Abstract
The retinal pigment epithelium (RPE), a monolayer of post-mitotic polarized epithelial cells, strategically situated between the photoreceptors and the choroid, is the primary caretaker of photoreceptor health and function. Dysfunction of the RPE underlies many inherited and acquired diseases that cause permanent blindness. Decades of research have yielded valuable insight into the cell biology of the RPE. In recent years, new technologies such as live-cell imaging have resulted in major advancement in our understanding of areas such as the daily phagocytosis and clearance of photoreceptor outer segment tips, autophagy, endolysosome function, and the metabolic interplay between the RPE and photoreceptors. In this review, we aim to integrate these studies with an emphasis on appropriate models and techniques to investigate RPE cell biology and metabolism, and discuss how RPE cell biology informs our understanding of retinal disease.
Keywords: Phagocytosis, Phagosome maturation, Autophagy, Organelles, Endosomes, Lysosomes, Metabolism
1. Introduction
The retinal pigment epithelium (RPE) forms a critical barrier between the retina and the systemic circulation, and performs many essential functions to support the neural retina. These include the daily phagocytosis of photoreceptor outer segment (OS) tips, recycling vitamin A, and maintaining the blood-retinal barrier. Here, we present an historical perspective of the seminal studies that first identified the central role of the RPE in OS membrane renewal, and discuss how they led to our current understanding of the different stages of OS phagosome formation and degradation by the RPE. We detail the molecular mechanisms involved in the ingestion, motility, and degradation of OS phagosomes, and examine the suggested role of LC3-associated phagocytosis in OS phagosome clearance. The turnover of the RPE’s own components is considered in a discussion of the importance of classical autophagy in RPE health and disease. Next, we explore mechanisms that regulate the biogenesis, transport, and function of various organelles (melanosomes, endosomes, lysosomes, and peroxisomes). These organelles are important for maintaining RPE homeostasis, participate in OS phagosome degradation and in RPE-photoreceptor communication. In the last section, we focus on metabolic coupling between the RPE and photoreceptors as a result of fatty acids released after lysosomal degradation of ingested OS disk membranes. Throughout the review, we consider experimental models that have been used (and misused) to study RPE cell biology and function.
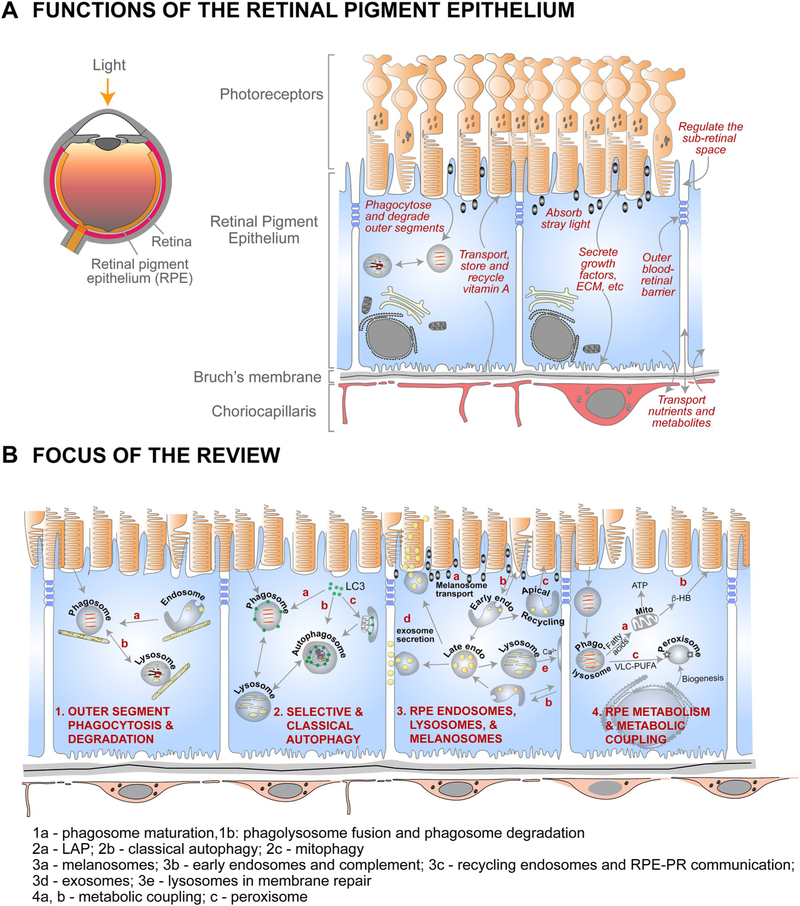
A summary of RPE functions and the focus of this review are illustrated in Fig. 1. Excellent reviews have been published elsewhere on the visual cycle and RPE physiology (Strauss, 2005; Wimmers et al., 2007; Travis et al., 2010; Kiser et al., 2014; Caceres and Rodriguez-Boulan, 2019).
Fig. 1.
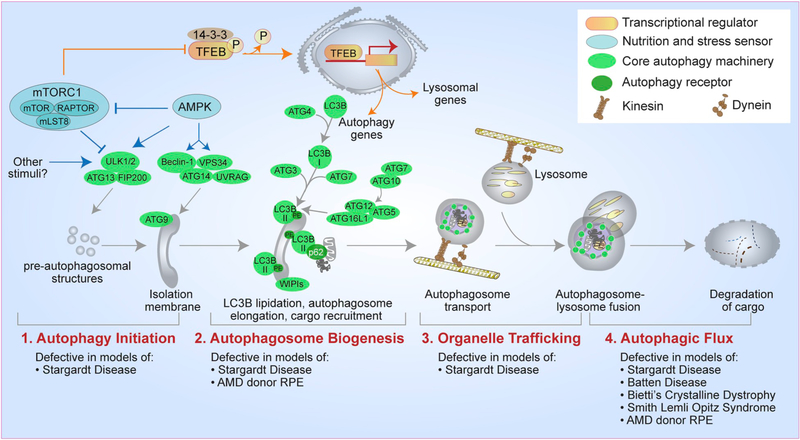
Organization and functions of the RPE and the focus of this review. (A) Left - cross-section of the eye; Right – organization of the outer retina and functions of the retinal pigment epithelium. (B) Summary of RPE functions that are the focus of the review. 1a – phagosome maturation; 1b – phagolysosome fusion and phagosome degradation; 2a – LC3-associated phagocytosis (LAP); 2b – classical autophagy; 2c – mitophagy; 3a – melanosome transport; 3b – early endosomes; 3c – recycling endosomes; 3d – late endosomes and exosome secretion; 3e – lysosomes and lysosome exocytosis; 4a and b – metabolic coupling between RPE and photoreceptors; 4c – peroxisomes in the RPE.
1.1. Organization and function of the RPE
From a developmental perspective, the RPE is a neuroepithelium, derived from the outer layer of the optic cup. Functionally, the RPE acts as a secretory epithelium, a regulatory barrier, a professional phagocyte, and an antigen-presenting cell. These multiple functions are made possible by the unique architecture, location, and specializations of this tissue. The RPE is characterized by apical-basal polarity, lateral junctional complexes between adjacent cells, and a basal surface that sits on an extracellular matrix known as the Bruch’s membrane. The apical microvilli of the RPE interface with the photoreceptor outer segments (OSs), the site of phototransduction. The polarized architecture of the RPE, with tight junctions between cells, enables the RPE to form the outer blood-retinal barrier, a selective barrier between the choroidal blood supply and the neural retina. Transport of nutrients and ions (and hence water) across the RPE, and directional secretion of growth factors and extracellular matrix components help maintain the structure and function of the photoreceptors and the choroid.
The RPE has several unique attributes that make it an active participant in the visual cycle. Moreover, expression of RPE-specific isoforms of key proteins are important for phototransduction. A striking example of this is expression of the β2 subunit of the Na+/K+-ATPase, which results in the localization of this pump to the apical surface, rather than the basolateral surface, as found in most other epithelia (Lobato-Alvarez et al., 2016). Apical localization of Na+/K+-ATPase is likely to be important for spatial buffering of ions in the subretinal space and for depolarizing the photoreceptor plasma membrane during phototransduction. In addition to Na+/K+-ATPase, polarized expression of other ion pumps and channels help regulate the environment around the photoreceptors, which is important for photoreceptor electrophysiology and viability (Wimmers et al., 2007). The RPE has melanosomes or pigment granules that absorb stray light and thus prevent the scattering of light, which would result in the deterioration of spatial resolution. The RPE absorbs vitamin A from the circulation and transports it to the photoreceptors to initiate the visual cycle. In rod photoreceptors, the G-protein-coupled receptor, rod opsin, needs to be bound to 11-cis-retinal for the absorption of light, which converts the 11-cis-retinal into all-trans-retinal. The isomerization of this all-trans-retinal back to 11-cis-retinal is a central event in the visual cycle that occurs in the RPE (Wald and Brown, 1956). The RPE also takes care of the catabolic phase of the turnover of OS disk membranes, by phagocytosing the distal OS disks and degrading them (Young and Bok, 1969). The products of this catabolism participate in metabolic coupling between the RPE and photoreceptors.
The RPE is a post-mitotic, terminally differentiated tissue. In the human eye, each RPE cell takes care of ~30 photoreceptors (Osterberg, 1935), which places a unique burden on the degradative machinery of the cell. As discussed below, crosstalk between lysosomal clearance of OSs, autophagic machinery and the endosomal network, as well as oxidative metabolism, is essential for maintaining RPE health and function.
2. RPE cell models
2.1. Experimental preparations for studying the RPE
Although recent advances in imaging have indicated the feasibility of live-imaging of the RPE in vivo (Gray et al., 2006; Palczewska et al., 2014; Liu et al., 2016, 2019b; Dysli et al., 2017), ex vivo preparations of the RPE offer a more tractable system for experimentation and mechanistic research. Various cell models have been utilized over the years to study aspects of RPE biology. Here, we will address the more common ones, and discuss their advantages and disadvantages.
A freshly-prepared RPE/choroid/sclera flatmount represents a close in situ approximation to in vivo RPE. Flatmounts can be collected and fixed at a specific time of day, thus preserving the effects of circadian rhythmicity. Recently, dynamic RPE intracellular activity has been imaged in live flat mounts, using scanning laser (Mao and Finnemann, 2016; Dejos et al., 2018) and spinning disk confocal microscopy (Umapathy and Williams, 2019), providing insight into phagosome maturation and organelle motility. A limitation of these flatmounts is that they maintain many of their characteristics in culture medium for only a matter of hours, so that many manipulations are not practicable.
Other experimental preparations include 1) primary RPE cells, most commonly isolated and cultured from rodent, pig, or human (fetal or adult donor) eyes, 2) RPE cells derived from mouse or human induced pluripotent stem cells (iPS-RPE) or embryonic stem cells (ES-RPE), and 3) cell lines from rodents and humans that have been transformed (RPE-J, hTERT-RPE1), or spontaneously immortalized (ARPE-19). These preparations can be cultured in dishes or, to allow fluid flow and enhance polarization, on semi-permeable Transwell filters. Cultures on Transwell filters or glass-bottomed dishes have recently been used for live-cell imaging of phagocytosis, autophagy, and endolysosome function (Jiang et al., 2015; Toops et al., 2015; Tan et al., 2016; Hazim et al., 2017; Kaur et al., 2018; Zhang et al., 2019).
2.2. Primary RPE cell cultures
Primary RPE cultures from laboratory rodents and farm animals have been used historically to exploit a readily available source, and to generate cultures of well-polarized cells.
RPE monolayers cultured from human donors, mice, pigs, and cattle reproduce several essential features of the RPE in situ.
An important characteristic of primary RPE, iPS-RPE, and ES-RPE cells is that they can form well-differentiated, RPE-like cultures, demonstrating a polarized epithelial organization (Fig. 2), close approximation of native RPE gene and protein expression, and the ability to perform RPE functions, such as the ingestion and degradation of OSs over a time-course that is comparable to that in vivo (Maminishkis et al., 2006; Strunnikova et al., 2010; Blenkinsop et al., 2013).
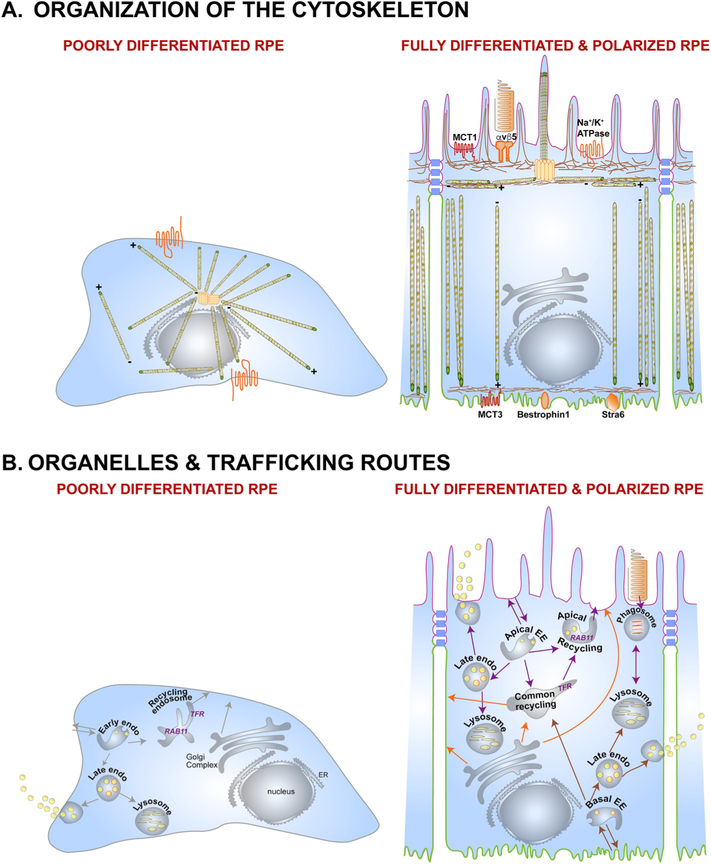
Fig. 2.
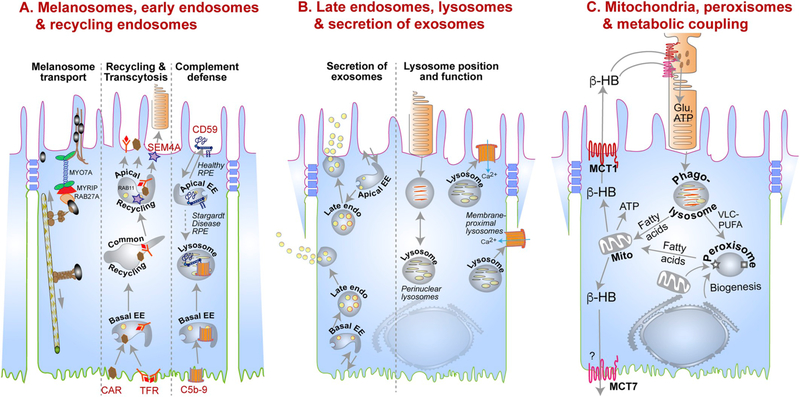
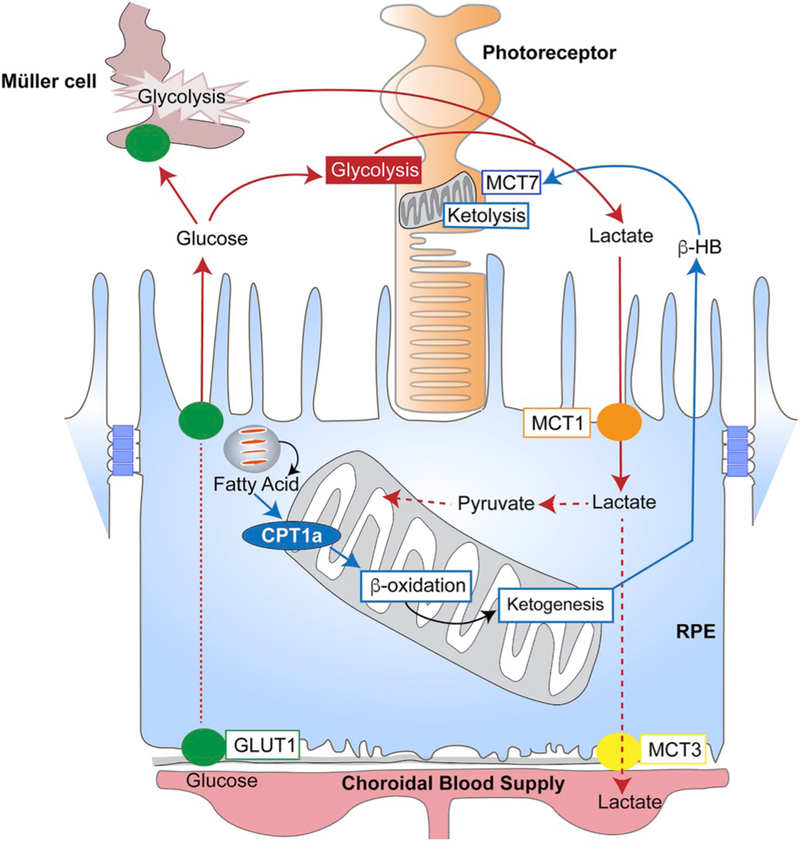
The polarized phenotype of the RPE. (A) Organization of the cytoskeleton in the RPE. In a non-polarized RPE cell (left), microtubules originate at the microtubule organizing center (MTOC), with the plus ends oriented towards the cell periphery. This poorly differentiated RPE cell shows non-polarized distribution of membrane proteins like the Na+/K+-ATPase (orange). A fully differentiated RPE cell (right) has well-defined apical (pink) and basolateral (green) membrane domains demarcated by tight junctions (blue). Microtubules are organized vertically with the plus ends oriented towards the basal surface. A lateral microtubule network and the cortical actin network are also found below the microvilli. This precisely organized cytoskeletal network provides structural support for the RPE and ensures accurate localization of apical (e.g., αvβ5, MCT1, Na+/K+-ATPase) and basolateral (e.g., MCT3, Bestrophin1, Stra6) membrane proteins. (B) Organelles and trafficking routes in the RPE. Polarized RPE have distinct biosynthetic (orange arrows), apical (purple arrows), and basolateral (brown arrows) trafficking routes that transport specific cargo destined for specific membrane domains, or for secretion into the apical or basolateral extracellular space. A key feature of polarized epithelia like the RPE is the apical recycling endosome, which is identified by the presence of RAB11, and distinct from the common recycling endosome, which has the transferrin receptor. In a non-polarized cell, RAB11 is found along with the transferrin receptor in the common recycling endosome. EE, early endosome; ER, endoplasmic reticulum.
Primary RPE cultures from human donors have been described since the 1970s (Mannagh et al., 1973). Since the early 2000s, human fetal RPE has been the most common source for primary human cultures. Such cultures exhibit clear epithelial morphology (Fig. 3A) and the expression of RPE and tight junction proteins. These cultures also have a high trans-epithelial electrical resistance (TER), from 500 to over 800 Ω cm2 (Hu and Bok, 2001; Maminishkis et al., 2006; Sonoda et al., 2009). The TER is a critical measure of the barrier function of the RPE, which is established by the expression and circumferential localization of tight junction proteins, such as zonula occludens (ZO)-1, occludin, and specific claudins (Rahner et al., 2004; Peng et al., 2011). A low TER is a criticism of some RPE cell lines (section 2.4, below). Nevertheless, a very high TER may be a characteristic of fetal RPE, and thus a potential drawback for modeling adult RPE. Detailed protocols to develop cultures from adult human donors have been established and are reported to yield highly polarized RPE monolayers after at least 4 weeks in culture (Blenkinsop et al., 2013). Drawbacks with both human fetal and adult RPE include the limited availability of tissue for RPE cell culture, and their tendency to dedifferentiate with subsequent passaging. Recent federal restrictions on usage of fetal tissues in NIH-funded research could likely further limit the availability and usage of human fetal RPE in the field.
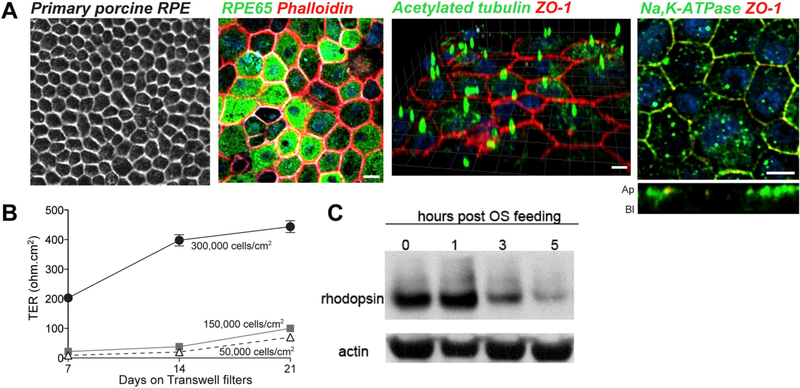
Fig. 3.
Primary porcine RPE cultures. (A) Expression of polarity and differentiation markers in primary porcine RPE cultured on semi-permeable Transwell inserts for 2 weeks. Left to right: brightfield image; RPE stained with antibodies to RPE65, acetylated tubulin, Na+,K+-ATPase (all green) and ZO-1 (red). Nuclei are stained with DAPI (blue); phalloidin was used to stain the actin cytoskeleton. (B) Polarity and trans-epithelial electrical resistance (TER) depends on density at the time of plating and weeks in culture. (C) Clearance of phagocytosed porcine outer segments by polarized porcine RPE cultures. Scale bar in A = 10 μm. Adapted from Toops et al. (2014).
The first report of primary rodent RPE culture was in 1977, in which the authors performed explant cultures of RPE from 1-week-old pigmented rats (Edwards, 1977). The subsequent development of methods for mouse primary RPE cultures (Gibbs et al., 2003) enabled generation of primary cultures from different lines of retinal degeneration mutants. These cultures can survive up to 10 days and are amenable to experimental manipulations including transfection, transduction, and live-cell imaging (Gibbs et al., 2003). A significant advantage of obtaining primary cultures of rodent RPE is that long post-mortem times can be avoided with these common laboratory animals. The presence of inbred lines also allows for a consistent product.
RPE from freshly harvested porcine and bovine eyes have been successfully used to generate well-polarized epithelia in several studies (Toops et al., 2014; Georgiannakis et al., 2015; Klingeborn et al., 2017b) (Fig. 3A). Primary porcine RPE cultures, grown on Transwell filters, exhibit correct localization of tight junction markers, such as ZO-1, and have a TER of ~400 Ω cm2, as early as 2 weeks in culture (Toops et al., 2014). Moreover, an advantage of porcine eyes is that humans and pigs share similarities with regards to their immune systems, which is an advantage for studying diseases with a strong immune component like age-related macular degeneration (AMD) (Mair et al., 2014). A disadvantage is that, because bovine and porcine eyes are obtained from local abattoirs, the quality of eyes is dependent on the procedures used at different facilities. Moreover, eyes from animals of different ages and sexes are often pooled, thus potentially increasing variability.
There are some disadvantages in using primary RPE cultures from animal models, in general. First, certain genes associated with human disease, such as the three different human-specific isoforms of apolipoprotein E, are not expressed in mice, pigs, or cattle, making it necessary to genetically modify these cells or to perform certain studies in human tissues. Second, non-viral lipid-based gene transfection is less efficient in primary RPE due to their post-mitotic nature. However, electroporation or nucleofection has been reported to yield transfection efficiencies of ~20–40% (Toops et al., 2014). Nevertheless, given the limited availability of human donor tissues, well-polarized primary RPE cultures from laboratory rodents and farm animals provide valuable models for studying RPE biology and disease pathogenesis. For example, studies on primary porcine RPE have contributed to the understanding of critical aspects of RPE biology, including response to complement attack (Georgiannakis et al., 2015; Tan et al., 2016), polarized secretion of exosomes (Klingeborn et al., 2017b), and RPE barrier functions (Ablonczy and Crosson, 2007; Shirasawa et al., 2013).
2.3. Stem cell-derived RPE cultures
RPE cultures differentiated from stem cells offer a tractable approach for the study of normal and disease-linked human RPE.
ESCs and iPSCs can spontaneously differentiate along the neural lineage, and then to RPE cells, which are readily identified by virtue of pigmentation and cobblestone appearance (Kawasaki et al., 2002; Buchholz et al., 2009) (Fig. 4). There are now published protocols using directed approaches that provide relatively efficient differentiation into cultures of cells, reported to possess a wide range of RPE characteristics, including gene expression, polarized organization, and physiological and cell biological functions (Idelson et al., 2009; Buchholz et al., 2013; Gong et al., 2015; Maruotti et al., 2015; Hazim et al., 2017). Thus, stem cell-derived RPE cultures appear to offer a tractable approach for the study of human RPE. In particular, human iPS-RPE cells offer the ability to develop ‘disease-in-a-dish’ models by obtaining iPSCs from patients with an inherited RPE pathology.
Fig. 4.
RPE cell culture differentiated from induced pluripotent stem cells (iPSCs). Phase contrast image shows cells in a tight cobblestone array, with a significant number of the cells showing dark pigmentation. iPSCs, in Matrigel-coated culture wells, were treated for 14 days, using culture medium, as described (Buchholz et al., 2013), except that 10 mM nicotinamide (Nic) was added for the first 4 days. After 14 days, the culture medium was changed to MEM-Nic with 5% FBS, as described (Hazim et al., 2019), for an additional 14 days. Scale bar = 100 μm.
Initial reports of stem cell-derived RPE cultures focused on the RPE characteristics that could be readily identified. With the widespread use of iPS-RPE cultures in the laboratory and, more recently, in proposed clinical trials of autologous cell transplantation to treat retinas damaged by RPE diseases such as AMD (Mandai et al., 2017; Sharma et al., 2019), it is important to address their potential shortcomings.
General disadvantages of stem cell-derived cultures, like those of primary cultures, include inefficient transfection (although not viral transduction), as well as the limited number of times they can be passaged before failing to re-differentiate fully into RPE cells (Adijanto and Philp, 2014). Although not explicitly reported in the literature, we have observed high passage-number cultures of RPE cells from human ESCs and iPSCs undergoing dedifferentiation.
One question, which also applies to primary RPE cultures from human fetuses, is whether the iPS-RPE cultures can be used to model age-related disease, such as AMD. Interestingly, under some conditions, these cultures have allowed disease discrimination. Human fetal RPE cultures carrying a complement factor H risk haplotype were found to have an increased susceptibility to oxidative stress and complement attack when fed OSs from Abca4−/− mice (Radu et al., 2011, 2014), and iPS-RPE from patients with different monogenic forms of macular degeneration were observed to accumulate drusen deposits after 90 days of culture (Galloway et al., 2017).
2.4. RPE cell lines
Until recently, methods routinely used for culturing RPE cell lines yielded cultures that did not exhibit critical features characteristic of the RPE in vivo.
Improved methods for generating well-differentiated ARPE-19 cultures have been recently established.
Cell lines offer the most tractable RPE cell models, as they are immortal, easily propagated and maintained, and readily transfected. The RPE-J line was the first of these to be described. It was generated by simian virus 40 (SV40) transformation of primary rat RPE cells. The cultures were shown to be polarized, based on circumferential localization of the tight-junction protein, ZO-1, and extensive apical microvilli, and possession of a significant TER (350 Ω cm2). However, due to the SV40 transformation, the cells are hypodiploid. Importantly, they do not exhibit essential polarity features that are characteristic of RPE, such as the specific apical localization of Na+/K+-ATPase and neural cell adhesion molecule (NCAM) (Nabi et al., 1993), which are critical for RPE and photoreceptor function.
The human telomerase (hTERT)-RPE1 cell line was derived from RPE cells of a 1-year old donor, using human telomerase reverse transcriptase activity. They have been reported to express some RPE-associated proteins (e.g. cellular retinaldehyde-binding protein [CRALBP]) and become pigmented in the absence of serum after 4 or 8 weeks in culture (Rambhatla et al., 2002). However, they do not develop polarity and a “cobblestone” organization that is typical of well-differentiated RPE monolayers. They have been used mainly for studies on the primary cilium. Ciliogenesis is robustly initiated after a few days of serum starvation, although, oddly, the cilium typically extends from the surface adjacent to the substrate, rather than an apical surface (Molla-Herman et al., 2010). For vision scientists, its most applicable use may be as a model for photoreceptors (Trivedi et al., 2012), rather than for the RPE, since its cilium arises from a pocket (Molla-Herman et al., 2010), similar to the way in which the connecting cilium of a photoreceptor arises from a recess in the inner segment (Liu et al., 2007).
When the adult RPE-19 (ARPE-19) and D407 lines were first described (Davis et al., 1995; Dunn et al., 1996), they were reported to differentiate readily into polarized RPE cells. However, these cell lines have genetic abnormalities, with triploidy in the D407 line (Davis et al., 1995), and an abnormal karyotype (most notably, a loss of one copy of chromosome 15, and an unbalanced translocation between the chromosome 15q and 19q) in the ARPE-19 line (Fasler-Kan et al., 2018; Hazim et al., 2019). Over the years, with numerous passages, the ability of cells of this line to demonstrate characteristics of differentiated RPE has diminished (Strunnikova et al., 2010; Lehmann et al., 2014). Nevertheless, the ARPE-19 cells remain a widely used cell line, with ~170 papers published annually that mention “ARPE-19” or “ARPE19” in the title or abstract (based on a current PubMed search), and, presumably, many others that still report experiments on the ARPE-19 cells, but may not mention the cell line by name in the abstract.
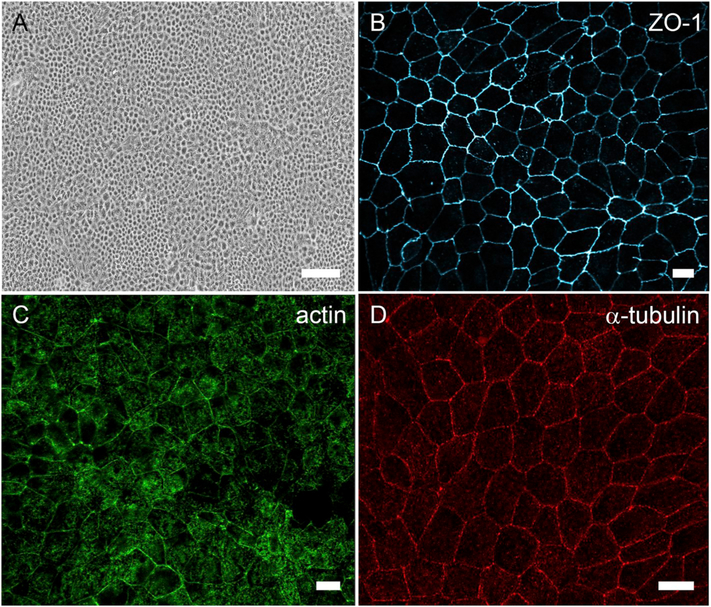
Recent reports have described new culture methods that can yield improvements in the phenotype of ARPE-19 cells, primarily using two means of initiating differentiation. The first method involves the addition of exogenous factors. With the addition of pyruvate in high-glucose DMEM, it was shown that ARPE-19 cells recapitulated key features of RPE cells including RPE-specific differentiation markers, polygonal morphology, polar expression of proteins, extensive microvilli, the ability to phagocytose, and secretion of VEGF (Ahmado et al., 2011). The expression of the premelanosome marker, Pmel17, and early melanization was observed, although, as with the original study from Dunn et al. (1996), characteristic cylindrical melanosomes of the RPE were not reported. The cells did not exhibit a TER greater than 51 Ω cm2; however, the same protocol employed by another group reported a TER of 126 Ω cm2 (Samuel et al., 2017). These protocols required 3–4 months for RPE differentiation, whereas, a more recent protocol was able to achieve a similar level of differentiation within 4 weeks through the addition of nicotinamide instead of pyruvate (Hazim et al., 2019) (Fig. 5).
Fig. 5.
Differentiated cultures of ARPE-19 cells. (A) Brightfield micrograph showing cobblestone morphology of ARPE-19 cells cultured on plastic for 2 weeks. (B) Immunofluorescence micrograph of zonula occludens (ZO)-1 localized to the apical junctions of ARPE-19 cells cultured on a laminin-coated Transwell filter for 2 weeks. (C) Phalloidin labeling in ARPE-19 cells cultured on a laminin-coated Transwell filter for 3 weeks shows cortical arrangement of the actin filaments. (D) Micrograph of α-tubulin immunolabeling demonstrating vertical microtubule arrangement in ARPE-19 cells cultured on a laminin-coated Transwell filter for 3 weeks. The cells depicted in all four panels were differentiated using the protocol described in (Hazim et al., 2019). Scale bar in A = 100 μm, (B–D) = 10 μm.
The second method of ARPE-19 cell differentiation is the growth of cells on extracellular matrix-rich membranes such as porcine lens capsule (Turowski et al., 2004). Such cells have been reported to have improved phagocytosis and TER values reaching 200 Ω cm2. However, the morphological features of these cells do not appear as RPE-like as those reported by others (Ahmado et al., 2011; Hazim et al., 2019).
Immortalized or transformed RPE cell lines, of course, differ in one major respect from RPE in vivo; that is, they must proliferate. This problem is exacerbated during in vitro culture as the cells that proliferate faster will have a higher representation in the culture. An important part of the protocol, reported by Hazim et al. for ARPE-19 cells, is a series of washes with trypsin that selectively removes the more proliferative cells, which tend to be more loosely adhered to the substrate, leaving behind firmly attached cells that more readily form a culture with a cobblestone appearance (Hazim et al., 2019).
Another concern for a cell line is the need for authentication. In an example from retinal research, the rat retinal ganglion cell (RGC)-5 cell line had been used as a model for retinal ganglion cells. However, this line was somehow replaced by cells from a line of mouse cells (Krishnamoorthy et al., 2013), sharing some properties with the 661W cell line, which expresses several markers of mouse cone photoreceptors (Tan et al., 2004). Sadly, researchers continued to use the line unwittingly as a retinal ganglion cell model for over a decade after the line no longer represented the RGC-5 cell line. A description of how cell lines will be authenticated is now a required part of any NIH research grant application, and this authentication should be performed regularly.
2.5. RPE cell cultures – basic standards
As with all models, an RPE cell culture should balance tractability with the possession of RPE characteristics found in vivo. However, a significant problem is that many published studies have used poor culture conditions, so that the cell cultures bear little resemblance to an epithelium, let alone the retinal pigment epithelium. The model of choice is dictated by the scientific question; to begin with, the cells should be RPE-like with respect to characteristics that are central to the mechanisms to be studied (Pfeffer and Philp, 2014). However, given the interplay among different mechanisms in a cell, we argue that the cultures used should conform to some basic standards of RPE morphology and physiology.
An RPE cell culture must be polarized, demonstrating a differentiated and well-demarcated apical-basal phenotype. It is essential to follow established culture methods that promote a polarized phenotype, especially for cell lines, but also for generating RPE from stem cells, and for maintaining primary RPE cultures. Polarity and differentiation are not only influenced by the source of the cells (transformed, primary, stem cell-derived), but also by plating density (Adijanto and Philp, 2014; Toops et al., 2014), and the substrate on which the cells are grown (Opas, 1989). In essence, however, the cells must be organized into an epithelium, with cobblestone-like packing, a barrier function that arises from the precise localization of junctional complexes at tight junctions (not throughout the cell), and a correctly organized microtubule and actin cytoskeleton (Fig. 2). Expression of “RPE signature” genes is important (Strunnikova et al., 2010); however, more importantly, the resulting proteins must be localized properly; e.g. the Na+/K+-ATPase should be in the apical membrane (not basal as is the case in RPE-J cells).
As discussed above, there are now reproducible protocols that generate RPE monolayers with a well-differentiated phenotype, not only for primary RPE cultures (Gibbs and Williams, 2003; Maminishkis et al., 2006; Strunnikova et al., 2010; Blenkinsop et al., 2013; Toops et al., 2014) and RPE cells derived from stem cells (Idelson et al., 2009; Buchholz et al., 2013; Gong et al., 2015; Maruotti et al., 2015; Hazim et al., 2017), but also for ARPE-19 cells (Hazim et al., 2019). Hence, our recommendation would be to follow these standardized cell culture protocols when using in vitro models of the RPE, which will be the essential first step to compare and correlate studies from different laboratories.
3. Phagocytosis of OS disk membranes
3.1. Turnover of phototransductive membrane
Initial observations on the turnover of OS disk membranes date back to the 1960s.
Whereas various types of cells are regularly replaced throughout the life of an organism, terminally-differentiated cells, such as neurons, must turn over their parts to avoid the accumulation of damaged macromolecules. In order to maximize sensitivity, photoreceptor cells typically have large amounts of membrane containing a high concentration of opsin. Turnover of this phototransductive membrane thus represents a major undertaking.
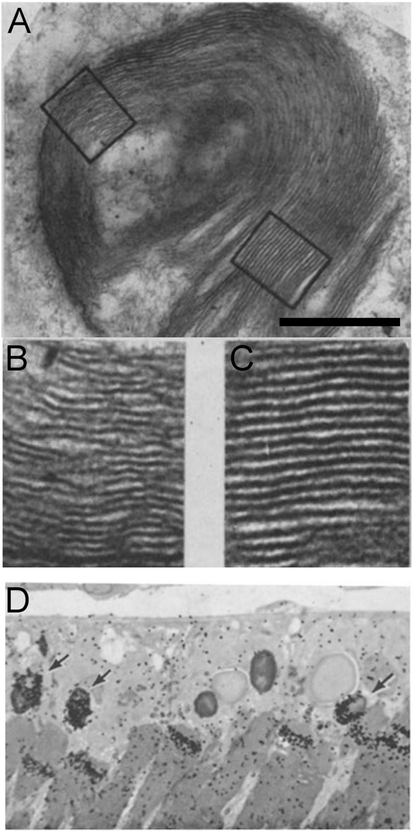
In the early 1960s, Dowling and co-workers reported electron microscope (EM) images of what we now know as phagosomes containing packets of OS disk membranes in sections of rat RPE (Dowling and Gibbons, 1962) (Fig. 6A–C). They described the phagosomes as “lamellar inclusion bodies”, and it was clear to them that the contents of the bodies bore a striking resemblance to the membranes of the outer segments. Noting that the RPE may be phagocytic, they wrote: “In the inclusion bodies we might be observing rod outer segment fragments engulfed in such a phagocytic process.” However, especially given concurrent observations on the Royal College of Surgeons (RCS) rat retina (Dowling and Sidman, 1962), and the inability to determine whether the perturbed membranes that accumulated in the subretinal space had originated from the photoreceptors or the RPE, these researchers also considered that the inclusion bodies may have been endogenous to the RPE. With the introduction of microscopic autoradiography, Droz (1963) was able to determine that proteins were synthesized in the photoreceptor inner segments, and, with time, migrated from the inner segments to the outer segments. The definitive demonstration that the RPE phagocytosed outer segment disks was not reported until 1969, when Young and Bok (1969) used microscopic autoradiography to trace radiolabeled disk membranes to phagosomes in the RPE (Fig. 6D). That defective phagocytic function of the RPE underlaid the retinal dystrophy of the RCS rat was subsequently resolved following an autoradiographic study (Bok and Hall, 1971), and observations of chimeric rats, generated to contain photoreceptor and RPE layers that consisted of mosaics of RCS and normal cells (Mullen and LaVail, 1976). With this phagocytic function, the RPE is responsible for the catabolic phase of the turnover of phototransductive membrane.
Fig. 6.
Early published images of OS phagosomes in the RPE. (A) Electron micrograph of an OS phagosome, described as a “lamellar particle” (Dowling and Sidman, 1962). (B and C) Higher magnification of the two boxed areas in A, showing disk membranes with their organization still evident. (D) Microscopic autoradiography image of the RPE and distal rod outer segments of a frog retina. The animal had been pulsed 59 days earlier with radiolabeled amino acids. A concentration of radiolabel is shown either near the tip of an outer segment or in OS phagosomes within the RPE (arrows) (Young and Bok, 1969). Scale bar in A = 0.5 μm. Adapted with permission from (A–C) ©1962 Dowling and Gibbons. Originally published in Journal of Cell Biology, https://doi.org/10.1083/jcb.14.3.459 and (D) ©1969 Young and Bok. Originally published in Journal of Cell Biology, https://doi.org/10.1083/jcb.42.2.392.
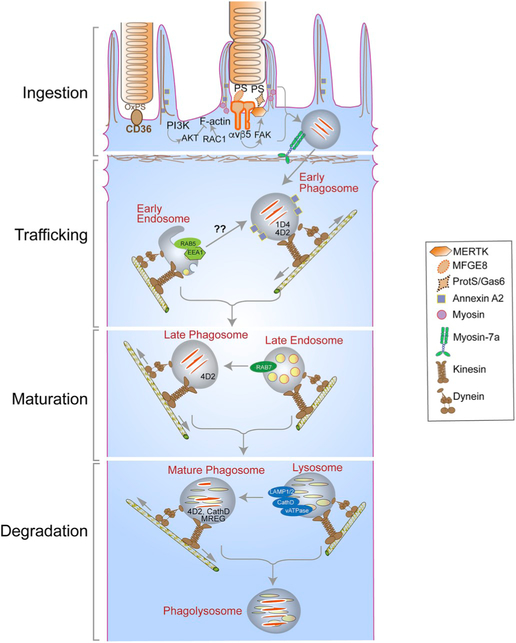
The overall process of phagocytosis involves a series of stages. A prerequisite binding between the OS plasma membrane and the apical membrane of the RPE is followed by the formation of a phagocytic cup around the distal OS. The distal tip of the OS is then ingested and transported into the cell. The phagosome is degraded following interactions with endolysosomes, a process that appears to involve a number of stages (Fig. 7). Here, we review our current understanding of these events.
Fig. 7.
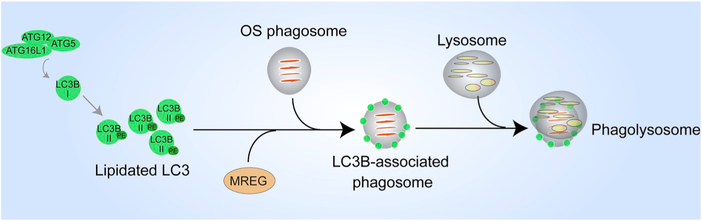
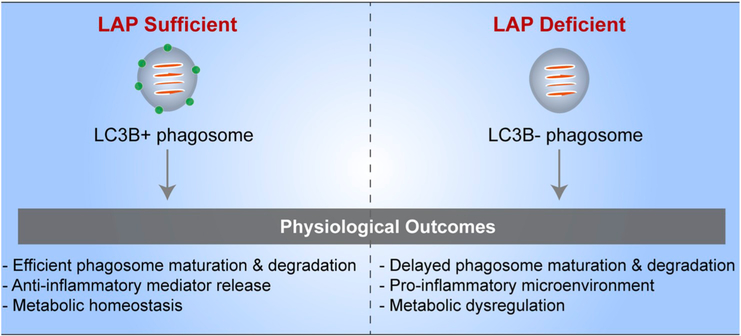
Major players and routes in the ingestion, degradation, and motility of photoreceptor outer segment (OS) phagosomes - Photoreceptor OS ingestion by the retinal pigment epithelium (RPE) occurs first, through the binding of exposed phosphatidylserine (PS) residues at the OS tips to the αvβ5 integrin on the apical RPE membrane, through its ligand milk fat globule protein E8 (MFGE8). OS recognition is then conveyed via focal adhesion kinase (FAK) to the ingestion receptor, Mer tyrosine kinase (MERTK), on the apical membrane, which also binds PS through its ligands, growth arrest-specific protein 6 (Gas6) or Protein S. CD36 binding to oxidized PS also serves as an additional ingestion pathway. OS binding results in actin remodeling to form the phagocytic cup that envelops and ingests the nascent phagosome, with the aid of a myosin motor. This remodeling is facilitated by the RAC1 GTPase and regulated by annexin A2 and, PI3K and AKT kinases. The ingested phagosome is first trafficked on apical actin filaments by myosin-7a, before being transferred to microtubule motors, kinesin and dynein, which mediate bidirectional motility with an overall apical to basal migration of phagosomes. These movements also facilitate phagosome interaction with early endosomes (indicated by RAB5 or early endosome antigen 1 [EEA1]), late endosomes (indicated by RAB7), and finally lysosomes (indicated by lysosome-associated membrane protein [LAMP] 1/2). During these interactions, the phagosome is gradually acidified through the vacuolar H+-ATPase (v-ATPase) and acquires degradative enzymes such as cathepsin D (CathD) which, in turn, requires melanoregulin (MREG) for enzyme maturation. The C-terminal epitope of rhodopsin, recognized by the mAb1D4 antibody, is lost early in phagosome maturation, whereas the N-terminus of rhodopsin remains mostly intact and recognizable by the mAb4D2 antibody.
3.2. The daily rhythm of disk membrane phagocytosis
Numbers of OS phagosomes in the RPE peak at specific times of day.
A characteristic of phagocytosis of OS disk membranes by the RPE is its daily rhythm. LaVail (1976) first reported a greater number of phagosomes in the rat RPE after light onset. Several laboratories have studied the cues that govern phagosome number in the RPE of various species. Most animals (including mouse, rat, cat, hamster, Xenopus laevis, goldfish, rabbit, and chick) appear to be entrained to a circadian rhythm, which is cued by the light/dark cycle and persists even when the animal is maintained in absolute darkness (LaVail, 1976; Besharse et al., 1977; Grace et al., 1996), but not in absolute light (Teirstein et al., 1980). In other animals, like the frog, Rana pipiens, there is a strong dependency on external light stimuli to initiate OS phagocytosis; complete darkness prevents phagocytosis (Basinger et al., 1976).
Studies conducted during the late 1970s investigated whether the daily phagocytosis rhythm was controlled by the superchiasmatic nucleus, which controls circadian rhythms in general. A few studies found that disruption of this region did not abrogate the daily schedule of the number of OS phagosomes and concluded that cues for OS phagocytosis were intrinsic to the eye (Currie et al., 1978; Hollyfield and Basinger, 1978; LaVail and Ward, 1978; Tamai et al., 1978; Besharse and Dunis, 1983; Terman et al., 1993). However, in his original paper, LaVail showed that blockage of the rat pineal gland with reserpine prevented the sudden increase in phagosome number that occurs after light onset (LaVail, 1976), suggesting some extraocular influence on circadian OS phagocytosis. It was also proposed that pineal influence might regulate the magnitude of the shedding response, as frog eyecups exposed to light in vitro had a greater number of phagosomes in the RPE compared with those eyes that were exposed to light at the same time of day in vivo (Flannery and Fisher, 1979). However, this difference may have been due to increased oxidation of OSs in culture, a condition that markedly increases the number of phagosomes in vitro (Williams and Roberts, 1992).
Interestingly, there appear to be differences in the peaks of rod and cone OS phagosome numbers. Across species, rod OS phagosomes are most abundant just after light onset, whereas cone OS phagosomes, depending on the species, appear at the same time as rod OS phagosomes, or demonstrate a peak of phagocytosis just after lights-off, as in lizard, chicken, goldfish, and ground squirrels (Young, 1977, 1978; Ruggiero and Finnemann, 2014). More recent work, however, has reported comparable phagosome numbers occurring at two peaks in the mouse and zebrafish RPE, with one peak after light onset and the other after lights-off (Lewis et al., 2018). Interestingly, cone and rod phagosomes were found in the RPE of both species at all times of day; although, in the cone-dominant zebrafish, there were more cone OS phagosomes during the dark peak and slightly more rod OS phagosomes during the light peak. While this report suggests reasons for the differences in their study compared with previous studies, including mouse strain or species differences, the authors also discuss the obvious, though previously neglected issue that phagosome number is the net result of not just phagocytosis, but also the rate of phagosome degradation. It is not known whether the kinetics of the latter, and thus phagosome half-life, varies according to time of day.
3.3. Turnover of phototransductive membrane in arthropods – a comparative viewpoint
Arthropod photoreceptors typically degrade their own shed phototransductive membrane.
The vast majority of phototransductive membrane is turned over each day in nocturnal arthropods.
Many investigators emphasize the large phagocytic load that the catabolic phase of disk membrane turnover places on the RPE. It is a major role for the RPE, but the amount of material ingested by each RPE cell is mainly due to the association of one RPE cell with many photoreceptor cells. In the central human retina, there are 33 photoreceptors associated with each RPE cell (Osterberg, 1935), and, in the center of a mouse retina, there are more than 200 photoreceptors for every RPE cell (Volland et al., 2015); the ratio decreases to ~12 in the periphery of the human retina and 100–120 in the periphery of a mouse retina (Volland et al., 2015). While each OS phagosome contains densely packed membranes, it represents only 10% of the OS in mammals, such as mouse and rat, and ~2% in the frog, Rana (Young, 1967; Young and Bok, 1969). Therefore, disk membrane proteins cannot be considered to have short lives. This rather slow renewal rate contrasts with the turnover of phototransductive membrane in some arthropods, which, in general, perform this task with some key differences in cell biology when compared with vertebrates.
In arthropods, phototransductive membrane is microvillar and forms rhabdomeres, which are comparable to the outer segments of vertebrate photoreceptors; the closely packed microvillar membrane provides an alternative way to form a structure that contains a high concentration of opsin and other phototransductive proteins. Like OSs, rhabdomeres undergo renewal of their membrane. Typically, the catabolic phase is initiated by endocytosis of the base of a microvillus, resulting in vesicles that aggregate in multivesicular bodies within the photoreceptor cell (Eguchi and Waterman, 1976; Satoh and Ready, 2005). There are some exceptions to this theme. In dipterans, where the individual rhabdomeres are separated (to form an open rhabdom), the tips of the microvilli may be phagocytosed by adjacent regions of the photoreceptor cells (Williams, 1982c), in addition to endocytosis of the microvillar bases. This is most noticeable in crepuscular dipterans (Williams and Blest, 1980; Blest et al., 1982). Both these routes, direct endocytosis and phagocytosis from extracellular space, lead to an accumulation of the phototransductive membrane in multivesicular bodies, and endolysosomal degradation within the photoreceptors themselves. Studies on Drosophila have shown that degradation also includes classical autophagy (Midorikawa et al., 2010).
Although an auxiliary cell type is not commonly involved in the degradation of shed rhabdomeral membrane, there is evidence from spider retinas that the tips of the microvilli are ingested by adjacent, non-pigmented glia (Blest and Maples, 1979), thus representing an event that is more akin to ingestion of OS membranes by the RPE.
As in vertebrates, phototransductive membrane turnover in arthropods occurs according to a daily rhythm. But, in crepuscular, and especially nocturnal arthropods, herein lies a most remarkable feat of this turnover. In a netcasting spider, which detects its prey by visual cues in dense rainforest at night, nearly all the phototransductive membrane is shed following dawn, and the rhabdomeres are then, essentially, entirely resynthesized at dusk (Blest, 1978). This complete separation of the two phases of turnover allows for an effective light adaptive mechanism. Analysis of two locust species that migrate at night found that the rhabdomeral cross-sectional area was 5-fold greater at night than during the day (Williams, 1982a, b), providing a concomitant increase in photoreceptor sensitivity at the expense of spatial resolution (Williams, 1983). Most of the phototransductive membrane of nocturnal arthropods therefore lasts half a day, a considerably shorter time than the 10–50 days for the disk membranes of vertebrates (Young, 1967; Young and Bok, 1969).
3.4. RPE cell models for phagocytosis studies
Kinetics of OS phagosome maturation and degradation should compare to that in vivo.
OS phagocytosis involves mechanisms that are specific for OSs.
Phagocytosis is a key function of the RPE that is significantly affected by RPE cell polarization. An apical membrane, containing receptors such as Mer tyrosine kinase (MERTK) and αvβ5 integrin, an actin-rich apical domain, and a cell body, with an epithelial microtubule organization and organelle distribution, are all polarity characteristics of a differentiated RPE cell, and are all important for the mechanisms and especially the kinetics of OS ingestion and degradation. For example, a well polarized RPE cell possesses vertical microtubules that do not emanate from the centrosome, and the rate of phagosome degradation is affected by motility along the RPE cell microtubules (Jiang et al., 2015) (Figs. 2 and 7).
Given that OS phagocytosis is an important function of RPE cells, measuring phagocytic capacity of these cells is a commonly-used metric. Flatmounts of the RPE, as noted earlier, provide an excellent in situ view of phagosomes, and, due to their fresh dissection, reflect the in vivo circadian rhythm of OS phagocytosis (LaVail, 1976). RPE cell cultures appear to be disconnected from in vivo rhythms, but are nevertheless amenable to experimentally-induced pulses of OS phagocytosis. The timing of ingestion can be controlled by lowering the temperature at which cells are fed OSs to 17–20 °C (Hall and Abrams, 1987). This cooler temperature permits binding but blocks ingestion. Once unbound, excess OSs are washed away, the cells can be incubated at 37 °C to initiate synchronized ingestion. This method provides much better temporal resolution of ingestion and phagosome maturation than that available by in vivo analysis.
In studying phagocytosis with RPE cell cultures, two points are important. The first concerns the type of RPE culture, along the lines discussed in Section 2. For quantitative studies, in particular, cell cultures should be homogeneous, ideally with all cells participating in the phagocytosis. Still, among different types of cultures, there is a large variation in the pulse time required for cells to achieve significant ingestion of OSs. Studies utilizing RPE-J cells routinely require pulses that are several hours long, with one study noting that the binding phase requires 2 h and subsequent ingestion another 2–3 h (Finnemann et al., 1997). ARPE-19 and primary RPE cells have been shown to ingest OSs in less than 1 h (Gibbs et al., 2003; Damek-Poprawa et al., 2009; Olchawa et al., 2013; Wavre-Shapton et al., 2014); although, with highly differentiated cultures, there appears to be a latency period prior to binding, likely due to the extensive microvilli impeding the association of suspended OSs with RPE membrane receptors. Importantly, however, maturation kinetics of OS phagosomes in well-differentiated RPE cell cultures, including cultures of primary RPE, human iPS-RPE, and ARPE-19 cells (Diemer et al., 2008; Hazim et al., 2017, 2019), is comparable to that in vivo.
A second point concerns what the cells are ingesting. Primary rat RPE cultures exhibited a specificity for phagocytosis of isolated rat OSs; compared with red blood cells, levels of OS binding and OS ingestion were 250 and 970 times, respectively, greater (Mayerson and Hall, 1986). In some studies, RPE cells have been incubated with latex beads, instead of isolated OSs, to investigate the mechanisms underlying phagocytosis in RPE. However, latex beads, while used extensively in phagocytosis studies of other systems (Desjardins and Griffiths, 2003), have been shown to use molecular mechanisms, involved in binding and ingestion by RPE cell cultures, that are distinct from those used by the RPE for OS phagocytosis (Edwards and Szamier, 1977; Chaitin and Hall, 1983a; Finnemann et al., 1997). A recent study has shown that the content of an OS phagosome influences its rate of degradation (Esteve-Rudd et al., 2018), emphasizing the importance of what the RPE cells are “fed”, in addition to the nature of the RPE cell culture itself. However, human, rat, and mouse RPE cells in culture can phagocytose OS membrane isolated from retinas of mice, rats, cows or pigs, with no obvious differences among species, suggesting mechanistic robustness across species (Mazzoni et al., 2014); although, a rigorous comparison of the phagocytosis and degradation rates of OS membrane from different species by a given RPE culture has yet to be reported.
3.5. Binding of OS membranes by the RPE
Externally-exposed phosphatidylserine at the OS tips and αvβ5 integrin in the RPE apical membrane facilitate OS binding to the RPE apical membrane.
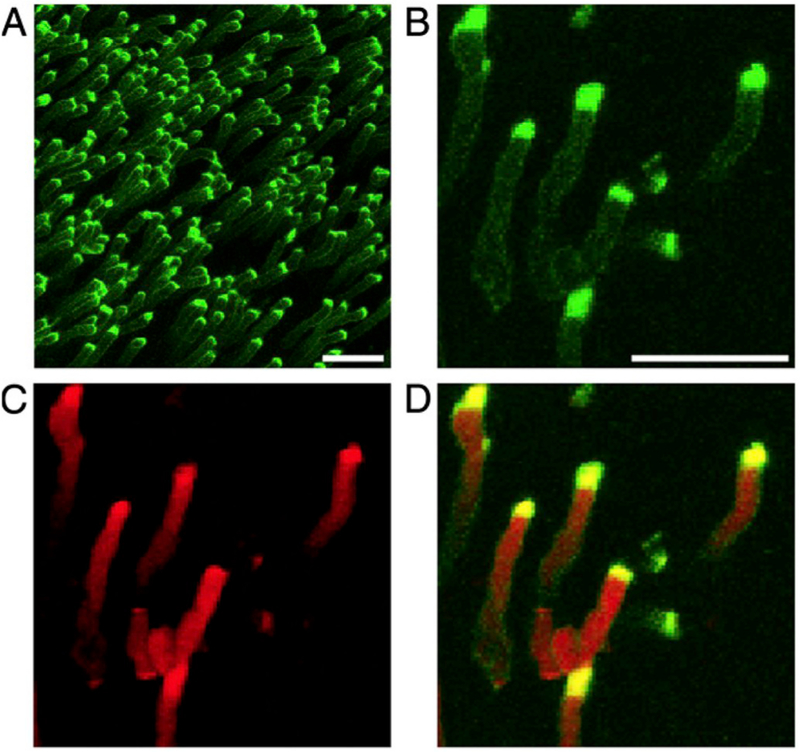
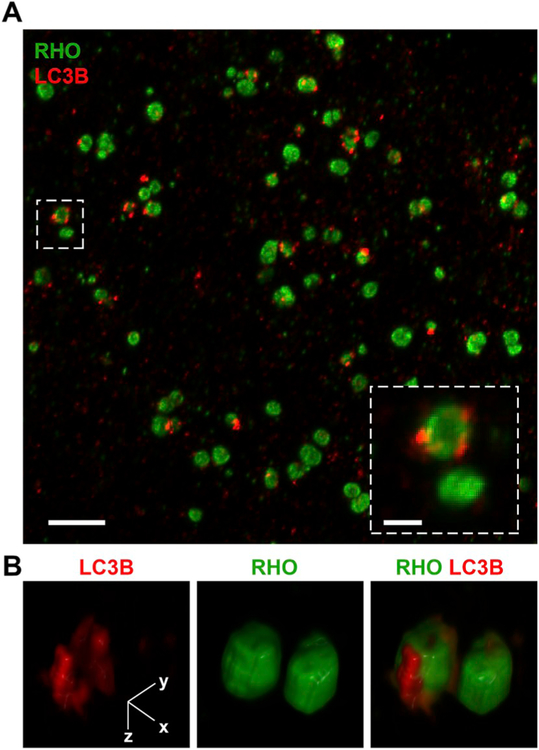
Early evidence suggested that different classes of phospholipids may be effective ligands for RPE phagocytic receptors (Effron et al., 1981). In this respect, phosphatidylserine (PS) has been widely studied as a phagocytic ligand; it is the classic ‘eat-me’ signal displayed by apoptotic cells and it is recognized by surface receptors on various phagocytic cells (Segawa and Nagata, 2015). PS is concentrated in the cytoplasmic leaflet of OS membranes, due to the activity of PS flippases. One such flippase, ATP8A2, has been localized to OS disk membranes and shown to translocate PS and, to a much lesser extent, phosphatidylethanolamine, implicating the enzyme in maintaining PS asymmetry in the OS (Coleman et al., 2009). Interestingly, the tips of OSs were found to contain PS exposed to the extracellular space, especially at light onset, using the PS-specific dye, pSIVA, a “polarity-sensitive indicator of viability and apoptosis” (Ruggiero et al., 2012) (Fig. 8).
Fig. 8.
Phosphatidylserine (PS) exposure at the tips of photoreceptor outer segments (OS). Wild type mouse retina dissected live at light onset are labeled and imaged with (A) a polarity-sensitive indicator of viability and apoptosis (pSIVA; green), which specifically binds to PS residues exposed to the extracellular space. (B–D) Higher magnification images show co-staining of (B) pSIVA and the (C) CellMask membrane stain (red), and (D) an overlay of both. Scale bar in A = 10 μm, B for B–D = 5 μm. Originally published in the Proceedings of the National Academy of Sciences (Ruggiero et al., 2012); permission granted by PNAS.
External PS on OSs is bound by milk fat globule E8 (MFGE8) which, in turn, serves as the ligand for αvβ5 integrin, residing in the apical RPE (Finnemann et al., 1997). MFGE8 protein levels in the retina are highest around light onset and decrease thereafter, thus coinciding with the phagocytic burst (Ruggiero and Finnemann, 2014). Adult rat RPE expresses two different αv integrins, αvβ3 and αvβ5 (Finnemann and Rodriguez-Boulan, 1999), although only αvβ5 facilitates OS binding and localizes to the apical surface (Finnemann et al., 1997). OS binding requires the formation of a complex between the tetraspanin CD81 and αvβ5 (Chang and Finnemann, 2007). The interaction between αvβ5 and OS binding is specific as antibodies against the integrin block OS binding, but not the binding or uptake of latex beads (Finnemann et al., 1997). In the absence of the β5 subunit, the daily rhythm of phagocytosis is lost and while OS ingestion is impaired, it is not absent (Nandrot et al., 2004), suggesting the existence of potential β5 compensatory mechanisms. OS phagocytosis is similarly arrhythmic, but not blocked in Mfge8−/− mice (Nandrot et al., 2007). β5−/− mice do not undergo retinal degeneration, but do demonstrate vision loss, as measured by electroretinograms (ERGs), from 6 months of age. They also accumulate fluorescent lipofuscin deposits, an inherent feature of the aging retina.
Negative regulation of OS binding via αvβ5 integrin likely maintains the acute phagocytic burst in the RPE. Through mechanisms that have yet to be characterized, the presence of the MERTK ingestion receptor (discussed in the section 3.5.1) limits OS binding via αvβ5 integrin, such that transient reduction of MERTK expression or use of cells lacking MERTK results in excessive OS binding (Nandrot et al., 2012).
αvβ5 has also been implicated in mediating retinal adhesion to the RPE, typically assessed by measuring the partitioning of apical melanosome-containing microvilli with the sensory retina following mechanical separation of the RPE and retina. The strength of adhesion peaked 3.5 h after light onset, i.e., past the phagocytic peak (Nandrot et al., 2006). Perhaps surprisingly, MFGE8, as the bridging molecule linking αvβ5 to the OS, plays only a minor role in retinal adhesion (Nandrot et al., 2007). It remains to be seen exactly how αvβ5 is mechanistically involved in retinal adhesion.
3.6. Ingestion of OS membranes by the RPE
MERTK is required for ingestion and is activated through αvβ5 integrin engagement and binding to Gas6/Protein S ligands.
TYRO3 and CD36 may be additional pathways mediating OS ingestion.
Actin rearrangement is required for formation of the phagocytic cup and phagosome ingestion.
Release of a packet of disk membranes from the OS tip requires the RPE.
3.6.1. Roles of MERTK and other ingestion-associated proteins
Engagement of the αvβ5 integrin initiates a signaling cascade that triggers OS ingestion, predominantly mediated by MERTK (Gal et al., 2000), a member of the Tyro-Axl-Mer (TAM) ingestion receptor family, that is present in the RPE apical membrane (Prasad et al., 2006). Indeed, β5−/− mice do not exhibit the characteristic peak of tyrosine phosphorylation seen in MERTK after light onset (Nandrot et al., 2004). As noted in section 3.5, however, OS ingestion does occur in these mice, suggesting that the basal level of phosphorylated MERTK that persists is sufficient to mediate phagocytosis. Focal adhesion kinase (FAK) appears to be the bridging step between OS recognition by αvβ5 integrin and OS engulfment mediated by MERTK (Finnemann, 2003). FAK operates downstream of αvβ5 integrin and shows a diurnal pattern of phosphorylation similar to that of MERTK, peaking after light onset and diminishing as phagosome degradation proceeds (Nandrot et al., 2004). A summary of known events in OS membrane ingestion is included in Fig. 7.
MERTK activation requires ligand binding of growth arrest-specific protein 6 (Gas6) or Protein S, which form the bridge between the RPE and exposed PS on OS. While deletion of either ligand alone does not affect retinal function in mice (Hall et al., 2005; Burstyn-Cohen et al., 2012), mice lacking both Gas6 and Protein S do develop moderate to severe retinal degeneration, demonstrating that at least one ligand is required for MERTK activation (Burstyn-Cohen et al., 2012).
Aside from MERTK, there are data to suggest that other TAM receptors, such as TYRO3, and the lipid scavenger receptor CD36, both of which are expressed in the RPE (Ryeom et al., 1996; Prasad et al., 2006), may play a supporting role in OS phagocytosis. Similar to MERTK, TYRO3 localizes to nascent phagosomes but not maturing ones (Feng et al., 2002). More recently, it was noted that adenoviral TYRO3 transduction induced OS phagocytosis in Mertk−/−;Tyro3−/− primary mouse RPE, although to a lesser extent than adenoviral MERTK transduction (Vollrath et al., 2015). Furthermore, TYRO3 exhibited gene dosage-dependent suppressive effects on the degree of retinal degeneration observed in Mertk−/− animals, but did not prevent it entirely, suggesting that MERTK is still indispensable. In contrast, overexpression of another PS-binding receptor, brain-specific angiogenesis inhibitor (BAI1), in Mertk−/− mice did not prevent retinal degeneration nor improve RPE phagocytic capacity prior to the onset of degeneration, although it did rescue phagocytosis of apoptotic germ cells by the phagocytic Sertoli cells of the testes, suggesting a more critical role for MERTK in RPE cells (Penberthy et al., 2017). The lipid receptor, CD36, is not required for OS binding but is involved in phagosome engulfment, possibly as a signaling molecule (Finnemann and Silverstein, 2001), as antibodies against CD36 inhibited OS membrane ingestion by RPE cells (Ryeom et al., 1996). Although CD36 functions in OS membrane uptake during normal conditions (Ryeom et al., 1996), it also recognizes oxidized phospholipids, such as oxidized PS and oxidized phosphatidylcholine, and may be functionally advantageous under oxidative stress (e.g., from intense light exposure) as an aid for the MERTK pathway (Greenberg et al., 2006; Sun et al., 2006).
Given the importance of MERTK to phagocytosis, mutations in the human MERTK gene can lead to retinitis pigmentosa, rod-cone dystrophies, and cone-rod dystrophies (Nandrot, 2014). The RCS rat was long used as a model of severe retinal degeneration (Bourne et al., 1938; Dowling and Sidman, 1962), before the identification of a genomic deletion in these animals that resulted in a truncated MERTK protein and lack of MERTK signaling (D’Cruz et al., 2000; Nandrot et al., 2000). Akin to the β5−/− mice for binding studies, the RCS rat or Mertk knockout mouse models have been instrumental in specifically investigating MERTK-related ingestion pathways. For example, inositol triphosphate (IP3) was identified as a secondary messenger downstream of MERTK, as RCS rats do no exhibit the characteristic increase in IP3 levels upon OS phagocytic challenge (Heth et al., 1995). The RCS rat also demonstrated that, unlike OS ingestion, MERTK was not required for latex bead ingestion (Edwards and Szamier, 1977). Therefore, careful consideration should be given to RPE studies employing latex beads, as the associated signaling pathways are likely to be different from those invoked by MERTK and OS. Indeed, wild type primary rat RPE cells do not exhibit the characteristic increase in IP3 levels upon latex bead phagocytic challenge (Heth et al., 1995). Due to defective phagocytosis, and thus a blockage of OS disk membrane turnover, the structure of OSs in RCS rats begins to deteriorate, leading to a complete loss of OSs within 3 months of age.
In general, MERTK plays a key role in a variety of phagocytic events, such as microglial phagocytosis to mediate synaptic pruning (Chung et al., 2013), macrophage phagocytosis to remove apoptotic immune cells (Scott et al., 2001), and phagocytosis of apoptotic germ cells and the residual body from maturing sperm by Sertoli cells in the testes (Breucker et al., 1985). Although the extent of its requirement may differ among different tissues, it nevertheless plays a central role, directly or indirectly affecting the regulation of a wide variety of genes including those involved in cytoskeletal rearrangement and phagosome maturation (Shelby et al., 2013; Penberthy et al., 2017).
3.6.2. Actin and associated proteins in the formation of the phagocytic cup
OS ingestion is mediated by rearrangement of the actin cytoskeleton to form an actin-rich phagocytic cup, first seen in early EM images of pseudopodia that were distinct from apical microvilli but in close apposition to the OS membrane (Steinberg et al., 1977; Nilsson, 1978; Matsumoto et al., 1987). In the amoeboid cells of Dictyostelium, the actin system of a phagocytic cup responds to changes in the shape of the surface of the particle being ingested (Clarke et al., 2010). Similarly, determination of the size of the phagocytic cup, and the size of the ensuing phagosome is likely to involve an interaction between the pseudopodia and regulated features of the OS, such as PS exposure (see section 3.4 (Ruggiero et al., 2012);) or the presence of open OS disks (Matsumoto and Besharse, 1985).
Though we have long known about actin involvement in OS phagosome ingestion, the molecular mechanisms underlying actin remodeling have only recently been investigated. RAC1 is a small GTP-binding protein in the Rho family of GTPases that destabilizes F-actin and facilitates its branching for the formation of the dynamic phagocytic cup. Upon OS feeding of cultured RPE, there is a brief burst of RAC1 activity together with colocalization of RAC1 with OS phagosomes. This activity is dependent upon αvβ5 integrin engagement by MFGE8 and is essential for phagosome ingestion (Mao and Finnemann, 2012). RAC1 inhibition prevents actin recruitment to the bound OS particle and therefore, prevents ingestion. MERTK activation is neither required for nor affected by RAC1 activation and subsequent actin remodeling (Mao and Finnemann, 2012), consistent with the observation that actin is recruited to bound OS in RPE cells of the RCS rat (Chaitin and Hall, 1983b). Annexin A2 (ANXA2) is a membrane-associated protein that regulates actin dynamics and binds signaling intermediates such as Ca2+, cholesterol-rich membranes, and phosphoinositides, serving as a direct or indirect linker between the phagosome membrane and actin cytoskeleton (Turowski et al., 2004; Law et al., 2009). ANXA2 likely functions upstream of FAK, as Anxa2−/− mice show delayed FAK phosphorylation following light onset. Consistent with its function, ANXA2 localizes to the phagocytic cup and the early phagosome but dissociates from fully ingested phagosomes (in vivo and in vitro) (Law et al., 2009), mirroring the association kinetics of actin. Treatment of differentiated ARPE-19 cells with ANXA2 siRNA leads to decreased ingestion (but not binding) of OS. Similarly, Anxa2−/− mice show phagosome accumulation in the apical microvilli (Law et al., 2009), an observation not noted in wild type mice, strongly suggesting delayed phagosome ingestion in the absence of ANXA2.
Once pseudopodia are extended, myosin motors are typically required for phagocytic cup closure, i.e., to provide the necessary force to retract extended pseudopodia and pull the ingested particle into the cell. To date, only myosin-2 has been shown to be necessary for OS ingestion in RPE (Strick et al., 2009). The mobilization of myosin-2 to the phagocytic cup was severely diminished in RPE cells from RCS rats, suggesting that while αvβ5 integrin converges on actin rearrangement pathways, MERTK signaling independently converges on the myosin force generation required for particle engulfment following phagocytic cup formation.
In addition to actin and actin-associated proteins, downstream kinases are also involved in modulating OS ingestion. MERTK phosphorylation is known to lead to its direct interaction with several proteins, including phosphoinositide 3-kinases (PI3K). Although a PI3K regulatory subunit was found to colocalize with early OS phagosomes (Shelby et al., 2013), the significance of this interaction or the downstream implications have not yet been investigated in the RPE. However, the opposing roles of PI3K and protein kinase B (PKB or AKT) in phagosome ingestion in the RPE have been investigated. Though they are both ubiquitous kinases involved in several cellular functions, PI3K is required for OS engulfment, possibly due to a role in promoting phagocytic cup formation, while AKT is not required for ingestion, and appears to restrict the size of the phagocytic cups and myosin-2 recruitment to the cup (Bulloj et al., 2013). Moreover, despite being a downstream effector of PI3K, AKT functions independently from PI3K during phagocytosis (Bulloj et al., 2013).
Given the importance of actin in phagocytosis, the polarity of the actin cytoskeleton can act as an indicator of RPE phagocytic capacity. RPE cells, differentiated from human adult RPE stem cells, that still had actin stress fibers, despite having junctional ZO-1 labeling and comparable apical expression of the αvβ5 integrin, exhibited a lower phagocytic capacity compared with more polarized cells, containing circumferential actin at the level of the junctional complexes and actin filaments within apical processes (Müller et al., 2018). Cells could be made to increase or decrease their phagocytic capacity by inhibiting or activating Rho kinase, which induced circumferential actin rearrangement or stress fiber formation, respectively.
Overall, compared with other professional phagocytes such as macrophages, our knowledge of actin dynamics during phagosome ingestion in the RPE is relatively unexplored and has been largely informed by just a few studies performed in recent years. Phagosome ingestion was visualized in vitro using live imaging of primary RPE (Jiang et al., 2015). However, the spatio-temporal relationship of actin and other molecular components of the phagocytic cup has still to be explored. Live-cell imaging offers an unprecedented level of insight into these dynamic processes and will be an important technique for advancing our understanding of OS phagosome ingestion.
3.6.3. Are OS membranes shed before ingestion by the RPE?
It is frequently mentioned in the literature that “disk membranes are shed and then phagocytosed by the RPE”. Yet, to our knowledge, there is no report of a convincing image of a detached packet of disk membranes waiting to be ingested. Indeed, to the contrary, it has been shown that the detachment of retinas in frog eyecups in vitro prevents the release of the distal disks from the region of detachment, demonstrating that OS membrane shedding does not occur in the absence of the RPE (Williams and Fisher, 1987). This evidence indicates that OS disk shedding and phagocytosis are not separate events, and, although the term, “disk shedding”, has been used historically, it is misleading and its use should probably be avoided in the context of OS disk membrane renewal.
Even molecular changes in the OS that lead to ingestion appear to require the RPE. The external PS localization at the tips of OSs, mentioned above, is stimulated only in the presence of the RPE, since it requires the RPE integrin, αvβ5, and its extracellular ligand, MFGE8 (Ruggiero et al., 2012). An earlier report showed that freshly isolated frog retinas incubated in the low molecular weight dye, Lucifer Yellow, incorporated the dye into the distal tips of the OSs, suggesting that the opening of some distal disks to the extracellular space is an early step that leads to phagocytosis of the OS tip (Matsumoto and Besharse, 1985). But, this, too, appears to be an event that could be regulated by the RPE, given that the frequency of stained OS tips was affected by the light exposure that the intact animal received prior to retinal dissection (while the OSs were still associated with the RPE) (Matsumoto and Besharse, 1985).
3.7. Degradation of OS phagosomes by the RPE
OS phagosomes mature by migrating from the apical to basal side of the RPE, while undergoing gradual acidification and degradation by hydrolases.
The RPE cytoskeleton and its motor proteins support rapid bidirectional motility of OS phagosomes, as well as basal migration, and are essential for normal phagosome degradation.
Phagosome degradation appears to involve a series of stages.
Proteins, such as RAB5 and RAB7, associate with phagosomes at different stages, thus marking as well as functioning in different stages of phagosome maturation.
Phagosome acidification mediated by v-ATPase is required for protease activation.
The lysosomal aspartyl protease, cathepsin D, is essential for phagosome degradation, together with cysteine proteases such as cathepsin S.
3.7.1. Role of the cytoskeleton and molecular motors
As early as Young and Bok’s paper on OS phagocytosis (Young and Bok, 1969), it was known that, once ingested, phagosomes traverse the RPE from the apical to basolateral domain, where they are ‘eliminated’. Herman and Steinberg found that, in the opossum RPE, the majority of phagosomes were located basally 1–2 h after light onset, with few remaining in the apical domain (Herman and Steinberg, 1982b). Their observation meant that, shortly after OS membrane ingestion, the resulting phagosomes had translocated ~100 μm, the distance between the apical and basal domains of the opossum RPE. This translocation appeared to require the RPE microtubule cytoskeleton, as intravitreal injections of colchicine, an inhibitor of microtubule polymerization, caused phagosomes to accumulate in the apical RPE. Similar treatments in albino rats prevented phagosome interactions with lysosomes, and thus acquisition of lysosomal acid phosphatase by the phagosomes (Beauchemin and Leuenberger, 1977).
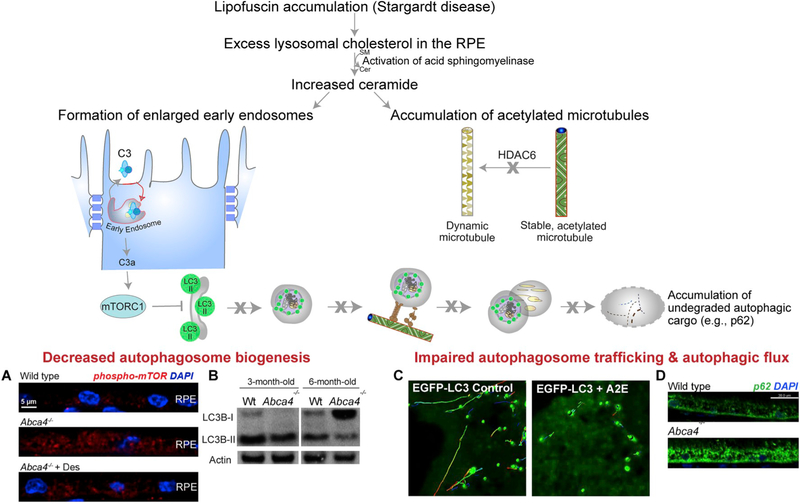
Imaging of OS phagosomes in live primary RPE cells showed that the phagosomes undergo rapid back and forth movements along labeled microtubules (see Video 2 in Jiang et al., 2015). Demonstrated association with subunits of both kinesin-1 (Jiang et al., 2015) and cytoplasmic dynein-1 (Esteve-Rudd et al., 2018), which are plus- and minus-end directed microtubule motors, respectively, is consistent with bidirectional motility along microtubules. It appears, then, that OS phagosomes are highly motile, undergoing rapid bidirectional movements, while maintaining an overall bias towards the basal domain of the RPE. The bidirectionality likely enhances encounters with endolysosomes (see next subsection). The importance of this motility has been illustrated by circumstances in which it is perturbed. OS phagosomes in the RPE of Klc1−/− mice (which lack a light chain of kinesin-1) have impaired motility and are degraded more slowly. Older Klc1−/− mice develop AMD-like pathology, such as a slow loss of photoreceptor cells, complement hyperactivation, increased oxidative stress, and increased accumulation of neutral lipids such as lipoproteins and basal laminar deposits. Some mutant retinas also showed structures resembling drusen, and neovascularization of the RPE and photoreceptor layers (Jiang et al., 2015).
In a second example, defective OS phagosome motility and degradation has been found in a mouse model of Stargardt macular degeneration (type 3) (Esteve-Rudd et al., 2018), leading to phagosome accumulation, RPE pathogenesis, and photoreceptor cell death (Karan et al., 2005). These mice express mutant human elongation of very long chain fatty acids (ELOVL)-4 protein that mislocalizes to the OS, producing OS phagosomes with altered content which, in turn, appear to be defective in their recruitment of motor linkers and motors, such as RAB7A and dynein (Esteve-Rudd et al., 2018).
Prior to association with the microtubule cytoskeleton, phagosomes must first pass through the apical actin meshwork of the RPE. There is evidence for two unconventional myosins functioning in this process. Both myosin-7a and myosin-6 have been reported to colocalize with OS phagosomes in the RPE (Jiang et al., 2015; Yu et al., 2018). The entry of phagosomes into the cell body region is retarded in Myo7a-null mice, as is the overall rate of OS phagosome degradation (Gibbs et al., 2003). It seems likely that this deficiency in myosin-7a activity may contribute to the inherited blindness in Usher syndrome type 1B, which is caused by mutations in the human orthologue, MYO7A (Weil et al., 1995). Myosin-6 downregulation was also shown to delay phagosome clearance (Yu et al., 2018).
Thus, the RPE cytoskeleton and its associated motors are not only key for the overall basal movement of OS phagosomes in the RPE, but also for the dynamic motility of phagosomes that appears to underlie their degradation.
3.7.2. Stages of OS phagosome degradation
The RPE literature has predominantly focused on the interaction between phagosomes and terminal degradative organelles known as lysosomes, particularly given that the latter possesses an array of hydrolases that are important for the degradation of OS phagosomal content. Lysosome involvement in phagosome degradation in the RPE was first noted by EM studies that identified acid phosphatase activity in both lysosomes and OS phagosomes (Ishikawa and Yamada, 1970). Moreover, these organelles could be seen in close apposition or fused to each other (Herman and Steinberg, 1982a). ‘Bridge-like structures’ were described between phagosomes and organelles thought to be lysosomes in the rat RPE as early as 30 min after light onset (Bosch et al., 1993). However, in recent years, emerging evidence, particularly from immunodetection of rhodopsin, suggests that phagosome maturation is likely more complex and proceeds in stages. For example, the monoclonal antibody, 1D4, whose epitope corresponds to the C-terminus of rod opsin (Molday and MacKenzie, 1983), primarily detects phagosomes in the apical, but not basal RPE, indicative of early phagosomes (Law et al., 2009; Esteve-Rudd et al., 2014; Wavre-Shapton et al., 2014). These phagosomes also possessed negligible cathepsin D (lysosomal protease) staining, suggesting that they had not yet encountered lysosomes and that loss of the 1D4 epitope occurred during a prelysosomal and pre-migratory processing step. In contrast, monoclonal antibodies, 4D2 and RET-P1, raised against the rod opsin N-terminus, labeled both mature and early phagosomes. An intermediate step may exist between 1D4 epitope loss and cathepsin D acquisition, as EM studies revealed fewer associated cathepsin D particles in 1D4-negative phagosomes that retained a distinct disk structure than in phagolysosomes (Wavre-Shapton et al., 2014).
Studies done with macrophages offer clues regarding the molecular mechanisms and organelle interactions that may be involved in the different stages of phagosome degradation. RAB GTPases mediate organelle trafficking and fusion, and, in doing so, facilitate interactions between phagosomes and endosomes. The presence of RAB5 and early endosome antigen 1 (EEA1) indicate interactions with early endosomes, whereas RAB7 is a marker for late endosomes, and the lysosome-associated membrane proteins, LAMP1 and LAMP2, are markers for lysosomes. Colocalization of OS phagosomes with these endolysosomal markers has suggested a trend such that early phagosomes associate with early endosome markers, RAB5 and EEA1, but this association wanes as LAMP1/2 association rises (Yu et al., 2018). While the molecular implications of the association of these markers are not well understood, the change in marker association indicates the presence of different stages in OS phagosome maturity. Moreover, it suggests a stepwise role for different endolysosomes in OS phagosome maturation. Alterations in endolysosomal marker association would likely indicate a defect in a particular maturation stage, which could contribute to inefficient OS phagosome degradation. A recent report has shown that deficient LAMP2 results in retarded OS phagosome degradation, and the accumulation of AMD-like deposits (Notomi et al., 2019).
Alterations in phagosome content itself may also affect phagosome association with RAB proteins. In a Stargardt macular degeneration type 3 mouse model, mutant ELOVL4 mislocalizes from the endoplasmic reticulum to the photoreceptor OSs. When these mutant OSs were fed to wild-type primary mouse RPE cells, the resulting OS phagosomes were found to associate with excessive RAB7A, and have affected motor protein recruitment and impaired motility, which likely underlies their observed slower degradation (Esteve-Rudd et al., 2018).
The importance of RAB proteins in orchestrating stages of OS phagosome degradation is also illustrated by models of choroideremia, an inherited choroidal and retinal degeneration. Choroideremia is caused by mutations in the REP1 (aka CHM) gene, which encodes RAB escort protein-1 (Cremers et al., 1990; Seabra et al., 1992). REPs are involved in RAB prenylation, a lipid modification that allows RAB membrane binding and function, and thus the association of RAB proteins with specific organelles. Although lack of REP1 function appears to be compensated by REP2 activity in most tissues, the outer retina including the RPE requires REP1. Rep1 knockout mice demonstrate delayed in vivo phagosome degradation (Wavre-Shapton et al., 2013). Moreover, human iPSC-derived RPE cells from choroideremia patients (Duong et al., 2018), and human fetal primary RPE cells, treated with REP1 siRNA (Gordiyenko et al., 2010), both demonstrate delayed OS phagosome clearance. Phagosomal RAB7 and subsequent LAMP1 acquisition were compromised in the cells treated with REP1 siRNA, thereby leading to fewer fusion events with lysosomes, which, coupled with defects in phagosomal acidification, resulted in slower phagosome clearance (Gordiyenko et al., 2010).
3.7.3. Phagosome acidification
One of the hallmarks of a maturing phagosome is increasing acidification. By immunoEM of RPE sections, OS phagosome acidification and cathepsin D acquisition occur concurrently with the loss of opsin antigenicity (Deguchi et al., 1994). The acidification is mediated by the vacuolar type H+-ATPase (v-ATPase), and is required for the proper functioning of lysosomal proteases. Intravitreal injection of bafilomycin, a v-ATPase inhibitor, resulted in the accumulation of large phagolysosomes containing cathepsin D and with preserved opsin antigenicity, demonstrating that enzyme acquisition alone was not sufficient for opsin degradation (Deguchi et al., 1994). Defects in lysosomal acidification through chronic administration of lysosomal alkalinizing agents result in extrusion of undigested OS phagocytic material from the basal RPE into the space between Bruch’s membrane and the RPE (Peters et al., 2006). Furthermore, pharmacological re-acidification of ARPE-19 cells, pretreated with lysosomal alkalinizing agents, increases their ability to degrade OS by reestablishing an acidic lysosomal environment more conducive to efficient protease activity (Liu et al., 2008). Moreover, these authors also demonstrate lysosome alkalinization in the albino Abca4−/− mouse (Liu et al., 2008) and have recently shown that restoration of lysosomal pH with P2Y12 receptor antagonist prevents photoreceptor loss in these mice (Lu et al., 2019). Endolysosomal acidification is thought to be regulated by direct interaction of βA3/A1-crystallin with lysosomal v-ATPase (Valapala et al., 2014). Indeed, RPE from βA3/A1-crystallin knockout mice have decreased v-ATPase activity, elevated lysosomal pH, and decreased cathepsin D activity. Additionally, the membrane organizer, caveolin-1, is recruited to maturing phagolysosomes and appears to be important for regulating lysosomal acidification and cathepsin D enzyme activity; increasing or decreasing caveolin-1 protein levels in vitro accelerated or decelerated phagosome degradation, respectively (Sethna et al., 2016), although the underlying mechanism remains unclear.
3.7.4. Role of cathepsin D
Cathepsin D was the first lysosomal protease specifically investigated in the RPE. It was reported to degrade OS proteins in vitro (Hayasaka et al., 1975; Rakoczy et al., 1997), including rhodopsin (Regan et al., 1980).
The importance of cathepsin D is well illustrated by the mcd/mcd transgenic mouse model which expresses a mutated form of cathepsin D rendering it enzymatically inactive (Rakoczy et al., 2002). These mice display features reminiscent of geographic atrophy in AMD, such as photoreceptor cell death, diminished ERG responses, accumulation of phagocytic material in the RPE, basal laminar and linear deposits, RPE pigmentary changes, and RPE cell atrophy and proliferation. Perhaps, unsurprisingly, there is strict regulation of cathepsin D activity using post-translational modifications (Lai et al., 2000). The presence of cathepsin D multimers, i.e., 46–60 kDa minimally active enzyme forms, in primary human RPE cultures correlate with the accumulation of autofluorescent debris derived from prolonged OS feeding (Rakoczy et al., 1996), suggesting a reduction in the degradative capacity of the culture. The incomplete maturation or mistargeting of cathepsin D is associated with perturbations in phagosome degradation. Cathepsin D maturation (Hoppe et al., 2004a), but not expression, is impaired in RPE cells treated with oxidized LDL, thereby leading to inefficient OS phagosome degradation (Hoppe et al., 2001). These cells also demonstrated increased secretion of the pro-enzyme into the extracellular space. Similarly, melanoregulin (MREG), a small, highly-charged protein that localizes to lysosomes (Frost et al., 2013), is important for cathepsin D maturation. RPE from Mreg−/− mice have decreased levels of the mature enzyme (Damek-Poprawa et al., 2009), and increased secretion of pro-cathepsin D into the extracellular medium (Frost et al., 2013). They also display delayed phagosome degradation, both in vivo and in vitro, increased laminin deposition in Bruch’s membrane, and greater accumulation of bisretinoids such as A2E in aged Mreg−/− mice compared to age-matched controls.
3.7.5. Cysteine proteases
While cathepsin D is a key lysosomal aspartyl protease for OS phagosome degradation, there are indications that cysteine proteases may be equally, if not more, important. Cysteine proteases are considerably more abundant than aspartyl proteases. Cysteine protease inhibition of RPE cells in vivo, through a single intravitreal injection, has been shown to increase the accumulation of phagosomes and autofluorescent material (Ivy et al., 1989; Katz and Shanker, 1989; Okubo et al., 2000); although, the inhibition is relieved within a week, and phagosome counts in the RPE return to near control levels (Okubo et al., 2000). In contrast, prolonged cysteine protease inhibition, through three daily intravitreal injections of inhibitor, resulted in phagosome accumulation in the RPE, even after a week (Okubo et al., 2000).
An allelic variant of the cystatin C gene, an inhibitor of cysteine cathepsins, is significantly associated with the risk of developing wet AMD (Zurdel et al., 2002). Cathepsin L has been detected at the transcript level in mouse RPE and can be upregulated in response to hyperoxia (Alizadeh et al., 2006). Cathepsin B has also been identified in the mouse (Alizadeh et al., 2006; Ahuja et al., 2008), bovine (Fröhlich and Klessen, 2001), and human (Hoppe et al., 2004b) RPE, although there appears to be some variation in the level of protein expression, at least in RPE from individual rats (Wassélius et al., 2003). In contrast to cathepsins L and B, whose specific roles in degradation have not been investigated, there is some evidence that cathepsin S is important for OS phagosome degradation. Primary human RPE cells, treated with anti-cathepsin S oligonucleotide over several days and then pulsed with isolated OS, showed more accumulation of autofluorescent material (used as an indicator of phagosome accumulation) compared with cells treated with either sense oligonucleotides or none at all (Rakoczy et al., 1994).
The relative importance of cysteine proteases in the degradation of OS phagosomes by the RPE is evident by comparing prolonged pharmacological cysteine and aspartyl protease inhibition of RPE cells in culture. Pan cysteine protease inhibition resulted in significantly greater autofluorescence accumulation, as a result of impaired OS degradation, compared with an aspartyl protease inhibitor (Rakoczy et al., 1994). It is possible that, given the high levels of cathepsin D in the RPE, aspartyl protease inhibition was insufficient to elicit significant autofluorescence accumulation. Additionally, it may also be possible that cysteine proteases are involved in the activation of aspartyl proteases, causing cysteine protease inhibition to result in a more pronounced delay of phagosome digestion due to a ‘combined’ inhibitory effect (Rakoczy et al., 1994). Most likely, phagosome degradation requires the combined action of a variety of lysosomal proteases, perhaps at different stages in degradation. Although cathepsin D has received the most attention with regard to OS phagosome degradation, cysteine proteases appear to be major players.
3.7.6. Lipase activity in the RPE
Given the high lipid content of OS phagosomes, it is not surprising that the RPE has the highest acid lipase activity of all ocular tissues (Hayasaka et al., 1977). Phospholipases generate the fatty acid substrates from ingested OS disk membrane phospholipids for mitochondrial β-oxidation (see section 6) (Reyes-Reveles et al., 2017). Phospholipase A activity in the RPE was reported in the early 1980s (Zimmerman et al., 1983). Phospholipase A2 (PLA2), the enzyme that cleaves the fatty acyl moiety from the number 2 position of phospholipids is of particular relevance in maintaining RPE homeostasis (Bazan, 2018; Knott et al., 2018).
4. Intersection of phagocytosis with autophagy: LC3-associated phagocytosis
In addition to the phagocytic machinery discussed in the preceding section, a portion of OS phagosomes in the RPE recruit microtubule-associated protein 1 light chain 3 (LC3) in a process known as LC3-associated phagocytosis (LAP) (Kim et al., 2013; Frost et al., 2015). Of the three LC3 isoforms, LC3A, LC3B, and LC3C, LAP requires LC3B. LAP appears to augment the clearance of phagosomes. The recruitment of LC3B to maturing phagosomes requires proteins involved in classical autophagy; however, LAP is distinct from classical autophagy both in initiation and function. In this section, we will discuss LAP in the RPE, thus following the phagocytic theme from the previous section. Classical autophagy in the RPE, particularly in relation to retinal degeneration, will be discussed in Section 5.
4.1. LC3-associated phagocytosis (LAP)
LAP was first identified about a decade ago in studies on phagocytosis of pathogens and dead cells by immune cells (Sanjuan et al., 2007; Martinez et al., 2011) (Fig. 9). It is distinguished from classical phagocytosis by the recruitment of lipidated LC3B (LC3B-II) to phagosomes. Conversion of non-lipidated LC3B–I to lipidated LC3B-II is necessary for binding of LC3B to the phagosome membrane. LAP is an autophagosome-independent process (Frost et al., 2015; Martinez et al., 2015; Klionsky et al., 2016), and does not require the upstream components needed for initiating or regulating classical autophagy. In addition, LC3B-II recruitment occurs on single-membraned phagosomes, unlike the double-membraned autophagosomes in classical autophagy (Frost et al., 2015; Martinez et al., 2015; Klionsky et al., 2016).
Fig. 9.
LC3-associated phagocytosis. ATG5 (in association with ATG12 and ATG16L1) lipidate LC3B by the addition of phosphatidylethanolamine (PE). In the RPE, lipidated LC3B is recruited to a population of OS phagosomes with the help of melanoregulin (MREG).
LAP is often considered a protective process, serving to dampen the immune response in macrophages in response to pathogens (for review see Heckmann et al., 2017), and delaying phagosome degradation during antigen presentation (Ligeon et al., 2017, 2018). In OS phagosome clearance within the RPE, many questions remain unanswered regarding the role of LAP relative to LC3B-independent endolysosomal degradation.
4.2. LC3-associated phagocytosis in OS phagosome clearance
A subpopulation of OS phagosomes recruits LC3B.
The Lc3b−/− mouse exhibits normal starvation-induced autophagy and delayed phagosome maturation.
LAP contributes to OS phagosome clearance.
4.2.1. RPE cell models to study LC3-associated phagocytosis
The most informative studies regarding the kinetics of LC3B association with opsin-positive phagosomes have been performed in polarized cultures of human RPE (Frost et al., 2015). Although some studies on LAP in the RPE have been performed with undifferentiated RPE-J cells (Kim et al., 2013; Muniz-Feliciano et al., 2017), it is well-established that the kinetics of OS uptake and OS phagosome degradation are sensitive to the state of RPE differentiation. As discussed in Section 3.7, degradation of OS phagosomes is dependent upon their motility. Phagosome motility is, in turn, determined by the organization of the cell’s actin and microtubule cytoskeleton. As illustrated in Fig. 2, this cytoskeletal organization changes dramatically as a non-polarized cell acquires the phenotype of a fully differentiated epithelial cell. Therefore, the extent of LC3 association with phagosomes, and the degradation of these LC3-associated phagosomes probably differs significantly between undifferentiated, proliferating cells in a cell line and bona fide polarized RPE (discussed below). In future studies, it seems imperative that researchers make the effort to utilize well-differentiated RPE cultures, as discussed in section 2.
Mutant mouse models have also been used in studies of LAP in the RPE. Combined with microscopy, they provide an in vivo perspective. However, as noted in the next subsection, unintended consequences, such as overexpression of transgenes or additional effects on pathways other than LAP, require caution in interpretation of results.
4.2.2. LC3-associated phagocytosis by RPE cells
Following the ingestion of OS membrane by the RPE, LC3B-II, its conjugation factor, ATG5, as well as the LC3 adaptor melanoregulin (MREG), are all found on a subpopulation of OS phagosomes within 15 min of ingestion (Kim et al., 2013; Frost et al., 2015). While LC3B-II-opsin association is clearly observed (Fig. 10), the extent of LC3B-II association with opsin-positive phagosomes remains unclear; in vitro (Frost et al., 2015) and in vivo (Dhingra et al., 2018) studies suggest that ~30–45% of ingested phagosomes are LC3B-positive. In those studies, the levels of endogenous LC3B associated with opsin-positive phagosomes were assessed by antibody labeling. Higher percentages of LC3B-opsin association are observed when GFP-LC3B is overexpressed in cultures of undifferentiated RPE-J cells, or, in mice, overexpressing GFP-LC3B (Kim et al., 2013); in both cases, 80–90% of opsin-containing structures are GFP-LC3B-positive. This discrepancy could be due to overexpressed GFP-LC3B, which would change the stoichiometry of LC3 in the cell available for competing processes (autophagy versus LAP). An assessment of the levels of LC3-associated phagosomes in models of age-related retinal disease may provide valuable insight into the balance between the two LC3B-requiring processes: basal, constitutive or stress-induced autophagosome formation (Section 5) and OS disk membrane lipid degradation (Section 7).
Fig. 10.
LC3B decorates opsin-positive phagosomes. (A) Maximum intensity confocal image showing a flatmount preparation of fixed mouse RPE/choroid labeled with antibodies raised to rhodopsin (RHO, green) and LC3B (red). Inset (lower right) shows a magnified view of the boxed region. (B) Volume rendering of a region containing the two phagosomes shown in the inset, after 3-D deconvolution of the 2.5 μm thick z-stack. Scale bars in x/y = 0.24 μm and z = 0.6 μm.
The Atg5ΔRPE mouse model has provided valuable insight into autophagy-associated pathways in the maintenance of RPE and photoreceptor health. In the Atg5ΔRPE mouse, rod and cone photoreceptor functions are diminished, although no cell loss is apparent. These mice exhibit a decrease in retinoid chromophore synthesis, including 11-cis retinal, suggesting a functional link between ATG5 phagosome association and visual pigment recycling (Kim et al., 2013). The Atg5ΔRPE mouse also exhibits elevated levels of oxidatively-modified lipids, proteins, and DNA with occasional RPE hypertrophy (Zhang et al., 2017). However, ATG5 is a component of both classical autophagy (Section 5) and LAP; therefore, loss of ATG5 alone does not allow researchers to delineate cause and effect relationships specific to either one of these two processes.
In contrast, the Lc3b−/− mouse shows a decrease in LC3-mediated OS phagosome clearance, with no change in classical autophagy (Dhingra et al., 2018). Therefore, the Lc3bΔRPE mouse is a model in which classical autophagy can be uncoupled from LAP. In the RPE, LAP is likely neuroprotective. Loss of LC3B (Dhingra et al., 2018) results in age-dependent deposition of lipid-rich structures between the RPE and choroid, an increase in lipid peroxidation adducts, and increases in the pro-inflammatory lipid 7-ketocholesterol. Compensatory, pro-survival, anti-inflammatory compounds, including docosahexaenoic acid (DHA)-derived neuroprotection and maresin-1, also decrease in the Lc3b−/− RPE. The resulting pro-inflammatory microenvironment leads to recruitment of microglia and contributes to RPE hypertrophy and loss of function (decreased c-wave). The physiological consequences of LAP-dependent degradation are illustrated schematically in Fig. 11. Loss of MREG, an LC3B binding partner contributes to a delay in phagosome maturation (Damek-Poprawa et al., 2009) and lysosomal exocytosis, as well as basal laminar thickening in older mice (Frost et al., 2013).
Fig. 11.
Physiological effects associated with LAP. LAP function has effects on phagosome maturation, the inflammatory microenvironment of the outer retina, and RPE metabolism.
With the establishment and validation of better animal and in vitro models, we can begin to answer several outstanding questions on the role of LAP in retinal health and disease. Is the critical balance between LAP and classical autophagy altered in response to oxidative stress? Can LAP components be stimulated to enhance OS degradation in aging or disease? It is important to consider the relative distribution of LC3-associated phagosomes across the entire retina, central versus periphery, as well as whether age or increased stress, such as light or cigarette smoke, stimulate LAP-mediated degradation. Interestingly, cigarette smoke stimulates LC3B (LAP)-dependent degradation of apoptotic cells in emphysema (Chen et al., 2010).
4.2.3. Regulation of LC3-associated phagocytosis
The regulation of LAP, particularly in relation to LC3-independent phagocytosis and classical autophagy, is likely critical for overall catabolism in the RPE. In macrophages, binding of pathogens and dead cells to specific cell surface receptors initiates a signaling cascade that recruits Rubicon (Run domain Beclin-1-interacting and cysteine-rich domain-containing protein) to the nascent phagosome. Rubicon, in turn, stimulates the lipidation machinery for LC3 decoration of phagosomes (Martinez et al., 2015). Rubicon-deficient macrophages undergo normal levels of phagocytic uptake yet fail to recruit LC3B-II to phagosomes (Kim et al., 2013; Martinez et al., 2015; Wong et al., 2018). Thus, Rubicon appears to function in balancing classical autophagy and LAP, by committing a cell to LAP while inhibiting classical autophagy (Wong et al., 2018).
Although a role for Rubicon in regulating LAP in the RPE has been suggested, experimental support for this notion is currently not strong. Rubicon-positive OS phagosomes were reported in undifferentiated RPE-J cells upon EGFR activation, but whether this is important for recruiting Rubicon-related downstream machinery is not known. Loss of Rubicon decreased OS degradation; however, these RPE-J cells also showed a dramatic loss of LAMP1-labeled lysosomes (Muniz-Feliciano et al., 2017), suggesting that knocking out Rubicon could delay OS clearance by interfering with multiple pathways including endosome maturation. Studies in other cell types suggest that Rubicon helps regulate endosome maturation by serving as a RAB7 effector (Sun et al., 2010). Thus, knocking out this multifunctional protein could very well impact LAP and autophagy via a combination of direct and indirect effects, rather than a single causal role in LAP.
Last, the relevance of these in vitro studies to RPE in vivo is unclear since there is no evidence that post-mitotic RPE express EGFR on the apical cell surface. The observation that Rubicon levels in the RPE are elevated at light onset (Muniz-Feliciano et al., 2017) suggests that synchronized OS phagocytosis is required for Rubicon activation. This prediction could be tested more directly with a model of asynchronous OS phagocytosis, such as the β5−/− mouse.
In conclusion, evidence supports that LAP functions in the clearance of OS phagosomes, but much remains to be understood regarding this role, especially relative to endolysosomal degradation that is independent of LC3. Given that only a subpopulation of OS phagosomes recruits LC3, what distinguishes these from other OS phagosomes? The use of well-defined cell culture and animal models will be important in addressing such questions.
5. Classical autophagy
Autophagy, a critical mechanism for recycling debris and damaged organelles, is upregulated in the RPE in response to cell stress.
Impaired autophagy is a feature of RPE from AMD donor tissue and multiple models of inherited retinal degenerations.
Live-cell imaging studies have helped identify mechanisms that drive autophagic defects in retinal diseases.
In autophagy, damaged organelles (e.g., mitochondria, lysosomes, peroxisomes) and cellular material (e.g., proteins, lipids, nucleic acids) are enclosed in a double-membraned autophagosome and delivered to lysosomes for degradation (Leidal et al., 2018; Levine and Kroemer, 2019). There are three principal routes of autophagy that differ primarily in the mechanism of cargo delivery to lysosomes: microautophagy, macroautophagy, and chaperone-mediated autophagy. Autophagy in ocular tissues and its role in visual development and disease have been extensively discussed in recent reviews (Frost et al., 2014; Boya et al., 2016). In this section, we will focus on macroautophagy, hereafter referred to as autophagy, where cargo to be degraded is sequestered within double-membraned autophagosomes that fuse with lysosomes containing hydrolytic enzymes. As discussed below, a growing body of evidence suggests that autophagy is crucial for maintaining RPE homeostasis, and impaired autophagy has been implicated in a plethora of retinal degenerations.
5.1. Molecular machinery and regulation of classical autophagy
Classical autophagy occurs at a basal, constitutive level in all eukaryotic cells, and is modulated by intrinsic and extrinsic stimuli. Its molecular machinery and regulation are illustrated in Fig. 12. Of the diverse signaling networks that regulate autophagy, those controlled by the mechanistic target of rapamycin (mTOR) and AMP-activated protein kinase (AMPK) have special relevance for the RPE. mTOR Complex 1 (mTORC1), which includes mTOR, regulatory-associated protein of mTOR (RAPTOR), and mammalian lethal with SEC13 protein 8 (MLST8), negatively regulates autophagy by two distinct mechanisms in nutrient-replete or stress-free conditions. First, it phosphorylates and inactivates ULK1 and ULK2 (unc-51-like kinases 1 & 2). Activation of the ULK complex (ULK1-ATG13-RB1CC1/FIP200) in turn activates the VPS34 complex (UVRAG-Beclin-VPS34-ATG14) to initiate biogenesis of the double-membraned autophagosome. Second, at the lysosomal membrane, mTOR phosphorylates TFEB and TFE3, master transcriptional regulators of autophagy and lysosome biogenesis, resulting in their sequestration within the cytosol. Starvation and other cellular stressors inhibit mTOR, which relieves the block on ULK1 and 2 to initiate autophagy, and allows nuclear translocation of TFEB/E3 to induce the transcription of lysosomal and autophagy genes. In contrast to mTOR, AMPK activates autophagy by activating ULK1/2, and by inhibiting RAPTOR (regulatory-associated protein of mTOR), which is necessary for mTOR kinase activity.
Fig. 12.
Classical autophagy. Inhibition of the mechanistic target of rapamycin (mTOR) and activation of AMP-activated protein kinase (AMPK) initiate macroautophagy by regulating the activity of specific multiprotein complexes. See Section 5.1 for a detailed description of the machinery involved in each step of autophagy and Sections 5.4–5.6 for a discussion of autophagy in retinal diseases.
At the nascent autophagosome, ATG9 delivers vesicles that provide membrane for autophagosome growth. ULK recruits the ATG5-ATG12-ATG16L1 complex to mediate conjugation of phosphatidylethanolamine to LC3B. Conversion of non-lipidated LC3B–I to lipidated LC3B-II is necessary for its binding to the membrane and is regulated by ATG4, ATG7, and ATG10. LC3B-II recruitment is essential for autophagosome elongation and for recruitment of cargo to the autophagosome. p62 and other autophagic receptors bind ubiquitinated proteins and organelles tagged for removal. These receptors also have LC3-interacting regions (LIRs) that bind LC3 at the nascent autophagosome, resulting in ubiquitinated cargo being enclosed within the growing autophagosome. At the final step, LC3B-II promotes autophagosome closure after dissociation of ATG proteins. Fusion of autophagosomes with lysosomes requires microtubule-based transport, tethering factors, and SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein [SNAP] receptor) proteins (Leidal et al., 2018; Levine and Kroemer, 2019).
5.2. RPE cell models to study autophagy
Autophagy is known to be upregulated in the RPE in response to a variety of stressors (see below); however, it has become increasingly clear that the ability of a particular stimulus to induce autophagy, and the extent of induction, both depend on the differentiation status of the RPE. First, whereas non-polarized RPE respond to “stereotypical” autophagy inducers such as serum starvation, well-polarized terminally differentiated primary RPE cultures do not (Toops et al., 2015). This is likely because primary RPE cultures are usually cultured in medium with 1% serum (Toops et al., 2014), and thus serum starvation is not as big a stressor as it would be for RPE cell lines that are cultured in high serum-containing medium (e.g., 10% fetal bovine serum [FBS] recommended by ATCC to culture ARPE-19 cells). Second, polarized primary RPE do not upregulate autophagy in response to the non-specific mTOR inhibitor rapamycin, but respond to the specific mTORC1 inhibitor Torin-1 (Toops et al., 2015). These differentiation-dependent responses to autophagic stimuli suggest that studies using poorly polarized or fibroblastic RPE cell lines and cultures cannot be extrapolated to RPE in vivo. Moreover, these discrepancies could partly explain the failure of rapamycin in clinical trials for AMD (Petrou et al., 2014).
5.3. Regulation of autophagy in the RPE
Autophagy is especially important for the post-mitotic RPE, where debris cannot be rendered harmless by dilution due to cell division. In highly differentiated primary RPE monolayers, basal, constitutive autophagy is crucial for the clearance of long-lived proteins such as p62/sequestosome-1 (SQSTM1), a specific autophagy substrate (Toops et al., 2015). Basal autophagy in the mouse retina has been shown to exhibit a bimodal circadian pattern that is regulated by distinct mechanisms in the photoreceptors and the RPE (Yao et al., 2014). In photoreceptors, light-dependent translocation of transducin and arrestin from the outer to inner segments leads to an increase in LC3B-II and ATG5-ATG12 expression. On the other hand, basal autophagy in the RPE is not regulated by light exposure, but is triggered by OS disk membrane phagocytosis. OS membrane phagocytosis is associated with the appearance of double-membrane autophagosomes, suggesting that like LAP, classical autophagy is also attuned to this major RPE function. However, whether OS membrane phagocytosis stimulates de novo autophagosome biogenesis, and how OS phagosomes interact with nascent autophagosomes, independent of LAP, is not yet known.
Based on current knowledge, it appears that there are two peaks of autophagy induction in response to OS membrane phagocytosis: in 6-month-old C57BL/6J mice RPE, the phagocytic peak occurs 30 min after light onset with a subsequent peak in LC3B-II and ATG5-ATG12 expression 2 h after light onset (Frost et al., 2015). In 2-month-old mice, peak LC3 and ATG5 expression occurs 7 h after light onset (Yao et al., 2014). This latter study did not measure ATG5 and LC3 levels at early time points (30–120 min) after light onset used in the Frost et al. study. This shift in peak expression of autophagy proteins agrees with the brief spurt of mTOR activation ~1 h after light onset reported in mouse RPE (Yu et al., 2018). Yao et al. also observed a second peak of LC3-II expression and double-membrane autophagosome formation 5 h before light onset (Yao et al., 2014). The mechanisms that drive the bimodal activation of autophagy and its relevance for OS clearance remain to be understood.
A central role for autophagy in maintaining RPE health, and consequently retinal function, is underscored by studies on mice with RPE-specific knockouts of autophagy genes. Loss of RB1CC1, a component of the ULK complex required to initiate autophagy, was shown to cause RPE atrophy and pigmentation defects as early as 4 months of age (Yao et al., 2015). This was accompanied by the accumulation of p62/SQSTM1, damaged mitochondria, and lipid droplets with increased autofluorescence in the RPE, all indicators of impaired autophagic flux. Decreased autophagy led to a secondary degeneration of photoreceptors, loss of scotopic and photopic vision, subretinal inflammation (microglial activation, complement activation), and sub-RPE accumulation of oxidative stress markers in 8-month-old mice. RPE-specific deletions of Atg5 or Atg7 cause multiple RPE abnormalities including pigmentation defects, vacuolization, accumulation of undigested cellular debris, and atrophy in aged mice (Zhang et al., 2017). Retinal degeneration was observed in 35–45% of mice between 8 and 24 months of age. The ULK complex is upstream of ATG5 and ATG7 in the autophagy cascade, which might explain why mice, lacking RB1CC1 in the RPE, have a more severe phenotype compared to those lacking Atg5 or Atg7. Interestingly, in the Abca4−/− mouse model of Stargardt disease, which is characterized by accelerated accumulation of lipofuscin bisretinoids, loss of ATG7 in the RPE did not increase lipofuscin formation, suggesting that the efficiency of autophagic flux does not impact bisretinoid formation (Perusek et al., 2015).
5.4. Autophagy in models of age-related macular degeneration
Like most other tissues, autophagy is upregulated in the RPE as an early stress response after exposure to varied insults such as intense light, oxidative stress, mitochondrial poisons, and cigarette smoke (Reme et al., 1999; Kunchithapautham and Rohrer, 2007; Chen et al., 2013; Wang et al., 2014). However, prolonged stress downregulates autophagy, which could send the cell into a spiral of increased debris, mitochondrial injury, and eventual metabolic reprogramming (Leidal et al., 2018; Levine and Kroemer, 2019). In mouse models that recapitulate features of AMD, such as the superoxide dismutase 2 (Sod2) knockout mouse, LC3, ATG7, and ATG9 expression is upregulated initially in all retinal layers including the RPE, but decreases with increasing degeneration (Mitter et al., 2014). A similar reciprocal relationship between autophagy and retinal degeneration was observed in mice with the targeted replacement of apolipoprotein E4 (APOE4) and fed a high fat diet: a decrease in the expression of proteins responsible for autophagosome biogenesis resulted in the accumulation of p62, indicative of decreased autophagic flux (Song et al., 2017).
Defective autophagy has been implicated in intractable age-related neurodegenerative diseases including Alzheimer’s and Parkinson’s diseases (Nixon, 2013). Studies on human donor tissue suggest that this could also be the case in AMD. Immunofluorescence staining of human retinal sections showed decreased expression of autophagy proteins (LC3, ATG7, or ATG9) with increasing severity of AMD, although the levels of p62 were not analyzed in this study (Mitter et al., 2014). RPE cultures established from AMD donors exhibited several features of disrupted autophagy, including decreased LC3B-II expression, swollen autolysosomes, and increased p62 (Golestaneh et al., 2017). AMD donor RPE cultures also accumulated lipid droplets, glycogen granules, and fragmented mitochondria, indicating a global breakdown of cellular clearance pathways. The presence of mitochondrial fragments confirms studies showing increased mitochondrial DNA damage (Fisher and Ferrington, 2018) in AMD donor RPE, and likely indicates a failure of mitophagy, a selective autophagic mechanism for removing damaged mitochondria (Pickles et al., 2018). During mitophagy, mitochondria destined for degradation are tagged with ubiquitin, which is recognized by autophagy receptors such as p62 and optineurin. These receptors have LIRs, which ensures the delivery of damaged mitochondria to autophagosomes. The mechanisms and machinery responsible for mitophagy in the RPE have yet to be elucidated. The mito-QC transgenic mouse, which expresses a pH-sensitive mitochondrial protein and allows for in vivo detection of mitophagy, was recently used to create a basal mitophagy and autophagy “map” in ocular tissues (McWilliams et al., 2019). Both photoreceptors and the RPE exhibited robust mitophagy; however, the authors could not establish whether the extent of mitophagy was altered as a function of light/dark cycle as was previously reported for classical autophagy (Yao et al., 2014).
5.5. Autophagy in models of inherited retinal degenerations
Autophagic abnormalities in the RPE have also been documented in several rodent and iPS-RPE models of inherited retinal degenerations. These include the pigmented Abca4−/− mouse model of Stargardt disease (Toops et al., 2015), the Cln3Δex1−6 mouse model of Batten disease or juvenile neuronal ceroid lipofuscinosis (Wavre-Shapton et al., 2015), and iPS-RPE models of Bietti’s crystalline dystrophy (Hata et al., 2018) and Smith-Lemli-Opitz (SLO) syndrome (Ramachandra Rao et al., 2018). In Abca4−/− RPE, both autophagosome biogenesis and autophagic flux are inhibited (Toops et al., 2015); however, in Batten, Bietti’s, and SLO diseases, autophagic defects manifest as an accumulation of LC3-positive autophagosomes and increased p62, suggestive of a block in autophagosome-lysosome fusion. Impaired autophagy is accompanied by defects in other organelles/functions such as delayed phagosome maturation (Bietti’s, Batten, SLO (Wavre-Shapton et al., 2015; Hata et al., 2018; Ramachandra Rao et al., 2018)), lysosomal dysfunction (Stargardt, Batten, Bietti’s (Liu et al., 2008; Wavre-Shapton et al., 2015; Tan et al., 2016; Hata et al., 2018)), and endosomal abnormalities (Stargardt (Kaur et al., 2018)) as discussed in subsequent sections.
5.6. Mechanisms underlying autophagic defects in retinal diseases
What do we know about the mechanisms underlying disrupted autophagy in these retinal degenerations? In Abca4−/− RPE cultures, highspeed live-cell imaging has helped identify defective microtubule-based autophagosome transport as a causal mechanism for impaired autophagy in the RPE (Toops et al., 2015) (Fig. 13). In normal, healthy RPE, autophagosomes are transported along dynamic microtubules to fuse with lysosomes, thereby enabling degradation of autophagic cargo. Post-translational modifications of tubulin such as acetylation and tyrosination/detyrosination can act either singly or in concert with other modifications to control motor recruitment in a cargo-specific manner (Hammond et al., 2008; Mackeh et al., 2013). Dynamic microtubules (marked by tyrosination) are required for efficient transport of autophagosomes, whereas stable microtubules (marked by acetylation) hinder autophagosome trafficking (Toops et al., 2015; Mohan et al., 2019). In Abca4−/− mice, lipofuscin bisretinoids such as A2E trap cholesterol and the anionic lipid bis-monoacylglycerophosphate (BMP) within RPE lysosomes (Lakkaraju et al., 2007; Toops et al., 2015). BMP is a co-factor for acid sphingomyelinase (ASMase), the enzyme that hydrolyzes sphingomyelin to ceramide. Aberrant activation of ASMase in Abca4−/− RPE leads to increased ceramide, which in turn inhibits histone deacetylase 6 (HDAC6), the major tubulin deacetylase, resulting in the accumulation of stable, acetylated microtubules (Toops et al., 2015). HDAC6 also regulates the autophagy-dependent clearance of defective mitochondria (Seigneurin-Berny et al., 2001), likely compromising lipid metabolism in Abca4−/− mice (discussed in more detail section 6). Live-cell imaging of RPE with lipofuscin bisretinoids showed significantly hindered autophagosome transport and impaired fusion with lysosomes. The antidepressant desipramine, which inhibits ASMase, decreased ceramide, and restored autophagosome trafficking and autophagic flux in Abca4−/− RPE.
Fig. 13.
Mechanisms underlying autophagic defects in retinal diseases. In the pigmented Abca4−/− mouse model of Stargardt disease, excess cholesterol that accumulates secondary to lipofuscin bisretinoids activates acid sphingomyelinase, the enzyme that hydrolyzes sphingomyelin to ceramide. (A and B) High levels of ceramide alter RPE membrane dynamics, resulting in the formation of giant early endosomes, which internalize the complement protein C3 into the cell. Within the RPE, C3 is cleaved to biologically active C3a, which activates mTOR (A). Chronic activation of mTOR inhibits autophagosome biogenesis (B). (C and D) Increased ceramide in Stargardt disease RPE results in the accumulation of acetylated microtubules, presumably by inhibiting histone deacetylase (HDAC) 6, which interferes with autophagosome trafficking (C). Impaired autophagosome transport delays autophagosome-lysosome fusion, and leads to the accumulation of autophagic cargo such as p62 (D). Adapted from Toops et al. (2015) and Kaur et al. (2018). Scale bar in A = 5 μm, D = 20 μm.
Since abnormal cholesterol storage is also a feature of Batten and Bietti’s diseases, it would be informative to investigate whether increased tubulin acetylation underlies impaired autophagic flux in these diseases, too. Excess cholesterol can also interfere with SNARE-mediated fusion of endosomes and lysosomes with autophagosomes and phagosomes (Fraldi et al., 2010). SNAREs are critical components of cellular fusion machinery. Two sets of cognate SNARE complexes are thought to mediate autophagosome-endosome/lysosome fusion. These include syntaxin-17 on autophagosomes and vesicle-associated membrane protein (VAMP) 7/8 on endolysosomal membranes (Levine and Kroemer, 2019). In lysosomal storage disorders, increased cholesterol sequesters VAMP7/8 within endolysosomal membranes and prevents their recycling for continued rounds of organelle fusion (Fraldi et al., 2010). If a similar mechanism is in play in RPE with excess lysosomal cholesterol, decreased VAMP7 or VAMP8 on endolysosomal membranes would slow down or inhibit fusion, and could explain the accumulation of autophagosomes seen in Batten, Bietti’s, and SLO RPE.
In contrast to these three diseases, which are characterized by an accumulation of autophagosomes, Abca4−/− Stargardt disease RPE exhibit a block in autophagosome biogenesis (Toops et al., 2015) as a result of chronic mTOR activation driven by intracellular C3 cleavage (Kaur et al., 2018) (Fig. 13). The mechanism points to an intriguing link between the complement pathway and autophagy in the RPE, and has parallels with the immune system: in T-cells, C3 is internalized via early endosomes and cleaved intracellularly into active C3a and C3b fragments. C3a activates mTOR, which is essential for driving metabolic reprogramming and cell fate decisions (Hess and Kemper, 2016). Studies have now established that this mechanism is also operational in the RPE. Intracellular C3a is almost undetectable in healthy RPE, likely due to efficient recycling or degradation; on the other hand, increased uptake of C3 in Abca4−/− RPE from the extracellular milieu into enlarged early endosomes (discussed in detail below) enables the generation of intracellular C3a, and consequently, sustained activation of mTOR (Kaur et al., 2018). Because mTOR regulates multiple metabolic and cellular pathways in addition to autophagy, aberrant mTOR activation can have severe consequences for RPE health. This is illustrated by studies in mouse models showing that sustained mTOR activation in the RPE via chemical or genetic approaches causes metabolic reprogramming, dedifferentiation, and hypertrophy of the RPE (Zhao et al., 2011). The resulting increase in RPE glycolytic flux decreases the amount of glucose available to photoreceptors, and could contribute to photoreceptor degeneration. These studies could also help explain the biphasic response of autophagy in aging and AMD discussed earlier in this section. In aging and early AMD, mTOR is well-regulated, and autophagosome biogenesis is not heavily impacted; in later stages of AMD, increased C3 expression in the retina would lead to increased generation of C3a fragments within the RPE, and consequently, activate mTOR chronically.
The studies discussed here establish that autophagy in the RPE is more than a simple house-keeping mechanism for “self-eating”. Rather, because it sits at the crossroads of metabolism, signaling, and inflammatory circuits in a highly metabolically active, post-mitotic tissue, autophagy plays a key role in maintaining the health of the RPE.
6. RPE organelles
RPE characteristics, function, and disease are intimately related to its organelles. First, of course, the RPE is typically pigmented, due to the presence of melanosomes. Other organelles include OS phagosomes (see Section 3), autophagosomes (see Section 4), and endosomes and lysosomes, whose functions includethe degradation of the two former organelles. Sequential interactions with early and late endosomes, and lysosomes are essential for the maturation and degradation of phagosomes and autophagosomes (Wavre-Shapton et al., 2014; Toops et al., 2015). In its most basic form, the endolysosomal system can be divided into a recycling circuit, involving early and recycling endosomes for recycling proteins and lipids, and a degradative circuit involving lysosomes to digest macromolecules down to their constitutive components (Huotari and Helenius, 2011). However, the reality is much more complex, especially for a polarized secretory epithelium such as the RPE, which has apical and basolateral endocytic/recycling routes, and transcytotic routes to transport cargo from one membrane domain to another (Rodriguez-Boulan et al., 2005; Lakkaraju and Rodriguez-Boulan, 2008) (Fig. 14). Further, as discussed below, lysosomes in the RPE perform various roles; in addition to their degradative functions, they participate in calcium signaling and membrane repair - mechanisms that are critical for maintaining RPE health and integrity (Liu et al., 2008; Tan et al., 2016).
Fig. 14.
Pathways of RPE organelles. (A) Apical localization of melanosomes in the RPE depends on the tripartite MYO7A-MYRIP-RAB27A complex (MYRIP, MYO7A and Rab interacting protein). Disruption of this complex leads to microtubule-mediated transport of melanosomes in the RPE cell body. Early and recycling endosomes participate in the transcytosis of the Coxsackie-adenovirus receptor (CAR) and the transferrin receptor (TfR) from the basolateral to the apical surface of the RPE. The RAB11-positive apical recycling endosome participates in transporting semaphorin 4A (SEM4A) to the photoreceptors. Endosomal trafficking is important for protecting the RPE from complement attack; the complement regulatory protein CD59 is recycled rapidly to the apical membrane in healthy RPE. In Stargardt disease, excess cholesterol reroutes CD59 towards lysosomes and makes the RPE susceptible to complement attack. Basal early endosomes (EE) endocytose the membrane attack complex (C5b-9) and sort it towards lysosomal degradation. (B) Apical and basal early endosomes mature into apical and basal multivesicular late endosomes, which release their intraluminal vesicles as exosomes either at the apical or basal surfaces. The position of lysosomes in the RPE determines their function. Perinuclear lysosomes are likely involved in digestion of phagocytosed OS disk membranes; whereas lysosomes near the plasma membrane participate in lysosome exocytosis necessary for membrane repair after complement attack. (C) Phagolysosomal digestion of OS releases fatty acids that are transported to mitochondria. Fatty acid oxidation releases β-hydroxybutyrate (β-HB), which is transported to photoreceptors for energy utilization. Very long chain polyunsaturated fatty acids (VLC-PUFA) products of OS catabolism are transported to peroxisomes. Both mitochondria and the ER participate in peroxisome biogenesis. MCT7, monocarboxylate transporter 7.
In addition to the above-mentioned organelles, this section will also discuss peroxisomes, which provide a link between OS membrane phagocytosis and RPE metabolism due to β-oxidation of the very long chain fatty acids in the ingested OS membranes. The function of the mitochondria will be discussed in Section 7, which focuses on metabolism.
6.1. RPE cell models to study the organization and function of organelles
The polarized phenotype of the RPE is characterized by a distinctive cytoskeletal arrangement, tight junctions that demarcate the apical and basolateral domains, and pertinent to this section, a spatially and functionally defined organization of melanosomes, endosomes, and lysosomes (Fig. 2). This organization is compromised in RPE cell lines that are not polarized or well-differentiated. For instance, in polarized RPE, like most other epithelia, RAB11 is found in apical recycling endosomes that are in close proximity to the apical membrane of the RPE. In fibroblastic RPE cultures, RAB11 and the transferrin receptor are both present in common recycling endosomes because there is no defined apical domain in these cells. RAB11 location is an important readout for cell health and for studies on RPE dedifferentiation and epithelial-mesenchymal transition (EMT), because loss of RAB11 from the apical recycling endosome in pre-cancerous epithelial cells is one of the first steps en route to EMT.
A highly differentiated, tightly packed RPE monolayer is especially important for studies on polarized secretion of exosomes. Poorly polarized, loosely packed, or sparsely plated cells would allow intermixing of apical and basolateral media, and make any conclusions about directional exosome secretion meaningless (Klingeborn et al., 2017a).
The subcellular location of endosomes and lysosomes helps the cell spatially segregate functions to specific regions of the RPE. As discussed in Section 3, phagosomes move to the interior of the cell to meet lysosomes with hydrolytic enzymes necessary for OS degradation. On the other hand, as discussed below, live-cell total internal reflectance fluorescence (TIRF) microscopy has shown that lysosomes close to the RPE plasma membrane participate in membrane repair after complement attack. The height of the RPE in vivo is ~10–15 μm, which is reproduced by human and porcine RPE cultures. Mouse RPE are ~6 μm tall. Most methods for culturing RPE cell lines result in rather flat cells that are well short of RPE in vivo; although, a recently published protocol for culturing ARPE-19 cells enables them to reach a height comparable to that of human RPE in vivo, 2–3 weeks after plating on Transwell filters (Hazim et al., 2019). These considerations should be kept in mind when deciding on models to study RPE organelle function.
6.2. Melanosomes
Eyes use melanin for screening light.
The RPE may lack melanosomes to accommodate a tapetum in some species.
Melanosomes function in light-dark adaptation in lower vertebrates.
Melanosomes protect against oxidative stress and may contribute to phagosome degradation.
Melanosome motility in the apical RPE involves myosins on actin filaments.
Melanosomes are lysosome-related organelles that contain melanin. The presence of eumelanin in eyes has been found to date back as far as 54 million years, as described recently in fossilized tipulid compound eyes (Lindgren et al., 2019), which show comparable organization and composition to present-day tipulid eyes (Williams, 1980; Lindgren et al., 2019).
Although pigmentation is a common characteristic of the RPE, it is not a universal one, as the RPE in some animals lacks melanosomes either completely or partially to make way for a tapetum lucidum. A tapetum reflects light back through the photoreceptors (effecting “eyeshine” when light is shone in the eye at night), in contrast to the absorption of light by melanosomes. Tapeta are found among vertebrates and invertebrates. They increase light absorption (light not absorbed by the photoreceptors on the first pass through may be absorbed on the second pass through) and are thus found in animals that are active in a low light environment. Tapeta may be within the retina, such as in some nocturnal spiders (Williams, 1979; Benson and Suter, 2013), and some teleosts (Arnott et al., 1970) and marsupials (Walls, 1939; Pirie, 1961). In each case, the tapetum lies behind the rhabdomeres or outer segments (with respect to the incoming light path). In vertebrates, retinal tapeta are thus contained within the RPE, and have been reported to consist of densely-packed granules of cholesterol (Pirie, 1961). In the opossum, Didelphis (Herman and Steinberg, 1981), the tapetum is contained within the superior RPE, which is extraordinarily thick, ~100 μm from apical to basal surfaces (Herman and Steinberg, 1982b). Tapeta in most nocturnal, placental mammals occur within the choroid; the underlying RPE cells simply lack melanosomes.
In the absence of a tapetum, the RPE contains melanosomes, which have a variety of potential functions. A clear role for melanosomes in the RPE (as well as melanocytes of the skin) is in the screening of light. During the day, when photoreceptor sensitivity is at less of a premium, it is important to limit the light scatter within the eye for increased spatial resolution. Thus, light screening by melanosomes can be a dynamic process, with melanosomes undergoing intracellular translocation.
Movements of melanosomes provide a means to alter visual sensitivity and resolution in a wide range of invertebrate and vertebrate eyes. Clear examples are found among mollusks (Daw and Pearlman, 1974), arthropods (Williams, 1983; Narendra et al., 2016), and fish and amphibians (Back et al., 1965; Burnside, 2001). In vertebrate eyes, cylindrically-shaped melanosomes enter the narrow apical processes of the RPE, and surround the OSs, with their long axis parallel to the OSs, and thus the direction of incoming light (Troutt and Burnside, 1989; Burgoyne et al., 2015). The pupils of lower vertebrate eyes do not dilate and constrict to alter the amount of light entering the eye. As compensation, the movement of melanosomes into the RPE apical processes, upon light exposure, and withdrawal from them, upon darkness, are major events, affording significant changes in the light-guiding properties of the OSs.
Although more muted, melanosomes have been observed to move into the RPE apical processes in response to light onset (Futter et al., 2004). The effect of this movement is unclear, but it may be related to functions other than light absorption that have been identified for RPE melanosomes. For example, melanosomes have been shown to have a cytoprotective effect in RPE cells under non-photic oxidative stress (Burke et al., 2011). Melanosomes may also contribute to degradation of the enormous phagocytic load incurred by RPE cells. RPE melanosomes contain proteases (Azarian et al., 2006), including cathepsin D, a major enzyme in the degradation of OS proteins (Hayasaka et al., 1975), and have been observed to fuse with phagosomes (e.g. Schraermeyer et al., 1999). Melanosomes tend to associate with more mature rod OS phagosomes; those that are not labeled with the mAb1D4 (Wavre-Shapton et al., 2014).
The light-dependent movements of melanosomes along the RPE apical processes in lower vertebrates are dependent on actin filaments, which are oriented with their plus ends biased apically (King-Smith et al., 1997). Studies on shaker1 mice identified the requirement of an unconventional myosin, myosin-7a, for the apical localization of melanosomes (Liu et al., 1998). Subsequently, it was shown that myosin-7a associates with RPE melanosomes by binding to myosin-7a- and Rab-interacting protein (MYRIP) (El-Amraoui et al., 2002), which in turn binds to RAB27A on the melanosome surface (Futter et al., 2004; Gibbs et al., 2004; Kuroda and Fukuda, 2005; Klomp et al., 2007) (Fig. 14A). In cultures of mouse and human RPE cells, lack of myosin-7a results in the inability to sequester melanosomes in the actin-rich apical domain; instead the melanosomes undergo more rapid bidirectional movements along microtubules in the cell body (Gibbs et al., 2004, 2010; Lopes et al., 2007; Jiang et al., 2019). In fish RPE, myosin-7a appears to be responsible for the dispersion of melanosomes along the apical processes in the light (McNeil et al., 2004), whereas, in the dark, retrograde movement out of the apical processes appears to require myosin-2 (Barsoum and King-Smith, 2007).
Melanosomes may be useful in retinal imaging of patients. Melanosomes are the dominant source of autofluorescence, when imaging the posterior region of the eye with near-infrared excitation (Gibbs et al., 2009). Near-infrared imaging has several advantages over excitation by short wavelength light, in which autofluorescence comes from endogenous lipofuscin, including less back scatter (Jacobson et al., 2008) and a better safety profile (Morgan et al., 2008). Melanosomes may also be detectable by other forms of clinical imaging, such as adaptive optics-optical coherence tomography (AO-OCT) (Liu et al., 2016). Diseases, such as Usher syndrome 1B, which is caused by mutant MYO7A, the gene that encodes myosin-7a, are predicted to involve mislocalized melanosomes in the RPE, based on observations of the mouse model (Liu et al., 1998). Detection of this mislocalization would offer an important surrogate indicator of correction in clinical trials. Moreover, in an exciting recent development, AO-OCT and speckle field dynamics may allow clinicians to detect defects in the motility of melanosomes (Liu et al., 2019b).
6.3. Early and recycling endosomes
Early and recycling endosomes participate in RPE-photoreceptor communication and photoreceptor maintenance.
The endosomal system can be co-opted to make the RPE susceptible to viruses and complement attack.
Abnormally enlarged early endosomes are seen in models of macular degeneration, suggesting a common etiology with other neurodegenerations such as Alzheimer’s disease.
6.3.1. Early endosomes
Early endosomes regulate mechanisms involved in signaling, growth, and defense, and can thus have far-reaching effects on cell health. In polarized epithelia, nascent vesicles that bud from the apical or basolateral plasma membrane coalesce to form apical or basal early endosomes, respectively. Membrane proteins and fluid-phase cargo destined for recycling are sorted into tubular recycling endosomes, whereas cargo destined to be degraded is sorted towards late endosomes and lysosomes (Rodriguez-Boulan et al., 2005; Lakkaraju and Rodriguez-Boulan, 2008) (Fig. 14A and B). In a recent study, highspeed live-cell imaging was used to visualize “newborn” early endosomes as they formed from the RPE plasma membrane with exquisite spatial and temporal detail. Membrane lipid composition influences the number and size of newborn endosomes. In RPE from wild type mice or young human donors, early endosomes are uniform in size; however, in Abca4−/− RPE and in RPE from aged human donors, lipofuscin bisretinoids drive the accumulation of ceramide - a cone-shaped lipid that promotes spontaneous negative curvature and inward budding - on the apical plasma membrane. This results in the formation of enlarged early endosomes from the apical surface, and these endosomes act as conduits for the entry of complement C3, as discussed above in section 5.6 (Kaur et al., 2018).
Abnormally enlarged early endosomes are an early sign of pathology in Alzheimer’s and Niemann-Pick Type C (NPC) diseases, and are thought to drive subsequent neurodegeneration (Small et al., 2017). Recent studies show that ceramide is increased in human brains as a function of age (Park et al., 2018) and in Alzheimer’s disease (Filippov et al., 2012), suggesting that a common mechanism could drive age-related early endosome defects in neurons and the RPE.
6.3.2. Recycling endosomes
Like other polarized epithelia, the RPE has common recycling endosomes, where apical and basolateral sorting routes meet, and RAB11-positive apical recycling endosomes (Rodriguez-Boulan et al., 2005). The importance of recycling endosomes in maintaining RPE health and function is illustrated by studies on mice lacking Slc9a8, the gene that encodes the sodium/hydrogen exchange protein, NHE8. This protein belongs to a large group of monovalent cation/hydrogen antiporters that transport Na+ in exchange for H+. They are involved in the regulation of intracellular pH, cell volume, cell adhesion, and organelle biogenesis. RPE lacking NHE8 have abnormally small recycling endosomes, and mice with an RPE-specific deletion of Slc9a8 show widespread retinal and RPE degeneration (Jadeja et al., 2015; Xia et al., 2015, 2018). Although the precise role of NHE8 in regulating RPE cellular pH or endosomal function remains to be established, these studies suggest that perturbing endosomal recycling in the RPE is sufficient to cause retinal dystrophy. A similar theme is echoed in studies on semaphorin 4A (Sema4A), an axon guidance molecule, that is implicated in causing retinal degenerations in humans. In the RPE, Sema4A regulates the sorting of proteins critical for photoreceptor survival and phototransduction into RAB11-positive apical recycling endosomes for eventual transport to photoreceptors. First, under conditions of oxidative stress, Sema4A reroutes anti-apoptotic prosaposins from lysosomal degradation towards apical recycling, and, second, it sorts cellular retinaldehyde-binding protein to RAB11-positive endosomes for transport to photoreceptors (Toyofuku et al., 2012). Sema4A mutant proteins that are associated with human disease are mislocalized in the RPE, leading to defective endosomal sorting of prosaposins and CRALBP, which makes the photoreceptors susceptible to degeneration (Nojima et al., 2013).
Common recycling endosomes are responsible for mediating transcytosis of the transferrin receptor and the Coxsackie-adenovirus receptor (CAR) from the basolateral to the apical surface in the RPE (Diaz et al., 2009; Perez Bay et al., 2013; Perez Bay et al., 2014) (Fig. 14A). The RPE is one of few epithelia that do not express the clathrin adaptor protein AP-1B that is required for basolateral localization of both transferrin receptor and CAR. In the absence of AP-1B, these proteins are initially sorted to the basolateral membrane from the Golgi network, but upon endocytosis, are transcytosed to the apical domain instead of being recycled back to the basolateral surface. Apical localization of CAR makes the RPE highly susceptible to adenovirus infection (Diaz et al., 2009). Studies on the molecular machinery involved in transferrin receptor transcytosis have identified specific roles for microtubule motors and apical sorting signals. Once internalized from the basolateral plasma membrane, the transferrin receptor is first transported to common recycling endosomes and then to apical recycling endosomes and apical early endosomes (Perez Bay et al., 2013). Apical transcytosis and delivery to the apical plasma membrane from these endosomes is regulated by RAB11A and requires galectin-4 (Perez Bay et al., 2014).
Endosomal defects that manifest as inefficient sorting or recycling can compromise the health of the RPE by making it susceptible to complement attack (Georgiannakis et al., 2015; Tan et al., 2016). Sequential recruitment of complement proteins C5b, C6, C7, C8, and C9 lead to the formation of the C5b-9 membrane attack complex (MAC), which forms pores on the plasma membrane. The complement-regulatory protein CD59, a glycophosphatidylinositol (GPI)-anchored protein on the apical surface of the RPE, prevents MAC assembly by inhibiting recruitment of C9 to the C5b-C8 complex. GPI-anchored proteins undergo rapid internalization and recycling to the plasma membrane in a cholesterol-dependent manner (Mayor et al., 1998). Recycling is rapid in cells depleted of cholesterol; whereas, in cells with cholesterol storage, GPI-anchored proteins are rerouted from the recycling to the degradative route. In normal RPE, complement attack accelerates CD59 recycling to the apical membrane to prevent MAC assembly. However, in the Abca4−/− mouse model of Stargardt disease, increased cholesterol prevents CD59 recycling in response to complement, and reroutes it to lysosomes. The resultant increase in MAC formation leads to increased intracellular calcium, mitochondrial fragmentation, and oxidative stress (Tan et al., 2016). At the basolateral surface, MAC is endocytosed into early endosomes and sorted towards lysosomal degradation. Inhibition of endocytosis or lysosomal function leads to mitochondrial damage in the RPE (Georgiannakis et al., 2015).
6.4. Late endosomes and lysosomes in the RPE
Late endosomes have unique cone-shaped lipids that drive the biogenesis of exosomes.
Lysosomes in the RPE are likely heterogeneous, with location determining the structure, content, and therefore function.
Maturation of early endosomes into multivesicular late endosomes begins with replacing RAB5 with RAB7, and culminates with the generation of intraluminal vesicles (ILVs) via inward budding of the endosomal limiting membrane (Huotari and Helenius, 2011). This inward budding is driven by a unique negatively-charged lipid called BMP that accumulates in late endosomal membranes. Like ceramide, BMP is a cone-shaped lipid that induces spontaneous negative curvature in membranes, resulting in endovesiculation and ILV formation (Matsuo et al., 2004). In lysosomal storage disorders such as Niemann Pick C1, cholesterol accumulation leads to a secondary accumulation of BMP, which could contribute to endolysosomal abnormalities. This cholesterol-induced BMP accumulation is a characteristic feature of Abca4−/− RPE, first reported by immunostaining (Toops et al., 2015) and later confirmed by imaging mass spectrometry (Anderson et al., 2017). In this model, as discussed in earlier sections, BMP activates ASMase, resulting in a secondary accumulation of ceramide in the late endosomes and lysosomes of the RPE.
6.4.1. Secretion of exosomes by the RPE
Fusion of multivesicular late endosomes with the plasma membrane results in the exocytosis of ILVs in the form of exosomes. Exosomes transfer proteins, lipids, and genetic material between cells, suggesting that they can participate in epithelial polarity, development, and tissue morphogenesis (Lakkaraju and Rodriguez-Boulan, 2008). These enigmatic vesicles are of intense research interest due to their potential roles in neurodegenerative diseases, cancer, and as disease biomarkers (Klionsky et al., 2016). Generally accepted criteria to distinguish exosomes from other vesicles include, mean diameters of 30–100 nm and the presence of late endosomal ILV proteins such as CD63, Flotillin-1, Alix, and Hsp70, since exosomes originate from late endosomes. The protein, lipid, and RNA (mRNA and microRNA) content, stimulus for secretion, and machinery involved in exosome biogenesis and release are all cell type-dependent (for an in-depth discussion of our current understanding of exosome biology see van Niel et al., 2018).
Analysis of exosomes released by polarized RPE cultures has been technically challenging due to the limited number of primary RPE cells available from human or porcine eyes, and the large volumes of media required for exosome harvest from the apical and basolateral compartments of cells cultured on semipermeable membrane filters. As a result, the ARPE-19 cell line has been mainly used to study the content and function of exosomes (reviewed in (Klingeborn et al., 2017a)). Because the ARPE-19 cells used were not equally differentiated, exosome purification and characterization methods are not comparable among these studies. However, a common theme that has emerged from these studies suggests that RPE-derived exosomes participate in inflammatory signaling and in immune modulation. Exosomes released by healthy RPE serve to decrease inflammation and downregulate the immune response, whereas exosomes released by stressed RPE promote inflammation and activate immune cells (Biasutto et al., 2013; Gangalum et al., 2016; Knickelbein et al., 2016; Shah et al., 2018). A handful of studies using polarized cultures of primary human (Sreekumar et al., 2010) or porcine RPE (Klingeborn et al., 2017a; Shah et al., 2018) support this relationship between the health of the RPE and the cargo packaged into exosomes. Much work still remains to be done to understand how polarized secretion of exosomes is regulated, mechanisms that control sorting of specific cargo into exosomes destined for apical and basal release, and their potential functions in vivo. It is, however, tempting to speculate that exosomes released by the RPE at the apical side could potentially communicate with the photoreceptors, Müller glia, and other cells in the neural retina; basally released exosomes could help maintain the Bruch’s membrane and the choroid by remodeling the extracellular matrix and modulating angiogenesis, respectively. During conditions of cellular stress, exosomes released by the RPE could then transmit these stress signals to photoreceptors, induce neovascularization, and promote drusen formation (Klingeborn et al., 2017a).
6.4.2. Location and function of lysosomes in the RPE
Late endosomes mature into lysosomes, which are the major degradative compartments in the cell. Lysosomes are dynamic organelles that receive cargo destined for clearance from the phagocytic, endocytic, secretory, and autophagic routes (Luzio et al., 2014). Lysosomes have over 50 hydrolases that are specific to a wide variety of targets, and RPE lysosomes are 7-fold more potent than liver lysosomes in degrading OS proteins (Zimmerman et al., 1983). EM data on cathepsin D recruitment to phagolysosomes after light onset and kinetic measurements of OS digestion (Bosch et al., 1993; Deguchi et al., 1994; Kennedy et al., 1994) suggest that lysosome hydrolase activities are likely coupled to diurnal OS phagocytosis. However, a temporal relationship between OS phagocytosis and enzyme activity per se has not been conclusively demonstrated. Lysosomal hydrolases are activated by the highly acidic luminal pH (4.5–5.0), which is maintained by the v-ATPase on lysosomal membranes (Mindell, 2012). Mouse models with disrupted RPE lysosome function such as those expressing a mutant form of cathepsin D (Rakoczy et al., 2002) (Section 3.7.4) or lacking the βA3/A1 crystallin (Sinha et al., 2016) show an accumulation of undegraded OS phagosomes and autophagosomes, accompanied by progressive retinal degeneration.
Lysosomal defects in the RPE have been documented in AMD donor tissue, mouse models of inherited retinal degenerations, and in a spectrum of lysosomal storage disorders. Lysosomes in cultured human RPE from AMD donors were found to be enlarged and annular; however, whether this abnormal lysosome morphology is associated with decreased hydrolytic function is not yet known (Golestaneh et al., 2017). A subtle shift in lysosomal pH and decreased transient receptor potential cation channel (TRPML) 1-mediated calcium release have been reported in the Abca4−/− mouse model of Stargardt disease (Gomez et al., 2018; Lu et al., 2018), but the underlying mechanisms are unclear. Circadian clearance of OS phagosomes by the RPE occurs normally in this model, suggesting that cathepsin D activity is not affected (Kaur et al., 2018). These seemingly contradictory findings could potentially be explained by lysosomal heterogeneity, i.e., all lysosomes are not created equal, and exhibit significant structural and functional differences. Studies in HeLa cells show that the position of lysosomes within the cell determines their luminal pH; peripheral lysosomes are less acidic than those in the perinuclear region. This is because peripheral lysosomes have less RAB7, which leads to decreased recruitment of the RAB7 effector RILP (RAB-interacting lysosomal protein). RILP, in turn, is responsible for recruiting specific subunits of the v-ATPase responsible for acidifying lysosomes (Johnson et al., 2016). Whether this relationship also holds in polarized post-mitotic cells like the RPE remains to be determined.
6.4.3. Membrane repair by lysosome exocytosis
Location of lysosomes within the cell is also important for membrane repair orchestrated by lysosome exocytosis. Fusion of lysosomes with the plasma membrane is a calcium-dependent process crucial for membrane repair, limiting pathogen entry, and clearing cellular debris (Idone et al., 2008). Calcium ionophores or pore-forming toxins cause a local increase in intracellular calcium, resulting in a conformational change in synaptotagmin 7, a lysosomal calcium sensor. This facilitates interactions between lysosomal v-SNAREs, such as VAMP7, with plasma membrane t-SNAREs, syntaxin 4 and SNAP-23. Lysosome exocytosis requires a population of peripheral lysosomes in close proximity to the plasma membrane that can be rapidly deployed for fusion in response to membrane damage. Long-range microtubule-mediated transport is required for peripheral localization of lysosomes and local depolymerization of the actin cytoskeleton is essential for the fusion of membrane proximal lysosomes with the cell membrane. In polarized RPE, calcium ionophores induce exocytosis at both the apical and basolateral domains (Xu et al., 2012), because syntaxin 4 is non-polar in the RPE (Low et al., 2002). This is in contrast to Madin-Darby canine kidney (MDCK) cells where basolateral localization of syntaxin 4 (Low et al., 1996) restricts lysosome exocytosis to the basolateral domain (Xu et al., 2012).
Live-cell TIRF imaging of polarized primary RPE cultures showed that complement attack stimulates peripheral lysosomes in the RPE to undergo rapid exocytosis, which helps eliminate cytotoxic C5b-9 terminal complement complex or MAC pores (Tan et al., 2016). Lysosome exocytosis also prevents intracellular calcium increase and protects RPE mitochondria from fragmentation. In RPE with defective microtubule transport such as in the Abca4−/− mouse, lysosomes are sequestered in the perinuclear region. The resulting decrease in membrane proximal lysosomes prevents membrane repair after complement attack, and makes the RPE susceptible to mitochondrial damage (Tan et al., 2016).
The studies discussed above add to the growing body of evidence that lysosomes perform multiple functions in the RPE – they help degrade phagocytosed and cellular material, act as signaling platforms, and maintain membrane integrity. Unsurprisingly, diseases that affect lysosomes often negatively impact the RPE. These include Danon disease, caused by mutations in LAMP2, and lysosomal storage disorders such as Niemann Pick Type C, caused by mutations in the cholesterol transporters Niemann Pick C1 or C2 (NPC1 or NPC2) (Phillips et al., 2008; Claudepierre et al., 2010; Thompson et al., 2016). Rodent and fruit fly models of NPC disease have extensive cholesterol accumulation in the RPE and retinal neurons, increased lipofuscin, and progressive photoreceptor degeneration (Phillips et al., 2008; Claudepierre et al., 2010). Although cardiomyopathy is the most common symptom of Danon disease, ~70% of patients have retinal abnormalities. These include chorioretinal atrophy and cone-rod dystrophy. Patients with Danon disease have increased fundus autofluorescence and abnormal full-field ERGs, indicative of decreased lysosome function and loss of RPE function (Thompson et al., 2016). Alternative splicing of LAMP2 results in three isoforms, LAMP2a, LAMP2b, and LAMP2c. Patients with mutant LAMP2b and LAMP2c have milder phenotypes, whereas those with LAMP2a present with severe disease (Thiadens et al., 2012). LAMP2a is critical for chaperone-mediated autophagy, because it binds cargo in the cytosol to be transported into the lysosome lumen (Kaushik and Cuervo, 2018). It will be interesting to explore how defective chaperone-mediated autophagy can lead to RPE atrophy.
6.5. Peroxisome biogenesis and implications for RPE health
Peroxisome number, but not necessarily function, is elevated during ageing.
The PEX11β-null mouse exhibits accumulation of sub-RPE deposits.
Peroxisomes are numerous within the RPE and often found adjacent to mitochondria, which is not surprising given the essential role of peroxisomes in β-oxidation of very long chain fatty acids (VLCFA) and the lipid catabolic demands placed on the RPE by a daily influx of VLCFA via phagocytic uptake and degradation of OS disk membranes. Peroxisomes are dynamic organelles, whose number and levels of function are regulated to maintain metabolic homeostasis in the face of a changing cellular milieu (e.g. shifts in nutritional supply). Surprisingly, despite diurnal peaks in phagocytosis and therefore peaks in substrate availability for β-oxidation, peroxisome numbers are stable throughout the day in mouse RPE. However, activity of the peroxisome antioxidant catalase is elevated during the early morning hours when numbers of ingested phagosomes are highest, suggesting enzyme induction (Daniele et al., 2019).
At any given time, the number of peroxisomes within a cell is a function of the rate of synthesis of new peroxisomal material, balanced by the rate of degradative removal. Peroxisomes are degraded by regulated autophagy (Deosaran et al., 2013; Nordgren et al., 2013) and to a lesser extent, peroxisomal Lon peptidase 2 (LONP2)-mediated proteolysis, and membrane disruption that is mediated by lipoxygenase 15 (Yokota et al., 2001; Yokota and Fahimi, 2009; Walker et al., 2018). Peroxisome biogenesis occurs by two distinct mechanisms, de novo synthesis (Ma et al., 2011; Agrawal and Subramani, 2016) or, self-replication through growth and fission (Schrader et al., 2012).
In de novo synthesis, mature peroxisomes are built incrementally, with the first step a budding of pre-peroxisomal vesicles bearing complementary proteins of the early import machinery, PEX16 and PEX3, from membranes of the endoplasmic reticulum and mitochondria, respectively (Schrader and Pellegrini, 2017; Sugiura et al., 2017). Heterotypic fusion of PEX3- and PEX16-bearing vesicles establish a population of pre-peroxisomes competent to import all other peroxisomal membrane proteins, including the critical luminal import machinery (PEX13, PEX14, etc.), that translocate metabolic and antioxidant enzymes into peroxisomes (Emmanouilidis et al., 2016; Platta et al., 2016). The import machinery is critical to peroxisomal function as all peroxisomal proteins are translated and folded in the cytoplasm.
Propagation of existing peroxisomes can also proceed through self-replication, initiated with PEX11β-dependent elongation of peroxisomal membranes, and followed by fission mediated by PEX11β activation of the GTPase dynamin-like protein 1, DLP-1 (Koch et al., 2003). There are three PEX11 isoforms, α, β, and γ, with β having ubiquitous, and α and γ having tissue-specific expression. PEX11β was shown to mediate peroxisome proliferation in the absence of external stimuli (Schrader et al., 1998). It facilitates peroxisome fission by promoting membrane deformation that depends on its self-dimerization (Bonekamp et al., 2013). DHA was recently shown to influence the degree of peroxisomal self-replication, by promoting dimerization of PEX11β in cells (Itoyama et al., 2011). DLP-1 has a shared function in peroxisomal and mitochondrial fission, and is recruited to peroxisomes by two mitochondrial-associated fission factors, mitochondrial fission factor (MFF) (Gandre-Babbe and van der Bliek, 2008), and mitochondrial fission 1 (FIS1), following PEX11β-mediated elongation (Koch et al., 2005; Itoyama et al., 2013).
6.5.1. Peroxisome proliferation
Peroxisome proliferation can occur in response to cellular stress. Oxidants and ultraviolet light stimulate the elongation of peroxisomes in cultured hepatic cells (Schrader et al., 1999). Peroxisome proliferation is induced through stimulation of peroxisome proliferator-activated receptor (PPAR) α activity in response to oxidative stress following ischemic injury in neurons (Young et al., 2015). Peroxisome proliferation has also been observed in aged cells, including late-passage cultured fibroblasts, and aged human RPE, where peroxisome number is increased, but antioxidant and enzyme activity is reduced (Beier et al., 1993). It was shown that import efficiency is reduced in aged cells, and since catalase has a weak peroxisomal import signal, this may disproportionately affect import of catalase relative to reactive oxygen species-producing enzymes, resulting in elevated oxidative stress (Terlecky et al., 2006). A recent proteomic study of C. elegans also showed an increase in peroxisome number, and reduced import efficiency in aged versus young worms (Narayan et al., 2016), which was explained by decreased expression of key members of the peroxisomal import machinery (e.g. PEX5). Finally, peroxisome numbers were shown to be elevated in AMD donor RPE, along with signs of disintegrated mitochondria, relative to age-matched unaffected donors (Feher et al., 2006; Bianchi et al., 2013), and it was postulated that this represented compensation, albeit incomplete, of peroxisomal β-oxidation for a primary dysfunction of mitochondria (Bianchi et al., 2013). However, the specific mechanisms responsible for elevated peroxisomes in ageing and in AMD have not been elucidated in the RPE. The possibility of impaired degradation rather than proliferation is suggested by a recent finding of elevated peroxisome numbers in mice lacking expression of LC3B (Daniele et al., 2019). Given the known role of LC3B in macroautophagic degradation of organelles, as well as decline in autophagy, impaired peroxisome turnover during aging could result in the elevated peroxisome numbers that have been observed.
6.5.2. Peroxisome proliferator-activated receptors and AMD
PPARs are nuclear hormone receptors that respond to binding of endogenous fatty acids and fatty acid derivatives such as eicosanoids, by regulating expression of genes within diverse cellular pathways including lipid metabolism and inflammation (Kliewer et al., 1997; Chinetti et al., 2000). Recently, there has been interest in the role of PPARs in the modulation of AMD disease progression (Dwyer et al., 2011; Malek and Lad, 2014). However, little is known of the role of PPARs in the regulation of RPE peroxisome homeostasis or proliferation. Expression of PPARα and β/δ was relatively high in freshly isolated human RPE; however, PPARγ was not detected. This was in contrast to ARPE-19 cells that showed moderate expression of all isoforms (Dwyer et al., 2011). Recently PPARβ/δ was implicated in modification of AMD disease severity, but distinct effects of PPARβ/δ modulation depended on cell type (Choudhary et al., 2016). PPAR activity in RPE cells in this study was found to modulate expression of extracellular matrix-related genes and genes involved in lipid metabolism such as apolipoprotein E (APOE), apolipoprotein A (APOA), and low-density lipoprotein receptor (LDLR). Some features of dry AMD were recapitulated in Pex11β−/− mice, such as increased accumulation of sub-RPE deposits and accumulation of lipofuscin (Choudhary et al., 2016). However, we are otherwise unaware of studies demonstrating elevated peroxisome numbers or function as a result of PPAR regulation in RPE cells.
6.6. Conclusions
Organelles in the RPE perform multiple defined functions that are essential for cellular homeostasis, and for healthy vision. These compartments form intricate networks that rely on their location within the cell, contacts between organelles, and functional cooperativity. Organelle networks in the RPE are exceedingly complex given its multiple functions as a professional phagocyte, a secretory epithelium, an antigen-presenting cell, and an active participant in the visual cycle. A further level of complexity is conferred by the polarized phenotype of the RPE, where it communicates with photoreceptors at the apical surface and with the choroid at the basal side. The advent of powerful imaging techniques (light sheet microscopy, super-resolution imaging, etc.) coupled with the use of fully differentiated, polarized RPE in corroboration with appropriate animal models will make it possible to deepen our insight into the RPE organelle “interactome” (Valm et al., 2017), to understand how they form, function, and communicate with one another, and how these relationships change with age and disease.
7. RPE metabolism
The RPE is the gatekeeper of the outer retina, and regulates the flux of nutrients and oxygen between the choroid and the subretinal space. Glucose, the major energy substrate of photoreceptor and Müller glial cells, is transported from the choroid to the subretinal space by the glucose transporter, GLUT1, which is expressed in the basolateral and apical membranes of the RPE (Swarup et al., 2019) (Fig. 15). The photoreceptors metabolize glucose through aerobic glycolysis, generating large amounts of lactate. In contrast, the RPE is oxidative and uses lactate produced by the photoreceptors and fatty acids from ingested OSs to fuel mitochondrial metabolism (Adijanto et al., 2014; Adijanto and Philp, 2014; Kanow et al., 2017; Reyes-Reveles et al., 2017). Critical aspects of the metabolic symbiosis between the RPE and neural retina are the focus of this section.
Fig. 15.
The RPE oxidizes lactate and fatty acids sparing glucose for the outer retina. The glucose transporter, GLUT1 (green), in the basolateral and apical membranes of the RPE transports glucose from the choroid to the subretinal space. In the outer neural retina, glucose is metabolized through aerobic glycolysis producing large amounts of lactate. The H+-coupled lactate transporter, MCT1, is polarized to the apical membrane of the RPE and facilitates the uptake of lactate from the subretinal space. Lactate is converted to pyruvate, which is transported into the mitochondria and oxidized. Excess lactate is transported out of the RPE across the basolateral membrane by MCT3. In addition to lactate, the RPE utilizes fatty acids from ingested outer segments to fuel fatty acid oxidation and ketogenesis, generating β-hydroxybutyrate (β-HB) that is transported to the subretinal space. β-HB is taken up by photoreceptors and used to support oxidative metabolism. CPT1a, carnitine palmitoyltransferase 1a.
7.1. RPE cell models to study metabolism
Cell culture and animal models have both been used to study RPE metabolism. While a number of aspects of RPE metabolism can be studied in culture, it is critical to assess the state of cell differentiation because there is a direct link between differentiation and metabolism (Adijanto and Philp, 2014). For example, undifferentiated RPE are more glycolytic than differentiated RPE, which is reflected in their transcriptomes and proteomes. Moreover, in vivo, the RPE is sandwiched between the choroid and photoreceptors; this arrangement can impact its metabolism, and has not yet been recapitulated in vitro. Culture media are usually high in glucose, but not lactate or fatty acids, which likely impacts the metabolome, transcriptome, proteome, and epigenome of cultured RPE.
Differentiated RPE express a unique set of solute transporters in their apical and basal membranes (Strunnikova et al., 2010; Adijanto and Philp, 2014). The expression and distribution of solute transporters have been investigated through histological studies, using polarized RPE cells cultured on Transwell filters, and transcriptomic studies, which have additionally identified RPE-specific solute transporters (Hellinen et al., 2019; Liu et al., 2019a). Metabolomic assays have been used to identify substrate utilization by the RPE in vitro and in vivo (Adijanto and Philp, 2014; Kanow et al., 2017; Yam et al., 2019).
While in vitro studies can provide some information about the metabolism of the RPE (Adijanto et al., 2014; Chao et al., 2017; Reyes-Reveles et al., 2017; Yam et al., 2019), it is important to keep in mind that the in vitro and the in vivo environments are very different. In vivo, the neural retina represents a large sink for oxygen and glucose, which influences the metabolism of the RPE.
A number of transgenic models have been used to study RPE metabolism, and the metabolic interaction between the choroid and the photoreceptors (Zhao et al., 2011; Kurihara et al., 2012, 2016; Swarup et al., 2019; Yam et al., 2019). These models have been made possible with the generation of the Best1-Cre transgenic line, allowing for RPE-specific knockout of regulators of RPE metabolism (Iacovelli et al., 2011).
7.2. Metabolism in the RPE and its relationship to the photoreceptors
The RPE relies on oxidative metabolism rather than glycolysis to support its metabolic needs.
RPE metabolism is linked to the metabolism of the outer retina through oxidation of fatty acids, from daily phagocytosis of OS tips, and lactate, produced through aerobic glycolysis in the outer retina.
Glycolysis is low in the RPE so that glucose is spared for the outer retina.
To facilitate the metabolic homeostasis in the outer retina, the RPE has made several adaptations that distinguish it from other epithelia. The polarized distribution of key solute transporters differs between the RPE, and the epithelia lining the gut and proximal convoluted tubule (Castorino et al., 2011). GLUT1 is localized to the basolateral membrane in most epithelia, but, in the RPE, it is found in both basolateral and apical membranes to facilitate trans-epithelial transport of glucose from the choroidal capillaries to the photoreceptor and Müller cells (Swarup et al., 2019) (Fig. 15). Since the outer retina produces large amounts of lactate through aerobic glycolysis, the RPE expresses two H+-coupled monocarboxylate transporters (MCT) whose coordinated activities facilitate lactate transport out of the subretinal space to the choroid (Fig. 15). MCT1, a high-affinity lactate transporter encoded by SLC16A1, is localized to the basolateral membrane in the epithelial lining of the gut and kidney. In contrast, in the RPE, MCT1 is localized to the apical membrane of the RPE to facilitate lactate transport out of the subretinal space to maintain the pH and osmolarity within the microenvironment of photoreceptor cells. Excess lactate is transported out of the RPE, across the basolateral membrane by MCT3, encoded by SLC16A8. Interestingly, SLC16A8 is only expressed in the RPE and choroid, with the highest expression in the RPE. A genome-wide association study (GWAS) showed that variants of SLC16A8 are risk factors for AMD (Fritsche et al., 2013, 2016). Further studies are needed to determine how SLC16A8 variants increase the risk of AMD.
The proline transporter SLC6A20 is also highly expressed in the RPE relative to other tissues, and its expression increases with maturation of the retina (Liu et al., 2019a; Yam et al., 2019). SLC6A20 variants have been found to be associated with macular thickening, suggesting it is important for the retina. It was recently shown using human fetal RPE that intermediates of proline metabolism are preferentially released across the apical membrane presumably to support photoreceptor cell metabolism (Yam et al., 2019). Interestingly, increases in dietary proline were found to protect visual function after sodium iodate-induced damage (Yam et al., 2019). Further in vivo studies using transgenic mice will need to be done in order to determine the importance of proline metabolism in the RPE.
The RPE has adapted its metabolism to be able to efficiently use substrates from ingested OSs. Like the liver, it actively engages in fatty acid oxidation (FAO) and ketogenesis. OSs are ~50% protein and 50% lipid by weight (Fliesler and Anderson, 1983). OS lipids, which are composed of 50% saturated fatty acids, 8–10% monounsaturated fatty acids, 30% polyunsaturated fatty acids, and 2% VLCFA (26C–34C in length, found predominately in phosphatidylcholine) (Fliesler and Anderson, 1983; Aveldano, 1987; Aveldano and Sprecher, 1987), provide a highly reduced form of fuel that, when oxidized, generates more energy than glucose, lactate, or amino acids. When human fetal RPE cells, cultured on Transwell filters, were incubated with 13C-palmitate, 13C-labeled TCA cycle intermediates, such as citrate and glutamate, were detected by gas chromatography mass spectrometry (GC-MS). Somewhat surprisingly, 13C-β-hydroxybutyrate (β-HB) was also generated, demonstrating that FAO was coupled to ketogenesis (Adijanto et al., 2014) (Figs. 14C and 15). A quantitative assessment of 13C-palmitate oxidation by human fetal RPE suggested that 50% of palmitate-derived acetyl-CoA was diverted towards ketogenesis to produce β-HB which was released apically, whereas the remaining acetyl-CoA entered the TCA cycle (Adijanto et al., 2014). Diverting acetyl-CoA to the ketogenic pathway ensures that there is no buildup of intermediates, which would inhibit carnitine palmitoyltransferase 1a (CPT1a) and result in lipid accumulation (Adijanto et al., 2014; Reyes-Reveles et al., 2017). β-HB is preferentially transported across the apical membrane by MCT1 (Adijanto et al., 2014; Reyes-Reveles et al., 2017).
The β-HB transporter MCT7, encoded by SLC16A6, is expressed by rod and cone photoreceptor cells (Fig. 15), and its expression is correlated with photoreceptor cell differentiation. Additionally, when the pyruvate transporter, mitochondrial pyruvate carrier (MPC) 1, was knocked out of the neural retina to inhibit pyruvate oxidation, β-HB was able to maintain ATP and nicotinamide adenine dinucleotide (NAD/NADH) levels for a short period of time (Grenell et al., 2019). Taken together, the expression of SLC16A6 and the metabolomics data suggest that rod and cone photoreceptors utilized β-HB as a metabolic substrate.
Since RPE generation of β-HB is linked to the diurnal schedule of OS shedding and degradation, it follows that the expression of key enzymes involved in FAO may be regulated as well. Consistent with this hypothesis, analysis of RNA-Seq data (Mustafi et al., 2013) also revealed that Hmgcs2 transcripts were 30% higher in mouse RPE isolated 1.5 h after light onset than that isolated 9 h after light onset. Additionally, PPAR-coactivator 1 (PGC-1, encoded by PPARGC1A), the master regulator of mitochondrial biogenesis and oxidative metabolism, is expressed at 50% higher levels in mouse RPE isolated 1.5 versus 9 h after light onset. In Lc3b−/− and Mreg−/− mice, that display delayed phagosome maturation, there was also a delay in β-HB production, thus coupling FAO and ketogenesis to phagosome maturation processes (Reyes-Reveles et al., 2017; Dhingra et al., 2018). In these models of delayed or defective phagocytosis, β-HB levels peaked later in the day, at 5–6 h after light onset for Abca4−/−, Mreg−/−, and Lc3b−/− mouse RPE (Dhingra et al., 2018). Moreover, in contrast to wild type (C57BL/6J) mouse RPE, in which HMGCS2 protein levels peak 2 h after light onset, in Lc3b−/−and Mreg−/− mouse RPE, HMGCS2 levels remain unchanged. Since β-HB can act as a signaling molecule as well as a metabolic substrate, the apical release of β-HB may be important for metabolically aligning photoreceptor cells with the RPE.
It is likely that cross talk between metabolic pathways regulates substrate utilization in the RPE. For example, oxidation of lactate and OSs generates intermediates that inhibit glycolysis, which may contribute to the ability of the RPE to spare glucose for the retina (Kanow et al., 2017; Wang et al., 2019). Both lactate and FAO can inhibit glucose utilization through regulation of NAD levels and the inhibition of phosphofructokinase (PFK) 1. This sequence of events triggered by FAO results in increases in mitochondrial acetyl-CoA and NADH both of which inhibit pyruvate dehydrogenase; these changes lead to increases in cytosolic citrate (Jenkins et al., 2011). Cytosolic citrate inhibits PFK1 leading to the accumulation of glucose-6-phosphate which leads to feedback inhibition of hexokinase (Jenkins et al., 2011). The net result of these regulatory processes is the sparing of glucose for the outer retina. As cytosolic citrate concentrations increase, this compound serves as an allosteric activator of acetyl-CoA carboxylase, thereby stimulating fatty acid synthesis. It is tempting to speculate that the 24-hr light/dark cycle of OS phagocytosis provides metabolic plasticity in the RPE/retina to maintain physiological function and provides the neural retina with the required energy and metabolic intermediates for OS renewal.
Support for the idea that the RPE is not reliant on glucose to sustain its metabolic needs comes from recent studies on a mouse model with an RPE-specific deletion of GLUT1 (Swarup et al., 2019). This deletion did not impact differentiation or functional properties of the RPE, demonstrating that glycolysis is not required to support the metabolic needs of the RPE (Swarup et al., 2019) nor, as predicted from early in vitro studies, is it required for late stage differentiation (Adijanto et al., 2014). In fact, a shift from oxidative to glycolytic metabolism leads to dedifferentiation of the RPE and a loss of expression of proteins required for RPE-specific functions. This was shown by genetically deleting Tfam (mitochondrial transcription factor A), which controls mitochondrial biogenesis, from the mouse RPE. The decrease in mitochondrial biogenesis and oxidative phosphorylation led to hypertrophy, followed by atrophy of the RPE. This was accompanied by loss of expression of RPE-specific genes, followed by loss of photoreceptor cell function and viability (Zhao et al., 2011).
There have been a number of studies in the literature which show that mitochondrial dysfunction contributes to AMD (Fisher and Ferrington, 2018; Kaarniranta et al., 2018). Since mitochondria have roles in energy production, calcium regulation, and generation of metabolites that regulate gene expression, it is easy to understand how mitochondrial dysfunction could contribute to pathological changes in the retina.
8. Future directions and open questions
In this review, we have focused on key areas of RPE cell biology and metabolism. We have endeavored to highlight seminal research over several decades, along with conceptual and technical advances made in recent years. These studies have significantly enhanced our insight into RPE biology, and how RPE dysfunction drives retinal pathology. However, several important questions remain open. Below are a few of these questions as they relate to the topics covered in this review.
8.1. Phagocytosis of OS disk membranes
What is the molecular interplay between OS and RPE in determining the excision of the OS tip and the size of an ingested phagosome?
Although key molecules in the binding and ingestion of OS membranes have been identified, imaging live RPE cells during these processes is needed to understand how the molecules are dynamically orchestrated.
Evidence points to OS phagosome degradation as a multistep process, involving different hydrolases and the coordination of different molecular motors, but the details of the cellular events and how the dynamics of the overall process is regulated remain as future challenges for discovery.
8.2. Intersection of phagocytosis with autophagy - LC3-associated phagocytosis
What distinguishes LC3-associated OS phagosomes from those without LC3?
Is the critical balance between LAP and classical autophagy altered during inflammation, oxidative stress, or disease?
8.3. Classical autophagy
How are autophagy and lysosome functions scaled to meet the requirements of diurnal OS phagocytosis and clearing endogenous RPE debris?
Which specific steps of autophagy (initiation, biogenesis, flux) are impaired in retinal diseases?
Can approaches to upregulate autophagy help preserve RPE homeostasis?
8.4. RPE organelles
Can imaging the localization and motility of RPE organelles, especially melanosomes, be optimized to provide valuable clinical information, and perhaps as an outcome measure in clinical trials of some forms of retinal degeneration?
Are organelle biogenesis and activity regulated by phagocytosis-, circadian-, or light-stimulated pathways?
Do exosomes released by the RPE function as biomarkers of RPE and photoreceptor health and disease?
How does the organelle interactome change during development, aging, and disease?
8.5. Metabolism in the RPE
What is the expression and distribution of solute transporters in the RPE?
What is the relationship between circadian rhythm, and the metabolic capacity of RPE and photoreceptors?
How does systemic metabolism contribute to RPE lipid balance?
The metabolism of the RPE has been adapted to accommodate ingestion of large amounts of lipid and proteins. Because of the complex interaction among the RPE, choroid, and photoreceptors, RPE metabolic studies need to be performed in vivo. Development of animal models should be a priority; such models will allow us to elucidate how genetic and environmental factors impact RPE/photoreceptor cell function.
How does daily oxidation of lipids and lactate direct and maintain RPE differentiation and photoreceptor cell health?
Acknowledgments
We thank Barry Burgess and Roni Hazim (Williams lab) for providing Figs. 4 and 5, respectively, and Dean Bok for comments on the manuscript. Work from the authors’ laboratories that is presented or discussed in this paper was supported by NIH NEI grants R01 EY023299 (AL), R01 EY026525 (NJP, KBB), R01 EY012042 to (NJP) R21 EY029826 (KBB), R01EY027442 and R01EY013408 (DSW) and a Foundation Fighting Blindness grant (DSW).
Abbreviations
- 1D4
clone number of the monoclonal antibody against the C-terminal nine amino acid epitope of bovine rod opsin
- 4D2
clone number of the monoclonal antibody against an N-terminal fragment of bovine rod opsin
- AMD
age-related macular degeneration
- ARPE-19
adult human RPE cell line
- D407
adult human RPE cell line
- EM
electron microscope
- ERG
electroretinogram
- ESC
embryonic stem cell
- FAO
fatty acid oxidation
- GWAS
genome-wide association studies
- hTERT-RPE1
human telomerase (hTERT)-immortalized retinal pigment epithelial cell line 1
- ILV
intraluminal vesicle
- iPSC
induced pluripotent stem cell
- LAP
LC3-associated phagocytosis
- LIR
LC3-interacting region
- MCT
monocarboxylate transporter
- NPC
Niemann-Pick Type C disease
- OCT
optical coherence tomography
- OS
outer segment
- pSIVA
polarity-sensitive indicator of viability and apoptosis
- PPAR
peroxisome proliferator-activated receptor
- RCS
Royal College of Surgeons
- RGC
retinal ganglion cell
- RNA-Seq
RNA sequencing
- RPE
retinal pigment epithelium
- RPE-J
rat RPE cell line
- SLO
Smith-Lemli-Opitz syndrome
- TIRF
total internal reflectance fluorescence
- TER
trans-epithelial electrical resistance
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ablonczy Z, Crosson CE, 2007. VEGF modulation of retinal pigment epithelium resistance. Exp. Eye Res. 85, 762–771. 10.1016/j.exer.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adijanto J, Philp NJ, 2014. Cultured primary human fetal retinal pigment epithelium (hfRPE) as a model for evaluating RPE metabolism. Exp. Eye Res. 126, 77–84. 10.1016/j.exer.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adijanto J, Du J, Moffat C, Seifert EL, Hurle JB, Philp NJ, 2014. The retinal pigment epithelium utilizes fatty acids for ketogenesis. J. Biol. Chem. 289, 20570–20582. 10.1074/jbc.M114.565457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal G, Subramani S, 2016. De novo peroxisome biogenesis: evolving concepts and conundrums. Bba-Mol Cell Res 1863, 892–901. 10.1016/j.bbamcr.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmado A, Carr AJ, Vugler AA, Semo M, Gias C, Lawrence JM, Chen LL, Chen FK, Turowski P, da Cruz L, Coffey PJ, 2011. Induction of differentiation by pyruvate and DMEM in the human retinal pigment epithelium cell line ARPE-19. Invest. Ophthalmol. Vis. Sci. 52, 7148–7159. 10.1167/iovs.10-6374. [DOI] [PubMed] [Google Scholar]
- Ahuja S, Ahuja-Jensen P, Johnson LE, Caffé AR, Abrahamson M, Ekström PA, van Veen T, 2008. rd1 Mouse retina shows an imbalance in the activity of cysteine protease cathepsins and their endogenous inhibitor cystatin C. Invest. Ophthalmol. Vis. Sci. 49, 1089–1096. 10.1167/iovs.07-0549. [DOI] [PubMed] [Google Scholar]
- Alizadeh P, Smit-McBride Z, Oltjen SL, Hjelmeland LM, 2006. Regulation of cysteine cathepsin expression by oxidative stress in the retinal pigment epithelium/choroid of the mouse. Exp. Eye Res. 83, 679–687. 10.1016/j.exer.2006.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DMG, Ablonczy Z, Koutalos Y, Hanneken AM, Spraggins JM, Calcutt MW, Crouch RK, Caprioli RM, Schey KL, 2017. Bis(monoacylglycero)phosphate lipids in the retinal pigment epithelium implicate lysosomal/endosomal dysfunction in a model of Stargardt disease and human retinas. Sci. Rep. 7, 17352. 10.1038/s41598-017-17402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott HJ, Maciolek NJ, Nicol JA, 1970. Retinal tapetum lucidum: a novel reflecting system in the eye of teleosts. Science 169, 478–480. 10.1126/science.169.3944.478. [DOI] [PubMed] [Google Scholar]
- Aveldano MI, 1987. A novel group of very long chain polyenoic fatty acids in dipolyunsaturated phosphatidylcholines from vertebrate retina. J. Biol. Chem. 262, 1172–1179. [PubMed] [Google Scholar]
- Aveldano MI, Sprecher H, 1987. Very long chain (C24 to C36) polyenoic fatty acids of the n-3 and n-6 series in dipolyunsaturated phosphatidylcholines from bovine retina. J. Biol. Chem. 262, 1180–1186. [PubMed] [Google Scholar]
- Azarian SM, McLeod I, Lillo C, Gibbs D, Yates JR, Williams DS, 2006. Proteomic analysis of mature melanosomes from the retinal pigmented epithelium. J. Proteome Res. 5, 521–529. 10.1021/pr0502323. [DOI] [PubMed] [Google Scholar]
- Back I, Donner KO, Reuter T, 1965. The screening effect of the pigment epithelium on the retinal rods in the frog. Vis. Res. 5, 101–111. [DOI] [PubMed] [Google Scholar]
- Barsoum IB, King-Smith C, 2007. Myosin II and Rho kinase activity are required for melanosome aggregation in fish retinal pigment epithelial cells. Cell Motil Cytoskeleton 64, 868–879. 10.1002/cm.20231. [DOI] [PubMed] [Google Scholar]
- Basinger S, Hoffman R, Matthes M, 1976. Photoreceptor shedding is initiated by light in the frog retina. Science 194, 1074–1076. 10.1126/science.1086510. [DOI] [PubMed] [Google Scholar]
- Bazan NG, 2018. Docosanoids and elovanoids from omega-3 fatty acids are prohomeostatic modulators of inflammatory responses, cell damage and neuroprotection. Mol. Aspect. Med. 64, 18–33. 10.1016/j.mam.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchemin M, Leuenberger P, 1977. Effects of colchicine on phagosome-lysosome interaction in retinal pigment epithelium. Albr. Graefes Arch. Klin. Exp. Ophthalmol. 203, 237–251. 10.1007/bf00409830. [DOI] [PubMed] [Google Scholar]
- Beier K, Volkl A, Fahimi HD, 1993. The impact of aging on enzyme proteins of rat liver peroxisomes: quantitative analysis by immunoblotting and immunoelectron microscopy. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 63, 139–146. [DOI] [PubMed] [Google Scholar]
- Benson K, Suter RB, 2013. Reflections on the tapetum lucidum and eyeshine in lycosoid spiders. J. Arachnol. 41, 43–52. [Google Scholar]
- Besharse JC, Dunis DA, 1983. Methoxyindoles and photoreceptor metabolism: activation of rod shedding. Science 219, 1341–1343. 10.1126/science.6828862. [DOI] [PubMed] [Google Scholar]
- Besharse JC, Hollyfield JG, Rayborn ME, 1977. Turnover of rod photoreceptor outer segments. II. Membrane addition and loss in relationship to light. J. Cell. Biol. 75, 507–527. 10.1083/jcb.75.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi E, Scarinci F, Ripandelli G, Feher J, Pacella E, Magliulo G, Gabrieli CB, Plateroti R, Plateroti P, Mignini F, Artico M, 2013. Retinal pigment epithelium, age-related macular degeneration and neurotrophic keratouveitis. Int. J. Mol. Med. 31, 232–242. 10.3892/ijmm.2012.1164. [DOI] [PubMed] [Google Scholar]
- Biasutto L, Chiechi A, Couch R, Liotta LA, Espina V, 2013. Retinal pigment epithelium (RPE) exosomes contain signaling phosphoproteins affected by oxidative stress. Exp. Cell Res. 319, 2113–2123. 10.1016/j.yexcr.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenkinsop TA, Salero E, Stern JH, Temple S, 2013. The culture and maintenance of functional retinal pigment epithelial monolayers from adult human eye. Methods Mol. Biol. 945, 45–65. 10.1007/978-1-62703-125-7_4. [DOI] [PubMed] [Google Scholar]
- Blest AD, 1978. Rapid synthesis and destruction of photoreceptor membrane by a dinopid spider - daily cycle. In: Proceedings of the Royal Society Series B-Biological Sciences 200. pp. 463. 10.1098/rspb.1978.0027. [DOI] [Google Scholar]
- Blest AD, Maples J, 1979. Exocytotic shedding and glial uptake of photoreceptor membrane by a salticid spider. Proc. R. Soc. Lond. B Biol. Sci. 204, 105–112. 10.1098/rspb.1979.0016. [DOI] [PubMed] [Google Scholar]
- Blest AD, Stowe S, Eddey W, Williams DS, 1982. The local deletion of a microvillar cytoskeleton from photoreceptors of tipulid flies during membrane turnover. Proc. R. Soc. Lond. B Biol. Sci. 215, 469–479. [DOI] [PubMed] [Google Scholar]
- Bok D, Hall MO, 1971. The role of the pigment epithelium in the etiology of inherited retinal dystrophy in the rat. J. Cell Biol. 49, 664–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonekamp NA, Grille S, Cardoso MJ, Almeida M, Aroso M, Gomes S, Magalhaes AC, Ribeiro D, Islinger M, Schrader M, 2013. Self-interaction of human Pex11p beta during peroxisomal growth and division. PloS One 8. 10.1371/journal.pone.0053424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch E, Horwitz J, Bok D, 1993. Phagocytosis of outer segments by retinal pigment epithelium: phagosome-lysosome interaction. J. Histochem. Cytochem. 41, 253–263. 10.1177/41.2.8419462. [DOI] [PubMed] [Google Scholar]
- Bourne MC, Campbell DA, Tansley K, 1938. Hereditary degeneration of the rat retina. Br. J. Ophthalmol. 22, 613–623. 10.1136/bjo.22.10.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boya P, Esteban-Martinez L, Serrano-Puebla A, Gomez-Sintes R, Villarejo-Zori B, 2016. Autophagy in the eye: development, degeneration, and aging. Prog. Retin. Eye Res. 55, 206–245. 10.1016/j.preteyeres.2016.08.001. [DOI] [PubMed] [Google Scholar]
- Breucker H, Schafer E, Holstein AF, 1985. Morphogenesis and fate of the residual body in human spermiogenesis. Cell Tissue Res. 240, 303–309. 10.1007/bf00222339. [DOI] [PubMed] [Google Scholar]
- Buchholz DE, Hikita ST, Rowland TJ, Friedrich AM, Hinman CR, Johnson LV, Clegg DO, 2009. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cell. 27, 2427–2434 10.1002/. [DOI] [PubMed] [Google Scholar]
- Buchholz DE, Pennington BO, Croze RH, Hinman CR, Coffey PJ, Clegg DO, 2013. Rapid and efficient directed differentiation of human pluripotent stem cells into retinal pigmented epithelium. Stem Cells Transl Med 2, 384–393. 10.5966/sctm.2012-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulloj A, Duan W, Finnemann SC, 2013. PI 3-kinase independent role for AKT in F-actin regulation during outer segment phagocytosis by RPE cells. Exp. Eye Res. 113, 9–18. 10.1016/j.exer.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne T, O’Connor MN, Seabra MC, Cutler DF, Futter CE, 2015. Regulation of melanosome number, shape and movement in the zebrafish retinal pigment epithelium by OA1 and PMEL. J. Cell Sci. 128, 1400–1407. 10.1242/jcs.164400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JM, Kaczara P, Skumatz CM, Zareba M, Raciti MW, Sarna T, 2011. Dynamic analyses reveal cytoprotection by RPE melanosomes against non-photic stress. Mol. Vis. 17, 2864–2877. [PMC free article] [PubMed] [Google Scholar]
- Burnside B, 2001. Light and circadian regulation of retinomotor movement. Prog. Brain Res. 131, 477–485. [DOI] [PubMed] [Google Scholar]
- Burstyn-Cohen T, Lew ED, Través PG, Burrola PG, Hash JC, Lemke G, 2012. Genetic dissection of TAM receptor-ligand interaction in retinal pigment epithelial cell phagocytosis. Neuron 76, 1123–1132. 10.1016/j.neuron.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres PS, Rodriguez-Boulan E, 2019. Retinal pigment epithelium polarity in health and blinding diseases. Curr. Opin. Cell Biol. 62, 37–45. 10.1016/j.ceb.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castorino JJ, Deborde S, Deora A, Schreiner R, Gallagher-Colombo SM, Rodriguez-Boulan E, Philp NJ, 2011. Basolateral sorting signals regulating tissue-specific polarity of heteromeric monocarboxylate transporters in epithelia. Traffic 12, 483–498. 10.1111/j.1600-0854.2010.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaitin M, Hall MO, 1983a. Defective ingestion of rod outer segments by cultured dystrophic rat pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 24, 812–820. [PubMed] [Google Scholar]
- Chaitin MH, Hall M, 1983b. The distribution of actin in cultured normal and dystrophic rat pigment epithelial cells during the phagocytosis of rod outer segments. Invest. Ophthalmol. Vis. Sci. 24, 821–831. [PubMed] [Google Scholar]
- Chang Y, Finnemann SC, 2007. Tetraspanin CD81 is required for the αvβ5-integrin-dependent particle-binding step of RPE phagocytosis. J. Cell Sci. 120, 3053–3063. 10.1242/jcs.006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao JR, Knight K, Engel AL, Jankowski C, Wang Y, Manson MA, Gu H, Djukovic D, Raftery D, Hurley JB, 2017. Human retinal pigment epithelial cells prefer proline as a nutrient and transport metabolic intermediates to the retinal side. J. Biol. Chem. 292, 12895–12905. 10.1074/jbc.M117.78842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Lam HC, Jin Y, Kim HP, Cao J, Lee SJ, Ifedigbo E, Parameswaran H, Ryter SW, Choi AM, 2010. Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. Proc. Natl. Acad. Sci. U. S. A 107, 18880–18885. 10.1073/pnas.1005574107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Sawada O, Kohno H, Le YZ, Subauste C, Maeda T, Maeda A, 2013. Autophagy protects the retina from light-induced degeneration. J. Biol. Chem. 288, 7506–7518. 10.1074/jbc.M112.439935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinetti G, Fruchart JC, Staels B, 2000. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm. Res. 49, 497–505. 10.1007/s000110050622. [DOI] [PubMed] [Google Scholar]
- Choudhary M, Ding JD, Qi X, Boulton ME, Yao PL, Peters JM, Malek G, 2016. PPARbeta/delta selectively regulates phenotypic features of age-related macular degeneration. Aging 8, 1952–1978. 10.18632/aging.101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W-S, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, 2013. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504, 394–400. 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M, Engel U, Giorgione J, Muller-Taubenberger A, Prassler J, Veltman D, Gerisch G, 2010. Curvature recognition and force generation in phagocytosis. BMC Biol. 8, 154. 10.1186/1741-7007-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudepierre T, Paques M, Simonutti M, Buard I, Sahel J, Maue RA, Picaud S, Pfrieger FW, 2010. Lack of Niemann-Pick type C1 induces age-related degeneration in the mouse retina. Mol. Cell. Neurosci. 43, 164–176. 10.1016/j.mcn.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Coleman JA, Kwok MC, Molday RS, 2009. Localization, purification, and functional reconstitution of the P4-ATPase Atp8a2, a phosphatidylserine flippase in photoreceptor disc membranes. J. Biol. Chem. 284, 32670–32679. 10.1074/jbc.M109.047415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers FP, van de Pol DJ, van Kerkhoff LP, Wieringa B, Ropers HH, 1990. Cloning of a gene that is rearranged in patients with choroideraemia. Nature 347, 674–677. 10.1038/347674a0. [DOI] [PubMed] [Google Scholar]
- Currie JR, Hollyfield JG, Rayborn ME, 1978. Rod outer segments elongate in constant light: darkness is required for normal shedding. Vis. Res. 18, 995–1003. 10.1016/0042-6989(78)90027-5. [DOI] [PubMed] [Google Scholar]
- Damek-Poprawa M, Diemer T, Lopes VS, Lillo C, Harper DC, Marks MS, Wu Y, Sparrow JR, Rachel RA, Williams DS, 2009. Melanoregulin (MREG) modulates lysosome function in pigment epithelial cells. J. Biol. Chem. 284, 10877–10889. 10.1074/jbc.M808857200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele LL, Caughey J, Volland S, Sharp RC, Dhingra A, Williams DS, Philp NJ, Boesze-Battaglia K, 2019. Peroxisome turnover and diurnal modulation of antioxidant activity in retinal pigment epithelia utilizes microtubule-associated protein 1 light chain 3B. Am. J. Physiol. Cell Physiol (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AA, Bernstein PS, Bok D, Turner J, Nachtigal M, Hunt RC, 1995. A human retinal pigment epithelial cell line that retains epithelial characteristics after prolonged culture. Invest. Ophthalmol. Vis. Sci. 36, 955–964. [PubMed] [Google Scholar]
- Daw NW, Pearlman AL, 1974. Pigment migration and adaptation in the eye of the squid, Loligo pealei. J. Gen. Physiol. 63, 22–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi J, Yamamoto A, Yoshimori T, Sugasawa K, Moriyama Y, Futai M, Suzuki T, Kato K, Uyama M, Tashiro Y, 1994. Acidification of phagosomes and degradation of rod outer segments in rat retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 35, 568–579. [PubMed] [Google Scholar]
- Dejos C, Kuny S, Han WH, Capel H, Lemieux H, Sauvé Y, 2018. Photoreceptor-induced RPE phagolysosomal maturation defects in Stargardt-like Maculopathy (STGD3). Sci. Rep. 8, 5944. 10.1038/s41598-018-24357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deosaran E, Larsen KB, Hua R, Sargent G, Wang Y, Kim S, Lamark T, Jauregui M, Law K, Lippincott-Schwartz J, Brech A, Johansen T, Kim PK, 2013. NBR1 acts as an autophagy receptor for peroxisomes. J. Cell Sci. 126, 939–952. 10.1242/jcs.114819. [DOI] [PubMed] [Google Scholar]
- Desjardins M, Griffiths G, 2003. Phagocytosis: latex leads the way. Curr. Opin. Cell Biol. 15, 498–503. 10.1016/s0955-0674(03)00083-8. [DOI] [PubMed] [Google Scholar]
- Dhingra A, Bell BA, Peachey NS, Daniele LL, Reyes-Reveles J, Sharp RC, Jun B, Bazan NG, Sparrow JR, Kim HJ, Philp NJ, Boesze-Battaglia K, 2018. Microtubule-associated protein 1 light chain 3B, (LC3B) is necessary to maintain lipid-mediated homeostasis in the retinal pigment epithelium. Front. Cell. Neurosci. 12, 351. 10.3389/fncel.2018.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz F, Gravotta D, Deora A, Schreiner R, Schoggins J, Falck-Pedersen E, Rodriguez-Boulan E, 2009. Clathrin adaptor AP1B controls adenovirus infectivity of epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 106, 11143–11148. 10.1073/pnas.0811227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diemer T, Gibbs D, Williams DS, 2008. Analysis of the Rate of Disk Membrane Digestion by Cultured RPE Cells, Recent Advances in Retinal Degeneration. Springer, pp. 321–326. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Gibbons IR, 1962. The fine structure of the pigment epithelium in the albino rat. J. Cell Biol. 14, 459–474. 10.1083/jcb.14.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JE, Sidman RL, 1962. Inherited retinal dystrophy in the rat. J. Cell Biol. 14, 73–109. 10.1083/jcb.14.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn K, Aotaki-Keen A, Putkey F, Hjelmeland L, 1996. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp. Eye Res. 62, 155–170. 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- Duong TT, Vasireddy V, Ramachandran P, Herrera PS, Leo L, Merkel C, Bennett J, Mills JA, 2018. Use of induced pluripotent stem cell models to probe the pathogenesis of choroideremia and to develop a potential treatment. Stem Cell Res. 27, 140–150. 10.1016/j.scr.2018.01.009. [DOI] [PubMed] [Google Scholar]
- Dwyer MA, Kazmin D, Hu P, McDonnell DP, Malek G, 2011. Research resource: nuclear receptor atlas of human retinal pigment epithelial cells: potential relevance to age-related macular degeneration. Mol. Endocrinol. 25, 360–372. 10.1210/me.2010-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dysli C, Wolf S, Berezin MY, Sauer L, Hammer M, Zinkernagel MS, 2017. Fluorescence lifetime imaging ophthalmoscopy. Prog. Retin. Eye Res. 60, 120–143. 10.1016/j.preteyeres.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D, 2000. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet. 9, 645–651. 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- Edwards RB, 1977. Culture of rat retinal pigment epithelium. In Vitro 13, 301–304. [DOI] [PubMed] [Google Scholar]
- Edwards RB, Szamier RB, 1977. Defective phagocytosis of isolated rod outer segments by RCS rat retinal pigment epithelium in culture. Science 197, 1001–1003. 10.1126/science.560718. [DOI] [PubMed] [Google Scholar]
- Effron J, Szamier R, Edwards R, 1981. Selective phagocytosis of liposomes by cultured RCS rat pigment epithelium. Invest. Ophthalmol. Vis. Sci. 21, 611–616. [PubMed] [Google Scholar]
- Eguchi E, Waterman TH, 1976. Freeze-etch and histochemical evidence for cycling in crayfish photoreceptor membranes. Cell Tissue Res. 169, 419–434. 10.1007/bf00218144. [DOI] [PubMed] [Google Scholar]
- El-Amraoui A, Schonn JS, Kussel-Andermann P, Blanchard S, Desnos C, Henry JP, Wolfrum U, Darchen F, Petit C, 2002. MyRIP, a novel Rab effector, enables myosin VIIa recruitment to retinal melanosomes. EMBO Rep. 3, 463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanouilidis L, Gopalswamy M, Passon DM, Wilmanns M, Sattler M, 2016. Structural biology of the import pathways of peroxisomal matrix proteins. Bba-Mol Cell Res 1863, 804–813. 10.1016/j.bbamcr.2015.09.034. [DOI] [PubMed] [Google Scholar]
- Esteve-Rudd J, Lopes VS, Jiang M, Williams DS, 2014. In Vivo and in Vitro Monitoring of Phagosome Maturation in Retinal Pigment Epithelium Cells, Retinal Degenerative Diseases. Springer, pp. 85–90. [DOI] [PubMed] [Google Scholar]
- Esteve-Rudd J, Hazim RA, Diemer T, Paniagua AE, Volland S, Umapathy A, Williams DS, 2018. Defective phagosome motility and degradation in cell nonautonomous RPE pathogenesis of a dominant macular degeneration. Proc. Natl. Acad. Sci. Unit. States Am. 5468–5473. 10.1073/pnas.1709211115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasler-Kan E, Aliu N, Wunderlich K, Ketterer S, Ruggiero S, Berger S, Meyer P, 2018. The retinal pigment epithelial cell line (ARPE-19) displays mosaic structural chromosomal aberrations. Methods Mol. Biol. 1745, 305–314. 10.1007/978-1-4939-7680-5_17. [DOI] [PubMed] [Google Scholar]
- Feher J, Kovacs I, Artico M, Cavallotti C, Papale A, Gabrieli CB, 2006. Mitochondrial alterations of retinal pigment epithelium in age-related macular degeneration. Neurobiol. Aging 27, 983–993. 10.1016/j.neurobiolaging.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Feng W, Yasumura D, Matthes MT, LaVail MM, Vollrath D, 2002. Mertk triggers uptake of photoreceptor outer segments during phagocytosis by cultured retinal pigment epithelial cells. J. Biol. Chem. 277, 17016–17022. 10.1074/jbc.M107876200. [DOI] [PubMed] [Google Scholar]
- Filippov V, Song MA, Zhang K, Vinters HV, Tung S, Kirsch WM, Yang J, Duerksen-Hughes PJ, 2012. Increased ceramide in brains with Alzheimer’s and other neurodegenerative diseases. J Alzheimers Dis 29, 537–547. 10.3233/JAD-2011-111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC, 2003. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J. 22, 4143–4154. 10.1093/emboj/cdg416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC, Rodriguez-Boulan E, 1999. Macrophage and retinal pigment epithelium phagocytosis: apoptotic cells and photoreceptors compete for alphavbeta3 and alphavbeta5 integrins, and protein kinase C regulates alphavbeta5 binding and cytoskeletal linkage. J. Exp. Med. 190, 861–874. 10.1084/jem.190.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC, Silverstein RL, 2001. Differential roles of CD36 and αvβ5 integrin in photoreceptor phagocytosis by the retinal pigment epithelium. J. Exp. Med. 194, 1289–1298. 10.1084/jem.194.9.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC, Bonilha VL, Marmorstein AD, Rodriguez-Boulan E, 1997. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires αvβ5 integrin for binding but not for internalization. In: Proceedings of the National Academy of Sciences 94. pp. 12932–12937. 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CR, Ferrington DA, 2018. Perspective on AMD pathobiology: a bioenergetic crisis in the RPE. Invest. Ophthalmol. Vis. Sci. 59, AMD41–AMD47. 10.1167/iovs.18-24289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery J, Fisher S, 1979. Light-triggered rod disc shedding in Xenopus retina in vitro. Invest. Ophthalmol. Vis. Sci. 18, 638–642. [PubMed] [Google Scholar]
- Fliesler SJ, Anderson RE, 1983. Chemistry and metabolism of lipids in the vertebrate retina. Prog. Lipid Res. 22, 79–131. [DOI] [PubMed] [Google Scholar]
- Fraldi A, Annunziata F, Lombardi A, Kaiser HJ, Medina DL, Spampanato C, Fedele AO, Polishchuk R, Sorrentino NC, Simons K, Ballabio A, 2010. Lysosomal fusion and SNARE function are impaired by cholesterol accumulation in lysosomal storage disorders. EMBO J. 29, 3607–3620. 10.1038/emboj.2010.237. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Fritsche LG, et al. , 2013. Seven new loci associated with age-related macular degeneration. Nat. Genet. 45, 433–439. 10.1038/ng.2578.439e431-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche LG, et al. , 2016. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat. Genet. 48, 134–143. 10.1038/ng.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich E, Klessen C, 2001. Enzymatic heterogeneity of bovine retinal pigment epithelial cells in vivo and in vitro. Graefe’s Arch. Clin. Exp. Ophthalmol. 239, 25–34. 10.1007/s004170000218. [DOI] [PubMed] [Google Scholar]
- Frost LS, Lopes VS, Stefano FP, Bragin A, Williams DS, Mitchell CH, Boesze-Battaglia K, 2013. Loss of melanoregulin (MREG) enhances cathepsin-D secretion by the retinal pigment epithelium. Vis. Neurosci. 30, 55–64. 10.1017/S0952523813000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost LS, Mitchell CH, Boesze-Battaglia K, 2014. Autophagy in the eye: implications for ocular cell health. Exp. Eye Res. 124, 56–66. 10.1016/j.exer.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost LS, Lopes VS, Bragin A, Reyes-Reveles J, Brancato J, Cohen A, Mitchell CH, Williams DS, Boesze-Battaglia K, 2015. The contribution of melanoregulin to microtubule-associated protein 1 light chain 3 (LC3) associated phagocytosis in retinal pigment epithelium. Mol. Neurobiol. 52, 1135–1151. 10.1007/s12035-014-8920-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter CE, Ramalho JS, Jaissle GB, Seeliger MW, Seabra MC, 2004. The role of Rab27a in the regulation of melanosome distribution within retinal pigment epithelial cells. Mol. Biol. Cell 15, 2264–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal A, Li Y, Thompson DA, Weir J, Orth U, Jacobson SG, Apfelstedt-Sylla E, Vollrath D, 2000. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat. Genet. 26, 270–271. [DOI] [PubMed] [Google Scholar]
- Galloway CA, Dalvi S, Hung SSC, MacDonald LA, Latchney LR, Wong RCB, Guymer RH, Mackey DA, Williams DS, Chung MM, Gamm DM, Pebay A, Hewitt AW, Singh R, 2017. Drusen in patient-derived hiPSC-RPE models of macular dystrophies. Proc. Natl. Acad. Sci. U. S. A. 114, E8214–E8223. 10.1073/pnas.1710430114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandre-Babbe S, van der Bliek AM, 2008. The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol. Biol. Cell 19, 2402–2412. 10.1091/mbc.E07-12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangalum RK, Bhat AM, Kohan SA, Bhat SP, 2016. Inhibition of the expression of the small heat shock protein alphaB-crystallin inhibits exosome secretion in human retinal pigment epithelial cells in culture. J. Biol. Chem. 291, 12930–12942. 10.1074/jbc.M115.698530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiannakis A, Burgoyne T, Lueck K, Futter C, Greenwood J, Moss SE, 2015. Retinal pigment epithelial cells mitigate the effects of complement attack by endocytosis of C5b-9. J. Immunol. 195, 3382–3389. 10.4049/jimmunol.1500937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs D, Williams DS, 2003. Isolation and culture of primary mouse retinal pigmented epithlelial cells. Adv. Exp. Med. Biol. 533, 347–352. [DOI] [PubMed] [Google Scholar]
- Gibbs D, Kitamoto J, Williams DS, 2003. Abnormal phagocytosis by retinal pigmented epithelium that lacks myosin VIIa, the Usher syndrome 1B protein. Proc. Natl. Acad. Sci. Unit. States Am. 100, 6481–6486. 10.1073/pnas.1130432100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs D, Azarian SM, Lillo C, Kitamoto J, Klomp AE, Steel KP, Libby RT, Williams DS, 2004. Role of myosin VIIa and Rab27a in the motility and localization of RPE melanosomes. J. Cell Sci. 117, 6473–6483. 10.1002/cm.20198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs D, Cideciyan AV, Jacobson SG, Williams DS, 2009. Retinal pigment epithelium defects in humans and mice with mutations in MYO7A: imaging melanosome-specific autofluorescence. Invest. Ophthalmol. Vis. Sci. 50, 4386–4393. 10.1167/iovs.09-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs D, Diemer T, Khanobdee K, Hu J, Bok D, Williams DS, 2010. Function of MYO7A in the human RPE and the validity of shaker1 mice as a model for Usher syndrome 1B. Invest. Ophthalmol. Vis. Sci. 51, 1130–1135. 10.1167/iovs.09-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golestaneh N, Chu Y, Xiao YY, Stoleru GL, Theos AC, 2017. Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Cell Death Dis. 8, e2537. 10.1038/cddis.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez NM, Lu W, Lim JC, Kiselyov K, Campagno KE, Grishchuk Y, Slaugenhaupt SA, Pfeffer BA, Fliesler SJ, Mitchell CH, 2018. Robust lysosomal calcium signaling through channel TRPML1 is impaired by lysosomal lipid accumulation. Faseb. J. 32, 782–794. 10.1096/fj.201700220RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Fields MA, Moreira EF, Bowrey HE, Gooz M, Ablonczy Z, Del Priore LV, 2015. Differentiation of human protein-induced pluripotent stem cells toward a retinal pigment epithelial cell fate. PloS One 10, e0143272. 10.1371/journal.pone.0143272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordiyenko NV, Fariss RN, Zhi C, MacDonald IMS, 2010. Silencing of the CHM gene alters phagocytic and secretory pathways in the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 51, 1143–1150. 10.1167/iovs.09-4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace MS, Wang LA, Pickard GE, Besharse JC, Menaker M, 1996. The tau mutation shortens the period of rhythmic photoreceptor outer segment disk shedding in the hamster. Brain Res. 735, 93–100. 10.1016/0006-8993(96)00600-2. [DOI] [PubMed] [Google Scholar]
- Gray DC, Merigan W, Wolfing JI, Gee BP, Porter J, Dubra A, Twietmeyer TH, Ahmad K, Tumbar R, Reinholz F, 2006. In vivo fluorescence imaging of primate retinal ganglion cells and retinal pigment epithelial cells. Optic Express 14, 7144–7158. 10.1364/oe.14.007144. [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL, 2006. Oxidized phosphatidylserine–CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J. Exp. Med. 203, 2613–2625. 10.1084/jem.20060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenell A, Wang Y, Yam M, Swarup A, Dilan TL, Hauer A, Linton JD, Philp NJ, Gregor E, Zhu S, Shi Q, Murphy J, Guan T, Lohner D, Kolandaivelu S, Ramamurthy V, Goldberg AFX, Hurley JB, Du J, 2019. Loss of MPC1 reprograms retinal metabolism to impair visual function. Proc. Natl. Acad. Sci. U. S. A. 116, 3530–3535. 10.1073/pnas.1812941116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MO, Abrams T, 1987. Kinetic studies of rod outer segment binding and ingestion by cultured rat RPE cells. Exp. Eye Res. 45, 907–922. 10.1016/s0014-4835(87)80105-7. [DOI] [PubMed] [Google Scholar]
- Hall MO, Obin MS, Heeb MJ, Burgess BL, Abrams TA, 2005. Both protein S and Gas6 stimulate outer segment phagocytosis by cultured rat retinal pigment epithelial cells. Exp. Eye Res. 81, 581–591. 10.1016/j.exer.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Hammond JW, Cai D, Verhey KJ, 2008. Tubulin modifications and their cellular functions. Curr. Opin. Cell Biol. 20, 71–76. 10.1016/j.ceb.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata M, Ikeda HO, Iwai S, Iida Y, Gotoh N, Asaka I, Ikeda K, Isobe Y, Hori A, Nakagawa S, Yamato S, Arita M, Yoshimura N, Tsujikawa A, 2018. Reduction of lipid accumulation rescues Bietti’s crystalline dystrophy phenotypes. Proc. Natl. Acad. Sci. U. S. A. 115, 3936–3941. 10.1073/pnas.1717338115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S, Hara S, Mizuno K, 1975. Degradation of rod outer segment proteins by cathepsin D. J. Biochem. 78, 1365–1367. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Hara S, Takaku Y, Mizuno K, 1977. Distribution of acid lipase in the bovine retinal pigment epithelium. Exp. Eye Res. 24, 1–6. 10.1016/0014-4835(77)90278-0. [DOI] [PubMed] [Google Scholar]
- Hazim RA, Karumbayaram S, Jiang M, Dimashkie A, Lopes VS, Li D, Burgess BL, Vijayaraj P, Alva-Ornelas JA, Zack JA, 2017. Differentiation of RPE cells from integration-free iPS cells and their cell biological characterization. Stem Cell Res. Ther. 8, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazim RA, Volland S, Yen A, Burgess BL, Williams DS, 2019. Rapid differentiation of the human RPE cell line, ARPE-19, induced by nicotinamide. Exp. Eye Res. 179, 18–24. 10.1016/j.exer.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann BL, Boada-Romero E, Cunha LD, Magne J, Green DR, 2017. LC3-Associated phagocytosis and inflammation. J. Mol. Biol. 429, 3561–3576. 10.1016/j.jmb.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellinen L, Sato K, Reinisalo M, Kidron H, Rilla K, Tachikawa M, Uchida Y, Terasaki T, Urtti A, 2019. Quantitative protein expression in the human retinal pigment epithelium: comparison between apical and basolateral plasma membranes with emphasis on transporters. Invest. Ophthalmol. Vis. Sci. 60, 5022–5034. 10.1167/iovs.19-27328. [DOI] [PubMed] [Google Scholar]
- Herman KG, Steinberg RH, 1982a. Phagosome degradation in the tapetal retinal pigment epithelium of the opossum. Invest. Ophthalmol. Vis. Sci. 23, 291–304. [PubMed] [Google Scholar]
- Herman KG, Steinberg RH, 1982b. Phagosome movement and the diurnal pattern of phagocytosis in the tapetal retinal pigment epithelium of the opossum. Investig. Ophthalmol. Vis. Sci. 23, 277–290. [PubMed] [Google Scholar]
- Hess C, Kemper C, 2016. Complement-mediated regulation of metabolism and basic cellular processes. Immunity 45, 240–254. 10.1016/j.immuni.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heth CA, Marescalchi PA, Ye L, 1995. IP3 generation increases rod outer segment phagocytosis by cultured Royal College of Surgeons retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 36, 984–989. [PubMed] [Google Scholar]
- Hollyfield JG, Basinger SF, 1978. Photoreceptor shedding can be initiated within the eye. Nature 274, 794. [DOI] [PubMed] [Google Scholar]
- Hoppe G, Marmorstein AD, Pennock EA, Hoff HF, 2001. Oxidized low density lipoprotein–induced inhibition of processing of photoreceptor outer segments by RPE. Invest. Ophthalmol. Vis. Sci. 42, 2714–2720. [PubMed] [Google Scholar]
- Hoppe G, O’Neil J, Hoff HF, Sears J, 2004a. Products of lipid peroxidation induce missorting of the principal lysosomal protease in retinal pigment epithelium. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 1689, 33–41. 10.1016/j.bbadis.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Hoppe G, O’Neil J, Hoff H, Sears J, 2004b. Accumulation of oxidized lipid-protein complexes alters phagosome maturation in retinal pigment epithelium. Cell. Mol. Life Sci. 61, 1664–1674. 10.1007/s00018-004-4080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Bok D, 2001. A cell culture medium that supports the differentiation of human retinal pigment epithelium into functionally polarized monolayers. Mol. Vis. 7, 14–19. [PubMed] [Google Scholar]
- Huotari J, Helenius A, 2011. Endosome maturation. EMBO J. 30, 3481–3500. 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovelli J, Zhao C, Wolkow N, Veldman P, Gollomp K, Ojha P, Lukinova N, King A, Feiner L, Esumi N, 2011. Generation of Cre transgenic mice with postnatal RPE-specific ocular expression. Invest. Ophthalmol. Vis. Sci. 52, 1378–1383. 10.1167/iovs.10-6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idelson M, Alper R, Obolensky A, Ben-Shushan E, Hemo I, Yachimovich-Cohen N, Khaner H, Smith Y, Wiser O, Gropp M, Cohen MA, Even-Ram S, Berman-Zaken Y, Matzrafi L, Rechavi G, Banin E, Reubinoff B, 2009. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell Stem Cell 5, 396–408. 10.1016/j.stem.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Idone V, Tam C, Andrews NW, 2008. Two-way traffic on the road to plasma membrane repair. Trends Cell Biol. 18, 552–559. 10.1016/j.tcb.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Yamada E, 1970. The degradation of the photoreceptor outer segment within the pigment epithelial cell of rat retina. J. Electron. Microsc. 19, 85–99. [PubMed] [Google Scholar]
- Itoyama A, Honsho M, Abe Y, Yoshida Y, Fujiki Y, 2011. Docosahexaenoic acid is required for peroxisomal elongation, a prerequisite for division of peroxisomes. Mol. Biol. Cell 22. [DOI] [PubMed] [Google Scholar]
- Itoyama A, Michiyuki S, Honsho M, Yamamoto T, Moser A, Yoshida Y, Fujiki Y, 2013. Mff functions with Pex11p beta and DLP1 in peroxisomal fission. Biology Open 2, 998–1006. 10.1242/bio.20135298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy G, Kanai S, Ohta M, Smith G, Sato Y, Kobayashi M, Kitani K, 1989. Lipofuscin-like substances accumulate rapidly in brain, retina and internal organs with cysteine protease inhibition. Adv. Exp. Med. Biol. 266, 31–45. 10.1007/978-1-4899-5339-1_3. discussion 45–37. [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Cideciyan AV, Aleman TS, Sumaroka A, Roman AJ, Gardner LM, Prosser HM, Mishra M, Bech-Hansen NT, Herrera W, Schwartz SB, Liu XZ, Kimberling WJ, Steel KP, Williams DS, 2008. Usher syndromes due to MYO7A, PCDH15, USH2A or GPR98 mutations share retinal disease mechanism. Hum. Mol. Genet. 17, 2405–2415. 10.1093/hmg/ddn140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadeja S, Barnard AR, McKie L, Cross SH, White JK, Sanger Mouse Genetics P, Robertson M, Budd PS, MacLaren RE, Jackson IJ, 2015. Mouse slc9a8 mutants exhibit retinal defects due to retinal pigmented epithelium dysfunction. Invest. Ophthalmol. Vis. Sci. 56, 3015–3026. 10.1167/iovs.14-15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins CM, Yang J, Sims HF, Gross RW, 2011. Reversible high affinity inhibition of phosphofructokinase-1 by acyl-CoA: a mechanism integrating glycolytic flux with lipid metabolism. J. Biol. Chem. 286, 11937–11950. 10.1074/jbc.M110.203661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Esteve-Rudd J, Lopes VS, Diemer T, Lillo C, Rump A, Williams DS, 2015. Microtubule motors transport phagosomes in the RPE, and lack of KLC1 leads to AMD-like pathogenesis. J. Cell Biol. 210, 595–611. 10.1083/jcb.201410112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Volland S, Wang H, Lopes VS, Balaji A, Li DG, Williams DS, 2019. Requirement of Microtubule Motor Transport in the Delivery of Melanosomes to the Actin-Rich, Apical Domain of the RPE. (submitted for publication). [DOI] [PMC free article] [PubMed]
- Johnson DE, Ostrowski P, Jaumouille V, Grinstein S, 2016. The position of lysosomes within the cell determines their luminal pH. J. Cell Biol. 212, 677–692. 10.1083/jcb.201507112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaarniranta K, Kajdanek J, Morawiec J, Pawlowska E, Blasiak J, 2018. PGC-1α protects RPE cells of the aging retina against oxidative stress-induced degeneration through the regulation of senescence and mitochondrial quality control. The significance for AMD pathogenesis. Int. J. Mol. Sci. 19, 2317. 10.3390/ijms1908231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanow MA, Giarmarco MM, Jankowski CS, Tsantilas K, Engel AL, Du J, Linton JD, Farnsworth CC, Sloat SR, Rountree A, Sweet IR, Lindsay KJ, Parker ED, Brockerhoff SE, Sadilek M, Chao JR, Hurley JB, 2017. Biochemical adaptations of the retina and retinal pigment epithelium support a metabolic ecosystem in the vertebrate eye. Elife 6. 10.7554/eLife.28899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karan G, Lillo C, Yang Z, Cameron DJ, Locke KG, Zhao Y, Thirumalaichary S, Li C, Birch DG, Vollmer-Snarr HR, Williams DS, Zhang K, 2005. Lipofuscin accumulation, abnormal electrophysiology, and photoreceptor degeneration in mutant ELOVL4 transgenic mice: a model for macular degeneration. Proc. Natl. Acad. Sci. U. S. A. 102, 4164–4169. 10.1073/pnas.0407698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz ML, Shanker MJ, 1989. Development of lipofuscin-like fluorescence in the retinal pigment epithelium in response to protease inhibitor treatment. Mech. Ageing Dev. 49, 23–40. 10.1016/0047-6374(89)90065-1. [DOI] [PubMed] [Google Scholar]
- Kaur G, Tan LX, Rathnasamy G, La Cunza N, Germer CJ, Toops KA, Fernandes M, Blenkinsop TA, Lakkaraju A, 2018. Aberrant early endosome biogenesis mediates complement activation in the retinal pigment epithelium in models of macular degeneration. Proc. Natl. Acad. Sci. U. S. A. 115, 9014–9019. 10.1073/pnas.1805039115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S, Cuervo AM, 2018. The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 19, 365–381. 10.1038/s41580-018-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H, Suemori H, Mizuseki K, Watanabe K, Urano F, Ichinose H, Haruta M, Takahashi M, Yoshikawa K, Nishikawa S, Nakatsuji N, Sasai Y, 2002. Generation of dopaminergic neurons and pigmented epithelia from primate ES cells by stromal cell-derived inducing activity. Proc. Natl. Acad. Sci. U. S. A. 99, 1580–1585. 10.1073/pnas.032662199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C, Rakoczy P, Robertson T, Papadimitriou J, Constable I, 1994. Kinetic studies on phagocytosis and lysosomal digestion of rod outer segments by human retinal pigment epithelial cells in vitro. Exp. Cell Res. 210, 209–214. 10.1006/excr.1994.1031. [DOI] [PubMed] [Google Scholar]
- King-Smith C, Paz P, Lee CW, Lam W, Burnside B, 1997. Bidirectional pigment granule migration in isolated retinal pigment epithelial cells requires actin but not microtubules. Cell Motil Cytoskeleton 38, 229–249 . [DOI] [PubMed] [Google Scholar]
- Kim JY, Zhao H, Martinez J, Doggett TA, Kolesnikov AV, Tang PH, Ablonczy Z, Chan CC, Zhou Z, Green DR, Ferguson TA, 2013. Noncanonical autophagy promotes the visual cycle. Cell 154, 365–376. 10.1016/j.cell.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiser PD, Golczak M, Palczewski K, 2014. Chemistry of the retinoid (visual) cycle. Chem. Rev. 114, 194–232. 10.1021/cr400107q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM, 1997. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc. Natl. Acad. Sci. U. S. A 94, 4318–4323. 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingeborn M, Dismuke WM, Bowes Rickman C, Stamer WD, 2017a. Roles of exosomes in the normal and diseased eye. Prog. Retin. Eye Res. 59, 158–177. 10.1016/j.preteyeres.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingeborn M, Dismuke WM, Skiba NP, Kelly U, Stamer WD, Bowes Rickman C, 2017b. Directional exosome proteomes reflect polarity-specific functions in retinal pigmented epithelium monolayers. Sci. Rep. 7, 4901. 10.1038/s41598-017-05102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, et al. , 2016. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12, 1–222. 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klomp AE, Teofilo K, Legacki E, Williams DS, 2007. Analysis of the linkage of MYRIP and MYO7A to melanosomes by RAB27A in retinal pigment epithelial cells. Cell Motil Cytoskeleton 64, 474–487. [DOI] [PubMed] [Google Scholar]
- Knickelbein JE, Liu B, Arakelyan A, Zicari S, Hannes S, Chen P, Li Z, Grivel JC, Chaigne-Delalande B, Sen HN, Margolis L, Nussenblatt RB, 2016. Modulation of immune responses by extracellular vesicles from retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 57, 4101–4107. 10.1167/iovs.15-18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott EJ, Gordon WC, Jun B, Do K, Bazan NG, 2018. Retinal pigment epithelium and photoreceptor preconditioning protection requires docosanoid signaling. Cell. Mol. Neurobiol. 38, 901–917. 10.1007/s10571-017-0565-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A, Thiemann M, Grabenbauer M, Yoon Y, McNiven MA, Schrader M, 2003. Dynamin-like protein 1 is involved in peroxisomal fission. J. Biol. Chem. 278, 8597–8605. 10.1074/jbc.211761200. [DOI] [PubMed] [Google Scholar]
- Koch A, Yoon Y, Bonekamp NA, McNiven MA, Schrader M, 2005. A role for Fis1 in both mitochondrial and peroxisomal fission in mammalian cells. Mol. Biol. Cell 16, 5077–5086. 10.1091/mbc.E05-02-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy RR, Clark AF, Daudt D, Vishwanatha JK, Yorio T, 2013. A forensic path to RGC-5 cell line identification: lessons learned. Invest. Ophthalmol. Vis. Sci. 54, 5712–5719. 10.1167/iovs.13-12085. [DOI] [PubMed] [Google Scholar]
- Kunchithapautham K, Rohrer B, 2007. Apoptosis and autophagy in photoreceptors exposed to oxidative stress. Autophagy 3, 433–441. [DOI] [PubMed] [Google Scholar]
- Kurihara T, Westenskow PD, Bravo S, Aguilar E, Friedlander M, 2012. Targeted deletion of Vegfa in adult mice induces vision loss. J. Clin. Invest. 122, 4213–4217. 10.1172/JCI65157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T, Westenskow PD, Gantner ML, Usui Y, Schultz A, Bravo S, Aguilar E, Wittgrove C, Friedlander MS, Paris LP, 2016. Hypoxia-induced metabolic stress in retinal pigment epithelial cells is sufficient to induce photoreceptor degeneration. Elife 5, e14319. 10.7554/eLife.14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda TS, Fukuda M, 2005. Functional analysis of Slac2-c/MyRIP as a linker protein between melanosomes and myosin VIIa. J. Biol. Chem. 280, 28015–28022. [DOI] [PubMed] [Google Scholar]
- Lai C-M, Robertson T, Papadimitriou J, Shen W-Y, Daw N, Constable IJ, Rakoczy PE, 2000. Controlled production of active Cathepsin D in Retinal Pigment Epithelial cells following adenovirus-mediated gene delivery. Mol. Ther. 2, 476–484. 10.1006/mthe.2000.0195. [DOI] [PubMed] [Google Scholar]
- Lakkaraju A, Rodriguez-Boulan E, 2008. Itinerant exosomes: emerging roles in cell and tissue polarity. Trends Cell Biol. 18, 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkaraju A, Finnemann SC, Rodriguez-Boulan E, 2007. The lipofuscin fluorophore A2E perturbs cholesterol metabolism in retinal pigment epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 104, 11026–11031. 10.1073/pnas.0702504104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM, 1976. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science 194, 1071–1074. 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- LaVail M, Ward P, 1978. Studies on the hormonal control of circadian outer segment disc shedding in the rat retina. Invest. Ophthalmol. Vis. Sci. 17 1189–1183. [PubMed] [Google Scholar]
- Law A-L, Ling Q, Hajjar KA, Futter CE, Greenwood J, Adamson P, Wavre-Shapton ST, Moss SE, Hayes MJ, 2009. Annexin A2 regulates phagocytosis of photoreceptor outer segments in the mouse retina. Mol. Biol. Cell 20, 3896–3904. 10.1091/mbc.e08-12-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann GL, Benedicto I, Philp NJ, Rodriguez-Boulan E, 2014. Plasma membrane protein polarity and trafficking in RPE cells: past, present and future. Exp. Eye Res. 126, 5–15. 10.1016/j.exer.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidal AM, Levine B, Debnath J, 2018. Autophagy and the cell biology of age-related disease. Nat. Cell Biol. 20, 1338–1348. 10.1038/s41556-018-0235-8. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G, 2019. Biological functions of autophagy genes: a disease perspective. Cell 176, 11–42. 10.1016/j.cell.2018.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TR, Kundinger SR, Link BA, Insinna C, Besharse JC, 2018. Kif17 phosphorylation regulates photoreceptor outer segment turnover. BMC Cell Biol. 19, 25. 10.1186/s12860-018-0177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligeon LA, Romao S, Munz C, 2017. Analysis of LC3-associated phagocytosis and antigen presentation. Methods Mol. Biol. 1519, 145–168. 10.1007/978-1-4939-6581-6_10. [DOI] [PubMed] [Google Scholar]
- Ligeon LA, Loi M, Munz C, 2018. LC3-Associated phagocytosis and antigen presentation. Curr. Protoc. Im. 123, e60. 10.1002/cpim.60. [DOI] [PubMed] [Google Scholar]
- Lindgren J, Nilsson DE, Sjovall P, Jarenmark M, Ito S, Wakamatsu K, Kear BP, Schultz BP, Sylvestersen RL, Madsen H, LaFountain JR Jr., Alwmark C, Eriksson ME, Hall SA, Lindgren P, Rodriguez-Meizoso I, Ahlberg P, 2019. Fossil insect eyes shed light on trilobite optics and the arthropod pigment screen. Nature. 10.1038/s41586-019-1473-z. [DOI] [PubMed] [Google Scholar]
- Liu X, Ondek B, Williams DS, 1998. Mutant myosin VIIa causes defective melanosome distribution in the RPE of shaker-1 mice. Nat. Genet. 19, 117–118. [DOI] [PubMed] [Google Scholar]
- Liu X, Bulgakov OV, Darrow KN, Pawlyk B, Adamian M, Liberman MC, Li T, 2007. Usherin is required for maintenance of retinal photoreceptors and normal development of cochlear hair cells. Proc. Natl. Acad. Sci. U. S. A. 104, 4413–4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lu W, Reigada D, Nguyen J, Laties AM, Mitchell CH, 2008. Restoration of lysosomal pH in RPE cells from cultured human and ABCA4−/− mice: pharmacologic approaches and functional recovery. Invest. Ophthalmol. Vis. Sci. 49, 772–780. 10.1167/iovs.07-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Kocaoglu OP, Miller DT, 2016. 3D imaging of retinal pigment epithelial cells in the living human retina. Invest. Ophthalmol. Vis. Sci. 57, OCT533–543. 10.1167/iovs.16-19106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Calton MA, Abell NS, Benchorin G, Gloudemans MJ, Chen M, Hu J, Li X, Balliu B, Bok D, Montgomery SB, Vollrath D, 2019a. Genetic analyses of human fetal retinal pigment epithelium gene expression suggest ocular disease mechanisms. Commun Biol 2, 186. 10.1038/s42003-019-0430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Kurokawa K, Hammer DX, Miller DT, 2019b. In vivo measurement of organelle motility in human retinal pigment epithelial cells. Biomed. Optic Express 10, 4142–4158. 10.1364/BOE.10.004142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobato-Alvarez JA, Roldan ML, Lopez-Murillo TD, Gonzalez-Ramirez R, Bonilla-Delgado J, Shoshani L, 2016. The apical localization of Na(+), K(+)-ATPase in cultured human retinal pigment epithelial cells depends on expression of the beta2 subunit. Front. Physiol. 7, 450. 10.3389/fphys.2016.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes VS, Ramalho JS, Owen DM, Karl MO, Strauss O, Futter CE, Seabra MC, 2007. The ternary Rab27a-Myrip-Myosin VIIa complex regulates melanosome motility in the retinal pigment epithelium. Traffic 8, 486–499. 10.1111/j.1600-0854.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low SH, Chapin SJ, Weimbs T, Komuves LG, Bennett MK, Mostov KE, 1996. Differential localization of syntaxin isoforms in polarized Madin-Darby canine kidney cells. Mol. Biol. Cell 7, 2007–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low SH, Marmorstein LY, Miura M, Li X, Kudo N, Marmorstein AD, Weimbs T, 2002. Retinal pigment epithelial cells exhibit unique expression and localization of plasma membrane syntaxins which may contribute to their trafficking phenotype. J. Cell Sci. 115, 4545–4553. [DOI] [PubMed] [Google Scholar]
- Lu W, Gomez NM, Lim JC, Guha S, O’Brien-Jenkins A, Coffey EE, Campagno KE, McCaughey SA, Laties AM, Carlsson LG, Mitchell CH, 2018. The P2Y12 receptor antagonist ticagrelor reduces lysosomal pH and autofluorescence in retinal pigmented epithelial cells from the ABCA4(−/−) mouse model of retinal degeneration. Front. Pharmacol. 9, 242. 10.3389/fphar.2018.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Campagno KE, Tso HY, Cenaj A, Laties AM, Carlsson LG, Mitchell CH, 2019. Oral delivery of the P2Y12 receptor antagonist ticagrelor prevents loss of photoreceptors in an ABCA4−/− mouse model of retinal degeneration. Invest. Ophthalmol. Vis. Sci. 60, 3046–3053. 10.1167/iovs.19-27241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio JP, Hackmann Y, Dieckmann NM, Griffiths GM, 2014. The biogenesis of lysosomes and lysosome-related organelles. Cold Spring Harb Perspect Biol 6, a016840. 10.1101/cshperspect.a016840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CL, Agrawal G, Subramani S, 2011. Peroxisome assembly: matrix and membrane protein biogenesis. JCB (J. Cell Biol.) 193, 7–16. 10.1083/jcb.201010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackeh R, Perdiz D, Lorin S, Codogno P, Pous C, 2013. Autophagy and microtubules - new story, old players. J. Cell Sci. 126, 1071–1080. 10.1242/jcs.115626. [DOI] [PubMed] [Google Scholar]
- Mair KH, Sedlak C, Kaser T, Pasternak A, Levast B, Gerner W, Saalmuller A, Summerfield A, Gerdts V, Wilson HL, Meurens F, 2014. The porcine innate immune system: an update. Dev. Comp. Immunol. 45, 321–343. 10.1016/j.dci.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek G, Lad EM, 2014. Emerging roles for nuclear receptors in the pathogenesis of age-related macular degeneration. Cell. Mol. Life Sci. 71, 4617–4636. 10.1007/s00018-014-1709-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maminishkis A, Chen S, Jalickee S, Banzon T, Shi G, Wang FE, Ehalt T, Hammer JA, Miller SS, 2006. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest. Ophthalmol. Vis. Sci. 47, 3612–3624. 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai M, et al. , 2017. Autologous induced stem-cell-derived retinal cells for macular degeneration. N. Engl. J. Med. 376, 1038–1046. 10.1056/NEJMoa1608368. [DOI] [PubMed] [Google Scholar]
- Mannagh J, Arya DV, Irvine AR Jr., 1973. Tissue culture of human retinal pigment epithelium. Invest. Ophthalmol. 12, 52–64. [PubMed] [Google Scholar]
- Mao Y, Finnemann SC, 2012. Essential diurnal Rac1 activation during retinal phagocytosis requires αvβ5 integrin but not tyrosine kinases focal adhesion kinase or Mer tyrosine kinase. Mol. Biol. Cell 23, 1104–1114. 10.1091/mbc.E11-10-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Finnemann SC, 2016. Live Imaging of LysoTracker-Labelled Phagolysosomes Tracks Diurnal Phagocytosis of Photoreceptor Outer Segment Fragments in Rat RPE Tissue Ex Vivo, Retinal Degenerative Diseases. Springer, pp. 717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Almendinger J, Oberst A, Ness R, Dillon CP, Fitzgerald P, Hengartner MO, Green DR, 2011. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc. Natl. Acad. Sci. U. S. A 108, 17396–17401. 10.1073/pnas.1113421108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Malireddi RK, Lu Q, Cunha LD, Pelletier S, Gingras S, Orchard R, Guan JL, Tan H, Peng J, Kanneganti TD, Virgin HW, Green DR, 2015. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat. Cell Biol. 17, 893–906. 10.1038/ncb3192. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Maruotti J, et al. , 2015. Small-molecule-directed, efficient generation of retinal pigment epithelium from human pluripotent stem cells. Proc. Natl. Acad. Sci. U. S. A. 112, 10950–10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto B, Besharse JC, 1985. Light and temperature modulated staining of the rod outer segment distal tips with Lucifer yellow. Invest. Ophthalmol. Vis. Sci. 26, 628–635. [PubMed] [Google Scholar]
- Matsumoto B, Defoe D, Besharse J, 1987. Membrane turnover in rod photoreceptors: ensheathment and phagocytosis of outer segment distal tips by pseudopodia of the retinal pigment epithelium. Proc. Roy. Soc. Lond. B 230, 339–354. 10.1098/rspb.1987.0023. [DOI] [PubMed] [Google Scholar]
- Matsuo H, Chevallier J, Mayran N, Le Blanc I, Ferguson C, Faure J, Blanc NS, Matile S, Dubochet J, Sadoul R, Parton RG, Vilbois F, Gruenberg J, 2004. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science 303, 531–534. [DOI] [PubMed] [Google Scholar]
- Mayerson PL, Hall MO, 1986. Rat retinal pigment epithelial cells show specificity of phagocytosis in vitro. J. Cell Biol. 103, 299–308. 10.1083/jcb.103.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S, Sabharanjak S, Maxfield FR, 1998. Cholesterol-dependent retention of GPI-anchored proteins in endosomes. EMBO J. 17, 4626–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni F, Safa H, Finnemann SC, 2014. Understanding photoreceptor outer segment phagocytosis: use and utility of RPE cells in culture. Exp. Eye Res. 126, 51–60. 10.1016/j.exer.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil EL, Tacelosky D, Basciano P, Biallas B, Williams R, Damiani P, Deacon S, Fox C, Stewart B, Petruzzi N, Osborn C, Klinger K, Sellers JR, Smith CK, 2004. Actin-dependent motility of melanosomes from fish retinal pigment epithelial (RPE) cells investigated using in vitro motility assays. Cell Motil Cytoskeleton 58, 71–82. 10.1002/cm.10179. [DOI] [PubMed] [Google Scholar]
- McWilliams TG, Prescott AR, Villarejo-Zori B, Ball G, Boya P, Ganley IG, 2019. A comparative map of macroautophagy and mitophagy in the vertebrate eye. Autophagy 1–13. 10.1080/15548627.2019.1580509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midorikawa R, Yamamoto-Hino M, Awano W, Hinohara Y, Suzuki E, Ueda R, Goto S, 2010. Autophagy-dependent rhodopsin degradation prevents retinal degeneration in Drosophila. J. Neurosci. 30, 10703–10719. 10.1523/JNEUROSCI.2061-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindell JA, 2012. Lysosomal acidification mechanisms. Annu. Rev. Physiol. 74, 69–86. 10.1146/annurev-physiol-012110-142317. [DOI] [PubMed] [Google Scholar]
- Mitter SK, Song C, Qi X, Mao H, Rao H, Akin D, Lewin A, Grant M, Dunn W, Ding J, Bowes Rickman C, Boulton M, 2014. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 10, 1989–2005. 10.4161/auto.36184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan N, Sorokina EM, Verdeny IV, Alvarez AS, Lakadamyali M, 2019. Detyrosinated microtubules spatially constrain lysosomes facilitating lysosome-autophagosome fusion. J. Cell Biol. 218, 632–643. 10.1083/jcb.201807124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday RS, MacKenzie D, 1983. Monoclonal antibodies to rhodopsin: characterization, cross-reactivity, and application as structural probes. Biochemistry 22, 653–660. [DOI] [PubMed] [Google Scholar]
- Molla-Herman A, Ghossoub R, Blisnick T, Meunier A, Serres C, Silbermann F, Emmerson C, Romeo K, Bourdoncle P, Schmitt A, Saunier S, Spassky N, Bastin P, Benmerah A, 2010. The ciliary pocket: an endocytic membrane domain at the base of primary and motile cilia. J. Cell Sci. 123, 1785–1795. 10.1242/jcs.059519. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Hunter JJ, Masella B, Wolfe R, Gray DC, Merigan WH, Delori FC, Williams DR, 2008. Light-induced retinal changes observed with high-resolution autofluorescence imaging of the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 49, 3715–3729. 10.1167/iovs.07-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen RJ, LaVail MM, 1976. Inherited retinal dystrophy: primary defect in pigment epithelium determined with experimental rat chimeras. Science 192, 799–801. 10.1126/science.1265483. [DOI] [PubMed] [Google Scholar]
- Müller C, Charniga C, Temple S, Finnemann SC, 2018. Quantified F-actin morphology is predictive of phagocytic capacity of stem cell-derived retinal pigment epithelium. Stem. Cell. Rep. 10, 1075–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniz-Feliciano L, Doggett TA, Zhou Z, Ferguson TA, 2017. RUBCN/rubicon and EGFR regulate lysosomal degradative processes in the retinal pigment epithelium (RPE) of the eye. Autophagy 13, 2072–2085. 10.1080/15548627.2017.1380124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafi D, Kevany BM, Genoud C, Bai X, Palczewski K, 2013. Photoreceptor phagocytosis is mediated by phosphoinositide signaling. Faseb. J. 27, 4585–4595. 10.1096/fj.13-237537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi IR, Mathews AP, Cohen-Gould L, Gundersen D, Rodriguez-Boulan E, 1993. Immortalization of polarized rat retinal pigment epithelium. J. Cell Sci. 104, 37–49. [DOI] [PubMed] [Google Scholar]
- Nandrot EF, 2014. Animal models. In: “the Quest to Decipher RPE Phagocytosis”, Retinal Degenerative Diseases. Springer, pp. 77–83. [DOI] [PubMed] [Google Scholar]
- Nandrot E, Dufour EM, Provost AC, Pequignot MO, Bonnel S, Gogat K, Marchant D, Rouillac C, Sepulchre de Conde B, Bihoreau MT, Shaver C, Dufier JL, Marsac C, Lathrop M, Menasche M, Abitbol MM, 2000. Homozygous deletion in the coding sequence of the c-mer gene in RCS rats unravels general mechanisms of physiological cell adhesion and apoptosis. Neurobiol. Dis. 7, 586–599. 10.1006/nbdi.2000.0328. [DOI] [PubMed] [Google Scholar]
- Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, Finnemann SC, 2004. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking αvβ5 integrin. J. Exp. Med. 200, 1539–1545. 10.1084/jem.20041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandrot EF, Anand M, Sircar M, Finnemann SC, 2006. Novel role for αvβ5-integrin in retinal adhesion and its diurnal peak. Am. J. Physiol. Cell Physiol. 290, C1256–C1262. 10.1152/ajpcell.00480.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandrot EF, Anand M, Almeida D, Atabai K, Sheppard D, Finnemann SC, 2007. Essential role for MFG-E8 as ligand for αvβ5 integrin in diurnal retinal phagocytosis. Proc. Natl. Acad. Sci. Unit. States Am. 104, 12005–12010. 10.1073/pnas.0704756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandrot EF, Silva KE, Scelfo C, Finnemann SC, 2012. Retinal pigment epithelial cells use a MerTK-dependent mechanism to limit the phagocytic particle binding activity of αvβ5 integrin. Biol. Cell. 104, 326–341. 10.1111/boc.201100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan V, Ly T, Pourkarimi E, Murillo AB, Gartner A, Lamond AI, Kenyon C, 2016. Deep proteome analysis identifies age-related processes in C. elegans. Cell Syst. 3, 144–159. 10.1016/j.cels.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra A, Greiner B, Ribi WA, Zeil J, 2016. Light and dark adaptation mechanisms in the compound eyes of Myrmecia ants that occupy discrete temporal niches. J. Exp. Biol. 219, 2435–2442. 10.1242/jeb.142018. [DOI] [PubMed] [Google Scholar]
- Nilsson SEG, 1978. Ultrastructural organization of the retinal pigment epithelium of the cynomolgus monkey. Acta Ophthalmol. 56, 883–901. 10.1111/j.1755-3768.1978.tb03809.x. [DOI] [PubMed] [Google Scholar]
- Nixon RA, 2013. The role of autophagy in neurodegenerative disease. Nat. Med. 19, 983–997. 10.1038/nm.3232. [DOI] [PubMed] [Google Scholar]
- Nojima S, et al. , 2013. A point mutation in Semaphorin 4A associates with defective endosomal sorting and causes retinal degeneration. Nat. Commun. 4, 1406. 10.1038/ncomms2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordgren M, Wang B, Apanasets O, Fransen M, 2013. Peroxisome degradation in mammals: mechanisms of action, recent advances, and perspectives. Front. Physiol. 4, 145. 10.3389/fphys.2013.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notomi S, et al. , 2019. Genetic LAMP2 deficiency accelerates the age-associated formation of basal laminar deposits in the retina. Proc. Natl. Acad. Sci. U. S. A. 116, 23724–23734. 10.1073/pnas.1906643116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo A, Sameshima M, Unoki K, Uehara F, Bird AC, 2000. Ultrastructural changes associated with accumulation of inclusion bodies in rat retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 41, 4305–4312. [PubMed] [Google Scholar]
- Olchawa MM, Herrnreiter AM, Skumatz CM, Zareba M, Sarna TJ, Burke JM, 2013. Photosensitized oxidative stress to ARPE-19 cells decreases protein receptors that mediate photoreceptor outer segment phagocytosis. Invest. Ophthalmol. Vis. Sci. 54, 2276–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opas M, 1989. Expression of the differentiated phenotype by epithelial cells in vitro is regulated by both biochemistry and mechanics of the substratum. Dev. Biol. 131, 281–293. 10.1016/s0012-1606(89)80001-6. [DOI] [PubMed] [Google Scholar]
- Osterberg GA, 1935. Topography of the layer of rods and cones in the human retina. Acta Ophthalmol. 6, 1–10. [Google Scholar]
- Palczewska G, Dong Z, Golczak M, Hunter JJ, Williams DR, Alexander NS, Palczewski K, 2014. Noninvasive two-photon microscopy imaging of mouse retina and retinal pigment epithelium through the pupil of the eye. Nat. Med. 20, 785. 10.1038/nm.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, Lee JY, Park KH, Jung IK, Kim KT, Lee YS, Ryu HH, Jeong Y, Kang M, Schwaninger M, Gulbins E, Reichel M, Kornhuber J, Yamaguchi T, Kim HJ, Kim SH, Schuchman EH, Jin HK, Bae JS, 2018. Vascular and neurogenic rejuvenation in aging mice by modulation of ASM. Neuron 100, 762. 10.1016/j.neuron.2018.10.038. [DOI] [PubMed] [Google Scholar]
- Penberthy KK, Rival C, Shankman LS, Raymond MH, Zhang J, Perry JS, Lee CS, Han CZ, Onengut-Gumuscu S, Palczewski K, 2017. Context-dependent compensation among phosphatidylserine-recognition receptors. Sci. Rep. 7, 14623. 10.1038/s41598-017-15191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Rao VS, Adelman RA, Rizzolo LJ, 2011. Claudin-19 and the barrier properties of the human retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 52, 1392–1403. 10.1167/iovs.10-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Bay AE, Schreiner R, Mazzoni F, Carvajal-Gonzalez JM, Gravotta D, Perret E, Lehmann Mantaras G, Zhu YS, Rodriguez-Boulan EJ, 2013. The kinesin KIF16B mediates apical transcytosis of transferrin receptor in AP-1B-deficient epithelia. EMBO J. 32, 2125–2139. 10.1038/emboj.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Bay AE, Schreiner R, Benedicto I, Rodriguez-Boulan EJ, 2014. Galectin-4-mediated transcytosis of transferrin receptor. J. Cell Sci. 127, 4457–4469. 10.1242/jcs.153437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perusek L, Sahu B, Parmar T, Maeno H, Arai E, Le YZ, Subauste CS, Chen Y, Palczewski K, Maeda A, 2015. Di-retinoid-pyridinium-ethanolamine (A2E) accumulation and the maintenance of the visual cycle are independent of atg7-mediated autophagy in the retinal pigmented epithelium. J. Biol. Chem. 290, 29035–29044. 10.1074/jbc.M115.682310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Reinthal E, Blitgen-Heinecke P, Bartz-Schmidt K-U, Schraermeyer U, 2006. Inhibition of lysosomal degradation in retinal pigment epithelium cells induces exocytosis of phagocytic residual material at the basolateral plasma membrane. Ophthalmic Res. 38, 83–88. 10.1159/000090268. [DOI] [PubMed] [Google Scholar]
- Petrou PA, Cunningham D, Shimel K, Harrington M, Hammel K, Cukras CA, Ferris FL, Chew EY, Wong WT, 2014. Intravitreal sirolimus for the treatment of geographic atrophy: results of a phase I/II clinical trial. Invest. Ophthalmol. Vis. Sci. 56, 330–338. 10.1167/iovs.14-15877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer BA, Philp NJ, 2014. Cell culture of retinal pigment epithelium: special Issue. Exp. Eye Res. 126, 1–4. 10.1016/j.exer.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Phillips SE, Woodruff EA 3rd, Liang P, Patten M, Broadie K, 2008. Neuronal loss of Drosophila NPC1a causes cholesterol aggregation and age-progressive neurodegeneration. J. Neurosci. 28, 6569–6582. 10.1523/JNEUROSCI.5529-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles S, Vigie P, Youle RJ, 2018. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr. Biol. 28, R170–R185. 10.1016/j.cub.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirie A, 1961. Cholesterol in the tapetum lucidum of the eye of of the opossum, Didelphis virginiana. Nature 191, 708–709. 10.1038/191708b0. [DOI] [PubMed] [Google Scholar]
- Platta HW, Brinkmeier R, Reidick C, Galiani S, Clausen MP, Eggeling C, 2016. Regulation of peroxisomal matrix protein import by ubiquitination. Bba-Mol Cell Res 1863, 838–849. 10.1016/j.bbamcr.2015.09.010. [DOI] [PubMed] [Google Scholar]
- Prasad D, Rothlin CV, Burrola P, Burstyn-Cohen T, Lu Q, de Frutos PG, Lemke G, 2006. TAM receptor function in the retinal pigment epithelium. Mol. Cell. Neurosci. 33, 96–108. 10.1016/j.mcn.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Radu RA, Hu J, Yuan Q, Welch DL, Makshanoff J, Lloyd M, McMullen S, Travis GH, Bok D, 2011. Complement system dysregulation and inflammation in the retinal pigment epithelium of a mouse model for stargardt macular degeneration. J. Biol. Chem. 286, 18593–18601. 10.1074/jbc.M110.191866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu RA, Hu J, Jiang Z, Bok D, 2014. Bisretinoid-mediated complement activation on retinal pigment epithelial cells is dependent on complement factor H haplotype. J. Biol. Chem. 289, 9113–9120. 10.1074/jbc.M114.548669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahner C, Fukuhara M, Peng S, Kojima S, Rizzolo LJ, 2004. The apical and basal environments of the retinal pigment epithelium regulate the maturation of tight junctions during development. J. Cell Sci. 117, 3307–3318. 10.1242/jcs.01181. [DOI] [PubMed] [Google Scholar]
- Rakoczy PE, Mann K, Cavaney DM, Robertson T, Papadimitreou J, Constable IJ, 1994. Detection and possible functions of a cysteine protease involved in digestion of rod outer segments by retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 35, 4100–4108. [PubMed] [Google Scholar]
- Rakoczy PE, Baines M, Kennedy CJ, Constable IJ, 1996. Correlation between autofluorescent debris accumulation and the presence of partially processed forms of cathepsin D in cultured retinal pigment epithelial cells challenged with rod outer segments. Exp. Eye Res. 63, 159–167. 10.1006/exer.1996.0104. [DOI] [PubMed] [Google Scholar]
- Rakoczy PE, Lai CM, Baines M, Di Grandi S, Fitton JH, Constable IJ, 1997. Modulation of cathepsin D activity in retinal pigment epithelial cells. Biochem. J. 324, 935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoczy PE, Zhang D, Robertson T, Barnett NL, Papadimitriou J, Constable IJ, Lai CM, 2002. Progressive age-related changes similar to age-related macular degeneration in a transgenic mouse model. Am. J. Pathol. 161, 1515–1524. 10.1016/s0002-9440(10)64427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandra Rao S, Pfeffer BA, Mas Gomez N, Skelton LA, Keiko U, Sparrow JR, Rowsam AM, Mitchell CH, Fliesler SJ, 2018. Compromised phagosome maturation underlies RPE pathology in cell culture and whole animal models of Smith-Lemli-Opitz Syndrome. Autophagy 14, 1796–1817. 10.1080/15548627.2018.1490851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambhatla L, Chiu CP, Glickman RD, Rowe-Rendleman C, 2002. In vitro differentiation capacity of telomerase immortalized human RPE cells. Invest. Ophthalmol. Vis. Sci. 43, 1622–1630. [PubMed] [Google Scholar]
- Regan C, De Grip W, Daemen F, Bonting S, 1980. Degradation of rhodopsin by a lysosomal fraction of retinal pigment epithelium: biochemical aspects of the visual process. XLI. Exp. Eye Res. 30, 183–191. 10.1016/0014-4835(80)90112-8. [DOI] [PubMed] [Google Scholar]
- Reme CE, Wolfrum U, Imsand C, Hafezi F, Williams TP, 1999. Photoreceptor autophagy: effects of light history on number and opsin content of degradative vacuoles. Invest. Ophthalmol. Vis. Sci. 40, 2398–2404. [PubMed] [Google Scholar]
- Reyes-Reveles J, Dhingra A, Alexander D, Bragin A, Philp NJ, Boesze-Battaglia K, 2017. Phagocytosis-dependent ketogenesis in retinal pigment epithelium. J. Biol. Chem. 292, 8038–8047. 10.1074/jbc.M116.770784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Kreitzer G, Musch A, 2005. Organization of vesicular trafficking in epithelia. Nat. Rev. Mol. Cell Biol. 6, 233–247. [DOI] [PubMed] [Google Scholar]
- Ruggiero L, Finnemann SC, 2014. Rhythmicity of the Retinal Pigment Epithelium, the Retina and Circadian Rhythms. Springer, pp. 95–112. [Google Scholar]
- Ruggiero L, Connor MP, Chen J, Langen R, Finnemann SC, 2012. Diurnal, localized exposure of phosphatidylserine by rod outer segment tips in wild-type but not Itgb5−/− or Mfge8−/− mouse retina. Proc. Natl. Acad. Sci. Unit. States Am. 109, 8145–8148. 10.1073/pnas.1121101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryeom SW, Sparrow JR, Silverstein RL, 1996. CD36 participates in the phagocytosis of rod outer segments by retinal pigment epithelium. J. Cell Sci. 109, 387–395. [DOI] [PubMed] [Google Scholar]
- Samuel W, Jaworski C, Postnikova OA, Kutty RK, Duncan T, Tan LX, Poliakov E, Lakkaraju A, Redmond TM, 2017. Appropriately differentiated ARPE-19 cells regain phenotype and gene expression profiles similar to those of native RPE cells. Mol. Vis. 23, 60–89. [PMC free article] [PubMed] [Google Scholar]
- Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, Green DR, 2007. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 450, 1253–1257. 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- Satoh AK, Ready DF, 2005. Arrestin1 mediates light-dependent rhodopsin endocytosis and cell survival. Curr. Biol. 15, 1722–1733. 10.1016/j.cub.2005.08.064. [DOI] [PubMed] [Google Scholar]
- Schrader M, Pellegrini L, 2017. The making of a mammalian peroxisome, version 2.0: mitochondria get into the mix. Cell Death Differ. 24, 1148–1152. 10.1038/cdd.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader M, Reuber BE, Morrell JC, Jimenez-Sanchez G, Obie C, Stroh TA, Valle D, Schroer TA, Gould SJ, 1998. Expression of PEX11 beta mediates peroxisome proliferation in the absence of extracellular stimuli. J. Biol. Chem. 273, 29607–29614. 10.1074/jbc.273.45.29607. [DOI] [PubMed] [Google Scholar]
- Schrader M, Wodopia R, Fahimi HD, 1999. Induction of tubular peroxisomes by UV irradiation and reactive oxygen species in HepG2 cells. J. Histochem. Cytochem. 47, 1141–1148. 10.1177/002215549904700906. [DOI] [PubMed] [Google Scholar]
- Schrader M, Bonekamp NA, Islinger M, 2012. Fission and proliferation of peroxisomes. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 1822, 1343–1357. 10.1016/j.bbadis.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Schraermeyer U, Peters S, Thumann G, Kociok N, Heimann K, 1999. Melanin granules of retinal pigment epithelium are connected with the lysosomal degradation pathway. Exp. Eye Res. 68, 237–245. [DOI] [PubMed] [Google Scholar]
- Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK, 2001. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411, 207–211. 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- Seabra MC, Brown MS, Slaughter CA, Sudhof TC, Goldstein JL, 1992. Purification of component A of Rab geranylgeranyl transferase: possible identity with the choroideremia gene product. Cell 70, 1049–1057. 10.1016/0092-8674(92)90253-9. [DOI] [PubMed] [Google Scholar]
- Segawa K, Nagata S, 2015. An apoptotic ‘eat me’ signal: phosphatidylserine exposure. Trends Cell Biol. 25, 639–650. 10.1016/j.tcb.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Seigneurin-Berny D, Verdel A, Curtet S, Lemercier C, Garin J, Rousseaux S, Khochbin S, 2001. Identification of components of the murine histone deacetylase 6 complex: link between acetylation and ubiquitination signaling pathways. Mol. Cell Biol. 21, 8035–8044. 10.1128/mcb.21.23.8035-8044.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethna S, Chamakkala T, Gu X, Thompson TC, Cao G, Elliott MH, Finnemann SC, 2016. Regulation of phagolysosomal digestion by caveolin-1 of the retinal pigment epithelium is essential for vision. J. Biol. Chem. 291, 6494–6506. 10.1074/jbc.M115.687004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N, Ishii M, Brandon C, Ablonczy Z, Cai J, Liu Y, Chou CJ, Rohrer B, 2018. Extracellular vesicle-mediated long-range communication in stressed retinal pigment epithelial cell monolayers. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 1864, 2610–2622. 10.1016/j.bbadis.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, et al. , 2019. Clinical-grade stem cell-derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci. Transl. Med. 11. 10.1126/scitranslmed.aat5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelby SJ, Colwill K, Dhe-Paganon S, Pawson T, Thompson DA, 2013. MERTK interactions with SH2-domain proteins in the retinal pigment epithelium. PloS One 8, e53964. 10.1371/journal.pone.0053964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasawa M, Sonoda S, Terasaki H, Arimura N, Otsuka H, Yamashita T, Uchino E, Hisatomi T, Ishibashi T, Sakamoto T, 2013. TNF-alpha disrupts morphologic and functional barrier properties of polarized retinal pigment epithelium. Exp. Eye Res. 110, 59–69. 10.1016/j.exer.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Sinha D, Valapala M, Shang P, Hose S, Grebe R, Lutty GA, Zigler JS Jr., Kaarniranta K, Handa JT, 2016. Lysosomes: regulators of autophagy in the retinal pigmented epithelium. Exp. Eye Res. 144, 46–53. 10.1016/j.exer.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Simoes-Spassov S, Mayeux R, Petsko GA, 2017. Endosomal traffic jams represent a pathogenic hub and therapeutic target in alzheimer’s disease. Trends Neurosci. 40, 592–602. 10.1016/j.tins.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Mitter SK, Qi X, Beli E, Rao HV, Ding J, Ip CS, Gu H, Akin D, Dunn WA Jr., Bowes Rickman C, Lewin AS, Grant MB, Boulton ME, 2017. Oxidative stress-mediated NFkappaB phosphorylation upregulates p62/SQSTM1 and promotes retinal pigmented epithelial cell survival through increased autophagy. PloS One 12, e0171940. 10.1371/journal.pone.0171940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda S, Spee C, Barron E, Ryan SJ, Kannan R, Hinton DR, 2009. A protocol for the culture and differentiation of highly polarized human retinal pigment epithelial cells. Nat. Protoc. 4, 662–673. 10.1038/nprot.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekumar PG, Kannan R, Kitamura M, Spee C, Barron E, Ryan SJ, Hinton DR, 2010. alphaB crystallin is apically secreted within exosomes by polarized human retinal pigment epithelium and provides neuroprotection to adjacent cells. PloS One 5, e12578. 10.1371/journal.pone.0012578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg R, Wood I, Hogan M, 1977. Pigment epithelial ensheathment and phagocytosis of extrafoveal cones in human retina. Philos. Trans. R. Soc. Lond. B Biol. Sci. 277, 459–471. 10.1098/rstb.1977.0028. [DOI] [PubMed] [Google Scholar]
- Strauss O, 2005. The retinal pigment epithelium in visual function. Physiol. Rev. 85, 845–881. 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- Strick DJ, Feng W, Vollrath D, 2009. Mertk drives myosin II redistribution during retinal pigment epithelial phagocytosis. Invest. Ophthalmol. Vis. Sci. 50, 2427–2435. 10.1167/iovs.08-3058. [DOI] [PubMed] [Google Scholar]
- Strunnikova N, Maminishkis A, Barb J, Wang F, Zhi C, Sergeev Y, Chen W, Edwards A, Stambolian D, Abecasis G, 2010. Transcriptome analysis and molecular signature of human retinal pigment epithelium. Hum. Mol. Genet. 19, 2468–2486. 10.1093/hmg/ddq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura A, Mattie S, Prudent J, McBride HM, 2017. Newly born peroxisomes are a hybrid of mitochondrial and ER-derived pre-peroxisomes. Nature 542, 251–254. 10.1038/nature21375. [DOI] [PubMed] [Google Scholar]
- Sun M, Finnemann SC, Febbraio M, Shan L, Annangudi SP, Podrez EA, Hoppe G, Darrow R, Organisciak DT, Salomon RG, 2006. Light-induced oxidation of photoreceptor outer segment phospholipids generates ligands for CD36-mediated phagocytosis by retinal pigment epithelium: a potential mechanism for modulating outer segment phagocytosis under oxidant stress conditions. J. Biol. Chem. 281, 4222–4230. 10.1074/jbc.M509769200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Westphal W, Wong KN, Tan I, Zhong Q, 2010. Rubicon controls endosome maturation as a Rab7 effector. Proc. Natl. Acad. Sci. U. S. A. 107, 19338–19343. 10.1073/pnas.1010554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup A, Samuels IS, Bell BA, Han JYS, Du J, Massenzio E, Abel ED, Boesze-Battaglia K, Peachey NS, Philp NJ, 2019. Modulating GLUT1 expression in retinal pigment epithelium decreases glucose levels in the retina: impact on photoreceptors and Muller glial cells. Am. J. Physiol. Cell Physiol. 316, C121–c133. 10.1152/ajpcell.00410.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai M, Teirstein P, Goldman A, O’Brien P, Chader G, 1978. The pineal gland does not control rod outer segment shedding and phagocytosis in the rat retina and pigment epithelium. Invest. Ophthalmol. Vis. Sci. 17, 558–562. [PubMed] [Google Scholar]
- Tan E, Ding XQ, Saadi A, Agarwal N, Naash MI, Al-Ubaidi MR, 2004. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Invest. Ophthalmol. Vis. Sci. 45, 764–768. 10.1167/iovs.03-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LX, Toops KA, Lakkaraju A, 2016. Protective responses to sublytic complement in the retinal pigment epithelium. Proc. Natl. Acad. Sci. U. S. A. 10.1073/pnas.1523061113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teirstein PS, Goldman AI, O’Brien P, 1980. Evidence for both local and central regulation of rat rod outer segment disc shedding. Invest. Ophthalmol. Vis. Sci. 19, 1268–1273. [PubMed] [Google Scholar]
- Terlecky SR, Koepke JI, Walton PA, 2006. Peroxisomes and aging. Biochim. Biophys. Acta 1763, 1749–1754. 10.1016/j.bbamcr.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman JS, Reme CE, Terman M, 1993. Rod outer segment disk shedding in rats with lesions of the suprachiasmatic nucleus. Brain Res. 605, 256–264. [DOI] [PubMed] [Google Scholar]
- Thiadens AA, Slingerland NW, Florijn RJ, Visser GH, Riemslag FC, Klaver CC, 2012. Cone-rod dystrophy can be a manifestation of Danon disease. Graefes Arch. Clin. Exp. Ophthalmol. 250, 769–774. 10.1007/s00417-011-1857-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DA, Constable PA, Liasis A, Walters B, Esteban MT, 2016. The physiology of the retinal pigment epithelium in Danon disease. Retina 36, 629–638. 10.1097/IAE.0000000000000736. [DOI] [PubMed] [Google Scholar]
- Toops KA, Tan LX, Lakkaraju A, 2014. A detailed three-step protocol for live imaging of intracellular traffic in polarized primary porcine RPE monolayers. Exp. Eye Res. 124, 74–85. 10.1016/j.exer.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toops KA, Tan LX, Jiang Z, Radu RA, Lakkaraju A, 2015. Cholesterol-mediated activation of acid sphingomyelinase disrupts autophagy in the retinal pigment epithelium. Mol. Biol. Cell 26, 1–14. 10.1091/mbc.E14-05-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T, Nojima S, Ishikawa T, Takamatsu H, Tsujimura T, Uemura A, Matsuda J, Seki T, Kumanogoh A, 2012. Endosomal sorting by Semaphorin 4A in retinal pigment epithelium supports photoreceptor survival. Genes Dev. 26, 816–829. 10.1101/gad.184481.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis GH, Kaylor J, Yuan Q, 2010. Analysis of the retinoid isomerase activities in the retinal pigment epithelium and retina. Methods Mol. Biol. 652, 329–339. 10.1007/978-1-60327-325-1_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi D, Colin E, Louie CM, Williams DS, 2012. Live-cell imaging evidence for the ciliary transport of rod photoreceptor opsin by heterotrimeric Kinesin-2. J. Neurosci. 32, 10587–10593. 10.1523/JNEUROSCI.0015-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troutt LL, Burnside B, 1989. Role of microtubules in pigment granule migration in teleost retinal pigment epithelial cells. Exp. Eye Res. 48, 433–443. [DOI] [PubMed] [Google Scholar]
- Turowski P, Adamson P, Sathia J, Zhang JJ, Moss SE, Aylward GW, Hayes MJ, Kanuga N, Greenwood J, 2004. Basement membrane-dependent modification of phenotype and gene expression in human retinal pigment epithelial ARPE-19 cells. Invest. Ophthalmol. Vis. Sci. 45, 2786–2794. 10.1167/iovs.03-0943. [DOI] [PubMed] [Google Scholar]
- Umapathy A, Williams DS, 2019. Live imaging of organelle motility in RPE flatmounts. Adv. Exp. Med. Biol. 1185, 389–393. 10.1007/978-3-030-27378-1_64. [DOI] [PubMed] [Google Scholar]
- Valapala M, Wilson C, Hose S, Bhutto IA, Grebe R, Dong A, Greenbaum S, Gu L, Sengupta S, Cano M, 2014. Lysosomal-mediated waste clearance in retinal pigment epithelial cells is regulated by CRYBA1/βA3/A1-crystallin via V-ATPase-MTORC1 signaling. Autophagy 10, 480–496. 10.4161/auto.27292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valm AM, Cohen S, Legant WR, Melunis J, Hershberg U, Wait E, Cohen AR, Davidson MW, Betzig E, Lippincott-Schwartz J, 2017. Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature 546, 162–167. 10.1038/nature22369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel G, D’Angelo G, Raposo G, 2018. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213–228. 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- Volland S, Esteve-Rudd J, Hoo J, Yee C, Williams DS, 2015. A comparison of some organizational characteristics of the mouse central retina and the human macula. PloS One 10, e0125631. 10.1371/journal.pone.0125631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath D, Yasumura D, Benchorin G, Matthes MT, Feng W, Nguyen NM, Sedano CD, Calton MA, LaVail MM, 2015. Tyro3 modulates Mertk-associated retinal degeneration. PLoS Genet. 11, e1005723. 10.1371/journal.pgen.1005723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald G, Brown PK, 1956. Synthesis and bleaching of rhodopsin. Nature 177, 174–176. [DOI] [PubMed] [Google Scholar]
- Walker CL, Pomatto LCD, Tripathi DN, Davies KJA, 2018. Redox regulation of homeostasis and proteostasis in peroxisomes. Physiol. Rev. 98, 89–115. 10.1152/physrev.00033.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls GL, 1939. Notes on the retinae of two opossum genera. J. Morphol. 64. [Google Scholar]
- Wang L, Cano M, Handa JT, 2014. p62 provides dual cytoprotection against oxidative stress in the retinal pigment epithelium. Biochim. Biophys. Acta 1843, 1248–1258. 10.1016/j.bbamcr.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, et al. , 2019. Metabolic deregulation of the blood-outer retinal barrier in retinitis pigmentosa. Cell Rep. 28, 1323–1334. 10.1016/j.celrep.2019.06.093.e1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassélius J, Wallin H, Abrahamson M, Ehinger B, 2003. Cathepsin B in the rat eye. Graefe’s Arch. Clin. Exp. Ophthalmol. 241, 934–942. 10.1007/s00417-003-0782-x. [DOI] [PubMed] [Google Scholar]
- Wavre-Shapton ST, Tolmachova T, da Silva ML, Futter CE, Seabra MC, 2013. Conditional ablation of the choroideremia gene causes age-related changes in mouse retinal pigment epithelium. PlLoS One 8, e57769. 10.1371/journal.pone.0057769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wavre-Shapton ST, Meschede IP, Seabra MC, Futter CE, 2014. Phagosome maturation during endosome interaction revealed by partial rhodopsin processing in retinal pigment epithelium. J. Cell Sci. 127, 3852–3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wavre-Shapton ST, Calvi AA, Turmaine M, Seabra MC, Cutler DF, Futter CE, Mitchison HM, 2015. Photoreceptor phagosome processing defects and disturbed autophagy in retinal pigment epithelium of Cln3Δex1–6 mice modelling juvenile neuronal ceroid lipofuscinosis (Batten disease). Hum. Mol. Genet. 24, 7060–7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil D, Blanchard S, Kaplan J, Guilford P, Gibson F, Walsh J, Mburu P, Varela A, Levilliers J, Weston MD, Kelley PM, Kimberling WJ, Wagenaar M, LeviAcobas F, Larget-Piet D, Munnich A, Steel KP, Brown SDM, Petit C, 1995. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature 374, 60–61. [DOI] [PubMed] [Google Scholar]
- Williams DS, 1979. The physiological optics of a nocturnal semi-aquatic spider, Dolomedes aquaticus (Pisauridae). Z. Naturforsch. 34c, 463–469. 10.1515/znc-1979-5-625. [DOI] [Google Scholar]
- Williams DS, 1980. Organisation of the compound eye of a tipulid fly during the day and night. Zoomorphologie 95, 85–104. [Google Scholar]
- Williams DS, 1982a. Ommatidial structure in relation to turnover of photoreceptor membrane in the locust. Cell Tissue Res. 225, 595–617. [DOI] [PubMed] [Google Scholar]
- Williams DS, 1982b. Photoreceptor membrane shedding and assembly can be initiated locally within an insect retina. Science 218, 898–900. 10.1126/science.7134980. [DOI] [PubMed] [Google Scholar]
- Williams DS, 1982c. Rhabdom size and photoreceptor membrane turnover in a muscoid fly. Cell Tissue Res. 226, 629–639. [DOI] [PubMed] [Google Scholar]
- Williams DS, 1983. Changes in photoreceptor performance associated with the daily turnover of photoreceptor membrane in locusts. J. Comp. Physiol. 150, 509–519. [Google Scholar]
- Williams DS, Blest AD, 1980. Extracellular shedding of photoreceptor membrane in the open rhabdom of a tipulid fly. Cell Tissue Res. 205, 423–438. [DOI] [PubMed] [Google Scholar]
- Williams DS, Fisher S, 1987. Prevention of rod disk shedding by detachment from the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 28, 184–187. [PubMed] [Google Scholar]
- Williams DS, Roberts EA, 1992. Modification of the daily photoreceptor membrane shedding response in vitro by antioxidants. Invest. Ophthalmol. Vis. Sci. 33, 3005–3008. [PubMed] [Google Scholar]
- Wimmers S, Karl MO, Strauss O, 2007. Ion channels in the RPE. Prog. Retin. Eye Res. 26, 263–301. 10.1016/j.preteyeres.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Wong SW, Sil P, Martinez J, 2018. Rubicon: LC3-associated phagocytosis and beyond. FEBS J. 285, 1379–1388. 10.1111/febs.14354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia CH, Liu H, Cheung D, Tang F, Chang B, Li M, Gong X, 2015. NHE8 is essential for RPE cell polarity and photoreceptor survival. Sci. Rep. 5, 9358. 10.1038/srep09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia CH, Ferguson I, Li M, Kim A, Onishi A, Li L, Su B, Gong X, 2018. Essential function of NHE8 in mouse retina demonstrated by AAV-mediated CRISPR/Cas9 knockdown. Exp. Eye Res. 176, 29–39. 10.1016/j.exer.2018.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Toops KA, Diaz F, Carvajal-Gonzalez JM, Gravotta D, Mazzoni F, Schreiner R, Rodriguez-Boulan E, Lakkaraju A, 2012. Mechanism of polarized lysosome exocytosis in epithelial cells. J. Cell Sci. 125, 5937–5943. 10.1242/jcs.109421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam M, Engel AL, Wang Y, Zhu S, Hauer A, Zhang R, Lohner D, Huang J, Dinterman M, Zhao C, 2019. Proline mediates metabolic communication between retinal pigment epithelial cells and the retina. J. Biol. Chem. 294, 10278–11028. 10.1074/jbc.RA119.007983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Jia L, Shelby SJ, Ganios AM, Feathers K, Thompson DA, Zacks DN, 2014. Circadian and noncircadian modulation of autophagy in photoreceptors and retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 55, 3237–3246. 10.1167/iovs.13-13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Jia L, Khan N, Lin C, Mitter SK, Boulton ME, Dunaief JL, Klionsky DJ, Guan JL, Thompson DA, Zacks DN, 2015. Deletion of autophagy inducer RB1CC1 results in degeneration of the retinal pigment epithelium. Autophagy 11, 939–953. 10.1080/15548627.2015.1041699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Fahimi HD, 2009. Degradation of excess peroxisomes in mammalian liver cells by autophagy and other mechanisms. Histochem. Cell Biol. 131, 455–458. 10.1007/s00418-009-0564-6. [DOI] [PubMed] [Google Scholar]
- Yokota S, Oda T, Fahimi HD, 2001. The role of 15-lipoxygenase in disruption of the peroxisomal membrane and in programmed degradation of peroxisomes in normal rat liver. J. Histochem. Cytochem. 49, 613–622. 10.1177/002215540104900508. [DOI] [PubMed] [Google Scholar]
- Young RW, 1967. The renewal of photoreceptor cell outer segments. J. Cell Biol. 33, 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW, 1977. The daily rhythm of shedding and degradation of cone outer segment membranes in the lizard retina. J. Ultra. Res. 61, 172–185. 10.1016/s0022-5320(77)80084-1. [DOI] [PubMed] [Google Scholar]
- Young RW, 1978. The daily rhythm of shedding and degradation of rod and cone outer segment membranes in the chick retina. Invest. Ophthalmol. Vis. Sci. 17, 105–116. [PubMed] [Google Scholar]
- Young RW, Bok D, 1969. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J. Cell. Biol. 42, 392–403. 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JM, Nelson JW, Cheng J, Zhang WR, Mader S, Davis CM, Morrison RS, Alkayed NJ, 2015. Peroxisomal biogenesis in ischemic brain. Antioxidants Redox Signal. 22, 109–120. 10.1089/ars.2014.5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Egbejimi A, Dharmat R, Xu P, Zhao Z, Long B, Miao H, Chen R, Wensel TG, Cai J, 2018. Phagocytosed photoreceptor outer segments activate mTORC1 in the retinal pigment epithelium. Sci. Signal. 11 eaag3315. 10.1126/scisignal.aag3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yang Y, Li S, Zhang S, Zhu X, Tai Z, Yang M, Liu Y, Guo X, Chen B, 2017. Loss of Tmem30a leads to photoreceptor degeneration. Sci. Rep. 7, 9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Presswalla F, Feathers K, Cao X, Hughes BA, Zacks DN, Thompson DA, Miller JM, 2019. A platform for assessing outer segment fate in primary human fetal RPE cultures. Exp. Eye Res. 178, 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Yasumura D, Li X, Matthes M, Lloyd M, Nielsen G, Ahern K, Snyder M, Bok D, Dunaief JL, LaVail MM, Vollrath D, 2011. mTOR-mediated dedifferentiation of the retinal pigment epithelium initiates photoreceptor degeneration in mice. J. Clin. Invest. 121, 369–383. 10.1172/jci44303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman WF, Godchaux W III, Belkin M, 1983. The relative proportions of lysosomal enzyme activities in bovine retinal pigment epithelium. Exp. Eye Res. 36, 151–158. 10.1016/0014-4835(83)90098-2. [DOI] [PubMed] [Google Scholar]
- Zurdel J, Finckh U, Menzer G, Nitsch RM, Richard G, 2002. CST3 genotype associated with exudative age related macular degeneration. Br. J. Ophthalmol. 86, 214–219. 10.1136/bjo.86.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]