Key Points
Del(Y) before transplantation was significantly associated with disease relapse in female-to-male allo-HCT.
A higher incidence of relapse in the del(Y) group might have been caused by attenuation of GVL due to a lack of H-Y antigens.
Visual Abstract
Abstract
The graft-versus-leukemia (GVL) effect is one of the curative mechanisms of allogeneic hematopoietic stem cell transplantation (allo-HCT). H-Y antigens, which are encoded by Y chromosome, are important targets of the GVL effect. Thus, deletion of the Y chromosome (del[Y]) might cause the GVL effect to deteriorate in a transplantation involving a female donor and male recipient, although the clinical significance of the del(Y) group remains to be elucidated. In this study, we evaluated adult male patients who underwent allo-HCT between 2010 and 2019 in Japan. There were 155 cases in the del(Y) group and 4149 cases without del(Y) who underwent female-to-male allo-HCT. Del(Y) was significantly associated with inferior overall survival (hazard ratio [HR], 1.24; 95% confidence interval [CI], 1.00-1.53; P = .049) and an increased risk of relapse (HR, 1.40; 95% CI, 1.08-1.80; P = .0098) in multivariate analyses. There was no significant difference in nonrelapse mortality between recipients with and without del(Y) (HR, 1.08; 95% CI, 0.769-1.51; P = .67). In contrast, del(Y) was not significantly associated with any clinical outcomes in the cohort of male-to-male allo-HCT. A higher incidence of relapse might have been caused by attenuation of the GVL effect resulting from a lack of H-Y antigens. Because a GVL effect resulting from sex mismatch may not be expected in men with del(Y) who undergo allo-HCT with a female donor, additional post–allo-HCT strategies might be required to prevent disease relapse.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HCT) is a curative treatment approach for hematologic malignancies, although it is associated with high morbidity and mortality.1 One of the curative mechanisms of allo-HCT involves the graft-versus-leukemia (GVL) effect, which is an immune reaction mediated by alloreactive donor lymphocytes,2 although a favorable effect of GVL has not been clearly elucidated because of its robust relationship with graft-versus-host disease (GVHD).3-7 Although the main targets of the GVL effect are human leukocyte antigens, minor histocompatibility antigens are also important.8
H-Y antigens, which are proteins encoded by the Y chromosome, are important minor histocompatibility antigens. The Y chromosome is the sex-determining chromosome and contains several genes that are involved in the differentiation of male-specific organs, spermatogenesis, various cytokines, and the cell cycle.9 Because the Y chromosome is specific to males, H-Y antigens are potential targets of GVHD in transplantations involving female donors and male recipients (female-to-male allo-HCT). The combination of a male recipient and female donor has been associated with an increased risk of GVHD and inferior survival but a lower relapse rate in female-to-male allo-HCT in selected situations, suggesting that H-Y antigens may play an important role as a target of the GVL effect.10,11 In this regard, deletion of the Y chromosome (del[Y]) in tumor cells might reduce this favorable effect of GVL. Del(Y) is a common mutation in somatic cells that increases with aging.12 However, the significance of del(Y) in the GVL effect remains to be elucidated. Moreover, del(Y) has been associated with age-related diseases, such as cancer, cardiovascular disease, Alzheimer disease, and diabetes.13 Although these results suggest a possible relationship between del(Y) and several allo-HCT complications, the clinical significance of the Y chromosome in allo-HCT beyond GVHD and the GVL effect has been poorly elucidated. In this study, we evaluated the clinical impact of del(Y) on clinical outcomes in female-to-male allo-HCT.
Methods
Data source and patient selection
Clinical data were provided by the Transplant Registry Unified Management Program of the Japanese Society for Transplantation and Cellular Therapy (JSTCT) and the Japanese Data Center for Hematopoietic Cell Transplantation.14,15
This study included adult male patients (age ≥16 years) diagnosed with acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), myelodysplastic syndrome (MDS), or myeloproliferative neoplasm (MPN) who underwent their first allo-HCT between January 2010 and December 2019 in Japan. Patients for whom information was lacking on karyotype at allo-HCT or diagnosis were excluded. This retrospective study was approved by the data management committee of the Transplant Registry Unified Management Program and by the institutional review board of Jichi Medical University Saitama Medical Center.
Definitions
Del(Y) was evaluated by G-banding karyotyping with a bone marrow specimen at allo-HCT or diagnosis, because karyotyping data at diagnosis only were reported in all patients except those with MDS. All patients with del(Y) were classified as the del(Y) group, and the others were classified as the Y-present group. Any additional chromosomal abnormalities were permitted.
The disease risk index (DRI) and hematopoietic cell transplantation comorbidity index (HCT-CI) were assessed based on a previous report.16,17 A low disease risk according to the DRI included AML with favorable cytogenetics, whereas a high disease risk included AML and MDS with adverse cytogenetics. The other diseases were classified as intermediate disease risk. A high stage risk according to the DRI included induction failure and active relapse at transplantation; any other disease status was classified as low stage risk. In terms of cytogenetics, for AML, t(8;21), inv(16), and t(15;17) were classified as favorable; complex karyotype (≥4 abnormalities) was classified as adverse; and other karyotypes were classified as intermediate. For MDS, abnormal chromosome 7 and complex karyotype (≥4 abnormalities) were classified as adverse, and the other karyotypes were considered intermediate.16 Conditioning regimens were classified as either myeloablative (MAC) or reduced intensity (RIC) according to the criteria from the Center for International Blood and Marrow Transplant Research.18 In brief, MAC regimens were defined by total-body irradiation at >8 Gy (fractionated) or ≥5 Gy (single dose), ≥7.2 mg/kg of IV busulfan, ≥9 mg/kg of oral busulfan, or >140 mg/m2 of melphalan, whereas other regimens were defined as RIC. T-cell in vivo depletion included antithymocyte globulin and alemtuzumab. A related donor with 6/6 antigen matches of HLA-A, -B, and -DR was considered to be an HLA-matched related donor, and any other related donors were considered to be HLA-mismatched related donors. In bone marrow transplantation and peripheral blood stem cell transplantation, an unrelated donor with 8/8 allelic matches of HLA-A, -B, -C, and -DR was classified as an HLA-matched unrelated donor, whereas any other voluntary donors were classified as HLA-mismatched unrelated donors. In cord blood transplantation, a cord blood unit with 6/6 antigen matches of HLA-A, -B, and -DR was considered an HLA-matched unrelated donor, and all other cord blood units were considered HLA-mismatched unrelated donors. Acute and chronic GVHD were diagnosed and graded based on standard criteria.19,20
Statistical analyses
Statistical analyses were mainly performed for female-to-male recipients. Patient characteristics were compared between the del(Y) and Y-present groups using Fisher’s exact test for categorical variables and the Mann-Whitney U test for continuous variables. The cumulative incidences of nonrelapse mortality (NRM), relapse, grade –2 to 4 acute GVHD, and chronic GVHD were estimated by Gray’s test and compared between the del(Y) and Y-present groups. Relapse and NRM were treated as competing risks for each other. Death resulting from any cause was treated as a competing risk for the other cumulative incidences. The cumulative incidence of chronic GVHD was evaluated among patients who survived >100 days after allo-HCT. Overall survival (OS) was estimated by the Kaplan-Meier method and compared by the log-rank test. A Cox proportional hazards regression model was used for multivariate analyses of survival outcomes and cumulative incidence of GVHD. Covariates included in the multivariate analyses were age, disease type, DRI, HCT-CI, performance status, donor type, conditioning intensity, GVHD prophylaxis, and T-cell in vivo depletion.
In addition, the impact of del(Y) was compared in a cohort matched for background factors such as age (>50 or ≤50 years), disease type (AML, ALL, MDS, or MPN), DRI (low, intermediate, high, or very high), HCT-CI (≥2 or 0-1), performance status (2-4 or 0-1), donor type (matched related, matched unrelated, mismatched related, or mismatched unrelated), donor source (bone marrow transplantation, peripheral blood stem cell transplantation, or cord blood transplantation), conditioning regimen (MAC or RIC), GVHD prophylaxis type (cyclosporin based, tacrolimus based, or other), and T-cell in vivo depletion (with or without antithymocyte globulin), using caliper widths equal to 0.2 standard deviation.
A 2-tailed P value <.05 was considered statistically significant. After analyses for female-to-male patients, analyses for male-to-male patients were performed in the same manner to validate the effect of del(Y) on patients undergoing female-to-male transplantation. All analyses in this study were performed with EZR (https://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html; Jichi Medical University Saitama Medical Center, Saitama, Japan), which is a graphical user interface for R (version 3.6.3; R Foundation for Statistical Computing, Vienna, Austria).21
Results
Characteristics of patients undergoing female-to-male allo-HCT
According to the eligibility criteria of this study, 155 cases in the del(Y) group and 4149 cases in the Y-present group were evaluated in the analyses for female-to-male allo-HCT. The median duration of follow-up for survivors was 41 months (range, 0-127). The median recipient age for the entire cohort was 53 years (range, 16-85); the median age was 56 years (range, 18-77) in the del(Y) group and 53 years (range, 16-85) in the Y-present group. Patient characteristics of the del(Y) and Y-present groups are shown in Table 1. In the del(Y) group, 18 cases lacked the Y chromosome, without any additional chromosomal abnormalities. The del(Y) group included more patients with AML and a higher HCT-CI score and fewer with ALL. The DRI of the del(Y) group was distributed equally among all risk groups, whereas the Y-present group included fewer patients at low and very high risk. There was no significant difference in age between the del(Y) and Y-present groups.
Table 1.
Characteristics of patients undergoing female-to-male allo-HCT
| Del(Y) (n = 155) | Y present (n = 4149) | P | |
|---|---|---|---|
| Age, y | .28 | ||
| ≤50 | 58 (37.4) | 1740 (41.9) | |
| >50 | 97 (62.6) | 2409 (58.1) | |
| Disease type | <.001 | ||
| AML | 116 (74.8) | 2350 (56.6) | |
| ALL | 15 (9.7) | 855 (20.6) | |
| MDS | 24 (15.5) | 790 (19.0) | |
| MPN | 0 (0.0) | 154 (3.7) | |
| DRI | <.001 | ||
| Low | 25 (16.1) | 139 (3.4) | |
| Intermediate | 44 (28.4) | 2379 (57.3) | |
| High | 45 (29.0) | 1363 (32.9) | |
| Very high | 41 (26.5) | 268 (6.5) | |
| HCT-CI | <.001 | ||
| 0-1 | 90 (58.1) | 2964 (71.4) | |
| ≥2 | 64 (41.3) | 1151 (27.7) | |
| PS | .11 | ||
| 0-1 | 135 (87.1) | 3773 (90.9) | |
| 2-4 | 20 (12.9) | 369 (8.9) | |
| Donor type | .93 | ||
| Matched related | 38 (24.5) | 982 (23.7) | |
| Matched unrelated | 24 (15.5) | 713 (17.2) | |
| Mismatched related | 14 (9.0) | 432 (10.4) | |
| Mismatched unrelated | 73 (47.1) | 1916 (46.2) | |
| Donor source | .44 | ||
| Bone marrow | 42 (27.1) | 1375 (33.1) | |
| Peripheral blood | 49 (31.6) | 1207 (29.1) | |
| Cord blood | 64 (41.3) | 1559 (37.6) | |
| Conditioning regimen | .33 | ||
| MAC | 100 (64.5) | 2835 (68.3) | |
| RIC | 55 (35.5) | 1313 (31.6) | |
| GVHD prophylaxis | .21 | ||
| CsA based | 38 (24.5) | 1251 (30.2) | |
| TAC based | 113 (72.9) | 2826 (68.1) | |
| Other | 4 (2.6) | 71 (1.7) | |
| T-cell in vivo depletion | .60 | ||
| Yes | 14 (9.0) | 443 (10.7) | |
| No | 141 (91.0) | 3706 (89.3) |
Data are presented as n (%).
CsA, cyclosporine; PS, performance status; TAC, tacrolimus.
Survival outcomes and incidence of GVHD for those undergoing female-to-male allo-HCT
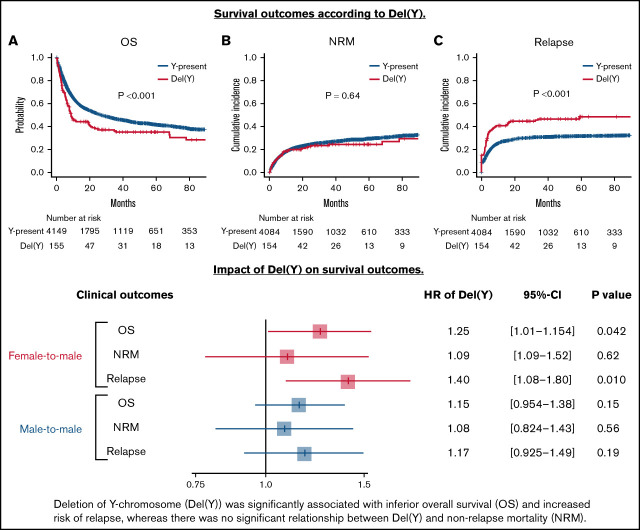
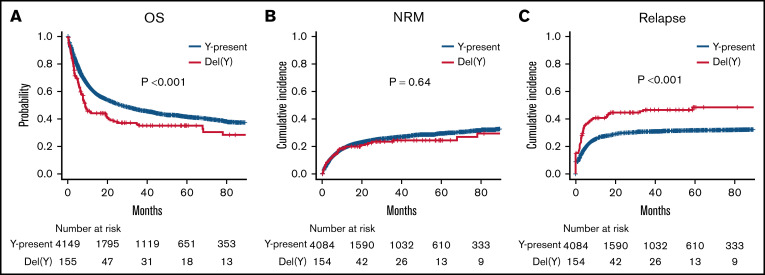
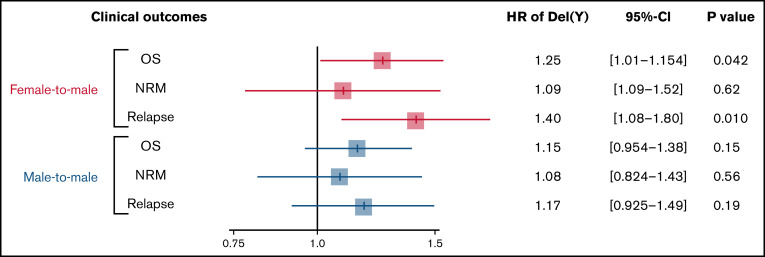
OS was significantly inferior in the del(Y) group (5-year OS, 35.1% vs 41.6%; P < .001; Figure 1A). Multivariate analysis confirmed a significant relationship between del(Y) and inferior OS (hazard ratio [HR], 1.25; 95% confidence interval [CI], 1.01-1.54; P = .042; Figure 2). Whereas NRM was comparable between the del(Y) and Y-present groups (5-year NRM, 24.4% vs 29.4%; P = .64; Figure 1B), the cumulative incidence of relapse (CIR) was significantly higher in the del(Y) group (5-year CIR, 48.4% vs 31.6%; P < .001; Figure 1C). Multivariate analysis of NRM and CIR was consistent with the results of the univariate analysis (HR of NRM, 1.09; 95% CI, 0.778-1.52; P = .62; HR of CIR, 1.40 95% CI, 1.08-1.80; P = .010; Figure 2). Multivariate analysis results are shown in Table 2.
Figure 1.
Clinical outcomes of female-to-male allo-HCT in a univariate analysis. (A) OS. (B) NRM. (C) CIR.
Figure 2.
Impact of del(Y) on clinical outcomes of female-to-male allo-HCT.
Table 2.
Multivariate analyses of allo-HCT outcomes in patients undergoing female-to-male allo-HCT
| OS | NRM | Relapse | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Del(Y) | 1.25 (1.01-1.54) | .042 | 1.09 (0.778-1.52) | .62 | 1.40 (1.08-1.80) | .010 |
| Age > 50 y | 1.70 (1.54-1.88) | <.001 | 2.08 (1.80-2.41) | <.001 | 1.19 (1.05-1.36) | .0090 |
| Disease | ||||||
| AML | Reference | 1.0 | Reference | 1.0 | Reference | 1.0 |
| ALL | 0.980 (0.864-1.11) | .75 | 1.01 (0.849-1.21) | .90 | 1.04 (0.884-1.23) | .61 |
| MDS | 0.880 (0.787-0.984) | .024 | 1.03 (0.881-1.20) | .74 | 0.750 (0.641-0.876) | <.001 |
| MPN | 1.24 (1.01-1.54) | .045 | 1.50 (1.13-1.99) | .0047 | 1.21 (0.901-1.63) | .20 |
| DRI | ||||||
| Low | Reference | 1.0 | Reference | 1.0 | Reference | 1.0 |
| Intermediate | 1.33 (1.00-1.76) | .0049 | 1.23 (0.857-1.77) | .26 | 1.39 (0.921-2.10) | .12 |
| High | 2.67 (2.02-3.54) | <.001 | 1.89 (1.31-2.72) | <.001 | 4.03 (2.68-6.05) | <.001 |
| Very high | 4.50 (3.33-6.06) | <.001 | 2.38 (1.56-3.61) | <.001 | 7.71 (5.04-11.8) | <.001 |
| HCT-CI ≥ 2 | 1.15 (1.05-1.27) | .0023 | 1.30 (1.15-1.48) | <.001 | 0.955 (0.841-1.09) | .48 |
| PS 2-4 | 1.92 (1.69-2.19) | <.001 | 1.95 (1.61-2.36) | <.001 | 1.64 (1.38-1.95) | <.001 |
| Donor type | ||||||
| Matched related | Reference | 1.0 | Reference | 1.0 | Reference | 1.0 |
| Matched unrelated | 1.37 (1.14-1.63) | <.001 | 1.42 (1.09-1.83) | .0083 | 1.03 (0.813-1.30) | .82 |
| Mismatched related | 1.35 (1.13-1.61) | .0011 | 1.64 (1.27-2.12) | <.001 | 0.891 (0.704-1.13) | .33 |
| Mismatched unrelated | 1.34 (1.09-1.64) | .0046 | 1.71 (1.29-2.26) | <.001 | 0.850 (0.650-1.11) | .23 |
| Donor source | ||||||
| Bone marrow | Reference | 1.0 | Reference | 1.0 | Reference | 1.0 |
| Peripheral blood | 1.15 (0.988-1.34) | .072 | 1.12 (0.900-1.40) | .31 | 1.08 (0.890-1.32) | .42 |
| Cord blood | 1.06 (0.913-1.22) | .47 | 0.935 (0.769-1.14) | .50 | 1.15 (0.938-1.40) | .18 |
| MAC | 1.13 (1.03-1.25) | .028 | 1.14 (0.998-1.31) | .054 | 0.974 (0.855-1.11) | .69 |
| GVHD prophylaxis | ||||||
| CsA based | Reference | 1.0 | Reference | 1.0 | Reference | 1.0 |
| TAC based | 0.846 (0.758-0.944) | .0027 | 0.787 (0.676-0.917) | .0021 | 0.945 (0.814-1.10) | .45 |
| Other | 1.05 (0.747-1.47) | .78 | 1.12 (0.701-1.79) | .63 | 1.19 (0.772-1.84) | .43 |
| T-cell in vivo depletion | 1.09 (0.940-1.26) | .26 | 1.06 (0.858-1.31) | .58 | 1.20 (0.987-1.45) | .067 |
CsA, cyclosporine; PS, performance status; TAC, tacrolimus.
The cumulative incidences of acute and chronic GVHD were similar in the del(Y) and Y-present groups (5-year grade –3-4 acute GVHD, 35.5% vs 33.5%; P = .63; 5-year chronic GVHD, 33.1% vs 39.4%; P = .12). The multivariate analysis did not show a significant relationship between del(Y) and either grade –2-4 acute GVHD (HR, 1.18; 95% CI 0.890-1.56; P = .25) or chronic GVHD (HR, 0.819; 95% CI, 0.576-1.16; P = .27; supplemental Table 1).
Death resulting from disease progression was more frequent in the del(Y) group (32.9% vs 19.0%; P < .001), whereas other causes of death, such as infection and acute GVHD, were comparable between the 2 groups (infection, 8.4% vs 10.0%; P = .59; acute GVHD, 1.3% vs 2.1%; P = .77; supplemental Table 2).
Subgroup analyses according to HLA disparity between male recipients and female donors
In the HLA-matched donor cohort (male recipients with HLA-matched related or unrelated female donors, n = 1757), the del(Y) group showed inferior OS and CIR than the Y-present group (5-year OS, 28.6% vs 46.4%; P < .001; 5-year CIR, 51.0% vs 31.8%; P < .001). Multivariate analyses also showed that del(Y) was significantly associated with inferior OS and an increased risk of CIR (HR for OS, 1.63; 95% CI, 1.19-2.24; P = .0026; HR for CIR, 1.61; 95% CI, 1.10-2.35; P = .015; supplemental Figure 1). NRM was comparable between the del(Y) and Y-present groups (5-year NRM, 23.7% vs 24.9%; P = .56). Multivariate analysis also showed no significant relationship between del(Y) and NRM (HR, 1.47; 95% CI, 0.87-2.49; P = .15).
In contrast, in the HLA-mismatched donor cohort (male recipients with HLA-mismatched related or unrelated female donors, n = 2435), the del(Y) group tended to show inferior OS than the Y-present group (5-year OS, 37.9% vs 38.4%; P = .098); CIR was also inferior in the del(Y) group (5-year CIR, 46.8% vs 31.2%; P = .0058). However, the differences did not remain significant in multivariate analyses (HR for OS, 1.05; 95% CI, 0.79-1.39; P = .75; HR for CIR, 1.31; 95% CI, 0.927-1.84; P = .13; supplemental Figure 1). NRM was comparable between the del(Y) and Y-present groups (5-year NRM, 25.6% vs 32.7%; P = .41; HR of del[Y], 0.89; 95% CI, 0.57-1.38; P = .60). In summary, the impact of del(Y) on survival and CIR seems apparent in HLA-matched female-to-male allo-HCT, and anti-HLA alloreactivity may outweigh responses against minor antigens in HLA-mismatched female-to-male allo-HCT.
Matched-pair analysis of survival outcomes and GVHD in those undergoing female-to-male allo-HCT according to del(Y)
Because there was a considerable difference in background between the del(Y) and Y-present groups, a matched-pair analysis was performed. As a result, 117 cases per group were matched (supplemental Table 3).
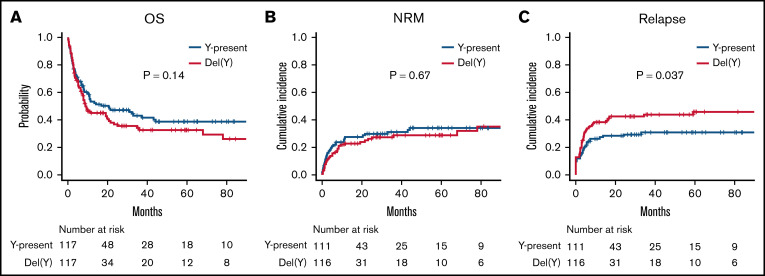
In the matched-pair analysis, OS was comparable between the del(Y) and Y-present groups (5-year OS, 32.5% vs 38.5%; P = .14; Figure 3A). There was also no significant difference in NRM between the del(Y) and Y-present groups (5-year NRM, 28.5% vs 34.1%; P = .67; Figure 3B), whereas CIR was significantly inferior in the del(Y) group (5-year CIR, 45.8% vs 30.8%; P = .037; Figure 3C). With regard to GVHD, there was no significant difference in the cumulative incidences of grade –2 to 4 acute GVHD and chronic GVHD between the 2 groups (5-year grade –2-4 acute GVHD, 41.9% vs 35.9%; P = .32; 5-year chronic GVHD, 31.9% vs 36.7%; P = .42).
Figure 3.
Clinical outcomes of female-to-male allo-HCT in a matched-pair cohort. (A) OS. (B) NRM. (C) CIR.
Survival outcomes and incidence of GVHD in those undergoing male-to-male allo-HCT
We additionally analyzed patients undergoing male-to-male allo-HCT to check whether the adverse impact of del(Y) on relapse was observed only in female-to-male allo-HCT.
The del(Y) group (n = 225) showed significantly inferior OS and CIR compared with the Y-present group (n = 6399) in a univariate analysis (5-year OS, 40.3% vs 46.5%; P = .0043; 5-year CIR, 35.3% vs 29.9%; P = .047), whereas NRM was comparable between the del(Y) and Y-present groups (5-year NRM, 26.5% vs 26.9%; P = .60). The cumulative incidences of acute and chronic GVHD in the del(Y) and Y-present groups were similar (5-year grade –2-4 acute GVHD, 38.0% vs 38.8%; P = .67; 5-year chronic GVHD, 32.4% vs 37.1%; P = .35). However, multivariate analyses did not show any significant relationship between del(Y) and clinical outcomes (HR of OS, 1.15; 95% CI, 0.954-1.38; P = .15; HR of NRM, 1.08; 95% CI, 0.824-1.43; P = .56; HR of CIR, 1.17; 95% CI, 0.925-1.49; P = .19; HR of grade –2-4 acute GVHD, 0.963; 95% CI, 0.772-1.20; P = .74; HR of chronic GVHD, 0.882; 95% CI, 0.669-1.16; P = .37; Figure 2).
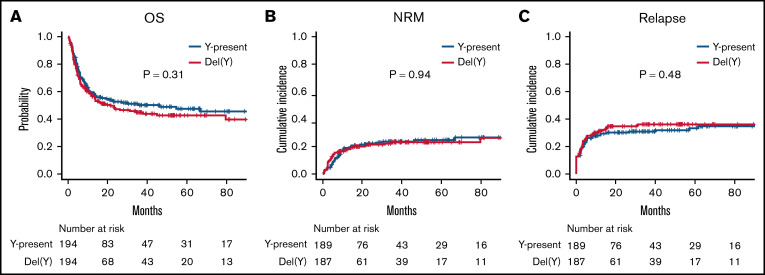
Furthermore, the matched-pair analyses according to del(Y) (194 cases per group; supplemental Table 3) demonstrated that there was no significant difference in survival outcomes between the del(Y) and Y-present groups (5-year OS, 42.7% vs 47.6%; P = .31; Figure 4A; 5-year NRM, 23.0% vs 24.7%; P = .94; Figure 4B; 5-year CIR, 36.2% vs 33.2%; P = .48; Figure 4C). The cumulative incidences of acute and chronic GVHD were also equivalent between the del(Y) and Y-present groups (5-year grade –2-4 acute GVHD, 38.9% vs 42.8%; P = .38; 5-year chronic GVHD, 34.0% vs 39.1%; P = .73).
Figure 4.
Clinical outcomes of male-to-male allo-HCT. (A) OS. (B) NRM. (C) CIR.
Additionally, we checked clinical outcomes according to sex mismatch separately in the Y-present and del(Y) cohorts (supplemental Figure 2). In the Y-present cohort, female-to-male allo-HCT was significantly associated with inferior OS (HR, 1.08; 95% CI, 1.02-1.15; P = .0073) and NRM (HR, 1.09; 95% CI, 1.01-1.19; P = .032) and an increased risk of chronic GVHD (HR, 1.16; 95% CI, 1.08-1.25; P < .001). In contrast, in the del(Y) cohort, female-to-male allo-HCT was not significantly associated with any clinical outcomes.
Discussion
The current study evaluated the clinical significance of del(Y) in male patients undergoing allo-HCT. In the analysis of female-to-male allo-HCT, del(Y) was significantly associated with an increased risk of relapse, and this adverse impact of del(Y) on relapse was also confirmed in a matched-pair analysis. Although del(Y) was also significantly associated with inferior OS in a multivariate analysis, this tendency was not confirmed in the matched-pair analysis. In contrast, del(Y) was not significantly associated with any clinical outcomes in the cohort of patients undergoing male-to-male allo-HCT.
H-Y antigens, which are encoded by the Y chromosome, are considered to be important targets of GVHD and the GVL effect, because female donor cells without the Y chromosome theoretically recognize male tissues/cells, which usually harbor the Y chromosome. In fact, previous studies have reported the presence of minor histocompatibility antigens including H-Y antigens in hematologic tumor cells.22-24 Additionally, the presence of H-Y antigen–specific B-cell and H-Y antibodies in sex-mismatched allo-HCT has also been reported previously.25,26 The incidence of chronic GVHD was higher than in the other sex combination and in recipients with H-Y antibodies.10,11,26 Conversely, lack of H-Y antigen might lead to attenuation of GVHD and the GVL effect, and an attenuated GVL effect would induce a high incidence of relapse. This hypothesis seems to be consistent with the results of the current study. The lack of reduced incidence of GVHD in this study might be explained by the tissue distribution of del(Y). Generally, del(Y) is considered to present mainly in blood cells, and most studies on del(Y) have used blood samples.12,13 Because there is limited information on del(Y) in peripheral tissues other than blood cells, donor cells are likely to be sensitized by H-Y antigens, and del(Y) might have only a limited impact on the incidence of GVHD. However, several studies have reported that del(Y) was found in tissues besides blood cells, such as buccal mucosa and brain.27,28 Therefore, it is possible that peripheral tissues other than blood cells may acquire del(Y), and this might contribute to modification of the GVHD mechanism and the distribution of involved organs in patients undergoing allo-HCT with del(Y). However, further investigation is essential to clarify this hypothesis.
Another explanation for the higher incidence of relapse in the del(Y) group is the effect of aging or additional chromosomal abnormalities. Aging is an important risk factor for del(Y); the incidence of del(Y) was reported to be only 0.05% in patients up to age 15 years and 1.34% in those age 76 to 80 years.27,29,30 Aging seems to be associated with additional chromosomal abnormalities, such as complex karyotype. In this study, the del(Y) group included more recipients with very high DRI than in the Y-present group, and this may have been due to the high frequency of complex karyotype in the del(Y) group. In addition, the impact of del(Y) itself should be considered. Several studies have reported a relationship between del(Y) and cancer risk, including both its incidence and mortality.31-34 However, the relationship was not consistent among different cancer types.33,35,36 In terms of hematologic malignancies, del(Y) was associated with a higher incidence and lower leukemic transformation of MDS.37,38 A high frequency of del(Y) cells was also observed in patients with AML and MPN.39 Moreover, a previous study reported a relationship between del(Y) and clonal hematopoiesis.40 Although large cohort studies did not reveal a significant relationship between del(Y) and hematologic malignancies,33,35 del(Y) might affect the mechanism of advanced progression in hematologic malignancies. However, the adverse impact of del(Y) in female-to-male allo-HCT was confirmed even after the effects of higher DRI, such as additional chromosomal abnormalities and older age, were adjusted for by a multivariate Cox model and matched-pair analysis. In contrast, the impact of del(Y) on relapse was not observed in the male-to-male allo-HCT cohort. These results suggest that the impact of del(Y) on relapse in female-to-male allo-HCT may be due to loss of the GVL effect of H-Y alloreaction and that additional chromosomal abnormalities or del(Y) itself has a very limited impact on the results of CIR.
We initially expected that del(Y) might be associated with some posttransplantation complications, because del(Y) is related to several age-related diseases, such as cardiovascular disease, Alzheimer disease, and diabetes.41,42 However, NRM and causes of nonrelapse death were comparable between the del(Y) and Y-present groups. Therefore, the clinical impact of del(Y) on posttransplantation complications was considered to be limited.
This study has several limitations as a result of its retrospective nature. First, the timing of the acquisition of karyotype information was heterogeneous. The registry data system we used did not collect karyotype information at transplantation, except for patients with MDS; most karyotype information was collected at the diagnosis. In some patients, the leukemic karyotype may have changed before transplantation after several chemotherapies or relapse events, and the impact of del(Y) might have been underestimated. Second, because information on the number of cells with del(Y) could not be extracted from this registry data, the impact of del(Y) might have been somewhat inaccurate and overestimated. Therefore, a quantitative method should be considered in additional studies.28,32,43 Third, there were only a limited number of patients with del(Y). Therefore, subgroup analyses stratified according to disease type and donor type could not be performed because of the small sample size. We applied matched-pair analyses in addition to multivariate analyses for the purpose of adjusting for heterogeneity in the study cohort as far as possible. However, information on gene mutations was not available in the current registry data–based study. Despite these limitations, to our knowledge, this is the first study to demonstrate the clinical significance of del(Y) in allo-HCT.
In conclusion, del(Y) was significantly associated with inferior CIR. This result was confirmed by multivariate Cox and matched-pair analyses. A higher incidence of relapse might have been caused by attenuation of the GVL effect as a result of a lack of H-Y antigens. Because a GVL effect by sex mismatch may not be expected in male recipients with del(Y) who undergo allo-HCT with a female donor, additional post-HCT strategies might be required to prevent disease relapse, such as consolidation/maintenance therapy. Additional studies are warranted to validate the results of this study.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the physicians and data managers for their work at the centers contributing valuable data on transplantation to the Japanese Society for Transplantation and Cellular Therapy (JSTCT). The authors also thank the members of the Transplant Registry Unified Management Committees of the JSTCT for their dedicated data management.
H.N. received a grant from the Japanese Society for the Promotion of Science KAKENHI (JP21K07070).
Authorship
Contribution: M. Tamaki and H.N. conceived the original idea; M. Tamaki designed the study, analyzed data, and wrote the manuscript; K.K., S.K., N.H., and K.Y. advised on methods and wrote the manuscript; N.U., N.D., M. Tanaka, K.I., M.S., Y. Katayama, S.M., T.A., and Y. Kanda collected data and revised the manuscript; J.K., M.O., and T.F. collected data, revised the manuscript, and were responsible for data management at Japanese Society for Transplantation and Cellular Therapy (JSTCT); Y.A. managed the unified registry database and revised the manuscript; H.N. designed the study, advised on the methods, analyzed data, wrote and revised the manuscript, and was responsible for this project of the JSTCT Transplant Complications Working Group; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: S.K. has received honoraria from Merck Sharp & Dohme, Sumitomo Dainippon Pharma, Pfizer, Astellas Pharma, Kyowa Kirin, Chugai Pharmaceutical, Bristol-Myers Squibb, Ono Pharmaceutical, Eisai, Nippon Kayaku, Takeda Pharmaceutical, and SymBio Pharmaceuticals. J.K. has consulted for Astellas Pharma, SymBio Pharmaceuticals, Takeda Pharmaceutical, Megakaryon, Janssen Pharmaceutical, and Daiichi Sankyo and received honoraria from Amgen Astellas Biopharma, Astellas Pharma, Bristol-Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Kyowa Kirin, Megakaryon, Novartis Pharma, Ono Pharmaceutical, Otsuka Pharmaceutical, Sanofi, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, and Teijin Pharma. Y.A. has received honoraria from Kyowa Kirin, AbbVie, Astellas Pharma, Mochida Pharmaceutical, Meiji Seika Pharma, and Chugai Pharmaceutical. H.N. has received honoraria from Takeda Pharmaceutical, Otsuka Pharmaceutical, Bristol-Myers Squibb, Celgene, Pfizer, Novartis, Janssen Pharmaceutical, Eisai, Chugai Pharmaceutical, and Nippon Shinyaku. The remaining authors declare no competing financial interests.
Correspondence: Masaharu Tamaki, Division of Hematology, Jichi Medical University Saitama Medical University Saitama Medical Center, 1-847 Amanuma-cho, Omiya-ku, Saitama city, Saitama 330-8503 Japan; e-mail: mtamaki@jichi.ac.jp; and Hideki Nakasone, Division of Hematology, Jichi Medical University Saitama Medical University Saitama Medical Center, 1-847 Amanuma-cho, Omiya-ku, Saitama city, Saitama 330-8503 Japan; e-mail: nakasone-tky@umin.ac.jp.
References
- 1.D’Souza A, Fretham C, Lee SJ, et al. Current use of and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2020;26(8):e177-e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loke J, Malladi R, Moss P, Craddock C. The role of allogeneic stem cell transplantation in the management of acute myeloid leukaemia: a triumph of hope and experience. Br J Haematol. 2020;188(1):129-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akahoshi Y, Igarashi A, Fukuda T, et al. ; Adult Acute Lymphoblastic Leukemia Working Group of the Japan Society for Hematopoietic Cell Transplantation . Impact of graft-versus-host disease and graft-versus-leukemia effect based on minimal residual disease in Philadelphia chromosome-positive acute lymphoblastic leukemia. Br J Haematol. 2020;190(1):84-92. [DOI] [PubMed] [Google Scholar]
- 4.Kato M, Kurata M, Kanda J, et al. Impact of graft-versus-host disease on relapse and survival after allogeneic stem cell transplantation for pediatric leukemia. Bone Marrow Transplant. 2019;54(1):68-75. [DOI] [PubMed] [Google Scholar]
- 5.Ringdén O, Labopin M, Ciceri F, et al. Is there a stronger graft-versus-leukemia effect using HLA-haploidentical donors compared with HLA-identical siblings? Leukemia. 2016;30(2):447-455. [DOI] [PubMed] [Google Scholar]
- 6.Terwey TH, Le Duc TM, Hemmati PG, et al. NIH-defined graft-versus-host disease and evidence for a potent graft-versus-leukemia effect in patients with acute lymphoblastic leukemia. Ann Oncol. 2013;24(5):1363-1370. [DOI] [PubMed] [Google Scholar]
- 7.Kanda Y, Izutsu K, Hirai H, et al. Effect of graft-versus-host disease on the outcome of bone marrow transplantation from an HLA-identical sibling donor using GVHD prophylaxis with cyclosporin A and methotrexate. Leukemia. 2004;18(5):1013-1019. [DOI] [PubMed] [Google Scholar]
- 8.Kolb HJ, Schmid C, Barrett AJ, Schendel DJ. Graft-versus-leukemia reactions in allogeneic chimeras. Blood. 2004;103(3):767-776. [DOI] [PubMed] [Google Scholar]
- 9.Lau YC. Y chromosome in health and diseases. Cell Biosci. 2020;10:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakasone H, Remberger M, Tian L, et al. Risks and benefits of sex-mismatched hematopoietic cell transplantation differ according to conditioning strategy. Haematologica. 2015;100(11):1477-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nannya Y, Kataoka K, Hangaishi A, Imai Y, Takahashi T, Kurokawa M. The negative impact of female donor/male recipient combination in allogeneic hematopoietic stem cell transplantation depends on disease risk. Transpl Int. 2011;24(5):469-476. [DOI] [PubMed] [Google Scholar]
- 12.Barros B, Morais M, Teixeira AL, Medeiros R. Loss of chromosome Y and its potential applications as biomarker in health and forensic sciences. Cytogenet Genome Res. 2020;160(5):225-237. [DOI] [PubMed] [Google Scholar]
- 13.Guo X, Dai X, Zhou T, et al. Mosaic loss of human Y chromosome: what, how and why. Hum Genet. 2020;139(4):421-446. [DOI] [PubMed] [Google Scholar]
- 14.Atsuta Y. Introduction of Transplant Registry Unified Management Program 2 (TRUMP2): scripts for TRUMP data analyses, part I (variables other than HLA-related data). Int J Hematol. 2016;103(1):3-10. [DOI] [PubMed] [Google Scholar]
- 15.Kanda J. Scripts for TRUMP data analyses. Part II (HLA-related data): statistical analyses specific for hematopoietic stem cell transplantation. Int J Hematol. 2016;103(1):11-19. [DOI] [PubMed] [Google Scholar]
- 16.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the disease risk index for allogeneic stem cell transplantation. Blood. 2014; 123(23):3664-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15(3):367-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825-828. [PubMed] [Google Scholar]
- 20.Sullivan KM, Agura E, Anasetti C, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28(3):250-259. [PubMed] [Google Scholar]
- 21.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosinski KV, Fujii N, Mito JK, et al. DDX3Y encodes a class I MHC-restricted H-Y antigen that is expressed in leukemic stem cells. Blood. 2008;111(9):4817-4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bleakley M, Otterud BE, Richardt JL, et al. Leukemia-associated minor histocompatibility antigen discovery using T-cell clones isolated by in vitro stimulation of naive CD8+ T cells. Blood. 2010;115(23):4923-4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brickner AG, Evans AM, Mito JK, et al. The PANE1 gene encodes a novel human minor histocompatibility antigen that is selectively expressed in B-lymphoid cells and B-CLL. Blood. 2006;107(9):3779-3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahaf B, Yang Y, Arai S, Herzenberg LA, Herzenberg LA, Miklos DB. H-Y antigen-binding B cells develop in male recipients of female hematopoietic cells and associate with chronic graft vs. host disease. Proc Natl Acad Sci USA. 2013;110(8):3005-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakasone H, Tian L, Sahaf B, et al. Allogeneic HY antibodies detected 3 months after female-to-male HCT predict chronic GVHD and nonrelapse mortality in humans. Blood. 2015;125(20):3193-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forsberg LA, Halvardson J, Rychlicka-Buniowska E, et al. Mosaic loss of chromosome Y in leukocytes matters. Nat Genet. 2019;51(1):4-7. [DOI] [PubMed] [Google Scholar]
- 28.Kimura A, Hishimoto A, Otsuka I, et al. Loss of chromosome Y in blood, but not in brain, of suicide completers. PLoS One. 2018;13(1):e0190667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guttenbach M, Koschorz B, Bernthaler U, Grimm T, Schmid M. Sex chromosome loss and aging: in situ hybridization studies on human interphase nuclei. Am J Hum Genet. 1995;57(5):1143-1150. [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou W, Machiela MJ, Freedman ND, et al. Mosaic loss of chromosome Y is associated with common variation near TCL1A. Nat Genet. 2016; 48(5):563-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forsberg LA, Rasi C, Malmqvist N, et al. Mosaic loss of chromosome Y in peripheral blood is associated with shorter survival and higher risk of cancer. Nat Genet. 2014;46(6):624-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noveski P, Madjunkova S, Sukarova Stefanovska E, et al. Loss of Y chromosome in peripheral blood of colorectal and prostate cancer patients. PLoS One. 2016;11(1):e0146264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson DJ, Genovese G, Halvardson J, et al. ; 23andMe Research Team . Genetic predisposition to mosaic Y chromosome loss in blood. Nature. 2019;575(7784):652-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machiela MJ, Dagnall CL, Pathak A, et al. Mosaic chromosome Y loss and testicular germ cell tumor risk. J Hum Genet. 2017;62(6):637-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terao C, Momozawa Y, Ishigaki K, et al. GWAS of mosaic loss of chromosome Y highlights genetic effects on blood cell differentiation. Nat Commun. 2019;10(1):4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loftfield E, Zhou W, Yeager M, Chanock SJ, Freedman ND, Machiela MJ. Mosaic Y loss is moderately associated with solid tumor risk. Cancer Res. 2019;79(3):461-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nomdedeu M, Pereira A, Calvo X, et al. ; Spanish MDS Group . Clinical and biological significance of isolated Y chromosome loss in myelodysplastic syndromes and chronic myelomonocytic leukemia. A report from the Spanish MDS Group. Leuk Res. 2017;63:85-89. [DOI] [PubMed] [Google Scholar]
- 38.Ganster C, Kämpfe D, Jung K, et al. New data shed light on Y-loss-related pathogenesis in myelodysplastic syndromes. Genes Chromosomes Cancer. 2015;54(12):717-724. [DOI] [PubMed] [Google Scholar]
- 39.Wiktor A, Rybicki BA, Piao ZS, et al. Clinical significance of Y chromosome loss in hematologic disease. Genes Chromosomes Cancer. 2000; 27(1):11-16. [PubMed] [Google Scholar]
- 40.Zink F, Stacey SN, Norddahl GL, et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood. 2017;130(6):742-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loftfield E, Zhou W, Graubard BI, et al. Predictors of mosaic chromosome Y loss and associations with mortality in the UK Biobank. Sci Rep. 2018;8(1):12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graham EJ, Vermeulen M, Vardarajan B, et al. Somatic mosaicism of sex chromosomes in the blood and brain. Brain Res. 2019;1721:146345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sullivan KM, Mannucci A, Kimpton CP, Gill P. A rapid and quantitative DNA sex test: fluorescence-based PCR analysis of X-Y homologous gene amelogenin. Biotechniques. 1993;15(4):636-638, 640-631. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.