Abstract
Background and Objectives
To describe the characteristics of patients with MS reporting cryptococcal meningitis (CM) while treated with fingolimod.
Methods
The Novartis safety database was searched for cases with CM between January 26, 2006, and February 28, 2020. The reporting rate of CM was estimated based on the case reports received and exposure to fingolimod in the postmarketing setting during the relevant period.
Results
A total of 60 case reports of CM were identified, mostly from the United States. The median age was 48 years, and 51.8% were women. Most of the patients had recovered or were recovering at the time of final report. A fatal outcome occurred in 13 cases. During the study period, the rate of CM in patients with MS receiving fingolimod was estimated to be 8 per 100,000 patient-years (95% CI: 6.0; 10.0). The incidence of CM seemed to increase with duration of treatment; however, this relationship remains uncertain due to wide CIs and missing data.
Discussion
The causal relationship between fingolimod treatment and CM is not yet fully understood. The CM mortality rate in fingolimod-treated patients is similar to that reported in HIV-negative patients. Vigilance for signs and symptoms of CM in patients receiving fingolimod, particularly the new onset of headaches and altered mental status, is essential. Early diagnosis and treatment are critical to reducing CM-associated mortality.
Cryptococcal meningitis (CM) is an opportunistic infection caused by Cryptococcus sp. that primarily occurs in patients with advanced immunosuppression. Cryptococcus sp. are ubiquitously found in the environment, most commonly associated with eucalyptus trees and bird feces.1,2 The 2 main species of Cryptococcus that are pathogenic to humans are C neoformans var. neoformans and C neoformans var. gattii. C neoformans is responsible for 82% of cryptococcal disease worldwide and chiefly affects immunosuppressed individuals. By contrast, Cryptococcus gattii is reported most frequently in immunocompetent individuals. Although C gatti is most commonly reported in tropical and subtropical regions, it has recently emerged as a human pathogen in the Pacific Northwest of the United States and southern Pacific coastal region of Canada.
Before the emergence of HIV in the mid-1980s, CM was a very rare complication seen in patients with advanced cancer or other serious illnesses/treatments associated with profound immunosuppression.3 Thereafter, HIV became the main risk factor of CM, which became a fairly common manifestation of AIDS until highly active antiretroviral therapies were introduced. Over the last 2 decades, CM has slowly increased above its low background rates in HIV-negative patients, primarily due to increased use of immunomodulatory therapies for a wide range of autoimmune and inflammatory conditions and the immunosuppression accompanying transplantation. Cryptococcal infections arising from pets and birds have also been reported.4,5 CM is among the rare opportunistic infections reported in patients with multiple sclerosis (MS) receiving a variety of disease-modifying treatments (DMTs), including natalizumab,6,7 dimethyl fumarate (DMF),8 and fingolimod.9-16
Fingolimod is a sphingosine 1-phosphate (S1P) receptor modulator approved for the treatment of relapsing forms of MS in patients aged 10 years and older. Fingolimod causes downregulation of S1P receptors expressed on the lymphocytes, thereby preventing the egress of autoreactive lymphocytes from the lymph nodes and reducing the infiltration of T and B lymphocytes into the CNS. This results in reduction in the number of circulating lymphocytes leading to peripheral lymphopenia.17 No association was observed between the nadir in lymphocyte count and the incidence of most common infections in the clinical trials of fingolimod in patients with MS.18 This may be attributed to the selective retention of only naive T cells and central memory cells in the lymph nodes and selective sparing of effector memory T cells by fingolimod.17 On rare occasions, opportunistic infections, including progressive multifocal leukoencephalopathy,19 CM,9-16 and other forms of cryptococcal infection including skin, lung, and disseminated infections,14,20,21 have been reported with fingolimod in the postmarketing setting. In this study, we describe the characteristics of CM case reports in patients with MS treated with fingolimod.
Methods
The Novartis safety database, which captures adverse events reported to Novartis by health-care professionals (HCPs), patients, and from a postmarketing surveillance program or from the literature, was searched for cases with CM using the following search terms: cryptococcal fungemia, cryptococcosis, disseminated cryptococcosis, meningitis cryptococcal, and neurocryptococcosis (Medical Dictionary for Regulatory Activities, version 22.1) from January 26, 2006, the inception of clinical development, to a data lock point of February 28, 2020, a total of 169 months. In cases where adverse event reports were received from a patient, Novartis followed up with the patient's physician if contact information and consent were provided by the patient.
Overall, patient exposure to fingolimod was estimated based on a combination of patient exposure to fingolimod in clinical trials and in the postmarketing setting (estimated from the worldwide sales volume of active substance sold during the period and the defined daily dose of 0.5 mg). The reporting rate of CM in patients treated with fingolimod was estimated using the number of cases with CM and estimated postmarketing exposure to fingolimod. Patient characteristics, details of treatment received, and disease outcome were compiled based on the pharmacovigilance reports received from consumers and HCPs held in the Novartis safety database, and patient identifiers were not revealed.
The risk of CM in relation to duration of fingolimod treatment was further analyzed. The fingolimod commercial drug exposure within each year of treatment was calculated from the average of estimated number of patients at risk at the beginning and end of each period. To avoid effects of small numbers, the annual exposure in the periods corresponding to years 6, 7, and 8 and beyond was combined to derive the number of patient-years of exposure beyond 5 years of exposure. For each period, the number of cases with CM attributed to fingolimod was divided by the corresponding exposure to estimate the incidence (hazard) within each period.
Data Availability
Anonymized data can be made available on request for research purposes by sending a request to the corresponding author.
Results
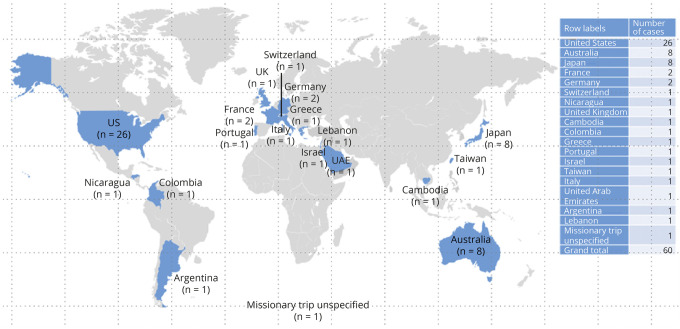
As of February 28, 2020, an estimated 299,600 patients have been treated with fingolimod, corresponding to 778,900 patient-years of exposure (34,300 patient-years in clinical trials and 744,600 patient-years in the postmarketing setting). At this cutoff date, a total of 60 cases with CM were identified in the Novartis safety database, of which 1 was identified in the long-term open-label extension study of fingolimod. Of the 60 cases, 56 cases were primarily reported by HCPs. Most of the cases (N = 26) were reported in the United States and 9 in Europe, followed by 8 cases each in Australia and Japan (Figure 1). One of the 26 cases reported in the United States had a travel history to Cambodia and was later diagnosed with CM. For the remaining 25 patients, no information was available on travel history to endemic areas before the diagnosis.
Figure 1. Geographic Distribution of Cryptococcal Meningitis Cases Reported With Fingolimod Treatment.
UAE = United Arab Emirates; UK = United Kingdom; US = the United States.
Patient Demographics and Baseline Characteristics
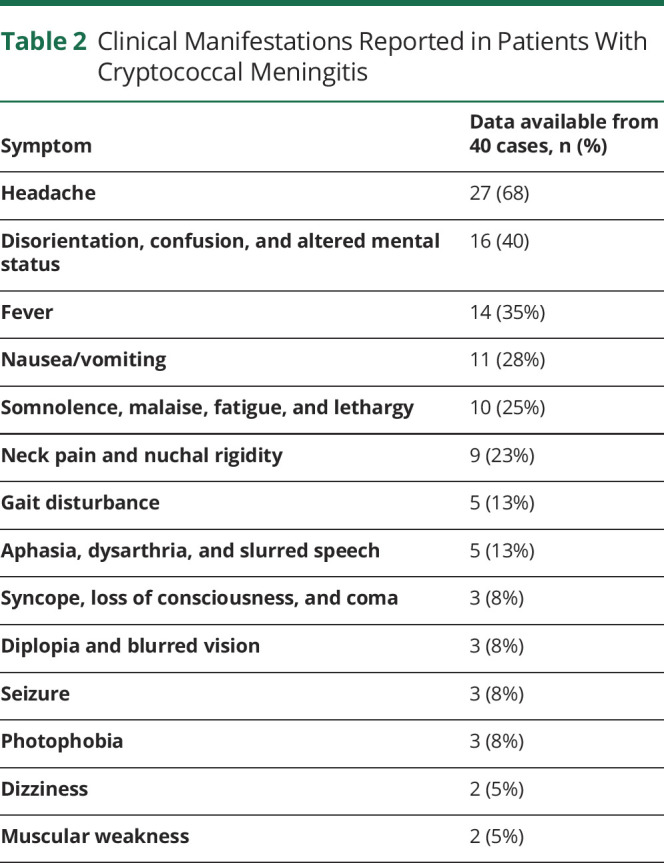
Owing to the postmarketing nature of the cases, information on patient demographics and other details was limited. The mean age based on 52 of 60 cases with known age was 48 years (range, 22–67 years; median age, 50), and 29 of the 56 patients with known sex were female individuals. Time-to-onset data were available in 44 of 60 cases, and in 4 additional cases, an estimate was provided. Most of the patients (45 of 48) for whom information about the duration of fingolimod therapy was provided had an exposure to fingolimod of 2 years or more. For the 44 cases with exact data, the mean time to onset was 48 months (range, 12–108 months; median, 44.5 months; Table 1). Because these cases were from the postmarketing setting, information on the HIV status was limited. However, none of the patients were reported to experience HIV. One patient had an aviary at home with several pet budgerigars.
Table 1.
Demographics and Overview of Cases
In 32 of the 60 cases, no information was provided regarding medical history/concurrent conditions. In the remaining 28 cases where information was available, 9 had medical history/concurrent conditions and/or concomitant therapy that may have been contributory: 2 patients experienced diabetes (both had fatal outcomes, and one of these patients also had concurrent early-stage lung cancer, chronic gastritis, and concomitant IV steroids); 5 patients reported concomitant use of steroids (1 patient also experienced obesity); 1 patient reported concurrent infection (disseminated Candida krusei with pneumonia and Acinetobacter urinary tract infection), and 1 patient experienced immune thrombocytopenic purpura and was treated with steroids.
Clinical Manifestations
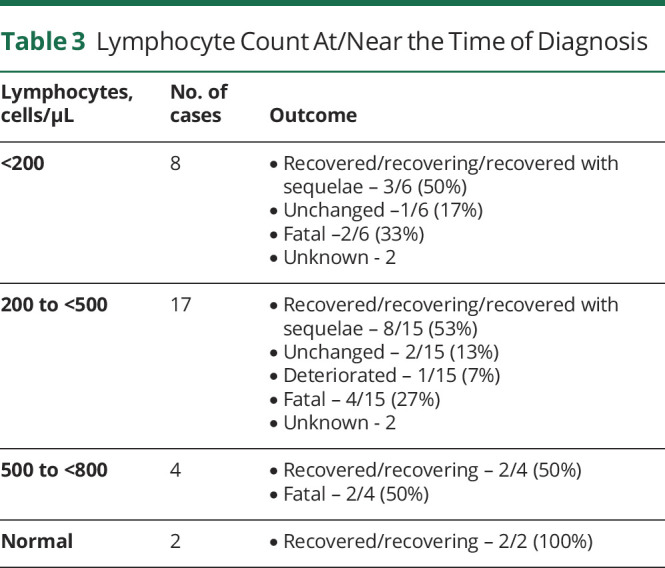
The clinical manifestations that heralded CM were provided in 67% (40 of 60) of the cases (Table 2). The most frequently reported symptoms were headache, disorientation/confusion, and fever. Paresis, agitation, vertigo, nystagmus, ataxia, body aches, memory impairment, decreased appetite, hiccups, tremors, chills, flu-like symptoms, and hallucinations were reported in 1 case each.
Table 2.
Clinical Manifestations Reported in Patients With Cryptococcal Meningitis
Lymphocyte Counts
Information on lymphocyte counts at/near the time of CM diagnosis and the outcome was available in 31 of 60 cases (Table 3). Owing to the small number of cases with information regarding lymphocyte counts, it is not possible to draw any correlations.
Table 3.
Lymphocyte Count At/Near the Time of Diagnosis
Treatment and Disease Outcome
Information on CM treatment medication was limited and was provided in 36 cases. In 31 of these 36 cases, the reported information on CM management seemed to be consistent with standard treatment (amphotericin, flucytosine, or fluconazole) for CM, including high-dose amphotericin-B (4–6 mg/kg/d) in 4 patients and flucytosine (200 mg/kg/d) in 1 patient. In 5 of the 36 cases, it was stated that unspecified antifungal treatment was prescribed. Among them, 58% (21 of the 36) patients recovered/were recovering or recovered with sequelae, and 22% (8 of the 36) had a fatal outcome. In the remaining patients, condition deteriorated in 1, unchanged in 2, and outcome was not reported in 4.
In the remaining 24 cases where treatment information was not provided, 21% (5 of the 24) recovered/recovering, 21% (5 of the 24) had a fatal outcome, condition was unchanged in 1 case, and in 54% (13 of the 24) cases, the outcome was unknown. None of the patients were reported to have developed immune reconstitution inflammatory syndrome.
Mortality
A fatal outcome was reported in 13 cases. Patient age was provided in 11 of these 13 cases, with a mean age of 54 years (range, 33–67 years), which is slightly older than the patients without fatal outcome (mean age, 47 years; range, 22–65 years; age was provided in 40 of 47 cases). These 13 cases included those with (1) poor documentation (n = 2), (2) confounding factors such as use of steroids, candida infection, and concurrent condition of diabetes (n = 6), (3) a history of CM 4 years before fingolimod initiation (n = 1), (4) other illnesses not related to CM (n = 1), (5) fingolimod discontinuation 7 months before onset of CM (n = 1), (6) noncompliance to fingolimod treatment (n = 1), and (7) experience of multifocal cerebral infactions reported to be secondary to meningitis.
Risk Analysis
As of February 28, 2020, the postmarketing exposure to fingolimod exceeded 744,600 patient-years. The estimated reporting rate of CM with fingolimod treatment based on 60 cases was 8 per 100,000 patient-years (95% CI: 6.0; 10.0). The risk of CM in relation to duration of fingolimod treatment was analyzed using 75% of the cases for which data on time to onset was available. Although the risk seemed higher with treatment duration (Figure 2), no definitive conclusion was made because of the large proportion of missing data, low case numbers, and wide 95% CIs.
Figure 2. Incidence of CM per 1,000 Patients by Year of Treatment.
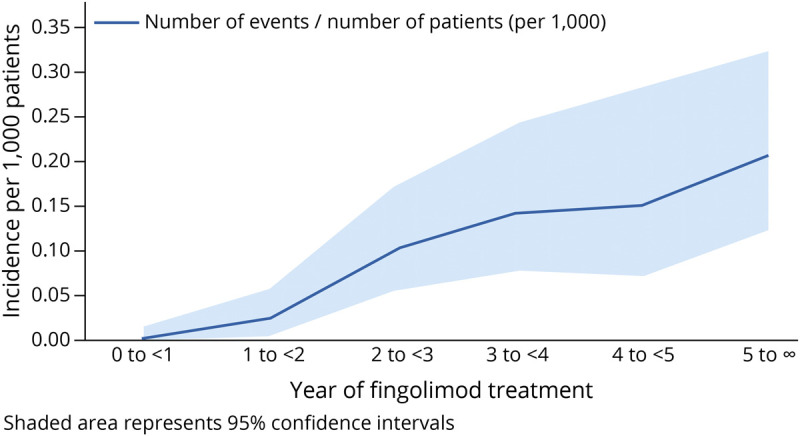
CM = Cryptococcal meningitis.
Discussion
In this study, we reported cases of CM in patients with MS receiving fingolimod treatment. As of February 28, 2020, a total of 60 cases were collected in the postmarketing setting with a reporting rate of 8 per 100,000 patient-years. The relationship between risk of CM and the duration of fingolimod treatment was investigated, but the large proportion of missing data, low case numbers, and wide 95% CIs prevented any definitive conclusions to be drawn on the risk of CM with fingolimod.
The clinical manifestations of CM can be quite subtle, and often, the diagnosis is not considered at the time of initial presentation. Among the cases collected for this study, the most common clinical features that heralded CM were headache (reported in 68% of patients), followed by disorientation, confusion, and altered mental status (reported in 40% of patients). There was limited information available regarding treatment. At the time of final report, however, most of the patients had recovered or were recovering.
Data on CM-associated mortality in patients with MS are scarce to compare the mortality rates in patients with CM on fingolimod. Based on the available data from HIV-associated cases with CM, 1-year mortality rates were high in patients who were not under care vs those who were receiving treatment. A 100% 1-year mortality rate was observed in patients from low-income countries who were not under care and 70% in patients who were under care. For middle-income countries, the 1-year mortality rates were presumed to be 40% and 60% in patients receiving conventional treatment and in those who were not under care, respectively. Similarly, in high-income countries such as Europe and North America, higher mortality rates were observed in patients who were not under care vs those who were under care (Europe: 45% vs 30%; North America: 30% vs 20%).22 Over the years, the mortality in cases with CM not associated with HIV ranged from 9% to 35% in various studies.23-26 A major reason for the high mortality associated with CM in all settings is initial failure to recognize the infection. Delays in the diagnosis and treatment of CM are associated with poor outcomes, especially in HIV-negative and nontransplant patients.27 In this study, 26 of the 43 cases with a known outcome had recovered or were recovering, and 13 of the 60 cases had a fatal outcome at the time of final report (28.2%). The mortality rate observed in our cases is consistent with that reported in CM in HIV-negative patients.26,27
The exact relationship between the mechanism of action of fingolimod and CM infections is not known. Fingolimod exerts its therapeutic effect by down-modulating the S1P1 receptors on T and B lymphocytes and preventing their egress from the lymphoid organs. This in turn prevents the autoimmune lymphocytes from attacking the CNS. In the FREEDOMS trial, 18% of patients who were receiving fingolimod treatment reached a nadir of <200 cells/µL.28 Despite such redistribution of the lymphocytes, no increase in the incidence of infections was observed with fingolimod treatment in the clinical trials.18 However, an increased risk of common infections such as herpes viral infections has been observed in the postmarketing setting.29 Similar to pivotal trial data, in this report, 8 of the 31 patients with available lymphocyte data reported lymphocyte counts <200 cells/µL and 17 patients reported a count of 200–500 cells/µL. Association between lymphocytes count and risk of CM infections cannot be further elucidated because of low case numbers and missing data. Recent preclinical research suggests that fingolimod could reactivate cryptococcosis from granulomas through S1PR3-dependent mechanisms, resulting in disorganization of macrophages and M2 polarization; however, this is likely not the only mechanism given siponimod's low affinity for the S1P3 receptor and association with CM.30,31
To the best of our knowledge, CM cases have been reported only rarely with other DMTs used in patients with MS, such as natalizumab6,7 and DMF.8 Two published case reports of CM with natalizumab were identified, and in both cases, patients were older than 45 years and had been receiving natalizumab infusions for approximately 2 years. Both patients had no history of an impaired immune system. CM was reported in a 46-year-old non-HIV patient who received DMF for 33 months. One confirmed case of CM was also reported with sipoimod, another S1P modulator approved for treatment of secondary progressive MS with active disease. This case occurred in an open-label extension part of a phase 3 trial and involved a 62-year-old woman with no other significant medical history who was treated with siponimod for 30 months31 Rare, nonmeningitic cryptococcal infections, including cutaneous, pulmonary, and disseminated, have also been reported in patients with MS receiving fingolimod in the postmarketing setting14,20,21 and other opportunistic infections, including progressive multifocal leukoencephalopathy, herpes, and varicella.
This report is inherently limited by the quality of the data typically available from spontaneous reports in the postmarketing setting. Cases reported in this study included spontaneous, voluntary reports, patient-only reported events not confirmed by HCPs, and cases found in the scientific literature. The risk estimates were based on the number of cases with CM reported in the Novartis pharmacovigilance database. The patient exposure data used for estimating the risk were derived from sales data. Moreover, this report is limited to cases of CM and not all cryptococcal infections. Cryptococcus can present with rash, and those cases were not included in this analysis.
CM is usually not considered in the initial differential diagnosis for patients with MS presenting with nonspecific neurologic complaints, and this omission can result in a serious delay in diagnosis. A higher index of suspicion is needed in the evaluation of patients treated with fingolimod who present with symptoms, even very subtle symptoms, suggestive of meningitis. Particularly concerning symptoms are new-onset headaches, altered mental status, and fever. Prompt diagnostic evaluation, including lumbar puncture, is mandated.28 Novartis implemented measures to minimize the risk of opportunistic infections including CM in patients taking fingolimod, such as information in the product label and other educational endeavors (educational materials and webinars) that promote greater awareness, earlier detection, diagnosis, and prompt treatment.
Glossary
- CM
cryptococcal meningitis
- DMF
dimethyl fumarate
- DMT
disease-modifying treatment
- HCP
health-care professional
- S1P
sphingosine 1-phosphate
Appendix. Authors
Contributor Information
Maurizio Del Poeta, Email: maurizio.delpoeta@stonybrook.edu.
Brian J. Ward, Email: brian.ward@mcgill.ca.
Benjamin Greenberg, Email: benjamin.greenberg@utsouthwestern.edu.
Bernhard Hemmer, Email: hemmer@tum.de.
Bruce A.C. Cree, Email: bruce.cree@ucsf.edu.
Sreelatha Komatireddy, Email: sreelatha.komatireddy@novartis.com.
Jitendriya Mishra, Email: jitendriya.mishra@novartis.com.
Roseanne Sullivan, Email: roseanne.sullivan@novartis.com.
Ajay Kilaru, Email: ajay.kilaru@novartis.com.
Alan Moore, Email: alan.moore@novartis.com.
Thomas Hach, Email: thomas.hach@novartis.com.
Study Funding
The study was funded by Novartis Pharma AG, Basel Switzerland.
Disclosure
M. Del Poeta is a cofounder and Chief Scientific Officer (CSO) of MicroRid Technologies, Inc. B.J. Ward serves on a scientific advisory board for Novartis and reports personal fees from Novartis for this activity. He is also a medical officer for Medicago Inc and holds parts of patents for vaccines targeting influenza, Clostridioides difficile, and Schistosoma mansoni. In the last 5 years, he has held academic industry awards with Medicago, MIT Canada and Aviex Technologies. B. Greenberg has received consulting fees from Alexion, Novartis, EMD Serono, Viela Bio, Genentech/Roche, Greenwich Biosciences, Axon Advisors, Rubin Anders, ABCAM, Signant, IQVIA, Sandoz, Druggability Technologies, Genzyme, Immunovant, and PRIME Education. He has received grant funding from PCORI, NIH, NMSS, The Siegel Rare Neuroimmune Association, Clene Nanomedicine, and the Guthy Jackson Charitable Foundation for NMO. He serves as an unpaid member of the board of the Siegel Rare Neuroimmune Association. He receives royalties from UpToDate. B. Hemmer has served on scientific advisory boards for Novartis; he has served as DMSC member for AllergyCare, Polpharma, and TG therapeutics; he or his institution have received speaker honoraria from Desitin; and his institution received research grants from Regeneron for MS research. He has been funded by the EU project Multiple MS, the excellence cluster Synergy and the BMBF-funded project Clinspect. He holds part of 2 patents; one for the detection of antibodies against KIR4.1 in a subpopulation of patients with MS and the other for genetic determinants of neutralizing antibodies to interferon. B.A.C. Cree reports personal fees for consulting from Alexion, Atara, Autobahn, EMD Serono, Novartis, Sanofi, Therini, and TG Therapeutics and received research support from Genentech. J.R. Berger reports grants from Biogen and Genentech/Roche; personal fees from Amgen, Biogen, Dr. Reddy's, Encycle, Excision-Bio, Genentech/Roche, Genzyme, Inhibikase, MAPI, Merck, Millennium/Takeda, Morphic, Novartis, Serono, and Shire. S. Komatireddy, J. Mishra, R. Sullivan, A. Kilaru, A. Moore, and T. Hach are employees of Novartis. Go to Neurology.org/NN for full disclosures.
References
- 1.Kozubowski L, Heitman J. Profiling a killer, the development of Cryptococcus neoformans. FEMS Microbiol Rev 2012;36(1):78-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao Y, Lin J, Fan Y, Lin X. Life cycle of Cryptococcus neoformans. Annu Rev Microbiol 2019;73:17-42. [DOI] [PubMed] [Google Scholar]
- 3.De Wytt CN, Dickson PL, Holt GW. Cryptococcal meningitis: a review of 32 years experience. J Neurol Sci 1982;53:283-292. [DOI] [PubMed] [Google Scholar]
- 4.Chang CC, Chen SC. Colliding epidemics and the rise of cryptococcosis. J Fungi (Basel) 2015;21. doi: 10.3390/jof2010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin YY, Shiau S, Fang CT. Risk factors for invasive Cryptococcus neoformans diseases: a case-control study. PLoS One 2015;10(3):e0119090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gundacker ND, Jordan SJ, Jones BA, Drwiega JC, Pappas PG. Acute cryptococcal immune reconstitution inflammatory syndrome in a patient on natalizumab. Open Forum Infect Dis 2016;3(1):ofw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valenzuela RM, Pula JH, Garwacki D, Cotter J, Kattah JC. Cryptococcal meningitis in a multiple sclerosis patient taking natalizumab. J Neurol Sci 2014;340(1-2):109-111. [DOI] [PubMed] [Google Scholar]
- 8.Workel HH, Wolfhagen MJHM, Bouwhuis JW, Kloosterziel ME. Cryptococcal meningitis in a patient with multiple sclerosis on dimethyl fumarate treatment: a case report. Mult Scler Relat Disord 2020;42:102137. [DOI] [PubMed] [Google Scholar]
- 9.Achtnichts L, Obreja O, Conen A, Fux CA, Nedeltchev K. Cryptococcal meningoencephalitis in a patient with multiple sclerosis treated with fingolimod. JAMA Neurol 2015;72(10):1203-1205. [DOI] [PubMed] [Google Scholar]
- 10.Chong I, Wang KY, Lincoln CM. Cryptococcal meningitis in a multiple sclerosis patient treated with Fingolimod: a case report and review of imaging findings. Clin Imaging 2019;54:53-56. [DOI] [PubMed] [Google Scholar]
- 11.Grebenciucova E, Reder AT, Bernard JT. Immunologic mechanisms of fingolimod and the role of immunosenescence in the risk of cryptococcal infection: a case report and review of literature. Mult Scler Relat Disord 2016;9:158-162. [DOI] [PubMed] [Google Scholar]
- 12.Huang D. Disseminated cryptococcosis in a patient with multiple sclerosis treated with fingolimod. Neurology 2015;85(11):1001-1003. [DOI] [PubMed] [Google Scholar]
- 13.Pham C, Bennett I, Jithoo R. Cryptococcal meningitis causing obstructive hydrocephalus in a patient on fingolimod. BMJ Case Rep 2017;2017:bcr-2017-220026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seto H, Nishimura M, Minamiji K, et al. . Disseminated cryptococcosis in a 63-year-old patient with multiple sclerosis treated with fingolimod. Intern Med 2016;55:3383-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anene-Maidoh TI, Paschall RM, Scott Graham R. Refractory cryptococcal meningoencephalitis in a patient with multiple sclerosis treated with fingolimod: a case report. Interdiscip Neurosurg 2018;12:8-9. [Google Scholar]
- 16.Ward MD, Jones DE, Goldman MD. Cryptococcal meningitis after fingolimod discontinuation in a patient with multiple sclerosis. Mult Scler Relat Disord 2016;9:47-49. [DOI] [PubMed] [Google Scholar]
- 17.Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol 2010;33:91-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francis G, Kappos L, O'Connor P, et al. Temporal profile of lymphocyte counts and relationship with infections with fingolimod therapy. Mult Scler 2014;20(4):471-480. [DOI] [PubMed] [Google Scholar]
- 19.Berger JR, Cree BA, Greenberg B, et al. Progressive multifocal leukoencephalopathy after fingolimod treatment. Neurology 2018;90(20):e1815–e1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forrestel AK, Modi BG, Longworth S, Wilck MB, Micheletti RG. Primary cutaneous Cryptococcus in a patient with multiple sclerosis treated with fingolimod. JAMA Neurol 2016;73(3):355-356. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter AF, Goodwin SJ, Bornstein PF, Larson AJ, Markus CK. Cutaneous cryptococcosis in a patient taking fingolimod for multiple sclerosis: here come the opportunistic infections?. Mult Scler 2017;23:297-299. [DOI] [PubMed] [Google Scholar]
- 22.Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017;17(8):873-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiertiburanakul S, Wirojtananugoon S, Pracharktam R, Sungkanuparph S. Cryptococcosis in human immunodeficiency virus-negative patients. Int J Infect Dis 2006;10(1):72-78. [DOI] [PubMed] [Google Scholar]
- 24.Moosa MY, Coovadia YM. Cryptococcal meningitis in Durban, South Africa: a comparison of clinical features, laboratory findings, and outcome for human immunodeficiency virus (HIV)-positive and HIV-negative patients. Clin Infect Dis 1997;24(2):131-134. [DOI] [PubMed] [Google Scholar]
- 25.Lu CH, Chang WN, Chang HW, Chuang YC. The prognostic factors of cryptococcal meningitis in HIV-negative patients. J Hosp Infect 1999;42(4):313-320. [DOI] [PubMed] [Google Scholar]
- 26.Lee YC, Wang JT, Sun HY, Chen YC. Comparisons of clinical features and mortality of cryptococcal meningitis between patients with and without human immunodeficiency virus infection. J Microbiol Immunol Infect 2011;44(5):338-345. [DOI] [PubMed] [Google Scholar]
- 27.Brizendine KD, Baddley JW, Pappas PG. Predictors of mortality and differences in clinical features among patients with Cryptococcosis according to immune status. PLoS One 2013;8(3):e60431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilenya [prescribing Information]. Novartis Pharmaceuticals Corporation; 2019. [Google Scholar]
- 29.Luna G, Alping P, Burman J, et al. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol 2020;77(2):184-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bryan AM, You JK, McQuiston T, et al. FTY720 reactivates cryptococcal granulomas in mice through S1P receptor 3 on macrophages. J Clin Invest 2020;130(9):4546-4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayzent [prescribing Information]. Novartis Pharmaceuticals Corporation; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data can be made available on request for research purposes by sending a request to the corresponding author.