Key Points
Modest responses and antitumor activity were observed with zanubrutinib monotherapy in patients with non-GCB DLBCL.
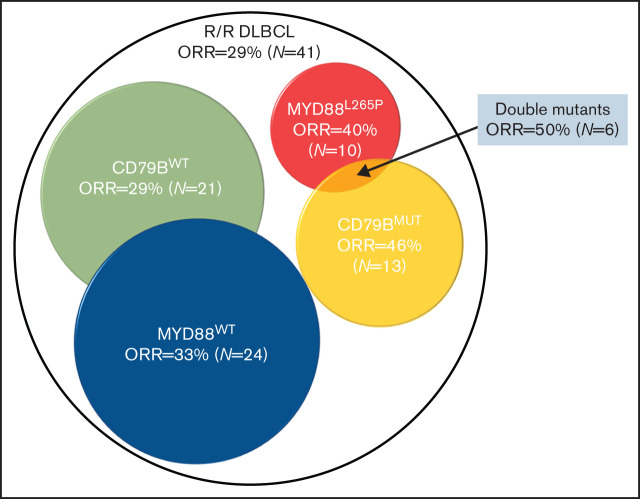
Potential antitumor activity was observed in DLBCL with mutations in both CD79B and MYD88 genes.
Visual Abstract
Abstract
The non-germinal center B-cell like (non-GCB) subtype of diffuse large B-cell lymphoma (DLBCL) has poor clinical outcomes. Bruton tyrosine kinase (BTK) inhibitors have established therapeutic activity in B-cell malignancies, with modest activity in DLBCL. Zanubrutinib, a potent and selective BTK inhibitor, was evaluated in patients with relapsed or refractory (R/R) non-GCB DLBCL. The BGB-3111-207 study (NCT03145064) was a multicenter single-arm phase 2 study. Patients received twice-daily oral zanubrutinib, 160 mg, until disease progression or unacceptable toxicity. The primary end point was the overall response rate (ORR). Secondary end points included progression-free survival (PFS) and duration of response (DOR). Overall survival (OS) was an exploratory end point. Forty-one patients were enrolled in China after having progressed or not responded to prior therapy. At data cutoff, 4 patients continued treatment with 37 discontinuations. The median follow-up was 6.8 months, the ORR was 29.3%, and the complete response rate was 17.1%. Median DOR, PFS, and OS were 4.5, 2.8, and 8.4 months, respectively. Adverse events (AEs) leading to treatment discontinuation were reported in 4 patients, and grade ≥ 3 AEs were reported in 48.8% of patients. Major hemorrhage, atrial fibrillation, and/or flutter were not observed. Zanubrutinib demonstrated modest antitumor activity in non-GCB DLBCL, like other BTK inhibitors, as well as a safety profile consistent with previous studies. Through retrospective biomarker testing, potential antitumor activity was observed in patients with both CD79B and MYD88 mutations, who have inferior outcomes to immunochemotherapy. Future studies of zanubrutinib in R/R non-GCB DLBCL will focus on developing mechanism-based treatment combinations and biomarker-driven patient selection.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma and accounts for 30% to 40% of new diagnoses.1,2 In recent years, biological studies have shown that DLBCL is not a single disease but a group of disorders with specific signaling programs. According to Hans’ algorithm, DLBCL can be categorized into germinal center B-cell–like (GCB) and non-GCB phenotypes by the immunohistochemical expression pattern of CD10, Bcl-6, and MUM1.3,4 Since 2004, rituximab, cyclophosphamide, doxorubicin, prednisolone immunochemotherapy has become first-line therapy for patients with DLBCL,5,6 with 3-year overall survival (OS) rates ranging from 59% to 91%. Unfortunately, a considerable proportion of patients are refractory to or relapse following first-line therapy, with very unfavorable outcomes especially for patients who are ineligible for intensive chemotherapy and hematopoietic stem cell transplant.
Bruton tyrosine kinase (BTK), an intermediary in the B-cell receptor signaling pathway, has been validated as a therapeutic target based on clinical data generated from BTK inhibitor–treated patients with a variety of B-cell malignancies characterized by constitutive B-cell receptor activation, such as non-GCB DLBCL.7 Ibrutinib, the first-in-class BTK inhibitor approved by the US Food and Drug Administration, has demonstrated modest activity in patients with relapsed DLBCL with an overall response rate (ORR) of 23%,8 similar to that of acalabrutinib, a second-generation BTK (ORR 24% in DLBCL, regardless of molecular subtype).9 Zanubrutinib (BGB-3111) is a novel small molecule, an oral BTK inhibitor that forms an irreversible covalent bond at a cysteine residue (Cys481) within the adenosine triphosphate binding pocket of the BTK protein. Zanubrutinib was shown to have a favorable safety profile and robust activity in several clinical studies treating B-cell malignancies.10-12
On the basis of the safety and efficacy of zanubrutinib in other B-cell malignancies, which showed preliminary data with more favorable pharmacokinetics and pharmacodynamics properties,13 in the current phase 2 study we aimed to evaluate the efficacy and safety of zanubrutinib in patients with relapsed or refractory (R/R) non-GCB DLBCL in China.
Methods
Patients and eligibility
The current study enrolled patients aged 18 years or older with histologically documented non-GCB DLBCL who had no response or had relapsed after ≥1 prior treatment regimen (eg, rituximab and anthracycline-based chemotherapy) and patients who were also ineligible for, or refused, intensive chemotherapy and bone marrow transplantation. Patients with current, or a history of, central nervous system lymphoma or prior exposure to a BTK inhibitor were excluded. DLBCL subtypes were designated as GCB or non-GCB, using the immunohistochemistry algorithm specified by Hans et al.14 Full inclusion and exclusion criteria are presented in the Data Supplement.
Study design and treatment
This open-label multicenter single-arm phase 2 study of zanubrutinib enrolled patients with R/R non-GCB DLBCL across 11 centers in China. They received zanubrutinib, 160 mg orally, twice a day in repeated 28-day cycles for up to ∼2 years or until progressive disease (PD), unacceptable toxicity, death, withdrawal of consent, or study termination, whichever came first. Zanubrutinib dose modification is provided in the Data Supplement.
This study was conducted in accordance with sponsor procedures and in compliance with the ethical principles of Good Clinical Practice, International Conference on Harmonization guidelines, the Declaration of Helsinki, and applicable local regulatory requirements. All patients provided written informed consent. The protocol, any amendments, and informed consent forms were reviewed and approved by the institutional review boards/independent ethics committees. This trial was registered at www.clinicaltrials.gov as #NCT03145064 (“Study of BTK Inhibitor BGB-3111 in Subjects with Relapsed/Refractory Non-GCB Type Diffuse Large B Cell Lymphoma”)
Study assessments
The primary end point was ORR, defined as a partial response (PR) or complete response (CR), according to the Revised International Working Group Criteria for Malignant Lymphomas (Lugano classification).15 Patients underwent positron-emission tomography and contrast computed tomography at screening, after 12 and 24 weeks of therapy, and at suspected complete remission. Additionally, contrast computed tomography was performed at weeks 36 and 48 and thereafter, once every 16 weeks. Endoscopy was mandatory to confirm CR in any patient with a documented history of gastrointestinal involvement at baseline. Bone marrow biopsies were required for confirmation of CR in patients with bone marrow tumor involvement at baseline. Confirmation of suspected disease progression (at any time) required a radiological assessment to be performed as soon as possible. The investigator assessed the severity of each adverse event (AE) reported during the study. When applicable, AEs were assessed and graded based upon the National Cancer Institute Common Terminology Criteria for Adverse Events v4.03 guidelines.
Statistical analysis
In this 1-stage single-arm phase 2 study, the sample size to be enrolled was calculated based on the level of precision of the estimated ORR. Assuming an observed ORR of 35% in 40 patients, the 95% exact confidence interval (CI) would be 20.6% to 51.7%.
The safety analysis set, which included all patients who received ≥1 dose of study drug, was the analysis set for the efficacy and safety analyses.
For the primary end point ORR, a 2-sided Clopper-Pearson 95% CI was constructed to assess the precision of the rate estimate. Response rates were also analyzed for specified subgroups: sex (male vs female), age group (<65 vs ≥ 65 years), disease stage (stage I/II vs III/IV), Eastern Cooperative Oncology Group Performance Status (ECOG PS; 0 vs ≥1), number of prior lines of therapy (1 vs ≥2), International Prognostic Index (IPI; <3 vs ≥3), and bulky disease (>7.5 vs ≤7.5 cm).
Duration of response (DOR), time to response, progression-free survival (PFS), and safety and tolerability profile were included as secondary end points. OS was included as the exploratory end point.
Median DOR, PFS, and OS, as well as the event-free rates at landmark time points, were estimated with the Kaplan-Meier method. Censoring rule for DOR and PFS followed the FDA’s Guidance document for industry: Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics.16 Median follow-up times for DOR, PFS, and OS were estimated by the reverse Kaplan-Meier method.
Biomarkers
Cell-of-origin assay by gene-expression profiling was performed using an HTG EdgeSeq DLBCL Cell of Origin Assay (HTG Molecular Diagnostics, Inc.)17 in formalin-fixed paraffin-embedded tumor samples before zanubrutinib treatment. Tumors were assigned to activated B-cell (ABC), GCB, or unclassified DLBCL using HTG EdgeSeq host software, based on the expression of 93 lymphoma-related genes.
CD79B and MYD88 mutations were detected by next-generation sequencing (Illumina HiSeq 4000) using formalin-fixed paraffin-embedded samples. Fisher’s exact test was applied to evaluate the ORR between different molecular groups.
Results
Patient characteristics
From June of 2017 to May of 2018, 41 patients were enrolled in the study, and all received ≥1 dose of the study drug. The median follow-up at the data cutoff date (24 May 2019) was 6.8 months (range, 0.5-20.2). The median age of patients was 62 years (range, 28-75). Most patients were male (n = 25, 61.0%) and had a baseline ECOG PS of 0 or 1 (n = 10 [24.4%] and n = 27 [65.9%], respectively). Most (n = 30, 73.2%) patients were relapsed from previous treatment, and more than a quarter (n = 11, 26.8%) had refractory disease per the investigator’s assessment. Patients with stage III or IV disease at study entry accounted for 24.4% (n = 10) and 51.2% (n = 21) of the total enrolled patients, respectively. A total of 48.8% of patients had high-intermediate (n = 17, 41.5%) or high (n = 3, 7.3%) IPI risk at study entry. Six (14.6%) patients had bulky disease, defined as having ≥1 lesion with longest diameter > 7.5 cm. Twenty-three (56.1%) patients had extranodal disease at study entry. Four (9.8%) patients had baseline bone marrow involvement, and 1 (2.4%) patient had unknown involvement. Three (7.3%) patients had confirmed gastrointestinal involvement. Most (n = 32, 78.0%) patients had elevated lactate dehydrogenase at baseline.
All patients had received prior systemic therapy. The median number of prior lines of anticancer therapies was 2 (range, 1-6); 31 (75.6%) patients had received ≥2 lines of prior therapy. The most common prior regimens contained rituximab; these included rituximab, cyclophosphamide, doxorubicin, and prednisolone (n = 40, 97.6%) and rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone, and etoposide (n = 39, 95.1%). Eleven patients (26.8%) were refractory to their last line of therapy. Four (9.8%) patients had undergone prior autologous stem cell transplantation. Details of prior therapy are summarized in Table 1.
Table 1.
Demographics and baseline characteristics (n = 41)
| Demographic or characteristic | Data |
|---|---|
| Sex | |
| Male | 25 (61.0) |
| Female | 16 (39.0) |
| Age, median (range), y | 62.0 (28-75) |
| Age ≥ 65 y | 13 (31.7) |
| ECOG PS | |
| 0 | 10 (24.4) |
| 1 | 27 (65.9) |
| 2 | 4 (9.8) |
| Disease status at study entry | |
| Relapsed | 30 (73.2) |
| Refractory | 11 (26.8) |
| Stage at study entry for non-GCB type DLBCL | |
| I | 6 (14.6) |
| II | 4 (9.8) |
| III | 10 (24.4) |
| IV | 21 (51.2) |
| IPI at study entry | |
| Low | 5 (12.2) |
| Low-intermediate | 16 (39.0) |
| High-intermediate | 17 (41.5) |
| High | 3 (7.3) |
| Bulky disease (any target lesion LDi > 7.5 cm) | 6 (14.6) |
| Baseline bone marrow involvement | 4 (9.8) |
| Extranodal disease at study entry | 23 (56.1) |
| Elevated LDH at baseline | 32 (78.0) |
| Prior lines of therapy, median (range) | 2 (1-6) |
Unless otherwise noted, data are n (%).
LDH, lactate dehydrogenase; LDi, longest diameter of a lesion.
Efficacy
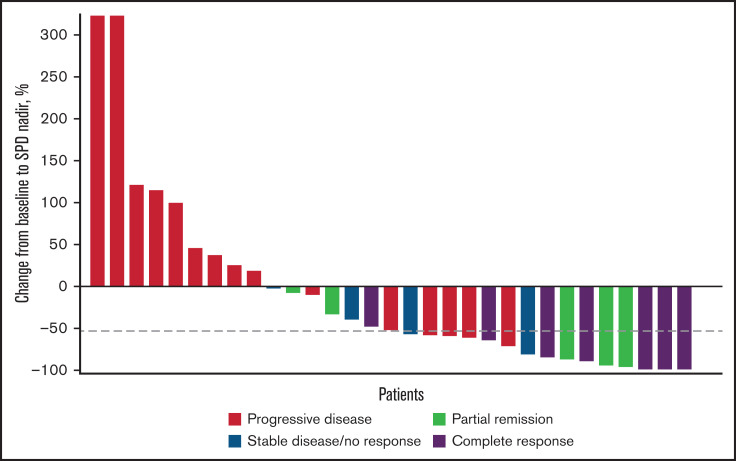
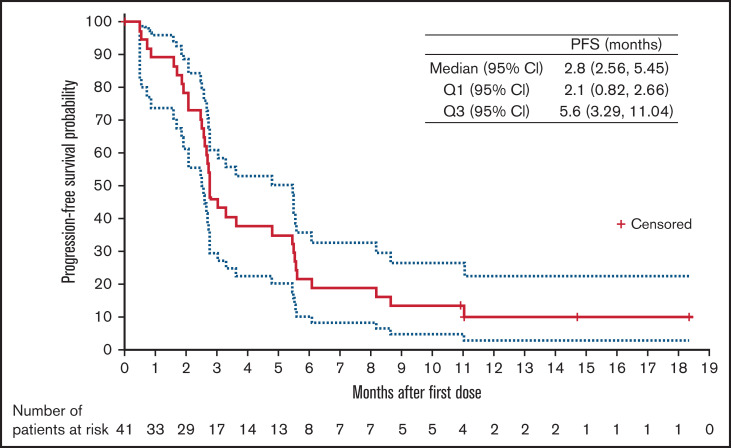
The ORR was 29.3% (95% CI, 16.1-45.5), and the CR rate was 17.1% (95% CI, 7.2-32.1). A waterfall plot of maximum percentage change from baseline in target lesion sum of perpendicular diameters (SPD) by best overall response is illustrated in Figure 1. After a median follow-up of 14.7 months (95% CI, 10.9-18.4), 33 (80.5%) patients had progressed or died. The estimated PFS event-free rates (progression or death) were 45.9%, 21.6%, and 10.1% at 3, 6, and 12 months, respectively. Median PFS for this study was 2.8 months (95% CI, 2.6-5.5); the PFS curve is shown in Figure 2. There were 12 responders. The median DOR was 4.5 months (95% CI, 2.1 months to not estimable). DOR event-free rate at 12 months was 31.3%. For the 12 responders, median time to response was 2.8 months (range, 2.6-3.2); all of them had achieved PR or CR at the first assessment. The median follow-up for OS was 14.7 months (95% CI, 13.9-17.9). Median OS for this study was 8.4 months (95% CI, 4.8 months to not estimable). The estimated OS at 12 months was 35.6%.
Figure 1.
Waterfall plot of percentage change from baseline to nadir in target lesion SPD by best overall response per investigator. Only patients with best overall response and percentage change from baseline SPD were included (n = 31). Dashed line represents the median reduction in SPD (−53%).
Figure 2.
PFS (solid red line: median; upper and lower dotted lines: Q3 and Q1, respectively).
Subgroup analyses show that treatment results, as assessed by the primary end point of ORR, were generally consistent across subgroups, as expected. Response rates were lower in patients previously treated with ≥2 lines of anticancer therapy. No response was observed in patients with bulky disease (longest diameter of a lesion >7.5 cm), although this subgroup had a small sample size. The results are displayed in supplemental Figure 1.
Treatment exposure and safety
As of 24 May 2019, 37 (90.2%) patients had discontinued treatment; only 4 (9.8%) patients were continuing treatment. The primary reason for discontinuing treatment was PD (29 patients, 70.7%); other reasons included AEs (4, 9.8%), consent withdrawal (3, 7.3%), and death (1, 2.4%). The median duration of treatment was 2.8 months (range: 0.4-19.8). Therapy was generally well tolerated, with few dose reductions (1 patient, 2.4%) or interruptions (6 patients, 14.6%). Treatment interruptions were mostly of short duration (median, 8 days [range, 3-21]).
Thirty-six (87.8%) patients experienced ≥1 treatment-emergent AE (TEAE); grade ≥ 3 and serious TEAEs were reported in 20 (48.8%) and 12 (29.3%) of patients, respectively. The most frequently reported TEAEs (in ≥10% of patients) were decreased neutrophil count (n = 9, 22.0%), hypokalemia (n = 7, 17.1%), and decreased platelet count (n = 5, 12.2%). The most common grade ≥3 TEAEs (in ≥2 patients) were decreased neutrophil count (n = 3, 7.3%), pneumonia (n = 2, 4.9%), abdominal pain (n = 2, 4.9%), and death from unknown cause (n = 2, 4.9%). A summary of grade ≥ 3 TEAEs reported in ≥1 patient is presented in Table 2. Serious TEAEs were reported in 12 (29.3%) patients. Major hemorrhage, atrial fibrillation and/or flutter, and tumor lysis syndrome were not observed.
Table 2.
Grade ≥ 3 TEAEs
| AE | Zanubrutinib (n = 41) |
|---|---|
| Patients with ≥1 grade ≥ 3 TEAE | 20 (48.8) |
| Infections and infestations | 6 (14.6) |
| Pneumonia | 2 (4.9) |
| Abdominal infection | 1 (2.4) |
| Herpes zoster | 1 (2.4) |
| Lung infection | 1 (2.4) |
| Otitis media chronic | 1 (2.4) |
| Urinary tract infection | 1 (2.4) |
| Laboratory investigations | 5 (12.2) |
| Decreased neutrophil count | 3 (7.3) |
| Increased alanine aminotransferase increased | 1 (2.4) |
| Increased γ-glutamyl transferase | 1 (2.4) |
| Decreased platelet count | 1 (2.4) |
| Decreased white blood cell count | 1 (2.4) |
| General disorders and administration site conditions | 3 (7.3) |
| Death | 2 (4.9) |
| Gait inability | 1 (2.4) |
| Musculoskeletal and connective tissue disorders | 3 (7.3) |
| Back pain | 1 (2.4) |
| Bone pain | 1 (2.4) |
| Synovitis | 1 (2.4) |
| Gastrointestinal disorders | 2 (4.9) |
| Abdominal pain | 2 (4.9) |
| Metabolism and nutrition disorders | 2 (4.9) |
| Hypokalemia | 1 (2.4) |
| Metabolic acidosis | 1 (2.4) |
| Blood and lymphatic system disorders | 1 (2.4) |
| Anemia | 1 (2.4) |
| Ear and labyrinth disorders | 1 (2.4) |
| Sudden hearing loss | 1 (2.4) |
| Eye disorders | 1 (2.4) |
| Cataract | 1 (2.4) |
| Hepatobiliary disorders | 1 (2.4) |
| Liver injury | 1 (2.4) |
| Renal and urinary disorders | 1 (2.4) |
| Proteinuria | 1 (2.4) |
All data are n (%).
Four (9.8%) patients experienced TEAEs leading to treatment discontinuation, including 1 patient each with urinary tract infection and pneumonia, increased alanine aminotransferase, synovitis, and pneumonia. Six (14.6%) patients experienced TEAEs leading to dose interruption. One TEAE (pneumonia) leading to dose interruption was reported in 2 patients (4.9%). One patient experienced TEAEs leading to dose reduction as a result of postherpetic neuralgia.
At the time of data cutoff, 24 patients (58.5%) had died, 19 from PD, 1 from unknown reasons (death after the safety follow-up and AE collection term), and 4 from AEs. Six deaths occurred within 30 days from the last dose of zanubrutinib: 2 from PD and 4 from AEs (2 from unknown cause, both occurring at home and considered unrelated or unlikely to be related to study treatment; 1 from pneumonia, considered possibly related to study treatment; and 1 from metabolic acidosis considered unlikely to be related to study treatment).
Biomarkers
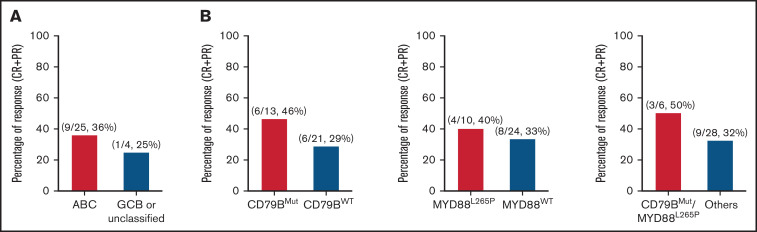
Of the 41 patients with non-GCB DLBCL, we were able to perform gene-expression profiling–based cell-of-origin classification in 29 patients at baseline. In the 25 patients classified with ABC DLBCL, the ORR was 36% (9/25), with a CR rate of 24% (6/25). Of the 4 patients with GCB or unclassified DLBCL, 1 patient had CR (Figure 3). The PFS curve is shown in supplemental Figure 2.
Figure 3.
Biomarker analysis. (A) Overall response by DLBCL subtype. (B) Overall response by CD79B and MYD88 mutation status. ORR between the CD79B ITAM mutant and nonmutant groups (P = .462), MYD88L265P mutant and nonmutant groups (P = .714), and CD79B ITAM and MYD88L265P double-mutant and others group (P = .641). The P values were calculated using Fisher’s exact test.
It was reported that patients with DLBCL with mutations in CD79B and MYD88 respond better to BTK inhibition.16 This prompted us to analyze the response rate in patients with CD79B ITAM or MYD88L265P mutations. Among 34 patients with available DNA sequencing results, 12 (35.3%) patients achieved an overall response. The ORR to zanubrutinib treatment was 46.2% (6/13) and 28.6% (6/21) in patients with mutant or wild-type CD79B, respectively. ORR in patients with mutant MYD88L265P was 40% (4/10), whereas it was 33.3% (8/24) in patients with wild-type MYD88. ORR was 50% (3/6) in patients with the CD79BMUT/MYD88L265P double mutation. The differences in ORR were not significant between different molecular groups as tested by Fisher’s exact test. The PFS curve for the different subgroups is shown in supplemental Figure 3.
Discussion
Zanubrutinib (BGB-3111) is a next-generation small-molecule inhibitor of BTK. In kinase inhibition and cell-based assays, it was more selective than ibrutinib for inhibition of BTK, exhibiting less off-target activity against EGFR, TEC, ITK, and other kinases.21,22 Non-GCB DLBCL has been associated with worse outcomes than GCB DLBCL in patients with newly diagnosed and R/R DLBCL, with shorter PFS and OS.18-20
In the current study, baseline demographic and disease characteristics were typical of patients with R/R non-GCB DLBCL and generally comparable to those reported in phase 2 studies of ibrutinib in R/R DLBCL.23 Subgroup analysis showed that responses were observed in almost all subgroups, but there was a trend toward lower response rates in more heavily pretreated patients (≥2 lines of prior anticancer therapy). The median PFS for this study was 2.8 months, and median OS was 8.4 months. Although PFS and OS were unsatisfactory for R/R DLBCL in the current study, the median DOR was 4.5 months, and 4 of 12 (33.3%) responders remained in remission with the median follow-up ∼ 12 months, suggesting that a given subtype of non-GCB R/R DLBCL may benefit more from zanubrutinib therapy.
A retrospective biomarker study was conducted to identify subsets of DLBCL that would potentially benefit from zanubrutinib treatment. Gene-expression profiling can classify DLBCL into ABC, GCB, and unclassified, according to the cell of origin. Ibrutinib showed modest activity in ABC DLBCL (ORR, 36.8%; 14/38) relative to GCB DLBCL (ORR, 5%; 1/20).23 Zanubrutinib resulted in a similar ORR in ABC DLBCL (ORR, 36%; 9/25). DLBCL displays a striking degree of genetic heterogeneity. Multiple efforts have been made to classify DLBCL based on shared genetic abnormalities and to explore therapeutic vulnerabilities based on tumor genetics. Two groups independently identified a genetic subtype of DLBCL with both CD79B and MYD88L265P mutations that had inferior outcomes in response to current standard immunochemotherapy.24,25 Interestingly, this specific type of DLBCL seemed to be responsive to ibrutinib treatment (ORR, 80%; 4/5).23 Our genetic analysis results also suggested the potential activity of zanubrutinib in these subsets of DLBCL (ORR, 50%; 3/6). An improved biomarker strategy beyond the GCB vs non-GCB classification may aid in targeting DLBCL more precisely.
As expected for this population of patients with R/R non-GCB DLBCL, most (n = 36, 87.8%) experienced ≥1 TEAE. Grade ≥3 TEAEs were reported in 20 (48.8%) patients. Serious TEAEs were reported in 12 (29.3%) patients. Four (9.8%) patients experienced an AE leading to death, all within 30 days of receiving the last dose of study drug. In this study, the AEs associated with BTK inhibitors that have the highest impact on the ability of patients to maintain their prescribed treatment are of special interest: hemorrhage, atrial fibrillation and/or flutter, hypertension, second primary malignancies, tumor lysis syndrome, infection, and cytopenia. These AEs are known to be associated with inhibition of off-target tyrosine kinases, such as EGFR, JAK3, and TEC. The AE profile in the current study was consistent with the class of BTK inhibitors; however, the incidence of select AEs of special interest seems lower with zanubrutinib than previously reported with ibrutinib16: grade ≥3 hemorrhage (ibrutinib 3%, zanubrutinib 0%), any grade of atrial fibrillation/flutter (ibrutinib 4%, zanubrutinib 0%) and hypertension (ibrutinib 12%, zanubrutinib 7.3%), and grade ≥3 infections (ibrutinib 24%, zanubrutinib 14.6%). However, the comparison above is indirect based on different studies. Although 24 deaths were reported, 19 (79%) were due to disease progression. Among 4 patients with AEs leading to death, only pneumonia was considered possibly related to study treatment; for the others, disease progression might have been a contributing factor. All of the safety information showed that zanubrutinib was generally well tolerated, and the safety profile was favorable for the treatment duration.
The current study has several limitations that impact interpretation and comparison against other studies. First, the sample size is small, limiting the generalizability of the results. In addition, there are variations in standard clinical practice, particularly with regard to prior therapies, that limit comparability; for example, only 4 patients (9.8%) with prior autologous stem cell transplantation were enrolled compared with the study evaluating ibrutinib, which enrolled ∼27% patients with prior autologous stem cell transplantation. This may be attributed to the differences in clinical practice in managing R/R DLBCL in different regions. Notwithstanding these limitations, the overall activity of zanubrutinib in R/R DLBCL appears similar to that of the other BTK inhibitors, but with a suggestion of improved tolerability compared with ibrutinib.
In conclusion, this phase 2 study in patients with R/R non-GCB DLBCL who had received ≥1 prior therapy, including rituximab and anthracycline-based chemotherapy, showed that administration of zanubrutinib (160 mg) twice a day was associated with a favorable safety and tolerability profile for the reported treatment duration. Antitumor activity observed in this patient population was modest and similar to the results from other studies that also evaluated the efficacy of BTK inhibitors as monotherapy. This pattern suggests that, in patients with R/R non-GCB DLBCL, additional studies should focus on the development of mechanism-based treatment combinations and biomarker-driven patient selection.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank the patients who participated in the study, their supporters, the investigators, and the clinical research staff from the study centers. Medical writing and editorial assistance were funded by BeiGene and provided by Gordon Bray and W2O arcus.
This work was supported by BeiGene Ltd. (Shanghai, China) and BeiGene USA, Inc. (San Mateo, CA).
Authorship
Contribution: J.H., H.G., W.N., and Z.T. designed the study; Z.T. and Y. Liu interpreted and analyzed data; H.Y., B.X., Y.S., H.Z., W.Z., D.Z., F.L., W.G., A.L., C.L., and Y. Li and their respective research teams reviewed patient records and collected data; J.H., H.G., W.N., Y. Liu, and Z.T. confirmed the accuracy of the data; and H.Y., Y. Li, and Y. Liu wrote the manuscript. All authors had full access to all data, carefully reviewed the manuscript, and approved the final version.
Conflict-of-interest disclosure: H.G., L.F., J.H., W.N., Z.T., and Y. Liu are employees of and own stock in BeiGene. The remaining authors declare no competing financial interests.
Correspondence: Yufu Li, 127 Dongming Rd, Jinshui District, Zhengzhou, Henan 450008, China; e-mail: liyufu439@126.com.
References
- 1.Al-Hamadani M, Habermann TM, Cerhan JR, Macon WR, Maurer MJ, Go RS. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: A longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am J Hematol. 2015;90(9): 790-795. [DOI] [PubMed] [Google Scholar]
- 2.Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443-459. [DOI] [PubMed] [Google Scholar]
- 3.Xie Y, Pittaluga S, Jaffe ES. The histological classification of diffuse large B-cell lymphomas. Semin Hematol. 2015;52(2):57-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer PN, Fu K, Greiner TC, et al. Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. J Clin Oncol. 2011;29(2):200-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24(19):3121-3127. [DOI] [PubMed] [Google Scholar]
- 6.Chaganti S, Illidge T, Barrington S, et al. ; British Committee for Standards in Haematology . Guidelines for the management of diffuse large B-cell lymphoma. Br J Haematol. 2016;174(1):43-56. [DOI] [PubMed] [Google Scholar]
- 7.Cameron F, Sanford M. Ibrutinib: first global approval. Drugs. 2014;74(2):263-271. [DOI] [PubMed] [Google Scholar]
- 8.Younes A, Ansell S, Fowler N, et al. The landscape of new drugs in lymphoma. Nat Rev Clin Oncol. 2017;14(6):335-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dyer MJ, De Vos S, Ruan J, et al. Acalabrutinib monotherapy in patients (pts) with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL). J Clin Oncol. 2018;36(15 suppl):7547. [Google Scholar]
- 10.Hillmen P, Brown JR, Eichhorst BF, et al. ALPINE: zanubrutinib versus ibrutinib in relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Future Oncol. 2020;16(10):517-523. [DOI] [PubMed] [Google Scholar]
- 11.Tam CS, Trotman J, Opat S, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134(11):851-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tam CS, Quach H, Nicol A, et al. Zanubrutinib plus obinutuzumab in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) or relapsed/refractory (R/R) follicular lymphoma (FL). Hematol Oncol. 2019;37(S2):121-122. https://onlinelibrary.wiley.com/doi/10.1002/hon.81_2629 [Google Scholar]
- 13.Guo Y, Liu Y, Hu N, et al. Discovery of zanubrutinib (BGB-3111), a novel, potent, and selective covalent inhibitor of Bruton’s tyrosine kinase. J Med Chem. 2019;62(17):7923-7940. [DOI] [PubMed] [Google Scholar]
- 14.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275-282. [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD, Fisher RI, Barrington SF, et al. ; United Kingdom National Cancer Research Institute . Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Food and Drug Administration. Clinical trial endpoints for the approval of cancer drugs and biologics. Guidance for industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-trial-endpoints-approval-cancer-drugs-and-biologics.
- 17.Schaffer M, Chaturvedi S, Alvarez JD, et al. Comparison of immunohistochemistry assay results with gene expression profiling methods for diffuse large B-cell lymphoma subtype identification in matched patient samples. J Mol Biomark Diagn. 2018;9:2. [Google Scholar]
- 18.Batlle-López A, González de Villambrosía S, Francisco M, et al. Stratifying diffuse large B-cell lymphoma patients treated with chemoimmunotherapy: GCB/non-GCB by immunohistochemistry is still a robust and feasible marker. Oncotarget. 2016;7(14):18036-18049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez-Ilizaliturri FJ, Deeb G, Zinzani PL, et al. Higher response to lenalidomide in relapsed/refractory diffuse large B-cell lymphoma in nongerminal center B-cell-like than in germinal center B-cell-like phenotype. Cancer. 2011;117(22):5058-5066. [DOI] [PubMed] [Google Scholar]
- 20.Thieblemont C, Briere J, Mounier N, et al. The germinal center/activated B-cell subclassification has a prognostic impact for response to salvage therapy in relapsed/refractory diffuse large B-cell lymphoma: a bio-CORAL study. J Clin Oncol. 2011;29(31):4079-4087. [DOI] [PubMed] [Google Scholar]
- 21.Li N, Sun Z, Liu Y, et al. Abstract 2597: BGB-3111 is a novel and highly selective Bruton’s tyrosine kinase (BTK) inhibitor. Cancer Res. 2015; 75(15 suppl):2597. [Google Scholar]
- 22.Tam CS, Grigg AP, Opat S, et al. The BTK inhibitor, BGB-3111, is safe, tolerable, and highly active in patients with relapsed/refractory B-cell malignancies: initial report of a phase 1 first-in-human trial. Blood. 2015;126(23):832. [Google Scholar]
- 23.Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21(8):922-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitz R, Wright GW, Huang DW, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018;378(15):1396-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chapuy B, Stewart C, Dunford AJ, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes [published corrections appear in Nat Med. 2018;24(8):1290-1291; Nat Med. 2018;24(8):1292]. Nat Med. 2018;24(5):679-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.