chirps’ or ‘trills’ on a longer time scale (greater than 100 ms). How female crickets evaluate the signal information content on the longer chirp time scale is less well understood than it is at the pulse time scale. Nonetheless, it appears that, given the correct frequency and pulse pattern, the output of pulse pattern recognition is integrated over time for stimulus power proportional to either number of pulses (with a purely differentiating filter) or total energy (with a purely integrating filter) [38,39]. That is, preference functions on the longer chirp time scale may not be the result of a separate neural filter, rather they may be an emergent property from integrating over time the output of the short pulse time scale filter.
Given serial processing of frequency → pulse pattern → chirp-scale energy, the order-of-operations model then predicts that frequency, pulse rate and pulse duty cycle processed first would show low coefficients of variation (SD/mean, hereafter CV), and closed preference functions corresponding to band pass filters and low phylogenetic signal, whereas chirp-scale features (pulses per chirp, chirp rate and chirp duty cycle) would show higher CVs and open preference functions corresponding to high pass filters and higher phylogenetic signal. Recent taxonomic [31] and phylogenetic [40] work provides the context for this study. For the first time, it is now possible to analyse signal evolution in the entire fauna of Gryllus crickets found in the United States and Canada, and make generalizable conclusions about signal recognition and mate choice. In this region, Weissman & Gray [31] recognize 35 named species and an additional six genetically distinct lineages of Gryllus crickets supported by multi-locus DNA (electronic supplementary material, figure S1). Two of the species do not call (Gryllus ovisopis [41,42] and G. cayensis [43]), but the calls of all other species and genetic lineages were analysed to characterize CVs and phylogenetic signal of the different call features.
2. Methods
For each species, I used Audacity software (audacityteam.org) to digitally analyse five exemplars of each call trait from each of five wild-caught male crickets recorded in the laboratory at close to 25°C (mean ± s.d. = 24.78 ± 0.86). Within this range, temperature effects on song are expected to be minimal [44]. Controlling for recording temperature was prioritized over controlling for the population of origin. Song traits analysed were dominant frequency (FFT size 1024), pulse duration, inter-pulse duration, number of pulses per chirp (or trill) and inter-chirp (or trill) interval. I calculated five measures of pulse rate and duty cycle from the five measures of pulse and inter-pulse durations per individual [rate = 1/(pulse + inter-pulse); duty cycle = pulse/(pulse + inter-pulse)]. Similarly, five measures of chirp rate and duty cycle were calculated from the five measures of chirp and inter-chirp interval durations per individual, with chirp duration = [(number of pulses per chirp × pulse duration) + (number of pulses per chirp – 1 × inter-pulse duration)]. For each trait, the five measurements per individual were averaged, and then, for each species, the five individuals' means were used to calculate species’ means, s.d. and CVs (as species s.d. divided by species mean) and used in subsequent analyses. Five individuals per species is a relatively small sample size for calculating species' means, s.d. and CVs; however, error is unlikely to be biased with respect to the comparison of CVs of different types of traits. The CVs for traits processed first (frequency, pulse rate and pulse duty cycle) were compared to the CVs for song traits processed later (pulses per chirp, chirp rate and chirp duty cycle) via nested ANOVA in SPSS v. 25 with traits nested within trait types.
Preference functions of female-field crickets (Gryllinae) were collected from the literature and summarized as ‘open’ or ‘closed’ [45,46]. Preference function data were collected for frequency, pulse rate, pulse duty cycle, number of pulses per chirp, chirp rate and chirp duty cycle. Some studies present data on rates per se while others report data on periods, but these are interchangeable as rate = 1/period.
I used the R package phylosignal [47], the species' mean trait values and the species-level phylogeny [40] to test for overall phylogenetic signal and to assess how phylogenetic autocorrelation varies with phylogenetic distance. Phylogenetic autocorrelation was assessed with two of the five metrics available in phylosignal: Moran's I and Abouheif's Cmean, because they are both based on an autocorrelation approach and do not assume a particular (i.e. Brownian motion) model of evolution [48]. In addition, local Moran's I was used to identify ‘hotspots’ of locally high values of positive or negative autocorrelation for traits which showed overall significant phylogenetic signal [47].
3. Results
Summarized song data for each species are available in the electronic supplemental material, appendix A1.
(a) . Patterns of variation
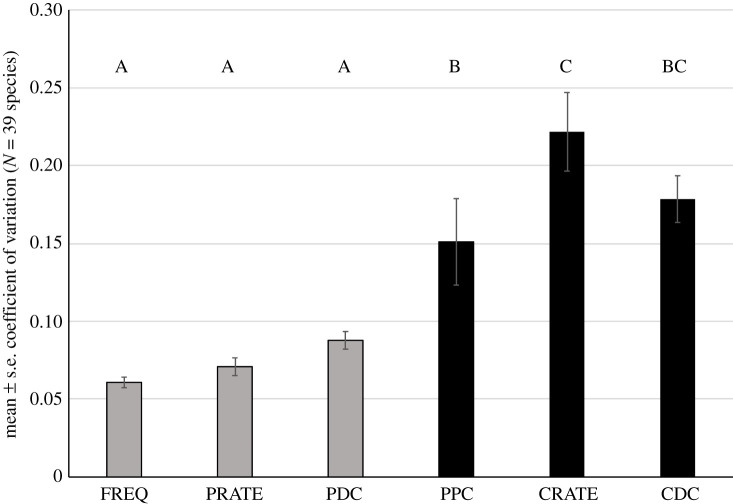
The pulse-scale signal traits processed first had lower CVs than did the chirp-scale signal traits processed later (n = 39 species or lineages, ANOVA with traits nested within trait type: trait type F1,4 = 64.383, p < 0.001). The magnitude of the difference was roughly 2 – 3 × higher CVs for the chirp-scale traits than for the pulse-scale traits (figure 2).
Figure 2.
Mean ± s.e. within-species variation in cricket call components, measured as coefficient of variation for 39 Gryllus species or genetic lineages. Call traits with grey bars are shorter time scale features of pulses: dominant frequency (FREQ), pulse rate (PRATE) and pulse duty cycle (PDC), which are evaluated first by females; those with black bars are longer time scale features of groups of pulses: pulses per chirp (PPC), chirp rate (CRATE) and chirp duty cycle (CDC) which are evaluated second. Traits with the same letter above the bars are not significantly different from each other.
(b) . Preference functions
A review of the literature on female preference functions in Gryllinae species (table 1) revealed that pulse-scale signal traits processed first are overwhelmingly subject to closed preference functions (frequency: 14 closed, 0 open; pulse rate: 16 closed, 1 open; pulse duty cycle: 11 closed, 3 open), whereas chirp-scale signal traits processed later are typically subject to open preference functions (pulses per chirp: 3 closed, 9 open; chirp rate: 3 closed, 7 open; chirp duty cycle: 0 closed, 12 open).
Table 1.
Summary of the literature that has addressed the shape or form of female preference functions for different parameters of male song in field crickets (Gryllinae). Numbers in the table are citations of individual studies which provide support for a particular type of preference function. Preferences are listed as either ‘closed’ or ‘open’, which correspond to a narrow band pass filter or a directional high pass filter, respectively.
| species | frequency |
pulse rate |
pulse duty cycle |
pulses per chirp |
chirp rate |
chirp duty cycle |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| closed | open | closed | open | closed | open | closed | open | closed | open | closed | open | |
| Acheta domesticus | [3,49] | [49,50] | [49]a | [49] | [51] | [49] | [51]b, [52] | |||||
| Gryllus bimaculatus | [3,53] | [54,55] | [55] | [53,56,57] | [56] | |||||||
| Melanogryllus desertus | [3] | |||||||||||
| Gryllus rubens | [58] | [58–60] | [58] | [58] | [58] | |||||||
| Gryllus texensis | [58] | [58,60,61] | [58] | [62,63] | [58,64] | [58] | ||||||
| Gryllus regularis [=G14] | [58] | [58] | [58] | [58] | [58] | |||||||
| Gryllus campestris | [65] | [65] | [65] | |||||||||
| Gryllus locorojo | [66]f | [66] | [66] | [66] | ||||||||
| Teleogryllus commodus | [67,68] | [67,68] | [67] | |||||||||
| Teleogryllus oceanicus | [67,68] | [67,68] | [67] | |||||||||
| Teleogryllus leo | [69]c | [69] | [69]e | [69]e | [69] | |||||||
| Gryllus firmus | [70] | [60,70,71] | [70] | [70] | [70] | |||||||
| Gryllus assimilis | [72] | [72] | ||||||||||
| Gryllus longicercus [=G13] | [70] | [70] | [70] | [70] | [70]a | |||||||
| Gryllus personatus | [26] | [26,60] | [26] | [26] | [26] | |||||||
| Gryllus lineaticeps | [26] | [26] | [26] | [73] | [26,73,74] | [26] | ||||||
| Gryllus staccato [=G15] | [26] | [60]c, [26] | [26] | [26] | [26] | |||||||
| Gryllus integerd | [75] | [75] | [75] | [76] | ||||||||
aDuty cycle tested indirectly, as function of durations and pauses.
bAt p < 0.06.
cBand pass but with broad tolerances.
dG. integer is a ‘stutter-trilling’ species, meaning that a series of chirps are concatenated into a trill, longer ‘trill’ durations [76] and fewer pulses per chirp and slower pulse rates [75] were favoured by females.
eDirectional favouring faster chirp rate if chirp duty cycle = 0.42 but stabilizing if chirp duration = 210 ms.
fWith typical two pulse chirps ([66], fig. 1F).
(c) . Phylogenetic signal
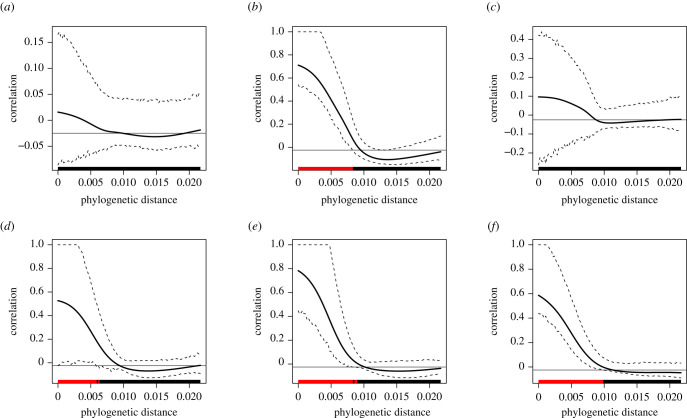
Two of the three pulse-scale signal traits, frequency and pulse duty cycle, showed no phylogenetic signal, whereas pulse rate and all three chirp-scale signal traits did show significant phylogenetic signal (table 2 and figure 3). Evaluation of local Moran's I is presented in electronic supplementary material, figures S2–S4. It revealed that the significant phylogenetic signal for pulse rate (electronic supplementary material, figure S2) derives from two transitions to faster pulse rates, one ancestral to the stutter-trillers (G. integer + G. armatus) and trillers (G. texensis + G. rubens + G. regularis) and one ancestral to (G. personatus + G. staccato + G. lineaticeps), plus one transition to slower pulse rates (G. vulcanus + G. longicercus); notably these taxa are either allopatric [31,77] or divergent in pulse rates at the species level [58,78].
Table 2.
Results of tests of phylogenetic signal in cricket song components.
| trait | Abouheif's Cmean | Moran's I |
|---|---|---|
| frequency | −0.003, p = 0.316 | −0.022, p = 0.337 |
| pulse rate | 0.479, p < 0.001 | 0.064, p < 0.001 |
| pulse duty cycle | 0.062, p = 0.126 | −0.008, p = 0.065 |
| pulses per chirp | 0.333, p < 0.002 | 0.014, p < 0.004 |
| chirp rate | 0.392, p < 0.007 | 0.011, p < 0.015 |
| chirp duty cycle | 0.449, p < 0.001 | 0.006, p < 0.027 |
Figure 3.
Phylocorrelograms indicating phylogenetic autocorrelation as a function of phylogenetic distance for each of the song traits. The solid black line is Moran's I autocorrelation index, with dashed lines indicating 95% confidence envelope. Significantly positive regions of autocorrelation are indicated in red. (a) Frequency, (b) pulse rate, (c) pulse duty cycle, (d) pulses per chirp, (e) chirp rate and (f) chirp duty cycle. (Online version in colour.)
4. Discussion
As pertains to crickets, in which serial processing of signal components was already well established [27,33], these results strongly support that signal components processed first are likely to be good indicators of broad-scale genetic compatibility (i.e. species-specific), because they (i) are static traits with low CVs, (ii) are subject to stabilizing selection via closed preference functions, (iii) change sufficiently rapidly during speciation that they tend to have low phylogenetic signal; components processed later are more likely to indicate quality—they (iv) are dynamic traits which have high CVs, (v) are subject to directional mate preferences and (vi) track phylogeny, suggesting stability of quality indicating signals relative to speciation rates. These results support the order-of-operations model and seem general in that they apply to an entire clade of North American Gryllus. Studies of other cricket families or subfamilies also lend support: closed preference functions have been found for pulse rate or frequency in Laupala cerasina in Trigoniidae [79], Oecanthus nigricornis and O. forbesi in Oecanthinae [80,81], and Scapteriscus acletus in Gryllotalpidae [82]; similar to my results with Gryllus, dominant frequency in Lebinthini cricket calls showed no phylogenetic signal, but longer time scale song structure did [83].
The only result obtained in this study which is somewhat at odds with the order-of-operations model was the finding of a significant phylogenetic signal in pulse rate. Local Moran's I analysis (electronic supplementary material, figure S2) showed that the significant phylogenetic signal in pulse rate resulted from transitions ancestral to clades which are currently allopatric (G. integer + G. armatus), (G. personatus + G. staccato + G. lineaticeps), (G. vulcanus + G. longicercus) [31] or, if partially sympatric, are strongly divergent in pulse rate (G. texensis + G. rubens + G. regularis) [58,78]. Sympatry is expected to influence the degree of phylogenetic signal because signal will tend to deteriorate with rapid changes at speciation, but no such changes are required among allopatric taxa. An interesting example is the Australian psyllids studied by Percy et al. [84], in which species from one clade are distributed among different host plant species of Eucalyptus and so can be considered allopatric, whereas another clade co-occurs micro-sympatrically within a single host plant Allocasuarina verticellata. The allopatric clade showed a significant phylogenetic signal in mating calls whereas the sympatric clade showed no relationship. That is, in the micro-sympatric clade for which signal recognition is functionally related to species identity, phylogenetic signal was absent. Sympatry versus allopatry has also been shown to affect gains versus losses of male secondary sex organs in hairstreak butterflies in a manner consistent with a species-isolating function [85].
In addition to the effect of sympatry versus allopatry, the predictions based on phylogenetic signal also depend upon the pattern of evolutionary change. If evolutionary change in recognition traits is gradual and proportional to time, then continuous stabilizing selection from closed preference functions would favour slower evolution and higher phylogenetic signal—the opposite of my predictions and of the empirical findings. This apparent contradiction is resolved, however, if recognition traits evolve via a punctuated rather than a gradual model of evolution (i.e. with rapid and reversible changes during speciation followed by relative stasis). Similarly, if attractive quality indicating traits have condition-dependent expression, then phenotypic selection on the signal traits primarily results in genetic changes in ‘condition’ rather than change in the traits themselves [86] (i.e. attractive traits with condition-dependent expression may evolve slowly, despite constant directional selection, leading to higher phylogenetic signal).
How generalizable are results from crickets? Crickets may be the ideal test case for the order-of-operations model in that the male long-distance calling song is restricted to a single sensory modality, and female reception of that signal has detailed neurobiological evidence of serial processing. Multi-modal mating signals seem much more likely to be processed in parallel simply because each sensory input starts with different types of receptors. Serial processing of multi-modal signals could still occur, however, if there is temporal separation between modalities. For example male Chinavia stinkbugs initiate pair formation via release of species-specific pheromones which attract receptive conspecific females to the male's plant; females then emit species-specific vibratory signals via the plant substrate to which males respond with species-specific substrate-borne vibrations and mate searching [87]; once the pair meets, antennation and physical courtship may proceed to mating [88]. Thus, in these stinkbugs, mating involves (at least) three sensory modalities with serial progression from species-specific chemical, to species-specific vibrational, to non-species-specific tactile assessment. Nonetheless, some of the iconic examples of complex sexual signalling systems involve simultaneous multi-modal signalling. For example, lek displays of manakins (Aves: Pipridae) often involve dynamic complex dances and visual feather ornamentation combined with vocal and mechanically produced sounds [89,90]. Such a complex multi-modal display does not preclude serial processing however: female manakins evaluate potential mates at leks of conspecific males, not at beehives, fig trees or waterfalls; evaluation of a pool of potential mates at leks suggests prior evaluation of an appropriate time and place to select among generally compatible potential mates. In various taxa, leks are often advertised by broadcast calls (hummingbirds [91]; flycatchers [92]; peafowl [93]; manakins [94,95]; frogs [96,97]; birds-of-paradise [98]). Therefore, even lek mating systems might be interpreted as following order-of-operations in mate choice, with the elaborate multi-modal on-lek displays constituting only the second step of discrimination. It is worth noting that in manakins more complex display elements show greater phylogenetic signal ([89], p. 223), potentially consistent with the ideas presented here.
The potential generality of the order-of-operations model is extended even further by considering serial processing to encompass the priority of information in addition to a linear temporal series of processing steps. Perhaps because I study crickets, I developed my thoughts more narrowly, but during the review of this paper, the reviewers and editor persuaded me that organisms with more complex nervous systems could also prioritize information processing in a manner consistent with an order-of-operations. This is harder to demonstrate empirically, and I am not aware of any examples with detailed neurobiological support, but neural gating [10] is a plausible mechanism by which an ‘attractiveness’ circuit is inhibited unless a ‘recognition’ circuit actively disinhibits it. Such a mechanism could apply to either unimodal or multi-modal signal integration.
In a broad sense, whether or not ‘sexual selection’ and ‘species recognition’ are distinct processes or part of a single unified process [7] may well be semantic. The order-of-operations model suggests a single continuum of mate discrimination, but that continuum for most species will involve two ordered steps: recognition of generally compatible mates followed by selection among them. That will typically require multi-component mating signals with different properties (i.e. relatively stereotyped ‘static’ species-specific signals evaluated first by band pass closed preference functions, followed by relatively dynamic, perhaps condition-dependent, signals evaluated second by high pass open preference functions). It makes little fitness sense for organisms to be overwhelmed by ‘sexiness’ prior to establishing general compatibility; if they were, more would end up like the unfortunate Julodimorpha bakewelli beetles copulating with beer bottles [13]. If signal recognition is typically tightly coupled with species identity, then signal recognition will usually lead to conspecific mating opportunities. Nonetheless, ‘signal recognition’ and ‘species recognition’ are not functionally equivalent, and Mendelson & Shaw [9] are correct to caution against ‘species recognition’ terminology, for example juveniles and non-breeding individuals do not produce mating signals, so despite being conspecific, they attract no special interest. The idea of signal recognition followed by mate choice among recognized potential mates does represent a single unified process of mate discrimination, but one that is ordered in accordance with the greatest marginal fitness gains associated with incremental investment in mate discrimination.
Supplementary Material
Acknowledgements
Two reviewers and the editor provided very helpful comments which greatly improved this work; in particular the idea of neural gating enabling ‘serial’ priority of information was raised during review. I am also grateful to D. B. Weissman for access to many of the cricket recordings.
Data accessibility
The data are provided in the electronic supplementary material [99].
Authors' contributions
D.A.G.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, visualization, writing—original draft and writing—review and editing.
Competing interests
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Emlen ST. 1972. An experimental analysis of the parameters of bird song eliciting species recognition. Behaviour 41, 130-171. ( 10.1163/156853972X00248) [DOI] [Google Scholar]
- 3.Popov AV, Shuvalov VF. 1977. Phonotactic behavior of crickets. J. Comp. Physiol. A 119, 111-126. ( 10.1007/bf00655876) [DOI] [Google Scholar]
- 4.Gerhardt HC. 1991. Female mate choice in treefrogs: static and dynamic acoustic criteria. Anim. Behav. 42, 615-635. ( 10.1016/s0003-3472(05)80245-3) [DOI] [Google Scholar]
- 5.Reinhold K. 2010. Variation in acoustic signalling traits exhibits footprints of sexual selection. Evolution 65, 738-745. ( 10.1111/j.1558-5646.2010.01130.x) [DOI] [PubMed] [Google Scholar]
- 6.Phelps SM, Rand AS, Ryan Michael J. 2006. A cognitive framework for mate choice and species recognition. Am. Nat. 167, 28-42. ( 10.1086/498538) [DOI] [PubMed] [Google Scholar]
- 7.Ryan MJ, Rand AS. 1993. Species recognition and sexual selection as a unitary problem in animal communication. Evolution 47, 647-657. ( 10.1111/j.1558-5646.1993.tb02118.x) [DOI] [PubMed] [Google Scholar]
- 8.Ryan MJ, Rand W, Hurd Peter L, Phelps Steve M, Rand AS. 2003. Generalization in response to mate recognition signals. Am. Nat. 161, 380-394. ( 10.1086/367588) [DOI] [PubMed] [Google Scholar]
- 9.Mendelson TC, Shaw KL. 2012. The (mis)concept of species recognition. Trends Ecol. Evol. 27, 421-427. ( 10.1016/j.tree.2012.04.001) [DOI] [PubMed] [Google Scholar]
- 10.Braganza O, Beck H. 2018. The circuit motif as a conceptual tool for multilevel neuroscience. Trends Neurosci. 41, 128-136. ( 10.1016/j.tins.2018.01.002) [DOI] [PubMed] [Google Scholar]
- 11.Friedman J, Barrett SCH. 2009. Wind of change: new insights on the ecology and evolution of pollination and mating in wind-pollinated plants. Ann. Bot. 103, 1515-1527. ( 10.1093/aob/mcp035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lotterhos KE, Levitan DR. 2010. Gamete release and spawning behavior in broadcast spawning marine invertebrates. In The evolution of primary sexual characters in animals (eds Leonard JL, Córdoba-Aguilar A), pp. 99-120. Oxford, UK: Oxford University Press. [Google Scholar]
- 13.Gwynne DT, Rentz DCF. 1983. Beetles on the bottle: male buprestids mistake stubbies for females (Coleoptera). Austral. J. Entomol. 22, 79-80. ( 10.1111/j.1440-6055.1983.tb01846.x) [DOI] [Google Scholar]
- 14.Schiestl FP. 2005. On the success of a swindle: pollination by deception in orchids. Naturwissenschaften 92, 255-264. ( 10.1007/s00114-005-0636-y) [DOI] [PubMed] [Google Scholar]
- 15.Lloyd JE. 1965. Aggressive mimicry in Photuris: firefly femmes fatales. Science 149, 653-654. ( 10.1126/science.149.3684.653) [DOI] [PubMed] [Google Scholar]
- 16.Marshall DC, Hill KBR. 2009. Versatile aggressive mimicry of cicadas by an Australian predatory katydid. PLoS ONE 4, e4185. ( 10.1371/journal.pone.0004185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfennig KS. 2007. Facultative mate choice drives adaptive hybridization. Science 318, 965-967. ( 10.1126/science.1146035) [DOI] [PubMed] [Google Scholar]
- 18.Cunningham EJA, Russell AF. 2000. Egg investment is influenced by male attractiveness in the mallard. Nature 404, 74-77. ( 10.1038/35003565) [DOI] [PubMed] [Google Scholar]
- 19.Sheldon BC. 2000. Differential allocation: tests, mechanisms and implications. Trends Ecol. Evol. 15, 397-402. ( 10.1016/s0169-5347(00)01953-4) [DOI] [PubMed] [Google Scholar]
- 20.Gerhardt HC, Huber F. 2002. Acoustic communication in insects and anurans: common problems and diverse solutions. Chicago, IL: University of Chicago Press. [Google Scholar]
- 21.Ritchie MG. 1996. The shape of female mating preferences. Proc. Natl Acad. Sci. USA 93, 14 628-14 631. ( 10.1073/pnas.93.25.14628) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardoso GC, Hu Y, Mota PG. 2012. Birdsong, sexual selection, and the flawed taxonomy of canaries, goldfinches and allies. Anim. Behav. 84, 111-119. ( 10.1016/j.anbehav.2012.04.015) [DOI] [Google Scholar]
- 23.Losos JB. 1999. Uncertainty in the reconstruction of ancestral character states and limitations on the use of phylogenetic comparative methods. Anim. Behav. 58, 1319-1324. ( 10.1006/anbe.1999.1261) [DOI] [PubMed] [Google Scholar]
- 24.Balakrishnan R. 2016. Behavioral ecology of insect acoustic communication. Insect Hearing 55, 49-80. ( 10.1007/978-3-319-28890-1_3) [DOI] [Google Scholar]
- 25.Greenfield MD. 1997. Acoustic communication in Orthoptera. In The bionoimics of grasshoppers, katydids and their kin (eds Gangwere SK, Muralirangan MC, Muralirangan M), pp. 197-230. Wallingford, UK: CAB International. [Google Scholar]
- 26.Hennig RM, Blankers T, Gray DA. 2016. Divergence in male cricket song and female preference functions in three allopatric sister species. J. Comp. Physiol. A 202, 347-360. ( 10.1007/s00359-016-1083-2) [DOI] [PubMed] [Google Scholar]
- 27.Schöneich S. 2020. Neuroethology of acoustic communication in field crickets - from signal generation to song recognition in an insect brain. Prog. Neurobiol. 194, 101882. ( 10.1016/j.pneurobio.2020.101882) [DOI] [PubMed] [Google Scholar]
- 28.Zuk M, Simmons LW. 1997. Reproductive strategies of the crickets (Orthoptera: Gryllidae). In The evolution of mating systems in insects and arachnids (eds Choe JC, Crespi BJ), pp. 89-109. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 29.Nischk F, Otte D. 2000. Bioacoustics, ecology and systematics of ecuadorian rainforest crickets (orthoptera: Gryllidae: Phalangopsinae), with a description of four new genera and ten new species. J. Orthoptera Res. 9, 229. ( 10.2307/3503651) [DOI] [Google Scholar]
- 30.Robillard T, Desutter-Grandcolas L. 2004. Phylogeny and the modalities of acoustic diversification in extant Eneopterinae (Insecta, Orthoptera, Grylloidea, Eneopteridae). Cladistics 20, 271-293. ( 10.1111/j.1096-0031.2004.00025.x) [DOI] [PubMed] [Google Scholar]
- 31.Weissman DB, Gray DA. 2019. Crickets of the genus Gryllus in the United States (Orthoptera: Gryllidae: Gryllinae). Zootaxa 4705, 1-277. ( 10.11646/zootaxa.4705.1.1) [DOI] [PubMed] [Google Scholar]
- 32.Baker CA, Clemens J, Murthy M. 2019. Acoustic pattern recognition and courtship songs: insights from insects. Annu. Rev. Neurosci. 42, 129-147. ( 10.1146/annurev-neuro-080317-061839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hedwig BG. 2016. Sequential filtering processes shape feature detection in crickets: a framework for song pattern recognition. Front. Physiol. 7, 46-46. ( 10.3389/fphys.2016.00046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hedwig B, Sarmiento-Ponce EJ. 2017. Song pattern recognition in crickets based on a delay-line and coincidence-detector mechanism. Proc. R. Soc. B 284, 20170745. ( 10.1098/rspb.2017.0745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schöneich S, Kostarakos K, Hedwig B. 2015. An auditory feature detection circuit for sound pattern recognition. Sci. Adv. 1, e1500325. ( 10.1126/sciadv.1500325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kostarakos K, Hedwig B. 2012. Calling song recognition in female crickets: temporal tuning of identified brain neurons matches behavior. J. Neurosci. 32, 9601-9612. ( 10.1523/JNEUROSCI.1170-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clemens J, Schöneich S, Kostarakos K, Hennig RM, Hedwig B. 2021. A small, computationally flexible network produces the phenotypic diversity of song recognition in crickets. eLife 10, e61475. ( 10.7554/eLife.61475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clemens J, Hennig RM. 2013. Computational principles underlying the recognition of acoustic signals in insects. J. Comput. Neurosci. 35, 75-85. ( 10.1007/s10827-013-0441-0) [DOI] [PubMed] [Google Scholar]
- 39.Hennig RM, Heller KG, Clemens J. 2014. Time and timing in the acoustic recognition system of crickets. Front. Physiol. 5, 286-286. ( 10.3389/fphys.2014.00286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gray DA, Weissman DB, Cole JA, Lemmon EM, Lemmon AR. 2020. Multilocus phylogeny of Gryllus field crickets (Orthoptera: Gryllidae: Gryllinae) utilizing anchored hybrid enrichment. Zootaxa 4750, 328-348. ( 10.11646/zootaxa.4750.3.2) [DOI] [PubMed] [Google Scholar]
- 41.Gray DA, Hormozi S, Libby FR, Cohen RW. 2018. Induced expression of a vestigial sexual signal. Biol. Lett. 14, 20180095. ( 10.1098/rsbl.2018.0095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker TJ. 1974. Gryllus ovisopis n. sp.: a taciturn cricket with a life cycle suggesting allochronic speciation. Florida Entomol. 57, 13. ( 10.2307/3493823) [DOI] [Google Scholar]
- 43.Walker TJ. 2001. Gryllus cayensis n. sp. (Orthoptera: Gryllidae), a taciturn wood cricket extirpated from the florida keys: songs, ecology and hybrids. Florida Entomol. 84, 700. ( 10.2307/3496404) [DOI] [Google Scholar]
- 44.Martin SD, Gray DA, Cade WH. 2000. Fine-scale temperature effects on cricket calling song. Can. J. Zool. 78, 706-712. ( 10.1139/z99-262) [DOI] [Google Scholar]
- 45.Kilmer JT, Fowler-Finn KD, Gray DA, Höbel G, Rebar D, Reichert MS, Rodríguez RL. 2017. Describing mate preference functions and other function-valued traits. J. Evol. Biol. 30, 1658-1673. ( 10.1111/jeb.13122) [DOI] [PubMed] [Google Scholar]
- 46.Rodríguez RL, Boughman JW, Gray DA, Hebets EA, Höbel G, Symes LB. 2013. Diversification under sexual selection: the relative roles of mate preference strength and the degree of divergence in mate preferences. Ecol. Lett. 16, 964-974. ( 10.1111/ele.12142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keck F, Rimet F, Bouchez A, Franc A. 2016. Phylosignal: an R package to measure, test, and explore the phylogenetic signal. Ecol. Evol. 6, 2774-2780. ( 10.1002/ece3.2051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Münkemüller T, Lavergne S, Bzeznik B, Dray S, Jombart T, Schiffers K, Thuiller W. 2012. How to measure and test phylogenetic signal. Methods Ecol. Evol. 3, 743-756. ( 10.1111/j.2041-210x.2012.00196.x) [DOI] [Google Scholar]
- 49.Stout JF, DeHaan CH, McGhee RW. 1983. Attractiveness of the male Acheta domesticus calling song to females. J. Comp. Physiol. A 153, 509-521. ( 10.1007/bf00612605) [DOI] [PubMed] [Google Scholar]
- 50.Walikonis R, Schoun D, Zacharias D, Henley J, Coburn P, Stout J. 1991. Attractiveness of the male Acheta domesticus calling song to females. J. Comp. Physiol. A 169. ( 10.1007/bf00194903) [DOI] [PubMed] [Google Scholar]
- 51.Gray DA. 1997. Female house crickets, Acheta domesticus, prefer the chirps of large males. Anim. Behav. 54, 1553-1562. ( 10.1006/anbe.1997.0584) [DOI] [PubMed] [Google Scholar]
- 52.Stout JF, McGhee R. 1988. Attractiveness of the male Acheta domestica calling song to females. J. Comp. Physiol. A 164, 277-287. ( 10.1007/BF00603958) [DOI] [PubMed] [Google Scholar]
- 53.Hirtenlehner S, Römer H. 2014. Selective phonotaxis of female crickets under natural outdoor conditions. J. Comp. Physiol. A 200, 239-250. ( 10.1007/s00359-014-0881-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doherty JA. 1985. Trade-off phenomena in calling song recognition and phonotaxis in the cricket, Gryllus bimaculatus (Orthoptera, Gryllidae). J. Comp. Physiol. A 156, 787-801. ( 10.1007/bf00610831) [DOI] [Google Scholar]
- 55.Schneider E, Hennig RM. 2011. Temporal resolution for calling song signals by female crickets, Gryllus bimaculatus. J. Comp. Physiol. A 198, 181-191. ( 10.1007/s00359-011-0698-6) [DOI] [PubMed] [Google Scholar]
- 56.Grobe B, Rothbart MM, Hanschke A, Hennig RM. 2012. Auditory processing at two time scales by the cricket Gryllus bimaculatus. J. Exp. Biol. 215, 1681-1690. ( 10.1242/jeb.065466) [DOI] [PubMed] [Google Scholar]
- 57.Trobe D, Schuster R, Römer H. 2011. Fast and reliable decisions for a dynamic song parameter in field crickets. J. Comp. Physiol. A 197, 131-135. ( 10.1007/s00359-010-0589-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blankers T, Hennig RM, Gray DA. 2015. Conservation of multivariate female preference functions and preference mechanisms in three species of trilling field crickets. J. Evol. Biol. 28, 630-641. ( 10.1111/jeb.12599) [DOI] [PubMed] [Google Scholar]
- 59.Doherty JA, Callos JD. 1991. Acoustic communication in the trilling field cricket, Gryllus rubens (Orthoptera: Gryllidae). J. Insect Behav. 4, 67-82. ( 10.1007/bf01092552) [DOI] [Google Scholar]
- 60.Gabel E, Gray DA, Matthias Hennig R. 2016. How females of chirping and trilling field crickets integrate the ‘what’ and ‘where’ of male acoustic signals during decision making. J. Comp. Physiol. A 202, 823-837. ( 10.1007/s00359-016-1124-x) [DOI] [PubMed] [Google Scholar]
- 61.Gray DA, Cade WH. 2000. Sexual selection and speciation in field crickets. Proc. Natl Acad. Sci. USA 97, 14 449-14 454. ( 10.1073/pnas.97.26.14449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gray DA, Cade WH. 1999. Sex, death, and genetic variation: natural and sexual selection on cricket song. Proc. R. Soc. Lond. B 266, 707-709. ( 10.1098/rspb.1999.0693) [DOI] [Google Scholar]
- 63.Gray DA, Cade WH. 1999. Quantitative genetics of sexual selection in the field cricket, Gryllus integer. Evolution 53, 848-854. ( 10.1111/j.1558-5646.1999.tb05378.x) [DOI] [PubMed] [Google Scholar]
- 64.Wagner JWE, Murray AM, Cade WH. 1995. Phenotypic variation in the mating preferences of female field crickets, Gryllus integer. Anim. Behav. 49, 1269-1281. ( 10.1006/anbe.1995.0159) [DOI] [Google Scholar]
- 65.Thorson J, Weber T, Huber F. 1982. Auditory behavior of the cricket. J. Comp. Physiol. A 146, 361-378. ( 10.1007/bf00612706) [DOI] [Google Scholar]
- 66.Rothbart MM, Hennig RM. 2012. The Steppengrille (Gryllus spec. assimilis): selective filters and signal mismatch on two time scales. PLoS ONE 7, e43975. ( 10.1371/journal.pone.0043975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bailey NW, Moran PA, Hennig RM. 2017. Divergent mechanisms of acoustic mate recognition between closely related field cricket species (Teleogryllus spp.). Anim. Behav. 130, 17-25. ( 10.1016/j.anbehav.2017.06.007) [DOI] [Google Scholar]
- 68.Hennig RM, Weber T. 1997. Filtering of temporal parameters of the calling song by cricket females of two closely related species: a behavioral analysis. J. Comp. Physiol. A 180, 621-630. ( 10.1007/s003590050078) [DOI] [Google Scholar]
- 69.Rothbart MM, Hennig RM. 2012. Calling song signals and temporal preference functions in the cricket Teleogryllus leo. J. Comp. Physiol. A 198, 817-825. ( 10.1007/s00359-012-0751-0) [DOI] [PubMed] [Google Scholar]
- 70.Gray DA, Gabel E, Blankers T, Hennig RM. 2016. Multivariate female preference tests reveal latent perceptual biases. Proc. R. Soc. B 283, 20161972. ( 10.1098/rspb.2016.1972) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Doherty JA, Storz MM. 1992. Calling song and selective phonotaxis in the field crickets, Gryllus firmus and G. pennsylvanicus (Orthoptera: Gryllidae). J. Insect Behav. 5, 555-569. ( 10.1007/bf01048004) [DOI] [Google Scholar]
- 72.Pollack GS, Kim JS. 2013. Selective phonotaxis to high sound-pulse rate in the cricket Gryllus assimilis. J. Comp. Physiol. A 199, 285-293. ( 10.1007/s00359-013-0792-z) [DOI] [PubMed] [Google Scholar]
- 73.Wagner WE. 1996. Convergent song preferences between female field crickets and acoustically orienting parasitoid flies. Behav. Ecol. 7, 279-285. ( 10.1093/beheco/7.3.279) [DOI] [Google Scholar]
- 74.Wagner WE, Basolo AL. 2007. The relative importance of different direct benefits in the mate choices of a field cricket. Evolution 61, 617-622. ( 10.1111/j.1558-5646.2007.00062.x) [DOI] [PubMed] [Google Scholar]
- 75.Hedrick A, Weber T. 1998. Variance in female responses to the fine structure of male song in the field cricket, Gryllus integer. Behav. Ecol. 9, 582-591. ( 10.1093/beheco/9.6.582) [DOI] [Google Scholar]
- 76.Hedrick AV. 1986. Female preferences for male calling bout duration in a field cricket. Behav. Ecol. Sociobiol. 19, 73-77. ( 10.1007/bf00303845) [DOI] [Google Scholar]
- 77.Gray DA, Gutierrez NJ, Chen TL, Gonzalez C, Weissman DB, Cole JA. 2015. Species divergence in field crickets: genetics, song, ecomorphology, and pre- and postzygotic isolation. Biol. J. Linn. Soc. 117, 192-205. ( 10.1111/bij.12668) [DOI] [Google Scholar]
- 78.Izzo AS, Gray DA. 2004. Cricket song in sympatry: species specificity of song without reproductive character displacement in Gryllus rubens. Ann. Entomol. Soc. Am. 97, 831-837. ( 10.1603/0013-8746(2004)097[0831:csisss]2.0.co;2) [DOI] [Google Scholar]
- 79.Shaw KL, Herlihy DP. 2000. Acoustic preference functions and song variability in the Hawaiian cricket Laupala cerasina. Proc. R. Soc. Lond. B 267, 577-584. ( 10.1098/rspb.2000.1040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Symes LB. 2018. Spatial and temporal variation in three call traits and preferences of the tree cricket Oecanthus forbesi. Behav. Ecol. Sociobiol. 72, 1-11. ( 10.1007/s00265-018-2442-5) [DOI] [Google Scholar]
- 81.Walker TJ. 1957. Specificity in the response of female tree crickets (Orthoptera, Gryllidae, Oecanthinae) to calling songs of the males. Ann. Entomol. Soc. Am. 50, 626-636. ( 10.1093/aesa/50.6.626) [DOI] [Google Scholar]
- 82.Ulagaraj SM, Walker TJ. 1975. Response of flying mole crickets to three parameters of synthetic songs broadcast outdoors. Nature 253, 530-532. ( 10.1038/253530a0) [DOI] [Google Scholar]
- 83.Tan MK, et al. 2021. Phylogeny, systematics and evolution of calling songs of the Lebinthini crickets (Orthoptera, Grylloidea, Eneopterinae), with description of two new genera. Syst. Entomol. 46, 1060-1087. ( 10.1111/syen.12510) [DOI] [Google Scholar]
- 84.Percy DM, Taylor GS, Kennedy M. 2006. Psyllid communication: acoustic diversity, mate recognition and phylogenetic signal. Invertebr. Syst. 20, 431-445. ( 10.1071/IS05057) [DOI] [Google Scholar]
- 85.Pereira MAR, Duarte M, Robbins RK. 2018. Hairstreak butterflies (Lepidoptera, Lycaenidae) and evolution of their male secondary sexual organs. Cladistics 35, 173-197. ( 10.1111/cla.12355) [DOI] [PubMed] [Google Scholar]
- 86.Rowe L, Houle D. 1996. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. Lond. B 263, 1415-1421. ( 10.1098/rspb.1996.0207) [DOI] [Google Scholar]
- 87.da Silveira S, Dias AM, Gomes Lagoa AC, Blassioli-Moraes MC, Borges M, Čokl A, Laumann RA.. 2019. Specificity of male responses to female vibratory signals in two Chinavia species (Hemiptera: Pentatomidae) is based on signal structure and narrow temporal parameters. Anim. Behav. Cogn. 6, 1-12. ( 10.26451/abc.06.01.01.2019) [DOI] [Google Scholar]
- 88.Laumann RA, Čokl A, Blassioli-Moraes MC, Borges M. 2016. Vibratory communication and its relevance to reproductive isolation in two sympatric stink bug species (Hemiptera: Pentatomidae: Pentatominae). J. Insect Behav. 29, 643-665. ( 10.1007/s10905-016-9585-x) [DOI] [Google Scholar]
- 89.Prum RO. 1990. Phylogenetic analysis of the evolution of display behavior in the neotropical manakins (Aves: Pipridae). Ethology 84, 202-231. ( 10.1111/j.1439-0310.1990.tb00798.x) [DOI] [Google Scholar]
- 90.Prum RO. 1998. Sexual selection and the evolution of mechanical sound production in manakins (Aves: Pipridae). Anim. Behav. 55, 977-994. ( 10.1006/anbe.1997.0647) [DOI] [PubMed] [Google Scholar]
- 91.Pizo MA, Silva WR. 2001. The dawn lek of the swallow-tailed hummingbird. Wilson Bullet. 113, 388-397. ( 10.1676/0043-5643(2001)113[0388:tdlots]2.0.co;2) [DOI] [Google Scholar]
- 92.Pizo MA, Aleixo A. 1998. Lek behavior of the gray-hooded flycatcher. Condor 100, 726-731. ( 10.2307/1369755) [DOI] [Google Scholar]
- 93.Anoop KR, Yorzinski JL. 2013. Peacock copulation calls attract distant females. Behaviour 150, 61-74. ( 10.1163/1568539X-00003037 [DOI] [Google Scholar]
- 94.Maynard DF, Ward KAA, Doucet SM, Mennill DJ. 2014. Telemetric and video assessment of female response to male vocal performance in a lek-mating manakin. Behav. Ecol. 26, 65-74. ( 10.1093/beheco/aru137) [DOI] [Google Scholar]
- 95.Schaedler LM, Ribeiro PHL, Guaraldo AC, Manica LT. 2019. Acoustic signals and repertoire complexity in swallow-tailed Manakins (Chiroxiphia caudata, Aves: Pipridae). Bioacoustics 29, 182-196. ( 10.1080/09524622.2018.1563870) [DOI] [Google Scholar]
- 96.Castellano S, Zanollo V, Marconi V, Berto G. 2009. The mechanisms of sexual selection in a lek-breeding anuran, Hyla intermedia. Anim. Behav. 77, 213-224. ( 10.1016/j.anbehav.2008.08.035) [DOI] [Google Scholar]
- 97.Grafe TU. 1997. Costs and benefits of mate choice in the lek-breeding reed frog, Hyperolius marmoratus. Anim. Behav. 53, 1103-1117. ( 10.1006/anbe.1996.0427) [DOI] [Google Scholar]
- 98.Beehler B, Pruett-Jones SG. 1983. Display dispersion and diet of birds of paradise: a comparison of nine species. Behav. Ecol. Sociobiol. 13, 229-238. ( 10.1007/bf00299927) [DOI] [Google Scholar]
- 99.Gray DA. 2022. Sexual selection and ‘species recognition’ revisited: serial processing and order-of-operations in mate choice. Figshare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Gray DA. 2022. Sexual selection and ‘species recognition’ revisited: serial processing and order-of-operations in mate choice. Figshare. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data are provided in the electronic supplementary material [99].