At the end of 2021, infections with the B.1.1.529 SARS-CoV-2 variant (Omicron) superseded those with the B.1.617.2 variant (Delta). This study compares baseline characteristics and in-hospital outcomes of patients who presented to 13 emergency departments in Paris from 29 November 2021 to 10 January 2022.
Visual Abstract. Patients With Delta Versus Omicron Variants in the Emergency Department.
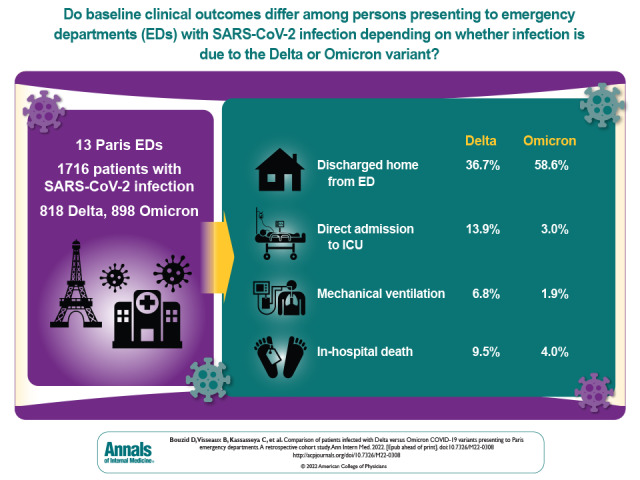
At the end of 2021, infections with the B.1.1.529 SARS-CoV-2 variant (Omicron) superseded those with the B.1.617.2 variant (Delta). This study compares baseline characteristics and in-hospital outcomes of patients who presented to 13 emergency departments in Paris from 29 November 2021 to 10 January 2022.
Abstract
Background:
At the end of 2021, the B.1.1.529 SARS-CoV-2 variant (Omicron) wave superseded the B.1.617.2 variant (Delta) wave.
Objective:
To compare baseline characteristics and in-hospital outcomes of patients with SARS-CoV-2 infection with the Delta variant versus the Omicron variant in the emergency department (ED).
Design:
Retrospective chart reviews.
Setting:
13 adult EDs in academic hospitals in the Paris area from 29 November 2021 to 10 January 2022.
Patients:
Patients with a positive reverse transcriptase polymerase chain reaction (RT-PCR) test result for SARS-CoV-2 and variant identification.
Measurements:
Main outcome measures were baseline clinical and biological characteristics at ED presentation, intensive care unit (ICU) admission, mechanical ventilation, and in-hospital mortality.
Results:
A total of 3728 patients had a positive RT-PCR test result for SARS-CoV-2 during the study period; 1716 patients who had a variant determination (818 Delta and 898 Omicron) were included. Median age was 58 years, and 49% were women. Patients infected with the Omicron variant were younger (54 vs. 62 years; difference, 8.0 years [95% CI, 4.6 to 11.4 years]), had a lower rate of obesity (8.0% vs. 12.5%; difference, 4.5 percentage points [CI, 1.5 to 7.5 percentage points]), were more vaccinated (65% vs. 39% for 1 dose and 22% vs. 11% for 3 doses), had a lower rate of dyspnea (26% vs. 50%; difference, 23.6 percentage points [CI, 19.0 to 28.2 percentage points]), and had a higher rate of discharge home from the ED (59% vs. 37%; difference, 21.9 percentage points [−26.5 to −17.1 percentage points]). Compared with Delta, Omicron infection was independently associated with a lower risk for ICU admission (adjusted difference, 11.4 percentage points [CI, 8.4 to 14.4 percentage points]), mechanical ventilation (adjusted difference, 3.6 percentage points [CI, 1.7 to 5.6 percentage points]), and in-hospital mortality (adjusted difference, 4.2 percentage points [CI, 2.0 to 6.5 percentage points]).
Limitation:
Patients with COVID-19 illness and no SARS-CoV-2 variant determination in the ED were excluded.
Conclusion:
Compared with the Delta variant, infection with the Omicron variant in patients in the ED had different clinical and biological patterns and was associated with better in-hospital outcomes, including higher survival.
Primary Funding Source:
None.
Several waves of infections related to the emergence of SARS-CoV-2, named the Alpha, Beta, and Delta variants, have emerged since December 2019 (1, 2). In November 2021, a new SARS-CoV-2 variant of concern—B.1.1.529 (Omicron)—was reported in South Africa. This variant triggered a fourth wave of infections that rapidly spread worldwide (3, 4). Early reports suggested that the large number of mutations in this variant were associated with immune escape and consequent reduced vaccine efficacy. In France, although 93% of persons aged 12 years and older had received at least 1 anti–COVID-19 vaccine dose by the end of 2021, the Omicron variant swiftly became the dominant variant (superseding the Delta variant), with subsequent increased demand on hospital services and the instigation of new population infection control measures (5).
Some preliminary studies quickly provided data on Omicron's infectiousness, but data on early presentation and patient-level characteristics at the time of emergency department (ED) visits are lacking (6, 7). Comparing the effects of Delta and Omicron infections may help health authorities better anticipate hospital needs, particularly intensive care unit (ICU) beds, during an ongoing Omicron wave. Compared with the Delta wave, the Omicron wave was associated with a different pattern of clinical characteristics and outcomes in patients hospitalized for COVID-19 in South Africa (8, 9). However, these associations were made without patient-level SARS-CoV-2 variant identification and without differentiating between patients admitted to the hospital for SARS-CoV-2 infection and those with incidental SARS-CoV-2 infection hospitalized for other reasons.
The aim of this study was to compare clinical and biological characteristics and hospital outcomes between patients with Omicron and Delta SARS-CoV-2 infections seeking care in 13 EDs in the Paris metropolitan area from 29 November 2021 to 10 January 2022.
Methods
Design
This was a retrospective observational multicenter study, which included 13 EDs in the Paris metropolitan area, France, caring for patients aged 16 years and older. All centers are academic hospitals affiliated with the Assistance Publique–Hôpitaux de Paris (APHP) network. Because of the study's retrospective nature on deidentified data, informed consent was waived. The study was approved by the ethics committee of Sorbonne Université (Comité d'éthique de la recherche de Sorbonne Université, Paris, France). The reporting of this study followed the recommendations of the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guideline (10).
Objectives and Outcomes
The primary objective of this study was to compare baseline clinical and biological characteristics of patients with Delta and Omicron SARS-CoV-2 infections in the ED. Baseline characteristics included demographic data, past medical history, vaccination status, physiologic variables at ED presentation, inflammatory biological markers (leukocytes, C-reactive protein, and D-dimer), oxygen therapy in the ED, and chest computed tomography imaging results (percentage of pulmonary involvement and presence of pulmonary embolism).
The secondary objectives were to determine the differences in vaccination status, ED management for respiratory failure, oxygen requirement, mechanical ventilation, discharge disposition, secondary ICU admission, and in-hospital mortality. The proportion of incidental COVID-19 was also compared between groups.
Secondary ICU admission was defined as an ICU admission during the hospital course in patients that were not admitted to an ICU directly from the ED. Incidental SARS-CoV-2 infection was defined by an expert group of physicians as either a SARS-CoV-2 infection that was diagnosed during the ED stay in patients that presented to the ED for a cause that was unrelated to COVID-19 or a patient in whom SARS-CoV-2 was not considered to be associated with a patient's clinical condition requiring hospital admission (http://aphp.aphp.fr/wp-content/blogs.dir/268/files/2022/01/APHP-COVID19-INF-0082-anglais-NOUVELLE-DEFINITION-POUR-LES-PATIENTS-COVID-HOSPITALISES-Covid-accessoire-V1.pdf)
Patients
Patients were included if they visited 1 of the participating EDs and had a positive reverse transcriptase polymerase chain reaction (RT-PCR) test result for SARS-CoV-2 during the 6 weeks between 29 November 2021 (week 48 of 2021) and 10 January 2022 (week 1 of 2022). To identify eligible patients, a list of positive RT-PCR tests done on patients in the ED were automatically extracted from the laboratory electronic file records of each center, and files were dismissed if there was no variant determination.
Patients were excluded if the SARS-CoV-2 variant was not sought or could not be determined. In cases of multiple ED visits during the inclusion period, only the first one was included and analyzed. As part of infection control procedures, staff members were routinely tested (without clinical assessment) if they were in close contact with a person with COVID-19, with no medical evaluation. Therefore, any staff member with a positive SARS-CoV-2 result was excluded from the study.
Data Collection
To reduce bias inherent to retrospective chart review studies, baseline characteristics, exposure, and outcomes were defined a priori. All terms in the case report form were predefined, and all abstractors were emergency physicians who had remote training by the primary investigator of this study. A standardized data collection instrument with clear criteria for recording both categorical and quantitative variables was shared. All data were collected on the integrated electronic patient record of the APHP trust (ORBIS, Agfa HealthCare). Medical records of patients that were transferred to another hospital in the APHP organization were available, which reduced the risk for loss to follow-up. Data about participant's inpatient hospital course were censored 10 days after the last inclusion—that is, on 20 January. When all data were locally collected in each ED, they were deidentified from the electronic case report form by removing the name, date of birth, and day of ED visit.
Variant Determination
Given that all patients with no variant determination were excluded, and because only 2 variants were detected during the study period, SARS-CoV-2 variants were classified as Delta (B.1.617.2) or Omicron (B.1.1.529). Identification of SARS-CoV-2 variants was undertaken for all positive nasopharyngeal swab results (<30 cycle threshold) using either a screening or full sequencing approach. The screening approach used both the detection of L452R mutation (VirSNiP Mutation Assays, TIB Molbiol) for Delta variant assessment and the S-gene target failure (TaqPath COVID-19, Thermo Fisher) and K417N or E484A mutation (VirSNiP Mutation Assays, TIB Molbiol) for Omicron variant assessment. Full genome assessment was done using the ARTIC V3 protocol on a GridION device (Oxford Nanopore) or the Respiratory Virus Oligos Panel V2 (ref: 20044311, Illumina).
Statistical Analysis
Patients' characteristics were described in the whole population and according to SARS-CoV-2 variant (Delta or Omicron). Categorical variables were expressed as number (percentage) and quantitative variables as mean (SD) or median (interquartile range [IQR]), depending on their distribution. Patients' characteristics differences and 95% CIs were calculated using the Wald method, with continuity correction for categorical variables and using normal approximation or the Brookmeyer and Crowley method for continuous variables, depending on their distribution (11). Logistic regression modeling was used to study the relationship between SARS-CoV-2 variant and the following outcomes: ICU hospitalization, mechanical ventilation, and death. Adjustment factors were age, sex, hypertension, obesity, diabetes, chronic respiratory disease, chronic kidney disease, immunosuppression, number of vaccine doses, and center. Results are expressed as differences and 2-sided 95% CIs. To address missing data, multiple imputations were done using the Fully Conditional Specification method of PROC MI (SAS/STAT, version 14.3 [SAS Institute]). The discriminative and logistic functions were used for categorical and binary variables, respectively, and 15 data sets were created. All results were combined using PROC MIANALYZE (SAS Institute).
Analyses were done with SAS, version 9.4 (SAS Institute).
Role of the Funding Source
Only institutional resources were used in this study.
Results
Among 3728 patients who had a positive RT-PCR test result in the ED during the study period, 1716 were included in the study (898 Omicron and 818 Delta) (Figure).
Figure. Study flow diagram.
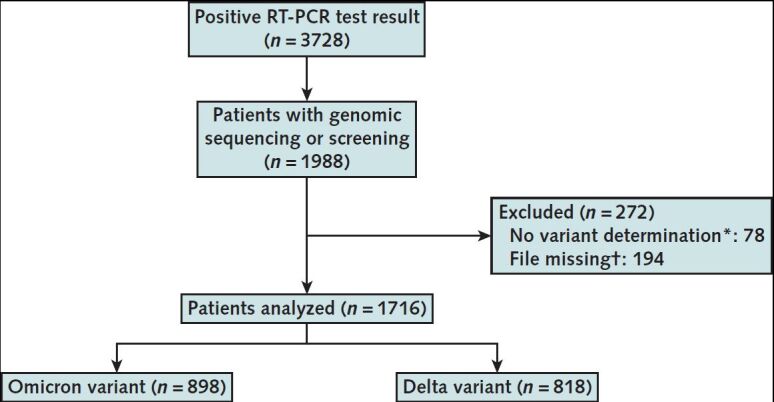
RT-PCR = reverse transcriptase polymerase chain reaction.
* Patients in whom the variant could not have been identified were excluded.
† The RT-PCR analyses in staff members were not included in the study.
Baseline Characteristics at ED Presentation
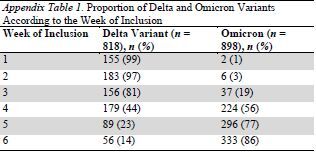
The median age of the analyzed population was 58 years (IQR, 38 to 75 years). and 49% were women. The proportion of Omicron increased from 1.3% in the first week of inclusion (week 48 of 2021) to 86% in the last week (week 1 of 2022) (Appendix Tables 1 and 2). Compared with the Delta variant, patients infected with Omicron were younger (54 years [IQR, 33 to 75 years] vs. 62 years [IQR, 45 to 75 years]; difference, 8.0 years [95% CI, 4.6 to 11.4 years]), more often female (53% vs. 45%; difference, 7.9 percentage points [CI, 3.1 to 12.7 percentage points]), and were less frequently overweight (8.0% vs. 12.5%; difference, 4.5 percentage points [CI, 1.5 to 7.5 percentage points]). Other comorbidities did not differ between groups (Table 1). There was a higher rate of patients that received at least 1, 2, or 3 doses of COVID-19 vaccine in the Omicron group (Table 1).
Appendix Table 1.
Proportion of Delta and Omicron Variants According to the Week of Inclusion
Appendix Table 2.
Proportion of Delta and Omicron Variants in Each Center
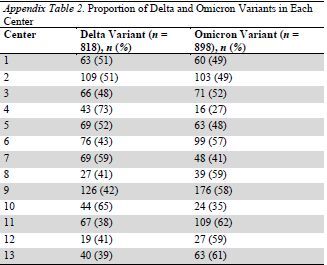
Table 1.
Baseline Characteristics at ED Presentation*
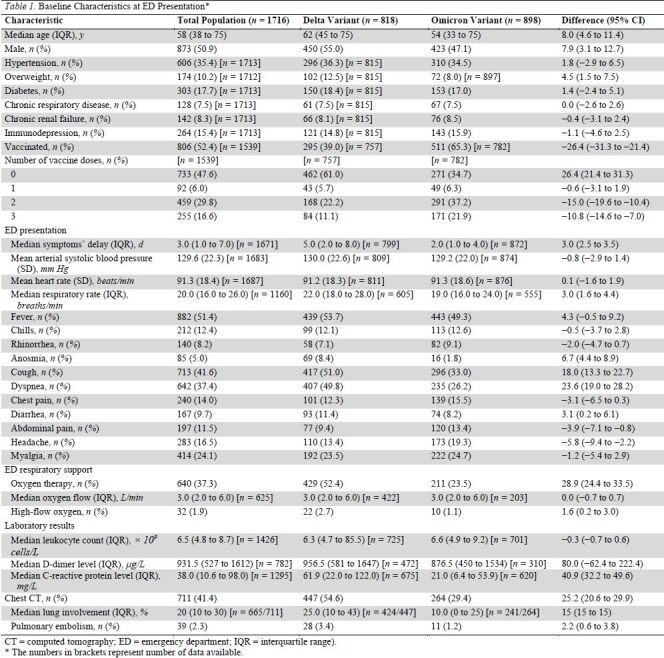
Patients with Omicron had an earlier ED presentation than those with Delta, with a median duration between first symptoms and ED visit of 2 days (IQR, 1 to 4 days) versus 5 days (IQR, 2 to 8 days) (difference, 3.0 days [CI, 2.5 to 3.5 days]). Overall, patients in the Omicron group had a lower rate of respiratory symptoms than those in the Delta group: 33% versus 51% for cough (difference, 18.0 percentage points [CI, 13.3 to 22.7 percentage points]) and 26% versus 50% (difference, 23.6 percentage points [CI, 19.0 to 28.2 percentage points]) for dyspnea. Biological markers of inflammation and pulmonary involvement on computed tomography were also lower in the Omicron group than in the Delta group (Table 1).
ED Discharge Disposition and In-Hospital Outcomes
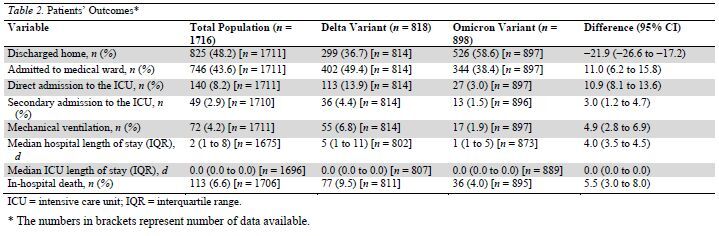
There were lower rates of hospital admission, ICU admission, mechanical ventilation, and in-hospital death in the Omicron group than in Delta group (Table 2). Incidental SARS-CoV-2 infection was diagnosed in 23% of patients with Omicron versus 13% of patients with Delta (difference, 10.4 percentage points [CI, 6.7 to 14.1 percentage points]).
Table 2.
Patients' Outcomes*
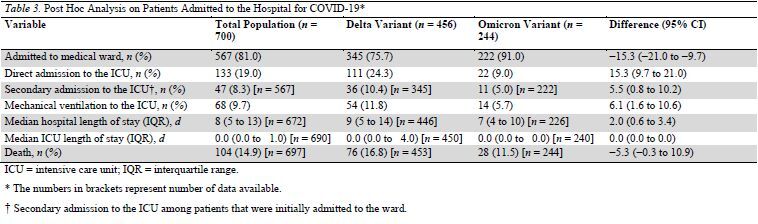
These results were confirmed in the prespecified sensitivity analysis of the 700 patients that were hospitalized for COVID-19 (that is, patients without incidental COVID-19) (Table 3). Among the 567 patients that were admitted into the ward for COVID-19 illness, 47 deteriorated and were secondarily transferred to the ICU (11 [5%] and 36 [10.4%] in the Omicron and Delta groups, respectively [difference, 5.5 percentage points {CI, 0.8 to 10.2 percentage points}]).
Table 3.
Post Hoc Analysis on Patients Admitted to the Hospital for COVID-19*
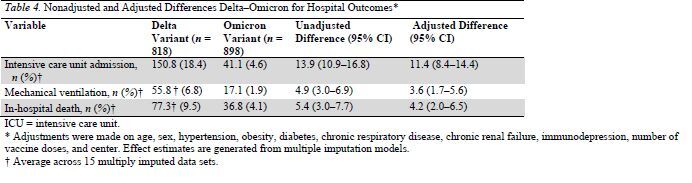
After adjustment, compared with the Delta variant, the Omicron variant was associated with a reduced risk for ICU admission (difference, 11.4 percentage points [CI, 8.4 to 14.4 percentage points]), mechanical ventilation (difference, 3.6 percentage points [CI, 1.7 to 5.6 percentage points]), and in-hospital mortality (difference, 4.2 percentage points [CI, 2.0 to 6.5 percentage points]) (Table 4).
Table 4.
Nonadjusted and Adjusted Differences Delta–Omicron for Hospital Outcomes*
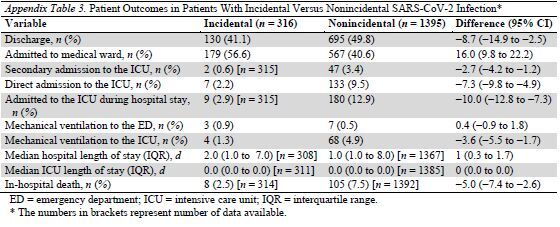
In a post hoc analysis, incidental COVID-19 was associated with lower risks for ICU admission, mechanical ventilation, and death (Appendix Table 3).
Appendix Table 3.
Patient Outcomes in Patients With Incidental Versus Nonincidental SARS-CoV-2 Infection*
Discussion
In this retrospective chart review of 1716 patients that visited an academic ED in the Paris area and had a variant identified, 52% were infected with Omicron, and 48% were infected with Delta. Omicron affected a younger population, with a higher rate of vaccination, a lower rate of respiratory symptoms, reduced levels of inflammation, and less extensive chest involvement on the CT scan. Adjusted analysis reported that Omicron was independently associated with improved hospital outcomes.
Since November 2021 and the first identification of the Omicron variant in South Africa, studies have reported that this variant is more infectious and that an immune escape leads to subsequent lower vaccine efficacy (12–14). The current study confirms these findings, with the higher proportion of vaccinated patients among those infected with Omicron, as was also observed by Brandal and colleagues in Denmark (15).
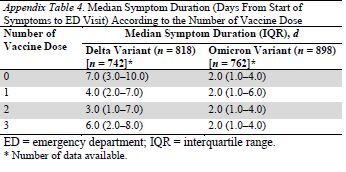
Another interesting finding was that the time from symptom onset to ED visit was shorter in patients with Omicron compared with those with Delta. This may be related to the fact that Omicron seems to have a tropism for the upper respiratory tract rather than the lower respiratory tract, which is also seen in cases of other respiratory viruses, including other coronaviruses (16, 17). It is possible that symptoms develop more quickly in the upper respiratory tract than in the lower, and therefore that time from first symptoms to decompensation requiring ED visit is shorter for Omicron than for Delta. Some other hypotheses can also be raised and will require further explorations, such as a quicker replication rate or an earlier immune response among vaccinated patients more frequently infected with Omicron than Delta. However, a post hoc analysis did not report any association between number of vaccine doses and the median symptom's delay for Omicron or Delta (Appendix Table 4, available at Annals.org).
Appendix Table 4.
Median Symptom Duration (Days From Start of Symptoms to ED Visit) According to the Number of Vaccine Dose*
Preliminary reports suggested that Omicron was associated with reduced severity (8, 9, 18, 19). After the first Omicron wave in South Africa, Maslo and colleagues (9) compared outcomes of hospitalized patients during the Omicron wave and during the Delta wave. They found different patterns of patients with infection admitted into hospital, with shorter lengths of stay and reduced mortality during the Omicron wave. However, this study only compared patients from different waves but not patients with confirmed different variants. In the current study, the variant was identified in each analyzed patient. We report that patients present to the ED with different patterns of disease dependent on SARS-CoV-2 variant and that their hospital course seems to be shorter and less severe for Omicron than for Delta.
The main limitation of this study is that we only included patients that had an identified variant. Of 3728 positive RT-PCR test results, only 1716 (46%) were analyzed for variant identification. In several EDs, a point-of-care RT-PCR device was available for rapid results, and positive results were not systematically followed by a control in the central laboratory where sequencing for variant identification would have been done. This group largely comprised patients that were discharged home from the ED because admitted patients usually had a control RT-PCR. Furthermore, during the last inclusion week (week 1 of 2022), a few centers experienced reagent shortages and did not proceed to variant identification in ambulatory patients. This bias may have led to an overrepresentation of the rate of Omicron patients hospitalized because Omicron was the predominant variant at that time. Because these patients were not eligible, we did not collect data on them, and consequently it is not possible to compare patients that had a variant determination with those that did not.
Another limitation is that comorbidities or clinical signs were recorded only if they were noted in the medical chart, without clear definition. For example, there was no specified threshold for “overweight,” and this was left to the discretion of the treating physician. We only recorded the number of vaccines the patient received and not whether patients had previous SARS-CoV-2 infection. Therefore, some patients may have had a full vaccination scheme with a booster dose (that is, COVID-19 illness plus 1 dose then a booster dose) but were recorded as having received only 2 vaccines shots. Pulmonary embolism was not systematically sought; therefore, it is possible that the reported lower rate of pulmonary embolism in the Omicron group may be biased because of a lower rate of chest imaging done in this group.
As a retrospective study, some data may not have been recorded, and there was no monitoring of the data collection method. This was mitigated by the use of a standardized case report form and abstractor training. Hospital follow-up ended on 18 January 2022, with subsequent censoring at a minimum of 9 days. Because the median length of stay of patients that were hospitalized for Omicron infection was 7 days (IQR, 4 to 10 days), we believe that this limitation is of limited effect. Furthermore, there was no follow-up after discharge from the hospital; therefore, any return visit to the hospital was not captured.
Finally, we analyzed patients when they presented to the ED, which incorporates a selection bias to evaluate the overall severity of a variant. It is likely that a smaller proportion of patients with Omicron sought ED care compared with those with Delta, which would further support a milder severity of Omicron. However, our study allows the comparison of characteristics of patients at the time of ED presentation and of prognosis in patients who are subsequently hospitalized. This will enable improved modeling and health care system planning for hospital and ICU resource allocation during an Omicron wave.
In conclusion, patients that visited the ED with SARS-CoV-2 infection exhibited different clinical characteristics when infected with the Omicron variant versus the Delta variant, with a younger age, fewer respiratory symptoms, and lower inflammatory response. Compared with Delta, infection with Omicron was independently associated with reduced risks for ICU admission, mechanical ventilation, and in-hospital death.
Supplementary Material
Appendix: Members of the IMProving Emergency Care (IMPEC) FHU Collaborators Group
Members of the IMProving Emergency Care (IMPEC) FHU Collaborators Group who contributed to this work but did not author it: Dr. Maud Salmona (Hôpital Saint-Louis, Paris), Pr. Jérôme Le Goff (Hôpital Saint-Louis, Paris), Dr. David Veyer (Hôpital Europeen George Pompidou, Paris), Dr. Richard Chocron (Hôpital Europeen George Pompidou, Paris), Dr. Clément Augustin (Hôpital Cochin, Paris), Pr. Flore Rozenberg (Hôpital Cochin, Paris), Dr. Jean-François Meritet (Hôpital Cochin, Paris), Dr. Aurélie Schnuriger (Hôpital Trousseau, Paris), Pr. Slim Fourati (Hôpital Henri Mondor, Créteil), Pr. Mehdi Khellaf (Hôpital Henri Mondor, Créteil), Dr. Audrey Coulomb (Hôpital Henri Mondor, Créteil), Dr. Frédéric Gindre (Hôpital Henri Mondor, Créteil), Dr. Khouloud Aloui (Hôpital Henri Mondor, Créteil), Dr. Hervé Jacquier (Hôpital Lariboisière, Paris), Dr. Xavier Eyer (Hôpital Lariboisière, Paris), Dr. Christophe Choquet (Hôpital Bichat, Paris), Dr. Vittiaroat Ing (Hôpital Bichat, Paris), Dr. Thomas Pavlovsky (Hôpital Bichat, Paris), Dr. Nadhira Houhou-Fidouh (Hôpital Bichat, Paris), Dr. Valentine Ferré (Hôpital Bichat, Paris), Dr. Luce Landraud (Hôpital Louis Mourier, Colombes), Dr. Hélène Goulet (Hôpital Tenon, Paris), Dr. Youri Yordanov (Hôpital Saint Antoine, Paris), Dr. Laurence Berard (Hôpital Saint Antoine, Paris), Alexandra Rousseau (Hôpital Saint Antoine, Paris), Théophile Morel (Assistance Publique – Hôpitaux de Paris, Paris), Theophile Vieux (Assistance Publique – Hôpitaux de Paris, Paris), Simon Cahen (Assistance Publique – Hôpitaux de Paris, Paris), Melkir Saib (Assistance Publique – Hôpitaux de Paris, Paris).
Footnotes
This article was published at Annals.org on 15 March 2022.
* For members of the IMProving Emergency Care (IMPEC) FHU Collaborators Group, see the Appendix (available at Annals.org).
Contributor Information
Maud Salmona, Hôpital Saint-Louis, Paris.
Jérôme Le Goff, Hôpital Saint-Louis, Paris.
David Veyer, Hôpital Europeen George Pompidou, Paris.
Richard Chocron, Hôpital Europeen George Pompidou, Paris.
Clément Augustin, Hôpital Cochin, Paris.
Flore Rozenberg, Hôpital Cochin, Paris.
Jean-François Meritet, Hôpital Cochin, Paris.
Aurélie Schnuriger, Hôpital Trousseau, Paris.
Slim Fourati, Hôpital Henri Mondor, Créteil.
Mehdi Khellaf, Hôpital Henri Mondor, Créteil.
Audrey Coulomb, Hôpital Henri Mondor, Créteil.
Frédéric Gindre, Hôpital Henri Mondor, Créteil.
Khouloud Aloui, Hôpital Henri Mondor, Créteil.
Hervé Jacquier, Hôpital Lariboisière, Paris.
Xavier Eyer, Hôpital Lariboisière, Paris.
Christophe Choquet, Hôpital Bichat, Paris.
Vittiaroat Ing, Hôpital Bichat, Paris.
Thomas Pavlovsky, Hôpital Bichat, Paris.
Nadhira Houhou-Fidouh, Hôpital Bichat, Paris.
Valentine Ferré, Hôpital Bichat, Paris.
Luce Landraud, Hôpital Louis Mourier, Colombes.
Hélène Goulet, Hôpital Tenon, Paris.
Youri Yordanov, Hôpital Saint Antoine, Paris.
Laurence Berard, Hôpital Saint Antoine, Paris.
Alexandra Rousseau, Hôpital Saint Antoine, Paris.
Théophile Morel, Assistance Publique – Hôpitaux de Paris, Paris.
Theophile Vieux, Assistance Publique – Hôpitaux de Paris, Paris.
Simon Cahen, Assistance Publique – Hôpitaux de Paris, Paris.
Melkir Saib, Assistance Publique – Hôpitaux de Paris, Paris.
Maud Salmona, Hôpital Saint-Louis, Paris.
Jérôme Le Goff, Hôpital Saint-Louis, Paris.
David Veyer, Hôpital Europeen George Pompidou, Paris.
Richard Chocron, Hôpital Europeen George Pompidou, Paris.
Clément Augustin, Hôpital Cochin, Paris.
Flore Rozenberg, Hôpital Cochin, Paris.
Jean-François Meritet, Hôpital Cochin, Paris.
Aurélie Schnuriger, Hôpital Trousseau, Paris.
Slim Fourati, Hôpital Henri Mondor, Créteil.
Mehdi Khellaf, Hôpital Henri Mondor, Créteil.
Audrey Coulomb, Hôpital Henri Mondor, Créteil.
Frédéric Gindre, Hôpital Henri Mondor, Créteil.
Khouloud Aloui, Hôpital Henri Mondor, Créteil.
Hervé Jacquier, Hôpital Lariboisière, Paris.
Xavier Eyer, Hôpital Lariboisière, Paris.
Christophe Choquet, Hôpital Bichat, Paris.
Vittiaroat Ing, Hôpital Bichat, Paris.
Thomas Pavlovsky, Hôpital Bichat, Paris.
Nadhira Houhou-Fidouh, Hôpital Bichat, Paris.
Valentine Ferré, Hôpital Bichat, Paris.
Luce Landraud, Hôpital Louis Mourier, Colombes.
Hélène Goulet, Hôpital Tenon, Paris.
Youri Yordanov, Hôpital Saint Antoine, Paris.
Laurence Berard, Hôpital Saint Antoine, Paris.
Alexandra Rousseau, Hôpital Saint Antoine, Paris.
Théophile Morel, Assistance Publique – Hôpitaux de Paris, Paris.
Theophile Vieux, Assistance Publique – Hôpitaux de Paris, Paris.
Simon Cahen, Assistance Publique – Hôpitaux de Paris, Paris.
Melkir Saib, Assistance Publique – Hôpitaux de Paris, Paris.
Collaborators: Maud Salmona, Jérôme Le Goff, David Veyer, Richard Chocron, Clément Augustin, Flore Rozenberg, Jean-François Meritet, Aurélie Schnuriger, Slim Fourati, Mehdi Khellaf, Audrey Coulomb, Frédéric Gindre, Khouloud Aloui, Hervé Jacquier, Xavier Eyer, Christophe Choquet, Vittiaroat Ing, Thomas Pavlovsky, Nadhira Houhou-Fidouh, Valentine Ferré, Luce Landraud, Hélène Goulet, Youri Yordanov, Laurence Berard, Alexandra Rousseau, Théophile Morel, Theophile Vieux, Simon Cahen, and Melkir Saib
References
- 1. Fontanet A , Autran B , Lina B , et al. SARS-CoV-2 variants and ending the COVID-19 pandemic. Lancet. 2021;397:952-954. [PMID: ] doi: 10.1016/S0140-6736(21)00370-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dyson L , Hill EM , Moore S , et al. Possible future waves of SARS-CoV-2 infection generated by variants of concern with a range of characteristics. Nat Commun. 2021;12:5730. [PMID: ] doi: 10.1038/s41467-021-25915-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization. Update on Omicron. Accessed at www.who.int/news/item/28-11-2021-update-on-omicron on 7 January 2022.
- 4. Karim SSA , Karim QA . Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398:2126-2128. [PMID: ] doi: 10.1016/S0140-6736(21)02758-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Santé publique France. InfoCovidFrance: Chiffres clés et évolution de la COVID-19 en France et dans le Monde. Accessed at www.santepubliquefrance.fr/dossiers/coronavirus-covid-19/coronavirus-chiffres-cles-et-evolution-de-la-covid-19-en-france-et-dans-le-monde on 7 January 2022.
- 6. Saxena SK , Kumar S , Ansari S , et al. Characterization of the novel SARS-CoV-2 Omicron (B.1.1.529) variant of concern and its global perspective. J Med Virol. 2022;94:1738-1744. [PMID: ] doi: 10.1002/jmv.27524 [DOI] [PubMed] [Google Scholar]
- 7. Torjesen I . Covid-19: Omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear. BMJ. 2021;375:n2943. [PMID: ] doi: 10.1136/bmj.n2943 [DOI] [PubMed] [Google Scholar]
- 8. Wolter N , Jassat W , Walaza S , et al. Early assessment of the clinical severity of the SARS-CoV-2 Omicron variant in South Africa: a data linkage study. Lancet. 2022;399:437-446. [PMID: ] doi: 10.1016/S0140-6736(22)00017-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maslo C , Friedland R , Toubkin M , et al. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 Omicron wave compared with previous waves. JAMA. 2022;327:583-584. [PMID: ] doi: 10.1001/jama.2021.24868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. von Elm E, Altman DG, Egger M, et al.. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453-7. [PMID: ] doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 11. Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics. 1982;38:29-41. doi: 10.2307/2530286 [DOI]
- 12. Dejnirattisai W , Shaw RH , Supasa P , et al; Com-COV2 study group. Reduced neutralisation of SARS-CoV-2 Omicron B.1.1.529 variant by post-immunisation serum [Letter]. Lancet. 2022;399:234-236. [PMID: ] doi: 10.1016/S0140-6736(21)02844-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cele S , Jackson L , Khoury DS , et al; NGS-SA. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602:654-656. [PMID: ] doi: 10.1038/s41586-021-04387-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meo SA , Meo AS , Al-Jassir FF , et al. Omicron SARS-CoV-2 new variant: global prevalence and biological and clinical characteristics. Eur Rev Med Pharmacol Sci. 2021;25:8012-8018. [PMID: ] doi: 10.26355/eurrev_202112_27652 [DOI] [PubMed] [Google Scholar]
- 15. Brandal LT , MacDonald E , Veneti L , et al. Outbreak caused by the SARS-CoV-2 Omicron variant in Norway, November to December 2021. Euro Surveill. 2021;26. [PMID: ] doi: 10.2807/1560-7917.ES.2021.26.50.2101147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Del Rio C, Omer SB, Malani PN.. Winter of Omicron-the evolving COVID-19 pandemic. JAMA. 2022;327:319-320. [PMID: ] doi: 10.1001/jama.2021.24315 [DOI] [PubMed] [Google Scholar]
- 17. Christie B . Covid-19: early studies give hope Omicron is milder than other variants. BMJ. 2021;375:n3144. [PMID: ] doi: 10.1136/bmj.n3144 [DOI] [PubMed] [Google Scholar]
- 18. Abdullah F , Myers J , Basu D , et al. Decreased severity of disease during the first global Omicron variant Covid-19 outbreak in a large hospital in Tshwane, South Africa. Int J Infect Dis. 2022;116:38-42. [PMID: ] doi: 10.1016/j.ijid.2021.12.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Diamond M , Halfmann P , Maemura T , et al. The SARS-CoV-2 B.1.1.529 Omicron virus causes attenuated infection and disease in mice and hamsters. Res Sq. 2021. [PMID: ] doi: 10.21203/rs.3.rs-1211792/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


