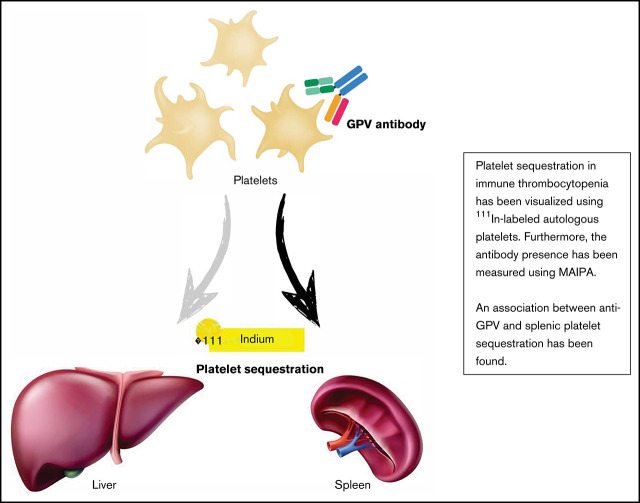
Key Points
Anti-GPV antibodies are associated with a splenic sequestration pattern in this cohort.
In the presence of antibodies, platelet clearance rate was associated with splenic sequestration.
Visual Abstract
Abstract
Antiglycoprotein (anti-GP) antibodies play an important role in the pathophysiology of immune thrombocytopenia (ITP). The sequestration pattern of platelets in the spleen and liver can be studied with 111In-labeled autologous platelet scans. No studies have investigated the role of anti-GP antibodies in sequestration patterns in ITP patients. In this study, we examined the association between antibodies and (1) platelet sequestration site and (2) clearance rate of platelets. All ITP patients receiving an 111In-labeled autologous platelet study between 2014 and 2018 were included. Antibodies were measured using the direct MAIPA method to determine the presence and titer of anti-GPIIb/IIIa, anti-GPIb/IX, and anti-GPV antibodies. Multivariate regression models were used to study the association between anti-GP antibodies, sequestration site, and clearance rate. Seventy-four patients were included, with a mean age of 36 years. Forty-seven percent of the patients showed a predominantly splenic sequestration pattern, 29% mixed, and 25% a hepatic pattern. In 53% of the patients, anti-GP antibodies were detected. Regression models showed a significant association between splenic sequestration and GPV autoantibodies. Furthermore, in patients where antibodies were present, the clearance rate was higher in patients with a splenic sequestration. Anti-GPV antibodies are associated with a splenic sequestration pattern in ITP patients. These associations provide insight into the possible pathophysiological mechanisms of ITP, which may lead to better detection and treatment of this partly idiopathic and prevalent disease.
Introduction
Immune thrombocytopenia (ITP) is an acquired autoimmune disorder characterized by low platelet numbers (peripheral blood platelet count <100 × 109/L). The thrombocytopenia results from increased clearance of platelets combined with impaired production of platelets.1 ITP is generally manifested by an increased bleeding tendency ranging from minor skin manifestations with petechiae and/or purpura to mucosal bleedings and possible fatal intracranial hemorrhages. In addition, patients with ITP experience a lower quality of life and difficulties in continuing their normal life routine, such as work.2
The pathophysiology of ITP is heterogeneous and partly unknown. Several pathways have been described in the development and chronicity of ITP. The most important mechanisms are (1) antibody-mediated platelet and/or megakaryocyte destruction, clearance, and inhibited production, and (2) T- and NK-cell activity against platelets and/or megakaryocytes.3-6 When autoantibodies are detected in ITP, the majority of those autoantibodies are directed against epitopes on glycoprotein (GP) IIb/IIIa, Ib/IX, and/or V.6-8 Megakaryocytes (MKs) are inhibited in their proplatelet formation and platelet-release by autoantibodies.9,10 These autoantibodies can lead to both splenic and hepatic clearance of platelets. Antibody-opsonized platelets may be recognized by FcγR-bearing phagocytes leading to mostly splenic clearing of platelets. Anti-GPIIb/IIIa is the most detected antibody in ITP, leading to splenic removal of platelets. It was reported that anti-GPIb/IX antibodies may lead to accelerated desialylation of platelets and subsequently earlier recognition by the Ashwell Morell Receptor on hepatocytes, thus increasing the otherwise physiologic hepatic clearance of platelets.11,12
It is not known which of the pathways are responsible for thrombopenia in the specific ITP patient. A better understanding of these mechanisms could have clinical implications, such as predicting the effectivity of splenectomy as a treatment of refractory ITP.
Imaging techniques using nuclear agents, such as 111In, are able to visualize and quantify platelet sequestration in splenic, hepatic, or mixed patterns. Studies moreover suggest that when a splenic sequestration is found, a splenectomy shows higher success rates compared with a mixed or hepatic pattern.13,14
In ITP, a possible association between platelet autoantibody specificity and sequestration site of platelets has not been studied previously.15,16 One of the reasons for this lack of data might be that the autoantibody detection in ITP has only recently become sensitive and specific enough.8,17-20
This study aims to investigate the association between GP autoantibodies with anti-GPIIb/IIIa, GPIb/IX, or GPV specificity and sequestration site of platelets in a cohort of treatment relapse and refractory ITP patients. Secondary objectives include investigating the role of platelet clearance rate calculated for both sequestration sites and in relation to the presence or absence of platelet autoantibodies.
Methods
Design and patients
A retrospective cohort study included all adult ITP patients who had an 111In-labeled platelet sequestration study in The Netherlands between 2014 and 2018. A sequestration study was performed if an ITP patient had an indication for successive therapy and splenectomy is considered as one of the therapeutic options. Therefore, the results of the sequestration studies were not used as a direct preoperative screening in this cohort.
Antibody measurements
Blood samples from all patients were taken at the first day of the 111In-labeled sequestration study as a routine diagnostic procedure and were sent to Sanquin Diagnostic Services in Amsterdam, The Netherlands, for autoantibody detection. Patient platelets, platelet eluates, and sera were tested within 24 hours after sampling, with the direct and indirect platelet immunofluorescence test (PIFT) as described by von dem Borne et al7,21 for the presence of platelet-associated and free-circulating autoantibodies of the IgG and IgM class. Furthermore, a modified direct and indirect MAIPA was used, as described by Kiefel et al,22 to investigate the presence of GPIIb/IIIa, GPIb/IX, and GPV-associated autoantibodies of the IgG class.7,8 The direct MAIPA was used in our primary analyses with a cutoff for positivity of AU = 0.130.
111In-labeled sequestration study
The 111In-labeled sequestration study is performed in accordance with the recommendations of The International Committee for Standardization in Hematology Panel on Diagnostic Application of Radionuclides.23 In short, 50 ml whole blood is derived from the patient and labeled with 111In tropolone. Autologous 111In-labeled platelets are reinjected in ITP patients. At t = 30 minutes, 3 hours, 24 hours, and 48 hours after reinjection of labeled platelets, new blood samples are taken for platelet survival studies. γ camera used for this study is 111In NMG, with peaks at 172 and 247 keV. Dynamic series are made for 25 minutes at t = 30 minutes. At t = 30 minutes, 3 hours, 24 hours, and 48 hours, static series are made for 5 minutes. At all series, both posterior and anterior scans are made. Categorization of platelet sequestration patterns was adopted from Najean et al9 where scan outcome is a ratio between liver and spleen in percentages at 30 minutes, 24 hours, and 48 hours after reinjection of labeled platelets. Percentages of sequestration in spleen and liver add up to 100%. In the clinical setting, the sequestration is categorized in splenic, mixed, and hepatic pattern based on the splenic:liver ratio (S:L ratio). S:L ratio >1.4 is described as a splenic sequestration pattern, 0.8 < 1.4 as mixed pattern, and <0.8 as hepatic platelet sequestration pattern. The percentage of splenic sequestration (0% to 100%) is used as a continuous variable in our primary analyses. The clearance rate is measured using the loss of radioactivity in the intervals between 0 hours and 24 hours and between 24 hours and 48 hours. At time point 0 hours, the radioactivity is 100%. Loss of radioactivity is used as a proxy for platelet clearance rate. The higher the loss of radioactivity, the higher the clearance rate is hypothesized to be. The Haga Teaching Hospital in The Hague, The Netherlands, performed all 111In-labeled sequestration studies.
Data and statistics
This study was approved by the central medical ethical review board in The Netherlands and by the local review board of the Haga Teaching Hospital in The Hague. Informed consent was waived due to the retrospective design of the study. Data on patient characteristics, antibody measurements, and 111In-labeled sequestration study were collected from the electronic patient files. Statistics were performed in IBM SPSS version 25. Patient characteristics were analyzed in a descriptive manner and stratified for antibody presence. We used linear regression models to investigate the associations between antibody presence and sequestration site. Age, sex, platelet count, and treatment lines prior to scan were used as covariates in the multivariate models.
Results
Baseline characteristics
The baseline (Table 1) shows the cohort consisting of 74 ITP patients, of whom 66% are females. Mean age at the time of the 111In-labeled sequestration study was 43 (±17) years; most patients (74%) were younger than 60 years. Mean age at the time of diagnosis of ITP was 36 (±18) years. Median platelet count at the time of the scan was 52 × 109/L (IQR 32-116), 22% of all patients had platelet counts lower than 30 × 109/L. The majority of the patients (69%) had successful medicinal treatment of their ITP at the time of scan and therefore had relatively high platelet counts. Bleeding scores were low, with only 7% of patients having a WHO bleeding score ≥3 from the time of diagnosis. 35% of the patients received 1 to 2 lines of therapy, 53% received 3 or 4 lines of therapy, and 13% received 5 or more lines of therapy prior to the scan.
Table 1.
Baseline characteristics
| All (n = 74), % | Anti-GP positive* (n = 30), % | Anti-GP negative (n = 27), % | |
|---|---|---|---|
| Age at scan, years | 43 ± 17 | 43 ± 17 | 44 ± 17 |
| <60 y | 74 | 73 | 74 |
| >60 y | 22 | 27 | 19 |
| Age at Dx ITP, years | 36 ± 18 | 39 ± 17 | 34 ± 20 |
| Gender, % female | 66 | 70 | 52 |
| Platelet count, x109/L | 52 [IQR 32,116] | 67[IQR 39,140] | 46[IQR 33,84] |
| Comorbidities >1 | 31 | 33 | 33 |
| Highest bleeding score | |||
| WHO 1 | 30 | 23 | 37 |
| WHO 2 | 49 | 60 | 44 |
| WHO 3 or higher | 7 | 0 | 11 |
| Treatment history | |||
| Initial wait & see | 10 | 10 | 15 |
| Corticosteroids | 84 | 93 | 82 |
| Rituximab | 23 | 33 | 15 |
| IVIG | 35 | 30 | 52 |
| TPO-ra | 43 | 43 | 44 |
| Number of treatment lines in history | |||
| 1-2 | 35 | 27 | 26 |
| 3-4 | 53 | 53 | 44 |
| 5 or more | 13 | 13 | 15 |
| Treatment strategy at time of scan | |||
| No treatment | 43 | 40 | 44 |
| Corticosteroid alone | 27 | 33 | 26 |
| TPO-ra alone | 19 | 17 | 15 |
| Other treatments† | 11 | 10 | 15 |
17/74 (23%) patients had no antibody testing.
Comorbidities was defined as relevant systemic comorbidity in 1 of the systems: heart disease, lung disease, kidney failure, malignancy or autoimmune disease.
WHO bleeding scale: 0 = no bleeding, 1 = petechiae, 2 = mild blood loss, 3 = gross blood loss, and 4 = debilitating.
Antibodies were considered present when the direct MAIPA was 0.130 or higher.
Other treatments consisted of either IVIG alone (n = 1 patient) or a combination of corticosteroid & IVIG (n = 4 patients), combination of corticosteroid and TPO-ra (n = 3 patients).
TPO-RA, thrombopoietin receptor agonist; IVIG, intravenous immune globulin.
Association between antibodies and sequestration pattern
Of the 57 tested patients, 30 (53%) showed 1 or more GP antibody specificity. Forty-seven percent of the patients had a splenic sequestration pattern, 29% mixed, and 25% had a hepatic pattern. Table 2 shows the number of patients with a splenic, mixed, and hepatic sequestration pattern, stratified by this presence of autoantibodies. In the patients where autoantibodies were present, 47% had a splenic pattern, 30% mixed, and 23% had a hepatic pattern. In the patients where no autoantibodies were found, 41% had a splenic pattern, 33% mixed, and 26% had a hepatic pattern. The various sequestration patterns were not different if the full cohort was compared with that of the patients tested for platelet autoantibodies (data not shown) or in the subgroups of patients with or without platelet autoantibodies.
Table 2.
Sequestration pattern at 48 hours stratified by antibody
| Pattern | Antibodies pos* (n = 30) n (%) | Antibodies neg (n = 27) n (%) |
|---|---|---|
| Splenic | 14 (47) | 11 (41) |
| Mixed | 9 (30) | 9 (33) |
| Hepatic | 7 (23) | 7 (26) |
Antibodies were considered present when the direct MAIPA was 0.130 or higher.
Table 3 shows the presence of autoantibodies stratified by sequestration pattern. Patients with a splenic pattern showed a higher percentage of anti-GPIIb/IIIa and GPV compared with patients with a hepatic pattern (71% vs 57% and 71% vs 29%, respectively). The opposite was found for anti-GPIb/IX, where patients with a hepatic pattern show a percentage of 71% vs 29% in the splenic pattern group.
Table 3.
Type of antibodies in patients where antibodies were found stratified by sequestration pattern at 48 hours
| Pattern | Anti-GPIIb/IIIa level* n (%) | Anti-GPIb/IX level* n (%) | Anti-GPV level* n (%) |
|---|---|---|---|
| Splenic, n = 14 | 10 (71) | 6 (43) | 10 (71) |
| Mixed, n = 9 | 5 (56) | 4 (44) | 7 (78) |
| Hepatic, n = 7 | 4 (57) | 6 (86) | 2 (29) |
Patients can have more than 1 antibody present; thus the groups are not mutually exclusive.
Table 4 shows the association between the sequestration site and antibody type in crude and multivariate models. The univariable association between platelet sequestration pattern and anti-GPV antibodies was 0.011 (95% CI 0.001-0.021), P = .034. The coefficient for anti-GPIIb/IIIa and anti-GPIb/IX antibodies was 0.003 (95% CI 0.009-0.015), P = .635; and 0.001 (95% CI 0.012-0.014), P = .853, respectively. The results from the multivariate models, including age, sex, platelet count, and treatments, did not show major differences compared with the crude associations.
Table 4.
Linear regression for the association between indium-labeled platelet scan and detected antibodies with direct MAIPA
| Anti-GPIIb/IIIa level β (95% CI), P value | Anti-GPIb/IX level β (95% CI), P value | Anti-GPV level β (95% CI), P value | |
|---|---|---|---|
| Splenic sequestration (%) at 48h | 0.003 (−0.009 – 0.015) | 0.001 (−0.012 – 0.014) | 0.011* (0.001 – 0.021) |
| crude effect | .635 | .853 | .034 |
| Splenic sequestration (%) pattern at 24h | 0.002 (−0.010 – 0.015) | 0.000 (−0.013 – 0.014) | 0.010 (0.000 – 0.021) |
| crude effect | .723 | .967 | .058 |
| Splenic sequestration (%) pattern at 30m | −0.001 (−0.017 – 0.014) | 0.003 (−0.014 – 0.019) | 0.014* (0.001 – 0.026) |
| crude effect | .890 | .731 | .034 |
| Multivariate models | |||
| Splenic sequestration (%) pattern at 48h | 0.003 (−0.009 – 0.016) | 0.003 (−0.011 – 0.016) | 0.010 (−0.001 – 0.021) |
| + age | .604 | .697 | .071 |
| Splenic sequestration (%) pattern at 48h | 0.002 (−0.009 − 0.014) | 0.002 (−0.010 − 0.015) | 0.011* (0.001 – 0.022) |
| + sex | .672 | .720 | .031 |
| Splenic sequestration (%) pattern at 48h | 0.002 (−0.010 – 0.015) | 0.001 (−0.012 – 0.015) | 0.011* (0.001 – 0.022) |
| + platelet count | .717 | .831 | .040 |
| Splenic sequestration (%) pattern at 48h | 0.001 (−0.012 – 0.014) | 0.001 (−0.010 – 0.011) | 0.010 (−0.001 – 0.022) |
| + treatment | .922 | .93 | .064 |
Association tested with univariable and multivariable linear regression models. Antibody variables were log transformed for normalization.
MAIPA, monoclonal antibody-specific immobilization of platelet antigen.
Association between sequestration site and clearance rate
We found an association between splenic sequestration and a faster clearance rate in patients where GP antibodies were present (β coefficient 0.186, SE 0.066, P = .009). We did not find an association between the sequestration site and the overall clearance rate (β coefficient 0.046, SE 0.029, P = .119). Supplemental Table S2 shows the results of the regression analyses for platelet sequestration patterns at different time points (30 minutes, 24 hours, 48 hours) and platelet clearance rate.
Discussion
The aim of this study was to investigate if GP autoantibodies are associated with a specific sequestration pattern or clearance rate of indium-labeled platelets in relapse/refractory ITP patients. This study showed no association between the overall presence of GP antibodies and a splenic sequestration pattern. However, within the cohort of GP-antibody-positive patients, this association was found in the presence of GPV autoantibodies. No significant associations were found between anti-GPIIb/IIIa or anti-GPIb/IX and sequestration pattern. The presence of anti-GPIb/IX seemed to be more pronounced in patients with a hepatic sequestration pattern but was not significant. Additionally, in the patients with GP antibodies, an association was observed between higher clearance rate and splenic sequestration. In patients without GP antibodies, no associations were found between clearance rate and a specific sequestration pattern.
Antibodies in ITP
Although the pathophysiology of ITP is not fully elucidated, autoantibodies are regarded to play an important role in the disease.12 Autoantibodies directed against GPIIb/IIIa, GPIb/IX, and GPV are the most frequently found in ITP, and held to be responsible for increased platelet clearance while possibly also for impaired platelet production and platelet function.7,8,24-27 Complement activation is more and more found to be possibly important in these mechanisms.18,28 Antibody positivity and load, in general, might be associated with more severe or refractory ITP, as shown in a study where a significant association between nonresponsiveness on rituximab treatment and lack of detectable platelet autoantibodies was found.20,29 Additionally, different antibody specificities were suggested to induce different mechanisms of platelet destruction, and this has been the subject of research for the past decades.30 Studies showed that GPIIb/IIIa antibodies are associated with clearance via Fc-receptor mediated clearance by splenic macrophages6,31 while GPIb/IX antibodies are associated with Fc-independent hepatic clearance via the Ashwell Morell receptor.32 Our observations in this cohort of ITP patients are in line with these associations. We found a higher percentage of patients with anti-GPIIb/IIIa (71%) and/or anti-GPV (71%) as compared with patients with anti-GPIb/IX (43%) if there was mainly splenic sequestration. In patients with hepatic sequestration, we find a higher number with anti-GPIb/IX (86%) as compared with patients with anti-GPIIb/IIIa (57%) and/or anti-GPV (29%). In regression models, only anti-GPV and a splenic sequestration pattern show a significant association. A limitation of our study is the small sample size which makes it underpowered to detect a significant association. Future studies are needed to build further on the associations and effect sizes found in this study.
Interestingly, we did find a significant association between the presence of anti-GPV and a splenic sequestration pattern. Until recently, the role of anti-GPV antibodies in ITP has not been described extensively. GPV is noncovalently linked to the GPIb/IX complex, and a major fragment, ie almost the entire extracellular part, of GPV is released after platelet activation by thrombin, elastase, and calpain.33 Modderman et al investigated GPV expression on the platelet membrane and showed that 11 000 intact GPV molecules are present on resting platelets from healthy individuals and that cleavage of the majority of GPV occurs after exposure to thrombin.33 We therefore assume that in the majority of ITP patients, sufficient GPV molecules are present on the platelet membrane for detecting platelet-associated autoantibodies and for autoantibody-induced platelet destruction. Recent publications hypothesized that anti-GPV may act in the same way as anti-GPIb/IX antibodies through binding on the GPIb/IX-V complex on the platelet surface.24 Vollenberg et al found anti-GPV antibodies in the majority of their autoantibody-positive patient population, with comparable prevalence to our cohort. GPV antibodies showed induction of platelet destruction in vivo (in NOD/SCID mouse models) and in vitro, irrespective of their avidity. Overall clearance of platelets is higher when patients also have GPV antibodies either (1) by increasing the overall IgG load, leading to overall more platelet clearance, or (2) specific functional effects of anti-GPV with changes in platelet reactivity.24,34 Our study suggests that GPV antibodies opsonize platelets for Fc-mediated clearance in the spleen and might have a distinct clearance pathway from other antibody specificities in ITP.
GPIb/IX on the surface is known to be an important aspect in platelet physiology, especially for its interaction with von Willebrand factor (VWF). However, more physiological roles are discovered with GPIb/IX activation by ligand binding under shear stress.35 One study suggests in animal model studies that specific antibodies against GPIbα N-terminus cause platelet clearance in the liver, and this may be associated with desialysation as part of the aging process of platelets, when aged platelets are cleared by the liver.11,32 In our cohort, we see relatively higher percentages of anti-GPIb/IX in patients with a hepatic sequestration pattern. This may incline a confirmation of the studies by Li et al in a first in vivo human study.32
Rate of clearance
In this study, we also examined the rate of clearance in association with sequestration site and found an increased rate of platelet clearance in antibody-positive patients with splenic sequestration. Antibody positivity and higher load may be leading to faster clearance, and faster clearance may to some extent be seen as a proxy for disease severity in ITP. This is in line with what Al-Samkari et al found.20 Their study showed that antibody positivity and load are associated with more disease severity.20 The impact of this finding is still unknown and should be investigated further together with other pathways, such as the role of T regulatory cells and direct CD8+ mediated cytotoxicity.36,37
Strengths and limitations
The strength of this study is that we, for the first time, collected data on platelet autoantibodies, autoantibody specificities in relation to sequestration in spleen, liver, or both in a group of ITP patients. Limitations were that although we included 74 patients, platelet antibodies were tested in 57 patients. Due to the lack of previous data on this subject, no power calculations could be performed, thus creating the possibility of low power to detect associations, especially when looking at subgroups of patients. This study provides the opportunity for future researchers to validate these new insights and study possible clinical implications for treatment choice. For generalizability, we included all patients who had scintigraphy in our hospital. Second, it is unknown which variables may confound the associations, partly due to the heterogeneous nature of ITP. In this study, we included the most used variables, such as age, sex, platelet count, and treatment history.
Third, in this study, we did not measure the IgA and IgM or IgG subclasses. It has been suggested that abnormalities in levels of IgA and IgM lead to more treatment-resistant ITP.38 Furthermore, this cohort lacked data on the presence of anti-GPIa/IIa, anti-GPIV, and anti-GPVI, which has been reported in some patients with ITP and may impact the site of platelet destruction but is not measured in the direct MAIPA that was used in this study.7,39,40 Fourth, the percentage of platelet autoantibody positivity in our cohort was lower than expected, given the sensitivity of the direct MAIPA. The sensitivity of MAIPA is 81% and the specificity of MAIPA is 98% for clinical diagnosis of ITP.7 In the investigated ITP cohort, platelet autoantibodies were detected in only 53% of the patients, which might be due to the selection of relapsed/refractory patients, but also some patients were treated successfully. Fifth, this cohort has no follow-up data upon the clinical outcome when splenectomy was performed. A longer follow-up is needed and will be insightful for the clinical implications of these findings.
Conclusion and future perspectives
In this study, we found in relapse/refractory ITP patients an association between splenic sequestration of platelets and the presence of GPV antibodies. Furthermore, our data suggest that patients with a hepatic pattern showed more often the presence of GPIb/IX antibodies. Moreover, this study found an association between clearance rate and a splenic sequestration pattern in anti-GP-positive patients. Future studies are needed to validate the associations and investigate the clinical implications. This study adds to the knowledge needed to better understand and individually diagnose and treat ITP.
Supplementary Material
The full-text version of this article contains a data supplement.
Authorship
Contribution: All authors interpreted the data, critically revised the manuscript, and approved the final draft; M.R.S., M.d.H., and L.P. contributed to the design of the study; S.N.A. developed the initial draft of the manuscript; M.R.S., J.J.Z., L.P., and M.d.H. provided critical direction during the development of the manuscript; and S.N.A. and A.S. performed the statistical analyses.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sufia Amini, Els Borst Eilersplein 275, 2545 AA The Hague, The Netherlands, e-mail: s.amini@hagaziekenhuis.nl.
References
- 1.Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393. [DOI] [PubMed] [Google Scholar]
- 2.Efficace F, Mandelli F, Fazi P, et al. Health-related quality of life and burden of fatigue in patients with primary immune thrombocytopenia by phase of disease. Am J Hematol. 2016;91(10):995-1001. [DOI] [PubMed] [Google Scholar]
- 3.Cooper N. State of the art - how I manage immune thrombocytopenia. Br J Haematol. 2017;177(1):39-54. [DOI] [PubMed] [Google Scholar]
- 4.Harrington WJ, Minnich V, Hollingsworth JW, Moore CV. Demonstration of a thrombocytopenic factor in the blood of patients with thrombocytopenic purpura. J Lab Clin Med. 1951;38(1):1-10. [PubMed] [Google Scholar]
- 5.McMillan R. The pathogenesis of chronic immune thrombocytopenic purpura. Semin Hematol. 2007;44(4 Suppl 5):S3-S11. [DOI] [PubMed] [Google Scholar]
- 6.McMillan R. Autoantibodies and autoantigens in chronic immune thrombocytopenic purpura. Semin Hematol. 2000;37(3):239-248. [DOI] [PubMed] [Google Scholar]
- 7.Porcelijn L, Huiskes E, Oldert G, Schipperus M, Zwaginga JJ, de Haas M. Detection of platelet autoantibodies to identify immune thrombocytopenia: state of the art. Br J Haematol. 2018;182(3):423-426. [DOI] [PubMed] [Google Scholar]
- 8.Porcelijn L, Schmidt DE, Oldert G, et al. Evolution and Utility of Antiplatelet Autoantibody Testing in Patients with Immune Thrombocytopenia. Transfus Med Rev. 2020;34(4):258-269. [DOI] [PubMed] [Google Scholar]
- 9.Iraqi M, Perdomo J, Yan F, Choi PY, Chong BH. Immune thrombocytopenia: antiplatelet autoantibodies inhibit proplatelet formation by megakaryocytes and impair platelet production in vitro. Haematologica. 2015;100(5):623-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaturvedi S, Arnold DM, McCrae KR. Splenectomy for immune thrombocytopenia: down but not out. Blood. 2018;131(11):1172-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan R, Chen M, Ma N, et al. Glycoprotein Ibα clustering induces macrophage-mediated platelet clearance in the liver. Thromb Haemost. 2017;113(01):107-117. [DOI] [PubMed] [Google Scholar]
- 12.Zufferey A, Kapur R, Semple JW. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP). J Clin Med. 2017;6(2):16.28208757 [Google Scholar]
- 13.Palandri F, Polverelli N, Catani L, et al. The choice of second-line therapy in steroid-resistant immune thrombocytopenia: role of platelet kinetics in a single-centre long-term study. Am J Hematol. 2014;89(11):1047-1050. [DOI] [PubMed] [Google Scholar]
- 14.Najean Y, Dufour V, Rain JD, Toubert ME. The site of platelet destruction in thrombocytopenic purpura as a predictive index of the efficacy of splenectomy. Br J Haematol. 1991;79(2):271-276. [DOI] [PubMed] [Google Scholar]
- 15.Heyns AP, Badenhorst PN, Lötter MG, Pieters H, Wessels P, Kotzé HF. Platelet turnover and kinetics in immune thrombocytopenic purpura: results with autologous 111In-labeled platelets and homologous 51Cr-labeled platelets differ. Blood. 1986;67(1):86-92. [PubMed] [Google Scholar]
- 16.Roca M, Muñiz-Diaz E, Mora J, et al. The scintigraphic index spleen/liver at 30 minutes predicts the success of splenectomy in persistent and chronic primary immune thrombocytopenia. Am J Hematol. 2011;86(11):909-913. [DOI] [PubMed] [Google Scholar]
- 17.Vrbensky JR, Moore JE, Arnold DM, Smith JW, Kelton JG, Nazy I. The sensitivity and specificity of platelet autoantibody testing in immune thrombocytopenia: a systematic review and meta-analysis of a diagnostic test. J Thromb Haemost. 2019;17(5):787-794. [DOI] [PubMed] [Google Scholar]
- 18.Najaoui A, Bakchoul T, Stoy J, et al. Autoantibody-mediated complement activation on platelets is a common finding in patients with immune thrombocytopenic purpura (ITP). Eur J Haematol. 2011;88(2):167-174. [DOI] [PubMed] [Google Scholar]
- 19.Brighton TA, Evans S, Castaldi PA, Chesterman CN, Chong BH. Prospective evaluation of the clinical usefulness of an antigen-specific assay (MAIPA) in idiopathic thrombocytopenic purpura and other immune thrombocytopenias. Blood. 1996;88(1):194-201. [PubMed] [Google Scholar]
- 20.Al-Samkari H, Rosovsky RP, Karp Leaf RS, et al. A modern reassessment of glycoprotein-specific direct platelet autoantibody testing in immune thrombocytopenia. Blood Adv. 2019;4(1):9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von dem Borne AE, Verheugt FW, Oosterhof F, von Riesz E, de la Rivière AB, Engelfriet CP. A simple immunofluorescence test for the detection of platelet antibodies. Br J Haematol. 1978;39(2):195-207. [DOI] [PubMed] [Google Scholar]
- 22.Kiefel V, Santoso S, Weisheit M, Müeller-Eckhardt C. Monoclonal antibody--specific immobilization of platelet antigens (MAIPA): a new tool for the identification of platelet-reactive antibodies. Blood. 1987;70(6):1722-1726. [PubMed] [Google Scholar]
- 23.International Committee for Standardization in Hematology. Panel on Diagnostic Applications of Radionuclides. Recommended method for indium-111 platelet survival studies. J Nucl Med. 1988;29(4):564-566. [PubMed] [Google Scholar]
- 24.Vollenberg R, Jouni R, Norris PAA, et al. Glycoprotein V is a relevant immune target in patients with immune thrombocytopenia. Haematologica. 2019;104(6):1237-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMillan R, Wang L, Tomer A, Nichol J, Pistillo J. Suppression of in vitro megakaryocyte production by antiplatelet autoantibodies from adult patients with chronic ITP. Blood. 2004;103(4):1364-1369. [DOI] [PubMed] [Google Scholar]
- 26.Chang M, Nakagawa PA, Williams SA, et al. Immune thrombocytopenic purpura (ITP) plasma and purified ITP monoclonal autoantibodies inhibit megakaryocytopoiesis in vitro. Blood. 2003;102(3):887-895. [DOI] [PubMed] [Google Scholar]
- 27.Houwerzijl EJ, Blom NR, van der Want JJ, Vellenga E, de Wolf JT. Megakaryocytic dysfunction in myelodysplastic syndromes and idiopathic thrombocytopenic purpura is in part due to different forms of cell death. Leukemia. 2006;20(11):1937-1942. [DOI] [PubMed] [Google Scholar]
- 28.Cheloff AZ, Kuter DJ, Al-Samkari H. Serum complement levels in immune thrombocytopenia: characterization and relation to clinical features. Res Pract Thromb Haemost. 2020;4(5):807-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porcelijn L, Huiskes E, Schipperus M, van der Holt B, de Haas M, Zwaginga JJ; Dutch HOVON 64 Study Group . Lack of detectable platelet autoantibodies is correlated with nonresponsiveness to rituximab treatment in ITP patients. Blood. 2017;129(25):3389-3391. [DOI] [PubMed] [Google Scholar]
- 30.Hoemberg M, Stahl D, Schlenke P, Sibrowski W, Pachmann U, Cassens U. The isotype of autoantibodies influences the phagocytosis of antibody-coated platelets in autoimmune thrombocytopenic purpura. Scand J Immunol. 2011;74(5):489-495. [DOI] [PubMed] [Google Scholar]
- 31.Kuwana M, Okazaki Y, Ikeda Y. Splenic macrophages maintain the anti-platelet autoimmune response via uptake of opsonized platelets in patients with immune thrombocytopenic purpura. J Thromb Haemost. 2009;7(2):322-329. [DOI] [PubMed] [Google Scholar]
- 32.Li J, van der Wal DE, Zhu G, et al. Desialylation is a mechanism of Fc-independent platelet clearance and a therapeutic target in immune thrombocytopenia. Nat Commun. 2015;6(1):7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Modderman PW, Admiraal LG, Sonnenberg A, von dem Borne AE. Glycoproteins V and Ib-IX form a noncovalent complex in the platelet membrane. J Biol Chem. 1992;267(1):364-369. [PubMed] [Google Scholar]
- 34.Nurden P, Nurden AT. Is the mysterious platelet receptor GPV an unsuspected major target for platelet autoantibodies? Haematologica. 2019;104(6):1103-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quach ME, Li R. Structure-function of platelet glycoprotein Ib-IX. J Thromb Haemost. 2020;18(12):3131-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu J, Liu X, Li X, et al. CD8(+) T cells induce platelet clearance in the liver via platelet desialylation in immune thrombocytopenia. Sci Rep. 2016;6(1):27445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma L, Simpson E, Li J, et al. CD8+ T cells are predominantly protective and required for effective steroid therapy in murine models of immune thrombocytopenia. Blood. 2015;126(2):247-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnason JE, Campigotto F, Neuberg D, Bussel JB. Abnormalities in IgA and IgM are associated with treatment-resistant ITP. Blood. 2012;119(21):5016-5020. [DOI] [PubMed] [Google Scholar]
- 39.He R, Reid DM, Jones CE, Shulman NR. Spectrum of Ig classes, specificities, and titers of serum antiglycoproteins in chronic idiopathic thrombocytopenic purpura. Blood. 1994;83(4):1024-1032. [PubMed] [Google Scholar]
- 40.Rabbolini DJ, Gardiner EE, Morel-Kopp MC, et al. Anti-glycoprotein VI mediated immune thrombocytopenia: an under-recognized and significant entity? Res Pract Thromb Haemost. 2017;1(2):291-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.