Abstract
The main aim of the present systematic review was to summarize the most frequently used telomerase regulators with an impact on aging and cancer that are referred to in in vitro and in vivo studies. For this purpose, a systematic review of the available literature on telomerase regulators referred to in articles from PubMed and Scopus libraries published from 2002 to 2021 and in accordance with PRISMA 2020 criteria, was conducted. Articles were included if they met the following criteria: They referred to telomerase modulators in aging and in cancer and were in vitro and/or in vivo studies, while studies that did not provide sufficient data or studies not written in English were excluded. In the present systematic review, 54 publications were included, of which 29 were full-text published studies, 11 were full-text reviews, 10 structure-based design studies and 4 abstracts are reported in this review. Telomerase regulators were then categorized as synthetic direct telomerase inhibitors, synthetic indirect telomerase inhibitors, synthetic telomerase activators, natural direct telomerase activators, natural telomerase inhibitors and natural indirect telomerase activators, according to their origin and their activity. On the whole, as demonstrated herein, telomerase regulators appear to be promising treatment agents in various age-related diseases. However, further in vivo and in vitro studies need to be performed in order to clarify the potentiality of telomerase as a therapeutic target.
Keywords: telomerase enzyme, inhibitors, activators, telomere length, regulators
Introduction
The ends of chromosomes, termed telomeres, consist of 5–15 kb double-stranded (ds) telomeric repeats 5′-TTAGGG-3′, which result in a single-stranded (ss) 3′ G-overhang. G-overhangs can form a G-quadruplex structure, which is crucial for telomeric stability (1). Telomeres are important for ensuring chromosomal integrity and their importance to cellular function is indicated by the evolutionary conservation of their repetitive sequences. However, due to the inability of DNA polymerase to replicate the end of the chromosome during lagging strand synthesis, there is a gradual loss of telomeric repeats in each cell division, with telomeres eventually becoming critically short, leading to cell senescence. If cells fail to undergo senescence, progressive telomere shortening can lead to the formation of chromosome fusions, genomic instability and eventually loss of cell viability and tumor development. This end replication issue is overcome with the enzyme telomerase, which is expressed in specific cell types (2).
Telomerase is a reverse transcriptase, responsible for the replication of telomeres. Telomerase binds to the 3′ G-overhang, which functions as its substrate, to begin elongating the telomeric sequence. When the first repeat is added, the 3′-end is repositioned, and telomerase is able to add more repeats. Telomerase consists of two subunits human (h)telomerase RNA (TR) and human (h)telomerase reverse transcriptase (TERT). The TR component contains the RNA template for telomeric amplification and specialized structures for the enzymatic stability. The TERT subunit, which is the catalytic one, consists of four functional domains, the telomerase N-terminal domain, the TR-binding domain, the reverse transcriptase domain and the C-terminal extension.
Telomeres are protected by the Shelterin complex which consists of six proteins TTAGGG repeat binding factor (TRF)1, TRF2, TPP1, RAP1, TIN2 and POT1, and binds to telomeres, playing a critical role in telomere stability and telomerase activity. TRF1 and TRF2 form homodimers and have a low affinity for DNA, while POT1 binds to ss telomeric DNA. TPP1 does not interact with DNA; however, it contributes to telomerase recruitment to the tip of the telomeres through interaction with hTERT and by base pairing between the template region in the hTR and the G-overhang. In the absence of certain Shelterin components, such as TRF1, TRF2 and POT1, telomeres become unprotected and often become critically short. This phenomenon triggers a DNA damage response [mainly through the ataxia-telangiectasia mutated (ATM)/ataxia telangiectasia and Rad3-related (ATR) kinases], in order to arrest the cell cycle and repair/protect telomeres preventing telomere fusions and breaks, a great source of genomic instability (3).
Telomerase has been a target for therapeutic interventions for several decades, as its regulation is associated with numerous diseases. More precisely, telomerase overexpression is related to cancer development, whereas telomerase inhibition is combined with age-related diseases (4–8). As a consequence, telomerase is a potential target in various diseases, and the investigation of telomerase modulators and regulators is of critical importance. In particular, since telomerase is not detected in the majority of normal tissues, telomerase inhibitors enable a more specific treatment of cancer compared with conventional chemotherapy drugs, which are not selective, acting also on healthy cells (9). On the other hand, telomerase activators can be potent drugs against age-related diseases associated with telomere shortening. Several studies have demonstrated that telomere shortening in humans is associated with an increased mortality rate due to heart disease, stroke, or infection, while individuals with chronic stress or infections have accelerated telomere shortening compared to healthy individuals of the same age (10).
The present systematic review summarizes all the known synthetic or non-synthetic telomerase-specific regulators, including genetic regulators, intracellular pathways controlling telomerase activity and natural components, which affect telomerase activity associated with cancer, chronic diseases and aging.
Data and methods
Aim and scope of the systematic review
The main aim of the present systematic review was to determine telomerase inhibitors and activators which impact aging and cancer that are referred to in in vitro and in vivo studies. Additionally, the present systematic review also provides a description of the actions of these inhibitors/activators and the type of substance (synthetic or not).
Search strategy
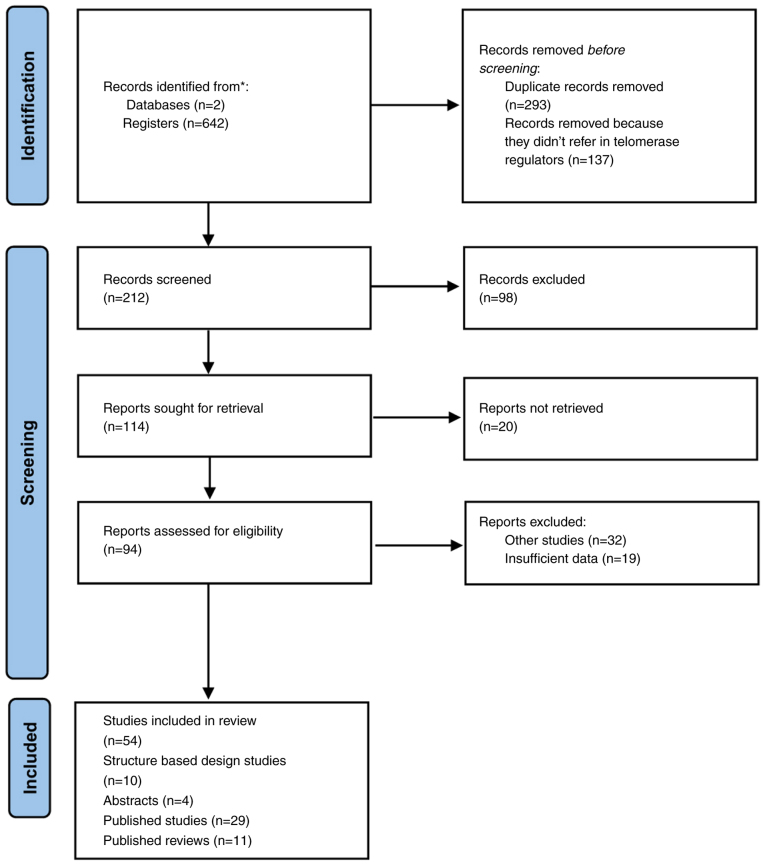
The review protocol followed PRISMA 2020 (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (11). Studies were identified through the PubMed and Scopus databases (between 2002 to 2021). The search strategy included the use of the following terms: ‘telomerase inhibitors in aging’, ‘telomerase inhibitors in cancer’, ‘telomerase activators in aging’, ‘telomerase activators in cancer’ ‘telomerase activators in cancer and in aging’ and ‘telomerase inhibitors in aging and in cancer’. Articles were included if they met the following criteria: They referred to telomerase modulators in aging and in cancer and were in vitro and/or in vivo studies, while studies that did not provide sufficient data or studies not written in the English language were excluded. A total of 293 duplicate records were removed prior to screening and 137 records were removed due to no reference to telomerase regulators. In total, 212 records were screened, 114 of which were sought for retrieval, while 94 reports were assessed for eligibility. Finally, 54 studies were included in the present systematic review, 10 of which are structure-based design studies, 4 were abstracts, 29 were published studies and 11 were published reviews (Fig. 1).
Figure 1.
PRISMA flow diagram for the identification and selection of studies.
Article analysis
According to the existing literature, there were 29 molecules, as reliable telomerase regulators that referred to aging and cancer. Taking into consideration their origin (synthetic or non-synthetic) and their mechanisms of action, these molecules were segmented into synthetic direct inhibitors, synthetic indirect inhibitors, synthetic activators, non-synthetic genetic inducers, non-synthetic natural inhibitors, non-synthetic natural activators. Telomerase inhibitors and telomerase activators are summarized in Tables I and II, respectively.
Table I.
Telomerase inhibitors.
| Authors/(Refs), year | Molecule | Mechanism of action |
|---|---|---|
| Wang and Yang (13), 2021; Seimiya et al (12), 2002 | MST-312 and MST-199a | The precise mechanism of telomerase inhibition by MST remains unclear |
| Altamura et al (14), 2021; Pandya et al (15), 2021; | BIBR1532a | Inhibits the formation of long reaction products, leading to an overall reduction in the number of added TTAGGG repeats |
| Pascolo et al (16), 2002 | ||
| Wang et al (17), 2016 | N-substituted-dihydropyrazole derivativesa | These compounds have an active site that binds to the hTERT subunit of telomerase and inhibit telomerase activity |
| Nasiri et al (19), 2013; Thelen et al (18), 2004 | Silibinina | Decreases telomerase catalytic subunit mRNA; combined with curcuminoids, downregulates hTERT gene expression in breast cancer cells |
| Taka et al (41), 2014 | Curcuminoid derivativesa | Play critical role in the affinity between telomerase and telomeric DNA |
| Chen et al (20), 2018 | Ethenesulfonyl fluoride derivativesa | These compounds exhibited potent inhibitory activities against telomerase though interaction with hTERT |
| Man et al (21), 2016 | Imidazole-4-one derivativesa | Alkyl group and the aliphatic substitutes with double bonds, are important moieties for telomerase inhibition, while longer carbon chains demonstrated inferior inhibition |
| Hu et al (22), 2020 | DIZ-3a | Is bound in G-quadruplex, inhibiting the ALT process as well as disrupting the T-loop structure |
| Thompson et al (23), 2018; Tremblay and Mascarenhas (25), 2021; Burchett et al (24), 2017 | GRN163La | Binds directly to the telomerase RNA component and inhibit telomerase activity |
| Ghareghomi et al (26), 2021 | siRNAsa | A novel strategy for silencing hTERT expression, following the RNAi method |
| Roe et al (27), 2015 | Acridine compoundsa | Bind to HSP90 chaperone, and form bifunctional acridine-HSP90 inhibitor ligands, acting as telomerase inhibitors |
| Meng et al (28), 2018 | Coumarinsa | Acts as telomerase inhibitor targeting c-myc promoter elements |
| Wei et al (38), 2016 | Oxoisoaporphineb | Binds to the G-quadruplex and stabilizes the telomeric structure preventing telomerase from replicating telomeres |
| Herz et al (39), 2014 | MTBITC, erucinb | The mechanism of telomerase inhibition remains unclear |
| Adler et al (40), 2011 | I3Cb | Inhibits telomerase activity and hTERT mRNA expression in prostatic cell lines |
| Adler et al (40), 2011 | DESb | DES combined with I3C and increases the inhibitory effect on telomerase activity, gene expression, and cell viability |
Referred to synthetic inhibitors;
referred to non-synthetic/natural inhibitors. MST-312, N,N'-1,3-phenylenebis[2,3-dihydroxy-benzamide; MST-199, N-[2-(3,4-dihydroxyphenyl)-4-oxo-4H-chromen-3-yl]-3,4 dihydroxybenzamide; DIZ-3, dimeric imidazole; GRN163L, imetelstat; MTBITC, 4-methylthio-3-butenyl isothiocyanate; I3C, indole-3-carbinol; DES, DES, diethylstilbestrol.
Table II.
Telomerase activators.
| Authors/(Refs), year | Molecule | Activity |
|---|---|---|
| Yu et al (42), 2018; Chu and Hickson (44), 2009 | Cycloastragenol or TA-65b | Upregulates the telomerase expression through the ERK pathway; induces the expression of JAK2; is a signal transducer and activator of STAT5b and modulates the telomerase expression through the JAK/STAT pathway |
| Kim et al (30), 2018 | PROX1b | Novel transcriptional activator of TERT with strong binding affinity for the mutant hTERT promoter |
| Li et al (31), 2017 | CDC5Lb | Novel hTERT promoter-binding protein and its knockdown inhibited tumor growth by down-regulating hTERT expression; it is also a transcriptional activator of hTERT |
| Chen et al (32), 2015 | SPT5b | Acts as a novel tumor-specific hTERT promoter-binding protein and activator in colon cancer cells |
| Qin et al (33), 2015 | RFPL3 and CBPb | RFPL3 binds to hTERT promoter; CBP is a co-activator promoting interaction between RFPL3 and hTERT promoter; RFPL3 act together with CBP to upregulate hTERT through the CBP-induced acetylation of RFPL3 protein and their co-anchoring at hTERT promoter region |
| Le Saux et al (29), 2013 | GRN510a | Induces the upregulation of TERT |
| Sanokawa-Akakura et al (36), 2016 | Hydrogen sulfideb | Maintains the expression of hTERT in a NAMPT-and SIRT1-dependent manner, delaying the onset of replicative senescence |
| Stefanou et al (34), 2010 | Leptinb | Modulates hTERT transcription by the binding of STAT3 and Myc/Max/Mad network proteins on the hTERT promoter |
| Yu et al (35), 2018 | ZEB1b | It binds to the hTERT promoter and upregulates hTERT transcription by activating the YAP co-activator |
| Tsoukalas et al (48), 2019 | Centella asiatica extract formulation (08AGTLF)b | Enhances telomerase activity, the mechanism remains unclear |
| Akiyama et al (37), 2011 | EPOb | Regulates telomerase via 2 separate mechanisms: i) On the transcriptional level, by regulating hTERT gene transcription through Janus tyrosine kinase 2/STAT5/c-Myc; and ii) on the post transcriptional level, by controlling hTERT protein phosphorylation by phosphatidylinositol 3-kinase/AKT |
| Uchiumi et al (45), 2011 | Resveratrolb | Increases Werner's syndrome gene promoter activity, and that expression of its gene and protein is accompanied by up-regulation of telomerase in HeLa S3 cells, without affecting cell viability |
Referred to synthetic inhibitors;
referred to non-synthetic/natural inhibitors. PROX, prospero homeobox protein; CDC5L, cell division cycle 5-like; SPT5, suppressor of Ty homolog-5; RFPL3, ret finger protein like 3; CBP, CREB binding protein; GRN163L, imetelstat; ZEB1, zinc finger E-box binding homeobox 1; EPO, erythropoietin.
Results
According to the PRISMA Statement Criteria 2020 (11), a systematic review was performed, by reclaiming data from 54 studies as follows: A total of 10 structure-based design studies, four abstracts, 29 published studies and 11 published reviews. Telomerase regulators are summarized into the following categories.
Synthetic direct telomerase inhibitors
Several synthetic telomerase inhibitors have been designed to downregulate its activity by directly binding to the enzyme and they are used in various diseases, including cancer. Specifically, 14 studies in the present systematic review referred to synthetic direct telomerase inhibitors. MST-312 [N,N'-bis(2,3-dihydroxybenzoyl)-1,2-phenylenediamine, dihydroxybenzoyl-1,3-phenylenediamine] and MST-199 (N-[2-(3,4-dihydroxyphenyl)-4-oxo-4H-chromen-3-yl]-3,4 dihydroxybenzamide) are synthesized on basis of epigallocatechin gallate, a major tea catechin that has been found to decrease telomerase activity. More specifically, the MST treatment of the U937 cell line has been shown to result in cell growth inhibition following a long-term period, which led to telomere shortening, as indicated by Southern blot analysis. In addition, treatment with MST-312 resulted in a high number of senescent cells, as measured by senescence-associated β-galactosidase staining (12). Furthermore, a recently published study indicated that MST-312 was a potent inhibitor of hepatocellular carcinoma (HCC) cells that overexpressed stathmin 1, an oncoprotein that promotes cancer cell migration and invasion (13). However, the precise mechanisms of telomerase inhibition by MST remain unclear (12).
BIBR1532 is a non-nucleosidic compound that is used as a selective telomerase inhibitor. It directly targets telomerase core components, inhibiting telomerase reconstitution from hTR and recombinant hTERT, similarly with the native enzyme derived from tumor cells. According to the analyzed inhibition profile, BIBR1532 is a mixed-type non-competitive inhibitor, since telomerase has different, yet functionally interdependent, binding sites for deoxyribonucleotides and BIBR1532. It inhibits the formation of long reaction products, leading to an overall reduction in the number of added TTAGGG repeats, as indicated by TRAP, telomerase repeated amplification protocol (TRAP) assays and RNA affinity chromatography. This suggests that BIBR1532 does not block the basic catalytic steps involved in template copying, but specifically impairs the elongation of the DNA substrate. A recent in vitro study demonstrated that BIBR1532 functions synergistically with anticancer treatment, such as chemotherapy or radiotherapy, which amplifies its anticancer effect (14). Nevertheless, it is important to state that the action of BIBR1532 is found to cause a severe toxic effect in various cell types. There is also evidence to indicate that in low, non-toxic doses, BIBR1532 is not effective against telomerase activity (15). However, a detailed understanding of the molecular basis of BIBR1532 inhibition will require the crystal structure analysis of the telomerase-inhibitor complex (16).
N-substituted-dihydropyrazole derivatives appear to inhibit telomerase activity and exert a potent antitumor effect against four types of cancer, namely gastric, breast and prostate cancer, and human hepatoma, while they exhibit good selectivity on tumor cells over somatic cells. Docking analysis has revealed that these compounds have an active site that binds to the hTERT subunit of telomerase. More specifically, dihydropyazole derivatives form a hydrogen bond with the lysine (Lys)710 residue of hTERT (17).
Silibinin, a polyphenolic flavonoid, is an anticancer drug that appears to downregulate telomerase activity and prostate-specific antigen (PSA) levels, together with the co-activator of the androgen receptor prostate epithelium specific Ets transcription factor. Silibinin has been shown to downregulate PSA mRNA expression and PSA secretion in conditioned medium, as shown by reverse transcription-quantitative PCR (RT-qPCR). Moreover, following silibinin treatment, telomerase catalytic subunit mRNA levels decreased significantly, while telomerase activity was downregulated (18). In addition, silibinin combined with curcuminoids, has been proven to downregulate hTERT gene expression in breast cancer cells (19). These results suggest the possible therapeutic use of silibinin as an anti-proliferative agent in intervention treatments for prostate cancer (18).
A structure-based design study of ethenesulfonyl fluoride derivatives, including certain 2-(hetero) arylethenesulfonyl fluoride and 1,3-dienylsulfonyl fluoride derivatives, indicates another group of chemical compounds that may be used as synthetic telomerase inhibitors and by extension in anti-cancer therapy. Some of these compounds exhibit potent inhibitory activities against telomerase though interaction with hTERT, as indicated by the modified TRAP assay (20).
Imidazole-4-one derivatives are selected telomerase inhibitors that have been shown to inhibit telomerase activity by TRAP analysis. Moreover, an extensive structure-activity association study demonstrated that the alkyl group and the aliphatic substitutes with double bonds, are important moieties for telomerase inhibition, while longer carbon chains demonstrated inferior inhibition (21). More specifically, dimeric imidazole is bound in the G-quadruplex, inhibiting the alternative lengthening of telomeres process, as well as a disruption in the T-loop structure (22).
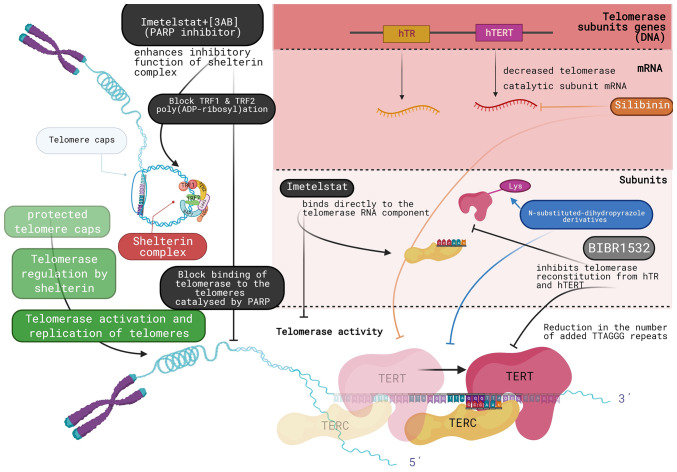
Another potent direct synthetic inhibitor of telomerase is imetelstat (GRN163L). Imetelstat is a 13-base oligonucleotide (5′-TAGGGTTAGACAA-3′), that binds directly to the telomerase RNA component. It is used as a treatment in numerous diseases, such as myelofibrosis, thrombocytopenia and various types of cancer. The treatment of a variety of cell lines with imetelstat has revealed that it is able to control cell cycle progression through the inhibition of telomerase activity. More specifically, telomerase inhibition by imetelstat attenuates the removal of DNA damage signals from telomeres in telomerase-positive cancer cells and affects the G2/M progression of the cell cycle, a mechanism that is common in response to telomere dysfunction (23). Another study analyzed the effects of imetelstat on two pancreatic cancer cell lines, AsPC1 and L3.6pl. In both cell lines, chronic exposure to imetelstat initially led to telomere shortening followed by the stabilization of critically short telomeres. In addition to the direct effects of imetelstat on telomerase activity, its function when combined with other drugs includes the further activation of intracellular pathways (24). Currently, imetelstat is being tested in a phase 3 clinical trial for myelofibrosis (25). The Shelterin complex, as aforementioned, is a telomere-associated complex that limits the access of telomerase to telomeres. The inhibitory function of this complex is enhanced by drugs that block the poly-ADP-ribosylation of TRF1 and TRF2, components of the Shelterin complex that are crucial for the binding of telomerase to the telomeres. This process is catalyzed by poly(ADP-ribose) polymerase (PARP) family enzymes, a group of enzymes involved in DNA repair and more specifically, in the ss break repair mechanism. The combined treatment of the GRN163L-resistant L3.6pl cells with imetelstat and 3-aminobenzamide (3AB), a general PARP inhibitor, has been shown to lead to additional telomere shortening and to limit the lifespan of the resistant cells (24) (Fig. 2).
Figure 2.
Mechanistic representation of telomerase inhibition by synthetic direct inhibitors (the image was drawn using BioRender). PARP, poly(ADP-ribose) polymerase; PARP, poly(ADP-ribose) polymerase; hTERT, human telomerase reverse transcriptase.
Finally, small interfering RNAs (siRNAs) are a novel strategy for silencing the hTERT expression, following the RNA interference method. More specifically, Ghareghomi et al indicated that siRNAs in combination with magnetic nanoparticles may be an effective treatment for ovarian cancer (26).
Synthetic indirect telomerase inhibitors
There are also additional synthetic compounds that act indirectly on telomerase activity by activating intracellular pathways. Acridine compounds, such as geldanamycin, GA alkyn and bis-amido chloroacridine do not bind directly to telomerase; however, they bind to heat shock protein (HSP)90 chaperone and form bifunctional acridine-HSP90 inhibitor ligands, further functioning as telomerase inhibitors. Acridine compounds can be used as potent anticancer drugs, since HSP90 is required for telomerase activity and as a consequence, the binding of acridine to HSP90 leads to the inhibition of telomerase activity and the downregulation of telomerase expression (27).
Other synthetic telomerase inhibitors are eight platinum complexes with substituted 3-(2′-benzimidazolyl) coumarins, which have been found to exhibit high cytotoxic activity in vitro against the cisplatin-resistant Sloan-Kettering ovarian cancer cisplatin-resistant cells (SK-OV-3/DDP) cancer cells, but low cytotoxicity one normal HL-7702 cells. Further analyses revealed that three of the eight complexes induced the apoptosis of SK-OV-3/DDP cancer cells via mitochondria dysfunction signaling pathways, while one of them acted as telomerase inhibitor targeting c-myc promoter elements. More specifically, it was demonstrated using western blot analysis and RT-qPCR that platinum complexes act on transcription and the expression of c-myc and hTERT, by inhibiting the c-myc promoter, leading to the induction of apoptosis via mitochondrial dysfunction. Therefore, platinum complexes are used as potent drugs in various cancer treatments (28).
Synthetic telomerase activators
The development and synthesis of telomerase activators has gained a increasing attention due to the emergence of age-related diseases or diseases associated with telomere dysfunction and shortening, including AIDS, aplastic anemia and pulmonary fibrosis. GRN510 is a novel small molecule activator, that was tested as a potential drug to limit fibrosis induced by bleomycin in mTERT heterozygous mice. Treatment with GRN510 was shown to lead to a 2–4 fold increase in telomerase activity both ex vivo and in vivo. GRN510 suppressed the development of fibrosis and the accumulation of senescent cells in the lungs via a mechanism dependent on telomerase activation, indicating that small molecule activators of telomerase can be used in therapies against idiopathic pulmonary fibrosis (29).
Non-synthetic-natural direct telomerase activators
Various genetic products function as regulators of telomerase on a transcriptional or posttranscriptional level and can serve as potential therapeutic targets in cancer and various diseases. In particular, somatic mutations in the hTERT promoter are related to telomerase activation and frequently occur at two hotspots in various types of cancer. Prospero homeobox protein 1 (PROX1) was identified as a novel transcriptional activator of TERT with strong binding affinity for the mutant hTERT promoter. TERT promoter mutations can enhance the promoter activity in HCC cells expressing PROX1, while PROX1 has been found to be necessary for the upregulation of TERT in Hep3B and HepG2 cells. These observations suggest that PROX1 may serve as potential target in therapy against HCC (30).
Another protein functioning as an activator of telomerase by binding to hTERT promoter is the cell division cycle 5-like (CDC5L). CDC5L is a protein encoded by CDC5L gene, and is highly expressed in certain types of cancer cells, particularly in colorectal cancer. CDC5L was identified as a novel hTERT promoter-binding protein and its knockdown has been found to inhibit tumor growth by downregulating hTERT expression. CDC5L has also been shown to be a transcriptional activator of hTERT in a luciferase reporter assay. These findings indicate that CDC5L may serve as a potential therapeutic target for human colorectal cancer (31).
Furthermore, suppressor of Ty homolog-5 (SPT5) is also a protein encoded by the suppressor of Ty 5 homolog (SUPT5H) gene and acts as a novel tumor-specific hTERT promoter-binding protein and activator in colon cancer cells. It has a tumor-specific binding activity to hTERT promoter in vivo and in vitro and it exhibits high expression levels in colorectal cancer cell lines and primary human colorectal cancer tissues. The inhibition of SUPT5H expression has been found to significantly suppress telomerase activity and telomere shortening, while it induces senescence in colon cancer cells, suppressing cancel cell growth and migration. According to these findings, since SPT5 contributes to the upregulation of hTERT, leading to tumor growth, its gene SUPT5H may serve as a potent therapeutic target and a novel tumor biomarker for colon cancer (32).
In the category of telomerase inducers through hTERT promoter regulation belong the ret finger protein like 3 (RFPL3) and CREB binding protein (CBP) proteins, leptin and zinc finger E-box binding homeobox 1 (ZEB1). RFPL3 and CBP co-localize in the nucleus and appear to be overexpressed in human lung cancer cells. They function synergistically to induce hTERT expression and activity and tumor cell growth. More precisely, RFPL3 binds to the hTERT promoter, whereas CBP is a co-activator, promoting the interaction between RFPL3 and hTERT promoter. It has also been found that RFPL3 act together with CBP to upregulate hTERT through the CBP-induced acetylation of RFPL3 protein and their co-anchoring at hTERT promoter region, suggesting that RFPL3/CBP/hTERT pathway may be used as a novel target for lung cancer treatment (33).
Leptin is encoded by human obese gene and was found to be associated with hTERT expression. It has been found to modulate hTERT transcription by the binding of signal transducer and activator of transcription (STAT)3 and Myc/Max/Mad network proteins on the hTERT promoter and may be a potent regulator of the malignancy of HCC (34).
Finally ZEB1 is a transcriptional regulator in various types of cancer, as it binds to the hTERT promoter and upregulates hTERT transcription by activating the YAP co-activator (35).
Further endogenous proteins exert an effect on telomerase activity not by directly binding to the hTERT promoter, but via the activation of intracellular pathways that can also serve as potential therapeutic targets. In a previous study, hydrogen sulfide (H2S) was shown to exert anti-aging effects and to protect against replicative senescence. In particular, the expression of H2S and its producing enzyme appeared to be diminished in adult human dermal fibroblasts undergoing replicative senescence. This reduced production coincided with the appearance of hallmarks of replicative senescence, such as an enhanced expression of p16, p21 and ribonucleotide-diphosphate reductase subunit M2 B. On the other hand, a decrease was observed in the levels of hTERT, sirtuin 1 (SIRT1), nicotinamide phosphoribosyltransferase (NAMPT) and the nicotinamide adenine dinucleotide (NAD)/NADH ratio, which was rescued by the addition of exogenous H2S. H2S maintained expression of hTERT in a NAMPT- and SIRT1-dependent manner, delaying the onset of replicative senescence (36).
Erythropoietin (EPO) also exerts an effect on telomerase activity. It has been found that in human erythroleukemic JAS-REN-A cells, EPO regulates telomerase via two separate mechanisms: i) On the transcriptional level, by regulating hTERT gene transcription through Janus tyrosine kinase 2/STAT5/c-Myc; and ii) on the post-transcriptional level, by controlling hTERT protein phosphorylation by phosphatidylinositol 3-kinase/AKT (37).
Non-synthetic-natural telomerase inhibitors
Several natural compounds that function as telomerase inhibitors are used as potent drugs in cancer therapy. Oxoisoaporphine, an alkaloid isolated from Menispermum dauricum, is known for its antitumor effects. Oxoisoaporphine metal complexes bind strongly to the G-quadruplex and stabilize the telomeric structure, preventing telomerase from replicating telomeres. The binding properties of these two oxoisaporphine compounds to telomerase were previously examined using molecular docking and TRAP-silver staining assay (38).
Another small molecule that is used as an anticancer drug, particularly in HCC, is 4-methylthiobutyl isothiocyanate (MTBITC, erucin), which is a metabolite of sulforaphane, a small molecule found in broccoli. Sulforaphane is known for its cancer therapeutic potential in vitro and in vivo, and is rapidly metabolized into MTBITC, which was previously identified as potent selective inducer of apoptosis in HCC cells. MTBITC was shown to be effective against telomerase and its function was independent from TP53, which is also a suppressor of telomerase (39).
Finally, another natural inhibitor of telomerase used in therapy against prostate cancer is Indole-3-carbinol (I3C), a phytochemical in cruciferous vegetables. A previous study demonstrated that I3C significantly inhibited telomerase activity and hTERT mRNA expression in prostate cancer cell lines. I3C becomes more potent when combined with diethylstilbestrol (DES), significantly enhancing the inhibitory effect on telomerase activity, gene expression and cell viability. These results suggested that I3C combined with DES may aid in the treatment of prostate cancer (40).
Non-synthetic-natural indirect telomerase activators
Several natural compounds acting as positive telomerase regulators are also used in age-related diseases associated with telomere dysfunction. Curcuminoids are natural polyphenol compounds derived from turmeric that appear to enhance telomerase activity. Although they are not considered to bind directly to the telomerase enzyme, they play a critical role in the affinity between telomerase and the telomeric DNA. A number of curcuminoid derivatives have been found to enhance telomerase activity in an in vitro TRAP assay and the minimal requirement for this enhanced telomerase activity is a curcuminoid core with at least one n-pentylpyridine side chain (41).
Another natural telomerase activator is cycloastragenol (CAG, GRN665 or TA65), a triterpenoid saponin compound and a hydrolytic product of the main active ingredient in Astragalus membranaceus. CAG is involved in two cell signaling pathways. One pathway includes the phosphorylation of extracellular signal-regulated kinase (ERK), and upregulates the telomerase expression through the ERK pathway, while the other induces the expression of Janus kinase 2 (JAK2) and STAT5b, and modulates telomerase expression through the JAK/STAT pathway (42). CAG has a variety of properties, such as in anti-hypoxic and anti-ischemic, anti-viral and anti-fibrotic properties, while it also ameliorates metabolism and wound healing. According to existing studies and clinical trials, CAG is safe and effective in telomere maintenance and lengthening, and has a broad range of applications (42). However, according to Akbarizare et al (43), CAG affected human dermal fibroblasts (HDFs) in a different manner. More specifically in HDFs, CAG interferes with TERT mRNA splicing, and inhibits telomerase activity. As a consequence, further studies are required to fully understand its efficacy and ensure the proper clinical use of CAG to treat diseases (42,43).
Finally, resveratrol is a natural polyphenol used in the treatment of Werner's syndrome (WS). Patients with WS undergo premature aging accompanied by chromosomal instability by mutations in the WRN gene, which is involved in DNA repair and telomere maintenance. It has been found that resveratrol increases WRN promoter activity, and that the expression of its gene and protein is accompanied by the upregulation of telomerase in HeLa S3 cells, without affecting cell viability. Therefore, its ability to maintain telomere length without affecting cell proliferation renders it an important tool in the treatment of WS (44,45).
In another study by Tsoukalas et al (46), an in vitro model was used and it was revealed that Centella asiatica extract formulation (08AGTLF) induced telomerase activation in peripheral blood mononuclear cells from healthy donors. Previous research has demonstrated that rats treated with Centella asiatica exhibited an enhanced cognitive ability and a higher expression of mitochondrial and antioxidant genes in the brain and liver, which may contribute to cognitive improvement (46). Furthermore it has been demonstrated that TERT expression in the brains of middle-aged rats was restored following the administration of a Centella asiatica containing supplement (Reverse™) for 3-month period (46). Behavioral effects have also been reported in a complementary behavioral study, where the administration of the same supplement (Reverse™) improved locomotor activity and significantly decreased stress in a dose-dependent manner in aged rats (47). However, future studies on humans are warranted to examine its effect on telomere length, aging and human health (48).
Discussion
Telomerase is the riboenzyme that contributes to the preservation of chromosomal integrity and stability by elongating the telomeric regions and eliminating the effects of DNA damage. Telomeric activity contributes to a prolonged cell life and this mechanism effectively serves the needs of malignant cells. Indeed, telomerase is highly expressed in tumorous cells of all cancer types, whereas it is normally absent in the vast majority of somatic cells (with an exception of the embryonic and stem cells that undergo fast differentiation and repetitive divisions) (49). However, the inefficient telomerase enzymatic performance has been linked to multiple age-related diseases (10).
In a time that the clinical community needs novel approaches for cancer treatment, telomerase appears to be a potent therapeutic target and biomarker. The pre-existing in-depth knowledge of the genetic and enzymatic background of telomerase is an important advantage for its use, as it will be easier to predict the possible pathways that need to be followed for the more effective controlling of its actions. It has now been a decade since scientists have been trying to take advantage of the regulation of telomerase for the treatment of cancer and other diseases (50).
There are three approaches to controlling telomerase. One is by blocking the expression of the enzyme. Telomerase consists of several different subunits, formed by the translation of genes found in different chromosomal regions. A gene therapy that includes regulators that target the desired loci can successfully lead to the suppression of the enzyme. The second effective method for telomerase inhibition depends upon the principle of targeting the catalytic subunits of enzyme. As long as the mechanisms of action and the intracellular pathways of this enzyme are understood, there is ample information provided on which of these steps could be blocked in order to prevent the catalytic activity of telomerase. The third alternative of telomerase inhibition is immunotherapy. Clinical cancer treatments have already been applied that can trigger an immune response in patients with h-TERT epitope injections (51). Over 25 known telomerase peptides induce an immune specific response process through human leukocyte antigens, which are finally destroyed. GV1001 and GRNVAC1 are two of the already released vaccines that improved the response of cancer patients and contributed to a prolonged survival rate (21).
It has been revealed that telomerase is active in 85–95% of cancers. TERT expression and telomerase activity in tumors are affected by TERT promoter mutations and the highest TERT promoter mutation rates occur in glioblastoma, melanoma, bladder urothelial carcinoma and lower-grade glioma (52). An intermediate mutation frequency range is observed in HCC and thyroid carcinoma. However, the lowest level of mutation frequency is detected in kidney, lung, prostate and gastrointestinal cancers, and in leukemia (52,53). In another study, it was found that the highest telomerase activity occurred ovarian, adrenal cortical, esophageal and lung cancer (54). In addition, it has been found that hTERT expression is increased in the early stages of colorectal cancer (55).
As aforementioned, telomerase is poorly expressed in somatic cells. This selective expression of the enzyme in malignancies could allow for novel cancer treatments to be more targeted into the desired tumors, while at the same time eliminating the side-effects. Synthetic and natural inhibitors are the molecules that play a significant role in telomerase inhibition, either by directly blocking the enzymatic subunits (such as MST-312, BIBR1532 etc.) or by blocking the formation of the enzymatic entity through the inhibition of the translation process (13,14). Synthetic drugs that bind to the template region of telomerase, such as imetelstat, have already begun to be tested in both in vivo and in vitro experiments; of note, impressive results have been obtained in several cancer types (melanoma, glioblastoma and pancreatic carcinoma, etc.) (23,52). Furthermore, systematic therapy by the intranasal administration of GRN163 and GRN163L was proven to be effective in brain tissue cases. The drugs overpass the brain blood barrier targeting only the cancer cells, with a low toxicity, so that the normal brain cells remain intact (56).
Possibly, there may be other alternatives when trying to affect the enzymatic activity of telomerase. For example, reactive oxygen species (ROS) scavengers have been widely used for years for attenuating the aging process. In HCC cells, the estimated rates of ROS are higher compared to normal cells. At the same time, ROS are involved in the Akt pathway, a regulatory telomerase system. Thus, probably NAC particles (antioxidants that directly affect ROS levels in the intracellular redox state) may aid telomerase inhibition through cell apoptosis (53).
As regards synthetic and natural factors, even everyday habits may have consequences on telomerase regulation. Food ingredients that enter the body can significantly affect the controlling process of telomerase. For example, catechin and sulfoquinorosyldiacylglycerol are molecules that block the activity of the enzyme, while retinoic acid and tocotrienol interfere with the pathways of telomerase regulation by negatively affecting the expression of the hTERT subunit. There are numerous xenobiotics and bioactive compounds, such as endocrine disrupting chemicals, that have been found to affect telomerase activity and have been linked to multiple age-related diseases (57).
The specificity of a drug is probably one of the most important qualifications that need to be considered when trying to develop alternative therapies for cancer and other diseases. From a clinical aspect, there are indications that telomerase is an effective target that results in the improvement of a number of human cancer types, while exerting minimal side-effects (58,59). Apart from its advantageous effects, there are some details that should not be neglected. It is always important to examine the in vivo telomerase enzymology in different cancer types. Clinicians should consider each patient as a separate incident and decide for the most appropriate treatment concerning the telomeric inhibition/activation in order to reach the highest potential for any disease.
Furthermore, there is always the possibility of telomeric resistance to specific types of synthetic and natural modulators (60). For example, due to additional mutation patterns on the genetic loci of our interest. This could be a parameter that enhances the stability of the enzyme. Finally, telomerase treatment should be an additional-if not a secondary-therapy for cancer and age-related diseases (61) until it will become efficiently reliable to replace traditionally applied therapies (as chemotherapy, radiation, and immunotherapy).
The present systematic review provides data regarding telomerase activators and inhibitors with effects on aging and cancer. Telomerase is not a universal target for cancer treatment and for aging prevention; however, targeting telomere maintenance mechanisms is crucial for future research in aging and cancer. Nevertheless, there are some limitations to the present study which should be mentioned. The majority of the research has been conducted using animal models; thus, there are no clinical data available regarding the use of these activators and inhibitors in human subjects. In addition, the present systematic review did not assess the risk of bias; however, the authors aim to perform such a risk analysis in the future. There is a need to search for unpublished data and to provide data other than those published in the Web of Science and PubMed databases. Data from clinical trial registries and regulatory agency websites were not included herein; thus, there was a lack of graphical and statistical analyses for bias assessment, such as funnel plot and Egger's test, as well the use the Cochrane collaboration risk of bias tool.
Future intervention studies on humans are warranted to examine the effects of telomerase modulators on aging and cancer since the treatment with telomerase activating procedures and an extended cell lifespan may accumulate rare genetic and epigenetic aberrations that may contribute to malignant transformation. To this end, it a potential therapeutic approach has been designed in which telomerase is induced temporarily and selectively in aged cells without promoting cancer growth by using a recombinant adeno-associated virus vector (62). In addition, the identification of novel telomerase modulators is an important aspect for increasing the tools that are currently available for telomerase approaches to cancer therapeutics and to aging prevention. For the past two decades, telomerase-based therapeutic cancer vaccines have been under clinical investigation since telomerase has a universal presence in cancer, and it is essential for tumor growth.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- ds
double-stranded
- ss
single-stranded
- (h)TR
(human) telomerase RNA
- (h)TERT
(human) telomerase reverse transcriptase
- TRF
TTAGGG repeat binding factor
- ATM kinase
ataxia-telangiectasia mutated kinase
- ATR kinase
ataxia telangiectasia and Rad3-related kinase
- TRAP
telomerase repeated amplification protocol
- Lys
lysine
- PSA
prostate-specific antigen
- PARP
poly(ADP-ribose) polymerase
- HSP90
heat shock protein 90
- PROX
prospero homeobox protein
- HCC
hepatocellular carcinoma
- CDC5L
cell division cycle 5-like
- SK-OV-3/DDP
Sloan-Kettering ovarian cancer cisplatin-resistant cells
- SPT5
suppressor of Ty homolog-5
- SUPT5H
suppressor of Ty 5 homolog
- RFPL3
ret finger protein like 3
- CBP
CREB binding protein
- STAT
signal transducer and activator of transcription
- SIRT1
sirtuin 1
- NAMPT
nicotinamide phosphoribosyltransferase
- EPO
erythropoietin
- MTBITC
4-methylthio-3-butenyl isothiocyanate
- I3C
indole-3-carbinol
- DES
diethylstilbestrol
- CAG
cycloastragenol
- ERK
extracellular signal-regulated kinase
- JAK2
Janus kinase 2
- EPO
erythropoietin
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
All authors (PF, ER, KK, EK, EA, EV, CM, DAS and AT) contributed to the conception and design of the study. PF and KK searched the literature for inclusion in the study that this was then examined and reviewed by EK, EA, EV and EV. PF, KK, EK, EA and EV drafted and wrote the manuscript. AT, DAS and CM provided advice on the experimental design, interpreted the results and critically revised the manuscript. ER and EK designed the figures. KK and EV designed the tables. PF and KK confirm the authenticity of all the raw data. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have no competing interests.
References
- 1.Lu W, Zhang Y, Liu D, Songyang Z, Wan M. Telomere-strucutre, function, and regulation. Exp Cell Res. 2013;319:133–141. doi: 10.1016/j.yexcr.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giardini MA, Segatto M, Da Silva MS, Nunes VS, Cano MIN. Telomere and telomerase biology. Prog Mol Biol Transl Sci. 2014;125:1–40. doi: 10.1016/B978-0-12-397898-1.00001-3. [DOI] [PubMed] [Google Scholar]
- 3.Sandin S, Rhodes D. Telomerase structure. Curr Opin Struct Biol. 2014;25:104–110. doi: 10.1016/j.sbi.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fragkiadaki P, Nikitovic D, Kalliantasi K, Sarandi E, Thanasoula M, Stivaktakis PD, Nepka C, Spandidos DA, Tosounidis T, Tsatsakis A. Telomere length and telomerase activity in osteoporosis and osteoarthritis. Exp Ther Med. 2020;19:1626–1632. doi: 10.3892/etm.2019.8370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fragkiadaki P, Tsoukalas D, Fragkiadoulaki I, Psycharakis C, Nikitovic D, Spandidos DA, Tsatsakis AM. Telomerase activity in pregnancy complications (Review) Mol Med Rep. 2016;14:16–21. doi: 10.3892/mmr.2016.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Razgonova MP, Zakharenko AM, Golokhvast KS, Thanasoula M, Sarandi E, Nikolouzakis K, Fragkiadaki P, Tsoukalas D, Spandidos DA, Tsatsakis A. Telomerase and telomeres in aging theory and chronographic aging theory (Review) Mol Med Rep. 2020;22:1679–1694. doi: 10.3892/mmr.2020.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vakonaki E, Tsiminikaki K, Plaitis S, Fragkiadaki P, Tsoukalas D, Katsikantami I, Vaki G, Tzatzarakis MN, Spandidos DA, Tsatsakis AM. Common mental disorders and association with telomere length. Biomed Rep. 2018;8:111–116. doi: 10.3892/br.2018.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasilopoulos E, Fragkiadaki P, Kalliora C, Fragou D, Docea AO, Vakonaki E, Tsoukalas D, Calina D, Buga AM, Georgiadis G, et al. The association of female and male infertility with telomere length (Review) Int J Mol Med. 2019;44:375–389. doi: 10.3892/ijmm.2019.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murofushi Y, Nagano S, Kamizono J, Takahashi T, Fujiwara H, Komiya S, Matsuishi T, Kosai K. Cell cycle-specific changes in hTERT promoter activity in normal and cancerous cells in adenoviral gene therapy: A promising implication of telomerase-dependent targeted cancer gene therapy. Int J Oncol. 2006;29:681–688. [PubMed] [Google Scholar]
- 10.Yeh JK, Lin MH, Wang CY. Telomeres as therapeutic targets in heart disease. JACC Basic to Transl Sci. 2019;4:855–865. doi: 10.1016/j.jacbts.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seimiya H, Oh-hara T, Suzuki T, Naasani I, Shimazaki T, Tsuchiya K, Tsuruo T. Telomere shortening and growth inhibition of human cancer cells by novel synthetic telomerase inhibitors MST-312, MST-295, and MST-1991. Mol Cancer Ther. 2002;1:657–665. [PubMed] [Google Scholar]
- 13.Wang SJ, Yang PM. A bioinformatics analysis identifies the telomerase inhibitor MST-312 for treating High-STMN1-expressing hepatocellular carcinoma. J Pers Med. 2021;11:332. doi: 10.3390/jpm11050332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altamura G, Degli Uberti B, Galiero G, De Luca G, Power K, Licenziato L, Maiolino P, Borzacchiello G. The small molecule BIBR1532 exerts potential anti-cancer activities in preclinical models of feline oral squamous cell carcinoma through inhibition of telomerase activity and down-regulation of TERT. Front Vet Sci. 2021;7:620776. doi: 10.3389/fvets.2020.620776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandya VA, Crerar H, Mitchell JS, Patani R. A non-toxic concentration of telomerase inhibitor BIBR1532 fails to reduce TERT expression in a feeder-free induced pluripotent stem cell model of human motor neurogenesis. Int J Mol Sci. 2021;22:3256. doi: 10.3390/ijms22063256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pascolo E, Wenz C, Lingner J, Hauel N, Priepke H, Kauffmann I, Garin-Chesa P, Rettig WJ, Damm K, Schnapp A. Mechanism of human telomerase inhibition by BIBR1532, a synthetic, non-nucleosidic drug candidate. J Biol Chem. 2002;277:15566–15572. doi: 10.1074/jbc.M201266200. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Cheng FX, Yuan XL, Tang WJ, Shi JB, Liao CZ, Liu XH. Dihydropyrazole derivatives as telomerase inhibitors: Structure-based design, synthesis, SAR and anticancer evaluation in vitro and in vivo. Eur J Med Chem. 2016;112:231–251. doi: 10.1016/j.ejmech.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Thelen P, Wuttke W, Jarry H, Grzmil M, Ringert RH. Inhibition of telomerase activity and secretion of prostate specific antigen by silibinin in prostate cancer cells. J Urol. 2004;171:1934–1938. doi: 10.1097/01.ju.0000121329.37206.1b. [DOI] [PubMed] [Google Scholar]
- 19.Nasiri M, Zarghami N, Koshki KN, Mollazadeh M, Moghaddam MP, Yamchi MR, Esfahlan RJ, Barkhordari A, Alibakhshi A. Curcumin and silibinin inhibit telomerase expression in T47D human breast cancer cells. Asian Pac J Cancer Prev. 2013;14:3449–3453. doi: 10.7314/APJCP.2013.14.6.3449. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Zha GF, Wang JQ, Liu XH. Ethenesulfonyl fluoride derivatives as telomerase inhibitors: structure-based design, SAR, and anticancer evaluation in vitro. J Enzyme Inhib Med Chem. 2018;33:1266–1270. doi: 10.1080/14756366.2018.1484735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Man RJ, Chen LW, Zhu HL. Telomerase inhibitors: A patent review (2010–2015) Expert Opin Ther Pat. 2016;26:679–688. doi: 10.1080/13543776.2016.1181172. [DOI] [PubMed] [Google Scholar]
- 22.Hu MH, Lin XT, Liu B, Tan JH. Dimeric aryl-substituted imidazoles may inhibit ALT cancer by targeting the multimeric G-quadruplex in telomere. Eur J Med Chem. 2020;186:111891. doi: 10.1016/j.ejmech.2019.111891. [DOI] [PubMed] [Google Scholar]
- 23.Thompson CAH, Gu A, Yang SY, Mathew V, Fleisig HB, Wong JMY. Transient telomerase inhibition with imetelstat impacts DNA damage signals and cell-cycle kinetics. Mol Cancer Res. 2018;16:1215–1225. doi: 10.1158/1541-7786.MCR-17-0772. [DOI] [PubMed] [Google Scholar]
- 24.Burchett KM, Etekpo A, Batra SK, Yan Y, Ouellette MM. Inhibitors of telomerase and poly(ADP-ribose) polymerases synergize to limit the lifespan of pancreatic cancer cells. Oncotarget. 2017;8:83754–83767. doi: 10.18632/oncotarget.19410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tremblay D, Mascarenhas J. Next generation therapeutics for the treatment of myelofibrosis. Cells. 2021;10:1034. doi: 10.3390/cells10051034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghareghomi S, Ahmadian S, Zarghami N, Hemmati S. hTERT-molecular targeted therapy of ovarian cancer cells via folate-functionalized PLGA nanoparticles co-loaded with MNPs/siRNA/wortmannin. Life Sci. 2021;277:119621. doi: 10.1016/j.lfs.2021.119621. [DOI] [PubMed] [Google Scholar]
- 27.Roe S, Gunaratnam M, Spiteri C, Sharma P, Alharthy RD, Neidle S, Moses JE. Synthesis and biological evaluation of hybrid acridine-HSP90 ligand conjugates as telomerase inhibitors. Org Biomol Chem. 2015;13:8500–8504. doi: 10.1039/C5OB01177A. [DOI] [PubMed] [Google Scholar]
- 28.Meng T, Qin QP, Wang ZR, Peng LT, Zou HH, Gan ZY, Tan MX, Wang K, Liang FP. Synthesis and biological evaluation of substituted 3-(2′-benzimidazolyl)coumarin platinum(II) complexes as new telomerase inhibitors. J Inorg Biochem. 2018;189:143–150. doi: 10.1016/j.jinorgbio.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Le Saux CJ, Davy P, Brampton C, Ahuja SS, Fauce S, Shivshankar P, Nguyen H, Ramaseshan M, Tressler R, Pirot Z, et al. A novel telomerase activator suppresses lung damage in a murine model of idiopathic pulmonary fibrosis. PLoS One. 2013;8:e58423. doi: 10.1371/journal.pone.0058423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YJ, Yoo JE, Jeon Y, Chong JU, Choi GH, Song DG, Jung SH, Oh BK, Park YN. Suppression of PROX1-mediated TERT expression in hepatitis B viral hepatocellular carcinoma. Int J Cancer. 2018;143:3155–3168. doi: 10.1002/ijc.31731. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Zhang N, Zhang R, Sun L, Yu W, Guo W, Gao Y, Li M, Liu W, Liang P, et al. CDC5L promotes hTERT expression and colorectal tumor growth. Cell Physiol Biochem. 2017;41:2475–2488. doi: 10.1159/000475916. [DOI] [PubMed] [Google Scholar]
- 32.Chen R, Zhu J, Dong Y, He C, Hu X. Suppressor of Ty homolog-5, a novel tumor-specific human telomerase reverse transcriptase promoter-binding protein and activator in colon cancer cells. Oncotarget. 2015;6:32841–32855. doi: 10.18632/oncotarget.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin Y, Chen W, Xiao Y, Yu W, Cai X, Dai M, Xu T, Huang W, Guo W, Deng W, Wu T. RFPL3 and CBP synergistically upregulate hTERT activity and promote lung cancer growth. Oncotarget. 2015;6:27130–27145. doi: 10.18632/oncotarget.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stefanou N, Papanikolaou V, Furukawa Y, Nakamura Y, Tsezou A. Leptin as a critical regulator of hepatocellular carcinoma development through modulation of human telomerase reverse transcriptase. BMC Cancer. 2010;10:442. doi: 10.1186/1471-2407-10-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu P, Shen X, Yang W, Zhang Y, Liu C, Huang T. ZEB1 stimulates breast cancer growth by up-regulating hTERT expression. Biochem Biophys Res Commun. 2018;495:2505–2511. doi: 10.1016/j.bbrc.2017.12.139. [DOI] [PubMed] [Google Scholar]
- 36.Sanokawa-Akakura R, Akakura S, Tabibzadeh S. Replicative senescence in human fibroblasts is delayed by hydrogen sulfide in a NAMPT/SIRT1 dependent manner. PLoS One. 2016;11:e0164710. doi: 10.1371/journal.pone.0164710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akiyama M, Kawano T, Mikami-Terao Y, Agawa-Ohta M, Yamada O, Ida H, Yamada H. Erythropoietin activates telomerase through transcriptional and posttranscriptional regulation in human erythroleukemic JAS-REN-A cells. Leuk Res. 2011;35:416–418. doi: 10.1016/j.leukres.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Wei ZZ, Qin QP, Chen JN, Chen ZF. Oxoisoaporphine as potent telomerase inhibitor. Molecules. 2016;21:1534. doi: 10.3390/molecules21111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herz C, Hertrampf A, Zimmermann S, Stetter N, Wagner M, Kleinhans C, Erlacher M, Schüler J, Platz S, Rohn S, et al. The isothiocyanate erucin abrogates telomerase in hepatocellular carcinoma cells in vitro and in an orthotopic xenograft tumour model of HCC. J Cell Mol Med. 2014;18:2393–4203. doi: 10.1111/jcmm.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adler S, Rashid G, Klein A. Indole-3-carbinol inhibits telomerase activity and gene expression in prostate cancer cell lines. Anticancer Res. 2011;31:3733–3737. [PubMed] [Google Scholar]
- 41.Taka T, Changtam C, Thaichana P, Kaewtunjai N, Suksamrarn A, Lee TR, Tuntiwechapikul W. Curcuminoid derivatives enhance telomerase activity in an in vitro TRAP assay. Bioorg Med Chem Lett. 2014;24:5242–5246. doi: 10.1016/j.bmcl.2014.09.059. [DOI] [PubMed] [Google Scholar]
- 42.Yu Y, Zhou L, Yang Y, Liu Y. Cycloastragenol: An exciting novel candidate for age-associated diseases. Exp Ther Med. 2018;16:2175–2182. doi: 10.3892/etm.2018.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akbarizare M, Ofoghi H, Hadizadeh M. Dual effect of sapogenins extracted from spirulina platensis on telomerase activity in two different cell lines. Mol Biol Res Commun. 2021;10:1–4. doi: 10.22099/mbrc.2020.38230.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu WK, Hickson ID. RecQ helicases: Multifunctional genome caretakers. Nat Rev Cancer. 2009;9:644–654. doi: 10.1038/nrc2682. [DOI] [PubMed] [Google Scholar]
- 45.Uchiumi F, Watanabe T, Hasegawa S, Hoshi T, Higami Y, Tanuma S. The effect of resveratrol on the Werner syndrome RecQ helicase gene and telomerase activity. Curr Aging Sci. 2011;4:1–7. doi: 10.2174/1874609811104010001. [DOI] [PubMed] [Google Scholar]
- 46.Tsoukalas D, Buga AM, Docea AO, Sarandi E, Mitrut R, Renieri E, Spandidos DA, Rogoveanu I, Cercelaru L, Niculescu M, et al. Reversal of brain aging by targeting telomerase: A nutraceutical approach. Int J Mol Med. 2021;48:199. doi: 10.3892/ijmm.2021.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsoukalas D, Zlatian O, Mitroi M, Renieri E, Tsatsakis A, Izotov BN, Burada F, Sosoi S, Burada E, Buga AM, et al. A novel nutraceutical formulation can improve motor activity and decrease the stress level in a murine model of middle-age animals. J Clin Med. 2021;10:624. doi: 10.3390/jcm10040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsoukalas D, Fragkiadaki P, Docea AO, Alegakis AK, Sarandi E, Thanasoula M, Spandidos DA, Tsatsakis A, Razgonova MP, Calina D. Discovery of potent telomerase activators: Unfolding new therapeutic and anti-aging perspectives. Mol Med Rep. 2019;20:3701–3708. doi: 10.3892/mmr.2019.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Looi LM, Ng MH, Cheah PL. Telomerase activation in neoplastic cell immortalization and tumour progression. Malays J Pathol. 2007;29:33–35. [PubMed] [Google Scholar]
- 50.Shay JW, Keith N. Targeting telomerase for cancer therapeutics. Br J Cancer. 2008;98:677–683. doi: 10.1038/sj.bjc.6604209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andrews LG, Tollefsbol TO. Methods of telomerase inhibition. Methods Mol Biol. 2008;405:1–7. doi: 10.1007/978-1-60327-070-0_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trybek T, Kowalik A, Góźdź S, Kowalska A. Telomeres and telomerase in oncogenesis. Oncol Lett. 2020;20:1015–1027. doi: 10.3892/ol.2020.11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bu X, Jia F, Wang W, Guo X, Wu M, Wei L. Coupled down-regulation of mTOR and telomerase activity during fluorouracil-induced apoptosis of hepatocarcinoma cells. BMC Cancer. 2007;7:208. doi: 10.1186/1471-2407-7-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barthel FP, Wei W, Tang M, Martinez-Ledesma E, Hu X, Amin SB, Akdemir KC, Seth S, Song X, Wang Q, et al. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nat Genet. 2017;49:349–357. doi: 10.1038/ng.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nikolouzakis TK, Vakonaki E, Stivaktakis PD, Alegakis A, Berdiaki A, Razos N, Souglakos J, Tsatsakis A, Tsiaoussis J. Novel prognostic biomarkers in metastatic and locally advanced colorectal cancer: Micronuclei frequency and telomerase activity in peripheral blood lymphocytes. Front Oncol. 2021;11:683605. doi: 10.3389/fonc.2021.683605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hashizume R, Ozawa T, Gryaznov SM, Bollen AW, Lamborn KR, Frey WH, II, Deen DF. New therapeutic approach for brain tumors: Intranasal delivery of telomerase inhibitor GRN163. Neuro Oncol. 2008;10:112–120. doi: 10.1215/15228517-2007-052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Renieri E, Vakonaki E, Karzi V, Fragkiadaki P, Tsatsakis AM. Telomere length: associations with nutrinets and xenobiotics. In: Toxicological Risk Assessment and Multi-System Health Impacts from Exposure. Academic Press. 2021:pp295–306. [Google Scholar]
- 58.Shay JW. Telomerase therapeutics: Telomeres recognized as a DNA damage signal: Commentary re: K. Kraemer et al, antisense-mediated hTERT inhibition specifically reduces the growth of human bladder cancer cells. Clin. Cancer Res. 2003;9:3794–3800. Clin Cancer Res 9: 3521–3525, 2003. [PubMed] [Google Scholar]
- 59.Martinez P, Blasco MA. Telomere-driven diseases and telomere-targeting therapies. J Cell Biol. 2017;216:875–887. doi: 10.1083/jcb.201610111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sugarman ET, Zhang G, Shay JW. In perspective: An update on telomere targeting in cancer. Mol Carcinog. 2019;58:1581–1588. doi: 10.1002/mc.23035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith-Sonneborn J. Telomerase biology associations offer keys to cancer and aging therapeutics. Curr Aging Sci. 2020;13:11–21. doi: 10.2174/1874609812666190620124324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bernardes de Jesus B, Vera E, Schneeberger K, Tejera AM, Ayuso E, Bosch F, Blasco MA. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol Med. 2012;4:691–704. doi: 10.1002/emmm.201200245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.