Key Points
The primary outcome, stroke, occurred in 6 of 23 patients randomized to apixaban compared with 0 of 25 patients randomized to warfarin.
This study with limitations suggests that apixaban is not an equitable substitute for warfarin to prevent thrombosis among TAPS patients.
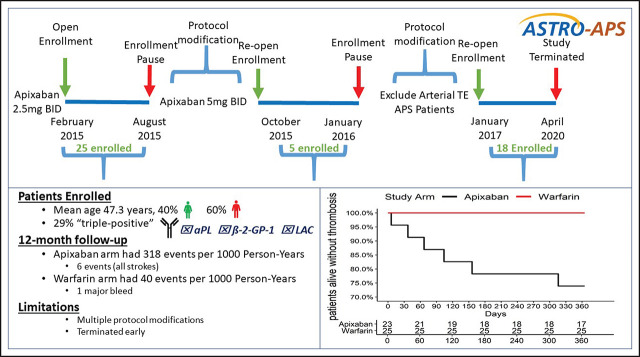
Visual Abstract
Abstract
Thrombotic antiphospholipid syndrome (TAPS) is characterized by venous, arterial, or microvascular thrombosis. Patients with TAPS merit indefinite anticoagulation, and warfarin has historically been the standard treatment. Apixaban is an oral factor Xa inhibitor anticoagulant that requires no dose adjustment or monitoring. The efficacy and safety of apixaban compared with warfarin for TAPS patients remain unknown. This multicenter prospective randomized open-label blinded endpoint study assigned anticoagulated TAPS patients to apixaban or warfarin (target international normalized ratio 2-3) for 12 months. The primary efficacy outcome was clinically overt thrombosis and vascular death. Apixaban was first given at 2.5 mg twice daily. Two protocol changes were instituted based on recommendations from the data safety monitoring board. After the twenty-fifth patient was randomized, the apixaban dose was increased to 5 mg twice daily, and after the thirtieth patient was randomized, subjects with prior arterial thrombosis were excluded. Primary outcomes were adjudicated by independent experts blinded to treatment allocation. Patients randomized between 23 February 2015 and 7 March 2019 to apixaban (n = 23) or warfarin (n = 25) were similar. Among the components of the primary efficacy outcome, only stroke occurred in 6 of 23 patients randomized to apixaban compared with 0 of 25 patients randomized to warfarin. The study ended prematurely after the forty-eighth patient was enrolled. Conclusions from our study are limited due to protocol modifications and low patient accrual. Despite these limitations, our results suggest that apixaban may not be routinely substituted for warfarin to prevent recurrent thrombosis (especially strokes) among patients with TAPS. This trial was registered at www.clinicaltrials.gov as #NCT02295475.
Background
Antiphospholipid syndrome (APS) is characterized by thrombosis involving the venous, arterial or microcirculation, pregnancy morbidity, and persistent characteristic antibodies.1 Patients with APS and thrombosis (thrombotic APS-TAPS) are at high risk for recurrent thrombosis, and indefinite anticoagulation is recommended.2,3 A vitamin K antagonist (VKA) such as warfarin is the historically preferred treatment of thrombotic antiphospholipid syndrome (TAPS) patients. However, the use of VKA is complicated by diet and multiple drug interactions and the need for frequent phlebotomy for dose adjustment. In addition, the rate of recurrent thrombosis among TAPS patients on warfarin remains high and approximates 25% within 5 years.4 The direct anticoagulant (DOAC) apixaban is a safe and effective alternative to VKA for the treatment and secondary prevention of venous thromboembolism (VTE)5,6 and is likewise effective in another hypercoagulable group, patients with cancer.7 Initial reports of DOAC administration among TAPS patients were encouraging.8 However, subsequent studies comparing rivaroxaban with VKA in TAPS patients suggested that rivaroxaban may be inferior to VKA9 for preventing recurrent thrombosis, especially ischemic strokes. Because of these observations, guidance from regulatory agencies10-12 and medical societies13,14 has emerged to recommend against the routine use of DOACs among patients with TAPS. However, the safety and efficacy of apixaban have not been reported in randomized control trials among TAPS patients.
We aimed to compare apixaban 2.5 mg twice daily with warfarin among TAPS patients using a prospective open-label blinded event (PROBE) study design. Our rationale for choosing apixaban 2.5 mg twice daily has been reported elsewhere.15 Briefly, this was based upon the comparative effectiveness of apixaban 2.5 mg twice daily and apixaban 5 mg twice daily among patients receiving extended phase treatment of VTE, with a favorable relative risk for the outcome of cardiovascular death, stroke, and myocardial infarction when compared with placebo.5
This pilot study was to randomize 200 TAPS patients to receive apixaban or warfarin and to report the outcomes of clinically overt thrombosis (venous and arterial thrombosis), bleeding events (major bleeding and clinically relevant nonmajor bleeding), and patient satisfaction with anticoagulation.
Methods
Study design
We report the full methods and study design elsewhere.15 Briefly, this is a US multicenter PROBE study in TAPS patients. At the beginning of the study, patients were randomized to either apixaban 2.5 mg twice daily or warfarin (target international normalized ratio [INR] 2-3) for 12 months. In 2016, to enhance recruitment, collaboration with the University of Utah Trial Innovation Center facilitated the identification of multiple external collaborators and activation of 1 site (The Ohio State University), which enrolled 1 patient prior to study closure. All other patients were enrolled at Intermountain Medical Center. The study was registered (ClinicalTrials.gov: NCT02295475), approved by the Intermountain Healthcare Institutional Review Board (IRB), and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice principles. An internal data safety monitoring board (DSMB) with expertise in cardiovascular and thrombotic disease and research was empaneled. The DSMB convened at predetermined intervals and provided recommendations regarding the management of the study to the investigators with reports filed to the IRB.
Patients
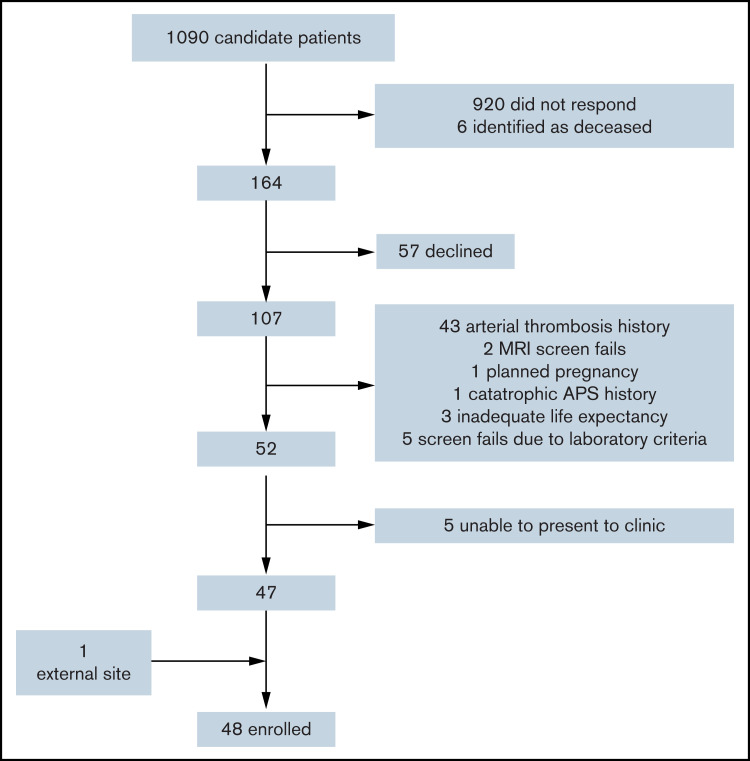
We implemented an innovative process for patient identification, including electronic medical record interrogation for eligible patients based upon historical laboratory values suggestive of APS receiving concomitant anticoagulation and direct patient mailing (Figure 1).15 We enrolled and randomized consenting patients with a history of TAPS receiving therapeutic anticoagulation for secondary prevention of thrombosis for at least 6 months. We classified patients as having definite, likely, or historical APS and gathered additional baseline demographics and clinical characteristics to understand the heterogeneity of APS patients that might inform future research. Definite APS was defined as radiologically verified thrombosis plus serial qualifying laboratory results by Sapporo criteria.1 Likely APS was defined as radiologically verified thrombosis plus at least 1 qualifying laboratory result, and historical APS was defined as a report of thrombosis along with patient-reported abnormal laboratory testing, but results were not available for confirmation. Patients were excluded if they (1) required anticoagulation for another nonapproved indication; (2) received dual antiplatelet therapy or aspirin >165 mg daily; (3) were pregnant (or with an intention to become pregnant); (4) had a life expectancy of <1 year; (5) had baseline hemoglobin <8 g/dL, platelets <50 × 109/L, creatinine >2.5 mg/dL, total bilirubin >1.5 times the upper limit of normal; or (6) had developed thrombosis while on warfarin with an INR ≥2.0. Randomization occurred using a computer-generated randomization tool with built-in random sequence generation that was applied with allocation sequence concealment without exception.
Figure 1.
Consort diagram of patients screened. One MRI screen fail was because of the identification of a brain tumor, and the other was because the patient had white matter changes disproportionate for age. MRI, magnetic resonance imaging.
Interventions
Patients randomized to apixaban received open-label apixaban provided by the study coordinator dispensed at the time of consent and randomization and at follow-up visits. Patients randomized to open-label warfarin were managed per clinical routine. All patients were instructed to contact study personnel immediately with any symptoms worrisome for thrombosis or bleeding, and these signs and symptoms were also reviewed at every encounter.
Follow-up
Following enrollment, patient encounters included in-person visits at months 6 and 12 and remote visits via e-mail portal link or telephone interview with standardized study questionnaires at months 1, 3, 9, and 13. At each visit, adherence to warfarin and apixaban were assessed by standard questionnaires and laboratory tests. Patient satisfaction with anticoagulation treatment was assessed at 1, 3, 6, 9, and 12 months with the Anti-Clot Treatment Scale (ACTS) survey,16 a 15-item patient-reported survey including 12 items assessing for burden and 3 items assessing for benefit. At the end of the study, all patients randomized to apixaban were transitioned to warfarin using a standardized protocol unless they elected to continue apixaban per routine clinical care at the discretion of the treating physician.
Outcomes
The primary clinical outcome was the combined rate of thrombosis (arterial and venous thrombosis) and vascular death. Arterial thrombosis included ischemic stroke, myocardial infarction, transient ischemic attack, or other arterial thromboembolism defined as we formerly reported.15 VTE was defined as proximal lower extremity deep vein thrombosis (involving popliteal vein and more proximally) and pulmonary embolism defined in a usual fashion.15 The primary safety outcome was major and clinically relevant nonmajor bleeding (CRNMB) by the International Society on Thrombosis and Haemostasis criteria.17,18 All primary efficacy and safety outcomes were assessed by a panel of physicians independent from the study with expertise in thrombosis and APS and who were blinded to the treatment arm. Each was provided outcome event definitions from the protocol to adjudicate all events of thrombosis, death, and bleeding. A κ coefficient of agreement among the independent adjudicators was calculated. We reported patients’ satisfaction using a standardized validated assessment tool.16
Protocol modifications
Following enrollment of the initial 25 patients, a routine DSMB review occurred. The DSMB observed that 3 strokes, all among patients in the apixaban arm, had occurred and recommended that all future patients randomized to receive apixaban, and all patients already enrolled, receive a higher dose of 5 mg twice daily, and this was adopted. In the subsequent 3 months, 5 additional patients were enrolled, and despite the higher apixaban dose, 3 additional strokes occurred in patients randomized to apixaban. At that time, an ad-hoc DSMB assessment was requested by the principal investigator. After reviewing the data, the DSMB recommended: (1) to continue enrollment, (2) that any patient previously enrolled with a history of arterial thrombosis and randomized to apixaban to be returned to warfarin, (3) that only patients without a history of arterial thrombosis to be considered eligible subsequently, and (4) that brain magnetic resonance imaging using a stroke detection protocol be obtained for all subsequent eligible candidates, and that only patients without radiographic evidence of prior stroke or white matter changes disproportionate for patient age can be enrolled. These recommendations were communicated to the IRB, and all recommendations were adopted, protocol modifications were published,19 and enrollment ensued until April 2019.
Statistical analysis
Based upon former work,20,21 we estimated a recurrent rate of thrombosis of 1.5% among patients receiving anticoagulation, and using a noninferiority design, we estimated that 4640 subjects would need to be enrolled to have 80% power to report a 1% difference in thrombosis rate. Given the large number of patients (with a rare condition) needed, we aimed to conduct a pilot study first by randomizing 200 patients to apixaban or warfarin to report primary outcome rates that could evaluate feasibility and inform future research. We hypothesized that apixaban would not differ from warfarin for the outcome of thrombosis and vascular death or major bleeding and CRNMB among patients with TAPS receiving therapeutic anticoagulation for secondary prevention of thrombosis. Descriptive demographics of the patient population were calculated for each arm as either mean and standard deviation or number of participants and percentage. Intention-to-treat analysis was used to report the primary efficacy outcome (thrombosis and vascular mortality) and primary safety outcome (major bleeding and CRNMB). Cox proportional-hazards models were used to assess differences in outcomes between the study arms. Person-time and Kaplan-Meier curves were calculated for each outcome separately and for the net clinical benefit upon combining the primary efficacy and safety outcomes. In a sensitivity analysis, the as-treated status of participants was considered for exposure to apixaban 2.5 mg twice daily dosing, apixaban 5.0 mg twice daily dosing, and warfarin. An additional sensitivity analysis was done to omit patients with a history of arterial thromboembolism. Quality of anticoagulation management was assessed by calculating the time in therapeutic range in a usual fashion.22 INR values used in the calculation were collected as part of the study protocol, as well as all laboratory values gathered from routine clinical care. Adherence to apixaban was calculated from the visit questionnaires that interrogated for elective interruption and missed doses and were reported as a percent of doses prescribed.
To analyze findings from the ACTS survey, benefit and burden questions were aggregated separately and then combined for a final score. Higher scores correlated with greater satisfaction. A Mann-Whitney U test was used to compare between study arms. All analyses were conducted with R version 4.0.3.
Role of the funding source
Bristol-Myers-Squibb/Pfizer Pharmaceuticals provided apixaban and funding for the study but had no role in the design or conduct of the study. Data collection, data management, analysis, data interpretation, and manuscript preparation were conducted independent of the funding source.
Results
Forty-eight patients were enrolled between 23 February 2015 and 7 March 2019. Twenty-three were randomized to apixaban and 25 to warfarin. Patient adherence to apixaban was 97.3%. Participants in the warfarin arm had a mean (standard deviation) of 301 (53.3) days with INR values, and the percent time in therapeutic range was 60% (23.8%). Baseline patient characteristics are reported in Table 1 and supplemental Table 1. A 12-month follow-up was completed for all participants.
Table 1.
Demographics of patients enrolled
| Characteristic | All, n (%) or mean (SD)* | Apixaban, n (%) or mean (SD)* | Warfarin, n (%) or mean (SD)* |
|---|---|---|---|
| n = 48 | n = 23 | n = 25 | |
| Demographics | |||
| Female | 40 (83.3) | 19 (82.6) | 21 (84) |
| Age, y | 47.3 (13)* | 46 (11.53)* | 48.5 (14.36)* |
| Body mass index, kg/m2 | 31.8 (6.99)* | 31.2 (8.06)* | 32.3 (5.96)* |
| Tobacco use status | |||
| Never | 39 (81.2) | 20 (87) | 19 (76) |
| Former | 5 (10.4) | 3 (13) | 2 (8) |
| Current | 0 (0) | 0 (0) | 0 (0) |
| Unknown | 3 (6.2) | 0 (0) | 3 (12) |
| Insurance | |||
| Private | 16 (33.3) | 3 (13) | 13 (52) |
| Public | 12 (25) | 8 (34.8) | 4 (16) |
| None | 19 (39.6) | 12 (52.2) | 7 (28) |
| Race | |||
| White | 46 (95.8) | 23 (100) | 23 (92) |
| African American | 1 (2.1) | 0 (0) | 1 (4) |
| Primary language | |||
| English | 42 (87.5) | 21 (91.3) | 21 (84) |
| Spanish | 5 (10.4) | 2 (8.7) | 3 (12) |
| Marital Status | |||
| Single | 9 (18.8) | 2 (8.7) | 7 (28) |
| Married | 32 (66.7) | 19 (82.6) | 13 (52) |
| Separated | 1 (2.1) | 0 (0) | 1 (4) |
| Divorced | 3 (6.2) | 2 (8.7) | 1 (4) |
| Unknown | 2 (4.2) | 0 (0) | 2 (8) |
| Religion | |||
| Christian | 30 (62.5) | 15 (65.2) | 15 (60) |
| No preference | 11 (22.9) | 4 (17.4) | 7 (28) |
| Unknown | 6 (12.5) | 4 (17.4) | 2 (8) |
| Clinical characteristics | |||
| Hemoglobin, g/dL | 13.8 (1.68)* | 13.2 (1.88)* | 14.3 (1.27)* |
| Platelet count | 254.6 (84.07)* | 265.7 (103.56)* | 243.9 (60.29)* |
| d-dimer | 569.4 (841.95)* | 412.3 (160.4)* | 713.5 (1160.61)* |
| Positivity | |||
| Triple | 14 (29.2) | 7 (30.4) | 7 (28) |
| Double | 6 (12.5) | 4 (17.4) | 2 (8) |
| Single | 12 (25) | 5 (21.7) | 7 (28) |
| APS status | |||
| Definite‡ | 20 (41.7) | 8 (34.8) | 12 (48) |
| Likely‡ | 12 (25) | 8 (34.8) | 4 (16) |
| Historical‡ | 16 (33.3) | 7 (30.4) | 9 (36) |
| Laboratory diagnostics | |||
| Lupus anticoagulant detected† | 20 (41.7) | 11 (47.8) | 9 (36) |
| Anticardiolipin IgG positive† | 18 (37.5) | 9 (39.1) | 9 (36) |
| Anticardiolipin IgM positive† | 11 (22.9) | 6 (26.1) | 5 (20) |
| Anti-β-2-glycoprotein-1 IgG positive† | 17 (35.4) | 10 (43.5) | 7 (28) |
| Anti-β-2-glycoprotein-1 IgM positive† | 6 (12.5) | 4 (17.4) | 2 (8) |
| Previous thrombotic events | 48 (100) | 23 (100) | 25 (100) |
| Arterial events | 17 (35.4) | 6 (26.1) | 11 (44) |
| Myocardial infarction | 2 (4.2) | 1 (4.3) | 1 (4) |
| Stroke | 12 (25) | 5 (21.7) | 7 (28) |
| Other | 4 (8.3) | 1 (4.3) | 3 (12) |
| Venous events | 38 (79.2) | 20 (87) | 18 (72) |
| Deep vein thrombosis | 34 (70.8) | 17 (73.9) | 17 (68) |
| Pulmonary embolism | 18 (37.5) | 11 (47.8) | 7 (28) |
| Pregnancy morbidity | 12 (25) | 7 (30.4) | 5 (20) |
| Risk factors | |||
| Smoking | 10 (20.8) | 4 (17.4) | 6 (24) |
| Hypertension | 7 (14.6) | 3 (13) | 4 (16) |
| Diabetes | 8 (16.7) | 4 (17.4) | 4 (16) |
| Dyslipidemia | 8 (16.7) | 4 (17.4) | 4 (16) |
| Heritable thrombophilia | 19 (39.6) | 10 (43.5) | 9 (36) |
| Charlson comorbidity index | 2.1 (2.31)* | 1.8 (1.83)* | 2.3 (2.7)* |
| Comorbidities | |||
| Systemic lupus erythematosus | 7 (14.6) | 2 (8.7) | 5 (20) |
| Autoimmune disease | 17 (35.4) | 8 (34.8) | 9 (36) |
| Depression/anxiety | 11 (22.9) | 7 (30.4) | 4 (16) |
| Migraine/headache | 12 (25) | 6 (26.1) | 6 (24) |
| GERD | 12 (25) | 7 (30.4) | 5 (20) |
| Reactive airway/COPD | 6 (12.5) | 5 (21.7) | 1 (4) |
| Chronic pain syndrome | 10 (20.8) | 6 (26.1) | 4 (16) |
| TIA/Stroke | 4 (8.3) | 1 (4.3) | 3 (12) |
| Medications | |||
| Aspirin | 4 (8.3) | 3 (13) | 1 (4) |
| Statin | 10 (20.8) | 6 (26.1) | 4 (16) |
| Hydroxychloroquine | 16 (33.3) | 6 (26.1) | 10 (40) |
| Other immunosuppressant | 12 (25) | 4 (17.4) | 8 (32) |
| Antihypertensive | 19 (39.6) | 10 (43.5) | 9 (36) |
| Calcium | 16 (33.3) | 9 (39.1) | 7 (28) |
| Vitamin D | 18 (37.5) | 10 (43.5) | 8 (32) |
| Antidepressant/anxiolytic | 15 (31.2) | 8 (34.8) | 7 (28) |
| Prescription analgesic | 22 (45.8) | 10 (43.5) | 12 (48) |
| Other vitamin supplement | 21 (43.8) | 11 (47.8) | 10 (40) |
| Diabetic medication | 2 (4.2) | 1 (4.3) | 1 (4) |
| Asthma/reactive airway | 12 (25) | 8 (34.8) | 4 (16) |
| Oral contraceptive | 2 (4.2) | 2 (8.7) | 0 (0) |
Demographics are reported as count (%), or when noted as mean (SD). APS, antiphospholipid syndrome; COPD, chronic obstructive pulmonary disease; GERD, gastroesophageal reflux disease; SD, standard deviation; TIA, transient ischemic attack.
Mean (SD).
At the time of enrollment.
Patients with APS where characterized as having definite APS defined as radiologically verified thrombosis plus a qualifying laboratory result, § likely APS was defined as radiologically verified thrombosis plus at least 1 qualifying laboratory result, or historical APS was defined as a report of a qualifying thrombosis event along with a reported history of abnormal laboratory testing, but results were not available for confirmation.
Derived from Sapporo Criteria: the presence of lupus anticoagulant or anticardiolipin IgG or IgM or anti-β-2-glycoprotein-1 IgG or IgM > 40 IgG phospholipid Units or IgM phospholipid units or >99 percentile, on 2 separate occasions at least 12 weeks apart.
Primary efficacy and safety outcomes
The Cox proportional-hazards models did not converge; therefore, comparisons of outcomes between study arms or subgroup analyses and their levels of statistical significance are not reported. The focus of the analysis shifted to a person-time analysis.
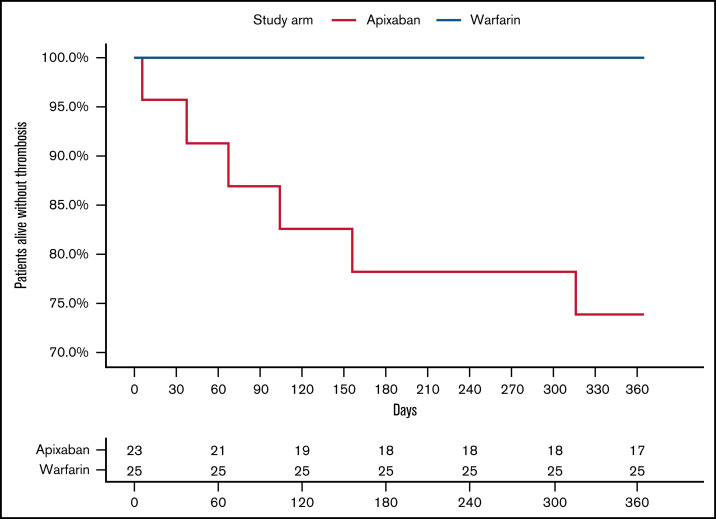
In the intention-to-treat analysis for the primary efficacy outcome, there were 6 thrombotic events among patients randomized to apixaban, all of which were strokes, at a rate of 318 events per 1000 person-years. No patient randomized to warfarin experienced a thrombotic event (Figure 2). There was 1 major bleeding event in the warfarin arm (vaginal bleeding with an INR = 2.9) and no CRNMB events, which resulted in a rate of 40 per 1000 person-years. No patient randomized to apixaban experienced a major bleed or CRNMB. When combining the primary efficacy (thrombosis and vascular death) and safety (major bleeding and CRNMB) outcomes, the rate of adverse outcomes per 1000 person-years was 318 for apixaban and 40 for warfarin. The characteristics of individual patients that experienced an outcome event are reported in Table 2, with additional details reported in supplemental Table 3. The κ coefficient of agreement among the independent adjudicators for the outcome events was 1.0.
Figure 2.
Kaplan-Meier cumulative event rate for thrombosis. The solid red line is for apixaban, and the solid blue line is for warfarin.
Table 2.
Details for each participant that experienced a thrombotic or major bleed event during the study
| ID | Age | Sex | BMI | Treatment | History | Positivity level* | Type | Event type | Days to event |
|---|---|---|---|---|---|---|---|---|---|
| 24 | 40 | Female | 39.3 | Apixaban | Stroke, DVT, PE, pregnancy loss | Single | Likely | Stroke | 156 |
| 16 | 43 | Female | 36.9 | Apixaban | DVT | Triple | Definite | Stroke | 67 |
| 12 | 47 | Female | 19.4 | Apixaban | Stroke, TIA, DVT, pregnancy loss | Double | Likely | Stroke | 37 |
| 2 | 51 | Female | 25.5 | Apixaban | Stroke, other arterial thrombosis, DVT, PE | Triple | Definite | Stroke | 316 |
| 32 | 66 | Male | 39.3 | Apixaban | DVT | N/A | Historical | Stroke | 104 |
| 3 | 69 | Female | 23.2 | Apixaban | Stroke, pregnancy loss | N/A | Historical | Stroke | 6 |
| 27 | 62 | Female | 30.5 | Warfarin | Stroke, DVT, PE | N/A | Historical | Major bleed† | 319 |
BMI, body mass index; DVT, deep vein thrombosis; N/A, not applicable as historical APS; PE, pulmonary embolism; TIA, transient ischemic attack.
Refers to whether the patient’s laboratory markers denote single-, double-, or triple-positivity for antiphospholipid syndrome.
Vaginal hemorrhage.
Secondary analyses
In the as-treated analysis, there were 3 thrombotic events among patients randomized to the apixaban 2.5 mg twice daily dosing and another 3 among patients randomized to apixaban 5 mg twice daily dosing. The 1000 person-year rates for thrombotic events were 603 events and 231 events for apixaban 2.5 mg and apixaban 5 mg dosing, respectively. The as-treated analysis for major and CRNMB events among patients randomized to warfarin was 34 per 1000 person-years and zero with apixaban. When combining thrombotic and bleeding events to report a net clinical outcome, the person-time rate per 1000 years was 318 for apixaban (any dose), 603, 231, and 40 for apixaban 2.5 mg, apixaban 5 mg, and warfarin, respectively.
After excluding patients with a history of arterial thrombosis, there were 17 remaining participants in the apixaban arm and 16 in the warfarin arm. Two thrombotic events occurred in the apixaban arm and none in the warfarin arm (supplement Figure 1). No major bleeding events occurred in either arm. The overall rate of thrombotic events for the apixaban arm was 129 per 1000 person-years, and the as-treated thrombotic event rates were 302 and 82 per 1000 person-years for apixaban 2.5 mg dosing and apixaban 5mg dosing, respectively.
Among patients randomized to apixaban 2.5 mg twice daily, 1 individual with a former history of stroke was transitioned to apixaban 5 mg twice daily upon the first protocol modification and then transitioned to warfarin with the second protocol modification. This patient experienced no outcome event. Detailed patient characteristics with study outcomes for all patients randomized are presented in supplement Table 1, adverse events and unscheduled encounters are reported in supplement Table 2, and stroke classification is reported in supplement Table 3.
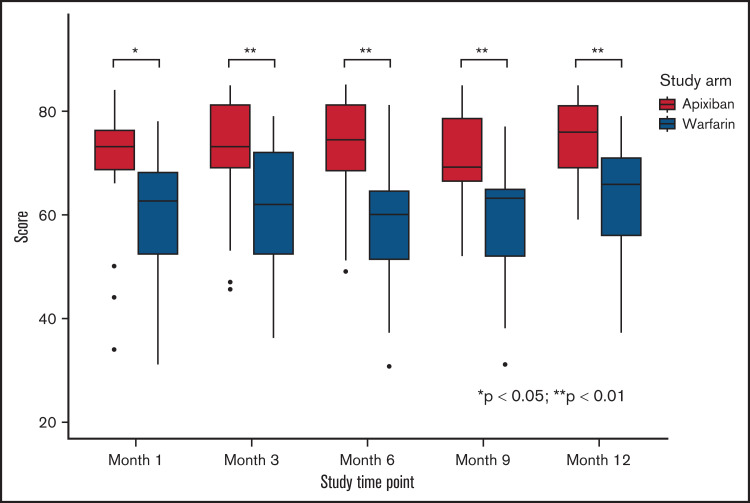
Patient satisfaction with anticoagulation was assessed using the ACTS survey. Patients on apixaban had significantly higher scores compared with patients on warfarin from month 1 through month 12, indicating that patients reported higher satisfaction with apixaban over warfarin (Figure 3).
Figure 3.
Assessment of patient satisfaction with anticoagulation treatment using ACTS among patients randomized to apixaban or warfarin. Shown is a comparison of patient satisfaction with anticoagulation treatment assessment measured with a validated assessment tool (ACTS) at the shown time points. The midline in each box represents the median, and the top and the bottom of each box represent the cutoffs of the interquartile range. The whiskers represent 1.5 times the interquartile range, and outliers are represented as dots. Apixaban was significantly favored over warfarin at every interval assessment.
Discussion
We present the results of the prospective randomized controlled pilot trial reporting rates of thrombosis and bleeding events among TAPS patients receiving either apixaban or warfarin. During the study duration of 12 months, we reported a high rate of thrombotic stroke in 6 patients randomized to apixaban (318 events per 1000 person-years) and in no patients randomized to warfarin. There was no bleeding event in the apixaban arm and 1 major bleeding event in the warfarin arm (rate: 40 events per 1000 person-years). Patients randomized to apixaban reported greater satisfaction with anticoagulation treatment.
Our observations align with those previously reported from prospective randomized clinical trials in TAPS patients comparing VKA to rivaroxaban. Pengo et al conducted a randomized control trial designed to enroll 536 triple-positive TAPS patients (positive for lupus anticoagulant, anticardiolipin, and anti-β-2-glycoprotein-I antibodies). The DSMB terminated the trial after 7 of 59 patients randomized to rivaroxaban experienced arterial thrombosis compared with none on warfarin.9 Ordi-Ros et al reported results of a noninferiority randomized control trial comparing rivaroxaban 20 mg daily with warfarin (target INR 2.5 or 3.5 among those with a history of recurrent thrombosis) in TAPS patients. After the study duration of 36 months, the rate of thrombosis was 3.9% in the rivaroxaban group compared with 2.1% in the VKA group (risk ratio 1.83 [95% CI, 0.71-4.76]), and did not meet the predefined noninferiority margin of 1.4. Stroke occurred among 9 patients randomized to rivaroxaban and in no patients randomized to VKA.23 Based on these results, in May 2019, European and US regulatory agencies changed the labeling of all DOACs to advise against use among patients with APS, especially triple-positive patients.10,12
We cannot make confident conclusions regarding the comparative efficacy and safety of apixaban and warfarin among TAPS patients based upon our study, which has significant limitations. Our study was limited by early termination, small sample size, and multiple protocol modifications. However, among the 48 patients enrolled, over 12 months we observed 6 thrombotic events (all ischemic strokes) in patients randomized to apixaban and in no patients randomized to warfarin. Our observations raise the concern that apixaban, like rivaroxaban in prior studies, may be associated with reduced efficacy compared with VKA in TAPS patients.
There are several possible reasons that warfarin may have greater efficacy in TAPS than apixaban and other oral Xa inhibitors. Oral Xa inhibitors have shorter half-lives than warfarin, and brief times of nonadherence may carry greater risk for thrombotic events.24 We did not measure anti-Xa activity levels to estimate therapeutic effect as this is not considered standard of care. However, we routinely solicited adherence to apixaban and systematically captured any missed doses. Patients’ reported adherence to apixaban was excellent (97.3%) and not dissimilar from that reported by others.9,23 Despite adherence, we cannot exclude that suboptimal drug concentration might contribute to the thrombotic events observed, especially among those patients that received apixaban 2.5 mg twice daily dosing.25 However, the same number of events (n = 3) were found among subjects that received 2.5 mg and 5 mg dosing based on the as-treated analysis. It is possible that an escalated dose of DOACs may be better among TAPS patients, and this is being investigated with rivaroxaban (ClinicalTrials.gov identifier: NCT03684564). APS is a syndrome and therefore includes a population that is heterogeneous in clinical history of thrombosis and laboratory manifestations of the disease. It is possible that there exists a subset of TAPS patients for which apixaban may be a reasonable alternative to VKA. To this end, it has been advised that all cases of DOAC use in APS patients should be reported to the ISTH-supported registry (ClinicalTrials.gov Identifier: NCT04262492).26 Our study design included a heterogeneous cohort of patients with a clinical diagnosis of TAPS and was intended to provide preliminary data to answer this question.
Significant limitations of our study include the need for 2 protocol modifications: (1) the dose of apixaban was increased from 2.5 mg twice daily to 5 mg twice daily (after the twenty-fifth patient) and (2) exclusion of patients with a history of arterial thrombosis or radiological evidence of stroke or white matter change disproportionate for patient age on brain magnetic resonance imaging (after the thirtieth patient). While we attempted to enlist a clinical trials network to improve recruitment, only 1 external site was able to enroll 1 additional patient. The study was terminated prior to target enrollment due to inadequate accrual and the resultant loss of funding.
Strengths of the study include the prospective randomized control study design and completion of 12-month follow-ups in all participants. All events were adjudicated by a panel of experts blinded to the treatment allocation.
In conclusion, we report our findings of a pilot study randomizing TAPS patients on long-term anticoagulation to apixaban or warfarin for the prevention of recurrent thrombosis. We observed an increased number of thrombotic strokes in patients receiving apixaban compared with those receiving warfarin, but our study was terminated early, and too few events occurred to make definitive conclusions. Nonetheless, our study is consistent with evidence for the role of DOACs among patients with TAPS and suggests that apixaban may not be an effective alternative to warfarin among patients with TAPS.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors would like to acknowledge Drs. J. Brent Muhlestein, Joseph Bledsoe, Robert Hoesch, Paul Asmar, Coleman Herrod, Logan McLean, Mark Dodson, Megan Donohue, Erica Mulciare-Jones, and Paul Johnson; the clinical research coordinator Abigail Bartosic; and Dr. J. Michael Dean and the staff of the University of Utah Center for Clinical & Translational Science.
Investigator-initiated funding from Bristol-Myers-Squibb-Pfizer Alliance was paid to Intermountain Healthcare.
Authorship
Contribution: S.C.W., S.M.S., M.T.R., D.K., C.G.E., T.-F.W., D.W.B., V.T.A., B.A., and E.L.W. were involved in conceptualization, data curation, formal analysis, investigation, methodology, supervision, visualization, writing of original draft, and writing, review, and editing of later drafts; S.C.W., S.M.S., C.G.E., J.F.L., E.L.W., D.G., V.T.A., and B.A. were involved in funding acquisition, project administration, resources, software, and validation; and S.C.W., D.G., and S.M.S. have accessed and verified the underlying data.
Conflict-of-interest disclosure: T.-F.W. reports research funding from Leo Pharma and advisory honoraria from Servier. The remaining authors declare no competing financial interests. Bristol Myers Squibb-Pfizer Alliance provided funding for this investigator-initiated study paid to Intermountain Healthcare with no financial support directed to the investigators.
Correspondence: Scott C. Woller, 5169 Cottonwood St. Suite #307, Murray, UT 84157; e-mail: scott.woller@imail.org.
References
- 1.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295-306. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz-Irastorza G, Cuadrado MJ, Ruiz-Arruza I, et al. Evidence-based recommendations for the prevention and long-term management of thrombosis in antiphospholipid antibody-positive patients: report of a task force at the 13th International Congress on antiphospholipid antibodies. Lupus. 2011;20(2):206-218. [DOI] [PubMed] [Google Scholar]
- 3.Keeling D, Mackie I, Moore GW, Greer IA, Greaves M; British Committee for Standards in Haematology . Guidelines on the investigation and management of antiphospholipid syndrome. Br J Haematol. 2012;157(1):47-58. [DOI] [PubMed] [Google Scholar]
- 4.Cervera R, Serrano R, Pons-Estel GJ, et al. ; Euro-Phospholipid Project Group (European Forum on Antiphospholipid Antibodies) . Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis. 2014;74(6):1011-1018. [DOI] [PubMed] [Google Scholar]
- 5.Agnelli G, Buller HR, Cohen A, et al. ; AMPLIFY-EXT Investigators . Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368(8):699-708. [DOI] [PubMed] [Google Scholar]
- 6.Agnelli G, Buller HR, Cohen A, et al. ; AMPLIFY Investigators . Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799-808. [DOI] [PubMed] [Google Scholar]
- 7.Agnelli G, Becattini C, Meyer G, et al. ; Caravaggio Investigators . Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382(17):1599-1607. [DOI] [PubMed] [Google Scholar]
- 8.Cohen H, Hunt BJ, Efthymiou M, et al. ; RAPS trial investigators . Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol. 2016;3(9):e426-e436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pengo V, Denas G, Zoppellaro G, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. 2018;132(13):1365-1371. [DOI] [PubMed] [Google Scholar]
- 10.(PRAC) PRAC. PRAC Recommendations on Signals. Domenico Scarlattilaan 6 @ 1083 HS Amsterdam @ The Netherlands. European Medicines Agency; 2019 [Google Scholar]
- 11.Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 2019;78(10):1296-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Services UDoHaH. Drug Safety-related Labeling Changes (SrLC). 2019. https://www.accessdata.fda.gov/scripts/cder/safetylabelingchanges/index.cfm?event=searchdetail.page&DrugNameID=238.
- 13.Zuily S, Cohen H, Isenberg D, et al. Use of direct oral anticoagulants in patients with thrombotic antiphospholipid syndrome: guidance from the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2020;18(9):2126-2137. [DOI] [PubMed] [Google Scholar]
- 14.Arachchillage DRJ, Gomez K, Alikhan R, Anderson JAM, Lester W, Laffan M; British Society for Haematology Haemostasis and Thrombosis Taskforce . Addendum to British Society for Haematology Guidelines on Investigation and Management of Antiphospholipid syndrome, 2012 (Br. J. Haematol. 2012; 157: 47-58): use of direct acting oral anticoagulants. Br J Haematol. 2020;189(2):212-215. [DOI] [PubMed] [Google Scholar]
- 15.Woller SC, Stevens SM, Kaplan DA, et al. Apixaban for the secondary prevention of thrombosis among patients with antiphospholipid syndrome: study rationale and design (ASTRO-APS). Clin Appl Thromb Hemost. 2015;22(3):239-247. [DOI] [PubMed] [Google Scholar]
- 16.Cano SJ, Lamping DL, Bamber L, Smith S. The Anti-Clot Treatment Scale (ACTS) in clinical trials: cross-cultural validation in venous thromboembolism patients. Health Qual Life Outcomes. 2012;10(1-11):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulman S, Angerås U, Bergqvist D, Eriksson B, Lassen MR, Fisher W; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2010;8(1):202-204. [DOI] [PubMed] [Google Scholar]
- 18.Granger CB, Alexander JH, McMurray JJV, et al. ; ARISTOTLE Committees and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992. [DOI] [PubMed] [Google Scholar]
- 19.Woller SC, Stevens SM, Kaplan DA, Rondina TM. Protocol modification of apixaban for the secondary prevention of thrombosis among patients with antiphospholipid syndrome study. Clin Appl Thromb Hemost. 2018;24(1):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finazzi G, Marchioli R, Brancaccio V, et al. A randomized clinical trial of high-intensity warfarin vs. conventional antithrombotic therapy for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome (WAPS). J Thromb Haemost. 2005;3(5):848-853. [DOI] [PubMed] [Google Scholar]
- 21.Crowther MA, Ginsberg JS, Julian J, et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med. 2003;349(12):1133-1138. [DOI] [PubMed] [Google Scholar]
- 22.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 2018;69(03):236-239. [PubMed] [Google Scholar]
- 23.Ordi-Ros J, Sáez-Comet L, Pérez-Conesa M, et al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome: a randomized noninferiority trial. Ann Intern Med. 2019;171(10):685-694. [DOI] [PubMed] [Google Scholar]
- 24.Abdou JK, Auyeung V, Patel JP, Arya R. Adherence to long-term anticoagulation treatment, what is known and what the future might hold. Br J Haematol. 2016;174(1):30-42. [DOI] [PubMed] [Google Scholar]
- 25.Schofield JR, Hassell K. Dosing considerations in the use of the direct oral anticoagulants in the antiphospholipid syndrome. J Clin Pharm Ther. 2017;43(1):104-106. [DOI] [PubMed] [Google Scholar]
- 26.Cohen H, Cuadrado MJ, Erkan D, et al. 16th International Congress on Antiphospholipid Antibodies Task Force Report on Antiphospholipid Syndrome Treatment Trends. Lupus. 2020;29(12):1571-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.