Key Points
In patients with newly diagnosed Ph+ ALL, ponatinib and prednisone therapy resulted in long molecular remissions and few resistance mutations.
The observed high rates of discontinuation and dose modification suggest that a lower dose may be more appropriate in older/unfit patients.
Visual Abstract
Abstract
Tyrosine kinase inhibitors have improved survival for patients with Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL). However, prognosis for old or unfit patients remains poor. In the INCB84344-201 (formerly GIMEMA LAL 1811) prospective, multicenter, phase 2 trial, we tested the efficacy and safety of ponatinib plus prednisone in newly diagnosed patients with Ph+ ALL ≥60 years, or unfit for intensive chemotherapy and stem cell transplantation. Forty-four patients received oral ponatinib 45 mg/d for 48 weeks (core phase), with prednisone tapered to 60 mg/m2/d from days-14-29. Prophylactic intrathecal chemotherapy was administered monthly. Median age was 66.5 years (range, 26-85). The primary endpoint (complete hematologic response [CHR] at 24 weeks) was reached in 38/44 patients (86.4%); complete molecular response (CMR) in 18/44 patients (40.9%) at 24 weeks. 61.4% of patients completed the core phase. As of 24 April 2020, median event-free survival was 14.31 months (95% CI 9.30-22.31). Median overall survival and duration of CHR were not reached; median duration of CMR was 11.6 months. Most common treatment-emergent adverse events (TEAEs) were rash (36.4%), asthenia (22.7%), alanine transaminase increase (15.9%), erythema (15.9%), and γ-glutamyltransferase increase (15.9%). Cardiac and vascular TEAEs occurred in 29.5% (grade ≥3, 18.2%) and 27.3% (grade ≥3, 15.9%), respectively. Dose reductions, interruptions, and discontinuations due to TEAEs occurred in 43.2%, 43.2%, and 27.3% of patients, respectively; 5 patients had fatal TEAEs. Ponatinib and prednisone showed efficacy in unfit patients with Ph+ ALL; however, a lower ponatinib dose may be more appropriate in this population. This trial was registered at www.clinicaltrials.gov as #NCT01641107.
Introduction
The Philadelphia (Ph) chromosome is the most frequent cytogenetic aberration in adults with acute lymphoblastic leukemia (ALL), with an incidence of up to 40% in adult patients with ALL and approximately 50% in patients ≥50 years.1-5 Treatment options for Ph+ ALL have expanded over the past 15 years, mainly due to the introduction of tyrosine kinase inhibitors (TKIs) in clinical practice.6-8 TKIs have been successfully combined with reduced-intensity9 chemotherapy and standard chemotherapy,10 with subsequent transplant, in fit adult patients. In adults with Ph+ ALL, induction with imatinib plus steroids, followed by imatinib plus chemotherapy and stem cell transplantation (SCT; where possible), was associated with an overall survival (OS) of 49% and disease-free survival of 46% at 5 years.10
Several GIMEMA study protocols have explored regimens that combine TKIs with steroids as an alternative to systemic chemotherapy.11-13 This approach, with prophylactic intrathecal chemotherapy reserved for central nervous system (CNS) disease, is of particular relevance for elderly or unfit patients who are not candidates for intensive chemotherapy and who may not be able to tolerate more intensive treatment.
The efficacy of induction therapy with high-dose imatinib (800 mg daily) plus prednisone for patients ≥60 years with de novo Ph+ ALL was explored previously in the GIMEMA LAL 0201-B trial.12 Twenty-nine patients >60 years were evaluable for response, and all achieved complete remission. Median OS from diagnosis was 20 months.12 Both the GIMEMA LAL 1205 trial (dasatinib plus prednisone)11 and the GIMEMA LAL 1408 trial (prednisone plus sequential nilotinib 400 mg and imatinib 300 mg)13 reported complete remission rates of >90% and median OS of approximately 30 months.
Other studies involving imatinib9,14-18 or a second-generation TKI19-23 in combination with chemotherapy have reported improved median survival with variable rates of induction mortality. No randomized studies comparing a TKI plus steroids or TKI plus chemotherapy have been conducted. As the incidence of Ph+ ALL increases with age,1 improving survival in elderly or unfit patients with Ph+ ALL who cannot receive chemotherapy is of paramount importance.
In trials with first- or second-generation TKIs, relapse was frequent and sometimes resulted in poor survival rates.9,11-24 Selection of cells with BCR-ABL1 kinase domain (KD) mutations was the main mechanism underlying relapses.25 In particular, the T315I mutation, which confers cross-resistance to imatinib, dasatinib, and nilotinib, was the most frequent mutation in relapsed/refractory ALL.26 A T315I mutation was detected in 12/17 patients (71%) who experienced relapse after dasatinib plus steroid treatment in the GIMEMA 1205 trial11 and in 18/24 (75%) patients who relapsed after dasatinib plus low-intensity chemotherapy treatment in the EWALL-PH-01 study (10/43 patients harbored T315I at diagnosis).23 Furthermore, patients who developed a mutation that conferred resistance to a TKI have a higher chance of developing further resistance.27 Patients who experience relapse after treatment with 1 TKI may respond to another TKI, but the duration of the second remission is usually shorter than their previous response.28 Subclones harboring mutations conferring TKI resistance have been detected at diagnosis,25,29 and resistant mutations at a low disease burden may predict a higher burden of BCR-ABL1 residual transcript and relapse.30
Ponatinib is a third-generation TKI with a wide spectrum of kinase inhibition.31,32 It is active against most known BCR-ABL1 mutations and is the only TKI with activity against Ph+ ALL subclones with the T315I mutation.31,33,34 Ponatinib has shown clinical activity in relapsed or refractory Ph+ ALL28,35,36 and in the first-line setting in combination with chemotherapy.37 Moreover, in a propensity score-matched analysis, first-line ponatinib in combination with chemotherapy conferred a better prognosis compared with dasatinib.38 These results, together with the pharmacologic characteristics of ponatinib, support the hypothesis of a beneficial role for ponatinib, not only in patients resistant to prior TKI therapy but also in untreated patients with Ph+ ALL. Ponatinib may also prevent the emergence of resistant clones, thus avoiding rapid disease progression.31
We investigated first-line ponatinib plus prednisone in patients ≥60 years or those unfit for intensive chemotherapy and SCT, with the aim of inducing deep and durable remissions. Endpoints included rate and quality of remission, measurable residual disease, survival, and the emergence of BCR-ABL1 mutations.
Methods
Study design and participants
INCB84344-201 (formerly GIMEMA LAL 1811; NCT01641107) was a phase 2, open-label, single-arm study performed at 23 study centers across Italy. Briefly, patients had new-onset Ph+ ALL according to the World Health Organization (WHO) classification,39 had no prior history of chronic myeloid leukemia, and were ≥60 years or were ≥18 years but unfit for a program of intensive chemotherapy and SCT (by the investigators’ judgment). Eligible patients had a WHO performance status of >50% (Karnofsky) or ≤2 (Eastern Cooperative Oncology Group [ECOG]) and adequate organ function. Key exclusion criteria included active hepatitis infection; any active infection; history of acute pancreatitis within 1 year or history of chronic pancreatitis; history of alcohol abuse; triglycerides >450 mg/dL; any clinically significant uncontrolled or active cardiovascular condition, including uncontrolled hypertension and history of deep venous or pulmonary embolism; and any impairment in gastrointestinal absorption of ponatinib. The study was undertaken in accordance with the ethical principles of the Declaration of Helsinki and the International Council on Harmonisation guidelines on Good Clinical Practice. The study protocol was reviewed and approved by the institutional review board or independent ethics committee at all participating centers. All patients provided written informed consent.
Procedures
Patients received a 7 to 14-day steroid pretreatment with oral prednisone at increasing doses (40-60 mg/m2 per day), during which the presence of the BCR-ABL1 transcript was confirmed by the GIMEMA central laboratory. Patients then received oral ponatinib 45 mg/d for 8 courses (6 weeks/course), defined as the core phase of the study. Prednisone 60 mg/m2 per day was administered from days 1 to 21 and then tapered and stopped at day 29. After course 8, continuation of ponatinib was offered in the extension phase of the study if investigators determined that the patient may continue to benefit from treatment. Intrathecal therapy with methotrexate, cytosine arabinoside, and dexamethasone was administered every 28 days in patients without clinical-cytologic evidence of meningeal involvement. In patients who developed CNS disease, intrathecal therapy was performed twice weekly until complete clearance of blast cells from cerebrospinal fluid was achieved, then once a week for 4 weeks, and then once a month thereafter. Aspirin, anti-infective therapies, and transfusion were offered to patients whenever clinically indicated.
Ponatinib dose reduction (to 30 mg or 15 mg) or suspension was planned after serious nonhematologic or hematologic toxicities. In the case of arterial or venous occlusive events, ponatinib treatment was not resumed unless the potential benefits outweighed the risk of recurrent events or the patient had no other treatment options. For serious nonhematologic adverse events (AEs) other than arterial or venous occlusions, ponatinib treatment was resumed only after resolution of the AE or when the potential benefit of resuming therapy was judged to outweigh the risks. In case of any hematologic AEs, dose reduction or therapy suspension was allowed only after confirmed complete hematologic response (CHR).
Outcomes
The primary endpoint was the proportion of patients who achieved at CHR at 6 months, defined as bone marrow blasts <5%, peripheral blood differential without blasts (neutrophils ≥1.5 × 109/L, platelets ≥100 × 109/L), and no evidence of extramedullary involvement from leukemia. Secondary endpoints included CHR rate at 6, 12, 24, 36, and 48 weeks; rate of complete cytogenetic response (CCyR), defined as the absence of Ph+ metaphases by chromosome banding analysis in at least 20 marrow cell metaphases,40,41 at 6, 12, 24, 36, and 48 weeks; duration of CCyR; rates of complete molecular response (CMR) and major molecular response (MMR), defined as BCR-ABL1/ABL1 ratio <0.01 and <0.10, respectively, by quantitative real-time polymerase chain reaction with a sensitivity of at least 30 000 molecules of ABL, at 12, 24, 36, and 48 weeks; duration of CMR; event-free survival (EFS) for the total study population, defined as time from enrollment to any event (failure of achieving CHR at week 6, treatment discontinuation, CHR lost, or death from any cause, whichever occurred first); and OS calculated for the entire study population as time from enrollment to death from any cause. Participants who were lost to follow-up or still alive at the time of analysis were right-censored at the date at which the participant was last known to be alive or the clinical data cutoff date for the analysis, whichever was earlier. The biologic endpoint was to describe the type and number of BCR-ABL1 KD mutations acquired during and after the study. Mutational analysis was performed by direct sequencing.25 AEs and serious AEs (SAEs) were recorded from the date when informed consent was obtained up to 30 days after the last administration of ponatinib and at any time if the events were suspected to be related to study medication. AEs and SAEs were classified using the Medical Dictionary for Regulatory Activities version 22.0 and graded using the Common Terminology Criteria for Adverse Events version 4.0. Analysis of AEs was limited to treatment-emergent adverse events (TEAEs), defined as any AE reported for the first time during treatment or worsening of a preexisting event after first administration of study drug (including pretreatment) and within 30 days of the last dose of study drug. Blood and bone marrow specimens were collected at diagnosis, at weeks 6, 12, 24, 36, and 48, and at any time in case of treatment failure or disease progression. Hematologic and cytogenetic response were assessed in local laboratories. Samples were analyzed in a centralized and certified laboratory for both BCR-ABL1 minimal residual disease and KD mutation analysis.
Permanent discontinuation criteria included lack of CHR by week 6, loss of CHR or CCyR at any time, pregnancy, patient or investigator decision, patient loss to follow-up, protocol violation, and AEs contraindicating further dosing (eg, myocardial infarction or stroke).
Statistical analysis
The study was designed to show a difference of 20% between the null hypothesis (55% of patients who had CHR at 6 months) and the alternative hypothesis fixed at 75%, with 80% power, using a single-stage phase 2 design with a 5% significance level (based on data of the historic control group GIMEMA LAL0201-B protocol9). Patients who received at least 1 dose of ponatinib were included in the efficacy and safety analysis. For categoric measurements, summary statistics included sample size, frequency, and percentages. For continuous measurements, summary statistics included sample size, mean, median, standard deviation, standard error of the mean, minimum, and maximum. Summary statistics for continuous measures were provided for baseline, the actual measurements at each visit, and the change and percentage change from baseline at each visit, if applicable. For time-to-event endpoints, including duration of CHR, duration of CCyR, duration of CMR, EFS, and OS, Kaplan-Meier curves were presented. Median survival time was estimated using the Kaplan-Meier method. Confidence intervals for median survival time were calculated using the method of Brookmeyer and Crowley.42 To be conservative, participants with missing post-baseline values were imputed as nonresponders. SAS software (SAS Institute Inc., Cary, NC; version 9.4) was used for the generation of all tables, graphs, and statistical analyses. Study data were initially collected and managed using the REDCap electronic data capture tools hosted at the GIMEMA Foundation and by InForm after change of sponsorship to Incyte.43 All authors had full access to the primary clinical trial data used to write the report.
Role of the funding source
GIMEMA, a nonprofit organization, performed computational and manual consistency checks on newly entered forms.
To ensure the study was conducted according to good clinical practice, GIMEMA data center provided investigators’ files to single centers and organized training meetings in which principal investigators and collaborative investigators participated.
At the time of study approval, the INCB84344-201 study (formerly GIMEMA LAL1811) was an investigator-initiated research trial and received support for pharmacovigilance and study drug distribution by Incyte. Incyte provided ponatinib to all study patients free of cost.
Results
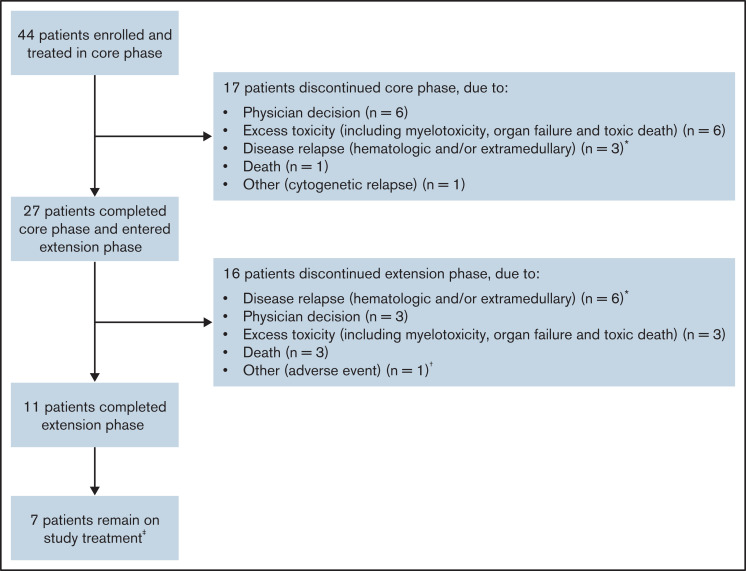
Forty-four patients were enrolled across 22 sites between December 2014 and January 2017 (Figure 1). The data cutoff was 24 April 2020. Median duration of follow-up was 34.9 months (range, 0.19-61.54).
Figure 1.
Patient disposition. *All patients had hematologic relapse, and 1 patient in the extension phase had both hematologic and extramedullary relapse. †Acute coronary syndrome leading to permanent drug withdrawal. ‡At the time of data cutoff (24 April 2020).
Overall, 17 patients (38.6%) discontinued treatment by week 48; 3 patients had documented hematological and/or extramedullary relapse, 6 patients withdrew due to excessive toxicity (including myelotoxicity, organ failure, and toxic death), 1 patient had cytogenetic relapse, and 1 patient died (Figure 1). Of note, 6 patients (13.6%) were discontinued due to physician decision during the core phase; 5 of these patients as well as 1 patient who discontinued the extension phase due to physician decision were reconsidered in terms of fitness and received an allogeneic SCT (alloSCT). In total, 27 patients (61.4%) entered the extension phase of the study; 11 patients completed the extension phase. Of the 16 patients who discontinued the extension phase, 6 had documented hematological and/or extramedullary relapse, 3 discontinued due to physician decision, 3 withdrew due to excessive toxicity, and 3 died. The reason for discontinuation in the remaining patient was documented on the case report form as “other (adverse event)”; this patient had acute coronary syndrome leading to permanent withdrawal of treatment.
Baseline characteristics and demographics are summarized in Table 1. Median age of patients at diagnosis was 66.5 years (range, 26-85), with 35 patients (79.5%) ≥60 years. Thirty-eight patients (86.4%) had a WHO performance status (ECOG) of 0 to 2 at baseline. The median white blood cell (WBC) count of patients enrolled at diagnosis was 4.3 × 106/mL (range, 0.33-115.9 × 106/mL); 1 patient had WBC >100 × 106/mL. CNS disease was reported for 6 patients (13.6%); 33 patients were negative for CNS involvement (75.0%; data missing for 5 patients [11.4%]). Medicated intrathecal prophylaxis was performed at baseline in 15 patients.
Table 1.
Baseline characteristics
| Characteristic | Full analysis set (N = 44) |
|---|---|
| Male/Female, n (%) | 22 (50.0)/22 (50.0) |
| Median age (range), y | 66.5 (26-85) |
| WHO performance status (ECOG PS), n (%)* 0 1 2 | 18 (40.9) 17 (38.6) 3 (6.8) |
| Median WBC count, × 106/mL (range)† | 4.3 (0.3-115.9) |
| Median hemoglobin, g/L (range)† | 98.5 (76.0-160.0) |
| Median platelets, 106/mL (range)† | 47.5 (10.0-269.0) |
| Median neutrophils/leukocytes, % (range)† | 30.9 (1.0-78.2) |
| Median lymphocytes/leukocytes, % (range)† | 36.0 (3.0-94.5) |
| Median left ventricular ejection fraction, % (range) | 63.0 (53.0-96.0) |
| Mean liver size, cm (SD) | 8.1 (25.68) |
| Mean spleen size, cm (SD) | 7.6 (21.63) |
| Adenomegaly (longest diameter >0 cm) Laterocervical, n (%)‡ Supraclavicular, n (%)§ Axillary, n (%)§ Inguinal, n (%)§ | 4 (9.1) 1 (2.3) 2 (4.5) 1 (2.3) |
| Mediastinal mass, n (%)§ | 1 (2.3) |
| CNS disease, n (%)¶ | 6 (13.6) |
| BCR rearrangement (BM) p190, n (%) p210, n (%) p190/p210, n (%) Missing, n (%) | 29 (65.9) 9 (20.5) 2 (4.5) 4 (9.1) |
| BCR rearrangement (peripheral blood) p190, n (%) p210, n (%) p190/210, n (%) Missing, n (%) | 5 (11.4) 3 (6.8) 2 (4.5) 34 (77.3) |
| BM p190/ABL ratio × 100, median (range) | 63.4 (0-172) |
| BM p210/ABL ratio × 100, median (range) | 36.0 (0-128) |
Sum of percentages may not be 100 due to rounding.
BM, bone marrow; ECOG PS, Eastern Cooperative Oncology Group performance status; SD, standard deviation.
Data missing from 6 patients.
Evaluated in 40 patients.
Data missing from 8 patients.
Data missing from 7 patients.
Data missing from 5 patients.
All 44 patients received steroid pretreatment, and pretreatment response was assessed in 34 patients; 19 patients (43.2%) showed ≥75% reduction in circulating blasts at the end of pretreatment. In the core phase, patients received a median 331 days (range, 2-341) of treatment, with a median daily dose of ponatinib of 34.16 mg (range, 10.1-46.0). During the extension phase, patients received a median 509 days (range, 52-1522) of treatment, with a median daily dose of ponatinib of 29.19 mg (range, 9.8-45.0). During the entire study, patients received a median 415 days (range, 2-1858) of treatment, with a median daily dose of ponatinib of 31.72 mg (range, 13.6-45.0). Thirty-three patients (75.0%) had at least 1 dose reduction, 24 patients (54.5%) had at least 1 dose interruption, and 23 (52.3%) had both (at least 1 dose reduction and 1 dose interruption).
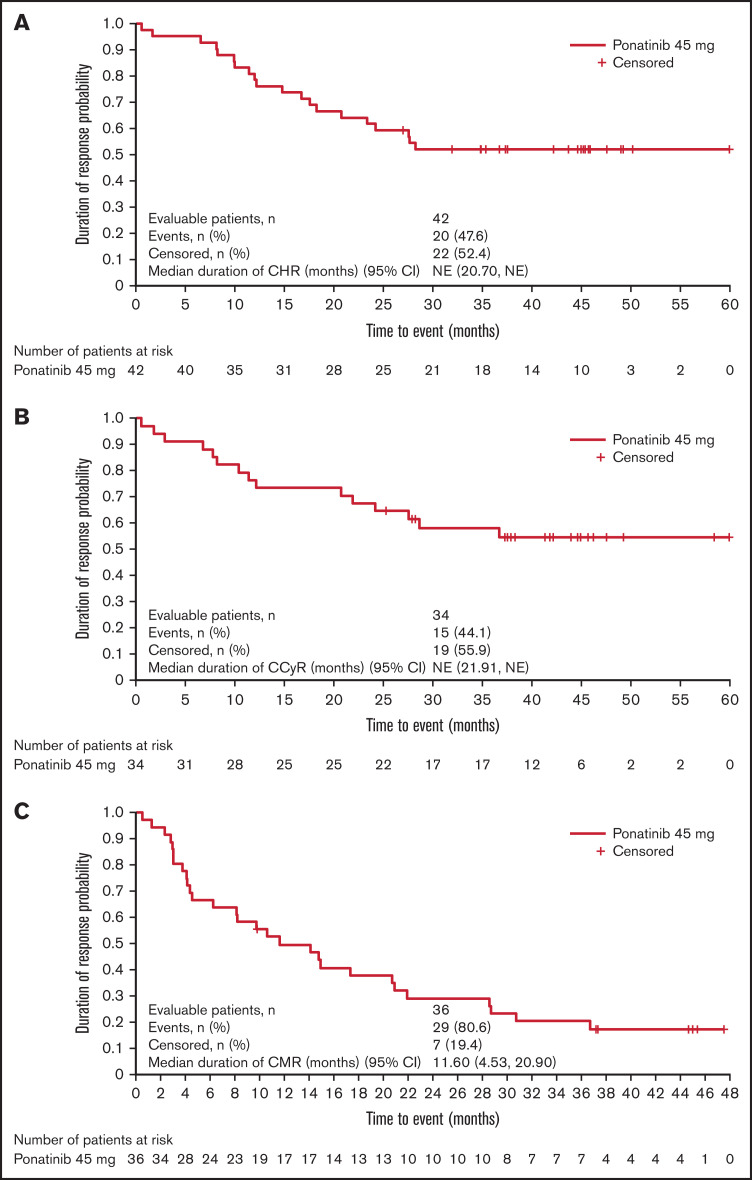
Of 44 patients treated with study drug, 42 patients (95.5%) achieved CHR at any time during treatment; 2 patients died before becoming evaluable for CHR (one patient died due to lung infection on day 6 [onset: day −6] and 1 patient died due to bilateral pneumonia on day 47 after experiencing severe neutropenia; both deaths were considered unrelated to study treatment). At week 24 (primary endpoint), 38 patients (86.4%) achieved CHR (Table 2). Median duration of CHR was not reached (95% CI 20.7, not evaluable [NE]; Figure 2A). At week 24, 24 patients (54.5%) had achieved CCyR, 18 patients (40.9%) were in CMR, and 14 patients (31.8%) were in MMR. Overall, 34 patients (77.3%) reached CCyR and 36 (81.8%) reached CMR at least once during treatment. Median (95% CI) duration of CCyR and CMR was not reached (21.91, NE) and 11.6 months (4.53, 20.90), respectively (Figure 2B-C). Overall, 6 patients experienced relapse while on ponatinib treatment (before/within 30 days of last dose of ponatinib); therefore, the relapse rate while on ponatinib was 14.3% (6/42 patients).
Table 2.
Hematologic, cytogenetic, and molecular response rates during the core phase of study
| Response | Week 6 | Week 12 | Week 24 | Week 36 | Week 48 |
|---|---|---|---|---|---|
| CHR, n (%) | 40 (90.9) | 37 (84.1) | 38 (86.4)* | 29 (65.9) | 25 (56.8) |
| CCyR, n (%) | 21 (47.7) | 19 (43.2) | 24 (54.5)* | 16 (36.4) | 15 (34.1) |
| CMR, n (%) | 21 (47.7) | 21 (47.7) | 18 (40.9) | 18 (40.9) | 16 (36.4) |
| MMR, n (%) | 15 (34.1) | 11 (25.0) | 14 (31.8)* | 8 (18.2) | 6 (13.6) |
Response rate was reported as the number of patients with response/number of total patients in the study. To be conservative, participants with missing postbaseline values were imputed as nonresponders.
Response at week 24 includes 1 participant who was assessed at week 20.
Figure 2.
Duration of response. Duration of CHR of patients who achieved CHR in the study, from diagnosis to death or loss of CHR (A); duration of CCyR of patients who achieved CCyR in the study, from diagnosis to death or loss of CCyR (B); and duration of CMR of the patients who achieved CMR in the study, from diagnosis to death or loss of CMR (C).
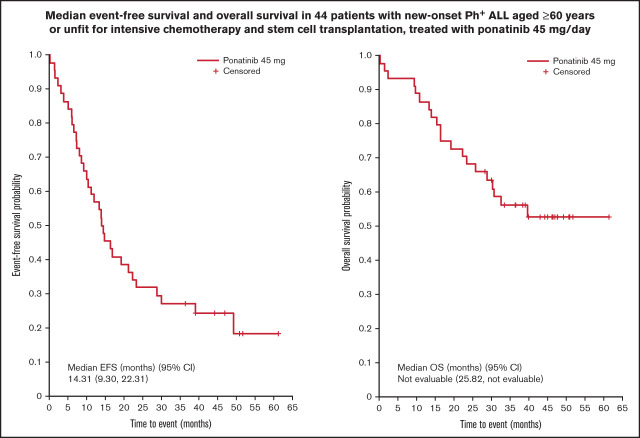
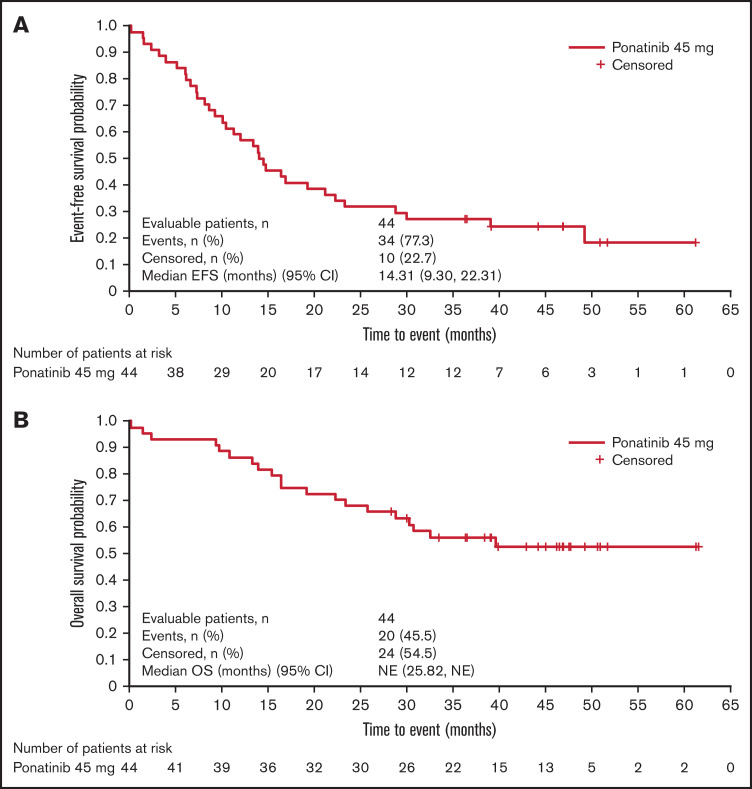
At data cutoff (24 April 2020), median EFS was 14.31 months (95% CI 9.30-22.31; Figure 3A). Median OS was not reached (95% CI 25.82, NE; Figure 3B). In total, 20 patients (45.5%) had died at the time of analysis due to ALL progression (n = 8); pneumonia (n = 2); cardiovascular disease (n = 2); lung infection (n = 1); bronchopulmonary aspergillosis with respiratory failure (n = 1); septic shock (n = 1); and sudden death (n = 1). The primary reason for death was indistinguishable for 1 patient (ALL progression and severe sepsis), and the reason for death was not recorded for the remaining 3 patients.
Figure 3.
Survival outcomes. EFS (A) and OS (B).
The most common TEAEs of any grade included rash (n = 16; 36.4%), asthenia (n = 10; 22.7%), alanine aminotransferase increase (n = 7; 15.9%), erythema (n = 7; 15.9%), and γ-glutamyltransferase increase (n = 7; 15.9%) (Table 3). Overall, 20/44 patients (45.5%) experienced an SAE. The most common SAEs were acute coronary syndrome (n = 3; 6.8%) and arterial occlusive disease, atrial fibrillation, cardiac failure, and pneumonia (each n = 2; 4.5%). Twelve patients reported TEAEs of special interest (of which 8 were grade ≥3): chest pain (n = 4), acute coronary syndrome (n = 3), arterial occlusive disease (n = 2), embolism (n = 2), and 1 each of angina pectoris, carotid arteriosclerosis, carotid artery stenosis, cerebrovascular accident, myocardial ischemia, peripheral arterial occlusive disease, and vascular graft occlusion. Thirty-seven patients had TEAEs considered related to treatment by a physician, and 13 patients had serious treatment-related TEAEs. The most common treatment-related TEAEs were skin and subcutaneous tissue disorders (23 patients). Cardiac and vascular disorders occurred in 13 (29.5%) and 12 (27.3%) patients, respectively; in 5 (cardiac) and 8 (vascular) patients, the event was considered to be related to the study drug. Dose reductions or interruptions due to TEAEs each occurred in 19 patients (43.2%). Twelve patients permanently discontinued study treatment due to TEAEs, and 5 patients had TEAEs that resulted in death (2 due to pneumonia, 1 due to bronchopulmonary aspergillosis and respiratory failure, 1 due to sudden death, and 1 due to septic shock); 3 of these 5 patients were in CHR at the time of death.
Table 3.
Treatment-emergent AEs of any grade occurring in at least 5% patients by MedDRA system organ class and preferred term
| TEAEs occurring in ≥ 5% patients, n (%) | Any grade | Grade ≥ 3 |
|---|---|---|
| Any TEAE | 41 (93.2) | 32 (72.7) |
| Cardiac disorders | 13 (29.5) | 8 (18.2) |
| Acute coronary syndrome | 3 (6.8) | 3 (6.8) |
| Atrial fibrillation | 3 (6.8) | 2 (4.5) |
| Gastrointestinal disorders | 15 (34.1) | 3 (6.8) |
| Constipation | 4 (9.1) | 0 (0.0) |
| Diarrhea | 3 (6.8) | 0 (0.0) |
| Dyspepsia | 3 (6.8) | 0 (0.0) |
| Hemorrhoids | 3 (6.8) | 0 (0.0) |
| General disorders | 18 (40.9) | 4 (9.1) |
| Asthenia | 10 (22.7) | 1 (2.3) |
| Pyrexia | 5 (11.4) | 1 (2.3) |
| Chest pain | 4 (9.1) | 0 (0.0) |
| Edema peripheral | 3 (6.8) | 0 (0.0) |
| Laboratory investigations | 14 (31.8) | 6 (13.6) |
| Alanine aminotransferase increased | 7 (15.9) | 1 (2.3) |
| γ-glutamyltransferase increased | 7 (15.9) | 3 (6.8) |
| Lipase increased | 4 (9.1) | 1 (2.3) |
| Amylase increased | 3 (6.8) | 0 (0.0) |
| Blood alkaline phosphatase increased | 3 (6.8) | 0 (0.0) |
| Metabolism and nutrition disorders | 13 (29.5) | 3 (6.8) |
| Hyperglycemia | 4 (9.1) | 1 (2.3) |
| Decreased appetite | 3 (6.8) | 0 (0.0) |
| Musculoskeletal and connective tissue disorders | 18 (40.9) | 1 (2.3) |
| Pain in extremity | 4 (9.1) | 0 (0.0) |
| Myalgia | 3 (6.8) | 0 (0.0) |
| Nervous system disorders | 11 (25.0) | 2 (4.5) |
| Headache | 6 (13.6) | 0 (0.0) |
| Respiratory, thoracic, and mediastinal disorders | 8 (18.2) | 2 (4.5) |
| Cough | 4 (9.1) | 0 (0.0) |
| Skin and subcutaneous tissue disorders | 26 (59.1) | 9 (20.5) |
| Rash | 16 (36.4) | 2 (4.5) |
| Erythema | 7 (15.9) | 2 (4.5) |
| Skin exfoliation | 3 (6.8) | 3 (6.8) |
| Skin ulcer | 3 (6.8) | 1 (2.3) |
| Surgical and medical procedures | 3 (6.8) | 0 (0.0) |
| Astringent therapy | 3 (6.8) | 0 (0.0) |
| Vascular disorders | 12 (27.3) | 7 (15.9) |
| Hypertension | 5 (11.4) | 3 (6.8) |
MedDRA, Medical Dictionary for Regulatory Activities.
Mutational analysis by direct sequencing was performed in 23 samples (17 bone marrow, 6 peripheral blood) at baseline. None of the 23 samples showed evidence of a BCR-ABL1 KD mutation at baseline. Furthermore, samples were analyzed during the study, including 6 samples (4 bone marrow, 2 peripheral blood) at week 6, 4 (3 bone marrow, 1 peripheral blood) at week 12, and 2 at week 24; 1 sample had evidence of an E459K mutation (abundance 26%) at week 6. One sample had evidence of a T315L mutation (abundance 100%) at week 12, and 1 bone marrow sample presented with a T315I mutation at week 48; these mutations were concurrent with disease relapse during ponatinib treatment.
Discussion
This study is the first to describe the activity and tolerability of ponatinib plus prednisone in unfit patients with Ph+ ALL. The regimen had promising clinical activity; at 24 weeks, 86.4% of patients were in CHR, and overall, 95.5% of patients experienced CHR during treatment. Median duration of CHR was not reached (95% CI 20.7, NE). After 24 weeks, 54.5%, 40.9%, and 31.8% of patients experienced CCyR, CMR, and MMR, respectively; median duration of CCyR and CMR was not reached (95% CI 21.91, NE) and 11.6 months (95% CI 4.53-20.90), respectively. The plateaus observed on the Kaplan-Meier plots of CHR, CCyR, and OS from ∼30 months onward are also encouraging.
This activity is notable with a nonchemotherapy regimen given the vulnerability of the patient population. Most of our patient population (79.5%) was ≥60 years, and the remaining 20.5% were considered unfit for SCT and unable to tolerate intensive chemotherapy based on the investigators’ judgment. Most patients (61.4%) completed the core phase, and 25.0% of the original population completed the extension phase. In this context, the long duration of CMR achieved is valuable and shows that a meaningful remission is still possible for elderly or frail patients, for whom more aggressive approaches are not available. Additionally, although all patients were unfit for SCT at enrollment, during ponatinib therapy, 5 patients in CHR were reevaluated and withdrawn from the study for alloSCT. Another patient who eventually received SCT was withdrawn while in CHR to receive immunotherapy, with SCT following.
Previous GIMEMA protocols have also achieved high levels of CHR without the use of intensive chemotherapy in Ph+ ALL, including CHR rates11,44,45 of 100% with imatinib in patients >60 years12 and 97% to 100% with dasatinib in adult patients.11,44,45 The CHR rate of 95.5% at any time during the present study is similar to the CHR rate with the sequential use of imatinib and nilotinib in a population of elderly or unfit patients (94% at 6 weeks),13 which may reflect the inclusion of ∼20% younger unfit patients in the patient population for each study. Although CMR rates were assessed at different timepoints, the CMR rate observed with ponatinib in older/unfit patients (41% at weeks 24 and 36) is higher than that observed in 2 separate studies of dasatinib plus steroids as induction therapy (19% and 29% at day 85).44,45 Median EFS in the current study was 14.3 months. This endpoint was not reported in the study of dasatinib plus prednisone, which instead reported disease-free survival (median 21.5 months).11 Of note, these 2 endpoints are not directly comparable because of differences in their definitions, particularly regarding the inclusion of treatment discontinuation in the definition of EFS given the high rate of discontinuations in the current study.
Promising results with another nonchemotherapeutic regimen of ponatinib in combination with venetoclax (a B-cell lymphoma-2 inhibitor) have also been observed. Preclinical studies suggest that the addition of ponatinib to venetoclax results in synergistic antileukemia activity in Ph+ ALL via inhibition of the Lck/Yes novel tyrosine kinase and prevention of downstream upregulation of antiapoptotic myeloid cell leukemia 1.46 Moreover, the combination of ponatinib, venetoclax, and dexamethasone has demonstrated early clinical efficacy in heavily pretreated patients with relapsed/refractory Ph+ ALL (complete remission or complete remission with incomplete hematologic recovery in 5/6 [83%] patients who received venetoclax 800 mg).47 Combinations of the bispecific anti-CD3/CD19 monoclonal antibody blinatumomab with TKIs (ponatinib, dasatinib, or bosutinib) have also shown efficacy in the treatment of Ph+ ALL, with high rates of CMR and favorable survival outcomes.45,48,49 Taken together, these findings suggest that combining TKIs with steroids and/or other nonchemotherapy agents may be an effective strategy to avoid chemotherapy without compromising efficacy in patients with Ph+ ALL.
Most AEs were manageable with ponatinib dose reduction. However, treatment discontinuations were observed during the core phase (n = 17/44; 38.6%) and extension phase (n = 16/27; 59.3%). The rate of discontinuations may reflect the general frailty of the patient population; however, 9/33 discontinuations (27.3%) were due to treatment toxicity, and nearly half of patients (43.2%) required dose reduction or interruption due to TEAEs, indicating that a proportion of patients could not tolerate this regimen. Overall, 5 patients died due to TEAEs, of whom 2 were in CHR. The main cause of permanent discontinuation due to TEAEs was cardiac toxicity (4/12 patients: acute coronary syndrome [n = 3] and myocardial ischemia [n = 1]). Several trials with ponatinib have reported a lower incidence of AEs, particularly cardiovascular events.35,37,50 The Ponatinib Ph+ ALL and Chronic Myeloid Leukemia Evaluation (PACE) trial reported cardiovascular toxicity in approximately 25% of cases, with exposure-adjusted incidence rates for newly occurring events decreasing over time.50 Furthermore, primary analysis results from the Optimizing Ponatinib Treatment in Chronic Phase CML (OPTIC) trial in patients with chronic myeloid leukemia reported incidence of treatment-emergent arterial occlusive events of 3.2% to 9.6% depending on the starting dose (15, 30, or 45 mg once daily).51 In our study, risk factors that include older age and age-related comorbidities, such as hypertension and dyslipidemia, may have played a role in the development of cardiovascular and other toxicities. Therefore, accurate cardiac screening is recommended before patients start ponatinib treatment. It is possible that prednisone treatment may also have contributed to some of the observed toxicities, although previous studies of TKIs administered with prednisone have reported relatively low rates of treatment discontinuation or interruption,11,12 suggesting that concomitant steroid use is unlikely to cause severe toxicity when used in combination with TKIs. Although based on induction phases only (45 days and 84 days, respectively), only 23% of participants treated with imatinib plus steroids experienced either a dose reduction or temporary discontinuation due to extrahematologic toxicities, whereas in the dasatinib plus prednisone study, permanent and temporary treatment discontinuations for any reason occurred in 8% and 21% of patients, respectively.11,12 In the current study, 6 patients (14%) permanently discontinued due to excessive toxicity during the 48-week core phase, and 9 (20%) discontinued during the entire study. Together with the high frequency of dose reductions or interruptions, these results suggest that a lower dose of ponatinib could potentially reduce the incidence of discontinuations and dose modifications in older or unfit patients.
A trial from the MD Anderson Cancer Center (MDACC) investigated the activity of ponatinib plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone52 and reported better outcomes for OS and lower rates of AEs compared with our study. Differences in survival between the MDACC study and our study could be due to the younger median age of the MDACC patient population (51 years vs 66.5 years), different biologic backgrounds of Ph+ ALL due to the difference in age, and the fact that patients in the MDACC study were fit enough to tolerate intensive chemotherapy, unlike our study patients. The lower incidence of AEs may also reflect the younger population in the MDACC study. In addition, the MDACC study used a different dosing strategy, whereby the protocol was amended to reduce the dose of ponatinib from 45 mg to 30 mg daily at cycle 2, with a further reduction to 15 mg once a CMR was achieved.52 A similar response-based strategy was also shown to be effective in the ponatinib dose-optimization study in patients with chronic myeloid leukemia.51 As such, it is possible that either a reduced starting dose of ponatinib or a dose-reduction strategy based on response may have an improved benefit–risk profile compared with continuous 45-mg daily dosing in older or unfit patients with Ph+ ALL.
Few mutated clones were detected among ponatinib-treated patients. Among the 23 patients evaluated at baseline, none presented with BCR-ABL1 KD mutation. Among the 14 patients evaluated during the study, only 3 patients harbored a BCR-ABL1 KD mutation (1 with E459K at week 6, 1 with T315L at week 12, and 1 with T315I at time of relapse on ponatinib). This observation is consistent with results from other studies,25,52 suggesting that a non–BCR-ABL1–dependent mechanism of resistance underlies most relapses. T315L was previously shown to confer resistance to ponatinib,53 and it is sensitive to axitinib in vitro.54,55 Future studies are needed to evaluate mechanisms of relapse for patients with Ph+ ALL treated with ponatinib-based regimens.
Because post-remission therapy with ponatinib alone may not be sufficient for preventing relapse and nonrelapse mortality in all patients, it is important to consider what consolidation options are available to decrease the likelihood of relapse, particularly because many patients are not eligible for myeloablative conditioning (MAC) alloSCT due to their age and comorbidities. In addition to MAC alloSCT, other consolidation approaches have been evaluated, including reduced-intensity conditioning (RIC) alloSCT, autologous SCT (autoSCT), and chemotherapy as well as newer drugs such as blinatumomab. Several retrospective studies suggest that survival outcomes are similar after RIC and MAC alloSCT, albeit at the cost of a higher risk of relapse with RIC alloSCT.56 Additionally, results of the Cancer and Leukemia Group B (CALGB) 10001 study demonstrated that autoSCT following imatinib plus sequential chemotherapy is a safe and effective alternative to alloSCT for Ph+ ALL patients without sibling donors.57 Likewise, the CALGB 10701 study found that patients undergoing alloSCT, autoSCT, or chemotherapy following dasatinib and dexamethasone induction therapy had similar treatment outcomes.58 Updated results of a phase 2 study investigating steroids plus dasatinib as induction therapy in adults with Ph+ ALL followed by 2 cycles of blinatumomab continued to show high molecular response rates and impressive survival outcomes, with 29/58 patients who started on blinatumomab undergoing subsequent transplantation.45,48 This latter finding suggests that nonchemotherapeutic regimens can be optimized further with other noncytotoxic therapies.
Our study had several limitations. The trial was a phase 2, single-arm study that recruited a small number of patients; 61.4% and 25.0% of patients completed the core phase and full protocol, respectively. Given the small patient sample, only limited conclusions can be drawn based on data collected during the extension phase. The drug regimen could be improved by including low-dose chemotherapy or new drugs such as inotuzumab and blinatumomab, which were shown to be effective in relapsed or refractory Ph+ ALL59,60 and in a measurable residual disease-positive setting.61 Reduction of the ponatinib dose is a promising strategy to reduce toxicities in a measurable minimal disease-driven strategy.
Our results, together with evidence in younger fit patients,38,52 suggest that ponatinib is an effective TKI across different populations of patients with new-onset Ph+ ALL and that the combination with prednisone appears to be a promising option for older and/or unfit patients, albeit with the potential consideration of a lower dose of ponatinib to minimize toxicity. The choice of ponatinib must also be tailored based on the cardiovascular risk of individual patients. To date, there are no results available from prospective, randomized trials comparing different TKIs in patients with Ph+ ALL; however, ponatinib plus prednisone showed promising efficacy in the current study in a frail patient population with few alternative options.
Acknowledgments
Medical writing support was provided by Alligent EU (Envision Pharma Group) and funded by Incyte Biosciences International.
Authorship
Contribution: All authors were involved in drafting of the manuscript and approved the final draft.
M. Baccarani, R.F., G. Martinelli, and M.V. designed the study; M.C.A., M. Baccarani, M. Bonifacio, M. Bocchia, A. Candoni, M.C., S.D’A., P.d.F., A.F., F.F., L.F., F.G., P.G., R.M.L., M.L., G. Marconi, G. Martinelli, M.P.M., A.P., C.P., S.P., C. Sartor, C. Selleri, F.S., A.T., P.T., S.T., and A.V. were responsible for patient care; P.F. coordinated the trial; M.C.A. and V.R. performed research; C.T. and V.R. performed quantification analysis and S.S. performed mutation analysis on study samples; M.G. contributed to database setup and transfer of data to Incyte, and reviewed and cleaned data; Z.Z. and M.W. contributed to data analysis.
Conflict-of-interest disclosure: M. Baccarani received honoraria and travel grants from Incyte, Novartis, Takeda, and Fusion Pharma. M. Bocchia received honoraria from Incyte, Celgene, and Janssen. M. Bonifacio received consultancy fees from Novartis, Pfizer, Incyte, and Amgen. A. Candoni received honoraria from Incyte. M.C. received honoraria from AbbVie, GlaxoSmithKline, Bristol-Myers Squibb, Adaptive Biotechnologies, Takeda, Janssen, Celgene, and Amgen. R.F. was a member of the board or received consultancy fees from Janssen, Amgen, Incyte, Novartis, Pfizer, and AbbVie. R.M.L. had an advisory role with AbbVie, Janssen, Jazz, Daiichi Sankyo, Servier, and Novartis and received research funding from Celgene. G. Martinelli provided consultation for Ariad/Incyte, Janssen, Pfizer, Celgene, Amgen, J&J, and Roche. M.P.M. received honoraria or consultancy fees from AbbVie, Amgen, Celgene, Janssen, Novartis, Pfizer, and Jazz Pharmaceuticals. C.P. received honoraria from Amgen, Astellas, Pfizer, and Novartis and participated in advisory boards with Pfizer, Janssen, AbbVie, and Novartis. A.T. participated in advisory boards with Janssen, AstraZeneca, AbbVie, and Beigene. S.S. was a consultant for Novartis, Bristol-Myers Squibb, and Incyte Biosciences. M.V. is a member of the GIMEMA Board of Directors. M.G. and M.W. are employees of Incyte. Z.Z. was an employee of Incyte at the time of manuscript preparation and owns stock in Incyte. The remaining authors declare no competing financial interests.
Correspondence: Giovanni Martinelli, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (I.R.S.T.) I.R.C.C.S., Meldola, Italy; e-mail: giovanni.martinelli@irst.emr.it.
References
- 1.Chiaretti S, Vitale A, Cazzaniga G, et al. Clinico-biological features of 5202 patients with acute lymphoblastic leukemia enrolled in the Italian AIEOP and GIMEMA protocols and stratified in age cohorts. Haematologica. 2013;98(11):1702-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gleissner B, Gökbuget N, Bartram CR, et al. ; German Multicenter Trials of Adult Acute Lymphoblastic Leukemia Study Group . Leading prognostic relevance of the BCR-ABL translocation in adult acute B-lineage lymphoblastic leukemia: a prospective study of the German Multicenter Trial Group and confirmed polymerase chain reaction analysis. Blood. 2002;99(5):1536-1543. [DOI] [PubMed] [Google Scholar]
- 3.Faderl S, Kantarjian HM, Thomas DA, et al. Outcome of Philadelphia chromosome-positive adult acute lymphoblastic leukemia. Leuk Lymphoma. 2000;36(3-4):263-273. [DOI] [PubMed] [Google Scholar]
- 4.Faderl S, Kantarjian HM, Talpaz M, Estrov Z. Clinical significance of cytogenetic abnormalities in adult acute lymphoblastic leukemia. Blood. 1998;91(11):3995-4019. [PubMed] [Google Scholar]
- 5.Ravandi F, Kebriaei P. Philadelphia chromosome-positive acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 2009;23(5):1043-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wassmann B, Pfeifer H, Scheuring U, et al. Therapy with imatinib mesylate (Glivec) preceding allogeneic stem cell transplantation (SCT) in relapsed or refractory Philadelphia-positive acute lymphoblastic leukemia (Ph+ALL). Leukemia. 2002;16(12):2358-2365. [DOI] [PubMed] [Google Scholar]
- 7.Wassmann B, Gökbuget N, Scheuring UJ, et al. ; GMALL Study Group . A randomized multicenter open label phase II study to determine the safety and efficacy of induction therapy with imatinib (Glivec, formerly STI571) in comparison with standard induction chemotherapy in elderly (>55 years) patients with Philadelphia chromosome-positive (Ph+/BCR-ABL+) acute lymphoblastic leukemia (ALL) (CSTI571ADE 10). Ann Hematol. 2003;82(11):716-720. [DOI] [PubMed] [Google Scholar]
- 8.Ottmann OG, Druker BJ, Sawyers CL, et al. A phase 2 study of imatinib in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoid leukemias. Blood. 2002;100(6):1965-1971. [DOI] [PubMed] [Google Scholar]
- 9.Chalandon Y, Thomas X, Hayette S, et al. ; Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) . Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. 2015;125(24):3711-3719. [DOI] [PubMed] [Google Scholar]
- 10.Chiaretti S, Vitale A, Vignetti M, et al. A sequential approach with imatinib, chemotherapy and transplant for adult Ph+ acute lymphoblastic leukemia: final results of the GIMEMA LAL 0904 study. Haematologica. 2016;101(12):1544-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foà R, Vitale A, Vignetti M, et al. ; GIMEMA Acute Leukemia Working Party . Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;118(25):6521-6528. [DOI] [PubMed] [Google Scholar]
- 12.Vignetti M, Fazi P, Cimino G, et al. Imatinib plus steroids induces complete remissions and prolonged survival in elderly Philadelphia chromosome-positive patients with acute lymphoblastic leukemia without additional chemotherapy: results of the Gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA) LAL0201-B protocol. Blood. 2007;109(9):3676-3678. [DOI] [PubMed] [Google Scholar]
- 13.Martinelli G, Papayannidis C, Piciocchi A, et al. Extremely high rate of complete hematological response of elderly Ph+ acute lymphoblastic leukemia (ALL) patients by innovative sequential use of Nilotinib and Imatinib. A GIMEMA Protocol LAL 1408 [abstract]. Cancer Res. 2014;74(19 suppl):5552. [Google Scholar]
- 14.Daver N, Thomas D, Ravandi F, et al. Final report of a phase II study of imatinib mesylate with hyper-CVAD for the front-line treatment of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. 2015;100(5):653-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassan R, Rossi G, Pogliani EM, et al. Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00. J Clin Oncol. 2010;28(22):3644-3652. [DOI] [PubMed] [Google Scholar]
- 16.Tanguy-Schmidt A, Rousselot P, Chalandon Y, et al. Long-term follow-up of the imatinib GRAAPH-2003 study in newly diagnosed patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: a GRAALL study. Biol Blood Marrow Transplant. 2013;19(1):150-155. [DOI] [PubMed] [Google Scholar]
- 17.Lickliter JD, Taylor K, Szer J, et al. ; Australasian Leukaemia and Lymphoma Group . An imatinib-only window followed by imatinib and chemotherapy for Philadelphia chromosome-positive acute leukemia: long-term results of the CMLALL1 trial. Leuk Lymphoma. 2015;56(3):630-638. [DOI] [PubMed] [Google Scholar]
- 18.Ribera JM, Oriol A, González M, et al. ; Grupo Español de Trasplante Hemopoyético Groups . Concurrent intensive chemotherapy and imatinib before and after stem cell transplantation in newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia: final results of the CSTIBES02 trial. Haematologica. 2010;95(1):87-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DY, Joo YD, Lim SN, et al. ; Adult Acute Lymphoblastic Leukemia Working Party of the Korean Society of Hematology . Nilotinib combined with multiagent chemotherapy for newly diagnosed Philadelphia-positive acute lymphoblastic leukemia. Blood. 2015;126(6):746-756. [DOI] [PubMed] [Google Scholar]
- 20.Ravandi F, O’Brien S, Thomas D, et al. First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia. Blood. 2010;116(12):2070-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon JH, Yhim HY, Kwak JY, et al. Minimal residual disease-based effect and long-term outcome of first-line dasatinib combined with chemotherapy for adult Philadelphia chromosome-positive acute lymphoblastic leukemia. Ann Oncol. 2016;27(6):1081-1088. [DOI] [PubMed] [Google Scholar]
- 22.Ravandi F, O’Brien SM, Cortes JE, et al. Long-term follow-up of a phase 2 study of chemotherapy plus dasatinib for the initial treatment of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer. 2015;121(23):4158-4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rousselot P, Coudé MM, Gokbuget N, et al. ; European Working Group on Adult ALL (EWALL) group . Dasatinib and low-intensity chemotherapy in elderly patients with Philadelphia chromosome-positive ALL. Blood. 2016;128(6):774-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ottmann OG, Wassmann B, Pfeifer H, et al. ; GMALL Study Group . Imatinib compared with chemotherapy as front-line treatment of elderly patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL). Cancer. 2007;109(10):2068-2076. [DOI] [PubMed] [Google Scholar]
- 25.Soverini S, De Benedittis C, Machova Polakova K, et al. Unraveling the complexity of tyrosine kinase inhibitor-resistant populations by ultra-deep sequencing of the BCR-ABL kinase domain. Blood. 2013;122(9):1634-1648. [DOI] [PubMed] [Google Scholar]
- 26.Soverini S, De Benedittis C, Papayannidis C, et al. Drug resistance and BCR-ABL kinase domain mutations in Philadelphia chromosome-positive acute lymphoblastic leukemia from the imatinib to the second-generation tyrosine kinase inhibitor era: the main changes are in the type of mutations, but not in the frequency of mutation involvement. Cancer. 2014;120(7):1002-1009. [DOI] [PubMed] [Google Scholar]
- 27.Soverini S, Gnani A, Colarossi S, et al. Philadelphia-positive patients who already harbor imatinib-resistant Bcr-Abl kinase domain mutations have a higher likelihood of developing additional mutations associated with resistance to second- or third-line tyrosine kinase inhibitors. Blood. 2009;114(10):2168-2171. [DOI] [PubMed] [Google Scholar]
- 28.Cortes JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012;367(22): 2075-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soverini S, Vitale A, Poerio A, et al. Philadelphia-positive acute lymphoblastic leukemia patients already harbor BCR-ABL kinase domain mutations at low levels at the time of diagnosis. Haematologica. 2011;96(4):552-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soverini S, De Benedittis C, Papayannidis C, et al. Clinical impact of low-burden BCR-ABL1 mutations detectable by amplicon deep sequencing in Philadelphia-positive acute lymphoblastic leukemia patients. Leukemia. 2016;30(7):1615-1619. [DOI] [PubMed] [Google Scholar]
- 31.O’Hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16(5):401-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Hare T, Deininger MW, Eide CA, Clackson T, Druker BJ. Targeting the BCR-ABL signaling pathway in therapy-resistant Philadelphia chromosome-positive leukemia. Clin Cancer Res. 2011;17(2):212-221. [DOI] [PubMed] [Google Scholar]
- 33.Seymour JF, Kim DW, Rubin E, et al. A phase 2 study of MK-0457 in patients with BCR-ABL T315I mutant chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood Cancer J. 2014;4(8):e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker WT, Yeung DT, Yeoman AL, et al. The impact of multiple low-level BCR-ABL1 mutations on response to ponatinib. Blood. 2016;127(15):1870-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah NP, Talpaz M, Deininger MW, et al. Ponatinib in patients with refractory acute myeloid leukaemia: findings from a phase 1 study. Br J Haematol. 2013;162(4):548-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Incyte Biosciences Distribution BV. Iclusig (ponatinib). Summary of product characteristics. Available at: https://www.ema.europa.eu/documents/product-information/iclusig-epar-product-information_en.pdf. Accessed 21 January 2021.
- 37.Jabbour E, Short NJ, Ravandi F, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: long-term follow-up of a single-centre, phase 2 study. Lancet Haematol. 2018;5(12):e618-e627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasaki K, Jabbour EJ, Ravandi F, et al. Hyper-CVAD plus ponatinib versus hyper-CVAD plus dasatinib as frontline therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: a propensity score analysis. Cancer. 2016;122(23):3650-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405. [DOI] [PubMed] [Google Scholar]
- 40.Baccarani M, Saglio G, Goldman J, et al. ; European LeukemiaNet . Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108(6):1809-1820. [DOI] [PubMed] [Google Scholar]
- 41.Baccarani M, Cortes J, Pane F, et al. ; European LeukemiaNet . Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27(35):6041-6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38(1):29-41. [Google Scholar]
- 43.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chiaretti S, Vitale A, Elia L, et al. Multicenter total therapy Gimema LAL 1509 protocol for de novo adult Ph+ acute lymphoblastic leukemia (ALL) patients. Updated results and refined genetic-based prognostic stratification. Blood. 2015;126(23):81. [Google Scholar]
- 45.Foà R, Bassan R, Vitale A, et al. ; GIMEMA Investigators . Dasatinib-blinatumomab for Ph-positive acute lymphoblastic leukemia in adults. N Engl J Med. 2020;383(17):1613-1623. [DOI] [PubMed] [Google Scholar]
- 46.Leonard JT, Rowley JS, Eide CA, et al. Targeting BCL-2 and ABL/LYN in Philadelphia chromosome-positive acute lymphoblastic leukemia. Sci Transl Med. 2016;8(354):354ra114. [DOI] [PubMed] [Google Scholar]
- 47.Short NJ, Konopleva M, Kadia T, et al. An effective chemotherapy-free regimen of ponatinib plus venetoclax for relapsed/refractory Philadelphia chromosome-positive acute lymphoblastic leukemia. Am J Hematol. 2021;96(7):E229-E232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiaretti S, Bassan R, Vitale A, et al. Updated results of the Gimema LAL2116, D-Alba trial, for newly diagnosed adults with Ph+ ALL. Presented at the European Hematology Association Virtual Congress; 17-19 June 2021. [Google Scholar]
- 49.Assi R, Kantarjian H, Short NJ, et al. Safety and efficacy of blinatumomab in combination with a tyrosine kinase inhibitor for the treatment of relapsed Philadelphia chromosome-positive leukemia. Clin Lymphoma Myeloma Leuk. 2017;17(12):897-901. [DOI] [PubMed] [Google Scholar]
- 50.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132(4):393-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cortes JE, Apperley J, Lomaia E, et al. OPTIC primary analysis: a dose-optimization study of 3 starting doses of ponatinib (PON) [abstract]. J Clin Oncol. 2021;39(15 suppl):7000. [Google Scholar]
- 52.Jabbour E, Kantarjian H, Ravandi F, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: a single-centre, phase 2 study. Lancet Oncol. 2015;16(15):1547-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang D, Pan H, Wang Y. T315L: a novel mutation within BCR-ABL kinase domain confers resistance against ponatinib. Leuk Lymphoma. 2017;58(7):1733-1735. [DOI] [PubMed] [Google Scholar]
- 54.Pemovska T, Johnson E, Kontro M, et al. Axitinib effectively inhibits BCR-ABL1(T315I) with a distinct binding conformation. Nature. 2015; 519(7541):102-105. [DOI] [PubMed] [Google Scholar]
- 55.Zabriskie MS, Eide CA, Yan D, et al. Extreme mutational selectivity of axitinib limits its potential use as a targeted therapeutic for BCR-ABL1-positive leukemia. Leukemia. 2016;30(6):1418-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leonard JT, Hayes-Lattin B. Reduced intensity conditioning allogeneic hematopoietic stem cell transplantation for acute lymphoblastic leukemia: current evidence, and improving outcomes going forward. Curr Hematol Malig Rep. 2018;13(4):329-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wetzler M, Watson D, Stock W, et al. Autologous transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia achieves outcomes similar to allogeneic transplantation: results of CALGB Study 10001 (Alliance). Haematologica. 2014;99(1):111-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wieduwilt MJ, Yin J, Wetzler M, et al. A phase II study of dasatinib and dexamethasone as primary therapy followed by transplantation for adults with newly diagnosed Ph/BCR-ABL1-positive acute lymphoblastic leukemia (Ph+ ALL): final results of Alliance/CALGB study 10701. Blood. 2018;132(suppl 1):309. [Google Scholar]
- 59.Martinelli G, Boissel N, Chevallier P, et al. Complete hematologic and molecular response in adult patients with relapsed/refractory Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia following treatment with blinatumomab: results from a phase II, single-arm, multicenter study. J Clin Oncol. 2017;35(16):1795-1802. [DOI] [PubMed] [Google Scholar]
- 60.Kantarjian H, Thomas D, Jorgensen J, et al. Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. Lancet Oncol. 2012;13(4):403-411. [DOI] [PubMed] [Google Scholar]
- 61.Topp MS, Gökbuget N, Zugmaier G, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 2012;120(26):5185-5187. [DOI] [PubMed] [Google Scholar]